Brugada Syndrome within Asian Populations: State-of-the-Art Review
Abstract
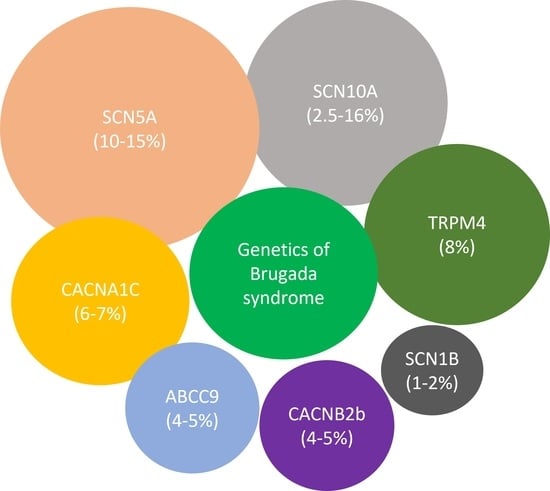
1. Introduction
2. Diagnostic and Risk Stratification Criteria
3. Prevalence of Brugada Syndrome in Asia
3.1. East Asia
3.2. Southeast Asia
3.3. South Asia and the Middle East
4. Pediatric and Young Populations
5. Elderly Population
6. Fever-Induced Brugada Electrocardiogram Pattern
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brugada, P.; Brugada, J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: A distinct clinical and electrocardiographic syndrome. A multicenter report. J. Am. Coll. Cardiol. 1992, 20, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Pappone, C.; Santinelli, V. Brugada Syndrome: Progress in Diagnosis and Management. Arrhythm. Electrophysiol. Rev. 2019, 8, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Kim, R.; Udupa, S.; Costain, G.; Jobling, R.; Liston, E.; Jamal, S.M.; Szybowska, M.; Morel, C.F.; Bowdin, S.; et al. Reappraisal of Reported Genes for Sudden Arrhythmic Death: Evidence-Based Evaluation of Gene Validity for Brugada Syndrome. Circulation 2018, 138, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Sarquella-Brugada, G.; Campuzano, O.; Arbelo, E.; Brugada, J.; Brugada, R. Brugada syndrome: Clinical and genetic findings. Genet. Med. 2016, 18, 3–12. [Google Scholar] [CrossRef]
- Mizusawa, Y.; Wilde, A.A. Brugada syndrome. Circ. Arrhythm. Electrophysiol. 2012, 5, 606–616. [Google Scholar] [CrossRef]
- Steinfurt, J.; Biermann, J.; Bode, C.; Odening, K.E. The Diagnosis, Risk Stratification, and Treatment of Brugada Syndrome. Dtsch. Ärzteblatt Int. 2015, 112, 394–401. [Google Scholar] [CrossRef]
- Vutthikraivit, W.; Rattanawong, P.; Putthapiban, P.; Sukhumthammarat, W.; Vathesatogkit, P.; Ngarmukos, T.; Thakkinstian, A. Worldwide Prevalence of Brugada Syndrome: A Systematic Review and Meta-Analysis. Acta Cardiol. Sin. 2018, 34, 267–277. [Google Scholar] [CrossRef]
- Antzelevitch, C.; Yan, G.X.; Ackerman, M.J.; Borggrefe, M.; Corrado, D.; Guo, J.; Gussak, I.; Hasdemir, C.; Horie, M.; Huikuri, H.; et al. J-Wave syndromes expert consensus conference report: Emerging concepts and gaps in knowledge. J. Arrhythm. 2016, 32, 315–339. [Google Scholar] [CrossRef]
- Surawicz, B. Brugada syndrome: Manifest, concealed, “asymptomatic,” suspected and simulated. J. Am. Coll. Cardiol. 2001, 38, 775–777. [Google Scholar] [CrossRef]
- Wilde, A.A.; Antzelevitch, C.; Borggrefe, M.; Brugada, J.; Brugada, R.; Brugada, P.; Corrado, D.; Hauer, R.N.; Kass, R.S.; Nademanee, K.; et al. Proposed diagnostic criteria for the Brugada syndrome: Consensus report. Circulation 2002, 106, 2514–2519. [Google Scholar] [CrossRef]
- Antzelevitch, C.; Brugada, P.; Borggrefe, M.; Brugada, J.; Brugada, R.; Corrado, D.; Gussak, I.; LeMarec, H.; Nademanee, K.; Perez Riera, A.R.; et al. Brugada syndrome: Report of the second consensus conference: Endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 2005, 111, 659–670. [Google Scholar] [CrossRef]
- Priori, S.G.; Wilde, A.A.; Horie, M.; Cho, Y.; Behr, E.R.; Berul, C.; Blom, N.; Brugada, J.; Chiang, C.E.; Huikuri, H.; et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: Document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013, 10, 1932–1963. [Google Scholar] [CrossRef]
- Priori, S.G.; Blomstrom-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Priori, S.G.; Gasparini, M.; Napolitano, C.; Della Bella, P.; Ottonelli, A.G.; Sassone, B.; Giordano, U.; Pappone, C.; Mascioli, G.; Rossetti, G.; et al. Risk stratification in Brugada syndrome: Results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) registry. J. Am. Coll. Cardiol. 2012, 59, 37–45. [Google Scholar] [CrossRef]
- Sroubek, J.; Probst, V.; Mazzanti, A.; Delise, P.; Hevia, J.C.; Ohkubo, K.; Zorzi, A.; Champagne, J.; Kostopoulou, A.; Yin, X.; et al. Programmed Ventricular Stimulation for Risk Stratification in the Brugada Syndrome: A Pooled Analysis. Circulation 2016, 133, 622–630. [Google Scholar] [CrossRef]
- Sieira, J.; Conte, G.; Ciconte, G.; Chierchia, G.B.; Casado-Arroyo, R.; Baltogiannis, G.; Di Giovanni, G.; Saitoh, Y.; Julia, J.; Mugnai, G.; et al. A score model to predict risk of events in patients with Brugada Syndrome. Eur. Heart J. 2017, 38, 1756–1763. [Google Scholar] [CrossRef]
- Probst, V.; Goronflot, T.; Anys, S.; Tixier, R.; Briand, J.; Berthome, P.; Geoffroy, O.; Clementy, N.; Mansourati, J.; Jesel, L.; et al. Robustness and relevance of predictive score in sudden cardiac death for patients with Brugada syndrome. Eur. Heart J. 2021, 42, 1687–1695. [Google Scholar] [CrossRef]
- Kawada, S.; Morita, H.; Antzelevitch, C.; Morimoto, Y.; Nakagawa, K.; Watanabe, A.; Nishii, N.; Nakamura, K.; Ito, H. Shanghai Score System for Diagnosis of Brugada Syndrome: Validation of the Score System and System and Reclassification of the Patients. JACC Clin. Electrophysiol. 2018, 4, 724–730. [Google Scholar] [CrossRef]
- Atarashi, H.; Ogawa, S.; Harumi, K.; Sugimoto, T.; Inoue, H.; Murayama, M.; Toyama, J.; Hayakawa, H. Three-year follow-up of patients with right bundle branch block and ST segment elevation in the right precordial leads. J. Am. Coll. Cardiol. 2001, 37, 1916–1920. [Google Scholar] [CrossRef]
- Erdogan, O.; Hunuk, B. Frequency of Brugada type ECG pattern in male subjects with fever. Int. J. Cardiol. 2013, 165, 562–563. [Google Scholar] [CrossRef] [PubMed]
- Gervacio-Domingo, G.; Isidro, J.; Tirona, J.; Gabriel, E.; David, G.; Amarillo, M.L.; Morales, D.; Dans, A. The Brugada type 1 electrocardiographic pattern is common among Filipinos. J. Clin. Epidemiol. 2008, 61, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Yano, K.; Chen, R.; He, Q.; Curb, J.D. The prevalence and prognosis of a Brugada-type electrocardiogram in a population of middle-aged Japanese-American men with follow-up of three decades. Am. J. Med. Sci. 2006, 331, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Makarawate, P.; Chaosuwannakit, N.; Ruamcharoen, Y.; Panthongviriyakul, A.; Pongchaiyakul, C.; Tharaksa, P.; Sripo, T.; Sawanyawisuth, K. Prevalence and associated factors of early repolarization pattern in healthy young northeastern Thai men: A correlation study with Brugada electrocardiography. J. Arrhythm. 2015, 31, 215–220. [Google Scholar] [CrossRef]
- Rattanawong, P.; Ngarmukos, T.; Chung, E.H.; Vutthikraivit, W.; Putthapiban, P.; Sukhumthammarat, W.; Vathesatogkit, P.; Sritara, P. Prevalence of Brugada ECG Pattern in Thailand From a Population-Based Cohort Study. J. Am. Coll. Cardiol. 2017, 69, 1355–1356. [Google Scholar] [CrossRef]
- Shen, X.; Tan, B.Y.Q.; Sia, C.H.; Lee, J.S.W.; Dalakoti, M.; Wang, K.; Lim, D.Y.Z.; Sng, G.G.R.; Lee, E.C.Y.; Chow, W.; et al. Prevalence of Brugada Syndrome in a Large Population of Young Singaporean Men. Circulation 2020, 141, 155–157. [Google Scholar] [CrossRef]
- Shin, S.C.; Ryu, H.M.; Lee, J.H.; Chang, B.J.; Shin, J.K.; Kim, H.S.; Heo, J.H.; Yang, D.H.; Park, H.S.; Cho, Y.; et al. Prevalence of the Brugada-type ECG recorded from higher intercostal spaces in healthy Korean males. Circ. J. 2005, 69, 1064–1067. [Google Scholar] [CrossRef]
- Uhm, J.S.; Hwang, I.U.; Oh, Y.S.; Choi, M.S.; Jang, S.W.; Shin, W.S.; Kim, J.H.; Lee, M.Y.; Rho, T.H.; Kim, Y.H.; et al. Prevalence of electrocardiographic findings suggestive of sudden cardiac death risk in 10,867 apparently healthy young Korean men. Pacing Clin. Electrophysiol. 2011, 34, 717–723. [Google Scholar] [CrossRef]
- Yoshinaga, M.; Anan, R.; Nomura, Y.; Tanaka, Y.; Tanaka, Y.; Sarantuya, J.; Oku, S.; Nishi, S.; Kawano, Y.; Tei, C.; et al. Prevalence and time of appearance of Brugada electrocardiographic pattern in young male adolescents from a three-year follow-up study. Am. J. Cardiol. 2004, 94, 1186–1189. [Google Scholar] [CrossRef]
- Chantapoh, W.; Rattanawong, P.; Ngamukos, T.; Simpson, R.J.; Thawepornpuripong, P.; Kunaporn, M.; Sritara, P.; Vathesatogkit, P.; Chung, E. Abstract 12273: High Prevalence of Brugada Syndrome Among Thai Migrant Workers in Israel. Circulation 2017, 136, A12273. [Google Scholar] [CrossRef]
- Juang, J.M.; Chen, C.Y.; Chen, Y.H.; Wu, I.C.; Hsu, C.C.; Chen, L.N.; Tang, F.C.; Wang, C.C.; Juan, C.C.; Chiu, H.C.; et al. Prevalence and prognosis of Brugada electrocardiogram patterns in an elderly Han Chinese population: A nation-wide community-based study (HALST cohort). Europace 2015, 17 (Suppl. 2), ii54–ii62. [Google Scholar] [CrossRef]
- Juang, J.M.; Phan, W.L.; Chen, P.C.; Lai, L.P.; Tsai, M.H.; Lin, J.W.; Cheng, P.H.; Chiu, W.Y.; Cheng, B.W.; Hwang, J.J.; et al. Brugada-type electrocardiogram in the Taiwanese population—Is it a risk factor for sudden death? J. Formos. Med. Assoc. 2011, 110, 230–238. [Google Scholar] [CrossRef]
- Tsuneoka, H.; Takagi, M.; Murakoshi, N.; Yamagishi, K.; Yokoyama, Y.; Xu, D.; Sekiguchi, Y.; Yamasaki, H.; Naruse, Y.; Ito, Y.; et al. Long-Term Prognosis of Brugada-Type ECG and ECG With Atypical ST-Segment Elevation in the Right Precordial Leads Over 20 Years: Results From the Circulatory Risk in Communities Study (CIRCS). J. Am. Heart Assoc. 2016, 5, e002899. [Google Scholar] [CrossRef]
- Tsuji, H.; Sato, T.; Morisaki, K.; Iwasaka, T. Prognosis of subjects with Brugada-type electrocardiogram in a population of middle-aged Japanese diagnosed during a health examination. Am. J. Cardiol. 2008, 102, 584–587. [Google Scholar] [CrossRef]
- Oe, H.; Takagi, M.; Tanaka, A.; Namba, M.; Nishibori, Y.; Nishida, Y.; Kawarabayashi, T.; Yoshiyama, M.; Nishimoto, M.; Tanaka, K.; et al. Prevalence and clinical course of the juveniles with Brugada-type ECG in Japanese population. Pacing Clin. Electrophysiol. 2005, 28, 549–554. [Google Scholar] [CrossRef]
- Yamakawa, Y.; Ishikawa, T.; Uchino, K.; Mochida, Y.; Ebina, T.; Sumita, S.; Kobayashi, T.; Matsushita, K.; Matsumoto, K.; Ohkusu, Y.; et al. Prevalence of right bundle-branch block and right precordial ST-segment elevation (Brugada-type electrocardiogram) in Japanese children. Circ. J. 2004, 68, 275–279. [Google Scholar] [CrossRef]
- Sakabe, M. Proportion and prognosis of healthy people with coved or saddle-back type ST segment elevation in the right precordial leads during 10 years follow-up. Eur. Heart J. 2003, 24, 1488–1493. [Google Scholar] [CrossRef]
- Miyasaka, Y.; Tsuji, H.; Yamada, K.; Tokunaga, S.; Saito, D.; Imuro, Y.; Matsumoto, N.; Iwasaka, T. Prevalence and mortality of the Brugada-type electrocardiogram in one city in Japan. J. Am. Coll. Cardiol. 2001, 38, 771–774. [Google Scholar] [CrossRef]
- Furuhashi, M.; Uno, K.; Tsuchihashi, K.; Nagahara, D.; Hyakukoku, M.; Ohtomo, T.; Satoh, S.; Nishimiya, T.; Shimamoto, K. Prevalence of asymptomatic ST segment elevation in right precordial leads with right bundle branch block (Brugada-type ST shift) among the general Japanese population. Heart 2001, 86, 161–166. [Google Scholar] [CrossRef]
- Rattanawong, P.; Ngarmukos, T.; Wisaratapong, T. Discovering Brugada syndrome during preoperative evaluation. J. Anesth. 2015, 29, 480. [Google Scholar] [CrossRef]
- Sidik, N.P.; Quay, C.N.; Loh, F.C.; Chen, L.Y. Prevalence of Brugada sign and syndrome in patients presenting with arrhythmic symptoms at a Heart Rhythm Clinic in Singapore. Europace 2009, 11, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Wajed, A.; Aslam, Z.; Abbas, S.F.; Irfan, M.; Bangash, K.; Rehman, S.; Amin, F. Frequency of Brugada-type ECG pattern (Brugada sign) in an apparently healthy young population. J. Ayub. Med. Coll. Abbottabad 2008, 20, 121–124. [Google Scholar]
- Bozkurt, A.; Yas, D.; Seydaoglu, G.; Acarturk, E. Frequency of Brugada-type ECG pattern (Brugada sign) in Southern Turkey. Int. Heart J. 2006, 47, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Bigi, M.A.; Aslani, A.; Shahrzad, S. Prevalence of Brugada sign in patients presenting with palpitation in southern Iran. Europace 2007, 9, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.; Topaz, G.; Heller, K.; Zeltser, D.; Ohayon, T.; Rozovski, U.; Halkin, A.; Rosso, R.; Ben-Shachar, S.; Antzelevitch, C.; et al. Fever-induced Brugada pattern: How common is it and what does it mean? Heart Rhythm. 2013, 10, 1375–1382. [Google Scholar] [CrossRef]
- Rattanawong, P.; Vutthikraivit, W.; Charoensri, A.; Jongraksak, T.; Prombandankul, A.; Kanjanahattakij, N.; Rungaramsin, S.; Wisaratapong, T.; Ngarmukos, T. Fever-Induced Brugada Syndrome Is More Common Than Previously Suspected: A Cross-Sectional Study from an Endemic Area. Ann. Noninvasive Electrocardiol. 2016, 21, 136–141. [Google Scholar] [CrossRef]
- Chockalingam, P.; Clur, S.A.; Breur, J.M.; Kriebel, T.; Paul, T.; Rammeloo, L.A.; Wilde, A.A.; Blom, N.A. The diagnostic and therapeutic aspects of loss-of-function cardiac sodium channelopathies in children. Heart Rhythm. 2012, 9, 1986–1992. [Google Scholar] [CrossRef]
- Porres, J.M.; Brugada, J.; Urbistondo, V.; Garcia, F.; Reviejo, K.; Marco, P. Fever unmasking the Brugada syndrome. Pacing Clin. Electrophysiol. 2002, 25, 1646–1648. [Google Scholar] [CrossRef]
- Shalev, A.; Zeller, L.; Galante, O.; Shimony, A.; Gilutz, H.; Illia, R. Symptomatic Brugada unmasked by fever. Isr. Med. Assoc. J. 2008, 10, 548–549. [Google Scholar]
- Viswanathan, S.; Aghoram, R. Brugada syndrome in patients with acute febrile illness. Indian Heart J. 2018, 70, 416–420. [Google Scholar] [CrossRef]
- Dumaine, R.; Towbin, J.A.; Brugada, P.; Vatta, M.; Nesterenko, D.V.; Nesterenko, V.V.; Brugada, J.; Brugada, R.; Antzelevitch, C. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ. Res. 1999, 85, 803–809. [Google Scholar] [CrossRef]
- Wilde, A.A.; Postema, P.G.; Di Diego, J.M.; Viskin, S.; Morita, H.; Fish, J.M.; Antzelevitch, C. The pathophysiological mechanism underlying Brugada syndrome: Depolarization versus repolarization. J. Mol. Cell. Cardiol. 2010, 49, 543–553. [Google Scholar] [CrossRef]
- Keller, D.I.; Rougier, J.S.; Kucera, J.P.; Benammar, N.; Fressart, V.; Guicheney, P.; Madle, A.; Fromer, M.; Schlapfer, J.; Abriel, H. Brugada syndrome and fever: Genetic and molecular characterization of patients carrying SCN5A mutations. Cardiovasc. Res. 2005, 67, 510–519. [Google Scholar] [CrossRef]
| Country | Author, Year | Study Design | Study Group | Sample | Mean Age ± SD (Years) | % Male | BrS n (%) | % Male | Type 2/3 BrP n (%) | % Male |
|---|---|---|---|---|---|---|---|---|---|---|
| Based on J-Wave Syndrome Expert Consensus Conference Report in 2016 (Proposed Shanghai Score System for Dx of BrS) | ||||||||||
| Taiwan, China | Juang, 2015 [31] | Cohort | Adults aged ≥ 55 years from Healthy Aging Longitudinal Study in Taiwan, China | 5214 | 69.3 ± 8 | 48.5 | 4 (0.08%) | 75 | 169 (3.24%) | 75 |
| Juang, 2011 [32] | Cross-Sectional | Hospital-based population seeking medical care for non-cardiovascular reasons in a tertiary medical center, Taiwan | 20,562 | 49 ± 21 | 38.8 | 1 (0.005%) | 0 | 25 (0.12%) | 0 | |
| Japan | Tsuneoka, 2016 [33] | Cohort | Health checkup in the Circulatory Risk in Community Study (CRICS), Osaka/ Akita/ Ibaraki, Japan | 7178 | 51.8 ± 7.1 | 40.2 | 8 (0.11%) | 87.5 | 84 (1.17%) | 88.1 |
| Tsuji, 2008 [34] | Cross-Sectional | Annual health examination Osaka, Japan | 13,904 | 58 ± 10 | 26.51 | 37 (0.27%) | 84 | 61 (0.44%) | 83.8 | |
| Ito, 2006 [23] | Cross-Sectional | Middle-aged or elderly Japanese-American men participated in theinitial examination of the Honolulu Heart Program, Oahu, Hawaii | 8006 | 54.1 ± 5.5 | 100 | 12 (0.15%) | 100 | 11(0.14%) | 100 | |
| Oe, 2005 [35] | Cohort | Health examination first-year elementary school children (aged six to seven years) in Izumi City, Osaka, Japan | 21,944 | 6–7 | 51.41 | 1(0.005%) | 0 | 3(0.015%) | 66.67 | |
| Yamakawa, 2004 [36] | Cross-Sectional | Health examinationKanagawa, Japan | 20,387 | 9.7 ± 3.2 | 51.18% | 1 (0.005%) | 100 | 3(0.015%) | 66.67 | |
| Yoshinaga, 2004 [29] | Cohort | Seventh-grade healthy male adolescent, Kagoshima, Japan | 7022 | 12 | 100 | 0 (0%) | N/A | 1 (0.014%) | 100 | |
| Tenth grade healthy male adolescent, Kagoshima, Japan | 15 | 1 (0.014%) | 100 | 2 (0.028%) | 100 | |||||
| Sakabe, 2003 [37] | Cohort | General healthcheckup | 3339 | N/A | 79.25 | 16 (0.48%) | N/A | 53 (1.5%) | N/A | |
| Atarashi, 2001 [20] | Cross-Sectional | Working adults Tokyo, Japan | 10,000 | 42 ± 9 | 89.1 | 54 (0.54%) | N/A | 51 (0.51%) | N/A | |
| Miyasaka, 2001 [38] | Cross-Sectional | Health examination Osaka, Japan | 13,929 | 58 ± 10 | 26.5 | 18 (0.13%) | N/A | 81 (0.58%) | N/A | |
| Furuhashi, 2001 [39] | Cross-Sectional | Health examination Asahikawa, Japan | 8612 | 49.2 (range 22 to 84) | 69.52 | 7 (0.08%) | 100 | 10 (0.15%) | 100 | |
| Korea | Uhm, 2011 [28] | Cross-Sectional | Healthy young Korean men | 10,867 | 20.9 ± 4.5 | 100 | 0 (0%) | N/A | 98 (0.9%) | 100 |
| Shin, 2005 [27] | Cross-Sectional | Healthy Korean men | 225 | 44 ± 13 | 100 | 0 (0%) | N/A | 3 (1.34%) | N/A | |
| Thailand | Rattanawong, 2017 [25] | Cohort | Thai workers in the central part of Thailand | 2446 | 40.8 ± 7 | 74.3 | 10 (0.4%) | 100 | 21 (0.85%) | 100 |
| Rattanawong, 2015 [40] | Cross-Sectional | Non-febrilepatients in an emergency department setting, Buriram, in the northeastern part of Thailand | 249 | 51.2 ± 18 | N/A | 2 (0.80%) | N/A | 7 (2.80%) | 100 | |
| Makarawate, 2015 [24] | Cross-Sectional | Healthy Thai men, Khon Kaen, in the northeastern part of Thailand | 282 | 27.82 ± 8.66 | 100 | 5 (1.77%) | 100 | 45 (15.96%) | 100 | |
| Singapore | Shen, 2020 [26] | Cross-Sectional | Health examinationbefore compulsory military service, Singapore | 54,599 | 18.7 ± 1.6 | 100 | 3 (0.005%) | 100 | 284 (0.52%) | 100 |
| Sidik, 2009 [41] | Cohort | Patients presented with pre-syncope, syncope, and/or palpitations without a known cause at an arrhythmia clinic, Singapore | 392 | 49.6 ± 19.1 | 55.9 | 19 (4.85%) | 94.7 | 9 (2.30%) | 100 | |
| Philippines | Gervacio-Domingo, 2008 [22] | Cross-Sectional | General population from the 2003 Philippine National Nutrition and Health Survey | 3907 | 50 ± N/A | 100 | 7 (0.18%) | 85.7 | 87 (2.22%) | 85.7 |
| Pakistan | Wajed, 2008 [42] | Cross-Sectional | Healthy young students in Hayatabad, Peshawar | 1100 | 20.7 ± 5.92 | 64.73 | 2 (0.18%) | 50 | 7 (0.64%) | 50 |
| Turkey | Bozkurt, 2006 [43] | Cross-Sectional | Healthy university students in Southern Turkey | 1238 | 38.9 ± 17.6 | 54.2 | 1 (0.08%) | 100 | 5 (0.40%) | 100 |
| Iran | Bigi, 2007 [44] | Cross-Sectional | Patients presentingwith palpitation in southern Iran | 3895 | 38.2 ± 11.9 | 46 | 14 (0.36%) | 78.6 | 86 (2.21%) | 78.6 |
| Israel | Adler, 2013 [45] | Cross-Sectional | Non-febrile patients in the emergency department. | 909 | 61 ± 19 | 9 | 1 (0.11%) | N/A | 4 (0.44%) | 100 |
| Country | Author, Year | Study Design | Study Group | Sample | Mean Age ± SD (Years) | % Male | Type 1 BrP n (%) | % Male | Type2/3 BrP n (%) | % Male |
|---|---|---|---|---|---|---|---|---|---|---|
| Thailand | Rattanawong, 2015 [40] | Cross-Sectional | Febrile patients, emergency department setting, Buriram, the northeastern part of Thailand | 152 | 54.8 ± 19.6 | N/A | 6 (4.0%) | 83.33% | 3 (2.0%) | N/A |
| India | Viswanathan, 2017 [50] | Cross-Sectional | Patients aged ≥ 13 years admitted with acute febrile illness (fever < three weeks) | 525 | 35.94 ± N/A | 72% | 11 (2.10%) | 100% | 12 (2.29%) | 91.67% |
| Turkey | Erdogan, 2012 [21] | Cross-Sectional | Febrile male patients in an emergency department | 103 | 37.7 ± 10.8 | 100% | 0 (0.00%) | N/A | 10 (9.70%) | 100% |
| Israel | Adler, 2013 [45] | Cross-Sectional | Febrile patients in an emergency department | 402 | 62 ± 22 | 60% | 8 (2%) | 87% | 7 (1.7%) | 71% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khawaja, M.; Qadeer, Y.K.; Siddiqui, R.; Chelu, M.G.; Aiumtrakul, N.; Pickett, J.K.; Brugada, R.; Brugada, J.; Brugada, P.; Krittanawong, C. Brugada Syndrome within Asian Populations: State-of-the-Art Review. Cardiogenetics 2023, 13, 61-74. https://doi.org/10.3390/cardiogenetics13020007
Khawaja M, Qadeer YK, Siddiqui R, Chelu MG, Aiumtrakul N, Pickett JK, Brugada R, Brugada J, Brugada P, Krittanawong C. Brugada Syndrome within Asian Populations: State-of-the-Art Review. Cardiogenetics. 2023; 13(2):61-74. https://doi.org/10.3390/cardiogenetics13020007
Chicago/Turabian StyleKhawaja, Muzamil, Yusuf Kamran Qadeer, Rehma Siddiqui, Mihail G. Chelu, Noppawit Aiumtrakul, June K. Pickett, Ramon Brugada, Josep Brugada, Pedro Brugada, and Chayakrit Krittanawong. 2023. "Brugada Syndrome within Asian Populations: State-of-the-Art Review" Cardiogenetics 13, no. 2: 61-74. https://doi.org/10.3390/cardiogenetics13020007
APA StyleKhawaja, M., Qadeer, Y. K., Siddiqui, R., Chelu, M. G., Aiumtrakul, N., Pickett, J. K., Brugada, R., Brugada, J., Brugada, P., & Krittanawong, C. (2023). Brugada Syndrome within Asian Populations: State-of-the-Art Review. Cardiogenetics, 13(2), 61-74. https://doi.org/10.3390/cardiogenetics13020007