Error in Figure
In the original publication [1], there was a mistake in Figure 4A as published. Figure 4A,C were unintentionally duplicated (identical images) and the corrected Figure 4 appears below. The authors state that the scientific conclusions are unaffected. This correction was approved by the Academic Editor. The original publication has also been updated.
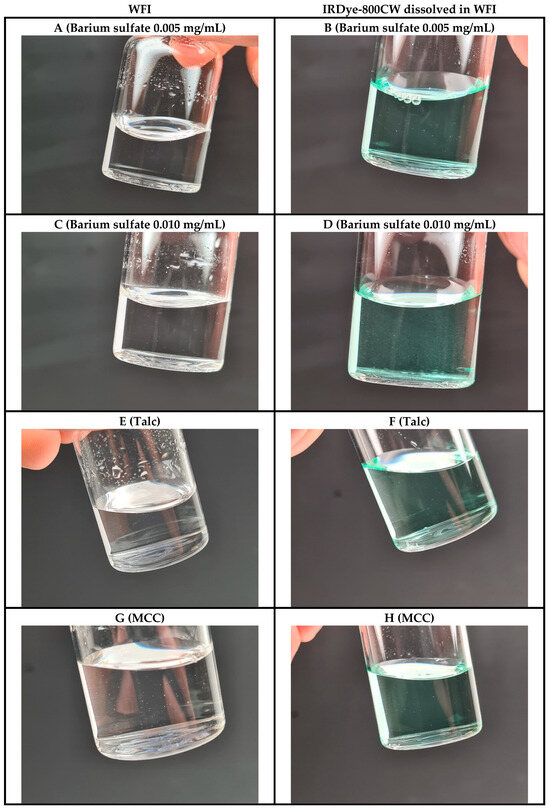
Figure 4.
Representative images of the manufactured GMP QTSs containing either WFI (left, clear solution) or IRDye-800CW dissolved in WFI (right, colored solution) simulating small intrinsic and/or extrinsic particulate matter as defects. (A,B) Barium sulfate 0.005 mg/mL; (C,D) barium sulfate 0.010 mg/mL; (E,F) talc; (G,H) microcrystalline cellulose (MCC).
Reference
- van den Born-Bondt, T.; Huizinga, H.P.S.; Kappert, K.R.; Westra, H.H.; van Zanten, J.; Woerdenbag, H.J.; Maurer, J.M.; Gareb, B. Development of an Adaptable Qualification Test Set for Personnel Involved in Visual Inspection Procedures of Parenteral Drug Products Manufactured Under Good Manufacturing Practice Conditions in Hospital Pharmacy Compounding Facilities. Pharmaceutics 2025, 17, 74. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).