Abstract
(1) Background: Currently available guidelines require upper gastrointestinal (GI) endoscopy with biopsy sampling for adult celiac disease (CD) diagnosis. Based on the pediatric experience, there has been a growing interest if serology-based diagnosis would be possible for adult CD also. Our aim was to analyze the associated upper GI tract lesions in newly diagnosed CD patients, to see if significant associated pathology is detected during index endoscopy, which might impact patient management not related to CD. (2) Methods: We performed a retrospective analysis of newly diagnosed CD cases diagnosed over a period of 7 years (2014–2020). Demographic, clinical, laboratory, endoscopy and histopathology data were collected from the patients’ charts. Diagnosis was set according to ACG Guideline 2013. (3) Results: Altogether 79 patients were recruited for this study purpose, 75.9% female, median age 39 years. All patients had positive CD-specific serology and atrophic mucosal injury in duodenal biopsy samples. Besides villous atrophy, associated endoscopic findings were detected in 42/79 (53.16%) of patients. Most of the gastric lesions were minor endoscopic findings—small sliding hiatal hernias, non-specific chronic gastritis, but we also found two cases of peptic ulcers, one case of metaplastic gastritis, six cases of atrophic gastritis and one subepithelial lesion. Only one patient had changes in the duodenum except CD-related findings—an inflammatory polyp in the duodenal bulb. No malignancies were found. (4) Conclusions: In our cohort, there was a significant number of newly diagnosed CD patients who had associated lesions during the index upper GI endoscopy, but most of them were minor endoscopic findings.
1. Introduction
Celiac disease (CD) is a lifelong, chronic autoimmune digestive disorder characterized by gluten intolerance in genetically susceptible individuals. In CD patients, gluten ingestion triggers an immune-mediated injury of the small bowel mucosa, which has a wide spectrum of clinical consequences, from intermittent irritable bowel-like symptoms to overt malabsorption and various extraintestinal features such a hematologic, rheumatologic, cutaneous, cardiovascular or neurologic manifestations [1,2,3,4,5,6,7,8]. These protean manifestations make clinical recognition of CD challenging and contribute to low diagnostic rates and significant diagnostic delay. Diagnosis relies on detection of CD-related autoantibodies and evidence of atrophic mucosal injury on endoscopic biopsy samples from the duodenum [9].
Due to the invasive nature of the endoscopic procedure, patients’ reluctance to undergo scoping and biopsy sampling, also several pitfalls related to histology, a growing interest has emerged in the last decades towards a non-biopsy diagnosis for CD. This has been already validated in pediatric CD and implemented since 2012 ESPHGAN guidelines, which allowed for a diagnosis of CD to be set without biopsies in European symptomatic children who fulfilled a triple diagnostic criteria: tissue transglutaminase antibodies over 10 times the upper limit of normal (ULN), positive antiendomysial antibodies (EMA) and positive HLA-DQ2/DQ8 haplotype [10]. This allowed for a significant reduction in the number of endoscopies needed to diagnose pediatric CD [11]. Moreover, the updated 2020 ESPHGAN guideline set even lighter rules for a non-bioptic strategy for CD diagnosis, not requiring HLA testing and presence of symptoms in children with IgA transglutaminase 2 (TG-2) antibodies with values 10 times the ULN and positive EMA in a second serum sample [12].
With the growing experience from pediatric CD, the scientific community has questioned the usefulness of endoscopy for CD diagnosis and pursued the idea if a serology-based diagnosis would also be possible for adult CD. Several papers approaching this diagnostic strategy have been reported in the last decade, but currently available guidelines still require histopathology evidence for CD diagnosis in adults [9], except the 2018 Finnish guidelines that allow a non-biopsy diagnostic approach [13]. An attempt to align adult to pediatric practice has been enforced in the 2019 AGA Clinical Practice Update, which stated that high levels of TG2 are accurate for diagnosing active CD and esophagogastroduodenoscopy may be performed for the purpose of differential diagnosis [14].
Although the pediatric experience has fueled adult CD researchers and clinicians to back-up the non-bioptic strategy, some have proposed more caution as there could be misdiagnosis with such rules, especially in primary care, and others are against omission of endoscopy and duodenal biopsies in adult CD as a baseline histology is needed for a comparison to follow-up biopsy in non-responders [15,16,17,18]. Additionally, while there is good correlation between serology and mucosal injury at diagnosis, CD-related antibodies do not accurately detect persistent villous atrophy in gluten-free diet treated CD patients [19].
Not least, upper gastrointestinal (GI) endoscopy in adults with suspected CD can also detect other pathologies, in the esophagus, stomach or even duodenum, which would otherwise be missed if patients would not be scoped for the indication to perform duodenal biopsies.
Our aim was to analyze the associated upper GI tract lesions in newly diagnosed CD patients, to see if significant pathology is detected and a management change is expected from the endoscopic procedure itself. This could build-up the arguments list to keep the endoscopy as a mandatory diagnostic tool for diagnosing CD.
2. Methods
We performed a retrospective analysis of newly diagnosed CD cases diagnosed over a period of 7 years (2014–2020). Demographic data, medical history, CD-specific serology, endoscopy and histopathology data were collected from the patients’ charts, at the time of diagnosis. CD diagnosis was set according to ACG Guideline 2013 [20].
All patients were tested for CD specific serology—IgA tissue transglutaminase (tTG) along with total serum IgA (if IgA-deficient then IgG tTG), and underwent upper GI endoscopy with multiple duodenal biopsies (1–2 from the duodenal bulb and 4 from the distal duodenum). Endoscopy was performed by two examiners and histology samples were assessed by one experienced pathologist and reported according to the Marsh–Oberhuber classification [21]. Associated lesions were extracted from the endoscopy report and grouped as esophageal, gastric and duodenal. Analysis of change in management according to endoscopy findings was carried out and included prescriptions, indications for further tests or follow-up.
Ethical approval was granted by the Local Ethics Committee. Statistical analysis was descriptive and carried out using Microsoft Excel version 16.35.
3. Results
Altogether 79 patients were recruited for this study purpose, 75.9% (60/79) female, median age 39 ± 11 years (range 19–83 years). Regarding the clinical presentation, most of our patients (57%) had typical presentation, while atypical forms represented 19% and screen-detected 13.9% of cases. Almost one-third (31.6%) of newly diagnosed CD had anemia. In total, 13 out of 79 patients (16.5%) had an associated autoimmune disease (type 1 diabetes mellitus—3.8%, autoimmune thyroid disease—8.9%, Sjogren syndrome—3.8%). Another 5.1% had dermatitis herpetiformis.
All patients had positive CD-specific serology, 96.2% IgA tTG positive while the remaining 3.8% were IgA deficient and IgG tTG positive. A total of 29 out of 79 patients included had tTG values over 10 times ULN. Histopathological assessment of duodenal biopsy samples revealed atrophic mucosal injury (Marsh 3) in all patients.
Besides villous atrophy, associated endoscopic findings (at least one) were detected in 42/79 (53.16%) of patients, as follows: esophageal (peptic esophagitis, esophageal candidiasis, esophageal heterotopic gastric mucosa, short segment Barrett’s esophagus)—10 cases; gastric (hiatal hernia, gastritis, gastric ulcer, subepithelial lesion)—36 cases; duodenal (diminutive sessile polyp)—1 case; Figure 1. Five patients had combined endoscopic findings. Most of the gastric lesions were minor endoscopic findings—small sliding hiatal hernias, non-specific chronic gastritis, but we also found two cases of peptic ulcers, one case of metaplastic gastritis, six cases of atrophic gastritis and one subepithelial lesion. Only one patient had changes in the duodenum except CD-related findings—an inflammatory polyp in the duodenal bulb. No malignancies were found.
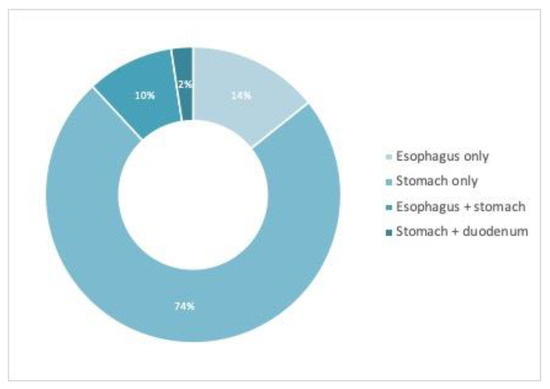
Figure 1.
Distribution of associated findings at upper endoscopy in the study cohort.
Considering the associated lesions detected at endoscopy, if omitting the indication for duodenal biopsy sampling, the examination would have yielded a change in management (prescriptions, further testing, follow-up indications) in 28 out of the 79 patients (35.44%).
4. Discussions
At present, diagnosing adult CD is based on clinical, serological and histopathological criteria, the latter requiring upper GI endoscopy for duodenal mucosal sampling. In contrast, a diagnosis of pediatric CD can be set by omitting endoscopic biopsies and histopathological assessment of duodenal mucosa, if certain serological criteria are met. This has been enforced in pediatric guidelines since 2012 [10] and successfully used in clinical practice [22], although there is a barrier due to low availability of EMA and genotyping in limited resource settings [23,24]. Of note, a specific non-biopsy protocol for diagnosing adult CD was recommended as interim guidance by the British Society of Gastroenterology in 2020, in the setting of COVID-19 pandemic [25]. However, a follow-up analysis after this interim guidance revealed that only 23% of patients fulfilled the criteria for a no-biopsy pathway [26]. In our study cohort, if considering tTG over 10 times the ULN as criteria for a non-biopsy strategy, 36.7% of patients would have been diagnosed based on serology only. However, we cannot consider this a full extrapolation of pediatric guidelines as we did not have EMA tested in a second serum sample in our study cohort.
Beyond the recommendation generated by the COVID-19 pandemic, the interest for a biopsy avoiding strategy in diagnosing adult CD has rapidly grown in the last decade, both among patients and the part of the scientific community, building on the successful experience from pediatric CD. In light of the current guideline recommendations for adult CD, it is well recognized that some of the patients are reluctant to undergo endoscopy and biopsy sampling for confirming a CD diagnosis and rely their diagnosis based on serology only. Additionally, histology in CD has been in the spotlight in recent literature with several issues from quality, orientation and readability of biopsy samples to high inter-observer variability and heterogeneity in reporting of abnormal findings [27,28,29,30,31].
On the other hand, other authors have supported the need for an initial endoscopy with histological evaluation of mucosa, in order to get a baseline assessment of the small bowel injury, which would be useful in for follow-up of patients. Not least, others also report on the low-titers of antibodies in ultra-short and mild-enteropathy CD, which would be missed by a serology-based diagnostic strategy [32]. Although bulb biopsies have been associated with several pitfalls [33,34], their importance has been reinforced by several studies [35,36,37].
Therefore, usefulness of endoscopy has been questioned in the last years, with the growing data in the literature supporting a non-biopsy diagnostic approach in adults also—Table 1 [38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57]. There are several limitations of these studies—the design and study population are heterogenous and most of them are retrospective in nature. Additionally, diagnostic accuracy of serology is known to be dependent on pre-test probability, meaning risk of having CD in different populations tested. A strength of the available research is that different TG2 assays have been reported in diagnosing CD without biopsies.

Table 1.
Studies evaluating the non-bioptic strategy in diagnosing adult CD.
In our study, we found a low prevalence of major endoscopic findings in newly diagnosed CD patients. However, some of the findings were clinically relevant, as their detection involved a change in management in about one-third of patients—whether establishing the need for endoscopic follow-up (e.g., metaplastic, atrophic gastritis, gastric ulcers), high dose proton pump inhibitors (e.g., ulcers), testing and eradication therapy for Helicobacter pylori infection, or further investigations (e.g., subepithelial lesions). Concurring results have been reported by Pacheco et al., who analyzed the frequency of interventions based on esophageal and gastric biopsies in patients undergoing endoscopy for elevated tTG: 7% of patients had therapy or referral related to esophageal pathology and 10% to gastric pathology [58]. A biopsy avoidance strategy for diagnosing CD would miss the clinical decisions driven by endoscopy findings, in a patient in whom upper endoscopy would otherwise not be performed, if not for the suspicion of CD. On the other hand, significant pathology on index endoscopy is more probable to be detected along with increasing age [59], and questions arise about the current difference regarding CD diagnosis in a teenager (in whom pediatric non-biopsy rules may apply) and a young adult (in whom currently available guidelines require small bowel biopsy). In this respect, an age threshold for serology-based diagnosis in adults might be considered.
Additionally, a significant proportion of our study patients had anemia at presentation, which is a well-known indication for endoscopic examination of the digestive tract [60]. Persisting anemia despite a strict gluten-free diet should raise suspicion of possible synchronous lesions in the GI tract [61].
Besides the possibility of detecting concurrent non-celiac lesions on index endoscopy, another important stake of not dropping endoscopy and biopsy-sampling in the diagnostic algorithm of adult CD, is the possibility of lymphoma occurrence, as opposed to pediatric CD. Refractory CD and the lymphoma risk is a well-recognized complication of CD and can be the first presentation of the disease [62].
Another drawback of a non-bioptic diagnosis for adult CD would be its inability to detect seronegative patients [63]. Not least, biopsy-avoiding diagnosis could have an impact on adherence to diet and follow-up, as some have suggested [64]. Patients who undergo a serological-based diagnosis only, lacking proof of bowel injury by histopathological assessment of duodenal biopsy samples, might perceive a lesser disease burden and be less adherent to diet compared to those who undergo a more thorough diagnosis. Histology proven mucosal damage of the small bowel remains a strong argument for a lifelong restrictive diet.
On the other side, we should take into consideration the costs associated with biopsy sampling and histopathology of bowel mucosa specimens, which burdens both the endoscopy and pathology departments. A routine duodenal biopsy in patients performing gastroscopy for refractory GERD or anemia is not a cost-effective approach, so the indication for biopsy should always have the right reasons, including to justify the cost [65,66]. Not least, there is low adherence to the current recommendation of multiple biopsy sampling, with some studies showing that some patients are diagnosed based on a single biopsy specimen [67,68,69].
In summary, there are several advantages but also some pitfalls for the current biopsy-based diagnosis in adult CD—Table 2.

Table 2.
Arguments list for biopsy-based, compared to serology-based criteria in diagnosing adult CD.
The current research has some limitations. First, we acknowledge the small sample size, single center experience and retrospective nature of the study. Second, the change in management might be overestimated because of some overlap of indications for upper endoscopy in some of the cases included, as scoping would have been performed due to other clinically driven reasons other than duodenal biopsy sampling for CD confirmation. Not least, we could not provide data regarding the cost-efficacy of the intervention studied.
Further studies with larger sample sizes should focus on identifying CD patients suitable for a biopsy sparing diagnostic strategy.
5. Conclusions
In our cohort, a significant number of newly diagnosed CD patients had associated lesions during the index upper GI endoscopy. Our results support the use of endoscopy in the diagnostic workup of suspected adult CD, which might generate a change in management by triggering further testing or treatment prescriptions.
Author Contributions
Conceptualization, D.V.B. and I.E.; Data curation, I.E., D.V.B., F.V., C.J., F.I.-R., A.P., D.M. and M.J.; Formal analysis, I.E., D.V.B., F.V., C.J., F.I.-R., A.P., D.M. and M.J.; Methodology, C.J., F.I.-R. and D.M.; Supervision, M.J.; Writing—original draft, D.V.B. and I.E.; Writing—review and editing, F.V., C.J., F.I.-R., A.P., D.M. and M.J. The first two authors share equal contribution for this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Central Military Emergency University Hospital. Local ethics approval was granted for this research project (414/19 October 2020).
Informed Consent Statement
Informed consent to use data for research purposes was given by patients during admission, in a specific paragraph on the standard hospital consent form.
Data Availability Statement
The dataset is available through the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Balaban, D.V.; Popp, A.; Ionita Radu, F.; Jinga, M. Hematologic Manifestations in Celiac Disease-A Practical Review. Med. Kaunas Lith. 2019, 55, 373. [Google Scholar] [CrossRef] [PubMed]
- Dima, A.; Jurcut, C.; Jinga, M. Rheumatologic manifestations in celiac disease: What should we remember? Rom. J. Intern. Med. Rev. Roum. Med. Interne 2019, 57, 3–5. [Google Scholar] [CrossRef]
- Durazzo, M.; Ferro, A.; Brascugli, I.; Mattivi, S.; Fagoonee, S.; Pellicano, R. Extra-Intestinal Manifestations of Celiac Disease: What Should We Know in 2022? J. Clin. Med. 2022, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Dumic, I.; Martin, S.; Salfiti, N.; Watson, R.; Alempijevic, T. Deep Venous Thrombosis and Bilateral Pulmonary Embolism Revealing Silent Celiac Disease: Case Report and Review of the Literature. Case Rep. Gastrointest. Med. 2017, 2017, 5236918. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, L.; Beteta-Gorriti, V.; Alvarez, N.; Gómez de Castro, C.; de Dios, A.; Palacios, L.; Santos-Juanes, J. Cutaneous and Mucosal Manifestations Associated with Celiac Disease. Nutrients 2018, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Mearns, E.S.; Taylor, A.; Thomas Craig, K.J.; Puglielli, S.; Leffler, D.A.; Sanders, D.S.; Lebwohl, B.; Hadjivassiliou, M. Neurological Manifestations of Neuropathy and Ataxia in Celiac Disease: A Systematic Review. Nutrients 2019, 11, 380. [Google Scholar] [CrossRef]
- Laurikka, P.; Nurminen, S.; Kivelä, L.; Kurppa, K. Extraintestinal Manifestations of Celiac Disease: Early Detection for Better Long-Term Outcomes. Nutrients 2018, 10, 1015. [Google Scholar] [CrossRef]
- Ciaccio, E.J.; Lewis, S.K.; Biviano, A.B.; Iyer, V.; Garan, H.; Green, P.H. Cardiovascular involvement in celiac disease. World J. Cardiol. 2017, 9, 652–666. [Google Scholar] [CrossRef]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef]
- Landman, M.; Theuns, S.D.M.; van Wering, H.M.; Tramper, G.; van Ledden, M.; Rietveld, E.; Vd Lelij, N.; Groeneweg, I.K.; Escher, J.; Groeneweg, M. Evaluation of the implementation of the 2012 ESPGHAN guideline of Coeliac disease in children: Results of a retrospective study in the Netherlands. Arch. Dis. Child. 2020, 105, 413. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef]
- Working Group Set Up by the Finnish Medical Society Duodecim and the Finnish Gastroenterology Society. Celiac Disease. Current Care Guidelines, 2018 [Internet]. Available online: https://www.kaypahoito.fi/hoi08001?tab=suositus#K1 (accessed on 1 January 2022).
- Husby, S.; Murray, J.A.; Katzka, D.A. AGA Clinical Practice Update on Diagnosis and Monitoring of Celiac Disease-Changing Utility of Serology and Histologic Measures: Expert Review. Gastroenterology 2019, 156, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.R.; Murray, J.A. Contemporary celiac disease diagnosis: Is a biopsy avoidable? Curr. Opin. Gastroenterol. 2016, 32, 80–85. [Google Scholar] [CrossRef]
- Kurien, M.; Ludvigsson, J.F.; Sanders, D.S.; The Authors of the BSG Guidelines. A no biopsy strategy for adult patients with suspected coeliac disease: Making the world gluten-free. Gut 2015, 64, 1003–1004. [Google Scholar] [CrossRef] [PubMed]
- Marks, L.J.; Kurien, M.; Sanders, D.S. The serological diagnosis of adult coeliac disease—A cautious step forward? Gastroenterol. Hepatol. Bed Bench 2018, 11, 175–177. [Google Scholar]
- Biagi, F.; Bianchi, P.I.; Campanella, J.; Zanellati, G.; Corazza, G.R. The impact of misdiagnosing celiac disease at a referral centre. Can. J. Gastroenterol. 2009, 23, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Silvester, J.A.; Kurada, S.; Szwajcer, A.; Kelly, C.P.; Leffler, D.A.; Duerksen, D.R. Tests for Serum Transglutaminase and Endomysial Antibodies Do Not Detect Most Patients With Celiac Disease and Persistent Villous Atrophy on Gluten-free Diets: A Meta-analysis. Gastroenterology 2017, 153, 689–701.e1. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. American College of Gastroenterology ACG clinical guidelines: Diagnosis and management of celiac disease. Am. J. Gastroenterol. 2013, 108, 656–677. [Google Scholar] [CrossRef]
- Oberhuber, G.; Granditsch, G.; Vogelsang, H. The histopathology of coeliac disease: Time for a standardized report scheme for pathologists. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1185–1194. [Google Scholar] [CrossRef]
- Donat, E.; Ramos, J.M.; Sánchez-Valverde, F.; Moreno, A.; Martinez, M.-J.; Leis, R.; Peña-Quintana, L.; Castillejo, G.; Fernández, S.; Garcia, Z.; et al. ESPGHAN 2012 Guidelines for Coeliac Disease Diagnosis: Validation Through a Retrospective Spanish Multicentric Study. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 284–291. [Google Scholar] [CrossRef]
- Smarrazzo, A.; Misak, Z.; Costa, S.; Mičetić-Turk, D.; Abu-Zekry, M.; Kansu, A.; Abkari, A.; Bouziane-Nedjadi, K.; Ben Hariz, M.; Roma, E.; et al. Diagnosis of celiac disease and applicability of ESPGHAN guidelines in Mediterranean countries: A real life prospective study. BMC Gastroenterol. 2017, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Araya, M.; Díaz, J.; Oyarzun, A.; Lucero, Y.; Alarcon, T.; González, M.; Canales, P.; Fierro, L.; Pérez-Bravo, F. Avoiding Small Intestinal Biopsies for Diagnosis of Celiac Disease in Children: A Reliable Strategy for All Patients? J. Pediatr. Gastroenterol. Nutr. 2018, 66, 785–788. [Google Scholar] [CrossRef] [PubMed]
- BSG Interim Guidance: COVID-19 Specific Non-Biopsy Protocol for Those with Suspected Coeliac Disease. 2020. Available online: https://www.bsg.org.uk/covid-19-advice/covid-19-specific-non-biopsy-protocol-guidance-for-those-with-suspected-coeliac-disease/ (accessed on 26 September 2021).
- Johnston, R.D.; Chan, Y.J.; Mubashar, T.; Bailey, J.R.; Paul, S.P. No-biopsy pathway following the interim BSG guidance reliably diagnoses adult coeliac disease. Frontline Gastroenterol. 2020, 13, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, R.P.; Marsh, M.N. From 2-dimensional to 3-dimensional: Overcoming dilemmas in intestinal mucosal interpretation. World J. Gastroenterol. 2019, 25, 2402–2415. [Google Scholar] [CrossRef]
- Collin, P.; Kaukinen, K.; Vogelsang, H.; Korponay-Szabó, I.; Sommer, R.; Schreier, E.; Volta, U.; Granito, A.; Veronesi, L.; Mascart, F.; et al. Antiendomysial and antihuman recombinant tissue transglutaminase antibodies in the diagnosis of coeliac disease: A biopsy-proven European multicentre study. Eur. J. Gastroenterol. Hepatol. 2005, 17, 85–91. [Google Scholar] [CrossRef]
- Arguelles-Grande, C.; Tennyson, C.A.; Lewis, S.K.; Green, P.H.R.; Bhagat, G. Variability in small bowel histopathology reporting between different pathology practice settings: Impact on the diagnosis of coeliac disease. J. Clin. Pathol. 2012, 65, 242–247. [Google Scholar] [CrossRef]
- Picarelli, A.; Borghini, R.; Donato, G.; Di Tola, M.; Boccabella, C.; Isonne, C.; Giordano, M.; Di Cristofano, C.; Romeo, F.; Di Cioccio, G.; et al. Weaknesses of histological analysis in celiac disease diagnosis: New possible scenarios. Scand. J. Gastroenterol. 2014, 49, 1318–1324. [Google Scholar] [CrossRef]
- Montén, C.; Bjelkenkrantz, K.; Gudjonsdottir, A.H.; Browaldh, L.; Arnell, H.; Naluai, Å.T.; Agardh, D. Validity of histology for the diagnosis of paediatric coeliac disease: A Swedish multicentre study. Scand. J. Gastroenterol. 2016, 51, 427–433. [Google Scholar] [CrossRef]
- Kurppa, K.; Collin, P.; Viljamaa, M.; Haimila, K.; Saavalainen, P.; Partanen, J.; Laurila, K.; Huhtala, H.; Paasikivi, K.; Mäki, M.; et al. Diagnosing mild enteropathy celiac disease: A randomized, controlled clinical study. Gastroenterology 2009, 136, 816–823. [Google Scholar] [CrossRef]
- Mooney, P.D.; Kurien, M.; Evans, K.E.; Rosario, E.; Cross, S.S.; Vergani, P.; Hadjivassiliou, M.; Murray, J.A.; Sanders, D.S. Clinical and Immunologic Features of Ultra-Short Celiac Disease. Gastroenterology 2016, 150, 1125–1134. [Google Scholar] [CrossRef]
- Taavela, J.; Popp, A.; Korponay-Szabo, I.R.; Ene, A.; Vornanen, M.; Saavalainen, P.; Lähdeaho, M.-L.; Ruuska, T.; Laurila, K.; Parvan, A.; et al. A Prospective Study on the Usefulness of Duodenal Bulb Biopsies in Celiac Disease Diagnosis in Children: Urging Caution. Am. J. Gastroenterol. 2016, 111, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Oberhuber, G.; Vogelsang, H. Clinical and Immunologic Features of Ultra-Short Celiac Disease. Gastroenterology 2016, 151, 773. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Özakıncı, H.; Kırmızı, A.; Tural, M.; Kiremitçi, S.; Savaş, B.; Kuloğlu, Z.; Kansu, A.; Ensari, A. Duodenal bulb biopsy in the diagnostic work-up of coeliac disease. Virchows Arch. Int. J. Pathol. 2020, 477, 507–515. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.R.; O’Brien, C.R.; Gremida, A.; Ling, C.; Rustagi, T. Efficacy of duodenal bulb biopsy for diagnosis of celiac disease: A systematic review and meta-analysis. Endosc. Int. Open 2018, 6, E1369–E1378. [Google Scholar] [CrossRef] [PubMed]
- Hill, P.G.; Holmes, G.K.T. Coeliac disease: A biopsy is not always necessary for diagnosis. Aliment. Pharmacol. Ther. 2008, 27, 572–577. [Google Scholar] [CrossRef]
- Sugai, E.; Moreno, M.L.; Hwang, H.J.; Cabanne, A.; Crivelli, A.; Nachman, F.; Vázquez, H.; Niveloni, S.; Argonz, J.; Mazure, R.; et al. Celiac disease serology in patients with different pretest probabilities: Is biopsy avoidable? World J. Gastroenterol. 2010, 16, 3144–3152. [Google Scholar] [CrossRef]
- Salmi, T.T.; Collin, P.; Reunala, T.; Mäki, M.; Kaukinen, K. Diagnostic methods beyond conventional histology in coeliac disease diagnosis. Dig. Liver Dis. 2010, 42, 28–32. [Google Scholar] [CrossRef]
- Alessio, M.G.; Tonutti, E.; Brusca, I.; Radice, A.; Licini, L.; Sonzogni, A.; Florena, A.; Schiaffino, E.; Marus, W.; Sulfaro, S.; et al. Correlation between IgA tissue transglutaminase antibody ratio and histological finding in celiac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 44–49. [Google Scholar] [CrossRef]
- Zanini, B.; Magni, A.; Caselani, F.; Lanzarotto, F.; Carabellese, N.; Villanacci, V.; Ricci, C.; Lanzini, A. High tissue-transglutaminase antibody level predicts small intestinal villous atrophy in adult patients at high risk of celiac disease. Dig. Liver Dis. 2012, 44, 280–285. [Google Scholar] [CrossRef]
- Wakim-Fleming, J.; Pagadala, M.R.; Lemyre, M.S.; Lopez, R.; Kumaravel, A.; Carey, W.D.; Zein, N.N. Diagnosis of celiac disease in adults based on serology test results, without small-bowel biopsy. Clin. Gastroenterol. Hepatol. 2013, 11, 511–516. [Google Scholar] [CrossRef]
- Beltran, L.; Koenig, M.; Egner, W.; Howard, M.; Butt, A.; Austin, M.R.; Patel, D.; Sanderson, R.R.; Goubet, S.; Saleh, F.; et al. High-titre circulating tissue transglutaminase-2 antibodies predict small bowel villous atrophy, but decision cut-off limits must be locally validated. Clin. Exp. Immunol. 2014, 176, 190–198. [Google Scholar] [CrossRef]
- Tortora, R.; Imperatore, N.; Capone, P.; De Palma, G.D.; De Stefano, G.; Gerbino, N.; Caporaso, N.; Rispo, A. The presence of anti-endomysial antibodies and the level of anti-tissue transglutaminases can be used to diagnose adult coeliac disease without duodenal biopsy. Aliment. Pharmacol. Ther. 2014, 40, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Oyaert, M.; Vermeersch, P.; De Hertogh, G.; Hiele, M.; Vandeputte, N.; Hoffman, I.; Bossuyt, X. Combining antibody tests and taking into account antibody levels improves serologic diagnosis of celiac disease. Clin. Chem. Lab. Med. 2015, 53, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Di Tola, M.; Marino, M.; Goetze, S.; Casale, R.; Di Nardi, S.; Borghini, R.; Donato, G.; Tiberti, A.; Picarelli, A. Identification of a serum transglutaminase threshold value for the noninvasive diagnosis of symptomatic adult celiac disease patients: A retrospective study. J. Gastroenterol. 2016, 51, 1031–1039. [Google Scholar] [CrossRef]
- Ganji, A.; Esmaeilzadeh, A.; Bahari, A.; Ghafarzadegan, K.; Afzal Aghayee, M.; Mosanen Mozafari, H.; Hayatbakhsh, A.; Ghavami Ghanbarabadi, V.; Ravarian, B.; Rahimi, L. Correlation Between Cut-off Level of Tissue Transglutaminase Antibody and Marsh Classification. Middle East J. Dig. Dis. 2016, 8, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Efthymakis, K.; Serio, M.; Milano, A.; Laterza, F.; Bonitatibus, A.; Di Nicola, M.; Neri, M. Application of the Biopsy-Sparing ESPGHAN Guidelines for Celiac Disease Diagnosis in Adults: A Real-Life Study. Dig. Dis. Sci. 2017, 62, 2433–2439. [Google Scholar] [CrossRef] [PubMed]
- Holmes, G.K.T.; Forsyth, J.M.; Knowles, S.; Seddon, H.; Hill, P.G.; Austin, A.S. Coeliac disease: Further evidence that biopsy is not always necessary for diagnosis. Eur. J. Gastroenterol. Hepatol. 2017, 29, 640–645. [Google Scholar] [CrossRef]
- Gülseren, Y.D.; Adiloğlu, A.K.; Yücel, M.; Dağ, Z.; Eyerci, N.; Berkem, R.; Filik, L.; Çaydere, M. Comparison of non-invasive tests with invasive tests in the diagnosis of celiac disease. J. Clin. Lab. Anal. 2019, 33, e22722. [Google Scholar] [CrossRef]
- Fuchs, V.; Kurppa, K.; Huhtala, H.; Laurila, K.; Mäki, M.; Collin, P.; Salmi, T.; Luostarinen, L.; Saavalainen, P.; Kaukinen, K. Serology-based criteria for adult coeliac disease have excellent accuracy across the range of pre-test probabilities. Aliment. Pharmacol. Ther. 2019, 49, 277–284. [Google Scholar] [CrossRef]
- Alharbi, I.S.; Sweid, A.M.; Memon, M.Y.; Alshieban, S.; Alanazi, A. Correlation of TTG IgA Level with Small Intestinal Histopathological Changes for Celiac Disease among Adult Saudi Patients. J. Transl. Intern. Med. 2020, 8, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Ylönen, V.; Lindfors, K.; Repo, M.; Huhtala, H.; Fuchs, V.; Saavalainen, P.; Musikka, A.; Laurila, K.; Kaukinen, K.; Kurppa, K. Non-Biopsy Serology-Based Diagnosis of Celiac Disease in Adults Is Accurate with Different Commercial Kits and Pre-Test Probabilities. Nutrients 2020, 12, 2736. [Google Scholar] [CrossRef] [PubMed]
- Penny, H.A.; Raju, S.A.; Lau, M.S.; Marks, L.J.; Baggus, E.M.; Bai, J.C.; Bassotti, G.; Bontkes, H.J.; Carroccio, A.; Danciu, M.; et al. Accuracy of a no-biopsy approach for the diagnosis of coeliac disease across different adult cohorts. Gut 2021, 70, 876–883. [Google Scholar] [CrossRef]
- Beig, J.; Rostami, K.; Hayman, D.T.S.; Hassan, S.; Gerred, S.; Ogra, R. Is duodenal biopsy always necessary for the diagnosis of coeliac disease in adult patients with high anti-tissue transglutaminase (TTG) antibody titres? Frontline Gastroenterol. 2021. [Google Scholar] [CrossRef]
- Nellikkal, S.S.; Hafed, Y.; Larson, J.J.; Murray, J.A.; Absah, I. High Prevalence of Celiac Disease Among Screened First-Degree Relatives. Mayo Clin. Proc. 2019, 94, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.C.; Green, N.; Dickerson, J.; Lee, D. Is Endoscopic Assessment of the Esophagus and Stomach Enough to Determine the Need for Biopsy at These Sites in Pediatric Patients Undergoing Endoscopy for Elevated TTG? Pediatr. Dev. Pathol. 2021, 24, 206–212. [Google Scholar] [CrossRef]
- Huang, Y.; Gui, Q.; Li, H.; Long, X.; Liang, X.; Lu, H. Age is the only predictor for upper gastrointestinal malignancy in Chinese patients with uncomplicated dyspepsia: A prospective investigation of endoscopic findings. BMC Gastroenterol. 2021, 21, 441. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.W.; Siddique, S.M.; Patel, A.; Harris, A.; Sultan, S.; Altayar, O.; Falck-Ytter, Y. AGA Clinical Practice Guidelines on the Gastrointestinal Evaluation of Iron Deficiency Anemia. Gastroenterology 2020, 159, 1085–1094. [Google Scholar] [CrossRef]
- Sanders, D.S.; Hurlstone, D.P. Synchronous and metachronous lesions may occur in patients with celiac disease anywhere in the GI tract. Gastrointest. Endosc. 2003, 57, 992. [Google Scholar] [CrossRef]
- Alcalde, M.; Carro, J.; Rivero, M.; Fernandez, J.J.; Saenz De Santamaria, J.S. Malt lymphoma as first clinical presentation of a celiac disease. Acta Gastro-Enterol. Belg. 1998, 61, 479–482. [Google Scholar]
- Schiepatti, A.; Sanders, D.S.; Biagi, F. Seronegative coeliac disease: Clearing the diagnostic dilemma. Curr. Opin. Gastroenterol. 2018, 34, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Joelson, A.M.; Geller, M.G.; Zylberberg, H.M.; Green, P.H.R.; Lebwohl, B. Numbers and Features of Patients With a Diagnosis of Celiac Disease Without Duodenal Biopsy, Based on a National Survey. Clin. Gastroenterol. Hepatol. 2019, 17, 1089–1097.e2. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Thanataveerat, A.; Green, P.H.R.; Lebwohl, B. Cost Effectiveness of Routine Duodenal Biopsy Analysis for Celiac Disease During Endoscopy for Gastroesophageal Reflux. Clin. Gastroenterol. Hepatol. 2015, 13, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Herrod, P.J.J.; Lund, J.N. Random duodenal biopsy to exclude coeliac disease as a cause of anaemia is not cost-efective and should be replaced with universally performed pre-endoscopy serology in patients on a suspected cancer pathway. Tech. Coloproctol. 2018, 22, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Rostami-Nejad, M.; Villanacci, V.; Hogg-Kollars, S.; Volta, U.; Manenti, S.; Reza-Zali, M.; Caio, G.; Giovenali, P.; Barakauskiene, A.; Kazenaite, E.; et al. Endoscopic and histological pitfalls in the diagnosis of celiac disease: A multicentre study assessing the current practice. Rev. Esp. Enferm. Dig. 2013, 105, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.A.; Blanshard, R.J.; Naylor, G.; Penny, H.A.; Mooney, P.D.; Sanders, D.S. Do gastroenterologists have medical inertia towards coeliac disease? A UK multicentre secondary care study. BMJ Open Gastroenterol. 2021, 8, e000544. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Kapel, R.C.; Neugut, A.I.; Green, P.H.R.; Genta, R.M. Adherence to Biopsy Guidelines Increases Celiac Disease Diagnosis. Gastrointest. Endosc. 2011, 74, 103–109. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).