Abstract
Microorganisms are ubiquitous and have been exploited for centuries to generate primary and secondary metabolites essential for human welfare and environmental sustainability. Microorganisms occupy a prominent position in the industrial sector due to their unique properties, such as the limited time and space required for their growth and proliferation, as well as their easy manipulation of the genetic material. Among all the microorganisms, probiotics have grabbed the attention of researchers because of their nonpathogenic nature and immersive application in treating digestive ailments and vitamin deficiency, boosting immunity, and detoxifying harmful chemicals. Furthermore, probiotics are widely used to treat various diseases such as constipation, colon cancer, type 2 diabetes mellitus, and obesity, as well as a range of intestinal disorders, including inflammatory bowel disease, among others. The updated information on these diseases and the role of probiotics has not been updated in the past few years. The present review covers updated information on the role of probiotics in these topics. The growth of populations around the globe has attracted the attention of scientists, primarily investigating diverse technologies to meet the gap between probiotic production and demand. With the support of standardized tools and techniques, researchers have explored the potent probiotic strains feasible for industrial production and treating health ailments. In the current review, we have curated the potential information essential for the screening, strain selection, production, and application necessary for probiotic researchers.
1. Introduction
Microorganisms, such as bacteria, fungi, archaea, protists, plankton, and amoebae, are prevalent in our day-to-day lives. The most recent estimate is that about 38 trillion (1012) microorganisms live in and on human individuals and play a crucial role in stimulating the immune system, detoxifying potential toxins, and synthesizing vitamins and amino acids essential for cellular metabolic functions. Among all the genera of microorganisms, Lactobacillus, Bifidobacterium, Escherichia coli, Clostridium, Streptococcus, Peptococcus, Ruminococcus, Fusobacterium, Bacteroidetes, Actinobacteria, Proteobacteria, Bacteroides, and Eubacterium are dominant in the regulation of human metabolic homeostasis. Human gut microbiome diversity and abundance are significantly reduced when exposed to therapeutic leads like antibiotics, proton pump inhibitors, non-steroidal anti-inflammatory drugs, antacids, antidepressants, sleeping pills, laxatives, and statins. This is followed by changes in the metabolic activity of the host gut microbiota [1]. The reduction or removal of these microbial flora causes toxic product accumulations that impair cellular processes and prevent vitamin synthesis, resulting in malnourishment and impairing the host system’s anabolic and catabolic reactions, which are crucial for the regulation of the biological system. As a result, there is a growing variety in the market of probiotic-containing foods and supplements. In 2001, the World Health Organization (WHO) and the Food and Agriculture Organization (FAO) organized an expert meeting that resulted in the definition of probiotics as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host”. Later, in 2014, this definition was modified for grammatical reasons [2].
Probiotics are becoming more and more popular in the healthcare industry, and by 2024, supplement sales are expected to reach USD 35–65 billion. In the 20th century, the field of probiotic research was investigating new strains of probiotics; however, Nobel laureate Élie Metchnikoff discovered that adding lactic acid-producing bacteria to dairy products improved the defense system’s performance and had a greater therapeutic effect on the host system. A few fermented food product reports stated that Brem [Bali, Indonesia], Rusip [Indonesia], Kimchi [Korea], Gochujang [Korea], Kefir [Russia], Gundruk [India], Khalpi [Nepal], Wine [America], Garris [Sudan], Yoghurt [Mesopotamia, Central Asia], and Ergo [Ethiopia] favor the growth of probiotic genera such as Streptococcus, Enterococcus, Alloiococcus, Aerococcus, Lactococcus, Oenococcus, Vagococcus, Lactobacillus, Carnobacterium, Pediococcus, Leuconostoc, Tetragenococcus, Weissella, Bifidobacterium, Symbiobacterium, and Atopobium [1].
Regarding the outcome of the probiotics research, a couple of guidelines about efficient strain design and development were introduced in the 1980s. As per these guidelines, therapeutic probiotics must meet all the following criteria: (a) strains must show a symbiotic, therapeutic effect; (b) they must be non-immunogenic and non-pathogenic; (c) strains should be compatible with the host system’s microbial environment, and be adaptable in the host system by keeping their variability; (d) strains should protect the healthy environment of the gut microbial flora; (e) during production, formulation, and storage, strains must be stable in their metabolic activities [2]. By keeping the standard guidelines, researchers explored the health benefits of potent probiotic strains ranging from gene to species level to avoid species-level variation effects in the treatment process. With the existing literature, tools, and technologies, probiotic researchers conducted experiments on human and animal models to prove the clinical potential and efficacy of various probiotic strains against numerous health ailments. The potential studies and clinically reported data confirmed that the probiotic strains are feasible for treating diarrhea, lactose intolerance, antimicrobial therapy, and anti-colorectal cancer activities. It was also reported that a few strains are also involved in reducing irritable bowel conditions and inflammations in the gut of the host system [3,4].
Selecting clinically important probiotic organisms with high durability is very crucial; previous reviews focused on any one of the probiotics and their applications. In this review we have broadly emphasized different probiotics and their applications, beginning with the screening, characterization, production, and application studies (with suitable examples). We also summarized recent findings for probiotic strain selection and the determination of their viability, production, and applications, which are essential for probiotic researchers in finding novel therapeutic probiotic strains.
2. Probiotic Strain Selection Criteria and Requirements
To meet the clinical requirements, EFSA (European Food Safety Authority), WHO, and FAO issued mandatory guidelines to probiotic researchers, stating that the strains must meet safety and functionality requirements, such as the route of strain selection, nonpathogenic, non-immunogenic nature, resistance to antibiotics, long durability in the gastrointestinal tract, and the ability to maintain their activity during production, processing, and preservation, which are crucial for patient safety [5,6]. The carriers or matrix employed in the formulation are also vital since they can impair the strain’s viability, lowering the product’s quality [7,8]. The following critical factors are tested during the initial screening and selection of probiotics:
- Stability of phenotypes and genotypes, including plasmid stability;
- Tolerance to bile and acid, as well as survival and growth;
- The adhesion characteristics of intestinal epithelial cells;
- Antimicrobial compound production;
- Patterns of antibiotic resistance;
- Inhibition of known gut pathogens;
- Immunogenicity, spoilage organisms, or both.
2.1. Probiotics with the Best Characterization: In Health Point
Before denoting beneficial microorganisms as probiotics, most culturable microbiota are promoted in fermented food products for their health-promoting activities (Figure 1). Among all the culturable microorganisms, lactic acid bacteria (LAB), used in yogurt, cheese, and pickles, attained a prominent position as the best probiotic supplement due to its unique properties (as mentioned by EFSA) and lack of lipopolysaccharides (LPS) and harmful extracellular proteases. During the research for efficient probiotics, researchers reported that Lactococcus and Streptococcus are predominant in the human ileum and jejunum, as well as, at lower densities, in the colon. This symbiotic relationship raised researchers’ attention to the molecular mechanisms that make these strains suitable for treating intestine-related ailments. With the advent of sophisticated technology, researchers found that these strains colonized the intestine for a limited time by releasing primary and secondary metabolites extracellularly without trapping them in the periplasm. This indigenous property prompted the researchers to develop engineered therapeutic probiotics, keeping Lactobacillus strains as reference strains to deliver molecules directly to the mucosa without any drawbacks or adverse effects on systemic distribution [9].

Figure 1.
Factors that influence the viability of probiotics in foods and the gastrointestinal system.
2.2. Probiotic Viability: What Factors Affect It?
To make the therapeutic formulation, it is also essential to consider the physiochemical parameters associated with the food products that influence the probiotic viability and functionality during production and preservation [Figure 1]. Intrinsic product parameters such as pH, salt, oxygen concentration, water, sugar content, and other elements such as fermentation settings and microbiological parameters, are among these factors [10]. On an industrial scale, including probiotics in meals poses several microbiological, technological, and economic problems.
Based on previous studies, the encapsulation of probiotics enhanced the cell viability of yogurt samples. Multilayer emulsion was an effective tool in preserving the viability of bacteria at the recommended effectiveness level [11,12]. Probiotic bacteria encapsulation is an emerging technology that facilitates the incorporation and protection of efficient strains in functional foods to meet therapeutic needs. Nonetheless, certain probiotic viability enhancement technologies, like microencapsulation, increase the cost of food manufacturing. To minimize the cost and meet the demands in the globalized market for functional products, it is necessary to explore inexpensive technologies to keep the product cost within accessible limits. The identification of appropriate bacterial strains, as well as the microencapsulation materials and technique, are significant problems that must be addressed further. The effectiveness, durability, and ecological acceptability of the microencapsulation techniques employed are crucial. Implementing microencapsulation on an industrial scale is hampered by several issues [13,14]. Different microencapsulation technologies have not been fully utilized yet and require more testing before they can be deployed correctly in real food matrices.
Microencapsulation industries are facing technological difficulties in maintaining optimistic, higher-beneficial-value goods. In this situation, the food industry will need additional resources and skills to successfully present the most innovative technologies to develop the next generation of food products [15]. To enhance the viability of probiotic strains during processing and preservation, and to overcome the adverse gastric conditions in the gastrointestinal system, extensive research should be conducted, providing appropriate technologies for microbial strain screening and encapsulation matrixes involved in the protection of probiotics in gastric conditions.
2.3. Physicians’ Guidance
In microbiome research, the discovery rate of new organisms with therapeutic potential for the human host is rapidly growing. Some microbial strains with systemic immunomodulatory functions are being studied in new ways [16], including food allergy diagnosis and treatment [17,18], regulation of the gastrointestinal–liver axis [19], neuroactive metabolite synthesis [20], and in regard to their antimicrobial action in the gastrointestinal system, skin, and urogenital tract [21]. Furthermore, microorganisms are increasingly recognized as being crucial to various metabolic functions [Figure 2] [22]. New microbial-based therapies will develop as a result of these discoveries, and physicians should review the following parameters before considering these strains for therapeutic applications:
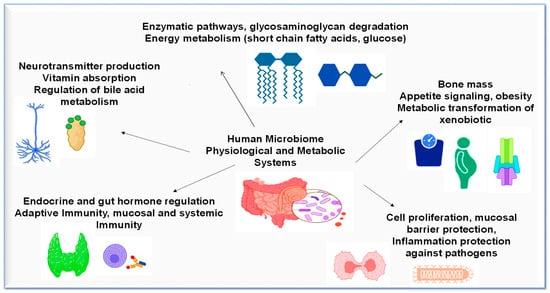
Figure 2.
Physiological and metabolic processes in human microbiome.
- Evidence that the strains were tested in a randomized, controlled, or comparable human experiment and categorized based on specific host or microbial genetic characteristics in a varied population.
- In the product, the dose and viability are the same as in the human experiment.
- There is whole-genome strain characterization and precise strain designation available.
However, many fundamental metabolic activities are maintained among individuals in a community, according to human microbiome research. Despite substantial interpersonal variability at the species level, many essential metabolic activities are carried out among individuals in a population [5]. As the subject of personalized medicine grows in popularity, proponents of personalized therapies must explicitly identify the foundation for group separation and validate the efficacy of the proposed sophisticated treatment in the targeted subpopulation [23]. The discovery and validation of microorganisms having significant and repeatable impacts across a varied population would be a more general strategy [24]. If the influence on human health is established in a controlled human study, therapies in a stratified and diverse population would meet the probiotic recommendations [22].
2.4. Dairy Starter Cultures of Probiotic for Manufacturing
With the available knowledge to get rid of any microbial-associated ailments, customers seek probiotic-supplemented dietary and dairy products. One such traditional dairy product is yogurt. Yogurts are made with Lactobacillus bulgaricus and Streptococcus thermophilus starter cultures, and they have become very popular among consumers as nutritious foods. Yogurt cultures are generally acknowledged to have been designated as probiotics because of their advantageous impacts on human health. Popović et al. characterized the functional yogurt starter cultures of the autochthonous strains S. thermophilus BGKMJ1-36 and L. bulgaricus BGVLJ1-21, which were isolated from artisanal sour milk and yogurt, respectively, and have health-promoting qualities. The strains BGKMJ1-36 and BGVLJ1-21 possess the capacity to hydrolyze αs1-, β-, and κ-casein, form curd after five hours at 42 °C, and exhibit antibacterial activity against Listeria monocytogenes. The strain BGKMJ1-36 generates exopolysaccharides that are crucial to the yogurt’s rheological characteristics. The strains of BGVLJ1-21 and BGKMJ1-36 colonies passed through the yogurt’s simulated gastrointestinal system [25]. In manufacturing, the targeted probiotic-supplemented product should have efficient and viable cells that can sustain the adverse condition, be stable, and work consistently in the various treatments [26]. Customers anticipate a product with a high count and long-lasting viability across a wide range of ambient humidity and temperatures, and so stretching on a high-quality functional food supplement should be considered in clinical research [27]. On the other hand, customers want quick and efficient strains involved in the rapid acidification of milk and milk products for more therapeutic benefits. The process methodologies are briefly detailed in the production process, and critical problems for manufacturing and troubleshooting uniform product performance are highlighted.
2.5. Production and Strains Development
It is critical to scale up to an intermediate level in the pilot to evaluate and mitigate these more typical production conditions before moving to economical assembly. Freeze-drying methodologies must be regularly assessed before formulation, from prototype to commercial production. The probiotic dose levels used in the final product should be based on those proven to be effective in human trials. Colony-forming units per gram of product are also a crucial factor. Although there is still a lack of knowledge regarding the minimum effective concentrations, it is generally agreed that probiotic products should have a minimum concentration of 106 cfu/g and that a total of 108 to 109 probiotic microorganisms should be consumed daily for the probiotic effect to be transferred to the consumer [27,28]. The strains should also be able to develop under standard manufacturing and commercial circumstances and should maintain viability throughout storage.
Maintaining optimum freeze-drying intervals while manufacturing the probiotic product is crucial. This is done by monitoring the pressure, temperatures, pellet bed thickness, water content, cell quality, and quantity, followed by viability stability [27]. To protect cell viability during processing, the cryoprotectant composition or dosage might be changed [28]. It is critical to figure out whether the freeze-drying system in the manufacturing unit scales up to an industrial scale. The frequency of freeze-drying cycles, condensing, and heat transfer rates must be evaluated to eliminate the moisture content generated during product drying and maintenance to obtain an efficient product.
A strain’s development qualities vary slightly from strain to strain as well. L. acidophilus is eosinophilic and exhibits strong acid and bile resistance. It may proliferate and develop in conditions that prevent other LABs from doing the same. Moreover, it can perform homotype fermentation using glucose, fructose, lactose, and sucrose, as well as ferment DL-lactic acid. During the development process, significant attention is paid to comprehending the production of probiotics on a commercial scale and evaluating the strains’ performance in the lab under identical circumstances. Every stage during the procedure depends on the one before, so detecting strain-dependent sensitivities and maintaining the cells’ viability during production and processing are critical. Harvesting the cells from the production medium might take several hours due to the enormous volume of the medium to be passed. In the initial downstream lab, scale centrifugation plays a significant role in separating small quantities. To keep cells in a viable mode during downstream processing, large-scale centrifugation, which generates heat and shear effects, must be avoided [28]. Additionally, during commercial-scale production, many processes where cells are pumped do not usually occur during counter-scale research and development. Furthermore, in economic manufacturing, cells are exposed to various situations, such as pH, temperature, and other conditions that remain challenging to replicate at the lab scale. For instance, Lactobacillus acidophilus essentially cannot grow at temperatures below 20 °C, and their ideal culture temperature is often 35–38 °C. L. acidophilus grows best at a pH of 5.5–6.0 and is not very heat resistant. Moreover, B. bifidum, B. breve, and B. longum are common Bifidobacterium strains employed to prevent and treat gastrointestinal disorders, including intestinal infections and cancers. For instance, the ideal growth temperature ranges for strains isolated from humans and animals are 36–38 °C and 41–43 °C, respectively. Furthermore, it has been discovered that B. animalis and B. thermacidophilum are also metabolically active at pH 3.5–4.0, with pH 6.5–7.0 being the ideal range for growth. With a few exceptions, like B. boum, B. thermophilum, B. dentium, and B. psychraerophilum, which may survive in microaerophilic environments, the majority of Bifidobacterial species are strict anaerobes [29,30].
2.6. Nutritional Necessities of the Strain
Nutritional needs for the growth and development of probiotic strains are crucial; carbon and nitrogen sources, especially amino acids, will facilitate the metabolic functions of the LAB and Bifidobacteria strains. Modifications to raw materials can significantly impact growth and productivity. Complex substances, like protein sources such as milk and yeast extract, simpler ingredients like salts, and simple carbohydrates, are commonly used as carbon sources. The nutritional microbes can adapt to the environment, and the source from which they were obtained is typically linked to the intricacy of these autotrophic and nutrient requirements [29]. Lactobacillus plantarum, for example, contains fewer autotrophs since it is generated from plant material [30]. Lactobacillus johnsonii, isolated from the upper gastrointestinal tract of a human, has more biosynthetic self-sufficiency and is found in an environment with more resources, such as tiny peptides, amino acids, and polysaccharides [31]. Creating a unique fermentation medium for microbial needs facilitates the high-performance end product [9,32,33,34,35,36,37]. Additionally, cane and beet molasses are available globally, offering high performance and quality that can be utilized in fermentations with peptones and yeast extracts to produce long-lasting results [38].
2.7. Raw Material Production
Since the fermentation medium is vital in producing bifidobacteria and LAB, alterations in the rare constituents can significantly impact performance and growth development. The supplier’s modifications to raw constituents could result from cost reductions to procedure enhancements, production method variations, or an ingredient source change. Compositional differences in intricate substances, such as milk, yeast extract, etc., and limited complex constituents, mainly salts and carbohydrates, are more evident. Based on the nutritional necessities and sensitivities of the strains being produced, a good amount of variation in intricate essential ingredients can go undiscovered, with some strains having consistent results. In contrast, the effectiveness of other strains is more negatively or positively impacted. With more complex substances like yeast extract, the strain performance changes more than the peptide size distribution, amino acid, nucleotide, carbohydrate, vitamin, and salt levels. Baker’s yeast is grown with beet, and cane molasses were isolated for yeast extract and peptone for food applications [38]. During manufacturing, the components used to culture the yeast to form the yeast extract and peptone cannot affect strain performance [39]. Furthermore, beet molasses and cane can be supplied, having demonstrated effective performances across the globe and a level of quality that can have long-term impacts when combined with yeast extracts and peptones in fermentations. Following investigations involving humans and demonstrating that bacteria qualify as probiotics, the next step is to investigate further to determine whether the strains can be cultivated on a large scale and effectively integrated into consumer items [40,41]. To avoid studying an uncommercialized strain, this stage of probiotic commercialization should occur concurrently with clinical trials. Pilot-scale culturing and industrial production have extremely diverse strain necessities compared to laboratory culturing [42,43]. The medium requirements also vary due to the production cost. A quality control program must be developed to assure the strain’s high efficiency, from inputs to the final product. It is advisable to maintain a quality assurance cell to conduct and produce dependable manufacturing procedures. All of this necessitates meticulous documentation of procedures and outcomes. After generating a sizeable probiotic bulk, the probiotic strains must be supplemented into consumer food products [44]. During the process, ranging from protecting shelf life to the storage conditions of the product, these products have various requirements. In any case, the consumer should receive a minimally effective dose at the end of the shelf life. Because probiotics are living microbes, this is a difficult task. It is essential to investigate and determine the health benefits for use with both humans and animals, then convey these benefits to the user by selecting the appropriate strains, culture conditions, and manufacturing processes [45].
3. Applications of Probiotics
Probiotics influence the intestinal microbiota, enhance the synthesis of short-chain fatty acids (SCFA), and reduce the chance of developing diseases [46]. The details are mentioned in Figure 3. Probiotics minimize the risk of several diseases, such as constipation, colon cancer, type 2 diabetes mellitus, and obesity, and they treat a range of intestinal disorders, including inflammatory bowel disease, among others [47].

Figure 3.
The probiotic clinical applications.
3.1. Probiotic’s Role in Colorectal Cancer
Probiotics are recognized bioactive compounds that can be used to cure a wide range of diseases. Research utilizing probiotics showed that digestive tract microbiota modulation inhibited the growth of malignancies. Similarly, probiotic supplements protect patients with colorectal cancer against side effects associated with their treatment [48]. According to the studies, a number of factors, including strain, host physiology, dosage, duration of the intervention, and other food supplements, can be used to assess the therapeutic efficacy of probiotic supplementation [49]. Probiotic therapy reduces carcinogens, boosts the microbiota, creates antimicrobials and anti-carcinogenic compounds, and enhances tight junction function, intestinal permeability, and enzyme activity in patients with colorectal cancer [49]. Numerous probiotic species, their metabolites, and other prebiotic components have been found to influence gut immunity and colon cancer incidence. Probiotics have been demonstrated to have immune system-regulating, anti-oxidative and anti-inflammatory properties. They can reduce lactose intolerance, minimize inflammation, and prevent diarrhoea [50].
Probiotics have been linked to colorectal cancer, according to research, and data from many trials suggests that a single-genus and multistrand probiotic may be an additional treatment option for patients with colorectal cancer [48]. Using in vitro and in vivo investigations, the inhibitory impact of probiotics on colorectal cancer has been demonstrated. Probiotic administration increased CD8 cell activation while decreasing and reducing the growth of CT26 tumors. Moreover, the probiotic supplements reduced the migration, proliferation, and invasion of CT26 cells. Research has demonstrated that a variety of probiotics can cooperate to boost the immune system and slow the growth of tumors, suggesting that probiotics may prove to be a potent new anticancer medication for use in future treatment plans [49]. Butyrate, a compound produced by the gut microbiota, is widely known for its positive effects on health. The effects of butyrate’s antiproliferative properties on colon cancer cell lines, HCT116 and SW620, were investigated. Butyrate has been shown to inhibit cancer cells from growing by decreasing the biomarkers. Moreover, catenin degradation was demonstrated by the Wnt/β-catenin signaling pathways, which further reduced the transcriptional activity of malignant cells [50]. When more thorough descriptions of gut biodiversity and precise assessments of changes in response to anticancer therapy are achievable, the significance of probiotics in avoiding cancer and minimizing its side effects will become evident. Furthermore, many probiotic strains, such as L. acidophilus HB56003, L. casei, L. paracasei IMPC2.1, L. rhamnosus GG, L. plantarum, Streptococcus thermophilus HB5621, Enterococcus faecalis HB62001, Bifidobacterium longum HB55020 are commercially available to treat colon cancer.
3.2. Probiotic’s Role in Type 2 Diabetes Mellitus and Obesity
Probiotic supplements with many strains were administered to type 2 diabetes mellitus (T2DM) patients who had not used medication for 12 to 13 weeks. This resulted in a small reduction in abdominal fat and a significant increase in homeostasis model assessment of insulin resistance (HOMA-IR) [51]. In type 2 diabetics, probiotics only slightly lowered fasting insulin levels and glycated hemoglobin (HbA1c). You might absorb fewer calories from food if you take probiotics; additionally, they affect the amounts of proteins and hormones linked to hunger and fat storage, potentially reducing inflammation, which lowers the risk of obesity. To fully assess the role of probiotics in T2DM patients, as well as establish the hypothetical background for probiotics to be widely used in clinical settings to treat fasting blood glucose (FBG), HbA1c, and T2DM homeostasis model evaluation of insulin resistance, research studies have demonstrated the impact of probiotics on three significant T2DM markers.
Tao et al. investigated the efficacy of probiotics in T2DM by meta-analysis. The outcome of the meta-analysis showed that probiotics treatment could decrease HbA1c, FBG, and insulin resistance levels in T2DM patients. Additional clinical data and research into the probiotic mechanism is needed to clarify the importance of probiotics in T2DM [52]. Kheirkhah et al. studied the therapeutic significance of a specialized probiotic supplementation to improve glycemic outcomes in T2DM patients, such as HbA1c, fasting plasma glucose, fasting plasma insulin, and HOMA-IR. The most notable effects on these glycemic parameters seem to be produced by multi-strain probiotics containing Lactobacillus acidophilus, Streptococcus thermophilus, Lactobacillus bulgaricus, and Bifidobacterium lactis, which are taken once daily for six to twelve weeks, and the colony forming unit (CFU) dosages of the probiotics varied from seven million to over 100 billion per day [53]. In addition, a few more strains, such as Bacteroides, Faecalibacterium, Akkermansia, Roseburia, Ruminococcus, Fusobacterium, and Blautia, were used to manage T2DM. Probiotic supplementation has potential and is becoming a more common option for treating type 2 diabetes; still, more studies, both in vivo and in vitro, are required to understand how it generally affects glycemic control.
3.3. Probiotic Role in Inflammatory Bowel Disease (IBD)
An additional source of evidence in support of probiotic use in inflammatory bowel disease is clinical. It is generally known that in patients with Crohn’s disease, diverting the fecal stream usually results in mucosal healing [54]. However, inflammation quickly develops after restoring intestinal continuity, and fecal contents are then returned to the healing bowel. Saccharomyces boulardii can be administered as maintenance for inflammatory bowel disease patients to provide more targeted support and obtain the benefits of robust anti-inflammatory actions [55]. Engineered probiotic ECN-pE(C/E)2 works as an effective chemical drug for inflammatory bowel disease. The oral administration of the drug improves the expression of tight junction associated proteins that protect the colon epithelial cells from inflammation induced apoptosis [56]. Also, it helps in eliminating reactive oxygen species and increases anti-inflammatory cytokines in IBD, which brings out the therapeutic use of probiotics in chronic diseases like IBD [57]. The gut microbiome plays an important role in IBD. Any imbalance in gut microbes damages the gut barrier and causes inflammatory responses, a condition known as dysbiosis. The person with chronic IBD experiences a progressive increase in pathogenic microbes while decreasing the beneficial bacteria. Mainly short chain producing bacteria like Faecalibacterium prausnitzii are observed to be reduced in those patients, which further impairs the immune responses and intestinal inflammation [58,59]. Certain probiotics, such as Lactobacillus sp. Are recognized as producing some antimicrobial components that activate aryl hydrocarbon receptors associated with IBD [60]. According to recent research, it has been observed that overexpression of TNF-α and other pro inflammatory substances contributes to the occurrence of IBD. In order to reduce the hyperactive signaling pathways, total gut restoration (TGR) is supplemented. TGR is a mixture of five strains of probiotics that improves the IL-6 and TNF-α signaling pathways [61]. Another study focused on the NF-kB signaling pathway in order to regulate the genes that are responsible for the inflammatory defense system. Probiotic supplements with Lactobacillus, S. boulardii, Lactobacillus GG, EcN 1917, and VSL#3, Escherichia coli Nissle 1917, and Bifidobacterium decrease the pro inflammatory activity of IL-6 and IL-1β that deal with inflammatory responses in GI epithelial cells [62,63].
3.4. Probiotics on Cholesterol Metabolism
Multifactorial diseases, neurological disorders, and liver-related diseases are becoming more common in today’s society due to unhealthy dietary consumption. Metabolic dysfunctions inhibit the majority of diseases. Obesity, high blood pressure, and heart disease are primarily caused by disturbances in lipid metabolism. Living bacteria, or probiotics, contribute to greater health advantages. It can also be included in dietary supplements to help regulate body weight. Research employing multi-strain probiotic supplementation demonstrates a noteworthy reduction in TNF-α and LDL-cholesterol in children who are obese [64]; additionally, it raises the body’s HDL cholesterol levels. The probiotics that are most frequently utilized, such as Streptococcus, Saccharomyces, Bacillus, Clostridium, Candida, lactobacillus and bifidobacterium spp., decrease the composition of the gut microbiome, which in turn causes lipid oxidation in the liver. However, they additionally minimize the body’s accumulation of fat [65]. According to a recent study, functional foods like probiotics have a significant effect on increasing the hydrolase enzyme, which increases intestinal absorption of cholesterol and causes the bile salts to hydrolyze. Despite being less effective than anti-obesity drugs, it has demonstrated a noteworthy impact on lipid metabolism in metabolic disorders [66]. Probiotics regulate the essential enzymes involved in cholesterol metabolism and production, such as phenyl transferase and HMG CoA synthetase [67]. Both de novo lipogenesis and lipid oxidation regulate the accumulation of fat in the liver. Probiotics increase the expression of PPAR alpha and SREBP1, which in turn increase lipid oxidation and de novo lipogenesis [68].
3.5. Probiotics in Lactose Metabolism
Due to decreased production of the lactose disaccharide, lactose intolerance has been found in over 60% of the world’s population. In the presence of the enzyme lactase, glucose and galactose combine to form lactose in the intestine [69]. Clinical effects of abnormal lactose production include bloating, cramping in the abdomen, nausea, diarrhea, and more [70,71]. This is carried out through microbial fermentation, where lactose is preserved in its undigested state. This lowers the hydrolytic activity and helps in the formation of short chain fatty acids in the colon, which can lead to lactose intolerance in some people. Probiotics are a good source of lactase, which is provided externally to promote lactose fermentation, and they also assist in increasing the amount of gut microbeam in the intestine [72]. Probiotics also have an antagonistic effect on the bacteria in the gastrointestinal tract lining that produce a lot of CO2 and methane [69,73]. In the absence of the lactase enzyme, β-galactosidase contributes to the breakdown and transgalactosulation of lactose. It generates continuous glycolysis and readily absorbs glucose and galactose by tissue. In the GI tract, probiotics have been shown to exhibit β-galactosidase activity. Probiotics with high levels of β-galactosidase activity include Lactobacillus acidophilus (strains W22 and W70), Lactobacillus delbrueckii spp., Lactic acid bulgaricus, Bifidobacteria, and Streptococcus thermophilus (W69) [74].
3.6. Probiotics in Immune Response
Studies have demonstrated the modulatory effects of probiotics on dendritic cells, macrophages, T-cells, and B-cells. Because probiotics have clinical significance in immune cell regulation, multiple studies have investigated the possibility of using them as an adjuvant therapy for immunological-related disorders. It has been demonstrated that probiotics significantly affect Toll-like receptors (TLRs) and their adaptors’ ability to distinguish between endogenous and foreign substances. Furthermore, it encompasses a broad range of pathogen recognition [75]. Studies have demonstrated that two probiotics produced from fish, Bacillus amyloliquefaciens, Bifidobacterium bifidum, Bifidobacterium breve, Lactobacillus, Saccharomyces, and Lactococcus lactis, can improve crayfish tolerance to pathogens and elicit an innate immune response. Further, they increase the expression of immunogens, boosting the gut microbiome [76]. The fermented product of probiotics, known as prebiotics, helps exhibit energy binding on exopolysaccharides. Probiotics stimulate macrophages to alter their cytokine production while suppressing the formation of short chain fatty acids and bacteriocins (pathogens). Additionally, it facilitates the production of additional antibodies (IgA) by B-cells by attaching microbe associated molecular patterns to dendritic cell pathogen recognition receptors. More IL-10 and TNF-β are then released, acting as a catalysts for the host immune response [77].
3.7. Antioxidant Activity of Probiotics
Oxidative stress is implicated in the development of various multifactorial and neurodegenerative diseases. GAPDH activity is suppressed at elevated levels of oxidative stress, and this inhibited glycolysis process lowers the amount of ATP produced by cells. Reduced ATP production hampers Na/K-ATPase and calcium pumps, which further create excess stress in cellular metabolism and prompt apoptosis. For that reason, the methods to reduce ROS species are a great challenge for all medical researchers [78,79]. Recently, probiotics have been implemented with natural antioxidants, increasing their safety and acceptability among the population. For example, phenolic antioxidants donate a hydrogen atom to radicals that prevent the formation of a radical chain reaction. Hence, they reduce the effectiveness of oxidative stress [80]. Studies have demonstrated that the flavonoid extracts have been effectively shown to inhibit lipid peroxidation, which is a major risk factor for certain cancers and cardiovascular diseases [81,82]. Various studies have shown that incorporating Lactobacillus acidophilus has successfully increased the antioxidants in Rosa roxburghii tratt by fermentation. Probiotics help to reduce undesirable components like aldehydes by enhancing the alcohols and esters that have a great impact on health benefits [83]. Additional investigation is warranted to examine the potential of microbial metabolites as antioxidants.
3.8. Probiotics and Oral Health
Oral health and general quality of life are closely related, according to the WHO Global Programme. According to the WHO, around 3.5 billion people worldwide will have been affected by oral disease in 2023 [84]. When taken in sufficient quantities, probiotics have positive effects. In the past ten years, research has shown that probiotics can also improve adaptive immune responses, reduce the risk of respiratory tract infections, and even treat oral disorders. Traditionally, they have been employed to treat issues related to gut health. Breast milk composition is influenced by specific probiotic bacteria that have been obtained from mothers [85], and research has revealed that some bacteria among all oral microorganisms may be beneficial for preserving dental health, such as Lactobacillus, Bifidobacterium, Streptococcus strains, L. reuteri strains, L. brevis (CD2), L. casei Shirota, L. salivarius WB21, Bacillus subtilis, L. bulgaricus, L. acidophilus, L. casei, L. helveticus, and L. lactis [86]. Their antagonistic effect on other commensal oral microbes is recognized to protect oral cavities from infections [87]. Furthermore, taking probiotics does not change IgA, which stops germs from sticking to tooth surfaces. In order to protect against oral infections, probiotics assist in preserving the balance of the oral microbiota. Probiotics have been shown to help preserve the balanced environment of excellent oral health by lowering the symptoms of gingivitis, periodontitis, and halitosis, even though the clinical data on their actual effectiveness in oral health is still uncertain [88,89]. Probiotics work in combination with conventional dental care practices to provide an effective preventive intervention. Because of their beneficial effects on oral health, probiotics have been recognized for incorporation into oral medications [90,91].
4. Summary and Future Directions
Probiotics are used for health and medical consumption, as new strains have been developed with probiotic products. The international scientific community has convened and discussed probiotics and prebiotics as being of potentially acute importance. Probiotics are targeted in clinical trials in health care and are near the quality standard for the patient’s health (cancer, gastrointestinal, and neuron disorders) [92]. Research into microbial delivery or changing the human microbiome to enhance health has advanced staggeringly since “probiotics” were discovered. The Human Microbiome Project has amplified this trend, supported by multidisciplinary initiatives outside of microbiology that have contributed to the rapid growth of microbiome research worldwide [93]. The ability to regulate these microbial communities holds considerable promise for new disease prevention and treatment strategies. The concept of swallowing or injecting live bacteria for medicinal purposes is gaining traction. Still, many microbiome studies in rats and humans, on the other hand, “oversell” their results. Misuse of the term “probiotic” has resulted in substantial misinformation. The basic principles presented here, which urge a proven scientific definition of “probiotics” and a detailed examination of their real-world clinical outcomes, are hoped to provide more clarity and create a deeper understanding of this dynamic subject [94].
In conclusion, probiotic research has recently gained much attention to fill the gap between production and demand. It aims to produce efficient strains supplemented with dietary formulations and will provide various health benefits. This review has emphasized the benefits of therapeutic bacterial strains, raising the likelihood of their usage today and in the future. According to research, probiotic bacteria control pathogen colonization and clear the gut through various mechanisms, including competition for limited resources in the intestine and modulation of the mucosal immune system. As a result of the findings of different in vivo and in vitro studies, which show a significant link between these beneficial microorganisms and adaptive immunity responses, more research has shifted to the need for probiotic strains in therapeutic applications to treat various ailments in recent years.
Author Contributions
Conceptualization, K.S. and V.D.R.; methodology, G.P.V., G.K., K.S. and V.D.R.; data curation, G.P.V., G.K., K.S. and V.D.R.; writing—original draft preparation, G.P.V., G.K., K.S. and V.D.R.; writing—review and editing, G.P.V., G.K., K.S. and V.D.R.; visualization, G.P.V., G.K., K.S. and V.D.R.; supervision, K.S. and V.D.R.; project administration, K.S. and V.D.R.; funding acquisition, K.S. and V.D.R. All authors have read and agreed to the published version of the manuscript.
Funding
Khajamohiddin Syed expresses sincere gratitude to the University of Zululand (Grant number P419), and Vaddi Damodara Reddy thanked SERB, New Delhi, India, for providing financial support under Ramanujan Fellowship schemes (SB/S2/RJN-043/2014 dated 17 December 2015).
Acknowledgments
Vaddi Damodara Reddy thanked the University of Zululand, South Africa, for appointing him as a visiting fellow.
Conflicts of Interest
The authors declare no conflicts of interest, and the funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Soemarie, Y.B.; Milanda, T.; Barliana, M.I. Fermented foods as probiotics: A review. J. Adv. Pharm. Technol. Res. 2021, 12, 335. [Google Scholar]
- Zolotukhin, P.; Prazdnova, E.; Chistyakov, V. Methods to assess the antioxidative properties of probiotics. Probiotics Antimicrob. Proteins 2018, 10, 589–599. [Google Scholar] [CrossRef]
- Kim, E.N.; Kim, M.Y.; Lim, J.H.; Kim, Y.; Shin, S.J.; Park, C.W.; Kim, Y.-S.; Chang, Y.S.; Yoon, H.E.; Choi, B.S. The protective effect of resveratrol on vascular aging by modulation of the renin–angiotensin system. Atherosclerosis 2018, 270, 123–131. [Google Scholar] [CrossRef]
- Silva, D.R.; Sardi, J.d.C.O.; de Souza Pitangui, N.; Roque, S.M.; da Silva, A.C.B.; Rosalen, P.L. Probiotics as an alternative antimicrobial therapy: Current reality and future directions. J. Funct. Foods 2020, 73, 104080. [Google Scholar] [CrossRef]
- Yoha, K.; Nida, S.; Dutta, S.; Moses, J.; Anandharamakrishnan, C. Targeted delivery of probiotics: Perspectives on research and commercialization. Probiotics Antimicrob. Proteins 2022, 14, 15–48. [Google Scholar] [CrossRef] [PubMed]
- Im, E.J.; Lee, H.H.-Y.; Kim, M.; Kim, M.-K. Evaluation of Enterococcal Probiotic Usage and Review of Potential Health Benefits, Safety, and Risk of Antibiotic-Resistant Strain Emergence. Antibiotics 2023, 12, 1327. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.; Slavin, J. Health benefits of fibre, prebiotics and probiotics: A review of intestinal health and related health claims. Qual. Assur. Saf. Crops Foods 2016, 8, 539–554. [Google Scholar] [CrossRef]
- Gheorghita, R.; Anchidin-Norocel, L.; Filip, R.; Dimian, M.; Covasa, M. Applications of biopolymers for drugs and probiotics delivery. Polymers 2021, 13, 2729. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, P.; Zhang, X. Probiotics regulate gut microbiota: An effective method to improve immunity. Molecules 2021, 26, 6076. [Google Scholar] [CrossRef]
- Fadl, M.G.; Kamel, Z. Cholesterol-lowering effects and safety assessment of Lactobacillus spp. in vivo and in vitro testing for human use as probiotic from the dairy product in Egypt. J. Genet. Eng. Biotechnol. 2022, 20, 1–11. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Bahrami, A.; Rehman, A.; Rezaei, A.; Babazadeh, A.; Singh, H.; Jafari, S.M. Co-encapsulation of probiotics with prebiotics and their application in functional/synbiotic dairy products. Crit. Rev. Food Sci. Nutr. 2022, 62, 2470–2494. [Google Scholar] [CrossRef]
- Varela-Pérez, A.; Romero-Chapol, O.O.; Castillo-Olmos, A.G.; García, H.S.; Suárez-Quiroz, M.L.; Singh, J.; Figueroa-Hernández, C.Y.; Viveros-Contreras, R.; Cano-Sarmiento, C. Encapsulation of Lactobacillus gasseri: Characterization, probiotic survival, in vitro evaluation and viability in apple juice. Foods 2022, 11, 740. [Google Scholar] [CrossRef]
- Kowalska, E.; Ziarno, M.; Ekielski, A.; Żelaziński, T. Materials used for the microencapsulation of probiotic bacteria in the food industry. Molecules 2022, 27, 3321. [Google Scholar] [CrossRef]
- Tan, L.L.; Ang, K.L.; Loo, S.C.J. Alginate encapsulation improves probiotics survival in carbonated sodas and beers. PLoS ONE 2023, 18, e0283745. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Fobofou, S.A.; Savidge, T. Microbial metabolites: Cause or consequence in gastrointestinal disease? Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G535–G552. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-G.; Sakamoto, K.; Seo, S.-U.; Pickard, J.M.; Gillilland III, M.G.; Pudlo, N.A.; Hoostal, M.; Li, X.; Wang, T.D.; Feehley, T. Neonatal acquisition of Clostridia species protects against colonization by bacterial pathogens. Science 2017, 356, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Feehley, T.; Plunkett, C.H.; Bao, R.; Choi Hong, S.M.; Culleen, E.; Belda-Ferre, P.; Campbell, E.; Aitoro, R.; Nocerino, R.; Paparo, L. Healthy infants harbor intestinal bacteria that protect against food allergy. Nat. Med. 2019, 25, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S. Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, T.; Li, Y.; Zhang, T.; Wang, Q.; He, J.; Wang, L.; Li, L.; Yang, N.; Fang, Y. Probiotics for the treatment of women with bacterial vaginosis: A systematic review and meta-analysis of randomized clinical trials. Eur. J. Pharmacol. 2019, 864, 172660. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Gadir, A.A.; Dhir, R. Probiotics: Reiterating what they are and what they are not. Front. Microbiol. 2019, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Kleerebezem, M.; Binda, S.; Bron, P.A.; Gross, G.; Hill, C.; van Hylckama Vlieg, J.E.; Lebeer, S.; Satokari, R.; Ouwehand, A.C. Understanding mode of action can drive the translational pipeline towards more reliable health benefits for probiotics. Curr. Opin. Biotechnol. 2019, 56, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Wandro, S.; Osborne, S.; Enriquez, C.; Bixby, C.; Arrieta, A.; Whiteson, K. The microbiome and metabolome of preterm infant stool are personalized and not driven by health outcomes, including necrotizing enterocolitis and late-onset sepsis. Msphere 2018, 3, e00104-18. [Google Scholar] [CrossRef] [PubMed]
- Popović, N.; Brdarić, E.; Đokić, J.; Dinić, M.; Veljović, K.; Golić, N.; Terzić-Vidojević, A. Yogurt produced by novel natural starter cultures improves gut epithelial barrier in vitro. Microorganisms 2020, 8, 1586. [Google Scholar] [CrossRef] [PubMed]
- Zommiti, M.; Feuilloley, M.G.; Connil, N. Update of probiotics in human world: A nonstop source of benefactions till the end of time. Microorganisms 2020, 8, 1907. [Google Scholar] [CrossRef] [PubMed]
- Kiepś, J.; Dembczyński, R. Current trends in the production of probiotic formulations. Foods 2022, 11, 2330. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Y.; Xia, Y.; Song, X.; Ai, L. Characteristics of probiotic preparations and their applications. Foods 2022, 11, 2472. [Google Scholar] [CrossRef]
- Gao, H.; Li, X.; Chen, X.; Hai, D.; Wei, C.; Zhang, L.; Li, P. The Functional Roles of Lactobacillus Acidophilus in Different Physiological and Pathological Processes. J. Microbiol. Biotechnol. 2022, 32, 1226. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Ho, C. Recent Development of Probiotic Bifidobacteria for Treating Human Diseases. Front. Bioeng. Biotechnol. 2021, 9, 770248. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Ray, R.; Zdolec, N. Lactobacillus plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. BioMed Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef] [PubMed]
- Davoren, M.J.; Liu, J.; Castellanos, J.; Rodríguez-Malavé, N.I.; Schiestl, R.H. A novel probiotic, Lactobacillus johnsonii 456, resists acid and can persist in the human gut beyond the initial ingestion period. Gut Microbes 2019, 10, 458–480. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Bangar, S.P.; Echegaray, N.; Suri, S.; Tomasevic, I.; Manuel Lorenzo, J.; Melekoglu, E.; Rocha, J.M.; Ozogul, F. The impacts of Lactiplantibacillus plantarum on the functional properties of fermented foods: A review of current knowledge. Microorganisms 2022, 10, 826. [Google Scholar] [CrossRef] [PubMed]
- Naibaho, J.; Jonuzi, E.; Butula, N.; Korzeniowska, M.; Föste, M.; Sinamo, K.N.; Chodaczek, G.; Yang, B. Fortification of milk-based yogurt with protein hydrolysates from brewers’ spent grain: Evaluation on microstructural properties, lactic acid bacteria profile, lactic acid forming capability and its physical behavior. Curr. Res. Food Sci. 2022, 5, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Kamzolova, S.V.; Morgunov, I.G. Optimization of medium composition and fermentation conditions for α-ketoglutaric acid production from biodiesel waste by Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2020, 104, 7979–7989. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, X.; Li, Q.; Peng, Z.; Li, J.; Zhang, J. Optimization of fermentation conditions for surfactin production by B. subtilis YPS-32. BMC Microbiol. 2023, 23, 117. [Google Scholar] [CrossRef]
- Fenster, K.; Freeburg, B.; Hollard, C.; Wong, C.; Rønhave Laursen, R.; Ouwehand, A. The Production and Delivery of Probiotics: A Review of a Practical Approach. Microorganisms 2019, 17, 83. [Google Scholar] [CrossRef]
- Shruthi, B.R.; Achur, R.N.H.; Nayaka Boramuthi, T. Optimized solid-state fermentation medium enhances the multienzymes production from Penicillium citrinum and Aspergillus clavatus. Curr. Microbiol. 2020, 77, 2192–2206. [Google Scholar] [CrossRef]
- Wu, D.; Fu, L.; Cao, Y.; Dong, N.; Li, D. Genomic insights into antimicrobial potential and optimization of fermentation conditions of pig-derived Bacillus subtilis BS21. Front. Microbiol. 2023, 14, 1239837. [Google Scholar] [CrossRef]
- Mamun-Or-Rashid, A.N.M.; Lucy, T.T.; Pramanik, M.K. Isolation, Identification, Optimization of Baker’s Yeast from Natural Sources, Scale-Up Production Using Molasses as a Cheap Carbohydrate Source, and Evaluation for Bread Production. Appl. Microbiol. 2022, 2, 516–533. [Google Scholar] [CrossRef]
- Chotani, G.; Dodge, T.; Hsu, A.; Kumar, M.; LaDuca, R.; Trimbur, D.; Weyler, W.; Samford, K. The commercial production of chemicals using pathway engineering. Biochem. Biophys. Acta 2000, 1543, 434–455. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zentek, J.; Vahjen, W. Optimization of production parameters for probiotic Lactobacillus strains as feed additive. Molecules 2019, 24, 3286. [Google Scholar] [CrossRef]
- Demirgul, F.; Simsek, O.; Bozkurt, F.; Dertli, E.; Sagdic, O. Production and characterization of yeast extracts produced by Saccharomyces cerevisiae, Saccharomyces boulardii and Kluyveromyces marxianus. Prep. Biochem. Biotechnol. 2022, 52, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Razali, W.A.W.; Pandhal, J. Outdoor pilot-scale cultivation and techno economic assessment of a novel omega-3 eicosapentaenoic acid over-producing Nannochloropsis oculata strain. Bioresour. Technol. Rep. 2023, 24, 101682. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 2023, 15, 2185034. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. The role of probiotics in colorectal cancer management. Evid.-Based Complement. Altern. Med. 2020, 2020, 3535982. [Google Scholar] [CrossRef]
- Shang, F.; Jiang, X.; Wang, H.; Chen, S.; Wang, X.; Liu, Y.; Guo, S.; Li, D.; Yu, W.; Zhao, Z. The inhibitory effects of probiotics on colon cancer cells: In vitro and in vivo studies. J. Gastrointest. Oncol. 2020, 11, 1224. [Google Scholar] [CrossRef]
- Peng, M.; Lee, S.-H.; Rahaman, S.O.; Biswas, D. Dietary probiotic and metabolites improve intestinal homeostasis and prevent colorectal cancer. Food Funct. 2020, 11, 10724–10735. [Google Scholar] [CrossRef]
- Ayesha, I.E.; Monson, N.R.; Klair, N.; Patel, U.; Saxena, A.; Patel, D.; Venugopal, S.; Ayesha, I.E. Probiotics and Their Role in the Management of Type 2 Diabetes Mellitus (Short-Term Versus Long-Term Effect): A Systematic Review and Meta-Analysis. Cureus 2023, 15, e46741. [Google Scholar] [CrossRef]
- Tao, Y.-W.; Gu, Y.-L.; Mao, X.-Q.; Zhang, L.; Pei, Y.-F. Effects of probiotics on type II diabetes mellitus: A meta-analysis. J. Transl. Med. 2020, 18, 30. [Google Scholar]
- Kheirkhah, A.H.; Forouzani-Moghaddam, M.J.; Afkhami-Ardekani, M. Relationship between probiotics and type 2 diabetes mellitus: A review. Iran. J. Diabetes Obes. 2023, 15, 119–128. [Google Scholar] [CrossRef]
- Toumi, R.; Samer, A.; Soufli, I.; Rafa, H.; Touil-Boukoffa, C. Role of probiotics and their metabolites in inflammatory bowel diseases (IBDs). Gastroenterol. Insights 2021, 12, 56–66. [Google Scholar]
- Pais, P.; Almeida, V.; Yılmaz, M.; Teixeira, M.C. Saccharomyces boulardii: What makes it tick as successful probiotic? J. Fungi 2020, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Ledder, O. Antibiotics in inflammatory bowel diseases: Do we know what we’re doing? Transl. Pediatr. 2019, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, Y.; Zhang, H.; Zhou, M.; Yu, Y.; Lin, S.; Jiang, B.; Zhao, X.; Miao, L.; Wei, C.-W. Integrated cascade nanozyme catalyzes in vivo ROS scavenging for anti-inflammatory therapy. Sci. Adv. 2020, 6, eabb2695. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, Y.; Liu, Q.; Li, S.; Cheng, Y.; Cheng, C.; Sun, Z.; Du, Y.; Butch, C.J.; Wei, H. An orally administered CeO2@ montmorillonite nanozyme targets inflammation for inflammatory bowel disease therapy. Adv. Funct. Mater. 2020, 30, 2004692. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.; Chen, Q.; Li, X.; Chen, L.; Dong, Z.; Zhu, W.; Yang, Y.; Liu, Z.; Chen, Q. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat. Commun. 2022, 13, 3432. [Google Scholar] [CrossRef]
- Xu, L.; Liu, B.; Huang, L.; Li, Z.; Cheng, Y.; Tian, Y.; Pan, G.; Li, H.; Xu, Y.; Wu, W.; et al. Probiotic Consortia and Their Metabolites Ameliorate the Symptoms of Inflammatory Bowel Diseases in a Colitis Mouse Model. Microbiol. Spectr. 2022, 10, e00622–e00657. [Google Scholar] [CrossRef]
- Najafi, S.; Sotoodehnejadnematalahi, F.; Amiri, M.M.; Pourshafie, M.R.; Rohani, M. Prophylactic vs. Therapeutic Effect of Probiotics on the Inflammation Mediated by the NF-κB Pathway in Inflammatory Bowel Conditions. Biomedicines 2023, 11, 1675. [Google Scholar] [CrossRef]
- Slack, R.; Macdonald, S.; Roper, J.; Jenkins, R.; Hatley, R. Emerging therapeutic opportunities for integrin inhibitors. Nat. Rev. Drug Discov. 2022, 21, 60–78. [Google Scholar] [CrossRef]
- Marzorati, M.; Bubeck, S.; Bayne, T.; Krishnan, K.; Giusto, M. Effects of combined prebiotic, probiotic, IgG and amino acid supplementation on the gut microbiome of patients with inflammatory bowel disease. Future Microbiol. 2022, 17, 1307–1324. [Google Scholar] [CrossRef]
- Chen, A.-C.; Fang, T.-J.; Ho, H.-H.; Chen, J.-F.; Kuo, Y.-W.; Huang, Y.-Y.; Tsai, S.-Y.; Wu, S.-F.; Lin, H.-C.; Yeh, Y.-T. A multi-strain probiotic blend reshaped obesity-related gut dysbiosis and improved lipid metabolism in obese children. Front. Nutr. 2022, 9, 922993. [Google Scholar] [CrossRef]
- Saarela, M.; Mättö, J.; Mattila-Sandholm, T. Safety Aspects of Lactobacillus and Bifidobacterium Species Originating from Human Oro-gastrointestinal Tract or from Probiotic Products. Microb. Ecol. Health Dis. 2002, 14, 234–241. [Google Scholar] [CrossRef]
- Momin, E.S.; Khan, A.A.; Kashyap, T.; Pervaiz, M.A.; Akram, A.; Mannan, V.; Sanusi, M.; Elshaikh, A.O.; Pervaiz Sr, M.A. The Effects of Probiotics on Cholesterol Levels in Patients with Metabolic Syndrome: A Systematic Review. Cureus 2023, 15, e37567. [Google Scholar] [CrossRef] [PubMed]
- Puttarat, N.; Kasorn, A.; Vitheejongjaroen, P.; Chantarangkul, C.; Tangwattanachuleeporn, M.; Taweechotipatr, M. Beneficial Effects of Indigenous Probiotics in High-Cholesterol Diet-Induced Hypercholesterolemic Rats. Nutrients 2023, 15, 2710. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Gu, M.; Werlinger, P.; Cho, J.-H.; Cheng, J.; Suh, J.-W. Lactobacillus sakei MJM60958 as a Potential Probiotic Alleviated Non-Alcoholic Fatty Liver Disease in Mice Fed a High-Fat Diet by Modulating Lipid Metabolism, Inflammation, and Gut Microbiota. Int. J. Mol. Sci. 2022, 23, 13436. [Google Scholar] [CrossRef]
- Oak, S.J.; Jha, R. The effects of probiotics in lactose intolerance: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, C.F.; Cailliez-Grimal, C.; Borges, F.; Revol-Junelles, A.-M. Review of lactose and galactose metabolism in lactic acid bacteria dedicated to expert genomic annotation. Trends Food Sci. Technol. 2019, 88, 121–132. [Google Scholar] [CrossRef]
- Catanzaro, R.; Sciuto, M.; Marotta, F. Lactose intolerance: An update on its pathogenesis, diagnosis, and treatment. Nutr. Res. 2021, 89, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Misselwitz, B.; Butter, M.; Verbeke, K.; Fox, M.R. Update on lactose malabsorption and intolerance: Pathogenesis, diagnosis and clinical management. Gut 2019, 68, 2080–2091. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Li, R.; Takala, T.M.; Tariq, M.; Zaidi, A.H.; Saris, P.E. Generation of lactose-and protease-positive probiotic Lacticaseibacillus rhamnosus GG by conjugation with Lactococcus lactis NCDO 712. Appl. Environ. Microbiol. 2021, 87, e02920–e02957. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Vinuesa, L.; Sánchez-Arroyo, A.; Moreno, F.J.; de Las Rivas, B.; Muñoz, R. Dual 6Pβ-Galactosidase/6Pβ-Glucosidase GH1 family for Lactose Metabolism in the probiotic bacterium lactiplantibacillus plantarum WCFS1. J. Agric. Food Chem. 2023, 71, 10693–10700. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Sun, Y.; Zhou, Y.; Men, X.; Wang, B.; Li, B.; Ren, Y. Effects of probiotics on growth, the toll-like receptor mediated immune response and susceptibility to Aeromonas salmonicida infection in rainbow trout Oncorhynchus mykiss. Aquaculture 2022, 561, 738668. [Google Scholar] [CrossRef]
- Zhu, L.; Kong, Y.; Chang, X.; Feng, J.; Wang, X.; Hou, L.; Zhao, X.; Pei, C.; Kong, X. Effects of two fish-derived probiotics on growth performance, innate immune response, intestinal health, and disease resistance of Procambarus clarkii. Aquaculture 2023, 562, 738765. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Wu, C. Modulation of gut microbiota and immune system by probiotics, pre-biotics, and post-biotics. Front. Nutr. 2022, 8, 634897. [Google Scholar] [CrossRef]
- Li, A.; Wang, Y.; Li, Z.; Qamar, H.; Mehmood, K.; Zhang, L.; Liu, J.; Zhang, H.; Li, J. Probiotics isolated from yaks improves the growth performance, antioxidant activity, and cytokines related to immunity and inflammation in mice. Microb Cell Fact 2019, 18, 112. [Google Scholar] [CrossRef]
- Hoffmann, A.; Kleniewska, P.; Pawliczak, R. Antioxidative activity of probiotics. Arch. Med. Sci. 2021, 17, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Muniandy, P.; Shori, A.B.; Baba, A.S. Influence of green, white and black tea addition on the antioxidant activity of probiotic yogurt during refrigerated storage. Food Packag. Shelf Life 2016, 8, 1–8. [Google Scholar] [CrossRef]
- Shori, A.B.; Baba, A.S. Comparative antioxidant activity, proteolysis and in vitro α-amylase and α-glucosidase inhibition of Allium sativum-yogurts made from cow and camel milk. J. Saudi Chem. Soc. 2014, 18, 456–463. [Google Scholar] [CrossRef]
- Shori, A.B.; Aljohani, G.S.; Al-zahrani, A.J.; Al-sulbi, O.S.; Baba, A.S. Viability of probiotics and antioxidant activity of cashew milk-based yogurt fermented with selected strains of probiotic Lactobacillus spp. LWT 2022, 153, 112482. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T.; Dai, Y.; Jiang, G.; Peng, Y.; Wang, J.; Song, Y.; Ding, Z. Effects of probiotics on antioxidant activity, flavor compounds and sensory evaluation of Rosa roxburghii Tratt. LWT 2023, 179, 114664. [Google Scholar] [CrossRef]
- Jain, N.; Dutt, U.; Radenkov, I.; Jain, S. WHO’s global oral health status report 2022: Actions, discussion and implementation. Oral Dis. 2023, 30, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Kekkonen, R.A.; Lummela, N.; Karjalainen, H.; Latvala, S.; Tynkkynen, S.; Jarvenpaa, S.; Kautiainen, H.; Julkunen, I.; Vapaatalo, H.; Korpela, R. Probiotic intervention has strain-specific anti-inflammatory effects in healthy adults. World J. Gastroenterol. 2008, 14, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Paineau, D.; Carcano, D.; Leyer, G.; Darquy, S.; Alyanakian, M.A.; Simoneau, G.; Bergmann, J.F.; Brassart, D.; Bornet, F.; Ouwehand, A.C. Effects of seven potential probiotic strains on specific immune responses in healthy adults: A double-blind, randomized, controlled trial. FEMS Immunol. Med. Microbiol. 2008, 53, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Haukioja, A.; Yli-Knuuttila, H.; Loimaranta, V.; Kari, K.; Ouwehand, A.C.; Meurman, J.H.; Tenovuo, J. Oral adhesion and survival of probiotic and other lactobacilli and bifidobacteria in vitro. Oral. Microbiol. Immunol. 2006, 21, 326–332. [Google Scholar] [CrossRef]
- Haukioja, A.; Loimaranta, V.; Tenovuo, J. Probiotic bacteria affect the composition of salivary pellicle and streptococcal adhesion in vitro. Oral. Microbiol. Immunol. 2008, 23, 336–343. [Google Scholar] [CrossRef]
- Haukioja, A. Probiotics and oral health. Eur. J. Dent. 2010, 4, 348–355. [Google Scholar] [CrossRef]
- Chen, W.; Ren, J.; Li, J.; Peng, S.; Zhang, C.; Lin, Y. Effects of probiotics on the oral health of patients undergoing orthodontic treatment: A systematic review and meta-analysis. Eur. J. Orthod. 2023, 45, 599–611. [Google Scholar] [CrossRef]
- Radhamanalan, G.; Dharumadurai, D. Prevalence of dental caries and knowledge of probiotics according to the Oral Health Monitoring Survey (OHMS) in Tamil Nadu, India. Preprint 2023. [Google Scholar] [CrossRef]
- Drago, L. Probiotics and colon cancer. Microorganisms 2019, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Mathipa, M.G.; Thantsha, M.S. Probiotic engineering: Towards development of robust probiotic strains with enhanced functional properties and for targeted control of enteric pathogens. Gut Pathog. 2017, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.C.; Masi, A.C.; Young, G.R.; Vatanen, T.; Lamb, C.A.; Smith, R.; Coxhead, J.; Butler, A.; Marsland, B.J.; Embleton, N.D. Strain-specific impacts of probiotics are a significant driver of gut microbiome development in very preterm infants. Nat. Microbiol. 2022, 7, 1525–1535. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).