Point-of-Care Electroencephalography in Acute Neurological Care: A Narrative Review
Abstract
1. Introduction
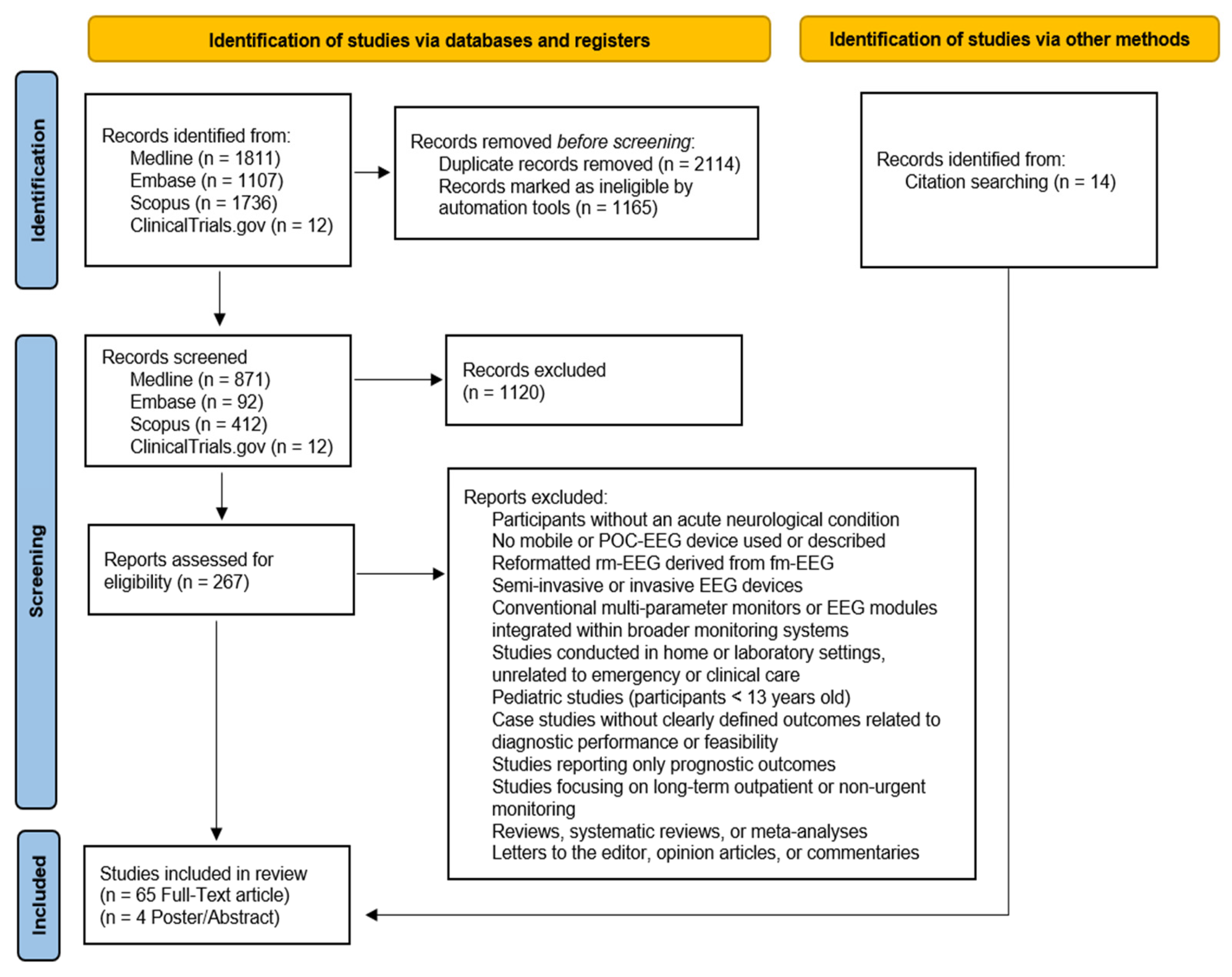
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Search Process
2.4. Data Extraction, Synthesis, Analysis, and Quality Appraisal
3. Results
3.1. POC-EEG Systems in the Assessment of NCSE
3.1.1. Evaluating Diagnostic Accuracy and Clinical Implications of POC-EEG Systems for NCSE Detection
3.1.2. Evaluating Feasibility of POC-EEG Systems for NCSE Detection
3.2. POC-EEG Systems in the Assessment of TBI
3.2.1. Evaluating Diagnostic Accuracy and Clinical Implications of POC-EEG Systems for TBI Evaluation
3.2.2. Evaluating Feasibility of POC-EEG Systems for TBI Evaluation
3.3. POC-EEG Systems in the Detection and Management of Strokes
3.3.1. Evaluating the Diagnostic Accuracy and Clinical Implications of POC-EEG Systems in Stroke Assessment
3.3.2. Evaluating Feasibility of POC-EEG Systems in Stroke Assessment
3.4. POC-EEG Systems in Delirium Detection
3.4.1. Evaluating the Diagnostic Accuracy and Clinical Implications of POC-EEG Systems for Delirium Identification
3.4.2. Evaluating the Feasibility of POC-EEG Systems for Delirium Identification
4. Discussion
4.1. Diagnostic Accuracy, Feasibility, and Clinical Implications of POC-EEG Systems in the Assessment of NCSE
4.2. Diagnostic Accuracy, Feasibility, and Clinical Implications of POC-EEG Systems in the Assessment of TBIs
4.3. Diagnostic Accuracy, Feasibility, and Clinical Implications of POC-EEG Systems in the Assessment of Strokes
4.4. Diagnostic Accuracy, Feasibility, and Clinical Implications of POC-EEG Systems in the Assessment of Delirium
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AIS | Acute ischemic stroke |
| AMS | Altered mental status |
| ASM | Anti-seizure medication |
| BAI | Brain Abnormality Index |
| BD | Brain death |
| BFI | Brain function index |
| BIS | Bispectral index |
| BSEEG | Bispectral EEG |
| BSI | Brain symmetry index |
| CCAT | Critical Care Air Transport |
| CCHR | Canadian CT Head Rule |
| CI | Concussion Index |
| CSs | Continuous slow waves |
| CT+ | Computed Tomography-positive |
| CT− | Computed Tomography-negative |
| DA | Drugs and alcohol |
| DAR | Delta/alpha ratio |
| DBATR | (Delta + Theta)/(Alpha + Beta) Ratio |
| DECIDE | Does Use of Rapid-Response EEG Impact Clinical Decision-Making |
| DRG | Diagnosis-related group |
| DSD | Delirium superimposed on dementia |
| DTI | Diffusion tensor imaging |
| EA | Epileptic activity |
| EDs | Emergency departments |
| ESE | Electrographic SE |
| ESz | Electrographic seizure |
| GA | Genetic algorithm |
| GCS | Glasgow Coma Scale |
| GPD | Generalized PD |
| HEP | Highly epileptiform patterns |
| ICU | Intensive care unit |
| IED | Interictal epileptiform discharge |
| LASSO | Least Absolute Shrinkage and Selection Operator |
| LPD | Lateralized periodic discharges |
| LVO | Large vessel occlusion |
| LVO-a | Anterior LVO |
| MTBI-DS | mTBI discriminant score |
| NCS | Non-convulsive seizure |
| NCSE | Non-convulsive status epilepticus |
| NEXUS | National Emergency X-Radiography Utilization Study |
| NOC | New Orleans Criteria |
| NPV | Negative predictive value |
| Non-EA | Non-epileptic activity |
| PABI | Post-anoxic brain injury |
| PD | Periodic discharges |
| POC-EEG | Point-of-care electroencephalography |
| PPV | Positive predictive value |
| QI | Quality improvement |
| RA | Rhythmic activity |
| RDA | Rhythmic delta activity |
| RTP | Return-to-play |
| SAFER-EEG | Seizure Assessment and Forecasting with Efficient Rapid-EEG |
| SBII | Structural Brain Injury Index |
| SE | Status epilepticus |
| SIC | Structural Injury Classifier |
| SRC | Sports-related concussion |
| SVM | Support Vector Machine |
| SW | Spikes and waves |
| SWLDA | Stepwise Linear Discriminant Analysis |
| SzB | Seizure burden |
| TAR | Theta–alpha ratio |
| TBI | Traumatic brain injury |
| UCH-L1 | Ubiquitin C-terminal hydrolase L1 |
| ViT | Vision Transformer |
| cEEG | Continuous EEG |
| conv-EEG | Conventional EEG |
| c-conv-EEG | Continuous conventional EEG |
| eBFI | Enhanced BFI |
| fm-EEG | Full-montage EEG |
| mTBI | Mild TBI |
| pESE | Possible ESE |
| pdBSI | Pairwise-derived BSI |
| qEEG | Quantitative EEG |
| rEEG | Routine EEG |
| rm-EEG | Reduced-montage EEG |
| rr-EEG | Rapid-response EEG |
| rsBSI | Revised BSI |
References
- Sharma, S.; Nunes, M.; Alkhachroum, A. Adult Critical Care Electroencephalography Monitoring for Seizures: A Narrative Review. Front. Neurol. 2022, 13, 951286. [Google Scholar] [CrossRef]
- Davey, Z.; Gupta, P.B.; Li, D.R.; Nayak, R.U.; Govindarajan, P. Rapid Response EEG: Current State and Future Directions. Curr. Neurol. Neurosci. Rep. 2022, 22, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Simma, L.; Romano, F.; Schmidt, S.; Ramantani, G.; Bölsterli, B.K. Integrating Neuromonitoring in Pediatric Emergency Medicine: Exploring Two Options for Point-of-Care Electroencephalogram (pocEEG) via Patient Monitors—A Technical Note. J. Pers. Med. 2023, 13, 1411. [Google Scholar] [CrossRef] [PubMed]
- Moctezuma, L.A.; Molinas, M. EEG Channel-Selection Method for Epileptic-Seizure Classification Based on Multi-Objective Optimization. Front. Neurosci. 2020, 14, 593. [Google Scholar] [CrossRef]
- Eberhard, E.; Beckerman, S.R. Rapid-Response Electroencephalography in Seizure Diagnosis and Patient Care: Lessons From a Community Hospital. J. Neurosci. Nurs. 2023, 55, 157–163. [Google Scholar] [CrossRef]
- Kozak, R.; Gururangan, K.; Dorriz, P.J.; Kaplan, M. Point-of-Care Electroencephalography Enables Rapid Evaluation and Management of Non-convulsive Seizures and Status Epilepticus in the Emergency Department. J. Am. Coll. Emerg. Physicians Open 2023, 4, e13004. [Google Scholar] [CrossRef]
- Wright, N.M.K.; Madill, E.S.; Isenberg, D.; Gururangan, K.; McClellen, H.; Snell, S.; Jacobson, M.P.; Gentile, N.T.; Govindarajan, P. Evaluating the Utility of Rapid Response EEG in Emergency Care. Emerg. Med. J. 2021, 38, 923–926. [Google Scholar] [CrossRef]
- Madill, E.S.; Gururangan, K.; Krishnamohan, P. Improved Access to Rapid Electroencephalography at a Community Hospital Reduces Inter-hospital Transfers for Suspected Non-convulsive Seizures. Epileptic Disord. 2022, 24, 507–516. [Google Scholar] [CrossRef]
- Vespa, P.M.; Olson, D.M.; John, S.; Hobbs, K.S.; Gururangan, K.; Nie, K.; Desai, M.J.; Markert, M.; Parvizi, J.; Bleck, T.P.; et al. Evaluating the Clinical Impact of Rapid Response Electroencephalography: The DECIDE Multicenter Prospective Observational Clinical Study*. Crit. Care Med. 2020, 48, 1249–1257. [Google Scholar] [CrossRef]
- Laccheo, I.; Sonmezturk, H.; Bhatt, A.B.; Tomycz, L.; Shi, Y.; Ringel, M.; DiCarlo, G.; Harris, D.; Barwise, J.; Abou-Khalil, B.; et al. Non-Convulsive Status Epilepticus and Non-Convulsive Seizures in Neurological ICU Patients. Neurocrit. Care 2015, 22, 202–211. [Google Scholar] [CrossRef]
- Young, G.B.; Jordan, K.G. Do Nonconvulsive Seizures Damage the Brain?—Yes. Arch. Neurol. 1998, 55, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Abend, N.S.; Arndt, D.H.; Carpenter, J.L.; Chapman, K.E.; Cornett, K.M.; Gallentine, W.B.; Giza, C.C.; Goldstein, J.L.; Hahn, C.D.; Lerner, J.T.; et al. Electrographic Seizures in Pediatric ICU Patients: Cohort Study of Risk Factors and Mortality. Neurology 2013, 81, 383. [Google Scholar] [CrossRef] [PubMed]
- Parikh, H.; Hoffman, K.; Sun, H.; Zafar, S.F.; Ge, W.; Jing, J.; Liu, L.; Sun, J.; Struck, A.; Volfovsky, A.; et al. Effects of Epileptiform Activity on Discharge Outcome in Critically Ill Patients in the USA: A Retrospective Cross-Sectional Study. Lancet Digit. Health 2023, 5, e495–e502. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, P.; Menon, D.K.; Citerio, G.; Vespa, P.; Bader, M.K.; Brophy, G.M.; Diringer, M.N.; Stocchetti, N.; Videtta, W.; Armonda, R.; et al. Consensus Summary Statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: A Statement for Healthcare Professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit. Care 2014, 21, 1–26. [Google Scholar] [CrossRef]
- Gavvala, J.; Abend, N.; LaRoche, S.; Hahn, C.; Herman, S.T.; Claassen, J.; Macken, M.; Schuele, S.; Gerard, E. Critical Care EEG Monitoring Research Consortium (CCEMRC) Continuous EEG Monitoring: A Survey of Neurophysiologists and Neuro-intensivists. Epilepsia 2014, 55, 1864–1871. [Google Scholar] [CrossRef]
- Beniczky, S.; Schomer, D.L. Electroencephalography: Basic Biophysical and Technological Aspects Important for Clinical Applications. Epileptic Disord. 2020, 22, 697–715. [Google Scholar] [CrossRef]
- MacDarby, L.; Healy, M.; McHugh, J.C. EEG Availability in the Intensive Care Setting: A Multicentre Study. Neurocrit. Care 2021, 34, 287–290. [Google Scholar] [CrossRef]
- Abend, N.S.; Topjian, A.A.; Williams, S. How Much Does It Cost to Identify a Critically Ill Child Experiencing Electrographic Seizures? J. Clin. Neurophysiol. 2015, 32, 257–264. [Google Scholar] [CrossRef]
- Ma, B.B.; Johnson, E.L.; Ritzl, E.K. Sensitivity of a Reduced EEG Montage for Seizure Detection in the Neurocritical Care Setting. J. Clin. Neurophysiol. 2018, 35, 256–262. [Google Scholar] [CrossRef]
- Kolls, B.J.; Husain, A.M. Assessment of Hairline EEG as a Screening Tool for Nonconvulsive Status Epilepticus. Epilepsia 2007, 48, 959–965. [Google Scholar] [CrossRef]
- Tanner, A.E.J.; Särkelä, M.O.K.; Virtanen, J.; Viertiö-Oja, H.E.; Sharpe, M.D.; Norton, L.; Davies-Schinkel, C.; Young, G.B. Application of Subhairline EEG Montage in Intensive Care Unit: Comparison With Full Montage. J. Clin. Neurophysiol. 2014, 31, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Gururangan, K. Diagnostic Utility of Reduced Electroencephalography. J. Clin. Neurophysiol. 2018, 35, 356. [Google Scholar] [CrossRef] [PubMed]
- Herta, J.; Koren, J.; Fürbass, F.; Hartmann, M.; Gruber, A.; Baumgartner, C. Reduced Electrode Arrays for the Automated Detection of Rhythmic and Periodic Patterns in the Intensive Care Unit: Frequently Tried, Frequently Failed? Clin. Neurophysiol. 2017, 128, 1524–1531. [Google Scholar] [CrossRef]
- Westover, M.B.; Gururangan, K.; Markert, M.S.; Blond, B.N.; Lai, S.; Benard, S.; Bickel, S.; Hirsch, L.J.; Parvizi, J. Diagnostic Value of Electroencephalography with Ten Electrodes in Critically Ill Patients. Neurocrit. Care 2020, 33, 479–490. [Google Scholar] [CrossRef]
- Tjepkema-Cloostermans, M.C.; Hofmeijer, J.; Hom, H.W.; Bosch, F.H.; Van Putten, M.J.A.M. Predicting Outcome in Post-anoxic Coma: Are Ten EEG Electrodes Enough? J. Clin. Neurophysiol. 2017, 34, 207–212. [Google Scholar] [CrossRef]
- Gururangan, K.; Razavi, B.; Parvizi, J. Diagnostic Utility of Eight-Channel EEG for Detecting Generalized or Hemispheric Seizures and Rhythmic Periodic Patterns. Clin. Neurophysiol. Pract. 2018, 3, 65–73. [Google Scholar] [CrossRef]
- LaMonte, M.P. Ceribell EEG Shortens Seizure Diagnosis and Workforce Time and Is Useful for COVID Isolation. Epilepsia Open 2021, 6, 331–338. [Google Scholar] [CrossRef]
- Hanley, D.F.; Chabot, R.; Mould, W.A.; Morgan, T.; Naunheim, R.; Sheth, K.N.; Chiang, W.; Prichep, L.S. Use of Brain Electrical Activity for the Identification of Hematomas in Mild Traumatic Brain Injury. J. Neurotrauma 2013, 30, 2051–2056. [Google Scholar] [CrossRef]
- Rittenberger, J.C.; Weissman, A.; Baldwin, M.; Flickinger, K.; Repine, M.J.; Guyette, F.X.; Doshi, A.A.; Dezfulian, C.; Callaway, C.W.; Elmer, J. Preliminary Experience with Point-of-Care EEG in Post-Cardiac Arrest Patients. Resuscitation 2019, 135, 98–102. [Google Scholar] [CrossRef]
- Mahmood, S.; El-Menyar, A.; Shabana, A.; Mahmood, I.; Asim, M.; Abdelrahman, H.; Al-Thani, H. Bispectral Index as a Predictor of Unsalvageable Traumatic Brain Injury. Brain Inj. 2017, 31, 1382–1386. [Google Scholar] [CrossRef]
- Parvizi, J.; Gururangan, K.; Razavi, B.; Chafe, C. Detecting Silent Seizures by Their Sound. Epilepsia 2018, 59, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Yazbeck, M.; Sra, P.; Parvizi, J. Rapid Response Electroencephalography for Urgent Evaluation of Patients in Community Hospital Intensive Care Practice. J. Neurosci. Nurs. 2019, 51, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Kamousi, B.; Karunakaran, S.; Gururangan, K.; Markert, M.; Decker, B.; Khankhanian, P.; Mainardi, L.; Quinn, J.; Woo, R.; Parvizi, J. Monitoring the Burden of Seizures and Highly Epileptiform Patterns in Critical Care with a Novel Machine Learning Method. Neurocrit. Care 2021, 34, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Dorriz, P.; Gururangan, K.; Kozak, R.; Kaplan, M. 583: Real-world performance of a seizure burden algorithm (clarity) in a community hospital. Crit. Care Med. 2024, 52, S265. [Google Scholar] [CrossRef]
- Kamousi, B.; Gupta, A.; Karunakaran, S.; Marjaninejad, A.; Woo, R.; Parvizi, J. 580: Improvements in a machine-learning algorithm for detecting status epilepticus. Crit. Care Med. 2024, 52, S263. [Google Scholar] [CrossRef]
- Ward, J.; Green, A.; Cole, R.; Zarbiv, S.; Dumond, S.; Clough, J.; Rincon, F. Implementation and Impact of a Point of Care Electroencephalography Platform in a Community Hospital: A Cohort Study. Front. Digit. Health 2023, 5, 1035442. [Google Scholar] [CrossRef]
- Hobbs, K.; Krishnamohan, P.; Legault, C.; Goodman, S.; Parvizi, J.; Gururangan, K.; Mlynash, M. Rapid Bedside Evaluation of Seizures in the ICU by Listening to the Sound of Brainwaves: A Prospective Observational Clinical Trial of Ceribell’s Brain Stethoscope Function. Neurocrit. Care 2018, 29, 302–312. [Google Scholar] [CrossRef]
- Rossini, P.M.; Cole, J.; Paulus, W.; Ziemann, U.; Chen, R. 1924–2024: First Centennial of EEG. Clin. Neurophysiol. 2025, 170, 132–135. [Google Scholar] [CrossRef]
- Faul, M.; Wald, M.M.; Xu, L.; Coronado, V.G. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths, 2002–2006; CDC: Centers for Disease Control and Prevention: Atlanta, GA, USA, 2010. [Google Scholar]
- Sariaslan, A.; Sharp, D.J.; D’Onofrio, B.M.; Larsson, H.; Fazel, S. Long-Term Outcomes Associated with Traumatic Brain Injury in Childhood and Adolescence: A Nationwide Swedish Cohort Study of a Wide Range of Medical and Social Outcomes. PLoS Med. 2016, 13, e1002103. [Google Scholar] [CrossRef]
- Elbin, R.J.; Sufrinko, A.; Schatz, P.; French, J.; Henry, L.; Burkhart, S.; Collins, M.W.; Kontos, A.P. Removal from Play After Concussion and Recovery Time. Pediatrics 2016, 138, e20160910. [Google Scholar] [CrossRef]
- Guskiewicz, K.M.; McCrea, M.; Marshall, S.W.; Cantu, R.C.; Randolph, C.; Barr, W.; Onate, J.A.; Kelly, J.P. Cumulative Effects Associated with Recurrent Concussion in Collegiate Football Players: The NCAA Concussion Study. JAMA 2003, 290, 2549–2555. [Google Scholar] [CrossRef] [PubMed]
- Anderson, V.; Godfrey, C.; Rosenfeld, J.V.; Catroppa, C. 10 Years Outcome from Childhood Traumatic Brain Injury. Int. J. Dev. Neurosci. 2012, 30, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Scholten, A.C.; Haagsma, J.A.; Cnossen, M.C.; Olff, M.; van Beeck, E.F.; Polinder, S. Prevalence of and Risk Factors for Anxiety and Depressive Disorders after Traumatic Brain Injury: A Systematic Review. J. Neurotrauma 2016, 33, 1969–1994. [Google Scholar] [CrossRef] [PubMed]
- Cole, W.R.; Arrieux, J.P.; Schwab, K.; Ivins, B.J.; Qashu, F.M.; Lewis, S.C. Test–Retest Reliability of Four Computerized Neurocognitive Assessment Tools in an Active Duty Military Population. Arch. Clin. Neuropsychol. 2013, 28, 732–742. [Google Scholar] [CrossRef]
- Tebano, M.T.; Cameroni, M.; Gallozzi, G.; Loizzo, A.; Palazzino, G.; Pezzini, G.; Ricci, G.F. EEG Spectral Analysis after Minor Head Injury in Man. Electroencephalogr. Clin. Neurophysiol. 1988, 70, 185–189. [Google Scholar] [CrossRef]
- Huff, J.S.; Naunheim, R.; Ghosh Dastidar, S.; Bazarian, J.; Michelson, E.A. Referrals for CT Scans in Mild TBI Patients Can Be Aided by the Use of a Brain Electrical Activity Biomarker. Am. J. Emerg. Med. 2017, 35, 1777–1779. [Google Scholar] [CrossRef]
- Hanley, D.; Prichep, L.S.; Badjatia, N.; Bazarian, J.; Chiacchierini, R.; Curley, K.C.; Garrett, J.; Jones, E.; Naunheim, R.; O’Neil, B.; et al. A Brain Electrical Activity Electroencephalographic-Based Biomarker of Functional Impairment in Traumatic Brain Injury: A Multi-Site Validation Trial. J. Neurotrauma 2018, 35, 41–47. [Google Scholar] [CrossRef]
- Hanley, D.; Prichep, L.S.; Bazarian, J.; Huff, J.S.; Naunheim, R.; Garrett, J.; Jones, E.B.; Wright, D.W.; O’Neill, J.; Badjatia, N.; et al. Emergency Department Triage of Traumatic Head Injury Using a Brain Electrical Activity Biomarker: A Multisite Prospective Observational Validation Trial. Acad. Emerg. Med. 2017, 24, 617–627. [Google Scholar] [CrossRef]
- Prichep, L.S.; Jacquin, A.; Filipenko, J.; Dastidar, S.G.; Zabele, S.; Vodencarevic, A.; Rothman, N.S. Classification of Traumatic Brain Injury Severity Using Informed Data Reduction in a Series of Binary Classifier Algorithms. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 806–822. [Google Scholar] [CrossRef]
- Rapp, P.E.; Keyser, D.O.; Albano, A.; Hernandez, R.; Gibson, D.B.; Zambon, R.A.; Hairston, W.D.; Hughes, J.D.; Krystal, A.; Nichols, A.S. Traumatic Brain Injury Detection Using Electrophysiological Methods. Front. Hum. Neurosci. 2015, 9, 11. [Google Scholar] [CrossRef]
- Thatcher, R.W.; North, D.M.; Curtin, R.T.; Walker, R.A.; Biver, C.J.; Gomez, J.F.; Salazar, A.M. An EEG Severity Index of Traumatic Brain Injury. J. Neuropsychiatry Clin. Neurosci. 2001, 13, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, R.W.; Biver, C.; McAlaster, R.; Camacho, M.; Salazar, A. Biophysical Linkage between MRI and EEG Amplitude in Closed Head Injury. NeuroImage 1998, 7, 352–367. [Google Scholar] [CrossRef] [PubMed]
- Wilde, E.A.; Goodrich-Hunsaker, N.J.; Ware, A.L.; Taylor, B.A.; Biekman, B.D.; Hunter, J.V.; Newman-Norlund, R.; Scarneo, S.; Casa, D.J.; Levin, H.S. Diffusion Tensor Imaging Indicators of White Matter Injury Are Correlated with a Multimodal Electroencephalography-Based Biomarker in Slow Recovering, Concussed Collegiate Athletes. J. Neurotrauma 2020, 37, 2093–2101. [Google Scholar] [CrossRef]
- Brooks, M.A.; Bazarian, J.J.; Prichep, L.S.; Dastidar, S.G.; Talavage, T.M.; Barr, W. The Use of an Electrophysiological Brain Function Index in the Evaluation of Concussed Athletes. J. Head Trauma Rehabil. 2018, 33, 1–6. [Google Scholar] [CrossRef]
- Prichep, L.S.; McCrea, M.; Barr, W.; Powell, M.; Chabot, R.J. Time Course of Clinical and Electrophysiological Recovery After Sport-Related Concussion. J. Head Trauma Rehabil. 2013, 28, 266–273. [Google Scholar]
- O’Neil, B.; Prichep, L.S.; Naunheim, R.; Chabot, R. Quantitative Brain Electrical Activity in the Initial Screening of Mild Traumatic Brain Injuries. West. J. Emerg. Med. 2012, 13, 394. [Google Scholar]
- Mulder, M.J.H.L.; Jansen, I.G.H.; Goldhoorn, R.-J.B.; Venema, E.; Chalos, V.; Compagne, K.C.J.; Roozenbeek, B.; Lingsma, H.F.; Schonewille, W.J.; van den Wijngaard, I.R.; et al. Time to Endovascular Treatment and Outcome in Acute Ischemic Stroke: MR CLEAN Registry Results. Circulation 2018, 138, 232–240. [Google Scholar] [CrossRef]
- Menon, B.K.; Sajobi, T.T.; Zhang, Y.; Rempel, J.L.; Shuaib, A.; Thornton, J.; Williams, D.; Roy, D.; Poppe, A.Y.; Jovin, T.G.; et al. Analysis of Workflow and Time to Treatment on Thrombectomy Outcome in the Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke (ESCAPE) Randomized, Controlled Trial. Circulation 2016, 133, 2279–2286. [Google Scholar] [CrossRef]
- Saver, J.L.; Goyal, M.; van der Lugt, A.; Menon, B.K.; Majoie, C.B.L.M.; Dippel, D.W.; Campbell, B.C.; Nogueira, R.G.; Demchuk, A.M.; Tomasello, A.; et al. Time to Treatment with Endovascular Thrombectomy and Outcomes from Ischemic Stroke: A Meta-Analysis. JAMA 2016, 316, 1279–1289. [Google Scholar] [CrossRef]
- Turc, G.; Maïer, B.; Naggara, O.; Seners, P.; Isabel, C.; Tisserand, M.; Raynouard, I.; Edjlali, M.; Calvet, D.; Baron, J.C.; et al. Clinical Scales Do Not Reliably Identify Acute Ischemic Stroke Patients with Large-Artery Occlusion. Stroke 2016, 47, 1466–1472. [Google Scholar] [CrossRef]
- Anadani, M.; Almallouhi, E.; Wahlquist, A.E.; Debenham, E.; Holmstedt, C.A. The Accuracy of Large Vessel Occlusion Recognition Scales in Telestroke Setting. Telemed. J. E-Health 2019, 25, 1071. [Google Scholar] [CrossRef] [PubMed]
- Antipova, D.; Eadie, L.; Macaden, A.; Wilson, P. Diagnostic Accuracy of Clinical Tools for Assessment of Acute Stroke: A Systematic Review. BMC Emerg. Med. 2019, 19, 49. [Google Scholar]
- Sharbrough, F.W.; Messick, J.M.; Sundt, T.M. Correlation of Continuous Electroencephalograms with Cerebral Blood Flow Measurements During Carotid Endarterectomy. Stroke 1973, 4, 674–683. [Google Scholar] [CrossRef] [PubMed]
- van Putten, M.J.A.M.; Hofmeijer, J. EEG Monitoring in Cerebral Ischemia: Basic Concepts and Clinical Applications. J. Clin. Neurophysiol. 2016, 33, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Srinivasan, R.; Burke Quinlan, E.; Solodkin, A.; Small, S.L.; Cramer, S.C. Utility of EEG Measures of Brain Function in Patients with Acute Stroke. J. Neurophysiol. 2016, 115, 2399–2405. [Google Scholar] [CrossRef]
- Shreve, L.; Kaur, A.; Vo, C.; Wu, J.; Cassidy, J.M.; Nguyen, A.; Zhou, R.J.; Tran, T.B.; Yang, D.Z.; Medizade, A.I.; et al. Electroencephalography Measures Are Useful for Identifying Large Acute Ischemic Stroke in the Emergency Department. J. Stroke Cerebrovasc. Dis. 2019, 28, 2280–2286. [Google Scholar] [CrossRef]
- Erani, F.; Zolotova, N.; Vanderschelden, B.; Khoshab, N.; Sarian, H.; Nazarzai, L.; Wu, J.; Chakravarthy, B.; Hoonpongsim-anont, W.; Yu, W.; et al. Electroencephalography Might Improve Diagnosis of Acute Stroke and Large Vessel Occlusion. Stroke 2020, 51, 3361–3365. [Google Scholar] [CrossRef]
- Finnigan, S.; Wong, A.; Read, S. Defining Abnormal Slow EEG Activity in Acute Ischaemic Stroke: Delta/Alpha Ratio as an Optimal QEEG Index. Clin. Neurophysiol. 2016, 127, 1452–1459. [Google Scholar] [CrossRef]
- Foreman, B.; Claassen, J. Quantitative EEG for the Detection of Brain Ischemia. Crit. Care 2012, 16, 216. [Google Scholar] [CrossRef]
- Finnigan, S.; Van Putten, M.J.A.M. EEG in Ischaemic Stroke: Quantitative EEG Can Uniquely Inform (Sub-)Acute Prognoses and Clinical Management. Clin. Neurophysiol. 2013, 124, 10–19. [Google Scholar] [CrossRef]
- van Putten, M.J.A.M.; Tavy, D.L.J. Continuous Quantitative EEG Monitoring in Hemispheric Stroke Patients Using the Brain Symmetry Index. Stroke 2004, 35, 2489–2492. [Google Scholar] [CrossRef] [PubMed]
- Van Kaam, R.C.; van Putten, M.J.A.M.; Vermeer, S.E.; Hofmeijer, J. Contralesional Brain Activity in Acute Ischemic Stroke. Cerebrovasc. Dis. 2018, 45, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Gottlibe, M.; Rosen, O.; Weller, B.; Mahagney, A.; Omar, N.; Khuri, A.; Srugo, I.; Genizi, J. Stroke Identification Using a Portable EEG Device—A Pilot Study. Neurophysiol. Clin. 2020, 50, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Van Meenen, L.C.C.; Van Stigt, M.N.; Marquering, H.A.; Majoie, C.B.L.M.; Roos, Y.B.W.E.M.; Koelman, J.H.T.M.; Potters, W.V.; Coutinho, J.M. Detection of Large Vessel Occlusion Stroke with Electroencephalography in the Emergency Room: First Results of the ELECTRA-STROKE Study. J. Neurol. 2022, 269, 2030–2038. [Google Scholar] [CrossRef]
- Shahrestani, S.; Wishart, D.; Han, S.M.J.; Strickland, B.A.; Bakhsheshian, J.; Mack, W.J.; Toga, A.W.; Sanossian, N.; Tai, Y.-C.; Zada, G. A Systematic Review of Next-Generation Point-of-Care Stroke Diagnostic Technologies. Neurosurg. Focus 2021, 51, E11. [Google Scholar] [CrossRef]
- Michelson, E.A.; Hanley, D.; Chabot, R.; Prichep, L.S. Identification of Acute Stroke Using Quantified Brain Electrical Activity. Acad. Emerg. Med. 2015, 22, 67–72. [Google Scholar] [CrossRef]
- Wilkinson, C.M.; Burrell, J.I.; Kuziek, J.W.P.; Thirunavukkarasu, S.; Buck, B.H.; Mathewson, K.E. Predicting Stroke Severity with a 3-Min Recording from the Muse Portable EEG System for Rapid Diagnosis of Stroke. Sci. Rep. 2020, 10, 18465. [Google Scholar] [CrossRef]
- Van Stigt, M.N.; Groenendijk, E.A.; Van Meenen, L.C.C.; Van De Munckhof, A.A.G.A.; Theunissen, M.; Franschman, G.; Smeekes, M.D.; Van Grondelle, J.A.F.; Geuzebroek, G.; Siegers, A.; et al. Prehospital Detection of Large Vessel Occlusion Stroke with EEG: Results of the ELECTRA-STROKE Study. Neurology 2023, 101, e2522–e2532. [Google Scholar] [CrossRef]
- Inouye, S.K.; Westendorp, R.G.J.; Saczynski, J.S. Delirium in Elderly People. Lancet 2014, 383, 911–922. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Jin, J. Poor Outcomes of Delirium in the Intensive Care Units Are Amplified by Increasing Age: A Retrospective Cohort Study. World J. Emerg. Med. 2021, 12, 117. [Google Scholar] [CrossRef]
- Khan, B.A.; Perkins, A.J.; Prasad, N.K.; Shekhar, A.; Campbell, N.L.; Gao, S.; Wang, S.; Khan, S.H.; Marcantonio, E.R.; Twigg, H.L., III; et al. Biomarkers of Delirium Duration and Delirium Severity in the ICU. Crit. Care Med. 2020, 48, 353. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.S.; Wang, S.; Perkins, A.J.; Gao, S.; Khan, S.; Lindroth, H.; Boustani, M.; Khan, B. Relationship Between Intensive Care Unit Delirium Severity and 2-Year Mortality and Health Care Utilization. Am. J. Crit. Care 2020, 29, 311–317. [Google Scholar] [CrossRef] [PubMed]
- DiLibero, J.; O’Donoghue, S.C.; DeSanto-Madeya, S.; Felix, J.; Ninobla, A.; Woods, A. An Innovative Approach to Improving the Accuracy of Delirium Assessments Using the Confusion Assessment Method for the Intensive Care Unit. Dimens. Crit. Care Nurs. 2016, 35, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Kobayashi, S.; Matsui, K.; Akaho, R.; Nishimura, K. The Accuracy of Delirium Assessment by Cardiologists Treating Heart Failure Inpatients: A Single Center Retrospective Survey. Biopsychosoc. Med. 2020, 14, 15. [Google Scholar] [CrossRef]
- Mulkey, M.; Albanese, T.; Kim, S.; Huang, H.; Yang, B. Delirium Detection Using GAMMA Wave and Machine Learning: A Pilot Study. Res. Nurs. Health 2022, 45, 652–663. [Google Scholar] [CrossRef]
- Tanabe, S.; Mohanty, R.; Lindroth, H.; Casey, C.; Ballweg, T.; Farahbakhsh, Z.; Krause, B.; Prabhakaran, V.; Banks, M.I.; Sanders, R.D. Cohort Study into the Neural Correlates of Postoperative Delirium: The Role of Connectivity and Slow-Wave Activity. Br. J. Anaesth. 2020, 125, 55–66. [Google Scholar] [CrossRef]
- Shinozaki, G.; Bormann, N.L.; Chan, A.C.; Zarei, K.; Sparr, N.A.; Klisares, M.J.; Jellison, S.S.; Heinzman, J.T.; Dahlstrom, E.B.; Duncan, G.N.; et al. Identification of Patients with High Mortality Risk and Prediction of Outcomes in Delirium by Bispectral EEG. J. Clin. Psychiatry 2019, 80, 19m12749. [Google Scholar] [CrossRef]
- Lee, S.; Yuki, K.; Chan, A.; Cromwell, J.; Shinozaki, G. The Point-of-Care EEG for Delirium Detection in the Emergency Department. Am. J. Emerg. Med. 2019, 37, 995–996. [Google Scholar] [CrossRef]
- Shinozaki, G.; Chan, A.C.; Sparr, N.A.; Zarei, K.; Gaul, L.N.; Heinzman, J.T.; Robles, J.; Yuki, K.; Chronis, T.J.; Ando, T.; et al. Delirium Detection by a Novel Bispectral Electroencephalography Device in General Hospital. Psychiatry Clin. Neurosci. 2018, 72, 856–863. [Google Scholar] [CrossRef]
- Ferrari, R. Writing Narrative Style Literature Reviews. Med. Writ. 2015, 24, 230–235. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Brenner, J.M.; Kent, P.; Wojcik, S.M.; Grant, W. Rapid Diagnosis of Nonconvulsive Status Epilepticus Using Reduced-Lead Electroencephalography. West J. Emerg. Med. 2015, 16, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Muraja-Murro, A.; Mervaala, E.; Westeren-Punnonen, S.; Lepola, P.; Töyräs, J.; Myllymaa, S.; Julkunen, P.; Kantanen, A.-M.; Kälviäinen, R.; Myllymaa, K. Forehead EEG Electrode Set versus Full-Head Scalp EEG in 100 Patients with Altered Mental State. Epilepsy Behav. 2015, 49, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Egawa, S.; Hifumi, T.; Nakamoto, H.; Kuroda, Y.; Kubota, Y. Diagnostic Reliability of Headset-Type Continuous Video EEG Monitoring for Detection of ICU Patterns and NCSE in Patients with Altered Mental Status with Unknown Etiology. Neurocrit. Care 2020, 32, 217–225. [Google Scholar] [CrossRef]
- Caricato, A.; Della Marca, G.; Ioannoni, E.; Silva, S.; Benzi Markushi, T.; Stival, E.; Biasucci, D.G.; Montano, N.; Gelormini, C.; Melchionda, I. Continuous EEG Monitoring by a New Simplified Wireless Headset in Intensive Care Unit. BMC Anesthesiol. 2020, 20, 298. [Google Scholar] [CrossRef]
- Meyer, M.; Fuest, S.; Krain, D.; Juenemann, M.; Braun, T.; Thal, S.C.; Schramm, P. Evaluation of a New Wireless Technique for Continuous Electroencephalography Monitoring in Neurological Intensive Care Patients. J. Clin. Monit. Comput. 2021, 35, 765–770. [Google Scholar] [CrossRef]
- Welte, T.M.; Janner, F.; Lindner, S.; Gollwitzer, S.; Stritzelberger, J.; Lang, J.D.; Reindl, C.; Sprügel, M.I.; Olmes, D.; Schwab, S.; et al. Evaluation of Simplified Wireless EEG Recordings in the Neurological Emergency Room. PLoS ONE 2024, 19, e0310223. [Google Scholar] [CrossRef]
- Kamousi, B.; Grant, A.M.; Bachelder, B.; Yi, J.; Hajinoroozi, M.; Woo, R. Comparing the Quality of Signals Recorded with a Rapid Response EEG and Conventional Clinical EEG Systems. Clin. Neurophysiol. Pract. 2019, 4, 69–75. [Google Scholar] [CrossRef]
- Chen, W.; Toprani, S.; Werbaneth, K.; Falco-Walter, J. Status Epilepticus and Other EEG Findings in Patients with COVID-19: A Case Series. Seizure 2020, 81, 198–200. [Google Scholar] [CrossRef]
- Kalkach-Aparicio, M.; Fatima, S.; Selte, A.; Sheikh, I.S.; Cormier, J.; Gallagher, K.; Avagyan, G.; Cespedes, J.; Krishnamurthy, P.V.; Elazim, A.A.; et al. Seizure Assessment and Forecasting with Efficient Rapid-EEG. Neurology 2024, 103, e209621. [Google Scholar] [CrossRef] [PubMed]
- Kurup, D.; Gururangan, K.; Desai, M.J.; Markert, M.S.; Eliashiv, D.S.; Vespa, P.M.; Parvizi, J. Comparing Seizures Captured by Rapid Response EEG and Conventional EEG Recordings in a Multicenter Clinical Study. Front. Neurol. 2022, 13, 915385. [Google Scholar] [CrossRef]
- Villamar, M.F.; Ayub, N.; Koenig, S.J. Automated Seizure Detection in Patients with Cardiac Arrest: A Retrospective Review of Ceribell™ Rapid-EEG Recordings. Neurocrit. Care 2023, 39, 505–513. [Google Scholar] [CrossRef]
- Desai, M.; Kalkach-Aparicio, M.; Sheikh, I.S.; Cormier, J.; Gallagher, K.; Hussein, O.M.; Cespedes, J.; Hirsch, L.J.; Westover, B.; Struck, A.F. Evaluating the Impact of Point-of-Care Electroencephalography on Length of Stay in the Intensive Care Unit: Subanalysis of the SAFER-EEG Trial. Neurocrit. Care 2024, 42, 108–117. [Google Scholar] [CrossRef]
- Gururangan, K.; Kozak, R.; Dorriz, P.J. Time Is Brain: Detection of Nonconvulsive Seizures and Status Epilepticus during Acute Stroke Evaluation Using Point-of-Care Electroencephalography. J. Stroke Cerebrovasc. Dis. 2025, 34, 108116. [Google Scholar] [CrossRef]
- Sheikh, Z.B.; Dhakar, M.B.; Fong, M.W.K.; Fang, W.; Ayub, N.; Molino, J.; Haider, H.A.; Foreman, B.; Gilmore, E.; Mizrahi, M.; et al. Accuracy of a Rapid-Response EEG’s Automated Seizure-Burden Estimator. Neurology 2025, 104, e210234. [Google Scholar] [CrossRef]
- Naunheim, R.S.; Treaster, M.; English, J.; Casner, T. Automated Electroencephalogram Identifies Abnormalities in the ED. Am. J. Emerg. Med. 2011, 29, 845–848. [Google Scholar] [CrossRef]
- Naunheim, R.S.; Treaster, M.; English, J.; Casner, T.; Chabot, R. Use of Brain Electrical Activity to Quantify Traumatic Brain Injury in the Emergency Department. Brain Inj. 2010, 24, 1324–1329. [Google Scholar] [CrossRef]
- McCrea, M.; Prichep, L.; Powell, M.R.; Chabot, R.; Barr, W.B. Acute Effects and Recovery After Sport-Related Concussion: A Neurocognitive and Quantitative Brain Electrical Activity Study. J. Head Trauma Rehabil. 2010, 25, 283–292. [Google Scholar]
- Barr, W.B.; Prichep, L.S.; Chabot, R.; Powell, M.R.; McCrea, M. Measuring Brain Electrical Activity to Track Recovery from Sport-Related Concussion. Brain Inj. 2012, 26, 58–66. [Google Scholar] [CrossRef]
- Prichep, L.S.; Ghosh-Dastidar, S.; Jacquin, A.; Koppes, W.; Miller, J.; Radman, T.; O’Neil, B.; Naunheim, R.; Huff, J.S. Classification Algorithms for the Identification of Structural Injury in TBI Using Brain Electrical Activity. Comput. Biol. Med. 2014, 53, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Prichep, L.S.; Naunheim, R.; Bazarian, J.; Mould, W.A.; Hanley, D. Identification of Hematomas in Mild Traumatic Brain Injury Using an Index of Quantitative Brain Electrical Activity. J. Neurotrauma 2015, 32, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, S.I.; Thomas, C.; Kulek, A.; Tolomello, R.; Mika, V.; Robinson, D.; Medado, P.; Pearson, C.; Prichep, L.S.; O’Neil, B.J. Comparison of Quantitative EEG to Current Clinical Decision Rules for Head CT Use in Acute Mild Traumatic Brain Injury in the ED. Am. J. Emerg. Med. 2015, 33, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Naunheim, R.; Covassin, T.; Jacquin, A.; Hanley, D.; Michelson, E. Using a Brain Electrical Activity Biomarker Could Aid in the Objective Identification of Mild Traumatic Brain Injury Patients. Am. J. Emerg. Med. 2018, 36, 142–143. [Google Scholar] [CrossRef]
- Vincent, A.S.; Bailey, C.M.; Cowan, C.; Cox-Fuenzalida, E.; Dyche, J.; Gorgens, K.A.; Krawczyk, D.C.; Young, L. Normative Data for Evaluating Mild Traumatic Brain Injury with a Handheld Neurocognitive Assessment Tool. Appl. Neuropsychol. Adult 2017, 24, 566–576. [Google Scholar] [CrossRef]
- Jacquin, A.; Kanakia, S.; Oberly, D.; Prichep, L.S. A Multimodal Biomarker for Concussion Identification, Prognosis and Management. Comput. Biol. Med. 2018, 102, 95–103. [Google Scholar] [CrossRef]
- Naunheim, R.; Konstantinovic Koscso, M.; Poirier, R. Reduction in Unnecessary CT Scans for Head-Injury in the Emergency Department Using an FDA Cleared Device. Am. J. Emerg. Med. 2019, 37, 1987–1988. [Google Scholar] [CrossRef]
- Michelson, E.; Huff, J.S.; Garrett, J.; Naunheim, R. Triage of Mild Head-Injured Intoxicated Patients Could Be Aided by Use of an Electroencephalogram-Based Biomarker. J. Neurosci. Nurs. 2019, 51, 62–66. [Google Scholar] [CrossRef]
- Covassin, T.; McGowan, A.L.; Bretzin, A.C.; Anderson, M.; Petit, K.M.; Savage, J.L.; Katie, S.L.; Elbin, R.J.; Pontifex, M.B. Preliminary Investigation of a Multimodal Enhanced Brain Function Index among High School and Collegiate Concussed Male and Female Athletes. Physician Sportsmed. 2020, 48, 442–449. [Google Scholar] [CrossRef]
- Bazarian, J.J.; Prichep, L.S. Validation of a Multimodal EEG-Based Index to Aid in Diagnosing and Tracking Concussion Among Athletes. Neurology 2022, 98, S20–S21. [Google Scholar] [CrossRef]
- Armañanzas, R.; Liang, B.; Kanakia, S.; Bazarian, J.J.; Prichep, L.S. Identification of Concussion Subtypes Based on Intrinsic Brain Activity. JAMA Netw. Open 2024, 7, e2355910. [Google Scholar] [CrossRef] [PubMed]
- Koreerat, N.R.; Giese, R. An Observation of Application of Structural Brain Injury Devices for Traumatic Brain Injury Evaluation in Austere Military Prehospital Settings. Mil. Med. 2021, 186, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Luster, J.D.; Hoffman, W.R.; Jordan, M.; Cacic, K.; Tchopev, Z.N.; Anderson, J.; Gissendanner, W.; Miranda, E.; Yuan, T.; Willis, A. Feasibility Assessment of Rapid Response EEG in the Identification of Nonconvulsive Seizures During Military Medical Air Transport. Mil. Med. 2024, 190, e479–e483. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.J.; Coppler, P.J.; Talia, N.N.; Charalambides, A.; Stancil, B.; Puccio, A.M.; Okonkwo, D.O.; Callaway, C.W.; Guyette, F.X.; Elmer, J. Prehospital Electroencephalography to Detect Traumatic Brain Injury during Helicopter Transport: A Pilot Observational Cohort Study. Prehosp. Emerg. Care 2024, 28, 405–412. [Google Scholar] [CrossRef]
- Numan, T.; Van den Boogaard, M.; Kamper, A.M.; Rood, P.J.; Peelen, L.M.; Slooter, A.J.; Abawi, M.; van den Boogaard, M.; Claassen, J.A.; Coesmans, M.; et al. Delirium Detection Using Relative Delta Power Based on 1-Minute Single-Channel EEG: A Multicentre Study. Br. J. Anaesth. 2019, 122, 60–68. [Google Scholar] [CrossRef]
- Wijnen, V.J.M.; Oudewortel, L.; Van Luijtelaar, G.; Witlox, J.; Slooter, A.J.C.; Van Gool, W.A. Feasibility and Potential of a Bedside Mini-EEG for Diagnosing Delirium Superimposed on Dementia. Clin. Neurophysiol. 2022, 142, 181–189. [Google Scholar] [CrossRef]
- Mulkey, M.A.; Huang, H.; Albanese, T.; Kim, S.; Yang, B. Supervised Deep Learning with Vision Transformer Predicts Delirium Using Limited Lead EEG. Sci. Rep. 2023, 13, 7890. [Google Scholar] [CrossRef]
| Study Design | Feasibility | Diagnostic Performance | Clinical Implications and Cost-Effectiveness | |
|---|---|---|---|---|
| 1. | First author: Brenner [94], 2015, USA Sample size: 12 adult patients (median age: 51.5) Conditions: AMS with seizure history or witnessed seizure Setting: ED EEG System: Portable Brainmaster EEG device Comparison: conv-EEG (reference standard) EEG Interpretation: neurophysiologist (POC-EEG); neurologist (conv-EEG) | POC-EEG:
| Agreement between POC-EEG and conv-EEG:
| POC-EEG device cost: ~USD 2500 conv-EEG cost: ~USD 50,000 |
| 2. | First author: Muraja-Murro [95], 2015, Finland Sample size: 100 patients (18–90 aged) Conditions: unexplained AMS from various etiologies Setting: ED EEG System: Grass Technologies Comparison: fm-EEG (reference standard) EEG Interpretation: Three expert neurophysiologists, blinded to EEG type | POC-EEG:
| POC-EEG performance:
| |
| 3. | First author: Rittenberger [29], 2019, USA Sample size: 95 patients (mean age: 59) Condition: PCA Setting: Tertiary care cardiac arrest hospital EEG system: Cadwell 6-electrode POC-EEG EEG Interpretation: Epileptologist and neurointensivist Comparison: First 30 min of c-conv-EEG, performed after POC-EEG EEG interpretation: Epileptologist and neurointensivist | POC-EEG:
| Agreement between POC-EEG and c-conv-EEG:
| Survival to hospital discharge:
|
| 4. | First author: Egawa [96], 2020, Japan Sample size: 50 patients (median age: 72) Conditions: AMS from various etiologies (subarachnoid hemorrhage, cerebral hemorrhage, post-cardiac arrest (PCA) syndrome, SE, TBI) Setting: neuro-ICU EEG system: AE-120A EEG headset Comparison: fm-cEEG (immediately subsequent) EEG Interpretation: One neurointensivist and one board-certified neurophysiologist | POC-EEG:
| POC-EEG findings:
| |
| 5. | First author: Caricato [97], 2020, Italy Sample size: 40 patients
EEG system: CerebAir headset (study group) Comparison: 8-electrode rm-EEG (control group) (continuous) EEG Interpretation: Expert neurologist | EEG Application:
| POC-EEG findings: EEG abnormalities classified as
| EEG-related ASM initiation:
|
| 6. | First author: Meyer [98], 2021, Germany Patients: 52 patients (mean age: 63 years) Conditions: AMS due to SE, ischemic stroke, intracranial bleeding, meningitis, encephalitis, metabolic encephalopathies Setting: neuro-ICU EEG system: CerebAir monitoring Comparison: Routine conv-EEG (delayed) EEG Interpretation: Resident physician, supervised by a board-certified senior physician | POC-EEG:
| Diagnostic Performance:
| |
| 7. | First author: Welte [99], 2024, Germany Sample size: 100 patients Setting: Neurological ED Conditions: AMS or suspected seizures EEG system: CerebAir (minimum 10 min) EEG Interpretation: Neurology resident, supervised by an EEG expert Comparison: conv-rEEG (55 patients) performed immediately hours to days after POC-EEG EEG Interpretation: Specialized neurology residents, supervised by senior board-certified EEG experts | POC-EEG:
| Agreement between swEEG and first rEEG results (55 patients):
| Potential therapeutic intervention:
|
| 8. | First author: Hobbs [37], 2018, USA Sample size: 34 patients (mean age: 61) Setting: ICU Condition: AMS (GCS <12) due to mixed etiologies (metabolic encephalopathy, AIS, intracerebral hemorrhage, TBI, and autoimmune encephalitis) and requiring EEG monitoring EEG system: Ceribell rr-EEG system EEG Interpretation: Sonified EEG by neurointensivists without epilepsy training Comparison: conv-EEG performed after POC-EEG; reference standard EEG Interpretation: Two epileptologists reviewed the entire Ceribell EEG recording and correlated findings with conv-EEG reports | POC-EEG:
| Diagnostic Performance:
| Sonification tool impact:
|
| 9. | First author: Parvizi [31], 2018, USA Sample size: 84 EEG samples selected from patients Condition: AMS EEG system: Ceribell (visual + sonification) EEG Interpretation:
| EEG sonification:
| Diagnostic Performance:
| |
| 10. | First author: Yazbeck [32], 2019, USA Sample size: 10 patients (mean age: 59.7 years) Setting: neuro-ICU Condition: AMS at risk for NCSE EEG system: Ceribell rr-EEG system sound application; interpreted on-site by treating physicians using real-time sonification and visual review on the rr-EEG device Comparison: conv-EEG performed after POC-EEG in six patients | POC-EEG:
| Concordance with conv-EEG:
| POC-EEG Impact on treatment decision:
|
| 11. | First author: Kamousi [100], 2019, USA Sample size:
Conditions: AMS (ICU study); healthy subject component (controlled laboratory setting) EEG system: Ceribell rr-EEG system Study design:
| Laboratory Study (Healthy Subject):
| ||
| 12. | First author: Chen [101], 2020, USA Sample size: 5 patients Setting: ICU Conditions: AMS or suspected seizures or SE in critically ill adult patients with confirmed COVID-19 infection EEG System: Ceribell rr-EEG Comparison: conv-EEG performed in 2 patients for extended monitoring |
| ||
| 13. | First author: LaMonte [27], 2021, USA Sample size:
EEG system:
| POC-EEG:
| POC-EEG Diagnostic Performance:
| POC-EEG implication:
|
| 14. | First author: Vespa [9], 2020, USA Sample size: 181 patients (mean age: 58.6) Setting: ICUs from five academic hospitals Condition: AMS suspected of NCS EEG system: Ceribell rr-EEG system (30 s sonification per hemisphere + 60 s visual EEG review; real-time interpretation: treating physician; remote neurologist review) Comparison: conv-EEG performed immediately after POC-EEG; reference standard | POC-EEG:
| POC-EEG diagnostic performance (vs. initial clinical suspicion):
| POC-EEG clinical impact (after vs. before):
|
| 15. | First author: Wright [7], 2021, USA Sample size: 38 patients Setting: ED, two hospital sites (Community hospital, Academic hospital) Condition: suspected NCSE due to various etiologies were identified (e.g., SE, stroke, TBI, toxic-metabolic encephalopathies, and idiopathic AMS) EEG system: Ceribell rr-EEG + Brain Stethoscope EEG Interpretation:
| POC-EEG:
| POC-EEG Diagnostic Performance:
| Overall impact of POC-EEG across both sites:
|
| 16. | First author: Kamousi [33], 2021, USA Sample size: 353 rr-EEG recordings Condition: Adults with AMS requiring rr-EEG monitoring for suspected seizures Settings: ICUs and EDs across six academic and community hospitals EEG system: Ceribell monitoring; Clarity machine learning algorithm Reference standard: Ceribell review by two independent neurologists | POC-EEG:
| POC-EEG Diagnostic Performance:
| Potential POC-EEG application:
|
| 17. | First author: Kalkach-Aparicio [102], 2024, USA Sample size: 240 patients (median age: 64) Conditions: Persistent altered AMS, clinical concern for NCS, patients at risk for SE Setting: University hospital EEG system: Ceribell rr-EEG; interpretation by EEG expert Comparison: conv-EEG performed after POC-EEG; interpretation by EEG expert | POC-EEG:
| Seizure detection using 2HELPS2B score on rr-EEG vs. cEEG:
| Seizure risk prediction:
|
| 18. | First author: Madill [8], 2022, USA Sample size: 74 patients (mean age: 61.7 years) Conditions: Clinical events concerning seizures (49%), PCA (24%), and unexplained encephalopathy (27%), Settings: ICU and ED, community hospital affiliated with a university hospital EEG system: Ceribell rr-EEG; interpretation by on-site neurology and remote epileptologist via tele-EEG Comparison: Historical practice before rr-EEG implementation | POC-EEG:
| POC-EEG Diagnostic Performance:
| Inter-hospital Transfers:
|
| 19. | First author: Kurup [103], 2022, USA Sample size: 19 patients Setting: ICU Condition: Suspected NCS or NCSE EEG system: Ceribell rr-EEG Comparison: conv-EEG; interpreted by experienced epileptologists | POC-EEG:
| EEG Findings:
| |
| 20. | First author: Eberhard [5], 2023, USA Sample size: 164 EEGs (35 conv-EEGs pre-QI; 115 rr-EEGs post-QI) Condition: Suspected seizures Setting: Community hospital EEG system: Ceribell rr-EEG, real-time sonification and cloud-based EEG interpretation: Remote review by on-call neurologist Comparison (Reference Standard): Historical control group (pre-QI) | POC-EEG:
| Seizure Detection Rates (diagnostic yield):
| Patients discharged:
|
| 21. | First author: Ward [36], 2023, USA Sample size: 88 patients (mean age: 57) Conditions: Concern for NCSE (19% exhibited hyperkinetic movements PCA, 46% had a history of seizures and 35% were unresponsive) Setting: ICU and ED at a community hospital EEG system:
| POC-EEG:
| POC-EEG Findings:
| Hospital transfer for emergent EEG:
Financial impact:
|
| 22. | First author: Villamar [104], 2023, USA Sample size: 21 patients (median age: 64) Condition: Comatose PCA patients Setting: ICU EEG system: Ceribell rr-EEG monitoring as part of routine clinical care; Clarity algorithm (version 4.0) for automated seizure detection EEG Interpretation: Board-certified epileptologist retrospective review Comparison:
| Raw POC-EEG review findings:
| ||
| 23. | First author: Kozak [6], 2023, USA Sample size: 157 adult patients (mean age: 57.7 years) Conditions: Clinical suspicion of seizures, unexplained encephalopathy, or PCA Setting: ED from a community hospital EEG system: Ceribell rr-EEG, Clarity, reviewed by intensivists and neurologists Comparison: conv-EEG performed after POC-EEG in 51.6% of cases; interpretation: EEG-trained neurologist (reference standard) | POC-EEG:
| POC-EEG Findings:
| Treatment changes based on POC-EEG findings: 59.2% of cases POC-EEG findings associated with ASM management changes (p < 0.001):
|
| 24. | First author: Kamousi [35], 2024, USA Sample size: 665 POC-EEG recordings Setting: 11 hospitals EEG system: Ceribell Clarity analysis (two versions tested) Reference standard: EEG reviewed post hoc by at least two blinded epileptologists | POC-EEG:
| Clarity Diagnostic Performance:
| |
| 25. | First author: Dorriz [34], 2024, USA Sample size: 317 POC-EEG recordings Setting: U.S. community hospital EEG system: Ceribell Clarity outputs (for SE detection); monitoring Reference standard: EEG-trained neurologist’s interpretation of POC-EEG recordings | POC-EEG:
| Clarity concordance with neurologist:
| |
| 26. | First author: Desai [105], 2024, USA Sample size: 283 patients
EEG system: Ceribell rr-EEG Comparison: At least 4 h conv-EEG | POC-EEG:
| POC-EEG impact on ICU stay:
| |
| 27. | First author: Gururangan [106], 2025, USA Sample size: 70 patients (mean age: 75.0 years) Conditions: 38 stroke patients (54.3%: 73.7% ischemic, 15.8% hemorrhagic, 10.5% TIA); 32 stroke mimics (45.7%: 46.9% seizures, 28.1% toxic-metabolic encephalopathy, 12.5% hypertensive encephalopathy) Setting: Tertiary care community hospital EEG system: Ceribell rr-EEG used during stroke codes Reference standard: Final stroke vs. stroke mimic diagnosis based on
| POC-EEG:
| POC-EEG findings:
| POC-EEG Seizure Detection in Stroke Codes:
|
| 28. | First author: Sheikh [107], 2025, USA Sample size: 235 rr-EEG Setting: Three hospitals Condition: Neurologic conditions with a high risk of seizures Setting: ICU or ED EEG system: ClarityPro (v 6.0) Setting: Three hospitals Reference standard: Expert neurophysiologist consensus review of EEGs | POC-EEG:
| Performance of Clarity at different SzB thresholds:
| Clarity application:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fratangelo, R.; Lolli, F.; Scarpino, M.; Grippo, A. Point-of-Care Electroencephalography in Acute Neurological Care: A Narrative Review. Neurol. Int. 2025, 17, 48. https://doi.org/10.3390/neurolint17040048
Fratangelo R, Lolli F, Scarpino M, Grippo A. Point-of-Care Electroencephalography in Acute Neurological Care: A Narrative Review. Neurology International. 2025; 17(4):48. https://doi.org/10.3390/neurolint17040048
Chicago/Turabian StyleFratangelo, Roberto, Francesco Lolli, Maenia Scarpino, and Antonello Grippo. 2025. "Point-of-Care Electroencephalography in Acute Neurological Care: A Narrative Review" Neurology International 17, no. 4: 48. https://doi.org/10.3390/neurolint17040048
APA StyleFratangelo, R., Lolli, F., Scarpino, M., & Grippo, A. (2025). Point-of-Care Electroencephalography in Acute Neurological Care: A Narrative Review. Neurology International, 17(4), 48. https://doi.org/10.3390/neurolint17040048






