Abstract
Continuous and personalized monitoring are beneficial for patients suffering from neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease and multiple sclerosis. However, such levels of monitoring are seldom ensured by traditional models of care. This paper presents NeuroPredict, a secure edge–cloud Internet of Medical Things (IoMT) platform that addresses this problem by integrating commercial wearables and in-house sensors with cognitive and behavioral evaluations. The NeuroPredict platform links high-frequency physiological signals with periodic cognitive tests through the use of a modular architecture with lightweight device connectivity, a semantic integration layer for timestamp alignment and feature harmonization across heterogeneous streams, and multi-timescale data fusion. Its use of encrypted transport and storage, role-based access control, token-based authentication, identifier separation, and GDPR-aligned governance addresses security and privacy concerns. Moreover, the platform’s user interface was built by considering human-centered design principles and includes role-specific dashboards, alerts, and patient-facing summaries that are meant to encourage engagement and decision-making for patients and healthcare providers. Experimental evaluation demonstrated the NeuroPredict platform’s data acquisition reliability, coherence in multimodal synchronization, and correctness in role-based personalization and reporting. The NeuroPredict platform provides a smart system infrastructure for eHealth and remote monitoring in neurodegenerative care, aligned with priorities on wearables/IoMT integration, data security and privacy, interoperability, and human-centered design.
1. Introduction
The aging of the global population [1] leads to the prevalence of chronic conditions requiring continuous and specialized monitoring. This challenges traditional models of care that are mostly unable to provide such monitoring, especially in real-time and at scale.
Neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and Multiple Sclerosis (MS) are chronic, degenerative, and currently incurable [2]. Their prevalence has led to a growing public health crisis, particularly in aging populations. They lead to significant impairments in cognitive, physical, and functional abilities. In spite of their diverse pathophysiologies, neurodegenerative diseases are all characterized by a prolonged preclinical phase, a varied course, and a significant amount of individual heterogeneity in response to treatment. These characteristics motivate the need for their early detection [3], continuous monitoring [4], and the use of personalized care strategies [5].
AD, the most frequent cause of dementia, is characterized by progressive cognitive decline with memory impairment [6] and loss of independence [7], with pathological changes being observable decades before the emergence of clinical symptoms. Early diagnosis and the monitoring of cognitive and functional changes in AD [8] can enable timely interventions and improve the patient’s quality of life.
PD is characterized by a loss of dopaminergic neurons, leading to motor symptoms including tremors, rigidity, and bradykinesia [9]. This is often accompanied by mental decline, with symptoms such as depression, sleep disturbances, and autonomic dysfunction frequently preceding motor symptoms. As the disease progresses, individuals face a variety of motor complications and treatment side effects [10]. This motivates the need for personalized monitoring.
MS is a chronic autoimmune disease that leads to demyelination in the central nervous system, with symptoms like vision and movement problems [11]. Its progression is difficult to predict and manage due to its variable pace for different individuals. This motivates the continuous monitoring of symptoms and the use of biomarkers [12].
Traditional healthcare models lack the real-time and decision-making capabilities needed to manage neurodivergent conditions like the ones described above. Artificial intelligence (AI), wearable sensors, telemedicine and other digital health advancements could offer a solution. Their non-invasive, real-time, and customized monitoring can support proactive data-driven treatment decisions. Their continuous use for early detection and personalized monitoring could contribute to a healthcare system’s response to the disease’s prevalence and cost.
This paper introduces NeuroPredict, a secure and flexible digital platform for personalized health monitoring and decision-making in the context of neurodegenerative diseases. It details the development of the platform’s underlying infrastructure, which integrates behavioral and physiological data collected from patient interfaces and connected devices. The NeuroPredict platform facilitates the remote, continuous, and customized monitoring of key patient health parameters such as vital signs, gait patterns, and cognitive performance. This raw health data can then be used by healthcare professionals to obtain actionable insights, helping them to detect disease progression early, develop more individualized treatment plans, and plan more timely interventions. While supporting these features, the platform also safeguards security and patient privacy and complies with healthcare regulations.
The NeuroPredict platform is proposed as a novel approach for the care of patients with neurodegenerative conditions, which combines adaptability, personalization, and security in facilitating data-informed decision-making. As opposed to most other solutions for the treatment of patients with neurodegenerative diseases, which either use only commercial wearables or focus on single-parameter monitoring, the NeuroPredict platform harmonizes heterogeneous data streams from both commercial and in-house IoMT devices and integrates multimodal physiological, cognitive, and behavioral data. The platform exposes role-based dashboards with alerts and physician reports to support decision-making and patient engagement. All of the above make this work relevant to broader research in eHealth and mHealth and especially contribute to recent directions in IoMT-enabled monitoring and smart system infrastructures for healthcare [13,14].
In this paper, the platform is evaluated as a proof-of-concept in laboratory conditions to inform from a technical and usability perspective, subsequent to clinical deployment.
This work aims to address the following research question: under controlled laboratory conditions, can a secure and modular IoMT platform reliably collect, synchronize, and semantically harmonize heterogeneous physiological, cognitive, and behavioral data that is useful for the monitoring of neurodegenerative diseases? To this end, it has the following objectives: (1) designing and developing a scalable edge–cloud architecture, which can integrate commercial and in-house IoMT devices; (2) developing a semantic and temporal harmonization layer, which can synchronize data from heterogenous streams; (3) developing user-specific dashboards and role-based interfaces, which can present the data to patients and healthcare providers; (4) conducting a laboratory proof-of-concept evaluation of the multimodal acquisition, synchronization, and role-based personalization. These objectives define the scope and contribution of this work within the ongoing development of the NeuroPredict platform.
The paper is structured as follows: Section 2 presents the platform architecture and how data integration, data management, and pre-processing and the user interfaces were designed. Section 3 presents the results of the proof-of-concept evaluation under controlled conditions in a laboratory. Section 4 discusses contributions, limitations, and future work. Section 5 concludes this work.
2. Materials and Methods
Multimodal Internet of Medical Things (IoMT) platforms that can integrate wearable sensing, behavioral monitoring, and cloud–edge processing pipelines for the continuous monitoring of neurodegenerative diseases are becoming a popular topic in the literature. Previous work has suggested that the physiological and behavioral data produced by commercial wearables is reliable enough for the purposes of health applications. For example, Zambotti et al. recently reported that the Oura Ring, Fitbit, and Withings devices deliver reasonably accurate sleep staging and nocturnal physiology when compared to polysomnography [15]. Fay-Karmon et al. showed that home-based gait analysis using inertial sensors can identify motor changes that are important when monitoring disease progression [16].
The more recent literature demonstrated that health trajectories can be identified more accurately by using multiple heterogeneous data inputs. For example, multi-modal time-series models using data from wearable sensors, speech analysis, and motor assessments have been shown to outperform single-source monitoring for the early detection of PD [3]. Using data from sleep metrics, daily activity patterns, and digital cognitive tasks led to superior predictive accuracy in monitoring AD and mild cognitive impairment compared to using unimodal approaches [17]. Combining accelerometry with patient-reported outcomes was found to result in more reliable digital phenotypes for a functional assessment for MS [18].
Hybrid cloud–edge architectures are used more and more frequently in IoMT platforms for chronic conditions because of their benefits in reducing latency, improving acquisition robustness, and preserving privacy. To reliably integrate heterogeneous data streams, secure device interoperability, semantic annotation, and timestamp harmonization have recently been identified as being essential [19]. Semantic integration frameworks defining specific semantic capabilities, sampling characteristics, and contextual descriptors have been shown to improve consistency in cross-device fusion [20]. Moreover, Fast Healthcare Interoperability Resources (FHIR)-based data models are increasingly being used to facilitate interoperability with electronic health records and ensure the maintainability of remote monitoring platforms [21].
The number of e-health and m-health digital monitoring platforms has been growing, and their monitoring capabilities have been expanding. However, most digital monitoring platforms now focus on a single disease, use a small number of sensors, or rely only on commercial wearables. Most mobile and app-centric platforms focus on capturing task-driven or self-reported measures, with physiological data streams only being partially integrated. This constrains their monitoring potential. Additionally, several existing solutions do not support unified multimodal data fusion or access for different types of users, which limits their scalability in clinical workflows. In contrast, new research into IoMT frameworks recommends the implementation of secure interoperability, multimodal signal integration, and structured role-based interfaces [22], which are all included in the NeuroPredict platform.
In conclusion, while existing IoMT and eHealth solutions show progress in wearable-based monitoring, mobile assessments, and cloud–edge data acquisition pipelines, they are limited by narrow disease domains and isolated sensor modalities. Most current systems only provide physiological or behavioral metrics, missing the opportunity to integrate them with cognitive evaluations, longitudinal behavioral markers, and contextual information in a coherent processing workflow. Many solutions handle heterogeneous data sources independently, which limits their scalability and longitudinal interpretability. The NeuroPredict platform addresses several of the above gaps through the following: consolidating diverse physiological, behavioral, and cognitive inputs into a unified processing and visualization pipeline; integrating heterogeneous sensing modalities under a single edge–cloud architecture; and including user-facing components that reflect multiple clinical perspectives.
Within this landscape, the NeuroPredict platform is positioned as a multimodal proof-of-concept system that consolidates diverse physiological, behavioral, and cognitive inputs into a unified processing and visualization pipeline. By integrating heterogeneous sensing modalities under a single edge–cloud architecture and designing user-facing components that reflect multiple clinical perspectives, the NeuroPredict platform addresses several gaps identified in prior work and offers a more comprehensive foundation for continuous monitoring of Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis.
The NeuroPredict platform integrates commercial and in-house IoMT-based devices for the continuous monitoring of neurodegenerative diseases. These devices capture physiological, behavioral, and cognitive signals, ranging from cardiovascular and respiratory parameters to gait variability, sleep quality, and cognitive performance scores. A modular architecture ensures that these heterogenous data streams are securely acquired, timestamped, and stored in an encrypted format in a database on the cloud.
2.1. Platform Architecture and Technological Foundation
2.1.1. Platform Architecture
Figure 1 presents the architecture of the NeuroPredict platform. It emphasizes the technology used for secure and efficient data processing, as well as how secure communication between the backend, data processing components, and frontend is ensured.
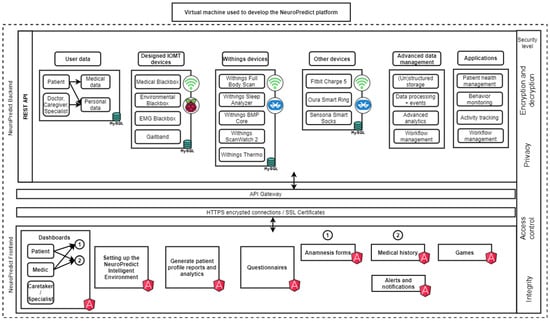
Figure 1.
Architecture of the NeuroPredict platform, illustrating modular backend–frontend design, IoMT device integration, secure communication, and analytics modules for real-time monitoring.
The frontend of NeuroPredict platform was built using modern technologies:
- Angular 17 and TypeScript 3.4 were used to build modular and scalable components (e.g., role-specific dashboards), which simplify data organization and user flow.
- Angular’s Reactive Extensions for JavaScript 7.8 library was used to handle data streams in real time, thus enabling health monitoring and the sending of alerts when thresholds are exceeded.
- Tailwnd CSS 3.4 and Sassy CSS (SCSS) 1.77 helped design coherent and visually attractive user interfaces.
- HTML5 and CSS3 were used to develop the structural foundation of the interface, facilitating navigation and good visual feedback.
These frontend technologies were integrated with the intention to support usability and accessibility for patients and healthcare providers, following established design principles. The current version of the platform is a proof of concept; thus, a formal usability evaluation has not been performed yet. The NeuroPredict platform contains custom dashboards containing useful information and operations for each user category. The UI/UX design includes features such as customization options for contrast and text size to improve accessibility for users with diverse requirements.
In its current version, the NeuroPredict platform presents different role-appropriate dashboards to clinicians, caregivers, and patients. This contributes to its security and traceability and reflects the RBAC model adopted throughout the platform.
The backend of the NeuroPredict platform integrates technologies that manage communication, data analysis, and storage:
- PHP 8.2 manages the interaction between the frontend and the database.
- C# 12.0 handles the core functionality for health data processing.
- C++ 20 was used for context, requiring high-performance computing like managing and analyzing complex physiological datasets.
- MySQL 8.0 handles structured data, while MongoDB handles semi-structured or unstructured data, ensuring flexibility and scalability in storing real-time and big data.
The platform’s backend architecture is organized into functional modules defining its logical structure, including: (1) the data ingestion and gateway communication module, which receives incoming IoMT streams; (2) the preprocessing and semantic harmonization module, which is responsible for validation, timestamp alignment, and feature-level normalization; (3) the data management module, which handles structured and unstructured storage; and (4) the analytics and reporting module, which manages rule-based event handling and the generation of role-specific summaries. PHP, C#, C++, MySQL, and MongoDB are technologies used for the implementation of the functionality included in these modules, but do not define the conceptual boundaries between the modules.
Figure 2 shows the progression of IoMT data streams from the ingestion layer to preprocessing, semantic harmonization, timestamp alignment, multimodal fusion, and event handling within the advanced data management subsystem. It also illustrates the interactions with the hybrid storage layer (MySQL for structured entities and MongoDB for high-frequency and semi-structured entities) and how the obtained unified data are inputted by the platform’s analytics and application layers.
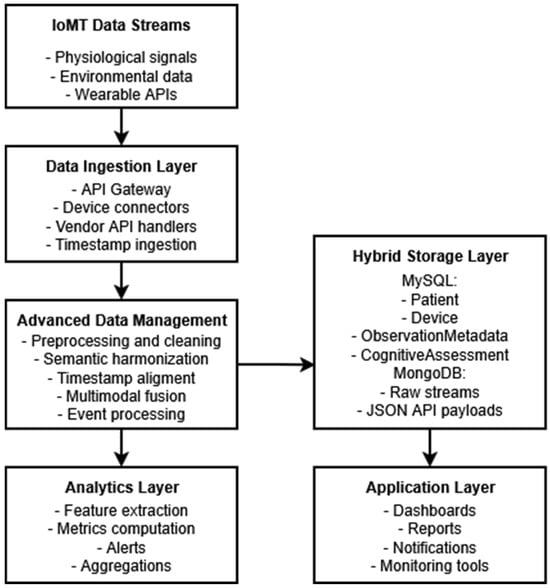
Figure 2.
Detailed architecture of the advanced data management subsystem on the NeuroPredict platform.
The modular design allows efficient integration with various medical devices and sensors, and so the NeuroPredict platform can evolve and scale with the changing clinical needs. Moreover, the platform can synchronously handle both continuous and episodic health data. This makes it particularly suitable for monitoring the subtle motor, vital, and cognitive changes occurring on different timescales in AD, PD, and MS.
2.1.2. Infrastructure
The different IoT devices are integrated within the NeuroPredict platform by using various communication protocols, including Message Queue Telemetry Transport (MQTT) for telemetry messaging and Hypertext Transfer Protocol Secure (HTTPS) for secure data transmission. Moreover, Raspberry Pi-based IoT gateways handle data collection and encryption from devices without default secure connections or needing local preprocessing.
The NeuroPredict platform uses Raspberry Pi 4 Model B units as edge gateways, which are chosen due to their balance between processing capability and energy efficiency during continuous monitoring. The model includes a quad-core ARM Cortex-A72 (1.5 GHz) CPU, 2–8 GB RAM (the 4 GB version being used on the NeuroPredict platform), dual-band Wi-Fi and Bluetooth 5.0 connectivity, and a gigabit Ethernet interface. These enable local preprocessing, encrypted data transfer, and stable operation when multiple IoMT data streams are ingested in parallel. An X729 UPS device shield ensures safe device shutdown and uninterrupted power during lengthy laboratory evaluations. The Raspberry Pi gateway supports secure sensor communication, timestamping, preliminary validation, and forwards harmonized data to the cloud layer.
Data are processed and stored using MySQL and NoSQL solutions, as mentioned in the previous section. Apache 2.4.62 enables fast efficient processing from the data streams.
Two cloud infrastructures are used:
- The Romanian national cloud service for public institutions (ICIPRO): A cloud service used for testing and experimentation within regulatory boundaries in Romanian public institutions.
- Microsoft Azure, under an infrastructure as a service (IaaS) model: A scalable cloud service for development, testing, and deployment.
This hybrid infrastructure is meant to ensure adaptability, scalability, and performance, while at the same time respecting national and international standards for digital health solutions [23].
All structured and semi-structured data for the current version of the NeuroPredict platform is stored in secure MySQL and MongoDB databases hosted on our institution’s (ICI Bucharest) dedicated cloud infrastructure (ICIPRO). Microsoft Azure is not used for storing patient-related data, but only for development, testing, and automated deployment as an infrastructure as a service.
2.1.3. Security and Privacy Principles
The NeuroPredict platform uses a multi-layered security architecture to protect sensitive medical data and maintain trust. Its security mechanisms include the following:
- Role-Based Access Control (RBAC), ensuring that users only have access to data and functionality that are relevant to their role, thus preventing unauthorized access.
- Encryption of all data exchanged between clients and servers using Transport Layer Security/Secure Sockets Layer (TLS/SSL) protocols, ensuring data integrity and confidentiality.
- Secure communication by using the HTTPS protocol, ensuring data integrity and confidentiality during transmission.
- Secure, stateless user authentication, using JSON Web Tokens (JWT).
To ensure privacy, the NeuroPredict platform processes personal health data in compliance with data privacy and security regulations, such the General Data Protection Regulation (GDPR), the Health Insurance Portability and Accountability Act, the Medical Device Regulation (MDR) and other applicable standard requirements [24]. This includes dedicated processes for data anonymization to protect privacy while supporting analytics and research.
The NeuroPredict platform has also been designed to include additional protection mechanisms as needed for various types of health data. Ongoing auditing and monitoring help to ensure that the NeuroPredict platform remains compliant with data privacy and security regulations, and is adaptable to changes in legislation and technology.
2.2. Integration of IoMT Devices in the NeuroPredict Platform
AD, PD, and MS often progress through subtle cross-domain interactions like changes in gait, joined by cognitive and sleep quality changes. This motivates the multimodal acquisition of physiological, behavioral, and cognitive markers. On the NeuroPredict platform, multimodal acquisition is achieved through the integration of a variety of IoMT devices and the collection, temporal synchronization, and standardization of data from them to ensure comparability across heterogenous sources. The data harmonization approach is meant to match, enhance, and evaluate multimodal data streams in a clinically reliable manner.
2.2.1. Commercial Wearables
Commercial wearable devices have become widely available, usable, and validated by research. They produce continuous and longitudinal data streams, which can help analyze subtle motor, cognitive, and sleep quality changes in AD, PD, and MS [25,26]. Therefore, a decision was made to integrate such devices from multiple vendors and collect different kinds of health data for the NeuroPredict platform: devices for activity tracking and accelerometry (Fitbit (Fitbit LLC, San Francisco, CA, USA)), sleep and circadian rhythms (Oura Ring (Ōura Health Oy, Oulu, Finland), Withings Sleep Analyzer (Withings S.A., Issy-les-Moulineaux, France)), vital signs and cardiovascular monitoring (Withings ScanWatch (Withings S.A., Issy-les-Moulineaux, France), Withings BPM Core (Withings S.A., Issy-les-Moulineaux, France)), and body composition (Withings Body + scale (Withings S.A., Issy-les-Moulineaux, France)).
Table 1 describes the commercial wearables integrated with the NeuroPredict platform, as grouped by medical domain. It presents their key measured parameters, relevance for monitoring AD, PD, and MS, and clinical validation.

Table 1.
Commercial wearable devices integrated with the NeuroPredict platform, grouped by medical domain. Clinical validation and access features are indicated where available.
The last row in Table 1 does not describe a standalone wearable device but refers to data-access capabilities; it was added because API-based data retrieval is a critical requirement for all integrated commercial devices, facilitating their interoperability within the NeuroPredict platform.
As can be noticed in Table 1, commercial wearables provide complementary datasets across the cardiovascular, metabolic, sleep, and mobility domains, which are essential for the monitoring of AD, PD, and MS.
2.2.2. In-House Developed Devices
The NeuroPredict platform additionally integrates several devices that were developed in-house to provide physiological, motor, and environmental parameters that are not otherwise covered by the integrated commercial solutions. These devices were meant to provide flexibility in sensor integration, high-frequency acquisition, and for experimental validation. They were developed internally as modular hardware systems but make use of commercially available sensing modules. These modules are certified and frequently used in biomedical applications, and include surface ECG sensors (e.g., AD8232), EMG units (e.g., MyoWare 2.0), inertial measurement units, SpO2 probes, and environmental sensors. The in-house development involved device enclosure design, hardware assembly, firmware development, data acquisition and synchronization logic, and secure transmission workflows, but not custom fabrication of sensing components. The decision to use commercially validated and certified sensors was motivated by their accuracy and reliability in producing physiological and environmental data, compounded with their flexible integration into in-house devices.
They each consist of a 3-D printed “Blackbox,” hosting modular sensors and electronics:
- The Medical “Blackbox” integrates electrocardiography (ECG) (AD8232), respiratory flow meters (SFM3300-D), peripheral capillary oxygen saturation (SpO2), and temperature probes [8,34]. It was developed on ESP32 and Raspberry Pi microcontrollers and supports local preprocessing and encrypted data transmission. On the NeuroPredict platform, it allows for continuous recording of cardiovascular and muscular signals.
- The Ambiental “Blackbox” records environmental data by integrating sensors for air quality (carbon dioxide (CO2), volatile organic compounds (VOC)), ambient temperature, humidity, light intensity, and motion. Such data are important when analyzing sleep quality, fatigue, and symptom changes in AD and PD [8,34].
- The EMG “Blackbox” is a dedicated electromyographic acquisition system which uses MyoWare 2.0 modules and wireless shields. It produces high-resolution EMG recordings, which are matched with accelerometer and gait signals and sent over ESP32 microcontrollers [35]. The EMG “Blackbox” has been tested for muscle activation analysis in a laboratory context.
- The Gaitband is a wearable device which includes accelerometers, gyroscopes, and pressure sensors to monitor gait in terms of its features [8,34]. It has a higher resolution and granularity than standard activity trackers, which allows it to be used to identify early changes in gait. This can help discover impairments that are not clinically visible and that are associated with disease progression in PD and MS [36,37,38].
Table 2 summarizes the characteristics of the in-house devices: their embedded sensors, key measured parameters, and clinical relevance for AD, PD, and MS. It also shows how the data that they capture complements that of the commercial devices, and that they also ensure that multimodal monitoring also covers the respiratory, muscular, and environmental dimensions. To date, they have only been used for testing interoperability, synchronization, and preprocessing pipelines in controlled laboratory conditions.

Table 2.
In-house developed devices integrated in the NeuroPredict platform, grouped by medical domain. Each prototype was designed as a modular, 3D-printed unit and validated under laboratory conditions.
2.2.3. Connectivity and Interoperability
The NeuroPredict platform uses a semantic integration layer, with each device including a self-describing metadata model with variables related to context, testing parameters, predicted data quality criteria, and measurement areas. This helps it distinguish comparable data from several devices like, for example, gait variability from an industry-standard accelerometer and a specially designed smart belt. All sensing modalities that were used are non-invasive and unobtrusive: the commercial wearables (e.g., wrist-worn devices, sleep mats, smart scales) do not penetrate the skin or require specialized clinical placement to collect physiological, sleep, activity, and cardiovascular signals; the Medical Blackbox uses surface electrodes for ECG, EMG, and SpO2; the Ambiental Blackbox passively records environmental parameters; and the Gaitband captures motion and posture through body-worn inertial sensors. In the current version of the platform, no clinician-applied sensors are used, and the sensing modalities are meant for short-term, in-laboratory use by the development team members. The semantic integration layer is a controlled version to ensure trace history and coherence when changes are made to the devices.
To address IoMT data heterogeneity, the NeuroPredict platform includes multi-layer timestamp synchronization and normalization procedures. As devices could face clock instability or connectivity issues, their time sources are aligned by a reference time protocol linked to the edge gateway’s Network Time Protocol (NTP)-synchronized clock. Incoming data streams are smoothed or resampled within customizable compliance periods, which ensures that underlying health events are interpreted in the correct order. This is particularly important for the monitoring of neurodegenerative diseases, in which subtle temporal variations should not be missed or misunderstood.
Feature-level harmonization is used to integrate measurements and dynamic values among sensors. For example, a standard frequency domain representation is used to harmonize heart rate calculations from different commercial devices, and acceleration measurements obtained in different g-forces are translated to m/s2. Such conversions ensure that consistent and comparable characteristics are provided to the predictive models. They require device capability identifiers from the semantic registry, which establish conversion variables and estimated sensor tolerances.
The NeuroPredict platform also includes multi-timescale data fusion. Low and high-frequency data (e.g., weekly cognitive tests, daily environmental exposures, 50 Hz gait signals) are coordinated by a data fusion process, using hierarchical temporal aggregation. The data are first aligned within short time frames (seconds to minutes) to help discover significant issues (e.g., freezing of gait). Identical characteristics are then summarized over hours and days to enable longitudinal trend analysis and the pursuit of chronic progression. This facilitates clear phenotyping and quick alerting.
Apart from quantitative information, the NeuroPredict platform also provides cognitive evaluation questionnaires and outcomes provided by patients. For example, the Montreal Cognitive Assessment (MoCA) cognitive evaluation is considered, alongside the previous week’s step count variability and sleep efficiency scores. The semantic integration layers timestamps and includes metadata for such information, thus giving it equal importance to objective data. This approach facilitates the building of an integrated risk profile for the patient.
Data segments received by the platform from any device are tagged with a session identifier, a unique patient alias, and a reference to the corresponding version of the data schema. This supports accurate interpretation, backward compatibility across NeuroPredict platform updates, robust data lineage audits, and traceable merging. The source metadata are stored in an append-only audit log that provides tamper-proof evidence of data origin and transformation phases. This allows downstream AI modules to differentiate the original data from its predictive outputs.
The NeuroPredict platform runs quality-check pipelines to verify data before analyzing it. Mechanisms included in these pipelines are complex anomaly detection procedures using autoencoders trained on normal signal distributions and rule-based plausibility filters. The management dashboard labels data segments that are deemed problematic and requests their manual checking. The use of this approach in pilot deployments reduced the amount of automatically analyzed incorrect data, thus improving clinical understanding of the platform’s outcomes.
The NeuroPredict platform uses a modular device acquisition framework to systematically enroll new IoMT devices. It includes semantic mapping, device capability assertion, and test-data validation against platform conformance requirements. It ensures that devices can integrate smoothly with the platform, provided they match the JavaScript Object Notation (JSON) schemas and semantic descriptors. A common data schema was developed for the experimental multimodal device integration, which does not derive from an established healthcare interoperability standard. To ensure interoperability with clinical systems, this schema is planned to be mapped to HL7/FHIR and constrained FHIR profiles as part of future work. The schema’s separation of entities, observations, and metadata enables future transformation into FHIR-compliant structures. Such a transformation will require rules and the definition of constrained profiles, which is expected for early-stage IoMT solutions and does not affect future interoperability. This supports the rapid development of new sensor varieties by just expanding the ontology and running mapping tests.
In initial trials with multiple sensors, including a commercial heart rate monitor and sleep monitor, or an in-house smart “gaitband” device, NeuroPredict platform successfully achieved device integration. Timestamp normalization and feature-level harmonization successfully enabled data fusion, as demonstrated by cross-device event synchronization reaching sub-second median offsets. Merging weekly MoCA results with daily step counts led to the platform identifying initial signs of cognitive–motor interaction, confirming the usefulness of combining different IoMT data streams.
In future work, the NeuroPredict platform will incorporate on-edge knowledge, in which only brief alerts are sent to the cloud. Instead, preliminary event detection (e.g., fall occurrences, significant arrhythmia) will be conducted locally at the gateway. This is meant to lower latency and bandwidth and enhance restrictions on confidentiality.
The NeuroPredict platform is designed to adopt Health Level Seven/Fast Healthcare Interoperability Resources (HL7/FHIR) as the canonical exchange model and to expose a FHIR-compatible API for core resources (patient, device, observation, questionnaire/questionnaire response, care plan). This facilitates its future clinical interoperability. On the short term, future work includes mapping current device payloads to FHIR and constrained profiles, and enabling a secure, bidirectional exchange with electronic medical record (EMR) modules. This approach will reduce vendor lock-in, enable traceable data lineage, and prepare the NeuroPredict platform for integration with existing clinical workflows.
2.3. Multisource Data Collection, Preprocessing, and Secure Management
The NeuroPredict platform relies on comprehensive and high-quality data collection, which directly influences its effectiveness in the monitoring and management of neurodegenerative diseases.
2.3.1. Multisource Data Collection and Integration
The NeuroPredict platform collects data from various sources: (1) IoMT sensors and smart devices tracking daily activities and behavior on an ongoing and unobtrusive basis; (2) clinical and medical history data, such as chronic conditions, treatments, surgeries, allergies, and family history, facilitating personalized monitoring and treatment; (3) patients and caregivers manually inputting data for devices that cannot send data automatically, such as glucometers (e.g., CareSens (i-SENS, Inc., Seoul, Republic of Korea)). Caregivers are seen by the NeuroPredict platform as having a role that is linked to—but distinct from—that of patients. This separation between roles contributes to the platform’s security and privacy by facilitating appropriate access control, clear acknowledgement of manual entries, and full traceability. Data are ingested both in structured formats (e.g., SQL-based clinical records) and semi-structured ones (JSON outputs from wearable devices), and they are harmonized in the database layer.
Data are integrated from in-house medical devices (e.g., medical and ambient “backboxes”, “gaitband” devices) and commercial medical devices (Withings, Fitbit, Oura, and Sensoria). The former produces physiological and behavioral metrics, while the latter provide additional biometric and lifestyle-related data.
The data that are provided by commercial devices such as Withings are first collected and encrypted on the manufacturer’s servers. NeuroPredict platform uses secure OAuth 2.0 authorization to retrieve this data (from Withings, through the Withings Health Data API) [39]. Only those de-identified physiological, sleep, and activity metrics that the user allowed access to are retrieved. They are obtained in JSON format, checked against the NeuroPredict platform’s semantic descriptors, and stored in the MySQL/MongoDB databases hosted on ICIPRO. They are processed exclusively in the platform’s environment hosted on ICIPRO. This approach is similar to the ones adopted for other systems developed by our team, involving collecting data originating from a Withings device from the Withings cloud through authenticated API calls and adding it to local processing pipelines [8,40]. Withings ensures GDPA compliance for all health data stored on its cloud infrastructure and maintains HIPAA-aligned safeguards for U.S. users, according to its regulatory and privacy policies.
The NeuroPredict platform includes two types of cognitive assessments: standardized cognitive tests (e.g., Mini-Mental State Exam (MMSE), MoCA), which objectively rate cognitive functions, and interactive cognitive games, which help evaluate memory, attention, and executive functions. The results of these assessments are saved in the patient’s profile and help monitor cognitive functions while also stimulating mental engagement.
The platform also monitors lifestyle-related risk factors such as smoking, alcohol consumption, and physical activity levels. These make it possible to gauge patient vulnerability and offer personalized preventative solutions.
To improve the quality of the outcomes and expand the platform’s applicability, the NeuroPredict platform integrates open-source datasets from public clinical studies and international initiatives related to neurodegenerative diseases. These open-source datasets are not mixed with data originating from the patient but used in isolated development pipelines solely for the purpose of algorithmic development: to pre-train and benchmark feature-extraction modules, validate data-quality filters, and test the robustness of the multimodal fusion procedures.
The platform supports both automatic and manual updates. This makes it possible to record in real time any new symptoms, medical test results, and treatment changes, and to react promptly as the patient’s disease progresses.
2.3.2. Data Transmission and Synchronization
The data collected by the platform from multimodal sources are transmitted through secure channels to prevent them from being corrupted before processing [41]. The Raspberry Pi interface used for transmission ensures that data are integrated and ready for analysis.
NeuroPredict platform uses lightweight protocols such as MQTT for telemetry and Bluetooth Low Energy for short-range connectivity with wearables, while all cloud communication is secured using HTTPS/TLS. Wearable devices typically use similar lightweight communication standards, because they ensure efficiency and interoperability with consumer health environments [41]. These standards offer the same benefits for continuous monitoring. For AD, PD, and MS, secure and lossless data transmission is a must, since missing or corrupted data may prevent the identification of events like subtle gait abnormalities, falls, or arrhythmic episodes.
2.3.3. Preprocessing Pipelines and Feature Extraction
Robust preprocessing is essential in the monitoring of neurodegenerative diseases, because even small artifacts or inaccuracies can affect the interpretation of the data. For example, motion artifacts in ECG could be misinterpreted as arrhythmic episodes, and inconsistent resampling of gait signals could mask early signs of mobility decline in PD or MS. To prevent such issues, the NeuroPredict platform includes normalization, timestamp harmonization, and integrity checks prior to feature extraction in all its preprocessing pipelines.
The data are preprocessed to ensure their quality and relevance before being analyzed. This consists of the following steps:
- Initial Data Validation and Normalization: The received data are logically validated and made into a consistent predefined format to ensure their integrity and compatibility.
- Encryption Plans (Secure Hashing Algorithm (SHA) + Advanced Encryption Standard (AES)): The NeuroPredict platform uses SHA for data integrity and identity verification and AER for encrypting health data before storage.
- JWT: The architecture of the NeuroPredict platform includes mechanisms to separate user identities from sensitive data. JWT allows for secure, stateless authentication and secure token storage mechanisms integrated into the design to manage session integrity and access control.
These design decisions share similarities with those made in recent work on IoMT-based preprocessing for the monitoring of chronic diseases [42] and multimodal feature extraction strategies for neurodegenerative disorders [43]. Likewise, the authors used normalization and encryption within preprocessing.
2.3.4. Cloud Storage and Data Security
All the collected structured data (e.g., personal details, health metrics, test results, access permissions) are stored in a MySQL cloud database, with only the backend having access to it. The database schema includes core entities such as Patient, Device, ObservationMetadata, CognitiveAssessment, AlertRule, UserRole, and AccessLog, represented as tables in the database. Each table stores pseudonymized user identifiers, device registration information, cognitive test scores, role-based permissions, and system events required for auditing. The schema was designed to support referential integrity, traceability, and secure linkage between multimodal data sources. The semi-structured or unstructured data are stored in a MongoDB database. They include high-frequency physiological payloads (e.g., accelerometry, ECG, EMG), environmental sensor packets, timestamped raw measurements from in-house devices, and JSON-based data retrieved through vendor APIs (e.g., sleep metrics, daily summaries). MongoDB collections maintain the original device-specific JSON structure while also supporting flexible indexing, rapid ingestion and retrieval during preprocessing, and synchronization. Personal patient identifiers are stored separately from health data, and the data are anonymized before harmonization, for data protection purposes. To make sure that users can only retrieve data that they are allowed according to their roles, data access is governed through RBAC [44]. Audit logs are produced frequently to track database changes and support accountability and reproducibility. Data encryption is used in transit using TLS/SSL, and at rest using AES-based storage encryption.
Figure 3 describes the NeuroPredict platform’s conceptual data model. It clarifies how MySQL and MongoDB support the hybrid storage architecture needed for real-time acquisition, secure traceability, multimodal fusion, and compliant data governance.
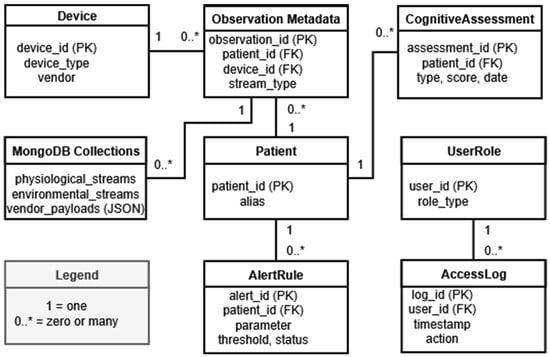
Figure 3.
Conceptual data model of the NeuroPredict platform, showing structured entities stored in MySQL and semi-structured high-frequency sensor streams stored in MongoDB. The symbol “*” indicates multiplicity (“zero or many”) in accordance with standard entity-relationship notation.
The relational layer implemented in MySQL is responsible for organizing all the structured data needed for multimodal monitoring. The Patient table represents its central entity and stores pseudonymized identifiers for each monitored patient. It has several dependent entities: CognitiveAssessment, storing timestamped cognitive test scores; ObservationMetadata, storing information about each multimodal acquisition, including device identifiers, stream types, and temporal intervals; and AlertRule, including personalized notifications based on thresholds. The Device table stores device-related information for both commercial and in-house devices. It is linked to the ObservationMetadata table, which helps ensure traceability, referential integrity, and consistent linkage between the different types of inputs. The UserRole table facilitates access control by storing permissions for patients, caregivers, clinicians, and administrators. The AccessLog table records timestamped actions performed by authenticated users to enable accountability. Together with UserRole, it supports privacy-aware data management, controlled access through RBAC, and detailed auditing of interactions with health data.
The high-frequency and semi-structured data (e.g., raw accelerometry, ECG, EMG, SpO2, environmental packets, vendor-specific JSON payloads) is recorded in MongoDB collections. The ObservationMetadata relational table has a storage reference to these collections, thus linking each structured data entry with its raw data stream. MongoDB maintains the original device-specific data structures, which enables rapid ingestion, flexible indexing, and efficient retrieval during the preprocessing and multimodal synchronization routines.
As a follow up to the description of the structured and semi-structured entities, the model specifies the cardinalities in the relational schema. The model specifically shows the one-to-many relationships of patients with cognitive assessments, multimodal observations, and alert rules, as well as the relationships of user roles and access logs. The model shows that every ObservationMetadata entry is related to one and only one patient and one and only one device, ensuring that all multimodal acquisitions are consistent and fully traceable. The MongoDB collections, described as document-oriented repositories, are referenced in ObservationMetadata to illustrate the hybrid relational–document data architecture of the NeuroPredict platform.
2.3.5. From Data Processing to Knowledge Extraction
The data are securely stored and processed on the cloud. The storage layer carries out replication and scheduled backups for relational data. Sharding is used to balance the load and maintain availability during high-volume ingestion. These approaches are typical for time series data in healthcare IoT and aim to improve resilience and prevent data loss [45].
Data processing involves algorithms and tools which analyze raw data and extract the following:
- Daily summaries on heart rate, heart rate variability, and sleep metrics.
- Gait analysis and EMG metrics: preliminary processing pipelines for them are currently being evaluated in terms of reliability and clinical relevance using pilot data
The resulting insights are used in several applications for monitoring and managing neurodegenerative diseases. These applications provide the information in real time and support clinical decision-making.
To date, the platform only supports rule-based and exploratory feature extraction. Plans are in place to introduce machine learning (ML) components, which can predict the evolution of neurodegenerative diseases once there is enough labeled data and validation.
The NeuroPredict platform includes explicit provenance tracking and metadata governance throughout the data lifecycle. Each phase (ingestion, preprocessing, feature generation) is traced using timestamps, software versions, and schema identifiers, which also makes it reproducible. The data lineage and transformation history are saved in a lightweight metadata registry, which ensures comparability, even if device formats and preprocessing routines change over time [46].
2.3.6. User Interaction Interfaces
The data insights can be visualized by healthcare providers and patients on user-friendly dashboards available for computers and tablets. The dashboard interfaces also provide alerts and notifications when anomalies occur in the data, e.g., unstable gait or variable heart rate. This is meant to offer proactive support on top of the descriptive information.
The multimodal dashboards were developed in line with the research recommendation of integrating digital monitoring for neurodegenerative conditions [47]. To date, the dashboard features have only been experimentally validated using simulated patient scenarios.
Figure 4 illustrates the entire integration pipeline of the NeuroPredict platform, emphasizing the role of each processing phase and showing how multimodal data streams are gradually transformed to become interpretable visual outputs presented on dashboards.
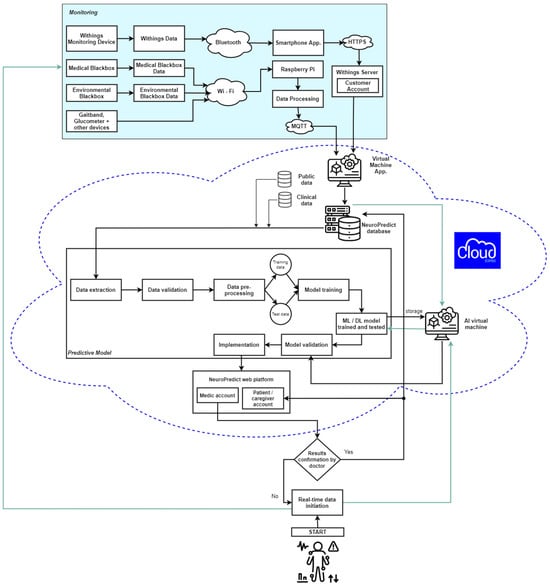
Figure 4.
Data flow in the NeuroPredict platform, showing IoMT device acquisition, edge preprocessing, secure transmission, cloud storage, and user dashboards.
2.4. Human-Centered Design Considerations
The NeuroPredict platform has been built to be focused on patients and healthcare providers in order to guarantee its acceptability in real-world medical settings. It aims to present therapeutically relevant findings in a usable and accessible manner, thus bridging the disparity between complex data processing pipelines and the needs of users. While the NeuroPredict platform’s dashboards have only undergone laboratory evaluations so far, embedding usability and accessibility into their interfaces paves the road to user trust and clinical acceptance.
To complement these human-centered design considerations, Figure 5 presents the UML use case diagram of the NeuroPredict platform.
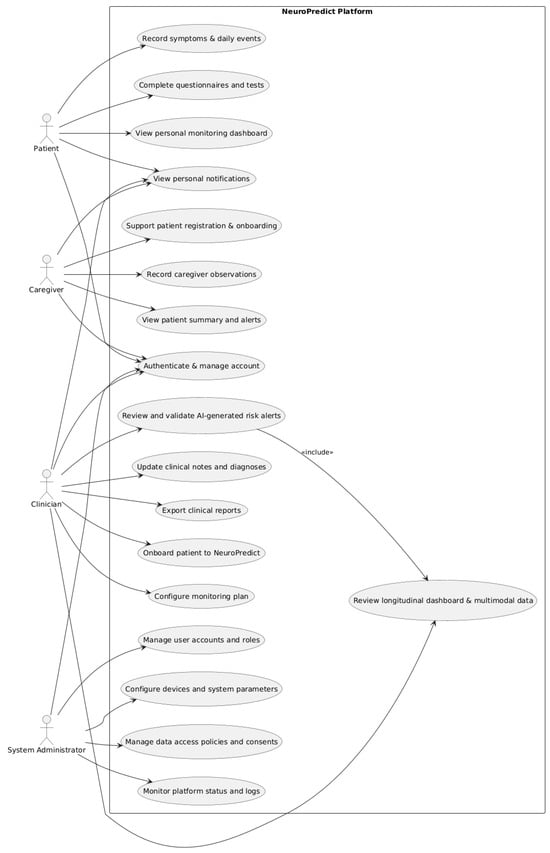
Figure 5.
Use case diagram of the NeuroPredict platform.
Figure 5 illustrates the specific ways in which the four user roles—patient, caregiver, clinician, and system admin—interact with the features of the platform. Conventional UML notations, including actor–use case connections and associations, are used to show the specific privileges and permissions of each role.
3. Results
Development and testing of the platform under laboratory conditions is ongoing. So far, the integration of commercial and in-house IoMT devices, personalized dashboards, cognitive and behavioral assessment modules, alert mechanisms, and patient engagement functionalities has been implemented and validated. The results presented in this paper describe the progress achieved to date, which gives the platform a proof-of-concept character. The NeuroPredict platform is expected to have been fully validated in the laboratory by the end of 2026. This will be followed by its preparation for subsequent clinical validation.
3.1. Dashboards
One of the key features of the NeuroPredict platform which has been validated under controlled laboratory conditions is its customizable monitoring dashboards. Built using modular frontend frameworks which make them device-compatible (on desktop, tablet, and smartphone), these dashboards integrate a variety of data streams to track a patient’s health pathway. They use rule-based data organization by clinical significance to indicate variations from patient-specific baselines, which can support timely awareness. For example, the NeuroPredict platform issues a high priority alert on the dashboard if over a seven-day period, the patient experienced increasing gait asymmetry which was associated with decreased quality of sleep. The displayed data are managed securely through secure transmission and access control mechanisms defined in the system’s architecture.
The NeuroPredict platform’s frontend has been developed using Django 4.2, which offered support for quick implementation via its templates, integration with backend logic, and access to secure databases. Responsive CSS frameworks were used to ensure usability across devices, and changes such as adjustable contrast and font sizes were added for increased accessibility. Django was also used to provide role-based authentication, so that users can access functionalities that are specific to their profiles: physicians’ longitudinal views of physiological and cognitive data, caregivers’ alerts and summarized patient trends, and patients’ simplified dashboards and preliminary self-assessment questionnaires.
The platform’s homepage, including the login interface for different user roles and a direct option to perform a preliminary self-assessment of neurodegenerative risk for unregistered users, is presented in Figure 6.
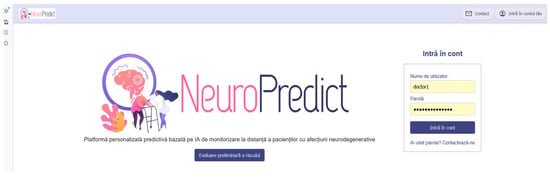
Figure 6.
Homepage of the NeuroPredict platform, illustrating the role-based login and access to the preliminary risk assessment module.
The interface displayed in Figure 6 represents the homepage page of the NeuroPredict platform, shown in its original implementation language (Romanian). The main interface elements include ”Platformă personalizată predictivă bazată pe IA de monitorizare la distanță a pacienților cu afecțiuni neurodegenerative” (Personalized predictive AI-based platform for remote monitoring of patients with neurodegenerative diseases), “Intră în cont” (Log in), “Nume de utilizator” (Username), “Parolă” (Password), “Ai uitat parola? Contactează-ne” (Forgot your password? Contact us), and “Evaluare preliminară a riscului” (Preliminary risk assessment). Additional navigation options visible in the header include “Contact” (Contact) and “Intră în contul tău” (Access your account). These labels correspond to standard authentication and navigation functions and illustrate the operational user interface of the platform.
Testing under laboratory conditions had positive outcomes: the role-based dashboards present correct parameters, accessibility settings work consistently across devices, and questionnaire results are securely transmitted and stored. Despite validation being only under controlled conditions, these outcomes support the feasibility of role-based personalization.
3.2. Cognitive and Behavioral Assessments
Healthcare providers can set up alert criteria such as the minimal time frame for irregular sequences, acceptable confidence intervals, and optimal aggregation periods to fit their patients’ risk profiles and receive customized alerts for their patients. The alert system is expandable to include more clinical guidelines or algorithmic criteria. Alerts can be set up to be sent as emails, secure in-app messages, or push notifications on an associated mobile application, to provide the flexibility required in current care settings.
The interactive cognitive assessment component provided on the platform’s frontend allows patients to complete cognitive assessments like MMSE and MoCA or shorter personalized cognitive tasks. Answers receive scores immediately and are saved in the patient’s longitudinal data profile. Cognitive assessments and optional reminders for them can be scheduled to allow patients to take assessments both under clinical supervision and at home while reducing the administrative burden.
A unified data model is used to put together questionnaire-based inputs and data streams that were obtained from the sensors. Cognitive and behavioral assessment results are automatically time-stamped, encrypted, and stored in the MySQL database, using Django ORM. Associated metadata (device ID, session identifier, role-based user token) is also maintained for traceability purposes. Python 3.13 routines ensure data alignment between assessment and continuous data streams (e.g., gait accelerometry, heart rate variability), supporting common indexing against the same temporal reference.
Figure 7 presents the questionnaire interface, where assessments are grouped into four categories: general cognitive screening tools like MMSE and MoCA (1), dedicated questionnaires on symptoms and functional difficulties for AD (2), PD (3), and MS (4). As questionnaire data are provided for visualization alongside physiological data, this allows the NeuroPredict platform to adapt its behavioral and cognitive monitoring to different patient profiles.
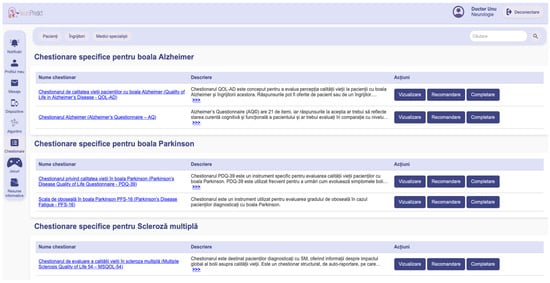
Figure 7.
Interface of the NeuroPredict platform showing the organization of self-assessment questionnaires into four categories: generic cognitive tests, AD-specific, PD-specific, and MS-specific questionnaires.
The interface displayed in Figure 7 represents the questionnaire management page of the NeuroPredict platform, shown in its original implementation language (Romanian). The page is structured by pathology-specific sections, including “Chestionare specifice pentru boala Alzheimer” (Questionnaires specific to Alzheimer’s disease), “Chestionare specifice pentru boala Parkinson” (Questionnaires specific to Parkinson’s disease), and “Chestionare specifice pentru Scleroza multiplă” (Questionnaires specific to Multiple Sclerosis). Table headers include “Nume chestionar” (Questionnaire name), “Descriere” (Description), and “Acțiuni” (Actions). The “Descriere” (Description) column contains short explanatory texts summarizing the purpose, clinical scope, and intended use of each questionnaire, indicating the targeted condition and the type of patient-reported or clinician-assisted assessment supported by the platform. Available actions for each questionnaire are “Vizualizare” (View), “Recomandare” (Recommendation), and “Completare” (Complete). The top navigation and filtering elements include “Pacienți” (Patients), “Îngrijitori” (Caregivers), “Medici specialiști” (Specialist physicians), “Căutare” (Search), “Notificări” (Notifications), “Profilul meu” (My profile), “Mesaje” (Messages), “Dispozitive” (Devices), “Chestionare” (Questionnaires), “Jocuri” (Games), and “Resurse informative” (Informational resources). The user role and session controls displayed in the header include “Doctor” (Doctor), “Neurologie” (Neurology), and “Deconectare” (Log out). These interface elements illustrate the operational dashboard used by clinicians to manage and assign standardized disease-specific questionnaires within the NeuroPredict platform.
3.3. Patient Empowerment and Engagement
User friendliness for patients was prioritized in the development of the NeuroPredict platform. To motivate their engagement, a streamlined overview interface provides them with short, easy-to-understand explanations of the current data levels. Preliminary studies with development team members playing the role of patients revealed increased understanding and motivation to self-monitor.
The patient dashboard is presented in Figure 8. It includes personalized activity reminders (about exercising, taking questionnaires, or cognitive games) and a medication schedule. These allow patients to manage their daily tasks and follow their treatment plan, thus encouraging them to engage actively in the management of their disease and not only rely on passive monitoring.
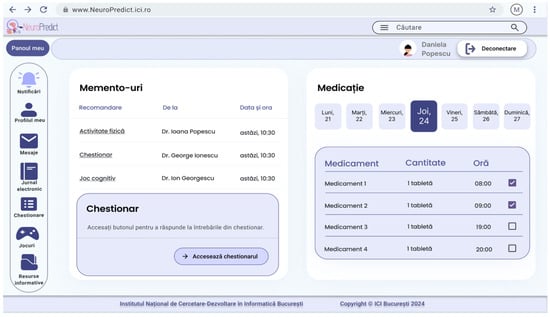
Figure 8.
Patient dashboard in the NeuroPredict platform, presenting personalized reminders and medication management tools to support self-monitoring and daily adherence.
The interface displayed in Figure 8 represents the patient dashboard of the NeuroPredict platform, shown in its original implementation language (Romanian). The main dashboard sections include “Panoul meu” (My dashboard), “Memento-uri” (Reminders), “Medicație” (Medication), and “Chestionar” (Questionnaire). The reminders panel includes the columns “Recomandare” (Recommendation), “De la” (From), and “Data șa ora” (Date and time), and displays entries such as “Activitate fizică” (Physical activity), “Chestionar” (Questionnaire), and “Joc cognitiv” (Cognitive game), together with the prescribing clinician and scheduled time. The questionnaire section contains the instruction “Accesați butonul pentru a răspunde la întrebările din chestionar” (Use the button to answer the questionnaire questions) and the action button “Accesează chestionarul” (Access the questionnaire). The medication management panel displays a calendar view with weekday labels “Luni” (Monday), “Marți” (Tuesday), “Miercuri” (Wednesday), “Joi” (Thursday), “Vineri” (Friday), “Sâmbătă” (Saturday), and “Duminică” (Sunday), and a table with the columns “Medicament” (Medication), “Cantitate” (Quantity), and “Oră” (Time). Navigation and account-related elements include “Notificări” (Notifications), “Profilul meu” (My profile), “Mesaje” (Messages), “Jurnal electronic” (Electronic journal), “Chestionare” (Questionnaires), “Jocuri” (Games), “Resurse informative” (Informational resources), “Căutare” (Search), the user name display, and “Deconectare” (Log out). These elements illustrate the operational patient-facing dashboard used for daily monitoring, medication adherence, and task reminders within the NeuroPredict platform.
Security is addressed through the encryption of all patient interactions and the use of the Hypertext Transfer Protocol Secure (HTTPS) when interactions are transmitted between the frontend and backend. Traceability is ensured by timestamping all patient interactions.
4. Discussion
The NeuroPredict platform is not only a proof of concept but reflects a shift to digital health platforms which have evolved from a mere focus on device performance to including multimodal integration with physiological, cognitive and environmental streams, semantic interoperability, and user-centered design. This shift corresponds to broader trends in eHealth and mHealth to link technical feasibility with clinical relevance, usability, and regulatory compliance.
The present NeuroPredict platform prototype exhibits several important differences when compared with existing digital health and IoMT-based monitoring solutions. While the literature emphasizes progress in wearable-driven monitoring and mobile health tools, most current solutions focus on single-domain physiological metrics or app-centric self-reported measures, with little view of cognitive, behavioral, and environmental data. Other systems continuously collect data from sensors, but not by integrating heterogeneous data streams into a unified, semantically coherent workflow that is available to users based on their role. The NeuroPredict platform, on the other hand, combines multimodal physiological, behavioral, and cognitive data within a single edge–cloud architecture, and presents it to users through role-specific dashboards. This approach is consistent with the current directions in the literature. It also addresses the gaps present in other solutions with regard to multimodal fusion, semantic coherence, and longitudinal interpretability.
The platform can be improved by incorporating advanced AI-driven multimodal fusion models. In particular, hypergraph neural networks have recently been promoted as being particularly effective in identifying multi-relational dependencies among diverse data modalities. For example, Zhu et al. [48] have shown that hypergraph structures can be used to model complex interactions because they encode higher-order relationships within a unified modeling space. This is useful for the NeuroPredict platform, because cognitive, behavioral, and physiological data co-evolve in non-linear ways, and so using graph-based or hypergraph-based fusion mechanisms would facilitate context-aware, semantically enriched reasoning across modalities. Incorporating such approaches is possible due to the platform’s modular architecture but would require clinically validated datasets.
Another useful enhancement of the NeuroPredict platform could be explainable predictive analytics for modeling disease trajectories. Recently, the early progression patterns of neurodegenerative diseases have been successfully characterized by explainable machine learning models using longitudinal digital biomarkers. Xia et al. [18] showed that the evolution of MS severity trends can be predicted accurately and in an explainable way by integrating passively sensed behaviors with ecological momentary assessments. Junaid et al. [3] developed explainable multimodal time-series models for early Parkinson’s disease detection, showing that combining physiological, motor, and behavioral data with interpretability can help identify clinically meaningful trends. On the NeuroPredict platform, we plan to add to existing rule-based reasoning transparent ML modules that can predict progression, identify high-risk transitions, and support data-driven clinical decision-making once a good number of clinically validated datasets are available.
4.1. Usability and Patient Empowerment
The NeuroPredict platform supports customization to encourage long-term patient engagement. Patients can set up health-related personal goals, such as sleep quality or daily activity goals, which the platform monitors and provides reports on. They are also provided with personalized educational resources which can help them self-manage. These are available through web-based applications which patients can integrate when needed to prevent them from becoming overwhelmed.
Patients and healthcare providers can communicate through secure bidirectional messaging using text and documents. Patients can make inquiries and share personal observations, and healthcare providers can proactively engage them to discuss their health trends. The data are saved in a centralized repository that is connected to the patient database.
Usability was prioritized and evaluated in laboratory conditions. It was the result of deliberate design decisions to facilitate patient empowerment and participatory medicine. Different users were provided with the level of detail that they need in their dashboards: patients were provided with simplified summaries which empowered them to manage their disease while not overwhelming them; caregivers were provided with adherence-monitoring data; and clinicians were provided with multimodal visualizations which support their supervision and decision-making. By connecting individual monitoring and feedback from the healthcare provider, patients were encouraged to engage even more.
4.2. Related Work and Comparison with Related eHealth Approaches
While continuous, home-centered monitoring using IoMT components is slowly being prioritized in eHealth nowadays, most eHealth solutions are still domain-specific. For example, in spite of its promising validation results for real-world symptom tracking and wearability, PDMonitor® mostly focuses on motor changes and the alignment of clinical scores [10].
Other IoMT-based platforms with wearable sensors and fog-enabled architectures have been evaluated in laboratory studies; however, most of them have never been broadly integrated into clinical workflows [49]. Consumer-grade wearables have only been partly validated. For example, clinical-grade accuracy has only been confirmed for some parameters, and issues with interoperability and usability were found for five consumer-grade devices for monitoring atrial fibrillation in the BASEL Wearable Study [25].
IoMT-based platform validation for sleep tracking has also shown variable results. Schyvens et al. evaluated six commercial wrist-worn devices by comparing their outputs with polysomnography and found that the devices were reasonably accurate in detecting sleep–wake but not as successful for finer-grained sleep analysis [26]. In a review of wearable sensors for sleep tracking in PD, Matos et al. reported their feasibility in free-living environments, but variable performance [30].
While edge and fog computing are increasingly being promoted and recent surveys have found evidence that they reduce latency and improve efficiency, the same surveys have highlighted long-term issues that they introduce concerning interoperability, security, and multi-stream synchronization [50].
Most of the above research involved evaluations that were restricted to few devices and limited user cohorts, suggesting a current lack of maturity of IoMT in healthcare [51].
Compared to previous research, the NeuroPredict platform integrates commercial and in-house developed devices, ambient sensors, and cognitive assessments. Secure and scalable data management is offered through a hybrid edge-to-cloud infrastructure (custom gateways with ICIPRO and Azure backends). Role-specific dashboards are provided to patients, caregivers, and providers, as specialized in their needs. The combination of multimodal IoMT integration, regulatory compliance, and usability makes the NeuroPredict platform a more coherent solution for the management of neurodegenerative diseases than current approaches, even if the platform has so far only been validated under controlled laboratory conditions.
4.3. Ethical and Regulatory Considerations
Consent for metadata and role-based access control ensure data privacy. Healthcare providers can only access data that patients have given consent for. Patients can only review their own data and do not have access to unauthorized raw data. They can update their consent with immediate effect on the frontend, which ensures compliance with the GDPR and upcoming European Union Artificial Intelligence Act (EU AI Act) frameworks.
As emphasized by recent regulatory instruments, usability evaluation must be built into the development of technical solutions, formative and summative, involving representative users and aligned with regulatory and guidance frameworks for safety and user experience. This can ensure user acceptability in the long term. The U.S. Food and Drug Administration (FDA)’s “Digital Health Technologies for Remote Data Acquisition in Clinical Investigations” (finalized December 2023) highlighted the need for digital health technologies to respect verification, validation, data quality, and risk management criteria when used in clinical trials [52]. The European Commission’s Medical Device Coordination Group (MDCG) 2025-4 Guidance on the safe making available of medical device software (MDSW) apps on online platforms (June 2025) emphasizes that healthcare apps must respect the responsibilities for usability, accessibility, and user feedback mechanisms mentioned under the MDR/In Vitro Diagnostic Regulation (IVDR) [53].
4.4. System Responsiveness and Future Extensions
Initial performance testing in the laboratory revealed that the interface of the NeuroPredict platform is adequately responsive, exhibiting error rates of under 0.5% during peak data refreshes and page loading speeds of under 500 ms in simulated network settings. Such positive results are essential to maintain clinical trust in care scenarios where latency and visualization delays are unacceptable. The repeatability of the performance results was checked for the long term, using a laptop with an Intel i5 CPU and a tablet with 4 GB RAM. While laboratory testing has confirmed adequate responsiveness for real-time use in controlled settings, future clinical validation is essential.
To quantify latency, we calculated the time (i.e., delay) between the server-side processing timestamp of each incoming data packet with the corresponding frontend rendering timestamp recorded in the angular-based dashboard. We measured this delay repeatedly under laboratory conditions using logged timestamps from backend and frontend components. For the device-to-backend transmission delay, we calculated the difference between the sensor timestamps and the server ingestion timestamps. To evaluate the synchronization between heterogeneous data streams, we checked whether data was ingested within the time expected by the internal scheduling mechanism (cross-timestamp alignment). These evaluations only used instrumentation available in the prototype and can be rerun for optimization or clinical testing purposes.
In addition to the evaluation outlined above, numerical benchmarks related to latency, data-flow stability, and inter-stream synchronization can be obtained from the platform’s logs for backend processing, device-to-server ingestion, and frontend rendering. The systematic extraction and reporting of such benchmarks is planned for future phases of this work. In this current phase, the focus was on validating architectural soundness and ensuring stable end-to-end operation.
The platform’s modular architecture supports real-time responsiveness but also aligns with research that systematically combines wearable sensor streams with clinical assessments to support continuous monitoring. The recent literature has demonstrated the superiority of integrating physiological signals from wearables with clinician-reported measures or standardized functional scores, as opposed to using either modality by itself. For example, Fay-Karmon et al. [16] showed that smartwatch-derived gait and activity signals can be successfully aligned with clinical symptom ratings to monitor the evolution of Parkinson’s disease in home environments. Xia et al. [18] emphasized that passively sensed behavioral data gain predictive value only when contextualized with ecological momentary assessments. These results support the long-term scalability of the NeuroPredict platform.
One of the planned enhancements of the platform is the extension of its explainable AI modules. This will include providing human-readable clarifications of risk outcomes, such as “this tendency is related to decreased gait regularity” when anomalies are detected. Recent empirical research shows that explainable interfaces with clear and clinically relevant explanations increase clinician trust in AI feedback and engagement with AI-enabled decision support, which is important for regulatory approval; similar outcomes were found for wearables in sensor-driven contexts [54,55].
Important future challenges to address in the context of AD and PD are those concerning false negatives and false positives, both of which can negatively impact clinical adoption. False negatives may result in missed opportunities for action and delayed treatment, while false positives can lead to unnecessary investigations and cause anxiety for patients and caregivers. They can directly affect the acceptance of biomarker systems in neurodegenerative disease monitoring, according to the literature. Rodríguez-Martín and Pérez-López found that wrist-worn sensors can lead to increased false positives when the user is performing random movements, and false negatives with regard to some dyskinetic symptoms when their wearing location is not the recommended one [56]. Lim et al. discuss the trade-offs between false negatives and false positives when using single-signal models [57]. Addressing challenges with false negatives and false positives in the NeuroPredict platform will involve strategies that ensure that the multiple modalities can check each other’s outputs to reduce error propagation.
4.5. Limitations and Current Maturity Level
The NeuroPredict platform is still under development. On the backend, secure data ingestion, basic preprocessing, and integration with commercial wearable biosensors have been implemented. Standardized APIs are provided to acquire data from the input devices, which makes early-stage interoperability testing possible. On the frontend, raw and partially processing physiological signals can be visualized. Testing has been conducted so far in laboratory settings to check the data format consistency and transmission reliability. Evaluation with users has so far involved a small preliminary patient cohort. Future work will involve scalability testing and evaluation with a larger and more diverse patient cohort in clinical settings.
The prototype AI/ML models that are currently integrated with the NeuroPredict platform support basic trend and anomaly detection in laboratory settings. This is in line with current eHealth approaches which integrate ML models to predict disease evolution and support early intervention. The platform’s analytical components are all part of a modular architecture built for scalability and reusability. Future work includes making the AI functionalities more explainable, as presented in Section 4.5.
Overall, the NeuroPredict platform is at early proof-of-concept maturity, at around TRL 3–4. While it has shown promising results during laboratory evaluation, the platform has not yet been evaluated in real-world clinical conditions or with diverse patient cohorts. A lack of real-life large-scale and longitudinal datasets prevented the testing of the current AI/ML models on their ability to generalize across disease stages and comorbidities. The analytical modules have only been tested with clean data, while testing with noisy data under clinical conditions is needed for future work.
Interoperability has only been tested for device-level APIs, while future work should also test integration with electronic health record systems and a standardized framework such as HL7/FHIR. Usability has only been evaluated in proof-of-concept sessions, while engagement of patients and healthcare providers in real clinical settings remains to be explored. The platform’s current deployment is restricted to research use, due to lack of MDR/IVDR certification and conformity assessment under the EU AI Act. The NeuroPredict platform has not been tested for longitudinal monitoring or been compared with clinical gold standards such as MoCA yet. Security has been considered when designing the architecture, but penetration testing and independent audits are pending.
The above limitations are typical of platforms at TRL 3–4, which emphasize technical feasibility and architectural soundness but have not been clinically adopted yet. To progress to higher TRL levels, extensive clinical validation, large annotated datasets, interoperability with hospital information systems, and structured evaluations of user trust and ethical compliance will be necessary.
To sum up, the NeuroPredict platform is meant to close the gap between cutting-edge data science and useful, real-world usage. By combining flexible, reliable data pipelines with responsive, usable, and evidence-based user interactions, it proposes a solution towards the proactive, personalized, and participative care of patients suffering from neurodegenerative diseases. Preliminary evaluation results are both technically and clinically promising; however, transition to clinical use will require large-scale trials, regulatory alignment, and sustained user acceptance.
5. Conclusions
This paper introduced the NeuroPredict platform, a proof-of-concept platform for the multimodal monitoring of neurodegenerative diseases like AD, PD, and MS. The platform brings together heterogenous data from wearable, in-house, ambient sensors and cognitive assessments to produce coherent digital biomarker profiles. Patients, caregivers, and clinicians can then access actionable insights suited to their needs using role-specific dashboards that were validated during proof-of-concept sessions. At its current level of development (TRL 3–4), the platform has been evaluated under controlled laboratory conditions, with positive results suggesting technical feasibility. Clinical validation and regulatory conformity have not been achieved yet.
Future development work involves the following:
- User interface/experience enhancements: Current plans include improving the platform’s responsiveness and its feedback mechanisms, such that the interaction between end-users across clinical and research domains can be facilitated. Ongoing usability testing will guide other improvements.
- Clinical validation planning: Once it reaches technical maturity, the NeuroPredict platform will undergo clinical evaluation at partner healthcare institutions. The aims will be to check its performance, diagnostic utility, and clinician acceptance.
- Regulatory compliance: The platform is already designed with compliance with the EU AI Act and certification as a high-risk AI system under healthcare classifications in mind. For example, for the latter, the platform already includes transparency mechanisms.
- EMR interoperability: The NeuroPredict platform will adopt healthcare data standards such as HL7 and FHIR, which will support its interoperability with EMR systems. This will enable its monitoring insights to be transmitted to the EMR, thus increasing its utility in clinical settings.
- Research platform for computational medicine: The NeuroPredict platform is also intended as a modular, extensible research platform that supports studies in computational medicine. It will be possible to extend it to integrate with other devices to collect different types of bio signal data and set it up with personalized health metrics for longitudinal patient monitoring.
The NeuroPredict platform has intentionally been designed as modular, to support its future incorporation of advanced AI analytics, including graph-based and hypergraph-based models. As the platform’s pipelines are independent and replaceable, new fusion or predictive modules can be added without needing to modify the architecture. Multimodal data streams already have an organization that is compatible with graph-structured representations. Overall, this architectural flexibility allows the platform to be enhanced with context-aware AI mechanisms as soon as clinically validated datasets become available.
In the long term, the NeuroPredict platform is intended to support AI-driven, data-centric research in neurology and related fields. In the short term, multiple randomized controlled trials are planned in order to demonstrate its long-term medical impact.
Author Contributions
Conceptualization, A.A. and M.I.; methodology, A.-V.V.; software, M.I. and A.A.; validation, A.-V.V., M.I. and A.A.; writing—original draft preparation, A.A. and M.I.; writing—review and editing A.-V.V., M.I. and A.A.; project administration, M.I. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the contribution of the Romanian Ministry of Research, Innovation, and Digitization for funding the development of the NeuroPredict platform within the project “Advanced Artificial Intelligence Techniques in Science and Applications” (Contract No. 13N/2023 (PN 23 38 05 01)) for the period 2023–2026.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AAMI | Association for the Advancement of Medical Instrumentation |
| AD | Alzheimer’s disease |
| AES | Advanced Encryption Standard |
| AI | Artificial Intelligence |
| API | Application Programming Interface |
| BMI | Body Mass Index |
| CO2 | Carbon Dioxide |
| ECG | Electrocardiography |
| EMG | Electromyography |
| EMR | Electronic Medical Record |
| EU AI Act | European Union Artificial Intelligence Act |
| FDA | U.S. Food and Drug Administration |
| FHIR | Fast Healthcare Interoperability Resources |
| GDPR | General Data Protection Regulation |
| GSM | Global System for Mobile Communications |
| HL7 | Health Level Seven |
| HTTPS | Hypertext Transfer Protocol Secure |
| IaaS | Infrastructure as a Service |
| ICIPRO | Romanian National Cloud Service for Public Institutions |
| IoMT | Internet of Medical Things |
| ISO | International Organization for Standardization |
| IVDR | In Vitro Diagnostic Regulation |
| JSON | JavaScript Object Notation |
| JWT | JSON Web Token |
| MDR | Medical Device Regulation |
| MDCG | Medical Device Coordination Group |
| ML | Machine Learning |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment |
| MS | Multiple Sclerosis |
| MQTT | Message Queuing Telemetry Transport |
| PD | Parkinson’s disease |
| RBAC | Role-Based Access Control |
| SHA | Secure Hash Algorithm |
| SpO2 | Peripheral Capillary Oxygen Saturation |
| SSL | Secure Sockets Layer |
| TLS | Transport Layer Security |
| TRL | Technology Readiness Level |
| VOC | Volatile Organic Compounds |
References
- European Commission. Directorate-General for Economic and Financial Affairs 2024 Ageing Report. Economic and Budgetary Projections for the EU Member States (2022–2070); European Commission: Brussels, Belgium, 2024. [Google Scholar]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Junaid, M.; Ali, S.; Eid, F.; El-Sappagh, S.; Abuhmed, T. Explainable Machine Learning Models Based on Multimodal Time-Series Data for the Early Detection of Parkinson’s Disease. Comput. Methods Programs Biomed. 2023, 234, 107495. [Google Scholar] [CrossRef]
- Paraschiv, E.; Petrache, C.; Bica, O.; Vasilevschi, A. Fall Detection System: Continuous in-Home Monitoring of Parkinson’s Patients. In Proceedings of the 2022 E-Health and Bioengineering Conference (EHB), Iași, Romania, 17–18 November 2022; pp. 1–4. [Google Scholar]
- Stara, V.; Soraci, L.; Takano, E.; Kondo, I.; Möller, J.; Maranesi, E.; Luzi, R.; Riccardi, G.R.; Browne, R.; Dacunha, S.; et al. Intrinsic Capacity and Active and Healthy Aging Domains Supported by Personalized Digital Coaching: Survey Study Among Geriatricians in Europe and Japan on EHealth Opportunities for Older Adults. J. Med. Internet Res. 2023, 25, e41035. [Google Scholar] [CrossRef] [PubMed]
- Ianculescu, M.; Paraschiv, E.-A.; Alexandru, A. Addressing Mild Cognitive Impairment and Boosting Wellness for the Elderly through Personalized Remote Monitoring. Healthcare 2022, 10, 1214. [Google Scholar] [CrossRef] [PubMed]
- Kurlan, R.; Richard, I.H.; Papka, M.; Marshall, F. Movement Disorders in Alzheimer’s Disease: More Rigidity of Definitions Is Needed. Mov. Disord. 2000, 15, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Sandulescu, V.; Ianculescu, M.; Valeanu, L.; Alexandru, A. Integrating IoMT and AI for Proactive Healthcare: Predictive Models and Emotion Detection in Neurodegenerative Diseases. Algorithms 2024, 17, 376. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Medikonda, J.; Namboothiri, P.K.; Natarajan, M. Role of Wearable Sensors with Machine Learning Approaches in Gait Analysis for Parkinson’s Disease Assessment: A Review. Eng. Sci. 2022, 19, 5–19. [Google Scholar] [CrossRef]
- Antonini, A.; Reichmann, H.; Gentile, G.; Garon, M.; Tedesco, C.; Frank, A.; Falkenburger, B.; Konitsiotis, S.; Tsamis, K.; Rigas, G.; et al. Toward Objective Monitoring of Parkinson’s Disease Motor Symptoms Using a Wearable Device: Wearability and Performance Evaluation of PDMonitor®. Front. Neurol. 2023, 14, 1080752. [Google Scholar] [CrossRef]
- Bakirtzis, C.; Ioannidis, P.; Messinis, L.; Nasios, G.; Konstantinopoulou, E.; Papathanasopoulos, P.; Grigoriadis, N. The Rationale for Monitoring Cognitive Function in Multiple Sclerosis: Practical Issues for Clinicians. Open Neurol. J. 2018, 12, 31–40. [Google Scholar] [CrossRef]
- Tea, F.; Groh, A.M.R.; Lacey, C.; Fakolade, A. A scoping review assessing the usability of digital health technologies targeting people with multiple sclerosis. npj Digit. Med. 2024, 7, 168. [Google Scholar] [CrossRef]
- Gallo, G.D.; Micucci, D. Internet of Medical Things Systems Review: Insights into Non-Functional Factors. Sensors 2025, 25, 2795. [Google Scholar] [CrossRef] [PubMed]
- Al Khatib, I.; Shamayleh, A.; Ndiaye, M. Healthcare and the Internet of Medical Things: Applications, Trends, Key Challenges, and Proposed Resolutions. Informatics 2024, 11, 47. [Google Scholar] [CrossRef]
- DE Zambotti, M.; Cellini, N.; Goldstone, A.; Colrain, I.M.; Baker, F.C. Wearable Sleep Technology in Clinical and Research Settings. Med. Sci. Sports Exerc. 2019, 51, 1538–1557. [Google Scholar] [CrossRef] [PubMed]
- Fay-Karmon, T.; Galor, N.; Heimler, B.; Zilka, A.; Bartsch, R.P.; Plotnik, M.; Hassin-Baer, S. Home-Based Monitoring of Persons with Advanced Parkinson’s Disease Using Smartwatch-Smartphone Technology. Sci. Rep. 2024, 14, 9. [Google Scholar] [CrossRef]
- Kourtis, L.C.; Regele, O.B.; Wright, J.M.; Jones, G.B. Digital Biomarkers for Alzheimer’s Disease: The Mobile/Wearable Devices Opportunity. npj Digit. Med. 2019, 2, 9. [Google Scholar] [CrossRef]
- Xia, Z.; Chikersal, P.; Venkatesh, S.; Walker, E.; Dey, A.K.; Goel, M. Longitudinal Digital Phenotyping of Multiple Sclerosis Severity Using Passively Sensed Behaviors and Ecological Momentary Assessments: Real-World Evaluation. J. Med. Internet Res. 2025, 27, e70871. [Google Scholar] [CrossRef]
- Huang, C.; Wang, J.; Wang, S.; Zhang, Y. Internet of Medical Things: A Systematic Review. Neurocomputing 2023, 557, 126719. [Google Scholar] [CrossRef]
- Rehman, A.U.; Lu, S.; Bin Heyat, M.B.; Iqbal, M.S.; Parveen, S.; Bin Hayat, M.A.; Akhtar, F.; Ashraf, M.A.; Khan, O.; Pomary, D.; et al. Internet of Things in Healthcare Research: Trends, Innovations, Security Considerations, Challenges and Future Strategy. Int. J. Intell. Syst. 2025, 2025, 8546245. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, S.; Kim, G. Convergence of Integrated Sensing and Communication (ISAC) and Digital-Twin Technologies in Healthcare Systems: A Comprehensive Review. Signals 2025, 6, 51. [Google Scholar] [CrossRef]
- Giansanti, D. The Future of Healthcare Is Digital: Unlocking the Potential of Mobile Health and E-Health Solutions. Healthcare 2025, 13, 802. [Google Scholar] [CrossRef]
- Matthew, P.; Mchale, S.; Deng, X.; Nakhla, G.; Trovati, M.; Nnamoko, N.; Pereira, E.; Zhang, H.; Raza, M. A Review of the State of the Art for the Internet of Medical Things. Science 2025, 7, 36. [Google Scholar] [CrossRef]
- Kalodanis, K.; Feretzakis, G.; Anastasiou, A.; Rizomiliotis, P.; Anagnostopoulos, D.; Koumpouros, Y. A Privacy-Preserving and Attack-Aware AI Approach for High-Risk Healthcare Systems Under the EU AI Act. Electronics 2025, 14, 1385. [Google Scholar] [CrossRef]
- Mannhart, D.; Lischer, M.; Knecht, S.; du Fay de Lavallaz, J.; Strebel, I.; Serban, T.; Vögeli, D.; Schaer, B.; Osswald, S.; Mueller, C.; et al. Clinical Validation of 5 Direct-to-Consumer Wearable Smart Devices to Detect Atrial Fibrillation. JACC Clin. Electrophysiol. 2023, 9, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Schyvens, A.-M.; Peters, B.; Van Oost, N.C.; Aerts, J.-M.; Masci, F.; Neven, A.; Dirix, H.; Wets, G.; Ross, V.; Verbraecken, J. A Performance Validation of Six Commercial Wrist-Worn Wearable Sleep-Tracking Devices for Sleep Stage Scoring Compared to Polysomnography. SLEEP Adv. 2025, 6, zpaf021. [Google Scholar] [CrossRef]
- Hakobyan, Z.; Zelveian, P.; Topouchian, J.; Hazarapetyan, L.; Asmar, R. Validation of the Withings BPM Core Device for Self-Blood Pressure Measurements in General Population According to the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization Universal Standard. Vasc. Health Risk Manag. 2023, 19, 391–398. [Google Scholar] [CrossRef]
- Withings. Withings ScanWatch, ScanWatch 2. Clinical Studies (EU & ROW). Withings: Paris, France, 2023.
- Ernstsson, J.; Svensson, B.; Liuba, P.; Weismann, C.G. Validation of Smartwatch Electrocardiogram Intervals in Children Compared to Standard 12 Lead Electrocardiograms. Eur. J. Pediatr. 2024, 183, 3915–3923. [Google Scholar] [CrossRef]
- Matos, J.; Ramos, B.; Fernandes, J.; Hansen, C.; Maetzler, W.; Vila-Chã, N.; Maia, L.F. Wearable Sensors for Sleep Monitoring in Free-Living Environments: A Scoping Review on Parkinson’s Disease. Biosensors 2025, 15, 212. [Google Scholar] [CrossRef]
- Withings. Withings Body+ Scale; Withings: Paris, France, 2023; Available online: https://www.withings.com/body-plus (accessed on 20 October 2025).
- Svensson, T.; Madhawa, K.; NT, H.; Chung, U.; Svensson, A.K. Validity and Reliability of the Oura Ring Generation 3 (Gen3) with Oura Sleep Staging Algorithm 2.0 (OSSA 2.0) When Compared to Multi-Night Ambulatory Polysomnography: A Validation Study of 96 Participants and 421,045 Epochs. Sleep. Med. 2024, 115, 251–263. [Google Scholar] [CrossRef]
- Withings. Withings Developer, Advanced Research API; Withings: Paris, France, 2023; Available online: https://developer.withings.com/api-reference (accessed on 20 October 2025).
- Băjenaru, O.L.; Băjenaru, L.; Ianculescu, M.; Constantin, V.-Ș.; Gușatu, A.-M.; Nuță, C.R. Geriatric Healthcare Supported by Decision-Making Tools Integrated into Digital Health Solutions. Electronics 2024, 13, 3440. [Google Scholar] [CrossRef]
- Ianculescu, M.; Constantin, V.-Ș.; Gușatu, A.-M.; Petrache, M.-C.; Mihăescu, A.-G.; Bica, O.; Alexandru, A. Enhancing Connected Health Ecosystems Through IoT-Enabled Monitoring Technologies: A Case Study of the Monit4Healthy System. Sensors 2025, 25, 2292. [Google Scholar] [CrossRef]
- De Pasquale, P.; Bonanno, M.; De Marchis, C.; Pergolizzi, L.; Lombardo Facciale, A.; Paladina, G.; Maggio, M.G.; Impellizzeri, F.; Ciancarelli, I.; Quartarone, A.; et al. Beyond Clinical Scales: An Observational Study on Instrumental Gait Analysis and Biomechanical Patterns in Patients with Parkinson’s Disease. Front. Bioeng. Biotechnol. 2025, 13, 1541240. [Google Scholar] [CrossRef] [PubMed]
- Villarón-Casales, C.; de Bernardo, N.; Alarcón-Jiménez, J.; López-Malo, D.; Proaño, B.; Martín-Ruiz, J.; de la Rubia Ortí, J.E. Amplitude of Lower Limb Muscle Activation in Different Phases of the Illinois Test in Parkinson’s Disease Patients: A Pilot Study. J. Clin. Med. 2024, 13, 5792. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-H.; Sun, S.-Y.; Li, G.; Gao, X.; Luo, W.; Tian, H.; Zhang, X.; Yin, X.; Liu, Z.; Chen, G.-C.; et al. Physical Activity and Sleep Pattern in Relation to Incident Parkinson’s Disease: A Cohort Study. Int. J. Behav. Nutr. Phys. Act. 2024, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Withings. Withings Health Data API—Developer Documentation; Withings: Paris, France, 2023; Available online: https://developer.withings.com/api-reference#tag/health-data (accessed on 20 October 2025).
- Nicolau, D.; Bica, O.; Băjenaru, L. Data Security Approach in Remote Healthcare Monitoring. Rom. Cyber Secur. J. 2023, 5, 45–55. [Google Scholar] [CrossRef]
- Saif, S.; Das, P.; Biswas, S.; Khan, S.; Haq, M.A.; Kovtun, V. A Secure Data Transmission Framework for IoT Enabled Healthcare. Heliyon 2024, 10, e36269. [Google Scholar] [CrossRef]
- Singh, A.K.; Krishnan, S. ECG Signal Feature Extraction Trends in Methods and Applications. Biomed. Eng. Online 2023, 22, 22. [Google Scholar] [CrossRef]
- Sergi, G.; Yekutieli, Z.; Meloni, M.; Bianchini, E.; Vivacqua, G.; Di Lazzaro, V.; Marano, M. Sympathetic Burden Measured Through a Chest-Worn Sensor Correlates with Spatiotemporal Gait Performances and Global Cognition in Parkinson’s Disease. Sensors 2025, 25, 5756. [Google Scholar] [CrossRef]
- Shammar, E.; Cui, X.; Zahary, A.; Alsamhi, S.H.; Al-qaness, M.A.A. Threat to Trust: A Systematic Review on Internet of Medical Things Security. J. Parallel Distrib. Comput. 2025, 206, 105172. [Google Scholar] [CrossRef]
- Alasmary, H. ScalableDigitalHealth (SDH): An IoT-Based Scalable Framework for Remote Patient Monitoring. Sensors 2024, 24, 1346. [Google Scholar] [CrossRef]
- Quiroga Gutierrez, A.C.; Lindegger, D.J.; Taji Heravi, A.; Stojanov, T.; Sykora, M.; Elayan, S.; Mooney, S.J.; Naslund, J.A.; Fadda, M.; Gruebner, O. Reproducibility and Scientific Integrity of Big Data Research in Urban Public Health and Digital Epidemiology: A Call to Action. Int. J. Environ. Res. Public Health 2023, 20, 1473. [Google Scholar] [CrossRef]
- Helminski, D.; Sussman, J.B.; Pfeiffer, P.N.; Kokaly, A.N.; Ranusch, A.; Renji, A.D.; Damschroder, L.J.; Landis-Lewis, Z.; Kurlander, J.E. Development, Implementation, and Evaluation Methods for Dashboards in Health Care: Scoping Review. JMIR Med. Inform. 2024, 12, e59828. [Google Scholar] [CrossRef]
- Zhu, P.; Chen, X.; Zhang, Z.; Li, P.; Cheng, X.; Dai, Y. AI-Driven Hypergraph Neural Network for Predicting Gasoline Price Trends. Energy Econ. 2025, 151, 108895. [Google Scholar] [CrossRef]
- Dabnichki, P.; Pang, T.Y. Wearable Sensors and Motion Analysis for Neurological Patient Support. Biosensors 2024, 14, 628. [Google Scholar] [CrossRef] [PubMed]
- Islam, U.; Alatawi, M.N.; Alqazzaz, A.; Alamro, S.; Shah, B.; Moreira, F. A Hybrid Fog-Edge Computing Architecture for Real-Time Health Monitoring in IoMT Systems with Optimized Latency and Threat Resilience. Sci. Rep. 2025, 15, 25655. [Google Scholar] [CrossRef] [PubMed]
- Rancea, A.; Anghel, I.; Cioara, T. Edge Computing in Healthcare: Innovations, Opportunities, and Challenges. Future Internet 2024, 16, 329. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Digital Health Technologies for Remote Data Acquisition in Clinical Investigations; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2023.
- European Commission. MDCG 2025-4 Guidance on the Safe Making Available of Medical Device Software (MDSW) Apps on Online Platforms; European Commission: Brussels, Belgium, 2025. [Google Scholar]
- Rosenbacke, R.; Melhus, Å.; McKee, M.; Stuckler, D. How Explainable Artificial Intelligence Can Increase or Decrease Clinicians’ Trust in AI Applications in Health Care: Systematic Review. JMIR AI 2024, 3, e53207. [Google Scholar] [CrossRef]
- Abdelaal, Y.; Aupetit, M.; Baggag, A.; Al-Thani, D. Exploring the Applications of Explainability in Wearable Data Analytics: Systematic Literature Review. J. Med. Internet Res. 2024, 26, e53863. [Google Scholar] [CrossRef]
- Rodríguez-Martín, D.; Pérez-López, C. Commercial Symptom Monitoring Devices in Parkinson’s Disease: Benefits, Limitations, and Trends. Front. Neurol. 2024, 15, 1470928. [Google Scholar] [CrossRef]
- Lim, W.-S.; Fan, S.-P.; Chiu, S.-I.; Wu, M.-C.; Wang, P.-H.; Lin, K.-P.; Chen, Y.-M.; Peng, P.-L.; Jang, J.-S.R.; Lin, C.-H. Smartphone-Derived Multidomain Features Including Voice, Finger-Tapping Movement and Gait Aid Early Identification of Parkinson’s Disease. npj Park. Dis. 2025, 11, 111. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).