Abstract
Background: Vonoprazan-based triple therapy has recently been reported as being more effective than proton pump inhibitors for the eradication of Helicobacter pylori (H. pylori), but it is apparent that the eradication rate could be further improved. Methods: We investigated the effect of the concomitant use of polaprezinc, a therapeutic agent for gastric ulcers, and vonoprazan-based seven-day triple therapy in patients with gastric ulcers compared to standard vonoprazan-based seven-day triple therapy in patients with atrophic gastritis. The regimen for the treatment of atrophic gastritis contained vonoprazan 20 mg, amoxicillin 750 mg, and clarithromycin 200 mg b.d. (VAC group) for seven days; and that for gastric ulcers contained VAC and polaprezinc 75 mg b.d. (VACP group) for seven days. Results: Between October 2021 and January 2023, 201 subjects were examined (VAC group, n = 165; VACP group, n = 36). In per-protocol (PP) analysis, the eradication rate was significantly higher in the VACP group (100%) than in the VAC group (88.2%) (p = 0.025). In patients with severe atrophic gastritis, eradication rates were significantly higher in the VACP group (100%) than in the VAC group (84.4%) in PP analysis. (p = 0.024). Conclusions: The concomitant use of polaprezinc and standard vonoprazan-based first-line eradication therapy is effective for H. pylori.
1. Introduction
Helicobacter pylori is a Gram-negative bacillus that causes several diseases, including gastric cancer, peptic ulcer, and atrophic gastritis. The eradication of H. pylori is very important for the prevention, cure, and prevention of recurrence of these diseases. Therefore, it is vital to eradicate H. pylori in positive individuals. In Japan, the eradication of H. pylori became covered by insurance in 2003, which was expanded in 2013 to patients with H. pylori-positive atrophic gastritis.
Numerous studies have reported the importance of eradicating H. pylori in preventing gastric cancer [1,2]. Eradication therapy has been shown to be effective in preventing the recurrence of early gastric cancer after endoscopic treatment [3]. However, the eradication rate has recently begun to decrease due to the prevalence of drug-resistant bacteria [4,5]. In particular, the resistance rate to clarithromycin (CAM) is reported to be around 40% in Japan, and the eradication rate with existing treatments is extremely low. Since CAM is a drug frequently used for pediatric, respiratory, and otorhinolaryngological infections, it is thought that H. pylori in the stomach may also develop resistance to it. The various regimens considered in attempts to increase the eradication rate include bismuth quadruple therapy [6], non-bismuth quadruple therapy [7], sequential therapy [8], regimens targeting CYP2C9 [9], and dual therapy [10], among others. It is very important to ensure successful first-line eradication; however, the eradication rates of these regimens are not fully satisfactory, and bismuth preparations are not available in all countries. It has also been shown that bismuth preparations have many side effects. There is a strong need to develop new regimens that are effective and have fewer side effects.
Vonoprazan, a new acid secretion inhibitor, became available in Japan in 2015. Compared to PPIs, this potassium-competitive acid blocker is reported to have higher accumulation in gastric glands due to its higher pKa, slower clearance from gastric glands, and slower dissociation from H+/K+-ATPase upon binding [11]. In addition, since it does not require acid activation, it has a rapid onset of action and is not metabolized by enzymes with polymorphisms [12]. This property is very effective in treating reflux esophagitis. It has also been reported to be more effective in eradicating H. pylori than PPIs [13]. The regimen of eradication is decided by the Japanese health insurance system. When CAM resistance is found in advance, it is recommended that CAM be changed to metronidazole (MNZ), but bacterial resistance to CAM has not always been checked in general clinical practice. A new and easier test for CAM-resistant bacteria that uses the PCR method has recently become available in Japan, and is covered by insurance [14]. It will be widely used in clinical practice in the near future. Therefore, the eradication rate has been limited, even with vonoprazan, and particularly so for CAM-resistant bacteria [15].
Polaprezinc is a chelated compound composed of zinc and L-carnosine. It is widely used in clinical practice in Japan and is approved by the Japanese insurance system as a therapeutic agent for gastric ulcers. The anti-ulcer effects of polaprezinc are thought to be due to its ability to scavenge active oxygen, act as an antioxidant, and promote wound healing [16,17,18,19]. In 1995, Kuwayama was the first to report the effectiveness of polaprezinc in eradicating H. pylori [20]. Subsequently, the efficacy of polaprezinc as an add-on to triple therapy comprising lansoprazole, amoxicillin (AMX), and CAM was reported [21,22]. These reports described eradication therapy using PPIs. Vonoprazan has replaced the main acid secretion inhibitor in eradication therapy, but the usefulness of its concomitant use with polaprezinc needs evaluation. Polaprezinc may be able to replace bismuth preparations. The aim of the present study was to investigate the efficacy of polaprezinc in concomitant use with vonoprazan-based first-line triple therapy.
2. Materials and Methods
2.1. Patients
From October 2021 to January 2023, we treated patients with gastric ulcer with concomitant use of polaprezinc and vonoprazan-based first-line triple therapy for H. pylori eradication, based on the previously reported finding that add-on polaprezinc could improve the eradication rate. Of note, polaprezinc is permitted for ulcer healing by the Japanese insurance system. We reviewed data from the same period and consecutively included the atrophic gastritis cases as controls. Although the diseases differ between the two groups, gastric ulcers are also accompanied by atrophic gastritis. In addition, no report has shown that drug-resistant bacteria that affect eradication are disease-specific. Accordingly, we compared a gastritis group and a gastric ulcer group. Eradication rates and data regarding adverse events were obtained from these two groups of patients. Patients with a history of eradication therapy and those who had undergone gastrectomy were excluded.
2.2. H. pylori Diagnosis
No patient had a history of eradication. H. pylori status was defined as positive in the case of positive rapid urease test (Helicocheck; Otsuka Co., Tokyo, Japan), histology, 13C-urea breath test (UBT tablets 100 mg; Otsuka Co.), or stool antigen test (Meridian HpSA ELISA2, Fujirebio, Tokyo, Japan). Successful eradication was defined as a negative 13C-urea breath test or stool antigen test. The test for assessing the success of eradication was performed at least six weeks after the completion of eradication therapy.
2.3. Esophagogastroduodenoscopy
All patients underwent esophagogastroduodenoscopy (GIF-H290Z) prior to eradication. Patients with gastric mucosal atrophy were classified into the atrophic gastritis group. The degree of atrophic gastritis was evaluated according to the Kimura–Takemoto classification, with C-Ⅰ to Ⅲ classified as mild atrophy and O-Ⅰ to Ⅲ as severe atrophy [23]. Patients with an open or closed ulcer in the stomach were classified into the gastric ulcer group. In the gastric ulcer group, the degree of atrophic gastritis was also evaluated. Two expert endoscopists retrospectively examined endoscopic findings. In this study, image-enhanced endoscopy was not used in all cases.
2.4. H. pylori Eradication Therapy
In Japan, the first-line eradication therapy is determined according to medical insurance guidelines, unless resistant bacteria are identified in advance. In the present patients with atrophic gastritis, H. pylori was eradicated with vonoprazan 20 mg b.i.d., AMX 750 mg b.i.d, and CAM 200 mg b.i.d. for seven days (VAC group). In those with gastric ulcer, H. pylori was eradicated with vonoprazan 20 mg b.i.d., AMX 750 mg b.i.d., CAM 200 mg b.i.d. for seven days, and the concomitant use of polaprezinc 75 mg b.i.d. for seven days (VACP group). Informed consent was obtained from patients in advance regarding the use of polaprezinc for the treatment of gastric ulcers in combination with eradication treatment. Eradication rates were compared between the VAC and VACP groups using intention-to-treat (ITT) and per-protocol (PP) analyses. To examine the effect of atrophic grade of gastritis on the success or failure of eradication, we investigated the eradication rates of severe atrophic gastritis in both the VAC and VACP groups. We also investigated the effect of age on eradication rate in the atrophic gastritis group.
2.5. Statistical Analysis
All statistical analyses were performed with JMP version 14.2 software (SAS Institute, Cary, NC, USA). Continuous variables are presented as the mean ± standard deviation and were compared using t-tests. Eradication rates were compared between the groups using Fisher’s exact test. The frequency of adverse events was also compared between groups using Fisher’s exact test. Statistical significance was set at p < 0.05.
2.6. Ethical Considerations
The Institutional Review Board of Dokkyo Medical University Saitama Medical Center approved this retrospective study (No. 23036, 9 May 2024).
3. Results
3.1. Patient Characteristics
During the study period, 201 patients received eradication treatment. There were 165 patients in the VAC group and 36 patients in the VACP group. There was no difference in mean age or sex ratio between the groups (Table 1).

Table 1.
Patient characteristics.
3.2. Eradication Rates in the VAC and VACP Groups
Figure 1 shows a flow chart of patient enrolment in the study.
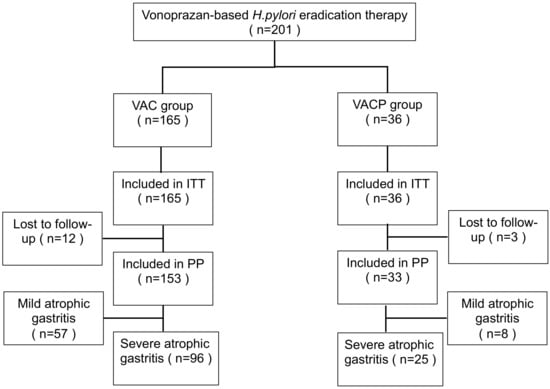
Figure 1.
Flow chart of patient enrollment. A total of 201 subjects were reviewed. Abbreviations: V, vonoprazan; A, amoxicillin; C, clarithromycin; P, polaprezinc; ITT, intention to treat; PP, per protocol.
The ITT analysis revealed eradication rates of 81.8% (135/165; 95% CI 75.2–87.0%) in the VAC group and 91.7% (33/36; 78.2–97.1%) in the VACP group (no significant difference) (Figure 2A). In the PP analysis, the eradication rates were significantly higher in the VACP group (100%, 33/33; 90.0–100%) than in the VAC group (88.2%, 135/153; 82.2–92.4%) (p = 0.025) (Figure 2B).
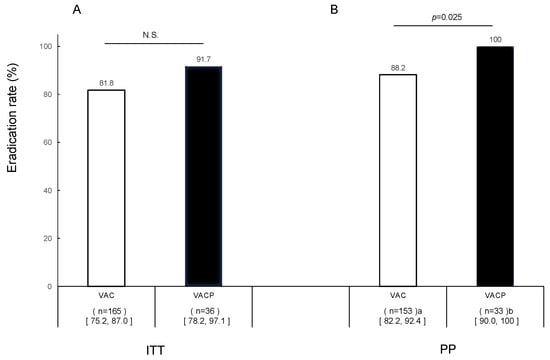
Figure 2.
Efficacy of first-line eradication therapy in all patients. (A) Intention-to-treat analysis and (B) per-protocol analysis. a Missing urea breath data (n = 12); b missing urea breath data (n = 3). Abbreviations: V, vonoprazan; A, amoxicillin; C, clarithromycin; P, polaprezinc; N.S., not significant.
3.3. Eradication Rates in the Severe Atrophic Gastritis Group
In the VAC group, the eradication rates in the ITT analysis were 85.7% (54/63; 95% CI 73.6–91.3%) and 79.4% (81/102; 70.6–86.1%) in the mild atrophic gastritis and severe atrophic gastritis groups, respectively. In the PP analysis, the eradication rates were significantly higher in the mild atrophic gastritis group (94.7%, 54/57; 85.6–98.2%) than in the severe atrophic gastritis group (84.4%, 81/96; 75.8–90.3%) (p = 0.044) In patients with severe atrophic gastritis, the eradication rate in the ITT analysis was 92.6% (25/27; 76.6–98.0%) in the VACP group; and the eradication rate in the PP analysis was significantly higher in the VACP group (100%, 25/25; 86.7%–100%) than in the VAC group (84.4%) (p = 0.024) (Figure 3). The eradication rate in the VACP group did not decrease even in those with severe gastritis.
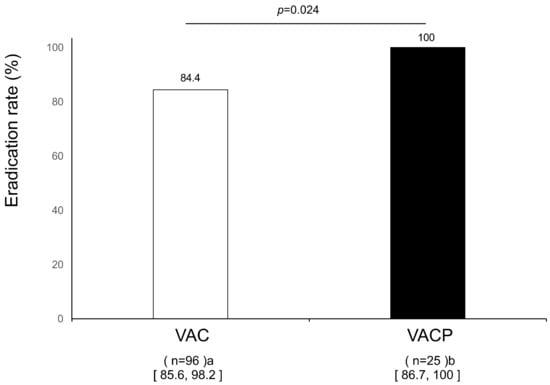
Figure 3.
Efficacy of first-line eradication therapy in severe gastritis patients in per-protocol analysis. a Missing urea breath data (n = 12); b missing urea breath data (n = 3). Abbreviations: V, vonoprazan; A, amoxicillin; C, clarithromycin; P, polaprezinc; N.S., not significant.
3.4. Effect of Age on Eradication Rate in the Atrophic Gastritis Group
After dividing patients with atrophic gastritis according to age (<60 years and ≥60 years), we found no effect of age in either the VAC or the VACP group (Table 2). In the severe atrophic gastritis group, the decreased eradication rates in the VAC group were not affected by age.

Table 2.
Effect of age on eradication rate in the severe atrophic gastritis group.
3.5. Adverse Events
The frequency of overall adverse events was 6.1% (10/165) and 11.1% (4/36) in the VAC and VACP groups, respectively (no significant difference) (Table 3).

Table 3.
Adverse events.
4. Discussion
Since the importance of eradication therapy was recognized, various regimens have been implemented. However, even with these eradication treatments, nearly 5–10% of cases require second-line eradication. According to the Maastricht VI/Florence consensus report, bismuth quadruple therapy is recommended as the first option for the first-line eradication of H. pylori when the proportion of clarithromycin-resistant bacteria is 15% or more or is unknown [24]. Non-bismuth quadruple therapy is recommended as a second option. In Japan, the rate of clarithromycin-resistant strains is reported to be approximately 40% [25]. Therefore, based on the Maastricht VI/Florence consensus report, bismuth quadruple therapy is recommended for eradication therapy in Japan, but bismuth preparations cannot be used for eradication in Japan. In addition, the first- and second-line eradication regimens are fundamentally determined by the Japanese health insurance system. When CAM resistance is found in advance, it is recommended to change CAM to MNZ. In Japan, resistance has not always been investigated in routine clinical practice; however, a simple test for CAM-resistant bacteria is now covered by insurance [15], and it may become more commonly used in the future.
First-line eradication regimens include PPIs that were standard before the vonoprazan era, but the eradication rate has been very low in recent years. In Japan, the proportion of CAM-resistant bacteria is increasing. Mori et al. reported an eradication rate of 69.4% in 2014 [26]. Since vonoprazan became available in Japan in 2015, triple therapy using vonoprazan instead of a PPI has been implemented. Although the eradication rate has improved, many studies have reported eradication rates of around 90% [27,28,29]. This figure is by no means satisfactory compared to other regimens. The ability of vonoprazan to suppress acid secretion is so strong that it is sufficient to activate antibiotics used for eradication. This suggests that further improvements in the eradication rate cannot be expected unless other components are devised. However, the types of antibiotics that are covered by Japanese health insurance are limited, and bismuth cannot be used. For satisfactory eradication therapy, it is necessary to consider drugs to which resistant bacteria are susceptible.
Polaprezinc, a chelate preparation of zinc and L-carnosine (Figure 4) [30], was launched in Japan in 1994 as a treatment for gastric ulcers and has been reported to have good anti-ulcer effects due to its ability to scavenge active oxygen, act as an antioxidant, and promote wound healing [17,18,19]. A multicenter, randomized, double-blind, double-dummy, positive-controlled clinical trial was recently conducted to examine the effects of polaprezinc on gastric ulcers [31].
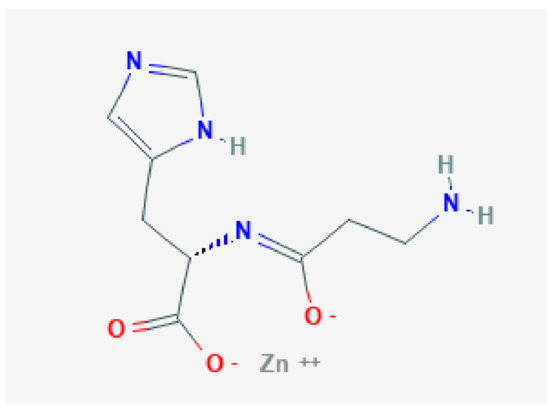
Figure 4.
Polaprezinc, a chelate preparation of zinc and l-carnosine.
Beneficial effects of eradication therapy have been reported using polaprezinc in combination with a PPI and two antibiotics [21,22]. Based on these results, the combined effect of vonoprazan, two antibiotics, and polaprezinc was assessed in the present study. On ITT analysis, there was no significant difference in results between the VAC and VACP groups overall, but on PP analysis, the eradication rate was significantly higher in the VACP group than in the VAC group. This finding indicates that the combined use of polaprezinc and standard vonoprazan-based therapy was effective for eradication, since polaprezinc has a known anti-H. pylori effect [32]. The eradication rate in the VAC group, in which standard triple therapy was administered, was comparable to that in previous reports. It also suggests that there is no great difference between the present rate of CAM-resistant bacteria and those reported in previous studies. There was no significant difference in side effects between the two groups. Accordingly, this regimen should be considered for future use. VACP regimens containing polaprezinc may be replaced by bismuth regimens recommended by the Maastricht VI/Florence consensus.
It is important to consider whether the eradication rate depends on the degree of atrophy. Based on a study that reported a low eradication rate in patients with severe atrophic gastritis [33], eradication rates were examined according to the grade of atrophy. In the VAC group, the eradication rate was significantly lower with severe atrophy than with mild atrophy, as previously reported. In severely atrophic gastric mucosa, the atrophy extends to the gastric body, the number of parietal cells is decreased, and acid secretion is reduced [34]. The pH in the stomach will therefore increase, and one would expect the effect of antibiotics such as AMX and CAM to be strengthened. In other words, severe atrophic gastritis may favor the eradication of H. pylori regardless of the type of acid-suppressing agent. However, since the present eradication rate in severe atrophic gastritis was low, the results are contradictory. In contrast, the eradication rate in the VACP group did not decrease even in those with severe gastritis, possibly because severe atrophic mucosa has been shown to have poorer mucosal blood flow than normal gastric mucosa [35], which may reduce the delivery of antibiotics. The mucus of the gastric mucosa may also be reduced. However, the details of this mechanism are still unknown. Polaprezinc acts directly on the gastric mucosa rather than through blood flow like bismuth, and the antibacterial effect of polaprezinc against H. pylori has been demonstrated [32]. Because of its direct effects, polaprezinc may become a new alternative to bismuth preparations. In addition, the antibacterial effect of polaprezinc might be the same regardless of the clarithromycin-resistance status of bacteria. It is necessary to verify the effect of polaprezinc against CAM-resistant bacteria in future studies.
It is also important to consider the association between age and eradication rate. A previous study reported lower eradication rates in elderly patients [36], whereas another reported low eradication rates in young people, which they considered to be due to low adherence. However, the present data showed no effect of age on the eradication rate in severe atrophic gastritis. In the VAC group, severe atrophy of the gastric mucosa may itself have affected the eradication rate. No effect of atrophy or age was observed in the VACP group.
This study had some limitations. First, this study was retrospective in design. Second, CAM-resistant bacteria were not investigated. However, the present results suggest that CAM-resistant bacteria do not affect polaprezinc. Third, the use of polaprezinc was limited to cases of gastric ulcer, and there were fewer such cases in the VACP group than in the VAC group. Fourth, eradication rates were compared in patients with gastritis and those with gastric ulcers. Although they are not the same disease, no report has shown that the eradication rate of H. pylori, especially CAM-resistant bacteria, differs depending on the disease. In addition, the background mucosa in the gastric ulcer group was also atrophic. Fifth, since gastric mucosal blood flow or mucus layer thickness were not measured directly, one can only speculate on the cause of the decreased eradication rate in the VAC group in patients with severe atrophy. However, since concomitant polaprezinc use improved the eradication rate, drug delivery seems to be involved.
5. Conclusions
In conclusion, this is the first study to examine the effect of the concomitant use of polaprezinc to the standard vonoprazan-based first-line eradication therapy in Japan, and to demonstrate its efficacy, particularly in cases of severe atrophy. These effects may be attributed to the direct effects of polaprezinc. A prospective randomized study that includes an antibiotic susceptibility test is needed to confirm the efficacy of polaprezinc with vonoprazan-based triple therapy in the future.
Author Contributions
Conceptualization, Y.K.; Data curation, Y.F. and K.T.; Investigation, Y.S.; Writing—original draft, Y.S.; Writing—review and editing, Y.K., M.T. (Morio Takahashi) and M.T. (Masaya Tamano). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Dokkyo Medical University Saitama Medical Center (No. 23036, 9 May 2024).
Informed Consent Statement
This study was approved by the Institutional Review Board of Dokkyo Medical University Saitama Medical Center, and the requirement for written informed consent was waived because of the retrospective study design.
Data Availability Statement
The data collected and/or analyzed during this study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lee, Y.C.; Chiang, T.H.; Chou, C.K.; Tu, Y.K.; Liao, W.C.; Wu, M.S.; Graham, D.Y. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology 2016, 150, 1113–1124.e5. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Chen, Y.; Chen, F.; Tao, T.; Hu, Z.; Wang, J.; You, J.; Wong, B.C.; Chen, J.; Ye, W. Effect of Helicobacter pylori Eradication on Gastric Cancer Prevention: Updated Report From a Randomized Controlled Trial With 26.5 Years of Follow-up. Gastroenterology 2022, 163, 154–162.e3. [Google Scholar] [CrossRef] [PubMed]
- Fukase, K.; Kato, M.; Kikuchi, S.; Inoue, K.; Uemura, N.; Okamoto, S.; Terao, S.; Amagai, K.; Hayashi, S.; Asaka, M. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: An open-label, randomised controlled trial. Lancet 2008, 372, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Suga, T.; Nagaya, T.; Arakura, N.; Matsumoto, T.; Nakayama, Y.; Tanaka, E. Antimicrobial resistance and characteristics of eradication therapy of Helicobacter pylori in Japan: A multi-generational comparison. Helicobacter 2014, 19, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Schubert, J.P.; Warner, M.S.; Rayner, C.K.; Roberts-Thomson, I.C.; Mangoni, A.A.; Costello, S.; Bryant, R.V. Increasing Helicobacter pylori clarithromycin resistance in Australia over 20 years. Intern. Med. J. 2021, 52, 1554–1560. [Google Scholar] [CrossRef]
- Ho, J.J.; Navarro, M.; Sawyer, K.; Elfanagely, Y.; Moss, S.F. Helicobacter pylori Antibiotic Resistance in the United States Between 2011 and 2021: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2022, 117, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Lee, S.Y. How to Effectively Use Bismuth Quadruple Therapy: The Good, the Bad, and the Ugly. Gastroenterol. Clin. North Am. 2015, 44, 537–563. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Calvet, X. Review article: Non-bismuth quadruple (concomitant) therapy for eradication of Helicobater pylori. Aliment. Pharmacol. Ther. 2011, 34, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Gatta, L.; Vakil, N.; Vaira, D.; Scarpignato, C. Global eradication rates for Helicobacter pylori infection: Systematic review and meta-analysis of sequential therapy. BMJ 2013, 347, f4587. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Shirai, N.; Takashima, M.; Xiao, F.; Hanai, H.; Sugimura, H.; Ohashi, K.; Ishizaki, T.; Kaneko, E. Effect of genotypic differences in CYP2C19 on cure rates for Helicobacter pylori infection by triple therapy with a proton pump inhibitor, amoxicillin, and clarithromycin. Clin. Pharmacol. Ther. 2001, 69, 158–168. [Google Scholar] [CrossRef]
- Suzuki, S.; Gotoda, T.; Kusano, C.; Ikehara, H.; Ichijima, R.; Ohyauchi, M.; Ito, H.; Kawamura, M.; Ogata, Y.; Ohtaka, M.; et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: A multicentre randomised trial in Japan. Gut 2020, 69, 1019–1026. [Google Scholar] [CrossRef]
- Sakurai, Y.; Nishimura, A.; Kennedy, G.; Hibberd, M.; Jenkins, R.; Okamoto, H.; Yoneyama, T.; Jenkins, H.; Ashida, K.; Irie, S.; et al. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Single Rising TAK-438 (Vonoprazan) Doses in Healthy Male Japanese/non-Japanese Subjects. Clin. Transl. Gastroenterol. 2015, 6, e94. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, H.; Sakurai, Y.; Nishimura, A.; Okamoto, H.; Hibberd, M.; Jenkins, R.; Yoneyama, T.; Ashida, K.; Ogama, Y.; Warrington, S. Randomised clinical trial: Safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment. Pharmacol. Ther. 2015, 41, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Sakurai, Y.; Shiino, M.; Funao, N.; Nishimura, A.; Asaka, M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: A phase III, randomised, double-blind study. Gut 2016, 65, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Kakiuchi, T.; Okuda, M.; Matsuo, M.; Fujimoto, K. Smart Gene as an effective non-invasive point-of-care test to detect Helicobacter pylori clarithromycin-resistant mutation. J. Gastroenterol. Hepatol. 2022, 37, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Mégraud, F.; Laine, L.; López, L.J.; Hunt, B.J.; Howden, C.W. Vonoprazan Triple and Dual Therapy for Helicobacter pylori Infection in the United States and Europe: Randomized Clinical Trial. Gastroenterology 2022, 163, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Shii, D.; Segami, T.; Kojima, R.; Suzuki, Y. Preventive actions of N-(3-aminopropionyl)-L-histidinato zinc (Z-103) through increases in the activities of oxygen-derived free radical scavenging enzymes in the gastric mucosa on ethanol-induced gastric mucosal damage in rats. JPN J. Pharmacol. 1992, 59, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, H.; Oinuma, T.; Sasai, T.; Yamaguchi, N.; Shimada, T.; Terano, A. Polaprezinc protects gastric mucosal cells from noxious agents through anti-oxidant properties in vitro. Gastroenterology 1998, 114, A149. [Google Scholar] [CrossRef]
- Ueda, K.; Ueyama, T.; Oka, M.; Ito, T.; Tsuruo, Y.; Ichinose, M. Polaprezinc (Zinc L-Carnosine) Is a Potent Inducer of Anti-oxidative Stress Enzyme, Heme Oxygenase (HO)-1—A New Mechanism of Gastric Mucosal Protection. J. Pharmacol. Sci. 2009, 110, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Kuwayama, H. Zinc compound is a novel, highly effective triple therapy for eradication of Helicobacter pylori. Gastroenterology 1995, 108, A139, (Abstract). [Google Scholar]
- Kashimura, H.; Suzuki, K.; Hassan, M.; Ikezawa, K.; Sawahata, T.; Watanabe, T.; Nakahara, A.; Mutoh, H.; Tanaka, N. Polaprezinc, a mucosal protective agent, in combination with lansoprazole, amoxycillin and clarithromycin increases the cure rate of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 1999, 13, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.; Abuelazm, M.; Ahmed, A.A.S.; Abdalshafy, H.; Abdelazeem, B.; Brašić, J.R. Efficacy and Safety of Polaprezinc-Based Therapy versus the Standard Triple Therapy for Helicobacter pylori Eradication: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 4126. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Takemoto, T. An Endoscopic Recognition of the Atrophic Border and its Significance in Chronic Gastritis. Endoscopy 1969, 1, 87–97. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Mégraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Mori, H. World trends for H. pylori eradication therapy and gastric cancer prevention strategy by H. pylori test-and-treat. J. Gastroenterol. 2017, 53, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Suzuki, H.; Omata, F.; Masaoka, T.; Asaoka, D.; Kawakami, K.; Mizuno, S.; Kurihara, N.; Nagahara, A.; Sakaki, N.; et al. Current status of first- and second-line Helicobacter pylori eradication therapy in the metropolitan area: A multicenter study with a large number of patients. Ther. Adv. Gastroenterol. 2019, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Gotoda, T.; Kusano, C.; Iwatsuka, K.; Moriyama, M. The Efficacy and Tolerability of a Triple Therapy Containing a Potassi-um-Competitive Acid Blocker Compared With a 7-Day PPI-Based Low-Dose Clarithromycin Triple Therapy. Am. J. Gastroenterol. 2016, 111, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Shichijo, S.; Hirata, Y.; Niikura, R.; Hayakawa, Y.; Yamada, A.; Mochizuki, S.; Matsuo, K.; Isomura, Y.; Seto, M.; Suzuki, N.; et al. Vonoprazan versus conventional proton pump inhibitor-based triple therapy as first-line treatment against Helicobacter pylori: A multicenter retrospective study in clinical practice. J. Dig. Dis. 2016, 17, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Noda, H.; Noguchi, S.; Yoshimine, T.; Goji, S.; Adachi, K.; Tamura, Y.; Izawa, S.; Ebi, M.; Yamamoto, S.; Ogasawara, N.; et al. A Novel Potassium-Competitive Acid Blocker Improves the Efficacy of Clarithromycin-containing 7-day Triple Therapy against Helicobacter pylori. J. Gastrointestin. Liver Dis. 2016, 25, 283–288. [Google Scholar] [CrossRef] [PubMed]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Promac#section=Structures (accessed on 18 December 2023).
- Shen, W.; Zhao, X.; Han, Z.; Miao, Y.; Huang, H.; Zhang, Z.; Dong, L.; Nie, Y.; Li, H.; Ni, R. Efficacy and safety of polaprezinc in the treatment of gastric ulcer: A multicenter, randomized, double-blind, double-dummy, positive-controlled clinical trial. Med. Eng. Phys. 2022, 110, 103860. [Google Scholar] [CrossRef] [PubMed]
- Sunairi, M.; Watanabe, K.; Suzuki, T.; Tanaka, N.; Kuwayama, H.; Nakajima, M. Effects of anti-ulcer agents on antibiotic activity against Helicobacter pylori. Eur. J. Gastroenterol. Hepatol. 1994, 6 (Suppl. 1), S121–S124. [Google Scholar] [PubMed]
- Kalkan, I.H.; Sapmaz, F.; Güliter, S.; Atasoy, P. Severe gastritis decreases success rate of Helicobacter pylori eradication. Wien. Klin. Wochenschr. 2015, 128, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Sipponen, P.; Kekki, M.; Seppala, K.; Siurala, M. The relationships between chronic gastritis and gastric acid secretion. Aliment. Pharmacol. Ther. 1996, 10 (Suppl. 1), 103–118. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Kimura, K.; Yoshida, Y.; Seki, M.; Kumakura, Y.; Satoh, K.; Kihira, K.; Ido, K.; Mato, M. Age-related changes in the microvessels of the human stomach: An ultra-structural study. J. Clin. Gastroenterol. 1993, 17 (Suppl. 1), S92–S98. [Google Scholar] [CrossRef] [PubMed]
- Kusunoki, M.; Yuki, M.; Ishitobi, H.; Kobayashi, Y.; Nagaoka, M.; Takahashi, Y.; Fukuba, N.; Komazawa, Y.; Shizuku, T.; Kinoshita, Y. Effect of Age on Effectiveness of Vonoprazan in Triple Therapy for Helicobacter pylori Eradication. Intern. Med. 2019, 58, 1549–1555. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).