Abstract
Thoracic aortic aneurysms (TAAs) are commonly seen in cardiovascular practice. Acquired and genetic conditions contribute to TAA formation. The natural history of genetically mediated TAA underscores the importance of early detection, regular monitoring, and prompt treatment to prevent complications, including dissection or rupture. The prognosis is poor in the event of acute dissection, with high rates of in-hospital mortality. Healthcare providers need to remain vigilant in their efforts to identify and surveil TAA to reduce the risk of complications. In this manuscript, we review the natural history of TAA, discuss the most common causes leading to the development of TAA, assess the value and limitations of diagnostic modalities, and review the management and long-term surveillance of patients with aortic disease.
Keywords:
aortic disease; aortic aneurysm; aortic dissection; genetics; Marfan syndrome; Loeys–Dietz 1. Introduction
Thoracic aortic aneurysms are clinically silent but may have lethal consequences. Natural history studies highlight the need for the appropriate diagnosis and timely management of thoracic disease to avoid aneurysmal expansion, leading to aortic dissection and potential rupture [1]. In the event of acute aortic dissection, the prognosis is poor, with overall mortality estimated at 1 to 2% per hour over the first 48 h, warranting timely surgical intervention or expeditious transfer to a tertiary care facility capable of providing acute aortic repair to improve survival [2]. However, even in cases when immediate surgical management can be achieved, the overall in-patient mortality remains high. The International Acute Aortic Dissection (IRAD) registry reported in-hospital mortality rates of ~30% for patients undergoing acute surgical management, while the mortality associated with medical management alone was greater than 50% [3]. The prognosis for patients sustaining an aortic rupture was worse and was often associated with immediate death due to catastrophic internal bleeding, poor distal perfusion to vital organs, and hypovolemic shock [3,4,5]. In this manuscript, we review the pathophysiology of thoracic aortic aneurysms along with the role of gender in aortic disease and discuss the most common genetic etiologies of thoracic aortic aneurysms. We also review the value and limitations of available diagnostic modalities and review the guidelines on the management and long-term surveillance of patients with aortic disease.
2. Overview of Thoracic Aortic Aneurysms
2.1. Epidemiology
Anatomically, aortic aneurysms are referred to as thoracic aortic aneurysms (TAAs), abdominal aortic aneurysms (AAAs), and thoraco-abdominal aortic aneurysms (TAAAs), the latter resulting from various degrees of the continuous dilatation of the descending aorta extending into the abdominal aorta [6]. Though both TAA and AAA share a number of similarities, each has its own unique characteristics contributing to pathophysiology, diagnosis, variations in management, and long-term surveillance. TAA refers to aortic disease in the thoracic cavity, which begins at the level of the aortic valve and extends to the 12th thoracic vertebra at the level of the diaphragmatic hiatus. TAA is an indolent process with an annual incidence of approximately 6 to 10 cases per 100,000 patient years, though the incidence is likely underestimated given the asymptomatic nature of the disease, lack of routine screening guidelines, and high pre-hospital mortality in cases of acute aortic dissection or rupture [5,6]. TAA can affect various aortic segments. The majority of TAAs (~60%) are known to occur in the aortic root or ascending aorta, while ~10% can affect the aortic arch [7,8]. It is not uncommon for patients with aortic disease to have aneurysms in multiple locations as TAA and AAA occur concomitantly in ~20% of individuals [9].
2.2. Pathophysiology of Thoracic Aortic Aneurysms
A pathognomonic feature of TAAs is cystic medial degeneration, a process characterized by the depletion of vascular smooth muscle cells (vSMCs), defects in collagen architecture, fragmentation of elastic fibers, and accumulation of proteoglycans in the aortic media [10]. Normally, the aorta is composed of three layers: intima, media, and adventitia. The tunica intima, the inner-most layer of the vessel, consists of a single row of vascular endothelium and supports the internal elastic lamina. The tunica media, the middle layer, is composed of elastic fibers that are oriented concentrically and longitudinally, and are both responsible for the aortic tensile strength and elasticity. The tunica adventitia, the outer-most layer of the aorta, is composed of abundant connective tissue, vasa vasorium, and nerve bundles supporting and anchoring the vessel from the outside [11]. In a healthy thoracic aorta, vSMCs produce the extracellular matrix (ECM), which is mainly composed of collagen and elastin, the essential scaffolding proteins of the aortic wall. The elastic fibers are distensible with a low tensile strength, and the collagen fibers provide tensile strength at higher pressures, allowing the aorta to act as an elastic reservoir capable of absorbing the systolic pulsatile blood flow from the left heart prior to distributing blood downstream during diastole. ECM proteins and vSMCs form a functional unit that maintains aortic tensile strength [12,13]. Histologically, as compared to a normal ascending aorta, TAAs exhibit more disorganization and fragmentation of the medial elastic fibers (Figure 1). In comparison, AAAs exhibit even more widespread destruction of the medial elastic fibers along with fibrocollagenous ECM replacement.
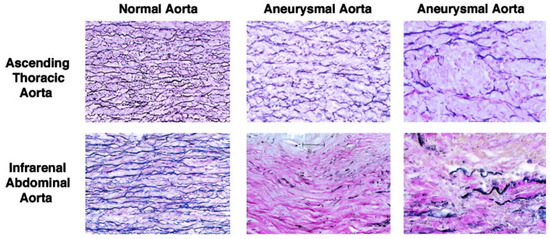
Figure 1.
Histopathology of aortic aneurysms. Reprinted with permission from “Altered patterns of gene expression distinguishing ascending aortic aneurysms from abdominal aortic aneurysms: complementary DNA expression profiling in the molecular characterization of aortic disease”. TS Absi et al., The Journal of Thoracic and Cardiovascular Surgery 2003, 126, 344–357.
A number of prior studies reported that vSMC dysfunction remains a key player in the formation and progression of aneurysmal disease. In both TAA and AAA, ECM homeostatic disruption promotes vSMC instability, detachment, subsequent migration, and apoptosis [14,15,16]. Moreover, aneurysmal disease affects the ability of vSMCs to undergo a switch between synthetic and contractile phenotypes. Contractile vSMCs play a central role in mechanotransduction and vessel integrity and express a higher number of contractile markers while synthetic vSMCs represent the proliferative phenotype. During aneurysm progression, the balance between contractile and proliferative vSMCs is shifted towards synthetic vSMCs, which is associated with increased proteolytic enzyme production by proliferative vSMCs and subsequent aneurysm formation, though the exact mechanism of this process remains unknown [16,17].
2.3. Gender Differences in the Pathophysiology of Thoracic Aortic Aneurysms
TAAs are common in males; however, the rate of aneurysm expansion is generally greater in females [18]. Females with TAA tend to have an atypical presentation of disease, which may contribute to worse clinical outcomes [19]. Prior studies have demonstrated that women with TAA experience higher mortality as compared to men, along with a 3-fold increase in the risk of dissection or rupture. Women are diagnosed with TAA later in life (up to a decade later), experience greater aneurysm growth rates, and may suffer aortic dissections or ruptures at smaller aortic diameters [19,20,21].
The underlying etiology of the gender differences in aortic disease progression remains poorly understood. Molecular and biomechanical factors may drive these differences as women with degenerative TAAs tend to have higher levels of matrix metalloproteinases (MMPs), a group of proteolytic enzymes capable of collagen, elastin, and extracellular matrix degradation [22,23,24]. In vitro studies suggest that prolonged exposure to the MMP-2 and MMP-9 enzymes may inhibit calcium smooth muscle entry, inhibiting muscle contraction and promoting aortic dilation and aneurysm formation [25]. Additionally, the expression of the tissue inhibitors of metalloproteinases 1 and 2 (TIMPs), enzymes that normally counteract the effects of MMPs, have been reported to be lower in women with TAA, which may result in more extensive proteolysis of the elastic and collagen fibers of the aortic wall and increased stiffness of the thoracic aorta [18,24]. This process may provide the basis for the deteriorated strength, progressive weakening, and stiffened stretch-stress response of the aortic wall, predisposing the vessel to dissection and rupture [26,27].
2.4. Risk Factors for the Formation of Thoracic Aortic Aneurysms
Acquired and genetic conditions contribute to TAA formation and pose significant risks for aortic dissections. Risk factors for TAA include older age, male gender, smoking, chronic obstructive pulmonary disease, hypertension, atherosclerosis, apolipoprotein concentrations, and high body mass index [28,29,30]. Besides these conventional risk factors, inflammatory, infectious, and genetic etiologies have also been implicated in TAA formation [18,31]. Generally, TAAs have a more robust genetic association than TAAA and AAA, with nearly 20% of all TAAs exhibiting a genetic predisposition. The recognition of genetic aortopathies is essential in the management of thoracic aortic disease, and treatment should be tailored based on specific genetic syndromes or non-syndromic variants.
3. Genetics of Thoracic Aortic Aneurysms
3.1. Marfan Syndrome
Marfan syndrome is an autosomal dominant connective tissue disorder with a prevalence of 2 to 3 per 10,000 persons [16]. The syndrome most commonly occurs due to a FBN1 mutation, located on chromosome 15q21.1, which encodes a cysteine-rich glycoprotein, Fibrillin-1. Fibrillin-1 is an essential component of the calcium-binding microfibrils embedded in the ECM and provides force-bearing structural support to various tissues. It is most prevalent in the tunica adventitia and tunica media of the aorta. To date, more than 1800 mutations with varying degrees of penetrance have been described [32,33]. Marfan syndrome is commonly inherited in an autosomal dominant pattern, but de novo mutations can occur and account for about a third of all cases. This disorder produces significant alterations in the connective fibers of the musculoskeletal, ocular, and cardiovascular systems, affecting the development and function of multiple organs. The most common cardiovascular manifestations include the formation of a sinus of Valsalva aortic aneurysm, descending thoracic and abdominal aortic aneurysm, mitral valve prolapse, and pulmonary artery dilatation. Aortic tortuosity is an uncommon manifestation and is considered to be a marker of worse cardiovascular outcomes [34].
Several mechanisms by which FBN1 mutations contribute to thoracic aortic disease have been proposed. FBN1 mutations may lead to increased susceptibility to MMP-driven proteolysis, though these effects may be counteracted by doxycycline administration, a known non-specific MMP inhibitor, as previously demonstrated in a mouse model (Fbn1C1039G/+). Moreover, the administration of doxycycline may be superior to beta-blockade due to its ability to normalize aortic stiffness, smooth muscle contractility, and aortic relaxation [35]. Another proposed mechanism of thoracic aortic disease in Marfan syndrome involves transforming growth factor-beta-1 (TGF-beta-1). The increased activation of TGF-beta-1 and subsequent mothers against decapentaplegic homolog 2 (SMAD2) signaling in Marfan syndrome may contribute to vSMC apoptosis and impede aortic tissue healing [36].
Neonatal Marfan syndrome, a distinct genotype and phenotype on the Marfan spectrum, is characterized by rapidly progressive, early-onset multi-valvular disease with severe tricuspid and mitral valve insufficiencies. The prognosis of neonatal Marfan syndrome is poor with nearly 95% mortality from congestive heart failure in the first year of life [37,38]. As compared to the neonatal type, the classic form of Marfan syndrome allows for survival into adulthood, yet the average lifespan is still significantly shortened when compared to the general population. The main cause of morbidity and mortality in this patient population is associated with acute aortic dissection or rupture, accounting for nearly 80% of deaths [39,40,41].
The management of Marfan syndrome revolves around the prevention of the primary manifestations of the disease and includes medical therapy with agents that reduce hemodynamic stress on the aortic wall. Prior studies have demonstrated substantial benefits of the long-term use of beta-adrenergic blockade in patients with Marfan syndrome across all age groups, including young children. Patients receiving beta-blockers were found to have slower aortic root growth and improved survival compared to the untreated population, though some still progressed to develop major cardiovascular complications warranting surgical intervention [42,43]. Similar results have been achieved in studies utilizing angiotensin receptor (ARB) blockade, including additive protection when used in combination with beta-blockers [44,45,46,47]. The American College of Cardiology/American Heart Association (ACC/AHA) guidelines on the management of aortic disease from 2022 recommends the initiation of beta-adrenergic blockade or an ARB administered in maximally tolerated doses as a Class I recommendation [48].
Cardiac imaging is essential, not only in establishing the diagnosis of Marfan syndrome, but also in following the progression of disease. The current ACC/AHA guidelines recommend diagnostic and surveillance imaging via a transthoracic echocardiogram (TTE) to assess for valvular pathology and to monitor changes in the size of the aortic root and ascending thoracic aorta, and/or a CT or MRI of the thoracic aorta if either the aortic root or ascending aorta is sub-optimally visualized (Class I recommendation) [48]. Imaging should take place 6 months following the initial diagnosis to assess the rate of aortic growth and may be followed with annual surveillance imaging if the aortic diameters are stable. The size of the aneurysm and the rate of aneurysmal growth are key factors in determining the timing of operative repair [49]. Surgical intervention to replace the aortic root and ascending aorta with a composite valve graft (CVG) is recommended when the aortic root diameter is ≥5 cm (Class I recommendation), though earlier surgical management may be considered in patients with an aortic root diameter ≥4.5 cm and high-risk features, such as a family history of aortic dissection, rapid aneurysmal growth (≥0.3 cm per year), diffuse aortic root and ascending aorta dilation, or marked vertebral artery tortuosity (Class IIa recommendation) or with a cross-sectional aortic root area to patient height ratio ≥10 cm2/m (Class IIa recommendation) [48,50]. Figure 2 depicts the specific mutations and ascending aortic dimensions for prophylactic surgical intervention. Genetic testing can be considered in patients with features of Marfan syndrome, early-onset thoracic aortic disease, those with a known family history of aortic, intracranial, or peripheral aneurysms or dissections, or a family history of unexplained sudden death in a first- or second-degree relative. Thoracic aortic imaging of the family members of affected individuals is also recommended.
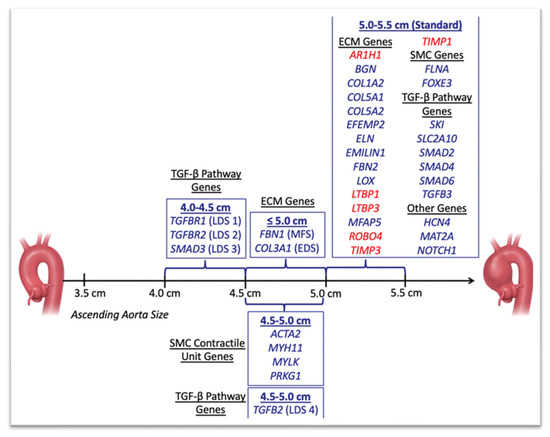
Figure 2.
Ascending aortic dimensions for prophylactic surgical intervention. Any gene newly reported during the year 2018 to be associated with TAAD is highlighted in red. ECM, extracellular matrix; SMC, smooth muscle cell; TGF, transforming growth factor. This image is obtained from Faggion Vinholo et al. [51]. Open access.
3.2. Loeys–Dietz Syndrome
Loeys–Dietz syndrome (LDS) is a rare, autosomal dominant connective tissue disorder associated with mutations in the transforming growth factor beta receptor (TGFβR1/TGFβR2) genes, SMAD3 gene, and TGFβ2/TGFβ3 genes [52]. De novo inheritance can also be observed. The disorder is characterized by widespread systemic involvement including craniofacial, musculoskeletal, neurologic, and vascular abnormalities. The existing phenotypes of LDS are divided into five types, reflective of their severity, with type 1 being the most severe phenotype and type 5 the least severe. All LDS syndrome phenotypes carry significant cardiovascular risks, though the full clinical spectrum of this disorder remains unknown. Similar to Marfan syndrome, aortic root aneurysms are common and can be associated with aortic insufficiency. Other cardiovascular features include diffuse arterial tortuosity, aneurysms affecting the arterial branches of the neck and intracranial vessels along with the thoracic and abdominal aorta and its distal branches, congenital heart defects, such as bicuspid aortic valve, patent ductus arteriosus, and atrial septal defects. Coronary artery aneurysms and spontaneous coronary artery dissections have also been reported [53,54]. Prior studies have reported the median survival of LDS patients to be around 37 years of age, and mortality was noted to occur primarily due to thoracic or abdominal aortic dissections, rupture, or major cerebral hemorrhage. Notably, aortic dissections can occur without marked arterial dilatation [55,56].
LDS demonstrates a similar prevalence of aortic aneurysm formation as compared to other inherited aortopathies, such as Marfan syndrome, vascular Ehlers–Danlos syndrome, or Turner Syndrome; however, the risk of dissections is much higher in patients with LDS and tends to occur at a younger age [57]. ACC/AHA guidelines recommend a baseline TTE to initially determine the diameter of the aortic root and ascending aorta, followed by a repeat TTE 6 months afterwards to assess the rate of aortic growth (Class I recommendation). If the disease is stable at 6 months, annual surveillance is recommended. In patients with a dilated or dissected aorta at baseline, annual surveillance with CT or MRI should be pursued (Class I recommendation). Additionally, given the propensity for aneurysms to affect the arterial branches ranging from the neck and intracranial vessels to the thoracic and abdominal aorta and its distal branches, patients with LDS benefit from a baseline head to pelvis screening with CT or MRI (Class I recommendation). Similar to the medical management of Marfan syndrome, ACC/AHA guidelines also recommend medical management with maximally tolerated doses of a beta-blocker or an ARB regimen (Class IIa), though, recommend lower thresholds for prophylactic surgical interventions on the aortic root and thoracic aorta. However, the surgical approach remains based on the specific genetic variant, aortic disease growth rate, extra-aortic features, and patient preferences (Figure 2). Notably, LDS cases with high-risk features (i.e., women with smaller body size with a TGFβR2 mutation, severe extra-aortic features, family history of aortic dissection, aortic growth rate > 0.3 cm per year) and mutations attributable to a pathogenic variant in TGFβR1 or TGFβR2 may benefit from prophylactic aortic root and ascending aortic replacement at a diameter of ≥4.0 cm; in patients with isolated TGFβR1 or TGFβR2 mutations without high-risk features or mutations in SMAD3 or TGFβ2, surgical replacement is recommended at a diameter of ≥4.5 cm [48,58,59,60,61].
3.3. Ehlers–Danlos Syndrome
Ehlers–Danlos syndrome (EDS) is a group of heterogeneous connective tissue disorders that occur due to abnormal collagen metabolism. The most recent international EDS classification recognizes 13 distinct EDS subtypes, but the classic and hypermobile subtypes account for over 90% of cases. The estimated prevalence of EDS ranges from 1:5000 to 1:250,000 births. Vascular EDS, formerly known as type IV EDS, results from an autosomal dominant mutation in the COL3A1 gene encoding the collagen alpha-1(III) chain, also known as the alpha 1 chain of type III collagen. To date, more than 700 COL3A1 mutations have been detected, and most of them represent missense mutations that interrupt the formation of the normal triple helical collagen structure [62]. Type III collagen is a major structural component of hollow organs, such as blood vessels and reproductive and gastrointestinal tracts. Other functions of type III collagen include direct intraluminal interaction with platelets in the coagulation cascade and wound healing. Type III collagen constitutes 5–20% of the entire collagen content in humans [63].
Of the EDS subtypes, vascular EDS has fewer classic features (skin hyperextensibility is not seen but is translucent with prominent superficial veins; joint hypermobility is uncommon) but carries the worst prognosis due to the increased risk of spontaneous arterial dissections (medium to large vessels are favored) and intestinal and uterine rupture at a young age. The proximal branches of the aortic arch, descending aorta, abdominal aorta, and distal branches such as the renal, mesenteric, iliac, and femoral arteries are particularly affected [64]. The life expectancy is severely limited, as up to 25% of patients with vascular EDS experience their first complication before the age of 20, and more than 80% of patients experience their first event by the age of 40 [65]. The median age of death is estimated to be approximately 50 years of age.
Specific management guidelines for vascular EDS are lacking but screening and medical management includes initial baseline head to pelvis imaging (CT/MRI) with subsequent annual surveillance imaging thereafter with aggressive blood pressure control and the prevention of major blood pressure fluctuations to reduce the risk of arterial dissections and ruptures. Beta-blockers are frequently utilized in patients with vascular EDS, though evidence regarding the benefits of beta-blockers and ARBs is lacking. A study assessing the use of celiprolol, a mixed β-1 antagonist and partial β-2 agonist in patients with vascular EDS, found that it could potentially extend the time to vascular complications [66]. A recent observational study revealed significantly lower mortality and clinical progression scores in patients treated with beta-blockers or ARBs [67]. Due to the tissue fragility and the high risk of perforations and subsequent bleeding complications, invasive surgical management carries a heightened risk in this patient population, though patients with rapid arterial aneurysmal growth should be considered for treatment [48,68]. All decisions on intervention should include shared decision-making via a multidisciplinary heart team approach [69,70].
3.4. Familial Thoracic Aortic Aneurysms
Familial thoracic aortic aneurysms represent a form of progressive thoracic aortic disease in the absence of known genetic syndromes. Unlike syndromic aneurysms known to be associated with pathogenic variants in the TGF-β signal and ECM-related genes, a significant proportion (~20%) of familial thoracic aortic disease results from altered components of the contractile apparatus of vSMCs, commonly encoded by MYLK, ACTA2, MYH11, and PRKG1 genes [71]. The MYLK gene encodes a myosin light chain kinase (MLCK), a ubiquitously present kinase, the target of which is to phosphorylate the regulatory light chain (RLC) of smooth and non-muscle myosin II. On the molecular level, RLC phosphorylation increases actin-activated myosin II ATPase and regulates many cellular cytoskeleton-mediated functions of muscle cells including endocytosis, secretion, cytokinesis, and migration [72]. MLCK is highly expressed in various SMCs, where RLC phosphorylation promotes smooth muscle contraction within hollow organs. An in vivo mice model demonstrated that the targeted deletion of smooth muscle-specific MLCK results in a significant reduction in RLC phosphorylation, contributing to varying degrees of arterial contractile impairment with resultant hypotension and smooth muscle dysfunction in the gastrointestinal and urinary tracts [73]. The histologic examination of the aortas of experimental mice revealed molecular changes consistent with TAA development, including notable pools of proteoglycans in the aortic media, the increased expression of lumican and decorin, increased collagen staining in the adventitial layer of the aorta, and increased MMP-2 expression.
The normal contractile function of the thoracic aorta is highly dependent on the interactions between thin and thick filaments, composed of the smooth muscle-specific isoform of alpha-actin proteins and β-myosin, encoded by the ACTA2 and MYH11 genes, respectively. Alpha-actin is the single most abundant protein in differentiated vSMCs, while ACTA2 is one of the most frequently mutated genes, responsible for about 12% to 21% of all familial cases of thoracic aortic aneurysms and dissections [74]. The mechanism leading to these events revolves around the impaired ability of vSMCs to contract in response to pulse pressure changes in the aorta. In addition to thoracic aortic disease, mutations in ACTA2 have also been associated with the premature onset of familial coronary artery disease and ischemic strokes, including Moyamoya disease, suggesting that a mutation in a single gene can trigger a variety of vascular pathologies in a familial cluster [75,76]. The contribution of ACTA2 mutations to non-familial vascular diseases has not been significant. In a large international case series of individuals with ACTA2 mutations (all heterozygous except for 1 homozygous missense mutation), nearly 50% of patients experienced an aortic event, with the vast majority (~88%) of these individuals presenting with thoracic aortic dissections [77]. Type A dissections were more prevalent (~54%) at an average age of 36 years, while type B dissections occurred at a younger age (~27 years). Moreover, aortic events were more prevalent in men (~62%) than in women (~38%), though the median age at the time of the first aortic event was not influenced by gender. The overall risk of mortality from an acute aortic event was noted to be 25% at a median age of 36 years. Despite significant variations in the diameter of the aortic root and ascending aorta at the time of dissection, a third of the patients experienced aortic dissections at a diameter < 5 cm. Notably, ACTA2-associated aneurysms involved the entire ascending aorta, from the sinuses of Valsalva to the aortic arch. These findings are of immense clinical value as they provide insight into the most suitable imaging modalities for the screening, diagnosis, and surveillance of aortic disease, and prompt discussions about timing of surgical aortic repair in these individuals.
Other studies have also recognized the role of genetic mutations associated with inherited predisposition to TAAs and dissections [78,79]. In a recent study involving the whole-exome sequencing of distant relatives affected by thoracic aortic disease, and the Sanger sequencing of individuals affected by known familial thoracic aortic disease, the presence of the same rare variant of the PRKG1 gene, PRKG1 c.530G>A (p.Arg177Gln), was identified [80]. In a healthy aorta, the PRKG1 gene encodes type I cGMP-dependent protein kinase (PKG-1) which controls vSMC relaxation. Alterations in the p.Arg177Gln trigger an increase in PKG-1 activity and subsequent reduction in RLC phosphorylation, decreasing the vSMC contraction. In this study, the majority of the mutation-positive individuals and relatives affected by the p.Arg177Gln defect (~63%) presented with acute aortic dissections, and 37% of the individuals had an aortic root enlargement. Acute aortic dissections were noted to occur as young as 17 years of age and were equally penetrant in men and women. Interestingly, women with p.Arg177Gln alterations presented with thoracic dissections at a younger age than they did with aortic root dilation (31 years vs. 43 years, p = 0.009), whereas men did not (31 years vs. 39 years, p = 0.18). A review of the aortic tissue pathology from the affected individuals was consistent with the typical pathology of thoracic aortic disease discussed earlier in this article. This analysis demonstrates the complexity of the genetic interactions between rare variants in PRKG1 and hereditary thoracic aortic disease and provides further evidence that healthy vSMC contractility is essential for maintaining the integrity of the thoracic aorta.
The predominant mode of inheritance in familial thoracic aortic aneurysms is autosomal dominant with varying degrees of penetrance and variable expressivity. Moreover, there are noticeable familial patterns of aneurysm clustering, with a distinct separation between ascending thoracic and descending thoracic aneurysms and AAAs. Familial aneurysms tend to occur at a later age but grow at a higher rate compared to the syndromic aortopathies [81,82]. The mean age of the affected individuals has previously been found to be 63.8 years for males and 67.0 years for women [83]. Interestingly, the first-degree relatives of patients with dissecting thoracic aortic aneurysms, particularly males, were also found to have a threefold increased risk of sudden death, likely from dissected aortic aneurysms. In the largest cross-sectional surveillance study of the at-risk relatives of individuals with familial aortopathies, up to half of the patients affected by familial thoracic aneurysms had affected relatives, including those with aortic root/ascending aorta diameter >5.0 cm [84]. Given the significant risks associated with the complications of acute aortic dissections, the current guidelines favor cascade genetic testing in patients with syndromic features; a known family history of aortic, intracranial, or peripheral aneurysms; unexplained sudden death, specifically in first-degree relatives; and in those diagnosed with an early onset aortopathy (<60 years). Genetic testing should be followed by aortic imaging in patients with identified pathologic variants. However, in cases when a pathogenic variant cannot be identified via genetic testing, thoracic aortic imaging becomes the only reliable method to diagnose thoracic aneurysms in high-risk families and should be pursued [85]. If negative, the ACC/AHA guidelines for the management of thoracic disease recommend repeat screening imaging in 5 years in young family members or 10 years in older family members, informed by their family history.
Prophylactic elective surgeries are often considered in patients with familial thoracic aortic aneurysms, given the higher risks of dissection and rupture compared to aneurysms that are not genetically mediated. Generally, prophylactic surgery is warranted when the maximal diameter of the aortic root reaches ≥ 5 cm. However, prophylactic aortic repair can be considered in those with diameters ≥ 4.5 cm and who have a known family history of aortic dissection at <5 cm. Similarly, patients with documented rapid aneurysmal growth (≥0.5 cm in 1 year or ≥0.3 cm in 2 consecutive years), or a family history of unexplained sudden death at <50 years of age may benefit from a repair by experienced surgeons in multidisciplinary aortic teams at a smaller diameter [86,87]. Figure 2 depicts the specific mutations and ascending aortic dimensions for prophylactic surgical intervention.
3.5. Bicuspid Aortic Valve
Bicuspid aortic valve (BAV) is the most common congenital heart anomaly and occurs in 1–2% of the population, with a strong male predominance [88]. BAV has been linked to a wide range of cardiovascular conditions including aortic valve insufficiency, aortic stenosis, infective endocarditis, aortic coarctation, and aortic aneurysms. Compared to the general population, patients with BAV, especially males, are more likely to develop aneurysmal disease, and various studies have shown that 20% to 30% of patients experience progressive aneurysmal enlargement [89,90]. While any segment of the ascending aorta can be involved, the most common location for BAV-related thoracic aneurysms is the tubular ascending aorta. The pathogenesis of this process is often ascribed to significant hemodynamic stress on the aortic wall from asymmetric high-velocity flow across the bicuspid valve leading to elastic fiber fragmentation, reduced collagen expression, and vSMC apoptosis. Additionally, significant variation in the expression of the MMP and TIMP enzymes has been noted across various aortic sites, with higher levels of MMP-2 and TIMP-3 concentrated in the concavity of the ascending aorta, suggestive of active, site-specific cellular processes [91,92]. It is not clear if hemodynamic forces are the only etiologic factor in the tubular ascending aortic phenotype. While less common, BAV-associated thoracic aortic aneurysms involving the aortic root at the sinuses of Valsalva have been reported to represent a more malignant phenotype with rapidly progressive disease.
It is important to note that, while the estimated risk of aortic dissections in this patient population has been reported to be eight times higher than in the general population, the overall risk remains low, at 0.03% per year [93]. A retrospective cohort study of patients with BAV living in Olmstead County, Minnesota, found that the incidence of aortic dissection was 3.1 per 10,000 patient years as compared to the general population of the county [93]. In patients 50 years of age or older, the incidence of aortic dissection and aortic aneurysms was 17.4 and 44.9 cases per 10,000 patient years, respectively. One of the most comprehensive longitudinal studies of individuals affected by BAV provided clinical reassurance since no significant hemodynamic deterioration was noted over a 20-year period [94]. The mortality rates in these patients were similar compared to the general population. Accelerated ascending aortic dilation was noted in about 40% of the patients. Despite a low overall mortality, the morbidity was significant but mainly associated with progressive valvular defects and the subsequent development of congestive heart failure requiring aortic valve replacement (AVR). The largest cohort study to date reinforced the concept that patients with BAV did not carry a significantly higher risk of fatal aortic dissections or ruptures, yet were at a higher risk of non-fatal cardiac events [95]. Notably, older age, moderate or severe aortic stenosis, and moderate or severe aortic insufficiency served as independent predictors of cardiac outcomes, and nearly 45% of the patients were found to have a dilated aortic sinus and/or ascending aorta at the last follow-up.
Despite the growing body of knowledge about the natural history of BAV, the data about definitive genetic and biological pathways leading to BAV pathogenesis remains limited. Similar to non-syndromic aortic aneurysms, the familial clustering has been noted to be around 9% in the first-degree relatives of affected individuals [96]. Thus, the ACC/AHA aortic disease guidelines recommend screening for BAV and dilation of the aortic root and/or ascending aorta in all the first-degree relatives of patients diagnosed with BAV with BAV-associated aortopathy (Class I recommendation) or in patients with isolated BAV without an associated aortopathy (Class IIa recommendation) [48]. TTE is favored as the initial screening modality, but CT or MRI may be utilized if TTE is unable to provide full anatomic assessment. The surgical replacement of the aortic root and/or ascending aorta is currently recommended at an aortic diameter ≥ 5.5 cm (Class I recommendation), or with a cross-sectional area to height ratio ≥ 10 cm2/m or with an aortic diameter of 5.0–5.4 cm if a high-risk feature is suspected (family history of aortic dissection, concomitant aortic coarctation, “root phenotype” aortopathy, or rapid aneurysmal expansion) (Class IIa recommendation). Additionally, the surgical replacement of the aortic root and/or ascending aorta can be considered during AVR if the aortic diameter is found to be ≥4.5 cm (Class IIa recommendation). It is important to note that, unlike in patients with a tricuspid aortic valve, the rate of aortic dilation does not decrease after AVR in patients with BAV, and late post-AVR aortic dissections can still occur.
3.6. Sporadic Thoracic Aortic Aneurysms
Although an increasing number of genetic associations contributing to TAA have been discovered, there still remains a significant proportion of thoracic aortopathies that cannot be explained by any specific underlying etiology. These TAAs are referred to as sporadic or degenerative aneurysms.
As previously described, various risk factors, particularly hypertension and older age, have been noted to promote TAA. Systemic hypertension is not only linked to thoracic aneurysm formation but also considered to be the most common cause of thoracic aortic dissection due to the prolonged mechanical stress on the aortic wall [97]. Age-related degeneration of the elastic lamina, on the other hand, is often considered a precursor for aneurysm formation. Other risk factors for TAA include smoking, atherosclerosis, and chronic obstructive pulmonary disease [98]. In addition, genetic variants can affect the susceptibility of hypertensive patients to thoracic aneurysmal disease. In a case–control study of 1351 subjects, a variant allele of the THBS2 gene was noted to serve as a risk factor for TAA formation, while the variant alleles of the HSPA8, GPX1, AGT, and TNF genes provided protection against aneurysm formation in patients with hypertension [99]. Besides genetic factors, vasoregulatory hormones, specifically angiotensin II, play an important role in both hypertensive and aneurysmal diseases. Various prior studies on animal models demonstrated that prolonged exposure to angiotensin II was associated with abdominal aortic aneurysm formation; angiotensin-induced ascending thoracic aneurysmal disease of Apo/E−/− mice models has also been described [100]. However, the pathological characteristics of aneurysmal disease varied significantly between abdominal and thoracic regions, suggestive of different mechanisms of disease. A mice model identified a causal relationship between systemic hypertension and pharmacologically induced degeneration of the elastic lamina favoring both thoracic and abdominal aneurysm formation with site-specific phenotypic differences [101]. Notably, the aneurysm formation in this animal model was dependent on hypertension and not on the direct effects of angiotensin II on the aortic wall. Systemic treatment with anti-hypertensive agents reduced the formation of both thoracic and abdominal aortic aneurysms in the experimental animals.
In the current ACC/AHA guidelines for the diagnosis and management of degenerative aortic disease, anti-hypertensive management plays a central role. The recommended blood pressure goals are strict (≤130/80 mmHg), and the initiation of beta-blockers and/or ARB therapy is recommended in the absence of contraindications [48]. Additionally, moderate- or high-intensity statin therapy and low-dose aspirin may be considered in patients with clinical or imaging evidence of atherosclerosis or concomitant evidence of penetrating aortic ulceration.
Once the diagnosis of degenerative TAA has been established, surveillance imaging with either TTE, CT, or MRI may be considered after 6–12 months. In the cases of aortic root or ascending aortic aneurysms, surgical intervention is recommended for an aortic diameter ≥ 5.5 cm, rapid growth as defined by aneurysmal growth of ≥0.5 cm/year or ≥0.3 cm/year for two consecutive years, or persistent clinical symptoms attributable to the aneurysm (Class I recommendation) [48]. Additionally, surgical intervention on the aorta may be considered at the time of aortic valve replacement with an aortic diameter ≥ 5.0 cm (Class IIa recommendation).
4. Diagnosis of Thoracic Aortic Aneurysms
4.1. Diagnostic Modalities of Thoracic Aortic Disease
A wide range of imaging modalities is available to help guide the diagnosis, surveillance, and management of thoracic aortic aneurysms. Chest X-rays can detect abnormalities in the size of the thoracic aorta or its contour, but lack specificity and are unable to provide an in-depth evaluation of the aorta. Other modalities, such as TTE, are more specific and can be used as a quick, cost-effective assessment of the aortic size at the sinus of Valsalva, sinotubular junction, and ascending aorta. However, the ability of a TTE to fully visualize the thoracic aorta is also limited by multiple factors that affect imaging quality. Computed tomography angiography (CTA) utilizes iodine-based contrast to visualize the aorta and its adjacent structures. A non-contrast CT may be obtained prior to contrast administration to assess for intramural hematoma in the setting of an acute thoracic syndrome or to evaluate the degree of aortic wall calcifications [102]. Magnetic resonance angiography (MRA) is another diagnostic tool that provides high-quality vascular imaging without the use of contrast nephrotoxicity and exposure to ionizing radiation. The disadvantages of this modality include a lower spatial resolution compared to CTA, artifacts in the setting of metallic implants and other hardware, the cost, and the time required to obtain the images. Both CTA and MRA scans are able to provide volume rendered three-dimensional reconstructions of aortic aneurysms and are useful in providing a visual overview of the extent and progression of the disease [103]. Per the current American and European guidelines for the management of aortic disease, the diameter of the aneurysm and rate of increase in size play a key role in the determination of operative intervention, and non-invasive imaging serves as an essential tool in planning for surgical repair [104].
While the above-mentioned modalities are useful in monitoring anatomic changes and providing risk stratification associated with the expansion of thoracic aneurysms, it is important to note that thoracic aortic disease is a dynamic pathophysiologic process. Beyond anatomic imaging, the primary pathologic mechanisms of TAA such as chronic inflammation leading to abnormal biomechanics of the aorta may be captured by positron emission tomographic (PET) imaging. The use of molecular imaging with PET scans can allow for the characterization of inflammatory activity in the aortic wall, and the use of the fluorine-2-deoxy-D-glucose (FDG) radiotracer with elevated avidity for highly metabolic cells has been previously correlated with the progression of aneurysmal disease in both animal and clinical models [105,106,107].
4.2. Role of Mock Loop Systems in Thoracic Aortic Disease
To better understand the mechanisms of aortic wall deterioration, various engineering setups have been developed to better illustrate specific hemodynamic conditions. In addition to aneurysm size and anatomic location, an understanding of the biomechanical wall shear stress distribution may fill an additive role in our understanding of the disease process and improve individual patient risk stratification [108,109,110]. Mock loop systems (MCLs), such as aortic MCLs, consist of active and passive elements, with the active elements designed to optimize aortic flow waveforms to grant physiologic vessel pulsatility. [111] Three-dimensional PC-MRI imaging is also utilized as an effective tool in mapping vascular geometry and quantifying blood velocities in various aortic segments. The use of these modalities allows for an accurate 3D printed reconstruction of the aorta, which can be used in the creation of patient-specific MCLs. These models have the potential to predict patient-specific outcomes and improve risk stratification.
5. Approach to Intervention
Despite the significant focus placed on the diagnosis and subsequent surveillance of TAA, surgical intervention represents the most definitive management, especially in high-risk patients. There are currently two principal approaches to TAA repair: thoracic endovascular aortic repair (TEVAR) and open surgical repair [58]. The current international (ESC/ACC/AHA) guidelines recommend that the decision to consider TEVAR or an open repair should be made on an individualized basis, taking into account factors such as vascular anatomy, pathology, and patient-specific comorbidities, utilizing a multidisciplinary approach (Class I recommendation).
TEVAR represents a minimally invasive approach, in which aortic repair is accomplished via the retrograde transarterial advancement of an expandable stent graft. The goal of this intervention is to prevent the further expansion and ultimate rupture of the TAA and is generally less invasive than an open repair. It has been reported to be associated with fewer complications as compared to open repair, though adverse events such as endoleaks, paraparesis/paraplegia (0.8–1.9%), stroke (2.1–3.5%) [112], and retrograde dissection of the ascending thoracic aorta (0.7–2.5%) [113] have been reported. In order to minimize the risk of complications, careful pre-procedural planning is essential. Contrast-enhanced CT imaging is the imaging modality of choice to assess the degree of aortic pathology and evaluate access sites. Despite the advances in minimally invasive techniques, concerns about the durability and safety of these interventions as compared to open surgical repair remain.
Open surgical repair is an alternative option in the management of advanced thoracic aortic disease. The goal of open surgical repair in ascending thoracic aortic aneurysms is to prevent the risk of aortic dissection and rupture, but techniques vary and it is highly dependent on the location and affected structures. It is important to note that, in patients with BAV, the blood flow is altered and will remain so after repair. As such, reconstructive aortic root surgery is often attempted to restore the natural hemodynamics; an aortic root replacement with either a mechanical composite graft or a xenograft, depending on the patient’s profile, may also be required. Surgical intervention on the aortic arch, whether repair or replacement, remains high-risk, but several emerging techniques have significantly lowered the risks associated with it. Among these techniques, the continuous use of anterograde cerebral perfusion provides cerebral protection during prolonged periods of circulatory arrest (Class IIa recommendation).
The optimal modality of TAA repair is patient-specific. In a recent systematic review comparing the outcomes of patients undergoing elective endovascular stent grafting, and open surgical repair in patients with aortic arch and ascending thoracic aneurysms, endovascular repair was initially associated with more favorable short-term outcomes, though open surgical repair yielded better long-term outcomes if patients survive the initial procedure [114]. The Gore TAG study found that the initial survival advantage associated with endovascular repair waned 5 years following the intervention [115,116,117,118]. Additional studies [119,120] suggested that the initial survival benefit from endovascular repair as compared to open aortic repair may wane earlier—up to 12 months post-intervention. Certainly, these outcomes may be influenced by selection bias, as the decision to pursue endovascular repair over open repair tends to be driven by patient-specific characteristics (i.e., age, medical comorbidities). The evolution of surgical techniques and patient selection over the years may also play a role in the reported long-term outcomes.
6. Prophylactic Surgical Intervention
The decision to proceed with prophylactic surgical intervention is based on the risk of TAA dissection and rupture as compared to the risk associated with surgical intervention. Driven by a significantly increased risk of in-hospital mortality in patients undergoing acute surgical management (up to 20 to 30%) [3] coupled with the relatively low risk of elective surgical intervention (~2.2%) [121], societal guidelines reflect a trend towards earlier prophylactic surgical intervention. The recent 2022 ACC/AHA guidelines now recommend a ≥5.0 cm threshold for surgical intervention for sporadic TAA when performed by experienced surgeons in a multidisciplinary aortic team, as compared to ≥5.5 cm recommended in the 2010 ACC/AHA guidelines [48,58,122]. However, prophylactic surgical intervention for sporadic TAA remains a contentious issue, as recommendations have mostly been based on retrospective, observational studies and expert consensus [123]. In comparison, the natural history and treatment course for abdominal aortic aneurysms is guided by robust randomized evidence [124,125].
The difficulty with relying on our current evidence base to guide prophylactic intervention for TAAs lies in the fact that only the patients who present to the medical system due to a complication of their TAA are included, excluding the large numbers of asymptomatic patients. Randomized controlled trials are needed to better confront this question and are currently enrolling patients [126]. Additionally, the ability to intervene prophylactically is dependent on an amenable medical system itself, which is difficult to accomplish in some European countries, such as the United Kingdom, where surgical intervention must be commissioned.
7. Conclusions
In conclusion, thoracic aortic diseases encompass a diverse spectrum of conditions that pose significant challenges in clinical practice. These disorders require a comprehensive understanding of their pathophysiology and accurate diagnosis, which often involves the genetic testing of family members. The potential for life-threatening complications mandates careful risk assessment and tailored management strategies. Advances in diagnostic imaging, genetic profiling, and research have contributed to better detection and risk prediction. Treatment options, ranging from conservative surveillance to endovascular repair or open surgical procedures have expanded therapeutic possibilities and personalized patient care. Nevertheless, challenges remain, including risk stratification and long-term surveillance for disease progression. Further research efforts and collaborative initiatives are necessary to deepen our understanding, enhance prevention strategies, and optimize the management of aortic diseases. By combining clinical expertise, technological advancements, and research endeavors, we can strive towards improved outcomes and a better quality of life for patients affected by these challenging conditions.
Author Contributions
Conceptualization: W.M., S.H. and C.L.; methodology: Y.P., W.M. and C.L.; resources, Y.P. and W.M.; data curation, Y.P. and W.M.; writing—original draft preparation: Y.P., W.M., M.T. and C.L.; writing—review and editing: Y.P., W.M., M.T., S.D., K.S., S.H. and C.L.; supervision, W.M., C.L. and S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors collectively declare no conflict of interest for this manuscript.
Abbreviations
| AAA | Abdominal aortic aneurysm |
| AAS | Acute aortic syndrome |
| ACC | American College of Cardiology |
| AHA | American Heart Association |
| ARB | Angiotensin receptor antagonist |
| AVR | Aortic valve replacement |
| BAV | Bicuspid aortic valve |
| CVG | Composite valve graft |
| CT | Computed tomography |
| CTA | Computed tomography angiography |
| ECG | Electrocardiogram |
| ECM | Extracellular matrix |
| EDS | Ehlers–Danlos syndrome |
| IMH | Intramural hematoma |
| IRAD | International Registry of Aortic Dissection |
| LDS | Loeys–Dietz syndrome |
| MCL | Mock Loop Systems |
| MLCK | Myosin light chain kinase |
| MMP | Matrix metalloproteinase |
| MRA | Magnetic resonance angiography |
| MRI | Magnetic resonance imaging |
| PAU | penetrating aortic ulcer |
| PET | Positron emission tomography |
| RLC | Regulatory light chain kinase |
| SMAD | Subsequent mothers against decapentaplegic homolog |
| TAAA | Thoraco-abdominal aortic aneurysm |
| TAA | Thoracic aortic aneurysm |
| TEVAR | Thoracic endovascular aortic repair |
| TGF-β | Transforming growth factor beta |
| TGFβR | Transforming growth factor beta receptor |
| TIMP | Tissue inhibitor of metalloproteinase |
| TTE | Transthoracic echocardiography |
References
- Yiu, R.S.; Cheng, S.W.K. Natural history and risk factors for rupture of thoracic aortic arch aneurysms. J. Vasc. Surg. 2016, 63, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.; Raymond, C.E. Therapeutic Goals in Patients with Acute Aortic Dissection. J. Am. Coll. Cardiol. 2015, 65, 1599–1600. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goldfinger, J.Z.; Halperin, J.L.; Marin, M.L.; Stewart, A.S.; Eagle, K.A.; Fuster, V. Thoracic Aortic Aneurysm and Dissection. J. Am. Coll. Cardiol. 2014, 64, 1725–1739. [Google Scholar] [CrossRef] [PubMed]
- Olsson, C.; Thelin, S.; Ståhle, E.; Ekbom, A.; Granath, F. Thoracic Aortic Aneurysm and Dissection: Increasing Prevalence and Improved Outcomes Reported in a Nationwide Population-Based Study of More than 14 000 Cases from 1987 to 2002. Circulation 2006, 114, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Cecchi, A.C.; Prakash, S.K.; Milewicz, D.M. Risk Factors for Thoracic Aortic Dissection. Genes 2022, 13, 1814. [Google Scholar] [CrossRef] [PubMed]
- Frederick, J.R.; Woo, Y.J. Thoracoabdominal aortic aneurysm. Ann. Cardiothorac. Surg. 2012, 1, 277–285. [Google Scholar] [PubMed]
- Clouse, W.D.; Hallett, J.W.J.; Schaff, H.V.; Gayari, M.M.; Ilstrup, D.M.; Melton, J.I. Improved prognosis of thoracic aortic aneurysms: A population-based study. JAMA 1998, 280, 1926–1929. [Google Scholar] [CrossRef]
- Kuzmik, G.A.; Sang, A.X.; Elefteriades, J.A. Natural history of thoracic aortic aneurysms. J. Vasc. Surg. 2012, 56, 565–571. [Google Scholar] [CrossRef]
- Gouveia e Melo, R.; Silva Duarte, G.; Lopes, A.; Alves, M.; Caldeira, D.; Fernandes e Fernandes, R.; Mendes Pedro, L. Synchronous and Metachronous Thoracic Aortic Aneurysms in Patients with Abdominal Aortic Aneurysms: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2020, 9, e017468. [Google Scholar] [CrossRef]
- Milewicz, D.M.; Guo, D.-C.; Tran-Fadulu, V.; Lafont, A.L.; Papke, C.L.; Inamoto, S.; Kwartler, C.S.; Pannu, H. Genetic Basis of Thoracic Aortic Aneurysms and Dissections: Focus on Smooth Muscle Cell Contractile Dysfunction. Annu. Rev. Genom. Hum. Genet. 2008, 9, 283–302. [Google Scholar] [CrossRef]
- Borges, L.F.; Gomez, D.; Quintana, M.; Touat, Z.; Jondeau, G.; Leclercq, A.; Meilhac, O.; Jandrot-Perrus, M.; Gutierrez, P.S.; Freymuller, E.; et al. Fibrinolytic activity is associated with presence of cystic medial degeneration in aneurysms of the ascending aorta. Histopathology 2010, 57, 917–932. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-J.; Lin, C.-Y.; Stitziel, N.O. Genetics of the extracellular matrix in aortic aneurysmal diseases. Matrix Biol. 2018, 71–72, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Du, W.; Ren, L.; Hamblin, M.H.; Becker, R.C.; Chen, Y.E.; Fan, Y. Vascular Smooth Muscle Cells in Aortic Aneurysm: From Genetics to Mechanisms. J. Am. Heart Assoc. 2021, 10, e023601. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Guo, D.-C.; Estrera, A.L.; Safi, H.J.; Huynh, T.T.; Yin, Z.; Cao, S.-N.; Lin, J.; Kurian, T.; Buja, L.M.; et al. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J. Thorac. Cardiovasc. Surg. 2006, 131, 671–678.e2. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhang, W.; Zhang, H.; Li, T.; Wang, Y.; Qin, Y.; Gu, H.; Du, J. Mechanical stretch-induced endoplasmic reticulum stress, apoptosis and inflammation contribute to thoracic aortic aneurysm and dissection. J. Pathol. 2015, 236, 373–383. [Google Scholar] [CrossRef]
- Gillis, E.; Van Laer, L.; Loeys, B.L. Genetics of Thoracic Aortic Aneurysm: At the Crossroad of Transforming Growth Factor-β Signaling and Vascular Smooth Muscle Cell Contractility. Circ. Res. 2013, 113, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, K.B.; van Merrienboer, T.A.R.; Ket, J.C.F.; Bogunovic, N.; van der Velden, J.; Yeung, K.K. The role of vascular smooth muscle cells in the development of aortic aneurysms and dissections. Eur. J. Clin. Investig. 2022, 52, e13697. [Google Scholar] [CrossRef]
- Boczar, K.; Boodwhani, M.; Beauchesne, L.; Chan, K.; Dennie, C.; Coutinho, T. Sex Differences in Thoracic Aortic Aneurysm Growth: Role of Aortic Stiffness. Hypertension 2019, 73, 190–196. [Google Scholar] [CrossRef]
- Huckaby, L.V.; Sultan, I.; Trimarchi, S.; Leshnower, B.; Chen, E.P.; Brinster, D.R.; Myrmel, T.; Estrera, A.L.; Montgomery, D.G.; Korach, A.; et al. Sex-Based Aortic Dissection Outcomes From the International Registry of Acute Aortic Dissection. Ann. Thorac. Surg. 2022, 113, 498–505. [Google Scholar] [CrossRef]
- Davies, R.R.; Goldstein, L.J.; Coady, A.M.; Tittle, S.L.; Rizzo, A.J.; Kopf, G.S.; Elefteriades, J.A. Yearly rupture or dissection rates for thoracic aortic aneurysms: Simple prediction based on size. Ann. Thorac. Surg. 2002, 73, 17–28. [Google Scholar] [CrossRef]
- Nienaber, C.A.; Fattori, R.; Mehta, R.H.; Richartz, B.M.; Evangelista, A.; Petzsch, M.; Cooper, J.V.; Januzzi, J.L.; Ince, H.; Sechtem, U.; et al. Gender-Related Differences in Acute Aortic Dissection. Circulation 2004, 109, 3014–3021. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.; Boodhwani, M.; Chan, K.L.; Beauchesne, L.; Dick, A.; Coutinho, T. Thoracic Aortic Aneurysm Growth: Role of Sex and Aneurysm Etiology. J. Am. Heart Assoc. 2017, 6, e003792. [Google Scholar] [CrossRef] [PubMed]
- Galis, Z.S.; Khatri, J.J. Matrix Metalloproteinases in Vascular Remodeling and Atherogenesis: The Good, the Bad, and the Ugly. Circ. Res. 2002, 90, 251–262. [Google Scholar] [CrossRef]
- Sokolis, D.P.; Iliopoulos, D.C. Impaired mechanics and matrix metalloproteinases/inhibitors expression in female ascending thoracic aortic aneurysms. J. Mech. Behav. Biomed. Mater. 2014, 34, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Chew, D.K.W.; Conte, M.S.; Khalil, R.A. Matrix metalloproteinase-specific inhibition of Ca2+ entry mechanisms of vascular contraction. J. Vasc. Surg. 2004, 40, 1001–1010. [Google Scholar] [CrossRef]
- Waddell, T.K.; Dart, A.M.; Gatzka, C.D.; Cameron, J.D.; Kingwell, B.A. Women exhibit a greater age-related increase in proximal aortic stiffness than men. J. Hypertens. 2001, 19, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Koullias, G.J.; Ravichandran, P.; Korkolis, D.P.; Rimm, D.L.; Elefteriades, J.A. Increased Tissue Microarray Matrix Metalloproteinase Expression Favors Proteolysis in Thoracic Aortic Aneurysms and Dissections. Ann. Thorac. Surg. 2004, 78, 2106–2110. [Google Scholar] [CrossRef]
- Landenhed, M.; Engström, G.; Gottsäter, A.; Caulfield, M.P.; Hedblad, B.; Newton-Cheh, C.; Melander, O.; Smith, J.G. Risk Profiles for Aortic Dissection and Ruptured or Surgically Treated Aneurysms: A Prospective Cohort Study. J. Am. Heart Assoc. 2015, 4, e001513. [Google Scholar] [CrossRef]
- Saliba, E.; Sia, Y.; Dore, A.; El Hamamsy, I. The ascending aortic aneurysm: When to intervene? IJC Heart Vasc. 2015, 6, 91–100. [Google Scholar] [CrossRef]
- Groenink, M. The influence of aging and aortic stiffness on permanent dilation and breaking stress of the thoracic descending aorta. Cardiovasc. Res. 1999, 43, 471–480. [Google Scholar] [CrossRef]
- Mathur, A.; Mohan, V.; Ameta, D.; Gaurav, B.; Haranahalli, P. Aortic aneurysm. J. Transl. Intern. Med. 2016, 4, 35–41. [Google Scholar] [CrossRef]
- Caglayan, A.O.; Dundar, M. Inherited diseases and syndromes leading to aortic aneurysms and dissections. Eur. J. Cardiothorac. Surg. 2009, 35, 931–940. [Google Scholar] [CrossRef]
- Meester, J.A.N.; Verstraeten, A.; Schepers, D.; Alaerts, M.; Van Laer, L.; Loeys, B.L. Differences in manifestations of Marfan syndrome, Ehlers-Danlos syndrome, and Loeys-Dietz syndrome. Ann. Cardiothorac. Surg. 2017, 6, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Bradley, T.J.; Bowdin, S.C.; Morel CF, J.; Pyeritz, R.E. The Expanding Clinical Spectrum of Extracardiovascular and Cardiovascular Manifestations of Heritable Thoracic Aortic Aneurysm and Dissection. Can. J. Cardiol. 2016, 32, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.W.Y.; Yang, H.H.C.; Radomski, M.W.; Van Breemen, C. Long-Term Doxycycline Is More Effective Than Atenolol to Prevent Thoracic Aortic Aneurysm in Marfan Syndrome Through the Inhibition of Matrix Metalloproteinase-2 and -9. Circ. Res. 2008, 102, 73–85. [Google Scholar] [CrossRef]
- Robinson, P.N.; Arteaga-Solis, E.; Baldock, C.; Collod-Beroud, G.; Booms, P.; De Paepe, A.; Dietz, H.C.; Guo, G.; Handford, A.P.; Judge, D.P.; et al. The molecular genetics of Marfan syndrome and related disorders. J. Med. Genet. 2006, 43, 769–787. [Google Scholar] [CrossRef]
- Maeda, J.; Kosaki, K.; Shiono, J.; Kouno, K.; Aeba, R.; Yamagishi, H. Variable severity of cardiovascular phenotypes in patients with an early-onset form of Marfan syndrome harboring FBN1 mutations in exons 24–32. Heart Vessels 2016, 31, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Carande, E.J.; Bilton, S.J.; Adwani, S. A Case of Neonatal Marfan Syndrome: A Management Conundrum and the Role of a Multidisciplinary Team. Case Rep. Pediatr. 2017, 2017, 8952428. [Google Scholar] [CrossRef] [PubMed]
- Porciani, M.C.; Attanasio, M.; Lepri, V.; Lapini, I.; Demarchi, G.; Padeletti, L.; Pepe, G.; Abbate, R.; Gensini, G.F. Prevalence of cardiovascular manifestations in Marfan syndrome. Ital. Heart J. Suppl. Off. J. Ital. Fed. Cardiol. 2004, 5, 647–652. [Google Scholar]
- Krause, K.J. Marfan syndrome: Literature review of mortality studies. J. Insur. Med. N. Y. 2000, 32, 79–88. [Google Scholar]
- Chan, Y.; Ting, C.; Ho, P.; Poon, J.; Cheung, G.; Cheng, S. Ten-Year Epidemiological Review of In-Hospital Patients with Marfan Syndrome. Ann. Vasc. Surg. 2008, 22, 608–612. [Google Scholar] [CrossRef]
- Shores, J.; Berger, K.R.; Murphy, E.A.; Pyeritz, R.E. Progression of Aortic Dilatation and the Benefit of Long-Term β-Adrenergic Blockade in Marfan’s Syndrome. N. Engl. J. Med. 1994, 330, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Rossi-Foulkes, R.; Roman, M.J.; Rosen, E.S.; Kramer-Fox, R.; Ehlers, K.H.; O’loughlin, J.E.; Davis, J.G.; Devereux, R.B. Phenotypic features and impact of Beta blocker or calcium antagonist therapy on Aortic lumen size in the Marfan syndrome. Am. J. Cardiol. 1999, 83, 1364–1368. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, A.; Spata, E.; Emberson, J.; Davies, K.; Halls, H.; Holland, L.; Wilson, K.; Reith, C.; Child, A.H.; Clayton, T.; et al. Angiotensin receptor blockers and β blockers in Marfan syndrome: An individual patient data meta-analysis of randomised trials. Lancet 2022, 400, 822–831. [Google Scholar] [CrossRef]
- Groenink, M.; Hartog, A.W.D.; Franken, R.; Radonic, T.; de Waard, V.; Timmermans, J.; Scholte, A.J.; Berg, M.P.v.D.; Spijkerboer, A.M.; Marquering, H.A.; et al. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: A randomized controlled trial. Eur. Heart J. 2013, 34, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Forteza, A.; Evangelista, A.; Sánchez, V.; Teixidó-Turà, G.; Sanz, P.; Gutiérrez, L.; Gracia, T.; Centeno, J.; Rodríguez-Palomares, J.; Rufilanchas, J.J.; et al. Efficacy of losartan vs. atenolol for the prevention of aortic dilation in Marfan syndrome: A randomized clinical trial. Eur. Heart J. 2016, 37, 978–985. [Google Scholar] [CrossRef]
- Chiu, H.H.; Wu, M.H.; Wang, J.K.; Lu, C.W.; Chiu, S.N.; Chen, C.A.; Lin, M.T.; Hu, F.C. Losartan Added to β-Blockade Therapy for Aortic Root Dilation in Marfan Syndrome: A Randomized, Open-Label Pilot Study. Mayo Clin. Proc. 2013, 88, 271–276. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Iii, J.H.B.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease. J. Am. Coll. Cardiol. 2022, 80, e223–e393. [Google Scholar] [CrossRef]
- Milewicz, D.M.; Dietz, H.C.; Miller, D.C. Treatment of Aortic Disease in Patients with Marfan Syndrome. Circulation 2005, 111, e150–e157. [Google Scholar] [CrossRef]
- Zeigler, S.M.; Sloan, B.; Jones, J.A. Pathophysiology and Pathogenesis of Marfan Syndrome. In Progress in Heritable Soft Connective Tissue Diseases; Halper, J., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; Volume 1348, pp. 185–206. [Google Scholar]
- Vinholo, T.F.; Brownstein, A.J.; Ziganshin, B.A.; Zafar, M.A.; Kuivaniemi, H.; Body, S.C.; Bale, A.E.; Elefteriades, J.A. Genes Associated with Thoracic Aortic Aneurysm and Dissection: 2019 Update and Clinical Implications. AORTA 2019, 7, 99–107. [Google Scholar]
- MacCarrick, G.; Black, J.H.; Bowdin, S.; El-Hamamsy, I.; Frischmeyer-Guerrerio, P.A.; Guerrerio, A.L.; Sponseller, P.D.; Loeys, B.; Dietz, H.C. Loeys–Dietz syndrome: A primer for diagnosis and management. Genet. Med. 2014, 16, 576–587. [Google Scholar] [CrossRef]
- Nickol, J.L.; Malik, S.A.; Yetman, A.T. Case report of Loeys-Dietz syndrome presenting with coronary artery aneurysm. Eur. Heart J. Case Rep. 2022, 6, ytac383. [Google Scholar] [CrossRef] [PubMed]
- Fattori, R.; Sangiorgio, P.; Mariucci, E.; Ritelli, M.; Wischmeijer, A.; Greco, C.; Colombi, M. Spontaneous coronary artery dissection in a young woman with Loeys-Dietz syndrome. Am. J. Med. Genet. A 2012, 158A, 1216–1218. [Google Scholar] [CrossRef]
- Cury, M.; Zeidan, F.; Lobato, A.C. Aortic Disease in the Young: Genetic Aneurysm Syndromes, Connective Tissue Disorders, and Familial Aortic Aneurysms and Dissections. Int. J. Vasc. Med. 2013, 2013, 267215. [Google Scholar] [CrossRef] [PubMed]
- Loeys, B.L.; Schwarze, U.; Holm, T.; Callewaert, B.L.; Thomas, G.H.; Pannu, H.; De Backer, J.F.; Oswald, G.L.; Symoens, S.; Manouvrier, S.; et al. Aneurysm Syndromes Caused by Mutations in the TGF-β Receptor. N. Engl. J. Med. 2006, 355, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Gouda, P.; Kay, R.; Habib, M.; Aziz, A.; Aziza, E.; Welsh, R. Clinical features and complications of Loeys-Dietz syndrome: A systematic review. Int. J. Cardiol. 2022, 362, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Erbel, R.; Germany, C.; Aboyans, V.; France, C.; France, C.B. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar]
- Writing Group Members; Hiratzka, L.F.; Bakris, G.L.; Beckman, J.A.; Bersin, R.M.; Carr, V.F.; Casey, D.E., Jr.; Eagle, K.A.; Hermann, L.K.; Isselbacher, E.M.; et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients with Thoracic Aortic Disease: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010, 121, e266–e3369. [Google Scholar]
- Appoo, J.J.; Bozinovski, J.; Chu, M.W.; El-Hamamsy, I.; Forbes, T.L.; Moon, M.; Ouzounian, M.; Peterson, M.D.; Tittley, J.; Boodhwani, M. Canadian Cardiovascular Society/Canadian Society of Cardiac Surgeons/Canadian Society for Vascular Surgery Joint Position Statement on Open and Endovascular Surgery for Thoracic Aortic Disease. Can. J. Cardiol. 2016, 32, 703–713. [Google Scholar] [CrossRef][Green Version]
- Boodhwani, M.; Andelfinger, G.; Leipsic, J.; Lindsay, T.; McMurtry, M.S.; Therrien, J.; Siu, S.C. Canadian Cardiovascular Society Position Statement on the Management of Thoracic Aortic Disease. Can. J. Cardiol. 2014, 30, 577–589. [Google Scholar] [CrossRef]
- Eagleton, M.J. Arterial complications of vascular Ehlers-Danlos syndrome. J. Vasc. Surg. 2016, 64, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Kuivaniemi, H.; Tromp, G. Type III collagen (COL3A1): Gene and protein structure, tissue distribution, and associated diseases. Gene 2019, 707, 151–171. [Google Scholar] [CrossRef]
- Germain, D.P. Ehlers-Danlos syndrome type IV. Orphanet J. Rare Dis. 2007, 2, 32. [Google Scholar] [CrossRef]
- Pepin, M.; Schwarze, U.; Superti-Furga, A.; Byers, P.H. Clinical and Genetic Features of Ehlers–Danlos Syndrome Type IV, the Vascular Type. N. Engl. J. Med. 2000, 342, 673–680. [Google Scholar] [CrossRef]
- Ong, K.-T.; Perdu, J.; De Backer, J.; Bozec, E.; Collignon, P.; Emmerich, J.; Fauret, A.-L.; Fiessinger, J.-N.; Germain, D.P.; Georgesco, G.; et al. Effect of celiprolol on prevention of cardiovascular events in vascular Ehlers-Danlos syndrome: A prospective randomised, open, blinded-endpoints trial. Lancet 2010, 376, 1476–1484. [Google Scholar] [CrossRef]
- Bowen, J.M.; Hernandez, M.; Johnson, D.S.; Green, C.; Kammin, T.; Baker, D.; Keigwin, S.; Makino, S.; Taylor, N.; Watson, O.; et al. Diagnosis and management of vascular Ehlers-Danlos syndrome: Experience of the UK national diagnostic service, Sheffield. Eur. J. Hum. Genet. 2023, 31, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Byers, P.H.; Belmont, J.; Black, J.; De Backer, J.; Frank, M.; Jeunemaitre, X.; Johnson, D.; Pepin, M.; Robert, L.; Sanders, L.; et al. Diagnosis, natural history, and management in vascular Ehlers-Danlos syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 40–47. [Google Scholar] [CrossRef]
- Lee, C.; Tully, A.; Fang, J.C.; Sugeng, L.; Elmariah, S.; Grubb, K.J.; Young, M.N. Building and Optimizing the Interdisciplinary Heart Team. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 101067. [Google Scholar] [CrossRef]
- Batchelor, W.B.; Anwaruddin, S.; Wang, D.D.; Perpetua, E.M.; Krishnaswami, A.; Velagapudi, P.; Wyman, J.F.; Fullerton, D.; Keegan, P.; Phillips, A.; et al. The Multidisciplinary Heart Team in Cardiovascular Medicine. JACC Adv. 2023, 2, 100160. [Google Scholar] [CrossRef]
- Takeda, N.; Komuro, I. Genetic basis of hereditary thoracic aortic aneurysms and dissections. J. Cardiol. 2019, 74, 136–143. [Google Scholar] [CrossRef]
- Wang, L.; Guo, D.-C.; Cao, J.; Gong, L.; Kamm, K.E.; Regalado, E.; Li, L.; Shete, S.; He, W.-Q.; Zhu, M.-S.; et al. Mutations in Myosin Light Chain Kinase Cause Familial Aortic Dissections. Am. J. Hum. Genet. 2010, 87, 701–707. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Peng, Y.; Zhang, W.; Lv, N.; Tang, J.; Chen, C.; Zhang, C.; Gao, S.; Chen, H.; Zhi, G.; et al. Myosin Light Chain Kinase Is Central to Smooth Muscle Contraction and Required for Gastrointestinal Motility in Mice. Gastroenterology 2008, 135, 610–620.e2. [Google Scholar] [CrossRef] [PubMed]
- Vinholo, T.F.; Brownstein, A.J.; Ziganshin, B.A.; Zafar, M.A.; Kuivaniemi, H.; Body, S.C.; Bale, A.E.; Elefteriades, J.A. Genes Associated with Thoracic Aortic Aneurysm and Dissection: An Update and Clinical Implications. AORTA 2017, 5, 11–20. [Google Scholar]
- Guo, D.-C.; Papke, C.L.; Tran-Fadulu, V.; Regalado, E.S.; Avidan, N.; Johnson, R.J.; Kim, D.H.; Pannu, H.; Willing, M.C.; Sparks, E.; et al. Mutations in Smooth Muscle Alpha-Actin (ACTA2) Cause Coronary Artery Disease, Stroke, and Moyamoya Disease, Along with Thoracic Aortic Disease. Am. J. Hum. Genet. 2009, 84, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.-C.; Pannu, H.; Tran-Fadulu, V.; Papke, C.L.; Yu, R.K.; Avidan, N.; Bourgeois, S.; Estrera, A.L.; Safi, H.J.; Sparks, E.; et al. Mutations in smooth muscle α-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat. Genet. 2007, 39, 1488–1493. [Google Scholar] [CrossRef]
- Regalado, E.S.; Guo, D.-C.; Prakash, S.; Bensend, T.A.; Flynn, K.; Estrera, A.; Safi, H.; Liang, D.; Hyland, J.; Child, A.; et al. Aortic Disease Presentation and Outcome Associated with ACTA2 Mutations. Circ. Cardiovasc. Genet. 2015, 8, 457–464. [Google Scholar] [CrossRef]
- Zhu, L.; Vranckx, R.; Van Kien, P.K.; Lalande, A.; Boisset, N.; Mathieu, F.; Wegman, M.; Glancy, L.; Gasc, J.-M.; Brunotte, F.; et al. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat. Genet. 2006, 38, 343–349. [Google Scholar] [CrossRef]
- Allaire, E.; Schneider, F.; Saucy, F.; Dai, J.; Cochennec, F.; Michineau, S.; Zidi, M.; Becquemin, J.-P.; Kirsch, M.; Gervais, M. New Insight in Aetiopathogenesis of Aortic Diseases. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 531–537. [Google Scholar] [CrossRef]
- Guo, D.-C.; Regalado, E.; Casteel, D.E.; Santos-Cortez, R.L.; Gong, L.; Kim, J.J.; Dyack, S.; Horne, S.G.; Chang, G.; Jondeau, G.; et al. Recurrent Gain-of-Function Mutation in PRKG1 Causes Thoracic Aortic Aneurysms and Acute Aortic Dissections. Am. J. Hum. Genet. 2013, 93, 398–404. [Google Scholar] [CrossRef]
- Albornoz, G.; Coady, M.A.; Roberts, M.; Davies, R.R.; Tranquilli, M.; Rizzo, J.A.; Elefteriades, J.A. Familial Thoracic Aortic Aneurysms and Dissections—Incidence, Modes of Inheritance, and Phenotypic Patterns. Ann. Thorac. Surg. 2006, 82, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.-G.; Chou, A.S.; Mok, S.C.; Ziganshin, B.A.; Charilaou, P.; Zafar, M.A.; Sieller, R.S.; Tranquilli, M.; Rizzo, J.A.; Elefteriades, J.A. Positive family history of aortic dissection dramatically increases dissection risk in family members. Int. J. Cardiol. 2017, 240, 132–137. [Google Scholar] [CrossRef]
- Biddinger, A.; Rocklin, M.; Coselli, J.; Milewicz, D.M. Familial thoracic aortic dilatations and dissections: A case control study. J. Vasc. Surg. 1997, 25, 506–511. [Google Scholar] [CrossRef]
- Robertson, E.N.; van der Linde, D.; Sherrah, A.G.; Vallely, M.P.; Wilson, M.; Bannon, P.G.; Jeremy, R.W. Familial non-syndromal thoracic aortic aneurysms and dissections—Incidence and family screening outcomes. Int. J. Cardiol. 2016, 220, 43–51. [Google Scholar] [CrossRef]
- Verhagen, J.M.; Kempers, M.; Cozijnsen, L.; Bouma, B.J.; Duijnhouwer, A.L.; Post, J.G.; Hilhorst-Hofstee, Y.; Bekkers, S.C.; Kerstjens-Frederikse, W.S.; van Brakel, T.J.; et al. Expert consensus recommendations on the cardiogenetic care for patients with thoracic aortic disease and their first-degree relatives. Int. J. Cardiol. 2018, 258, 243–248. [Google Scholar] [CrossRef]
- Weinsaft, J.W.; Devereux, R.B.; Preiss, L.R.; Feher, A.; Roman, M.J.; Basson, C.T.; Geevarghese, A.; Ravekes, W.; Dietz, H.C.; Holmes, K.; et al. Aortic Dissection in Patients with Genetically Mediated Aneurysms. J. Am. Coll. Cardiol. 2016, 67, 2744–2754. [Google Scholar] [CrossRef] [PubMed]
- Saeyeldin, A.; Zafar, M.A.; Li, Y.; Tanweer, M.; Abdelbaky, M.; Gryaznov, A.; Brownstein, A.J.; Velasquez, C.A.; Buntin, J.; Thombre, K.; et al. Decision-making algorithm for ascending aortic aneurysm: Effectiveness in clinical application? J. Thorac. Cardiovasc. Surg. 2019, 157, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Hinton, R.B. Bicuspid Aortic Valve and Thoracic Aortic Aneurysm: Three Patient Populations, Two Disease Phenotypes, and One Shared Genotype. Cardiol. Res. Pract. 2012, 2012, 926975. [Google Scholar] [CrossRef] [PubMed]
- Borger, M.A.; Fedak, P.W.; Stephens, E.H.; Gleason, T.G.; Girdauskas, E.; Ikonomidis, J.S.; Khoynezhad, A.; Siu, S.C.; Verma, S.; Hope, M.D.; et al. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve–related aortopathy: Full online-only version. J. Thorac. Cardiovasc. Surg. 2018, 156, e41–e74. [Google Scholar] [CrossRef] [PubMed]
- Michelena, H.I.; Prakash, S.K.; Della Corte, A.; Bissell, M.M.; Anavekar, N.; Mathieu, P.; Bossé, Y.; Limongelli, G.; Bossone, E.; Benson, D.W.; et al. Bicuspid Aortic Valve: Identifying Knowledge Gaps and Rising to the Challenge From the International Bicuspid Aortic Valve Consortium (BAVCon). Circulation 2014, 129, 2691–2704. [Google Scholar] [CrossRef] [PubMed]
- Yassine, N.M.; Shahram, J.T.; Body, S.C. Pathogenic Mechanisms of Bicuspid Aortic Valve Aortopathy. Front. Physiol. 2017, 8, 687. [Google Scholar] [CrossRef]
- Cotrufo, M.; Della Corte, A.; De Santo, L.S.; Quarto, C.; De Feo, M.; Romano, G.; Amarelli, C.; Scardone, M.; Di Meglio, F.; Guerra, G.; et al. Different patterns of extracellular matrix protein expression in the convexity and the concavity of the dilated aorta with bicuspid aortic valve: Preliminary results. J. Thorac. Cardiovasc. Surg. 2005, 130, 504.e1–504.e9. [Google Scholar] [CrossRef]
- Michelena, H.I.; Khanna, A.D.; Mahoney, D.; Margaryan, E.; Topilsky, Y.; Suri, R.M.; Eidem, B.; Edwards, W.D.; Sundt, T.M.; Enriquez-Sarano, M. Incidence of Aortic Complications in Patients with Bicuspid Aortic Valves. JAMA 2011, 306, 1104. [Google Scholar] [CrossRef]
- Michelena, H.I.; Desjardins, V.A.; Avierinos, J.F.; Russo, A.; Nkomo, V.T.; Sundt, T.M.; Pellikka, P.A.; Tajik, A.J.; Enriquez-Sarano, M. Natural History of Asymptomatic Patients with Normally Functioning or Minimally Dysfunctional Bicuspid Aortic Valve in the Community. Circulation 2008, 117, 2776–2784. [Google Scholar] [CrossRef] [PubMed]
- Tzemos, N. Outcomes in Adults with Bicuspid Aortic Valves. JAMA 2008, 300, 1317. [Google Scholar] [CrossRef] [PubMed]
- Siu, S.C.; Silversides, C.K. Bicuspid Aortic Valve Disease. J. Am. Coll. Cardiol. 2010, 55, 2789–2800. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, S.W. Accentuating and Opposing Factors Leading to Development of Thoracic Aortic Aneurysms Not Due to Genetic or Inherited Conditions. Front. Cardiovasc. Med. 2015, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Danyi, P.; Elefteriades, J.A.; Jovin, I.S. Medical therapy of thoracic aortic aneurysms. Trends Cardiovasc. Med. 2012, 22, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Oguri, M.; Kato, N.; Hibino, T.; Yajima, K.; Yoshida, T.; Metoki, N.; Yoshida, H.; Satoh, K.; Watanabe, S.; et al. Assessment of Genetic Risk Factors for Thoracic Aortic Aneurysm in Hypertensive Patients. Am. J. Hypertens. 2008, 21, 1023–1027. [Google Scholar] [CrossRef]
- Daugherty, A.; Rateri, D.L.; Charo, I.F.; Owens, A.P.; Howatt, D.A.; Cassis, L.A. Angiotensin II infusion promotes ascending aortic aneurysms: Attenuation by CCR2 deficiency in apoE−/− mice. Clin. Sci. 2010, 118, 681–689. [Google Scholar] [CrossRef]
- Kanematsu, Y.; Kanematsu, M.; Kurihara, C.; Tsou, T.L.; Nuki, Y.; Liang, E.I.; Makino, H.; Hashimoto, T. Pharmacologically Induced Thoracic and Abdominal Aortic Aneurysms in Mice. Hypertension 2010, 55, 1267–1274. [Google Scholar] [CrossRef]
- Kallianos, K.G.; Burris, N.S. Imaging Thoracic Aortic Aneurysm. Radiol. Clin. N.Am. 2020, 58, 721–731. [Google Scholar] [CrossRef]
- Ho, D.; Squelch, A.; Sun, Z. Modelling of aortic aneurysm and aortic dissection through 3D printing. J. Med. Radiat. Sci. 2017, 64, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Bhave, N.M.; Nienaber, C.A.; Clough, R.E.; Eagle, K.A. Multimodality Imaging of Thoracic Aortic Diseases in Adults. JACC Cardiovasc. Imaging 2018, 11, 902–919. [Google Scholar] [CrossRef]
- Kato, K.; Nishio, A.; Kato, N.; Usami, H.; Fujimaki, T.; Murohara, T. Uptake of 18 F-FDG in Acute Aortic Dissection: A Determinant of Unfavorable Outcome. J. Nucl. Med. 2010, 51, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Almarzooq, Z.; Salata, B.; Devereux, R.B. Role of molecular imaging with positron emission tomographic in aortic aneurysms. J. Thorac. Dis. 2017, 9, S333–S342. [Google Scholar] [CrossRef] [PubMed]
- Hyafil, F.; Cornily, J.-C.; Rudd, J.H.; Machac, J.; Feldman, L.J.; Fayad, Z.A. Quantification of Inflammation within Rabbit Atherosclerotic Plaques Using the Macrophage-Specific CT Contrast Agent N1177: A Comparison with 18 F-FDG PET/CT and Histology. J. Nucl. Med. 2009, 50, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Gallo, D.; De Santis, G.; Negri, F.; Tresoldi, D.; Rizzo, G.; Ponzini, R.; Massai, D.; Deriu, M.; Cadioli, M.; Verhegghe, B.; et al. On the Use of In Vivo Measured Flow Rates as Boundary Conditions for Image-Based Hemodynamic Models of the Human Aorta. In Proceedings of the ASME 2011 Summer Bioengineering Conference, Parts A and B, Farmington, PA, USA, 22–25 June 2011; American Society of Mechanical Engineers: New York, NY, USA, 2011; pp. 15–16. [Google Scholar] [CrossRef]
- Morbiducci, U.; Ponzini, R.; Gallo, D.; Bignardi, C.; Rizzo, G. Inflow boundary conditions for image-based computational hemodynamics: Impact of idealized versus measured velocity profiles in the human aorta. J. Biomech. 2013, 46, 102–109. [Google Scholar] [CrossRef]
- Xuan, Y.; D’souza, S.N.; Wang, Z.; Pierre, A.S.; Lawton, J.S.; Ge, L.; Tseng, E.E. Patient-Specific Biomechanics in Marfan Ascending Thoracic Aortic Aneurysms. Ann. Thorac. Surg. 2022, 114, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Vignali, E.; Gasparotti, E.; Mariotti, A.; Haxhiademi, D.; Ait-Ali, L.; Celi, S. High-Versatility Left Ventricle Pump and Aortic Mock Circulatory Loop Development for Patient-Specific Hemodynamic In Vitro Analysis. ASAIO J. 2022, 68, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.; Benjamin, E.; Boutouyrie, P.; Cameron, J.; Chen, C.-H.; Cruickshank, J.K.; et al. Aortic Pulse Wave Velocity Improves Cardiovascular Event Prediction. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef]
- Grabenwöger, M.; Alfonso, F.; Bachet, J.; Bonser, R.; Czerny, M.; Eggebrecht, H.; Evangelista, A.; Fattori, R.; Jakob, H.; Lönn, L.; et al. Thoracic Endovascular Aortic Repair (TEVAR) for the treatment of aortic diseases: A position statement from the European Association for Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 2012, 33, 1558–1563. [Google Scholar]
- McCarthy, A.; Gray, J.; Sastry, P.; Sharples, L.; Vale, L.; Cook, A.; Mcmeekin, P.; Freeman, C.; Catarino, P.; Large, S. Systematic review of endovascular stent grafting versus open surgical repair for the elective treatment of arch/descending thoracic aortic aneurysms. BMJ Open 2021, 11, e043323. [Google Scholar] [CrossRef] [PubMed]
- Bavaria, J.E.; Appoo, J.J.; Makaroun, M.S.; Verter, J.; Yu, Z.-F.; Mitchell, R.S. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: A multicenter comparative trial. J. Thorac. Cardiovasc. Surg. 2007, 133, 369–377.e4. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-S.; Haider, S.; Makaroun, M.S. Endovascular Therapy of Thoracic Aneurysms: Gore TAG Trial Results. Semin. Vasc. Surg. 2006, 19, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-S.; Haider, S.; Makaroun, M.S. US multicenter trials of endoprostheses for the endovascular treatment of descending thoracic aneurysms. J. Vasc. Surg. 2006, 43, A12–A19. [Google Scholar] [CrossRef][Green Version]
- Go, M.R.; Cho, J.-S.; Makaroun, M.S. Mid-term Results of a Multicenter Study of Thoracic Endovascular Aneurysm Repair Versus Open Repair. Perspect. Vasc. Surg. Endovasc. Ther. 2007, 19, 124–130. [Google Scholar] [CrossRef]
- Von Allmen, R.S.; Anjum, A.; Powell, J.T. Outcomes after endovascular or open repair for degenerative descending thoracic aortic aneurysm using linked hospital data. Br. J. Surg. 2014, 101, 1244–1251. [Google Scholar] [CrossRef]
- Goodney, P.P.; Travis, L.; Lucas, F.L.; Fillinger, M.F.; Goodman, D.C.; Cronenwett, J.L.; Stone, D.H. Survival After Open Versus Endovascular Thoracic Aortic Aneurysm Repair in an Observational Study of the Medicare Population. Circulation 2011, 124, 2661–2669. [Google Scholar] [CrossRef]
- Mori, M.; Shioda, K.; Wang, X.; Mangi, A.A.; Yun, J.J.; Darr, U.; Elefteriades, J.A.; Geirsson, A. Perioperative Risk Profiles and Volume-Outcome Relationships in Proximal Thoracic Aortic Surgery. Ann. Thorac. Surg. 2018, 106, 1095–1104. [Google Scholar] [CrossRef]
- Writing Group Members; Hiratzka, L.F.; Bakris, G.L.; Beckman, J.A.; Bersin, R.M.; Carr, V.F.; Casey, D.E., Jr.; Eagle, K.A.; Hermann, L.K.; Isselbacher, E.M.; et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients with Thoracic Aortic Disease. J. Am. Coll. Cardiol. 2010, 55, e27–e129. [Google Scholar] [CrossRef]
- Guo, M.H.; Appoo, J.J.; Saczkowski, R.; Smith, H.N.; Ouzounian, M.; Gregory, A.J.; Herget, E.J.; Boodhwani, M. Association of Mortality and Acute Aortic Events with Ascending Aortic Aneurysm: A Systematic Review and Meta-analysis. JAMA Netw. Open 2018, 1, e181281. [Google Scholar] [CrossRef] [PubMed]
- Ashton, H.A.; Buxton, M.J.; Day, N.E.; Kim, L.G.; Marteau, E.M.; Scott, R.A.P.; Thompson, S.G.; Walker, N.M. Multicentre Aneurysm Screening Study Group. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: A randomised controlled trial. Lancet 2002, 360, 1531–1539. [Google Scholar] [PubMed]
- Lederle, F.A.; Wilson, S.E.; Johnson, G.R.; Reinke, D.B.; Littooy, F.N.; Acher, C.W.; Ballard, D.J.; Messina, L.M.; Gordon, I.L.; Chute, E.P.; et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N. Engl. J. Med. 2002, 346, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.H.; Appoo, J.J.; Wells, G.A.; Chu, M.; Ouzounian, M.; Fortier, J.; Boodhwani, M. Protocol for a randomised controlled trial for Treatment in Thoracic Aortic Aneurysm: Surgery versus Surveillance (TITAN: SvS). BMJ Open 2021, 11, e052070. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).