Nanoparticles and Nanomaterials: A Review from the Standpoint of Pharmacy and Medicine
Abstract
1. Introduction
2. Classification of NMs
3. Classification of NPs
3.1. Organic NPs
3.1.1. Liposomal NPs
3.1.2. VLPs as Contemporary Immunobiological Nanostructures
3.1.3. Polymeric and Dendrimeric NPs
3.2. Inorganic NPs
3.3. Carbon-Based NPs
3.4. Quantum Dots
4. Physicochemical Properties of NPs
4.1. Morphological Properties of NPs
4.2. Mechanical Properties of NPs
4.3. Specific Surface Area
4.4. Types of Intermolecular Interactions
4.4.1. Van der Waals Forces
4.4.2. Electrostatic Interaction Forces
4.5. Electric Double Layer (EDL)
4.5.1. Derjaguin–Landau–Verwey–Overbeek Theory (DLVO)
4.5.2. Johnson–Kendall–Roberts Theory (JKR)
- Not applicable for solid objects;
- The particles are assumed to possess a symmetrical shape and uniform force distribution;
- Dynamic factors, such as the rate of convergence of particles and the influence of external forces, are not taken into consideration.
4.5.3. Derjaguin–Muller–Toporov Theory (DMT)
4.6. Surface Plasmon Resonance
4.7. The Phenomenon of Intrinsic Radiothermal Emission
4.8. Magnetic Properties of NPs
4.9. Thermal Properties of NPs
5. Methods of NPs Synthesis
5.1. Synthesis of LNPs
5.2. Biosynthesis of VLP
5.3. Synthesis of Polymeric NPs
5.4. Synthesis of Dendrimer NPs
5.5. Preparation of Inorganic NPs
5.6. Synthesis of Carbon NPs and Quantum Dots
6. Contemporary Approaches to NPs Modification
7. Contemporary Application of NPs
7.1. Application of LNPs in Pharmacy
7.2. Application of Modern Nanovaccines—VLPs
7.3. Applications of Polymeric NPs and Dendrimers
7.4. Application of Inorganic NPs
7.5. Carbon Particles and Quantum Dots in Pharmacy
7.6. Applications of Peptide Modified NPs
8. Quality Control of NPs as Pharmaceuticals
8.1. Evaluation of the Morphological and Topographic Characteristics of NPs
8.1.1. Electron Microscopy
8.1.2. Dynamic Light Scattering
8.1.3. ζ-Potential
8.1.4. The Brunauer-Emmett-Teller (BET) and Barrett-Joyner-Halenda (BJH) Methods
8.2. Control of the Chemical Structure of NPs
8.2.1. IR-Spectroscopy
8.2.2. Raman Spectroscopy
8.2.3. X-Ray Fluorescence Analysis
8.3. Evaluation of the Optical Properties of NPs
8.3.1. UV-Spectroscopy
8.3.2. Fluorimetry
8.4. Evaluation of the Thermal Properties of NPs
8.4.1. Differential Scanning Calorimetry
8.4.2. Thermogravimetry
8.5. Intrinsic Radiothermal Emission of NPs
9. Discussion and Conclusions
10. Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Einstein, A. Eine Neue Bestimmung Der Moleküldimensionen Inaugural-Dissertation. 1905. Available online: https://www.research-collection.ethz.ch/bitstream/handle/20.500.11850/139872/eth-30378-01.pdf;jsessionid=483E0F97C497CEEF5230D3257BF2E6CD?sequence=1 (accessed on 12 December 2024).
- Knoll, V.M.; Ruska, E. Das Elektronenmikroskop. 1931. Available online: https://iubemcenter.indiana.edu/doc/ruska/ruska-the-electron-microscope.pdf (accessed on 12 December 2024).
- Feynman, R.P. Plenty of Room at the Bottom. 1959. Available online: https://web.pa.msu.edu/people/yang/RFeynman_plentySpace.pdf (accessed on 12 December 2024).
- Hansma, P.K.; Elings, V.B.; Marti, O.; Bracker, C.E. Scanning Tunneling Microscopy and Atomic Force Microscopy: Application to Biology and Technology. Science 1988, 242, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Binnig, G.; Rohrer, H. Scanning Tunneling Microscopy-From Birth to Adolescene. Rev. Mod. Phys. 1987, 59, 615. [Google Scholar] [CrossRef]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic Force Microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef]
- Sandhu, R.; Singh, N.; Dhankhar, J.; Gandhi, K.; Sharma, R. Dynamic Light Scattering (DLS) Technique, Principle, Theoretical and Applications. Biophys. Rev. 2018, 8, 409–427. [Google Scholar] [CrossRef]
- Kreuter, J. Nanoparticles and Nanocapsules—New Dosage Forms in the Nanometer Size Range. Pharm. Acta Helv. 1978, 53, 33–39. [Google Scholar]
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A Systematic Review on Silver Nanoparticles-Induced Cytotoxicity: Physicochemical Properties and Perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Agnihotri, R.; Gaur, S.; Albin, S. Nanometals in Dentistry: Applications and Toxicological Implications—A Systematic Review. Biol. Trace Elem. Res. 2020, 197, 70–88. [Google Scholar] [CrossRef]
- Carvalho, A.P.A.; Conte-Junior, C.A. Recent Advances on Nanomaterials to COVID-19 Management: A Systematic Review on Antiviral/Virucidal Agents and Mechanisms of SARS-CoV-2 Inhibition/Inactivation. Glob. Chall. 2021, 5, 2000115. [Google Scholar] [CrossRef]
- Csáki, A.; Möller, R.; Fritzsche, W. Gold Nanoparticles as Novel Label for DNA Diagnostics. Expert Rev. Mol. Diagn. 2002, 2, 187–193. [Google Scholar] [CrossRef]
- Altammar, K.A. A Review on Nanoparticles: Characteristics, Synthesis, Applications, and Challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef] [PubMed]
- Rezić, I. Nanoparticles for Biomedical Application and Their Synthesis. Polymers 2022, 14, 4961. [Google Scholar] [CrossRef] [PubMed]
- Geoffrion, L.D.; Guisbiers, G. Quantum Confinement: Size on the Grill! J. Phys. Chem. Solids 2020, 140, 109320. [Google Scholar] [CrossRef]
- Kolahalam, L.A.; Kasi Viswanath, I.V.; Diwakar, B.S.; Govindh, B.; Reddy, V.; Murthy, Y.L.N. Review on Nanomaterials: Synthesis and Applications. Mater. Today Proc. 2019, 18, 2182–2190. [Google Scholar] [CrossRef]
- Paras; Yadav, K.; Kumar, P.; Teja, D.R.; Chakraborty, S.; Chakraborty, M.; Mohapatra, S.S.; Sahoo, A.; Chou, M.M.C.; Liang, C.-T.; et al. A Review on Low-Dimensional Nanomaterials: Nanofabrication, Characterization and Applications. Nanomaterials 2022, 13, 160. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Wong, X.Y.; Sena-Torralba, A.; Álvarez-Diduk, R.; Muthoosamy, K.; Merkoçi, A. Nanomaterials for Nanotheranostics: Tuning Their Properties According to Disease Needs. ACS Nano 2020, 14, 2585–2627. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, X. Gold Nanoparticles for Skin Drug Delivery. Int. J. Pharm. 2022, 625, 122122. [Google Scholar] [CrossRef]
- Kazemzadeh, H.; Mozafari, M. Fullerene-Based Delivery Systems. Drug Discov. Today 2019, 24, 898–905. [Google Scholar] [CrossRef]
- Belal, F.; Mabrouk, M.; Hammad, S.; Ahmed, H.; Barseem, A. Recent Applications of Quantum Dots in Pharmaceutical Analysis. J. Fluoresc. 2024, 34, 119–138. [Google Scholar] [CrossRef]
- Singh, S.; Raina, D.; Rishipathak, D.; Babu, K.R.; Khurana, R.; Gupta, Y.; Garg, K.; Rehan, F.; Gupta, S.M. Quantum Dots in the Biomedical World: A Smart Advanced Nanocarrier for Multiple Venues Application. Arch. Pharm. 2022, 355, 2200299. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.A.; Singh, A.K.; Sharma, P.; Brown, S.C.; Moudgil, B.M. Nanoparticles as Contrast Agents for In-Vivo Bioimaging: Current Status and Future Perspectives. Anal. Bioanal. Chem. 2011, 399, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Ulanova, M.; Poljak, A.; Wen, W.; Bongers, A.; Gloag, L.; Gooding, J.; Tilley, R.; Sachdev, P.; Braidy, N. Nanoparticles as Contrast Agents for The Diagnosis of Alzheimer’s Disease: A Systematic Review. Nanomedicine 2020, 15, 725–743. [Google Scholar] [CrossRef]
- Fernandes, N.B.; Shenoy, R.U.K.; Kajampady, M.K.; DCruz, C.E.M.; Shirodkar, R.K.; Kumar, L.; Verma, R. Fullerenes for the Treatment of Cancer: An Emerging Tool. Environ. Sci. Pollut. Res. 2022, 29, 58607–58627. [Google Scholar] [CrossRef]
- Sell, M.; Lopes, A.R.; Escudeiro, M.; Esteves, B.; Monteiro, A.R.; Trindade, T.; Cruz-Lopes, L. Application of Nanoparticles in Cancer Treatment: A Concise Review. Nanomaterials 2023, 13, 2887. [Google Scholar] [CrossRef]
- Masoomzadeh, S.; Gholikhani, T.; Aminroaia, P.; Taghvimi, A.; Javadzadeh, Y. How Can Fullerenes Help in the Treatment of Diseases? A Review Article on Pharmaceutical Usage of Fullerenes as Carriers. Comb. Chem. High Throughput Screen. 2023, 26, 2607–2613. [Google Scholar] [CrossRef] [PubMed]
- Latyshev, O.E.; Zaykova, O.N.; Eliseeva, O.V.; Savochkina, T.E.; Chernoryzh, Y.Y.; Syroeshkin, A.V.; Petrov, G.V.; Vorkunova, G.K.; Larichev, V.F.; Fediakina, I.T.; et al. Development, Production and Characterization of SARS-CoV-2 Virus-like Particles (Coronaviridae: Orthocoronavirinae: Betacoronavirus: Sarbecovirus). Probl. Virol. 2024, 69, 175–186. [Google Scholar] [CrossRef]
- Chartuprayoon, N.; Zhang, M.; Bosze, W.; Choa, Y.-H.; Myung, N.V. One-Dimensional Nanostructures Based Bio-Detection. Biosens. Bioelectron. 2015, 63, 432–443. [Google Scholar] [CrossRef]
- Iijima, S.; Ajayan, P.M.; Ichihashi, T. Growth Model for Carbon Nanotubes. Phys. Rev. Lett. 1992, 69, 3100–3103. [Google Scholar] [CrossRef]
- Negri, V.; Pacheco-Torres, J.; Calle, D.; López-Larrubia, P. Carbon Nanotubes in Biomedicine. Top. Curr. Chem. 2020, 378, 15. [Google Scholar] [CrossRef]
- Tarasiuk, O.; Scuteri, A. Role of Tunneling Nanotubes in the Nervous System. Int. J. Mol. Sci. 2022, 23, 12545. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Aoki, K.; Haniu, H.; Kamanaka, T.; Takizawa, T.; Sobajima, A.; Yoshida, K.; Okamoto, M.; Kato, H.; Saito, N. Applications of Carbon Nanotubes in Bone Regenerative Medicine. Nanomaterials 2020, 10, 659. [Google Scholar] [CrossRef]
- Saha, T.; Dash, C.; Jayabalan, R.; Khiste, S.; Kulkarni, A.; Kurmi, K.; Mondal, J.; Majumder, P.K.; Bardia, A.; Jang, H.L.; et al. Intercellular Nanotubes Mediate Mitochondrial Trafficking between Cancer and Immune Cells. Nat. Nanotechnol. 2022, 17, 98–106. [Google Scholar] [CrossRef]
- Khan, S.; Jawlikar, P.; Lahoti, S.; Bhusnure, O.; Chitlange, S.; Sangshetti, J. Application of Carbon Nanotubes In Drug Delivery of Non-Cancerous Diseases: A Review. Curr. Pharm. Des. 2021, 27, 2454–2467. [Google Scholar] [CrossRef]
- Roldo, M. Carbon Nanotubes in Drug Delivery: Just a Carrier? Ther. Deliv. 2016, 7, 55–57. [Google Scholar] [CrossRef]
- Komane, P.; Kumar, P.; Choonara, Y. Functionalised Carbon Nanotubes: Promising Drug Delivery Vehicles for Neurovascular Disorder Intervention. AAPS PharmSciTech 2023, 24, 201. [Google Scholar] [CrossRef]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon Nanotubes: Smart Drug/Gene Delivery Carriers. Int. J. Nanomed. 2021, 16, 1681–1706. [Google Scholar] [CrossRef]
- De Carvalho Lima, E.N.; Diaz, R.S.; Justo, J.F.; Castilho Piqueira, J.R. Advances and Perspectives in the Use of Carbon Nanotubes in Vaccine Development. Int. J. Nanomed. 2021, 16, 5411–5435. [Google Scholar] [CrossRef]
- Kim, C.G.; Kye, Y.-C.; Yun, C.-H. The Role of Nanovaccine in Cross-Presentation of Antigen-Presenting Cells for the Activation of CD8+ T Cell Responses. Pharmaceutics 2019, 11, 612. [Google Scholar] [CrossRef]
- Lin, J.; Miao, L.; Zhong, G.; Lin, C.-H.; Dargazangy, R.; Alexander-Katz, A. Understanding the Synergistic Effect of Physicochemical Properties of Nanoparticles and Their Cellular Entry Pathways. Commun. Biol. 2020, 3, 205. [Google Scholar] [CrossRef]
- Eker, F.; Duman, H.; Akdaşçi, E.; Bolat, E.; Sarıtaş, S.; Karav, S.; Witkowska, A.M. A Comprehensive Review of Nanoparticles: From Classification to Application and Toxicity. Molecules 2024, 29, 3482. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Marioli, M.; Zhang, K. Analytical Characterization of Liposomes and Other Lipid Nanoparticles for Drug Delivery. J. Pharm. Biomed. Anal. 2021, 192, 113642. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.F.; Lao, L.L.; Xiong, G.M.; Venkatraman, S. Controlled-Release Nanotherapeutics: State of Translation. J. Control. Release 2018, 284, 39–48. [Google Scholar] [CrossRef]
- Kędra, K.; Oledzka, E.; Sobczak, M. Self-Immolative Domino Dendrimers as Anticancer-Drug Delivery Systems: A Review. Pharmaceutics 2024, 16, 668. [Google Scholar] [CrossRef]
- Bangham, A.D.; Horne, R.W. Negative Staining of Phospholipids and Their Structural Modification by Surface-Active Agents as Observed in the Electron Microscope. J. Mol. Biol. 1964, 8, 660–668. [Google Scholar] [CrossRef]
- Jeong, M.; Lee, Y.; Park, J.; Jung, H.; Lee, H. Lipid Nanoparticles (LNPs) for in Vivo RNA Delivery and Their Breakthrough Technology for Future Applications. Adv. Drug Deliv. Rev. 2023, 200, 114990. [Google Scholar] [CrossRef]
- Khare, P.; Edgecomb, S.X.; Hamadani, C.M.; Tanner, E.E.L.; S Manickam, D. Lipid Nanoparticle-Mediated Drug Delivery to the Brain. Adv. Drug Deliv. Rev. 2023, 197, 114861. [Google Scholar] [CrossRef]
- Dilliard, S.A.; Cheng, Q.; Siegwart, D.J. On the Mechanism of Tissue-Specific MRNA Delivery by Selective Organ Targeting Nanoparticles. Proc. Natl. Acad. Sci. USA 2021, 118, e2109256118. [Google Scholar] [CrossRef]
- Yasmin, F.; Najeeb, H.; Shaikh, S.; Hasanain, M.; Naeem, U.; Moeed, A.; Koritala, T.; Hasan, S.; Surani, S. Novel Drug Delivery Systems for Inflammatory Bowel Disease. World J. Gastroenterol. 2022, 28, 1922–1933. [Google Scholar] [CrossRef]
- Barenholz, Y. (Chezy) Doxil®—The First FDA-Approved Nano-Drug: Lessons Learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Lin, W.; Li, A.; Qiu, L.; Huang, H.; Cui, P.; Wang, J. Albumin Nanoparticles Increase the Efficacy of Doxorubicin Hydrochloride Liposome Injection Based on Threshold Theory. Mol. Pharm. 2024, 21, 2970–2980. [Google Scholar] [CrossRef] [PubMed]
- El Moukhtari, S.H.; Garbayo, E.; Amundarain, A.; Pascual-Gil, S.; Carrasco-León, A.; Prosper, F.; Agirre, X.; Blanco-Prieto, M.J. Lipid Nanoparticles for SiRNA Delivery in Cancer Treatment. J. Control. Release 2023, 361, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Gutkin, A.; Kedmi, R.; Ramishetti, S.; Veiga, N.; Jacobi, A.M.; Schubert, M.S.; Friedmann-Morvinski, D.; Cohen, Z.R.; Behlke, M.A.; et al. CRISPR-Cas9 Genome Editing Using Targeted Lipid Nanoparticles for Cancer Therapy. Sci. Adv. 2020, 6, eabc9450. [Google Scholar] [CrossRef]
- Zhang, W.; Gong, C.; Chen, Z.; Li, M.; Li, Y.; Gao, J. Tumor Microenvironment-Activated Cancer Cell Membrane-Liposome Hybrid Nanoparticle-Mediated Synergistic Metabolic Therapy and Chemotherapy for Non-Small Cell Lung Cancer. J. Nanobiotechnol. 2021, 19, 339. [Google Scholar] [CrossRef]
- Zhu, X.; Li, S. Nanomaterials in Tumor Immunotherapy: New Strategies and Challenges. Mol. Cancer 2023, 22, 94. [Google Scholar] [CrossRef]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced Targeted Therapies in Cancer: Drug Nanocarriers, the Future of Chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef]
- Bayer, M.E.; Blumberg, B.S.; Werner, B. Particles Associated with Australia Antigen in the Sera of Patients with Leukaemia, Down’s Syndrome and Hepatitis. Nature 1968, 218, 1057–1059. [Google Scholar] [CrossRef]
- Lagoutte, P.; Mignon, C.; Donnat, S.; Stadthagen, G.; Mast, J.; Sodoyer, R.; Lugari, A.; Werle, B. Scalable Chromatography-Based Purification of Virus-like Particle Carrier for Epitope Based Influenza A Vaccine Produced in Escherichia coli. J. Virol. Methods 2016, 232, 8–11. [Google Scholar] [CrossRef]
- Uddin, M.N.; Henry, B.; Carter, K.D.; Roni, M.A.; Kouzi, S.S. A Novel Formulation Strategy to Deliver Combined DNA and VLP Based HPV Vaccine. J. Pharm. Pharm. Sci. 2019, 22, 536–547. [Google Scholar] [CrossRef]
- Huber, B.; Wang, J.W.; Roden, R.B.S.; Kirnbauer, R. RG1-VLP and Other L2-Based, Broad-Spectrum HPV Vaccine Candidates. J. Clin. Med. 2021, 10, 1044. [Google Scholar] [CrossRef]
- Andersson, A.-M.; Schwerdtfeger, M.; Holst, P. Virus-Like-Vaccines against HIV. Vaccines 2018, 6, 10. [Google Scholar] [CrossRef]
- Tarrés-Freixas, F.; Aguilar-Gurrieri, C.; Rodríguez de la Concepción, M.L.; Urrea, V.; Trinité, B.; Ortiz, R.; Pradenas, E.; Blanco, P.; Marfil, S.; Molinos-Albert, L.M.; et al. An Engineered HIV-1 Gag-Based VLP Displaying High Antigen Density Induces Strong Antibody-Dependent Functional Immune Responses. NPJ Vaccines 2023, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Abouleila, Y.; Saris, A.; Shimizu, Y.; Ottenhoff, T.H.M.; Mashaghi, A. Ebola Virus–like Particles Reprogram Cellular Metabolism. J. Mol. Med. 2023, 101, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Carra, J.H.; Martins, K.A.O.; Schokman, R.D.; Robinson, C.G.; Steffens, J.T.; Bavari, S. A Thermostable, Chromatographically Purified Ebola Nano-VLP Vaccine. J. Transl. Med. 2015, 13, 228. [Google Scholar] [CrossRef]
- Thoresen, D.; Matsuda, K.; Urakami, A.; Ngwe Tun, M.M.; Nomura, T.; Moi, M.L.; Watanabe, Y.; Ishikawa, M.; Hau, T.T.T.; Yamamoto, H.; et al. A Tetravalent Dengue Virus-like Particle Vaccine Induces High Levels of Neutralizing Antibodies and Reduces Dengue Replication in Non-Human Primates. J. Virol. 2024, 98, e00239-24. [Google Scholar] [CrossRef]
- Boigard, H.; Cimica, V.; Galarza, J.M. Dengue-2 Virus-like Particle (VLP) Based Vaccine Elicits the Highest Titers of Neutralizing Antibodies When Produced at Reduced Temperature. Vaccine 2018, 36, 7728–7736. [Google Scholar] [CrossRef]
- Kostina, L.V.; Filatov, I.E.; Eliseeva, O.V.; Latyshev, O.E.; Chernoryzh, Y.Y.; Yurlov, K.I.; Lesnova, E.I.; Khametova, K.M.; Cherepushkin, S.A.; Savochkina, T.E.; et al. Study of the Safety and Immunogenicity of VLP-Based Vaccine for the Prevention of Rotavirus Infection in Neonatal Minipig Model. Probl. Virol. 2023, 68, 415–427. [Google Scholar] [CrossRef]
- Tse Sum Bui, B.; Haupt, K. Molecularly Imprinted Polymer Hydrogel Nanoparticles: Synthetic Antibodies for Cancer Diagnosis and Therapy. ChemBioChem 2022, 23, e202100598. [Google Scholar] [CrossRef]
- Lu, X.-Y.; Wu, D.-C.; Li, Z.-J.; Chen, G.-Q. Polymer Nanoparticles. In Progress in Nucleic Acid Research and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 104, pp. 299–323. [Google Scholar] [CrossRef]
- Lima, A.L.; Gratieri, T.; Cunha-Filho, M.; Gelfuso, G.M. Polymeric Nanocapsules: A Review on Design and Production Methods for Pharmaceutical Purpose. Methods 2022, 199, 54–66. [Google Scholar] [CrossRef]
- Purohit, D.; Jalwal, P.; Manchanda, D.; Saini, S.; Verma, R.; Kaushik, D.; Mittal, V.; Kumar, M.; Bhattacharya, T.; Rahman, M.H.; et al. Nanocapsules: An Emerging Drug Delivery System. Recent. Pat. Nanotechnol. 2023, 17, 190–207. [Google Scholar] [CrossRef]
- Ebeling, B.; Belal, K.; Stoffelbach, F.; Woisel, P.; Lansalot, M.; D’Agosto, F. Polymer Nanospheres with Hydrophobic Surface Groups as Supramolecular Building Blocks Produced by Aqueous PISA. Macromol. Rapid Commun. 2019, 40, 1800455. [Google Scholar] [CrossRef]
- Campión, R.; Gonzalez-Navarro, C.J.; Luisa Martínez López, A.; Cristina Martínez-Oharriz, M.; Matías, C.; Sáiz-Abajo, M.-J.; Collantes, M.; Peñuelas, I.; Irache, J.M. Zein-Based Nanospheres and Nanocapsules for the Encapsulation and Oral Delivery of Quercetin. Int. J. Pharm. 2023, 643, 123216. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Baeza, A.; Guisasola, E.; Torres-Pardo, A.; González-Calbet, J.M.; Melen, G.J.; Ramirez, M.; Vallet-Regí, M. Hybrid Enzyme-Polymeric Capsules/Mesoporous Silica Nanodevice for In Situ Cytotoxic Agent Generation. Adv. Funct. Mater. 2014, 24, 4625–4633. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Barros, M.T. Polymeric Nanoparticles: A Study on the Preparation Variables and Characterization Methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef]
- Roberts, J.C.; Adams, Y.E.; Tomalia, D.; Mercer-Smith, J.A.; Lavallee, D.K. Using Starburst Dendrimers as Linker Molecules to Radiolabel Antibodies. Bioconjug. Chem. 1990, 1, 305–308. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Naylor, A.M.; Goddard, W.A. Starburst Dendrimers: Molecular-Level Control of Size, Shape, Surface Chemistry, Topology, and Flexibility from Atoms to Macroscopic Matter. Angew. Chem. Int. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- Alshammari, B.H.; Lashin, M.M.A.; Mahmood, M.A.; Al-Mubaddel, F.S.; Ilyas, N.; Rahman, N.; Sohail, M.; Khan, A.; Abdullaev, S.S.; Khan, R. Organic and Inorganic Nanomaterials: Fabrication, Properties and Applications. RSC Adv. 2023, 13, 13735–13785. [Google Scholar] [CrossRef]
- Choi, J.; Kim, G.; Cho, S.B.; Im, H.J. Radiosensitizing High-Z Metal Nanoparticles for Enhanced Radiotherapy of Glioblastoma Multiforme. J. Nanobiotechnol. 2020, 18, 122. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Singh, S.P. Drug Encapsulating Polysaccharide-loaded Metal Nanoparticles: A Perspective Drug Delivery System. Drug Dev. Res. 2021, 82, 145–148. [Google Scholar] [CrossRef]
- Tweney, R.D. Discovering Discovery: How Faraday Found the First Metallic Colloid. Perspect. Sci. 2006, 14, 97–121. [Google Scholar] [CrossRef]
- Desia, N.; Momin, M.; Khan, T.; Gharat, S.; Ningthoujam, R.S.; Omri, A. Metallic Nanoparticles as Drug Delivery System for the Treatment of Cancer. Expert Opin. Drug Deliv. 2021, 18, 1261–1290. [Google Scholar] [CrossRef] [PubMed]
- Vasile, B.S.; Birca, A.C.; Musat, M.C.; Holban, A.M. Wound Dressings Coated with Silver Nanoparticles and Essential Oils for The Management of Wound Infections. Materials 2020, 13, 1682. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Kotrange, H.; Najda, A.; Bains, A.; Gruszecki, R.; Chawla, P.; Tosif, M.M. Metal and Metal Oxide Nanoparticle as a Novel Antibiotic Carrier for the Direct Delivery of Antibiotics. Int. J. Mol. Sci. 2021, 22, 9596. [Google Scholar] [CrossRef]
- Agreles, M.A.A.; Cavalcanti, I.D.L.; Cavalcanti, I.M.F. Synergism between Metallic Nanoparticles and Antibiotics. Appl. Microbiol. Biotechnol. 2022, 106, 3973–3984. [Google Scholar] [CrossRef]
- Panáček, D.; Hochvaldová, L.; Bakandritsos, A.; Malina, T.; Langer, M.; Belza, J.; Martincová, J.; Večeřová, R.; Lazar, P.; Poláková, K.; et al. Silver Covalently Bound to Cyanographene Overcomes Bacterial Resistance to Silver Nanoparticles and Antibiotics. Adv. Sci. 2021, 8, 2003090. [Google Scholar] [CrossRef]
- Gharpure, S.; Akash, A.; Ankamwar, B. A Review on Antimicrobial Properties of Metal Nanoparticles. J. Nanosci. Nanotechnol. 2020, 20, 3303–3339. [Google Scholar] [CrossRef]
- Ansari, A.; Pervez, S.; Javed, U.; Abro, M.I.; Nawaz, M.A.; Qader, S.A.U.; Aman, A. Characterization and Interplay of Bacteriocin and Exopolysaccharide-Mediated Silver Nanoparticles as an Antibacterial Agent. Int. J. Biol. Macromol. 2018, 115, 643–650. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Vernaya, O.I.; Melnikov, M.Y. Hybrid Nanosystems of Antibiotics with Metal Nanoparticles—Novel Antibacterial Agents. Molecules 2023, 28, 1603. [Google Scholar] [CrossRef]
- Peng, H.; Huang, Q.; Wu, T.; Wen, J.; He, H. Preparation of Porous γ-Fe2O3@mWO3 Multifunctional Nanoparticles for Drug Loading and Controlled Release. Curr. Drug Deliv. 2018, 15, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, S.; Goyal, N.; Gupta, S. Synthesizing and Optimizing Rutile TiO2 Nanoparticles for Magnetically Guided Drug Delivery. Int. J. Nanomed. 2022, 17, 3147–3161. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Gun’ko, Y.; Vallet-Regí, M. ZnO Nanostructures for Drug Delivery and Theranostic Applications. Nanomaterials 2018, 8, 268. [Google Scholar] [CrossRef]
- Palanisamy, S.; Wang, Y.-M. Superparamagnetic Iron Oxide Nanoparticulate System: Synthesis, Targeting, Drug Delivery and Therapy in Cancer. Dalton Trans. 2019, 48, 9490–9515. [Google Scholar] [CrossRef]
- Wahajuddin; Arora, S. Superparamagnetic Iron Oxide Nanoparticles: Magnetic Nanoplatforms as Drug Carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, H. Daunorubicin-TiO2 Nanocomposites as a “Smart” PH-Responsive Drug Delivery System. Int. J. Nanomed. 2012, 7, 235–242. [Google Scholar] [CrossRef][Green Version]
- Yin, M.; Ju, E.; Chen, Z.; Li, Z.; Ren, J.; Qu, X. Upconverting Nanoparticles with a Mesoporous TiO2 Shell for Near-Infrared-Triggered Drug Delivery and Synergistic Targeted Cancer Therapy. Chem. Eur. J. 2014, 20, 14012–14017. [Google Scholar] [CrossRef]
- Anjum, S.; Hashim, M.; Malik, S.A.; Khan, M.; Lorenzo, J.M.; Abbasi, B.H.; Hano, C. Recent Advances in Zinc Oxide Nanoparticles (ZnO NPs) for Cancer Diagnosis, Target Drug Delivery, and Treatment. Cancers 2021, 13, 4570. [Google Scholar] [CrossRef]
- Costa, B.A.; Abuçafy, M.P.; Barbosa, T.W.L.; da Silva, B.L.; Fulindi, R.B.; Isquibola, G.; da Costa, P.I.; Chiavacci, L.A. ZnO@ZIF-8 Nanoparticles as Nanocarrier of Ciprofloxacin for Antimicrobial Activity. Pharmaceutics 2023, 15, 259. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Aquib, M.; Farooq, M.A.; Banerjee, P.; Akhtar, F.; Filli, M.S.; Boakye-Yiadom, K.O.; Kesse, S.; Raza, F.; Maviah, M.B.J.; Mavlyanova, R.; et al. Targeted and Stimuli–Responsive Mesoporous Silica Nanoparticles for Drug Delivery and Theranostic Use. J. Biomed. Mater. Res. A 2019, 107, 2643–2666. [Google Scholar] [CrossRef] [PubMed]
- Kirla, H.; Henry, D.J.; Jansen, S.; Thompson, P.L.; Hamzah, J. Use of Silica Nanoparticles for Drug Delivery in Cardiovascular Disease. Clin. Ther. 2023, 45, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Tiburcius, S.; Krishnan, K.; Yang, J.; Hashemi, F.; Singh, G.; Radhakrishnan, D.; Trinh, H.T.; Verrills, N.M.; Karakoti, A.; Vinu, A. Silica-Based Nanoparticles as Drug Delivery Vehicles for Prostate Cancer Treatment. Chem. Rec. 2021, 21, 1535–1568. [Google Scholar] [CrossRef]
- Igaz, N.; Bélteky, P.; Kovács, D.; Papp, C.; Rónavári, A.; Szabó, D.; Gácser, A.; Kónya, Z.; Kiricsi, M. Functionalized Mesoporous Silica Nanoparticles for Drug-Delivery to Multidrug-Resistant Cancer Cells. Int. J. Nanomed. 2022, 17, 3079–3096. [Google Scholar] [CrossRef]
- Qu, Z.; Wong, K.Y.; Moniruzzaman, M.; Begun, J.; Santos, H.A.; Hasnain, S.Z.; Kumeria, T.; McGuckin, M.A.; Popat, A. One-Pot Synthesis of PH-Responsive Eudragit-Mesoporous Silica Nanocomposites Enable Colonic Delivery of Glucocorticoids for the Treatment of Inflammatory Bowel Disease. Adv. Ther. 2021, 4, 2000165. [Google Scholar] [CrossRef]
- Friedman, S.H.; DeCamp, D.L.; Sijbesma, R.P.; Srdanov, G.; Wudl, F.; Kenyon, G.L. Inhibition of the HIV-1 Protease by Fullerene Derivatives: Model Building Studies and Experimental Verification. J. Am. Chem. Soc. 1993, 115, 6506–6509. [Google Scholar] [CrossRef]
- Sengupta, J.; Hussain, C.M. The Emergence of Carbon Nanomaterials as Effective Nano-Avenues to Fight against COVID-19. Materials 2023, 16, 1068. [Google Scholar] [CrossRef]
- Gupta, I.; Azizighannad, S.; Farinas, E.T.; Mitra, S. Antiviral Properties of Select Carbon Nanostructures and Their Functionalized Analogs. Mater. Today Commun. 2021, 29, 102743. [Google Scholar] [CrossRef]
- Alipour, E.; Alimohammady, F.; Yumashev, A.; Maseleno, A. Fullerene C60 Containing Porphyrin-like Metal Center as Drug Delivery System for Ibuprofen Drug. J. Mol. Model. 2020, 26, 7. [Google Scholar] [CrossRef]
- Yukawa, H.; Sato, K.; Baba, Y. Theranostics Applications of Quantum Dots in Regenerative Medicine, Cancer Medicine, and Infectious Diseases. Adv. Drug Deliv. Rev. 2023, 200, 114863. [Google Scholar] [CrossRef]
- Ekimov, A.I.; Efros, A.L.; Onushchenko, A.A. Quantum Size Effect in Semiconductor Microcrystals. Solid State Commun. 1985, 56, 921–924. [Google Scholar] [CrossRef]
- Brus, L.E. Electron–Electron and Electron-Hole Interactions in Small Semiconductor Crystallites: The Size Dependence of the Lowest Excited Electronic State. J. Chem. Phys. 1984, 80, 4403–4409. [Google Scholar] [CrossRef]
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and Characterization of Nearly Monodisperse CdE (E = Sulfur, Selenium, Tellurium) Semiconductor Nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Olivos, H.J.; Bachhawat-Sikder, K.; Kodadek, T. Quantum Dots As A Visual Aid For Screening Bead-Bound Combinatorial Libraries. ChemBioChem 2003, 4, 1242–1245. [Google Scholar] [CrossRef]
- Pohanka, M. Quantum Dots in the Therapy: Current Trends and Perspectives. Mini-Rev. Med. Chem. 2017, 17, 650–656. [Google Scholar] [CrossRef]
- Yang, Y.; Mao, G.; Ji, X.; He, Z. DNA-Templated Quantum Dots and Their Applications in Biosensors, Bioimaging, and Therapy. J. Mater. Chem. B 2020, 8, 9–17. [Google Scholar] [CrossRef]
- Sarkar, K.; Bank, S.; Chatterjee, A.; Dutta, K.; Das, A.; Chakraborty, S.; Paul, N.; Sarkar, J.; De, S.; Ghosh, S.; et al. Hyaluronic Acid-Graphene Oxide Quantum Dots Nanoconjugate as Dual Purpose Drug Delivery and Therapeutic Agent in Meta-Inflammation. J. Nanobiotechnol. 2023, 21, 246. [Google Scholar] [CrossRef]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance Properties of Nano-Sized Particles and Molecules as Imaging Agents: Considerations and Caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef]
- Schleh, C.; Semmler-Behnke, M.; Lipka, J.; Wenk, A.; Hirn, S.; Schäffler, M.; Schmid, G.; Simon, U.; Kreyling, W.G. Size and Surface Charge of Gold Nanoparticles Determine Absorption across Intestinal Barriers and Accumulation in Secondary Target Organs after Oral Administration. Nanotoxicology 2012, 6, 36–46. [Google Scholar] [CrossRef]
- Zha, S.; Liu, H.; Li, H.; Li, H.; Wong, K.-L.; All, A.H. Functionalized Nanomaterials Capable of Crossing the Blood–Brain Barrier. ACS Nano 2024, 18, 1820–1845. [Google Scholar] [CrossRef]
- Patil, A.; Mishra, V.; Thakur, S.; Riyaz, B.; Kaur, A.; Khursheed, R.; Patil, K.; Sathe, B. Nanotechnology Derived Nanotools in Biomedical Perspectives: An Update. Curr. Nanosci. 2019, 15, 137–146. [Google Scholar] [CrossRef]
- Syroeshkin, A.V.; Petrov, G.V.; Taranov, V.V.; Pleteneva, T.V.; Koldina, A.M.; Gaydashev, I.A.; Kolyabina, E.S.; Galkina, D.A.; Sorokina, E.V.; Uspenskaya, E.V.; et al. Radiothermal Emission of Nanoparticles with a Complex Shape as a Tool for the Quality Control of Pharmaceuticals Containing Biologically Active Nanoparticles. Pharmaceutics 2023, 15, 966. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Devarajan, P.V.; Jindal, A.B.; Patil, R.R.; Mulla, F.; Gaikwad, R.V.; Samad, A. Particle Shape: A New Design Parameter for Passive Targeting In Splenotropic Drug Delivery. J. Pharm. Sci. 2010, 99, 2576–2581. [Google Scholar] [CrossRef]
- Amodeo, J.; Pizzagalli, L. Modeling the Mechanical Properties of Nanoparticles: A Review. C. R. Phys. 2021, 22, 1–32. [Google Scholar] [CrossRef]
- Wu, Q.; Miao, W.; Zhang, Y.; Gao, H.; Hui, D. Mechanical Properties of Nanomaterials: A Review. Nanotechnol. Rev. 2020, 9, 259–273. [Google Scholar] [CrossRef]
- Li, H.; Xiao, H.; Ou, J. A Study on Mechanical and Pressure-Sensitive Properties of Cement Mortar with Nanophase Materials. Cem. Concr. Res. 2004, 34, 435–438. [Google Scholar] [CrossRef]
- Al Ghabban, A.; Al Zubaidi, A.B.; Jafar, M.; Fakhri, Z. Effect of Nano SiO2 and Nano CaCO3 on The Mechanical Properties, Durability and Flowability of Concrete. IOP Conf. Ser. Mater. Sci. Eng. 2018, 454, 012016. [Google Scholar] [CrossRef]
- Teng, X.; Liu, H.; Huang, C. Effect of Al2O3 Particle Size on the Mechanical Properties of Alumina-Based Ceramics. Mater. Sci. Eng. 2007, 452, 545–551. [Google Scholar] [CrossRef]
- Naguib, G.; Maghrabi, A.A.; Mira, A.I.; Mously, H.A.; Hajjaj, M.; Hamed, M.T. Influence of Inorganic Nanoparticles on Dental Materials’ Mechanical Properties. A Narrative Review. BMC Oral Health 2023, 23, 897. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Liu, D.; Subramanyam, K.; Wang, B.; Yang, J.; Huang, J.; Auguste, D.T.; Moses, M.A. Nanoparticle Elasticity Directs Tumor Uptake. Nat. Commun. 2018, 9, 130. [Google Scholar] [CrossRef]
- Stefaniak, A.B. Principal Metrics and Instrumentation for Characterization of Engineered Nanomaterials. In Metrology and Standardization of Nanotechnology; Wiley: Hoboken, NJ, USA, 2017; pp. 151–174. [Google Scholar]
- Commission Recommendation on the Definition of Nanomaterial. 2011, pp. 38–40. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011H0696 (accessed on 11 January 2025).
- Bleeker, E.A.J.; de Jong, W.H.; Geertsma, R.E.; Groenewold, M.; Heugens, E.H.W.; Koers-Jacquemijns, M.; van de Meent, D.; Popma, J.R.; Rietveld, A.G.; Wijnhoven, S.W.P.; et al. Considerations on the EU Definition of a Nanomaterial: Science to Support Policy Making. Regul. Toxicol. Pharmacol. 2013, 65, 119–125. [Google Scholar] [CrossRef]
- Kiaee, G.; Dimitrakakis, N.; Sharifzadeh, S.; Kim, H.; Avery, R.K.; Moghaddam, K.M.; Haghniaz, R.; Yalcintas, E.P.; de Barros, N.R.; Karamikamkar, S.; et al. Laponite-Based Nanomaterials for Drug Delivery. Adv. Healthc. Mater. 2022, 11, 2102054. [Google Scholar] [CrossRef]
- Keesom, W.H. On the Deduction of the Equation of State from Boltzmann’s Entropy Principle. 1912. Available online: https://dwc.knaw.nl/DL/publications/PU00012940.pdf (accessed on 11 January 2025).
- Power, G.; Nagaraj, M.; Vij, J.K.; Johari, G.P. Debye Process and Dielectric State of an Alcohol in a Nonpolar Solvent. J. Chem. Phys. 2011, 134, 044525. [Google Scholar] [CrossRef]
- London, F. The General Theory of Molecular Forces. Trans. Faraday Soc. 1937, 33, 8b-26. [Google Scholar] [CrossRef]
- Tang, Z.; Kotov, N.A.; Giersig, M. Spontaneous Organization of Single CdTe Nanoparticles into Luminescent Nanowires. IEEE J. Quantum Electron. 2002, 282, 491. [Google Scholar] [CrossRef]
- Walker, D.A.; Kowalczyk, B.; De La Cruz, M.O.; Grzybowski, B.A. Electrostatics at the Nanoscale. Nanoscale 2011, 3, 1316–1344. [Google Scholar] [CrossRef]
- Leighton, R.B. Static and Dynamic Electricity. Eng. Sci. 1951, 14, 2. [Google Scholar] [CrossRef][Green Version]
- Spavieri, G.; Gillies, G.T.; Rodriguez, M. Physical Implications of Coulomb’s Law. Metrologia 2004, 41, S159–S170. [Google Scholar] [CrossRef]
- Man, Z.; Bian, J.; Xing, X.; Lu, Z.; Zhang, W. Unexpected Coulomb Interactions in Nonpolar Solvent for Highly Efficient Nanoxerography of Perovskite Quantum Dots. J. Phys. Chem. Lett. 2021, 12, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wu, J.; Yarin, A.L. Electrospun Membranes Filtering 100 Nm Particles from Air Flow by Means of the van Der Waals and Coulomb Forces. J. Memb. Sci. 2022, 644, 120138. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. Understanding the Electric Double-Layer Structure, Capacitance, and Charging Dynamics. Chem. Rev. 2022, 122, 10821–10859. [Google Scholar] [CrossRef]
- He, Y.; Yang, Y.; Nie, S.; Liu, R.; Wan, Q. Electric-Double-Layer Transistors for Synaptic Devices and Neuromorphic Systems. J. Mater. Chem. C Mater. 2018, 6, 5336–5352. [Google Scholar] [CrossRef]
- Gschwend, G.C.; Girault, H.H. Discrete Helmholtz Model: A Single Layer of Correlated Counter-Ions. Metal Oxides and Silica Interfaces, Ion-Exchange and Biological Membranes. Chem. Sci. 2020, 11, 10304–10312. [Google Scholar] [CrossRef]
- Gouy, M. Sur La Constitution de La Charge Électrique à La Surface d’un Électrolyte. J. Phys. Théorique Appliquée 1910, 9, 457–468. [Google Scholar] [CrossRef]
- Chapman, D.L. A Contribution to the Theory of Electrocapillarity. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1913, 25, 475–481. [Google Scholar] [CrossRef]
- Moya, A.A. Theory of the Formation of the Electric Double Layer at the Ion Exchange Membrane–Solution Interface. Phys. Chem. Chem. Phys. 2015, 17, 5207–5218. [Google Scholar] [CrossRef]
- Becker, M.; Loche, P.; Rezaei, M.; Wolde-Kidan, A.; Uematsu, Y.; Netz, R.R.; Bonthuis, D.J. Multiscale Modeling of Aqueous Electric Double Layers. Chem. Rev. 2024, 124, 1–26. [Google Scholar] [CrossRef]
- Agmo Hernández, V. An Overview of Surface Forces and the DLVO Theory. ChemTexts 2023, 9, 10. [Google Scholar] [CrossRef]
- Ohshima, H. The Derjaguin–Landau–Verwey–Overbeek (DLVO) Theory of Colloid Stability. In Electrical Phenomena at Interfaces and Biointerfaces; Wiley: Hoboken, NJ, USA, 2012; pp. 27–34. [Google Scholar]
- Benedini, L.; Antollini, S.; Fanani, M.L.; Palma, S.; Messina, P.; Schulz, P. Study of the Influence of Ascorbyl Palmitate and Amiodarone in the Stability of Unilamellar Liposomes. Mol. Membr. Biol. 2014, 31, 85–94. [Google Scholar] [CrossRef]
- Guo, D.; Xie, G.; Luo, J. Mechanical Properties of Nanoparticles: Basics and Applications. J. Phys. D Appl. Phys. 2014, 47, 013001. [Google Scholar] [CrossRef]
- Umstätter, P.; Urbassek, H.M. Influence of Elastic Stiffness and Surface Adhesion on Bouncing of Nanoparticles. Nanoscale Res. Lett. 2017, 12, 637. [Google Scholar] [CrossRef]
- Chu, Y.-S.; Dufour, S.; Thiery, J.P.; Perez, E.; Pincet, F. Johnson-Kendall-Roberts Theory Applied to Living Cells. Phys. Rev. Lett. 2005, 94, 028102. [Google Scholar] [CrossRef]
- Johnson, K.L.; Kendall, K.; Roberts, A.D. Surface Energy and the Contact of Elastic Solids. Proc. R. Soc. Lond. A Math. Phys. Sci. 1971, 324, 301–313. [Google Scholar] [CrossRef]
- Derjaguin, B.V.; Muller, V.M.; Toporov, Y.P. Effect of Contact Deformations on the Adhesion of Particles. J. Colloid Interface Sci. 1975, 53, 314–326. [Google Scholar] [CrossRef]
- Tan, S.; Sherman, R.L.; Ford, W.T. Nanoscale Compression of Polymer Microspheres by Atomic Force Microscopy. Langmuir 2004, 20, 7015–7020. [Google Scholar] [CrossRef]
- Sun, W.; Zeng, Q.; Yu, A.; Kendall, K. Calculation of Normal Contact Forces between Silica Nanospheres. Langmuir 2013, 29, 7825–7837. [Google Scholar] [CrossRef]
- Khlebtsov, N.G.; Dykman, L.A. Optical Properties and Biomedical Applications of Plasmonic Nanoparticles. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 1–35. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Kosame, S.; Josline, M.J.; Lee, J.-H.; Ju, H. Anomalous Spectral Shift of Localized Surface Plasmon Resonance. Nanoscale Adv. 2024, 6, 2636–2643. [Google Scholar] [CrossRef] [PubMed]
- Tomaev, V.V.; Polishchuk, V.A.; Vartanyan, T.A.; Vasiliev, E.A. Surface Plasmon Resonance in Zinc Nanoparticles. Phys. Chem. Glass 2019, 45, 259–264. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.R.; Noor, A.S.; Moksin, M.M. Application of Surface Plasmon Resonance Based on a Metal Nanoparticle. In Plasmonics—Principles and Applications; InTech: Houston, TX, USA, 2012. [Google Scholar]
- Abd Elhaleem, S.M.; Elsebaei, F.; Shalan, S.; Belal, F. Utilization of Localized Surface Plasmon Resonance of Silver Nanoparticles for the Spectrofluorimetric Estimation of Oxymetazoline in Dosage Forms: Application to Aqueous Humor. J. Fluoresc. 2021, 31, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Juma, M.W.; Birech, Z.; Mwenze, N.M.; Ondieki, A.M.; Maaza, M.; Mokhotjwa, S.D. Localized Surface Plasmon Resonance Sensing of Trenbolone Acetate Dopant Using Silver Nanoparticles. Sci. Rep. 2024, 14, 5721. [Google Scholar] [CrossRef]
- Arcas, A.S.; Jaramillo, L.; Costa, N.S.; Allil, R.C.S.B.; Werneck, M.M. Localized Surface Plasmon Resonance-Based Biosensor on Gold Nanoparticles for Taenia Solium Detection. Appl. Opt. 2021, 60, 8137. [Google Scholar] [CrossRef]
- Mahmudin, L.; Wulandani, R.; Riswan, M.; Kurnia Sari, E.; Dwi Jayanti, P.; Syahrul Ulum, M.; Arifin, M.; Suharyadi, E. Silver Nanoparticles-Based Localized Surface Plasmon Resonance Biosensor for Escherichia coli Detection. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 311, 123985. [Google Scholar] [CrossRef]
- Fong, K.E.; Yung, L.-Y.L. Localized Surface Plasmon Resonance: A Unique Property of Plasmonic Nanoparticles for Nucleic Acid Detection. Nanoscale 2013, 5, 12043. [Google Scholar] [CrossRef]
- Petrov, G.V.; Galkina, D.A.; Koldina, A.M.; Grebennikova, T.V.; Eliseeva, O.V.; Chernoryzh, Y.Y.; Lebedeva, V.V.; Syroeshkin, A.V. Controlling the Quality of Nanodrugs According to Their New Property—Radiothermal Emission. Pharmaceutics 2024, 16, 180. [Google Scholar] [CrossRef]
- Tang, K.; Qi, W.; Wei, Y.; Ru, G.; Liu, W. High-Throughput Calculation of Interlayer van Der Waals Forces Validated with Experimental Measurements. Research 2022, 2022, 9765121. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Duan, X. Van Der Waals Integration before and beyond Two-Dimensional Materials. Nature 2019, 567, 323–333. [Google Scholar] [CrossRef]
- Petrov, G.V.; Gaidashev, I.A.; Syroeshkin, A.V. Physical and Chemical Characteristic of Aqueous Colloidal Infusions of Medicinal Plants Containing Humic Acids. Int. J. Appl. Pharm. 2024, 16, 76–82. [Google Scholar] [CrossRef]
- Roduner, E. Size Matters: Why Nanomaterials Are Different. Chem. Soc. Rev. 2006, 35, 583. [Google Scholar] [CrossRef] [PubMed]
- Moein Najafabadi, S.; Safaei Ghomi, J. Synthesis of COF-SO3H Immobilized on Manganese Ferrite Nanoparticles as an Efficient Nanocomposite in the Preparation of Spirooxindoles. Sci. Rep. 2023, 13, 22731. [Google Scholar] [CrossRef]
- Hu, M.; Butt, H.-J.; Landfester, K.; Bannwarth, M.B.; Wooh, S.; Thérien-Aubin, H. Shaping the Assembly of Superparamagnetic Nanoparticles. ACS Nano 2019, 13, 3015–3022. [Google Scholar] [CrossRef]
- Hori, H.; Teranishi, T.; Nakae, Y.; Seino, Y.; Miyake, M.; Yamada, S. Anomalous Magnetic Polarization Effect of Pd and Au Nano-Particles. Phys. Lett. A 1999, 263, 406–410. [Google Scholar] [CrossRef]
- Farzin, A.; Etesami, S.A.; Quint, J.; Memic, A.; Tamayol, A. Magnetic Nanoparticles in Cancer Therapy and Diagnosis. Adv. Healthc. Mater. 2020, 9, 1901058. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Stanicki, D.; Laurent, S. Magnetic Iron Oxide Nanoparticles for Drug Delivery: Applications and Characteristics. Expert Opin. Drug Deliv. 2019, 16, 69–78. [Google Scholar] [CrossRef]
- Andrievski, R.A. Review of Thermal Stability of Nanomaterials. J. Mater. Sci. 2014, 49, 1449–1460. [Google Scholar] [CrossRef]
- Angayarkanni, S.A.; Philip, J. Review on Thermal Properties of Nanofluids: Recent Developments. Adv. Colloid Interface Sci. 2015, 225, 146–176. [Google Scholar] [CrossRef]
- Qiu, L.; Zhu, N.; Feng, Y.; Michaelides, E.E.; Żyła, G.; Jing, D.; Zhang, X.; Norris, P.M.; Markides, C.N.; Mahian, O. A Review of Recent Advances in Thermophysical Properties at the Nanoscale: From Solid State to Colloids. Phys. Rep. 2020, 843, 1–81. [Google Scholar] [CrossRef]
- Rio, I.S.R.; Rodrigues, A.R.O.; Rodrigues, C.P.; Almeida, B.G.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Development of Novel Magnetoliposomes Containing Nickel Ferrite Nanoparticles Covered with Gold for Applications in Thermotherapy. Materials 2020, 13, 815. [Google Scholar] [CrossRef] [PubMed]
- Dheyab, M.A.; Aziz, A.A.; Khaniabadi, P.M.; Jameel, M.S.; Oladzadabbasabadi, N.; Rahman, A.A.; Braim, F.S.; Mehrdel, B. Gold Nanoparticles-Based Photothermal Therapy for Breast Cancer. Photodiagnosis Photodyn. Ther. 2023, 42, 103312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Thin-Film Hydration Followed by Extrusion Method for Liposome Preparation. In Liposomes; Part of the Book Series: Methods in Molecular Biology; Springer: New York, NY, USA, 2023; Volume 2622, pp. 57–63. [Google Scholar]
- Szoka, F.; Papahadjopoulos, D. Comparative Properties and Methods of Preparation of Lipid Vesicles (Liposomes). Annu. Rev. Biophys. Bioeng. 1980, 9, 467–508. [Google Scholar] [CrossRef]
- Tejera-Garcia, R.; Ranjan, S.; Zamotin, V.; Sood, R.; Kinnunen, P.K.J. Making Unilamellar Liposomes Using Focused Ultrasound. Langmuir 2011, 27, 10088–10097. [Google Scholar] [CrossRef]
- Panahi, Y.; Farshbaf, M.; Mohammadhosseini, M.; Mirahadi, M.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A. Recent Advances on Liposomal Nanoparticles: Synthesis, Characterization and Biomedical Applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 788–799. [Google Scholar] [CrossRef]
- Al-Amin, M.; Bellato, F.; Mastrotto, F.; Garofalo, M.; Malfanti, A.; Salmaso, S.; Caliceti, P. Dexamethasone Loaded Liposomes by Thin-Film Hydration and Microfluidic Procedures: Formulation Challenges. Int. J. Mol. Sci. 2020, 21, 1611. [Google Scholar] [CrossRef]
- Piunti, C.; Cimetta, E. Microfluidic Approaches for Producing Lipid-Based Nanoparticles for Drug Delivery Applications. Biophys. Rev. 2023, 4, 031304. [Google Scholar] [CrossRef]
- Hemmati, F.; Hemmati-Dinarvand, M.; Karimzade, M.; Rutkowska, D.; Eskandari, M.H.; Khanizadeh, S.; Afsharifar, A. Plant-Derived VLP: A Worthy Platform to Produce Vaccine against SARS-CoV-2. Biotechnol. Lett. 2022, 44, 45–57. [Google Scholar] [CrossRef]
- Rodríguez-Limas, W.A.; Tyo, K.E.J.; Nielsen, J.; Ramírez, O.T.; Palomares, L.A. Molecular and Process Design for Rotavirus-like Particle Production in Saccharomyces cerevisiae. Microb. Cell Fact. 2011, 10, 33. [Google Scholar] [CrossRef]
- Hua, T.; Zhang, D.; Tang, B.; Chang, C.; Liu, G.; Zhang, X. The Immunogenicity of the Virus-like Particles Derived from the VP2 Protein of Porcine Parvovirus. Vet. Microbiol. 2020, 248, 108795. [Google Scholar] [CrossRef]
- Mohsen, M.; Gomes, A.; Vogel, M.; Bachmann, M. Interaction of Viral Capsid-Derived Virus-Like Particles (VLPs) with the Innate Immune System. Vaccines 2018, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Fuenmayor, J.; Gòdia, F.; Cervera, L. Production of Virus-like Particles for Vaccines. New Biotechnol. 2017, 39, 174–180. [Google Scholar] [CrossRef]
- Safari, H.; Felder, M.L.; Kaczorowski, N.; Eniola-Adefeso, O. Effect of the Emulsion Solvent Evaporation Technique Cosolvent Choice on the Loading Efficiency and Release Profile of Anti-CD47 from PLGA Nanospheres. J. Pharm. Sci. 2022, 111, 2525–2530. [Google Scholar] [CrossRef]
- Ren, Y.; Qi, C.; Ruan, S.; Cao, G.; Ma, Z.; Zhang, X. Selenized Polymer-Lipid Hybrid Nanoparticles for Oral Delivery of Tripterine with Ameliorative Oral Anti-Enteritis Activity and Bioavailability. Pharmaceutics 2023, 15, 821. [Google Scholar] [CrossRef]
- Pieper, S.; Onafuye, H.; Mulac, D.; Cinatl, J.; Wass, M.N.; Michaelis, M.; Langer, K. Incorporation of Doxorubicin in Different Polymer Nanoparticles and Their Anticancer Activity. Beilstein J. Nanotechnol. 2019, 10, 2062–2072. [Google Scholar] [CrossRef]
- Miller, M.K.; Chapa-Villarreal, F.A.; Oldenkamp, H.F.; Elder, M.G.; Venkataraman, A.K.; Peppas, N.A. Stimuli-Responsive Self-Assembled Polymer Nanoparticles for the Oral Delivery of Antibodies. J. Control. Release 2023, 361, 246–259. [Google Scholar] [CrossRef]
- Chen, H.; Celik, A.E.; Mutschler, A.; Combes, A.; Runser, A.; Klymchenko, A.S.; Lecommandoux, S.; Serra, C.A.; Reisch, A. Assembly of Fluorescent Polymer Nanoparticles Using Different Microfluidic Mixers. Langmuir 2022, 38, 7945–7955. [Google Scholar] [CrossRef]
- Schoffelen, S.; van Hest, J.C.M. Multi-Enzyme Systems: Bringing Enzymes Together in Vitro. Soft Matter 2012, 8, 1736–1746. [Google Scholar] [CrossRef]
- Dueber, J.E.; Wu, G.C.; Malmirchegini, G.R.; Moon, T.S.; Petzold, C.J.; Ullal, A.V.; Prather, K.L.J.; Keasling, J.D. Synthetic Protein Scaffolds Provide Modular Control over Metabolic Flux. Nat. Biotechnol. 2009, 27, 753–759. [Google Scholar] [CrossRef]
- Liu, Y.; Du, J.; Yan, M.; Lau, M.Y.; Hu, J.; Han, H.; Yang, O.O.; Liang, S.; Wei, W.; Wang, H.; et al. Biomimetic Enzyme Nanocomplexes and Their Use as Antidotes and Preventive Measures for Alcohol Intoxication. Nat. Nanotechnol. 2013, 8, 187–192. [Google Scholar] [CrossRef]
- Gupta, V.; Nayak, S. Dendrimers: A Review on Synthetic Approaches. J. Appl. Pharm. Sci. 2015, 5, 117–122. [Google Scholar] [CrossRef]
- Patil, N.G.; Augustine, R.; Zhang, Y.; Hong, S.C.; Kim, I. Synthesis of Stimuli-Responsive Heterofunctional Dendrimer by Passerini Multicomponent Reaction. ACS Omega 2019, 4, 6660–6668. [Google Scholar] [CrossRef] [PubMed]
- Kreye, O.; Kugele, D.; Faust, L.; Meier, M.A.R. Divergent Dendrimer Synthesis via the Passerini Three-Component Reaction and Olefin Cross-Metathesis. Macromol. Rapid Commun. 2014, 35, 317–322. [Google Scholar] [CrossRef]
- Jee, J.-A.; Spagnuolo, L.A.; Rudick, J.G. Convergent Synthesis of Dendrimers via the Passerini Three-Component Reaction. Org. Lett. 2012, 14, 3292–3295. [Google Scholar] [CrossRef]
- García-Álvarez, F.; Martínez-García, M. Click Reaction in the Synthesis of Dendrimer Drug-Delivery Systems. Curr. Med. Chem. 2022, 29, 3445–3470. [Google Scholar] [CrossRef]
- Numai, S.; Yoto, R.; Kimura, M.; Simanek, E.E.; Kitano, Y. Click Chemistry of Melamine Dendrimers: Comparison of “Click-and-Grow” and “Grow-Then-Click” Strategies Using a Divergent Route to Diversity. Molecules 2022, 28, 131. [Google Scholar] [CrossRef]
- Guven, Z.P.; Silva, P.H.J.; Luo, Z.; Cendrowska, U.B.; Gasbarri, M.; Jones, S.T.; Stellacci, F. Synthesis and Characterization of Amphiphilic Gold Nanoparticles. J. Vis. Exp. 2019, 149, e58872. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of Thiol-Derivatised Gold Nanoparticles in a Two-Phase Liquid–Liquid System. J. Chem. Soc. Chem. Commun. 1994, 801–802. [Google Scholar] [CrossRef]
- Chai, O.J.H.; Xie, J. Unraveling the Mechanism of the Brust-Schiffrin Formation of Au25(SR)18 through Mass Spectrometry. J. Phys. Chem. Lett. 2024, 15, 5137–5142. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Chen, X.; Ma, Y.; Dai, C.; Yang, H.; Li, Q.; Tao, J.; Wu, T. Ball Milling Synthesis of Fe3O4 Nanoparticles-Functionalized Porous Boron Nitride with Enhanced Cationic Dye Removal Performance. RSC Adv. 2024, 14, 7124–7130. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, L.; Huang, H.; He, W.; Ming, W. Progress in Laser Ablation and Biological Synthesis Processes: “Top-Down” and “Bottom-Up” Approaches for the Green Synthesis of Au/Ag Nanoparticles. Int. J. Mol. Sci. 2022, 23, 14658. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, A.; Sergievskaya, A.; El Mel, A.-A.; Fucikova, A.; Antunes Corrêa, C.; Vesely, J.; Duverger-Nédellec, E.; Cornil, D.; Cornil, J.; Tessier, P.-Y.; et al. Co-Sputtering of Gold and Copper onto Liquids: A Route towards the Production of Porous Gold Nanoparticles. Nanotechnology 2020, 31, 455303. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, H. A Review of Methods for Synthesis of Al Nanoparticles. Orient. J. Chem. 2014, 30, 1941–1949. [Google Scholar] [CrossRef]
- Cai, F.; Li, S.; Huang, H.; Iqbal, J.; Wang, C.; Jiang, X. Green Synthesis of Gold Nanoparticles for Immune Response Regulation: Mechanisms, Applications, and Perspectives. J. Biomed. Mater. Res. A 2022, 110, 424–442. [Google Scholar] [CrossRef]
- Dubey, R.K.; Shukla, S.; Hussain, Z. Green Synthesis of Silver Nanoparticles; A Sustainable Approach with Diverse Applications. Chin. J. Appl. Physiol. 2023, 39, e20230007. [Google Scholar] [CrossRef]
- Gonçalves, M.C. Sol-Gel Silica Nanoparticles in Medicine: A Natural Choice. Design, Synthesis and Products. Molecules 2018, 23, 2021. [Google Scholar] [CrossRef]
- Finnie, K.S.; Bartlett, J.R.; Barbé, C.J.A.; Kong, L. Formation of Silica Nanoparticles in Microemulsions. Langmuir 2007, 23, 3017–3024. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid. Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Rangaraj, S.; Venkatachalam, R. A Lucrative Chemical Processing of Bamboo Leaf Biomass to Synthesize Biocompatible Amorphous Silica Nanoparticles of Biomedical Importance. Appl. Nanosci. 2017, 7, 145–153. [Google Scholar] [CrossRef]
- Chen, Q.; Ge, Y.; Granbohm, H.; Hannula, S.-P. Effect of Ethanol on Ag@Mesoporous Silica Formation by In Situ Modified Stöber Method. Nanomaterials 2018, 8, 362. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Feng, N. Mesoporous Silica Nanoparticles: Synthesis, Classification, Drug Loading, Pharmacokinetics, Biocompatibility, and Application in Drug Delivery. Expert Opin. Drug Deliv. 2019, 16, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Eklund, P.C. Fullerenes. J. Mater. Res. 1993, 8, 2054–2097. [Google Scholar] [CrossRef]
- Marek, J.; Buchta, V.; Soukup, O.; Stodulka, P.; Cabal, J.; Ghosh, K.K.; Musilek, K.; Kuca, K. Chemistry of Fullerene Epoxides: Synthesis, Structure, and Nucleophilic Substitution-Addition Reactivity. Molecules 2012, 17, 6395. [Google Scholar] [CrossRef]
- Yasuno, T.; Ohe, T.; Ikeda, H.; Takahashi, K.; Nakamura, S.; Mashino, T. Synthesis and Antitumor Activity of Novel Pyridinium Fullerene Derivatives. Int. J. Nanomed. 2019, 14, 6325–6337. [Google Scholar] [CrossRef]
- Sargazi, S.; ER, S.; Mobashar, A.; Gelen, S.S.; Rahdar, A.; Ebrahimi, N.; Hosseinikhah, S.M.; Bilal, M.; Kyzas, G.Z. Aptamer-Conjugated Carbon-Based Nanomaterials for Cancer and Bacteria Theranostics: A Review. Chem. Biol. Interact. 2022, 361, 109964. [Google Scholar] [CrossRef]
- Cetinkaya, A.; Kaya, S.I.; Ozcelikay, G.; Budak, F.; Ozkan, S.A. Carbon Nanomaterials-Based Novel Hybrid Platforms for Electrochemical Sensor Applications in Drug Analysis. Crit. Rev. Anal. Chem. 2024, 54, 1227–1242. [Google Scholar] [CrossRef]
- Sajid, M.I.; Jamshaid, U.; Jamshaid, T.; Zafar, N.; Fessi, H.; Elaissari, A. Carbon Nanotubes from Synthesis to in Vivo Biomedical Applications. Int. J. Pharm. 2016, 501, 278–299. [Google Scholar] [CrossRef]
- Awasthi, K.; Srivastava, A.; Srivastava, O.N. Synthesis of Carbon Nanotubes. J. Nanosci. Nanotechnol. 2005, 5, 1616–1636. [Google Scholar] [CrossRef]
- Dugam, S.; Nangare, S.; Patil, P.; Jadhav, N. Carbon Dots: A Novel Trend in Pharmaceutical Applications. Ann. Pharm. Françaises 2021, 79, 335–345. [Google Scholar] [CrossRef]
- Ranjbar-Navazi, Z.; Eskandani, M.; Johari-Ahar, M.; Nemati, A.; Akbari, H.; Davaran, S.; Omidi, Y. Doxorubicin-Conjugated D-Glucosamine- and Folate-Bi-Functionalised InP/ZnS Quantum Dots for Cancer Cells Imaging and Therapy. J. Drug Target. 2018, 26, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, H.; Sagar, L.K.; Geraili, A.; Chang, D.; García de Arquer, F.P.; Flynn, C.D.; Lee, S.; Sargent, E.H.; Kelley, S.O. Highly Stable Biotemplated InP/ZnSe/ZnS Quantum Dots for In Situ Bacterial Monitoring. ACS Appl. Mater. Interfaces 2024, 16, 55086–55096. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hwang, D.W.; Jung, H.S.; Kim, K.W.; Pham, X.-H.; Lee, S.-H.; Byun, J.W.; Kim, W.; Kim, H.-M.; Hahm, E.; et al. High-Quantum Yield Alloy-Typed Core/Shell CdSeZnS/ZnS Quantum Dots for Bio-Applications. J. Nanobiotechnol. 2022, 20, 22. [Google Scholar] [CrossRef]
- Lin, T.N.; Chih, K.H.; Yuan, C.T.; Shen, J.L.; Lin, C.A.J.; Liu, W.R. Laser-Ablation Production of Graphene Oxide Nanostructures: From Ribbons to Quantum Dots. Nanoscale 2015, 7, 2708–2715. [Google Scholar] [CrossRef]
- Liu, M.; Xu, Y.; Niu, F.; Gooding, J.J.; Liu, J. Carbon Quantum Dots Directly Generated from Electrochemical Oxidation of Graphite Electrodes in Alkaline Alcohols and the Applications for Specific Ferric Ion Detection and Cell Imaging. Analyst 2016, 141, 2657–2664. [Google Scholar] [CrossRef]
- Rempel, A.A.; Ovchinnikov, O.V.; Weinstein, I.A.; Rempel, S.V.; Kuznetsova, Y.V.; Naumov, A.V.; Smirnov, M.S.; Eremchev, I.Y.; Vokhmintsev, A.S.; Savchenko, S.S. Quantum Dots: Modern Methods of Synthesis and Optical Properties. Russ. Chem. Rev. 2024, 93, RCR5114. [Google Scholar] [CrossRef]
- Chemseddine, A.; Weller, H. Highly Monodisperse Quantum Sized CdS Particles by Size Selective Precipitation. Berichte Bunsenges. Phys. Chem. 1993, 97, 636–638. [Google Scholar] [CrossRef]
- Manikandan, V.; Lee, N.Y. Green Synthesis of Carbon Quantum Dots and Their Environmental Applications. Env. Res. 2022, 212, 113283. [Google Scholar] [CrossRef]
- Caglayan, M.O.; Mindivan, F.; Şahin, S. Sensor and Bioimaging Studies Based on Carbon Quantum Dots: The Green Chemistry Approach. Crit. Rev. Anal. Chem. 2022, 52, 814–847. [Google Scholar] [CrossRef]
- Alavi, M.; Hamidi, M. Passive and Active Targeting in Cancer Therapy by Liposomes and Lipid Nanoparticles. Drug Metab. Pers. Ther. 2019, 34, 20180032. [Google Scholar] [CrossRef]
- Almeida, B.; Nag, O.K.; Rogers, K.E.; Delehanty, J.B. Recent Progress in Bioconjugation Strategies for Liposome-Mediated Drug Delivery. Molecules 2020, 25, 5672. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, P. Experimental Researches on Specific Therapy. In The Collected Papers of Paul Ehrlich; Elsevier: Amsterdam, The Netherlands, 1960; pp. 106–117. [Google Scholar]
- Riaz, M.; Riaz, M.; Zhang, X.; Lin, C.; Wong, K.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A Review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Chauhan, M.; Sonali; Yadav, B.; Dutt, R.; Hu, L.; Muthu, M.S.; Singh, R.P. Enhanced Permeability and Retention Effect-Focused Tumor-Targeted Nanomedicines: Latest Trends, Obstacles and Future Perspective. Nanomedicine 2022, 17, 1213–1216. [Google Scholar] [CrossRef]
- Luo, F.; Yu, Y.; Li, M.; Chen, Y.; Zhang, P.; Xiao, C.; Lv, G. Polymeric Nanomedicines for the Treatment of Hepatic Diseases. J. Nanobiotechnol. 2022, 20, 488. [Google Scholar] [CrossRef]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current Trends and Challenges in Cancer Management and Therapy Using Designer Nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef]
- Pagels, R.F.; Pinkerton, N.M.; York, A.W.; Prud’homme, R.K. Synthesis of Heterobifunctional Thiol-poly(lactic acid)-b-poly(ethylene glycol)-hydroxyl for Nanoparticle Drug Delivery Applications. Macromol. Chem. Phys. 2020, 221, 1900396. [Google Scholar] [CrossRef]
- Rea, I.; Martucci, N.M.; De Stefano, L.; Ruggiero, I.; Terracciano, M.; Dardano, P.; Migliaccio, N.; Arcari, P.; Taté, R.; Rendina, I.; et al. Diatomite Biosilica Nanocarriers for SiRNA Transport inside Cancer Cells. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 3393–3403. [Google Scholar] [CrossRef]
- Jung, H.-S.; Moon, D.-S.; Lee, J.-K. Quantitative Analysis and Efficient Surface Modification of Silica Nanoparticles. J. Nanomater. 2012, 2012, 593471. [Google Scholar] [CrossRef]
- Baeza, A.; Vallet-Regí, M. Mesoporous Silica Nanoparticles as Theranostic Antitumoral Nanomedicines. Pharmaceutics 2020, 12, 957. [Google Scholar] [CrossRef]
- Villegas, M.R.; Baeza, A.; Vallet-Regí, M. Nanotechnological Strategies for Protein Delivery. Molecules 2018, 23, 1008. [Google Scholar] [CrossRef] [PubMed]
- Banihashem, S.; Nezhati, M.N.; Panahia, H.A. Synthesis of Chitosan-Grafted-Poly(N-vinylcaprolactam) Coated on the Thiolated Gold Nanoparticles Surface for Controlled Release of Cisplatin. Carbohydr. Polym. 2020, 227, 115333. [Google Scholar] [CrossRef] [PubMed]
- Korpany, K.V.; Mottillo, C.; Bachelder, J.; Cross, S.N.; Dong, P.; Trudel, S.; Friščić, T.; Blum, A.S. One-Step Ligand Exchange and Switching from Hydrophobic to Water-Stable Hydrophilic Superparamagnetic Iron Oxide Nanoparticles by Mechanochemical Milling. Chem. Commun. 2016, 52, 3054–3057. [Google Scholar] [CrossRef] [PubMed]
- Benbenishty-Shamir, H.; Gilert, R.; Gotman, I.; Gutmanas, E.Y.; Sukenik, C.N. Phosphonate-Anchored Monolayers for Antibody Binding to Magnetic Nanoparticles. Langmuir 2011, 27, 12082–12089. [Google Scholar] [CrossRef]
- Sainz-Urruela, C.; Vera-López, S.; San Andrés, M.P.; Díez-Pascual, A.M. Graphene-Based Sensors for the Detection of Bioactive Compounds: A Review. Int. J. Mol. Sci. 2021, 22, 3316. [Google Scholar] [CrossRef]
- Shim, G.; Kim, D.; Lee, S.; Chang, R.S.; Byun, J.; Oh, Y.-K. Staphylococcus aureus-Mimetic Control of Antibody Orientation on Nanoparticles. Nanomedicine 2019, 16, 267–277. [Google Scholar] [CrossRef]
- Maddahfar, M.; Wen, S.; Hosseinpour Mashkani, S.M.; Zhang, L.; Shimoni, O.; Stenzel, M.; Zhou, J.; Fazekas de St Groth, B.; Jin, D. Stable and Highly Efficient Antibody–Nanoparticles Conjugation. Bioconjug. Chem. 2021, 32, 1146–1155. [Google Scholar] [CrossRef]
- Filbrun, S.L.; Filbrun, A.B.; Lovato, F.L.; Oh, S.H.; Driskell, E.A.; Driskell, J.D. Chemical Modification of Antibodies Enables the Formation of Stable Antibody–Gold Nanoparticle Conjugates for Biosensing. Analyst 2017, 142, 4456–4467. [Google Scholar] [CrossRef]
- Ho, K.-W.; Liu, Y.-L.; Liao, T.-Y.; Liu, E.-S.; Cheng, T.-L. Strategies for Non-Covalent Attachment of Antibodies to PEGylated Nanoparticles for Targeted Drug Delivery. Int. J. Nanomed. 2024, 19, 10045–10064. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, K.; Zhang, X.; Chung, E.J. The Effect of Size, Charge, and Peptide Ligand Length on Kidney Targeting by Small, Organic Nanoparticles. Bioeng. Transl. Med. 2020, 5, e10173. [Google Scholar] [CrossRef]
- Duro-Castano, A.; Moreira Leite, D.; Forth, J.; Deng, Y.; Matias, D.; Noble Jesus, C.; Battaglia, G. Designing Peptide Nanoparticles for Efficient Brain Delivery. Adv. Drug Deliv. Rev. 2020, 160, 52–77. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.Y.; Panganiban, B.; Xu, T. Peptide-Polymer Conjugates: From Fundamental Science to Application. Annu. Rev. Phys. Chem. 2013, 64, 631–657. [Google Scholar] [CrossRef] [PubMed]
- Kulhari, H.; Telukutla, S.R.; Pooja, D.; Shukla, R.; Sistla, R.; Bansal, V.; Adams, D.J. Peptide Grafted and Self-Assembled Poly(γ-glutamic acid)-phenylalanine Nanoparticles Targeting Camptothecin to Glioma. Nanomedicine 2017, 12, 1661–1674. [Google Scholar] [CrossRef]
- Shao, K.; Zhang, Y.; Ding, N.; Huang, S.; Wu, J.; Li, J.; Yang, C.; Leng, Q.; Ye, L.; Lou, J.; et al. Functionalized Nanoscale Micelles with Brain Targeting Ability and Intercellular Microenvironment Biosensitivity for Anti-Intracranial Infection Applications. Adv. Heal. Mater. 2015, 4, 291–300. [Google Scholar] [CrossRef]
- Miura, Y.; Takenaka, T.; Toh, K.; Wu, S.; Nishihara, H.; Kano, M.R.; Ino, Y.; Nomoto, T.; Matsumoto, Y.; Koyama, H.; et al. Cyclic RGD-Linked Polymeric Micelles for Targeted Delivery of Platinum Anticancer Drugs to Glioblastoma through the Blood–Brain Tumor Barrier. ACS Nano 2013, 7, 8583–8592. [Google Scholar] [CrossRef]
- Merrifield, R.B. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Noki, S.; de la Torre, B.G.; Albericio, F. Safety-Catch Linkers for Solid-Phase Peptide Synthesis. Molecules 2024, 29, 1429. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, A.; de la Torre, B.G.; Albericio, F. Liquid-Phase Peptide Synthesis (LPPS): A Third Wave for the Preparation of Peptides. Chem. Rev. 2022, 122, 13516–13546. [Google Scholar] [CrossRef]
- Liu, T.; Peng, Z.; Lai, M.; Hu, L.; Zhao, J. Inverse Peptide Synthesis Using Transient Protected Amino Acids. J. Am. Chem. Soc. 2024, 146, 4270–4280. [Google Scholar] [CrossRef]
- Zhao, Q.; Xu, W.; Xing, L.; Lin, Z. Recombinant Production of Medium- to Large-Sized Peptides in Escherichia coli Using a Cleavable Self-Aggregating Tag. Microb. Cell Fact. 2016, 15, 136. [Google Scholar] [CrossRef]
- Gregoriadis, G.; Swain, C.P.; Wills, E.J.; Tavill, A.S. Drug-Carrier Potential of Liposomes in Cancer Chemotherapy. Lancet 1974, 303, 1313–1316. [Google Scholar] [CrossRef]
- Rahman, Y.E.; Rosenthal, M.W.; Cerny, E.A.; Moretti, E.S. Preparation and Prolonged Tissue Retention of Liposome-Encapsulated Chelating Agents. J. Lab. Clin. Med. 1974, 83, 640–647. [Google Scholar] [PubMed]
- Paolino, D.; d’Avanzo, N.; Canato, E.; Ciriolo, L.; Grigoletto, A.; Cristiano, M.C.; Mancuso, A.; Celia, C.; Pasut, G.; Fresta, M. Improved Anti-Breast Cancer Activity by Doxorubicin-Loaded Super Stealth Liposomes. Biomater. Sci. 2024, 12, 3933–3946. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles—From Liposomes to MRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Cheong, M.S.; Lee, J.; Bang, E.-K.; Park, S.I.; Park, H.-J.; Bae, S.-H.; Yoon, S.; Roh, G.; Lee, S.; et al. Immunogenicity and Protection of a Triple Repeat Domain III MRNA Vaccine Against Zika Virus. Vaccine 2025, 43, 126518. [Google Scholar] [CrossRef]
- Chatzikleanthous, D.; O’Hagan, D.T.; Adamo, R. Lipid-Based Nanoparticles for Delivery of Vaccine Adjuvants and Antigens: Toward Multicomponent Vaccines. Mol. Pharm. 2021, 18, 2867–2888. [Google Scholar] [CrossRef]
- Freyn, A.W.; Ramos da Silva, J.; Rosado, V.C.; Bliss, C.M.; Pine, M.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; de Souza Ferreira, L.C.; Weissman, D.; et al. A Multi-Targeting, Nucleoside-Modified MRNA Influenza Virus Vaccine Provides Broad Protection in Mice. Mol. Ther. 2020, 28, 1569–1584. [Google Scholar] [CrossRef]
- Poria, R.; Kala, D.; Nagraik, R.; Dhir, Y.; Dhir, S.; Singh, B.; Kaushik, N.K.; Noorani, M.S.; Kaushal, A.; Gupta, S. Vaccine Development: Current Trends and Technologies. Life Sci. 2024, 336, 122331. [Google Scholar] [CrossRef]
- Pushko, P.; Pumpens, P.; Grens, E. Development of Virus-like Particle Technology from Small Highly Symmetric to Large Complex Virus-like Particle Structures. Intervirology 2013, 56, 141–165. [Google Scholar] [CrossRef]
- Kim, H.J.; Cho, S.Y.; Park, M.-H.; Kim, H.-J. Comparison of the Size Distributions and Immunogenicity of Human Papillomavirus Type 16 L1 Virus-like Particles Produced in Insect and Yeast Cells. Arch. Pharm. Res. 2018, 41, 544–553. [Google Scholar] [CrossRef]
- Cheng, K.; Du, T.; Li, Y.; Qi, Y.; Min, H.; Wang, Y.; Zhang, Q.; Wang, C.; Zhou, Y.; Li, L.; et al. Dual-Antigen-Loaded Hepatitis B Virus Core Antigen Virus-like Particles Stimulate Efficient Immunotherapy Against Melanoma. ACS Appl. Mater. Interfaces 2020, 12, 53682–53690. [Google Scholar] [CrossRef] [PubMed]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like Particles: Preparation, Immunogenicity and Their Roles as Nanovaccines and Drug Nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Cai, H.; Steinmetz, N.F. Viral Nanoparticles for Drug Delivery, Imaging, Immunotherapy, and Theranostic Applications. Adv. Drug Deliv. Rev. 2020, 156, 214–235. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Vogel, M.; Riether, C.; Muller, J.; Salatino, S.; Ternette, N.; Gomes, A.C.; Cabral-Miranda, G.; El-Turabi, A.; Ruedl, C.; et al. Targeting Mutated Plus Germline Epitopes Confers Pre-Clinical Efficacy of an Instantly Formulated Cancer Nano-Vaccine. Front. Immunol. 2019, 10, 1015. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Speiser, D.E.; Michaux, J.; Pak, H.; Stevenson, B.J.; Vogel, M.; Inchakalody, V.P.; de Brot, S.; Dermime, S.; Coukos, G.; et al. Bedside Formulation of a Personalized Multi-Neoantigen Vaccine against Mammary Carcinoma. J. Immunother. Cancer 2022, 10, e002927. [Google Scholar] [CrossRef]
- Mainini, F.; Eccles, M.R. Lipid and Polymer-Based Nanoparticle SiRNA Delivery Systems for Cancer Therapy. Molecules 2020, 25, 2692. [Google Scholar] [CrossRef]
- Yu, L.; Luo, Z.; Chen, T.; Ouyang, Y.; Xiao, L.; Liang, S.; Peng, Z.; Liu, Y.; Deng, Y. Bioadhesive Nanoparticles for Local Drug Delivery. Int. J. Mol. Sci. 2022, 23, 2370. [Google Scholar] [CrossRef]
- Wang, B.; Wang, S.; Zhang, Q.; Deng, Y.; Li, X.; Peng, L.; Zuo, X.; Piao, M.; Kuang, X.; Sheng, S.; et al. Recent Advances in Polymer-Based Drug Delivery Systems for Local Anesthetics. Acta Biomater. 2019, 96, 55–67. [Google Scholar] [CrossRef]
- Pan, Y.; Li, Y.; Zhao, H.; Zheng, J.; Xu, H.; Wei, G.; Hao, J.; Cui, F. Bioadhesive Polysaccharide in Protein Delivery System: Chitosan Nanoparticles Improve the Intestinal Absorption of Insulin In Vivo. Int. J. Pharm. 2002, 249, 139–147. [Google Scholar] [CrossRef]
- Sgorla, D.; Lechanteur, A.; Almeida, A.; Sousa, F.; Melo, E.; Bunhak, É.; Mainardes, R.; Khalil, N.; Cavalcanti, O.; Sarmento, B. Development and Characterization of Lipid-Polymeric Nanoparticles for Oral Insulin Delivery. Expert Opin. Drug Deliv. 2018, 15, 213–222. [Google Scholar] [CrossRef]
- Chellathurai, M.S.; Yong, C.L.; Sofian, Z.M.; Sahudin, S.; Hasim, N.B.M.; Mahmood, S. Self-Assembled Chitosan-Insulin Oral Nanoparticles—A Critical Perspective Review. Int. J. Biol. Macromol. 2023, 243, 125125. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Yao, Y.; Zhang, J.; Liu, W.; Ji, K.; Wei, X.; Wang, Y.; Liu, X.; Zhang, S.; et al. Glucose-Responsive Charge-Switchable Lipid Nanoparticles for Insulin Delivery. Angew. Chem. Int. Ed. 2023, 62, e202303097. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Li, Z.; Hsu, C.-H.; Hwang, L.-P.; Lin, Y.-C.; Chou, P.-T.; Lin, Y.-Y. Dendrimer- and Copolymer-Based Nanoparticles for Magnetic Resonance Cancer Theranostics. Theranostics 2018, 8, 6322–6349. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Santos, S.; Igne Ferreira, E.; Giarolla, J. Dendrimer Prodrugs. Molecules 2016, 21, 686. [Google Scholar] [CrossRef]
- Zhao, T.; Zhou, M.; Wu, R.; Wang, H.; Zouboulis, C.C.; Zhu, M.; Lee, M. Dendrimer-Conjugated Isotretinoin for Controlled Transdermal Drug Delivery. J. Nanobiotechnol. 2023, 21, 285. [Google Scholar] [CrossRef]
- Guerra, R.O.; do Carmo Neto, J.R.; de Albuquerque Martins, T.; Farnesi de-Assunção, T.S.; Junior, V.R.; de Oliveira, C.J.F.; Silva, A.C.A.; da Silva, M.V. Metallic Nanoparticles: A New Frontier in the Fight Against Leishmaniasis. Curr. Med. Chem. 2022, 29, 4547–4573. [Google Scholar] [CrossRef]
- Alphandéry, E. Natural Metallic Nanoparticles for Application in Nano-Oncology. Int. J. Mol. Sci. 2020, 21, 4412. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Qiu, X.; Zuo, F.; Wang, B. Mesoporous Silica Nanoparticles as a Drug Delivery Mechanism. Open Life Sci. 2024, 19, 20220867. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Sarkis, M.; Minassian, G.; Mitri, N.; Rahme, K.; Fracasso, G.; El Hage, R.; Ghanem, E. D2B-Functionalized Gold Nanoparticles: Promising Vehicles for Targeted Drug Delivery to Prostate Cancer. ACS Appl. Bio Mater. 2023, 6, 819–827. [Google Scholar] [CrossRef]
- Elbialy, N.S.; Fathy, M.M.; Khalil, W.M. Doxorubicin Loaded Magnetic Gold Nanoparticles for In Vivo Targeted Drug Delivery. Int. J. Pharm. 2015, 490, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Urso, A.; Meloni, F.; Malatesta, M.; Latorre, R.; Damoci, C.; Crapanzano, J.; Pandolfi, L.; Giustra, M.D.; Pearson, M.; Colombo, M.; et al. Endotracheal Nebulization of Gold Nanoparticles for Noninvasive Pulmonary Drug Delivery. Nanomedicine 2023, 18, 317–330. [Google Scholar] [CrossRef]
- Sakthi Devi, R.; Girigoswami, A.; Siddharth, M.; Girigoswami, K. Applications of Gold and Silver Nanoparticles in Theranostics. Appl. Biochem. Biotechnol. 2022, 194, 4187–4219. [Google Scholar] [CrossRef]
- Li, W.; Chen, X. Gold Nanoparticles for Photoacoustic Imaging. Nanomedicine 2015, 10, 299–320. [Google Scholar] [CrossRef]
- Proniewicz, E. Metallic Nanoparticles as Effective Sensors of Bio-Molecules. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 288, 122207. [Google Scholar] [CrossRef]
- Chota, A.; George, B.P.; Abrahamse, H. Recent Advances in Green Metallic Nanoparticles for Enhanced Drug Delivery in Photodynamic Therapy: A Therapeutic Approach. Int. J. Mol. Sci. 2023, 24, 4808. [Google Scholar] [CrossRef]
- Cavassin, E.D.; de Figueiredo, L.F.P.; Otoch, J.P.; Seckler, M.M.; de Oliveira, R.A.; Franco, F.F.; Marangoni, V.S.; Zucolotto, V.; Levin, A.S.S.; Costa, S.F. Comparison of Methods to Detect the in Vitro Activity of Silver Nanoparticles (AgNP) Against Multidrug Resistant Bacteria. J. Nanobiotechnol. 2015, 13, 64. [Google Scholar] [CrossRef]
- Parra-Nieto, J.; del Cid, M.A.G.; de Cárcer, I.A.; Baeza, A. Inorganic Porous Nanoparticles for Drug Delivery in Antitumoral Therapy. Biotechnol. J. 2021, 16, 2000150. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous Silica Nanoparticles in Drug Delivery and Biomedical Applications. Nanomedicine 2015, 11, 313–327. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Alsehli, M.; Al-Enizi, A.; Nafady, A. Recent Advances in Mesoporous Silica Nanoparticles for Targeted Drug Delivery Applications. Curr. Drug Deliv. 2022, 19, 436–450. [Google Scholar] [CrossRef]
- Kienzle, A.; Kurch, S.; Schlöder, J.; Berges, C.; Ose, R.; Schupp, J.; Tuettenberg, A.; Weiss, H.; Schultze, J.; Winzen, S.; et al. Dendritic Mesoporous Silica Nanoparticles for PH-Stimuli-Responsive Drug Delivery of TNF-Alpha. Adv. Healthc. Mater. 2017, 6, 1700012. [Google Scholar] [CrossRef] [PubMed]
- Zaltariov, M.-F.; Ciubotaru, B.-I.; Ghilan, A.; Peptanariu, D.; Ignat, M.; Iacob, M.; Vornicu, N.; Cazacu, M. Mucoadhesive Mesoporous Silica Particles as Versatile Carriers for Doxorubicin Delivery in Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 14687. [Google Scholar] [CrossRef] [PubMed]
- Priya, V.; Vikas; Mehata, A.K.; Jain, D.; Singh, S.K.; Muthu, M.S. Efficient Delivery of Abciximab Using Mesoporous Silica Nanoparticles: In-Vitro Assessment for Targeted and Improved Antithrombotic Activity. Colloids Surf. B Biointerfaces 2022, 218, 112697. [Google Scholar] [CrossRef] [PubMed]
- Galhano, J.; Marcelo, G.A.; Duarte, M.P.; Oliveira, E. Ofloxacin@Doxorubicin-Epirubicin Functionalized MCM-41 Mesoporous Silica–Based Nanocarriers as Synergistic Drug Delivery Tools for Cancer Related Bacterial Infections. Bioorg Chem. 2022, 118, 105470. [Google Scholar] [CrossRef]
- Gudiol, C.; Carratalà, J. Antibiotic Resistance in Cancer Patients. Expert Rev. Anti Infect. Ther. 2014, 12, 1003–1016. [Google Scholar] [CrossRef]
- Alphandéry, E. Nano Dimensions/Adjuvants in COVID-19 Vaccines. J. Mater. Chem. B 2022, 10, 1520–1552. [Google Scholar] [CrossRef]
- Masalova, O.V.; Lesnova, E.I.; Andreev, S.M.; Shershakova, N.N.; Kozlov, V.V.; Permyakova, K.Y.; Demidova, N.A.; Valuev-Elliston, V.T.; Turetskiy, E.A.; Ivanov, A.V.; et al. Adjuvant Effect of Dispersed Fullerene C60 on the Immune Response to Constructs Harboring Amino Acid and Nucleotide Sequences of Hepatitis C Virus Nonstructural NS5B Protein. Probl. Virol. 2022, 67, 516–526. [Google Scholar] [CrossRef]
- Shershakova, N.; Baraboshkina, E.; Andreev, S.; Purgina, D.; Struchkova, I.; Kamyshnikov, O.; Nikonova, A.; Khaitov, M. Anti-Inflammatory Effect of Fullerene C60 in a Mice Model of Atopic Dermatitis. J. Nanobiotechnol. 2016, 14, 8. [Google Scholar] [CrossRef]
- Al-Anbari, H.H.; Mahdi, Z.A.-A.; Zandi, H.; Karimi, M. Investigating a Nickel-Decorated Fullerene for Adsorbing Tespa Anticancer: Drug Delivery Assessments. J. Mol. Model. 2022, 28, 390. [Google Scholar] [CrossRef]
- Sukumar, A.N.; Duraisamy, P.D.; Paul, S.P.M.; Gopalan, P.; Angamuthu, A. Pristine B40 Fullerene as a Potential Gemcitabine Drug Carrier for Anti-Lung Cancer Properties: A DFT and QTAIM Study. J. Biomol. Struct. Dyn. 2024, 1–14. [Google Scholar] [CrossRef]
- Abdellatif, A.A.; Younis, M.A.; Alsharidah, M.; Al Rugaie, O.; Tawfeek, H.M. Biomedical Applications of Quantum Dots: Overview, Challenges, and Clinical Potential. Int. J. Nanomed. 2022, 17, 1951–1970. [Google Scholar] [CrossRef] [PubMed]
- Mukerabigwi, J.F.; Tang, R.; Cao, Y.; Mohammed, F.; Zhou, Q.; Zhou, M.; Ge, Z. Mitochondria-Targeting Polyprodrugs to Overcome the Drug Resistance of Cancer Cells by Self-Amplified Oxidation-Triggered Drug Release. Bioconjug. Chem. 2023, 34, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Johari-Ahar, M.; Barar, J.; Alizadeh, A.M.; Davaran, S.; Omidi, Y.; Rashidi, M.-R. Methotrexate-Conjugated Quantum Dots: Synthesis, Characterisation and Cytotoxicity in Drug Resistant Cancer Cells. J. Drug Target. 2016, 24, 120–133. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Yu, L.; Wu, L.; Hao, X.; Liu, Q.; Lin, L.; Huang, Z.; Ruan, Z.; Weng, S.; et al. Quaternized Carbon Quantum Dots with Broad-Spectrum Antibacterial Activity for the Treatment of Wounds Infected with Mixed Bacteria. Acta Biomater. 2022, 138, 528–544. [Google Scholar] [CrossRef]
- Dong, X.; Liang, W.; Meziani, M.J.; Sun, Y.-P.; Yang, L. Carbon Dots as Potent Antimicrobial Agents. Theranostics 2020, 10, 671–686. [Google Scholar] [CrossRef]
- Wu, Q.; Song, J.; Wang, Y.; Li, H.; Zhao, L.; Lv, H.; Zhao, X.-E. Carbon Quantum Dots–Functionalized Silica Stationary Phase for Pharmaceutical Analysis by a Green Liquid Chromatography Mode. Microchim. Acta 2022, 189, 175. [Google Scholar] [CrossRef]
- Elnaggar, M.M.; El-Yazbi, A.F.; Belal, T.S.; Elbardisy, H.M. White Sustainable Luminescent Determination of Nifuroxazide Using Nitrogen–Sulphur Co-Doped Carbon Quantum Dots Nanosensor in Bulk and Various Pharmaceutical Matrices. RSC Adv. 2023, 13, 29830–29846. [Google Scholar] [CrossRef]
- Tan, J.; Wu, S.; Cai, Q.; Wang, Y.; Zhang, P. Reversible Regulation of Enzyme-like Activity of Molybdenum Disulfide Quantum Dots for Colorimetric Pharmaceutical Analysis. J. Pharm. Anal. 2022, 12, 113–121. [Google Scholar] [CrossRef]
- Dai, J.; Ashrafizadeh, M.; Aref, A.R.; Sethi, G.; Ertas, Y.N. Peptide-Functionalized, -Assembled and -Loaded Nanoparticles in Cancer Therapy. Drug Discov. Today 2024, 29, 103981. [Google Scholar] [CrossRef]
- Rajabi, M.; Adeyeye, M.; Mousa, S.A. Peptide-Conjugated Nanoparticles as Targeted Anti-Angiogenesis Therapeutic and Diagnostic in Cancer. Curr. Med. Chem. 2019, 26, 5664–5683. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, J.; Yan, J.; You, W.; Hou, P.; He, W.; Zhao, X. Modulating Protein–Protein Interactions In Vivo via Peptide-Lanthanide-Derived Nanoparticles for Hazard-Free Cancer Therapy. J. Biomed. Nanotechnol. 2019, 15, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, G.; Brambilla, L.; Rossi, D. Cell-Penetrating Peptides: From Basic Research to Clinics. Trends Pharmacol. Sci. 2017, 38, 406–424. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Liu, J.; He, Q.; Wang, L.; Shi, J. Overcoming Multidrug Resistance of Cancer Cells by Direct Intranuclear Drug Delivery Using TAT-Conjugated Mesoporous Silica Nanoparticles. Biomaterials 2013, 34, 2719–2730. [Google Scholar] [CrossRef] [PubMed]
- Miauton, A.; Audran, R.; Besson, J.; Maby-El Hajjami, H.; Karlen, M.; Warpelin-Decrausaz, L.; Sene, L.; Schaufelberger, S.; Faivre, V.; Faouzi, M.; et al. Safety and Immunogenicity of a Synthetic Nanoparticle-Based, T Cell Priming Peptide Vaccine against Dengue in Healthy Adults in Switzerland: A Double-Blind, Randomized, Vehicle-Controlled, Phase 1 Study. EBioMedicine 2024, 99, 104922. [Google Scholar] [CrossRef]
- Rahman, N.A.A.; Fuaad, A.A.-H.A.; Azami, N.A.M.; Amin, M.C.I.M.; Azmi, F. Next-Generation Dengue Vaccines: Leveraging Peptide-Based Immunogens and Advanced Nanoparticles as Delivery Platforms. J. Pharm. Sci. 2024, 113, 2044–2054. [Google Scholar] [CrossRef]
- Qiao, L.; Chen, M.; Li, S.; Hu, J.; Gong, C.; Zhang, Z.; Cao, X. A Peptide-Based Subunit Candidate Vaccine against SARS-CoV-2 Delivered by Biodegradable Mesoporous Silica Nanoparticles Induced High Humoral and Cellular Immunity in Mice. Biomater. Sci. 2021, 9, 7287–7296. [Google Scholar] [CrossRef]
- Rajchakit, U.; Sarojini, V. Recent Developments in Antimicrobial-Peptide-Conjugated Gold Nanoparticles. Bioconjug. Chem. 2017, 28, 2673–2686. [Google Scholar] [CrossRef]
- Falciani, C.; Zevolini, F.; Brunetti, J.; Riolo, G.; Gracia, R.; Marradi, M.; Loinaz, I.; Ziemann, C.; Cossío, U.; Llop, J.; et al. Antimicrobial Peptide-Loaded Nanoparticles as Inhalation Therapy for Pseudomonas aeruginosa Infections. Int. J. Nanomed. 2020, 15, 1117–1128. [Google Scholar] [CrossRef]
- Vladár, A.E.; Hodoroaba, V.-D. Characterization of Nanoparticles by Scanning Electron Microscopy. In Characterization of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 7–27. [Google Scholar]
- Padilla-Hernández, C.I.; Silva-Jara, J.M.; Reyes-Becerril, M.; Fonseca-García, A.; Anaya-Esparza, L.M.; Orozco-Sánchez, P.R.; Rivera-Valdés, J.J.; López-Orozco, M.; Velázquez-Carriles, C.A.; Macías-Rodríguez, M.E. Green Synthesis of Zinc Oxide Nanoparticles with Psidium Cattleianum Leaves Extracts as Reducing Agent: Influence of Extraction Method on Physicochemical and Biological Activities. Physchem 2025, 5, 17. [Google Scholar] [CrossRef]
- Sung, J.; Bae, Y.; Park, H.; Kang, S.; Choi, B.K.; Kim, J.; Park, J. Liquid-Phase Transmission Electron Microscopy for Reliable In Situ Imaging of Nanomaterials. Annu. Rev. Chem. Biomol. Eng. 2022, 13, 167–191. [Google Scholar] [CrossRef]
- Duan, J.; Wang, J.; Hou, L.; Ji, P.; Zhang, W.; Liu, J.; Zhu, X.; Sun, Z.; Ma, Y.; Ma, L. Application of Scanning Tunneling Microscopy and Spectroscopy in the Studies of Colloidal Quantum Qots. Chem. Rec. 2023, 23, e202300120. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dixit, C.K. Methods for Characterization of Nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Elsevier: Amsterdam, The Netherlands, 2017; pp. 43–58. [Google Scholar]
- Rodríguez-Galván, A.; Reyes, M.; Ávila-Cruz, M.; Rivera, M.; Basiuk, V.A. Scanning Tunneling Microscopy Study of Lipoic Acid, Mannose, and cRGD@AuNPs Conjugates. Nanomaterials 2023, 13, 2596. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Kumar, V.; Randhawa, S.; Verma, S.K. Preparation and Characterization of Extracellular Vesicles. Am. J. Reprod. Immunol. 2021, 85, e13367. [Google Scholar] [CrossRef]
- La Verde, G.; Sasso, A.; Rusciano, G.; Capaccio, A.; Fusco, S.; Mayol, L.; Biondi, M.; Silvestri, T.; Netti, P.A.; La Commara, M.; et al. Characterization of Hyaluronic Acid-Coated PLGA Nanoparticles by Surface-Enhanced Raman Spectroscopy. Int. J. Mol. Sci. 2022, 24, 601. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. In Characterization of Nanoparticles Intended for Drug Delivery; Part of the Book Series: Methods in Molecular Biology; Springer: New York, NY, USA, 2011; pp. 63–70. [Google Scholar]
- Öztürk, K.; Kaplan, M.; Çalış, S. Effects of Nanoparticle Size, Shape, and Zeta Potential on Drug Delivery. Int. J. Pharm. 2024, 666, 124799. [Google Scholar] [CrossRef]
- Musielak, E.; Feliczak-Guzik, A.; Nowak, I. Optimization of the Conditions of Solid Lipid Nanoparticles (SLN) Synthesis. Molecules 2022, 27, 2202. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental Methods in Chemical Engineering: Specific Surface Area and Pore Size Distribution Measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Khanmohammadi, M.; Garmarudi, A.B.; Khoddami, N.; Shabani, K.; Khanlari, M. A Novel Technique Based on Diffuse Reflectance Near-Infrared Spectrometry and Back-Propagation Artificial Neural Network for Estimation of Particle Size in TiO2 Nano Particle Samples. Microchem. J. 2010, 95, 337–340. [Google Scholar] [CrossRef]
- Ock, K.-S.; Dembereldorj, U.; Park, J.; Ganbold, E.-O.; Kim, S.; Shin, H.-C.; Joo, S.-W. Temperature-Dependent Structural Change of d-Penicillamine-Capped Chiral Gold Nanoparticles Investigated by Infrared Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 102, 419–424. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Mizaikoff, B. Recent Advances on the Characterization of Nanoparticles Using Infrared Spectroscopy. Trends Anal. Chem. 2016, 84, 97–106. [Google Scholar] [CrossRef]
- Ueya, Y.; Umezawa, M.; Kobayashi, Y.; Ichihashi, K.; Kobayashi, H.; Matsuda, T.; Takamoto, E.; Kamimura, M.; Soga, K. Effects of Hydrophilic/Hydrophobic Blocks Ratio of PEG-b-PLGA on Emission Intensity and Stability of over-1000 Nm near-Infrared (NIR-II) Fluorescence Dye-Loaded Polymeric Micellar Nanoparticles. Anal. Sci. 2022, 38, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Demin, A.M.; Koryakova, O.V.; Krasnov, V.P. Quantitative Determination of 3-Aminopropylsilane on the Surface of FE3O4 Nanoparticles by Attenuated Total Reflection Infrared Spectroscopy. J. Appl. Spectrosc. 2014, 81, 565–569. [Google Scholar] [CrossRef]
- Li, Y.-S.; Church, J.S. Raman Spectroscopy in the Analysis of Food and Pharmaceutical Nanomaterials. J. Food Drug Anal. 2014, 22, 29–48. [Google Scholar] [CrossRef]
- Yamamoto, T.; Murakami, Y.; Motoyanagi, J.; Fukushima, T.; Maruyama, S.; Kato, M. An Analytical System for Single Nanomaterials: Combination of Capillary Electrophoresis with Raman Spectroscopy or with Scanning Probe Microscopy for Individual Single-Walled Carbon Nanotube Analysis. Anal. Chem. 2009, 81, 7336–7341. [Google Scholar] [CrossRef]
- Prasad, C.; Yuvaraja, G.; Venkateswarlu, P. Biogenic Synthesis of Fe3O4 Magnetic Nanoparticles Using Pisum Sativum Peels Extract and Its Effect on Magnetic and Methyl Orange Dye Degradation Studies. J. Magn. Magn. Mater. 2017, 424, 376–381. [Google Scholar] [CrossRef]
- Qin, Y.; Yin, S.; Chen, M.; Yao, W.; He, Y. Surface-Enhanced Raman Spectroscopy for Detection of Fentanyl and Its Analogs by Using Ag-Au Nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 285, 121923. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, J.; Yim, G.; Ahn, H.J.; Lee, M.; Kim, T.-H.; Park, C.; Min, J.; Jang, H.; Lee, T. Fabrication of a Surface-Enhanced Raman Spectroscopy-Based Analytical Method Consisting of Multifunctional DNA Three-Way Junction-Conjugated Porous Gold Nanoparticles and Au-Te Nanoworm for C-Reactive Protein Detection. Anal. Bioanal. Chem. 2022, 414, 3197–3204. [Google Scholar] [CrossRef]
- Potts, P.J. X-Ray Fluorescence Analysis: Principles and Practice of Wavelength Dispersive Spectrometry. In A Handbook of Silicate Rock Analysis; Springer: Dordrecht, The Netherlands, 1987; pp. 226–285. [Google Scholar]
- Habeche, F.; Boukoussa, B.; Issam, I.; Mokhtar, A.; Lu, X.; Iqbal, J.; Hacini, S.; Hachemaoui, M.; Bengueddach, A.; Hamacha, R. Catalytic Reduction of Organic Pollutants, Antibacterial and Antifungal Activities of AgNPs@CuO Nanoparticles–Loaded Mesoporous Silica. Environ. Sci. Pollut. Res. 2022, 30, 30855–30873. [Google Scholar] [CrossRef]
- Ikram, M.; Umar, E.; Raza, A.; Haider, A.; Naz, S.; Ul-Hamid, A.; Haider, J.; Shahzadi, I.; Hassan, J.; Ali, S. Dye Degradation Performance, Bactericidal Behavior and Molecular Docking Analysis of Cu-Doped TiO2 Nanoparticles. RSC Adv. 2020, 10, 24215–24233. [Google Scholar] [CrossRef]
- Nazemi, Z.; Mehdikhani-Nahrkhalaji, M.; Haghbin-Nazarpak, M.; Staji, H.; Kalani, M.M. Antibacterial Activity Evaluation of Bioactive Glass and Biphasic Calcium Phosphate Nanopowders Mixtures. Appl. Phys. A 2016, 122, 1063. [Google Scholar] [CrossRef]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro Story and the Clinical Translation of Nanomedicines Containing Nucleic Acid-Based Drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Jung, S. Monte Carlo Modeling of Gold Nanoparticles Detection Limits of Benchtop Three-dimensional L- and K-shell X-ray Fluorescence Mapping Systems. X-Ray Spectrom. 2023, 52, 28–37. [Google Scholar] [CrossRef]
- Nzilu, D.M.; Madivoli, E.S.; Makhanu, D.S.; Wanakai, S.I.; Kiprono, G.K.; Kareru, P.G. Green Synthesis of Copper Oxide Nanoparticles and Its Efficiency in Degradation of Rifampicin Antibiotic. Sci. Rep. 2023, 13, 14030. [Google Scholar] [CrossRef]
- Cesur, S.; Cam, M.E.; Sayın, F.S.; Su, S.; Harker, A.; Edirisinghe, M.; Gunduz, O. Metformin-Loaded Polymer-Based Microbubbles/Nanoparticles Generated for the Treatment of Type 2 Diabetes Mellitus. Langmuir 2022, 38, 5040–5051. [Google Scholar] [CrossRef]
- Khramtsov, P.; Kalashnikova, T.; Bochkova, M.; Kropaneva, M.; Timganova, V.; Zamorina, S.; Rayev, M. Measuring the Concentration of Protein Nanoparticles Synthesized by Desolvation Method: Comparison of Bradford Assay, BCA Assay, Hydrolysis/UV Spectroscopy and Gravimetric Analysis. Int. J. Pharm. 2021, 599, 120422. [Google Scholar] [CrossRef]
- Qiu, Y.; Yuan, B.; Cao, Y.; He, X.; Akakuru, O.U.; Lu, L.; Chen, N.; Xu, M.; Wu, A.; Li, J. Recent Progress on Near-infrared Fluorescence Heptamethine Cyanine Dye-based Molecules and Nanoparticles for Tumor Imaging and Treatment. WIREs Nanomed. Nanobiotechnol. 2023, 15, e1910. [Google Scholar] [CrossRef]
- Ghaderi, S.; Ramesh, B.; Seifalian, A.M. Fluorescence Nanoparticles “Quantum Dots” as Drug Delivery System and Their Toxicity: A Review. J. Drug Target. 2011, 19, 475–486. [Google Scholar] [CrossRef]
- Bakalova, R.; Zhelev, Z.; Jose, R.; Nagase, T.; Ohba, H.; Ishikawa, M.; Baba, Y. Role of Free Cadmium and Selenium Ions in the Potential Mechanism for the Enhancement of Photoluminescence of CdSe Quantum Dots Under Ultraviolet Irradiation. J. Nanosci. Nanotechnol. 2005, 5, 887–894. [Google Scholar] [CrossRef]
- Borlan, R.; Focsan, M.; Maniu, D.; Astilean, S. Interventional NIR Fluorescence Imaging of Cancer: Review on Next Generation of Dye-Loaded Protein-Based Nanoparticles for Real-Time Feedback During Cancer Surgery. Int. J. Nanomed. 2021, 16, 2147–2171. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Z.; Huang, C.; Zhao, S.; Zhu, S.; Liu, R.; Zhu, H. Self-Assembled BODIPY Nanoparticles for Near-Infrared Fluorescence Bioimaging. Molecules 2023, 28, 2997. [Google Scholar] [CrossRef] [PubMed]
- Morozova, M.A.; Tumasov, V.N.; Kazimova, I.V.; Maksimova, T.V.; Uspenskaya, E.V.; Syroeshkin, A.V. Second-Order Scattering Quenching in Fluorescence Spectra of Natural Humates as a Tracer of Formation Stable Supramolecular System for the Delivery of Poorly Soluble Antiviral Drugs on the Example of Mangiferin and Favipiravir. Pharmaceutics 2022, 14, 767. [Google Scholar] [CrossRef] [PubMed]
- Lichota, A.; Szabelski, M.; Krokosz, A. Quenching of Protein Fluorescence by Fullerenol C60(OH)36 Nanoparticles. Int. J. Mol. Sci. 2022, 23, 12382. [Google Scholar] [CrossRef]
- Bunjes, H.; Unruh, T. Characterization of Lipid Nanoparticles by Differential Scanning Calorimetry, X-Ray and Neutron Scattering. Adv. Drug Deliv. Rev. 2007, 59, 379–402. [Google Scholar] [CrossRef]
- Castelli, F.; Puglia, C.; Sarpietro, M.G.; Rizza, L.; Bonina, F. Characterization of Indomethacin-Loaded Lipid Nanoparticles by Differential Scanning Calorimetry. Int. J. Pharm. 2005, 304, 231–238. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, T.; Gao, L.; Zhao, W.; Wang, P. Preparation of Azithromycin Nanosuspensions by High Pressure Homogenization and Its Physicochemical Characteristics Studies. Drug Dev. Ind. Pharm. 2007, 33, 569–575. [Google Scholar] [CrossRef]
- Paredes da Rocha, N.; de Souza, A.; Nishitani Yukuyama, M.; Lopes Barreto, T.; Macedo, L.d.O.; Löbenberg, R.; Lima Barros de Araújo, G.; Ishida, K.; Araci Bou-Chacra, N. Highly Water-Soluble Dapsone Nanocrystals: Towards Innovative Preparations for an Undermined Drug. Int. J. Pharm. 2023, 630, 122428. [Google Scholar] [CrossRef]
- Saadat, A.; Banaei, A.; Sattarifar, M.; Pargolghasemi, P. Preparation 2-Hydroxy-1-Naphthaldehyde Cross-Linked Fe3O4@chitosan-Polyacrylamide Nanocomposite for Removal of Everzol Black from Aqueous Solutions. Sci. Rep. 2023, 13, 10618. [Google Scholar] [CrossRef]
- Matusiak, J.; Maciołek, U.; Kosińska-Pezda, M.; Sternik, D.; Orzeł, J.; Grządka, E. Textural and Thermal Properties of the Novel Fucoidan/Nano-Oxides Hybrid Materials with Cosmetic, Pharmaceutical and Environmental Potential. Int. J. Mol. Sci. 2022, 23, 805. [Google Scholar] [CrossRef]
- Budnyak, T.M.; Aminzadeh, S.; Pylypchuk, I.V.; Riazanova, A.V.; Tertykh, V.A.; Lindström, M.E.; Sevastyanova, O. Peculiarities of Synthesis and Properties of Lignin–Silica Nanocomposites Prepared by Sol-Gel Method. Nanomaterials 2018, 8, 950. [Google Scholar] [CrossRef]
- Paiva, M.R.B.; Andrade, G.F.; Dourado, L.F.N.; Castro, B.F.M.; Fialho, S.L.; Sousa, E.M.B.; Silva-Cunha, A. Surface Functionalized Mesoporous Silica Nanoparticles for Intravitreal Application of Tacrolimus. J. Biomater. Appl. 2021, 35, 1019–1033. [Google Scholar] [CrossRef] [PubMed]
- Hafez, M.; Popov, A.I.; Zelenkov, V.N.; Teplyakova, T.V.; Rashad, M. Humic Substances as an Environmental- Friendly Organic Wastes Potentially Help as Natural Anti-Virus to Inhibit COVID-19. Sci. Arch. 2020, 1, 53–60. [Google Scholar] [CrossRef]
- Uspenskaya, E.V.; Syroeshkin, A.V.; Pleteneva, T.V.; Kazimova, I.V.; Grebennikova, T.V.; Fedyakina, I.T.; Lebedeva, V.V.; Latyshev, O.E.; Eliseeva, O.V.; Larichev, V.F.; et al. Nanodispersions of Polyelectrolytes Based on Humic Substances: Isolation, Physico-Chemical Characterization and Evaluation of Biological Activity. Pharmaceutics 2021, 13, 1954. [Google Scholar] [CrossRef] [PubMed]
- Mobasser, S.; Akbar Firoozi, A. Review of Nanotechnology Applications in Science and Engineering. J. Civ. Eng. Urban. 2016, 6, 84–93. [Google Scholar]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. MRNA-Lipid Nanoparticle COVID-19 Vaccines: Structure and Stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Alvi, M.; Yaqoob, A.; Rehman, K.; Shoaib, S.M.; Akash, M.S.H. PLGA-Based Nanoparticles for the Treatment of Cancer: Current Strategies and Perspectives. AAPS Open 2022, 8, 12. [Google Scholar] [CrossRef]
- Kumarasamy, R.V.; Natarajan, P.M.; Umapathy, V.R.; Roy, J.R.; Mironescu, M.; Palanisamy, C.P. Clinical Applications and Therapeutic Potentials of Advanced Nanoparticles: A Comprehensive Review on Completed Human Clinical Trials. Front. Nanotechnol. 2024, 6, 1479993. [Google Scholar] [CrossRef]
- Soliman, S. Nanomedicine: Advantages and Disadvantages of Nanomedicine. J. Nanomed. Nanotechnol. 2023, 14, 666. [Google Scholar] [CrossRef]
- Lopes, J.; Lopes, D.; Pereira-Silva, M.; Peixoto, D.; Veiga, F.; Hamblin, M.R.; Conde, J.; Corbo, C.; Zare, E.N.; Ashrafizadeh, M.; et al. Macrophage Cell Membrane-Cloaked Nanoplatforms for Biomedical Applications. Small Methods 2022, 6, 2200289. [Google Scholar] [CrossRef]
- Gheibi Hayat, S.M.; Bianconi, V.; Pirro, M.; Sahebkar, A. Stealth Functionalization of Biomaterials and Nanoparticles by CD47 Mimicry. Int. J. Pharm. 2019, 569, 118628. [Google Scholar] [CrossRef]
- Mathur, P.; Jha, S.; Ramteke, S.; Jain, N.K. Pharmaceutical Aspects of Silver Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum Dots versus Organic Dyes as Fluorescent Labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Kajani, A.A.; Zarkesh-Esfahani, S.H.; Bordbar, A.-K.; Khosropour, A.R.; Razmjou, A.; Kardi, M. Anticancer Effects of Silver Nanoparticles Encapsulated by Taxus baccata Extracts. J. Mol. Liq. 2016, 223, 549–556. [Google Scholar] [CrossRef]
- Khan, F.; Hashmi, M.U.; Khalid, N.; Hayat, M.Q.; Ikram, A.; Janjua, H.A. Controlled Assembly of Silver Nano-Fluid in Heliotropium crispum Extract: A Potent Anti-Biofilm and Bactericidal Formulation. Appl. Surf. Sci. 2016, 387, 317–331. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as Drug Delivery Systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef]
- Wen, J.; Moloney, E.B.; Canning, A.; Donohoe, E.; Ritter, T.; Wang, J.; Xiang, D.; Wu, J.; Li, Y. Synthesized Nanoparticles, Biomimetic Nanoparticles and Extracellular Vesicles for Treatment of Autoimmune Disease: Comparison and Prospect. Pharmacol. Res. 2021, 172, 105833. [Google Scholar] [CrossRef]
- Qiao, L.; Dou, X.; Song, X.; Xu, C. Green Synthesis of Nanoparticles by Probiotics and Their Application. Adv. Appl. Microbiol. 2022, 119, 83–128. [Google Scholar]
- Romano, I.; Vitiello, G.; Gallucci, N.; Di Girolamo, R.; Cattaneo, A.; Poli, A.; Di Donato, P. Extremophilic Microorganisms for the Green Synthesis of Antibacterial Nanoparticles. Microorganisms 2022, 10, 1885. [Google Scholar] [CrossRef]
- Hano, C.; Abbasi, B.H. Plant-Based Green Synthesis of Nanoparticles: Production, Characterization and Applications. Biomolecules 2021, 12, 31. [Google Scholar] [CrossRef]
- Cazenave, J.; Ale, A.; Bacchetta, C.; Rossi, A.S. Nanoparticles Toxicity in Fish Models. Curr. Pharm. Des. 2019, 25, 3927–3942. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; da Silva, C.F.; Andrade, L.N.; de Lima Oliveira, D.; Campos, J.; Souto, E.B. Alginate Nanoparticles for Drug Delivery and Targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. [Google Scholar] [CrossRef]
- Rannard, S.; Owen, A. Nanomedicine: Not a Case of “One Size Fits All”. Nano Today 2009, 4, 382–384. [Google Scholar] [CrossRef]
- Foulkes, R.; Man, E.; Thind, J.; Yeung, S.; Joy, A.; Hoskins, C. The Regulation of Nanomaterials and Nanomedicines for Clinical Application: Current and Future Perspectives. Biomater. Sci. 2020, 8, 4653–4664. [Google Scholar] [CrossRef]
- Leong, H.S.; Butler, K.S.; Brinker, C.J.; Azzawi, M.; Conlan, S.; Dufés, C.; Owen, A.; Rannard, S.; Scott, C.; Chen, C.; et al. On the Issue of Transparency and Reproducibility in Nanomedicine. Nat. Nanotechnol. 2019, 14, 629–635. [Google Scholar] [CrossRef]
- Halamoda-Kenzaoui, B.; Geertsma, R.; Pouw, J.; Prina-Mello, A.; Carrer, M.; Roesslein, M.; Sips, A.; Weltring, K.M.; Spring, K.; Bremer-Hoffmann, S. Future Perspectives for Advancing Regulatory Science of Nanotechnology-Enabled Health Products. Drug Deliv. Transl. Res. 2022, 12, 2145–2156. [Google Scholar] [CrossRef]
- Siegrist, S.; Cörek, E.; Detampel, P.; Sandström, J.; Wick, P.; Huwyler, J. Preclinical Hazard Evaluation Strategy for Nanomedicines. Nanotoxicology 2019, 13, 73–99. [Google Scholar] [CrossRef]
- Kondor, A.C.; Molnár, É.; Jakab, G.; Vancsik, A.; Filep, T.; Szeberényi, J.; Szabó, L.; Maász, G.; Pirger, Z.; Weiperth, A.; et al. Pharmaceuticals in Water and Sediment of Small Streams under the Pressure of Urbanization: Concentrations, Interactions, and Risks. Sci. Total Environ. 2022, 808, 152160. [Google Scholar] [CrossRef]
- Fekadu, S.; Alemayehu, E.; Dewil, R.; Van der Bruggen, B. Pharmaceuticals in Freshwater Aquatic Environments: A Comparison of the African and European Challenge. Sci. Total Environ. 2019, 654, 324–337. [Google Scholar] [CrossRef]
- Desbiolles, F.; Malleret, L.; Tiliacos, C.; Wong-Wah-Chung, P.; Laffont-Schwob, I. Occurrence and Ecotoxicological Assessment of Pharmaceuticals: Is There a Risk for the Mediterranean Aquatic Environment? Sci. Total Environ. 2018, 639, 1334–1348. [Google Scholar] [CrossRef]
- Berkner, S.; Schwirn, K.; Voelker, D. Too Advanced for Assessment? Advanced Materials, Nanomedicine and the Environment. Environ. Sci. Eur. 2022, 34, 71. [Google Scholar] [CrossRef] [PubMed]
- Drug Products, Including Biological Products, That Contain Nanomaterials Guidance for Industry Contains Nonbinding Recommendations. 2022. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/drug-products-including-biological-products-contain-nanomaterials-guidance-industry (accessed on 20 April 2025).
- Pita, R.; Ehmann, F.; Papaluca, M. Nanomedicines in the EU—Regulatory Overview. AAPS J. 2016, 18, 1576–1582. [Google Scholar] [CrossRef]
- Malyshev, V.; Gab, A.; Kovalenko, V.; Pryshedko, O.; Shakhnin, D. Estimation of Global Nanomedicine Market: Status, Segment Analysis, Dynamics, Competition and Prospects. Technol. Audit. Prod. Reserves 2024, 1, 48–59. [Google Scholar] [CrossRef]
- Limaye, V.; Fortwengel, G.; Limaye, D. Regulatory Roadmap for Nanotechnology Based Medicines. Int. J. Drug Regul. Aff. 2018, 2, 33–41. [Google Scholar] [CrossRef]
- Li, X.; Lai, Y.; Wan, G.; Zou, J.; He, W.; Yang, P. Approved Natural Products-Derived Nanomedicines for Disease Treatment. Chin. J. Nat. Med. 2024, 22, 1100–1116. [Google Scholar] [CrossRef]
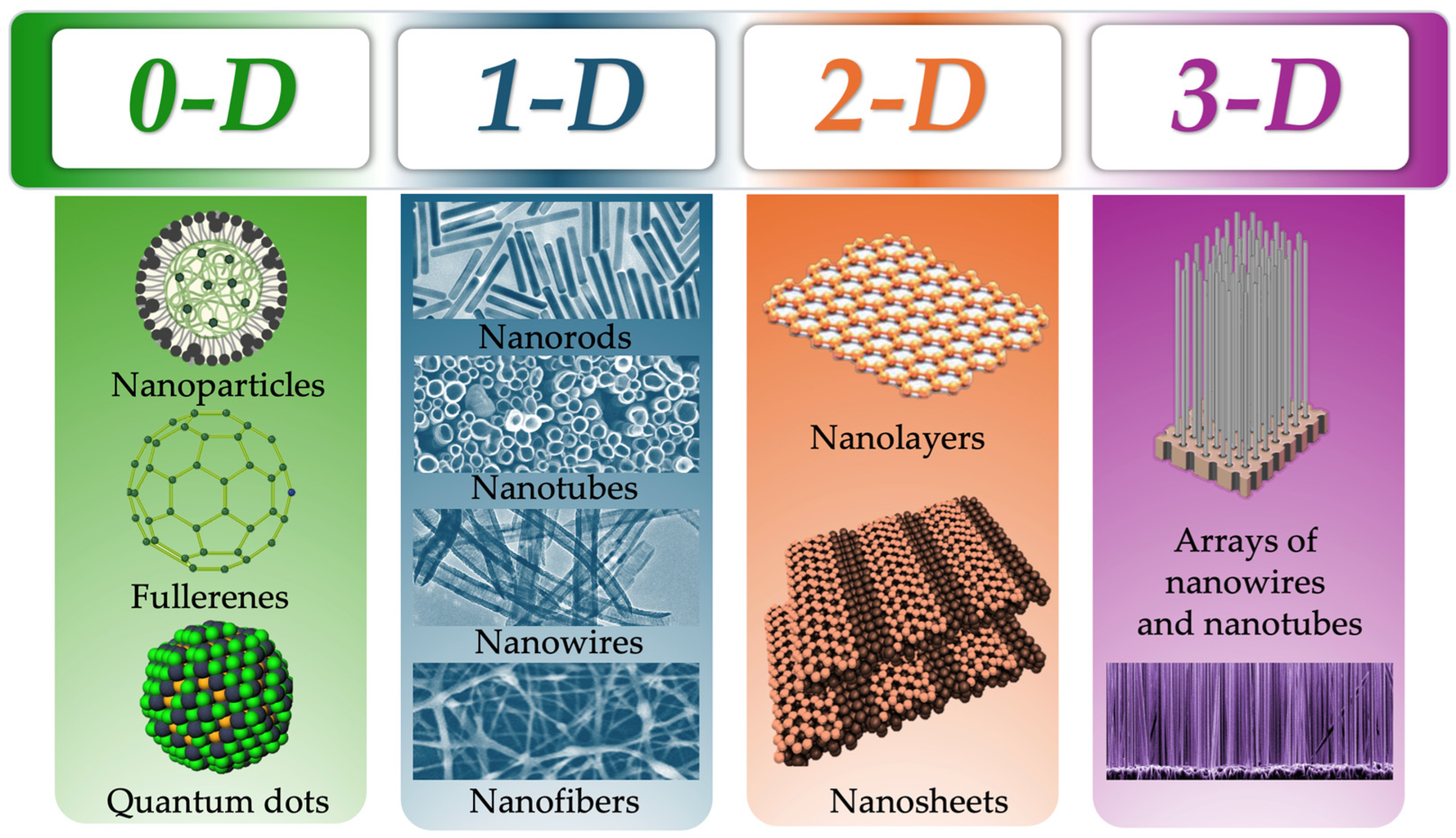
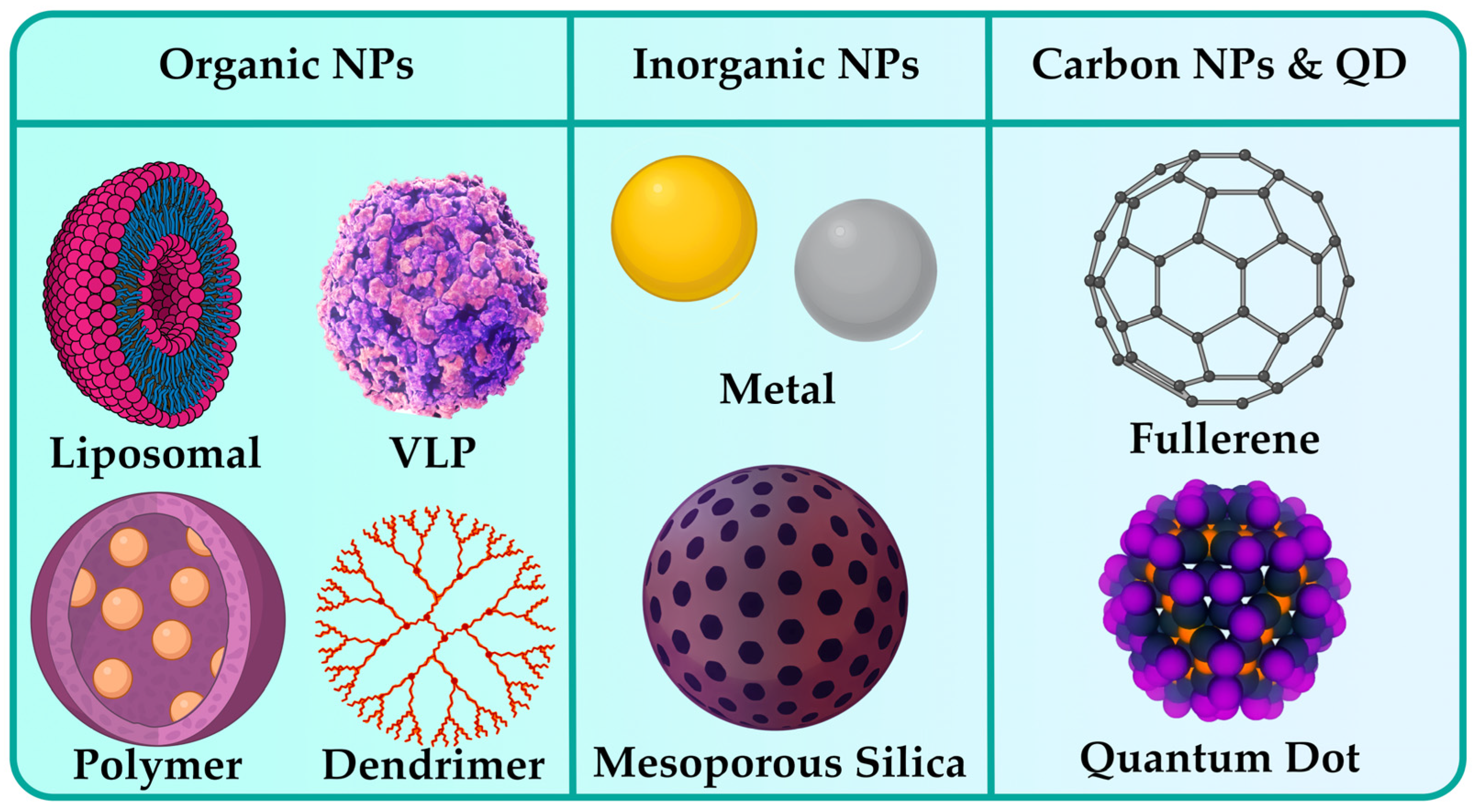
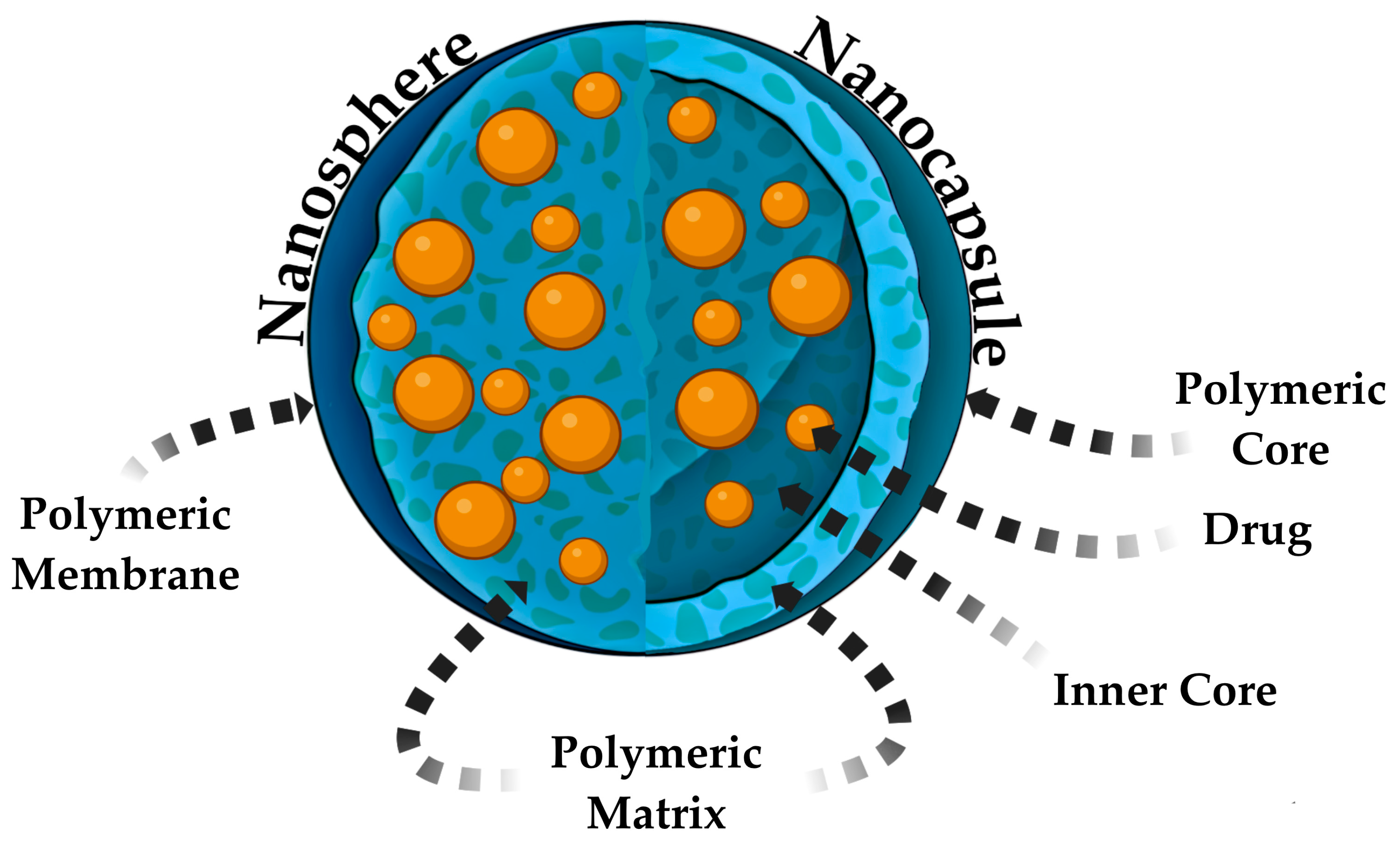
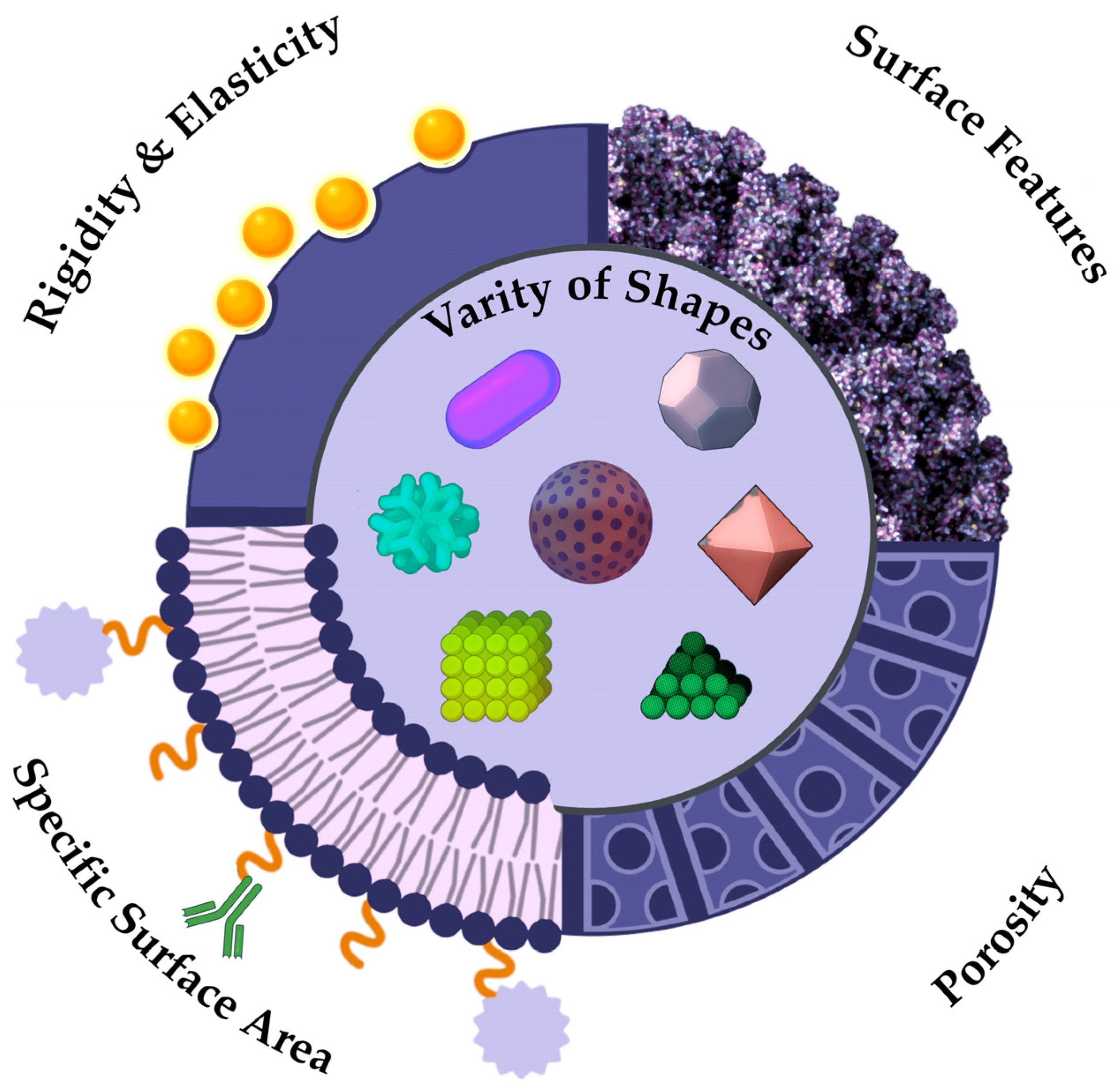
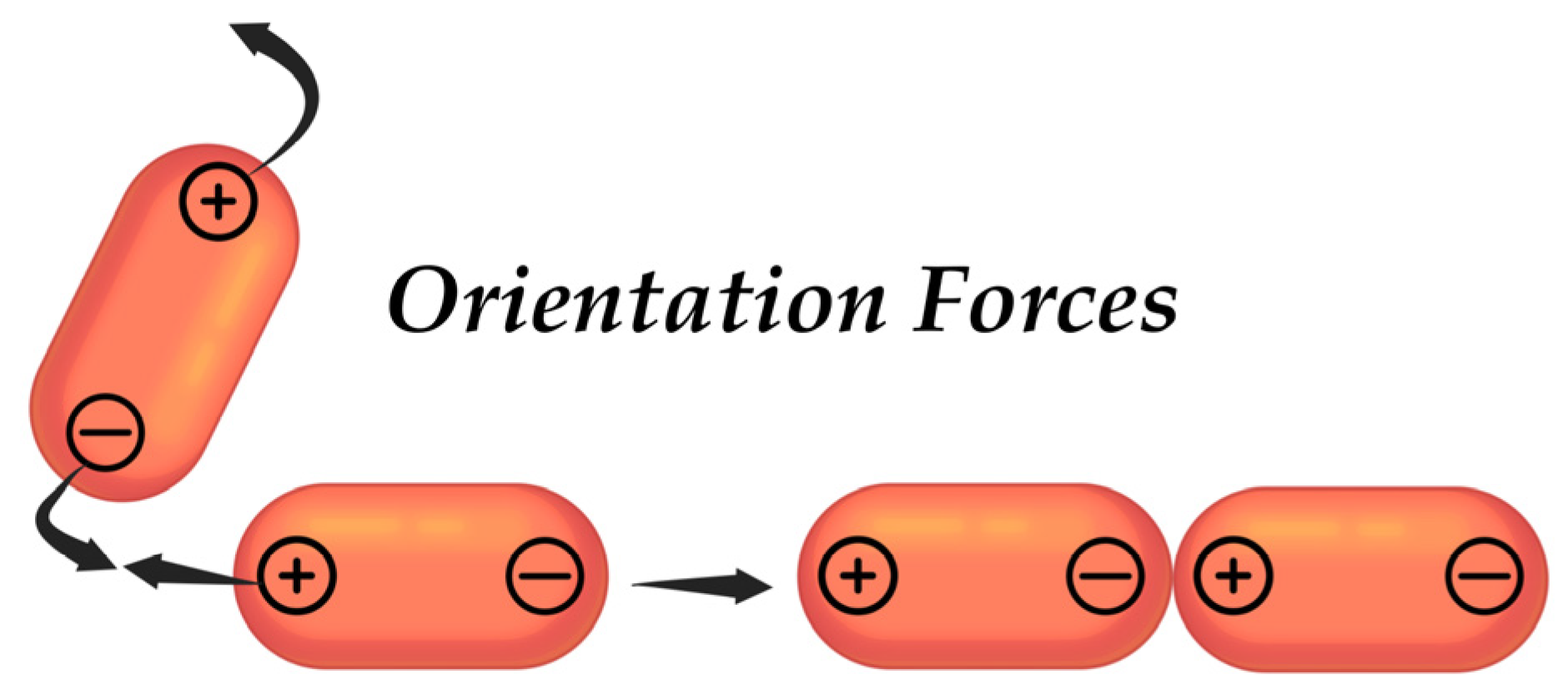
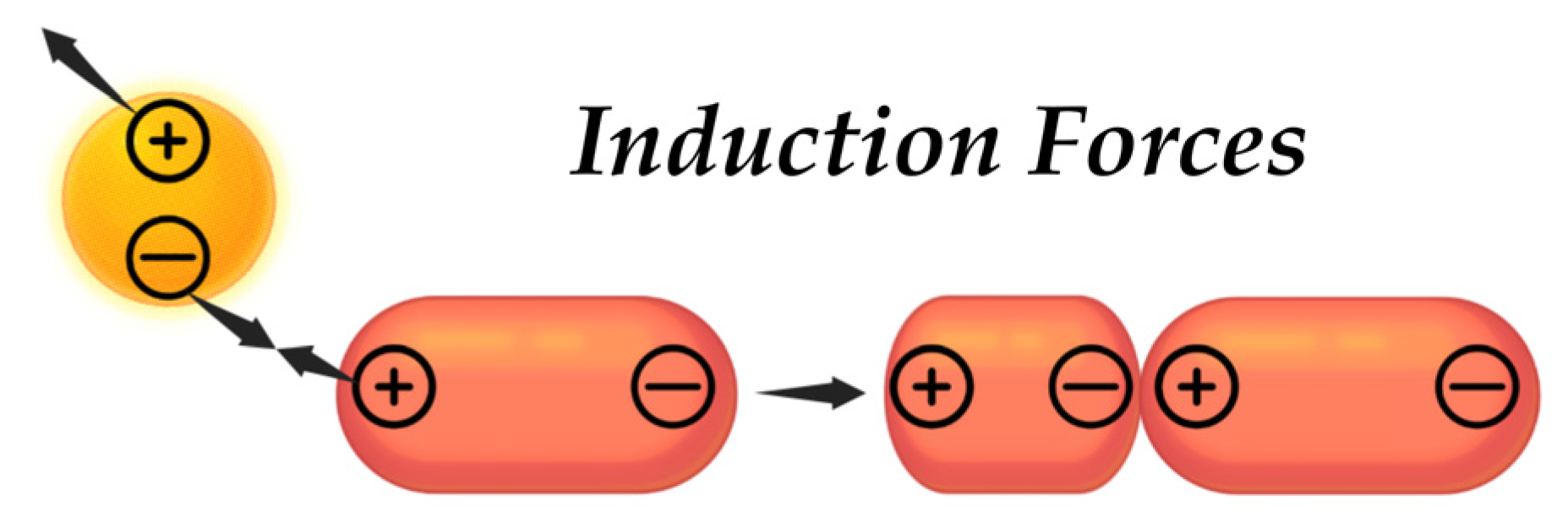
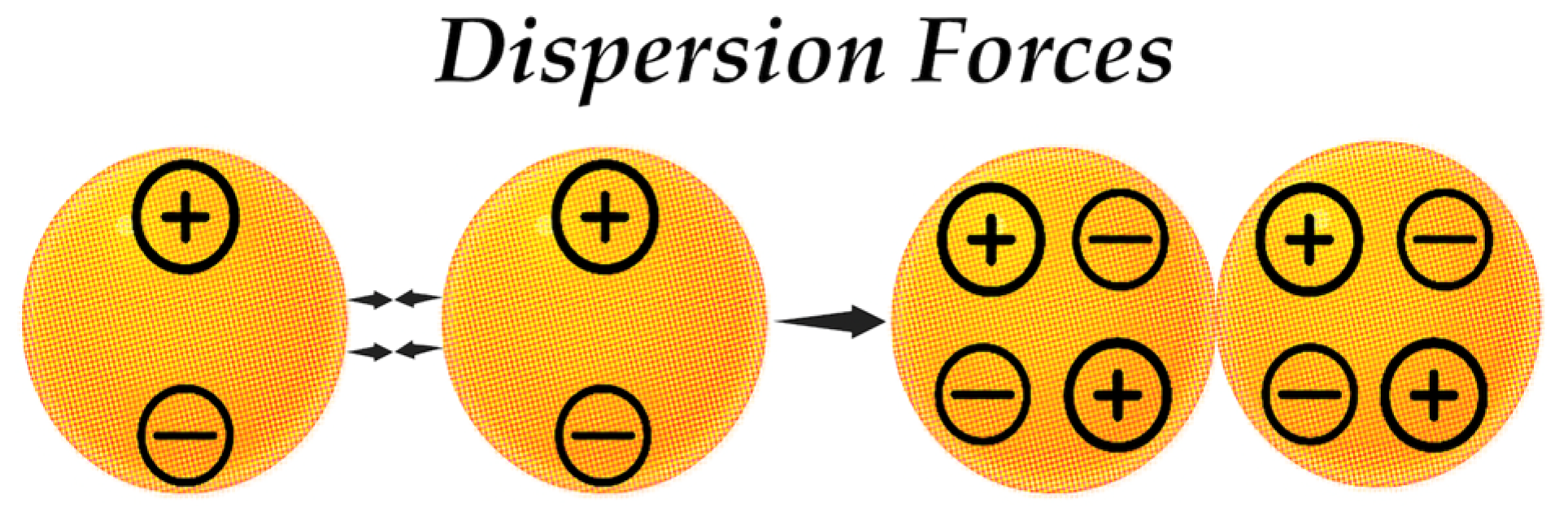
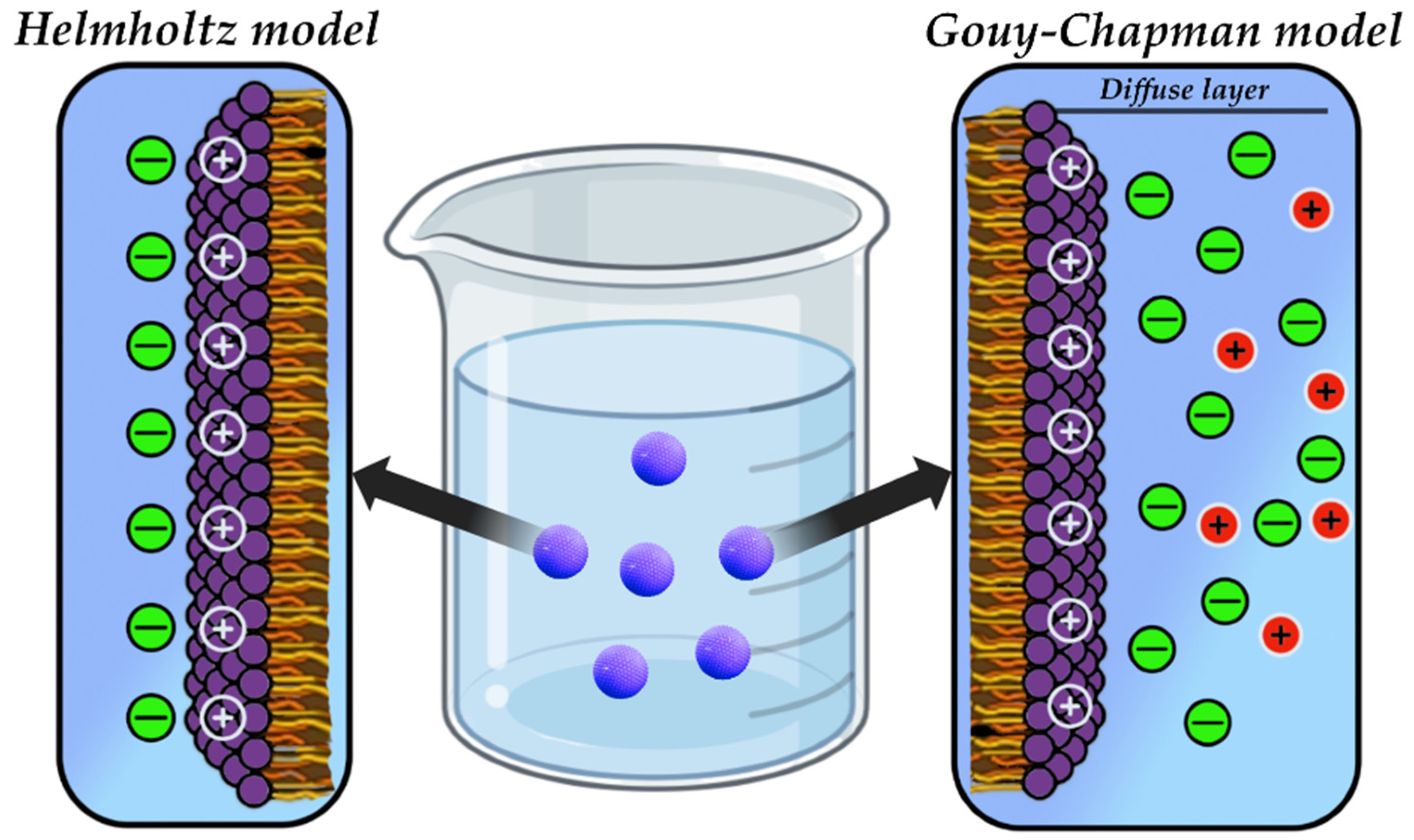
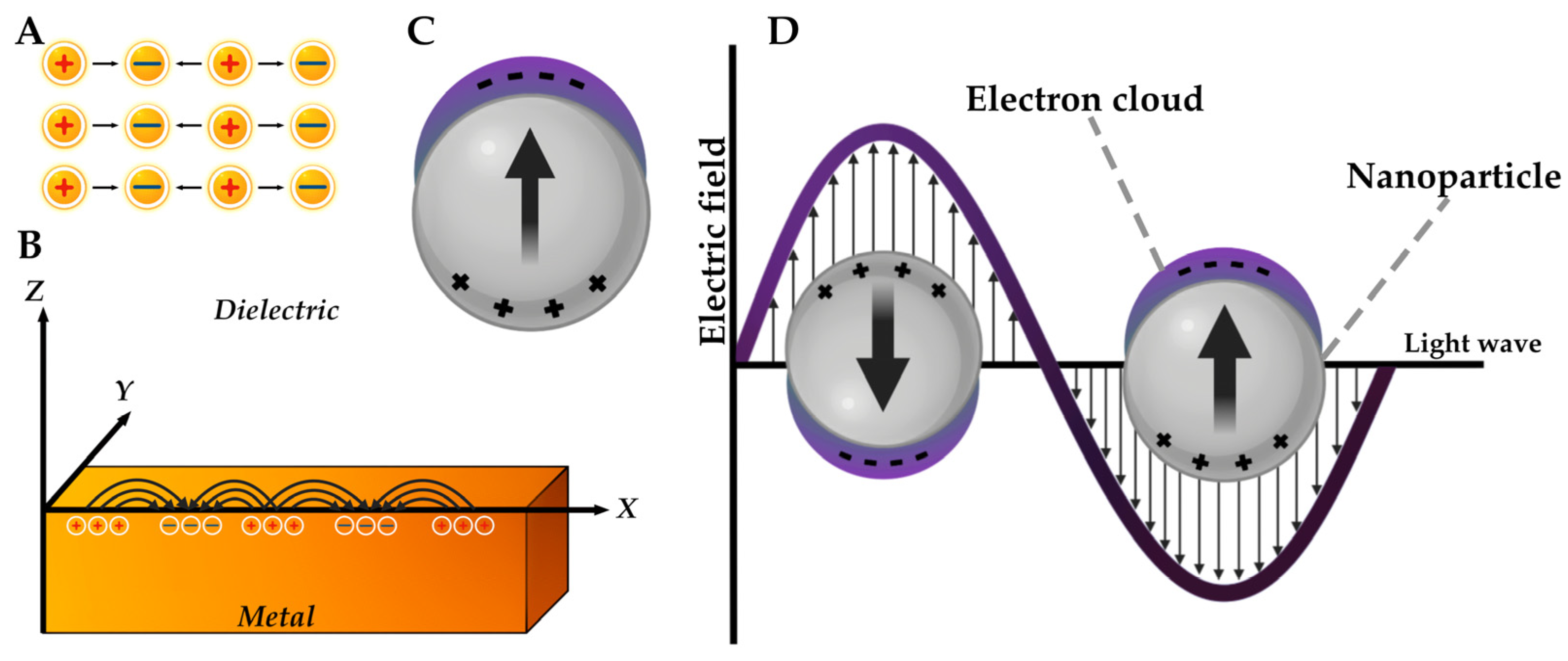
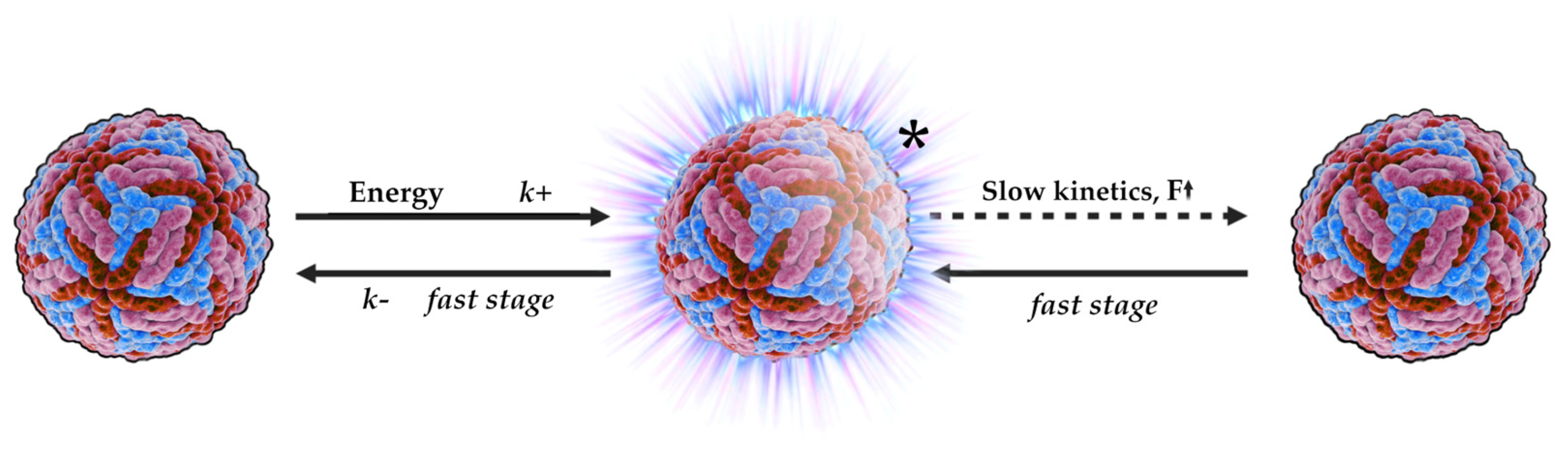
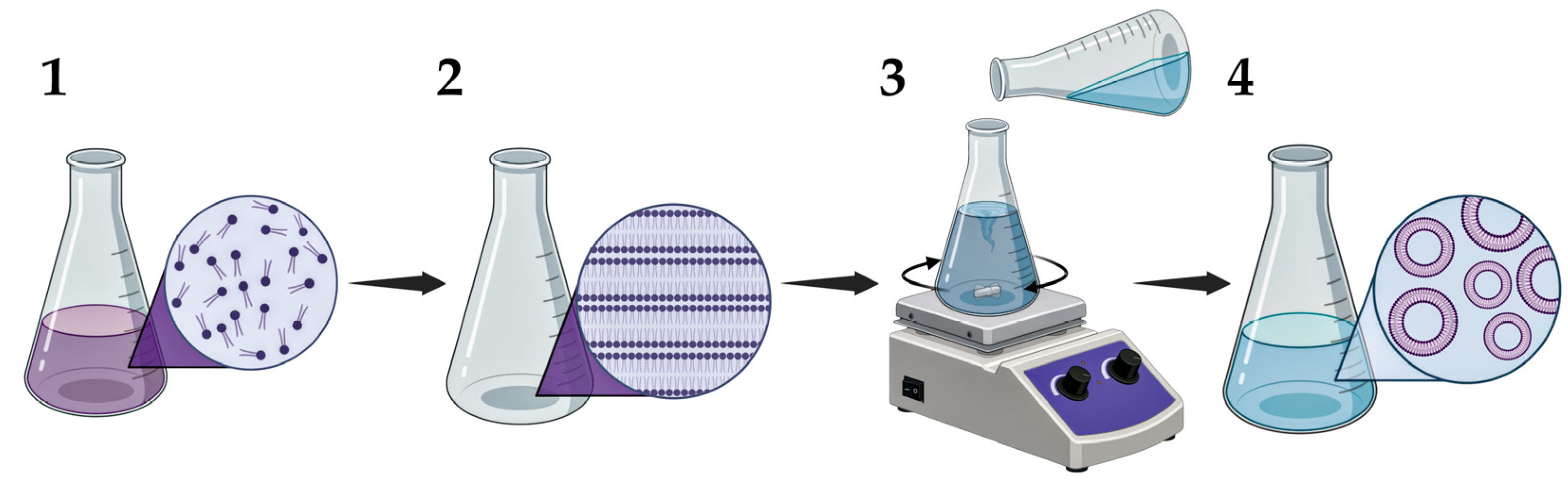
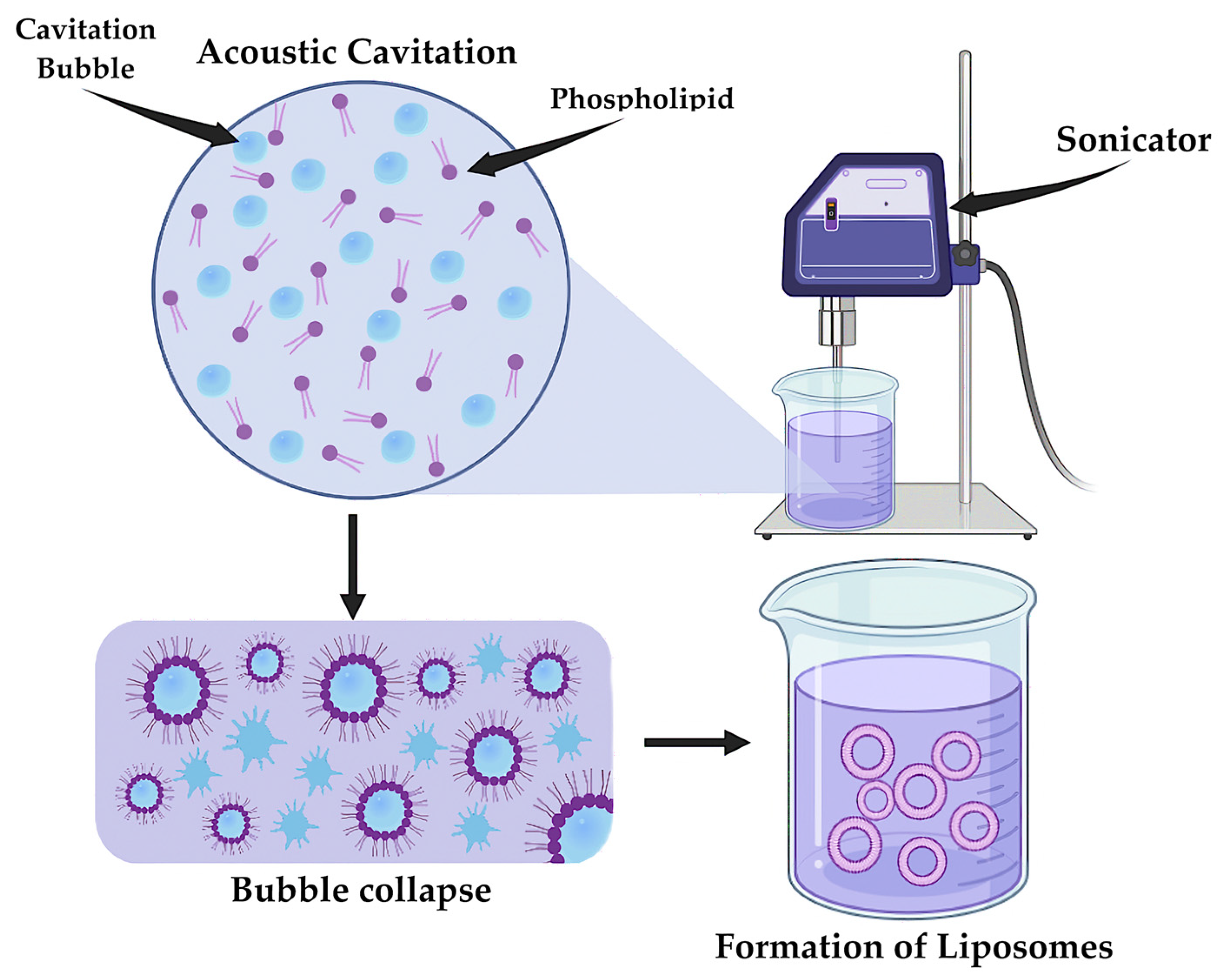
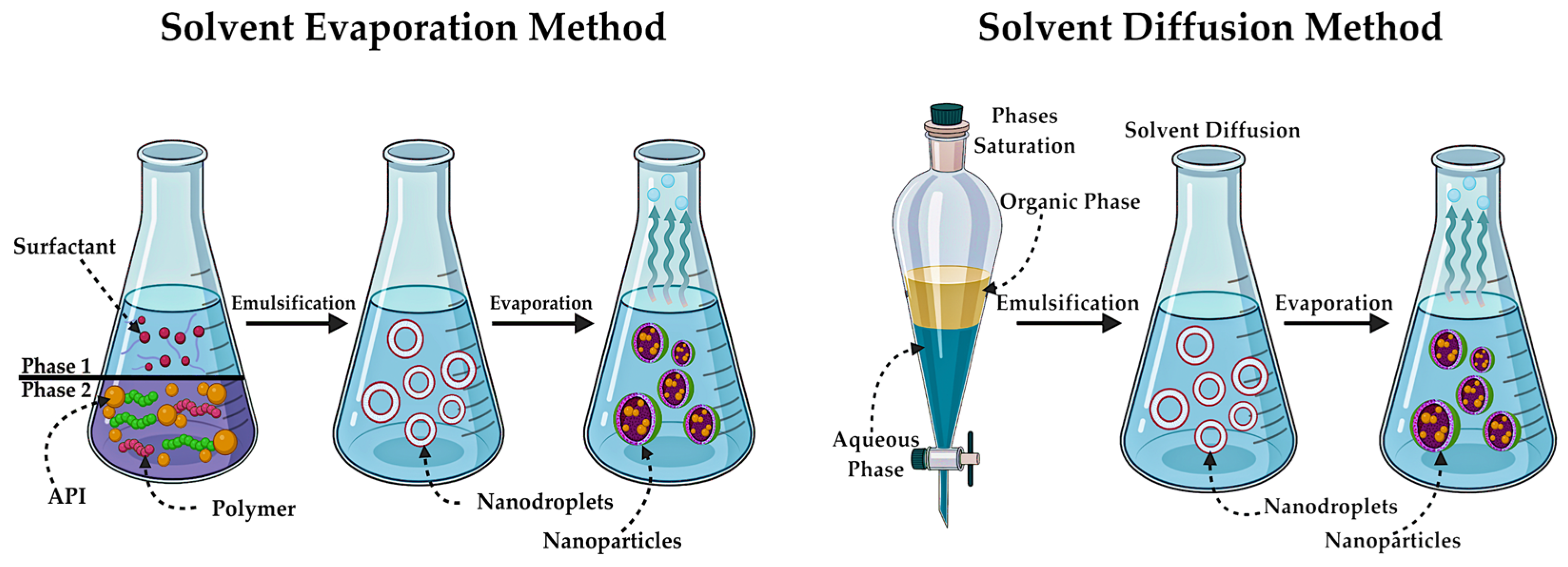
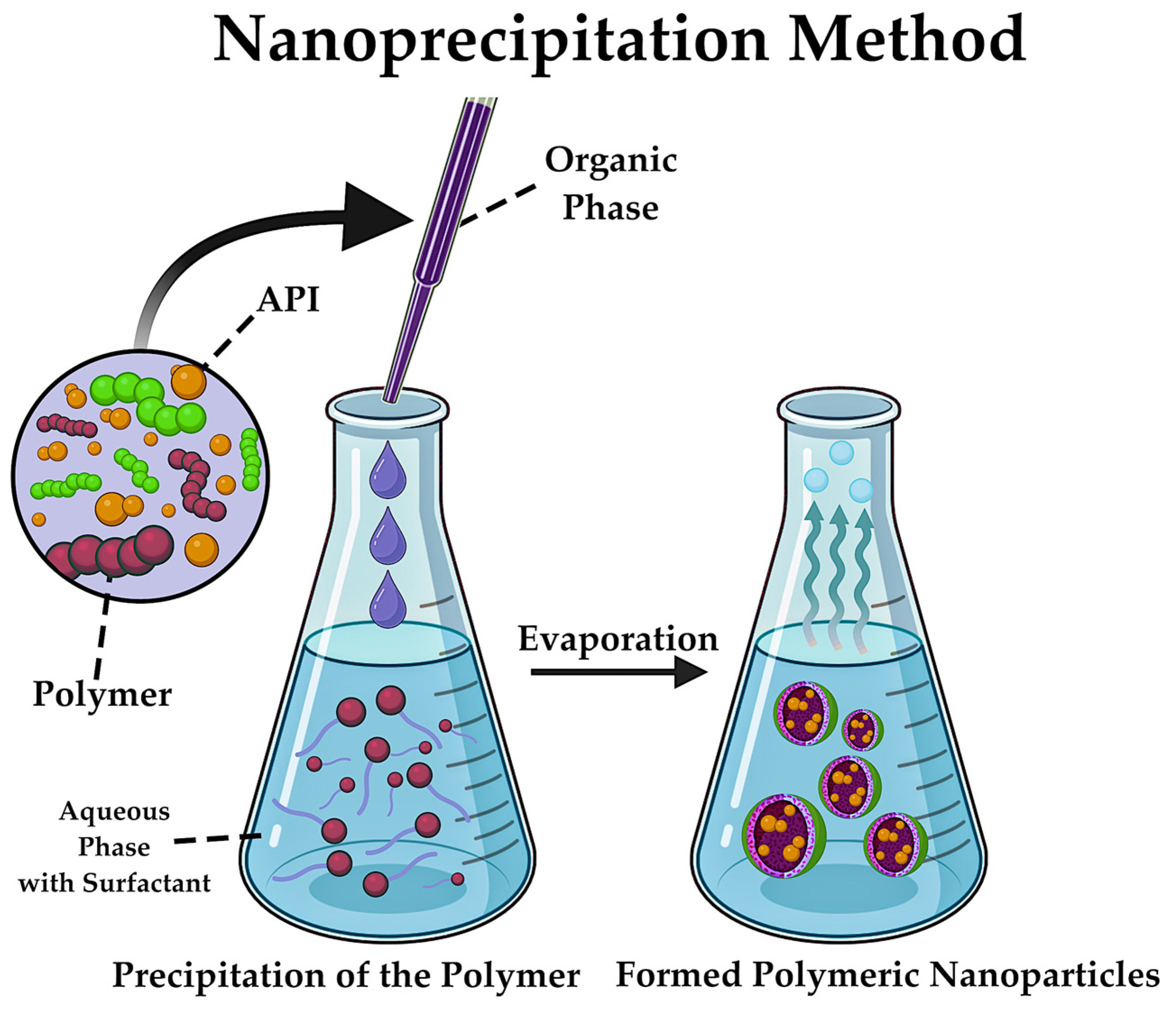
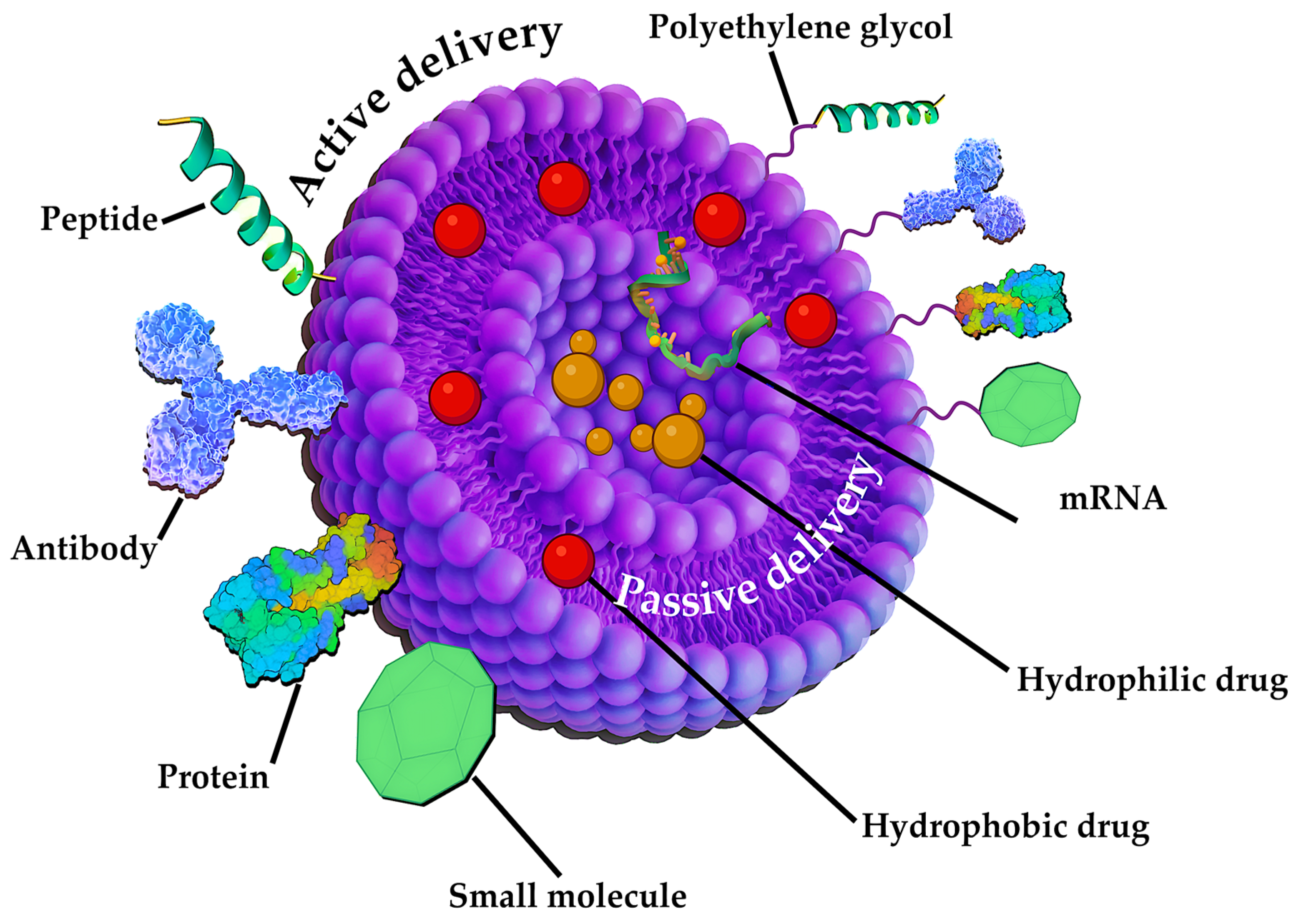
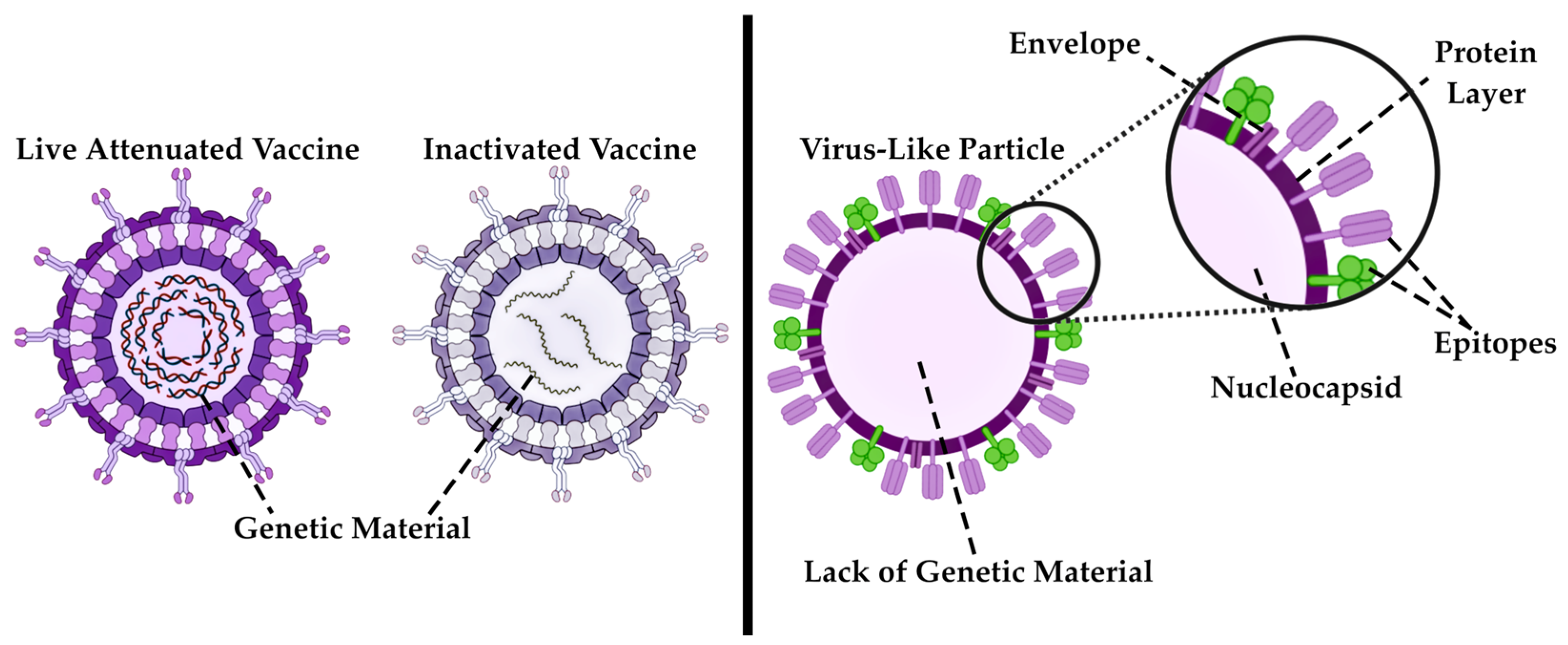
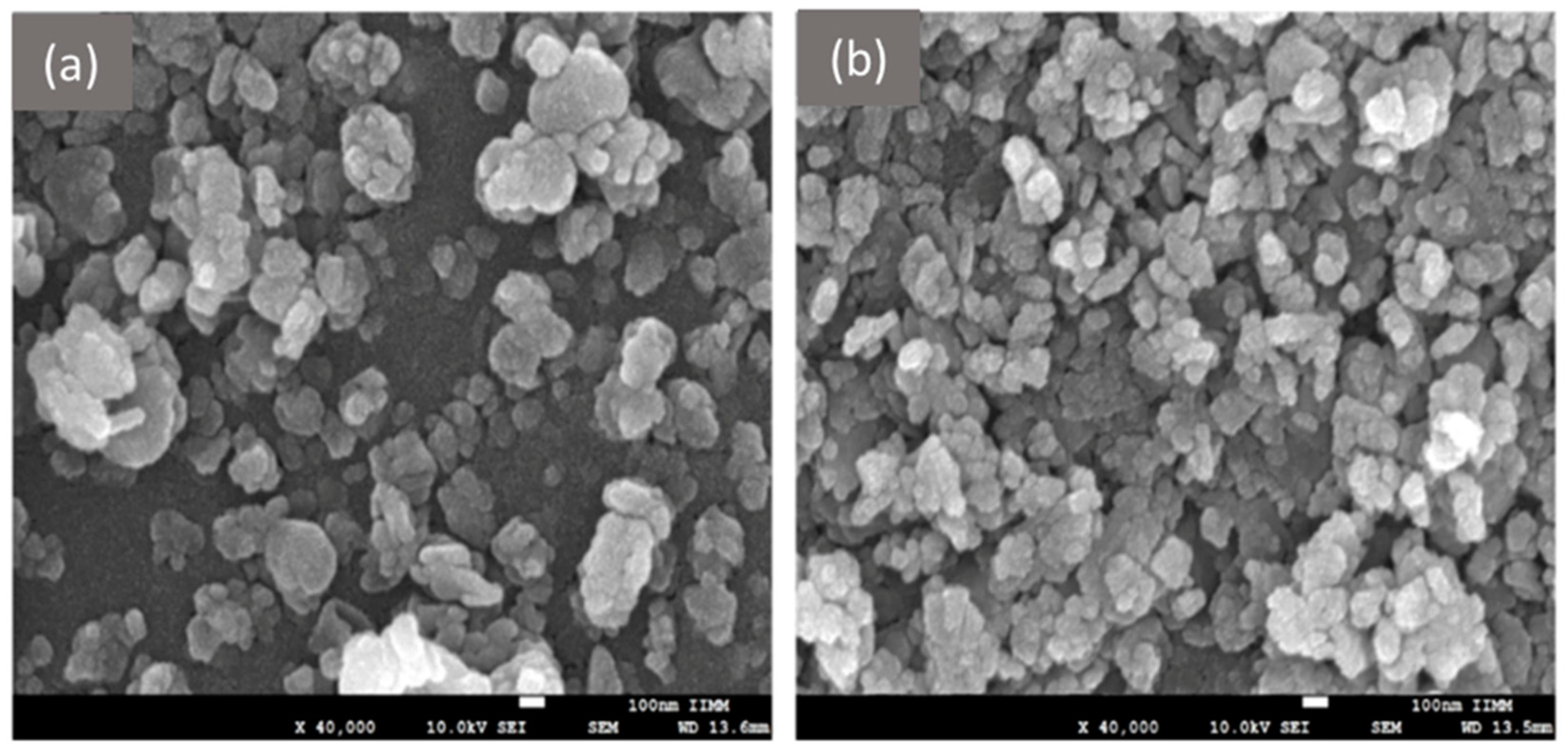
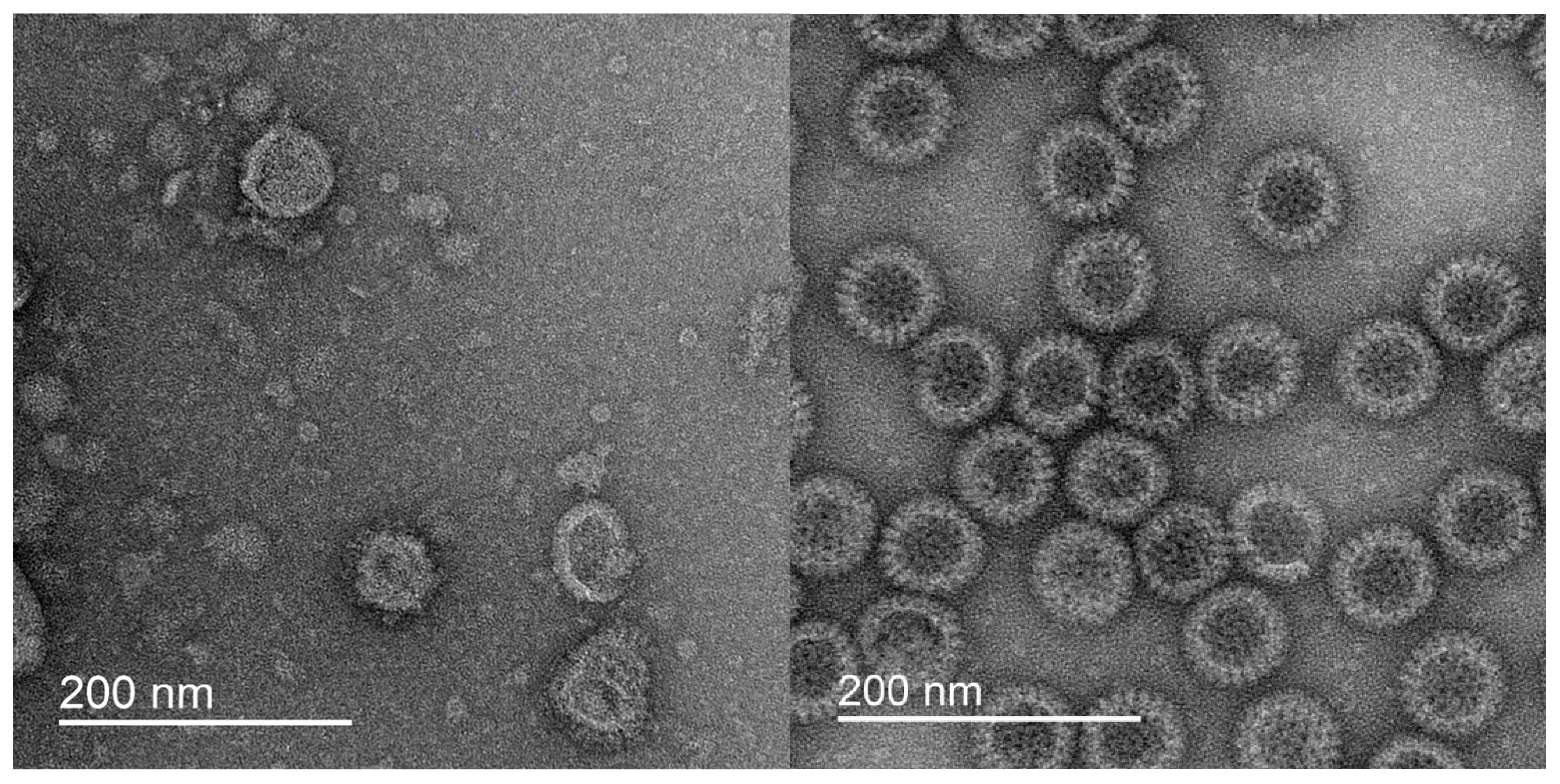
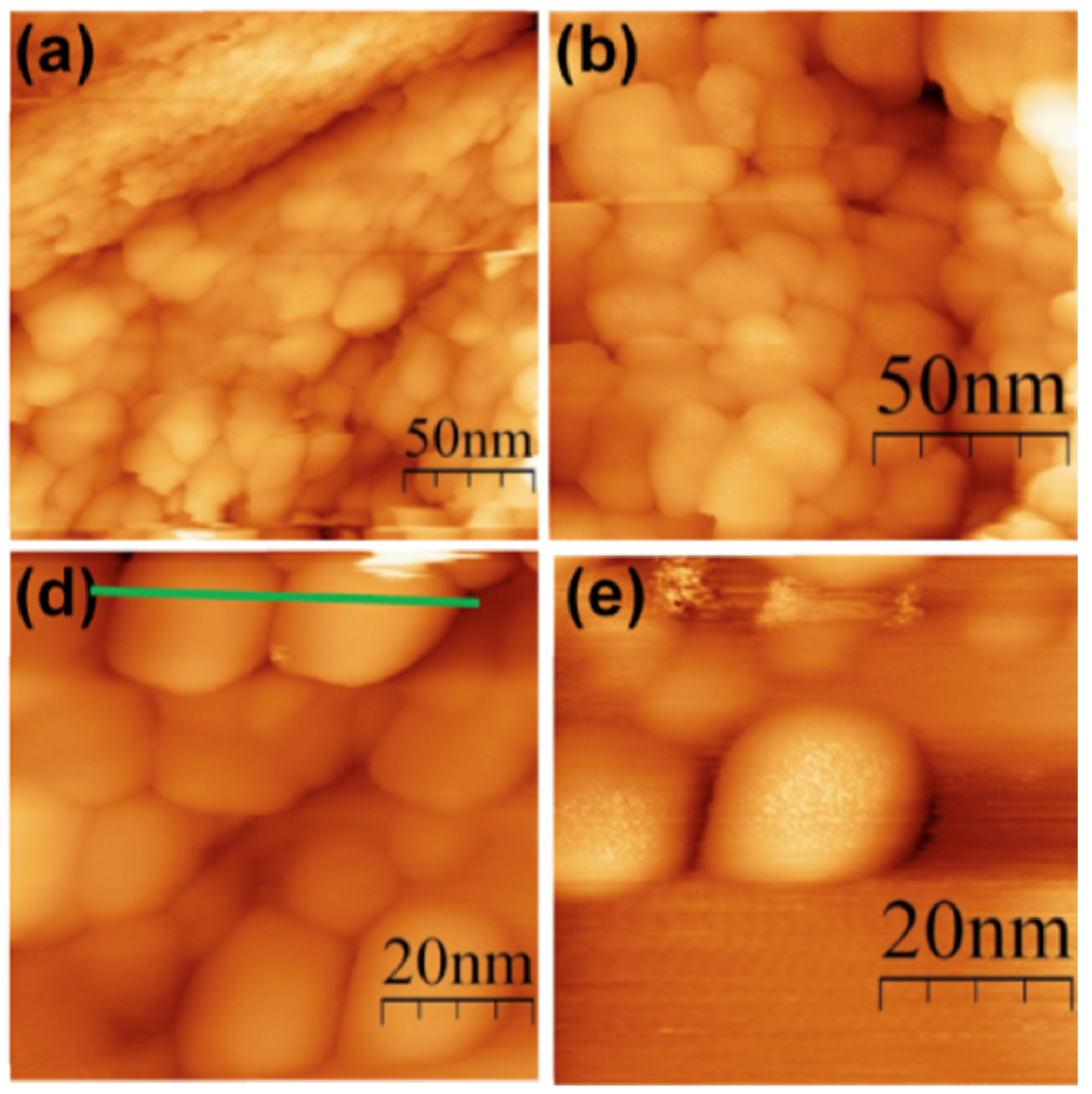
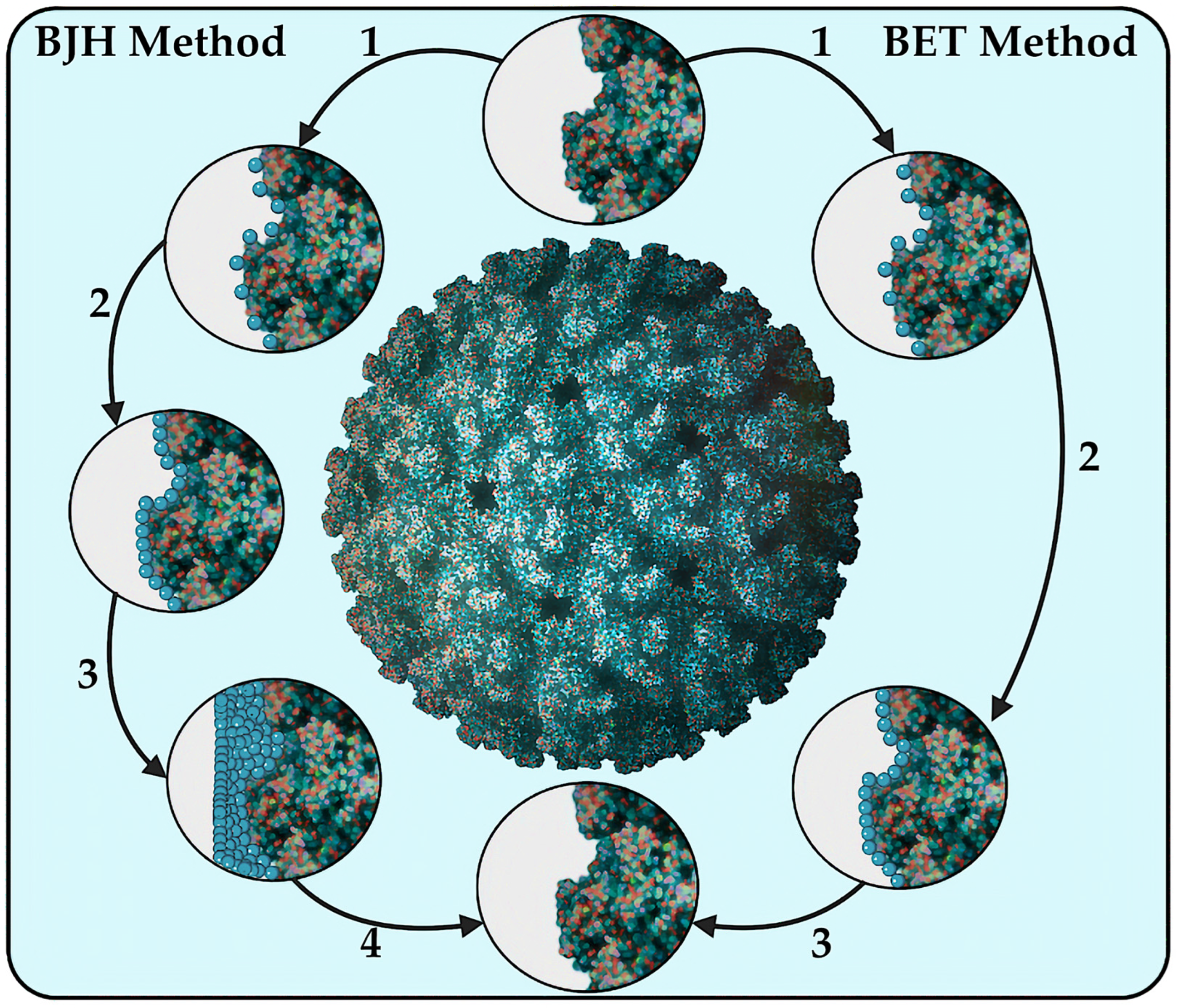
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrov, G.V.; Koldina, A.M.; Ledenev, O.V.; Tumasov, V.N.; Nazarov, A.A.; Syroeshkin, A.V. Nanoparticles and Nanomaterials: A Review from the Standpoint of Pharmacy and Medicine. Pharmaceutics 2025, 17, 655. https://doi.org/10.3390/pharmaceutics17050655
Petrov GV, Koldina AM, Ledenev OV, Tumasov VN, Nazarov AA, Syroeshkin AV. Nanoparticles and Nanomaterials: A Review from the Standpoint of Pharmacy and Medicine. Pharmaceutics. 2025; 17(5):655. https://doi.org/10.3390/pharmaceutics17050655
Chicago/Turabian StylePetrov, Gleb V., Alena M. Koldina, Oleg V. Ledenev, Vladimir N. Tumasov, Aleksandr A. Nazarov, and Anton V. Syroeshkin. 2025. "Nanoparticles and Nanomaterials: A Review from the Standpoint of Pharmacy and Medicine" Pharmaceutics 17, no. 5: 655. https://doi.org/10.3390/pharmaceutics17050655
APA StylePetrov, G. V., Koldina, A. M., Ledenev, O. V., Tumasov, V. N., Nazarov, A. A., & Syroeshkin, A. V. (2025). Nanoparticles and Nanomaterials: A Review from the Standpoint of Pharmacy and Medicine. Pharmaceutics, 17(5), 655. https://doi.org/10.3390/pharmaceutics17050655









