Abstract
Background: Ischemic stroke (IS) remains a leading global cause of mortality, recurrence, and long-term disability, with survivors also at risk of post-stroke dementia (PSD) and cognitive impairment (PSCI). The precise impact of statin therapy across different IS populations, including those with cardioembolic/atrial fibrillation (CE/AF) strokes and patients with low-baseline low-density lipoprotein (LDL) cholesterol, remains unclear, as does the influence of statin timing, intensity, type, and solubility. Methods: We conducted the Impact of Statin Therapy on the Risk of Stroke Recurrence, Mortality, and Dementia After Ischemic Stroke (ISMARDD) meta-analysis, synthesizing evidence from 51 studies (n = 521,126), to evaluate the association between post-stroke statin therapy and key outcomes: all-cause mortality, stroke recurrence, cognition, and C-reactive protein (CRP). PSD was defined as new, persistent cognitive decline meeting standard diagnostic criteria, and PSCI as measurable but sub-threshold cognitive deficits. Random-effects models were used, and certainty was assessed with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework. Results: Statin therapy significantly reduced all-cause mortality within 3 months (OR 0.32), at 1 year (OR 0.35), and beyond 1 year (OR 0.56). Stroke recurrence was modestly reduced both within 1 year (OR 0.77) and after 1 year (OR 0.76). Statin use was associated with a lower risk of PSD (OR 0.74) but not PSCI overall. Benefits extended to CE/AF-related strokes and patients with low-baseline LDL cholesterol, both showing significantly lower mortality with statin use. Early initiation (<24 h) was linked with reduced recurrence, though effects of statin intensity, type, and solubility were inconsistent. Statins also significantly reduced CRP levels, underscoring anti-inflammatory and pleiotropic mechanisms. Conclusions: The ISMARDD study demonstrates that statins confer survival benefit and selective cognitive protection (notably reduced PSD risk) after ischemic stroke, with modest recurrence benefit, supporting their broad use in secondary prevention. These findings highlight the need for precision-guided approaches tailored to stroke subtype, pharmacogenomics, and treatment timing to optimize therapeutic outcomes.
1. Introduction
Ischemic stroke (IS) remains a leading global cause of death and long-term disability, with rising incidence particularly among ageing populations []. Despite advances in acute stroke interventions such as thrombolysis and mechanical thrombectomy, many survivors remain at elevated risk of recurrent stroke, cognitive impairments, and death [,]. While this epidemiological burden is well established, the critical question for clinical pharmacology is how pharmacological interventions, particularly statins, modify these risks beyond lipid lowering.
Statins, or 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, are central to cardiovascular and cerebrovascular risk reduction due to their low-density lipoprotein (LDL) cholesterol-lowering effects []. Beyond lipid modulation, accumulating evidence supports statins’ pleiotropic actions, including anti-inflammatory, endothelial-stabilizing, and neuroprotective effects [], which may be especially relevant in the complex post-ischemic environment. Their ability to lower C-reactive protein (CRP) provides a biomarker-based link between statin exposure and anti-inflammatory benefit, suggesting mechanisms that could explain mortality and cognitive protection across diverse stroke subtypes.
The pivotal Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial demonstrated the efficacy of high-dose atorvastatin (80 mg daily) in reducing stroke recurrence in non-cardioembolic IS patients []. However, the SPARCL trial excluded patients with atrial fibrillation (AF), cardioembolic strokes (CE), and coronary artery disease (CAD)—populations that constitute a large proportion of real-world IS cases [,,]. Current guideline recommendations, derived largely from SPARCL, therefore fail to fully reflect the heterogeneity of IS subtypes and comorbidities seen in practice, including CE/AF-related strokes and patients with low baseline LDL-cholesterol [,,,,,,,].
While previous meta-analyses have investigated the impact of statin therapy on stroke recurrence and mortality, they often have inconsistent inclusion criteria, limited subtype representation, and a lack of stratification by pharmacological parameters such as statin intensity, solubility, or timing of initiation [,]. Moreover, few reviews have systematically integrated these drug-related factors with outcomes such as post-stroke cognitive impairment (PSCI) (referred to as measurable but sub-threshold cognitive deficits within 3–6 months of the stroke) or dementia (PSD) (defined as persistent cognitive decline meeting standard diagnostic criteria (Diagnostic and Statistical Manual of Mental Disorders [DSM] or International Classification of Diseases [ICD]) within 6 months of stroke) []—conditions increasingly recognized as critical long-term sequelae of IS [,]. Incorporating CRP and pharmacogenomic considerations provides a translational bridge between statin pharmacology and clinical outcomes, directly informing precision prescribing.
The Impact of Statin Therapy on the Risk of Stroke Recurrence, Mortality, and Dementia After Ischemic Stroke (ISMARDD) study was undertaken to address these critical gaps. We conducted a comprehensive systematic review and meta-analysis to evaluate the effects of post-stroke statin therapy on all-cause mortality, stroke recurrence, and cognitive outcomes across a broad range of IS populations. We further examined how these outcomes vary by statin intensity, type, solubility, and timing of initiation, and assessed the impact on CRP levels as a mechanistic marker, to provide insight into their pleiotropic and anti-inflammatory mechanisms. By synthesizing data from over 500,000 patients, the ISMARDD study provides an updated, evidence-based foundation for optimizing statin therapy in post-stroke management and advancing precision-based prevention strategies.
Objectives
This study aims to address the following research questions:
- Primary Questions
- (a)
- In the broader IS cohort:
- i.
- What is the prevalence of all-cause mortality, stroke recurrence and PSD/PSCI in statin users, nonusers and overall?
- ii.
- Is post-stroke statin use associated with all-cause mortality, stroke recurrence and PSD/PSCI?
- (b)
- Variations in statin parameters:
- i.
- Is increasing statin intensity associated with all-cause mortality and stroke recurrence?
- ii.
- What is the prevalence of all-cause mortality and stroke recurrence by statin intensity, type, timing of initiation and solubility?
- Secondary Questions
- (a)
- In CE/AF-related IS subgroups:
- i.
- What is the prevalence of all-cause mortality and stroke recurrence in statin users, nonusers and overall?
- ii.
- Is post-stroke statin use associated with all-cause mortality and stroke recurrence?
- (b)
- In IS patients with low baseline LDL-cholesterol:
- i.
- What is the prevalence of all-cause mortality in statin users, nonusers, and overall?
- ii.
- Is post-stroke statin use associated with all-cause mortality?
- (c)
- Is post-stroke statin use associated with changes in CRP levels in IS patients?
2. Materials and Methods
2.1. Literature Search and Study Selection
A comprehensive literature search for studies published between January 2005 to June 2025 was conducted in PubMed, EMBASE, Scopus, Web of Science and Cochrane Library databases. Key terms in the search strategy included: “stroke”, “brain infarction”, “cerebrovascular event”, “cerebrovascular accident”, “statin” and “hmg-coa reductase inhibitor”. Searches were limited to human studies and those published in or translated to English. A detailed search strategy is available in Supplemental Table S1.
All identified titles and abstracts were initially screened and filtered using EndNote Version 21.5 (Clarivate Analytics, London, UK). The eligibility of remaining articles was thoroughly examined against the inclusion and exclusion criteria outlined below. Additional relevant studies were identified by examining the reference list of systematic reviews and meta-analyses and via Google Scholar. These processes were conducted independently by two researchers, and any discrepancies were resolved through discussion.
The systematic flow of the literature search and study selection is depicted in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart (Figure 1). This report aligns with the PRISMA 2020 and the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) checklists, which are presented in Supplemental Tables S2 and S3. This study was also registered in Open Science, registration number “mqnk5” (https://osf.io/mqnk5/ [accessed on 20 July 2025]).
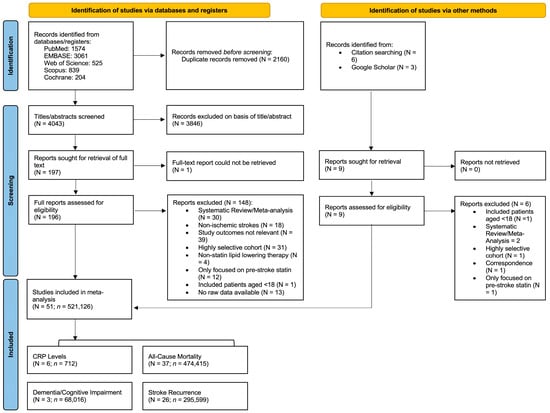
Figure 1.
Preferred Reporting System for Systematic Reviews and Meta-Analyses (PRISMA) Flowchart showing the studies included in the meta-analysis. Abbreviations: N, number of studies; n, number of patients; CRP, C-reactive protein.
2.2. Inclusion and Exclusion Criteria
Studies were included in this meta-analysis if they satisfied the following criteria:
- (a)
- participants aged 18 years or older;
- (b)
- patients receiving statin therapy after IS (either pre-stroke statin continued or newly initiated statin after stroke);
- (c)
- studies recruiting patients with all IS subtypes, or those focused on CE/AF-related strokes or patients with low baseline LDL-cholesterol;
- (d)
- included data on the outcomes of PSD/PSCI, stroke recurrence, all-cause mortality; or reported mean and standard deviation or median and interquartile ranges of CRP levels;
- (e)
- English language publications or translated to English; and
- (f)
- sample size of at least 20 patients.
Studies were excluded if:
- (a)
- included hemorrhagic stroke patients;
- (b)
- restricted to highly select cohorts, such as those with cancer or excluded key IS subtypes, such as those focused only on non-CE strokes;
- (c)
- did not contain raw data for outcomes;
- (d)
- only reported on pre-stroke statin use;
- (e)
- case reports, small case series, or studies with insufficient sample size;
- (f)
- systematic reviews and meta-analyses;
- (g)
- non-English or not translated.
2.3. Data Extraction
An Excel spreadsheet was utilized to extract the following key data from the selected studies:
- Study demographics—author, publication year, country, study design, cohort size.
- Control and intervention characteristics—statin type, dose, and timing of initiation.
- Patient demographics—age, sex, National Institute of Health Stroke Scale (NIHSS) score, IS subtype according to Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification, comorbidities (hypertension, diabetes mellitus, atrial fibrillation, coronary artery disease, dyslipidemia, previous stroke) and lifestyle factors (smoking and alcohol consumption).
- Clinical outcomes—all-cause mortality (within 3 months, 1 year and after 1 year), stroke recurrence (within and after 1 year), any PSD/PSCI diagnosis and CRP levels (within 3–7 days and after 7 days).
When required, Wan et al.’s method was employed to estimate means and standard deviations (SDs) from medians and interquartile ranges (IQR) []. Statin solubility was determined based on the statin characteristics defined by Climent et al. []. Statin intensity was based on the American College of Cardiology & American Heart Association (ACC/AHA) Classification of Intensity [].
2.4. Methodological Quality Assessment of Included Studies
The methodological quality of the included studies was assessed using the Modified Jadad Analysis (Supplemental Table S4). We also assessed non-randomized studies with the Risk of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool and randomized controlled trials (RCTs) with the Risk of Bias (RoB 2) tool (Supplemental Tables S5 and S6); two reviewers assessed each study independently. The potential risk of bias due to funding was also evaluated based on the declaration of funding sources and conflicts of interest reported in each individual study (Supplemental Table S7).
2.5. Statistical Analyses
Statistical analyses were conducted using STATA version 13.0 (StataCorp, College Station, TX, USA). Summary analyses of patient characteristics were determined from the patient demographic data extracted from the included studies. In the analysis of statin parameter variations, any study that specified a statin parameter (such as timing of initiation, type or dose) was included in the analyses. In some instances, the number of patients included in the analysis were fewer than the initial total of patients due to missing data or lack of follow-up. Where possible, analyses were stratified by time-period, to determine both short-term and long-term effects of statin therapy, as well as by study design.
2.5.1. Prevalence Estimations
The “metaprop” package in STATA was used to perform a random-effects meta-analysis of proportions in studies to determine the pooled prevalence of the clinical outcomes in statin users and nonusers. The “cimethod(exact)” and “ftt” commands were used to obtain the 95% confidence intervals (CIs). Pooled prevalence estimates were calculated using all available studies, with no minimum number of studies required for inclusion in each analysis.
2.5.2. Outcome Associations
The “metan” package in STATA was used to perform a DerSimonian and Laird (DL) random-effects meta-analysis and construct forest plots to determine the association between post-stroke statin use and clinical outcomes. The DL model was selected given its ability to account for heterogeneity between and within studies. A minimum of three studies were required to conduct association analyses.
The analysis involved the calculation of odds ratios (ORs) between post-stroke statin exposure and outcomes such as all-cause mortality, stroke recurrence and PSD/PSCI. For zero-event studies, we used a continuity correction of 0.5. An OR < 1.0 indicated lower odds of the adverse outcome. Standardized mean differences (SMDs) were also calculated to determine the association between post-stroke statin use and changes CRP levels. A negative SMD indicated lower CRP levels in the statin exposed group.
2.5.3. Statistical Significance, Heterogeneity and Variance
Statistical significance was considered when the p-value was <0.05. Heterogeneity was quantified with τ2, I2, H2, and Cochran’s Q. Similarly to Shen et al., heterogeneity was considered low when I2 < 30%, moderate when I2 = 30–50%, substantial when I2 = 51–75%, and severe when I2 > 75% []. We explored heterogeneity sources through subgroup analyses by stroke subtype, statin parameters, and study design.
2.5.4. Bias and Sensitivity Analyses
The “metaninf” package in STATA was used to conduct sensitivity analyses which assessed the impact on the pooled OR when individual studies were excluded. We assessed small-study effects using Egger’s and Peters’ tests and inspected funnel plots.
2.6. Certainty of Evidence Assessment
We assessed certainty of evidence for each primary outcome using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework, considering risk of bias, inconsistency, indirectness, imprecision, and publication bias. Certainty ratings were presented as high, moderate, low, or very low. For each outcome, we provided the relative effect, assumed control risk, and calculated absolute effect estimates where baseline risk was available.
3. Results
3.1. Description of Included Studies
From 4052 identified articles, 206 full-text articles were assessed for eligibility. Of these, 144 were excluded for various reasons such as irrelevant study outcomes and highly selective or non-IS focused cohorts (Figure 1). A total number (N) of 51 studies, encompassing a total of (n) 521,126 patients, were included in this meta-analysis [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,]. Among these, 31 studies were focused on all IS subtypes, of which 3 studies looked at PSD/PSCI [,,], 21 looked at all-cause mortality [,,,,,,,,,,,,,,,,,,,,], 12 looked at stroke recurrence [,,,,,,,,,,,], and 6 studies looked at CRP levels [,,,,,]. 10 studies were focused on CE/AF-related stroke, of which 8 looked at all-cause mortality [,,,,,,,] and 8 looked at stroke recurrence [,,,,,,,]. Three studies looked at all-cause mortality in IS patients with low baseline LDL-cholesterol [,,]. Finally, 9 studies purely focused on statin parameters, including 6 studies on statin intensity [,,,,,], 2 on statin type [,], and 1 on statin timing of initiation []. Of the 51 studies included in the analysis, 22 studies provided data for multiple research questions.
Characteristics of the studies included in this meta-analysis—including study design, patient demographics as well as comorbidities and lifestyle factors—are detailed in Table 1 and Table 2, respectively. The meta-analysis incorporated studies from a diverse range of countries and age groups. Patients had high prevalence of cardiovascular risk factors and comorbidities such as hypertension, coronary artery disease and smoking. Variation in stroke treatment protocols was observed as the proportion of patients initiated on statin therapy after stroke ranged from 9.7% to 83.7%. A broad range of stroke severities were included, as evidenced by the wide spread of NIHSS scores.

Table 1.
Clinical characteristics of studies included in meta-analysis of post-stroke statin use and clinical outcomes after ischemic stroke.

Table 2.
Comorbidities and lifestyle factors in cohorts of ischemic stroke patients included in meta-analysis of post-stroke statin use and clinical outcomes.
3.2. Primary Analysis
3.2.1. Impact of Post-Stroke Statin Use on Clinical Outcomes in Broader Ischemic Stroke Cohorts
Prevalence Estimations
- All-Cause Mortality
The overall prevalence of all-cause mortality was 12% within 3 months (95% CI: [0.09, 0.16]; z = 11.67; p < 0.01; n = 11,233; N = 10) [,,,,,,,,,], 14% within 1 year (95% CI: [0.08, 0.21]; z = 7.82; p < 0.01; n = 84,567; N = 14) [,,,,,,,,,,,,,] and 23% after 1 year (95% CI: [0.07, 0.44]; z = 4.20; p = 0.01; n = 163,348; N = 8) [,,,,,,,] of IS (Table 3; Supplemental Figure S1). There was severe heterogeneity among these studies (I2 = 94.83%, 99.73% and 99.98%, respectively, for each analysis).

Table 3.
Pooled prevalence of clinical outcomes in all ischemic stroke subtypes.
In statin users, the prevalence of all-cause mortality within 3 months was 8% (95% CI: [0.05, 0.11]; z = 8.38; p < 0.01; n = 5052; N = 10) compared to 18% (95% CI: [0.13, 0.24]; z = 10.38, p < 0.01; n = 6181; N = 10) in statin non-users) [,,,,,,,,,]. Within 1 year after stroke, statin users had a prevalence of 9% (95% CI: [0.06, 0.14]; z = 7.30, p < 0.01; n = 55,421; N = 14) compared to nonusers who had a 21% prevalence (95% CI: [0.14, 0.30]; z = 8.49; p < 0.01; n = 29,146; N = 14) [,,,,,,,,,,,,,]. After 1 year statin users had a prevalence of 19% (95% CI: [0.05, 0.41]; z = 3.58; p < 0.01; n = 97,171; N = 8) compared to 30% (95% CI: [0.14, 0.50]; z = 5.30, p < 0.01; n = 66,177; N = 8) in nonusers [,,,,,,,] (Table 3; Supplemental Figure S2).
- Stroke Recurrence
The overall prevalence of stroke recurrence was 4% (95% CI: [0.02, 0.08]; z = 4.38; p < 0.01; n = 60,026; N = 5) [,,,,] within 1 year and 17% (95% CI: [0.11, 0.24]; z = 9.02; p < 0.01; n = 144,993; N = 8) [,,,,,,,] after 1 year of IS (Table 3; Supplemental Figure S3). There was severe heterogeneity among these studies, (I2 = 81.38% and 99.86%, respectively, for each analysis).
In statin users, the prevalence of stroke recurrence within 1 year was 3% (95% CI: [0.01, 0.07]; z = 3.41; p < 0.01; n = 43,631; N = 5) compared to 5% (95% CI: [0.02, 0.10]; z = 4.59; p < 0.01; n = 16,395; N = 5) in nonusers [,,,,]. After 1 year of IS, statin users had a prevalence of 13% (95% CI: [0.08, 0.20]; z = 7.69; p < 0.01; n = 101,244; N = 8) compared to 20% (95% CI: [0.13, 0.28]; z = 9.31; p < 0.01; n = 43,749; N = 8) in nonusers [,,,,,,,] (Table 3; Supplemental Figure S4).
- Post-Stroke Dementia/Cognitive Impairment
The overall prevalence of PSD/PSCI was 18% (95% CI: [0.09, 0.30]; z = 6.16; p < 0.01; n = 68,016; N = 3) [,,] (Table 3; Supplemental Figure S5). There were insufficient studies to calculate heterogeneity between the studies.
The prevalence of PSD/PSCI was 19% in both statin users (95% CI: [0.09, 0.31]; z = 5.67, p < 0.01; n = 46,831; N = 3) and nonusers (95% CI: [0.09, 0.31]; z = 6.05; p < 0.01; n = 21,185; N = 3) [,,] (Table 3; Supplemental Figure S6).
Outcome Associations
- All-Cause Mortality
Statin use was associated with significantly decreased odds of all-cause mortality within 3 months with OR 0.32 (95% CI: [0.26, 0.39]; p < 0.01; z = −12.44; n = 11,233; N = 10) [,,,,,,,,,], within 1 year with OR 0.35 (95% CI: [0.28, 0.44]; p < 0.01; z = −9.30; n = 84,567; N = 14) [,,,,,,,,,,,,,] and after 1 year with OR 0.56 (95% CI: [0.44, 0.72]; p < 0.01; z = −4.51; n = 163,348; N = 8) [,,,,,,,] of IS (Table 4; Figure 2). The direction of effect remained consistent when stratified by study design, although was not significant for the RCT subgroups. Heterogeneity of studies was mixed, with low heterogeneity within the 3-month analysis (I2 = 19.4%), but severe heterogeneity for within 1-year (I2 = 86.8%) and after 1-year (I2 = 97.9%) analyses.

Table 4.
Summary effects and heterogeneity obtained from meta-analysis of statin use and clinical outcomes after ischemic stroke.
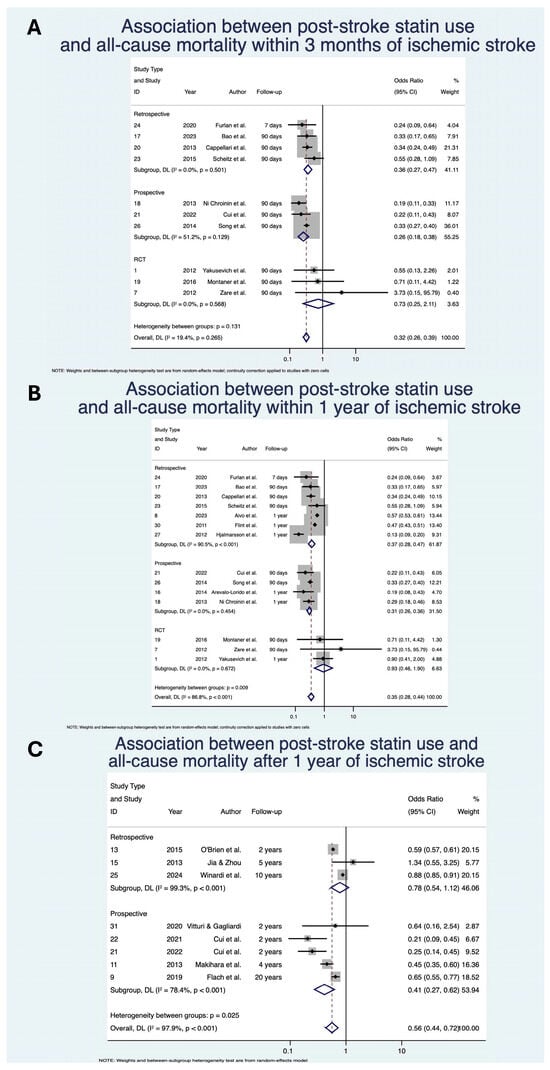
Figure 2.
Association between post-stroke statin use and all-cause mortality (A) within 3 months of ischemic stroke; (B) within 1 year of ischemic stroke; (C) after 1 year of ischemic stroke [,,,,,,,,,,,,,,,,,,,,]. Abbreviations: CI, confidence interval; DL, DerSimonian and Laird; p, p-value.
- Stroke Recurrence
Statin use was associated with significantly decreased odds of stroke recurrence within 1 year with OR 0.77 (95% CI: [0.72, 0.82], z = −7.64; p < 0.01; n = 60,026; N = 5) [,,,,] and after 1 year with OR 0.76 (95% CI: [0.66, 0.87], z = −3.88; p < 0.01; n = 144,993; N = 8) [,,,,,,,] of IS (Table 4; Figure 3). The direction of effect remained consistent when stratified by study design, although was not significant for the RCT subgroup in the within 1 year analysis. Heterogeneity of studies was mixed, with low heterogeneity for the within 1 year analysis (I2 = 0.0%) but severe heterogeneity (I2 = 85.1%) for the post 1-year analysis.
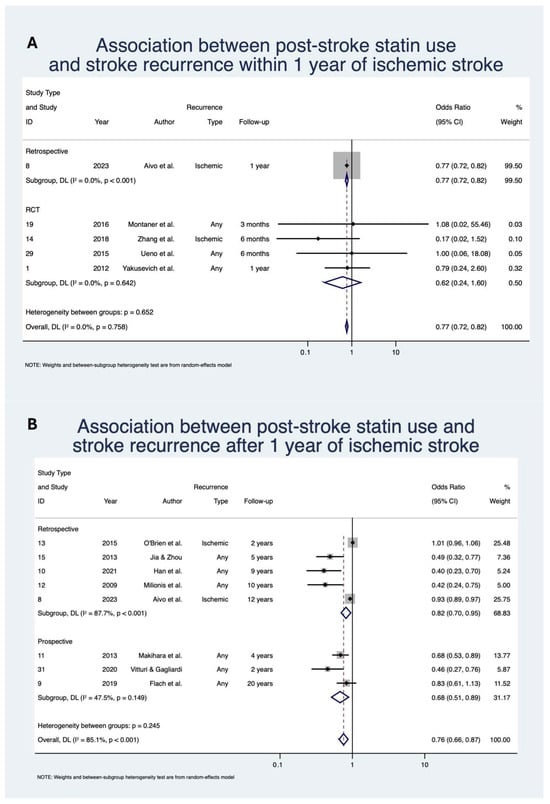
Figure 3.
Association between post-stroke statin use and stroke recurrence: (A) within 1 year of ischemic stroke; (B) after 1 year of ischemic stroke [,,,,,,,,,,,]. Abbreviations: CI, confidence interval; DL, DerSimonian and Laird; p, p-value; Due to model limitations, studies with 0 events were replaced with 0.5 to allow for OR calculation.
- Dementia/Cognitive Impairment
Statin use was not significantly associated with decreased odds of overall PSD or PSCI after IS with OR 0.83 (95% CI: [0.66, 1.05]; z = −1.54; p = 0.12; n = 68,016; N = 3) [,,] (Table 4; Figure 4). When looking at PSD alone, statin use was associated with significantly decreased odds with OR 0.74 (95% CI: [0.60, 0.90]; z = −2.94; p < 0.01; n = 67,666; N = 2) [,]. Heterogeneity was severe for the overall analysis (I2 = 90.9%) and the PSD subgroup (I2 = 92.1%).
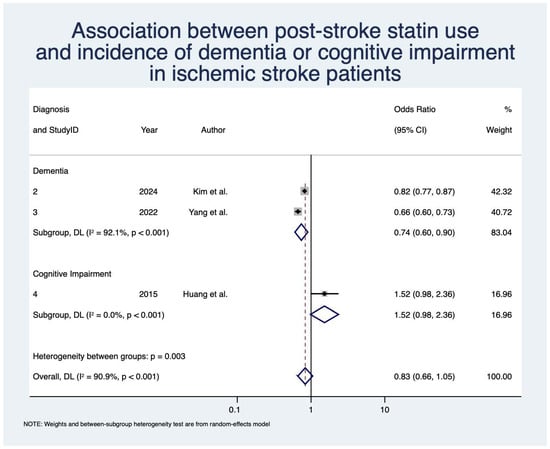
Figure 4.
Association between post-stroke statin use and incidence of dementia/cognitive impairment in ischemic stroke patients [,,]. Abbreviations: CI, confidence interval; DL, DerSimonian and Laird; p, p-value.
3.2.2. Impact of Variations in Statin Parameters on Clinical Outcomes
- Statin Timing
The prevalence of all-cause mortality in patients initiating statin within 24 h was 10% (95% CI: [0.06, 0.12]; z = 7.13; p < 0.01; n = 316; N = 3) [,,] compared to 6% (95% CI: [0.03, 0.09]; z = 7.45; p < 0.01; n = 1169; N = 3) [,,] in those initiating statin therapy after 24 h (Table 5; Supplemental Figure S7).

Table 5.
Pooled prevalence and summary effects of clinical outcomes after ischemic stroke by statin parameters.
The prevalence of stroke recurrence, in patients initiating statin within 24 h, was 5% (95% CI: [0.01, 0.11]; z = 3.44; p < 0.01; n = 95; N = 2) [,] compared to 9% (95% CI: [0.06, 0.14]; z = 8.45; p < 0.01; n = 239; N = 2) [,] in those initiating statin therapy after 24 h (Table 5; Supplemental Figure S7).
- Statin Type
The prevalence of all-cause mortality was lowest amongst rosuvastatin users (4%; 95% CI: [0.01, 0.07]; z = 4.55; p < 0.01; n = 6919; N = 2) [,] and highest amongst simvastatin users (10%; 95% CI: [0.06, 0.16]; z = 6.44; p < 0.01; n = 136; N = 2) [,] (Table 5; Supplemental Figure S8).
The prevalence of stroke recurrence was lowest amongst rosuvastatin users, though not statistically significant (2%; 95% CI: [0.00, 0.07]; z = 1.47; p = 0.14; n = 6681; N = 3) [,,]. Stroke recurrence was highest amongst atorvastatin users (4%; 95% CI: [0.03, 0.05]; z = 10.93; p < 0.01; n = 37,080; N = 3) [,,] (Table 5; Supplemental Figure S8).
- Statin Solubility
The prevalence of all-cause mortality was similar between hydrophilic (6%, 95% CI: [0.05, 0.06]; z = 36.84; p < 0.01; n = 6919; N = 2) [,] and lipophilic (6%; 95% CI: [0.03, 0.10]; z = 5.88; p < 0.01; n = 37,181; N = 5) statin users [,,,,] (Table 5; Supplemental Figure S9).
The prevalence of stroke recurrence was lower amongst hydrophilic statin users (2%; 95% CI: [0.00, 0.07]; z = 1.47; p < 0.01; n = 6681; N = 3) [,,] compared to lipophilic (4%; 95% CI: [0.02, 0.06]; z = 6.37; p < 0.01; n = 37,216; N = 5) statin users [,,,,] (Table 5; Supplemental Figure S9).
- Statin Intensity
The prevalence of all-cause mortality was lowest in the moderate-high intensity statin group (4%; 95% CI: [0.02, 0.06]; z = 7.54; p < 0.01; n = 471; N = 2) [,] and was highest in the low-moderate intensity statin group (19%; 95% CI: [0.12, 0.28]; z = 8.31; p < 0.01; n = 21,001; N = 4) [,,,] (Table 5; Supplemental Figure S10).
The prevalence of stroke recurrence was lowest in the moderate intensity statin group (4%; 95% CI: [0.00, 0.10]; z = 2.40; p = 0.02; n = 205; N = 4) [,,,] and was highest in the moderate-high intensity statin group (17%; 95% CI: [0.13, 0.22]; z = 14.90; p < 0.01; n = 344; N = 1) []. The prevalence of stroke recurrence was similar between the low-moderate (10%; 95% CI: [0.10, 0.11]; z = 93.47; p = 0.01; n = 20,486; N = 1) [] and high intensity (10%; 95% CI: [0.10, 0.11]; z = 55.10; p = 0.12; n = 9205; N = 2) [,] statin groups (Table 5; Supplemental Figure S10).
Increasing intensity of statin was not significantly associated with all-cause mortality with OR 0.92 (95% CI: [0.76, 1.12]; z = −0.80; p = 0.42; n = 103,236; N = 6) [,,,,,] or stroke recurrence with OR 1.10 (95% CI [0.88, 1.38]; z = −0.82; p = 0.41; n = 102,647; N = 4) [,,,] (Table 5; Supplemental Figure S11).
3.3. Secondary Analysis
3.3.1. Impact of Post-Stroke Statin Use in Cardioembolic/Atrial Fibrillation-Related Strokes
Prevalence Estimations
- All-Cause Mortality
The overall prevalence of all-cause mortality was 23% (95% CI: [0.18, 0.28]; z = 15.74; p < 0.01; n = 51,280; N = 3) [,,] within 1 year and 10% (95% CI: [0.04, 0.17]; z = 4.94; p < 0.01; n = 24,805; N = 5) [,,,,] after 1 year of cardioembolic or AF-related stroke (Supplemental Table S8; Supplemental Figure S12).
In statin users, the prevalence of all-cause mortality within 1 year was 19% (95% CI: [0.14, 0.24]; z = 12.97; p < 0.01; n = 7107; N = 3) compared to 30% (95% CI: [0.19, 0.42]; z = 8.66, p < 0.01; n = 44,373; N = 3) in nonusers [,,]. This was again observed after 1 year with a prevalence of 5% (95% CI: [0.02, 0.10]; z = 4.07, p < 0.01; n = 9497; N = 5) in statin users compared to 10% (95% CI: [0.03, 0.20]; z = 3.95, p < 0.01; n = 14,591; N = 5) in nonusers [,,,,] (Supplemental Table S8; Supplemental Figure S13).
- Stroke Recurrence
The overall prevalence of stroke recurrence was 6% (95% CI: [0.05, 0.06]; z = 31.22; p < 0.01; n = 4630; N = 2) [,] within 1 year and 13% (95% CI: [0.10, 0.18]; z = 11.60; p < 0.01; n = 28,723; N = 6) [,,,,,] after 1 year of cardioembolic or AF-related stroke (Supplemental Table S8; Supplemental Figure S14).
In statin users, the prevalence of stroke recurrence within 1 year was 5% (95% CI: [0.04, 0.06]; z = 22.78, p < 0.01; n = 2761; N = 2) compared to 6% (95% CI: [0.05, 0.07]; z = 20.53, p < 0.01; n = 1869; N = 2) in nonusers [,]. 1 year after stroke, prevalence of stroke recurrence was 12% (95% CI: [0.08, 0.17]; z = 8.94; p < 0.01; n = 11,040; N = 6) among statin users compared to 13% (95% CI: [0.10, 0.16]; z = 13.35; p < 0.01; n = 17,683; N = 6) in nonusers [,,,,,] (Supplemental Table S8; Supplemental Figure S15).
Outcome Associations
- All-Cause Mortality
Statin use was associated with significantly decreased odds of all-cause mortality both within 1 year with OR 0.57 (95% CI: [0.42, 0.78]; z = −3.49; p < 0.01; n = 51,280; N = 3) [,,] and after 1 year with OR 0.47 (95% CI: [0.25, 0.88]; z = −2.37; p = 0.02; n = 24,805; N = 5) [,,,,] in CE/AF-related stroke patients (Supplemental Table S9; Supplemental Figure S16). Heterogeneity for both analyses was severe, (I2 = 88.5% and 90.5%, respectively).
- Stroke Recurrence
Due to insufficient data, association analyses were unable to be conducted for stroke recurrence within 1 year. However, statin use was not significantly associated with decreased odds of stroke recurrence after 1 year with OR 0.84 (95% CI: [0.63, 1.12]; z = −1.23, p = 0.22; n = 28,723; N = 6) [,,,,,] in CE/AF-related stroke patients (Supplemental Table S9; Supplemental Figure S17). Heterogeneity of studies was severe (I2 = 82.3%).
3.3.2. Impact of Post-Stroke Statin Use in Ischemic Stroke Patients with Low Baseline LDL-Cholesterol Levels
- Prevalence Estimations
The overall prevalence of all-cause mortality was 11% (95% CI: [0.07, 0.16]; z = 8.94; p < 0.01; n = 4824; N = 3) [,,] (Supplemental Table S8; Supplemental Figure S12).
In statin users, the prevalence of all-cause mortality was 6% (95% CI: [0.04, 0.09]; z = 8.18; p < 0.01; n = 2799; N = 3) compared to 17% (95% CI: [0.11, 0.23]; z = 9.68; p < 0.01; n = 2025; N = 3) in statin non-users [,,] (Supplemental Table S8; Supplemental Figure S13).
- Outcome Associations
In patients with low baseline LDL-cholesterol, statin use was associated with significantly lower odds of all-cause mortality in IS patients with low baseline LDL-cholesterol levels with OR 0.32 (95% CI: [0.17, 0.58]; z = −3.708; p < 0.001; n = 4824; N = 3) [,,] (Supplemental Table S9; Supplemental Figure S16). Heterogeneity of studies was severe (I2 = 85.6%).
3.3.3. Impact of Post-Stroke Statin Use on CRP Levels in Ischemic Stroke Patients
Statin use was associated with significantly lower CRP levels within 3–7 days after IS (SMD = −0.41, 95% CI: [−0.75, −0.06]; z = −2.33; p = 0.02; n = 157; N = 3) [,,] and after 7 days of IS (SMD = −2.64, 95% CI: [−5.26, −0.03]; z = −1.98; p < 0.01; n = 410; N = 4) [,,,] (Table 4; Supplemental Figure S18). Heterogeneity of studies was mixed, with low heterogeneity for the 3–7-day analysis (I2 = 0.0%) and severe for the post 7-day analysis (I2 = 98.6%).
3.4. Bias & Sensitivity Analysis
Sensitivity analysis demonstrated that the exclusion of outliers and high-risk studies did not significantly alter the pooled estimates for most analyses (Supplemental Figure S19). The p-values in Egger’s and Peter’s tests were insignificant for most analyses (Supplemental Tables S10 and S11; Supplemental Figure S20). Inspection of funnel plots, however, revealed asymmetry, suggesting potential for publication bias (Supplemental Figure S21).
4. Discussion
This meta-analysis, the ISMARDD Study, significantly contributes to the evolving understanding of statin therapy’s role in secondary prevention following IS. Beyond confirming reduced mortality and recurrence risks, ISMARDD highlights the clinical pharmacology relevance of statin therapy. The mortality benefit likely reflects pleiotropic actions, anti-inflammatory, antioxidant, and endothelial-stabilizing, beyond LDL reduction. The observed reduction in CRP provides a biomarker-based link between statin exposure and anti-inflammatory benefit, strengthening mechanistic plausibility and translational relevance.
Pooled prevalence rates were 13–23% for all-cause mortality, 4–17% for stroke recurrence, and 19% for cognitive impairment or dementia. Statin users consistently experienced lower mortality and dementia risk compared to non-users, though effects on overall cognitive impairment were neutral. No consistent trends were identified concerning statin intensity, type, or solubility. Early initiation of statins (within 24 h) was linked with a lower prevalence of stroke recurrence. While early initiation of statin therapy (<24 h) demonstrated benefit for recurrence reduction, evidence regarding statin intensity, type, and solubility remains heterogeneous and should be interpreted with caution. These pharmacologic parameters warrant validation in controlled, phenotype-stratified cohorts. Pooled association analyses showed that statin use significantly reduced the odds of all-cause mortality by 44–68%, a benefit that extended across key subgroups, including patients with CE/AF-related strokes and those with low baseline LDL-cholesterol, who showed 43–53% and 68% lower odds of mortality, respectively. The effect on stroke recurrence was modest and mixed. While the broader IS group saw a 23–24% reduction in both short- and long-term recurrence, this effect did not reach significance in the CE/AF subgroup. Despite substantial heterogeneity (I2 > 75%), sensitivity analyses confirmed the robustness and consistency of the mortality and recurrence findings, underscoring the generalizability of the benefit across varied study populations and methods. No significant differences were observed for PSCI and composite cognitive impairment. However, statin use was associated with a 26% lower risk of post-stroke dementia. These findings extend the evidence base beyond the SPARCL trial [], demonstrating benefit even in real-world populations excluded from that trial, including CE/AF-related strokes and patients with low baseline LDL-cholesterol, and align with prior meta-analyses reporting consistent mortality reductions but variable effects on recurrence [,,,]. Key clinical insights relating to post-stroke statin use, generated by the ISMARDD study and by previous studies, are summarized in Figure 5, and the certainty of the findings is summarized in Table 6. Substantial heterogeneity (I2 > 75%) across several analyses reflects the inclusion of diverse populations, study designs, and statin regimens. Nevertheless, sensitivity analyses confirmed the consistency of direction and robustness of pooled estimates.
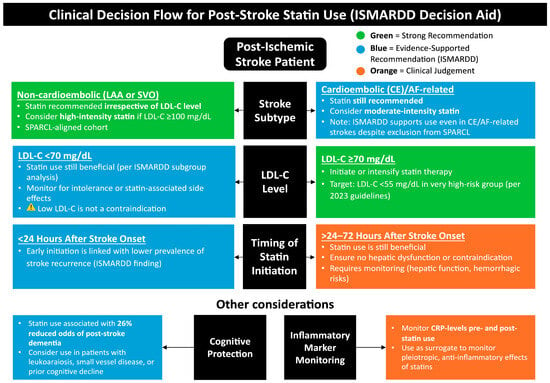
Figure 5.
Clinical decision flow for post-stroke statin use (ISMARDD Decision Aid). This framework summarizes the recommended approach to post-stroke statin therapy based on stroke subtype, LDL-cholesterol (LDL-C) level, and timing of initiation. High-intensity statins (e.g., atorvastatin 80 mg or rosuvastatin 20 mg daily) are recommended for most patients with ischemic stroke or TIA to reduce recurrent vascular events, particularly in those with large-artery atherosclerosis (LAA) or mixed etiologies. In cardioembolic or AF-related stroke, statins may be considered to lower overall vascular risk but do not substitute for oral anticoagulation [], which remains the cornerstone of secondary prevention. Lipid-lowering therapy should target LDL-C < 70 mg/dL or a ≥50% reduction from baseline, with lipid reassessment 4–12 weeks after initiation. Baseline liver enzyme testing is recommended, with repeat testing if clinically indicated; creatine kinase (CK) measurement is reserved for symptomatic cases. Statins are contraindicated in active liver disease or pregnancy, and dose adjustments may be required in renal impairment or when used with interacting medications [,,,,]. Abbreviations: ISMARDD, Impact of Statin Therapy on the Risk of Stroke Recurrence, Mortality, and Dementia After Ischemic Stroke; LAA, large artery atherosclerosis; SVO, small vessel occlusion; LDL-C, low-density lipoprotein cholesterol; AF, atrial fibrillation; CE, cardioembolic; CRP, C-reactive protein.

Table 6.
Grading of Recommendations Assessment, Development and Evaluation (GRADE) Summary of Findings: Statin Therapy After Ischemic Stroke.
To clarify the clinical guidance depicted in Figure 5, in accordance with the 2021 AHA/ASA secondary prevention guideline [], high-intensity statin therapy (e.g., atorvastatin 80 mg or rosuvastatin 20 mg daily) is preferred for most ischemic stroke or TIA patients to reduce recurrent cardiovascular and cerebrovascular events. The recommendation for statin use in CE or AF-related ischemic stroke primarily addresses comorbid atherosclerotic and systemic vascular risk rather than the embolic mechanism itself. Moderate-intensity regimens may be considered only when high-intensity therapy is contraindicated or poorly tolerated. Evidence from trials such as SPARCL supports the long-term benefit of statin therapy for secondary prevention [], though not specifically within the first 24–48 h post-onset. All patients should have lipid profiles reassessed 4–12 weeks after initiation, with ongoing monitoring for hepatic or muscle-related adverse effects.
- Mechanistic Insights and Subgroup Effects
The mortality benefit, observed in post-stroke populations, likely reflects statin’s dual mechanism of action. While fatality after IS often arises from direct neurological damage, cardiovascular complications, or systemic infections [], statins offer pleiotropic effects—anti-inflammatory, antioxidant, and endothelial-stabilizing—that extend beyond lipid lowering []. These effects may explain the consistent mortality benefit observed even in subgroups such as CE/AF-related stroke and patients with low baseline LDL-cholesterol, where hyperlipidemia is not central to pathophysiology. These findings are supported by our meta-analysis of CRP, a systemic inflammation marker, which was significantly lower among statin users compared to nonusers.
- Stroke Recurrence and Etiology-Specific Effects
While statin use was associated with reduced stroke recurrence in the broader IS group, this benefit did not extend to the CE/AF subgroup. This may reflect etiological differences, especially since stroke recurrence tends to follow the same mechanism as the index event []. Patients with large artery atherosclerosis may benefit more from statin use due to their plaque-stabilizing effects [], whereas CE/AF-related strokes, which are typically driven by cardiac dysrhythmia, may require alternative strategies []. Although statins exhibit antithrombotic properties, they likely do not modify the electrophysiological mechanisms of dysrhythmias [].
- Cognitive Outcomes: Differentiating PSD and PSCI
Statin therapy was associated with a 26% reduction in PSD risk but showed no clear effect on PSCI. This divergence may reflect underlying pathophysiological differences. While PSCI is often linked to neurodegeneration [], PSD involves vascular injury, neuroinflammation, and possible acceleration of Alzheimer’s pathology [,,]. Statins’ neuroprotective and anti-inflammatory properties may better align with the pathogenesis of PSD, explaining the differential benefit [].
- Optimizing Statin Therapy in Ischemic Stroke: Toward Precision Neurology
Statins are well established in the secondary prevention of IS and transient ischemic attack (TIA). Emerging evidence, including from this meta-analysis, suggests that statins may also confer mortality and cognitive benefits. The optimal regimen, including type, dose, and timing of initiation, remains to be clearly defined to inform evidence-based clinical guidelines. Given that the majority of included studies were observational, causality cannot be inferred. Nonetheless, the consistency of effect direction across study designs supports a strong associative signal warranting further randomized validation. Although our inclusion criteria focused on post-stroke statin therapy, several studies distinguished between pre-existing and newly initiated users. Evidence suggests that continuation of pre-stroke statins may further enhance survival and reduce early neurological deterioration, consistent with the concept of ‘statin preconditioning’ []. Future analyses differentiating continuation versus de novo initiation are warranted.
In our analysis, early statin initiation (within 24 h of stroke onset) was linked with a lower prevalence of stroke recurrence, though this was not seen with a reduction in all-cause mortality. This finding contrasts with several trials that reported no difference in outcomes between early and delayed initiation [,]. Similarly, no significant association was found between statin intensity and reduced adverse outcomes, mirroring mixed results in the literature. While some studies suggest high-intensity statins yield better outcomes, others report no clear advantage [,]. No consistent trends emerged regarding statin type or solubility. Lipophilic statins (such as atorvastatin, simvastatin) have greater blood–brain barrier penetration, which may enhance neuroprotective effects, though their use has also been linked to potential cognitive decline [], underscoring the need for further clarification regarding their role in stroke recovery. The ISMARDD meta-analysis also revealed variable outcome profiles among individual statins. While simvastatin demonstrated higher prevalence of mortality and recurrence, rosuvastatin exhibited the most favorable outcomes, with atorvastatin showing intermediate or inconsistent effects. These apparent differences are likely attributable to variations in study design, sample sizes, and population characteristics rather than inherent pharmacologic disparities. In contrast to cardiology trials, where lipid-lowering and plaque-stabilizing effects dominate [,,,], post-stroke outcomes may be influenced by additional factors such as blood–brain barrier penetration [,,], central nervous system distribution [], and pleiotropic anti-inflammatory mechanisms []. Rosuvastatin’s higher hydrophilicity and potent CRP-lowering capacity may enhance cerebrovascular protection [], whereas variability in atorvastatin findings may reflect differential inclusion of cardioembolic and mixed-etiology cohorts. These findings underscore the need for head-to-head, stroke-specific comparative studies to delineate the neurovascular effects of individual statins beyond their cardiovascular efficacy.
Responses to statin therapy vary depending on stroke subtype and individual patient characteristics such as age, ethnicity, comorbidities, and baseline LDL-cholesterol levels. The current study showed consistent mortality benefits across subgroups, but recurrence benefits differed by stroke etiology. CE/AF-related strokes showed less consistent recurrence benefit. Genetic variability further contributes to differential statin response [,]; Asian patients may achieve similar outcomes with lower doses due to altered metabolism [], while some individuals exhibit pharmacogenomic resistance to statins [,]. These interindividual differences underscore the importance of pharmacogenomic modifiers of statin metabolism and transport, such as SLCO1B1 and ABCG2 variants, which alter bioavailability and treatment response. Patients with variations in SLCO1B1 gene have lower hepatic uptake of statins [], or those with ABCG2 variants have impaired statin transport and lower bioavailability []. In such cases, adjunctive therapies (such as ezetimibe, PCSK9 inhibitors, bempedoic acid) warrant exploration. This highlights how precision-guided statin prescribing, integrating pharmacogenomics, solubility, and inflammatory markers, can optimize secondary prevention and cognitive protection after stroke.
These interindividual variations challenge the utility of a one-size-fits-all model, which is currently reflected in guidelines. A shift toward precision neurology, tailoring therapy to stroke subtype, pharmacogenomic profile, and inflammatory or lipid biomarkers, may enhance statin efficacy and safety []. This is particularly relevant for low- and middle-income countries (LMICs), where access to reperfusion therapies is limited and cost-effective interventions like statins may offer critical clinical value [].
4.1. Strengths and Limitations
To our knowledge, this is the first meta-analysis to comprehensively evaluate the effects of post-stroke statin therapy on key clinical outcomes across short- and long-term time points. It is also the first to meta-analyze outcomes related to PSCI and PSD, as well as in patients with low baseline LDL-cholesterol levels—populations historically underrepresented in prior trials.
Nonetheless, several limitations warrant consideration. First, variability in treatment protocols across included studies may have influenced outcomes. Although only studies evaluating post-stroke statin use were included, differences in treatment duration, adherence, and concurrent use of thrombolysis, thrombectomy, or antiplatelet agents may introduce confounding. Second, the analysis primarily relied on observational data, inherently subject to selection bias and unmeasured confounders. Third, substantial statistical heterogeneity (I2 > 75%) was observed in most outcomes, likely reflecting differences in patient populations and local management practices, which may limit generalizability. Fourth, limited data were available on statin parameters (type, intensity, solubility), CE/AF-related stroke outcomes, and PSCI/PSD, restricting our ability to conduct association and robust subgroup analyses. Notably, data on lacunar strokes, an important etiology particularly in Asian populations [], were insufficient for inclusion. Fifth, analyses involving statin-specific parameters were descriptive in nature and could not adjust for confounders, precluding causal inference. Lastly, evidence of publication bias may have inflated effect estimates, impacting the reliability of conclusions.
4.2. Future Directions
This meta-analysis highlights key gaps that should inform future research. First, RCTs are needed to evaluate the impact of statin regimen variations—including intensity, type, and timing—on clinical outcomes to support more tailored, evidence-based recommendations. Second, longitudinal studies assessing cognitive outcomes, particularly PSCI and PSD, are essential to better understand statins’ role in long-term brain health. Future longitudinal studies should employ standardized cognitive batteries, e.g., Montreal Cognitive Assessment (MoCA), Mini Mental State Examination (MMSE), or National Institute of Neurological Disorders and Stroke and the Canadian Stroke Network (NINDS-CSN), to uniformly quantify post-stroke cognitive trajectories []. Third, focused research on the efficacy of statins in lacunar strokes is needed, especially given their prevalence in non-Western populations []. Fourth, meta-regression was limited by inadequate stratified data but will be considered in future pooled analyses. Fifth, exploring combination strategies involving statins and non-statin agents (e.g., PCSK9 inhibitors, ezetimibe) in statin-resistant individuals may help optimize outcomes in precision-guided, post-stroke lipid management. Serial measurement of CRP and related inflammatory mediators could clarify the temporal dynamics of statins’ pleiotropic effects and guide optimal initiation timing. Finally, future trials integrating genetic, lipidomic, and inflammatory biomarkers will enable precision-guided statin therapy tailored to stroke subtype and pharmacogenomic profile.
5. Conclusions
This comprehensive meta-analysis, the ISMARDD Study, demonstrates that post-stroke statin therapy is associated with significant reductions in all-cause mortality and PSD, with modest benefits on stroke recurrence. These effects were observed consistently across diverse IS populations, including patients with CE or AF-related strokes and those with low-baseline LDL cholesterol levels—subgroups historically excluded from key trials. Early initiation of statin therapy (within 24 h of stroke onset) was linked with reduced prevalence of recurrence, reinforcing the importance of timing in maximizing therapeutic outcomes. Although heterogeneity was present across included studies, sensitivity analyses confirmed the stability of key findings. No consistent variations were noted based on statin intensity, type, or solubility, suggesting that other biological and clinical factors may better guide individualized statin strategies. The reduction in CRP levels among statin users supports the role of anti-inflammatory and pleiotropic mechanisms in their neuroprotective effects. Taken together, these findings support a broad recommendation for statin use after IS. However, they also highlight the limitations of a one-size-fits-all approach. A shift toward precision neurology, where treatment is tailored to stroke subtype, comorbidities, LDL thresholds, cognitive risk, and inflammatory markers, may help unlock the full therapeutic potential of statins in post-stroke care []. Future trials should prioritize individualized treatment models and investigate optimal statin regimens for improving survival and long-term brain health in diverse stroke populations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/neurolint17110176/s1, Supplemental Tables: Table S1. Search Strategy (Keywords/MeSH Terms); Table S2. Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) (2020) Checklist; Table S3. Meta-analysis of Observational Studies in Epidemiology (MOOSE) Checklist; Table S4. Modified Jadad Analysis for Methodological Quality; Table S5. ROBINS-I: Risk of Bias in Non-Randomized Studies of Interventions; Table S6. RoB-2: Risk of Bias in Randomized Trials; Table S7. Funding Bias Scores for Studies; Table S8. Pooled prevalence of clinical outcomes in ischemic stroke patient subgroups with post-stroke statin use vs. no post-stroke statin use; Table S9. Summary effects and heterogeneity obtained from meta-analysis of statin use and clinical outcomes after ischemic stroke in sub-groups; Table S10. Outputs of Peters’ test; Table S11. Outputs of Egger’s test; Supplemental Figures: Figure S1. Estimated prevalence of all-cause mortality within 3 months, 1 year and after 1 year of ischemic stroke; Figure S2. Estimated prevalence of all-cause mortality within 3 months, 1 year and after 1 year of ischemic stroke in statin users vs. nonusers, as shown in Figure S3. Estimated prevalence of stroke recurrence within 1 year and after 1 year of ischemic stroke; Figure S4. Estimated prevalence of stroke recurrence within 1 year and after 1 year of ischemic stroke in statin users vs. nonusers; Figure S5. Estimated prevalence of dementia/cognitive impairment after ischemic stroke; Figure S6. Estimated prevalence of dementia/cognitive impairment after ischemic stroke in statin users vs. nonusers; Figure S7. Estimated prevalence of all-cause mortality and stroke recurrence by statin timing of initiation; Figure S8. Estimated prevalence of all-cause mortality and stroke recurrence by statin type; Figure S9. Estimated prevalence of all-cause mortality and stroke recurrence by statin solubility; Figure S10. Estimated prevalence of all-cause mortality and stroke recurrence by statin intensity; Figure S11. Association between increasing statin intensity and all-cause mortality and stroke recurrence after ischemic stroke; Figure S12. Estimated prevalence of all-cause mortality within and after 1 year in cardioembolic/atrial fibrillation stroke patients and patients with low baseline low-density lipoprotein cholesterol; Figure S13. Estimated prevalence of all-cause mortality within and after 1 year in cardioembolic/atrial fibrillation stroke patients and patients with low baseline low-density lipoprotein cholesterol in statin users vs. nonusers; Figure S14. Estimated prevalence of stroke recurrence within and after 1 year in cardioembolic/atrial fibrillation stroke patients; Figure S15. Estimated prevalence of stroke recurrence within and after 1 year in cardioembolic/atrial fibrillation stroke patients in statin users vs. nonusers; Figure S16. Association between statin use and all-cause mortality within and after 1 year in cardioembolic and atrial-fibrillation related stroke patients and patients with low baseline low-density lipoprotein cholesterol; Figure S17. Association between statin use and stroke recurrence after 1 year in cardioembolic and atrial-fibrillation related stroke patients; Figure S18. Difference in CRP levels (mg/L) within 3–7 days and after 7 days of ischemic stroke between statin users and nonusers; Figure S19. Graphs of Sensitivity Analysis; Figure S20. Graphs of Egger’s Regression Test; Figure S21. Graphs of Funnel Plots.
Author Contributions
S.B. conceptualized and led the ISMARDD study, developing the overarching framework and supervising the Global Health Neurology Lab team. He provided intellectual leadership, validated key concepts, and oversaw all aspects of study design and manuscript development. S.B. encouraged M.G. to explore this topic and guided the synthesis and interpretation of findings. M.G. and S.B. jointly conducted the literature review, data collection, drafting of the manuscript, and critical revisions. R.G.B. and K.J.S. contributed to the discussion of the study design and provided critical feedback during the drafting and revision process. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no direct funding. S.B. received separate financial support through the Grant-in-Aid for Scientific Research (KAKENHI) funded by the Japan Society for the Promotion of Science (JSPS), Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan (Grant ID: 23KF0126). S.B. was also awarded the JSPS International Fellowship supported by MEXT and the Australian Academy of Science for the period 2023–2025 (Grant ID: P23712).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
S.B. reports leadership or fiduciary roles with the following organizations: National Cerebral and Cardiovascular Center (Osaka, Japan) as Visiting Director (2023–2025); Rotary District 9675 (Sydney, Australia) as District Chair for Diversity, Equity, and Inclusion; the Global Health and Migration Hub Community, Global Health Hub Germany (Berlin, Germany) as Chair and Founding Member; and editorial board memberships at PLOS One, BMC Neurology, Frontiers in Neurology, Frontiers in Stroke, Frontiers in Public Health, Journal of Aging Research, Neurology International, Diagnostics, and BMC Medical Research Methodology. He also serves as a Member of the College of Reviewers for the Canadian Institutes of Health Research (CIHR), Government of Canada; Director of Research for the World Headache Society (Bengaluru, India); Scientific Review Committee Member at Cardiff University Biobank (UK); Chair of the Rotary Reconciliation Action Plan (RAP), Rotary District 9675 (NSW, Australia); Healthcare and Medical Adviser for Japan Connect (Osaka, Japan); and Expert Adviser/Reviewer for the Cariplo Foundation (Milan, Italy). These roles are unrelated to the submitted work. The other authors (M.G., R.G.B. and K.J.S.) declare no conflicts of interest. The funding bodies had no role in the design, data collection, interpretation, or preparation of this manuscript. The content is solely the responsibility of the authors and does not represent the official views of any affiliated or funding organizations.
References
- Feigin, V.L.; Abate, M.D.; Abate, Y.H.; Abd ElHafeez, S.; Abd-Allah, F.; Abdelalim, A.; Abdelkader, A.; Abdelmasseh, M.; Abd-Elsalam, S.; Abdi, P.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 973–1003. [Google Scholar] [CrossRef]
- Craig, L.; Hoo, Z.L.; Yan, T.Z.; Wardlaw, J.; Quinn, T.J. Prevalence of dementia in ischaemic or mixed stroke populations: Systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2022, 93, 180. [Google Scholar] [CrossRef]
- Skajaa, N.; Adelborg, K.; Horváth-Puhó, E.; Rothman, K.J.; Henderson, V.W.; Thygesen, L.C.; Sørensen, H.T. Risks of stroke recurrence and mortality after first and recurrent strokes in Denmark. Neurology 2022, 98, e329–e342. [Google Scholar] [CrossRef]
- Taylor, F.; Huffman, M.D.; Macedo, A.F.; Moore, T.H.M.; Burke, M.; Davey Smith, G.; Ward, K.; Ebrahim, S.; Gay, H.C. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2013, 1, CD004816. [Google Scholar] [CrossRef]
- German, C.A.; Liao, J.K. Understanding the molecular mechanisms of statin pleiotropic effects. Arch. Toxicol. 2023, 97, 1529–1545. [Google Scholar] [CrossRef]
- Amarenco, P.; Bogousslavsky, J.; Callahan, A.; Goldstein, L.B.; Hennerici, M.; Rudolph, A.E.; Sillesen, H.; Simunovic, L.; Szarek, M.; Welch, K.M.; et al. High-dose atorvastatin after stroke or transient ischemic attack. N. Engl. J. Med. 2006, 355, 549–559. [Google Scholar] [CrossRef]
- Bhatia, R.; Sharma, G.; Patel, C.; Garg, A.; Roy, A.; Bali, P.; Singh, N.; Sisodia, P.; Sreenivas, V.; Srivastava, M.V.P.; et al. Coronary artery disease in patients with ischemic stroke and TIA. J. Stroke Cerebrovasc. Dis. 2019, 28, 104400. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Martínez, I.; Morales-Caba, L.; Segura, T. Atrial fibrillation and stroke: A review and new insights. Trends Cardiovasc. Med. 2023, 33, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Australian and New Zealand Living Clinical Guidelines for Stroke Management. Available online: https://informme.org.au/en/Guidelines/Clinical-Guidelines-for-Stroke-Management (accessed on 1 April 2025).
- Dawson, J.; Béjot, Y.; Christensen, L.M.; De Marchis, G.M.; Dichgans, M.; Hagberg, G.; Heldner, M.R.; Milionis, H.; Li, L.; Pezzella, F.R.; et al. European Stroke Organisation (ESO) guideline on pharmacological interventions for long-term secondary prevention after ischaemic stroke or transient ischaemic attack. Eur. Stroke J. 2022, 7, I–II. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, D.J.; Lindsay, M.P.; Douketis, J.; Smith, E.E.; Dowlatshahi, D.; Wein, T.; Bourgoin, A.; Cox, J.; Falconer, J.B.; Graham, B.R.; et al. Canadian Stroke Best Practice Recommendations: Secondary prevention of stroke update 2020. Can. J. Neurol. Sci. 2022, 49, 315–337. [Google Scholar] [CrossRef]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline from the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef]
- Liu, L.; Li, Z.; Zhou, H.; Duan, W.; Huo, X.; Xu, W.; Li, S.; Nie, X.; Liu, H.; Liu, J.; et al. Chinese Stroke Association guidelines for clinical management of ischaemic cerebrovascular diseases: Executive summary and 2023 update. Stroke Vasc. Neurol. 2023, 8, e3. [Google Scholar] [CrossRef]
- Miyamoto, S.; Ogasawara, K.; Kuroda, S.; Itabashi, R.; Toyoda, K.; Itoh, Y.; Iguchi, Y.; Shiokawa, Y.; Takagi, Y.; Ohtsuki, T.; et al. Japan Stroke Society guideline 2021 for the treatment of stroke. Int. J. Stroke 2022, 17, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Stroke and Transient Ischaemic Attack in over 16s: Diagnosis and Initial Management. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542436/ (accessed on 1 April 2025).
- National Clinical Guideline for Stroke for the UK and Ireland. Available online: www.strokeguideline.org (accessed on 1 April 2025).
- Hong, K.S.; Lee, J.S. Statins in acute ischemic stroke: A systematic review. J. Stroke 2015, 17, 282–301. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, L.; Marshall, I.; Wolfe, C.; Wang, Y. Statin therapy for preventing recurrent stroke in patients with ischemic stroke: A systematic review and meta-analysis of randomized controlled trials and observational cohort studies. Neuroepidemiology 2022, 56, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Rost, N.S.; Brodtmann, A.; Pase, M.P.; van Veluw, S.J.; Biffi, A.; Duering, M.; Hinman, J.D.; Dichgans, M. Post-Stroke Cognitive Impairment and Dementia. Circ. Res. 2022, 130, 1252–1271. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, H.; Edwards, D.; Ding, C.; Yan, L.; Brayne, C.; Mant, J. Association of blood lipids, atherosclerosis and statin use with dementia and cognitive impairment after stroke: A systematic review and meta-analysis. Ageing Res. Rev. 2020, 57, 100962. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Climent, E.; Benaiges, D.; Pedro-Botet, J. Hydrophilic or lipophilic statins? Front. Cardiovasc. Med. 2021, 8, 687585. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2019, 139, e1046–e1081. [Google Scholar] [CrossRef]
- Shen, H.; Killingsworth, M.C.; Bhaskar, S.M.M. Comprehensive meta-analysis of futile recanalization in acute ischemic stroke patients undergoing endovascular thrombectomy: Prevalence, factors, and clinical outcomes. Life 2023, 13, 1965. [Google Scholar] [CrossRef]
- Aivo, J.; Ruuskanen, J.O.; Tornio, A.; Rautava, P.; Kyto, V. Lack of statin therapy and outcomes after ischemic stroke: A population-based study. Stroke 2023, 54, 781–790. [Google Scholar] [CrossRef]
- Arevalo-Lorido, J.C.; Carretero-Gomez, J.; Fernandez-Recio, J.M.; Alvarez-Oliva, A.; Gutierrez-Montano, C.; Najarro-Diez, F.; Martin-Sanchez, M.J. Lowering C-reactive protein with statins after an ischemic stroke avoids mortality and readmissions. A prospective cohort study. Ann. Med. 2015, 47, 226–232. [Google Scholar] [CrossRef]
- Bach, F.; Skajaa, N.; Esen, B.O.; Fuglsang, C.H.; Horvath-Puho, E.; Sorensen, H.T.; Adelborg, K. High-intensity versus moderate-intensity statin treatment for patients with ischemic stroke: Nationwide cohort study. Eur. Stroke J. 2023, 8, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.J.; Zhang, Y.; Li, Y.B.; Guo, J.; He, L. Low-to-moderate dose statins improve the functional outcome of acute ischemic stroke with conventional medication treatment. Cardiovasc. Diagn. Ther. 2023, 13, 686. [Google Scholar] [CrossRef]
- Beer, C.; Blacker, D.; Bynevelt, M.; Hankey, G.J.; Puddey, I.B. A randomized placebo controlled trial of early treatment of acute ischemic stroke with atorvastatin and irbesartan. Int. J. Stroke 2012, 7, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.-J.; Zhang, X.-F.; Zheng, K.-D. Effects of atorvastatin and rosuvastatin on blood lipids, platelet aggregation rate and inflammatory factors in patients with cerebral infarction. Afr. J. Online 2017, 16, 2507–2513. [Google Scholar] [CrossRef]
- Cappellari, M.; Bovi, P.; Moretto, G.; Zini, A.; Nencini, P.; Sessa, M.; Furlan, M.; Pezzini, A.; Orlandi, G.; Paciaroni, M.; et al. The THRombolysis and STatins (THRaST) study. Neurology 2013, 80, 655–661. [Google Scholar] [CrossRef]
- Chen, X.; Zhuang, X.; Peng, Z.; Yang, H.; Chen, L.; Yang, Q. Intensive statin therapy for acute ischemic stroke to reduce the number of microemboli: A preliminary, randomized controlled study. Eur. Neurol. 2018, 80, 163–170. [Google Scholar] [CrossRef]
- Choi, J.Y.; Seo, W.K.; Kang, S.H.; Jung, J.M.; Cho, K.H.; Yu, S.; Oh, K. Statins improve survival in patients with cardioembolic stroke. Stroke 2014, 45, 1849. [Google Scholar] [CrossRef]
- Choi, K.H.; Seo, W.K.; Park, M.S.; Kim, J.T.; Chung, J.W.; Bang, O.Y.; Kim, G.M.; Song, T.J.; Kim, B.J.; Heo, S.H.; et al. Effect of statin therapy on outcomes of patients with acute ischemic stroke and atrial fibrillation. J. Am. Heart Assoc. 2019, 8, e013941. [Google Scholar] [CrossRef]
- Choi, S.E.; Bucci, T.; Huang, J.Y.; Yiu, K.H.; Tsang, C.T.; Lau, K.K.; Hill, A.; Irving, G.; Lip, G.Y.H.; Abdul-Rahim, A.H. Early statin use is associated with improved survival and cardiovascular outcomes in patients with atrial fibrillation and recent ischaemic stroke: A propensity-matched analysis of a global federated health database. Eur. Stroke J. 2024, 10, 116–127. [Google Scholar] [CrossRef]
- Ni Chróinín, D.; Callaly, E.L.; Duggan, J.; Merwick, A.; Hannon, N.; Sheehan, O.; Marnane, M.; Horgan, G.; Williams, E.B.; Harris, D.; et al. Association between acute statin therapy, survival, and improved functional outcome after ischemic stroke The North Dublin Population Stroke Study. Stroke 2011, 42, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Dong, S.; Liu, Q.; Bao, J.; Gao, L.; Li, Y.; He, L. Low-dose statins improve prognosis of patients with ischaemic stroke undergoing intra-arterial thrombectomy: A prospective cohort study. J. Clin. Neurosci. 2022, 103, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Li, Y.; Bao, J.; Dong, S.; Gao, L.; He, L. Low dose statins improve prognosis of ischemic stroke patients with intravenous thrombolysis. BMC Neurol. 2021, 21, 220. [Google Scholar] [CrossRef]
- Flach, C.; Elstad, M.; Muruet, W.; Wolfe, C.D.A.; Rudd, A.G.; Douiri, A. The impact of pre- and post-stroke statin use on stroke severity and long-term outcomes: A population-based cohort study. Cerebrovasc. Dis. 2019, 47, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.C.; Kamel, H.; Navi, B.B.; Rao, V.A.; Faigeles, B.S.; Conell, C.; Klingman, J.G.; Sidney, S.; Hills, N.K.; Sorel, M.; et al. Statin use during ischemic stroke hospitalization is strongly associated with improved poststroke survival. Stroke 2012, 43, 147–154. [Google Scholar] [CrossRef]
- Furlan, N.E.; de Souza, J.T.; Bazan, S.G.Z.; Franco, R.J.D.; Luvizutto, G.J.; Gut, A.L.; Modolo, G.P.; Winckler, F.C.; Martin, L.C.; Bazan, R. Association between statin use and mortality risks during the acute phase of ischemic stroke in patients admitted to an intensive care unit. Arq. Neuro-Psiquiatr. 2020, 78, 158–162. [Google Scholar] [CrossRef]
- Gong, C.; Liu, C.; Wang, Y.; Chen, L.; Yuan, J.; Zhang, J.; Xiaoming, L.; Chen, Y.; Huang, L.; Xu, T.; et al. Effect of statin treatment on clinical outcomes in cardioembolic stroke with endovascular thrombectomy. J. Neurointerv. Surg. 2024, 16, 947–954. [Google Scholar] [CrossRef]
- Han, J.; Choi, Y.K.; Leung, W.K.; Hui, M.T.; Leung, M.K.W. Long term clinical outcomes of patients with ischemic stroke in primary care—A 9-year retrospective study. BMC Fam. Pract. 2021, 22, 164. [Google Scholar] [CrossRef]
- Hjalmarsson, C.; Bokemark, L.; Manhem, K.; Mehlig, K.; Andersson, B. The effect of statins on acute and long-term outcome after ischemic stroke in the elderly. Am. J. Geriatr. Pharmacother. 2012, 10, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.H.; Zhou, L.C. Effect of 20 mg/day atorvastatin: Recurrent stroke survey in chinese ischemic stroke patients with prior intracranial hemorrhage. J. Clin. Neurol. 2013, 9, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kim, N.; Park, T.H.; Bang, O.Y.; Lee, J.S.; Lee, J.; Han, M.K.; Park, S.H.; Gorelick, P.B.; Bae, H.J. Early statin use in ischemic stroke patients treated with recanalization therapy: Retrospective observational study. BMC Neurol. 2015, 15, 122. [Google Scholar] [CrossRef]
- Kim, J.T.; Lee, J.S.; Kim, B.J.; Kang, J.; Lee, K.J.; Park, J.M.; Kang, K.; Lee, S.J.; Kim, J.G.; Cha, J.K.; et al. Statin treatment in patients with stroke with low-density lipoprotein cholesterol levels below 70 mg/dL. J. Am. Heart Assoc. 2023, 12, e030738. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.T.; Lee, J.S.; Kim, H.; Kim, B.J.; Kang, J.H.; Lee, K.J.; Park, J.M.; Kang, K.Y.S.; Lee, S.J.; Kim, J.G.; et al. Comparative effectiveness of rosuvastatin versus atorvastatin in acute ischemic stroke treatment. J. Am. Heart Assoc. 2025, 14, e038080. [Google Scholar] [CrossRef]
- Kim, T.J.; Lee, J.S.; Oh, M.S.; Park, S.H.; Lee, K.B.; Kyung-Ho, Y.; Lee, B.C.; Yoon, B.W.; Ko, S.B. Risk of long-term post-stroke dementia using a linked dataset of patients with ischemic stroke without a history of dementia. Int. J. Stroke 2024, 20, 601–610. [Google Scholar] [CrossRef]
- Kytö, V.; Åivo, J.; Ruuskanen, J.O. Intensity of statin therapy after ischaemic stroke and long-term outcomes: A nationwide cohort study. Stroke Vasc. Neurol. 2025, 10, 142–145. [Google Scholar] [CrossRef]
- Lee, K.P.; Huang, H.C.; Tsai, J.Y.; Hsu, L.C. Statin treatment in stroke patient with low-density lipoprotein cholesterol levels below 70 mg/dL. J. Stroke Cerebrovasc. Dis. 2024, 33, 107645. [Google Scholar] [CrossRef]
- Lin, H.C.; Tsai, W.C.; Lin, J.R.; Chang, W.N.; Huang, C.C.; Wang, H.C.; Kung, C.T.; Su, C.M.; Su, Y.J.; Lin, W.C.; et al. Adjunctive statin therapy reduces intracranial hemorrhage and 1-year mortality in patients with atrial fibrillation after acute ischemic stroke: A population-based epidemiological study from Taiwan. J. Clin. Neurosci. 2019, 69, 224–229. [Google Scholar] [CrossRef]
- Makihara, N.; Kamouchi, M.; Hata, J.; Matsuo, R.; Ago, T.; Kuroda, J.; Kuwashiro, T.; Sugimori, H.; Kitazono, T. Statins and the risks of stroke recurrence and death after ischemic stroke: The Fukuoka Stroke Registry. Atherosclerosis 2013, 231, 211–215. [Google Scholar] [CrossRef]
- Marvardi, M.; Paciaroni, M.; Caso, V. Statin therapy in ischemic stroke patients with atrial fibrillation: Efficacy and safety outcomes. Eur. Stroke J. 2025, 10, 775–783. [Google Scholar] [CrossRef]
- Milionis, H.J.; Giannopoulos, S.; Kosmidou, M.; Panoulas, V.; Manios, E.; Kyritsis, A.P.; Elisaf, M.S.; Vemmos, K. Statin therapy after first stroke reduces 10-year stroke recurrence and improves survival. Neurology 2009, 72, 1816–1822. [Google Scholar] [CrossRef]
- Montaner, J.; Bustamante, A.; Garcia-Matas, S.; Martinez-Zabaleta, M.; Jimenez, C.; De La Torre, J.; Rubio, F.R.; Segura, T.; Masjuan, J.; Canovas, D.; et al. Combination of thrombolysis and statins in acute stroke is safe: Results of the STARS randomized trial (Stroke Treatment with Acute Reperfusion and Simvastatin). Stroke 2016, 47, 2870–2873. [Google Scholar] [CrossRef]
- Muscari, A.; Puddu, G.M.; Santoro, N.; Serafini, C.; Cenni, A.; Rossi, V.; Zoli, M. The atorvastatin during ischemic stroke study: A pilot randomized controlled trial. Clin. Neuropharmacol. 2011, 34, 141–147. [Google Scholar] [CrossRef]
- Ntaios, G.; Papavasileiou, V.; Makaritsis, K.; Milionis, H.; Manios, E.; Michel, P.; Lip, G.Y.; Vemmos, K. Statin treatment is associated with improved prognosis in patients with AF-related stroke. Int. J. Cardiol. 2014, 177, 129–133. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.C.; Greiner, M.A.; Xian, Y.; Fonarow, G.C.; Olson, D.M.; Schwamm, L.H.; Bhatt, D.L.; Smith, E.E.; Maisch, L.; Hannah, D.; et al. Clinical effectiveness of statin therapy after ischemic stroke: Primary results from the statin therapeutic area of the Patient-Centered Research Into Outcomes Stroke Patients Prefer and Effectiveness Research (PROSPER) Study. Circulation 2015, 132, 1404–1413. [Google Scholar] [CrossRef]
- Park, H.K.; Lee, J.S.; Hong, K.S.; Cho, Y.J.; Park, J.M.; Kang, K.; Lee, S.J.; Kim, J.G.; Cha, J.K.; Kim, D.H.; et al. Statin therapy in acute cardioembolic stroke with no guidance-based indication. Neurology 2020, 94, e1984–e1995. [Google Scholar] [CrossRef]
- Sakurai, K.; Ishaya, K.; Takaishi, S.; Kato, B.; Shimizu, K.; Shimomura, K.; Tokuyama, Y.; Hasegawa, Y. Effects of early statin treatment on inflammatory biomarkers and clinical deterioration in patients with acute ischemic stroke. Clin. Neurol. 2011, 51, 6–13. [Google Scholar] [CrossRef]
- Scheitz, J.F.; Endres, M.; Heuschmann, P.U.; Audebert, H.J.; Nolte, C.H. Reduced risk of poststroke pneumonia in thrombolyzed stroke patients with continued statin treatment. Int. J. Stroke 2015, 10, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Wang, Y.; Zhao, X.; Liu, L.; Wang, C.; Wang, A.; Du, W.; Wang, Y.; Xu, Y. Inpatient statin use Is associated with decreased mortality of acute stroke patients with very low low-density lipoprotein cholesterol. J. Stroke Cerebrovasc. Dis. 2015, 24, 2369–2374. [Google Scholar] [CrossRef]
- Song, B.; Wang, Y.L.; Zhao, X.Q.; Liu, L.P.; Wang, C.X.; Wang, A.X.; Du, W.L.; Wang, Y.J. Association between statin use and short-term outcome based on severity of ischemic stroke: A cohort study. PLoS ONE 2014, 9, e84389. [Google Scholar] [CrossRef]
- Ueno, Y.; Yamashiro, K.; Tanaka, Y.; Watanabe, M.; Miyamoto, N.; Shimada, Y.; Kuroki, T.; Tanaka, R.; Miyauchi, K.; Daida, H.; et al. Rosuvastatin may stabilize atherosclerotic aortic plaque: Transesophageal echocardiographic study in the EPISTEME trial. Atherosclerosis 2015, 239, 476–482. [Google Scholar] [CrossRef]
- Vitturi, B.K.; Gagliardi, R.J. The role of statins in cardioembolic stroke. J. Clin. Neurosci. 2019, 72, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Vitturi, B.K.; Gagliardi, R.J. The influence of statin withdrawal and adherence on stroke outcomes. Neurol. Sci. 2020, 42, 2317–2323. [Google Scholar] [CrossRef]
- Winardi, W.; Moi, S.H.; Winardi, T.; Cheng, Y.W.; Chen, P.Y.; Lin, C.K. Nationwide big data analysis of statin use and intracerebral hemorrhage risk in acute ischemic stroke patients in Taiwan. Medicina 2024, 60, 939. [Google Scholar] [CrossRef]
- Wu, Y.L.; Saver, J.L.; Chen, P.C.; Lee, J.D.; Wang, H.H.; Rao, N.M.; Lee, M.; Ovbiagele, B. Effect of statin use on clinical outcomes in ischemic stroke patients with atrial fibrillation. Medicine 2017, 96, e5918. [Google Scholar] [CrossRef]
- Yakusevich, V.V.; Malygin, A.Y.; Lychenko, S.V.; Petrochenko, A.S.; Kabanov, A.V. The efficacy of high-dose simvastatin in acute period of ischemic stroke. Ration. Pharmacother. Cardiol. 2012, 8, 4–16. [Google Scholar] [CrossRef]
- Yang, W.Y.; Li, Y.F.; Wang, Z.R.; Yu, T.X.; Xu, D.J.; Yang, N.; Niu, X.Y.; Cai, X.L.; Zhuo, W.Y.; Wu, X.M.; et al. Combined therapy of intensive statin plus intravenous rt-PA in acute ischemic stroke: The INSPIRE randomized clinical trial. J. Neurol. 2021, 268, 2560–2569. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Toh, S.; Li, X.; Edwards, D.; Brayne, C.; Mant, J. Statin use is associated with lower risk of dementia in stroke patients: A community-based cohort study with inverse probability weighted marginal structural model analysis. Eur. J. Epidemiol. 2022, 37, 615–627. [Google Scholar] [CrossRef]
- Zare, M.; Saadatnia, M.; Mousavi, S.A.; Keyhanian, K.; Davoudi, V.; Khanmohammadi, E. The effect of statin therapy in stroke outcome: A double blind clinical trial. Int. J. Prev. Med. 2012, 3, 68–72. [Google Scholar]
- Zhang, Z.; Yao, X.; Wang, M.; Huang, Y.; Shen, T.; Zhang, W.; Liu, Y. Therapeutic effects of aspirin combined with atorvastatin on ischemic strokes. Int. J. Clin. Exp. Med. 2018, 11, 11104–11111. [Google Scholar]
- Huang, Y.; Yang, S.; Jia, J. Factors related to long-term post-stroke cognitive impairment in young adult ischemic stroke. Med. Sci. Monit. 2015, 21, 654–660. [Google Scholar] [CrossRef]
- Eun, M.; Jung, J.; Choi, K.; Seo, W. Statin effects in atrial fibrillation-related stroke: A systematic review and meta-analysis. Front. Neurol. 2020, 11, 589684. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Y.; Yuan, J.; Chen, Y. The effect of statin treatment on outcomes of cardioembolic stroke: A systematic review and meta-analysis of real-world Studies. CNS Drugs 2021, 35, 717–726. [Google Scholar] [CrossRef]
- Patel, J.; Bhaskar, S.M.M. Diagnosis and Management of Atrial Fibrillation in Acute Ischemic Stroke in the Setting of Reperfusion Therapy: Insights and Strategies for Optimized Care. J. Cardiovasc. Dev. Dis. 2023, 10, 458. [Google Scholar] [CrossRef]
- Guasti, L.; Lupi, A. Lipidology Update: Targets and Timing of Well-Established Therapies. Available online: https://www.escardio.org/Councils/Council-for-Cardiology-Practice-(CCP)/Cardiopractice/lipidology-update-targets-and-timing-of-well-established-therapies (accessed on 1 April 2025).
- Rajesh, K.; Spring, K.J.; Smokovski, I.; Upmanyue, V.; Mehndiratta, M.M.; Strippoli, G.F.M.; Beran, R.G.; Bhaskar, S.M.M. The impact of chronic kidney disease on prognosis in acute stroke: Unraveling the pathophysiology and clinical complexity for optimal management. Clin. Exp. Nephrol. 2025, 29, 149–172. [Google Scholar] [CrossRef]
- Singh, R.J.; Chen, S.; Ganesh, A.; Hill, M.D. Long-term neurological, vascular, and mortality outcomes after stroke. Int. J. Stroke 2018, 13, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Kolmos, M.; Christoffersen, L.; Kruuse, C. Recurrent ischemic stroke—A systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 2021, 30, 105935. [Google Scholar] [CrossRef] [PubMed]
- Herder, M.; Arntzen, K.A.; Johnsen, S.H.; Eggen, A.E.; Mathiesen, E.B. Long-term use of lipid-lowering drugs slows progression of carotid atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic effects of statins on the cardiovascular system. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef]
- Stephan, B.C.M.; Hunter, S.; Harris, D.; Llewellyn, D.J.; Siervo, M.; Matthews, F.E.; Brayne, C. The neuropathological profile of mild cognitive impairment (MCI): A systematic review. Mol. Psychiatry 2012, 17, 1056–1076. [Google Scholar] [CrossRef]
- Doyle, K.P.; Buckwalter, M.S. Immunological mechanisms in poststroke dementia. Curr. Opin. Neurol. 2020, 33, 30–36. [Google Scholar] [CrossRef]
- Du, S.; Wang, X.; Xiao, L.; Tu, J.; Zhu, W.; He, T.; Liu, C. Molecular mechanisms of vascular dementia: What can be learned from animal models of chronic cerebral hypoperfusion? Mol. Neurobiol. 2017, 54, 3670–3682. [Google Scholar] [CrossRef]
- Moskowitz, M.A.; Lo, E.H.; Iadecola, C. The science of stroke: Mechanisms in search of treatments. Neuron 2010, 67, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Rajab, H.A.; Al-Kuraishy, H.M.; Shokr, M.M.; Al-Gareeb, A.I.; Al-Harchan, N.A.; Alruwaili, M.; Papadakis, M.; Alexiou, A.; Batiha, G.E. Statins for vascular dementia: A hype or hope. Neuroscience 2025, 567, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Arboix, A.; García-Eroles, L.; Oliveres, M.; Targa, C.; Balcells, M.; Massons, J. Pretreatment with statins improves early outcome in patients with first-ever ischaemic stroke: A pleiotropic effect of statins or a beneficial effect of hypercholesterolemia? BMC Neurol. 2010, 10, 47. [Google Scholar] [CrossRef]
- Azizi, E.; Prabhakaran, S.; Brorson, J.R. Statin initiation and early stroke recurrence in the Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke Trial (POINT) trial population. J. Stroke Cerebrovasc. Dis. 2025, 34, 108349. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, L.; Pan, Y.; Chen, W.; Jing, J.; Wang, C.; Johnston, S.C.; Amarenco, P.; Bath, P.M.; Yang, Y.; et al. Immediate- or delayed-intensive statin in acute cerebral ischemia: The INSPIRES randomized clinical trial. JAMA Neurol. 2024, 81, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.M.; Lobo, K.; Lalor Tavares, J.L.; da Silva Gomes, R.P.; Santos, L.; Oliveira, R.C.S. Intensive versus non-intensive statin therapy in patients with ischemic stroke: A systematic review and meta-analysis. J. Clin. Neurosci. 2025, 138, 111361. [Google Scholar] [CrossRef]
- Lee, M.; Cheng, C.; Wu, Y.; Lee, J.; Hsu, C.; Ovbiagele, B. Association between intensity of low-density lipoprotein cholesterol reduction with statin-based therapies and secondary stroke prevention: A meta-analysis of randomized clinical trials. JAMA Neurol. 2022, 79, 349–358. [Google Scholar] [CrossRef]
- Fong, C.W. Statins in therapy: Understanding their hydrophilicity, lipophilicity, binding to 3-hydroxy-3-methylglutaryl-CoA reductase, ability to cross the blood brain barrier and metabolic stability based on electrostatic molecular orbital studies. Eur. J. Med. Chem. 2014, 85, 661–674. [Google Scholar] [CrossRef]
- Chrispin, J.; Martin, S.S.; Hasan, R.K.; Joshi, P.H.; Minder, C.M.; McEvoy, J.W.; Kohli, P.; Johnson, A.E.; Wang, L.; Blaha, M.J.; et al. Landmark lipid-lowering trials in the primary prevention of cardiovascular disease. Clin. Cardiol. 2013, 36, 516–523. [Google Scholar] [CrossRef]
- Nissen, S.E.; Tuzcu, E.M.; Schoenhagen, P.; Brown, B.G.; Ganz, P.; Vogel, R.A.; Crowe, T.; Howard, G.; Cooper, C.J.; Brodie, B.; et al. Effect of Intensive Compared with Moderate Lipid-Lowering Therapy on Progression of Coronary AtherosclerosisA Randomized Controlled Trial. JAMA 2004, 291, 1071–1080. [Google Scholar] [CrossRef]
- McPherson, R.; Adreak, N.; Sharma, A. Medications for Lipid Control: Statins vs Newer Drugs. Can. J. Cardiol. 2024, 40, S26–S34. [Google Scholar] [CrossRef]
- Temporelli, P.L.; Arca, M.; D’Erasmo, L.; De Caterina, R. Lipid-Lowering Therapy in Patients with Coronary Heart Disease and Prior Stroke: Mission Impossible? J. Clin. Med. 2021, 10, 886. [Google Scholar] [CrossRef]
- Sierra, S.; Ramos, M.C.; Molina, P.; Esteo, C.; Vázquez, J.A.; Burgos, J.S. Statins as neuroprotectants: A comparative in vitro study of lipophilicity, blood-brain-barrier penetration, lowering of brain cholesterol, and decrease of neuron cell death. J. Alzheimers Dis. 2011, 23, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-C.; Lei, S.-Y.; Zhang, D.-H.; He, Q.-Y.; Sun, Y.-Y.; Zhu, H.-J.; Qu, Y.; Zhou, S.-Y.; Yang, Y.; Li, C.; et al. The pleiotropic effects of statins: A comprehensive exploration of neurovascular unit modulation and blood–brain barrier protection. Mol. Med. 2024, 30, 256. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Liao, J.K. The Pleiotropic Effects of Statins—From Coronary Artery Disease and Stroke to Atrial Fibrillation and Ventricular Tachyarrhythmia. Curr. Vasc. Pharmacol. 2019, 17, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Fracassi, A.; Marangoni, M.; Rosso, P.; Pallottini, V.; Fioramonti, M.; Siteni, S.; Segatto, M. Statins and the Brain: More than Lipid Lowering Agents? Curr. Neuropharmacol. 2019, 17, 59–83. [Google Scholar] [CrossRef]
- Luvai, A.; Mbagaya, W.; Hall, A.S.; Barth, J.H. Rosuvastatin: A review of the pharmacology and clinical effectiveness in cardiovascular disease. Clin. Med. Insights Cardiol. 2012, 6, 17–33. [Google Scholar] [CrossRef]
- Reiner, Ž. Resistance and intolerance to statins. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1057–1066. [Google Scholar] [CrossRef]
- Gupta, M.; Smokovski, I.; Chatzis, D.G.; Spring, K.J.; Mehndiratta, M.M.; Beran, R.G.; Bhaskar, S.M.M. Statins in acute ischemic stroke: Mechanisms, resistance, and precision strategies for neurovascular and cognitive protection. CNS Drugs 2025, 39, 1083–1107. [Google Scholar] [CrossRef]
- Liao, J.K. Safety and efficacy of statins in Asians. Am. J. Cardiol. 2007, 99, 410–414. [Google Scholar] [CrossRef]
- Link, E.; Parish, S.; Armitage, J.; Bowman, L.; Heath, S.; Matsuda, F.; Gut, I.; Lathrop, M.; Collins, R. SLCO1B1 variants and statin-induced myopathy—A genomewide study. N. Engl. J. Med. 2008, 359, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Wei, B.; Li, H.; Peng, Y.; Luo, Z. Impacts of ABCG2 loss of function variant (p. Gln141Lys, c.421 C > A, rs2231142) on lipid levels and statin efficiency: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2024, 24, 202. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Wijeratne, T.; Zelenak, K.; Huasen, B.B.; Iacobucci, M.; Killingsworth, M.C.; Beran, R.G.; Gebreyohanns, M.; Sekhar, A.; Khurana, D.; et al. Disparities in Access to Reperfusion Therapy for Acute Ischemic Stroke (DARTS): A comprehensive meta-analysis of ethnicity, socioeconomic status, and geographical factors. CNS Drugs 2025, 39, 417–442. [Google Scholar] [CrossRef]
- Venketasubramanian, N. Stroke demographics, risk factors, subtypes, syndromes, mechanisms and inter-ethnic differences between Chinese, Malays and Indians in Singapore—A hospital-based study. J. Cardiovasc. Dev. Dis. 2024, 11, 180. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Bhaskar, S. Unifying Vascular Injury and Neurodegeneration: A Mechanistic Continuum in Cerebral Small Vessel Disease and Dementia. Eur. J. Neurosci. 2025, 62, e70246. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).