Abstract
Lung cancer is the leading cause of cancer-related deaths worldwide. Non-small cell lung cancer (NSCLC) is the major subtype, accounting for more than 85% of all lung cancer cases. Recent advances in precision oncology have allowed NSCLC patients bearing specific oncogenic epidermal growth factor receptor (EGFR) mutations to respond well to EGFR tyrosine kinase inhibitors (TKIs). Due to the high EGFR mutation frequency (up to more than 50%) observed particularly in Asian NSCLC patients, EGFR-TKIs have produced unprecedented clinical responses. Depending on their binding interactions with EGFRs, EGFR-TKIs are classified as reversible (first-generation: gefitinib and erlotinib) or irreversible inhibitors (second-generation: afatinib and dacomitinib; third-generation: osimertinib). While the discovery of osimertinib represents a breakthrough in the treatment of NSCLC, most patients eventually relapse and develop drug resistance. Novel strategies to overcome osimertinib resistance are urgently needed. In Asian countries, the concomitant use of Western medicine and traditional Chinese medicine (TCM) is very common. Ganoderma lucidum (Lingzhi) and Coriolus versicolor (Yunzhi) are popular TCMs that are widely consumed by cancer patients to enhance anticancer efficacy and alleviate the side effects associated with cancer therapy. The bioactive polysaccharides and triterpenes in these medicinal mushrooms are believed to contribute to their anticancer and immunomodulating effects. This review presents the latest update on the beneficial combination of Lingzhi/Yunzhi and EGFR-TKIs to overcome drug resistance. The effects of Lingzhi/Yunzhi on various oncogenic signaling pathways and anticancer immunity, as well as their potential to overcome EGFR-TKI resistance, are highlighted. The potential risk of herb–drug interactions could become critical when cancer patients take Lingzhi/Yunzhi as adjuvants during cancer therapy. The involvement of drug transporters and cytochrome P450 enzymes in these herb–drug interactions is summarized. Finally, we also discuss the opportunities and future prospects regarding the combined use of Lingzhi/Yunzhi and EGFR-TKIs in cancer patients.
1. Introduction
EGFR-mutated NSCLC represents a clinically significant subset of lung cancers, characterized by oncogenic driver mutations that promote tumor growth, survival, and progression. While EGFR-TKIs such as gefitinib, erlotinib, and osimertinib have revolutionized the treatment of EGFR-mutated NSCLC by targeting these mutations, therapeutic resistance remains a major challenge. Mechanisms of resistance, including secondary mutations (e.g., T790M and C797S), bypass pathway activation (e.g., MET amplification), and phenotypic transformations (e.g., epithelial–mesenchymal transition (EMT) or small cell lung cancer (SCLC) transformation), often limit the long-term efficacy of EGFR-TKIs [1,2,3,4]. Furthermore, the tumor microenvironment (TME) of EGFR-mutated NSCLC, characterized by a low tumor mutation burden (TMB), impaired T cell activity, and high levels of immunosuppressive cytokines, further complicates treatment outcomes [5,6].
Ganoderma lucidum (Lingzhi) and Coriolus versicolor (Yunzhi), two medicinal mushrooms widely used in traditional Chinese medicine (TCM) in Asian countries, have emerged as promising complementary therapies in cancer treatment. Their bioactive compounds, including polysaccharides and triterpenoids, exhibit a broad range of antitumor activities, such as the inhibition of oncogenic signaling pathways, suppression of angiogenesis, reversal of multidrug resistance (MDR), and modulation of tumor immunity [7,8]. They enhance immune function by activating natural killer (NK) cells, T cells, and macrophages while reducing immunosuppressive cells such as regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) [9,10,11,12]. Lingzhi and Yunzhi are also known to enhance the efficacy of conventional cancer therapies, including chemotherapy, radiotherapy, and targeted therapies, by inhibiting various oncogenic signaling pathways and reversing MDR [13,14,15]. The two medicinal mushrooms are also known to reduce the immunosuppressive and oxidative stress effects caused by chemotherapeutic drugs while enhancing their efficacy [10]. Yunzhi has also been demonstrated to reduce fatigue and enhance immune recovery in cancer patients [16]. Moreover, both mushrooms alleviate treatment-related side effects, such as fatigue, nausea, and immunosuppression, thus improving the quality of life of cancer patients [10,17,18,19]. Recent studies suggest that Lingzhi/Yunzhi may augment the efficacy of EGFR-TKIs by targeting EGFR-associated resistance mechanisms, reducing immunosuppression in the TME, and enhancing immune responses [15,20,21,22].
This review summarizes the latest advances in the beneficial therapeutic effects of Lingzhi/Yunzhi in overcoming drug resistance, strengthening immunity, and improving clinical outcomes in EGFR-mutated NSCLC. These medicinal mushrooms may be used as adjuvant therapies to address the current challenges in the management of EGFR-mutated NSCLC [23].
2. Drug Resistance to EGFR-TKIs
2.1. Resistance Mechanisms to First- and Second-Generation EGFR-TKIs
The resistance mechanisms of first- and second-generation EGFR-TKIs can be categorized into three main types: EGFR-dependent changes, activation of bypass or downstream pathways, and phenotypic transformations. The most prominent mechanism is the EGFR T790M mutation, which accounts for 50–60% of resistance cases against the first- and second-generation competitive EGFR-TKIs. This mutation leads to drug resistance by increasing the affinity of the EGFR kinase for ATP. Other EGFR mutations, such as L747S and T854A, contribute to resistance but are less common. The activation of bypass pathways, including HER2 amplification, MET amplification, and PIK3CA/KRAS mutations, promotes tumor survival and proliferation. Moreover, phenotypic transformations, such as EMT or transformation to SCLC, further drive resistance [2,3,4].
2.2. Drug Resistance to Osimertinib in First-Line and Second-Line Treatment
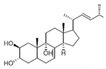
Osimertinib is the only third-generation EGFR-TKI clinically approved by major regulatory authorities for the treatment of EGFR T790M-positive patients who have progressed following treatment with first- or second-generation EGFR-TKIs. Osimertinib was also approved in 2018 as a first-line therapy for advanced EGFR-mutated NSCLC, regardless of T790M mutation status. Figure 1 shows the chemical structures of US Food and Drug Administration (FDA)-approved EGFR TKIs. While the discovery of osimertinib represents a breakthrough in the treatment of NSCLC, most patients eventually relapse and develop resistance to treatment. Osimertinib resistance arises from EGFR-dependent mechanisms (including tertiary mutations, such as C797S, and EGFR amplification) or EGFR-independent mechanisms (such as MET/HER2 amplification, KRAS/BRAF mutations, and oncogenic fusions) [1]. Histological transformations (e.g., SCLC) and EMT also contribute, subsequently activating bypass pathways (MAPK, PI3K-Akt) and promoting tumor survival and progression [1,3].
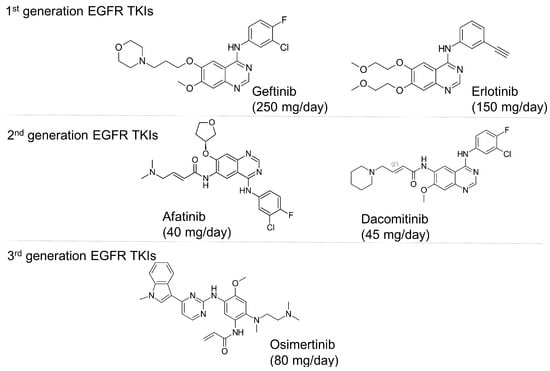
Figure 1.
Chemical structures and recommended daily dosage of the US Food and Drug Administration (FDA)-approved EGFR TKIs.
2.2.1. EGFR-Dependent Resistance Mechanisms [24]
Tertiary EGFR mutations, particularly C797S (10–26% in second-line; 7% in first-line settings), disrupt osimertinib binding and confer resistance, with treatment effectiveness depending on its allelic context (cis versus trans with T790M). Rare EGFR mutations like L792H, L718Q, G796R, and G724S also hinder osimertinib binding, while L718Q retains sensitivity to earlier-generation TKIs when T790M is absent. EGFR amplification, observed mainly in second-line settings, enhances EGFR signaling and bypasses osimertinib inhibition. Additionally, T790M dynamics show that its loss during progression is linked to alternative resistance mechanisms (e.g., MET amplification, KRAS mutations) and poorer outcomes.
2.2.2. EGFR-Independent Resistance Mechanisms
Bypass pathway activation is a pivotal osimertinib resistance mechanism that involves the emergence of alternative signaling pathways or histological transformations to drive cancer growth [25]. Among these mechanisms, MET amplification represents the most commonly reported one, occurring in 15% of first-line and 19% of second-line cases. This could co-occur with EGFR mutations such as C797S, thereby stimulating downstream pathways (MAPK, PI3K-Akt). HER2 amplification (2–5%) and RAS–MAPK pathway mutations (e.g., KRAS G12D, BRAF V600E) further drive resistance, while PI3K pathway activation via PIK3CA mutations or PTEN loss promotes tumor survival. Rare oncogenic fusions (e.g., BRAF, RET, ALK) are found in 3–10% of cases and can co-occur with EGFR mutations. Histological transformation to SCLC, associated with TP53 and RB1 inactivation, occurs in 4–15% of cases. Additionally, EMT, characterized by reduced E-cadherin and increased vimentin, contributes to resistance through transcription factors such as ZEB1 and TWIST1.
3. Potentiation of Antitumor Efficacy of EGFR-TKIs by Medicinal Mushrooms
3.1. Lingzhi Inhibits Various Oncogenic Pathways and Overcomes Drug Resistance in EGFR-Mutated NSCLC
Lingzhi demonstrates potent antitumor effects in EGFR-mutated NSCLC by downregulating EGFR expression, inhibiting key oncogenic pathways (PI3K/AKT/mTOR, ERK, Wnt/β-catenin), and suppressing EMT and angiogenesis. It addresses resistance to EGFR-TKIs by targeting bypass mechanisms, reactivating p53, and reversing MDR. Moreover, Lingzhi has also been reported to inhibit telomerase activity, thus limiting cancer cell immortality [14,15,26]. The multifaceted biological activities of Lingzhi in EGFR-mutated NSCLC are described below.
3.1.1. Inhibition of EGFR and Its Downstream Signaling Pathways
Numerous active components have been identified from Lingzhi that can inhibit EGFR pathways to suppress tumor growth, induce apoptosis, and block angiogenesis (Figure 2 and Table 1). WSG, a water-soluble polysaccharide isolated from Lingzhi with an average molecular mass of approximately 1000 kDa, was shown to significantly inhibit lung tumor growth, reduce the size of metastatic nodules in the lungs, and prolong the survival of LLC1-bearing mice [7]. Mechanistically, WSG was found to inhibit the phosphorylation of ERK1/2 in NSCLC cells upon either EGF or TGFβ stimulation. It also inhibited the phosphorylation of several other downstream signaling molecules, including FAK, AKT, and Smad2. Furthermore, WSG was reported to induce the degradation of TGFβ and EGF receptors via the proteasomal and lysosomal pathways, respectively [7]. In tongue cancer cells, WSG was shown to dramatically reduce cell viability and colony formation, increase sub-G1 and G2/M populations, and induce apoptotic responses [27]. The enhanced apoptosis was associated with an increase in the Bax/Bcl2 ratio and reduction in EGFR/AKT phosphorylation. Furthermore, the WSG + cisplatin combination was found to produce synergistic anticancer effects [27].
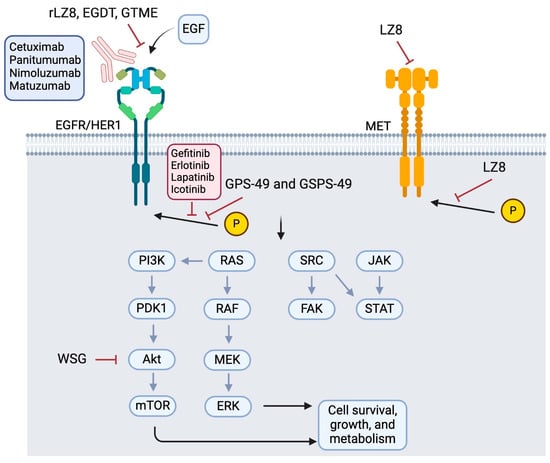
Figure 2.
Reported molecular targets of Lingzhi in the EGFR signaling pathway. Abbreviations: rLZ8: recombinant Ling Zhi-8 (a protein isolated from Ganoderma lucidum); EGDT: Ergosta-7,22-diene-2β,3α,9α-triol (a sterol); GTME: G. tsugae methanol extract; GPS-49 and GSPS-49: Ganoderma lucidum polysaccharides or sulfated polysaccharides isolated on day 49; WSG: a water-soluble glucose-enriched polysaccharide from Ganoderma lucidum; LZ-8: a medicinal peptide of Lingzhi.

Table 1.
Modulation of EGFR signaling by Lingzhi.
rLZ-8 (Ling Zhi-8), an immunoregulatory protein extracted from Lingzhi [33], regulates multiple immune cells, including T cells. It was shown to induce cell cycle arrest and apoptosis by downregulating the expression of wild-type and mutated EGFR and inhibiting EGFR downstream effectors (AKT and ERK1/2) in lung cancer cells in vitro [15]. In a lung tumor-bearing mouse model, rLZ-8 was also shown to effectively inhibit cancer progression and reduce tumoral EGFR expression in vivo. Mechanistically, rLZ-8 was found to decrease the plasma membrane expression of EGFR by altering EGFR localization and to enhance the EGF-induced degradation of EGFR [15]. rLZ-8 could bind to EGFR to induce EGFR autophosphorylation at tyrosine-1045 and trigger ubiquitination by inducing the formation of EGFR/Cbl complexes, subsequently leading to EGFR protein degradation. This working model was further supported by the fact that Cbl-shRNA abolished rLZ-8-induced EGFR degradation [15]. In other human cancer types besides NSCLC, rLZ-8 (0.2–2 mg/mL) was shown to significantly inhibit cancer cell proliferation including breast (MDA-MB-468), hepatocellular carcinoma (HCC) (Hep3B), and melanoma (B16F10). It also exhibited the dose-dependent inhibition (4–8 mg/kg) of tumor xenograft growth in an orthotopic HCC NOG-mouse model [28]. In the HCC cell line Hep3B, rLZ-8 was shown to specifically bind to EGFR and readily penetrate the cell membrane, subsequently disrupting endosomal recycling and inducing apoptosis. Interestingly, the antitumor efficacy of rLZ-8 on five patient-derived tumor xenograft (PDX) models of HCC (LI6280, LI1097, LI0050, LI0334, LI6611) was directly correlated with tumoral EGFR expression levels, suggesting the mechanistic involvement of EGFR. Molecular docking and protein cross-linking studies revealed that rLZ-8 directly blocks the EGFR dimerization interface via an epitope (S222/K269) on the extracellular domain of EGFR [28]. The formation of functional EGFR dimers and downstream EGFR signaling was therefore inhibited by rLZ-8 [28].
Ergosta-7-22-diene-2β,3α,9α-triol (EGDT), a sterol isolated from the mycelia of Lingzhi, demonstrated significant concentration-dependent (10–50 μM) anti-proliferative effects on nasopharyngeal carcinoma (NPC) cells, induced G0/G1 phase cell cycle arrest, and promoted apoptosis in vitro. EGDT was shown to downregulate EGFR at both mRNA and protein levels, which was associated with the significant inhibition of EGFR downstream RAF/MEK/ERK and PI3K/AKT signaling pathways. Additionally, EGDT (10 mg/kg) exhibited robust antitumor activity in NPC xenograft models in immunocompromised mice [29].
The methanol extract of Ganoderma tsugae (GTME), a polypore mushroom closely related to Lingzhi, was also reported to inhibit the proliferation of an EGFR-high-expressing epidermoid carcinoma cell line (A-431) and capillary-like tube formation in human umbilical vein endothelial cells (HUVECs). GTME (50–200 μg/mL) inhibited EGFR and VEGF expression, thereby reducing VEGF secretion from A-431 cells [30]. Since VEGF plays a pivotal role in angiogenesis, tumor growth, and metastasis, GTME may be useful for antitumor treatment by inhibiting tumor vasculature.
Lingzhi contains numerous biologically active components, among which polysaccharides (GPS) and their sulfated form (GSPS) are known to possess potent antitumor, immunomodulatory, anti-inflammatory, and neuroprotective effects. GPS is produced from the fruiting bodies, spores, or mycelia of Lingzhi. Accumulating evidence suggests that GPS is more abundant in the mycelia than in the fruiting bodies and spores. Therefore, commercially available GPS is usually obtained from Lingzhi mycelia. During industrial production, different supplements can be added to the culture medium of Lingzhi mycelia to increase the yield of mycelial biomass and derived GPS. Interestingly, the physicochemical characteristics and antitumor activities of GPS/GSPS from Lingzhi mycelium have been shown to vary with the harvest time of the liquid Lingzhi mycelial culture [32]. The optimal time to harvest GPS and GSPS was found to be 49 days from the mycelial culture. GPS and GSPS (100–300 μg/mL), isolated on day 49, demonstrated significant anticancer effects without impacting normal fibroblasts. The inhibitory effect of GPS-49 and GSPS-49 on the signaling networks mediated by EGFR and the transforming growth factor beta receptor (TGFβR) was found to be the most optimal [32].
3.1.2. Modulation of VEGF-Dependent Angiogenesis Program by Lingzhi/Yunzhi
Angiogenesis supports tumor growth and progression by providing the oxygen and nutrients needed by rapidly growing cancer cells. Vascular endothelial growth factor (VEGF) represents the major angiogenesis inducer, facilitating the development of tumor vasculature. To this end, Lingzhi and Yunzhi have been reported as natural VEGF inhibitors (Figure 3 and Table 2), targeting tumor angiogenesis through multiple mechanisms. Lingzhi inhibits angiogenesis by downregulating VEGF expression and secretion through the suppression of the EGFR and PI3K/AKT/mTOR pathways [30]. It also reduces endothelial cell proliferation and capillary formation, thereby limiting tumor vascularization and oxygen/nutrient supply, which are crucial for tumor survival and metastasis [26,34,35,36].
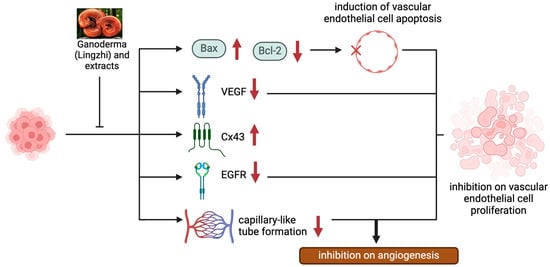
Figure 3.
The anti-angiogenic effects of Lingzhi and its molecular mechanisms.

Table 2.
Modulation of VEGF-dependent angiogenesis program by Lingzhi/Yunzhi.
Lingzhi polysaccharides (GLPS) have demonstrated the concentration-dependent inhibition of VEGF expression at both the mRNA and protein levels. In mouse melanoma cells (B16F10), GLPS reduced VEGF mRNA expression at concentrations as low as 0.2 µg/mL and protein levels at 0.8 µg/mL. A similar effect was also observed in human lung carcinoma cells (LA795) and hepatocarcinoma cells (HepG2), where GLPS significantly suppressed VEGF expression [11,37,38]. Additionally, Ganoderma lucidum polysaccharide peptide (GLPP) has also been reported to reduce VEGF secretion in human lung carcinoma cells (PG) at 200 µg/mL [21].
Ganoderma lucidum spore oil (GLSO) also demonstrated remarkable anti-VEGF activity. GLSO significantly decreased VEGF mRNA expression in HepG2 cells, human lung carcinoma cells (LTEP-a2), and breast carcinoma cells (MCF-7), with an approximately 50% reduction in VEGF expression in LTEP-a2 cells at 5–20 µg/mL [39,40,41]. In animal models, GLSO and spore powder effectively reduced tumor weight, volume, and VEGF levels in mice implanted with breast cancer cells (MDA-MB-231) and HCC cells (H22) at doses of 50–200 mg/kg [42,43,44,45]. These findings indicate the broad-spectrum anti-angiogenic properties of Lingzhi.
Similarly, polysaccharides derived from Yunzhi also demonstrated potent VEGF-inhibitory effects. Protein-bound polysaccharides (PBPs) were shown to regulate cytokine secretion to suppress VEGF production in murine breast cancer cells (4T1) at 50–200 µg/mL [47]. PSP significantly decreased tumor vascular density and VEGF mRNA expression in murine sarcoma models (S180) at 100–300 µg/mL [48]. In tumor xenograft models, Coriolus versicolor polysaccharide extract (CVE; 50–150 mg/kg) was shown to substantially reduced tumor weight and VEGF expression [49].
3.1.3. Inhibition of Other Aberrant Oncogenic Pathways by Lingzhi/Yunzhi
Besides EGFR, Lingzhi was also reported to inhibit other oncogenic pathways, such as c-Met and Wnt/β-catenin, which are often involved in bypass resistance in EGFR-mutated NSCLC. Lingzhi-derived peptides (e.g., Lingzhi-8; LZ8) were found to suppress c-Met signaling to inhibit downstream pathways such as AKT and ERK [14], whereas Ganoderma extract was shown to block Wnt/β-catenin signaling by inhibiting LRP6 phosphorylation [50]. On the other hand, Lingzhi was also reported to reduce MMP-2 and MMP-9 expression, which are critical for tumor invasion and metastasis [51].
LZ8 and GLE, two bioactive components from Ganoderma lucidum, exhibited distinct antitumor mechanisms in different cancer types. LZ8 is an immunomodulatory protein isolated from the mycelial extract of Lingzhi [52]. It was shown to effectively inhibit cell migration and growth in both c-Met-overexpressing (HCC372) and c-Met-negative (HCC329) HCC at concentrations of 10–50 μg/mL. In immunocompromised SCID mice, LZ8 (10–20 mg/kg) significantly reduced primary HCC329 tumor growth and intrahepatic metastasis, with comparable potency to a c-Met-specific antagonist (JNJ-38877605). Mechanistically, LZ8 (20–100 μg/mL) effectively suppressed the phosphorylation of JNK, ERK, and AKT in c-Met-negative HCC329 cells. On the other hand, LZ8 was also found to reduce c-Met expression and phosphorylation, as well as the downstream p-ERK and p-AKT (but not p-JNK), in the c-Met-overexpressing HCC372 cells. Based on a receptor array screening of the major receptor tyrosine kinases, LZ8 demonstrated the most potent inhibitory effect on EGFR. In HepG2 cells, LZ8 suppressed HGF-induced c-Met signaling (p-c-Met, p-JNK, p-ERK, and p-paxillin) and cancer cell migration at concentrations of 25–100 μg/mL, outperforming JNJ-38877605 (a c-Met-specific antagonist). These findings highlight the anti-HCC potential of LZ8 via both c-Met-dependent and -independent pathways [14].
A commercially available whole-mushroom Ganoderma lucidum extract (GLE; manufactured by Pharmanex) has been reported to inhibit cancer proliferation and stemness in triple-negative breast cancer (TNBC) by targeting the STAT3 signaling pathway [53]. GLE was characterized as containing 5% polysaccharides, 6% triterpenes, and 1% cracked spores. At 50–200 μg/mL, GLE reduced the phosphorylation of STAT3 at Tyr705, a critical modification for STAT3 activation, in TNBC cell lines (MDA-MB-231 and SUM-149). This downregulation led to the decreased expression of a few key STAT3-regulated self-renewal transcription factors, including OCT4, NANOG, and SOX2. Additionally, GLE (50–250 μg/mL) also inhibited upstream components of the STAT3 pathway, such as JAK1 and JAK2. By disrupting STAT3 signaling, GLE (100–250 μg/mL) reduced the abundance of breast cancer stem cells (i.e., ALDH1-positive and CD44+/CD24− cell population) and inhibited mammosphere formation [53].
Lingzhi also suppressed oncogene-driven metastasis in EGFR-mutated NSCLC by targeting EMT and migration-related pathways. rLZ-8 inhibited EMT by downregulating a key transcription factor, Slug, but increasing E-cadherin expression, thereby suppressing cell mobility [54]. Furthermore, Lingzhi inhibited FAK-SRC-paxillin signaling, which disrupts cytoskeletal reorganization and focal adhesion formation, resulting in reduced tumor migration and invasion [26,55].
3.1.4. Circumvention of Other EGFR TKI Resistance Mechanisms
Lingzhi was also reported to reactivate mutant p53, restoring its tumor-suppressive functions, and to reverse MDR by inhibiting P-gp, thereby increasing intracellular drug accumulation. These effects enhance the efficacy of chemotherapy and targeted therapies in resistant EGFR-mutant NSCLC [26,36,56] (summarized in Table 3).

Table 3.
Lingzhi/Yunzhi enhanced antitumor activity of EGFR-TKIs in preclinical studies.
Telomerase activation is associated with uncontrolled cell proliferation and represents a key cancer hallmark. Interestingly, the inhibition of telomerase, via the c-Myc-dependent downregulation of hTERT, has been recently shown to contribute to the antitumor activity of osimertinib [62]. The combination of a telomere-targeting inhibitor (6-Thio-dG) and osimertinib was shown to inhibit the growth of osimertinib-resistant NSCLC and to delay the emergence of acquired resistance to osimertinib. In this context, Lingzhi has been shown to inhibit telomerase activity by downregulating hTERT (the catalytic subunit of telomerase). This inhibition is mediated by suppressing the c-Myc transcription factor, which binds to the hTERT promoter [63]. In addition, some Lingzhi-derived compounds stabilize G-quadruplex structures in telomeric DNA, further inhibiting telomerase activity and reducing the ability of cancer cells to maintain their telomere length [26,64,65].
The Chinese herbal prescription Xiaoji decoction (XJD), which contains 15 g of Yunzhi, has been traditionally used for cancer therapy. Wu et al. reported that XJD effectively inhibited the growth of NSCLC cells through the ERK1/2-mediated reciprocal repression of HOTAIR and SP1 protein expression, subsequently leading to reduced EP4 gene expression. XJD also displayed synergistic antitumor effects with gefitinib in drug-resistant NSCLC cells, both in vitro and in vivo [61].
4. Involvement of Lingzhi/Yunzhi-Mediated Antitumor Immunity
4.1. Immunological Characteristics of EGFR-Mutated NSCLC
The TME of EGFR-mutated NSCLC is characterized by a highly immunosuppressive and complex network of cells, cytokines, and signaling pathways that hinder effective antitumor immune responses (Figure 4) [5,66]. Compared to tumors harboring wild-type EGFR, EGFR-driven NSCLC tumors exhibit low tumor immunogenicity, including reduced TMB, fewer neoantigens, impaired T cell clonality, and lower PD-L1 expression.
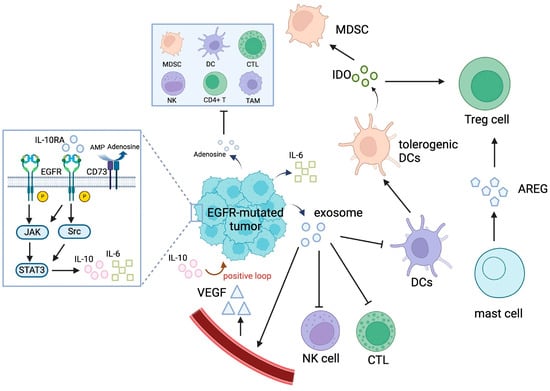
Figure 4.
The immunosuppressive TME in EGFR-mutated NSCLC. Abbreviations: AMP, adenosine monophosphate; AREG, amphiregulin; CTL, cytotoxic T lymphocyte; DC, dendritic cell; GSK-3β, glycogen synthase kinase 3β; IDO, indoleamine 2,3-dioxygenase; IL-10, interleukin 10; IL-6, interleukin 6; MDSC, myeloid-derived suppressor cell; NK, natural killer; TAM, tumor-associated macrophage; TIL, tumor-infiltrating lymphocyte; TME, tumor microenvironment; Treg, regulatory T cell; VEGF, vascular endothelial growth factor.
4.1.1. Low Tumor Immunogenicity
EGFR-mutated tumors typically have a lower TMB compared to EGFR wild-type tumors, resulting in fewer neoantigens and reduced immune recognition [67,68]. Moreover, EGFR-mutated tumors show impaired T cell expansion and weaker clonal diversity, reducing the ability of T cells to mount an effective antitumor response [69]. Furthermore, although EGFR mutations may upregulate PD-L1 through intrinsic signaling pathways (e.g., PI3K/AKT/mTOR, JAK/STAT), PD-L1 expression in EGFR-mutated tumors is generally lower than in EGFR wild-type tumors [70].
4.1.2. EGFR-Mutated Tumors Are Infiltrated with Immunosuppressive Immune Cells
EGFR activation promotes the infiltration and activation of immunosuppressive regulatory T cells (Tregs), which suppress antitumor immunity [71,72]. Despite the presence of dendritic cells (DCs) in EGFR-mutated tumors, their maturation is suppressed by the IL-6/STAT3 signaling pathway, leading to impaired antigen presentation [6,73]. Moreover, tumor-associated macrophages (TAMs) in EGFR-mutated NSCLC exhibit an immunosuppressive M2 phenotype, secreting factors like IL-10 and VEGF, which promote tumor progression and immune evasion [6,72]. In addition, EGFR-mutated tumors often lack the sufficient infiltration of cytotoxic CD8+ T cells, and the T cells present are frequently inactivated or dysfunctional [74,75]. Furthermore, natural killer (NK) cells are less abundant and phenotypically anergic (i.e., lacking the ability to react to antigens) in EGFR-mutated tumors, partly due to high levels of TGF-β in the TME [76,77].
4.1.3. EGFR-Mutated NSCLC Secretes Immunosuppressive Cytokines
IL-6 and IL-10 are known to be upregulated in EGFR-mutated tumors with acquired resistance to TKIs [73,76]. These immunosuppressive cytokines impair antitumor immunity by promoting Treg and myeloid-derived suppressor cell (MDSC) recruitment and inhibiting T and NK cells. EGFR-mutated tumors with activated EGFR signaling are known to secrete VEGF, thereby promoting angiogenesis and creating an immunosuppressive TME [6,78]. Moreover, EGFR activation has been reported to synergize with TGF-β signaling to promote EMT and suppress T cell infiltration [76,77]. Furthermore, the overexpression of CD73 in EGFR-mutated tumors is known to increase adenosine levels, thereby suppressing CD8+ T cell and NK cell activity [79,80]. Recently, exosomes secreted from EGFR-mutated tumors were shown to contain oncogenic EGFR and various immunosuppressive factors, which induce the apoptosis of CD8+ T cells, impair DC function, and promote Treg development [6,81].
4.1.4. EGFR-TKI Treatment Reinforces the Immunosuppressive TME in EGFR-Mutated Tumors
Recent research has revealed a dynamic shift toward a more immunosuppressive TME in EGFR-mutated tumors upon EGFR-TKI therapy [73]. When treatment-naïve EGFR-mutated NSCLC was treated with TKIs, the initial treatment response was accompanied by an increase in cytotoxic CD8+ T cell infiltration but a reduction in immunosuppressive Tregs and M2-like TAMs [6,73,74]. EGFR-TKIs also upregulated MHC class I molecules on tumor cells, improving antigen presentation to T cells and enhancing immune recognition [74,76]. Initial treatment with EGFR-TKIs also reduced levels of immunosuppressive cytokines such as IL-6 and increased levels of interferon-gamma (IFN-γ), which supported immune activation [6,73]. However, this immune-permissive TME was only transient and gradually shifted to a more immunosuppressive state as treatment continued.
Intriguingly, upon extended EGFR-TKI treatment, EGFR-mutated tumors developed an immunosuppressive TME. There was an accumulation of MDSCs in the TME after prolonged EGFR-TKI therapy, which suppressed T cell activity [73]. Continuous EGFR-TKI treatment also increased the levels of IL-10 and CCL2, both of which contribute to an immunosuppressive environment [73]. While intratumoral CD8+ T cell infiltration initially increased, prolonged EGFR-TKI treatment led to decreased T cell density and activity, particularly in tumors with low PD-L1 expression [74]. Moreover, sustained EGFR-TKI therapy may also result in T cell exhaustion, reducing their ability to sustain effective antitumor immune responses [73].
4.2. Immunomodulatory Effects of Lingzhi/Yunzhi and Their Potential Role in Treating EGFR-Mutated NSCLC
The stimulatory effect of Lingzhi/Yunzhi on host immune systems plays a vital role in their antitumor effects (Figure 5). As there are overwhelmingly more investigations into the antitumor effect of Lingzhi than Yunzhi in the literature, most examples described below are devoted to Lingzhi. The relevance of EGFR-mutated tumors is discussed.
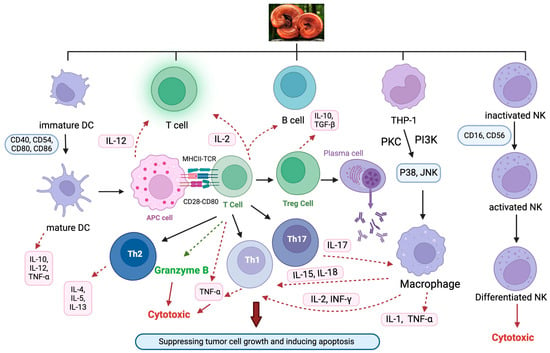
Figure 5.
The immunomodulatory effects of Ganoderma on various immune cells. Abbreviations: APCs, antigen-presenting cells; DCs, dendritic cells; IL, interleukin; IFN-γ, interferon-γ; NK, natural killer cells; PKC, protein kinase C; Th, T helper cells; TNF-α, tumor necrosis factor-α; Treg, regulatory T cells.
4.2.1. Ganoderma Boosts Antitumor Immunity
Enhancing Antitumor Activity of Macrophages
Macrophages are the most abundant immune cells bearing multifaceted roles in regulating tumor growth in the TME. They are characterized by cellular plasticity, which can switch from an anti-inflammatory/tumor-permissive (M2) to a pro-inflammatory/antitumor (M1) phenotype, depending on the changes in the TME [82]. Therefore, macrophages represent an attractive target for cancer immunotherapy. It is feasible to inhibit tumor proliferation by reprogramming the macrophages within the TME towards the antitumor M1 phenotype or preventing the adoption of the pro-tumor M2 phenotype [83].
Macrophages are known to eliminate tumor cells by phagocytosis, after recognizing the “eat-me” plasma membrane proteins on tumor cells [84]. To this end, Lingzhi has been reported to enhance the recognition of pathogens by macrophages through the increased expression of various surface receptors. Wei et al. reported that G. lucidum polysaccharides (EORP, 50–200 μg/mL) upregulated the expressions of two surface receptors on macrophages (toll-like receptor 4 (TLR4) and CD14), thus improving their ability to recognize and respond to LPS to kill tumor cells [85]. In a mouse study, G. lucidum extract and polysaccharide D6 were shown to markedly improve the phagocytic ability of peritoneal macrophages [86]. A specific bioactive proteoglycan fraction from the fruiting body of G. lucidum (GLIS) was also reported to enhance macrophage phagocytosis in tumor-bearing mice [87]. GLIS (200 mg/kg) was found to increase macrophage-mediated phagocytosis and antitumor activity in a sarcoma S180 mouse model, resulting in a 60% reduction in tumor growth [88].
Moreover, Lingzhi was also known to stimulate the secretion of pro-inflammatory cytokines from macrophages. Various Ganoderma extracts and G. lucidum spore polysaccharides (50–200 μg/mL) have been shown to promote IL-1 production in mice [89,90]. GLIS was also reported to stimulate the production of TNF-α, IL-1, and nitric oxide (NO) in the TME of tumor-bearing mice [87]. Recently, GLIS (50–200 μg/mL) was also reported to activate bone marrow-derived macrophages in tumor-bearing mice, which secreted the pro-inflammatory cytokines IL-1β, TNF-α, and NO to trigger higher antitumor activities [88]. PSG-1, a polysaccharide derived from G. atrum, was found to promote TNF-α transcription and macrophage activation via the NF-κB, PI3K/Akt, and MAPK pathways in a concentration-dependent manner (50–200 μg/mL) [91].
On the other hand, polysaccharide from Ganoderma lucidum (PS-G) was known to activate key intracellular signaling pathways (PKC, p38 MAPK, and Src-family kinases such as Lyn) in macrophages, which enhanced phagocytosis and the migration of neutrophils [92,93]. PS-G also inhibited apoptosis via Akt-regulated signaling pathways. Moreover, Ganoderma atrum polysaccharide (PSG-1) (50–200 μg/mL) was reported to activate macrophages through TLR4-dependent pathways, leading to NF-κB activation and increased cytokine production [91]. Furthermore, triterpene-rich extracts from G. lucidum (AF) (50–100 μg/mL) enhanced TNF-α production by modulating p38 and JNK MAPK pathways [94].
Lingzhi was also reported to promote the M1 polarization of macrophages to enhance antitumor immunity. Sun et al. observed that G. lucidum polysaccharides (GL-PS) promoted M1 polarization in the presence of LPS, which was accompanied by an increase in TNF-α, IL-6, and IL-12 production but a decrease in IL-10 secretion [95]. Similarly, β-glucans from G. lucidum (50–200 μg/mL) were shown to upregulate the M1 phenotype markers (e.g., IL-12, IFN-γ) in tumor-associated macrophages but reduce the M2 phenotype markers (e.g., TGF-β, IL-10) in the TME [96].
Last but not least, upon the exposure of macrophages to tert-butyl hydroperoxide and alloxan-induced oxidative damage, G. lucidum polysaccharide peptide (GLPP, 50–200 μg/mL) prevents macrophage necrosis by reducing free radical levels and preserving mitochondrial membrane potential [17,19].
Enhancing the Antitumor Activity of NK Cells
NK cells are specialized immune effector cells that play critical roles in tumor immunosurveillance by producing cytokines and secreting lytic granules [97]. Lingzhi has been shown to promote NK cell activation and differentiation of other immune cells under immunosuppressed conditions. The oral administration of crude Lingzhi extract (3–6 mg/kg) was reported to significantly increase NK cell activity in mouse models of WEHI-3 leukemia cells [98]. The extract promoted the activation and proliferation of NK and T cells and significantly reduced the abundance of leukemia cells in the spleen. Lingzhi polysaccharides were also reported to activate NK cells through TLR4 signaling [99]. Tsai et al. demonstrated that acid-hydrolyzed polysaccharide fragments of Lingzhi (GLPS-SF1 and GLPS-SF2) stimulated NK cell activation and proliferation. GLPS-SF1 (50–200 μg/mL) was found to induce the production of antitumor cytokines (IL-12, TNF-α, and IFN-γ) via TLR4 signaling. On the other hand, GLPS-SF2 (an oligosaccharide fraction; 50–200 μg/mL) was shown to promote NK and T cell activation and proliferation. The unique sugar structures of these polysaccharides were shown to mediate immune-stimulating effects [99].
Lingzhi also enhanced NK cell cytotoxicity by increasing their functional activity. Won et al. demonstrated that alcohol-insoluble components (GL-AI) of Lingzhi significantly enhanced the cytotoxic activity of spleen NK cells in C3H/HeN mice when administered through oral (100–500 mg/kg), intraperitoneal (i.p., 4–200 mg/kg), or intravenous (i.v., 1–50 mg/kg) routes. Similarly, water-soluble extracts of Ganoderma tsugae mycelium (GT) and its alcohol-insoluble fraction (GTI, 1–50 mg/kg) increased NK cell activity in a dose-dependent manner, along with elevated blood interferon (IFN) levels. Neutralization studies confirmed that both IFN-α/β and IFN-γ were critical to this enhanced NK activity [9,100]. Ning et al. reported that the intraperitoneal injection of Lingzhi polysaccharides (1 mg/day) for 7 days significantly increased NK cell activity, lymphocyte proliferation, and serum levels of TNF-α and IL-2 in tumor-bearing mice. These cytokines are essential for activating NK cells and promoting antitumor immunity [101].
Under immunosuppressive conditions, Lingzhi was shown to restore the antitumor functions of NK cells. In mice treated with the chemotherapeutic drug cyclophosphamide, low doses of Lingzhi polysaccharides (2.5 mg/kg; daily for 7 days) were found to enhance NK cell cytotoxicity, lymphokine-activated killer (LAK) cell activity, and macrophage phagocytosis [10]. Lingzhi polysaccharides also accelerated the recovery of bone marrow cells and T and B lymphocyte proliferation without side effects, suggesting its potential as an adjunct therapy to mitigate chemotherapy-induced immunosuppression [10].
Modulating T Cells to Enhance Antitumor Response
Lingzhi is known to promote antitumor immunity by modulating T cells through various mechanisms, including enhancing T cell activation, promoting the differentiation of T cell subsets, and regulating cytokine production. Studies in glioma-bearing rats demonstrated that Lingzhi polysaccharides (Gl-PS) significantly elevated these cytokines, which are critical for T cell activation and immune responses [102]. A few other studies also revealed that Gl-PS boosted the mRNA and protein expression of IL-2, IFN-γ, and TNF-α in splenic T cells while augmenting their antitumor cytotoxicity [103,104,105]. Moreover, GLE (oral administration at 200–400 mg/kg) promoted T cell receptor signaling pathways and increased CD3+, CD4+, and CD8+ T cell subsets in tumor-bearing mice [106].
Intriguingly, Lingzhi was also found to restore the balance between CD4+ helper T cells and CD8+ cytotoxic T cells. In NSCLC patients undergoing chemotherapy, Ganoderma lucidum spores (oral administration at 1.5 g/day) increased CD4+ T cell levels and the CD4+/CD8+ ratio, suggesting improved cellular immunity [107]. Similarly, in HCC patients after surgery, Lingzhi spores (1.5 g/day) were also found to elevate CD4+ T cells and restore immune homeostasis [108]. The polysaccharide purified from Ganoderma lucidum (PS-G, 10 µg/mL) was reported to promote antitumor Th1 responses by increasing IFN-γ production through dendritic cell activation and IL-12 secretion [109].
Lingzhi modulates cytokine production by promoting the release of pro-inflammatory cytokines while reducing immunosuppressive ones. Lingzhi polysaccharides (50–200 μg/mL) stimulated T lymphocytes to release IL-2 and IFN-γ, thereby enhancing T cell activation and cytotoxicity against tumor cells [110]. In tumor-bearing mice, Lingzhi extract (GLE; 200–400 mg/kg) was known to upregulate cytokines such as IFN-γ, IL-2, and TNF-α, thus producing T cell-mediated antitumor immunity [106]. Furthermore, Ganoderma lucidum polysaccharides (Gl-PS; at concentrations of 0.8, 3.2, and 12.8 µg/mL) have been reported to reduce the levels of two immunosuppressive cytokines (IL-10 and TGF-β1), which are often secreted by tumors to evade immune surveillance [11,12].
It is particularly noteworthy that Lingzhi could restore T cell function in immunosuppressed cancer patients. In chemotherapy-treated patients, Lingzhi extract (16 capsules/day) prevented reductions in CD3+, CD4+, and CD8+ T cells, which are crucial for cellular immunity [111]. Similarly, in HCC patients after surgery, Lingzhi spores (1.5 g/day) have been shown to improve T cell numbers and restore immune balance, presumably preventing tumor recurrence [108]. The metabolic or functional exhaustion of T cells represents an important drug resistance mechanism to immune checkpoint blockade therapy [112]. Recently, a water extract from sporoderm-breaking spores of Ganoderma lucidum (ESG) (oral administration, 400 mg/kg) was shown to downregulate the immune checkpoint molecules PD-1 and CTLA-4, thus restoring T cell functionality and enhancing tumor immune surveillance in tumor-bearing mice [113].
Collectively, Lingzhi modulates T cells by enhancing their activation, regulating cytokine production, balancing T cell subsets, restoring immune function in immunosuppressed conditions, and suppressing immune checkpoint molecules. Thus, Lingzhi represents a promising adjunct therapy for enhancing antitumor immunity during cancer treatment.
4.3. Potential Role of Lingzhi in EGFR-Mutated NSCLC: A Tumor Immunology Perspective
As discussed above, EGFR-mutated NSCLC is characterized by an immunosuppressive TME, leading to tumor evasion from immune surveillance. Accumulating evidence suggests that Lingzhi could be used as an immunomodulatory adjuvant in EGFR-mutated NSCLC to enhance therapeutic outcomes. By enhancing dendritic cell function, activating T cells and NK cells, suppressing immunosuppressive cells, and regulating cytokines, the integration of Lingzhi into existing therapies could enhance immune responses, reduce resistance, and improve clinical outcomes for patients with EGFR-mutated NSCLC [11,109,113]. In addition, Lingzhi was also found to promote macrophage polarization toward the M1 phenotype and facilitate antitumor immunity, but it inhibited pro-tumor M2 macrophage polarization [95].
In EGFR-mutated NSCLC, immunosuppressive cytokines such as IL-6, VEGF, and TGF-β are elevated, which contribute to immune evasion and tumor progression. Lingzhi was able to restore immune balance by increasing pro-inflammatory cytokines like IFN-γ, IL-12, and TNF-α, which activate cytotoxic T cells and NK cells while decreasing immunosuppressive cytokines like IL-10, TGF-β, and VEGF [11,12,106]. By modulating cytokine levels, Lingzhi creates a more favorable immune environment for antitumor activity.
NK cells play a critical role in tumor suppression but are often phenotypically inactive in EGFR-mutated NSCLC due to high levels of TGF-β in the TME [114]. Lingzhi has been shown to increase NK cell numbers, boost their cytotoxic functions by increasing perforin and granzyme production, and restore NK cell effectiveness, which is critical for overcoming immune evasion [9,10,100].
The adenosine signaling pathway, driven by CD73 overexpression, is a key immunosuppressive mechanism against T cells and NK cells in EGFR-mutated NSCLC [78]. Lingzhi has been shown to downregulate CD73 expression and reduce adenosine levels, thereby restoring antitumor immunity [12,115].
EGFR-mutated NSCLC patients generally respond poorly to immune checkpoint inhibitors (ICIs) due to the immunosuppressive TME [116]. Lingzhi’s immunomodulatory properties, including enhanced T cell infiltration, reduced Tregs and MDSCs, and cytokine modulation, may improve the efficacy of ICIs. These effects position Lingzhi as a potential adjuvant therapy to overcome resistance and enhance immune responses in EGFR-mutated NSCLC [106,113]. While EGFR TKIs are standard treatment for EGFR-mutated NSCLC, their prolonged use can lead to an immunosuppressive TME [117]. Lingzhi may complement EGFR TKIs by enhancing immune surveillance, preventing the accumulation of suppressive immune cells like MDSCs, and maintaining an active immune response [11,113]. Therefore, Lingzhi may be used as an adjuvant to facilitate the efficacious antitumor combination of EGFR TKIs and immune checkpoint blockade therapy. Recently, Lingzhi was also reported to counteract other immunosuppressive mechanisms in EGFR-mutated NSCLC, such as exosome-mediated immune modulation and impaired antigen presentation. By enhancing MHC-I expression and reducing T cell apoptosis, Lingzhi could improve tumor immune recognition [11,103]. Furthermore, the capacity of Lingzhi to modulate immune cell markers and cytokine profiles may reduce the risk of hyperprogressive disease, which is characterized by a paradoxical rapid tumor growth after initial treatment with PD-1/PD-L1 immunotherapy [118].
5. Pharmacokinetics Consideration
5.1. Potential Lingzhi–EGFR TKI Interaction via Drug Transporters
EGFR-TKIs are substrates of key drug transporters including P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and multidrug resistance proteins (MRPs) [119]. These transporters restrict drug accumulation in various organs/tissues, such as the brain. The effects of Lingzhi on various drug transporters are summarized in Table 4. Cao et al. conducted a detailed investigation on the effects of Ganoderma lucidum extract (GLE), Ganoderma lucidum polysaccharides (GLP), or Ganoderma lucidum triterpenoids (GLT) (in particular, ganoderic acids; GAAs) on the transport activity of P-gp, MRP, and BCRP [119]. The results from the cellular uptake experiment showed that GLE (100 μg/mL) could significantly increase the cellular accumulation of P-gp or MRP substrate (Rhodamine 123 (Rho) and Calcein (Cal), respectively) by inhibiting the efflux transporter functions. In contrast, GLE was found to significantly decrease the cellular accumulation of BCRP substrate Hoechst 33342 (Hoe) by inducing BCRP transport activity. On the other hand, GLP did not show any significant effects on P-gp or MRP but induced the drug efflux activity of BCRP. GAA did not affect the activity of the three efflux transporters at the experimental concentrations [119].

Table 4.
Effects of Lingzhi on various ABC drug transporters.
Using a bidirectional Caco-2 permeability assay, the transport of specific transporter probe substrate (Rho or Cal) was measured in the apical to the basolateral direction (AP→BL) in the presence or absence of GLE [119]. The apparent permeability (Papp A-B) values could be calculated to assess the inhibition of specific drug transporters by GLE. GLE was shown to significantly increase (AP→BL) transport as well as Papp A-B values for the P-gp substrate (Rho) and MRP substrate (Cal), suggesting its inhibition of P-gp/MRP-mediated drug efflux [119]. Among all EGFR-TKIs, osimertinib displays the lowest efflux ratio, suggesting that it is less affected by drug transporters [123]. While gefitinib, erlotinib, afatinib, and osimertinib are all considered to be P-gp substrates in vitro, patient data indicated that clinically relevant drug–drug interactions are only prominent for the second-generation EGFR TKI afatinib [124].
The overexpression of ABC transporters in cancer cells is known to mediate MDR to numerous chemotherapeutic drugs [125]. Recently, a triterpenoid (Ganoderiol F) isolated from Lingzhi was reported to inhibit P-gp and reverse P-gp-mediated multidrug resistance by increasing the intracellular accumulation of a P-gp substrate anticancer drug doxorubicin [126]. While EGFR-TKIs are known substrates for major ABC drug transporters, the circumvention of ABC transporter-mediated drug resistance to EGFR-TKIs by Lingzhi/Yunzhi in experimental cancer models has not been reported.
5.2. Potential Lingzhi/Yunzhi-EGFR TKI Interaction via Metabolic Enzymes
The four clinically approved EGFR-TKIs (gefitinib, erlotinib, afatinib, and osimertinib) are metabolized by cytochrome P450 (CYP) enzymes, particularly CYP3A4, thus making them susceptible to drug–drug interactions involving CYP modulators [124]. The potential modulatory effect of Lingzhi/Yunzhi on various CYP enzymes is summarized in Table 5. While Lingzhi/Yunzhi polysaccharides (such as GLP and PSP) display limited CYP inhibition, the triterpenoids component (e.g., ganoderic acid A (GAA)) has been reported to exhibit a more significant CYP inhibitory effect [127]. In general, the likelihood of herb–drug interactions appears low for most Lingzhi/Yunzhi preparations but may be clinically relevant for drugs metabolized by CYP3A4 or when using concentrated triterpenoids like GAA.

Table 5.
Modulation of CYP isoenzymes by Lingzhi/Yunzhi.
In human liver microsomes (HLMs), GLE exhibited weak inhibitory effects on CYP2C19, 2D6, 3A4, and 2C9 with IC50 values of 131.2, 164.4, 150.5, and 142.2 μg/mL, respectively. The inhibitory effect of GLT was slightly stronger than that of GLE with IC50 values of 102.5, 116.1, 136.4, and 82.2 μg/mL, respectively [119]. On the other hand, GLP did not significantly affect the six CYP450 enzymes. In rat liver microsomes (RLM), GLT showed a weak inhibitory effect on CYP2C9 with an IC50 value of 163.1 μg/mL but did not affect the activity of other enzymes. In a Sprague Dawley rat study, the inhibitory effect of GLP on CYP1A2, CYP3A4, and CYP2E1 was evaluated by analyzing the metabolism of the corresponding enzyme substrate drugs (phenacetin, nifedipine, and chlorzoxazone, respectively) [128]. GLP was shown to inhibit the three CYP enzymes in a concentration-dependent manner, suggesting possible herb–drug interactions between GLP and other concomitantly administered CYP substrate drugs.
GAA inhibited the activity of CYP3A4, 2D6, and 2E1 but did not affect other isoforms. The inhibition of CYP3A4, 2D6, and 2E1 was concentration-dependent with IC50 values of 15.05, 21.83, and 28.35 μM, respectively. Additionally, GAA was not only a non-competitive inhibitor of CYP3A4 but also a competitive inhibitor of CYP2D6 and 2E1, with Ki values of 7.16, 10.07, and 13.45 μM. Meanwhile, the inhibition of CYP3A4 was time-dependent, with a KI /Kinact value of 7.91/0.048 μM/min. This in vitro study indicated that GAA has the potential to result in drug–drug interactions with other drugs metabolized by CYP3A4, 2D6, and 2E1. Further clinical studies are needed for the identification of this interaction [130].
Polysaccharide peptide (PSP), isolated from the COV-1 strain of Coriolus versicolor (Yunzhi), can competitively inhibit tolbutamide 4-hydroxylation in both pooled human liver microsomes and specific human CYP2C9 in vitro. However, considering the comparatively high Ki values observed for PSP interaction with human CYP2C9, it is unlikely to significantly contribute to herb–drug interactions [129,132]. “I’m-Yunity” is a herbal supplement produced from a specific strain of Yunzhi. PSP is believed to be the active ingredient in I’m-Yunity. To assess the impact of I’m-Yunity on CYP3A4, Nicandro et al. conducted an open-label study involving 12 healthy adult volunteers, providing participants with a 14-day supply of I’m-Yunity and instructing them to take 1200 mg thrice daily with meals. The results indicated that the short-term use of I’m-Yunity for 14 days did not lead to any significant inhibition or induction of CYP3A4. The concurrent administration of I’m-Yunity with medications primarily metabolized by CYP3A4 is unlikely to result in significant herb–drug interactions [131].
Collectively, Lingzhi exhibits pharmacokinetic interactions with EGFR-TKIs by modulating efflux transporters and CYP enzymes, potentially enhancing drug efficacy but requiring careful monitoring to avoid adverse effects. On the other hand, Yunzhi offers a lower risk profile for pharmacokinetic interactions, making it an attractive complementary therapy. Further clinical studies are necessary to validate these findings and optimize the use of Lingzhi and Yunzhi in combination with EGFR-TKIs for improved cancer treatment outcomes.
6. Conclusions and Future Perspectives
This review highlights the significant potential of Lingzhi and Yunzhi as complementary therapeutic agents in managing EGFR-mutated NSCLC. The bioactive components in Lingzhi and Yunzhi, such as polysaccharides and triterpenoids, exhibit multifaceted antitumor properties. These include the inhibition of oncogenic signaling pathways (e.g., PI3K/AKT/mTOR, ERK, Wnt/β-catenin), suppression of EMT, reactivation of tumor suppressors (p53), and reversal of multidrug resistance [14,15,56]. Moreover, the immunomodulating effects of Lingzhi/Yunzhi, including enhanced activity of T cells and NK cells, reduced Tregs and MDSCs, and suppression of immunosuppressive cytokines (e.g., IL-6, VEGF, and TGF-β) further support their potential to complement immune checkpoint blockade therapy in EGFR-mutated NSCLC [9,10,11,12].
Despite these promising findings, challenges and knowledge gaps remain. Current preclinical and limited clinical studies highlight the efficacy of Lingzhi and Yunzhi, but large-scale, randomized clinical trials are needed to confirm their safety, efficacy, and optimal integration with EGFR-TKIs and ICIs in NSCLC treatment. Importantly, potential pharmacokinetic interactions between these medicinal mushrooms and conventional therapies require further exploration to ensure safe and effective combination regimens [15,131,133]. Lingzhi, with its triterpenoids, inhibits key efflux transporters like P-gp and MRPs, which could increase the tissue retention of concurrently administered antitumor drugs [13,119]. Moreover, Lingzhi also exhibits mild inhibitory effects on CYP enzymes (e.g., CYP3A4, CYP2D6), which may alter drug metabolism and clearance of co-administered EGFR-TKIs [130]. In contrast, the bioactive ingredient of Yunzhi (polysaccharopeptides, PSP) exhibits negligible effects on drug transporters and CYP enzymes, offering a safer pharmacokinetic profile [129,131].
Future research should prioritize well-designed clinical trials to validate the efficacy and safety of Lingzhi and Yunzhi as adjuvants in NSCLC treatment. An excellent systematic review of clinical studies investigating medicinal mushroom supplements for cancer therapy has been published recently [134]. There were 12 different mushroom preparations reported in the 39 trials included in that systematic review. In two hepatocellular carcinoma studies [135,136] and one breast cancer study [137] investigating Huaier granules (Trametes robiniophila Murr), patient survival benefit was reported. In combination with chemotherapy, polysaccharide K (PSK) was also shown to produce survival benefit in four gastric cancer studies [138,139,140,141]. Some of these trials only investigate the antitumor effect of the mushrooms alone, not their combinations with other chemotherapeutic drugs. Moreover, the sample size was small in some trials, and they did not use a randomized controlled trial design. Therefore, the evidence so far is inconclusive to recommend the routine use of medicinal mushrooms for treating cancer patients. These trials should evaluate key clinical endpoints, such as progression-free survival (PFS), overall survival (OS), and quality of life (QoL), in patients receiving combinations of EGFR-TKIs and these medicinal mushrooms. Indeed, a multicenter, randomized, double-blind, placebo-controlled clinical trial (China Clinical Trial Registry ChiCTR2300072786) is currently recruiting patients with an aim to investigate the potential synergistic antitumor effect of de-walled Ganoderma Lucidum spore powder on targeted therapy in advanced NSCLC patients [142]. Further mechanistic studies are needed to elucidate the molecular interactions between Lingzhi/Yunzhi and EGFR pathways, resistance mechanisms (e.g., MET/HER2 amplification), the TME, and systemic immunity.
Moreover, personalized approaches should be explored, focusing on genetic polymorphisms in metabolic enzymes and drug transporters to predict individual patient responses to Lingzhi/Yunzhi-based combination therapy with osimertinib. Predictive biomarkers of therapeutic response should also be identified to optimize treatment for specific patient subgroups. To this end, a recent clinical study demonstrated that a few single nucleotide polymorphisms (SNPs) in CYP enzymes (CYP2A6*4 and CYP2C9*3) were associated with the increased incidence of adverse drug reactions and also reduced median PFS in Thai patients with NSCLC on osimertinib therapy [143]. Intriguingly, another recent clinical study revealed that SNPs of two efflux transporters (ABCB1 3435C>T and ABCG2 34G>A) were significantly correlated with the development of brain metastases in patients with NSCLC on osimertinib therapy, probably due to diminished intracerebral drug levels [144]. On the other hand, the result from a retrospective observational multicenter study showed that the ABCG2 421C>A genotype was associated with a higher incidence of diarrhea in patients with NSCLC on osimertinib therapy [145]. As Lingzhi and Yunzhi may modulate the pharmacokinetic properties of osimertinib via these CYP enzymes and/or ABC transporters, pharmacogenetic testing may facilitate the selection of a patient subgroup to benefit from the herb–drug combination. In addition, combination regimens involving Lingzhi/Yunzhi, EGFR-TKIs, ICIs, or other targeted therapies should be evaluated. Such synergistic approaches may overcome drug resistance, enhance antitumor immunity, reduce toxicities, and improve outcomes in EGFR-mutated NSCLC.
Standardization and quality control of Lingzhi and Yunzhi products are critical to ensure consistent pharmacological effects in clinical applications. Future research should focus on identifying active components, optimizing extraction methods, and maintaining rigorous quality standards [146,147]. Given their broad-spectrum antitumor and immunomodulatory effects, Lingzhi/Yunzhi may be applicable to other EGFR-driven malignancies, such as head and neck cancers and inflammatory breast cancer [14,15,23].
In conclusion, Lingzhi and Yunzhi represent a promising advancement in integrative cancer therapy, particularly for addressing treatment resistance and immunosuppression in EGFR-mutated NSCLC. Their successful clinical integration will require concerted efforts in clinical research, standardization, and mechanistic studies to ensure efficacy, safety, and quality as adjuvant therapies.
Author Contributions
Conceptualization, K.K.W.T., Z.Z., W.C.C. and Y.W.C.; writing—original draft preparation, H.Z.; writing—review and editing, H.Z., L.W., Y.W.C., W.C.C., Z.Z. and K.K.W.T.; supervision, K.K.W.T.; project administration, K.K.W.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Food and Health Bureau HKSAR (Health and Medical Research Fund 21223421), Guangdong-Hong Kong-Macao New Drug Screening Joint Laboratory (Project 8326200), and Institute of Chinese Medicine CUHK (SKL Open Fund SKLOF-X).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Conflicts of Interest
There are no conflicts of interest to report for this work.
References
- Leonetti, A.; Sharma, S.; Minari, R.; Perego, P.; Giovannetti, E.; Tiseo, M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br. J. Cancer 2019, 121, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Hou, Z.; Zhang, Y.; Jiang, B. Drug resistance mechanisms and progress in the treatment of EGFR-mutated lung adenocarcinoma. Oncol. Lett. 2022, 24, 408. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Gu, Z.; Yu, X.; Cheng, T.; Liu, B. Research progress on the role of bypass activation mechanisms in resistance to tyrosine kinase inhibitors in non-small cell lung cancer. Front. Oncol. 2024, 14, 1447678. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zeng, L.; Huang, Z.; Ruan, Z.; Yan, H.; Zou, C.; Xu, S.; Zhang, Y. Strategies beyond 3rd EGFR-TKI acquired resistance: Opportunities and challenges. Cancer Med. 2025, 14, e70921. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.W.; Fong, W.; Cho, W.C.S. Immunotherapy in treating EGFR-mutant lung cancer: Current challenges and new strategies. Front. Oncol. 2021, 11, 635007. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Sha, H.; Qin, X.; Chen, Y.; Li, X.; Shi, M.; Feng, J. EGFR E746-A750 deletion in lung cancer represses antitumor immunity through the exosome-mediated inhibition of dendritic cells. Oncogene 2020, 39, 2643–2657. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Qiu, W.L.; Tsao, S.M.; Tseng, A.J.; Lu, M.K.; Hua, W.J.; Cheng, H.C.; Hsu, H.Y.; Lin, T.Y. Effects of WSG, a polysaccharide from Ganoderma lucidum, on suppressing cell growth and mobility of lung cancer. Int. J. Biol. Macromol. 2020, 165 Pt A, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, L. Cellular and molecular mechanism of Ganoderma (Lingzhi) against tumor. Adv. Exp. Med. Biol. 2019, 1182, 79–118. [Google Scholar] [PubMed]
- Won, S.J.; Lee, S.S.; Ke, Y.H.; Lin, M.T. Enhancement of splenic NK cytotoxic activity by extracts of Ganoderma lucidum mycelium in mice. J. Biomed. Lab. Sci. 1989, 2, 201–213. [Google Scholar]
- Zhu, X.L.; Chen, A.F.; Lin, Z.B. Ganoderma lucidum polysaccharides enhance the function of immunological effector cells in immunosuppressed mice. J. Ethnopharmacol. 2007, 11, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.X.; Lin, Z.B.; Duan, X.S.; Qi, H.H.; Yang, N.; Li, M.; Xing, E.H.; Sun, Y.; Yu, M.; Li, W.D.; et al. Suppression of the production of transforming growth factor β1, interleukin-10, and vascular endothelial growth factor in the B16F10 cells by Ganoderma lucidum polysaccharides. J. Interferon Cytokine Res. 2014, 34, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.X.; Li, W.D.; Lin, Z.B.; Duan, X.S.; Xing, E.H.; Jiang, M.M.; Yang, N.; Qi, H.H.; Sun, Y.; Li, M.; et al. Cytokine production suppression by culture supernatant of B16F10 cells and amelioration by Ganoderma lucidum polysaccharides in activated lymphocytes. Cell Tissue Res. 2015, 360, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.R.; Huo, X.K.; Dong, P.P.; Wang, C.; Huang, S.S.; Zhang, B.J.; Zhang, H.L.; Deng, S.; Liu, K.X.; Ma, X.C. Inhibitory effects of highly oxygenated lanostane derivatives from the fungus Ganoderma lucidum on P-glycoprotein and α-glucosidase. J. Nat. Prod. 2015, 78, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.R.; Hu, C.T.; You, R.I.; Ma, P.L.; Pan, S.M.; Lee, M.C.; Wu, W.S. Preclinical trials for prevention of tumor progression of hepatocellular carcinoma by LZ-8 targeting c-Met dependent and independent pathways. PLoS ONE 2015, 10, e0114495. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Hsu, H.Y.; Sun, W.H.; Wu, T.H.; Tsao, S.M. Induction of Cbl-dependent epidermal growth factor receptor degradation in Ling Zhi-8 suppressed lung cancer. Int. J. Cancer 2017, 140, 2596–2607. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Yan, P.; Lam, W.C.; Yao, L.; Bian, Z. Coriolus versicolor and Ganoderma lucidum related natural products as an adjunct therapy for cancers: A systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 2019, 10, 703. [Google Scholar] [CrossRef] [PubMed]
- You, Y.H.; Lin, Z.B. Protective effects of Ganoderma lucidum polysaccharides peptide on injury of macrophages induced by reactive oxygen species. Acta Pharmacol. Sin. 2002, 23, 787–791. [Google Scholar] [PubMed]
- You, Y.H.; Lin, Z.B. Free radical scavenging activity by Ganoderma lucidum polysaccharides peptide on peritoneal macrophages in mice. Chin. J. Clin. Pharmacol. Ther. 2004, 9, 52–55. [Google Scholar]
- You, Y.H.; Lin, Z.B. Influence of Ganoderma lucidum polysaccharides peptide on free radical induced peritoneal macrophage injury in early stage. Chin. J. Pharmacol. Toxicol. 2004, 19, 137–139. [Google Scholar]
- Stanley, G.; Harvey, K.; Slivova, V.; Jiang, J.; Sliva, D. Ganoderma lucidum suppresses angiogenesis through the inhibition of secretion of VEGF and TGF-β1 from prostate cancer cells. Biochem. Biophys. Res. Commun. 2005, 330, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.Z.; Lin, Z.B. Ganoderma lucidum polysaccharides peptide inhibits the growth of vascular endothelial cell and the induction of VEGF in human lung cancer cell. Life Sci. 2006, 78, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.Y.; Liu, J.J.; Sun, X.F.; Wang, N. Ganoderma lucidum inhibits proliferation of human ovarian cancer cells by suppressing VEGF expression and up-regulating the expression of connexin 43. BMC Complement. Altern. Med. 2014, 14, 434. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Arroyo, I.J.; Rios-Fuller, T.J.; Feliz-Mosquea, Y.R.; Lacourt-Ventura, M.; Leal-Alviarez, D.J.; Maldonado-Martinez, G.; Cubano, L.A.; Martinez-Montemayor, M.M. Ganoderma lucidum combined with the EGFR tyrosine kinase inhibitor, erlotinib synergize to reduce inflammatory breast cancer progression. J. Cancer 2016, 7, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Vendrell, J.A.; Quantin, X.; Aussel, A.; Solassol, I.; Serre, I.; Solassol, J. EGFR-dependent mechanisms of resistance to osimertinib determined by ctDNA NGS analysis identify patients with better outcome. Transl. Lung Cancer Res. 2021, 10, 4084–4094. [Google Scholar] [CrossRef] [PubMed]
- Zalaquett, Z.; Hachem, M.C.R.; Kassis, Y.; Hachem, S.; Eid, R.; Kourie, H.R.; Planchard, D. Acquired resistance mechanism to osimertinib: The constant battle. Cancer Treat. Rev. 2023, 116, 102557. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.C.; Shen, Y.C.; Kao, M.C.; Mathew, D.C.; Karuppaiya, P.; Li, M.L.; Yang, H.L. Ganoderma tsugae induced ROS-independent apoptosis and cytoprotective autophagy in human chronic myeloid leukemia cells. Food Chem. Toxicol. 2019, 124, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Hua, W.J.; Qiu, W.L.; Tseng, A.J.; Cheng, H.C.; Lin, T.Y. WSG, a glucose-enriched polysaccharide from Ganoderma lucidum, suppresses tongue cancer cells via inhibition of EGFR-mediated signaling and potentiates cisplatin-induced apoptosis. Int. J. Biol. Macromol. 2021, 193, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Fan, Q.; Liu, Z.; Zhang, S.; Huang, W.; Li, H.; Liang, C.; Sun, F. An epitope on EGFR loading catastrophic internalization serves as a novel oncotarget for hepatocellular carcinoma therapy. Cancers 2020, 12, 456. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.W.; Ye, Z.J.; Xu, Y.M.; Zhang, J.; Tang, J.S. Ergosta-7,22-diene-2β,3α,9α-triol (EGDT) from Ganoderma lucidum inhibits nasopharyngeal carcinoma cells by blocking EGFR signaling pathway. Chin. Herb. Med. 2018, 10, 27–33. [Google Scholar] [CrossRef]
- Hsu, S.C.; Ou, C.C.; Chuang, T.C.; Li, J.W.; Lee, Y.J.; Wang, V.; Liu, J.Y.; Chen, C.S.; Lin, S.C.; Kao, M.C. Ganoderma tsugae extract inhibits expression of epidermal growth factor receptor and angiogenesis in human epidermoid carcinoma cells: In vitro and in vivo. Cancer Lett. 2009, 281, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Mahran, Y.F.; Hassan, H.M. Ganoderma lucidum prevents cisplatin-induced nephrotoxicity through inhibition of epidermal growth factor receptor signaling and autophagy-mediated apoptosis. Oxid. Med. Cell. Longev. 2020, 2020, 4932587. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.L.; Lo, H.C.; Lu, M.K.; Lin, T.Y. Significance of culture period on the physiochemistry and anti-cancer potentials of polysaccharides from mycelia of Ganoderma lucidum. Int. J. Biol. Macromol. 2023, 242 Pt 4, 125181. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qiu, F.; Wang, Y.; Liang, F.; Liang, J.; Lin, C.; Liang, J.; Gong, B.; Chan, S.; De Zhang, Z.; et al. A recombinant protein rLZ-8, originally extracted from Ganoderma lucidum, ameliorates OVA-induced lung inflammation by regulating Th17/Treg balance. J. Leukoc. Biol. 2020, 108, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Taniguchi, M.; Baba, K. Antitumor and antimetastatic effects on liver of triterpenoid fractions of Ganoderma lucidum: Mechanism of action and isolation of an active substance. Anticancer Res. 2002, 22, 3309–3318. [Google Scholar] [PubMed]
- Guo, P.R.; Sheng, Y.W.; Liu, B.; Wang, C.; Liu, Y.D.; Fu, D.W.; Wang, C.C. Influence of Ganoderma lucidum polysaccharide on the inhibitory effects of cisplatin on the tumor growth and angiogenesis in bladder cancer (T24) cells-bearing nude mice. Med. J. Chin. PLA 2014, 39, 470–474. [Google Scholar]
- Sadava, D.; Still, D.W.; Mudry, R.R.; Kane, S.E. Effect of Ganoderma on drug-sensitive and multidrug-resistant small-cell lung carcinoma cells. Cancer Lett. 2009, 277, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lu, J.; Duan, X.S.; Qi, H.H.; Sun, L.X. Inhibitory Effects of Ganoderma Lucidum Polysaccharides on VEGF Secretion in B16F10 Melanoma Cells. J. Chengde Med. Coll. 2017, 34, 276–278. [Google Scholar]
- Chen, Y.H.; Zou, J.J.; Li, S.B.; Huang, G.R.; Zheng, Z.L.; Dong, Y.X.; Yang, M.X.; Luo, Y.H. The inhibiting effects of Ganoderma lucidum polysaccharides on proliferation and migration of HepG2 liver cancer cells. Guangdong Med. J. 2018, 39, 1625–1628. [Google Scholar]
- Sun, L.; Hua, C.Y.; Hou, Y.Y. Effect of glossy Ganoderma spore oil on human hepatoma cell line HepG2. J. Pract. Oncol. 2011, 26, 128–133. [Google Scholar]
- Song, Y.X.; Dou, H.; Liu, X.Q.; Gong, W.; Hou, Y.Y. The effects of Ganoderma lucidum spore oil on MCF-7 cell apoptosis and migration. Inf. Tradit. Chin. Med. 2011, 28, 91–94. [Google Scholar]
- Wang, Y.P.; Zhao, G.F.; Liu, L.; Li, P.F.; Hou, Y.Y. Glossy Ganoderma spore oil induced apoptosis of human lung adenocarcinoma LTEP-a2 cells through up-regulating miR-16 expression. Herald Med. 2011, 30, 570–573. [Google Scholar]
- Zhang, J.; Niu, M.M.; Yang, L.; Fan, L.S.; Wu, L.; Zhan, J.; Zhang, H.Q. Effects of Ganoderma lucidum spore oil and Ganoderma lucidum extraction spore oil on angiogenesis regulatory factors. Acta Anat. Sin. 2014, 45, 525–530. [Google Scholar]
- Wang, X.Y.; Chen, G.Y.; Su, J.; Lv, G.Y.; Chen, S.H. Effects of Ganoderma lucidum spore powders and broken Ganoderma lucidum spore powders on tumor growth and VEGF expression of mice with Lewis lung cancer. Pharmacol. Clin. Chin. Mater. Med. 2017, 33, 118–122. [Google Scholar]
- Wang, X.J.; Xu, J.P.; Cheng, Y.F. Inhibitory and antiangiogenetic effects of lucid Ganoderma spore on the human hepatocarcinoma transplanted in nude mice. Acta Acad. Med. Xuzhou 2006, 26, 115–119. [Google Scholar]
- Bian, S.; Xu, L.; Yu, Y.; Dun, W.L. LZBZY’s regulatory effect on the growth of H22 tumor and the expression of VEGF in transplanted H22 tumor of mice. Anhui Med. Pharm. J. 2007, 11, 1067–1068. [Google Scholar]
- El-Khashab, I. Antiangiogenic and proapoptotic activities of atorvastatin and Ganoderma lucidum in tumor mouse model via VEGF and caspase-3 pathways. Asian Pac. J. Cancer Prev. 2021, 22, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Jędrzejewski, T.; Pawlikowska, M.Ł.; Sobocińska, J.; Wrotek, S. Protein-bound polysaccharides from Coriolus versicolor fungus disrupt the crosstalk between breast cancer cells and macrophages through inhibition of angiogenic cytokines production and shifting tumour-associated macrophages from the M2 to M1 subtype. Cell. Physiol. Biochem. 2020, 54, 615–628. [Google Scholar] [PubMed]
- Ho, J.; Konerding, M.; Gaumann, A.; Groth, M.; Liu, W. Fungal polysaccharopeptide inhibits tumor angiogenesis and tumor growth in mice. Life Sci. 2004, 75, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hou, Y.Y.; Zhang, W.Y.; Han, X.D. Research on the antitumor actions of extracts from the fruiting body of Coriolus versicolor. J. Med. Postgrad. 2004, 17, 413–415+419. [Google Scholar]
- Zhang, Y. Ganoderma lucidum (Reishi) suppresses proliferation and migration of breast cancer cells via inhibiting Wnt/β-catenin signaling. Biochem. Biophys. Res. Commun. 2017, 488, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Li, S.; Zhuo, Y.; Chen, J.; Qin, X.; Guo, G. Anticancer effect of triterpenes from Ganoderma lucidum in human prostate cancer cells. Oncol. Lett. 2017, 14, 7467–7472. [Google Scholar] [PubMed]
- Kino, K.; Yamashita, A.; Yamaoka, K.; Watanabe, J.; Tanaka, S.; Ko, K.; Shimizu, K.; Tsunoo, H. Isolation and characterization of a new immunomodulatory protein, Ling Zhi-8 (LZ-8), from Ganoderma lucidum. J. Biol. Chem. 1989, 264, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Rios-Fuller, T.J.; Ortiz-Soto, G.; Lacourt-Ventura, M.; Maldonado-Martinez, G.; Cubano, L.A.; Schneider, R.J.; Martinez-Montemayor, M.M. Ganoderma lucidum extract (GLE) impairs breast cancer stem cells by targeting the STAT3 pathway. Oncotarget 2018, 9, 35907–35921. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Hsu, H.Y. Ling Zhi-8 reduces lung cancer mobility and metastasis through disruption of focal adhesion and induction of MDM2-mediated Slug degradation. Cancer Lett. 2016, 375, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.S.; Song, Y.L.; Yin, Z.Q.; Guo, J.J.; Wang, S.P.; Zhao, W.W.; Chen, X.P.; Zhang, Q.W.; Lu, J.J.; Wang, Y.T. Ganoderiol A-enriched extract suppresses migration and adhesion of MDA-MB-231 cells by inhibiting FAK-SRC-paxillin cascade pathway. PLoS ONE 2013, 8, e76620. [Google Scholar] [CrossRef] [PubMed]
- Li, W.D.; Zhang, B.D.; Wei, R.; Liu, J.H.; Lin, Z.B. Reversal effect of Ganoderma lucidum polysaccharide on multidrug resistance in K562/ADM cell line. Acta Pharmacol. Sin. 2008, 29, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Liu, J.; Wang, Y.; Dong, J. Co-administration of MDR1 and BCRP or EGFR/PI3K inhibitors overcomes lenvatinib resistance in hepatocellular carcinoma. Front. Oncol. 2022, 12, 944537. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Chang, C.L.; Chen, T.H.; Chang, Y.W.; Lin, S.B. Colossolactone H, a new Ganoderma triterpenoid, exhibits cytotoxicity and potentiates drug efficacy of gefitinib in lung cancer. Fitoterapia 2016, 114, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Feliz-Mosquea, Y.; Suárez-Arroyo, I.; Cubano, L.A. Synergistic effect between Ganoderma lucidum (Reishi) and lapatinib in HER2+ inflammatory breast cancer cells. Cancer Res. 2014, 74 (Suppl. S19), 3219. [Google Scholar] [CrossRef]
- Liu, G.; Wang, K.; Kuang, S.; Cao, R.; Bao, L.; Liu, R.; Liu, H.; Sun, C. The natural compound GL22, isolated from Ganoderma mushrooms, suppresses tumor growth by altering lipid metabolism and triggering cell death. Cell Death Dis. 2018, 9, 689. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tang, Q.; Ren, X.; Zheng, F.; He, C.; Chai, X.; Li, L.; Hann, S.S. Reciprocal interaction of HOTAIR and SP1 together enhance the ability of Xiaoji decoction and gefitinib to inhibit EP4 expression. J. Ethnopharmacol. 2019, 237, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Vallega, K.A.; Wang, D.; Quan, Z.; Fan, S.; Wang, Q.; Leal, T.; Ramalingam, S.S.; Sun, S.Y. Inhibition of hTERT/telomerase/telomere mediates therapeutic efficacy of osimertinib in EGFR mutant lung cancer. J. Exp. Med. 2024, 221, e20240435. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Hsiao, Y.M.; Hsu, C.P.; Lin, M.Y.; Wang, J.C.; Huang, Y.L.; Ko, J.L. Transcriptionally mediated inhibition of telomerase of fungal immunomodulatory protein from Ganoderma tsugae in A549 human lung adenocarcinoma cell line. Mol. Carcinog. 2006, 45, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Sillapapongwarakorn, S.; Yanarojana, S.; Pinthong, D.; Thithapandha, A.; Ungwitayatorn, J.; Supavilai, P. Molecular docking based screening of triterpenoids as potential G-quadruplex stabilizing ligands with anti-cancer activity. Bioinformation 2017, 13, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.S.; Chen, L.S. Triterpenoids from Ganoderma lucidum inhibit the activation of EBV antigens as telomerase inhibitors. Exp. Ther. Med. 2017, 14, 3273–3278. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Li, Z.; Sun, Y.; Liu, T.; Yin, R. Persist or resist: Immune checkpoint inhibitors in EGFR-mutated NSCLC. Cancer Sci. 2025, 116, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.Y.; Zhang, J.T.; Liu, S.Y.; Su, J.; Zhang, C.; Xie, Z.; Zhou, Q.; Tu, H.Y.; Xu, C.R.; Yan, L.X.; et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology 2017, 6, e1356145. [Google Scholar] [CrossRef] [PubMed]
- Vokes, N.; Jimenez Alguilar, E.; Adeni, A.; Umeton, R.; Sholl, L.; Rizvi, H.; Hellmann, M.; Awad, M.; Van Allen, E. MA19.01 efficacy and genomic correlates of response to anti-PD1/PD-L1 blockade in non-small cell lung cancers harboring targetable oncogenes. J. Thorac. Oncol. 2018, 13 (Suppl. S10), S422. [Google Scholar] [CrossRef]
- Negrao, M.V.; Lam, V.K.; Reuben, A.; Rubin, M.L.; Landry, L.L.; Roarty, E.B.; Rinsurongkawong, W.; Lewis, J.; Roth, J.A.; Swisher, S.G.; et al. PD-L1 expression, tumor mutational burden, and cancer gene mutations are stronger predictors of benefit from immune checkpoint blockade than HLA class I genotype in non-small cell lung cancer. J. Thorac. Oncol. 2019, 14, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Ota, K.; Kawahara, A.; Hattori, S.; Iwama, E.; Harada, T.; Matsumoto, K.; Takayama, K.; Takamori, S.; Kage, M.; et al. Association of PDL1 overexpression with activating EGFR mutations in surgically resected non-small-cell lung cancer. Ann Oncol. 2014, 25, 1935–1940. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, N.; Li, L.; Chen, X.; Liu, X.; Zhang, Y.; Cui, J. Favorable immune microenvironment in patients with EGFR and MAPK co-mutations. Lung Cancer 2020, 11, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Chen, J.; Cheng, J.N.; Sun, C.; Jia, Q.; Diao, X.; Zhu, B. Tumor microenvironment properties are associated with low CD68-positive cell infiltration and favorable disease-free survival in EGFR-mutant lung adenocarcinoma. Clin. Lung Cancer 2018, 19, e551–e558. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, X.; Jiang, T.; Zhao, S.; Zhao, C.; Zhang, L.; Liu, X.; Shi, J.; Qiao, M.; Luo, J.; et al. EGFR-targeted therapy alters the tumor microenvironment in EGFR-driven lung tumors: Implications for combination therapies. Int. J. Cancer 2019, 145, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Isomoto, K.; Haratani, K.; Hayashi, H.; Shimizu, S.; Tomida, S.; Niwa, T.; Yokoyama, T.; Fukuda, Y.; Chiba, Y.; Kato, R.; et al. Impact of EGFR-TKI treatment on the tumor immune microenvironment in EGFR mutation-positive non-small cell lung cancer. Clin. Cancer Res. 2020, 26, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Toki, M.I.; Mani, N.; Smithy, J.W.; Liu, Y.; Altan, M.; Wasserman, B.; Tuktamyshov, R.; Schalper, K.; Syrigos, K.N.; Rimm, D.L. Immune marker profiling and programmed death ligand 1 expression across NSCLC mutations. J. Thorac. Oncol. 2018, 13, 1884–1896. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.M.; Traeger, L.; Eusebio, J.; Simon, N.M.; Sequist, L.V.; Greer, J.A.; Temel, J.S.; Pirl, W.F. Depression, inflammation, and epidermal growth factor receptor (EGFR) status in metastatic non-small cell lung cancer: A pilot study. J. Psychosom. Res. 2017, 99, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Sunaga, N.; Imai, H.; Shimizu, K.; Shames, D.S.; Kakegawa, S.; Girard, L.; Sato, M.; Kaira, K.; Ishizuka, T.; Gazdar, A.F.; et al. Oncogenic KRAS-induced interleukin-8 overexpression promotes cell growth and migration and contributes to aggressive phenotypes of non-small cell lung cancer. Int. J. Cancer 2012, 130, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Negrao, M.V.; Reuben, A.; Federico, L.; Diao, L.; McGrail, D.; Nilsson, M.; Robichaux, J.; Munoz, I.G.; Patel, S.; et al. Characterization of the immune landscape of EGFR-mutant NSCLC identifies CD73/adenosine pathway as a potential therapeutic target. J. Thorac. Oncol. 2021, 16, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Park, L.C.; Rhee, K.; Kim, W.B.; Cho, A.; Song, J.; Anker, J.F.; Oh, M.; Bais, P.; Namburi, S.; Chuang, J.; et al. Immunologic and clinical implications of CD73 expression in non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2018, 36 (Suppl. S15), 12050. [Google Scholar] [CrossRef]
- Ishii, H.; Azuma, K.; Kawahara, A.; Kinoshita, T.; Matsuo, N.; Naito, Y.; Tokito, T.; Yamada, K.; Akiba, J.; Hoshino, T. Predictive value of CD73 expression in EGFR-mutation positive non-small-cell lung cancer patients received immune checkpoint inhibitors. J. Clin. Oncol. 2018, 36 (Suppl. S15), 9065. [Google Scholar] [CrossRef]
- Simoni, Y.; Becht, E.; Fehlings, M.; Loh, C.Y.; Koo, S.L.; Teng, K.W.W.; Yeong, J.P.S.; Nahar, R.; Zhang, T.; Kared, H.; et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 2018, 557, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, T.D.; Prieto-Dominguez, N.; Gowda, P.S.; Ubil, E. Mechanisms of macrophage plasticity in the tumor environment: Manipulating activation state to improve outcomes. Front. Immunol. 2021, 12, 642285. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Feng, L.; Schmid, F. Macrophage-based cancer immunotherapy: Challenges and opportunities. Exp. Cell Res. 2024, 442, 114198. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Han, B.; Qi, M.; Li, Y.; Duan, Y.; Yao, Y. Modulating macrophage-mediated programmed cell removal: An attractive strategy for cancer therapy. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189172. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.T.; Hua, K.F.; Hsu, J.; Karmenyan, A.; Tseng, K.Y.; Wong, C.H.; Chiou, A. The interaction of lipopolysaccharide with membrane receptors on macrophages pretreated with extract of Reishi polysaccharides measured by optical tweezers. Opt. Express 2007, 15, 11020–11032. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.B.; Zhang, Z.L.; Ruan, Y.; Wu, Y.C.; Cong, Z. The pharmacological study of Ganoderma lucidum part VI: Effects of different extract fractions from Ganoderma lucidum fruiting bodies on the phagocytic activity of mouse peritoneal macrophages. Edib. Fungi 1980, 3, 5–6. [Google Scholar][Green Version]
- Tang, Q.J.; Zhang, J.S.; Pan, Y.J.; Martin, Z.K.; Werner, R.; Fan, H. Activation of normal and tumor-bearing mouse macrophages by active polysaccharide from Ganoderma lucidum. Curr. Immunol. 2005, 25, 49–52. [Google Scholar][Green Version]
- Zhang, J.; Tang, Q.; Zhou, C.; Jia, W.; Da Silva, L.; Nguyen, L.D.; Reutter, W.; Fan, H. GLIS, a bioactive proteoglycan fraction from Ganoderma lucidum, displays antitumour activity by increasing both humoral and cellular immune response. Life Sci. 2010, 87, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.Z.; Lin, Z.B. Comparison of the effects of polysaccharides from wood-cultured and bag-cultured Ganoderma lucidum on murine spleen lymphocyte proliferation in vitro. Acta Pharm. Sin. 2003, 38, 92–97. [Google Scholar]
- Tang, Q.J.; Zhang, J.S.; Pan, Y.J.; Werner, R.; Fan, H. Activation of mouse macrophages by the alkali extracted polysaccharide from spore of Ganoderma lucidum. Chin. J. Cell Mol. Immunol. 2004, 20, 142–144. [Google Scholar]
- Huang, J.Q.; Nie, Q.X.; Liu, X.Z.; Zhang, S.S.; Nie, S.P.; Huang, D.F.; Xie, M.Y. Ganoderma atrum polysaccharide modulates TNF-α secretion and mRNA expression in macrophages of S-180 tumor-bearing mice. Food Hydrocoll. 2016, 53, 24–30. [Google Scholar] [CrossRef]
- Hsu, M.J.; Lee, S.S.; Lee, S.T.; Lin, W.W. Signaling mechanisms of enhanced neutrophil phagocytosis and chemotaxis by the polysaccharide purified from Ganoderma lucidum. Br. J. Pharmacol. 2003, 139, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.J.; Lee, S.S.; Lin, W.W. Polysaccharide purified from Ganoderma lucidum inhibits spontaneous and Fas-mediated apoptosis in human neutrophils through activation of the phosphatidylinositol 3 kinase/Akt signaling pathway. J. Leukoc. Biol. 2002, 72, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Shuto, T.; Sato, M.; Onuki, K.; Mizunoe, S.; Suzuki, S.; Sato, T.; Koga, T.; Suico, M.A.; Kai, H.; et al. Lucidenic acids-rich extract from antlered form of Ganoderma lucidum enhances TNF-α induction in THP-1 monocytic cells possibly via its modulation of MAP kinases p38 and JNK. Biochem. Biophys. Res. Commun. 2011, 408, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.X.; Lin, Z.B.; Lu, J.; Li, W.D.; Niu, Y.D.; Yu, S.; Hu, C.Y.; Zhang, G.Q.; Duan, X.S. The improvement of M1 polarization in macrophages by glycopeptide derived from Ganoderma lucidum. Immunol. Res. 2017, 65, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Wu, Y.S.; Chen, S.; Liu, C.F.; Chen, S.N. Mushroom β-glucan may immunomodulate the tumor-associated macrophages in the Lewis lung carcinoma. Biomed. Res. Int. 2015, 2015, 604385. [Google Scholar]
- Saadh, M.J.; Rasulova, I.; Khalil, M.; Farahim, F.; Sarbu, I.; Ciongradi, C.I.; Omar, T.M.; Alhili, A.; Jawad, M.J.; Hani, T.; et al. Natural killer cell-mediated immune surveillance in cancer: Role of tumor microenvironment. Pathol. Res. Pract. 2024, 254, 155120. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Yang, J.S.; Yang, J.L.; Wu, C.L.; Chang, S.J.; Lu, K.W.; Lin, J.J.; Hsia, T.C.; Lin, Y.T.; Ho, C.C.; et al. Ganoderma lucidum extracts inhibited leukemia WEHI-3 cells in BALB/c mice and promoted an immune response in vivo. Biosci. Biotechnol. Biochem. 2009, 73, 2589–2594. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Yang, F.L.; Huang, Z.Y.; Chen, C.S.; Yang, Y.L.; Hua, K.F.; Li, J.; Chen, S.T.; Wu, S.H. Oligosaccharide and peptidoglycan of Ganoderma lucidum activate the immune response in human mononuclear cells. J. Agric. Food Chem. 2012, 60, 2830–2837. [Google Scholar] [CrossRef] [PubMed]
- Won, S.J.; Lin, M.T.; Wu, W.L. Ganoderma tsugae mycelium enhances splenic natural killer cell activity and serum interferon production in mice. Jpn. J. Pharmacol. 1992, 59, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Ning, A.H.; Cao, J.; Huag, M.; Zhang, W. The antitumor action and influence of immune system of Ganoderma polysaccharides in mice. Chin. J. Microecol. 2004, 16, 13–14. [Google Scholar]
- Wang, C.H.; Shi, S.S.; Chen, Q.; Lin, S.Q.; Wang, R.; Wang, S.Z.; Chen, C.M. Antitumor and immunomodulatory activities of Ganoderma lucidum polysaccharides in glioma-bearing rats. Integr. Cancer Ther. 2018, 17, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.X.; Lin, Z.B.; Li, X.J.; Li, M.; Lu, J.; Duan, X.S.; Ge, Z.H.; Song, Y.X.; Xing, E.H.; Li, W.D. Promoting effects of Ganoderma lucidum polysaccharides on B16F10 cells to activate lymphocytes. Basic Clin. Pharmacol. Toxicol. 2011, 108, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.X.; Lin, Z.B.; Duan, X.S.; Lu, J.; Ge, Z.H.; Li, X.F.; Li, X.J.; Li, M.; Xing, E.H.; Song, Y.X.; et al. Enhanced MHC class I and costimulatory molecules on B16F10 cells by Ganoderma lucidum polysaccharides. J. Drug Target 2012, 20, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.X.; Lin, Z.B.; Duan, X.S.; Lu, J.; Ge, Z.H.; Li, X.J.; Li, M.; Xing, E.H.; Jia, J.; Lan, T.F.; et al. Ganoderma lucidum polysaccharides antagonize the suppression on lymphocytes induced by culture supernatants of B16F10 melanoma cells. J. Pharm. Pharmacol. 2011, 63, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Chen, Q.; He, Y.-M. The effect of Ganoderma lucidum extract on immunological function and identify its anti-tumor immunostimulatory activity based on the biological network. Sci. Rep. 2018, 8, 12680. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Qu, Z.H.; Zhao, Z.Y. Effect of Ganoderma lucidum spores on immune function in patients with non-small cell lung cancer during chemotherapy. China Pract. Med. 2014, 9, 20–21. [Google Scholar]
- Zhen, Z.J.; Wang, F.J.; Fan, G.Y.; Chen, H.W. Effect of Ganoderma lucidum spore to the immunological function of patients with hepatocellular carcinoma after operation. Chin. J. Hepat. Surg. 2013, 2, 171–174. [Google Scholar]
- Lin, Y.L.; Liang, Y.C.; Lee, S.S.; Chiang, B.L. Polysaccharide purified from Ganoderma lucidum induced activation and maturation of human monocyte-derived dendritic cells by the NF-κB and p38 mitogen-activated protein kinase pathways. J. Leukoc. Biol. 2005, 78, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Hsu, M.L.; Hsu, H.C.; Tzeng, C.H.; Lee, S.S.; Shiao, M.S. The anti-tumor effect of Ganoderma lucidum is mediated by cytokines released from activated macrophages and T lymphocytes. Int. J. Cancer 1997, 70, 699–705. [Google Scholar] [CrossRef]
- Lin, N.D.; Su, J.N.; Zhu, Z.; Gao, Y.H.; Gao, H.; Xie, G.Y. Analysis on 66 cases of cancer patients treated by chemotherapy with extract of Ganoderma lucidum. J. Pract. Tradit. Chin. Intern. Med. 2004, 18, 457–458. [Google Scholar]
- Wang, M.M.; Coupland, S.E.; Aittokallio, T.; Figueiredo, C.R. Resistance to immune checkpoint therapies by tumor-induced T-cell desertification and exclusion: Key mechanisms, prognostication and new therapeutic opportunities. Br. J. Cancer 2023, 129, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Su, L.; Li, D.; Shuai, O.; Zhang, Y.; Liang, H.; Jiao, C.; Xu, Z.; Lai, Y.; Xie, Y. Antitumor activity of extract from the sporoderm-breaking spore of Ganoderma lucidum: Restoration on exhausted cytotoxic T cell with gut microbiota remodeling. Front. Immunol. 2018, 9, 1765. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; He, Y.T.; Dong, S.; Wei, X.W.; Chen, Z.H.; Zhang, B.; Chen, W.D.; Yang, X.R.; Wang, F.; Shang, X.M.; et al. Single-cell transcriptome analysis revealed a suppressive tumor immune microenvironment in EGFR mutant lung adenocarcinoma. J. Immunother. Cancer 2022, 10, e003534. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Sun, L.X.; Lin, Z.B.; Duan, X.S.; Ge, Z.H.; Xing, E.H.; Lan, T.F.; Yang, N.; Li, X.J.; Li, M.; et al. Antagonism by Ganoderma lucidum polysaccharides against the suppression by culture supernatants of B16F10 melanoma cells on macrophage. Phytother. Res. 2014, 28, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Huang, D.; Wang, Y.; Li, D.; Yang, X.; Fu, Y.; Du, N.; Zhao, Y.; Li, X.; Ma, J.; et al. The efficacy of immune checkpoint inhibitors in advanced EGFR-mutated non-small cell lung cancer after resistance to EGFR-TKIs: Real-world evidence from a multicenter retrospective study. Front. Immunol. 2022, 13, 975246. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Gao, Z.; Wu, D.; Zheng, J.; Hu, C.; Huang, D.; He, C.; Liu, Y.; Lin, C.; Peng, T.; et al. Understanding the dynamics of TKI-induced changes in the tumor immune microenvironment for improved therapeutic effect. J. Immunother. Cancer 2024, 12, e009165. [Google Scholar] [CrossRef] [PubMed]
- Angelicola, S.; Giunchi, F.; Ruzzi, F.; Frascino, M.; Pitzalis, M.; Scalambra, L.; Semprini, M.S.; Pittino, O.M.; Cappellp, C.; Siracusa, I.; et al. PD-L1 and IFN-γ modulate non-small cell lung cancer (NSCLC) cell plasticity associated to immune checkpoint inhibitor (ICI)-mediated hyperprogressive disease (HPD). J. Transl. Med. 2025, 23, 2. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.R.; Feng, L.; Ye, L.H.; Wang, L.S.; Xiao, B.X.; Tao, X.; Chang, Q. Ganoderic Acid A Metabolites and Their Metabolic Kinetics. Front. Pharmacol. 2017, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Tang, W. Ganoderic acid T improves sensitivity of multidrug-resistant KB-A-1/dox cells to doxorubicin. Nat. Prod. Res. Dev. 2013, 25, 1052–1055. [Google Scholar]
- Tang, W. Ganoderic acid Me improves the sensitivity of multidrug-resistant KB-A-1/Dox cells to doxorubicin. Sci. Technol. Food Ind. 2013, 34, 97–100. [Google Scholar]
- Tang, Y.; Gao, Q.H.; Xu, W.T.; Wu, Z.; Li, F.R. The active section of Ganoderma lucidum on reversion of multidrug resistance in multidrug-resistant gastric carcinoma cell line SGC7901/ADR. J. Chin. Med. Mater. 2013, 36, 2007–2010. [Google Scholar]
- Ballard, P.; Yates, J.W.; Yang, Z.; Kim, D.W.; Yang, J.C.H.; Cantarini, M.; Pickup, K.; Jordan, A.; Hickey, M.; Grist, M.; et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin. Cancer Res. 2016, 22, 5130–5140. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Y.; Li, J.L. Comparative review of drug-drug interactions with epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small-cell lung cancer. Onco Targets Ther. 2019, 12, 5467–5484. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Shen, C.; Lin, Q.F.; Zhong, J.Y.; Zhou, Y.F.; Liu, B.C.; Xu, J.H.; Zhang, Z.Q.; Li, P. Sterols and triterpenoids from Ganoderma lucidum and their reversal activities of tumor multidrug resistance. Nat. Prod. Res. 2022, 36, 1396–1399. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lin, Y.; Lv, X.; Wu, Y.; Han, C.; Cao, P.; Zhang, G.; Leng, A.; Zhou, J.; Wang, C. Triterpenoids from Ganoderma lucidum inhibit cytochrome P450 enzymes interfering with the metabolic process of specific clinical drugs. Front. Pharmacol. 2024, 15, 1485209. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, X.; Li, D.; Lou, Y.Q.; Lin, Z.B.; Zhang, G.L. Effects of Ganoderma lucidum polysaccharide on CYP2E1, CYP1A2 and CYP3A activities in BCG-immune hepatic injury in rats. Biol. Pharm. Bull. 2007, 30, 1702–1706. [Google Scholar] [CrossRef] [PubMed]
- Yeung, J.H.; Or, P.M. Polysaccharide peptides from Coriolus versicolor competitively inhibit model cytochrome P450 enzyme probe substrates metabolism in human liver microsomes. Phytomedicine 2012, 19, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, F.; Chen, D.; Su, K.; Zhang, L.; Jiang, R. In vitro inhibitory effects of ganoderic acid A on human liver cytochrome P450 enzymes. Pharm. Biol. 2020, 58, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Nicandro, J.P.; Tsourounis, C.; Frassetto, L.; Guglielmo, B.J. In vivo effect of I’m-Yunity on hepatic cytochrome P450 3A4. J. Herb. Pharmacother. 2007, 7, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Yeung, J.H.; Or, P.M. Polysaccharide peptides from Coriolus versicolor competitively inhibit tolbutamide 4-hydroxylation in specific human CYP2C9 isoform and pooled human liver microsomes. Phytomedicine 2011, 18, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Rodseeda, C.; Yamanont, P.; Pinthong, D.; Korprasertthaworn, P. Inhibitory effects of Thai herbal extracts on the cytochrome P450 3A-mediated metabolism of gefitinib, lapatinib and sorafenib. Toxicol. Rep. 2022, 9, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; de Mores, A.R.; Cohen, L.; Anwar, M.M.; Lazar, F.; Hicklen, R.; Lopez, G.; Yang, P.; Bruera, E. Medicinal mushroom supplements in cancer: A systematic review of clinical studies. Curr. Oncol. Rep. 2023, 25, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Pan, L.C.; Zheng, Y.G.; Du, G.S.; Fu, X.Q.; Zhu, Z.D.; Song, J.Y.; Liu, Z.J.; Su, X.Z.; Chen, W.; et al. Novel strategy of sirolimus plus thymalfasin and Huaier granule on tumor recurrence of hepatocellular carcinoma beyond the UCSF criteria following liver transplantation: A single center experience. Oncol. Lett. 2018, 16, 4407–4417. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, X.P. Adjuvant Huaier granule for hepatocellular carcinoma after curative resection: A phase IV, multicenter, randomized, and parallel-group controlled clinical trial. J. Hepatol. 2017, 66 (Suppl. S1), S622. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Chen, T. Efficacy of Huaier granule in patients with breast cancer. Clin. Transl. Oncol. 2019, 21, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Akagi, J.; Baba, H. PSK may suppress CD57(+) T cells to improve survival of advanced gastric cancer patients. Int. J. Clin. Oncol. 2010, 15, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Ito, G.; Tanaka, H.; Ohira, M.; Yoshii, M.; Muguruma, K.; Kubo, N.; Yashiro, M.; Yamada, N.; Maeda, K.; Sawada, T.; et al. Correlation between efficacy of PSK postoperative adjuvant immunochemotherapy for gastric cancer and expression of MHC class I. Exp. Ther. Med. 2012, 3, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.S.; Kang, S.Y.; Lee, H.W.; Jeong, S.H.; Park, J.S.; Lee, K.J.; Cho, Y.K.; Han, S.U.; Lee, S.Y.; Lim, H.Y.; et al. 5-Fluorouracil, mitomycin-c, and polysaccharide-k versus uracil-ftorafur and polysaccharide-K as adjuvant chemoimmunotherapy for patients with locally advanced gastric cancer with curative resection. Onkologie 2013, 36, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, M.; Mochiki, E.; Ishiguro, T.; Ogura, T.; Sobajima, J.; Kumagai, Y.; Ishibashi, K.; Ishida, H. Improved efficacy by addition of protein-bound polysaccharide K to adjuvant chemotherapy for advanced gastric cancer. Anticancer Res. 2016, 36, 4237–4241. [Google Scholar] [PubMed]
- Wu, T.T.; Chen, Y.Y.; Yuan, Z.C.; Yang, G.W.; Zhang, G.L. Synergy of de-walled Ganoderma Lucidum spore powder (GLSP) on targeted therapy in advanced non-squamous non-small cell lung cancer with epidermal growth factor receptor (EGFR) mutant: Protocol for a randomized, double-blind, placebo-controlled study. BMC Complement. Med. Ther. 2024, 24, 125. [Google Scholar] [CrossRef] [PubMed]
- Majam, T.; Sukasem, C.; Reungwetwattana, T.; Chansriwong, P.; Atasilp, C.; Trachu, N.; Thamrongjjrapat, T.; Sukprasong, R.; Meanwatthana, J. CYP450 and drug efflux transporters polymorphism influence clinical outcomes of Thai osimertinib-treated non-small cell lung cancer patients. Front. Pharmacol. 2023, 14, 1222435. [Google Scholar] [CrossRef] [PubMed]
- Veerman, G.D.; Boosman, R.J.; Jebbink, M.; Oomen-de Hoop, E.; van der Wekken, A.J.; Bahce, I.; Hendriks, L.E.L.; Croes, S.; Steendam, C.M.J.; de Jonge, E.; et al. Influence of germline variations in drug transporters ABCB1 and ABCG2 on intracerebral osimertinib efficacy in patients with non-small cell lung cancer. EClinicalMedicine 2023, 59, 101955. [Google Scholar] [CrossRef] [PubMed]
- Tanda, M.; Yamamoto, K.; Hori, T.; Nishiguchi, H.; Yagi, M.; Shimizu, M.; Konishi, T.; Ozaki, T.; Yoshioka, N.; Tachihara, M.; et al. Association of STAT3, CYP3A5, and ABCG2 polymorphisms with osimerinib-induced adverse events in NSCLC patients. Anticancer Res. 2023, 43, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, K.; Chen, S.; Wang, L.; Zhang, X.; Xu, W.; Yam, M.F.; Wu, C.; Xu, W.; Lin, Y. Quality control of Ganoderma lucidum by using C, H, O, and N stable isotypes and C and N contents for geographical traceability. Front. Plant Sci. 2023, 14, 1234729. [Google Scholar]
- Yeung, S.; Chen, Q.; Yu, Y.; Zhou, B.; Wu, W.; Li, X.; Huang, Y.; Wang, Z. Quality evaluation of commercial products of Ganoderma lucidum made from its fruiting body and spore. Acta Chromatogr. 2021, 34, 100–113. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).