Abstract
Extracellular vesicles (EVs) are small membrane-bound particles that play a vital role in intercellular communication by facilitating the transfer of molecular cargo. In this review, we provide an overview of EV biology in corneal diseases, along with current approaches to therapeutic uses of EVs. Since EVs generally retain surface markers indicative of their cell of origin, they possess a degree of tissue specificity, which benefits drug delivery systems and highlights their potential as biomarkers to study disease processes. Further advances in technology and methodology will accelerate our understanding of EVs and help guide the field towards improved diagnostic techniques and therapeutic targets. We summarize EVs and their potential impact in medicine with a discussion of the limitations that remain in current approaches, as well as areas to focus on for future growth.
1. Introduction
When a child is born, they are introduced to a whole new world of sensations. There are now loud noises, cold environments, and having to breathe for the first time. The child opens their eyes, but the world is blurry due to their undeveloped retina. As the child grows, their eyesight improves day by day as the retina forms. Once the child can see clearly, visual perception becomes an essential part of their daily life. They experience the delight of seeing their parents’ faces and their favorite toy clearly, as well as dangers like stairs and obstacles. This person is also able to use the computer, drive, and experience a normal existence. But what if all of this were taken away due to corneal dystrophies?
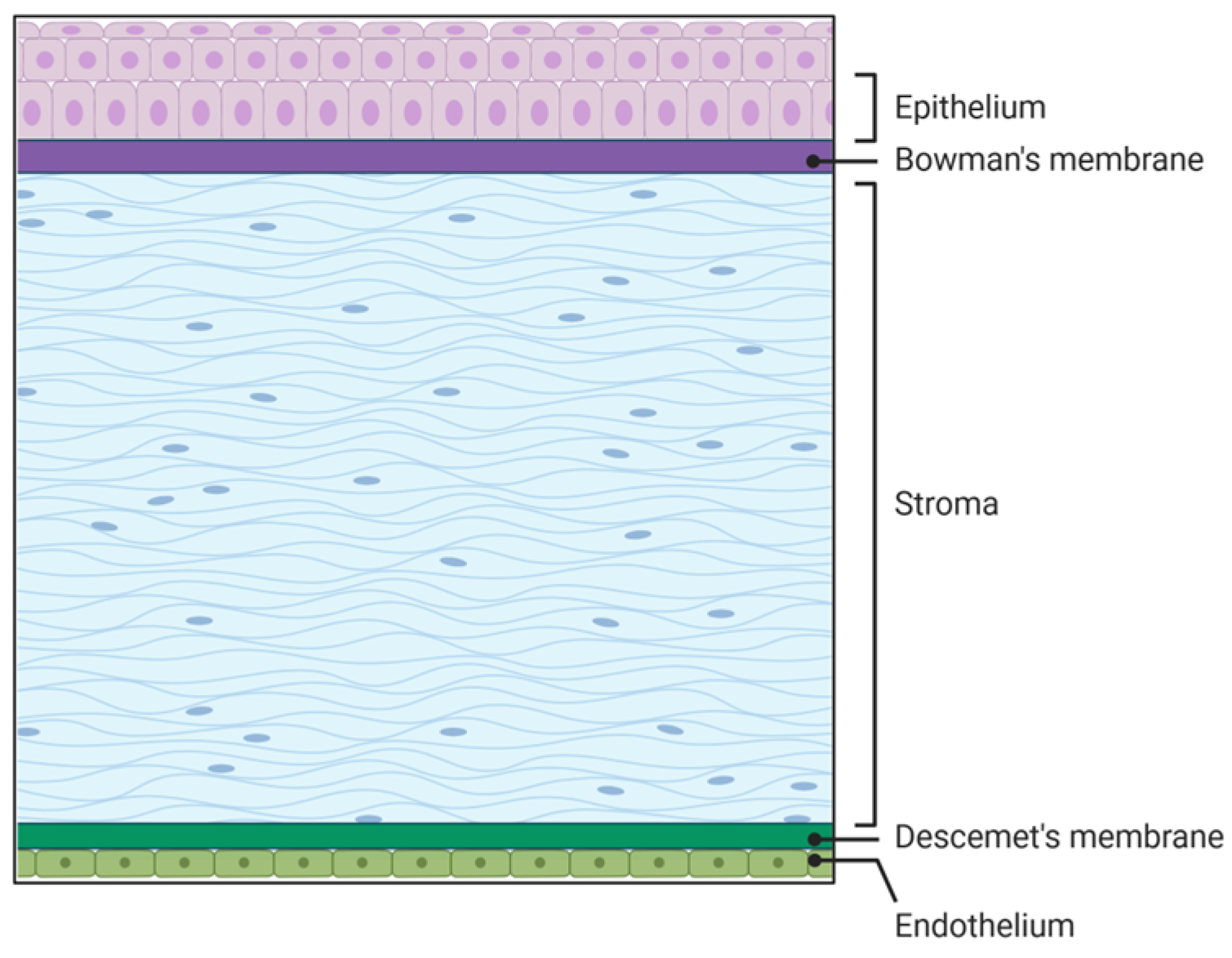
The cornea is the transparent part of the eye located on the anterior segment of the eye. The human cornea plays a crucial role in vision by providing two-thirds of the refractive power of the eye [1,2]. It comprises three cellular and two acellular layers. The epithelial layer is the outermost corneal layer that can regenerate after being wounded in healthy individuals, but has delayed wound healing in diseased corneas such as those with diabetes [3,4,5]. The next layer is the acellular Bowman’s layer, which separates the epithelium from the stromal layer. The stromal layer is the thickest layer of the cornea and is made up of resident cells termed keratocytes as well as extracellular matrix (ECM) components like collagen, decorin, lumican, and keratocan, to name a few. The collagen fibrils run parallel to each other, which is essential for corneal transparency and enhances its refractive power [1]. The fourth layer is the Descemet’s membrane, which is the second acellular layer and acts as a barrier against infection. It divides the stromal layer from the endothelial layer, which is the final layer of the cornea that is in contact with the aqueous humor (Figure 1).
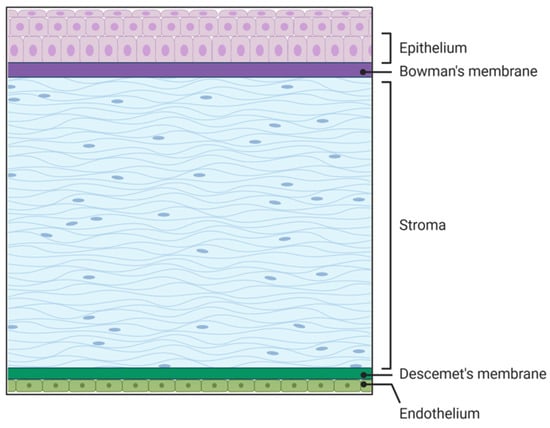
Figure 1.
Five layers of the cornea. Source: Created in BioRender. Hefley, B. (2026) https://BioRender.com/r1otvqv.
The cornea is a complex, highly innervated [6,7,8], and immune-privileged tissue [9,10,11] that requires cell–cell communication between the various layers. One structure that the cornea uses to achieve this communication is by using EVs.
EVs are membrane-bound particles that facilitate intercellular communication by passing cargo such as DNA, RNA, and proteins from one cell to another [12,13,14,15]. Here, we provide an overview of EV research in corneal dystrophies and discuss current approaches for potential therapeutic use. There is a growing interest in EVs as biomarkers of disease and drug carriers, which will also be discussed in this review.
2. Extracellular Vesicles (EVs)
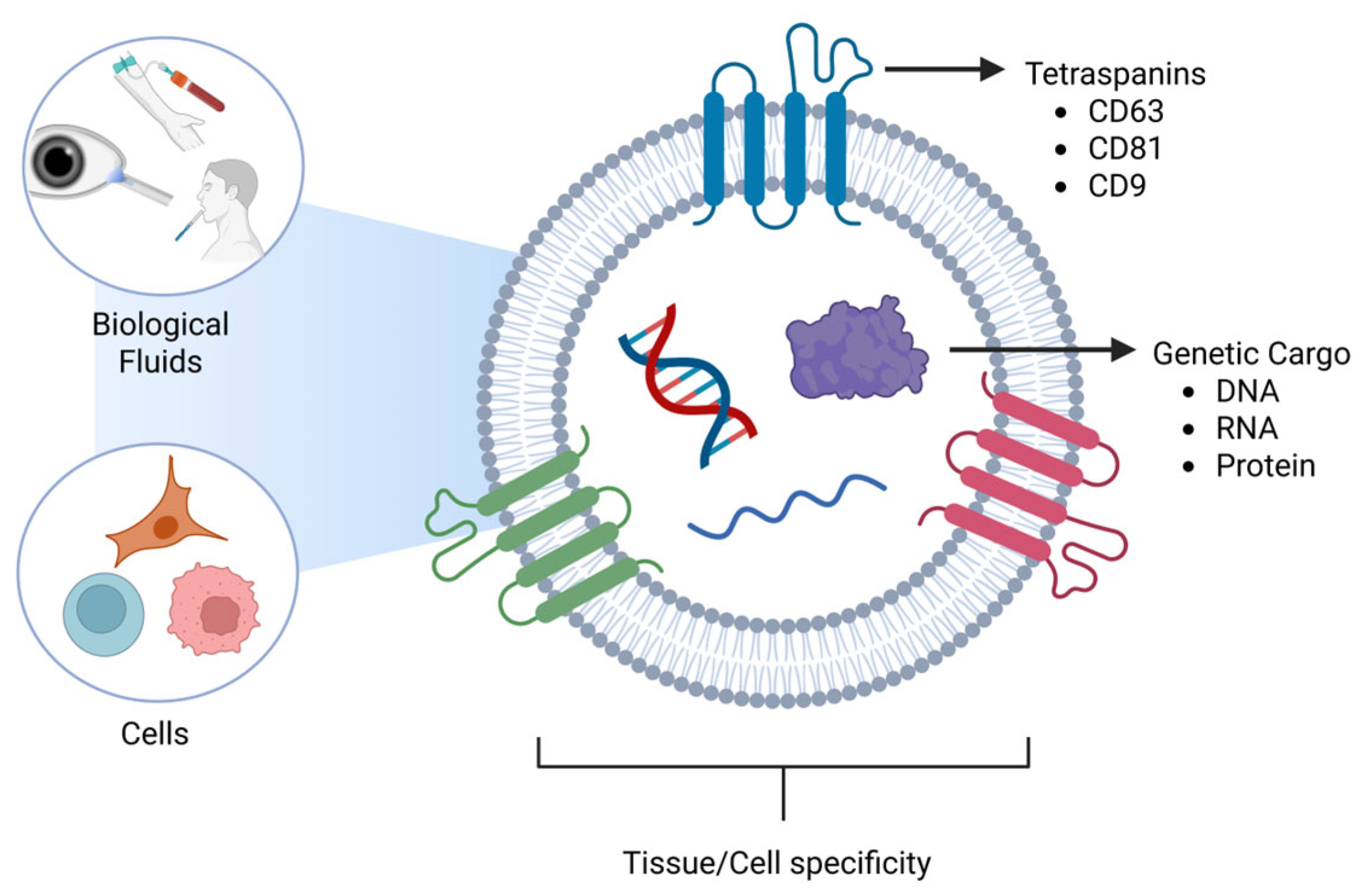
Early studies of EVs began in the 1960s [16], as EVs were initially thought to be vesicles for the release of unwanted cellular material. The history of their discovery and study has been well-reviewed in sentinel papers in the field [17,18,19]. There are three major categories of EVs: exosomes, microvesicles, and apoptotic bodies. Exosomes are the smallest EVs whose diameters range from 30–150 nm [20,21,22], and are produced by the endosomal membrane system [18,23,24]. Microvesicles are the intermediate vesicles, which range from 100 to 1000 nm [25,26,27] and are formed from the budding of the plasma membrane [24,28,29]. Apoptotic vesicles are the largest of the EVs, which are 1000–5000 nm [30,31,32] and also bud off the plasma membrane [31,32,33]. These size ranges do not definitively determine the subtype of EVs due to overlapping size ranges. There is a large debate in the field about the actual size range of exosomes, with some reports stating that they range between 30 nm and 150 nm [20,22,34], while other reports state a range of 50 nm–150 nm [35,36,37]. These differences in reporting the size can be due to several factors, such as isolation techniques or even the measuring equipment. While isolating the exosomes, different techniques, such as ultracentrifugation with its high speed, could cause the squishing of exosomes, making them smaller, while the gentler process, like size-exclusion chromatography (SEC), will provide integrity and preserve their size to 50–150 nm. Mitochondria-derived EVs are another subclass of EVs that originate from mitochondria and contain selective proteins, including TOM20 and OPA1, as well as mitochondrial DNA [38]. Intact extracellular mitochondria have also been detected in blood and in vitro [39,40]. Cancer research has expanded our knowledge of EVs by discovering that they not only travel short distances between cells, but they can also travel large distances in the body via the bloodstream [41,42,43]. EVs have been found in various biological fluids such as blood [44,45,46], tears [47,48,49], saliva [50,51,52], breast milk [53,54,55,56,57,58], and cerebrospinal fluid, to name a few (Figure 2).
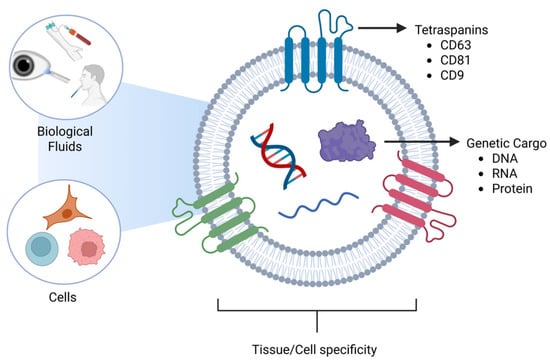
Figure 2.
Schematic diagram of Extracellular vesicles with tetraspanins and genetic cargo in different biological fluids. Source: Created in BioRender. Hefley, B. (2026) https://BioRender.com/x18k556.
EVs can be isolated by utilizing various methods such as ultracentrifugation, flow cytometry, size exclusion chromatography (SEC), exosome precipitation, and microfluidics-based isolation techniques [59,60]. Regular centrifugation is not sufficient in isolating EVs due to their small nature. This process requires the user to centrifuge their samples at various speeds to remove cells and cell debris, and then centrifuge the supernatant at an even higher speed to pellet the EVs [61]. Ultracentrifugation is the most widely used method for EV isolation [62], but prolonged use of the centrifuge can alter the shape of the EVs [27]. Ultrafiltration is one of the popular size-based exosome isolation methods along with SEC. It does not require special equipment, as membrane filters can be used with defined molecular weight or size exclusion limits, making it faster compared to ultracentrifugation [59].
Flow cytometry is another commonly used method to isolate EVs, which has the advantage of evaluating EVs at multiple time points and end points, as well as single EV analysis. This method has some limitations, in which the EV signal can be weak [63] compared to ultracentrifugation, and can cause the clogging of the filter pores in the flow cytometer [64]. Microfluidics-based isolation is considered to be the next generation of exosome research, as it uses precision engineering in manipulating tiny volumes of fluids using the physical and biochemical properties of exosomes [59]. Despite having their advantages, all these methods of isolation have their own limitations based on their unique characteristics and cause low to moderate purities in isolated exosomes.
Technological advances in recent years have markedly improved the methods available for EV phenotyping. Super-resolution microscopy [65] and fluorescent tags that are covalently bound to the EV membrane have enabled imaging and tracking of EVs at or near the single-particle level in vitro and in vivo [66,67]. Of these methods, fluorescent reporters (red tdTomato or GFP) mediated via palmitoylation permit dynamic monitoring of EVs that are derived from the host cell plasma membrane and secreted into the extracellular space [68]. Cell-type-specific markers have been used to enrich for neuronal-derived EVs found in blood based on neural cell adhesion molecules (NCAM or LCAM), allowing for the study of acute changes occurring in the central and peripheral nervous systems [69,70]. Nanoparticle tracking analysis (NTA) can also be used to measure the size and total EV concentration of the sample [71,72,73]. EV cargo can be analyzed by isolating the vesicle and applying molecular methods, such as polymerase chain reaction [74,75,76] (PCR) and western blotting [77,78,79] to analyze the genetic content.
EVs may be tissue-specific due to proteins located on their surface [80]. One group of proteins that is heavily studied in EV research is tetraspanins, which span the plasma membrane four times. The three tetraspanins commonly observed in EVs are CD63, CD81, and CD9. In the body, CD63 is located in the endosomal membrane system [81,82,83] and plays a role in the immune system, as well as binding and sorting cholesterol into endosomes for storage and distribution via EVs [84,85,86]. CD81 and CD9 play a role in inflammation [87,88], cell growth [89,90], cell proliferation [91,92], and are located on the plasma membrane [88,93].
EV Heterogeneity
Although EVs can be produced from the same cell type, for example, fibroblasts, the EVs can experience heterogeneity, which can alter the surface protein expressions as well as the molecular cargo [94]. The changes in the molecular cargo can give insight into the pathological and physiological state of their cell of origin, which allows tracking of disease progression [94]. EVs have been shown to carry multiple kinds of DNA, which include double-stranded DNA, single-stranded DNA, mitochondrial DNA, and circular DNA. This cargo can then be transferred horizontally to other cells, which promotes genetic diversity but can also influence a healthy cell to develop a diseased phenotype.
EV heterogeneity is vastly understudied, and further studies are warranted in this field. Mastering this concept could lead to developing EVs for clinical trials as well as mass production of therapeutics.
3. EVs in the Cornea
3.1. Corneal-Derived EVs
Since the cornea plays a critical role in vision, it is imperative that it remains transparent and uninjured. Unfortunately, there are many environmental factors and diseases that threaten the integrity of this tissue. Studies have investigated various cellular components and processes that keep the cornea healthy. One process that has been explored is the properties and functions of EVs as mediators of cell–cell communication in the cornea. Table 1 provides a summary of all corneal diseases and their EV therapeutics discussed in this review.

Table 1.
Summary of corneal EV therapeutics.
EVs derived from the cornea have been shown to influence corneal wound healing. Yeung et al. found that CD81 expression was similar between corneal epithelial cells and corneal stromal cells [121]. The authors measured the size and surface potential of epithelial and stromal EVs and found no significant differences between these two groups [121]. They also showed subtle differences in the EV protein expression between epithelial and stromal cells alluded to unique phenotypes [121].
EVs secreted by the corneal epithelium increase alpha-smooth muscle actin (α-SMA) expression by keratocytes, with the epithelial basement membrane limiting their travel into the stroma [103,122,123]. An in vitro study revealed that exosomes released from cultured corneal epithelial fibroblasts and endothelial cells promote epithelial cell migration via activation of the MAPK and JAK/STAT pathways [124]. EVs derived from corneal epithelial cells have been found to contain Thrombospondin-1 (TSP-1) as well as fibronectin, which are provisional matrix proteins that influence cell contractility and migration [125]. This work suggests that EVs derived from the epithelium may be important mediators of corneal scarring and provides some evidence for a protective effect of an intact basement membrane in blunting scar formation by inhibiting EV diffusion from the epithelium following mild debridement.
It has been shown that corneal fibroblasts secrete EVs rich in MMP-2 and 14, which may be taken up by vascular endothelial cells and play a role in corneal neovascularization [126]. Parekh et al. showed that human corneal endothelial cell-derived EVs decrease the proliferation rate and wound healing response in vitro and ex vivo of endothelial cells in human, porcine, and rabbit models, as well as an increase in the cell size and apoptosis [127].
Studies have also shown that EVs derived from other origins may have beneficial effects on the cornea as well. Platelet-derived EVs (PEV) have been shown to decrease blood vessel formation in an injured cornea in an alkali-burn mouse model [128]. PEVs can also increase the wound closure rate of murine corneal endothelial cells in vitro [128]. Serum-derived EVs have been found to promote human corneal epithelial cells during an in vivo scratch assay [129]. Milk-derived EVs have been shown to increase the proliferation and migration rate of corneal epithelial cells after an alkali burn in vitro [130]. Salivary EVs have also been found to increase the proliferation and migration rate of corneal epithelial cells, both in vitro and in vivo, in a mouse model [131].
In terms of other bioactive membrane-bound particles in the cornea, extracellular mitochondria derived from injured sensory nerve axons are readily taken up by corneal epithelial cells via macropinocytosis and appear to be respiratory-competent [132]. The intercellular transport of mitochondria has been studied in different cell types in the body, including astrocytes and neurons [133], adipocytes [134], cancer, and immune cells [135] suggesting that this interplay may provide a conserved mechanism for preserving metabolic function and cell survival in times of stress [136]. Engulfed mitochondria by the corneal epithelium have been found to accelerate wound healing in a chemical burn model, providing supporting evidence that endocytosed mitochondria activate pro-reparative signaling responses [137]. Uptake of exogenous mitochondria by corneal endothelial cells in vitro has shown similar findings in a Fuch’s corneal endothelial model, with a reduction in oxidative stress and an increase in the mitochondrial membrane potential as a measure of energy production [138]. While extracellular mitochondria appear to retain respiratory function, it remains unclear if transferred mitochondria fully integrate into the host cell and remain respiratory competent in the long term.
EVs and Corneal Immune-Privilege Interplay
As mentioned, the cornea is an immune-privileged tissue that rapidly clears foreign bodies that enter. Resident EVs are allowed to play their role and do not trigger the cornea’s immune response. When the eye is injured, the resident immune cells are activated to remove the irritant from the eye. If treated with EVs from an external source, the cornea may work to clear the foreign EVs from the area.
Corneal grafts are an effective treatment to restore a person’s eyesight, but unfortunately, there is a high rate of graft rejection in conditions associated with inflammation or infection [139]. Rejection may be exacerbated by the introduction of EVs from an external source, which contain foreign antigens [140,141,142]. These antigens can induce inflammation and accelerate EV clearance by activating the innate and adaptive immune response [140,141,142]. This immune system activation can then lead to graft rejection in high-risk cases [140,141,142].
3.2. Stem Cell-Derived EV in the Corneas
EVs can be derived not only from established cell types but also from multipotent stem cells, which have the potential to serve as therapeutics for corneal injuries. Wang et al. found that induced pluripotent stem cell (iPSC)- and mesenchymal stem cell (MSC)- derived exosomes facilitate corneal wound closure after injury by increasing cell proliferation and migration. Their data suggested that iPSC-derived exosomes have a stronger therapeutic effect compared to MSC-derived exosomes [96,143]. MSCs can also be used to prolong corneal transplant graft survival in rats [143]. Shojaati et al. found that corneal stromal stem cells (CSSC)-derived EVs may play a crucial role in inhibiting corneal scarring and initiate regeneration of transparency to wounded corneal tissue [102].
MSCs derived from different sources can also provide beneficial effects to the cornea. Yu et al. introduced EVs derived from bone marrow-derived MSCs (BMSCs) to wounded epithelial cells on a cornea-on-a-chip and found that these EVs accelerated corneal epithelial wound healing [99]. Saccu et al. reported similar findings by treating wounded human corneal epithelial cells with BMSC-derived EVs and found that the cells recovered faster with modulation of angiogenesis, cell death, and inflammation in a wounded cornea [95]. BMSCs-derived exosomes can also be used as a vehicle to transport specific miRNAs to an injured cornea [143]. Adipose MSC (ASC)-derived exosomes have also been shown to have beneficial effects on rat corneal endothelial cells that underwent a freeze injury [144]. Tao et al. investigated the effects of EVs derived from human placenta-derived MSCs for alkaline injuries in a mouse model. They found that the hp-MSC EVs provided an ameliorative effect on the injured cornea by increasing anti-inflammation and proliferation, and decreasing apoptosis in the epithelial cells [101]. Hp-MSCs also provided ameliorative effects of inhibited inflammation and angiogenesis in the affected cornea [101].
3.3. Corneal Fibrosis
Corneal fibrosis, following injury, arises from the excessive accumulation of aberrant extracellular matrix produced by myofibroblasts [97,98]. This condition compromises the cornea’s transparency and structural integrity, significantly altering light-scattering properties and consequently reducing visual acuity [100,145].
In the cornea, communication between the corneal epithelium and stroma is critical for corneal wound repair, and EVs have been shown to influence physiological and pathological responses during this process [122]. Corneal wounds induce the release of EVs in rat epithelial debridement and rabbit keratectomy injury models [105]. While EVs released from the corneal epithelium after debridement were obstructed from entering the stroma by Bowman’s layer, a keratectomy wound, which involved the removal of Bowman’s layer and the anterior stroma, permitted the diffusion of EVs into the stromal tissue [105]. Mouse epithelial cell-derived EVs have also been reported to induce mouse corneal fibroblast proliferation and differentiation into myofibroblast [103,105]. Mesenchymal stem cells from corneal stromal stem cells-derived EVs have been reported to inhibit corneal scarring during wound healing in mice [102]. Human saliva-EVs have also been observed by our group to regulate stromal cell migration and wound healing [112]. Overall, these findings suggest that EVs may serve as a novel therapeutic target or be applied as therapeutic carriers of cargo that could prevent scar formation during the corneal wound healing process.
3.4. Keratoconus
Keratoconus (KC) is a corneal disease that forms the cornea into a cone shape [146,147]. It affects 1:400–1:2000 [104,147] individuals worldwide and is known to appear in puberty, extending through the third and fourth decades of life. Some common techniques to diagnose KC are by using slit lamp and tomography, but these techniques require physical manifestations to be present in order to make a diagnosis. Studies have shown molecular changes in this disease with altered levels of hormones and inflammatory factors in the blood [111,148,149,150], tears [111,148,151,152], and saliva [111,148,153] of KC patients. EVs have also been observed to have unique differences between healthy and KC samples.
Hefley et al. analyzed tear-derived EVs from healthy and KC donors. The authors found that the size and total particle counts did not differ between healthy and KC tEVs [107]. They also found that KC males showed a significant downregulation of CD63/CD9 and CD63/CD81/CD9 compared to healthy males [107]. Additional studies for KC have found that the protein cargo from corneal stromal cell-derived EVs altered levels of adhesion and migration proteins [110] as well as an overexpression of miR-184 [154]. Lozano et al. found a decreased expression of CD9 in corneal stromal cells, which suggests that KC individuals produce fewer EVs compared to healthy individuals [110]. EVs from the fibroblasts of patients with KC have been shown to contain miRNAs that have the role of silencing apoptosis, which has been found to be absent in healthy fibroblast EVs [106,155].
Escandon et al. introduced salivary EVs to human keratoconic stromal cells (HKCs) in an attempt to achieve therapeutic results in KC. They found that the salivary EVs were able to reduce the fibronectin protein expression as well as increase the thrombospondin-1 and cleaved vimentin [112]. These results support that salivary EVs could be a potential therapeutic treatment for KC.
Common treatments for this disease include collagen crosslinking, which strengthens the cornea by relinking the collagen fibrils in the cornea, and corneal transplantation (keratoplasty). One research group conducted a clinical trial to treat KC using autologous adipose tissue-derived mesenchymal stem cells (MSC(AT)). There were no post-operative complications, along with improved uncorrected and corrected distance in visual acuity at the 1- and 3-year follow-ups [36]. Considering KC as one of the leading causes of visual impairment in the cornea, more EV studies are needed in order to find potential therapeutic markers as well as to establish a biomarker for this disease.
3.5. Diabetes
Diabetes Mellitus (DM) is a metabolic disorder that is recognized as a major global disease, causing structural and functional ocular complications [108,156]. The prevalence of DM is increasing rapidly, contributing to a corresponding rise in corneal complications. The cornea is one of the most densely innervated and metabolically active tissues in the body. Hyperglycemia disrupts cellular and extracellular balance, leading to diabetic keratopathy. Primary complications associated with diabetic keratopathy include delayed corneal wound healing, loss of corneal sensitivity, reduced epithelial thickness, oxidative stress, and inflammation [108,109,156,157].
Roles of EVs have been identified in nearly every compartment of the eye, including the aqueous humor, vitreous humor, retina, and corneal microenvironment. EVs are essential for mediating cell–cell communication, signal transduction, and extracellular matrix remodeling in the cornea [125]. Due to its exposed location, the cornea is highly susceptible to injuries and epithelial breakdown. EVs, which are secreted by nearly all cells in the body, hold potential as therapies for treating corneal diseases owing to their key role in cell-to-cell communication [143]. Damage to epithelial cells triggers migration to heal the wound area, a process accelerated by corneal-derived exosomes and contributions from other layers [103,105,124]. Studies have shown that EVs are secreted in the cornea following injury [105,123,158]. EVs from isolated human corneal epithelial cells promote myofibroblast differentiation by corneal fibroblasts, suggesting that Bowman’s layer may provide an important barrier to pro-fibrotic EVs following wounding [103]. Supporting this idea, corneal injuries with an intact basement membrane are not generally associated with scarring compared to penetrating injuries, which occur with permeation of EVs and soluble factors into the stroma from the corneal epithelium and tear film [159].
Apart from their healing role in the cornea, EVs have been found to be significant in maintaining retinal cell function and homeostasis [160]. The role of EVs has been investigated in diabetic retinopathy (DR), which is a complication of diabetes that damages retinal blood vessels and the light-sensitive layer at the back of the eye. Studies have observed elevated levels of EVs due to inflammation, oxidative stress, and high blood glucose levels [160,161]. The potential of EVs as biomarkers has been explored in DR, where higher plasma EV numbers were observed in severe stages compared to mild cases [162]. Zhu et al. suggest that this difference in EV numbers may provide predictors for assessing the severity of DR in patients [160]. EVs also act as crucial mediators in the progression of DR, contributing to endothelial dysfunction, blood-retinal barrier breakdown, and neovascularization. In addition to this, EVs carry miRNAs, proteins, and nucleic acids that trigger pro-inflammatory and pro-angiogenic cascades, contributing to retinal damage [163]. Additionally, EVs have been investigated for their role in retinal pigment epithelium (RPE), where they induce an immunoregulatory CD14++CD16++ phenotype that inhibits T-cell proliferation without affecting cell survival [163].
Several studies have explored the therapeutic benefits of exogenous EVs as therapeutics in pre-clinical models of diabetes. Hefley et al. investigated EVs derived from healthy and diabetic corneal stromal constructs, revealing larger sizes and higher total particle counts in EVs derived from healthy constructs compared to diabetic ones, suggesting that diabetes may be associated with differential EV-mediated signaling in the corneal stroma [114]. Therapeutic applications of EVs derived from mesenchymal stem cells from adipose tissue have been demonstrated, showing corneal nerve regeneration and sensation recovery in streptozotocin (STZ)-induced diabetic keratopathy (DK) mice, correlating with activation of pro-reparative responses [116]. EVs have also been found to significantly promote corneal epithelial wound healing through activation of Nerve Growth Factor (NGF)/Tropomyosin receptor kinase A (TrkA) pathway, which is an essential pathway involved in the maintenance of corneal sensory nerves that penetrate the epithelium and stroma [116]. Studies involving EVs derived from healthy corneal epithelial cells, MSCs, or limbal stem cells show potential in reversing diabetic corneal damage through restoration of normal epithelial wound healing, reduction of oxidative stress and inflammation, and delivery of beneficial miRNAs to corneal cells. This area can be further explored to better understand the role of EVs in corneal diabetes, along with other corneal diseases.
3.6. Herpes Simplex Virus
The Herpes Simplex Virus (HSV) is a common virus that can be transmitted through bodily fluids and can cause inflammation of the cornea and loss of corneal innervation. HSV type 1 (HSV-1) can lead to corneal infection, which can cause the infected individuals to have Herpes Simplex Keratitis (HSK), which is the leading factor for infectious blindness [118]. HSV-2 can cause lesions on the genital area. HSK can travel to the trigeminal ganglion after the first exposure and remain latent. Once activated, HSK can affect the epithelium, stroma, and endothelium layer of the cornea, in which recurring infections can lead to corneal thinning, neovascularization, and scarring of the corneal epithelium [118]. This disease can also affect other areas of the eye, such as the iris, lens, vitreous humor, and retina, which leads to iridocyclitis, anterior uveitis, vitritis, cataracts, and retinal detachment if not caught in time.
Slit lamp evaluations are the first step in diagnosing HSK. If the slit lamp evaluation is inconclusive, the clinician may take a sample and perform PCR for a confirmation test. Current treatments for HSK include topical antibacterials and antivirals.
Little is known about EVs in the study of HSK. Ma et al. identified 339 metabolites from tear EVs collected from patients with HSK and found altered levels of amino acid and energy metabolism, providing insight into the effects of HSV on metabolic activity in host cells [118].
Even though HSV is one of the more prevalent sexually transmitted diseases, the spread of HSK is not entirely known. A recent study suggests that tear exosomes could be the latent site of the HSV-1 virus in recurring HSK, which could lead to transmission of this virus. This study confirmed that HSV-1 genes can be transferred intercellularly by the exosomal pathway, highlighting EVs as both biomarkers and potential mediators of infection [120].
3.7. Dry Eye Disease
The cornea is protected from environmental factors, such as dirt and dust, by the tear film, which also keeps the cornea moist. The tear film is comprised of three layers: the lipid layer, aqueous layer, and finally the mucin layer. In a healthy individual, this film is equally spread across the anterior of the eye and is replaced quickly after each blink. Individuals with dry eye have a reduction in this tear film, which will lead the affected individual to experience itching, glare, eye irritation, blurry vision, and discomfort. There are two different types of dry eye. The first type is evaporative dry eye (EDE), in which the mucosal layer is diminished in the tear film, leading to the evaporation of the aqueous layer. The second type of dry eye is aqueous tear deficiency (ATD), in which not enough of the aqueous layer is produced.
Studies are hoping to determine whether EVs can be used as a diagnostic tool to identify dry eye and to decrease the number of tests needed to diagnose an individual with dry eye. Studies evaluating small RNA cargo in EVs have revealed notable differences in microRNAs in dry eye disease. Cross et al. analyzed RNA transcripts from dry eye EVs and found 26,000+ different transcripts [48]. Of these transcripts, 6% showed significantly altered levels, such as sodium channel modifier 1 (SCNM1) and microRNA-130b, between healthy individuals and individuals with dry eye [48]. Pucker et al. collected tear samples from healthy and dry eye individuals and found an upregulation of inflammatory markers, including miR-127-5p, miR-1273h-3p, miR-1288-5p, miR-139-3p, miR-1910-5p, miR-203b-5p, miR-22-5p, miR-4632-3p, and miR-130b-5p, which is what Cross et al. also observed [113].
The current treatment for dry eye is artificial tears, which only provides a temporary solution. Current research is being conducted in search of a better therapeutic option for this disease. Yu et al. used a dry-eye mouse model, which induced corneal damage, to test the therapeutic effects of EVs derived from human adipose tissue stem cells (hADSC-EVs). They found that eye drops containing hADSC-EVs effectively alleviate ocular surface damage by decreasing the NLRP3 inflammatory response in dry eye [115]. Yi et al. investigated EVs derived from human amniotic epithelial cells (hAEC-EVs) and observed decreased inflammation in the ocular surface in an in vivo mouse model [117]. Ocular surface epithelial cells treated with hAEC-EVs had reduced inflammatory cytokines, as well as reduced corneal epithelial cell migration and proliferation [117]. Wang et al. found that when a mouse model that has dry eye is treated with EVs derived from human umbilical cord-derived MSCs (huMSC-EVs), the IRAK/TAB2/NF-κB pathway is targeted, reducing inflammation and restoring the corneal surface [119].
3.8. Bioengineering Applications
One of the challenges that limits the therapeutic application of EVs introduced to the cornea has the potential to be rapidly cleared due to the immune-privileged properties of the cornea, such as tear turnover, blinking, nasolacrimal drainage, and limited epithelial permeability [164,165]. Hydrogels, with their features including biocompatibility, high water content, and adjustable mechanical properties, help sustain the release of corneal epithelial cell-derived EVs [166,167,168]. Tang et al. have also implemented iPSC-MSC-derived EVs into hydrogels and have observed a similar sustained EV release in a damaged cornea [169]. The iPSC-MSC-derived EVs have been observed to reduce scar formation in vivo by decreasing the mRNA expression that codes for collagen type 1 alpha 1, collagen type V alpha 1, and collagen type V alpha 2, which are highly expressed collagens in the cornea [169]. Additionally, studies on biopolymer-based hydrogels loaded with MSC-derived exosomes demonstrate increased corneal adhesion and sustained bioavailability in vivo, further supporting the application of a hydrogel delivery platform for improving EVs residence time in corneal applications [170,171]. In corneal epithelial injury models, Sun et al. showed that exosome-loaded DEGMA-modified hyaluronic acid hydrogel stayed on the corneal surface much longer than free exosomes and enhanced prolonged local exposure of miRNA cargo on the ocular surface, which enhanced epithelial regeneration and reduced inflammation [172].
3.9. Challenges in Clinical Translation
As we have presented in this review, there are many sources and methods used to introduce EVs to the cornea. There is currently no standardization of EV research in place, which may lead to varying results between labs using the same techniques; therefore, there is a need for standardization practices in this field. MISEV is attempting to provide standardization techniques to scientists in the field to ensure uniformity in EV research. They also describe terminology to spread awareness that there are not only three types of EVs, but numerous types of vesicles and particles present in samples.
Another challenge that needs to be overcome in moving forward with using EVs as therapeutics is the manufacturing processes, as well as the stability of these vesicles. EVs are produced from cells, so there needs to be quality control to ensure they are following the MISEV guidelines. EVs are also produced from cells, which can be a challenge in itself since cells are a precious resource. EVs also have to be stored at a certain temperature to maintain stability, and freeze-thaw cycles may impact their characteristics and effectiveness. The EVs would also have to contain self-antigens to avoid detection by the cornea’s immune system.
4. Conclusions
EVs could be used as a powerful tool to treat corneal diseases since it has been suggested that they have a beneficial impact on the wound-healing cascade. Since EVs are derived from cells and can be found in biological fluids, they may provide valuable insight into the health of the cornea by utilizing them as biomarkers for various corneal diseases. EVs can also serve as a natural drug delivery system, which can bypass the body’s immune system.
There are numerous challenges that must be overcome for EVs to be safe to use as therapeutics. Some of these challenges include EV heterogeneity, the immune-privileged properties of the cornea, delivery of EVs, and mass-production methods, to name a few. Further research on EVs is warranted to determine their use as biomarkers, as well as therapeutic options for the corneal diseases described in this review.
Author Contributions
Conceptualization, D.K.; methodology, B.S.H., P.S., T.B.M., P.N. and D.K.; software, B.S.H., P.S., T.B.M., P.N. and D.K.; validation, B.S.H., P.S., T.B.M., P.N. and D.K.; formal analysis, B.S.H., P.S., T.B.M., P.N. and D.K.; investigation, B.S.H., P.S., T.B.M., P.N. and D.K.; resources, B.S.H., P.S., T.B.M., P.N. and D.K.; data curation, B.S.H., P.S., T.B.M., P.N., Y.M. and D.K.; writing—original draft preparation, B.S.H., P.S., T.B.M., P.N., S.E.N. and D.K.; writing—review and editing, B.S.H., P.S., T.B.M., P.N., Y.M., S.E.N. and D.K.; visualization, B.S.H., P.S., T.B.M., P.N., S.E.N. and D.K.; supervision, B.S.H., P.S., T.B.M., P.N. and D.K.; project administration, D.K.; funding acquisition, D.K. All authors have read and agreed to the published version of the manuscript.
Funding
EY030028 (NIH/NEI).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| EV | Extracellular Vesicles |
| ECM | Extracellular Matrix |
| NCAM | Neural Cell Adhesion Molecules |
| NTA | Nanoparticle Tracking Analyzer |
| PCR | Polymerase Chain Reaction |
| α-SMA | Alpha Smooth Muscle Actin |
| PEV | Platelet-derived EVs |
| iPSCs | Induced Pluripotent Stem Cells |
| MSCs | Mesenchymal Stem Cells |
| CSSCs | Corneal Stromal Stem Cells |
| BMSCs | Bone Marrow-derived MSCs |
| ASCs | Adipose MSCs |
| KC | Keratoconus |
| HKC | Keratoconic Stromal Cells |
| MSC(AT) | Autologous Adipose Tissue-derived MSCs |
| DM | Diabetes Mellitus |
| DR | Diabetic Retinopathy |
| RPE | Retinal Pigment Epithelium |
| STZ | Streptozotocin |
| DK | Diabetic Keratopathy |
| NGF | Nerve Growth Factor |
| TrkA | Tropomyosin Receptor Kinase A |
| HSV | Herpes Simplex Virus |
| HSV-1 | Herpes Simplex Virus Type 1 |
| EDE | Evaporative Dry Eye |
| ATD | Aqueous Tear Deficiency |
| SCNM1 | Sodium Channel Modifier 1 |
| hADSC-EVs | Human Adipose Tissue Stem-Cells derived EVs |
| hAEC-EVs | Human Amniotic Epithelial Cells-derived EVs |
| huMSC-EVs | Human Umbilical Cord-derived MSCs-derived EVs |
References
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef]
- Meek, K.M.; Knupp, C.; Lewis, P.N.; Morgan, S.R.; Hayes, S. Structural control of corneal transparency, refractive power and dynamics. Eye 2025, 39, 644–650. [Google Scholar] [CrossRef]
- Yu, F.X.; Lee, P.S.Y.; Yang, L.; Gao, N.; Zhang, Y.; Ljubimov, A.V.; Yang, E.; Zhou, Q.; Xie, L. The impact of sensory neuropathy and inflammation on epithelial wound healing in diabetic corneas. Prog. Retin. Eye Res. 2022, 89, 101039. [Google Scholar] [CrossRef]
- Ladea, L.; Zemba, M.; Calancea, M.I.; Caltaru, M.V.; Dragosloveanu, C.D.M.; Coroleuca, R.; Catrina, E.L.; Brezean, I.; Dinu, V. Corneal Epithelial Changes in Diabetic Patients: A Review. Int. J. Mol. Sci. 2024, 25, 3471. [Google Scholar] [CrossRef]
- Ljubimov, A.V. Diabetic complications in the cornea. Vision. Res. 2017, 139, 138–152. [Google Scholar] [CrossRef]
- Schwend, T. Wiring the ocular surface: A focus on the comparative anatomy and molecular regulation of sensory innervation of the cornea. Differentiation 2023, 132, 24–40. [Google Scholar] [CrossRef]
- Lum, E.; Corbett, M.C.; Murphy, P.J. Corneal Sensitivity After Ocular Surgery. Eye Contact Lens 2019, 45, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.C.B.; Tajbakhsh, Z.; Wolffsohn, J.S. The clinical impact of contact lens wear on neural structure and function of the cornea. Clin. Exp. Optom. 2025, 108, 408–418. [Google Scholar] [CrossRef]
- Hori, J.; Kunishige, T.; Nakano, Y. Immune Checkpoints Contribute Corneal Immune Privilege: Implications for Dry Eye Associated with Checkpoint Inhibitors. Int. J. Mol. Sci. 2020, 21, 3962. [Google Scholar] [CrossRef] [PubMed]
- Maharana, P.K.; Mandal, S.; Kaweri, L.; Sahay, P.; Lata, S.; Asif, M.I.; Nagpal, R.; Sharma, N. Immunopathogenesis of corneal graft rejection. Indian J. Ophthalmol. 2023, 71, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Gurnani, B.; Czyz, C.N.; Mahabadi, N.; Havens, S.J. Corneal Graft Rejection. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Sun, M.; Zhang, H.; Liu, J.; Chen, J.; Cui, Y.; Wang, S.; Zhang, X.; Yang, Z. Extracellular Vesicles: A New Star for Gene Drug Delivery. Int. J. Nanomed. 2024, 19, 2241–2264. [Google Scholar] [CrossRef]
- Kim, H.I.; Park, J.; Zhu, Y.; Wang, X.; Han, Y.; Zhang, D. Recent advances in extracellular vesicles for therapeutic cargo delivery. Exp. Mol. Med. 2024, 56, 836–849. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Huang, Z.; Zhong, H.; Lei, Q.; Ai, Y.; Xie, Z.; Zhang, T.; Jiang, B.; Zhu, W.; Sheng, Y.; et al. Analysis and Biomedical Applications of Functional Cargo in Extracellular Vesicles. ACS Nano 2022, 16, 19980–20001. [Google Scholar] [CrossRef]
- Fonseka, P.; Mathivanan, S. Extracellular Vesicles Biogenesis, Cargo Sorting and Implications in Disease Conditions. Cells 2023, 12, 280. [Google Scholar] [CrossRef]
- Wolf, P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef]
- Couch, Y.; Buzas, E.I.; Di Vizio, D.; Gho, Y.S.; Harrison, P.; Hill, A.F.; Lotvall, J.; Raposo, G.; Stahl, P.D.; Thery, C.; et al. A brief history of nearly EV-erything—The rise and rise of extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12144. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Thery, C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J. Extracell. Vesicles 2019, 8, 1648167. [Google Scholar] [CrossRef] [PubMed]
- Mu, N.; Li, J.; Zeng, L.; You, J.; Li, R.; Qin, A.; Liu, X.; Yan, F.; Zhou, Z. Plant-Derived Exosome-Like Nanovesicles: Current Progress and Prospects. Int. J. Nanomed. 2023, 18, 4987–5009. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Li, C.; Yu, Y.; Yi, Y.; Wang, J.; Chen, D. Exosome-Induced Regulation in Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 1464. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, B.; Yang, Y.; Jiang, Q.; Li, T.; Gong, J.; Tang, H.; Zhang, Q. Stem cell-derived exosomes: Emerging therapeutic opportunities for wound healing. Stem Cell Res. Ther. 2023, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Mondal, J.; Pillarisetti, S.; Junnuthula, V.; Saha, M.; Hwang, S.R.; Park, I.K.; Lee, Y.K. Hybrid exosomes, exosome-like nanovesicles and engineered exosomes for therapeutic applications. J. Control Release 2023, 353, 1127–1149. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Mahmoudi, K.; Ezrin, A.; Hadjipanayis, C. Small extracellular vesicles as tumor biomarkers for glioblastoma. Mol. Asp. Med. 2015, 45, 97–102. [Google Scholar] [CrossRef]
- Sarkar, R.; Xu, Z.; Perera, C.J.; Apte, M.V. Emerging role of pancreatic stellate cell-derived extracellular vesicles in pancreatic cancer. Semin. Cancer Biol. 2023, 93, 114–122. [Google Scholar] [CrossRef]
- Brennan, K.; Martin, K.; FitzGerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef]
- Clancy, J.W.; Schmidtmann, M.; D’Souza-Schorey, C. The ins and outs of microvesicles. FASEB Bioadv. 2021, 3, 399–406. [Google Scholar] [CrossRef]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2017, 8, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Lei, Q.; Luo, X.; Yin, J.; Chen, S.; Hao, C.; Shiyu, L.; Ma, D. Advances in biological functions and applications of apoptotic vesicles. Cell Commun. Signal. 2023, 21, 260. [Google Scholar] [CrossRef]
- Jiang, L.; Paone, S.; Caruso, S.; Atkin-Smith, G.K.; Phan, T.K.; Hulett, M.D.; Poon, I.K.H. Determining the contents and cell origins of apoptotic bodies by flow cytometry. Sci. Rep. 2017, 7, 14444. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Sui, B.; Xiang, L.; Yan, X.; Wu, D.; Shi, S.; Hu, X. Emerging understanding of apoptosis in mediating mesenchymal stem cell therapy. Cell Death Dis. 2021, 12, 596. [Google Scholar] [CrossRef]
- Kakarla, R.; Hur, J.; Kim, Y.J.; Kim, J.; Chwae, Y.J. Apoptotic cell-derived exosomes: Messages from dying cells. Exp. Mol. Med. 2020, 52, 1–6. [Google Scholar] [CrossRef]
- Barzin, M.; Bagheri, A.M.; Ohadi, M.; Abhaji, A.M.; Salarpour, S.; Dehghannoudeh, G. Application of plant-derived exosome-like nanoparticles in drug delivery. Pharm. Dev. Technol. 2023, 28, 383–402. [Google Scholar] [CrossRef]
- Shu, S.; Allen, C.L.; Benjamin-Davalos, S.; Koroleva, M.; MacFarland, D.; Minderman, H.; Ernstoff, M.S. A Rapid Exosome Isolation Using Ultrafiltration and Size Exclusion Chromatography (REIUS) Method for Exosome Isolation from Melanoma Cell Lines. Methods Mol. Biol. 2021, 2265, 289–304. [Google Scholar] [CrossRef]
- Alio, J.L.; Alio Del Barrio, J.L.; El Zarif, M.; Azaar, A.; Makdissy, N.; Khalil, C.; Harb, W.; El Achkar, I.; Jawad, Z.A.; De Miguel, M.P. Regenerative Surgery of the Corneal Stroma for Advanced Keratoconus: 1-Year Outcomes. Am. J. Ophthalmol. 2019, 203, 53–68. [Google Scholar] [CrossRef]
- Rahbari, M.; Rahbari, N.; Reissfelder, C.; Weitz, J.; Kahlert, C. Exosomes: Novel implications in diagnosis and treatment of gastrointestinal cancer. Langenbecks Arch. Surg. 2016, 401, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Todkar, K.; Chikhi, L.; Desjardins, V.; El-Mortada, F.; Pepin, G.; Germain, M. Selective packaging of mitochondrial proteins into extracellular vesicles prevents the release of mitochondrial DAMPs. Nat. Commun. 2021, 12, 1971. [Google Scholar] [CrossRef] [PubMed]
- Al Amir Dache, Z.; Otandault, A.; Tanos, R.; Pastor, B.; Meddeb, R.; Sanchez, C.; Arena, G.; Lasorsa, L.; Bennett, A.; Grange, T.; et al. Blood contains circulating cell-free respiratory competent mitochondria. FASEB J. 2020, 34, 3616–3630. [Google Scholar] [CrossRef]
- Sanz-Ros, J.; Mas-Bargues, C.; Romero-Garcia, N.; Huete-Acevedo, J.; Dromant, M.; Borras, C. The Potential Use of Mitochondrial Extracellular Vesicles as Biomarkers or Therapeutical Tools. Int. J. Mol. Sci. 2023, 24, 7005. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, M.A. Overview and Update on Extracellular Vesicles: Considerations on Exosomes and Their Application in Modern Medicine. Biology 2022, 11, 804. [Google Scholar] [CrossRef]
- Joshi, B.S.; de Beer, M.A.; Giepmans, B.N.G.; Zuhorn, I.S. Endocytosis of Extracellular Vesicles and Release of Their Cargo from Endosomes. ACS Nano 2020, 14, 4444–4455. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular vesicles and nanoparticles: Emerging complexities. Trends Cell Biol. 2023, 33, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N.; Dalirfardouei, R.; Dias, T.; Lotvall, J.; Lasser, C. Tetraspanins distinguish separate extracellular vesicle subpopulations in human serum and plasma—Contributions of platelet extracellular vesicles in plasma samples. J. Extracell. Vesicles 2022, 11, e12213. [Google Scholar] [CrossRef]
- Lucien, F.; Gustafson, D.; Lenassi, M.; Li, B.; Teske, J.J.; Boilard, E.; von Hohenberg, K.C.; Falcon-Perez, J.M.; Gualerzi, A.; Reale, A.; et al. MIBlood-EV: Minimal information to enhance the quality and reproducibility of blood extracellular vesicle research. J. Extracell. Vesicles 2023, 12, e12385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Takeuchi, T.; Takeda, A.; Mochizuki, H.; Nagai, Y. Comparison of serum and plasma as a source of blood extracellular vesicles: Increased levels of platelet-derived particles in serum extracellular vesicle fractions alter content profiles from plasma extracellular vesicle fractions. PLoS ONE 2022, 17, e0270634. [Google Scholar] [CrossRef] [PubMed]
- Aqrawi, L.A.; Galtung, H.K.; Guerreiro, E.M.; Ovstebo, R.; Thiede, B.; Utheim, T.P.; Chen, X.; Utheim, O.A.; Palm, O.; Skarstein, K.; et al. Proteomic and histopathological characterisation of sicca subjects and primary Sjogren’s syndrome patients reveals promising tear, saliva and extracellular vesicle disease biomarkers. Arthritis Res. Ther. 2019, 21, 181. [Google Scholar] [CrossRef]
- Cross, T.; Ovstebo, R.; Brusletto, B.S.; Troseid, A.S.; Olstad, O.K.; Aspelin, T.; Jackson, C.J.; Chen, X.; Utheim, T.P.; Haug, K.B.F. RNA Profiles of Tear Fluid Extracellular Vesicles in Patients with Dry Eye-Related Symptoms. Int. J. Mol. Sci. 2023, 24, 15390. [Google Scholar] [CrossRef]
- Shiju, T.M.; Yuan, A. Extracellular vesicle biomarkers in ocular fluids associated with ophthalmic diseases. Exp. Eye Res. 2024, 241, 109831. [Google Scholar] [CrossRef]
- Chiabotto, G.; Gai, C.; Deregibus, M.C.; Camussi, G. Salivary Extracellular Vesicle-Associated exRNA as Cancer Biomarker. Cancers 2019, 11, 891. [Google Scholar] [CrossRef]
- Feng, J.; Xiao, B.L.; Zhang, L.Z.; Zhang, Y.H.; Tang, M.; Xu, C.M.; Chen, G.; Zhang, Z.L. Simultaneous Detection of Two Extracellular Vesicle Subpopulations in Saliva Assisting Tumor T Staging of Oral Squamous Cell Carcinoma. Anal. Chem. 2023, 95, 7753–7760. [Google Scholar] [CrossRef]
- Sjoqvist, S.; Otake, K. Saliva and Saliva Extracellular Vesicles for Biomarker Candidate Identification-Assay Development and Pilot Study in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2023, 24, 5237. [Google Scholar] [CrossRef]
- Chutipongtanate, S.; Morrow, A.L.; Newburg, D.S. Human Milk Extracellular Vesicles: A Biological System with Clinical Implications. Cells 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, D.; Dorodnykh, D.; Avdeenko, N.V.; Nekliudov, N.A.; Garssen, J.; Elolimy, A.A.; Petrou, L.; Simpson, M.R.; Yeruva, L.; Munblit, D. Perspective: The Role of Human Breast-Milk Extracellular Vesicles in Child Health and Disease. Adv. Nutr. 2021, 12, 59–70. [Google Scholar] [CrossRef]
- Vahkal, B.; Altosaar, I.; Ariana, A.; Jabbour, J.; Pantieras, F.; Daniel, R.; Tremblay, E.; Sad, S.; Beaulieu, J.F.; Cote, M.; et al. Human milk extracellular vesicles modulate inflammation and cell survival in intestinal and immune cells. Pediatr. Res. 2025, 98, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.E.; Vorn, R.; Chimenti, M.; Crouch, K.; Shaoshuai, C.; Narayanaswamy, J.; Harken, A.; Schmidt, R.; Gill, J.; Lee, H. Extracellular vesicle miRNAs in breast milk of obese mothers. Front. Nutr. 2022, 9, 976886. [Google Scholar] [CrossRef] [PubMed]
- Bozack, A.K.; Colicino, E.; Rodosthenous, R.S.; Bloomquist, T.R.; Baccarelli, A.A.; Wright, R.O.; Wright, R.J.; Lee, A.G. Breast milk-derived extracellular vesicle miRNAs are associated with maternal asthma and atopy. Epigenomics 2022, 14, 727–739. [Google Scholar] [CrossRef]
- Galley, J.D.; Besner, G.E. The Therapeutic Potential of Breast Milk-Derived Extracellular Vesicles. Nutrients 2020, 12, 745. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef]
- Huang, J.; Chen, H.; Li, N.; Liu, P.; Yang, J.; Zhao, Y. Emerging technologies towards extracellular vesicles large-scale production. Bioact. Mater. 2025, 52, 338–365. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Getting, S.J.; Moschos, S.A. Extracellular vesicles and their nucleic acids for biomarker discovery. Pharmacol. Ther. 2018, 192, 170–187. [Google Scholar] [CrossRef]
- Tiwari, S.; Kumar, V.; Randhawa, S.; Verma, S.K. Preparation and characterization of extracellular vesicles. Am. J. Reprod. Immunol. 2021, 85, e13367. [Google Scholar] [CrossRef]
- Gul, B.; Syed, F.; Khan, S.; Iqbal, A.; Ahmad, I. Characterization of extracellular vesicles by flow cytometry: Challenges and promises. Micron 2022, 161, 103341. [Google Scholar] [CrossRef]
- Safford, H.R.; Bischel, H.N. Flow cytometry applications in water treatment, distribution, and reuse: A review. Water Res. 2019, 151, 110–133. [Google Scholar] [CrossRef]
- Schermelleh, L.; Ferrand, A.; Huser, T.; Eggeling, C.; Sauer, M.; Biehlmaier, O.; Drummen, G.P. Super-resolution microscopy demystified. Nat. Cell Biol. 2019, 21, 72–84. [Google Scholar] [CrossRef]
- Liebel, M.; Ortega Arroyo, J.; Beltrán, V.S.; Osmond, J.; Jo, A.; Lee, H.; Quidant, R.; van Hulst, N.F. 3D tracking of extracellular vesicles by holographic fluorescence imaging. Sci. Adv. 2020, 6, eabc2508. [Google Scholar] [CrossRef]
- O’Brien, K.; Ughetto, S.; Mahjoum, S.; Nair, A.V.; Breakefield, X.O. Uptake, functionality, and re-release of extracellular vesicle-encapsulated cargo. Cell Rep. 2022, 39, 110651. [Google Scholar] [CrossRef]
- Lai, C.P.; Kim, E.Y.; Badr, C.E.; Weissleder, R.; Mempel, T.R.; Tannous, B.A.; Breakefield, X.O. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat. Commun. 2015, 6, 7029. [Google Scholar] [CrossRef]
- Mustapic, M.; Eitan, E.; Werner, J.K., Jr.; Berkowitz, S.T.; Lazaropoulos, M.P.; Tran, J.; Goetzl, E.J.; Kapogiannis, D. Plasma extracellular vesicles enriched for neuronal origin: A potential window into brain pathologic processes. Front. Neurosci. 2017, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Badhwar, A.; Haqqani, A.S. Biomarker potential of brain-secreted extracellular vesicles in blood in Alzheimer’s disease. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2020, 12, e12001. [Google Scholar] [CrossRef] [PubMed]
- Comfort, N.; Cai, K.; Bloomquist, T.R.; Strait, M.D.; Ferrante, A.W., Jr.; Baccarelli, A.A. Nanoparticle Tracking Analysis for the Quantification and Size Determination of Extracellular Vesicles. J. Vis. Exp. 2021. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.; Ferreira, Y.J.; Dragovic, R.A.; Redman, C.W.; Sargent, I.L. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. J. Extracell. Vesicles 2013, 2, 19671. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.; Sayle, S.; Karow, A.R.; Bakowsky, U.; Garidel, P. Nanoparticle tracking analysis of particle size and concentration detection in suspensions of polymer and protein samples: Influence of experimental and data evaluation parameters. Eur. J. Pharm. Biopharm. 2016, 104, 30–41. [Google Scholar] [CrossRef]
- Xu, D.; Di, K.; Fan, B.; Wu, J.; Gu, X.; Sun, Y.; Khan, A.; Li, P.; Li, Z. MicroRNAs in extracellular vesicles: Sorting mechanisms, diagnostic value, isolation, and detection technology. Front. Bioeng. Biotechnol. 2022, 10, 948959. [Google Scholar] [CrossRef]
- Lee, H.; He, X.; Le, T.; Carnino, J.M.; Jin, Y. Single-step RT-qPCR for detection of extracellular vesicle microRNAs in vivo: A time- and cost-effective method. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 318, L742–L749. [Google Scholar] [CrossRef]
- Kim, J.A.; Park, C.; Sung, J.J.; Seo, D.J.; Choi, S.J.; Hong, Y.H. Small RNA sequencing of circulating small extracellular vesicles microRNAs in patients with amyotrophic lateral sclerosis. Sci. Rep. 2023, 13, 5528. [Google Scholar] [CrossRef]
- Bagci, C.; Sever-Bahcekapili, M.; Belder, N.; Bennett, A.P.S.; Erdener, S.E.; Dalkara, T. Overview of extracellular vesicle characterization techniques and introduction to combined reflectance and fluorescence confocal microscopy to distinguish extracellular vesicle subpopulations. Neurophotonics 2022, 9, 021903. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- van Maanen, J.C.; Bach, F.C.; Braun, T.S.; Giovanazzi, A.; van Balkom, B.W.M.; Templin, M.; Wauben, M.H.M.; Tryfonidou, M.A. A Combined Western and Bead-Based Multiplex Platform to Characterize Extracellular Vesicles. Tissue Eng. Part. C Methods 2023, 29, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, X.; Li, Q.; Lai, H.; Zhang, H.; Hu, Z.; Li, Y.; Huang, S. EV-origin: Enumerating the tissue-cellular origin of circulating extracellular vesicles using exLR profile. Comput. Struct. Biotechnol. J. 2020, 18, 2851–2859. [Google Scholar] [CrossRef]
- Kobayashi, T.; Vischer, U.M.; Rosnoblet, C.; Lebrand, C.; Lindsay, M.; Parton, R.G.; Kruithof, E.K.; Gruenberg, J. The tetraspanin CD63/lamp3 cycles between endocytic and secretory compartments in human endothelial cells. Mol. Biol. Cell 2000, 11, 1829–1843. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; Charrin, S.; Simoes, S.; Romao, M.; Rochin, L.; Saftig, P.; Marks, M.S.; Rubinstein, E.; Raposo, G. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev. Cell 2011, 21, 708–721. [Google Scholar] [CrossRef]
- Hendricks, E.L.; Smith, I.R.; Prates, B.; Barmaleki, F.; Liebl, F.L.W. The CD63 homologs, Tsp42Ee and Tsp42Eg, restrict endocytosis and promote neurotransmission through differential regulation of synaptic vesicle pools. Front. Cell Neurosci. 2022, 16, 957232. [Google Scholar] [CrossRef] [PubMed]
- Im, Y.; Yoo, H.; Ko, R.E.; Lee, J.Y.; Park, J.; Jeon, K. Exosomal CD63 in critically ill patients with sepsis. Sci. Rep. 2021, 11, 20300. [Google Scholar] [CrossRef]
- Carmo, L.A.; Bonjour, K.; Ueki, S.; Neves, J.S.; Liu, L.; Spencer, L.A.; Dvorak, A.M.; Weller, P.F.; Melo, R.C. CD63 is tightly associated with intracellular, secretory events chaperoning piecemeal degranulation and compound exocytosis in human eosinophils. J. Leukoc. Biol. 2016, 100, 391–401. [Google Scholar] [CrossRef]
- Palmulli, R.; Couty, M.; Piontek, M.C.; Ponnaiah, M.; Dingli, F.; Verweij, F.J.; Charrin, S.; Tantucci, M.; Sasidharan, S.; Rubinstein, E.; et al. CD63 sorts cholesterol into endosomes for storage and distribution via exosomes. Nat. Cell Biol. 2024, 26, 1093–1109. [Google Scholar] [CrossRef]
- Hasezaki, T.; Yoshima, T.; Mine, Y. Anti-CD81 antibodies reduce migration of activated T lymphocytes and attenuate mouse experimental colitis. Sci. Rep. 2020, 10, 6969. [Google Scholar] [CrossRef]
- Brosseau, C.; Colas, L.; Magnan, A.; Brouard, S. CD9 Tetraspanin: A New Pathway for the Regulation of Inflammation? Front. Immunol. 2018, 9, 2316. [Google Scholar] [CrossRef]
- Vences-Catalan, F.; Rajapaksa, R.; Kuo, C.C.; Miller, C.L.; Lee, A.; Ramani, V.C.; Jeffrey, S.S.; Levy, R.; Levy, S. Targeting the tetraspanin CD81 reduces cancer invasion and metastasis. Proc. Natl. Acad. Sci. USA 2021, 118, e2018961118. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.M.; Jang, B.G.; Lee, D.H.; Hyun, C.L. Increased CD9 expression predicts favorable prognosis in human cancers: A systematic review and meta-analysis. Cancer Cell Int. 2021, 21, 472. [Google Scholar] [CrossRef]
- Mizoshiri, N.; Shirai, T.; Terauchi, R.; Tsuchida, S.; Mori, Y.; Hayashi, D.; Kishida, T.; Arai, Y.; Mazda, O.; Nakanishi, T.; et al. The tetraspanin CD81 mediates the growth and metastases of human osteosarcoma. Cell. Oncol. 2019, 42, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Kodam, S.P.; Baghban, N.; Ullah, M. CD9 role in proliferation, rejuvenation, and therapeutic applications. Genes Dis. 2024, 11, 101008. [Google Scholar] [CrossRef]
- Na, K.; Lee, S.; Kim, D.K.; Kim, Y.S.; Hwang, J.Y.; Kang, S.S.; Baek, S.; Lee, C.Y.; Yang, S.M.; Han, Y.J.; et al. CD81 and CD82 expressing tumor-infiltrating lymphocytes in the NSCLC tumor microenvironment play a crucial role in T-cell activation and cytokine production. Front. Immunol. 2024, 15, 1336246. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, X.; Greening, D.W.; Huang, Y.; Li, B.; Li, Z.; Long, G.; Lotvall, J.; Lv, L.; Poon, I.K.H.; et al. Unveiling Heterogeneity: Innovations and Challenges in Single-Vesicle Analysis for Clinical Translation. J. Extracell. Vesicles 2025, 14, e70209. [Google Scholar] [CrossRef]
- Saccu, G.; Menchise, V.; Gai, C.; Bertolin, M.; Ferrari, S.; Giordano, C.; Manco, M.; Dastru, W.; Tolosano, E.; Bussolati, B.; et al. Bone Marrow Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles Promote Corneal Wound Repair by Regulating Inflammation and Angiogenesis. Cells 2022, 11, 3892. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hou, Y.; Li, X.; Song, Z.; Sun, B.; Li, X.; Zhang, H. Comparison of exosomes derived from induced pluripotent stem cells and mesenchymal stem cells as therapeutic nanoparticles for treatment of corneal epithelial defects. Aging 2020, 12, 19546–19562. [Google Scholar] [CrossRef]
- Funderburgh, J.L.; Mann, M.M.; Funderburgh, M.L. Keratocyte phenotype mediates proteoglycan structure: A role for fibroblasts in corneal fibrosis. J. Biol. Chem. 2003, 278, 45629–45637. [Google Scholar] [CrossRef]
- Zieske, J.D. Extracellular matrix and wound healing. Curr. Opin. Ophthalmol. 2001, 12, 237–241. [Google Scholar] [CrossRef]
- Yu, Z.; Hao, R.; Du, J.; Wu, X.; Chen, X.; Zhang, Y.; Li, W.; Gu, Z.; Yang, H. A human cornea-on-a-chip for the study of epithelial wound healing by extracellular vesicles. iScience 2022, 25, 104200, Erratum in iScience 2025, 28, 112247. [Google Scholar] [CrossRef]
- Wilson, S.E.; Marino, G.K.; Torricelli, A.A.M.; Medeiros, C.S. Injury and defective regeneration of the epithelial basement membrane in corneal fibrosis: A paradigm for fibrosis in other organs? Matrix Biol. 2017, 64, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Chen, X.; Cao, H.; Zheng, L.; Li, Q.; Zhang, K.; Han, Z.; Han, Z.C.; Guo, Z.; Li, Z.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Corneal Wound Repair. Stem Cells Int. 2019, 2019, 5738510. [Google Scholar] [CrossRef] [PubMed]
- Shojaati, G.; Khandaker, I.; Funderburgh, M.L.; Mann, M.M.; Basu, R.; Stolz, D.B.; Geary, M.L.; Dos Santos, A.; Deng, S.X.; Funderburgh, J.L. Mesenchymal Stem Cells Reduce Corneal Fibrosis and Inflammation via Extracellular Vesicle-Mediated Delivery of miRNA. Stem Cells Transl. Med. 2019, 8, 1192–1201. [Google Scholar] [CrossRef]
- McKay, T.B.; Hutcheon, A.E.K.; Zieske, J.D.; Ciolino, J.B. Extracellular Vesicles Secreted by Corneal Epithelial Cells Promote Myofibroblast Differentiation. Cells 2020, 9, 1080. [Google Scholar] [CrossRef]
- Karamichos, D. Keratoconus: Challenges and Emerging Trends. J. Mol. Genet. Med. 2018, 12, 367. [Google Scholar] [CrossRef]
- Han, K.Y.; Tran, J.A.; Chang, J.H.; Azar, D.T.; Zieske, J.D. Potential role of corneal epithelial cell-derived exosomes in corneal wound healing and neovascularization. Sci. Rep. 2017, 7, 40548. [Google Scholar] [CrossRef]
- Hadvina, R.; Lotfy Khaled, M.; Akoto, T.; Zhi, W.; Karamichos, D.; Liu, Y. Exosomes and their miRNA/protein profile in keratoconus-derived corneal stromal cells. Exp. Eye Res. 2023, 236, 109642. [Google Scholar] [CrossRef] [PubMed]
- Hefley, B.S.; Deighan, C.; Vasini, B.; Khan, A.; Hjortdal, J.; Riaz, K.M.; Liu, Y.; Karamichos, D. Revealing the presence of tear extracellular vesicles in Keratoconus. Exp. Eye Res. 2022, 224, 109242. [Google Scholar] [CrossRef]
- Shrestha, P.; Whelchel, A.E.; Nicholas, S.E.; Liang, W.; Ma, J.X.; Karamichos, D. Monocarboxylate Transporters: Role and Regulation in Corneal Diabetes. Anal. Cell. Pathol. 2022, 2022, 6718566. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yang, L.; Wang, Q.; Li, Y.; Wei, C.; Xie, L. Mechanistic investigations of diabetic ocular surface diseases. Front. Endocrinol. 2022, 13, 1079541. [Google Scholar] [CrossRef] [PubMed]
- Lozano, V.; Martin, C.; Blanco, N.; Alcalde, I.; Fernandez-Vega Cueto, L.; Merayo-Lloves, J.; Quiros, L.M. Exosomes Released by Corneal Stromal Cells Show Molecular Alterations in Keratoconus Patients and Induce Different Cellular Behavior. Biomedicines 2022, 10, 2348. [Google Scholar] [CrossRef]
- Sharif, R.; Bak-Nielsen, S.; Sejersen, H.; Ding, K.; Hjortdal, J.; Karamichos, D. Prolactin-Induced Protein is a novel biomarker for Keratoconus. Exp. Eye Res. 2019, 179, 55–63. [Google Scholar] [CrossRef]
- Escandon, P.; Liu, A.; Nicholas, S.E.; Khan, A.; Riaz, K.M.; Karamichos, D. Unravelling Novel Roles of Salivary Exosomes in the Regulation of Human Corneal Stromal Cell Migration and Wound Healing. Int. J. Mol. Sci. 2022, 23, 4330. [Google Scholar] [CrossRef]
- Pucker, A.D.; Ngo, W.; Postnikoff, C.K.; Fortinberry, H.; Nichols, J.J. Tear Film miRNAs and Their Association With Human Dry Eye Disease. Curr. Eye Res. 2022, 47, 1479–1487. [Google Scholar] [CrossRef]
- Hefley, B.S.; McKay, T.B.; Hutcheon, A.E.K.; Ciolino, J.B.; Karamichos, D. Corneal epithelial-stromal constructs to study differences associated with diabetes mellitus. Exp. Eye Res. 2024, 248, 110100. [Google Scholar] [CrossRef]
- Yu, C.; Chen, P.; Xu, J.; Liu, Y.; Li, H.; Wang, L.; Di, G. hADSCs derived extracellular vesicles inhibit NLRP3inflammasome activation and dry eye. Sci. Rep. 2020, 10, 14521. [Google Scholar] [CrossRef]
- Wang, G.; Zeng, L.; Gong, C.; Gong, X.; Zhu, T.; Zhu, Y. Extracellular vesicles derived from mouse adipose-derived mesenchymal stem cells promote diabetic corneal epithelial wound healing through NGF/TrkA pathway activation involving dendritic cells. Exp. Eye Res. 2023, 231, 109484. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Kim, J.; Kim, M.J.; Yae, C.G.; Kim, K.H.; Kim, H.K. Development of human amniotic epithelial cell-derived extracellular vesicles as cell-free therapy for dry eye disease. Ocul. Surf. 2024, 34, 370–380. [Google Scholar] [CrossRef]
- Ma, H.; Chen, T.; Li, C.; Xu, H.; Feng, Q.; Su, Y.; Cai, J.; Zhu, Q.; Liu, F.; Hu, L. Metabolic signatures of tear extracellular vesicles caused by herpes simplex keratitis. Ocul. Surf. 2024, 31, 21–30. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Chen, Q.; Wei, Z.; Xu, X.; Han, D.; Zhang, Y.; Chen, Z.; Liang, Q. MicroRNAs of extracellular vesicles derived from mesenchymal stromal cells alleviate inflammation in dry eye disease by targeting the IRAK1/TAB2/NF-kappaB pathway. Ocul. Surf. 2023, 28, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, S.; Zhao, X.; Zhao, P.; Jia, Q.; Ma, H.; Lin, Q. Role of tear exosomes in the spread of herpes simplex virus type 1 in recurrent herpes simplex keratitis. Eye 2023, 37, 3180–3185. [Google Scholar] [CrossRef] [PubMed]
- Yeung, V.; Boychev, N.; Kanu, L.N.; Ng, V.; Ross, A.E.; Hutcheon, A.E.K.; Ciolino, J.B. Proteomic Characterization of Corneal Epithelial and Stromal Cell-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2024, 25, 10338. [Google Scholar] [CrossRef]
- Yeung, V.; Boychev, N.; Farhat, W.; Ntentakis, D.P.; Hutcheon, A.E.K.; Ross, A.E.; Ciolino, J.B. Extracellular Vesicles in Corneal Fibrosis/Scarring. Int. J. Mol. Sci. 2022, 23, 5921. [Google Scholar] [CrossRef] [PubMed]
- Zieske, J.D.; Hutcheon, A.E.K.; Guo, X. Extracellular Vesicles and Cell-Cell Communication in the Cornea. Anat. Rec. 2020, 303, 1727–1734. [Google Scholar] [CrossRef]
- Desjardins, P.; Berthiaume, R.; Couture, C.; Le-Bel, G.; Roy, V.; Gros-Louis, F.; Moulin, V.J.; Proulx, S.; Chemtob, S.; Germain, L.; et al. Impact of Exosomes Released by Different Corneal Cell Types on the Wound Healing Properties of Human Corneal Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 12201. [Google Scholar] [CrossRef]
- McKay, T.B.; Yeung, V.; Hutcheon, A.E.K.; Guo, X.; Zieske, J.D.; Ciolino, J.B. Extracellular Vesicles in the Cornea: Insights from Other Tissues. Anal. Cell. Pathol. 2021, 2021, 9983900. [Google Scholar] [CrossRef]
- Pedersen, C.; Chen, V.T.; Herbst, P.; Zhang, R.; Elfert, A.; Krishan, A.; Azar, D.T.; Chang, J.H.; Hu, W.Y.; Kremsmayer, T.P.; et al. Target specification and therapeutic potential of extracellular vesicles for regulating corneal angiogenesis, lymphangiogenesis, and nerve repair. Ocul. Surf. 2024, 34, 459–476. [Google Scholar] [CrossRef] [PubMed]
- Parekh, M.; Rhys, H.; Ramos, T.; Ferrari, S.; Ahmad, S. Extracellular Vesicles Derived From Human Corneal Endothelial Cells Inhibit Proliferation of Human Corneal Endothelial Cells. Front. Med. 2021, 8, 753555. [Google Scholar] [CrossRef]
- Liu, G.S.; Chen, H.A.; Chang, C.Y.; Chen, Y.J.; Wu, Y.Y.; Widhibrata, A.; Yang, Y.H.; Hsieh, E.H.; Delila, L.; Lin, I.C.; et al. Platelet-derived extracellular vesicle drug delivery system loaded with kaempferol for treating corneal neovascularization. Biomaterials 2025, 319, 123205. [Google Scholar] [CrossRef]
- Saraf, N.; Ramachandran, R.A.; Cao, M.; Lemoff, A.; Baniasadi, H.; Robertson, D.M. Serum-derived extracellular vesicles for the treatment of severe ocular surface disease. Ocul. Surf. 2024, 34, 317–325. [Google Scholar] [CrossRef]
- Wang, M.; Yang, X.; Ye, Y.; Fan, K.; Chen, C.; Zheng, L.; Li, X.; Dong, C.; Li, C.; Dong, N. Anti-inflammatory and Restorative effects of milk exosomes and Dexamethasone-Loaded exosomes in a corneal alkali burn model. Int. J. Pharm. 2024, 666, 124784. [Google Scholar] [CrossRef]
- Liang, W.; Huang, L.; Clayton, J.M.; Nicholas, S.E.; Hefley, B.S.; Ma, J.X.; Karamichos, D. Exploring the Therapeutic Potential of Salivary Exosomes in Corneal Epithelial Wound Healing. Investig. Ophthalmol. Vis. Sci. 2025, 66, 8. [Google Scholar] [CrossRef] [PubMed]
- Pal-Ghosh, S.; Datta-Majumdar, H.; Datta, S.; Dimri, S.; Hally, J.; Wehmeyer, H.; Chen, Z.; Watsky, M.; Ma, J.X.; Liang, W.; et al. Corneal epithelial cells upregulate macropinocytosis to engulf metabolically active axonal mitochondria released by injured axons. Ocul. Surf. 2025, 37, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Luo, Z.; Lin, X.; Wang, W.; Aschner, M.; Cai, P.; Wang, Y.L.; Shao, W.; Yu, G.; Guo, Z.; et al. Intercellular transfer of mitochondria via tunneling nanotubes protects against cobalt nanoparticle-induced neurotoxicity and mitochondrial damage. Nanotoxicology 2021, 15, 1358–1379. [Google Scholar] [CrossRef]
- Crewe, C.; Funcke, J.B.; Li, S.; Joffin, N.; Gliniak, C.M.; Ghaben, A.L.; An, Y.A.; Sadek, H.A.; Gordillo, R.; Akgul, Y.; et al. Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes. Cell Metab. 2021, 33, 1853–1868.e11. [Google Scholar] [CrossRef]
- Chun, S.; An, J.; Kim, M.S. Mitochondrial Transfer Between Cancer and T Cells: Implications for Immune Evasion. Antioxidants 2025, 14, 1008. [Google Scholar] [CrossRef]
- Liu, H.; Mao, H.; Ouyang, X.; Lu, R.; Li, L. Intercellular Mitochondrial Transfer: The Novel Therapeutic Mechanism for Diseases. Traffic 2024, 25, e12951. [Google Scholar] [CrossRef]
- Raz, D.; Ben-Yaakov, K.; Levi, M.; Bertolin, M.; Ferrari, S.; Ponzin, D.; Busin, M.; Leiba, H.; Marcovich, A.L.; Eisenberg-Lerner, A.; et al. Mitochondria Transplantation Promotes Corneal Epithelial Wound Healing. Investig. Ophthalmol. Vis. Sci. 2024, 65, 14. [Google Scholar] [CrossRef]
- Methot, S.; Proulx, S.; Brunette, I.; Rochette, P.J. Rescuing cellular function in Fuchs endothelial corneal dystrophy by healthy exogenous mitochondrial internalization. Sci. Rep. 2023, 13, 3380. [Google Scholar] [CrossRef]
- Feizi, S.; Hatami, F.; Mirzaei, S.K.; Bayat, K. Medical and surgical approaches to prevent corneal graft rejection in high-risk recipients. Surv. Ophthalmol. 2025, 71, 674–684. [Google Scholar] [CrossRef]
- Kolenc, A.; Dimnik, Z.; Marzidovsek, M.; Schollmayer, P.; Hawlina, M.; Malicev, E.; Luznik Marzidovsek, Z. Extracellular Vesicle-Derived Bioactive Molecules for Corneal and Ocular Surface Regeneration. J. Clin. Med. 2025, 14, 5594. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhang, J.; Liu, G.; Wolfram, J. Immunogenicity of Extracellular Vesicles. Adv. Mater. 2024, 36, e2403199. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Hayashi, T.; Kurita, J.; Yuda, K.; Mizuki, N.; Yamagami, S. Antigen Presentation by Extracellular Vesicles in the Acute Phase of Corneal Transplantation. Investig. Ophthalmol. Vis. Sci. 2025, 66, 38. [Google Scholar] [CrossRef]
- Hernandez, B.J.; Robertson, D.M. Exosomes in Corneal Homeostasis and Wound Healing. Curr. Eye Res. 2025, 50, 1240–1248. [Google Scholar] [CrossRef]
- Ryu, Y.; Hwang, J.S.; Bo Noh, K.; Park, S.H.; Seo, J.H.; Shin, Y.J. Adipose Mesenchymal Stem Cell-Derived Exosomes Promote the Regeneration of Corneal Endothelium Through Ameliorating Senescence. Investig. Ophthalmol. Vis. Sci. 2023, 64, 29. [Google Scholar] [CrossRef]
- Kivanany, P.B.; Grose, K.C.; Tippani, M.; Su, S.; Petroll, W.M. Assessment of Corneal Stromal Remodeling and Regeneration after Photorefractive Keratectomy. Sci. Rep. 2018, 8, 12580. [Google Scholar] [CrossRef]
- Asimellis, G.; Kaufman, E.J. Keratoconus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Sharif, R.; Bak-Nielsen, S.; Hjortdal, J.; Karamichos, D. Pathogenesis of Keratoconus: The intriguing therapeutic potential of Prolactin-inducible protein. Prog. Retin. Eye Res. 2018, 67, 150–167. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; D’Souza, S.; Khamar, P.; Ghosh, A.; Nuijts, R.; Sethu, S. Biochemical Markers and Alterations in Keratoconus. Asia Pac. J. Ophthalmol. 2020, 9, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Sobrino, T.; Regueiro, U.; Malfeito, M.; Vieites-Prado, A.; Perez-Mato, M.; Campos, F.; Lema, I. Higher Expression of Toll-Like Receptors 2 and 4 in Blood Cells of Keratoconus Patiens. Sci. Rep. 2017, 7, 12975. [Google Scholar] [CrossRef] [PubMed]
- Zarei-Ghanavati, S.; Yahaghi, B.; Hassanzadeh, S.; Mobarhan, M.G.; Hakimi, H.R.; Eghbali, P. Serum 25-Hydroxyvitamin D, Selenium, Zinc and Copper in Patients with Keratoconus. J. Curr. Ophthalmol. 2020, 32, 26–31. [Google Scholar] [CrossRef]
- Ionescu, I.C.; Corbu, C.G.; Tanase, C.; Ionita, G.; Nicula, C.; Coviltir, V.; Potop, V.; Constantin, M.; Codrici, E.; Mihai, S.; et al. Overexpression of Tear Inflammatory Cytokines as Additional Finding in Keratoconus Patients and Their First Degree Family Members. Mediat. Inflamm. 2018, 2018, 4285268. [Google Scholar] [CrossRef]
- Shetty, R.; Ghosh, A.; Lim, R.R.; Subramani, M.; Mihir, K.; Reshma, A.R.; Ranganath, A.; Nagaraj, S.; Nuijts, R.M.; Beuerman, R.; et al. Elevated expression of matrix metalloproteinase-9 and inflammatory cytokines in keratoconus patients is inhibited by cyclosporine A. Investig. Ophthalmol. Vis. Sci. 2015, 56, 738–750. [Google Scholar] [CrossRef]
- McKay, T.B.; Hjortdal, J.; Sejersen, H.; Asara, J.M.; Wu, J.; Karamichos, D. Endocrine and Metabolic Pathways Linked to Keratoconus: Implications for the Role of Hormones in the Stromal Microenvironment. Sci. Rep. 2016, 6, 25534. [Google Scholar] [CrossRef]
- Hughes, A.E.; Bradley, D.T.; Campbell, M.; Lechner, J.; Dash, D.P.; Simpson, D.A.; Willoughby, C.E. Mutation altering the miR-184 seed region causes familial keratoconus with cataract. Am. J. Hum. Genet. 2011, 89, 628–633. [Google Scholar] [CrossRef]
- Blanco-Agudin, N.; Ye, S.; Gonzalez-Fernandez, S.; Alcalde, I.; Merayo-Lloves, J.; Quiros, L.M. Exosomes in Ocular Health: Recent Insights into Pathology, Diagnostic Applications and Therapeutic Functions. Biomedicines 2025, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; He, Y.; Ren, Y.R.; Chen, B.H. Corneal alteration and pathogenesis in diabetes mellitus. Int. J. Ophthalmol. 2019, 12, 1939–1950. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Whelchel, A.; Nicholas, S.; Sharif, R.; Riaz, K.; Karamichos, D. Diabetic keratopathy: Insights and challenges. Surv. Ophthalmol. 2020, 65, 513–529. [Google Scholar] [CrossRef]
- de Oliveira, R.C.; Tye, G.; Sampaio, L.P.; Shiju, T.M.; DeDreu, J.; Menko, A.S.; Santhiago, M.R.; Wilson, S.E. TGFbeta1 and TGFbeta2 proteins in corneas with and without stromal fibrosis: Delayed regeneration of apical epithelial growth factor barrier and the epithelial basement membrane in corneas with stromal fibrosis. Exp. Eye Res. 2021, 202, 108325. [Google Scholar] [CrossRef]
- Medeiros, C.S.; Marino, G.K.; Santhiago, M.R.; Wilson, S.E. The Corneal Basement Membranes and Stromal Fibrosis. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4044–4053. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, J.; Sun, Y.; Xu, W.; Qian, H. Emerging role of extracellular vesicles in diabetic retinopathy. Theranostics 2024, 14, 1631–1646. [Google Scholar] [CrossRef] [PubMed]
- Andres-Blasco, I.; Gallego-Martinez, A.; Machado, X.; Cruz-Espinosa, J.; Di Lauro, S.; Casaroli-Marano, R.; Alegre-Ituarte, V.; Arevalo, J.F.; Pinazo-Duran, M.D. Oxidative Stress, Inflammatory, Angiogenic, and Apoptotic molecules in Proliferative Diabetic Retinopathy and Diabetic Macular Edema Patients. Int. J. Mol. Sci. 2023, 24, 8227. [Google Scholar] [CrossRef]
- Su, Y.; Chen, J.; Dong, Z.; Zhang, Y.; Ma, R.; Kou, J.; Wang, F.; Shi, J. Procoagulant Activity of Blood and Endothelial Cells via Phosphatidylserine Exposure and Microparticle Delivery in Patients with Diabetic Retinopathy. Cell Physiol. Biochem. 2018, 45, 2411–2420. [Google Scholar] [CrossRef]
- Martins, B.; Pires, M.; Ambrosio, A.F.; Girao, H.; Fernandes, R. Contribution of extracellular vesicles for the pathogenesis of retinal diseases: Shedding light on blood-retinal barrier dysfunction. J. Biomed. Sci. 2024, 31, 48. [Google Scholar] [CrossRef]
- Mobaraki, M.; Soltani, M.; Zare Harofte, S.; Zoudani, E.L.; Daliri, R.; Aghamirsalim, M.; Raahemifar, K. Biodegradable Nanoparticle for Cornea Drug Delivery: Focus Review. Pharmaceutics 2020, 12, 1232. [Google Scholar] [CrossRef]
- Imperiale, J.C.; Acosta, G.B.; Sosnik, A. Polymer-based carriers for ophthalmic drug delivery. J. Control Release 2018, 285, 106–141. [Google Scholar] [CrossRef]
- Rosenquist Lybecker, J.; Van de Ven, A.; Braesch-Andersen, K.; Juriga, D.; Norein, N.; Hansson, P.; Samanta, A. Hydrogel-Mediated Sustained Delivery of Corneal Epithelial Extracellular Vesicles: A Strategy for Enhanced Corneal Regeneration. ACS Omega 2025, 10, 37081–37095. [Google Scholar] [CrossRef]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef]
- Cooper, R.C.; Yang, H. Hydrogel-based ocular drug delivery systems: Emerging fabrication strategies, applications, and bench-to-bedside manufacturing considerations. J. Control Release 2019, 306, 29–39. [Google Scholar] [CrossRef]
- Tang, Q.; Lu, B.; He, J.; Chen, X.; Fu, Q.; Han, H.; Luo, C.; Yin, H.; Qin, Z.; Lyu, D.; et al. Exosomes-loaded thermosensitive hydrogels for corneal epithelium and stroma regeneration. Biomaterials 2022, 280, 121320. [Google Scholar] [CrossRef]
- Wei, R.; Wang, Y.; Feng, Z.; Liu, R.; Liu, C.; Hu, X.; Liu, Y.; Kong, B.; Zhou, X.; Li, M. Self-healing adhesive oxidized guar gum hydrogel loaded with mesenchymal stem cell exosomes for corneal wound healing. J. Nanobiotechnol. 2025, 23, 321. [Google Scholar] [CrossRef]
- Sabbagh, F.; Zargarian, S.S.; Kosik-Koziol, A.; Nakielski, P.; Pierini, F. Hydrogel-based ocular drug delivery systems. J. Mater. Chem. B 2025, 13, 14982–15006. [Google Scholar] [CrossRef]
- Sun, X.; Song, W.; Teng, L.; Huang, Y.; Liu, J.; Peng, Y.; Lu, X.; Yuan, J.; Zhao, X.; Zhao, Q.; et al. MiRNA 24-3p-rich exosomes functionalized DEGMA-modified hyaluronic acid hydrogels for corneal epithelial healing. Bioact. Mater. 2023, 25, 640–656. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.