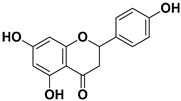
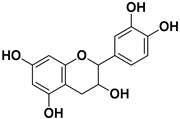
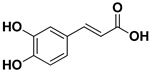
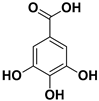
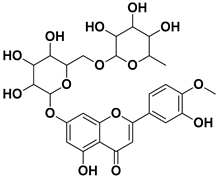
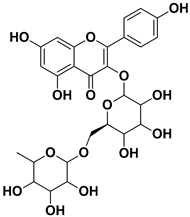
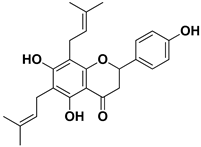
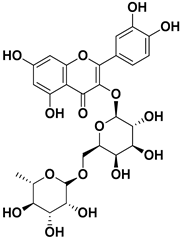
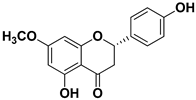
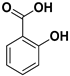
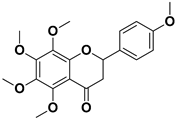
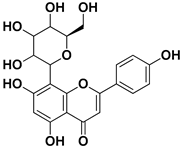
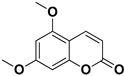
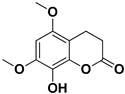
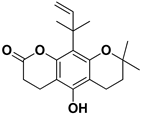
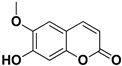
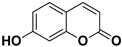
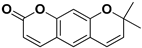
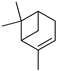
Abstract
The recent COVID-19 pandemic highlighted the significant challenge of insufficient antiviral pharmacological options. Edible plants offer a promising avenue for developing novel antiviral drugs. Etrog citron (Citrus medica L.), which is a valuable edible and medicinal plant, contains various antiviral phytochemicals, mainly flavonoids, coumarins, and terpenes. However, the therapeutic application of these compounds remains limited by factors such as poor solubility, limited bioavailability, and unclear mechanisms of action. The aim of the present article is to offer a comprehensive analysis of the antiviral phytochemicals extracted from various parts of Citrus medica, emphasizing their mode of action and delivery strategies that may allow turning these compounds into new antiviral drugs.
1. Introduction
Medicinal and edible plants and their constituents present a promising avenue for developing effective antiviral drugs [1,2,3,4]. All these plants deserve much attention because their active phytochemicals are not only potential antiviral drugs, but they also display a wide range of biomedical applications [5,6,7].
Etrog citron (Citrus medica L.) is a valuable edible and medicinal plant. We have recently thoroughly discussed its antimicrobial phytochemicals [8]. However, C. medica is not only an important source of antimicrobial compounds but also contains various antiviral phytochemicals as well [9]. Although the antiviral properties of this plant have been mentioned [10,11,12], an in-depth overview of the antiviral phytochemicals, their mechanisms of action, and strategies for their delivery is much needed. The antiviral phytochemicals of C. medica are of special interest because viral conditions remain a leading worldwide cause of morbidity and mortality [13]. In addition, the COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), impacted the everyday life of many people around the world because of historic numbers of cases and deaths [14]. Although vaccine development was successful, along with a high level of vaccination and reduction in transmission, SARS-CoV-2 tended to mutate, as has been noted with the South African variant (variant B.1.351), ‘Epsilon’ (B.1.429) in Taiwan, and ‘Mu’ (B.1.621) in Colombia [15].
Unfortunately, no vaccine or effective specific treatment is currently available in many viral infection cases; there are only some drugs for treating herpesviruses, influenza, hepatitis C, and HIV [16]. These drugs are costly and often ineffective because of viral resistance and adverse side effects. Therefore, naturally derived agents could serve as a promising alternative for treating viral infections. Plant-derived compounds represent broadly acting antivirals [17,18].
Knowledge of the molecular mechanisms of phytochemical antiviral actions is particularly important in planning an effective therapeutic approach. The application of antiviral compounds of C. medica in the pharmaceutical industry faces challenges such as low yield, solubility, and bioavailability. This review aims to highlight the most important antiviral compounds derived from various parts of C. medica L., along with their mechanisms of action and delivery strategies.
2. Methods
While several studies reported the chemical composition of C. medica L. [10,12,19], data on the antiviral phytochemicals are relatively scarce. A systematic, structured search of several electronic databases (PubMed, Google Scholar, Scopus, and Science Direct) was conducted using names of chemical compounds and widespread species of viruses. The multiple criteria sorting methods were applied [20]. We have extracted data regarding inclusion/exclusion criteria since 2002.
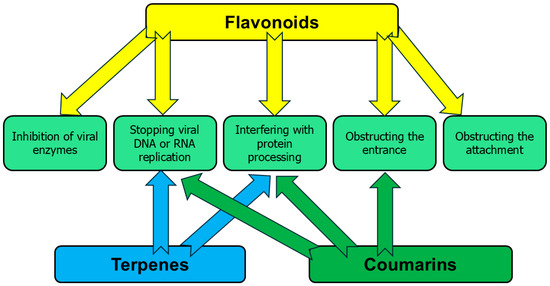
4. Applicability of Delivery Systems
As mentioned above, all parts of C. medica are abundant with various antiviral chemicals, and these compounds are characterized by low water solubility and low bioavailability, which significantly limit their practical application. Although all these components are edible, their direct application with food significantly decreases their activity because proteins and lipids interact with them [98]. In addition, metabolism, chemical structures, chemical heterogeneity, and forms of intake are also responsible for affecting the bioavailability of antiviral phytochemicals. Modern nanotechnology tackles the above-mentioned problems by incorporating the antiviral phytochemicals of C. medica into various nanomaterials. Certain delivery approaches are recognized as capable to ensure optimal delivery of antiviral compounds with the abovementioned properties [8,13]. These include phytosomes, nanoparticles, self-microemulsifying drug delivery systems (SMEDDS), and self-nanoemulsifying drug delivery systems (SNEDDS hydrogels, microspheres, transferosomes and ethosomes) [13]. The bioavailability challenges and the available approaches to deal with them are summarized in Figure 2.
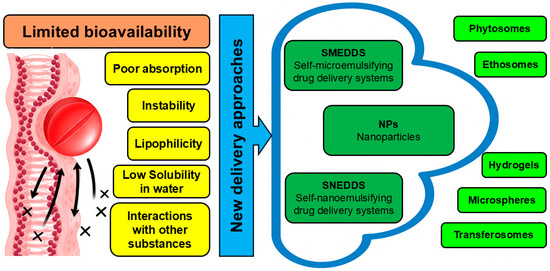
Figure 2.
Delivery strategies that amplify the antiviral activity of phytochemicals identified in C. medica.
While the vast majority of the available delivery research studied isolated phytochemicals, one work investigated the fruit and leaf extract of C. medica within zinc oxide nanoparticles and demonstrated its effectiveness against the pathogenic avian influenza H5N1 virus [9]. Other studies associated with delivery systems were performed using antiviral phytocomponents derived from C. medica.
Phytosomes represent different forms of phospholipid-based delivery systems, combining either plant extracts or phytochemicals and phospholipids [99], with phosphate groups of phospholipids binding to hydroxyl parts of phytochemicals through H-bonds [100]. It is interesting that quercetin phytosomes increased the bioavailability rate of this compound twenty times in comparison with total quercetin in human plasma [101]. Furthermore, quercetin phytosomes were effective against SARS-CoV-2 viruses in the initial stages of COVID-19 infection [102]. Although phytosomes containing hesperidin enhanced solubility in a basic buffer system, there was excellent membrane permeation efficiency and better results in antioxidant activity than hesperidin [103]. Their antiviral properties were not investigated, and the antiviral phytosomal formulations were not released to the market because they were not developed deeply. In addition, their drawbacks include unstable nature, short life, and so on, which must be overcome. The electrosprayed nanoparticles in polyvinyl alcohol, which were loaded with chlorogenic acid, demonstrated significant antiviral activity against coronavirus (HCoV-229E) and Middle East respiratory syndrome coronavirus (MERS-CoV) and NRCEHKU270) [104].
In vitro experiments were performed with hesperidin in combination with favipiravir liposomal nanoformulations against SARS-CoV-2. As a result, this combination inhibited the replication of SARS-CoV-2 better than liposomes loaded by individual components or free favipiravir and hesperidin [105].
Metallic nanoparticles gained attention due to their contribution to targeted drug delivery [106]. These nanoparticles, containing silver, gold, zinc oxide, iron oxide, titanium dioxide, and so on, are effective antiviral agents without phytochemicals, depending on size ranges, morphologies, surface chemistry, and charges [107]. The possible antiviral mechanisms are associated with the attachment of nanoparticles to surface moieties of viruses with production of reactive oxygen species and the disruption of viral proteins [108]. Two antiviral terpenes of C. medica, carvacrol and geraniol, enhanced antiviral activity of nanoparticles of zinc oxide when several viruses were assessed, including SARS-Co-V-2 [109].
Silver nanoparticles with a mixture of flavonoids, gallic acid, chlorogenic acid, and naringenin significantly inhibited infectious laryngotracheitis virus and infectious bronchitis virus in chickens, with a possible mechanism of antiviral activity being interaction between the antiviral agent and the external viral envelope proteins [110].
Highly monodispersed gold nanoparticles, which were synthesized using gallic acid, were effective against two species of Herpes simplex virus due to blocking viral attachment and penetration into the host cells [111]. Antiviral effects of gold and silver nanoparticles with gallic acid against SARS-CoV-2 were presented in another research, with the presence of gallic acid decreasing the toxicity of nanoparticles [112].
It was reported that farnesol-containing nanoparticles caused inhibition of the spike proteins of SARS-CoV-2 by up to 83%, obstructing the attachment and entrance of viruses into the host cells due to the lipophilic structure of farnesol and its ability to interact with the double lipid layer of the viral envelope [113].
It is known that polymeric biomaterials are the most innovative and enticing possibility of delivery systems, especially biodegradable and biocompatible polymers. In fact, removal of the carrier when release of the drug occurs is an essential aspect of the system. The most widespread polymeric biomaterials for the formulation are polysaccharides, polypeptides, or phospholipids [114].
For example, chitosan is a cationic biocompatible polysaccharide [115]. It was reported that chitosan nanoparticles containing either gallic acid or quercetin significantly inhibited SARS-CoV-2 entry into the host cells [116].
Gallic acid was successfully transformed into biocompatible graphene quantum dots which enhanced antiviral activity against pseudorabies virus both in vitro and in vivo, inhibiting the viral adsorption, invasion and replication [117].
The antiviral compounds of Citrus medica are similar to other edible plants; it has been proposed that they are likely to have minimal toxicity. Indeed, the toxicity of nanocarriers in any shape or form needs additional research.
For the clinical use of C. medica nanotechnological products, it is necessary to study their interaction with human cells, tissues, and organs following long-term administration courses. Thus, in vitro and in vivo studies for the potential cytotoxic impact of these products are required prior to clinical trials.
Although laboratory studies in cell models show antiviral activity of active compounds of C. medica at certain concentrations (e.g., 20–50 µM), embodiment of these findings into human therapy requires thorough pharmacokinetic estimations. Due to their low bioavailability, such concentrations of flavonoids, coumarins and terpenes are often unrealistic through oral administration. Indeed, without advanced delivery systems or chemical modifications, these treatment concentrations may not be clinically realistic.
As described throughout this article, the clinical translation of active compounds derived from Citrus medica is impeded by challenges such as an incomplete understanding of antiviral mechanisms, and the need for comprehensive pharmacokinetic modeling long-term safety evaluation, and large-scale clinical validation. Our proposed roadmap for the development of Citrus medica-derived antivirals is depicted in Figure 3.
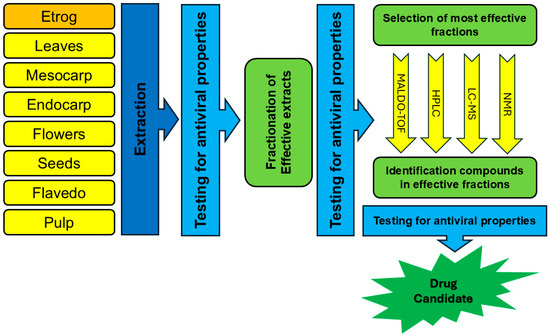
Figure 3.
Roadmap for development of C. medica-derived antivirals.
5. Conclusions
Accumulating data shows that phytochemicals of C. medica could become valuable antiviral material. Unfortunately, the chemical compositions of many varieties of etrog citron are unknown, and many important antiviral compounds have yet to be identified and estimated. Figure 3 represents a possible roadmap for the development of C. medica-derived antivirals. Comparative metabolomics methods may highlight a wide range of secondary metabolites. In addition, SAR-guided phytochemical modifications are effective means to enhance antiviral properties of flavonoids, coumarins, and terpenes of C. medica.
Preclinical studies in animal models and clinical trials were almost not performed using antiviral compounds of C. medica. Validation studies in vivo are very important to test antiviral compounds, extracts, juices, and essential oils of C. medica, providing crucial data on pharmacokinetics and pharmacodynamics in a comprehensive way.
The broad use of antiviral compounds of C. medica has been hampered by its low water solubility and low bioavailability. Emerging studies have suggested that phytosomes, nanoparticles, self-microemulsifying drug delivery systems (SMEDDS), and self-nanoemulsifying drug delivery systems (SNEDDS hydrogels, microspheres, transferosomes, and ethosomes) may enhance the solubility and physiological efficacy of antiviral compounds of C. medica. Appropriate nanocarriers can amplify the antiviral efficacy of active compounds derived from C. medica.
Despite the growing interest in nanotechnology and natural products, major pharmaceutical companies are generally reluctant to invest in these areas. As a result, the development of innovative antiviral products in this field is largely driven by start-ups and small pharmaceutical companies, which often face significant challenges in securing investment [118]. We hope that our proposed roadmap for the development of Citrus medica-derived antivirals (presented in Figure 3) will aid in overcoming these challenges.
Author Contributions
Conceptualization, F.N., A.D. and S.B.-S.; methodology, F.N.; validation, F.N., A.D., L.Y., B.K. and S.B.-S.; formal analysis, F.N., A.D., L.Y., B.K., S.F.-B. and S.B.-S.; investigation, L.Y., F.N., B.K., A.D., S.F.-B. and S.B.-S.; resources, S.B.-S.; data curation, B.K.; writing—original draft preparation, L.Y., F.N., O.S. and B.K.; writing—review and editing, L.Y., S.F.-B., F.N., B.K., O.S., A.D. and S.B.-S.; visualization, B.K.; supervision, F.N., A.D. and S.B.-S.; project administration, S.B.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zitterl-Eglseer, K.; Marschik, T. Antiviral Medicinal Plants of Veterinary Importance: A Literature Review. Planta Medica 2020, 86, 1058–1072. [Google Scholar] [CrossRef]
- Dilip Bhandare, S. Exploration of Medicinal Plants as Potential Therapeutics against COVID-19: Molecular Insights and Drug Development Prospects with Other Significant Medicinal Information a Retrospective Exposition. J. Pharm. Pharmacol. 2025, 77, 18–31. [Google Scholar] [CrossRef]
- Lowe, H.; Steele, B.; Bryant, J.; Fouad, E.; Toyang, N.; Ngwa, W. Antiviral Activity of Jamaican Medicinal Plants and Isolated Bioactive Compounds. Molecules 2021, 26, 607. [Google Scholar] [CrossRef]
- Malekmohammad, K.; Rafieian-Kopaei, M.; Sardari, S.; Sewell, R.D.E. Effective Antiviral Medicinal Plants and Biological Compounds Against Central Nervous System Infections: A Mechanistic Review. Curr. Drug Discov. Technol. 2020, 17, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Rana, J.N.; Gul, K.; Mumtaz, S. Isorhamnetin: Reviewing Recent Developments in Anticancer Mechanisms and Nanoformulation-Driven Delivery. Int. J. Mol. Sci. 2025, 26, 7381. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S. Prunin: An Emerging Anticancer Flavonoid. Int. J. Mol. Sci. 2025, 26, 2678. [Google Scholar] [CrossRef]
- Dahan, A.; Yarmolinsky, L.; Budovsky, A.; Khalfin, B.; Ben-Shabat, S. Therapeutic Potential of Ficus benjamina: Phytochemical Identification and Investigation of Antimicrobial, Anticancer, Pro-Wound-Healing, and Anti-Inflammatory Properties. Molecules 2025, 30, 1961. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Yarmolinsky, L.; Nakonechny, F.; Semenova, O.; Khalfin, B.; Ben-Shabat, S. Etrog Citron (Citrus medica) as a Novel Source of Antimicrobial Agents: Overview of Its Bioactive Phytochemicals and Delivery Approaches. Pharmaceutics 2025, 17, 761. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, A.M.; El-Abd, E.A.W.; Afifi, A.H.; Hashim, F.A.; Kutkat, O.; Ali, M.A.; El Raey, M.A.; El Hawary, S.S. Comparative Metabolomics Analysis of Citrus medica Var. Sarcodactylis swingle and Limonia acidissima Linn. Fruits and Leaves Cultivated in Egypt in Context to Their Antiviral Effects. Heliyon 2024, 10, e32335. [Google Scholar] [CrossRef]
- Benedetto, N.; Carlucci, V.; Faraone, I.; Lela, L.; Ponticelli, M.; Russo, D.; Mangieri, C.; Tzvetkov, N.T.; Milella, L. An Insight into Citrus medica Linn.: A Systematic Review on Phytochemical Profile and Biological Activities. Plants 2023, 12, 2267. [Google Scholar] [CrossRef]
- Tundis, R.; Xiao, J.; Silva, A.S.; Carreiró, F.; Loizzo, M.R. Health-Promoting Properties and Potential Application in the Food Industry of Citrus medica L. and Citrus × clementina Hort. Ex Tan. Essential Oils and Their Main Constituents. Plants 2023, 12, 991. [Google Scholar] [CrossRef] [PubMed]
- Chhikara, N.; Kour, R.; Jaglan, S.; Gupta, P.; Gat, Y.; Panghal, A. Citrus medica: Nutritional, Phytochemical Composition and Health Benefits—A Review. Food Funct. 2018, 9, 1978–1992. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Yarmolinsky, L.; Porat, D.; Dahan, A. Antiviral Effect of Phytochemicals from Medicinal Plants: Applications and Drug Delivery Strategies. Drug Deliv. Transl. Res. 2020, 10, 354–367. [Google Scholar] [CrossRef]
- Wang, H.; Paulson, K.R.; Pease, S.A.; Watson, S.; Comfort, H.; Zheng, P.; Aravkin, A.Y.; Bisignano, C.; Barber, R.M.; Alam, T.; et al. Estimating Excess Mortality Due to the COVID-19 Pandemic: A Systematic Analysis of COVID-19-Related Mortality, 2020–21. Lancet 2022, 399, 1513–1536. [Google Scholar] [CrossRef]
- Farhat, A.; Hajer, B.H.; Bassem, K.; Youssef, B.H.; Philippe, M.; Slim, A.; Fendri, I. Apigenin Analogues as SARS-CoV-2 Main Protease Inhibitors: In-Silico Screening Approach. Bioengineered 2022, 13, 3350–3361. [Google Scholar] [CrossRef]
- Kausar, S.; Said Khan, F.; Ishaq Mujeeb Ur Rehman, M.; Akram, M.; Riaz, M.; Rasool, G.; Hamid Khan, A.; Saleem, I.; Shamim, S.; Malik, A. A Review: Mechanism of Action of Antiviral Drugs. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211002620. [Google Scholar] [CrossRef]
- Huang, R.-L.; Tang, W.; Wang, C.; Yan, C.; Hu, Y.; Yang, H.-X.; Xiang, H.-Y.; Huang, X.-J.; Hu, L.-J.; Ye, W.-C.; et al. Antiviral C-Geranylated Flavonoids from Artocarpus Communis. Phytochemistry 2024, 225, 114165. [Google Scholar] [CrossRef]
- Du, H.; Cui, C.; Zhang, T.; Cai, Q.; Zhang, Y.; Hou, H. Mechanistic Insights into the Delivery and Pharmacodynamic Enhancement of Baicalin Nanoparticles in Traditional Chinese Plant Medicine-Based Antiviral Therapies. Ind. Crops Prod. 2025, 235, 121690. [Google Scholar] [CrossRef]
- Arias, B.Á.; Ramón-Laca, L. Pharmacological Properties of Citrus and Their Ancient and Medieval Uses in the Mediterranean Region. J. Ethnopharmacol. 2005, 97, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sotoudeh-Anvari, A. The Applications of MCDM Methods in COVID-19 Pandemic: A State of the Art Review. Appl. Soft Comput. 2022, 126, 109238. [Google Scholar] [CrossRef] [PubMed]
- Ramadugu, C.; Keremane, M.L.; Hu, X.; Karp, D.; Federici, C.T.; Kahn, T.; Roose, M.L.; Lee, R.F. Genetic Analysis of Citron (Citrus medica L.) Using Simple Sequence Repeats and Single Nucleotide Polymorphisms. Sci. Hortic. 2015, 195, 124–137. [Google Scholar] [CrossRef]
- Yuan, Y.; Geng, X.; Wu, H.; Kumar, R.; Wang, J.; Xiao, J.; Tian, H. Chemical Composition, Antimicrobial Activities, and Microencapsulation by Complex Coacervation of Tea Tree Essential Oils. J. Food Process. Preserv. 2022, 46, e16585. [Google Scholar] [CrossRef]
- Gao, Z.; Zhong, W.; Chen, K.; Tang, P.; Guo, J. Chemical Composition and Anti-Biofilm Activity of Essential Oil from Citrus medica L. Var. sarcodactylis Swingle Against Listeria monocytogenes. Ind. Crops Prod. 2020, 144, 112036. [Google Scholar] [CrossRef]
- Ninfali, P.; Antonelli, A.; Magnani, M.; Scarpa, E.S. Antiviral Properties of Flavonoids and Delivery Strategies. Nutrients 2020, 12, 2534. [Google Scholar] [CrossRef]
- Ahmad, A.; Kaleem, M.; Ahmed, Z.; Shafiq, H. Therapeutic Potential of Flavonoids and Their Mechanism of Action Against Microbial and Viral Infections—A Review. Food Res. Int. 2015, 77, 221–235. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Orrù, G.; Fais, A.; Fantini, M.C. Quercetin and Its Derivates as Antiviral Potentials: A Comprehensive Review. Phytother. Res. 2022, 36, 266–278. [Google Scholar] [CrossRef]
- Metwaly, A.M.; El-Fakharany, E.M.; Alsfouk, A.A.; Ibrahim, I.M.; Elkaeed, E.B.; Eissa, I.H. Integrated Study of Quercetin as a Potent SARS-CoV-2 RdRp Inhibitor: Binding Interactions, MD Simulations, and In Vitro Assays. PLoS ONE 2024, 19, e0312866. [Google Scholar] [CrossRef]
- Yarmolinsky, L.; Huleihel, M.; Zaccai, M.; Ben-Shabat, S. Potent Antiviral Flavone Glycosides from Ficus benjamina Leaves. Fitoterapia 2012, 83, 362–367. [Google Scholar] [CrossRef]
- Corcoran, M.P.; McKay, D.L.; Blumberg, J.B. Flavonoid Basics: Chemistry, Sources, Mechanisms of Action, and Safety. J. Nutr. Gerontol. Geriatr. 2012, 31, 176–189. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Beşler, Z.N.; Bayraktar, D.Z.; Koçak, M.C.; Kızıltan, G. Investigation of Potential Effects of Quercetin on COVID-19 Treatment: A Systematic Review of Randomized Controlled Trials. Clin. Sci. Nutr. 2024, 6, 107–117. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Miolo, G.; Innocenti, G.; Caffieri, S. The Photodegradation of Quercetin: Relation to Oxidation. Molecules 2012, 17, 8898–8907. [Google Scholar] [CrossRef]
- Zou, H.; Ye, H.; Kamaraj, R.; Zhang, T.; Zhang, J.; Pavek, P. A Review on Pharmacological Activities and Synergistic Effect of Quercetin with Small Molecule Agents. Phytomedicine 2021, 92, 153736. [Google Scholar] [CrossRef]
- Veckenstedt, A.; Güttner, J.; Béládi, I. Synergistic Action of Quercetin and Murine Alpha/Beta Interferon in the Treatment of Mengo Virus Infection in Mice. Antivir. Res. 1987, 7, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I.; Raleva, S.; Genova, P.; Argirova, R. Structure-Activity Relationships of Synthetic Coumarins as HIV-1 Inhibitors. Bioinorg. Chem. Appl. 2006, 2006, 68274. [Google Scholar] [CrossRef]
- Torres, F.; Brucker, N.; Andrade, S.; Kawano, D.; Garcia, S.; Poser, G.; Eifler-Lima, V. New Insights into the Chemistry and Antioxidant Activity of Coumarins. Curr. Top. Med. Chem. 2014, 14, 2600–2623. [Google Scholar] [CrossRef]
- Pan, L.; Li, X.; Yan, Z.; Guo, H.; Qin, B. Phytotoxicity of Umbelliferone and Its Analogs: Structure–Activity Relationships and Action Mechanisms. Plant Physiol. Biochem. 2015, 97, 272–277. [Google Scholar] [CrossRef]
- Xiao, S.; Tian, Z.; Wang, Y.; Si, L.; Zhang, L.; Zhou, D. Recent Progress in the Antiviral Activity and Mechanism Study of Pentacyclic Triterpenoids and Their Derivatives. Med. Res. Rev. 2018, 38, 951–976. [Google Scholar] [CrossRef] [PubMed]
- Shintre, M.S.; Gaonkar, T.A.; Modak, S.M. Evaluation of an Alcohol-Based Surgical Hand Disinfectant Containing a Synergistic Combination of Farnesol and Benzethonium Chloride for Immediate and Persistent Activity Against Resident Hand Flora of Volunteers and with a Novel In Vitro Pig Skin Model. Infect. Control Hosp. Epidemiol. 2007, 28, 191–197. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, F.-J.; Cao, X.; Li, M.-Y. Research Progress in Biosynthesis and Regulation of Plant Terpenoids. Biotechnol. Biotechnol. Equip. 2021, 35, 1799–1808. [Google Scholar] [CrossRef]
- Hagvall, L.; Karlberg, A.-T.; Christensson, J.B. Contact Allergy to Air-Exposed Geraniol: Clinical Observations and Report of 14 Cases. Contact Dermat. 2012, 67, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Sallem, O.W.; Abdelhassib, M.R.; Eldahshan, O.A. Potentiation of Anti-Helicobacter pylori Activity of Clarithromycin by Pelargonium graveolens Oil. Arab. J. Gastroenterol. 2021, 22, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.A.; Nagarajan, S.K.; Ramesh, V.; Palaniyandi, V.; Selvam, S.P.; Madhavan, T. Computational Evaluation of Major Components from Plant Essential Oils as Potent Inhibitors of SARS-CoV-2 Spike Protein. J. Mol. Struct. 2020, 1221, 128823. [Google Scholar] [CrossRef]
- Sivaraman, D.; Pradeep, P. Revealing Anti-Viral Potential of Bio-Active Therapeutics Targeting SARS-CoV2-Polymerase (RdRp) in Combating COVID-19: Molecular Investigation on Indian Traditional Medicines. arXiv 2020. [Google Scholar] [CrossRef][Green Version]
- Kumar, A.; Choudhir, G.; Shukla, S.K.; Sharma, M.; Tyagi, P.; Bhushan, A.; Rathore, M. Identification of Phytochemical Inhibitors Against Main Protease of COVID-19 Using Molecular Modeling Approaches. J. Biomol. Struct. Dyn. 2021, 39, 3760–3770. [Google Scholar] [CrossRef]
- Besli, N.; Ercin, N.; Carmena-Bargueño, M.; Sarikamis, B.; Kalkan Cakmak, R.; Yenmis, G.; Pérez-Sánchez, H.; Beker, M.; Kilic, U. Research into How Carvacrol and Metformin Affect Several Human Proteins in a Hyperglycemic Condition: A Comparative Study In Silico and In Vitro. Arch. Biochem. Biophys. 2024, 758, 110062. [Google Scholar] [CrossRef]
- Akermi, S.; Smaoui, S.; Chaari, M.; Elhadef, K.; Gentile, R.; Hait, M.; Roymahapatra, G.; Mellouli, L. Combined In Vitro/In Silico Approaches, Molecular Dynamics Simulations and Safety Assessment of the Multifunctional Properties of Thymol and Carvacrol: A Comparative Insight. Chem. Biodivers. 2024, 21, e202301575. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Manumol, M.; Alagarasu, K.; Parashar, D.; Cherian, S. Phytochemicals of Different Medicinal Herbs as Potential Inhibitors Against Dengue Serotype 2 Virus: A Computational Approach. Mol. Biotechnol. 2025, 3599–3612. [Google Scholar] [CrossRef]
- Dantas, L.B.R.; Alcântara, I.S.; Júnior, C.P.S.; de Oliveira, M.R.C.; Martins, A.O.B.P.B.; Dantas, T.M.; Ribeiro-Filho, J.; Coutinho, H.D.M.; Passos, F.R.S.; Quintans-Júnior, L.J.; et al. In Vivo and In Silico Anti-Inflammatory Properties of the Sesquiterpene Valencene. Biomed. Pharmacother. 2022, 153, 113478. [Google Scholar] [CrossRef]
- Chan, Y.-Y.; Li, C.-H.; Shen, Y.-C.; Wu, T.-S. Anti-Inflammatory Principles from the Stem and Root Barks of Citrus medica. Chem. Pharm. Bull. 2010, 58, 61–65. [Google Scholar] [CrossRef]
- Sarowska, J.; Wojnicz, D.; Jama-Kmiecik, A.; Frej-Mądrzak, M.; Choroszy-Król, I. Antiviral Potential of Plants Against Noroviruses. Molecules 2021, 26, 4669. [Google Scholar] [CrossRef]
- Obaid, R.J.; Mughal, E.U.; Naeem, N.; Sadiq, A.; Alsantali, R.I.; Jassas, R.S.; Moussa, Z.; Ahmed, S.A. Natural and Synthetic Flavonoid Derivatives as New Potential Tyrosinase Inhibitors: A Systematic Review. RSC Adv. 2021, 11, 22159–22198. [Google Scholar] [CrossRef]
- Khandelwal, N.; Chander, Y.; Kumar, R.; Riyesh, T.; Dedar, R.K.; Kumar, M.; Gulati, B.R.; Sharma, S.; Tripathi, B.N.; Barua, S.; et al. Antiviral Activity of Apigenin Against Buffalopox: Novel Mechanistic Insights and Drug-Resistance Considerations. Antivir. Res. 2020, 181, 104870. [Google Scholar] [CrossRef]
- Manvar, D.; Mishra, M.; Kumar, S.; Pandey, V.N. Identification and Evaluation of Anti Hepatitis C Virus Phytochemicals from Eclipta alba. J. Ethnopharmacol. 2012, 144, 545–554. [Google Scholar] [CrossRef]
- Wu, C.-C.; Fang, C.-Y.; Cheng, Y.-J.; Hsu, H.-Y.; Chou, S.-P.; Huang, S.-Y.; Tsai, C.-H.; Chen, J.-Y. Inhibition of Epstein-Barr Virus Reactivation by the Flavonoid Apigenin. J. Biomed. Sci. 2017, 24, 2. [Google Scholar] [CrossRef]
- Yi, B.; Chew, B.X.Z.; Chen, H.; Lee, R.C.H.; Fong, Y.D.; Chin, W.X.; Mok, C.K.; Chu, J.J.H. Antiviral Activity of Catechin Against Dengue Virus Infection. Viruses 2023, 15, 1377. [Google Scholar] [CrossRef] [PubMed]
- You, H.-L.; Huang, C.-C.; Chen, C.-J.; Chang, C.-C.; Liao, P.-L.; Huang, S.-T. Anti-Pandemic Influenza A (H1N1) Virus Potential of Catechin and Gallic Acid. J. Chin. Med. Assoc. 2018, 81, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Saivish, M.V.; Pacca, C.C.; da Costa, V.G.; de Lima Menezes, G.; da Silva, R.A.; Nebo, L.; da Silva, G.C.D.; de Aguiar Milhim, B.H.G.; da Silva Teixeira, I.; Henrique, T.; et al. Caffeic Acid Has Antiviral Activity against Ilhéus Virus In Vitro. Viruses 2023, 15, 494. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Shirasago, Y.; Tanida, I.; Kakuta, S.; Uchiyama, Y.; Shimojima, M.; Hanada, K.; Saijo, M.; Fukasawa, M. Structural Basis of Antiviral Activity of Caffeic Acid Against Severe Fever with Thrombocytopenia Syndrome Virus. J. Infect. Chemother. 2021, 27, 397–400. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.; Jia, Y.; He, L.; Li, J.; Yu, C.; Liao, C.; Yu, Z.; Zhang, C. Research Note: Anti-Inflammatory Effects and Antiviral Activities of Baicalein and Chlorogenic Acid against Infectious Bursal Disease Virus in Embryonic Eggs. Poult. Sci. 2021, 100, 100987. [Google Scholar] [CrossRef]
- Galochkina, A.V.; Anikin, V.B.; Babkin, V.A.; Ostrouhova, L.A.; Zarubaev, V.V. Virus-Inhibiting Activity of Dihydroquercetin, a Flavonoid from Larix sibirica, Against Coxsackievirus B4 in a Model of Viral Pancreatitis. Arch. Virol. 2016, 161, 929–938. [Google Scholar] [CrossRef]
- Yarmolinsky, L.; Nakonechny, F.; Budovsky, A.; Zeigerman, H.; Khalfin, B.; Sharon, E.; Yarmolinsky, L.; Ben-Shabat, S.; Nisnevitch, M. Antimicrobial and Antiviral Compounds of Phlomis viscosa Poiret. Biomedicines 2023, 11, 441. [Google Scholar] [CrossRef]
- AbuBakar, U.; Low, Z.X.; Aris, M.Z.M.; Lani, R.; Abidin, S.A.Z.; Abdullah-Zawawi, M.-R.; Hassandarvish, P.; Karsani, S.A.; Khairat, J.E. Antiviral Potential of Diosmin Against Influenza A Virus. Sci. Rep. 2025, 15, 17192. [Google Scholar] [CrossRef]
- Huang, L.; Kuang, J.; Yu, J.; Yu, Q.; Xu, W.; Liu, M.; Wei, Y.; Han, S.; Huang, Y.; Li, P. Antiviral Activity of Epicatechin Against Singapore Grouper Iridovirus In Vitro and In Vivo. Fish Shellfish. Immunol. 2025, 162, 110331. [Google Scholar] [CrossRef] [PubMed]
- Al-Shuhaib, M.B.S.; Hashim, H.O.; Al-Shuhaib, J.M.B. Epicatechin Is a Promising Novel Inhibitor of SARS-CoV-2 Entry by Disrupting Interactions between Angiotensin-Converting Enzyme Type 2 and the Viral Receptor Binding Domain: A Computational/Simulation Study. Comput. Biol. Med. 2022, 141, 105155. [Google Scholar] [CrossRef]
- Lee, J.-H.; Oh, M.; Seok, J.H.; Kim, S.; Lee, D.B.; Bae, G.; Bae, H.-I.; Bae, S.Y.; Hong, Y.-M.; Kwon, S.-O.; et al. Antiviral Effects of Black Raspberry (Rubus coreanus) Seed and Its Gallic Acid Against Influenza Virus Infection. Viruses 2016, 8, 157. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, W.; Sun, J.; Ou, G.; Zhong, N.-S.; Liu, Z. Exploring the Potential Pharmacological Mechanism of Hesperidin and Glucosyl Hesperidin against COVID-19 Based on Bioinformatics Analyses and Antiviral Assays. Am. J. Chin. Med. 2022, 50, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.; Dubey, K. Molecular Docking Studies of Bioactive Nicotiflorin against 6W63 Novel Coronavirus 2019 (COVID-19). Comb. Chem. High Throughput Screen. 2021, 24, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Meragelman, K.M.; McKee, T.C.; Boyd, M.R. Anti-HIV Prenylated Flavonoids from Monotes africanus. J. Nat. Prod. 2001, 64, 546–548. [Google Scholar] [CrossRef]
- Tripathi, P.K.; Singh, J.; Gaurav, N.; Garg, D.K.; Patel, A.K. In-Silico and Biophysical Investigation of Biomolecular Interaction between Naringin and NsP2 of the Chikungunya Virus. Int. J. Biol. Macromol. 2020, 160, 1061–1066. [Google Scholar] [CrossRef]
- Babaeekhou, L.; Ghane, M.; Abbas-Mohammadi, M. In Silico Targeting SARS-CoV-2 Spike Protein and Main Protease by Biochemical Compounds. Biologia 2021, 76, 3547–3565. [Google Scholar] [CrossRef]
- Lin, S.-C.; Chen, M.-C.; Li, S.; Lin, C.-C.; Wang, T.T. Antiviral Activity of Nobiletin Against Chikungunya Virus In Vitro. Antivir. Ther. 2017, 22, 689–697. [Google Scholar] [CrossRef]
- Chojnacka, K.; Witek-Krowiak, A.; Skrzypczak, D.; Mikula, K.; Młynarz, P. Phytochemicals Containing Biologically Active Polyphenols as an Effective Agent Against COVID-19-Inducing Coronavirus. J. Funct. Foods 2020, 73, 104146. [Google Scholar] [CrossRef]
- Bachmetov, L.; Gal-Tanamy, M.; Shapira, A.; Vorobeychik, M.; Giterman-Galam, T.; Sathiyamoorthy, P.; Golan-Goldhirsh, A.; Benhar, I.; Tur-Kaspa, R.; Zemel, R. Suppression of Hepatitis C Virus by the Flavonoid Quercetin Is Mediated by Inhibition of NS3 Protease Activity. J. Viral Hepat. 2012, 19, e81–e88. [Google Scholar] [CrossRef]
- Choi, H.-J. In Vitro Antiviral Activity of Sakuranetin Against Human Rhinovirus 3. Osong Public Health Res. Perspect. 2017, 8, 415–420. [Google Scholar] [CrossRef]
- Kwon, D.-H.; Ji, J.-H.; Yim, S.-H.; Kim, B.-S.; Choi, H.-J. Suppression of Influenza B Virus Replication by Sakuranetin and Mode of Its Action. Phytother. Res. 2018, 32, 2475–2479. [Google Scholar] [CrossRef]
- Jamshidinia, N.; Saadatpour, F.; Arefian, E.; Mohammadipanah, F. Augmented Antiviral Activity of Chlorhexidine Gluconate on Herpes Simplex Virus Type 1, H1N1 Influenza A Virus, and Adenovirus in Combination with Salicylic Acid. Arch. Virol. 2023, 168, 302. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-J.; Wu, X.; Li, M.-M.; Li, G.-Q.; Yang, Y.-T.; Luo, H.-J.; Huang, W.-H.; Chung, H.Y.; Ye, W.-C.; Wang, G.-C.; et al. Antiviral Activity of Polymethoxylated Flavones from “Guangchenpi”, the Edible and Medicinal Pericarps of Citrus reticulata ‘Chachi’. J. Agric. Food Chem. 2014, 62, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, N.M.; Al-Sayed, E.; Moghannem, S.; Azam, F.; El-Shazly, M.; Singab, A.N. Breaking Down the Barriers to a Natural Antiviral Agent: Antiviral Activity and Molecular Docking of Erythrina Speciosa Extract, Fractions, and the Major Compound. Chem. Biodivers. 2020, 17, e1900511. [Google Scholar] [CrossRef]
- Yang, Q.-Y.; Tian, X.-Y.; Fang, W.-S. Bioactive Coumarins from Boenninghausenia sessilicarpa. J. Asian Nat. Prod. Res. 2007, 9, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Sunthitikawinsakul, A.; Kongkathip, N.; Kongkathip, B.; Phonnakhu, S.; Daly, J.W.; Spande, T.F.; Nimit, Y.; Napaswat, C.; Kasisit, J.; Yoosook, C. Anti-HIV-1 Limonoid: First Isolation from Clausena excavata. Phytother. Res. 2003, 17, 1101–1103. [Google Scholar] [CrossRef]
- Su, C.-R.; Yeh, S.F.; Liu, C.M.; Damu, A.G.; Kuo, T.-H.; Chiang, P.-C.; Bastow, K.F.; Lee, K.-H.; Wu, T.-S. Anti-HBV and Cytotoxic Activities of Pyranocoumarin Derivatives. Bioorganic Med. Chem. 2009, 17, 6137–6143. [Google Scholar] [CrossRef]
- Baggieri, M.; Gioacchini, S.; Borgonovo, G.; Catinella, G.; Marchi, A.; Picone, P.; Vasto, S.; Fioravanti, R.; Bucci, P.; Kojouri, M.; et al. Antiviral, Virucidal and Antioxidant Properties of Artemisia Annua Against SARS-CoV-2. Biomed. Pharmacother. 2023, 168, 115682. [Google Scholar] [CrossRef]
- Sotoudeheian, M.; Mirahmadi, S.-M.-S.; Pirhayati, M.; Farahmandian, N.; Azarbad, R.; Toroudi, H.P. Targeting SIRT1 by Scopoletin to Inhibit XBB.1.5 COVID-19 Life Cycle. Curr. Rev. Clin. Exp. Pharmacol. 2025, 20, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Mendis, W.R.H.; Lim, J.-W.; Jung, S.-J.; Kang, S.Y. Antiviral Effects of Umbelliferone against Viral Hemorrhagic Septicemia Virus in Olive Flounder (Paralichthys olivaceus). Fish Shellfish. Immunol. 2024, 152, 109767. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, N.; Zu, Y.; Fu, Y. Comparative Anti-Infectious Bronchitis Virus (IBV) Activity of (-)-Pinene: Effect on Nucleocapsid (N) Protein. Molecules 2011, 16, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative Study on the Antiviral Activity of Selected Monoterpenes Derived from Essential Oils. Phytother. Res. 2010, 24, 673–679. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Wu, X.; Xu, R.; Li, Y. Antiviral Mechanism of Carvacrol on HSV-2 Infectivity Through Inhibition of RIP3-Mediated Programmed Cell Necrosis Pathway and Ubiquitin-Proteasome System in BSC-1 Cells. BMC Infect. Dis. 2020, 20, 832. [Google Scholar] [CrossRef]
- Sánchez, C.; Aznar, R.; Sánchez, G. The Effect of Carvacrol on Enteric Viruses. Int. J. Food Microbiol. 2015, 192, 72–76. [Google Scholar] [CrossRef]
- Gómez, L.A.; Stashenko, E.; Ocazionez, R.E. Comparative Study on In Vitro Activities of Citral, Limonene and Essential Oils from Lippia citriodora and L. Alba on Yellow Fever Virus. Nat. Prod. Commun. 2013, 8, 249–252. [Google Scholar] [CrossRef]
- Ornan, İ.E.; Özcelik, B.; Kartal, M.; Kan, Y. Antimicrobial and Antiviral Effects of Essential Oils from Selected Umbelliferae and Labiatae Plants and Individual Essential Oil Components. Turk. J. Biol. 2012, 36, 239–246. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Screening for Antiviral Activities of Isolated Compounds from Essential Oils. Evid. Based Complement. Altern. Med. 2011, 2011, 253643. [Google Scholar] [CrossRef]
- Shoji, Y.; Ishige, H.; Tamura, N.; Iwatani, W.; Norimatsu, M.; Shimada, J.; Mizushima, Y. Enhancement of Anti-Herpetic Activity of Antisense Phosphorothioate Oligonucleotides 5′ End Modified with Geraniol. J. Drug Target. 1998, 5, 261–273. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, L.; Li, R.; Yan, Y.; Yin, J.; Dai, Q.; Guo, X.; Li, W.; Li, Y.; Liu, M.; et al. In Vitro and In Vivo Antiviral Activity of Maqian (Zanthoxylum myriacanthum Var. Pubescens) Essential Oil and Its Major Constituents Against Strains of Influenza Virus. Ind. Crops Prod. 2022, 177, 114524. [Google Scholar] [CrossRef]
- Battinelli, L.; Mengoni, F.; Lichtner, M.; Mazzanti, G.; Saija, A.; Mastroianni, C.M.; Vullo, V. Effect of Limonin and Nomilin on HIV-1 Replication on Infected Human Mononuclear Cells. Planta Medica 2003, 69, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.-C.; Ng, L.-T.; Cheng, P.-W.; Chiang, W.; Lin, C.-C. Antiviral Activities of Extracts and Selected Pure Constituents of Ocimum basilicum. Clin. Exp. Pharmacol. Physiol. 2005, 32, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, S.; Sahoo, S.K. In Silico ADMET and Molecular Docking Study on Searching Potential Inhibitors from Limonoids and Triterpenoids for COVID-19. Comput. Biol. Med. 2020, 124, 103936. [Google Scholar] [CrossRef]
- Silva, L.M.; Hill, L.E.; Figueiredo, E.; Gomes, C.L. Delivery of Phytochemicals of Tropical Fruit By-Products Using Poly (Dl-Lactide-Co-Glycolide) (PLGA) Nanoparticles: Synthesis, Characterization, and Antimicrobial Activity. Food Chem. 2014, 165, 362–370. [Google Scholar] [CrossRef]
- Kanojiya, D.; Parmar, G.; Chauhan, B.; Gondalia, S.; Rakholiya, M. Phytosomes: A Contemporary Method for Delivering Novel Herbal Drugs. J. Nat. Remedies 2024, 24, 239–253. [Google Scholar] [CrossRef]
- Khan, J.; Alexander, A.; Ajazuddin; Saraf, S.; Saraf, S. Recent Advances and Future Prospects of Phyto-Phospholipid Complexation Technique for Improving Pharmacokinetic Profile of Plant Actives. J. Control. Release 2013, 168, 50–60. [Google Scholar] [CrossRef]
- Dipierro, F.; Khan, A.; Bertuccioli, A.; Maffioli, P.; Derosa, G.; Khan, S.; Khan, B.A.; Nigar, R.; Ujjan, I.; Devrajani, B.R. Quercetin Phytosome® as a Potential Candidate for Managing COVID-19. Minerva Gastroenterol. 2021, 67, 19–195. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, M.; Dinda, S.; Ghosh, P.S.; Das, S.K. Anti-SARS-CoV-2, Antioxidant and Immunomodulatory Potential of Dietary Flavonol Quercetin: Focus on Molecular Targets and Clinical Efficacy. Eur. J. Med. Chem. Rep. 2024, 10, 100125. [Google Scholar] [CrossRef]
- Kalita, B.; Patwary, B.N. Formulation and In Vitro Evaluation of Hesperidin-Phospholipid Complex and Its Antioxidant Potential. Curr. Drug Ther. 2020, 15, 28–36. [Google Scholar] [CrossRef]
- Saleh, A.; Abdelkader, D.H.; El-Masry, T.A.; Eliwa, D.; Alotaibi, B.; Negm, W.A.; Elekhnawy, E. Antiviral and Antibacterial Potential of Electrosprayed PVA/PLGA Nanoparticles Loaded with Chlorogenic Acid for the Management of Coronavirus and Pseudomonas Aeruginosa Lung Infection. Artif. Cells Nanomed. Biotechnol. 2023, 51, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Elimam, H.; El-Sawy, H.S.; Fayed, M.A.A.; Mahmoud, S.H.; Bakr, R.O.; Saleh, R.M.; Mostafa, A.; Elshal, M.F. Antiviral Potential of Rosuvastatin and Hesperidin in Combination with Favipiravir Liposomal Nanoformulations in Targeting the Main Protease (Mpro) of SARS-CoV-2: Molecular Docking, Molecular Dynamics and in-Vitro Studies. J. Drug Deliv. Sci. Technol. 2024, 97, 105799. [Google Scholar] [CrossRef]
- Khursheed, R.; Dua, K.; Vishwas, S.; Gulati, M.; Jha, N.K.; Aldhafeeri, G.M.; Alanazi, F.G.; Goh, B.H.; Gupta, G.; Paudel, K.R.; et al. Biomedical Applications of Metallic Nanoparticles in Cancer: Current Status and Future Perspectives. Biomed. Pharmacother. 2022, 150, 112951. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Sarkar, J.; Das, S.; Aich, S.; Bhattacharyya, P.; Acharya, K. Antiviral Potential of Nanoparticles for the Treatment of Coronavirus Infections. J. Trace Elem. Med. Biol. 2022, 72, 126977. [Google Scholar] [CrossRef] [PubMed]
- Mizielińska, M.; Nawrotek, P.; Stachurska, X.; Ordon, M.; Bartkowiak, A. Packaging Covered with Antiviral and Antibacterial Coatings Based on ZnO Nanoparticles Supplemented with Geraniol and Carvacrol. Int. J. Mol. Sci. 2021, 22, 1717. [Google Scholar] [CrossRef]
- Abo-El-Yazid, Z.H.; Ahmed, O.K.; El-Tholoth, M.; Ali, M.A.-S. Green Synthesized Silver Nanoparticles Using Cyperus rotundus L. Extract as a Potential Antiviral Agent Against Infectious Laryngotracheitis and Infectious Bronchitis Viruses in Chickens. Chem. Biol. Technol. Agric. 2022, 9, 55. [Google Scholar] [CrossRef]
- Halder, A.; Das, S.; Ojha, D.; Chattopadhyay, D.; Mukherjee, A. Highly Monodispersed Gold Nanoparticles Synthesis and Inhibition of Herpes Simplex Virus Infections. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 413–421. [Google Scholar] [CrossRef]
- Pilaquinga, F.; Bosch, R.; Morey, J.; Bastidas-Caldes, C.; Torres, M.; Toscano, F.; Debut, A.; Pazmiño-Viteri, K.; de Las Nieves Piña, M. High In Vitro activity of Gold and Silver Nanoparticles from Solanum mammosum L. Against SARS-CoV-2 Surrogate Phi6 and Viral Model PhiX174. Nanotechnology 2023, 34, 175705. [Google Scholar] [CrossRef]
- Ivanova, A.; Ivanova, K.; Fiandra, L.; Mantecca, P.; Catelani, T.; Natan, M.; Banin, E.; Jacobi, G.; Tzanov, T. Antibacterial, Antibiofilm, and Antiviral Farnesol-Containing Nanoparticles Prevent Staphylococcus Aureus from Drug Resistance Development. Int. J. Mol. Sci. 2022, 23, 7527. [Google Scholar] [CrossRef]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted Delivery of Low Molecular Drugs Using Chitosan and Its Derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef]
- Pramanik, S.; Sali, V. Connecting the Dots in Drug Delivery: A Tour d’horizon of Chitosan-Based Nanocarriers System. Int. J. Biol. Macromol. 2021, 169, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Emam, M.H.; Mahmoud, M.I.; El-Guendy, N.; Loutfy, S.A. Establishment of In-House Assay for Screening of Anti-SARS-CoV-2 Protein Inhibitors. AMB Express 2024, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Su, F.; Li, J.; Yu, B.; Xu, L.; Xiong, T.; Shao, K.; Yuan, X. Enhanced In Vivo Antiviral Activity against Pseudorabies Virus Through Transforming Gallic Acid into Graphene Quantum Dots with Stimulation of Interferon-Related Immune Responses. J. Mater. Chem. B 2023, 12, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, R. Cost–Effectiveness of Nanomedicine: The Path to a Future Successful and Dominant Market? Nanomedicine 2015, 10, 1851–1853. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).