Abstract
Background/objectives: The increasing prevalence of multidrug-resistant (MDR) bacteria, particularly Klebsiella pneumoniae, calls for the development of new antimicrobial agents. This study investigates a series of fluorinated azolium salts and their rhodium(I) complexes for antibacterial activity against clinical and reference strains of K. pneumoniae. Methods: Eleven fluorinated azolium salts and their corresponding Rh(I) complexes (22 compounds total) were synthesized and tested against several K. pneumoniae strains, including three MDR clinical isolates (U–13685, H–9871, U–13815) and ATCC reference strains. Minimum inhibitory concentrations (MICs) were determined. In silico ADMET analyses were conducted to evaluate intestinal absorption, oral bioavailability, Caco-2 permeability, carcinogenicity, solubility, and synthetic accessibility. Results: Among the Rh(I) complexes, Rh–1, Rh–3, and Rh–11 showed activity against the three MDR isolates (MIC = 62.5–250 µg/mL), while Rh–1, Rh–4, Rh–6, and Rh–11 were active against all ATCC strains (MIC = 3.9–250 µg/mL). The corresponding azolium salts displayed weak or no activity, highlighting the critical role of the metal center. ADMET predictions indicated that most Rh complexes had good intestinal absorption, and all except Rh–3, Rh–4, and Rh–9 were predicted to be orally bioavailable. Compounds Rh–1 to Rh–7 showed Caco-2 permeability, and all were classified as non-carcinogenic. Rh–8 to Rh–11 exhibited lower solubility and synthetic accessibility. Conclusions: The results underscore the potential of fluorinated Rh(I) complexes as antibacterial agents against MDR K. pneumoniae, with Rh–1 and Rh–11 emerging as promising leads based on activity and favorable predicted pharmacokinetics.
1. Introduction
Multidrug-resistant (MDR) bacterial infections in hospitalized patients have become a major global concern due to the high mortality rate and increasing hospital costs [1,2,3,4]. According to the World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC), the ever–increasing antimicrobial resistance poses an imminent threat to human health, potentially leading us back to the pre-antibiotic era. Among the bacterial pathogens, a significant number have been identified as extremely drug-resistant pathogens, including members of the ESKAPE group (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter), which are of particular concern in relation to emerging nosocomial infections [5,6,7,8,9]. Klebsiella pneumoniae, for instance, is one of the most important members of the ESKAPE group. This bacterium frequently acquires and transfers resistance traits through horizontal gene transfer (HGT), enabling it to successfully evade antibiotic treatments [10,11,12]. This poses a serious problem in hospitals, as K. pneumoniae exhibits an extraordinary ability to colonize the skin of patients and medical staff, as well as the hospital environment. Furthermore, K. pneumoniae is the main cause of acute infections such as urinary tract infection (UTI), pneumonia, and wound infections [13,14]. Indeed, K. pneumoniae poses a significant burden on the healthcare system worldwide, serving as the main causative agent of nosocomial infections with MDR phenotypes and production of extended-spectrum β-lactamases (ESBLs). It is well known that the latter enzyme confers high resistance to certain antibiotics, such as penicillins and cephalosporins [6,8,15,16,17].
In addition to the serious health implications, infections caused by bacteria with MDR have significant economic and epidemiological consequences. Over the past two decades, these microorganisms have been involved in numerous outbreaks worldwide, with K. pneumoniae extended-spectrum β-lactamase producers (KPN–ESBL) being the causative agent in most cases. Studies have reported varying percentages of KPN–ESBL depending on the geographical location. For example, in some large cities in Mexico with populations of around one million, such as Monterrey, Guadalajara, and Merida, KPN–ESBL and multi-resistant strains were reported at rates of 35.9%, 26.9%, and 78.8%, respectively [18,19]. Since the introduction of antibiotics, β-lactams have been widely used as antimicrobial agents for the treatment of serious infections. However, the emergence of bacteria strains that produce ESBL and lead to MDR has severely limited the therapeutic options available to doctors [20], motivating research groups to develop and improve new antibiotics. Organometallic compounds have garnered significant attention due to their potent antimicrobial activity and pharmaceutical properties [21,22,23]. For instance, rhodium-based complexes have demonstrated the ability to disrupt the formation of antibiotic-resistant biofilms, exhibit synergistic bactericidal activity with other biocides, selectively inhibit metabolic pathways, and kill MDR bacteria. Furthermore, various mechanisms of metal-induced microbial activity have been identified, including the production of reactive oxygen species (ROS) and the depletion of antioxidants, which can cause DNA damage and inhibit enzymatic activities crucial for bacterial growth. In addition, some in vitro studies have shown that ruthenium and rhodium complexes can inhibit the activity of acetylcholinesterase and exhibit a high affinity for bovine serum albumin (BSA), transferrin, and serum. Thus, it is likely that nucleic acids and/or proteins serve as intracellular targets of these complexes in bacterial cells [24].
Based on the above, we sought to evaluate a series of fluorinated compounds (Figure 1) against multi-resistant K. pneumoniae, which is a significant public health issue. To do so, we synthesized a family of compounds with different features. Our approach was based on previous research showing that Rhodium(I) can act as an antibacterial active center against a range of bacteria, including E. coli, S. aurum, B. subtilis, B. thuringiensis, and P. aeruginosa [25,26,27,28,29]. In 2019, Whitehead et al. reported that Rh(III) solutions had a MIC of 7.81 µg/mL against MDR K. pneumoniae. However, the antibiotic resistance of the strains was not mentioned [30]. The mechanism of action of the metal ions involves the direct contact of the metal with the bacterial cell wall and subsequent internalization in the cell, which may cause oxidation of the cellular components, generating reactive oxygen species (ROS) and disruption of the transmembrane electron transport chain [30,31]. We reasoned that the internalization of the metal center may be enhanced by the presence of a ligand. Therefore, we employed N-heterocyclic carbene (NHC) ligands, which stabilize the metal center by forming a strong M–C bond [32] and have also demonstrated significant activity against a range of bacteria, including E. faecalis, E. faecium, S. aureus, S. epidermidis, B. subtilis, and M. smegmatis [33,34,35,36]. We selected fluorinated NHC ligands for our study because of the flexibility and ease of functionalization that NHC ligands provide, allowing us to incorporate different functionalities into the compounds. Fluorine atoms were specifically incorporated, as they are known to modulate the biological activity of drugs by controlling acidity, lipophilicity, conformation, and metabolism [37,38,39,40,41]. While fluorinated NHC complexes have attracted attention for their antitumor properties [42], their use as antibiotics remains underexplored.
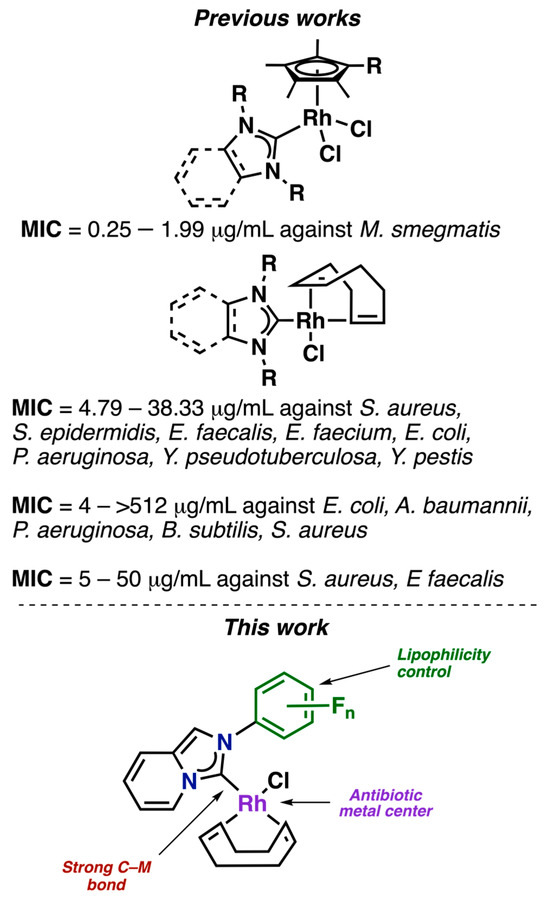
Figure 1.
Previous works of Rh–NHC complexes with antibiotic properties [33,34,35,36]. In this work, fluorinated NHC ligands were selected for their structural versatility and ease of functionalization, allowing the introduction of diverse functionalities into the compounds.
2. Results and Discussion
2.1. Synthesis and Characterization of the Rh(I)–NHC Complexes
Figure 2 illustrates the fluorinated compounds evaluated in this study, which were previously described in our recent publications [43,44]. Additionally, we determined the molecular structures of three complexes (Rh–1, Rh–6, and Rh–8) through X–ray diffraction analyses (Figure 3). The crystals were obtained by slowly diffusing hexane into a concentrated dichloromethane solution of the respective complex. These complexes were isostructural, exhibiting coordination of the NHC ligand to the metal center and completing their coordination sphere with one COD and one chlorine ligand. The geometry around the metal was slightly distorted square–planar, with angles around the metal near 90°. The lengths between C (carbene)–Rh bonds were very similar, at approximately ~2.02 Å.
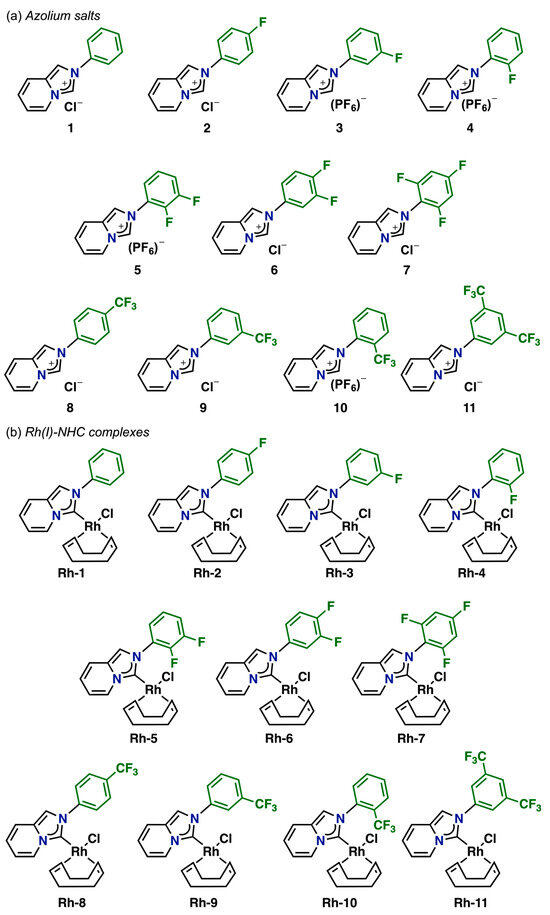
Figure 2.
(a) Azolium salts and (b) Rh(I)–NHC complexes with fluorinated fragments.
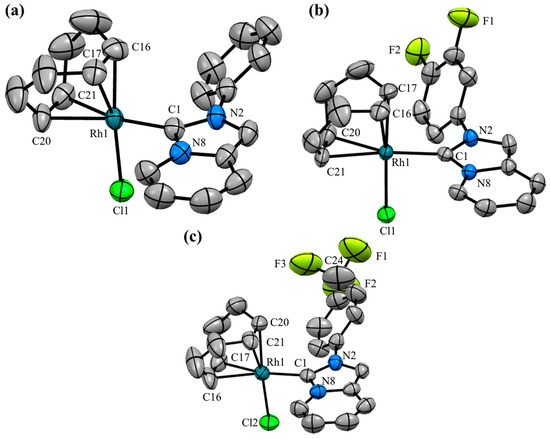
Figure 3.
Molecular structures of (a) Rh–1, (b) Rh–6, and (c) Rh–8. Hydrogen atoms have been omitted for clarity. Ellipsoids are at the 50% probability level. Selected bond lengths (Å): Rh–1: Rh1–C1 2.028(3), Rh1–Cl1 2.3922(9), Rh1–C16 2.098(4), Rh1–C17 2.100(4), Rh1–C20 2.191(4), Rh1–C21 2.197(3). Rh–6: Rh1–C1 2.022(3), Rh1–Cl1 2.3845(9), Rh1–C16 2.118(4), Rh1–C17 2.112(4), Rh1–C20 2.199(4), Rh1–C21 2.211(4). Rh–8: Rh1–C1 2.020(6), Rh1–Cl2 2.389(2), Rh1–C16 2.191(4), Rh1–C17 2.211(6), Rh1–C20 2.094(6), Rh1–C21 2.118(6). Selected angles (°): Rh–1: C1–Rh1–Cl1: 88.68(9). Rh–6: C1–Rh1–Cl1: 89–41(9). Rh–8: C1–Rh1–Cl2: 90.1(2).
2.2. Isolation and Characterization of MDR Klebsiella pneumoniae
Fifteen Klebsiella pneumoniae isolates were obtained from different clinical samples (blood, sputum, abscesses, and urine) and characterized in selective media (i.e., MacConkey agar). All isolates were positive for urease, lysine, Simmon’s citrate, and ornithine, and negative for motility and indole production. Of the fifteen isolates, eleven were identified as MDR and ESBL producers. The MDR- and ESBL-positive (+) isolates exhibited higher resistance to all antibiotic groups than the non-MDR and ESBL-negative (–) isolates, as expected.
The antibiotic susceptibility test results revealed that eleven isolates were resistant to multiple drugs, while four isolates were exclusively resistant to Ampicillin. Among the multiple drug-resistant isolates, the highest frequency of resistance was observed for β-lactams, with 55.3%, followed by tetracyclines (50.0%), sulfonamides (46.7%), furans (46.7%), quinolones (43.3%), and aminoglycosides (28.9%).
2.3. Antibacterial Activity Evaluation (MIC/MBC)
In our initial experiments, we assessed the efficacy of the 22 prepared compounds by determining their minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) against six different control strains, including Staphylococcus aureus mecA (–) (ATCC 25923), Staphylococcus aureus mecA (–) (ATCC 29213), Staphylococcus aureus mecA (+) (ATCC 43300), Escherichia coli (ATCC 25922), Enterococcus faecalis (ATCC 29212), and ESBL Klebsiella pneumoniae (ATCC 700603), as well as an isolate of Streptococcus spp. obtained from nasopharyngeal exudate. Our findings, summarized in Table 1, revealed that the azolium salts exhibited very low activity against all the strains, with MIC/MBC > 250 μg/mL. However, the presence of the metal fragment resulted in significantly lower values. For instance, complex Rh–1 displayed MIC/MBC values of 62.5/62.5 μg/mL against S. aureus (ATCC 29213) and E. faecalis (ATCC 29212). Intriguingly, the presence of a single fluorine atom did not impact the activity of the complexes, whereas the presence of two fluorine atoms at positions 3 and 4 (Rh–6) led to a decrease in MIC/MBC concentration to 62.5 μg/mL against S. aureus (ATCC 25923), S. aureus (ATCC 43300), and Streptococcus spp. In comparison, the complex with three fluorine atoms (Rh–7) was highly selective against S. aureus (ATCC 29213), exhibiting a MIC of 3.9 μg/mL. Notably, complex Rh–11, which contains two –CF3 groups, displayed the lowest MIC/MBC concentrations, ranging from 0.97 to 62.5 μg/mL, depending on the strain, with the lowest values observed against E. faecalis (ATCC 29212) (MIC/MBC = 0.97/15.6 μg/mL). These values are similar to those obtained for commonly used antibiotics such as linezolid (LIN), gentamicin (GEN), or amoxicillin (AMOX).

Table 1.
Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC) for fluorinated compounds against ATCC strains.
To develop new antibiotics against MDR and ESBL-producing Klebsiella pneumoniae, we assessed the MIC and MBC values for all fluorinated compounds against our clinical K. pneumoniae isolates: U–13685, H–9871, U–13815, H–9866, H–166, and S–401 (Table 2). Azolium salts were inactive against all strains (MIC/MBC >250/250 µg/mL). However, complexes Rh–1, Rh–3, Rh–4, Rh–5, Rh–6, and Rh–11 exhibited activity against isolates U–13685, H–9871, U–13815, and H–9866, with MIC values ranging from 62.5 to 250 µg/mL. Moreover, most of these complexes were bactericidal against these strains, except for Rh–1 and Rh–6, which showed a bacteriostatic effect against isolate U–13815. However, these complexes exhibited MIC values >250 µg/mL against clinical isolates H–9866, H–166, and S–401. Interestingly, complex Rh–3, containing a fluorine atom at the 3-position, displayed the most potent activity against our clinical isolates, with the lowest MIC/MBC values (62.5 µg/mL).

Table 2.
Minimal Inhibitory Concentration and Minimal Bactericidal Concentration (µg/mL) for fluorinated compounds against isolates of MDR Klebsiella pneumoniae.
The results described above are consistent with the fact that Gram-positive bacteria have a simpler cell wall structure, which facilitates the transfer of the complexes inside the cells. In contrast, Gram-negative bacteria have an outer lipopolysaccharide membrane that makes it more difficult for the complexes to penetrate the cell wall. However, it is interesting to note that despite this barrier, complexes Rh–1, Rh–3, Rh–4, Rh–5, Rh–6, and Rh–11 were still active against isolates U–13685, H–9871, U–13815, and H–9866. This suggests that the different resistance phenotypes of these isolates may play a role in the ability of the complexes to penetrate the cell wall.
2.4. Time–Killing Kinetics of Rh(I)–NHC Complexes
To determine the bactericidal kinetics of the organometallic compounds, we utilized the broth microdilution method. We focused on those complexes that exhibited the lowest MIC values, which were previously determined using the broth microdilution method. As shown in Figure 4, complex Rh–1 demonstrated a concentration-dependent bactericidal effect at 1X MIC and 4X MIC (Figure 4A,B). Specifically, Rh–1 showed the same germicidal speed against S. aureus (ATCC 29213) at concentrations of 1X MIC (62.5 µg/mL) and 4X MIC (250 µg/mL) after 4 h (5 log10 CFU/mL). Furthermore, Rh–1 exhibited a bacteriostatic effect against the clinical isolate of Klebsiella pneumoniae (U–13815), reducing bacterial growth (1 log10 CFU/mL) during the initial 6 h at a concentration of 1X MIC (250 µg/mL) (Figure 4E).
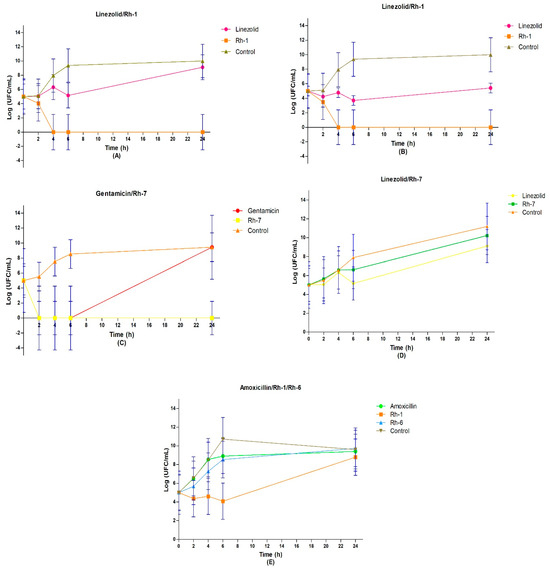
Figure 4.
Time–kill curves of: (A) S. aureus (ATCC 29213) treated with Rh–1 at 1 X MIC ** and (B) 4 X MIC *; (C) E. coli (ATCC 25922) and (D) S. aureus (ATCC 29213) treated with Rh–7 at 1 X MIC *; (E) K. pneumoniae U–13815 (clinical isolate) treated with Rh–1 and Rh–6 at 1 X MIC. All the time–kill curves were recorded during 24 h. The blue bars in each value indicate the standard deviation of the mean. One-way ANOVA followed by Tukey’s multiple comparisons test was applied. * p < 0.05, ** p < 0.01. (A) (linezolid vs. Rh-1 (1 MIC)) *, (A) (Rh-1 vs. control (1 MIC)) **, (B) (Rh-1 vs. control (4 MIC)) * and (C) (Rh-7 vs. control) *. The control compounds are described in the Supplementary Information.
Compound Rh–7 displayed remarkable bactericidal activity against E. coli (ATCC 25922) at 1 MIC after only 2 h of incubation, reducing the bacterial load by 5 log10 CFU/mL (Figure 4D). Conversely, it exhibited a bacteriostatic effect against S. aureus (ATCC 29213) at 1 MIC, causing a difference of 1 log10 CFU/mL until 6 h of incubation (Figure 4D). The growth index of S. aureus (ATCC 29213) remained unchanged between long incubation times (6–24 h) and short times, exhibiting a growth rate of 3 log10 CFU/mL until 6 h (Figure 4D). Interestingly, gentamicin demonstrated a similar effect to Rh–7 in the first 6 h, reducing the bacterial load by 5 log10 CFU/mL, but the growth of E. coli (ATCC 25922) increased considerably afterward, matching the growth control at 24 h at 1 MIC. As shown in Figure 4D, the growth rate of E. coli (ATCC 25922) treated with 1 MIC was higher in shorter incubation times (0–6 h), reaching 3–4 log10 CFU/mL, whereas in longer times, it reduced to 1 log10 CFU/mL from 6 to 24 h. Conversely, linezolid exhibited a bacteriostatic effect, reducing the bacterial load by 1 log10 CFU/mL at 1X MIC (1 µg/mL) and by 2 log10 CFU/mL at 4X MIC (4 µg/mL).
Complex Rh–6 showed a similar effect on bacterial growth (3 log10 CFU/mL) to that of amoxicillin against the clinical isolate of K. pneumoniae (U–13815). Specifically, the growth rate of K. pneumoniae (U–13815) was 6 log10 CFU/mL during the 0–6 h incubation period and 0 log10 CFU/mL during the 6–24 h period (Figure 4E). Moreover, there was an observed decline in the growth of U–13815 of approximately 1 log10 CFU/mL at 24 h in the growth control (250 µg/mL). Notably, a noticeable difference in bacterial presence was observed after 24 h following contact with Rh–1 and Rh–6 in K. pneumoniae (U–13815), compared to amoxicillin, using the Gram stain technique (Figure 4E).
2.5. ADMET Prediction/Pharmacokinetic Parameters
We performed ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicity) predictions for the azolium salts and complexes. Zolpidem and cisplatin were included as reference drugs for comparative purposes (Table 3). The ADMET profile of a compound is a critical determinant of its pharmacological efficacy and potential toxicity. Predictions were carried out using the admetSAR and SwissADME online platforms [45,46]. The results indicate that all the azolium salts are orally bioavailable in humans and can cross the blood–brain barrier and Caco-2 cells. Both the azolium salts and the reference drugs fall under category III of acute oral toxicity and are not considered carcinogenic. Additionally, compounds 1, 2, 3, 6, 8, 9, 10, and 11 do not exhibit hepatotoxicity, while compounds 1, 2, 5, 6, 7, 8, 9, 10, and 11 show intestinal absorption in humans.

Table 3.
List of ADMET properties for the fluorinated compounds.
The ADMET analysis predicted that all the metal complexes in the study were permeable to the blood–brain barrier. Additionally, Complexes Rh–1, Rh–2, Rh–3, Rh–4, Rh–6, Rh–7, Rh–8, and Rh–10 showed intestinal absorption, similar to cisplatin. Most of the Rh(I)–NHC complexes and cisplatin were found to be orally bioavailable in humans, with the exception of Rh–3, Rh–4, and Rh–9. Interestingly, all the complexes and drugs studied were classified as having acute oral toxicity category III and were found to be non-carcinogenic, with the exception of cisplatin. Furthermore, Rh(I)–NHC complexes (excluding Rh–5) were found to be non-mutagenic and non-hepatotoxic. Moreover, complexes Rh–1 to Rh–7 were found to be permeable to Caco–2 cells, as was cisplatin. However, complexes Rh–8 to Rh–11 were not permeable to Caco–2 cells. All organometallic compounds and cisplatin were found to produce aquatic toxicity in fish and crustaceans, but they did not show similarities with lead.
According to the SILICOS–IT model, the solubility of all Rh(I)–NHC complexes in water is categorized as moderately to slightly soluble. However, most of the compounds comply with the chemical rules of “LIPINSKI” and are similar to commercial drugs, with the exception of Rh–8, Rh–9, Rh–10, and Rh–11. These compounds were predicted to have a low synthetic accessibility, which means that they are more difficult to synthesize than the other complexes. On the other hand, the rest of the Rh(I)–NHC complexes have a synthetic accessibility close to 1, indicating that they are very easy to synthesize.
3. Conclusions
We investigated the antibiotic activity of a series of fluorinated compounds against various Gram-positive and -negative bacteria. While the azolium salts displayed minimal activity against the tested strains, the Rh(I) complexes demonstrated highly promising results. Notably, the complex containing two-CF3 groups displayed a remarkable MIC value of up to 0.97 μg/mL against E. faecalis (ATCC 29212). Additionally, we observed that the presence of a single fluorine atom in the complexes produced an active species that exhibited comparable antibacterial activity to amoxicillin when tested against our clinical isolates of Klebsiella pneumoniae. These encouraging results suggest that the Rh(I) complexes could serve as useful models for the development of novel antibacterial drugs. Other potential biological applications using these complexes are currently under study in our laboratories.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics17080973/s1, Supplementary material includes, full descriptions of the materials and methods employed for the isolation and characterization of Klebsiella pneumoniae, the antibacterial activity evaluation (MIC/MBC), the time–killing kinetics of Rh(I)–NHC complexes and the ADMET prediction/Pharmacokinetic parameters. As well as full description of the data collection and refinement as well as crystallographic data for compounds Rh–1 (CCDC 2280627), Rh–6 (CCDC 2280628) and Rh–8 (CCDC 2280629). The later been deposited at the Cambridge Crystallographic Data Centre. Copies of this information are available free of charge on request from The Director, CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK (Fax: +44-1223-336033; e-mail deposit@ccdc.cam.ac.uk or www: http://www.ccdc.cam.ac.uk (accessed on 1 June 2025)).
Author Contributions
Conceptualization, D.M.-M.; data curation, L.Á.T.-G., R.P.-U., H.V., Y.P.A.-S., V.R.-M., and D.M.-M.; formal analysis, R.P.-U., S.H.-O., Y.S.-L., V.R.-M., and D.M.-M.; funding acquisition, D.M.-M.; investigation, L.Á.T.-G., R.P.-U., H.V., G.G.V.-R., Y.P.A.-S., and Y.S.-L.; project administration, D.M.-M.; resources, D.M.-M.; software, S.H.-O.; supervision, D.M.-M.; validation, D.M.-M.; writing—original draft, R.P.-U., H.V., V.R.-M., and D.M.-M.; writing—review and editing, D.M.-M. All authors have read and agreed to the published version of the manuscript.
Funding
The financial support of this research by PAPIIT–DGAPA–UNAM (PAPIIT IN223323) and CONACYT A1–S–33933 is gratefully acknowledged.
Institutional Review Board Statement
This project was submitted for evaluation and subsequently approved by the Research and Ethics Review Committee of the Culiacán General Hospital “Dr. Bernardo J. Gastelum”, during the period 01February 2019–28 February 2020, with ethical concession number 20160708.
Data Availability Statement
The data that support the findings of this study are available in the Supplementary Materials of this article.
Acknowledgments
We would like to thank Francisco Javier Pérez Flores, Q. Eréndira García Ríos, M.Sc. Lucia del Carmen Márquez Alonso, M.Sc. Lucero Ríos Ruiz, Q. María de la Paz Orta Pérez, Q. Roció Patiño–Maya, Beatriz Quiroz García, and Nuria Esturau Escofet for technical assistance. L. A. T.–G. would like to thank Programa de Becas CONACyT (número de becario: 626610).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- van Duin, D.; Paterson, D.L. Multidrug-Resistant Bacteria in the Community: Trends and Lessons Learned. Infect. Dis. Clin. N. Am. 2016, 30, 377–390. [Google Scholar] [CrossRef]
- Spellberg, B.; Blaser, M.; Guidos, R.J.; Boucher, H.W.; Bradley, J.S.; Eisenstein, B.I.; Gerding, D.; Lynfield, R.; Reller, L.B.; Rex, J.; et al. Combating antimicrobial resistance: Policy recommendations to save lives. Clin. Infect. Dis. 2011, 52 (Suppl. S5), S397–S428. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Villodres, A.; Martin-Gandul, C.; Penalva, G.; Guisado-Gil, A.B.; Crespo-Rivas, J.C.; Pachon-Ibanez, M.E.; Lepe, J.A.; Cisneros, J.M. Prevalence and Risk Factors for Multidrug-Resistant Organisms Colonization in Long-Term Care Facilities Around the World: A Review. Antibiotics 2021, 10, 680. [Google Scholar] [CrossRef]
- Medina, E.; Pieper, D.H. Tackling Threats and Future Problems of Multidrug-Resistant Bacteria. Curr. Top. Microbiol. Immunol. 2016, 398, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Tigabu, A.; Getaneh, A. Staphylococcus aureus, ESKAPE Bacteria Challenging Current Health Care and Community Settings: A Literature Review. Clin. Lab. 2021, 67, 1539–1549. [Google Scholar] [CrossRef]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed. Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- Bush, K.; Jacoby, G.A. Updated functional classification of beta-lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef]
- Rice, L.B. Progress and challenges in implementing the research on ESKAPE pathogens. Infect. Control Hosp. Epidemiol. 2010, 31 (Suppl. S1), S7–S10. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Cataldo, M.A.; Dancer, S.J.; De Angelis, G.; Falcone, M.; Frank, U.; Kahlmeter, G.; Pan, A.; Petrosillo, N.; Rodriguez-Bano, J.; et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin. Microbiol. Infect. 2014, 20 (Suppl. S1), 1–55. [Google Scholar] [CrossRef]
- CDC. Antibiotic Resistance Threats in the United States, 2019; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019.
- Jacoby, G.A. AmpC beta-lactamases. Clin. Microbiol. Rev. 2009, 22, 161–182. [Google Scholar] [CrossRef]
- Gonzalez, A.C.; Nieves, B.; Solorzano, M.; Cruz, J.; Puig, J.; Moreno, M. Characterization of extended-spectrum beta-lactamases-producing Klebsiella pneumoniae isolates of two intensive care units. Rev. Chilena Infectol. 2013, 30, 374–380. [Google Scholar] [CrossRef]
- Ho, J.; Tambyah, P.A.; Paterson, D.L. Multiresistant Gram-negative infections: A global perspective. Curr. Opin. Infect. Dis. 2010, 23, 546–553. [Google Scholar] [CrossRef]
- Zhao, W.H.; Hu, Z.Q. Epidemiology and genetics of CTX-M extended-spectrum beta-lactamases in Gram-negative bacteria. Crit. Rev. Microbiol. 2013, 39, 79–101. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, R. Growing group of extended-spectrum beta-lactamases: The CTX-M enzymes. Antimicrob. Agents Chemother. 2004, 48, 1–14. [Google Scholar] [CrossRef]
- Babic, M.; Hujer, A.M.; Bonomo, R.A. What’s new in antibiotic resistance? Focus on beta-lactamases. Drug. Resist. Updat. 2006, 9, 142–156. [Google Scholar] [CrossRef]
- Uc-Cachon, A.H.; Gracida-Osorno, C.; Luna-Chi, I.G.; Jimenez-Guillermo, J.G.; Molina-Salinas, G.M. High Prevalence of Antimicrobial Resistance Among Gram-Negative Isolated Bacilli in Intensive Care Units at a Tertiary-Care Hospital in Yucatan Mexico. Medicina 2019, 55, 588. [Google Scholar] [CrossRef] [PubMed]
- de Kraker, M.E.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef]
- Durdu, B.; Meric Koc, M.; Hakyemez, I.N.; Akkoyunlu, Y.; Daskaya, H.; Sumbul Gultepe, B.; Aslan, T. Risk Factors Affecting Patterns of Antibiotic Resistance and Treatment Efficacy in Extreme Drug Resistance in Intensive Care Unit-Acquired Klebsiella pneumoniae Infections: A 5-Year Analysis. Med. Sci. Monit. 2019, 25, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Frei, A. Metal Complexes, an Untapped Source of Antibiotic Potential? Antibiotics 2020, 9, 90. [Google Scholar] [CrossRef]
- Liang, J.; Sun, D.; Yang, Y.; Li, M.; Li, H.; Chen, L. Discovery of metal-based complexes as promising antimicrobial agents. Eur. J. Med. Chem. 2021, 224, 113696. [Google Scholar] [CrossRef]
- Patra, M.; Gasser, G.; Metzler-Nolte, N. Small organometallic compounds as antibacterial agents. Dalton Trans. 2012, 41, 6350–6358. [Google Scholar] [CrossRef]
- Ma, D.L.; Wang, M.; Mao, Z.; Yang, C.; Ng, C.T.; Leung, C.H. Rhodium complexes as therapeutic agents. Dalton Trans. 2016, 45, 2762–2771. [Google Scholar] [CrossRef]
- Chandra, S.; Tyagi, M.; Agrawal, S. Spectral and antimicrobial studies on tetraaza macrocyclic complexes of PdII, PtII, RhIII and IrIII metal ions. J. Saudi Chem. Soc. 2011, 15, 49–54. [Google Scholar] [CrossRef]
- Beloglazkina, E.K.; Manzheliy, E.A.; Moiseeva, A.A.; Maloshitskaya, O.A.; Zyk, N.V.; Skvortsov, D.A.; Osterman, I.A.; Sergiev, P.V.; Dontsova, O.A.; Ivanenkov, Y.A.; et al. Synthesis, characterisation, cytotoxicity and antibacterial activity of ruthenium(II) and rhodium(III) complexes with sulfur-containing terpyridines. Polyhedron 2016, 107, 27–37. [Google Scholar] [CrossRef]
- Fandzloch, M.; Augustyniak, A.W.; Dobrzanska, L.; Jedrzejewski, T.; Sitkowski, J.; Wypij, M.; Golinska, P. First dinuclear rhodium(II) complexes with triazolopyrimidines and the prospect of their potential biological use. J. Inorg. Biochem. 2020, 210, 111072. [Google Scholar] [CrossRef] [PubMed]
- Aradhyula, B.P.R.; Joshi, N.; Poluri, K.M.; Kollipara, M.R. Synthesis and antibacterial studies of rhodium and iridium complexes comprising of dipyridyl hydrazones. J. Mol. Struct. 2018, 1164, 191–199. [Google Scholar] [CrossRef]
- Mansouri, G.; Heidarizadi, F.; Naghipour, A.; Notash, B. Synthesis, characterization and antibacterial study of cyclometalated rhodium(III) complex containing dithiocarbamate. J. Mol. Struct. 2016, 1121, 128–134. [Google Scholar] [CrossRef]
- Vaidya, M.; McBain, A.J.; Banks, C.E.; Whitehead, K.A. Single and combined antimicrobial efficacies for nine metal ion solutions against Klebsiella pneumoniae, Acinetobacter baumannii and Enterococcus faecium. Int. Biodeterior. Biodegrad. 2019, 141, 39–43. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Ott, I. Metal N-heterocyclic carbene complexes in medicinal chemistry. Adv. Inorg. Chem. 2020, 75, 121–148. [Google Scholar] [CrossRef]
- Bernier, C.M.; DuChane, C.M.; Martinez, J.S.; Falkinham, J.O.; Merola, J.S. Synthesis, Characterization, and Antimicrobial Activity of RhIII and IrIII N-Heterocyclic Carbene Piano-Stool Complexes. Organometallics 2021, 40, 1670–1681. [Google Scholar] [CrossRef]
- Simpson, P.V.; Schmidt, C.; Ott, I.; Bruhn, H.; Schatzschneider, U. Synthesis, cellular uptake and biological activity against pathogenic microorganisms and cancer cells of rhodium and iridium N-heterocyclic carbene complexes bearing charged substituents. Eur. J. Inorg. Chem. 2013, 2013, 5547–5554. [Google Scholar] [CrossRef]
- Streciwilk, W.; Terenzi, A.; Cheng, X.; Hager, L.; Dabiri, Y.; Prochnow, P.; Bandow, J.E.; Wolfl, S.; Keppler, B.K.; Ott, I. Fluorescent organometallic rhodium(I) and ruthenium(II) metallodrugs with 4-ethylthio-1,8-naphthalimide ligands: Antiproliferative effects, cellular uptake and DNA-interaction. Eur. J. Med. Chem. 2018, 156, 148–161. [Google Scholar] [CrossRef]
- Cetinkaya, B.; Cetinkaya, E.; Küçükbay, H.; Durmaz, R. Antimicrobial activity of carbene complexes of rhodium(I) and ruthenium(II). Arzneimittelforschung 1996, 46, 821–823. [Google Scholar]
- Wang, J.; Sánchez-Roselló, M.; Aceña, J.L.; Del Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in pharmaceutical industry: Fluorine-containing drugs introduced to the market in the last decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Acenã, J.L.; Soloshonok, V.A.; Izawa, K.; Liu, H. Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II-III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev. 2016, 116, 422–518. [Google Scholar] [CrossRef] [PubMed]
- Isanbor, C.; O’Hagan, D. Fluorine in medicinal chemistry: A review of anti-cancer agents. J. Fluorine Chem. 2006, 127, 303–319. [Google Scholar] [CrossRef]
- Kirk, K.L. Fluorine in medicinal chemistry: Recent therapeutic applications of fluorinated small molecules. J. Fluorine Chem. 2006, 127, 1013–1029. [Google Scholar] [CrossRef]
- Müller, K.; Faeh, C.; Diederich, F. Fluorine in pharmaceuticals: Looking beyond intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef]
- Rufino-Felipe, E.; Colorado-Peralta, R.; Reyes-Márquez, V.; Valdés, H.; Morales-Morales, D. Fluorinated-NHC transition metal complexes: Leading characters as potential anticancer metallodrugs. Anti-Cancer Agents Med. Chem. 2021, 21, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Turcio-García, L.Á.; Valdés, H.; Hernández-Ortega, S.; Canseco-Gonzalez, D.; Morales-Morales, D. Arylation of aldehydes catalyzed by fluorinated NHC–Rh(I) complexes. New J. Chem. 2022, 46, 16789–16800. [Google Scholar] [CrossRef]
- Turcio-García, L.Á.; Valdés, H.; Arenaza-Corona, A.; Hernández-Ortega, S.; Morales-Morales, D. Electronic properties and supramolecular study of selenoureas with fluorinated-NHC ligands derived from imidazo [1,5-a]pyridines. New J. Chem. 2023, 47, 2090–2095. [Google Scholar] [CrossRef]
- Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. admetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 2019, 35, 1067–1069. [Google Scholar] [CrossRef]
- SwissADME. Available online: http://www.swissadme.ch/index.php#/ (accessed on 1 December 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).