Therapeutic Evaluation Punica granatum Peel Powder for the Ailment of Inflammatory Bowel Disorder in NCM460 Cell Line and in Albino Rats
Abstract
1. Introduction
2. Materials and Method
2.1. Peel Powder of Punica Granatum (PPPG) Sample Preparation
2.2. Preliminary Phytochemical Analysis
2.3. Estimation of Total Phenolic Content
2.4. Animal Experiment
2.5. Primary Selection of Extraction for the Effective Anti-Inflammatory Activity in HRBC Membrane Stabilization Method
2.6. Cell Culture for the Anti-Inflammatory Activity
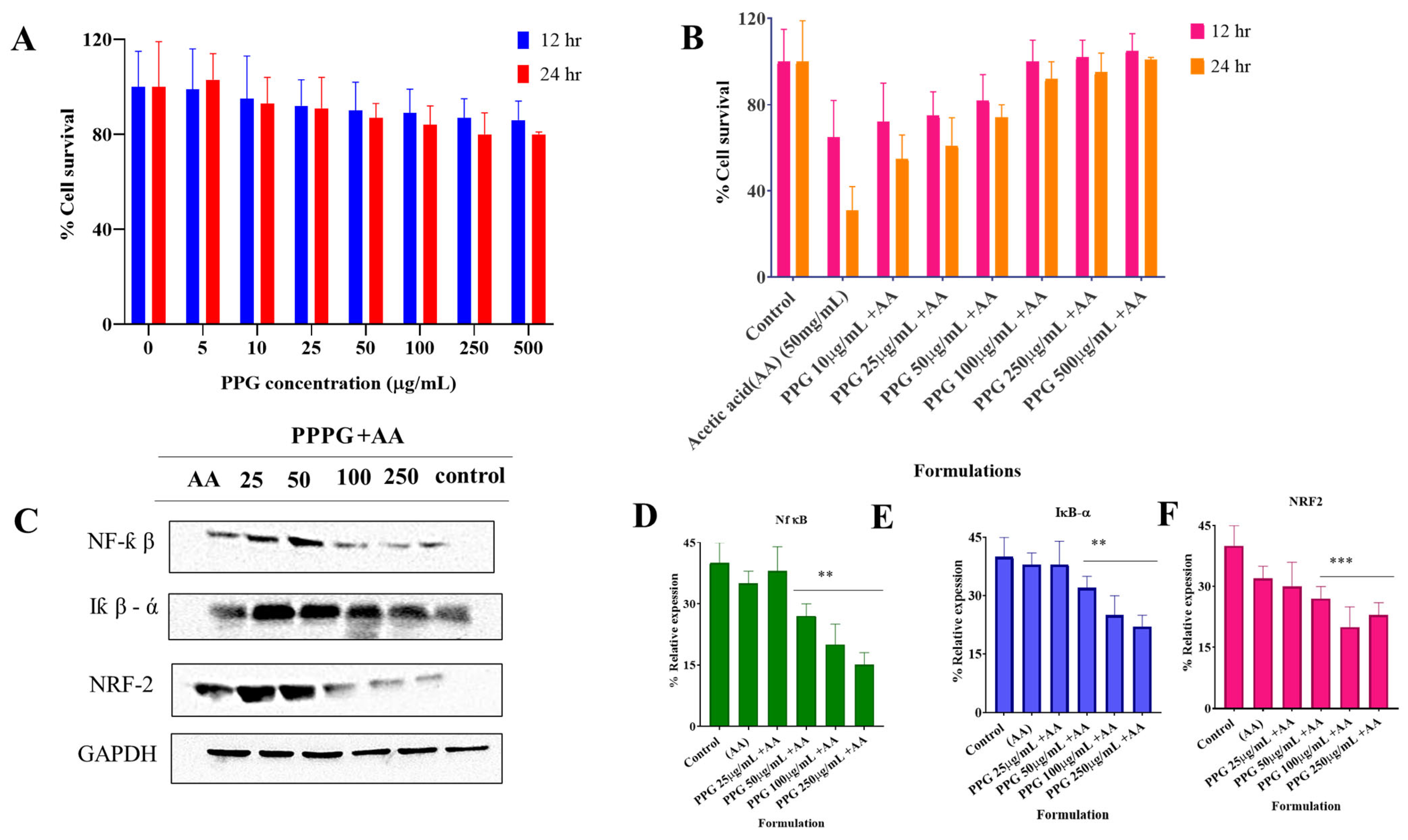
2.6.1. Cellular Toxicity Study
2.6.2. Western Blot Analysis
2.7. Induction of Colitis by Acetic Acid and Experimental Design
- (a)
- Control group: Received the same experimental handling as the test group but without acetic acid treatment. Drug treatment was substituted with vehicle (water for injection) administration, orally (5 mL/kg).
- (b)
- PPPG group: Treated orally or i.p. with PPPG (100 mg/kg) without acetic acid treatment, this group was maintained to observe any untoward effect or other than therapeutic effect of the peel powder.
- (c)
- Acetic acid treatment group: Received oral (5 mL/kg) or i.p. (2 mL/kg) vehicle after induction of colitis with acetic acid.
- (d)
- Acetic acid treatment plus low-dose PPPG group: Treated orally or i.p. with 3 mg/kg PPPG after acetic acid treatment.
- (e)
- Acetic acid treatment plus moderate-dose PPPG group: Treated orally or i.p. with 30 mg/kg PPPG after acetic acid treatment.
- (f)
- Acetic acid treatment plus high-dose PPPG group: Treated orally or i.p. with 100 mg/kg PPPG after acetic acid treatment.
- (g)
- Reference group: Treated intrarectally with 100 mg/kg 5-aminosalicylic acid (5-ASA) as a standard treatment after acetic acid treatment.
2.8. Biomarker Estimation
2.8.1. Estimation of Myeloperoxidase (MPO)
2.8.2. Estimation of Lipid Peroxidation (Malondialdehyde) (MDA) in the Inflamed Colon
- -
- OD of T represents the optical density of the sample at 532 nm;
- -
- Total volume of the reaction mixture is the total volume of the reaction mixture in mL;
- -
- The nanomolar extinction coefficient of MDA (1.56 × 105 M−1 cm−1) is used for conversion;
- -
- Sample volume (mg protein/mL) refers to the volume of the sample containing protein in mg per mL.
2.9. Histopathological Study: Excised Colon of Representative Sample from Various Roups Were Observed After H&E Staining by a Person Blind to the Treatment Protocol
- -
- Score of 0: normal intestinal mucosa observed in the visual field.
- -
- Score of 1: mild inflammation and edema of the mucosal layer with disappearance of 1/3 of the crypts at the basal part.
- -
- Score of 2: moderate inflammation of the mucosal layer with disappearance of 2/3 of the crypts at the basal part.
- -
- Score of 3: moderate inflammation of the mucosal layer with complete disappearance of crypts, while the epithelial layer remains intact.
- -
- Score of 4: severe inflammation involving the mucosa, submucosa, and myometrium, with complete disappearance of crypts and epithelium.
2.10. Statistical Analysis
3. Result
3.1. Anti-Inflammatory Activity
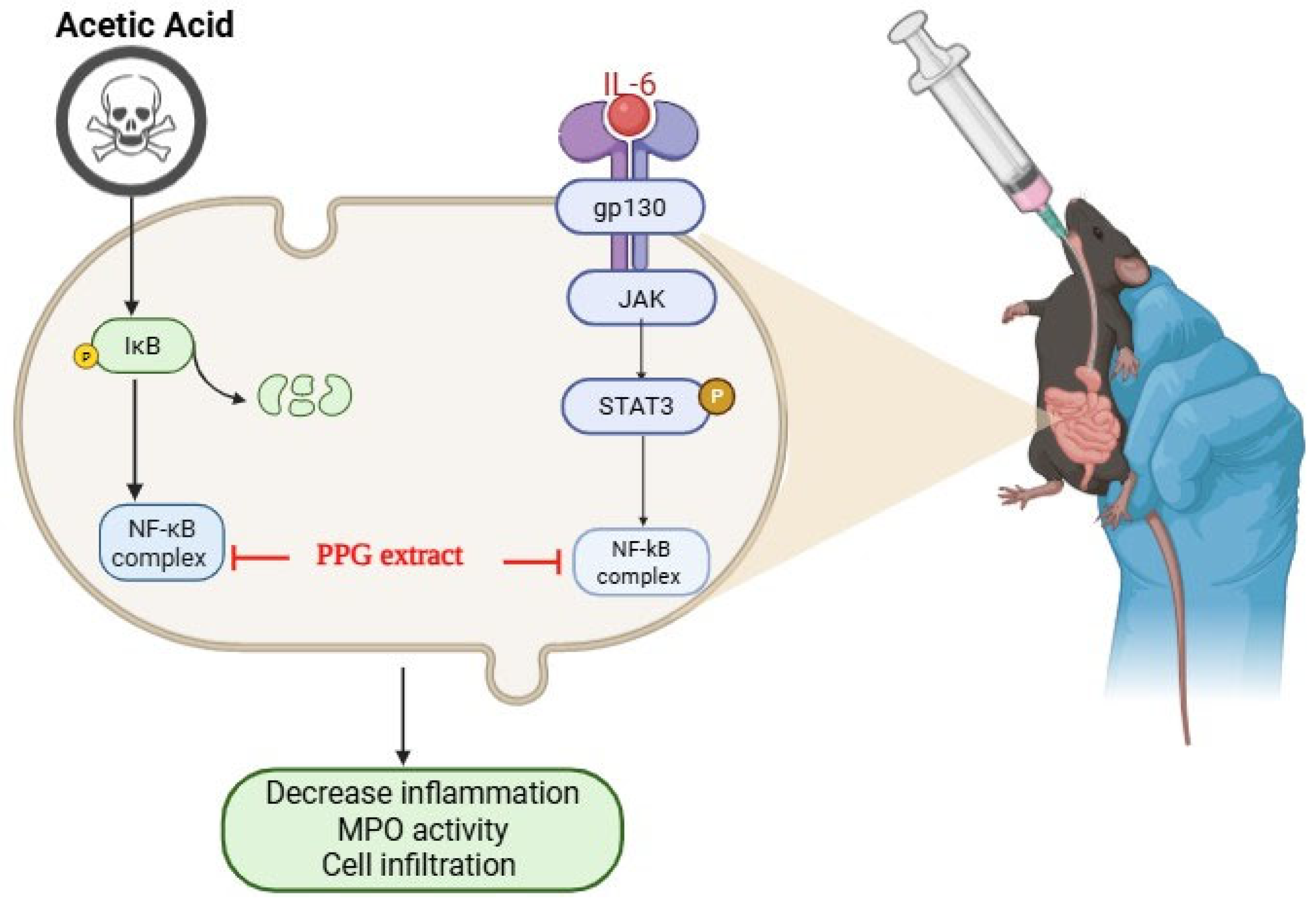
3.2. PPG Alleviated Acetic Acid Induced Injury in NCM460 Cells
Effect of PPG on NCM460 Cell Viability and Inflammatory Signaling Pathways
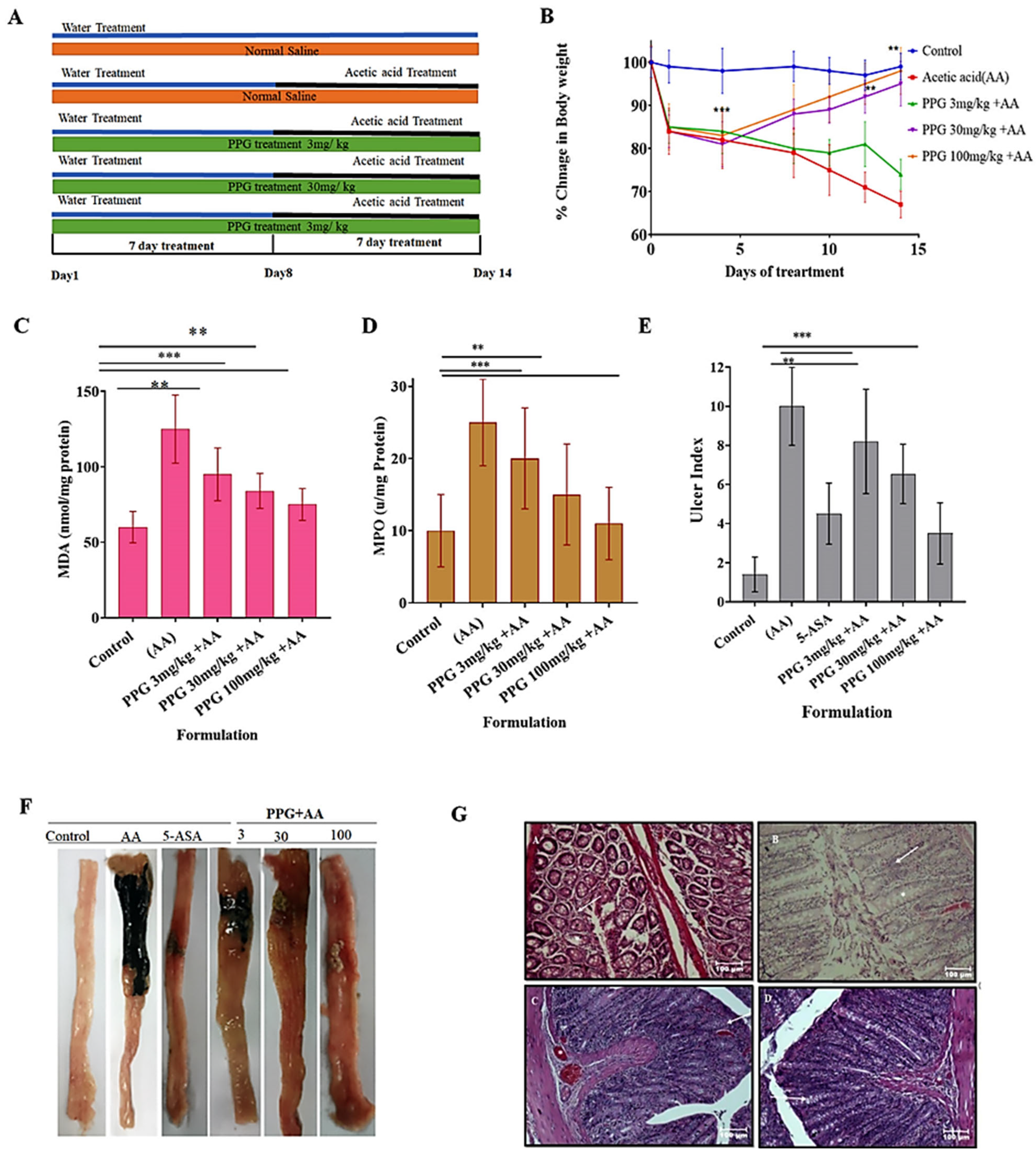
3.3. Acute Toxicity Studies In Vivo
3.4. Recording of Body Weight of Animals from Various Groups
3.5. Change in the Level of Malondialdehyde (MDA) in the Inflamed Colon of Experimental Animals
3.6. Change in the Level of Myeloperoxidase (MPO) in the Inflamed Colon of Experimental Animals
3.7. Ulcer Index of the Inflamed Colon of Experimental Animals from Various Groups
Colitis Control Group of Animals Recorded a Significant Increase in Ulcer Index
3.8. Gross Morphological Changes in the Parts of GIT (Colon) Experimental Animals
3.9. Histopathological Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loddo, I.; Romano, C. Inflammatory bowel disease: Genetics, epigenetics, and pathogenesis. Front. Immunol. 2015, 6, 551. [Google Scholar] [CrossRef] [PubMed]
- Kővári, B.; Báthori, Á.; Friedman, M.S.; Lauwers, G.Y. Histologic diagnosis of inflammatory bowel diseases. Adv. Anat. Pathol. 2022, 29, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, J.; Escudier, M.P. Diseases of the Gastrointestinal Tract. In Burket’s Oral Medicine; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 553–577. [Google Scholar]
- Mathias, C.B.; McAleer, J.P.; Szollosi, D.E. Inflammatory Diseases of the Gastrointestinal Tract and Pharmacological Treatments. In Pharmacology of Immunotherapeutic Drugs; Springer: Cham, Switzerland, 2020; pp. 175–205. [Google Scholar]
- Rubenstein, J.H.; Waljee, A.; Jeter, J.; Velayos, F.; Ladabaum, U.; Higgins, P.D. Cost-effectiveness of ulcerative colitis surveillance in the setting of 5-aminosalicylates. Am. J. Gastroenterol. 2009, 104, 2222. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.; Mahon, B.; Jones, T.; Griffin, P. An assessment of the human health impact of seven leading foodborne pathogens in the United States using disability adjusted life years. Epidemiol. Infect. 2015, 143, 2795–2804. [Google Scholar] [CrossRef]
- Rafeeq, M.; Murad, H.A.S.; Abdallah, H.M.; El-Halawany, A.M. Protective effect of 6-paradol in acetic acid-induced ulcerative colitis in rats. BMC Complement. Med. Ther. 2021, 21, 28. [Google Scholar]
- Sartor, R.B.; Wu, G.D. Roles for intestinal bacteria, viruses, and fungi in pathogenesis of inflammatory bowel diseases and therapeutic approaches. Gastroenterology 2017, 152, 327–339.e4. [Google Scholar] [CrossRef]
- Shanahan, F. Probiotics and inflammatory bowel disease: Is there a scientific rationale? Inflamm. Bowel Dis. 2000, 6, 107–115. [Google Scholar] [CrossRef]
- Khalili, H.; Chan, S.S.; Lochhead, P.; Ananthakrishnan, A.N.; Hart, A.R.; Chan, A.T. The role of diet in the aetiopathogenesis of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 525–535. [Google Scholar] [CrossRef]
- Joshi, C.; Patel, P.; Kothari, V. Anti-infective potential of hydroalcoholic extract of Punica granatum peel against gram-negative bacterial pathogens. F1000Research 2019, 8, 70. [Google Scholar] [CrossRef]
- Suthar, V. Therapeutic Effects of Pomegranate (Punica granatum). J. Pharm. Res. Innov. (JPRI) 2022, 2, 23–28. [Google Scholar] [CrossRef]
- Bhandari, P.R. Pomegranate (Punica granatum L.). Ancient seeds for modern cure? Review of potential therapeutic applications. Int. J. Nutr. Pharmacol. Neurol. Dis. 2012, 2, 171–184. [Google Scholar] [CrossRef]
- Ramya, S.; Narayanan, V.; Ponnerulan, B.; Saminathan, E.; Veeranan, U. Potential of peel extracts of Punica granatum and Citrus aurantifolia on alloxan-induced diabetic rats. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 24. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.-F.; Al Askar, A.A. In vitro evaluation of biological activities and phytochemical analysis of different solvent extracts of Punica granatum L. (Pomegranate) peels. Plants 2021, 10, 2742. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Harborne, J.B. Comparative biochemistry of the flavonoids-IV.: Correlations between chemistry, pollen morphology and systematics in the family plumbaginaceae. Phytochemistry 1967, 6, 1415–1428. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Nikolaeva, T.; Lapshin, P.; Zagoskina, N. Method for Determining the Total Content of Phenolic Compounds in Plant Extracts with Folin–Denis Reagent and Folin–Ciocalteu Reagent: Modification and Comparison. Russ. J. Bioorganic Chem. 2022, 48, 1519–1525. [Google Scholar] [CrossRef]
- Hotta, S.K.; Neelima, N. In Vitro Evaluation of Anti Inflammatory Activity of Methanolic And Ethanolic Leaf Extract of Psidium guajava. World J. Curr. Med. Pharm. Res. 2020, 2, 159–165. [Google Scholar] [CrossRef]
- Huangfu, S.; Dou, R.; Zhong, S.; Guo, M.; Gu, C.; Jurczyszyn, A.; Yang, Y.; Jiang, B. Modified Pulsatillae decoction inhibits DSS-induced ulcerative colitis in vitro and in vivo via IL-6/STAT3 pathway. BMC Complement. Med. Ther. 2020, 20, 179. [Google Scholar] [CrossRef]
- De, A.; Ko, Y.T. Why mRNA-ionizable LNPs formulations are so short-lived: Causes and way-out. Expert Opin. Drug Deliv. 2023, 20, 175–187. [Google Scholar] [CrossRef]
- De, A.; Kang, J.H.; Lee, O.H.; Ko, Y.T. Optimizing long-term stability of siRNA using thermoassemble ionizable reverse pluronic-Bcl2 micelleplexes. Int. J. Biol. Macromol. 2024, 264, 130783. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, S.; Wang, M.; Chen, X.; Tian, L.; Wang, L.; Yang, W.; Chen, L.; He, F.; Yin, W. Flavonoid-rich extracts from okra flowers exert antitumor activity in colorectal cancer through induction of mitochondrial dysfunction-associated apoptosis, senescence and autophagy. Food Funct. 2020, 11, 10448–10466. [Google Scholar] [CrossRef] [PubMed]
- De, A.; Roychowdhury, P.; Bhuyan, N.R.; Ko, Y.T.; Singh, S.K.; Dua, K.; Kuppusamy, G. Folic Acid Functionalized Diallyl Trisulfide–Solid Lipid Nanoparticles for Targeting Triple Negative Breast Cancer. Molecules 2023, 28, 1393. [Google Scholar] [CrossRef]
- Cruz-Muñoz, J.R.; Barrios-García, T.; Valdez-Morales, E.E.; Durán-Vazquez, M.F.; Méndez-Rodríguez, K.B.; Barajas-Espinosa, A.; Ochoa-Cortes, F.; Martínez-Saldaña, M.C.; Gómez-Aguirre, Y.A.; Alba, R.G. Ethanolic extract from Lepidium virginicum L. ameliorates DNBS-induced colitis in rats. J. Ethnopharmacol. 2022, 289, 115056. [Google Scholar] [CrossRef]
| Constituents | Water | Ethanol | Methanol | Ethyl Acetate |
|---|---|---|---|---|
| Carbohydrate | + | + | + | − |
| Phytosterol | − | − | − | − |
| Fixed oil | − | − | − | − |
| Alkaloid | − | − | − | − |
| Glycoside | − | + | + | − |
| Saponin | + | − | − | − |
| Flavonoid | − | − | + | + |
| Tannin | + | + | + |
| Treatment | 100 µg/mL | 200 µg/mL | 300 µg/mL |
|---|---|---|---|
| Control (Distilled Water) | 100.00 ± 0.00 (0.00%) | – | – |
| Water Extract | 46.76 ± 2.03 (53.24%) | 41.18 ± 1.06 (58.82%) | 36.26 ± 2.05 (63.74%) |
| Ethanolic Extract | 39.12 ± 1.02 (60.88%) | 34.55 ± 1.19 (65.45%) | 30.76 ± 0.06 (69.24%) |
| Methanolic Extract | 08.75 ± 0.03 (91.25%) * | 05.16 ± 1.11 (94.84%) * | 00.74 ± 2.04 (99.26%) * |
| Ethyl Acetate Extract | 12.27 ± 0.62 (87.73%) * | 04.26 ± 0.98 (95.74%) * | 02.87 ± 1.54 (97.13%) * |
| Diclofenac Sodium | 02.87 ± 1.20 (97.13%) * | 02.38 ± 0.90 (97.62%) * | 00.97 ± 0.54 (99.03%) * |
| Dose (mg/kg) | Skin Color | Diarrhea | Lacrimation | Sedation | Respiration |
|---|---|---|---|---|---|
| 3 | No change | Not observed | No | No | Normal |
| 30 | No change | Not observed | No | No | Normal |
| 100 | No change | Not observed | No | No | Normal |
| 250 | No change | Little loose tools (20% animals) | Frequently (50% animals) | No | Abnormal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roychowdhury, P.; Prajapati, G.K.; Singh, R.; Gurunath, P.; C, R.; Kuppuswamy, G.; De, A. Therapeutic Evaluation Punica granatum Peel Powder for the Ailment of Inflammatory Bowel Disorder in NCM460 Cell Line and in Albino Rats. Pharmaceutics 2025, 17, 843. https://doi.org/10.3390/pharmaceutics17070843
Roychowdhury P, Prajapati GK, Singh R, Gurunath P, C R, Kuppuswamy G, De A. Therapeutic Evaluation Punica granatum Peel Powder for the Ailment of Inflammatory Bowel Disorder in NCM460 Cell Line and in Albino Rats. Pharmaceutics. 2025; 17(7):843. https://doi.org/10.3390/pharmaceutics17070843
Chicago/Turabian StyleRoychowdhury, Parikshit, Gyanendra Kumar Prajapati, Rupesh Singh, Prasanna Gurunath, Ramesh C, Gowthamarajan Kuppuswamy, and Anindita De. 2025. "Therapeutic Evaluation Punica granatum Peel Powder for the Ailment of Inflammatory Bowel Disorder in NCM460 Cell Line and in Albino Rats" Pharmaceutics 17, no. 7: 843. https://doi.org/10.3390/pharmaceutics17070843
APA StyleRoychowdhury, P., Prajapati, G. K., Singh, R., Gurunath, P., C, R., Kuppuswamy, G., & De, A. (2025). Therapeutic Evaluation Punica granatum Peel Powder for the Ailment of Inflammatory Bowel Disorder in NCM460 Cell Line and in Albino Rats. Pharmaceutics, 17(7), 843. https://doi.org/10.3390/pharmaceutics17070843








