Abstract
Substantial increase in the production of agri-food commodities over the past years has resulted in the generation of enormous volumes of wastes and by-products, thus contributing to increased environmental pollution. Being an under-exploited raw material which are rich in bioactive compounds (e.g., polyphenols, dietary fibre, oils, essential vitamins, minerals, etc), novel strategies and initiatives have been proposed and implemented for the effective management and valorization of these wastes and by-products. The proposed initiatives and strategies support the concepts of EU circular economy and green biorefinery, thus promoting sustainability. One of the strategies of management of waste and by-products includes the effectual development of nutritious low-cost sustainable animal feed. Currently, in the world market, there are a range of fruit and vegetable wastes and by-products that have been effectively introduced in animal diets. Within this context, this systematic review focuses on a diversified group of agri-food wastes (and the industrial by-products), their bioactive components, the opportunities for the development of animal feed or feed supplements (for Ruminants, Non-Ruminants and as Poultry feed) and conclusively the health benefits imparted. In addition, the safety issues and regulations aspects are also covered.
1. Introduction
Recent years have seen significant growth in the production of horticulture-based agri-food commodities. One of the reasons for the increased production is to fulfil the needs of the ever-growing population as well as to meet the changed dietary habits of consumers who have shifted to vegetarian-based diets []. Today, fruit and vegetable-based food industries are making upright progress wherein a wide range of products are produced and marketed such as jellies, syrups, juice, and chips. However, the production has also resulted in large quantities of waste and by-products (fruit and vegetable wastes; FVWs) being generated that go either as a landfill or are discarded in an unsustainable way. Owing to unsustainable disposal methods and practices, a significantly higher increase in environmental pollution is being witnessed [,]. As per the latest reports of the Eurostat, annually in the EU, approximately 57 million tons of food waste is generated []. Nearly 18 % of the total share of waste came from the processing and manufacturing sectors and the amount was nearly 10 million tons of fresh mass []. In the EU alone, an estimated €143 billion loss due to food waste is known, leading to approximately 6% of the total greenhouse gas emissions. According to the FAO (2014), 1.3 billion tons of food waste is generated annually, and 60% of this comes from fruit and vegetables. The amount of fruit and vegetable loss exceeds all other types of food wastes []. This can be the result of poor processing, poor infrastructure and/or handling, as well as the behaviour of retailers and consumers []. Processing of agri-food materials tends to transform the raw material into stable products [] and involves the application of selective processing techniques like drying, freezing, canning, peeling, pressing, etc. [,]. Recently, the main focus has been laid towards the management of food industrial wastes and by-products, their management and valorization [,,]. Pomace represents the solid remains of processed raw material (fruit or vegetable) and it usually consists of the pulp, skin, stem and seeds. It is referred to as a by-products of the fruit and vegetables [,,]. In Figure 1, fruit and vegetable processing leading to pomace generation is depicted.
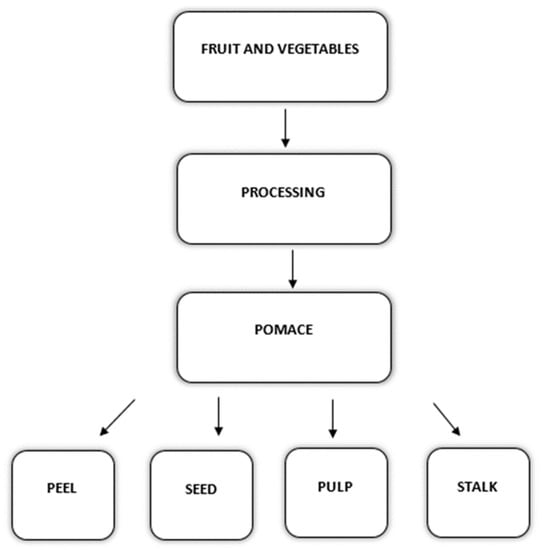
Figure 1.
Fruit and vegetable processing leading to pomace generation.
Novel waste management strategies and initiatives have been implemented for the valorization of FVWs. One of these approaches includes green extraction techniques for extracting bioactive compounds and the development of animal feed. FVWs represent a highly under-exploited cheap raw material source, rich in bioactive compounds [,,,] and it holds high potential in animal feeds production. Exploiting these FVWs has been proven highly beneficial in animal nutrition and health, specifically in the livestock industry []. In addition, bioactive compounds obtained from FVWs have been proven useful in applications in food, cosmetics, paper, pharmaceutical industries, and others [,].
Today, the manufacturing of animal feed is already facing several issues and challenges due to a shortage of available fertile land, fresh water, ongoing climate problems, coupled with food-fuel-feed competition and a shortage of livestock feedstuff [,,,,]. These challenges are especially prominent in middle- and low-income generating countries []. In addition to this, the cost of currently utilized ingredients for the development of animal feeds such as maize, wheat, soya and other commodities has recently increased []. Therefore, it has become a necessity to explore low-cost nutritious raw materials as well as develop novel low-cost feed in order to maintain the sustainability of livestock production []. In this regard, FVWs have been evaluated as one of the potential and profitable substitutes/ingredients to produce animal feed due to their relatively low cost, easy availability and rich content of bioactive compounds, which could have a positive impact on animal welfare, growth and health [,,]. Utilization of FVWs as animal feed may seem practical and economically useful, however, it meets certain limitations.
The digestibility of the feed is an important factor to be considered when it comes to including wastes as feed material for livestock. Feed digestibility is related to the nutritive value of feed and it predicts potential animal performance []. Adding high levels of FVWs in animal diets can lead to potential low digestibility of feed. In addition, increased amount of FVWs can result in decreased nutrient intake and growth performance of animals [,]. Low digestibility is attributed to a high content of neutral detergent fibre, especially lignin and also high concentrations of dietary phenolic compounds in FVWs. The high content of lignin in waste negatively affects the digestion of fibers as it compromises the access of fibrolytic enzymes to cellulose and hemicellulose which results in slower digesta passage rate thus causing a decline of dry matter intake (DMI). Dietary polyphenolic compounds are believed to inhibit the growth and activity or ruminal microbes such as Bacteroides fibrisolvens and Ruminococcus albus and several microbial enzymes too. Dietary phenolic compounds also have the ability to irreversibly bind certain nutrients such as fibre and crude protein [,,].
Supplementing animal feed with FVWs at certain proportions could lead to adverse effects on animal performance, expressed as a decrease in milk yield or inhibited weight gain both in ruminant and monogastric animals [,,]. Therefore, it is essential to evaluate the nutritional value, the content of active compounds and bioavailability of FVWs. It is important to evaluate their effect on animal performance and health too. Another limitation on FVW’s use as feed ingredients can be attributed to insufficient research activities undertaken on the influences of FVWs on animal welfare.
Further, a consequential limiting factor of FVWs valorization in production of animal feed is the potential presence of heavy metals, chemical residuals, pesticides, toxins and anti-nutritional factors in high levels, which could have an adversarial effect on animal health or can even be fatal []. In order to avoid toxic agents, the manufacturing of animal feed must follow safety guidelines and good practices for use of agricultural wastes as animal feed []. Using FVWs as feed resources requires compliance with legislation and requirements of feed safety. The final product must meet chemical and microbiological safety standards [,,]. However, complicated safety regulations and legislation of the utilization of FVW can sometimes intimidate farmers and feed technologists from using them as feed material []. Another aspect that needs to be considered is the hygienic quality during storage. Not enough information is available on handling, storage, processing conditions and production costs which can discourage animal nutritionists from including FVWs in feed development [].
Even though, FVWs are of low-cost, their transport, treatment and processing can sometimes be very expensive and economically inefficient []. For example, the high moisture content of FVW is a problem, as it can lead to microbial deterioration and spoilage. In order to prevent the decay of FVW, it has to be transported urgently to the facilities, where water reduction strategies are performed on the waste. This results in high transportation costs especially if the facility is far away or transported waste material is heavy []. In addition, water reduction strategies can be pricey as well []. One of the solutions could be drying the waste and producing the feed at the same spot as where the waste originates in order to reduce transportation costs []. In addition, treatment and processing of FVWs can result in unknown effects on the nutrient content of the FVWs []. For example, drying methods can have a negative impact and decrease the level of bioactive compounds of FVWs. Among drying methods, freeze-drying has proven to be a suitable method for retaining the maximum amounts of bioactive compounds [,,].
Other limiting factors for effective utilization of FVWs would be their seasonality and full-time availability. For manufacturing animal feeds, fruit and vegetables which are produced throughout the year and are available in large quantities are always a better option. Low availability of certain fruit and vegetables also means inadequate product quantity, which further-on fails to support the supply chain. Therefore, a detailed evaluation of the selection of FVWs needs to be performed before their use as feed material []. In addition to this, the nutrient composition of FVWs varies depending on the season and this is important to consider while evaluating their use for feed development. []. Animals’ response to FVWs is a very important factor as well. Animals responding poorly to feed with selectively supplemented or fortified FVWs could limit their further use as feed ingredient [].
There are number of factors which need to be considered when it comes to the utilization of FVWs, but these should not outshine their positive impact on animal welfare, health and animal products. Bioactive compounds present in FVWs have been shown to have a high potential for enhancing animal well-being. In addition, the utilization of FVWs for the development of animal feed corresponds to the EU circular economy concepts created with the goal of reducing the production of waste and supporting the continued use of waste and by-products as resource material. The circular economy concept is an excellent alternative to the current inefficient linear economic model. Its principles offer tools and help to create sustainable feed and food systems []. With this background information, this systematic review focuses on a diversified group of agri-food wastes (and the industrial by-products), their bioactive components, the opportunities for the development of animal feed/feed supplements (for ruminants, non-ruminants and as poultry feed) and conclusively the health benefits imparted. In addition, the safety issues and regulations aspects are also covered in this review.
2. Methodology
For the current review, relevant research articles in international databases (Pubmed, Scopus, Science Direct, Google scholar) were explored, evaluated and compared. The literature survey was conducted in 2021 and 2022 and it focused on articles with keywords as fruit and vegetable waste, pomace, animal feeds, circular economy, safety and valorization technologies. The aim of writing this review is mainly focused to discuss the bioactive contents of selected pomace (waste and by-products) as well as to explore for opportunities on their role in animal nutrition and performance after processing it as animal or livestock feed. In addition, some of the current studies/reports wherein pomace has been studied as animal feed have been critically evaluated (Figure 2).
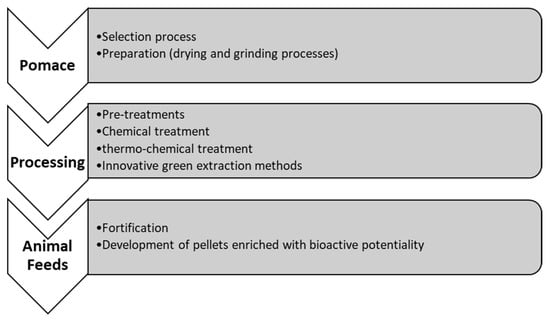
Figure 2.
General processes involved for conversion of pomace to animal feed.
3. Composition of Fruit and Vegetable Wastes
Fruit and vegetable wastes (FVWs) encompass high amounts of various bioactive compounds with established bioactivities [,,,,]. Nowadays, these compounds are frequently used in a range of industries, including paper, textile, pharmaceuticals, and food industries [,,,,,]. Therefore, the extraction of these bioactive compounds has recently gained a lot of attention and has been studied extensively. Green extraction methods are constantly being improved and modified so optimum yield and quality of bioactive compounds can be achieved. Environment-friendly procedures and green biorefinery techniques are being chosen over traditional methods.
3.1. Proteins and Enzymes
Proteins are an essential component in both human and animal diets influencing their growth. Proteins play an essential role in forming the muscles and are components of other molecules too. A deficiency in essential proteins often leads to various types of diseases in livestock, and therefore an adequate and high-quality source of protein is necessary. Soybean, rapeseed and crop legumes are the main sources of protein both for ruminant and monogastric animals. FVWs are an excellent source of protein too. For this reason, protein in FVWs is often used as valuable ingredients for the manufacture of feed components for livestock. Some examples include apple pomace, cabbage leaves, cauliflower stalk, radish leaves, pea pods, snow peas, potato, beetroot and carrot pomace [,]. In Table 1, the protein content of certain FVWs has been compared to protein content of conventional animal feed.
Enzymes are proteins which regulate chemical reactions in living organisms []. Enzymes such as pectinase, invertase, cellulase and amylases are extracted from FVWs with the help of certain bacteria or fungi [,,,]. Enzymes are used in a large number of industries, especially in the pharmaceutical sector and food industry [,]. Exogenous enzymes are often added to animal feed to improve digestion, animal performance and growth []. This is often seen in post-weaning pigs whose gastrointestinal tract, immune system and enzyme secretory capacity are not fully developed [,,]. In the first few days after weaning, piglets often suffer from growth checks. In order to prevent growth check in weaned piglets, piglets’ diets were supplemented with antibiotics, zinc oxide and copper, however, with the growth of antimicrobial resistance, new solutions had to be implemented, and exogenous enzymes have been successfully given instead []. In the research articles that have discussed the addition of exogenous enzymes to animal feed, there is limited information on whether enzymes were derived from FVWs. However, it is well-known that many enzymes, such as cellulase, hemicellulase, xylanase and invertase can be efficiently produced using FVWs. Commonly used FVWs for recovery of mentioned enzymes are peels of banana, orange, pineapple, pomegranate and citrus waste and by-products [,,,,].
Based on the above-mentioned features, it is evident that protein plays a significant role in animal diets, however, protein content in feed does not describe the protein quality. Hence, it is necessary for the measurement of digestibility and degradability of protein in FVW to be evaluated prior to including them in the animal diet as this is more important compared to protein content. In addition, crude protein content is no longer considered a valuable parameter in the evaluation of feed quality. Crude protein content represents the amount of nitrogen present in feed which is then used to determine the amount of protein in feed []. However, protein is not the only component which contains nitrogen as nitrogen is also present in components such as nucleic acids and nucleotides, vitamins, amines, urea and amides. Nitrogen which is provided by these components is referred to as non-protein nitrogen (NPN). NPN fraction usually makes up a considerable percentage of crude protein content []. And even though ruminants can use non-protein nitrogen in their bodies [] there are still problems with using crude protein as a measure of feed’s protein quality. In addition, crude protein does not provide information on the actual nutritional value of protein, but the composition and the ratio of essential amino acids (EAA) does give more information []. This emphasizes the importance of analyzing amino acids content in raw materials used for animal feed production. In addition, a deficit in any one of the essential amino acids can result in the interruption of protein synthesis which further negatively affects the performance of the animal [,]. A shortage of one amino acid (AA) can be limiting the absorption of others. The amount of AA in feeds is crucial as it could limit the growth and production in young stock [].

Table 1.
The protein content of selected FVW in comparison with typical protein feeds.
Table 1.
The protein content of selected FVW in comparison with typical protein feeds.
| Feed | Crude Protein (% of DM) | Metabolizable Protein (g/kg DM) | Protein Degradability (% DM) | Reference |
|---|---|---|---|---|
| Soybean meal | 53. 60 | 95 | 58.50 | [] |
| Heat treated Rapeseed cake | 36.30 | 166 | 53.40 | [] |
| Cold pressed Rapeseed cake | 33.20 | 102 | 89.20 | [] |
| Maize grain | 10.28 | 95.26 | 6.73 | [] |
| Wheat bran | 15.68 | 107.11 | 9.23 | [] |
| Maize fodder | 9.77 | 72.01 | 5.37 | [] |
| Canola meal | 40.10 | 92 | 4.75 | [,] |
| Tomato pomace | 22.21 | 6.30 | 9.74% | [] |
| Beetroot pulp | 93.40 | 4.8 | 3.46% | [] |
3.2. Dietary Fibre
Dietary fibre plays a vital role in livestock nutrition as their source and fractions affect physiological functions of the gastrointestinal tract, gut health, gut microflora, and performance in ruminants [], monogastric animals [] and poultry []. Dietary fibre represents non-starch carbohydrate polymers, which cannot be digested in the small intestine of non-ruminant species [,]. Dietary fibres are commonly divided into insoluble and soluble dietary fibre based on their water solubility []. However, recent research on the contribution of dietary fibre to a monogastric diet, argues against this classification because the solubility of polymers depends on more factors than just solubility in water []. These factors include molecular properties and conformational entropy. Therefore, certain polymers which are considered soluble can sometimes be in fact poorly soluble in water. The study has debated that the current classification of dietary fibre into soluble and insoluble is not enough to clarify how dietary fibre positively affects the health of monogastric animals []. More information on the classification of dietary fibre based on their chemical properties is discussed by Arranz et al. [].
Dietary fibre includes cellulose (as the main macromolecule in vegetable waste), hemicelluloses, lignin, pectin, inulin, β-glucans, gums and non-digestible oligosaccharides [,]. Today, many sources of dietary fibre such as hay and silage are being partially replaced by FVWs in the production of animal or livestock feed. This is because many FVWs are rich in dietary fibre, and they represent an affordable and easily available source of needed nutrients.
Regarding the role of dietary fibre in monogastric animals’ diets, Montagne and co-workers undertook a study to evaluate the effects of dietary fibre on health and gut development in young non-ruminants such as piglets and chicken []. The study concluded that some components of dietary fibre improve gut health and play a role in prevention of reducing duration of diarrhea in young non-ruminant animals.
A difference between the effects of soluble and insoluble dietary fibre has also been established. For example, when it comes to general effects on intestinal pathology of non-ruminant animals, soluble fibre increases intestinal transit time, delays glucose absorption, delays gastric emptying, increases pancreatic secretion and slows absorption while insoluble fibre decreases intestinal transit time, improves water holding capacity and it helps in faecal bulking. Dietary fibre can positively affect the reproductive performance in pigs []. In weaned piglets, DF (dietary fibre) helps the GIT (gastrointestinal) function and development mostly by changing the microbial composition, and the activity of microbials and by stimulating the production of volatile fatty acids (VFA). VFAs, and especially butyrate, help the proliferation and differentiation of epithelial cells []. In growing pigs, even though only partly digested, dietary fibre is an inevitable part of the feed. Dietary fibres’ contribution to energy supply is almost negligible in growing pigs; however, its contribution to energy supply increases in mature pigs and especially sows. The digestibility of dietary fibre depends highly on the source as it has been shown that dietary fibre extracted from beet pulp and soybean hulls is more digestible by pigs than the one coming from wheat straw [].
Further, dietary fibre is established to play an important role in ruminants’ diets. Dietary fibre influences the intake and digestion of nutrients which affects the animal’s performance. A high dietary fibre diet increases mastication and rumen fermentation time and therefore salivation as well. Reducing the particle size through mastication and rumination is an important part of digesting forage. Mastication and rumination reduce the size of particles and increase the surface available for rumen enzymes and microbes. Increased salivation time plays a significant role in maintaining the normal rumen function as it affects the buffering capacity of rumen fluid and provides optimal ruminal pH for the growth of cellulolytic microbes. The growth of cellulolytic microbes tends to stimulate the production of acetic acid, a precursor for milk fat, and hence high-fibre diets can prevent milk fat depression in lactating ruminants []. Animals’ performance depends on the intake of digestible nutrients so the digestibility of fibre is an important aspect which needs to be considered []. For example, 20–70 % of cellulose might not be digested by an animal which further influences net energy being significantly decreased compared to energy intake []. There are a number of factors affecting the digestion of dietary fibre and those include plant structure, plant species and maturity, nature of the predominant microbial fibre-digesting microorganisms, factors that control the adhesion of hydrolytic enzymes of microbial population, and animal factors which influence mastication and salivation []. The composition and amount of animal feed also play a role in the digestion of dietary fibre, especially the concentration of non-structural carbohydrates, N supply of feed and supplementation of diet with fat and fatty acids []. For example, an increased supply of non-structural carbohydrates decreases fibre digestion as this leads to lower ruminal pH, which might not be suitable for cellulolytic bacteria. In addition, the increased availability of amino acids showed an increased rate of fibre digestion while an increased supply of fats and fatty acids negatively influence fibre digestion due to their toxic effect on rumen bacteria []. Increased feed intake decreases rumen residence time which leads to incomplete digestion of digestible neutral detergent fibre [].
Several FVWs such as apple pomace, grape pomace, pumpkin pomace and potato peels, contain significant amounts of dietary fibre (see Table 2) [,,,]. For example, apple pomace is a superior source of soluble (pectin) and insoluble fractions of dietary fibre with the proportions of these two fractions being well-balanced. Grape pomace are also a rich source of dietary fibre, mostly hemicelluloses, cellulose and in small amounts, pectin [].

Table 2.
Dietary fibre in some of the fruit and vegetable pomace.
The aforementioned studies have shown the positive effect of dietary fibre in animal nutrition. A well-balanced diet involving high content of both protein and dietary fibre contributes to animal health with an emphasis on the positive impact on GIT. Fruit and vegetable-derived pomace remain a highly under-exploited source of many important bioactive compounds including dietary fibre. Their disposal means meagre wasting of numerous important bioactive compounds, which could be utilized and could contribute to animal and human nutrition. Therefore, dietary fibre from agri-food waste needs to become efficiently utilized in order for the sustainability of food and feed systems to be achieved.
3.3. Polyphenolic Compounds
Polyphenolic compounds are bioactive compounds that can be easily obtained from a large number of plant products, including those of FVWs and by-products. It is opined that the amount of these secondary metabolites are higher in the waste portion of many fruits and vegetables compared to their edible fractions [,]. Owed to the positive health properties that phenolic compounds extracted from FVW exhibit, they are often added to animal diets. In Table 3, research activities undertaken on the phenolic contents in selected fruit and vegetable pomace are depicted.

Table 3.
Phenolic compounds in some of the selected fruit and vegetable pomace.
Phenolic Compounds in Wastes and By-Products
The content of polyphenolic compounds in FVWs are associated with the nutritional quality of plant material. For example, grape waste (pomace), generated in wine or juice industries consists of large quantities of grape pomace, which includes grape seeds, pulp, skins and stalks and is rich in phenolic compounds. It is commonly disposed-off creating environmental issues, so its utilization in developing animal feed is one of the better alternatives. Grape pomace is easily available, and of low cost which makes it a good and cheap source of important nutrients []. Grape seeds contain high amounts of polyphenolic compounds, in particular pro-anthocyanidins, the class of phenols which exhibit antioxidant activity and free radicals scavenging capacity []. The amount of phenolic compounds in grape seeds is higher than in grape skins and stems [,]. Grape seeds also manifest the highest anti-oxidant, cytotoxic and antibacterial (against Gram-positive bacteria) activities []. In addition to pro-anthocyanidins, grape wastes contain flavonoids. Grape seeds and grape marc have been shown to prevent fatty liver disease and ketosis when added to the diet of dairy cows by reducing inflammation and stress in the endoplasmic reticulum in the liver. This has been attributed to high content of flavonoids. In addition, the high content of tannins in grape seeds and grape marc influences ruminal metabolism. Tannins have also shown to improve milk yield []. In addition, phenolic compounds extracted from grape pomace play a role in inhibiting the growth of certain bacteria such as Gram-positive Staphylococcus aureus, Listeria monocytogenes and Gram negative Pseudomonas aeruginosa []. Due to their health-beneficial properties, the recovery of phenolic compounds has received a lot of attention.
Sea buckthorn (SBT) berries (fresh and processed) are a common food ingredient rich in nutrients and health-promoting compounds [,,]. SBT pomace and leaves are rich source of polyphenolic compounds. Moreover, berries, leaves and twigs have equal cytotoxic activity which makes the whole plant of sea buckthorn attractive to be used at the industrial level []. In addition to phenolic compounds, they contain numerous minerals, vitamins, carotenoids and fatty acids [,,]. However, the content of phenolic components is highly related to a variety [], cultivation techniques [], and processing methods adopted []. Recent years have witnessed extensive research with regard to the recovery of bioactive compounds from SBT pomace. SBT leaves have a higher content of phenolic compounds compared to berries. Leaves are rich in flavonoid glycosides, especially isorhamnetin and quercetin derivatives, and ellagitannins []. In addition, the young shoots of SBT are considered to have similar levels of bioactive compounds as leaves []. Several studies on livestock have shown that SBT leaves or leaf extract have a beneficial effect on the growth performance in calves [], piglets [] and in poultry []. Moreover, the leaves could have specific or general health-promoting effects on farm animals [,,,].
Significant amounts of olive pomace are obtained from olive oil industries and their disposal in landfills and rivers negatively affects the environment. There are several alternatives recommended for olive pomace utilization and one of them is the extraction of polyphenolic compounds that have wide applications in the food and pharma sectors. Efficient valorization of olive pomace can also provide financial support to farmers and animal feed producers. Oleuropein represents the most profuse phenolic compound in olive pomace [,]. In addition to oleuropein, hydroxytyrosol is the most important phenolic compound derived from olive pomace and especially olive leaves. Recent years have seen a great interest in recovering phenolic compounds from olive leaves. Olive leaves are rich in polyphenolic compounds such as gallic acid, egallic acid, caffeic acid, salicylic acid, pyrogallol, catechin, catechol, syringic acid, chlorogenic acid, coumarin, ferulic acid, vannillic acid. Olive leaves are also rich in flavonoids like hesperidin, naringin, hesperidin, rutin, quercetin, luteolin, apigenin 7-O-glucoside, kaempherol, rosmarinic acid, rhamnetin and apigenin [,,]. They are often utilized for the production of ruminant feed and as feed supplements. In the diet of ewes, polyphenols from olive pomace influence the rumen metabolism at various levels as they affect the microorganisms related to bio-hydrogenesis, Food and feed supplemented with phenolic compounds extracted from olive waste showed increased nutritional content [].
Pumpkin wastes are also a rich source of bioactive polyphenolic compounds. It contains protocatechuic acid, caffeic acid, chromogenic acid, p-coumaric acid, gallic acid, vanilic acid, 4-hydrxybenzoic acid, sinapic acid, kaempferol, isoquercetin, myricetin, rutin, astragalin, and quercetin []. The application and uses of pumpkin waste as a feed supplement is discussed later on in the text.
Apricots pomace also has high contents of phenolic compounds, specifically - neo-chlorogenic and chlorogenic acids, proanthocyanidins, kaempferol glycosides, cyanidin 3-glucoside and certain quercetin derivatives. The phenolic content in apricot pomace depends on the cultivar of the apricot. The content also differs between apricot skin and flesh []. Citrus fruits pomace contains phenolic compounds, especially flavonoids like narirutin, hesperidin, naringin, and eriocitrin, and the quantity is comparable to edible portions []. These fruits exhibit strong bioactivity and are often used in livestock diets. Phenolic compounds in banana peels include benzoquinones, hydroxyl-benzoic acids, and acetophenones, phenylacetic acids, anthraquinones, naphthoquinones, iso-flavanoids and flavonoids, lignins, lignans, and tannins. In addition, banana peels contain gallocatechin and dopamine, which are natural antioxidants as well and are used in pharmaceutical industries or in food industries as a natural food preservative []. Apple pomace, frequently obtained during post-processing of apples for the production of fruit juice, has been proven as a good source of polyphenolic compounds. Pomace contains apple seeds, a little amount of stem, residual flesh and apple peel. Apple pomace is especially rich in pro-anthocyanidins and flavonoids []. In addition, non-specific poly-phenolics such as chlorogenic acid were also detected in apple pomace. Apple seeds are a good source of polyphenols. In fact, polyphenols represent a predominant component of seeds. The most abundant polyphenol in apple seed is phloridzin but its content is very high in other portions of this fruit as well.
3.4. Essential Oils and Lipids
FVWs have also been explored for the recovery of bioactive essential oils, lipids and organic acids. Some of them are discussed in the below text.
Essential oils (EO) are extracted from a number of fruits and vegetables wastes, especially citrus fruits [,,]. There are numerous studies reporting on the effect of addition of EO to animal feed. The study by de Souza and co-researchers reported the effect of clove, eugenol, thymol, vanillin and rosemary EO on animal performance, in situ digestibility, behaviour activities, feed intake and carcass characteristics of heifers []. EO was added to a high-grain diet and the study reported improved daily weight gain, in situ dry matter, neutral detergent fibre (NDF) digestibility, behaviour activities and feed efficiency of animals. EOs had negligible or no effects on the muscle, fat or bone percentage of the carcass. In the study by Nanon [], it was reported that lemongrass oil and a mixture of garlic and ginger oil to improve the dry matter digestibility of fibrous feed due to improved NDF digestibility. The reported work showed that the EO can be used as rumen modifiers to improve the digestion of feed, especially roughage feeds. Furthermore, adding EO to animals’ diet had no effect on methane production. The addition of baccharis, tamarind, cashew nutshell liquid and clove oil to high grain diet of beef cattle did not alter feed intake, nutrient digestibility, animals’ performance or feeding behaviour. In fact, supplementing EOs to the diet resulted in improved dry matter, organic matter and NDF digestibility. The addition of mentioned EO increased the concentration of propionate causing the reduction of the acetate /propionate ratio []. Furthermore, in the study by Mottin and co-workers, the inclusion of clove, cashew, castor oil and a blend of eugenol, thymol and vanillin did not alter carcass characteristics, but it did alter the body composition of fat and muscle. EO did not modify drip losses, pH or fat thickness []. In addition, supplementation of these oils affected thawing/ageing and cooking losses, it increased water loss and altered the colour, texture, antioxidant activity and lipid oxidation of the meat. It has been concluded that EO can be added to animals’ diets in the right dose as the high doses can present oxidative effects.
In addition, FVW contain a high amount of lipids as well. Some of the waste that contains high amounts of lipids include mango kernels (stearic acid, oleic acid and palmitic acid), tomato seeds, apple seeds, pomegranates seeds and apricot seeds [,,].
3.5. Organic Acids
Citric and lactic acids can be obtained from various types of FVWs. Owed to citric acid´s multiple usage and demand, its production and utilization have been thoroughly studied. The strategy of obtaining acids from FVW is opined to reduce production costs, as FVW is readily available and of no cost, besides being environmental-friendly [,]. Organic acids can be obtained from apple pomace [,], white grape pomace [], pomegranate peel [], pineapple waste [], banana peel [], mango peel [] and orange peel [,].
4. FVWs in Animal Feeds
4.1. Utilization of FVW in Ruminant Feed
Today, the livestock sector contributes significantly to global warming and the production of greenhouse gas emissions mainly through processes of growing livestock, and processing and transportation of animal feed and products of animal origin [,].
The quest for exploitation of novel feed resources could be one of the solutions for sustainable animal feed production. Their utilization could lead to a reduced negative impact on the environment and promote zero-waste horticulture [,]. It is believed that FVWs can up to a certain extent replace the current feed resources, that are already in shortage, and additionally enhance animal health [,,,]. Utilization of FVWs in animal feed development could also be a solution to the shortage of available land and water, current food-feed-fuel competition and continued increase of the price of currently used ruminant feed material. In addition, not only does the utilization of FVWs mitigate environmental pollution by not being disposed in landfills or via other unsustainable ways, but also feeding of selective FVWs has been noted to decrease nitrogen (N) and methane (CH4) emissions. For example, condensed tannins from grape pomace have been shown to adjust the metabolism of nutrients in ruminants. They have the ability to shift the excretion of N from urine to faeces. The excretion of N through faeces contributes less to N emission compared to urine N []. In addition, condensed tannins have the ability to also change microbiomes in rumen and fermentation process resulting in a decrease of CH4 emissions [,].
As production-related waste in fruits and vegetables ranges between 30 to 70% of the proceeded material [], there have been many studies evaluating the use of certain FVWs for the production of ruminant feed [,,]. Moreover, the utilization of FVWs initiates the use of less food-competing materials in ruminant diets []. A number of factors influence the use of FVWs as alternative feeds for a ruminant. These include the nutritional value of FVWs and their effect on the production, performance and quality of milk and meat. In addition, the digestive physiology of ruminants and the high content of fibre in FVW needs to be considered when adding a large amount of FVWs to the animal diets []. It is important to consider various costs involved in the production too. In addition, it is necessary that these feed resources are not suitable for human consumption or production of energy so that food-feed-fuel competition can be avoided [,].
Pumpkin waste has been characterized as a potential feed resource for livestock feed. In the review by Valdez-Arjona & Ramírez-Mella [] the researchers evaluated its addition to diet of ruminants and concluded that pumpkin wastes possess good nutritional value and bioactive compounds such as polyphenolic compounds (carotenoids especially), polyunsaturated fatty acids, proteins, vitamins, minerals, pigments and polysaccharides which positively affects the milk and meat composition of ruminants. In addition, study reported increased amounts of α- and β-carotene, violaxanthin and lutein in milk []. Further, Boldea and co-workers concluded that addition of pumpkin seed cake to the diet does not alter milk yield or milk composition in dairy goats []. Replacing 30% of corn stubble (stover) with pumpkin wastes in the diet increased the ruminal digestibility of dry matter, but decreased the digestibility of NDF. The addition of pumpkin waste also enhanced the overall health and welfare of ruminants as the waste manifests a number of health activities such as anti-parasitic, antioxidant, antimicrobial, antifungal, and anti-inflammatory activities [].
In the study by Mannelli and co-workers, supplementing olive pomace to ewe’s diet increased the content of oleic acid and α–linoleic acid (α-LNA) in milk. It also increased milk yield and improved its nutritional quality []. Adding olive pomace to the feed did not alter the rumen functionality of ewes and there was no negative influence on animals’ welfare. In the study by García-Rodríguez and co-researchers it was shown that the inclusion of olive cake increased diet degradability but did not alter rumen fermentation, synthesis of a microbial protein nor microbial growth []. Replacing barley straw and maize silage with olive cake showed increased diet disappearance, decreased methane production and there were minimal changes in the microbial population in dairy sheep. Dried stones of olive pomace added to the diet of lactating buffalos showed no negative effect on the quantity or quality of milk as the chemical properties of milk remained unchanged. The nutrient quality of milk however has increased as it showed a higher content of tocopherols, retinol, the presence of hydroxytyrosol, and an improved fatty acid composition [].
Apple pomace has been routinely added to ruminants’ diets without any detrimental effects on animal’s growth, productivity or health. For example, feeding dairy goats with tomato and apple pomace silage, which replaced berseem hay up to 50%, improved milk yield and milk composition, digestibility and feed efficiency. This study concluded that the addition of tomato and apple pomace to ruminant feed is economically beneficial. It showed no negative effects on the animal performance or health of dairy goats and their offspring []. In a study by Fang et al. it was reported that the addition of apple pomace improves silage fermentation. It also enhanced ruminant productivity []. In addition, ensiled apple pomace has been successfully supplemented up to 30% in the feed of dairy cows []. The incorporation of 15% of ensiled apple pomace in the diet of dairy cows along with wheat bran, milled rice bran and chopped alfalfa resulted in enhanced production of milk with less milk fat []. However, even though apple pomace showed high potential and is widely used, its utilization can be limited due to the alcohol content that is produced during fermentation.
Incorporation of banana peels into ruminant diet shows immense potential as the banana peel is rich in bioactive compounds, it is highly nutritious and a good source of protein, fibre, minerals and phenolic compounds, including tannin, which is often used as a feed additive to improve performance of ruminants. In addition, banana peel is a good feeding material for ruminants since the rumen microbes are capable of digesting it and valuable bioactive components are therefore easily absorbed in the small intestine. Their presence in blood increases the production and quality of milk and meat. The addition of banana peel to the sheep diet increased degradability and VFA production []. Dairy cows fed with ripe banana peel showed increased milk productivity []. In many countries, banana foliage has been used in ruminant feed, and it has been reported that it contains high amounts of tannins, flavonoids and terpenoids which manifest various health benefits including anti-parasitic activities [].
Sea buckthorn (SBT) pomace has been also considered as a supplement to the ruminant diet. It is rich in valuable components, mostly phenolic compounds—flavonoids, tocopherols, vitamins, proteins, highly digestible carbohydrates, etc. In a study reported by Hao and co-workers, SBT pomace was added to the sheep diet to evaluate its effect on digestion physiology and growth performance [,]. The study showed that the addition of SBT pomace to a certain percentage in sheep diet increased dry matter intake, average daily gain and in situ NDF degradation, without adverse effects on feed efficiency [] or energy metabolism []. In addition, Qin et al. showed a positive effect of the administration of SBT pomace on meat quality characteristics and increased muscle mass in ram lambs []. Furthermore, in another study conducted on ram lambs [], it was concluded that SBT pomace could act in various molecular targets regulating the browning of white adipose tissue. Similar results have been reported in studies with monogastric animals. Further, up to 12% of SBT pomace was administered in pigs without any negative effect on growth performance and overall meat quality []. In a study by Dannenberger and co-researchers. supplementation of 12% of dried SBT pomace was shown to have a moderate effect on immunity and the metabolism of fatty acids in growing pigs []. In addition, there are several other aspects which support SBT’s potential in ruminant nutrition. Firstly, there was a finding that SBT meals could be a bio-sorbent to inhibit the negative effect of mycotoxins in feed []. Secondly, similar to apple pomace, sea buckthorn pomace at the level of 5% has the potential to improve the fermentation of alfalfa silage [] and therefore could be considered as a silage additive.
Grape pomace can also be added to the ruminant diet and can be a partial replacement to forage in small ruminant diets []. It can improve BMI, animal growth, health, and overall welfare. A study conducted on addition of grape pomace in the sheep diet by Guerra-Rivas et al. [], revealed increased quality of milk due to higher content of antioxidants and fatty acids. These acids also have a beneficial effect on sheep meat. However, many researchers have pointed out certain constraints and issues related to the utilization of grape pomace for the production of ruminant feed [,]. It has been established that high content of tannins and anthocyanins can negatively affect utilization of nutrients. On the other hand, tannins (along with other phenolic compounds) can enhance the metabolism in rumen by reducing methanogenesis (they can inhibit the growth and activity of certain methanogen bacteria) and by supplying small intestine with proteins due to decreased ruminal degradability []. Grape seeds are believed to possess immunomodulatory effect in sheep due to high amount of flavonoids and pro-anthocyanidins [,]. The supplementation of grape and citrus by-products to ruminant feed can also decrease the food production costs and prolong the shelf life of ruminant products. Their addition to the diet in the study showed no detrimental effect on animal growth, it improved DMI, the fatty acid profile and overall quality of ruminant meat. Grape and citrus fruits by-products also exhibit various bioactivities such as antioxidant, anti-inflammatory and anti-helmintic activities along with enhancing and modulating the immune system of animals. Adding flavonoids extracted from grape pomace and citrus pulp below 200 g/kg DM can result in decrease of occurrence of parakeratosis and acidosis in ruminants. In addition, they can reduce oxidative stress due to high content of phenolic compounds [].
4.2. Utilization of FVW in Poultry Feed
The predicted growth of the global population challenges the production of poultry feed resources as well. FVWs can be a representative of an excellent alternative to the current poultry feed resource in the market []. Presently, poultry are given xenobiotic drugs, which have proven to enhance animal productivity [,]. However, the long-term use of xenobiotic drugs frequently leads to excessive amounts of undesired chemical residues in the bodies of animals, which could be a threat to human health too. Xenobiotic drugs also have a negative impact on the reproductive performance of animals. It is believed that fruit and vegetable waste, apart from benefiting the health and performance of animals, can be a good alternative to synthetic growth hormones and antibiotics [].
Apple pomace is rich source of dietary fibre, pectin, vitamin C and phenolic compounds. Previous studies have showed that the inclusion of apple pomace in a poultry diet improves the productivity and reproduction performances of chickens. However, there are several limitations to its inclusion. These include low digestibility due to the high rate of lignin/cellulose and small amounts of minerals and proteins []. On the other side, apple pulp has a high content of pectin, polyphenols and carbohydrates and it can be used to decrease the amount of uric acid and increase blood glucose levels in chicken []. According to Yitbarek and co-workers, apple pomace can replace 10% of maize bran without a negative impact on performance and production in broilers []. The addition of more than 10% can lead to reduced feed efficiency due to low fibre content. In a study where dried apple pomace was added to the broiler diet in different extent [], the evaluation of gut development, antioxidant capacity, growth, immune response and blood biochemical parameters was performed. The study showed detrimental effects on the mentioned parameters when incremental levels of dried apple pomace were added to the diet. In a study conducted by Heidarisafar and co-researchers, the inclusion of processed apple peel waste on broiler chickens under heat stress was evaluated []. Heat stress can often lead to oxidative stress and decreased productivity of animals. Antioxidants are often used to mitigate oxidative stress, however, the traditional poultry feed, which contains maize and soybean lacks antioxidants. Therefore, there is a vast scope on using feed resources rich in antioxidants such as apple pomace. The study concluded that the inclusion of apple pomace into the feed of broilers from 28 to 49 days of age, increased high-density lipoproteins (HDL) cholesterol, decreased low density lipoprotein (LDL) cholesterol and did not have any adverse effect on broilers’ performance, productivity or carcass characteristics.
Banana waste has the ability to manage blood pressure and prevent the development of cancerous cells, diabetes and certain gastrointestinal diseases due to its high oligosaccharides content. It provides additional energy and has a positive effect on the growth, development, and reproductive performance of poultry due to its high starch levels []. In the study conducted on banana peel, Yitbarek and co-workers concluded that the addition of 10% banana peel enhanced poultry feed efficiency and feed conversion and the quality of poultry eggs and meat. However, its incorporation of more than 10% could result in a decreased growth rate of poultry [].
Grape pomace has also been incorporated into poultry feed. Its high content of polyphenolic compounds has potential to prevent the negative impacts of pathogens and free radicals. A study was conducted on grape pomace inclusion in the feed of broiler chicks with the aim of evaluating its effects on their growth, digestibility of nutrients, blood parameters and meat quality []. Grape pomace supplementation showed positive effects on body weight gain during early growth stages, it reduced serum cholesterol levels and improved meat quality in broilers.
In addition, Kara & Kocaouglu-guclu evaluated grape pomace’s supplementation in the diet of egg-laying hens and evaluated the egg production and quality parameters []. The study results indicated that grape pomace enhanced the overall chicken productivity as well as the production and quality of eggs. Also, overall blood levels of proteins, triglycerides and total serum cholesterol were improved in egg-laying hens []. In a study made by Lichovnikova and co-researchers, it was shown that supplementation of broiler feed with 1.5% red grape pomace did not lead to detrimental effects on their growth []. However, it increased the digestion of nutrients and the metabolisable energy and it also increased levels of antioxidants in the blood and the number of “good” bacteria Lactobacillus spp. in the ileum of broiler chicken.
Pumpkin waste inclusion in poultry feed has been shown to prevent certain degenerative diseases and oxidative stress. It is believed it also helps in the treatment of diabetes []. In regard to pumpkin seed, when added 6% into the diet of broiler chicken, it shows increased weight gain of broiler chicken, a decrease in abdominal fat and improved quality of breast meat []. Also, pumpkin seed oil does not have an adverse effect on productive performance []. Adding pumpkin leaf meal in the diet of broilers results in increased body weight gain and in a decrease of total serum cholesterol and fat content in the heart, gastrus and muscles. Chickens´ diet can also be supplemented with pumpkin seed oil, which extends the animals’ life by decreasing phospholipids, triglyceride and cholesterol concentrations in the blood. In addition, it increases the chicken’s weight and it can result in more eggs per hen weekly. This can be explained by the presence of phytogens in pumpkin, which stimulate the secretion of gastric juices, and enzymes, improve the intestinal mucosa, increase feed intake by stimulating olfactory receptors and enhance reproductive performances [].
Dried tomato pomace is often used in poultry feed, however, it has low energy value which needs to be taken into account when added into feed formulations so that adverse effects are minimized. It is recommended that tomato pomace is added at the level of 15 or 20% in grown chicken. In the diet of layers that require less energy, it can be more successfully incorporated and replace wheat bran. Tomato pomace has a high amount of lycopene, a natural antioxidant, which is health beneficial for poultry. Lycopene and other carotenoids can also positively affect egg yolks´ colour [,].
It is reported that sea buckthorn (SBT) pomace influences the colour, pH and rheological properties of eggs []. It can be used as a natural preservative rich in antioxidants and to inhibit lipid oxidation in breast muscles without altering the physicochemical properties []. In a study undertaken by Sharma et al. 20% of the crude protein of the traditional concentrate has been replaced with sea buckthorn cake in layer birds to evaluate its effect on egg production []. The inclusion of SBT cake in poultry feed by 20% improved egg production and had no negative impact on the quality traits of eggs. Contrary to this, Dvořák and co-workers showed that supplementation of SBT pomace could result in a more intense and darker colour of egg yolk []. In addition, SBT leaves, cake and pomace were added to broiler feed at different levels in the study conducted by Mushtaq and co-researchers []. This study showed that broilers’ growth weight, feed intake and feed conversion ratio were not altered with the inclusion of sea buckthorn pomace, cake, and leaves in the poultry diet. It also did not alter mortality or serum mineral levels. It has been concluded that SBT leaves, cakes and pomace can be exploited as a non-conventional feed resource that can be included in the broiler diet without any adverse side effects.
5. Safety and Regulations
Materials which are not specifically produced for the purpose of developing animal feed can sometimes have unacceptable levels of residuals that can negatively affect animals’ health and animal products. Feeding animals with these materials therefore has to be done considering regional regulations and with caution. FVWs can often be contaminated by heavy metals, toxins, mycotoxins, pesticides and chemical residues. If FVWs are used for the development of animal feed, their presence can negatively affect animal health []. Therefore, following safety regulations, quality control systems and good practices for use of agricultural wastes as animal feed are necessary for ensuring the quality and safety of animal feed []. There are also legal restrictions regarding feeding animals with raw materials in order to prevent the spread of various pathogenic diseases. However, complicated safety regulations and legislation regarding the utilization of FVWs can sometimes intimidate farmers and feed technologists from using them as reliable feed material []. Worldwide, there are various systems used for ensuring the safety of feed ingredients as well as lists of ingredients which can be used and their limits and lists of ingredients which ought to be avoided []. Strict adherence to these safety norms and regulations is necessary.
6. Conclusions
Agri-food industrial wastes and by-products represent an unconventional but very promising alternative to current feed materials available in the market. They are a rich source of valuable bioactive compounds that exhibit a range of health-promoting properties that can contribute to overall animal welfare. In addition, certain bioactive compounds have also shown the ability to decrease both nitrogen (N) and methane (CH4) emissions, therefore, mitigating environmental pollution. However, there are certain limitations in the valorization of wastes and by-products for the development of animal feed that still need to be considered such as a possible decrease in nutrient digestibility, complicated safety regulation and the high cost of waste treatment and transportation involved. In addition, there may always be concerns over high chances of microbial contamination, the occurrence of mycotoxins, the presence of heavy metals, pesticides or other chemical residuals, toxins and unwarranted anti-nutrient compounds. Besides, the cost-effectiveness of converting waste biomass to value-added products like that of animal feed also needs to be carefully designed.
Author Contributions
D.M.: collecting literature, writing, original draft preparation; M.K., writing, and editing; R.B., Conceptualization, visualization, supervision, writing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This review articles theme is based on our ongoing project: ERA-Chair in VALORTECH at the Estonian University of Life Sciences, which has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 810630. The first author gratefully acknowledges the PhD study funding received from this grant.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Eurostat. 2022. Available online: https://food.ec.europa.eu/safety/food-waste_en (accessed on 4 December 2022).
- Coman, V.; Teleky, B.E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.F.; Vodnar, D.C. Bioactive potential of fruit and vegetable wastes. Adv. Food Nutr. Res. 2020, 91, 157–225. [Google Scholar] [PubMed]
- FAO. Definitional Framework of Food Losses and Waste; FAO: Rome, Italy, 2014; Available online: https://www.fao.org/3/at144e/at144e.pdf (accessed on 2 November 2022).
- Salunkhe, D.K.; Kadam, S. (Eds.) Handbook of Fruit Science and Technology: Production, Composition, Storage, and Processing; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Barta, J.; Balla, C.; Vatai, G. Dehydration preservation of fruits. In Handbook of Fruits and Fruit Processing; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 133–151. [Google Scholar]
- Sinha, N.K.; Sidhu, J.; Barta, J.; Wu, J.; Cano, M.P. (Eds.) Handbook of Fruits and Fruit Processing; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Plazzotta, S.; Manzocco, L.; Nicoli, M.C. Fruit and vegetable waste management and the challenge of fresh-cut salad. Trends Food Sci. Technol. 2017, 63, 51–59. [Google Scholar] [CrossRef]
- Bhat, R. (Ed.) Valorization of Agri-Food Wastes and By-Products: Recent Trends, Innovations and Sustainability Challenges; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Hussain, S.; Joudu, I.; Bhat, R. Dietary fiber from underutilized plant resources—A positive approach for valorization of fruit and vegetable wastes. Sustainability 2020, 12, 5401. [Google Scholar] [CrossRef]
- Perussello, C.A.; Zhang, Z.; Marzocchella, A.; Tiwari, B.K. Valorization of apple pomace by extraction of valuable compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 776–796. [Google Scholar] [CrossRef]
- Quiles, A.; Campbell, G.M.; Struck, S.; Rohm, H.; Hernando, I. Fiber from fruit pomace: A review of applications in cereal-based products. Food Rev. Int. 2018, 34, 162–181. [Google Scholar] [CrossRef]
- Schmid, V.; Trabert, A.; Keller, J.; Bunzel, M.; Karbstein, H.P.; Emin, M.A. Defined shear and heat treatment of apple pomace: Impact on dietary fiber structures and functional properties. Eur. Food Res. Technol. 2021, 247, 2109–2122. [Google Scholar] [CrossRef]
- Kasapidou, E.; Sossidou, E.; Mitlianga, P. Fruit and vegetable co-products as functional feed ingredients in farm animal nutrition for improved product quality. Agriculture 2015, 5, 1020–1034. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.H.; Pintea, A. Phenolic compounds, flavonoids, lipids and antioxidant potential of apricot (Prunus armeniaca L.) pomace fermented by two filamentous fungal strains in solid state system. Chem. Cent. J. 2017, 11, 1–10. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, Y.; Wang, Y.; Shi, T.; Zhang, L.; Chen, Y.; Xie, M. Comparison of (poly) phenolic compounds and antioxidant properties of pomace extracts from kiwi and grape juice. Food Chem. 2019, 271, 425–432. [Google Scholar] [CrossRef]
- Achilonu, M.; Shale, K.; Arthur, G.; Naidoo, K.; Mbatha, M. Phytochemical benefits of agroresidues as alternative nutritive dietary resource for pig and poultry farming. J. Chem. 2018, 2018, 1035071. [Google Scholar] [CrossRef]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Mahato, N.; Sharma, K.; Koteswararao, R.; Sinha, M.; Baral, E.; Cho, M.H. Citrus essential oils: Extraction, authentication and application in food preservation. Crit. Rev. Food Sci. Nutr. 2019, 59, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Halmemies-Beauchet-Filleau, A.; Rinne, M.; Lamminen, M.; Mapato, C.; Ampapon, T.; Wanapat, M.; Vanhatalo, A. Alternative and novel feeds for ruminants: Nutritive value, product quality and environmental aspects. Animal 2018, 12, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Arjona, L.P.; Ramírez-Mella, M. Pumpkin waste as livestock feed: Impact on Nutrition and Animal Health and on Quality of Meat, Milk, and Egg. Animals 2019, 9, 769. [Google Scholar] [CrossRef] [PubMed]
- Tayengwa, T.; Mapiye, C. Citrus and winery wastes: Promising dietary supplements for sustainable ruminant animal nutrition, health, production, and meat quality. Sustainability 2018, 10, 3718. [Google Scholar] [CrossRef]
- Shah, F.; Wu, W. Soil and crop management strategies to ensure higher crop productivity within sustainable environments. Sustainability 2019, 11, 1485. [Google Scholar] [CrossRef]
- Balehegn, M.; Duncan, A.; Tolera, A.; Ayantunde, A.A.; Issa, S.; Karimou, M.; Zampaligré, N.; Kiema, A.; Gnanda, I.; Varijakshapanicker, P.; et al. Improving adoption of technologies and interventions for increasing supply of quality livestock feed in low- and middle-income countries. Glob. Food Secur. 2020, 26, 100372. [Google Scholar] [CrossRef]
- Yitbarek, M.B. Some Selected Vegetable and Fruit Wastes for Poultry Feed. J. Vet. Anim. Res. 2019, 1, 1. [Google Scholar]
- Rauw, W.M.; Rydhmer, L.; Kyriazakis, I.; Øverland, M.; Gilbert, H.; Dekkers, J.C.; Hermesch, S.; Bouquet, A.; Gómez Izquierdo, E.; Louveau, I.; et al. Prospects for sustainability of pig production in relation to climate change and novel feed resources. J. Sci. Food Agric. 2020, 100, 3575–3586. [Google Scholar] [CrossRef]
- Wadhwa, M.; Bakshi, M.P.; Makkar, H.P. Waste to worth: Fruit wastes and by-products as animal feed. CAB Rev. 2015, 10, 1–26. [Google Scholar] [CrossRef]
- Bakshi MP, S.; Wadhwa, M.; Makkar, H.P. Waste to worth: Vegetable wastes as animal feed. CAB Rev. 2016, 11, 1–26. [Google Scholar] [CrossRef]
- Adesogan, A.T. What are feeds worth? A critical evaluation of selected nutritive value methods. In Proceedings of the 13th Annual Florida Ruminant Nutrition Symposium, Gainesville, FL, USA, 10–11 January 2002; pp. 33–47. [Google Scholar]
- Chikwanha, O.C.; Raffrenato, E.; Muchenje, V.; Nolte JV, E.; Mapiye, C. Effect of grape (Vitis vinifera L. cv. Pinotage) pomace supplementation on nutrient utilization in finisher lambs. Small Rumin. Res. 2019, 179, 48–55. [Google Scholar] [CrossRef]
- Vinyard, J.R.; Myers, C.A.; Murdoch, G.K.; Rezamand, P.; Chibisa, G.E. Optimum grape pomace proportion in feedlot cattle diets: Ruminal fermentation, total tract nutrient digestibility, nitrogen utilization, and blood metabolites. J. Anim. Sci. 2021, 99, skab044. [Google Scholar] [CrossRef] [PubMed]
- Hanušovský, O.; Gálik, B.; Bíro, D.; Šimko, M.; Juráček, M.; Rolinec, M.; Zábranský, L.; Philipp, C.; Puntigam, R.; Slama, J.A.; et al. The Nutritional Potential of Grape By-Products from the Area of Slovakia and Austria. Emir. J. Food Agric. 2020, 32, 1–10. [Google Scholar] [CrossRef]
- Cassinerio, C.A.; Fadel, J.G.; Asmus, J.; Heguy, J.M.; Taylor, S.J.; DePeters, E.J. Tomato seeds as a novel by-product feed for lactating dairy cows. J. Dairy Sci. 2015, 98, 4811–4828. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, S.; Viveros, A.; Centeno, C.; Romero, C.; Arija, I.; Brenes, A. Effects of dietary grape seed extract on growth performance, amino acid digestibility and plasma lipids and mineral content in broiler chicks. Animal 2013, 7, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Tsiplakou, E.; Zervas, G. The effect of dietary inclusion of olive tree leaves and grape marc on the content of conjugated linoleic acid and vaccenic acid in the milk of dairy sheep and goats. J. Dairy Res. 2008, 75, 270. [Google Scholar] [CrossRef] [PubMed]
- FAO; IFIF. Good practices for the feed industry—Implementing the Codex Alimentarius Code of Practice on Good Animal Feeding. In FAO Animal Production and Health Manual No. 9; FAO: Rome, Italy, 2010. [Google Scholar]
- Kasza, G.; Szabó-Bódi, B.; Lakner, Z.; Izsó, T. Balancing the desire to decrease food waste with requirements of food safety. Trends Food Sci. Technol. 2019, 84, 74–76. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Jörundsdóttir, H.Ó. Food in the bioeconomy. Trends Food Sci. Technol. 2019, 84, 4–6. [Google Scholar] [CrossRef]
- Tedesco, D.E.A.; Scarioni, S.; Tava, A.; Panseri, S.; Zuorro, A. Fruit and Vegetable Wholesale Market Waste: Safety and Nutritional Characterisation for Their Potential Re-Use in Livestock Nutrition. Sustainability 2021, 13, 9478. [Google Scholar] [CrossRef]
- Silva-Espinoza, M.A.; Ayed, C.; Foster, T.; Camacho MD, M.; Martínez-Navarrete, N. The impact of freeze-drying conditions on the physico-chemical properties and bioactive compounds of a freeze-dried orange puree. Foods 2020, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Mphahlele, R.R.; Fawole, O.A.; Makunga, N.P.; Opara, U.L. Effect of drying on the bioactive compounds, antioxidant, antibacterial and antityrosinase activities of pomegranate peel. BMC Complement. Altern. Med. 2016, 16, 1–12. [Google Scholar] [CrossRef]
- Mbondo, N.N.; Owino, W.O.; Ambuko, J.; Sila, D.N. Effect of drying methods on the retention of bioactive compounds in African eggplant. Food Sci. Nutr. 2018, 6, 814–823. [Google Scholar] [CrossRef]
- Jurgilevich, A.; Birge, T.; Kentala-Lehtonen, J.; Korhonen-Kurki, K.; Pietikäinen, J.; Saikku, L.; Schösler, H. Transition towards circular economy in the food system. Sustainability 2016, 8, 69. [Google Scholar] [CrossRef]
- Gabriela MM, I.; Ganjyal, G. Fruit Processing By-Products: A Rich Source for Bioactive Compounds and Value-Added Products. In Food Processing By-Products and their Utilization; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 11–26. [Google Scholar]
- Gowe, C. Review on potential use of fruit and vegetables by-products as a valuable source of natural food additives. Food Sci. Qual. Manag. 2015, 45, 47–61. [Google Scholar]
- De la Torre, I.; Acedos, M.G.; Ladero, M.; Santos, V.E. On the use of resting L. delbrueckii spp. delbrueckii cells for D-lactic acid production from orange peel wastes hydrolysates. Biochem. Eng. J. 2019, 145, 162–169. [Google Scholar] [CrossRef]
- Majerska, J.; Michalska, A.; Figiel, A. A review of new directions in managing fruit and vegetable processing by-products. Trends Food Sci. Technol. 2019, 88, 207–219. [Google Scholar] [CrossRef]
- Krasnova, I.; Segliņa, D. Content of Phenolic Compounds and Antioxidant Activity in Fresh Apple, Pomace and Pomace Water Extract—Effect of Cultivar. Proc. Latv. Acad. Sci. Sect. B. Nat. Exact Appl. Sci. 2019, 73, 513–518. [Google Scholar] [CrossRef]
- Kruczek, M.; Gumul, D.; Kačániová, M.; Ivanišhová, E.; Mareček, J.; Gambuś, H. Industrial Apple Pomace By-Products as A Potential Source Of Pro-Health Compounds In Functional Food. J. Microbiol. Biotechnol. Food Sci. 2017, 7, 22. [Google Scholar] [CrossRef]
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and vegetable waste management: Conventional and emerging approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Anwar, Z.; Irshad, M.; Asad, M.J.; Ashfaq, H. Cellulase production from species of fungi and bacteria from agricultural wastes and its utilization in industry: A review. Adv. Enzym. Res. 2016, 4, 44–55. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Pandey, A. Novel enzymatic processes applied to the food industry. Curr. Opin. Food Sci. 2016, 7, 64–72. [Google Scholar] [CrossRef]
- Uma, C.; Gopalakrishnan, V.K. Utilization of pomegranate peel waste for the production and characterization of invertase using Cladosporium sp. Biosci. Biotechnol. Res. Asia 2016, 5, 277–282. [Google Scholar]
- Uygut, M.A.; Tanyildizi, M.Ş. Optimization of alpha-amylase production by Bacillus amyloliquefaciens grown on orange peels. Iran. J. Sci. Technol. Trans. A Sci. 2018, 42, 443–449. [Google Scholar] [CrossRef]
- Oyedeji, O.; Bakare, M.K.; Adewale, I.O.; Olutiola, P.O.; Omoboye, O.O. Optimized production and characterization of thermostable invertase from Aspergillus niger IBK1, using pineapple peel as alternate substrate. Biocatal. Agric. Biotechnol. 2017, 9, 218–223. [Google Scholar] [CrossRef]
- Bajaj, P.; Mahajan, R. Cellulase and xylanase synergism in industrial biotechnology. Appl. Microbiol. Biotechnol. 2019, 103, 8711–8724. [Google Scholar] [CrossRef]
- Torres-Pitarch, A.; Hermans, D.; Manzanilla, E.G.; Bindelle, J.; Everaert, N.; Beckers, Y.; Torrallardona, D.; Bruggeman, G.; Gardiner, G.E.; Lawlor, P.G. Effect of feed enzymes on digestibility and growth in weanedpigs: A systematic review and meta-analysis. Anim. Feed. Sci. Technol. 2017, 233, 145–159. [Google Scholar] [CrossRef]
- Zhang, T.; Deng, B.; Zhang, R.; Qin, X.; Zhang, J.; Zhao, J. Dietary Sea buckthorn Pomace induces beige adipocyte formation in inguinal white adipose tissue in lambs. Animals 2019, 9, 193. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Jiang, X.; Chen, L.; Hong, L.; Zhuo, Y.; Lin, Y.; Fang, Z.; Che, L.; Feng, B.; et al. Effects of dietary supplementation with exogenous catalase on growth performance, oxidative stress, and hepatic apoptosis in weaned piglets challenged with lipopolysaccharide. J. Anim. Sci. 2020, 98, skaa067. [Google Scholar] [CrossRef]
- Lallès, J.P.; Montoya, C.A. Dietary alternatives to in-feed antibiotics, gut barrier function and inflammation in piglets post-weaning: Where are we now? Anim. Feed. Sci. Technol. 2021, 274, 114836. [Google Scholar] [CrossRef]
- Mandal, M.; Ghosh, U. Statistical Optimization of Fermentation Parameters for Cellulase Production Utilizing Banana Peel. J. Adv. Biol. Biotechnol. 2016, 1–9. [Google Scholar] [CrossRef]
- Uday, U.S.P.; Majumdar, R.; Tiwari, O.N.; Mishra, U.; Mondal, A.; Bandyopadhyay, T.K.; Bhunia, B. Isolation, screening and characterization of a novel extracellular xylanase from Aspergillus niger (KP874102. 1) and its application in orange peel hydrolysis. Int. J. Biol. Macromol. 2017, 105, 401–409. [Google Scholar] [CrossRef]
- Mashetty, S.B.; Biradar, V. Orange peel as novel substrate for enhanced invertase production by a. niger in solid state fermentation. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1114–1121. [Google Scholar] [CrossRef]
- Pacheco, D.; Waghorn, G.C. Dietary nitrogen-definitions, digestion, excretion and consequences of excess for grazing ruminants. Proc. New Zld Grassl. Assoc. 2008, 70, 107–116. [Google Scholar] [CrossRef]
- Tadele, Y.; Amha, N. Use of different non protein nitrogen sources in ruminant nutrition: A review. Adv. Life Sci. Technol. 2015, 29, 100–105. [Google Scholar]
- Friedman, M. Nutritional value of proteins from different food sources. A review. J. Agric. Food Chem. 1996, 44, 6–29. [Google Scholar] [CrossRef]
- Tesseraud, S.; Temim, S.; Le Bihan-Duval, E.; Chagneau, A.M. Increased responsiveness to dietary lysine deficiency of pectoralis major muscle protein turnover in broilers selected on breast development. J. Anim. Sci. 2001, 79, 927–933. [Google Scholar] [CrossRef]
- Maynard, C.W.; Kidd, M.T.; Chrystal, P.V.; McQuade, L.R.; McInerney, B.V.; Selle, P.H.; Liu, S.Y. Assessment of limiting dietary amino acids in broiler chickens offered reduced crude protein diets. Anim. Nutr. 2022, 10, 1–11. [Google Scholar] [CrossRef]
- Boisen, S.; Hvelplund, T.; Weisbjerg, M.R. Ideal amino acid profiles as a basis for feed protein evaluation. Livest. Prod. Sci. 2000, 64, 239–251. [Google Scholar] [CrossRef]
- Huhtanen, P.; Hetta, M.; Swensson, C. Evaluation of canola meal as a protein supplement for dairy cows: A review and a meta-analysis. Can. J. Anim. Sci. 2011, 91, 529–543. [Google Scholar] [CrossRef]
- Kaldmäe, H.; Leming, R.; Kass, M.; Lember, A.; Tölp, S.; Kärt, O. Chemical composition and nutritional value of heat-treated and cold-pressed rapeseed cake. Vet. Ir. Zootech. 2010, 49. [Google Scholar]
- Das, L.K.; Kundu, S.S.; Kumar, D.; Datt, C. The evaluation of metabolizable protein content of some indigenous feedstuffs used in ruminant nutrition. Vet. World 2014, 7, 257–261. [Google Scholar] [CrossRef][Green Version]
- Maxin, G.; Ouellet, D.R.; Lapierre, H. Ruminal degradability of dry matter, crude protein, and amino acids in soybean meal, canola meal, corn, and wheat dried distillers grains. J. Dairy Sci. 2013, 96, 5151–5160. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, A.; Safamehr, A.; Palangi, V.; Mehmannavaz, Y.M. The Determination of metabolizable protein of some feedstuffs used in ruminant. Res. J. Biol. Sci. 2008, 3, 804–806. [Google Scholar]
- Zebeli, Q.; Aschenbach, J.R.; Tafaj, M.; Boguhn, J.; Ametaj, B.N.; Drochner, W. Invited review: Role of physically effective fiber and estimation of dietary fiber adequacy in high-producing dairy cattle. J. Dairy Sci. 2012, 95, 1041–1056. [Google Scholar] [CrossRef]
- Mateos, G.G.; Fondevila, G.; Cámara, L. The importance of the fibre fraction of the feed in non-ruminant diets. In The Value of Fibre. Engaging the Second Brain for Animal Nutrition; González-Ortiz, G., Bedford, M.R., Knudsen, K.E.B., Courtin, C.M., Classen, H.L., Eds.; Wageningen Academic Publisher: Wageningen, The Netherlands, 2019; pp. 61–83. [Google Scholar]
- Kheravii, S.K.; Morgan, N.K.; Swick, R.A.; Choct, M.; Wu, S.B. Roles of dietary fibre and ingredient particle size in broiler nutrition. Worlds Poult. Sci. J. 2018, 74, 301–316. [Google Scholar] [CrossRef]
- Slominski, B.A. Advances in the understanding of dietary fibre and its components in relation to the use of alternative feed ingredients in modern poultry and livestock production. In Proceedings of the 2018 Animal Nutrition Conference of Canada, Cutting Edge Nutritionalstrategies for Improving Performance, Profitability and Sustainability, Edmonton, AL, Canada, 2–3 May 2018; pp. 107–130. [Google Scholar]
- Williams, B.A.; Mikkelsen, D.; Flanagan, B.M.; Gidley, M.J. “Dietary fibre”: Moving beyond the “soluble/insoluble” classification for monogastric nutrition, with an emphasis on humans and pigs. J. Anim. Sci. Biotechnol. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Arranz, S.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Effects of Dietary Fiber Intake on Cardiovascular Risk Factors. In Recent Advances in Cardiovascular Risk Factors; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Mudgil, D. The interaction between insoluble and soluble fiber. In Dietary Fiber for the Prevention of Cardiovascular Disease; Academic Press: Cambridge, MA, USA, 2017; pp. 35–59. [Google Scholar]
- Montagne, L.; Pluske, J.R.; Hampson, D.J. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed. Sci. Technol. 2003, 108, 95–117. [Google Scholar] [CrossRef]
- Van Hees, H.M.J.; Davids, M.; Maes, D.; Millet, S.; Possemiers, S.; Den Hartog, L.A.; van Kempen, T.A.T.G.; Janssens, G.P.J. Dietary fibre enrichment of supplemental feed modulates the development of the intestinal tract in suckling piglets. J. Anim. Sci. Biotechnol. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Noblet, J.; Le Goff, G. Effect of dietary fibre on the energy value of feeds for pigs. Anim. Feed. Sci. Technol. 2001, 90, 35–52. [Google Scholar] [CrossRef]
- Lu, C.D.; Kawas, J.R.; Mahgoub, O.G. Fibre digestion and utilization in goats. Small Rumin. Res. 2005, 60, 45–52. [Google Scholar] [CrossRef]
- Huhtanen, P.; Ahvenjärvi, S.; Weisbjerg, M.R.; Nørgaard, P. Digestion and Passage of Fibre in Ruminants. In Ruminant Physiology: Digestion, metabolism and Impact of Nutrition on Gene Expression, Immunology and Stress; Wageningen Academic Publisher: Wageningen, The Netherlands, 2006; pp. 87–138. [Google Scholar]
- Varga, G.A.; Kolver, E.S. Microbial and animal limitations to fiber digestion and utilization. J. Nutr. 1997, 127, 819S–823S. [Google Scholar] [CrossRef]
- Kuchtová, V.; Karovičová, J.; Kohajdová, Z.; Minarovičová, L. Chemical composition and functional properties of pumpkin pomace-incorporated crackers. Acta Chim. Slovaca 2016, 9, 54–57. [Google Scholar] [CrossRef]
- Gil-Sánchez, I.; Cueva, C.; Sanz-Buenhombre, M.; Guadarrama, A.; Moreno-Arribas, M.V.; Bartolomé, B. Dynamic gastrointestinal digestion of grape pomace extracts. Bioacessible phenolic metabolites and impacto on human gut microbiota. J. Food Compos. Anal. 2018, 68, 41–52. [Google Scholar] [CrossRef]
- Wang, S.; Gu, B.J.; Ganjyal, G.M. Impacts of the inclusion of various fruit pomace types on the expansion of corn starch extrudates. Lebensm. -Wiss. Technol. 2019, 110, 223–230. [Google Scholar] [CrossRef]
- He, C.; Sampers, I.; Raes, K. Dietary fiber concentrates recovered from agro-industrial by-products: Functional properties and application as physical carriers for probiotics. Food Hydrocoll. 2021, 111, 106175. [Google Scholar] [CrossRef]
- Sharoba, A.M.; Farrag, M.A.; Abd El-Salam, A.M. Utilization of some fruits and vegetables waste as a source of dietary fiber and its effect on the cake making and its quality attributes. J. Agroaliment. Process. Technol. 2013, 19, 429–444. [Google Scholar]
- Bender, A.B.B.; Speroni, C.S.; Moro, K.I.B.; Morisso, F.D.P.; dos Santos, D.R.; da Silva, L.P.; Penna, N.G. Effects of micronization on dietary fiber composition, physicochemical properties, phenolic compounds, and antioxidant capacity of grape pomace and its dietary fiber concentrate. Lebensm. -Wiss. Technol. 2020, 117, 108652. [Google Scholar] [CrossRef]
- Turksoy, S.; Özkaya, B. Pumpkin and carrot pomace powders as a source of dietary fiber and their effects on the mixing properties of wheat flour dough and cookie quality. Food Sci. Technol. Res. 2011, 17, 545–553. [Google Scholar] [CrossRef]
- Shao, D.; Bartley, G.E.; Yokoyama, W.; Pan, Z.; Zhang, H.; Zhang, A. Plasma and hepatic cholesterol-lowering effects of tomato pomace, tomato seed oil and defatted tomato seed in hamsters fed with high-fat diets. Food Chem. 2013, 139, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C. Grape pomace as a source of phenolic compounds and diverse bioactive properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.M.; Tou, J.C. A comprehensive analysis of the composition, health benefits, and safety of apple pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Moilanen, J.; Laaksonen, O.; Yang, W.; Tenhu, E.; Yang, B. Phenolic compounds and antioxidant activities of tea-type infusions processed from sea buckthorn (Hippophaë rhamnoides) leaves. Food Chem. 2019, 272, 1–11. [Google Scholar] [CrossRef]
- Pasini, F.; Chinnici, F.; Caboni, M.F.; Verardo, V. Recovery of oligomeric proanthocyanidins and other phenolic compounds with established bioactivity from grape seed by-products. Molecules 2019, 24, 677. [Google Scholar] [CrossRef]
- Gessner, D.K.; Ringseis, R.; Siebers, M.; Keller, J.; Kloster, J.; Wen, G.; Eder, K. Inhibition of the pro-inflammatory NF-κB pathway by grape seed and grape marc meal extract in intestinal epithelial cells. J. Anim. Physiol. Anim. Nutr. 2012, 96, 1074–1083. [Google Scholar] [CrossRef]
- Hassan, Y.I.; Kosir, V.; Yin, X.; Ross, K.; Diarra, M.S. Grape pomace as a promising antimicrobial alternative in feed: A critical review. J. Agric. Food Chem. 2019, 67, 9705–9710. [Google Scholar] [CrossRef]
- Tian, Y.; Liimatainen, J.; Alanne, A.; Lindstedt, A.; Liu, P.; Sinkkonen, J.; Kallio, H.; Yang, B. Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berry plants. Food Chem. 2017, 220, 266–281. [Google Scholar] [CrossRef]
- Tian, Y.; Puganen, A.; Alakomi, H.; Uusitupa, A.; Saarela, M.; Yang, B. Antioxidative and antibacterial activities of aqueous ethanol extracts of berries, leaves, and branches of berry plants. Food Res. Int. 2018, 106, 291–303. [Google Scholar] [CrossRef]
- Sytařová, I.; Orsavová, J.; Snopek, L.; Mlček, J.; Byczyński, Ł.; Mišurcová, L. Impact of phenolic compounds and vitamins C and E on antioxidant activity of sea buckthorn (Hippophaë rhamnoides L.) berries and leaves of diverse ripening times. Food Chem. 2020, 310, 125784. [Google Scholar] [CrossRef]
- Marciniak, B.; Kontek, R.; Żuchowski, J.; Stochmal, A. Novel bioactive properties of low-polarity fractions from sea-buckthorn extracts (Elaeagnus rhamnoides (L.) A. Nelson)—(in vitro). Biomed. Pharmacother. 2021, 135, 111141. [Google Scholar] [CrossRef] [PubMed]
- Kolk, K.; Pällin, R.; Kärner, T.; Oraste, O. Sea buckthorn as a home garden crop. Agraarteadus 2004, 15, 102–106. [Google Scholar]
- Piir, R. Hippophae rhamnoides L. in Estonia for fruit growing. Agraarteadus 1996, 7, 162–175. [Google Scholar]
- Nemzer, B.V.; Kalita, D.; Yashin, A.Y.; Yashin, Y.I. Bioactive Compounds, Antioxidant Activities, and Health Beneficial Effects of Selected Commercial Berry Fruits: A Review. J. Food Res. 2020, 9, 78–101. [Google Scholar] [CrossRef]
- Dienaitė, L.; Pukalskas, A.; Pukalskienė, M.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Phytochemical Composition, Antioxidant and Antiproliferative Activities of Defatted Sea Buckthorn (Hippophaë rhamnoides L.) Berry Pomace Fractions Consecutively Recovered by Pressurized Ethanol and Water. Antioxidants 2020, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Destandau, E.; Le Floch, G.; Lucchesi, M.E.; Elfakir, C. Antimicrobial, antioxidant and phytochemical investigations of sea buckthorn (Hippophaë rhamnoides L.) leaf, stem, root and seed. Food Chem. 2012, 131, 754–760. [Google Scholar] [CrossRef]
- Liepa, L.; Zolnere, E.; Dūrītis, I.; Segliņa, D. Preliminary results of the effect of the seabuckthorn leaves and fruit marc extract on the health indices of calves. In Proceedings of the 4th European Workshop on Seabuckthorn EuroWorks 2016 RPD Abstracts, Riga, Latvia, 17–19 August 2016; Volume 2, p. 83. [Google Scholar]
- Morar, R.; Cimpeanu, S.; Morar, E.; Mărghitaș, L.; Rozalia, Z. Results of the use of certain phytotherapeutic preparations in the feeding of weaned piglets. Bul. Inst. Agron. Cluj-Napoca Ser. Zooteh. Și Med. Vet. 1990, 44, 101–108. [Google Scholar]
- Pathak, G.P.; Sharma, N.; Mane, B.G.; Sharma, D.; Krofa, D.; Khurana, S.K. Effect of Sea buckthorn (Hippophae rhamnoides)-leaves, pulp and oil on growth performance, carcass characteristics and meat quality of broilers chicken. J. Poult. Sci. Technol. 2015, 3, 20–23. [Google Scholar]
- Huff, N.K.; Auer, A.D.; Garza Jr, F.; Keowen, M.L.; Kearney, M.T.; McMullin, R.B.; Andrews, F.M. Effect of sea buckthorn berries and pulp in a liquid emulsion on gastric ulcer scores and gastric juice pH in horses. J. Vet. Intern. Med. 2012, 26, 1186–1191. [Google Scholar] [CrossRef]
- Ositis, U.; Seglina, D.; Strikauska, S.; Bula, S. Influence of sea buckthorn by-products premix feeding on the mare and foal blood biochemical indices. In Proceedings of the 1st Nordic Feed Science Conference, Uppsala, Sweden, 22–23 June 2010; pp. 137–141. [Google Scholar]
- Jain, A.; Varshneya, C.; Bharadwaj, P. Ochratoxin induced immunosupression and its protection by seabuckthorn (Hippophae rhamnoides) and glucomannan in Japanese quail (Courtunix Courtunix Japonica). Indian Vet. J. 2013, 90, 44–46. [Google Scholar]
- Patial, V.; Asrani, R.K.; Patil, R.D.; Kumar, N.; Sharma, R. Protective effect of sea buckthorn (Hippophae rhamnoides L.) leaves on ochratoxin-A induced hepatic injury in Japanese quail. Vet. Res. 2015, 3, 98–108. [Google Scholar]
- Souilem, S.; Fki, I.; Kobayashi, I.; Khalid, N.; Neves, M.A.; Isoda, H.; Sayadi, S.; Nakajima, M. Emerging technologies for recovery of value-added components from olive leaves and their applications in food/feed industries. Food Bioprocess Technol. 2017, 10, 229–248. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Ultrasound increases the aqueous extraction of phenolic compounds with high antioxidant activity from olive pomace. Lebensm. -Wiss. Technol. 2018, 89, 284–290. [Google Scholar] [CrossRef]
- Morsi, M.K.E.S.; Galal, S.M.; Alabdulla, O. Ultrasound assisted extraction of polyphenols with high antioxidant activity from olive pomace (Olea europaea L.). Carpathian J. Food Sci. Technol. 2019, 11, 193–202. [Google Scholar]
- Irakli, M.; Chatzopoulou, P.; Ekateriniadou, L. Optimization of ultrasound-assisted extraction of phenolic compounds: Oleuropein, phenolic acids, phenolic alcohols and flavonoids from olive leaves and evaluation of its antioxidant activities. Ind. Crops Prod. 2018, 124, 382–388. [Google Scholar] [CrossRef]
- Hilali, S.; Fabiano-Tixier, A.S.; Ruiz, K.; Hejjaj, A.; Ait Nouh, F.; Idlimam, A.; Bily, A.; Mandi, L.; Chemat, F. Green extraction of essential oils, polyphenols, and pectins from orange peel employing solar energy: Toward a zero-waste biorefinery. ACS Sustain. Chem. Eng. 2019, 7, 11815–11822. [Google Scholar] [CrossRef]
- Wadhwa, M.; Bakshi, M.P.S. Utilization of fruit and vegetable wastes as livestock feed and as substrates for generation of other value-added products. Rap Publ. 2013, 4, 1–67. [Google Scholar]
- Azhdarzadeh, F.; Hojjati, M. Chemical composition and antimicrobial activity of leaf, ripe and unripe peel of bitter orange (Citrus aurantium) essential oils. Nutr. Food Sci. Res. 2016, 3, 43–50. [Google Scholar] [CrossRef]
- Bustamante, J.; van Stempvoort, S.; García-Gallarreta, M.; Houghton, J.A.; Briers, H.K.; Budarin, V.L.; Matharu, A.S.; Clark, J.H. Microwave assisted hydro-distillation of essential oils from wet citrus peel waste. J. Clean. Prod. 2016, 137, 598–605. [Google Scholar] [CrossRef]
- de Souza, K.A.; de Oliveira Monteschio, J.; Mottin, C.; Ramos, T.R.; de Moraes Pinto, L.A.; Eiras, C.E.; Guerrero, A.; do Prado, I.N. Effects of diet supplementation with clove and rosemary essential oils and protected oils (eugenol, thymol and vanillin) on animal performance, carcass characteristics, digestibility, and ingestive behavior activities for Nellore heifers finished in feedlot. Livest. Sci. 2019, 220, 190–195. [Google Scholar]
- Nanon, A.; Suksombat, W.; Yang, W.Z. Effects of essential oils supplementation on in vitro and in situ feed digestion in beef cattle. Anim. Feed. Sci. Technol. 2014, 196, 50–59. [Google Scholar] [CrossRef]
- Carvalho, V.M.; Ávila, V.A.D.; Bonin, E.; Matos, A.M.; do Prado, R.M.; Castilho, R.A.; Silva, R.R.; de Abreu Filho, B.A.; do Prado, I.N. Effect of extracts from baccharis, tamarind, cashew nut shell liquid and clove on animal performance, feed efficiency, digestibility, rumen fermentation and feeding behavior of bulls finished in feedlot. Livest. Sci. 2021, 244, 104361. [Google Scholar] [CrossRef]
- Mottin, C.; Ornaghi, M.G.; Carvalho, V.M.; Guerrero, A.; Vital, A.C.P.; Ramos, T.R.; Bonin, E.; Lana de Araújo, F.; de Araújo Castilho, R.; do Prado, I.N. Carcass characteristics and meat evaluation of cattle finished in temperate pasture and supplemented with natural additive containing clove, cashew oil, castor oils, and a microencapsulated blend of eugenol, thymol, and vanillin. J. Sci. Food Agric. 2022, 102, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Monrroy, M.; Rueda, L.; Aparicio, A.L.; García, J.R. Fermentation of Musa paradisiaca Peels to Produce Citric Acid. J. Chem. 2019, 2019, 8356712. [Google Scholar] [CrossRef]
- Roukas, T.; Kotzekidou, P. Pomegranate peel waste: A new substrate for citric acid production by Aspergillus niger in solid-state fermentation under non-aseptic conditions. Environ. Sci. Pollut. Res. 2020, 12, 13105–13113. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.R.; Anwar, Z.; Irshad, M.; Mukhtar, S.; Warraich, N.T. Bio-synthesis of citric acid from single and co-culture-based fermentation technology using agro-wastes. J. Radiat. Res. Appl. Sci. 2016, 9, 57–62. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Ayeni, A.O.; Daramola, M.O. Parametric optimization of citric acid production from apple pomace and corn steep liquor by a wild type strain of Aspergillus niger: A Response surface methodology approach. Int. J. Eng. Res. Afr. 2018, 36, 98–113. [Google Scholar] [CrossRef]
- Papadaki, E.; Mantzouridou, F.T. Citric acid production from the integration of Spanish-style green olive processing wastewaters with white grape pomace by Aspergillus niger. Bioresour. Technol. 2019, 280, 59–69. [Google Scholar] [CrossRef]
- Kareem, S.; Akpan, I.; Alebiowu, O. Production of citric acid by Aspergillus niger using pineapple waste. Malays. J. Microbiol. 2010, 6, 161. [Google Scholar]
- Abbas, N.; Safdar, W.; Ali, S.; Choudhry, S.; Elahi, S. Citric acid production from Aspergillus niger using mango (Mangifera indica L.) and sweet orange (Citrus sinensis) peels as substrate. Int. J. Sci. Eng. Res. 2016, 7, 868–872. [Google Scholar] [CrossRef]
- Torrado, A.M.; Cortés, S.; Salgado, J.M.; Max, B.; Rodríguez, N.; Bibbins, B.P.; Converti, A.; Domínguez, J.M. Citric acid production from orange peel wastes by solid-state fermentation. Braz. J. Microbiol. 2011, 42, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Correddu, F.; Lunesu, M.F.; Buffa, G.; Atzori, A.S.; Nudda, A.; Battacone, G.; Pulina, G. Can Agro-Industrial By-Products Rich in Polyphenols be Advantageously Used in the Feeding and Nutrition of Dairy Small Ruminants. Animals 2020, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Soltan, M.; Elsamadony, M.; Mostafa, A.; Awad, H.; Tawfik, A. Harvesting zero waste from co-digested fruit and vegetable peels via integrated fermentation and pyrolysis processes. Environ. Sci. Pollut. Res. 2019, 26, 10429–10438. [Google Scholar] [CrossRef] [PubMed]
- Olmo-García, L.; Monasterio, R.P.; Sánchez-Arévalo, C.M.; Fernández-Gutiérrez, A.; Olmo-Peinado, J.M.; Carrasco-Pancorbo, A. Characterization of new olive fruit derived products obtained by means of a novel processing method involving stone removal and dehydration with zero waste generation. J. Agric. Food Chem. 2019, 67, 9295–9306. [Google Scholar] [CrossRef]
- Schader, C.; Muller, A.; Scialabba, N.E.H.; Hecht, J.; Isensee, A.; Erb, K.H.; Smith, P.; Makkar, H.P.; Klocke, P.; Leiber, F.; et al. Impacts of feeding less food-competing feedstuffs to livestock on global food system sustainability. J. R. Soc. Interface 2015, 12, 20150891. [Google Scholar] [CrossRef]
- Gerlach, K.; Pries, M.; Tholen, E.; Schmithausen, A.J.; Büscher, W.; Südekum, K.H. Effect of condensed tannins in rations of lactating dairy cows on production variables and nitrogen use efficiency. Animal 2018, 12, 1847–1855. [Google Scholar] [CrossRef]
- Koenig, K.M.; Beauchemin, K.A. Effect of feeding condensed tannins in high protein finishing diets containing corn distillers grains on ruminal fermentation, nutrient digestibility, and route of nitrogen excretion in beef cattle. J. Anim. Sci. 2018, 96, 4398–4413. [Google Scholar] [CrossRef]
- Salami, S.A.; Luciano, G.; O’Grady, M.N.; Biondi, L.; Newbold, C.J.; Kerry, J.P.; Priolo, A. Sustainability of feeding plant by-products: A review of the implications for ruminant meat production. Anim. Feed. Sci. Technol. 2019, 251, 37–55. [Google Scholar] [CrossRef]
- Boldea, I.M.; Dragomir, C.; Gras, M.A.; Ropotă, M. Inclusion of rapeseed and pumpkin seed cakes in diets for Murciano-Granadina goats alters the fatty acid profile of milk. South Afr. J. Anim. Sci. 2021, 51, 262–270. [Google Scholar] [CrossRef]
- Mannelli, F.; Cappucci, A.; Pini, F.; Pastorelli, R.; Decorosi, F.; Giovannetti, L.; Mele, M.; Minieri, S.; Conte, G.; Pauseli, M.; et al. Effect of different types of olive oil pomace dietary supplementation on the rumen microbial community profile in Comisana ewes. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- García-Rodríguez, J.; Mateos, I.; Saro, C.; González, J.S.; Carro, M.D.; Ranilla, M.J. Replacing forage by crude olive cake in a dairy sheep diet: Effects on ruminal fermentation and microbial populations in Rusitec Fermenters. Animals 2020, 10, 2235. [Google Scholar] [CrossRef]
- Terramoccia, S.; Bartocci, S.; Taticchi, A.; Di Giovanni, S.; Pauselli, M.; Mourvaki, E.; Urbani, S.; Servili, M. Use of dried stoned olive pomace in the feeding of lactating buffaloes: Effect on the quantity and quality of the milk produced. Asian-Australas. J. Anim. Sci. 2013, 26, 971–980. [Google Scholar] [CrossRef]
- Fayed, A. Influence of feeding mixture of tomato and apple pomace silage to lactating goats on productive performance. Egypt. J. Sheep Goats Sci. 2019, 11, 1–13. [Google Scholar]
- Fang, J.; Cao, Y.; Matsuzaki, M.; Suzuki, H. Effects of apple pomace proportion levels on the fermentation quality of total mixed ration silage and its digestibility, preference and ruminal fermentation in beef cows. Anim. Sci. J. 2016, 87, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Ramdani, D.; Hernaman, I.; Nurmeidiansyah, A.A.; Heryadi, D.; Nurachma, S. Potential Use of Banana Peels Waste at Different Ripening Stages for Sheep Feeding on Chemical, Tannin, and In Vitro Assessments. IOP Conf. Ser. Earth Environ. Sci. 2019, 334, 012003. [Google Scholar] [CrossRef]
- Hao, X.; Diao, X.; Yu, S.; Ding, N.; Mu, C.; Zhao, J.; Zhang, J. Nutrient digestibility, rumen microbial protein synthesis, and growth performance in sheep consuming rations containing sea buckthorn pomace. J. Anim. Sci. 2018, 96, 3412–3419. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.Y.; Ding, N.; Mu, C.T.; Zhang, C.X.; Zhao, J.X.; Zhang, J.X. Effects of sea buckthorn pomace supplementation on energy partitioning and substrate oxidation in male lambs. Anim. Feed. Sci. Technol. 2019, 247, 149–156. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, T.; Cao, Y.; Deng, B.; Zhang, J.; Zhao, J. Effects of dietary sea buckthorn pomace supplementation on skeletal muscle mass and meat quality in lambs. Meat Sci. 2020, 166, 108141. [Google Scholar] [CrossRef]
- Nuernberg, K.; Nuernberg, G.; Priepke, A.; Dannenberger, D. Sea buckthorn pomace supplementation in the finishing diets of pigs—Are there effects on meat quality and muscle fatty acids? Arch. Anim. Breed. 2015, 58, 107–113. [Google Scholar] [CrossRef]
- Dannenberger, D.; Tuchscherer, M.; Nürnberg, G.; Schmicke, M.; Kanitz, E. Sea Buckthorn Pomace Supplementation in the Diet of Growing Pigs—Effects on Fatty Acid Metabolism, HPA Activity and Immune Status. Int. J. Mol. Sci. 2018, 19, 596. [Google Scholar] [CrossRef] [PubMed]
- Palade, L.M.; Dore, M.I.; Marin, D.E.; Rotar, M.C.; Taranu, I. Assessment of Food By-Products’ Potential for Simultaneous Binding of Aflatoxin B1 and Zearalenone. Toxins 2021, 13, 2. [Google Scholar] [CrossRef]
- Chen, L.; Qu, H.; Bai, S.; Yan, L.; You, M.; Gou, W.; Li, P.; Gao, F. Effect of wet sea buckthorn pomace utilized as an additive on silage fermentation profile and bacterial community composition of alfalfa. Bioresour. Technol. 2020, 314, 123773. [Google Scholar] [CrossRef]
- Manso, T.; Gallardo, B.; Salvá, A.; Guerra-Rivas, C.; Mantecón, A.R.; Lavín, P.; De la Fuente, M.A. Influence of dietary grape pomace combined with linseed oil on fatty acid profile and milk composition. J. Dairy Sci. 2016, 99, 1111–1120. [Google Scholar] [CrossRef]
- Guerra-Rivas, C.; Gallardo, B.; Mantecón, Á.R.; del Álamo-Sanza, M.; Manso, T. Evaluation of grape pomace from red wine by-product as feed for sheep. J. Sci. Food Agric. 2017, 97, 1885–1893. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic content and vitro antioxidant characteristics of wine industry and otheragri-food solid waste extracts. J. Food Compos. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Abarghuei, M.J.; Rouzbehan, Y.; Alipour, D. The influence of the grape pomace on the ruminal parameters of sheep. Livest. Sci. 2010, 132, 73–79. [Google Scholar] [CrossRef]
- Achilonu, M.C.; Nwafor, I.C.; Umesiobi, D.O.; Sedibe, M.M. Biochemical proximates of pumpkin (Cucurbitaeae spp.) and their beneficial effects on the general well-being of poultry species. J. Anim. Physiol. Anim. Nutr. 2018, 102, 5–16. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Francis, G.; Becker, K. Bioactivity of phytochemicals in some lesser-known plants and their effects and potential applications in livestock and aquaculture production systems. Animal 2007, 1, 1371–1391. [Google Scholar] [CrossRef]
- Aghili, A.H.; Toghyani, M.; Tabeidian, S.A. Effect of incremental levels of apple pomace and multi enzyme on performance, immune response, gut development and blood biochemical parameters of broiler chickens. Int. J. Recycl. Org. Waste Agric. 2019, 8, 321–334. [Google Scholar] [CrossRef]
- Heidarisafar, Z.; Sadeghi, G.; Karimi, A.; Azizi, O. Apple peel waste as a natural antioxidant for heat-stressed broiler chickens. Trop. Anim. Health Prod. 2016, 48, 831–835. [Google Scholar] [CrossRef]
- Aditya, S.; Ohh, S.J.; Ahammed, M.; Lohakare, J. Supplementation of grape pomace (Vitis vinifera) in broiler diets and its effect on growth performance, apparent total tract digestibility of nutrients, blood profile, and meat quality. Anim. Nutr. 2018, 4, 210–214. [Google Scholar] [CrossRef]
- Kara, K.; Kocaoglu-guclu, B. The effects of different molting methods and supplementation of grape pomace to the diet of molted hens on post-molt performance, egg quality and peroxidation of egg lipids. J. Fac. Vet. Med. Erciyes Univ. 2012, 9, 183–196. [Google Scholar]
- Lichovnikova, M.; Kalhotka, L.; Adam, V.; Klejdus, B.; Anderle, V. The effects of red grape pomace inclusion in grower diet on amino acid digestibility, intestinal microflora, and sera and liver antioxidant activity in broilers. Turk. J. Vet. Anim. Sci. 2015, 39, 406–412. [Google Scholar] [CrossRef]
- Martínez, Y.; Valdivié, M.; Martínez, O.; Estarrón, M.; Córdova, J. Utilization of pumpkin (Cucurbita moschata) seed in broiler chicken diets. Cuba. J. Agric. Sci. 2010, 44, 387–392. [Google Scholar]
- Hajati, H.; Hasanabadi, A.; Waldroup, P.W. Effects of dietary supplementation with pumpkin oil (Cucurbita pepo) on performance and blood fat of broiler chickens during finisher period. Am. J. Anim. Vet. Sci. 2011, 6, 40–44. [Google Scholar] [CrossRef]
- Mansoori, B.; Modirsanei, M.; Kiaei, M.M. Influence of dried tomato pomace as an alternative to wheat bran in maize or wheat based diets, on the performance of laying hens and traits of produced eggs. Iran. J. Vet. Res. 2008, 9, 341–346. [Google Scholar]
- Panaite, T.D.; Mironeasa, S.; Iuga, M.; Vlaicu, P.A. Liquid egg products characterization during storage as a response of novel phyto-additives added in hens diet. Emir. J. Food Agric. 2019, 31, 304–314. [Google Scholar] [CrossRef]
- Orczewska-Dudek, S.; Pietras, M.; Nowak, J. The effect of amaranth seeds, sea buckthorn pomace and black chokeberry pomace in feed mixtures for broiler chickens on productive performance, carcass characteristics and selected indicators of meat quality. Ann. Anim. Sci. 2018, 18, 501–523. [Google Scholar] [CrossRef]
- Sharma, V.K.; Rana, D.; Wadhwa, D.; Hasanuzzaman, M. Effect of feeding seabuckthorn cake (Hippophae L.) on egg production and egg quality in poultry birds. J. Anim. Feed. Sci. Technol. 2017, 45–53. [Google Scholar]
- Dvořák, P.; Suchý, P.; Straková, E.; Doležalová, J. The effect of a diet supplemented with sea-buckthorn pomace on the colour and viscosity of the egg yolk. Acta Vet. Brno 2017, 86, 303–308. [Google Scholar] [CrossRef][Green Version]
- Mushtaq, M.; Sharma, V.K.; Daisy, R.; Sharma, A. Effect of dietary replacement of protein with seabuckthorn products alone and in combination on the performance of broiler birds. J. Anim. Feed. Sci. Technol. 2017, 5, 61–64. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).