Ferroptosis Among the Antiproliferative Pathways Activated by a Lipophilic Ruthenium(III) Complex as a Candidate Drug for Triple-Negative Breast Cancer
Abstract
1. Introduction
2. Experimental Section
2.1. Cancer and Healthy Cell Cultures
2.2. Human TNBC Cell Lines
2.3. Human Healthy Control Cell Lines
2.4. Cell Survival Assessment
2.5. IC50 Calculation
2.6. ROS Detection
2.7. LIP Measurement
2.8. Proteins Extraction
2.9. Western Blot Analysis
2.10. Glutathione (GSH/GSSG) Ratio Assay
2.11. Lipid Peroxidation
2.12. MDA Determination
2.13. Mitochondria Morphological Analysis by Confocal Microscopy
2.14. RCD Pathway Activation
2.15. Statistical Data Analysis
3. Results
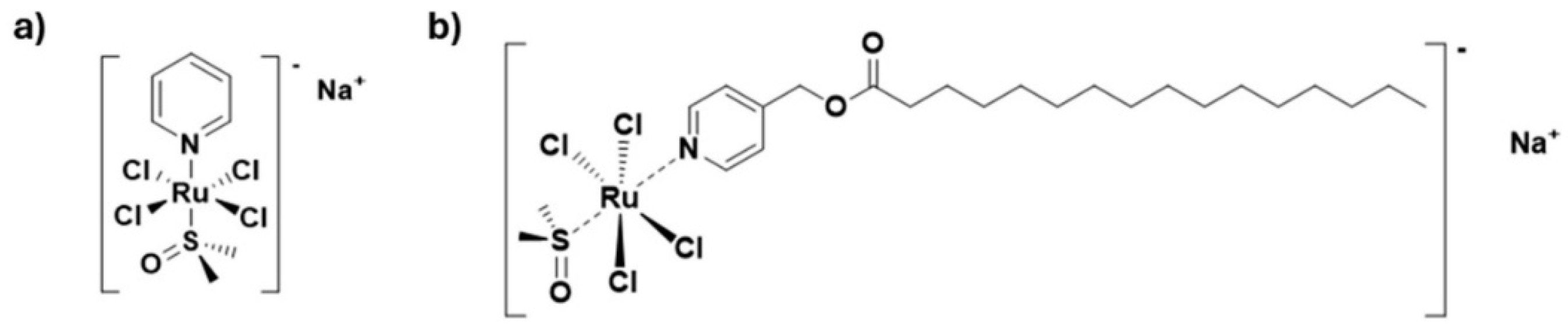
3.1. PalmiPyRu: Evidence of Anticancer Efficacy and Selectivity In Vitro
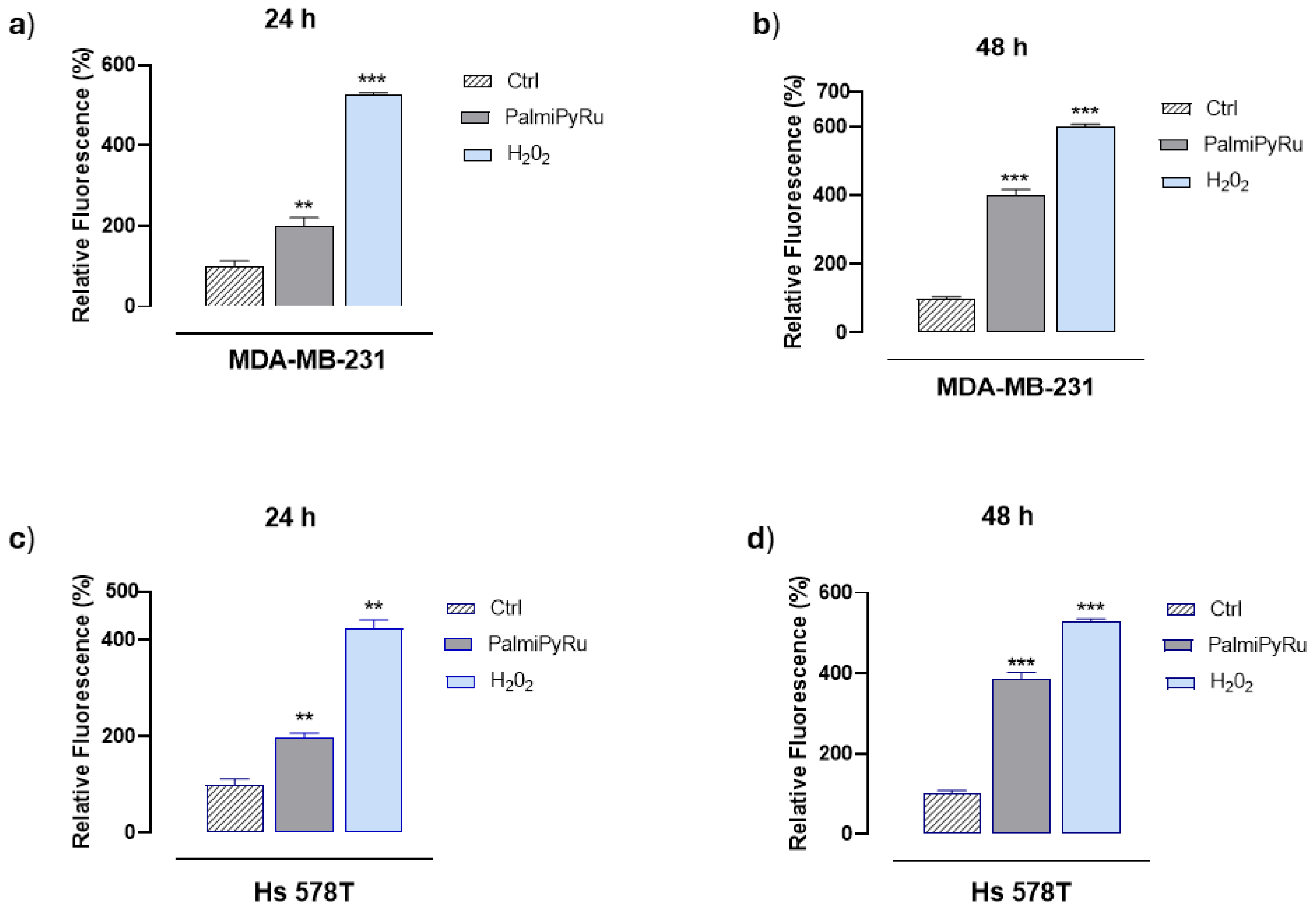
3.2. PalmiPyRu Triggers Oxidative Stress and ROS Production in TNBC Models
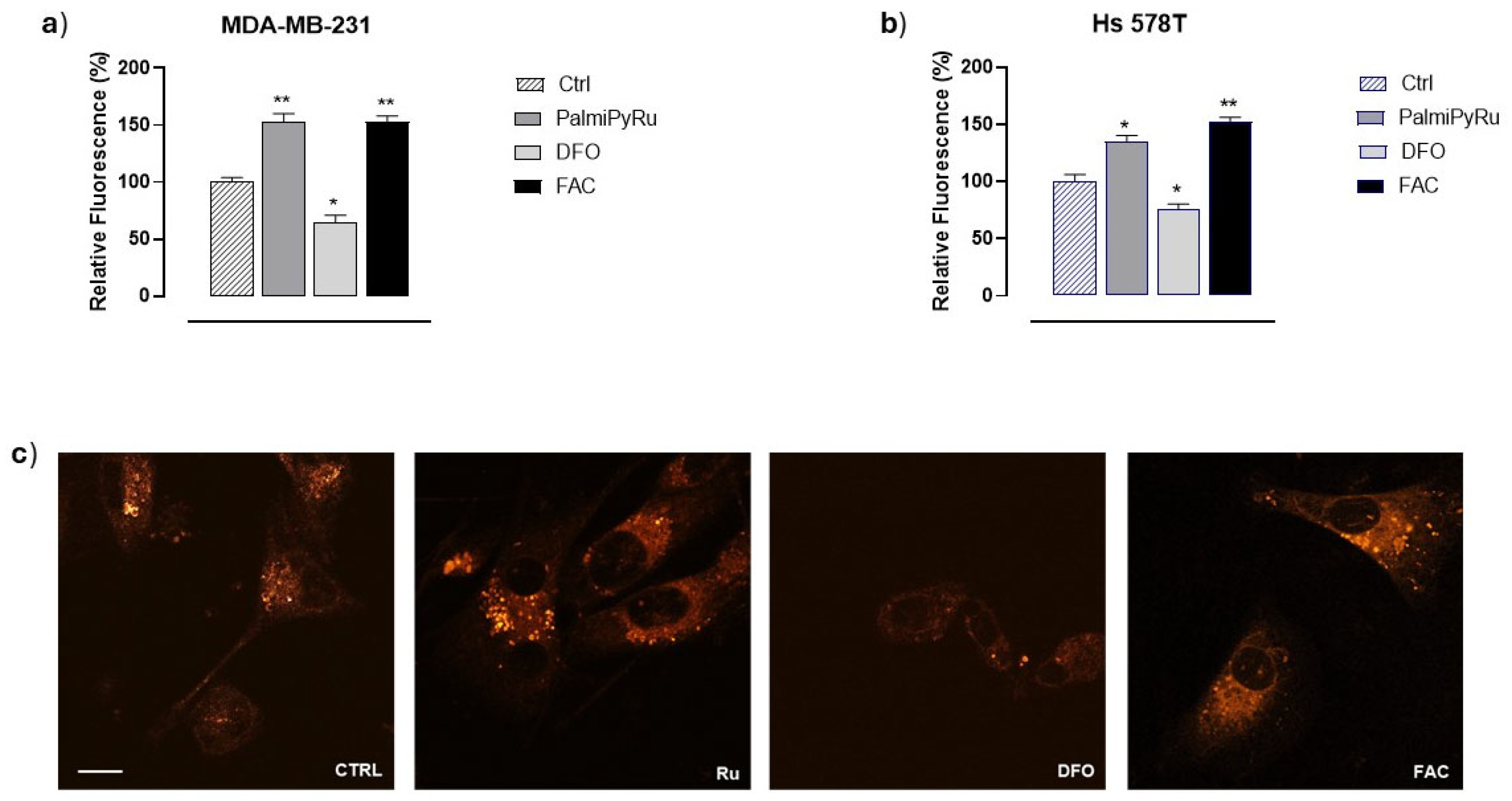
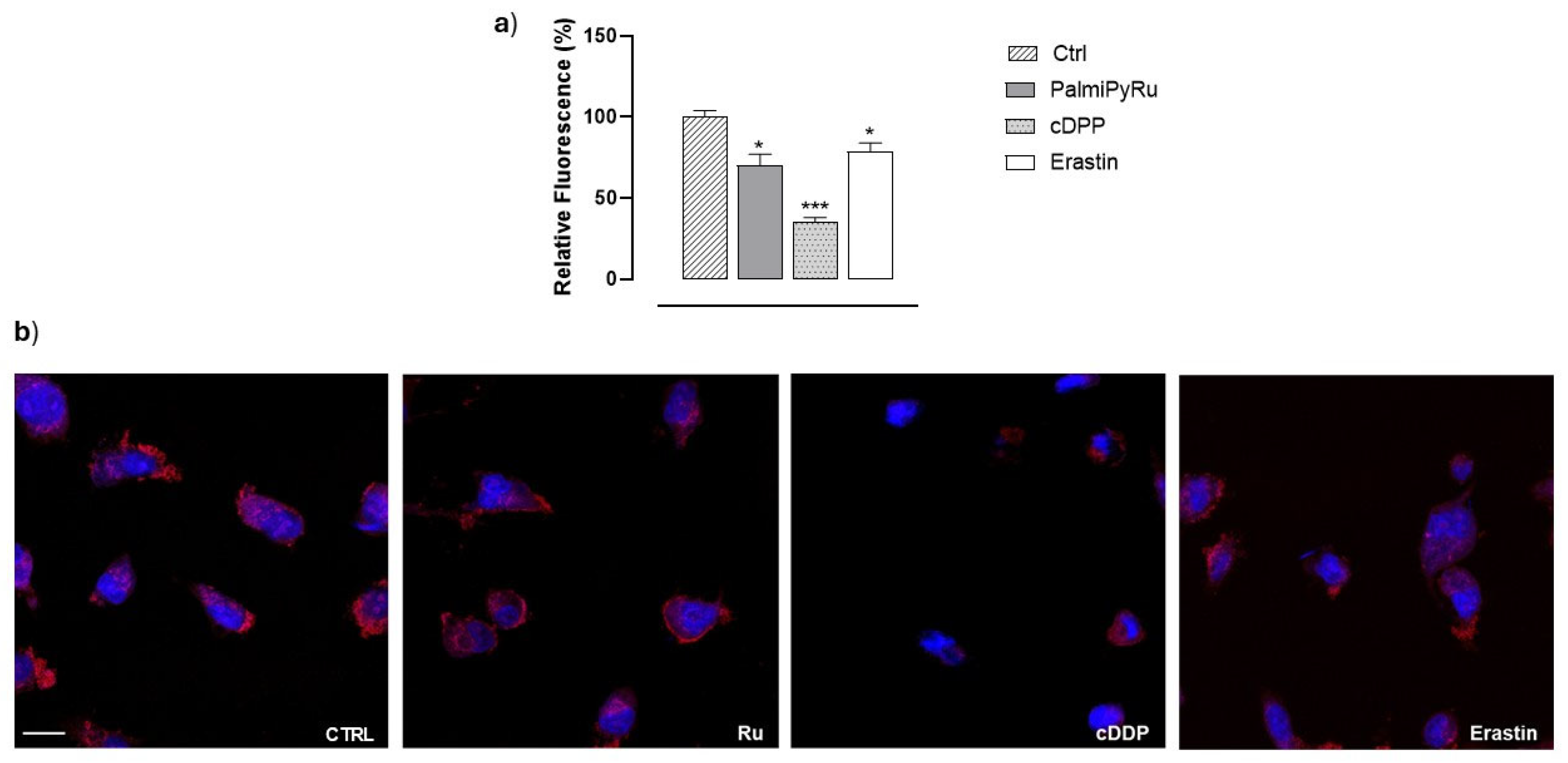
3.3. Disruption of Iron Homeostasis
3.4. PalmiPyRu Modulates Key Proteins Involved in Iron Homeostasis in TNBC Model
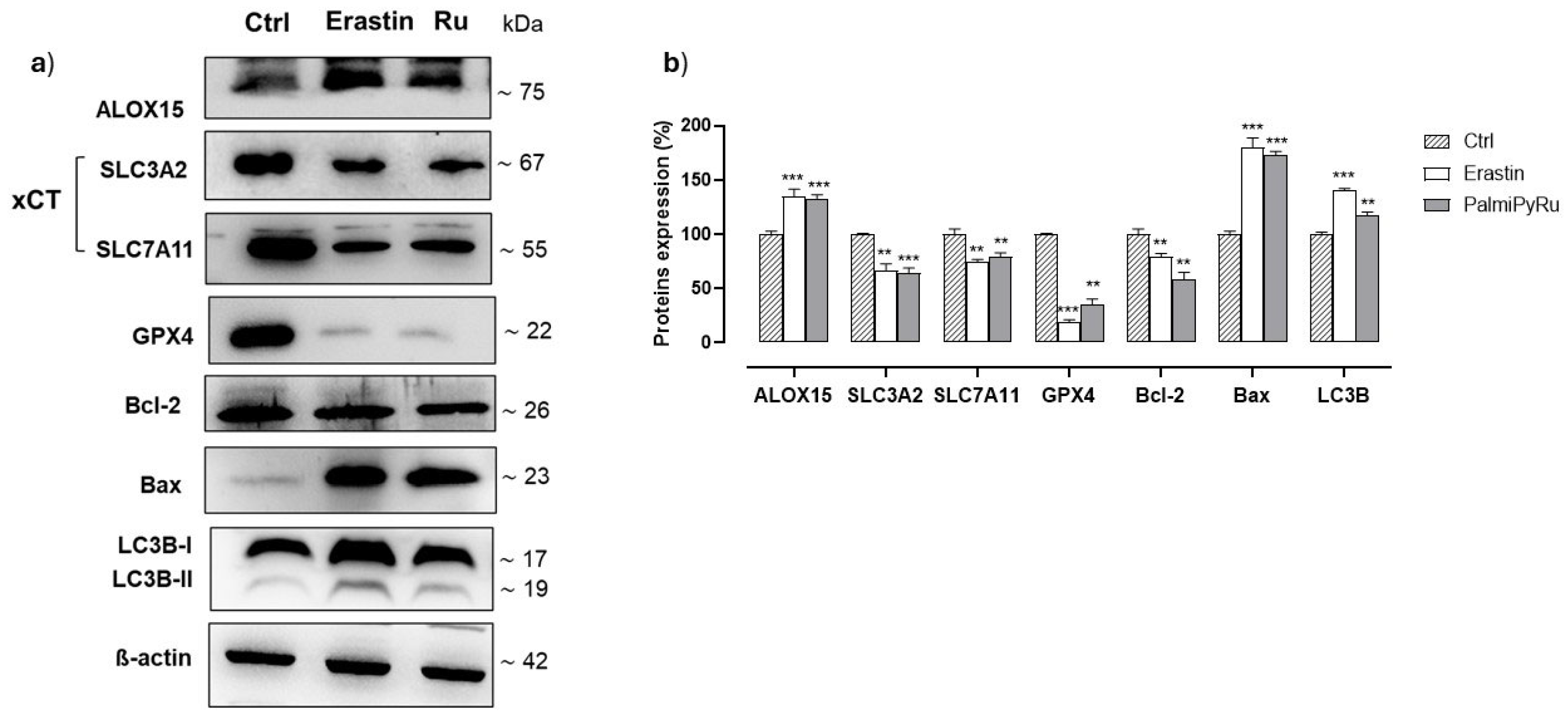
3.5. PalmiPyRu Interferes with the Ferroptotic Protein Pathway
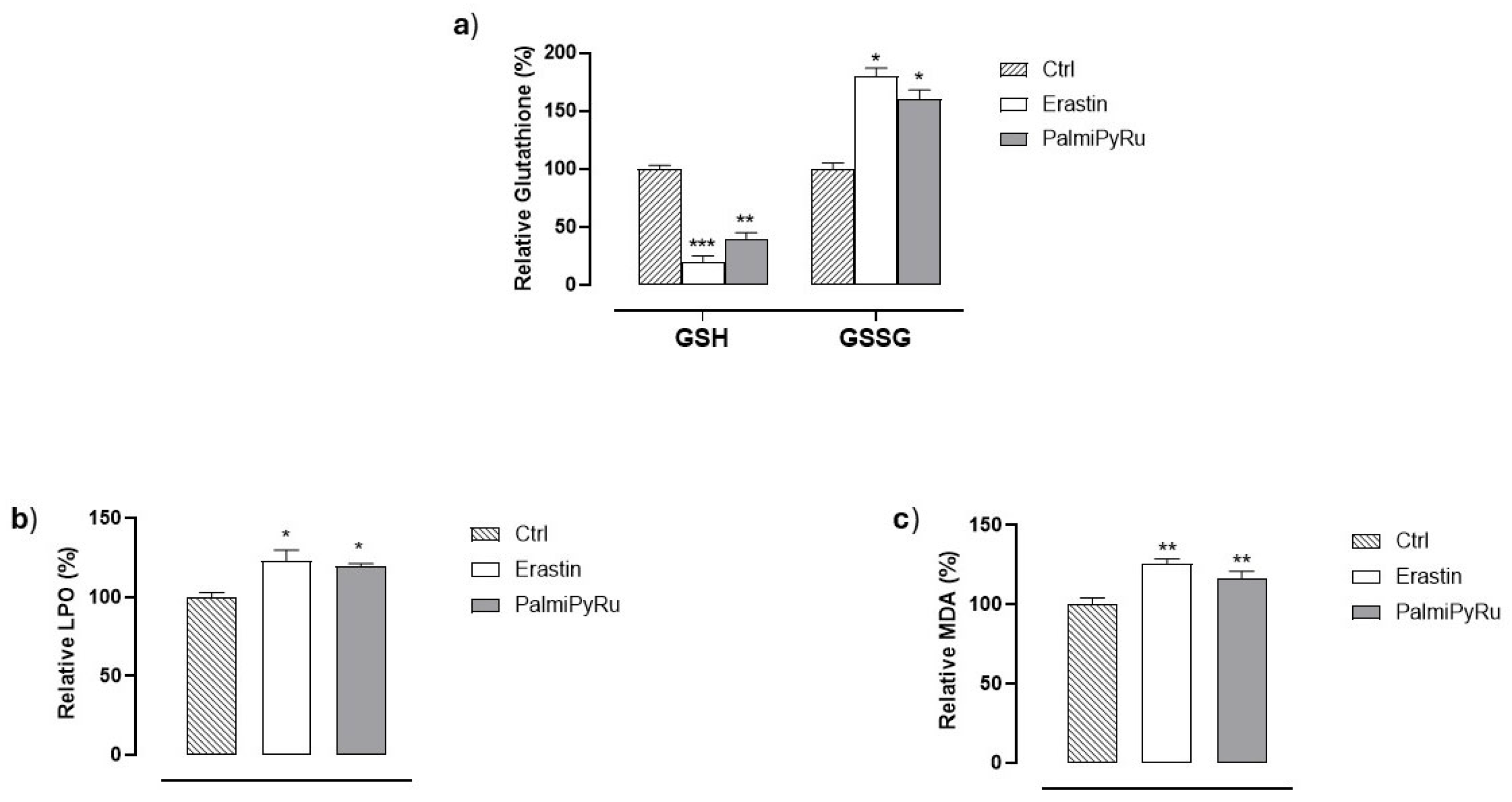
3.6. Glutathione (GSH) Depletion and Lipid Peroxidation
3.7. PalmiPyRu Effect on Mitochondria in MDA-MB-231 Cells
3.8. PalmiPyRu Triggers Multiple RCD Pathways in MDA-MB-231 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piccolo, M.; Ferraro, M.G.; Raucci, F.; Riccardi, C.; Saviano, A.; Russo Krauss, I.; Trifuoggi, M.; Caraglia, M.; Paduano, L.; Montesarchio, D.; et al. Safety and Efficacy Evaluation In Vivo of a Cationic Nucleolipid Nanosystem for the Nanodelivery of a Ruthenium(III) Complex with Superior Anticancer Bioactivity. Cancers 2021, 13, 5164. [Google Scholar] [CrossRef]
- Ferraro, M.G.; Bocchetti, M.; Riccardi, C.; Trifuoggi, M.; Paduano, L.; Montesarchio, D.; Misso, G.; Santamaria, R.; Piccolo, M.; Irace, C. Triple Negative Breast Cancer Preclinical Therapeutic Management by a Cationic Ruthenium-Based Nucleolipid Nanosystem. Int. J. Mol. Sci. 2023, 24, 6473. [Google Scholar] [CrossRef]
- Riccardi, C.; Musumeci, D.; Trifuoggi, M.; Irace, C.; Paduano, L.; Montesarchio, D. Anticancer Ruthenium(III) Complexes and Ru(III)-Containing Nanoformulations: An Update on the Mechanism of Action and Biological Activity. Pharmaceuticals 2019, 12, 146. [Google Scholar] [CrossRef]
- Mangiapia, G.; Vitiello, G.; Irace, C.; Santamaria, R.; Colonna, A.; Angelico, R.; Radulescu, A.; D’Errico, G.; Montesarchio, D.; Paduano, L. Anticancer cationic ruthenium nanovectors: From rational molecular design to cellular uptake and bioactivity. Biomacromolecules 2013, 14, 2549–2560. [Google Scholar] [CrossRef][Green Version]
- Simeone, L.; Mangiapia, G.; Vitiello, G.; Irace, C.; Colonna, A.; Ortona, O.; Montesarchio, D.; Paduano, L. Cholesterol-based nucleolipid-ruthenium complex stabilized by lipid aggregates for antineoplastic therapy. Bioconjugate Chem. 2012, 23, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, G.; Luchini, A.; D’Errico, G.; Santamaria, R.; Capuozzo, A.; Irace, C.; Montesarchio, D.; Paduano, L. Cationic liposomes as efficient nanocarriers for the drug delivery of an anticancer cholesterol-based ruthenium complex. J. Mater. Chem. B 2015, 3, 3011–3023. [Google Scholar] [CrossRef]
- Riccardi, C.; Musumeci, D.; Capuozzo, A.; Irace, C.; King, S.; Russo Krauss, I.; Paduano, L.; Montesarchio, D. “Dressing up” an Old Drug: An Aminoacyl Lipid for the Functionalization of Ru(III)-Based Anticancer Agents. ACS Biomater. Sci. Eng. 2018, 4, 163–174. [Google Scholar] [CrossRef]
- Irace, C.; Misso, G.; Capuozzo, A.; Piccolo, M.; Riccardi, C.; Luchini, A.; Caraglia, M.; Paduano, L.; Montesarchio, D.; Santamaria, R. Antiproliferative effects of ruthenium-based nucleolipidic nanoaggregates in human models of breast cancer in vitro: Insights into their mode of action. Sci. Rep. 2017, 7, 45236. [Google Scholar] [CrossRef]
- Piccolo, M.; Misso, G.; Ferraro, M.G.; Riccardi, C.; Capuozzo, A.; Zarone, M.R.; Maione, F.; Trifuoggi, M.; Stiuso, P.; D’Errico, G.; et al. Exploring cellular uptake, accumulation and mechanism of action of a cationic Ru-based nanosystem in human preclinical models of breast cancer. Sci. Rep. 2019, 9, 7006. [Google Scholar] [CrossRef]
- Ferraro, M.G.; Piccolo, M.; Misso, G.; Maione, F.; Montesarchio, D.; Caraglia, M.; Paduano, L.; Santamaria, R.; Irace, C. Breast Cancer Chemotherapeutic Options: A General Overview on the Preclinical Validation of a Multi-Target Ruthenium(III) Complex Lodged in Nucleolipid Nanosystems. Cells 2020, 9, 1412. [Google Scholar] [CrossRef]
- Riccardi, C.; Piccolo, M.; Ferraro, M.G.; Graziano, R.; Musumeci, D.; Trifuoggi, M.; Irace, C.; Montesarchio, D. Bioengineered lipophilic Ru(III) complexes as potential anticancer agents. Biomater. Adv. 2022, 139, 213016. [Google Scholar] [CrossRef]
- Ferraro, M.G.; Piccolo, M.; Misso, G.; Santamaria, R.; Irace, C. Bioactivity and Development of Small Non-Platinum Metal-Based Chemotherapeutics. Pharmaceutics 2022, 14, 954. [Google Scholar] [CrossRef] [PubMed]
- Kenny, R.G.; Marmion, C.J. Toward Multi-Targeted Platinum and Ruthenium Drugs—A New Paradigm in Cancer Drug Treatment Regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef] [PubMed]
- Alessio, E.; Mestroni, G.; Bergamo, A.; Sava, G. Ruthenium antimetastatic agents. Curr. Top. Med. Chem. 2004, 4, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.; Carson, R.; Moss, D.; Sessler, T.; Lavin, D.; Tiwari, V.K.; Karelia, S.; Kennedy, R.; Savage, K.I.; McDade, S.; et al. Ruthenium Drug BOLD-100 Regulates BRAFMT Colorectal Cancer Cell Apoptosis through AhR/ROS/ATR Signaling Axis Modulation. Mol. Cancer Res. 2024, 22, 1088–1101. [Google Scholar] [CrossRef]
- Swaminathan, S.; Haribabu, J.; Karvembu, R. From Concept to Cure: The Road Ahead for Ruthenium-Based Anticancer Drugs. ChemMedChem 2024, 19, e202400435. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, D.; Le, Q.; Wang, Y.; Wang, W. Ruthenium-based antitumor drugs and delivery systems from monotherapy to combination therapy. Nanoscale 2022, 14, 16339–16375. [Google Scholar] [CrossRef]
- Park, B.J.; Raha, P.; Pankovich, J.; Bazett, M. Utilization of Cancer Cell Line Screening to Elucidate the Anticancer Activity and Biological Pathways Related to the Ruthenium-Based Therapeutic BOLD-100. Cancers 2022, 15, 28. [Google Scholar] [CrossRef]
- Alessio, E.; Messori, L. NAMI-A and KP1019/1339, Two Iconic Ruthenium Anticancer Drug Candidates Face-to-Face: A Case Story in Medicinal Inorganic Chemistry. Molecules 2019, 24, 1995. [Google Scholar] [CrossRef]
- Bytzek, A.K.; Koellensperger, G.; Keppler, B.K.; GHartinger, C. Biodistribution of the novel anticancer drug sodium trans-[tetrachloridobis(1H-indazole)ruthenate(III)] KP-1339/IT139 in nude BALB/c mice and implications on its mode of action. J. Inorg. Biochem. 2016, 160, 250–255. [Google Scholar] [CrossRef]
- Piccolo, M.; Ferraro, M.G.; Iazzetti, F.; Santamaria, R.; Irace, C. Insight into Iron, Oxidative Stress and Ferroptosis: Therapy Targets for Approaching Anticancer Strategies. Cancers 2024, 16, 1220. [Google Scholar] [CrossRef]
- Dixon, S.J.; Olzmann, J.A. The cell biology of ferroptosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 424–442. [Google Scholar] [CrossRef] [PubMed]
- Foo, B.J.; Eu, J.Q.; Hirpara, J.L.; Pervaiz, S. Interplay between Mitochondrial Metabolism and Cellular Redox State Dictates Cancer Cell Survival. Oxid. Med. Cell. Longev. 2021, 2021, 1341604. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Swargiary, G.; Ralph, S.J. Targeting the redox imbalance in mitochondria: A novel mode for cancer therapy. Mitochondrion 2022, 62, 50–73. [Google Scholar] [CrossRef]
- Morales, M.; Xue, X. Targeting iron metabolism in cancer therapy. Theranostics 2021, 11, 8412–8429. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Emadi, A. Ruthenium-based chemotherapeutics: Are they ready for prime time? Cancer Chemother. Pharmacol. 2010, 66, 1–9. [Google Scholar] [CrossRef]
- Kozieł, S.; Wojtala, D.; Szmitka, M.; Kędzierski, P.; Bieńko, D.; Komarnicka, U.K. Insights into the binding of half-sandwich phosphino Ir(III) and Ru(II) complexes to deoxyribonucleic acid, albumin and apo-transferrin: Experimental and theoretical investigation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 304, 123289. [Google Scholar] [CrossRef]
- Simovic, A.R.; Masnikosa, R.; Bratsos, I.; Alessio, E. Chemistry and reactivity of ruthenium(II) complexes: DNA/protein binding mode and anticancer activity are related to the complex structure. Coord. Chem. Rev. 2019, 398, 113011. [Google Scholar] [CrossRef]
- Piccioli, F.; Sabatini, S.; Messori, L.; Orioli, P.; Hartinger, C.G.; Keppler, B.K. A comparative study of adduct formation between the anticancer ruthenium(III) compound HInd trans-[RuCl4(Ind)2] and serum proteins. J. Inorg. Biochem. 2004, 98, 1135–1142. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, X.; Duan, D.; Zhao, L. Organelle-Specific Mechanisms in Crosstalk Between Apoptosis and Ferroptosis. Oxid. Med. Cell. Longev. 2023, 2023, 3400147. [Google Scholar] [CrossRef]
- Suchiita, A.; Gupta, N.; Nandi, K.; Goswami, B. Navigating the crossroads of cell death interplay and intersections among ferroptosis, apoptosis and autophagy. Drug Metab. Pers. Ther. 2025, 40, 89–105. [Google Scholar] [CrossRef]
- Shen, H.; Wei, Y.; Yang, Q.; Cai, Y.; Zhu, K.; Chen, X. Scoparone induces both apoptosis and ferroptosis via multiple mechanisms in non-small-cell lung cancer cells. Toxicol. Vitr. 2023, 91, 105627. [Google Scholar] [CrossRef]
- Wu, L.; Xu, G.; Li, N.; Zhu, L.; Shao, G. Curcumin Analog, HO-3867, Induces Both Apoptosis and Ferroptosis via Multiple Mechanisms in NSCLC Cells with Wild-Type p53. Evid. Based Complement Altern. Med. 2023, 2023, 8378581. [Google Scholar] [CrossRef] [PubMed]
- Palabiyik, A.A. The role of Bcl-2 in controlling the transition between autophagy and apoptosis (Review). Mol. Med. Rep. 2025, 32, 172. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Liao, M.; Qin, R.; Zhu, S.; Peng, C.; Fu, L.; Chen, Y.; Han, B. Regulated cell death (RCD) in cancer: Key pathways and targeted therapies. Signal Transduct. Target. Ther. 2022, 7, 286. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.; Welsh, C.M.; He, Y.W. Targeting regulated cell death pathways in cancers for effective treatment: A comprehensive review. Front. Cell Dev. Biol. 2024, 12, 1462339. [Google Scholar] [CrossRef]
- Piccolo, M.; Russo, C.; Arciuolo, V.; Ferraro, M.G.; Abbate, V.; Di Porzio, A.; Cinquegrana, E.; Di Leva, F.S.; Pagano, B.; Randazzo, A.; et al. Design, Synthesis, and Anticancer Activity of Drug-like Iron Chelators/G-Quadruplex Binders as Synergic Dual Targeting Agents. J. Med. Chem. 2025, 68, 1245–1259. [Google Scholar] [CrossRef]
- Miniaci, M.C.; Irace, C.; Capuozzo, A.; Piccolo, M.; Di Pascale, A.; Russo, A.; Lippiello, P.; Lepre, F.; Russo, G.; Santamaria, R. Cysteine Prevents the Reduction in Keratin Synthesis Induced by Iron Deficiency in Human Keratinocytes. J. Cell. Biochem. 2016, 117, 402–412. [Google Scholar] [CrossRef]
- Santamaria, R.; Irace, C.; Festa, M.; Maffettone, C.; Colonna, A. Induction of ferritin expression by oxalomalate. Biochim. Biophys. Acta. 2004, 1691, 151–159. [Google Scholar] [CrossRef][Green Version]
- Arosio, P.; Ingrassia, R.; Cavadini, P. Ferritins: A family of molecules for iron storage, antioxidation and more. Biochim. Biophys. Acta. 2009, 1790, 589–599. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Zhang, R.; Wang, F.; Wang, T.; Jiao, Y. The Role of Erastin in Ferroptosis and Its Prospects in Cancer Therapy. Onco Targets Ther. 2020, 13, 5429–5441. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, Y.; Jiang, Y.; Zhang, L.; Cheng, W. GPX4: The hub of lipid oxidation, ferroptosis, disease and treatment. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, F.; Ding, X.; Qin, J.; Wang, W.; Luo, L. Identifying ALOX15-initiated lipid peroxidation increases susceptibility to ferroptosis in asthma epithelial cells. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167176. [Google Scholar] [CrossRef] [PubMed]
- Benatzy, Y.; Palmer, M.A.; Brüne, B. Arachidonate 15-lipoxygenase type B: Regulation, function, and its role in pathophysiology. Front. Pharmacol. 2022, 13, 1042420. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Chung, S.W. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019, 10, 822. [Google Scholar] [CrossRef]
- Khatun, J.; Gelles, J.D.; Chipuk, J.E. Dynamic death decisions: How mitochondrial dynamics shape cellular commitment to apoptosis and ferroptosis. Dev. Cell 2024, 59, 2549–2565. [Google Scholar] [CrossRef]
- Costigan, A.; Hollville, E.; Martin, S.J. Discriminating Between Apoptosis, Necrosis, Necroptosis, and Ferroptosis by Microscopy and Flow Cytometry. Curr. Protoc. 2023, 3, e951. [Google Scholar] [CrossRef]
- Zagami, P.; Carey, L.A. Triple negative breast cancer: Pitfalls and progress. NPJ Breast Cancer 2022, 8, 95. [Google Scholar] [CrossRef]
- Jie, H.; Ma, W.; Huang, C. Diagnosis, Prognosis, and Treatment of Triple-Negative Breast Cancer: A Review. Breast Cancer Dove Med. Press 2025, 17, 265–274. [Google Scholar] [CrossRef]
- Bianchini, G.; De Angelis, C.; Licata, L.; Gianni, L. Treatment landscape of triple-negative breast cancer—Expanded options, evolving needs. Nat. Rev. Clin. Oncol. 2022, 19, 91–113. [Google Scholar] [CrossRef]
- Domínguez-Jurado, E.; Lara-Sánchez, A.; Bravo, I.; Ocaña, A.; Alonso-Moreno, C. State of the art in organometallic ruthenium metallodrugs for breast cancer treatment: Advances and innovations. Coord. Chem. Rev. 2025, 523, 216252. [Google Scholar] [CrossRef]
- Golbaghi, G.; Castonguay, A. Rationally Designed Ruthenium Complexes for Breast Cancer Therapy. Molecules 2020, 25, 265. [Google Scholar] [CrossRef] [PubMed]
- Das, U.; Basu, U.; Paira, P. Recent trends in the design and delivery strategies of ruthenium complexes for breast cancer therapy. Dalton Trans. 2024, 53, 15113–15157. [Google Scholar] [CrossRef] [PubMed]
- Côrte-Real, L.; Matos, A.P.; Alho, I.; Morais, T.S.; Tomaz, A.I.; Garcia, M.H.; Santos, I.; Bicho, M.P.; Marques, F. Cellular uptake mechanisms of an antitumor ruthenium compound: The endosomal/lysosomal system as a target for anticancer metal-based drugs. Microsc. Microanal. 2013, 19, 1122–1130. [Google Scholar] [CrossRef]
- Musumeci, D.; Rozza, L.; Merlino, A.; Paduano, L.; Marzo, T.; Massai, L.; Messori, L.; Montesarchio, D. Interaction of anticancer Ru(III) complexes with single stranded and duplex DNA model systems. Dalton Trans. 2015, 44, 13914–13925. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, L.; Ding, J.; Chen, Y. Iron Metabolism in Cancer. Int. J. Mol. Sci. 2018, 20, 95. [Google Scholar] [CrossRef]
- An, X.; Yu, W.; Liu, J.; Tang, D.; Yang, L.; Chen, X. Oxidative cell death in cancer: Mechanisms and therapeutic opportunities. Cell Death Dis. 2024, 15, 556. [Google Scholar] [CrossRef]
- Averill-Bates, D.A. The antioxidant glutathione. Vitam. Horm. 2023, 121, 109–141. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Lewerenz, J.; Hewett, S.J.; Huang, Y.; Lambros, M.; Gout, P.W.; Kalivas, P.W.; Massie, A.; Smolders, I.; Methner, A.; Pergande, M.; et al. The cystine/glutamate antiporter system x(c)(-) in health and disease: From molecular mechanisms to novel therapeutic opportunities. Antioxid. Redox Signal. 2013, 18, 522–555. [Google Scholar] [CrossRef]
- Liu, J.; Xia, X.; Huang, P. xCT: A Critical Molecule That Links Cancer Metabolism to Redox Signaling. Mol. Ther. 2020, 28, 2358–2366. [Google Scholar] [CrossRef] [PubMed]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021, 12, 599–620. [Google Scholar] [CrossRef] [PubMed]
- Polewski, M.D.; Reveron-Thornton, R.F.; Cherryholmes, G.A.; Marinov, G.K.; Cassady, K.; Aboody, K.S. Increased Expression of System xc- in Glioblastoma Confers an Altered Metabolic State and Temozolomide Resistance. Mol. Cancer Res. 2016, 14, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Xie, E.; Li, Y.; Li, J.; Zhang, Y.; Chi, X.; Hu, X.; Xu, L.; Hou, T.; Stockwell, B.R.; et al. The structure of erastin-bound xCT-4F2hc complex reveals molecular mechanisms underlying erastin-induced ferroptosis. Cell Res. 2022, 32, 687–690. [Google Scholar] [CrossRef]
- Larraufie, M.H.; Yang, W.S.; Jiang, E.; Thomas, A.G.; Slusher, B.S.; Stockwell, B.R. Incorporation of metabolically stable ketones into a small molecule probe to increase potency and water solubility. Bioorg. Med. Chem. Lett. 2015, 25, 4787–4792. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, B.; Xiong, Q.; Feng, Y.; Du, H. Erastin-induced ferroptosis causes physiological and pathological changes in healthy tissues of mice. Mol. Med. Rep. 2021, 24, 713. [Google Scholar] [CrossRef]
- Patel, D.; Kharkar, P.S.; Gandhi, N.S.; Kaur, E.; Dutt, S.; Nandave, M. Novel analogs of sulfasalazine as system xc− antiporter inhibitors: Insights from the molecular modeling studies. Drug Dev. Res. 2019, 80, 758–777. [Google Scholar] [CrossRef]
- Zheng, J.; Sato, M.; Mishima, E.; Sato, H.; Proneth, B.; Conrad, M. Sorafenib fails to trigger ferroptosis across a wide range of cancer cell lines. Cell Death Dis. 2021, 12, 698. [Google Scholar] [CrossRef]
- Abbasi, U.; Abbina, S.; Gill, A.; Kizhakkedathu, J.N. Development of an iron overload HepG2 cell model using ferrous ammonium citrate. Sci. Rep. 2023, 13, 21915. [Google Scholar] [CrossRef]
- Kotla, N.K.; Dutta, P.; Parimi, S.; Das, N.K. The Role of Ferritin in Health and Disease: Recent Advances and Understandings. Metabolites 2022, 12, 609. [Google Scholar] [CrossRef]
- Wang, J.; Wu, N.; Peng, M.; Oyang, L.; Jiang, X.; Peng, Q.; Zhou, Y.; He, Z.; Liao, Q. Ferritinophagy: Research advance and clinical significance in cancers. Cell Death Discov. 2023, 9, 463. [Google Scholar] [CrossRef]
- Latunde-Dada, G.O. Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1893–1900. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Z.; Dong, Z.; Liu, S.; Kan, H.; Zhang, S. Multifaceted interplays between the essential players and lipid peroxidation in ferroptosis. J. Genet. Genom. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ueta, E.; Kimura, T.; Yamamoto, T.; Osaki, T. Reactive oxygen species (ROS) control the expression of Bcl-2 family proteins by regulating their phosphorylation and ubiquitination. Cancer Sci. 2004, 95, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Susnow, N.; Zeng, L.; Margineantu, D.; Hockenbery, D.M. Bcl-2 family proteins as regulators of oxidative stress. Semin. Cancer Biol. 2009, 19, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Marquez, R.T.; Xu, L. Bcl-2:Beclin 1 complex: Multiple, mechanisms regulating autophagy/apoptosis toggle switch. Am. J. Cancer Res. 2012, 2, 214–221. [Google Scholar]
- Wu, Z.; Su, M.; Chen, H.; Chen, X.; Chen, C.Y.; An, L.; Shao, Z.; Liu, X.; Lin, Y.; OuYang, A.J.; et al. Sinularin Exerts Anti-cancer Effects by Inducing Oxidative Stress-mediated Ferroptosis, Apoptosis, and Autophagy in Prostate Cancer Cells. Anticancer Agents Med. Chem. 2023, 23, 1457–1468. [Google Scholar] [CrossRef]



| IC50 Values (µM) | |||||||
|---|---|---|---|---|---|---|---|
| Drugs | TNBC Cell Lines | Healthy Cell Lines | |||||
| Hs578T | MDA-MB-231 | BT-549 | HDFa | HHFK | HaCaT | MCF-10A | |
| cDDP | 20 ± 4 | 11 ± 3 | 15 ± 2 | 72 ± 4 | 93 ± 3 | 50 ± 3 | 88 ± 3 |
| PalmiPyRu | 24 ± 3 | 18 ± 2 | 37 ± 5 | >150 | >150 | >150 | >150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraro, M.G.; Iazzetti, F.; Bocchetti, M.; Riccardi, C.; Montesarchio, D.; Santamaria, R.; Misso, G.; Piccolo, M.; Irace, C. Ferroptosis Among the Antiproliferative Pathways Activated by a Lipophilic Ruthenium(III) Complex as a Candidate Drug for Triple-Negative Breast Cancer. Pharmaceutics 2025, 17, 918. https://doi.org/10.3390/pharmaceutics17070918
Ferraro MG, Iazzetti F, Bocchetti M, Riccardi C, Montesarchio D, Santamaria R, Misso G, Piccolo M, Irace C. Ferroptosis Among the Antiproliferative Pathways Activated by a Lipophilic Ruthenium(III) Complex as a Candidate Drug for Triple-Negative Breast Cancer. Pharmaceutics. 2025; 17(7):918. https://doi.org/10.3390/pharmaceutics17070918
Chicago/Turabian StyleFerraro, Maria Grazia, Federica Iazzetti, Marco Bocchetti, Claudia Riccardi, Daniela Montesarchio, Rita Santamaria, Gabriella Misso, Marialuisa Piccolo, and Carlo Irace. 2025. "Ferroptosis Among the Antiproliferative Pathways Activated by a Lipophilic Ruthenium(III) Complex as a Candidate Drug for Triple-Negative Breast Cancer" Pharmaceutics 17, no. 7: 918. https://doi.org/10.3390/pharmaceutics17070918
APA StyleFerraro, M. G., Iazzetti, F., Bocchetti, M., Riccardi, C., Montesarchio, D., Santamaria, R., Misso, G., Piccolo, M., & Irace, C. (2025). Ferroptosis Among the Antiproliferative Pathways Activated by a Lipophilic Ruthenium(III) Complex as a Candidate Drug for Triple-Negative Breast Cancer. Pharmaceutics, 17(7), 918. https://doi.org/10.3390/pharmaceutics17070918












