Correction: Yu et al. Non-Cationic RGD-Containing Protein Nanocarrier for Tumor-Targeted siRNA Delivery. Pharmaceutics 2021, 13, 2182
Affiliation Update
Author’s Email Update
Funding Update
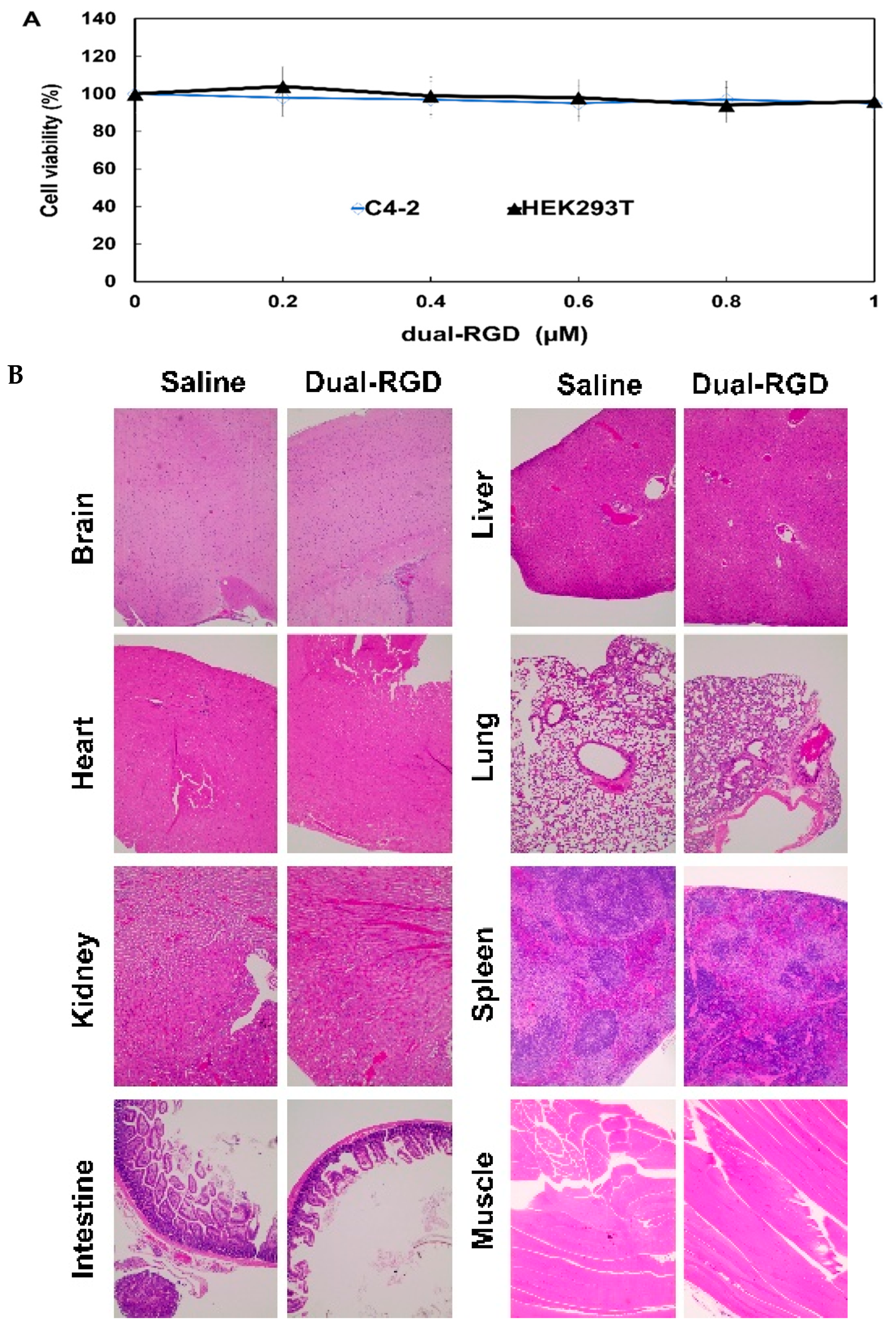
Error in Figure
References
- Yu, X.; Xue, L.; Zhao, J.; Zhao, S.; Wu, D.; Liu, H.Y. Non-Cationic RGD-Containing Protein Nanocarrier for Tumor-Targeted siRNA Delivery. Pharmaceutics 2021, 13, 2182. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Maihle, N.J.; Yu, X.; Tang, S.-C.; Liu, H.Y. Synergistic targeting HER2 and EGFR with bivalent aptamer-siRNA chimera efficiently inhibits HER2-positive tumor growth. Mol. Pharm. 2018, 15, 4801–4813. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Xue, L.; Zhao, J.; Zhao, S.; Wu, D.; Liu, H.Y. Correction: Yu et al. Non-Cationic RGD-Containing Protein Nanocarrier for Tumor-Targeted siRNA Delivery. Pharmaceutics 2021, 13, 2182. Pharmaceutics 2025, 17, 1462. https://doi.org/10.3390/pharmaceutics17111462
Yu X, Xue L, Zhao J, Zhao S, Wu D, Liu HY. Correction: Yu et al. Non-Cationic RGD-Containing Protein Nanocarrier for Tumor-Targeted siRNA Delivery. Pharmaceutics 2021, 13, 2182. Pharmaceutics. 2025; 17(11):1462. https://doi.org/10.3390/pharmaceutics17111462
Chicago/Turabian StyleYu, Xiaolin, Lu Xue, Jing Zhao, Shuhua Zhao, Daqing Wu, and Hong Yan Liu. 2025. "Correction: Yu et al. Non-Cationic RGD-Containing Protein Nanocarrier for Tumor-Targeted siRNA Delivery. Pharmaceutics 2021, 13, 2182" Pharmaceutics 17, no. 11: 1462. https://doi.org/10.3390/pharmaceutics17111462
APA StyleYu, X., Xue, L., Zhao, J., Zhao, S., Wu, D., & Liu, H. Y. (2025). Correction: Yu et al. Non-Cationic RGD-Containing Protein Nanocarrier for Tumor-Targeted siRNA Delivery. Pharmaceutics 2021, 13, 2182. Pharmaceutics, 17(11), 1462. https://doi.org/10.3390/pharmaceutics17111462




