Abstract
Polymeric micelles are widely studied as nanocarriers for hydrophobic drugs, yet their structural stability under physiological conditions remains a major limitation. This review provides a comparative evaluation of synthetic and natural polymeric micelles with a focus on their stability under dilution and in protein-rich environments. The discussion integrates thermodynamic and kinetic factors governing micelle integrity and examines how molecular composition, hydrophobic segment length, and core–shell modifications influence disintegration behavior. While synthetic micelles commonly collapse below their critical micelle concentration during intravenous administration, natural polymeric micelles, such as those derived from chitosan, alginate, or heparin, exhibit improved resistance to dilution but remain vulnerable to protein-induced destabilization. Strategies such as core or shell cross-linking, surface functionalization, and natural polymer coatings are reviewed as promising approaches to enhance circulation stability and controlled drug release. The work provides a framework for designing micellar systems with balanced biocompatibility, biodegradability, and robustness suitable for clinical drug-delivery applications.
1. Introduction
Therapeutic compounds with low water solubility often require carriers to ensure stable transport in aqueous fluids. Among the various delivery systems explored in the literature, polymeric beads, liposomal, and micellar systems have been extensively studied [1].
Surfactant-based micelles, in particular, are widely used due to their versatility and commercial availability [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16]. In copolymeric micelles, the hydrophobic core serves as a nanoscale reservoir for lipophilic drug molecules, while the hydrophilic shell (corona) ensures water solubility [17,18,19,20,21,22,23,24]. Their relatively large size, tunable chemical structure, biocompatibility, and ability to mimic natural carriers such as viruses make copolymeric micelles excellent drug-delivery vehicles for various pharmaceutical applications [25].
Applications include medical diagnostic imaging, drug delivery and targeting, immunology, and gene therapy. For instance, polymeric micelles enhance medical imaging by enabling the accumulation of contrast agents at targeted sites, thereby improving signal intensity [9]. When used as blood-pool agents (BPAs) in computed tomography (CT), their extended circulation times allow for high-resolution arterial and venous mapping [26,27,28].
The use of copolymers in drug delivery is well-established. In a comprehensive review on micelle stability, Owen et al. [29] trace the use of polymeric micelles back to early work by Ringsdorf et al. [30,31]. The wide range of commercially available polymers provides extensive opportunities to design copolymers with tailored properties.
Hydrophilic blocks can range from polyethylene glycol (PEG) to polypeptide mimics such as poly-L-lysine (PLL) and poly(lactic-co-glycolic acid) (PLGA). Lipophilic blocks include poly(lactide)/polylactic acid (PLA), poly(ε-caprolactone) (PCL), and functional polymers like poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) and poly(2-allyloxymethyl-2-ethyltrimethylene carbonate) (PAOMEC) [31]. This diversity of building blocks allows for the design of micelles with a wide variety of structural and functional properties.
2. Formation of Micellar Drug-Delivery Vehicles
The tendency of polar water molecules to exclude nonpolar molecules leads to attraction and spontaneous association (self-assembly) of the hydrophobic sections of a copolymer in an aqueous environment. A balance between this attraction and the affinity of the polar hydrophilic sections of a copolymer towards water leads to the creation of self-assembled micellar structures of well-defined size and morphology. The position of the equilibrium on the concentration scale is termed the critical micelle concentration (CMC). The CMC is basically the minimum concentration required for the molecules to self-assemble and is typically around the micromolar concentration range.
This equilibrium means that micellization is a reversible process. At concentrations lower than the CMC, the molecules of the surfactant are in the form of monomers and tend to accumulate at the air–water (or oil–water) interface. As the surfactant concentration is increased, both the bulk and the interface are saturated at a specific concentration, and the increased activity leads to self-aggregation (or self-assembly). However, for the polymeric micelles, the transition is not sharp and is highlighted by the gradual formation of dimers, trimers, etc. [32]. Hence, around the CMC, the micelles are loose and may contain some water in their core. They become more compact, durable, and smaller in size when the surfactant concentration is increased. Further increase in surfactant concentration may lead to changes in micellar morphology such as a transition from a spherical to cylindrical configuration. Micelle formation characteristics, and hence the CMC of a surfactant, are strongly influenced by the molecular structure, temperature, pH, presence of electrolytes, or other co-surfactants (Figure 1) [29,33,34,35].
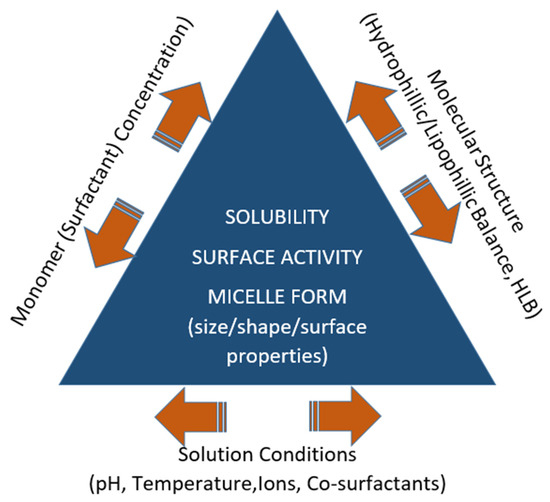
Figure 1.
Factors that determine micelle formation and properties.
The morphology of a micelle can differ depending on the chemical structure, chain length, and concentration of the copolymer, which permits tuning the size and shape of the micelle for a specific application. For example, rod-shaped micelles have better mechanical stability; round-shaped micelles provide good colloidal stability; lamella-shaped micelles allow better control of shape and size distribution; worm-shaped micelles have a high tendency to bind and coalesce [35,36,37,38]; and cylindrically shaped micelles of simple surfactants are widely employed to prepare soft templates in manufacturing micro-porous structures [39].
3. Requirements for Micellar Drug-Delivery Vehicles
For a micelle to function as a successful drug carrier, it must fulfill several requirements functions. The micelle must be biocompatible and benign to the biological environment. In other words, it must properly perform its intended functions in the tissue through induced reactions without inducing unacceptable toxic, immunogenic, thrombogenic, or carcinogenic responses. To achieve this, the carrier system should possess physicochemical properties that render it non-immunogenic and compatible with the host immune system. It must show the right amounts of immunostimulatory (e.g., to prevent secondary tumor formation by stimulating the immune system) and immunosuppressive (e.g., to allow the acceptance of foreign tissues in organ transplant patients) properties. The main factor in determining the immuno-compatibility of a carrier is basically related to its surface properties [40], which form the basis of devising various methods such as polyethylene glycol addition or PEGylation [41] to modify the carrier system to achieve compatibility. The surface properties of the carrier also determine its interaction, and hence compatibility, with native blood components which may influence its efficiency and may even render it inoperative.
The biological response and overall suitability of a given material are context-dependent and may differ across applications and tissue environments. For example, biodegradable poly(lactic-co-glycolic acid) (PLGA) polymeric-based nano-and microspheres, which offer a well-characterized, subjectively mild tissue reaction, may cause fairly strong acute inflammations when introduced into the loose connective tissue surrounding nerves [42]. The term ‘subjectively’ enters in the above sentence because biocompatibility is a relative concept which is determined by the risk–benefit ratio, which again may be different for different applications. Therefore, the use of the term bio-compatible should be used very cautiously since it may have misleading implications depending on the application. The reviews by Naahidi et al. [42] and Sundar et al. [43] should be referred to for more details on bio-compatible and biodegradable nanostructures.
Secondly, a drug carrier should be biodegradable. The carrier should be properly eliminated from the tissue after performing its function without requiring further treatment for removal. Even if it fulfills the requirements of a bio-compatible material, if it is not biodegradable, the drug carrier may trigger a reaction from the immune system or accumulate in organs such as the liver, kidney, and spleen, leading to toxic or potentially life-long side effects. Some examples of widely employed biodegradable polymers for drug delivery are PLGA, PLA (poly-lactic acid), and PCL (poly-ε-caprolactone) [44]. Well-known PEO-polypropylene oxide (PPO)-PEO tri-block copolymers made up of hydrophilic polyethylene oxide and hydrophobic polypropylene oxide groups (e.g., the Pluronic® series) have also found wide use due to their excellent biodegradability, relatively small critical micelle concentrations, and better drug loading properties. Chitosan and Gelatin are examples of natural polymers which offer great biocompatibility and biodegradability.
Thirdly, the hydrophobic cores of the micelles must be large enough to be able to contain enough smaller drug molecules, while being small enough to penetrate the target cells. Micelles of copolymers and natural polymers with sizes in the order of 10–100 nm perfectly satisfy these criteria. Some examples of direct measurements carried out in our labs are given in Figure 2 and Figure 3. The left-hand-side graph in Figure 2 gives the in situ dynamic light scattering size distribution of barren micelles of the well-known Pluronic® brand tri-block copolymer P-123 in distilled water. The figure shows that the micelles have an average size of around 18 and a maximum size of around 40 nm (DLS, dynamic light scattering). The average size observed agrees with the reported values in the literature [45,46]. A scanning transmission electron microscope (STEM) picture of the same micelles after immobilization on carbon grids is given in the right-hand-side graph of Figure 2. The image clearly shows the size and the spherical shape of the micelles. Scanning transmission electron microscope (STEM) images of the same micelles loaded with strongly hydrophobic drugs are presented in Figure 3.
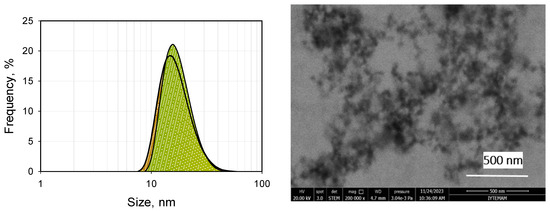
Figure 2.
Size distribution by DLS (left) and STEM (right) images of 10−3 M P-123 (barren micelles).
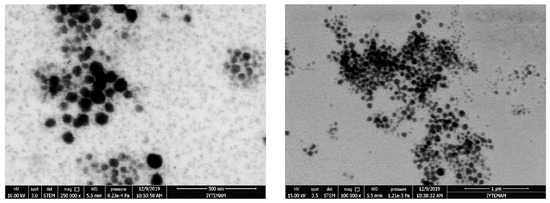
Figure 3.
STEM images of curcumin-loaded P-123 micelles (left), probucol-loaded P-123 micelles (right).
Lastly and most importantly, the micelles must have sufficient stability to reach the target site intact and have sensitivity to respond to the target site for delivery of the load and display a controlled release profile. Placement of the active molecules within a micelle’s core is not sufficient for success in drug delivery for two main reasons. Micelle formation, which corresponds to a thermodynamic equilibrium at a specific surfactant concentration, is a reversible process with concentration changes. Considering that dilution of the micellar solution during intravenous injection is unavoidable, the stability of the drug-carrying micelles should be a serious concern in real applications. On the other hand, assuming that the micelles reach the target site intact, they must also show the proper response at the target site for the successful delivery of their chemical load. Therefore, the vast number of copolymeric building blocks available must be modified by some means to form micelles with proper stability and response characteristics to be able to function as dependable drug-delivery vehicles. Due to the large variety of options available, the routes for forming micelles like drug-delivery vehicles with different features can be classified under those which provide improved stability for successful transport and those which lead to the proper response at the target site.
In drug-delivery applications, the hydrophobic cores of the micelles of polymeric molecules act as a solvent for lipophilic drug molecules and store the drug until it is released at the target site [32,47,48,49,50,51,52,53,54,55,56,57,58,59]. Therefore, it is imperative that polymeric micelles should maintain their structural integrity and not disintegrate in an untimely manner in the bloodstream until they reach the target site. Unfortunately, deterioration during intravenous injection is a serious threat, since the micellar solutions necessarily become diluted below CMC in addition to being exposed to pH and salt changes and contact with native proteins and cells in body fluid [29,60,61].
To clarify the underlying causes of this instability, it is instructive to contrast the dominant stability mechanisms of natural and synthetic micelles. Hydrophobically modified chitosan micelles, as natural polymers in which N-acylation introduces long-chain hydrophobic substituents on amino groups, exhibit cooperative hydrophobic associations between these chains, which will be discussed in the following sections. This forms a cohesive, interlocked matrix that encapsulates lipophilic drugs and resists disassembly under dilution, since breakup would require simultaneous disruption of multiple hydrophobic contacts—an energetically unfavorable process. In contrast, synthetic block copolymer micelles such as Pluronic P-123 adopt a core–corona architecture, with a hydrophobic PPO core and a hydrophilic PEO corona. When the concentration falls below the CMC, favorable corona–water interactions drive de-association into unimers as the system returns to thermodynamic equilibrium, leading to dilution-induced disintegration.
Micelle stability can be examined from two perspectives: thermodynamic stability, which describes how the system acts as micelles are formed and reach equilibrium, and kinetic stability, which describes the behavior of the system over time in solution. The CMC is one of the parameters used to characterize the thermodynamic stability of micelles and is related to thermal energy and the effective interaction energy between polymers and bulk solution. Since polymer chains of polymeric micelles have more points of interaction, they have a lower CMC than the low-molar-mass surfactant micelles. It is believed that the lower the CMC value, the higher the micellization ability and micellar stability [3,33,62,63,64]. The length of the hydrophobic segment is also shown to be one of the factors affecting stability [65,66]. Other than these, various modifications can be devised to increase the thermodynamic stability of the micelles [64,67,68,69].
The authors of this paper investigated the thermodynamic and kinetic stability of barren and drug-loaded Pluronic® P-123 micelles in systematic re-dilution steps with DW and simulated body fluid (SBF). They observed through STEM, TEM, and DLS measurements that the drug-loaded micelles exhibited significant disintegration upon dilution in DW and SBF. The left-hand-side graphs in Figure 4 show the effect of dilution on barren micelles when they are diluted from a stock solution of full-grown micelles. The average micelle size at the solution surfactant concentration of 10−3 M (16 nm) became progressively smaller and reached a value close to that of the P-123 monomer (around 4 nm) at a surfactant concentration of 10−7 M P-123 following dilution. Disintegration due to dilution could not be prevented when the micelles were formed in SBF solutions or when they were loaded with lipophilic drug molecules. This behavior is consistent with the core–corona equilibrium mechanism outlined above, where corona–water interactions dominate near and below the CMC, favoring micelle disassembly.
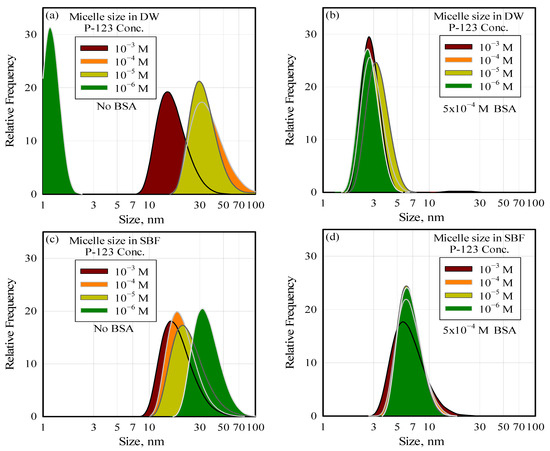
Figure 4.
The effect of dilution and the presence of protein on the integrity (size) of the P-123 micelles in water (a,b) and in SBF (c,d).
Their more recent work conclusively demonstrates that native plasma components (in this case, the protein BSA) significantly affect micelle integrity. The graph on the left-hand side of Figure 4 shows how micelles are affected by BSA in solution. It can be observed that the average micelle size of 18 nm, in the absence of BSA, decreases with dilution, reaching less than 3 nm at a BSA concentration of 5 × 10−4 M. BSA-induced micelle disintegration also cannot be prevented in SBF or in the presence of encapsulated lipophilic drug molecules [47]. It should be noted that the BSA concentration at which the micelles disintegrate is within the range of the plasma protein concentration. It is suggested that dilution and the presence of native plasma components must be considered in devising micelles as drug carriers.
Micelle formation is a reversible process that is highly dependent on weak intermolecular interactions. These weak bonds can be strengthened by cross-linking strategies within the shell or core regions [10,70,71,72,73,74,75,76]. Wang et al. [77] state that redox-sensitive cross-linking of the micelles within the shell reduces their CMC and enhances their stability against severe conditions encountered during the intravenous injection. Similarly, Lee et al. [78] suggest pH-hydrolysable cross-linking within the shell improved the micellar physical stability even in the presence of micelle-disrupting surfactants, SDS. Lu et al. [71] list several covalent cross-linking methods in the shell or the core regions such as photo/ultraviolet-induced dimerization, di-functional cross-linkers, click cross-linking, silicon chemistry methods, and reversible boronate ester bonds. In addition, other cross-linking methods based on non-covalent molecular interactions, including static electric interaction and hydrogen bonding, have been applied to increase the stability of micelles [79]. However, shell cross-linking may alter the surface and hydrophilicity characteristics of the micelles, which in turn affect their solvation properties in the bloodstream.
The CMC is inversely proportional to the length of the hydrophobic block; as the chain becomes longer, the hydrophobic interactions within the micelle core strengthen, thereby lowering the CMC and enhancing micellar stability. To reduce CMC, hydrophobic interactions can be increased by adding hydrophobic segments to the micelle core. Similarly, the presence of highly hydrophobic drugs has been suggested to increase micelle stability due to hydrophobic interactions between the encapsulated drug and the polymer [29,80,81,82,83]. In a few cases reported in the literature, interactions between the encapsulated drug and the micellar core have been shown to lower the CMC and decrease the drug release rate. The authors also state that the methods applied to form micelles, such as dialysis and co-solvent evaporation, or changes in solvent conditions or temperature also have an effect on the stability of micelles. Another potential method for improving micelle stability of synthetic polymers is to wrap the drug-loaded micelles with a natural polymeric structure such as chitosan by cross-linking the chitosan molecules around the individual micelles [14].
Modifications for improving the response at the target site are carried out to provide the micelles with functionalities such as pH-, light-, or redox-responsive stimuli sensitivity, or charge-converted and core/shell cross-linked micelles for proper intracellular delivery at the target site [69,77,84,85,86,87,88,89]. Stimulus-sensitive polymeric micelles are customized to release drugs by changing their composition or conformation after exposure to extracellular or intracellular triggers. These structural changes can occur in different ways, such as by cleaving the specific linker between the transported substance and the polymer, cleaving the shielding polymer block, affecting changes in the charged groups on the polymer, or solubilizing the polymer [56,74,90,91]. There are excellent review papers [69,92] which summarize the details of stimuli-responsive polymeric micelles.
4. Natural Polymers (Biopolymers)
Micelles can also be prepared from natural biopolymers that are produced by the cells of living organisms and derived from a wide variety of sources, including plants, animals, and microorganisms. Due to their similarity to the extracellular matrix, mechanical tunability, high biocompatibility, and high water-holding capacity, biopolymers have been employed in a variety of biomedical applications such as pharmaceuticals, drug-delivery applications, tissue regeneration scaffolds, and imaging agents. They help prevent chronic inflammation, immune responses, and associated toxic effects [93,94,95], and their environmental impact is minimal (during production and application) [96,97].
Natural biopolymers can be generally grouped into three origins: polysaccharides, proteins, and microbially fermented biopolymers [96] (Table 1). They may originate from plants, animals (terrestrial or marine), or algae, or be produced through microbial fermentation. Cellulose, starch, pectin, and mannan are polysaccharides originating from plants, whereas chitin, heparin, and hyaluronan are animal-based polysaccharides. Agarose, alginate, carrageenan, and fucoidan are non-animal groups of polysaccharides extracted from algae. A brief description of each group along with its best-known, most used principal member will be presented in the following paragraphs for completeness. For a more detailed discussion, refer to the recent papers by O’Brien [94], Garg et al. [98], Nikolova and Chavali [99], Qu et al. [100], Shick et al. [101], and Williams [102], which provide extensive reviews on natural materials.

Table 1.
Classification of some common natural biomaterials.
Table 1.
Classification of some common natural biomaterials.
| Type | Origin | Examples | References |
|---|---|---|---|
| Polysaccharides | Plant | Cellulose, Starch, Pectin, Mannan | [103,104,105,106,107,108,109,110] |
| Animal | Chitin/Chitosan, Heparin, Hyaluronan | [20,64,85,111,112,113,114] | |
| Microbial | Dextran, Pullulan | [84,115,116,117,118,119,120] | |
| Algal | Fucoidan, Agar/Agarose, Carrageenan, Alginate | [121,122,123,124,125,126,127,128] | |
| Proteins | Animal | Collagen, Gelatin, Fibrin, Silk | [129,130,131,132,133,134,135,136,137] |
| Microbially fermented | Microbial | Polyhydroxy-alkanoates | [138,139,140,141] |
5. Modification of Biopolymers for Micellization to Use as Drug Delivery Vehicles
Manufacturing biopolymeric structures homogeneously and reproducibly, especially in specific forms such as spherical nanoparticles, films, foams, or scaffolds, is a hot field of recent research. The micelles of biopolymers have also started to attract significant interest in recent years as drug-delivery agents in the pharmaceutical field. However, most of the time a biopolymer must be subjected to some form of modification to form micelles with specific properties in order to be able to find a wide application area in drug delivery and tissue engineering [142,143,144,145].
Specifically, biopolymers in their natural form are not suitable for self-assembly; micelle formation requires assistance through the chemical modification of biopolymer molecules to obtain hydrophobic functional groups. Once they are made to self-assemble, biopolymeric micelles present great potential for controlled release, drug targeting, and hydrophobic active material solubilization with the added advantage of intrinsic biocompatibility and biodegradability, which is always a question with synthetic polymers. The important steps/factors involved in their modification and micellization is generalized by the following flowsheet (Figure 5).
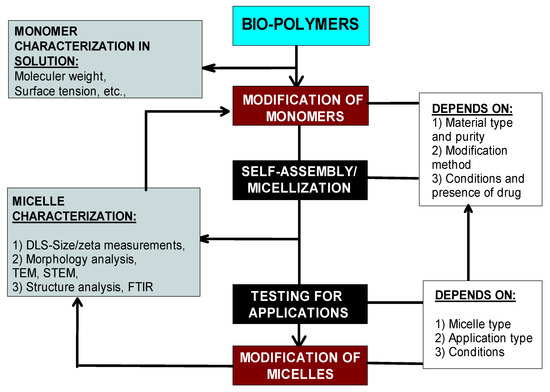
Figure 5.
The general methodology of biopolymer modification and its relation to micellization.
Biopolymers can be modified through various methods depending on their functional groups. Uronic acid-rich polysaccharides like alginate are typically modified via carboxyl group amidation or esterification, while aminated polysaccharides undergo modifications such as quaternization, N-acylation, or N-alkylation [146,147,148,149,150]. The following summarizes key studies on the modification and micellization of widely used biopolymers.
Cellulose possesses rich functional groups, allowing for self-assembly through modifications including esterification [151,152,153,154,155,156,157,158], etherification [103,159], backbone grafting [160,161,162,163], ROP [162,164,165], ATRP [166,167,168], and RAFT polymerization [169,170,171]. Stimuli-responsive self-assembly has been achieved by tuning molecular structures or external conditions like temperature [172,173,174], light [74,175], pH [176], or multiple triggers [177,178]. For example, pH-responsive micelles have been formed from HEC-graft poly(acrylic acid) [179], and pH-sensitive molecular brushes have also been reported.
Amphiphilic cellulose derivatives have been developed to self-assemble into micelles or vesicles [180,181], with sizes ranging from 20 to 430 nm [103]. These include QC-g-OCL polymers, cellulose-g-PCL systems, and cellulose derivatives carrying long alkyl chains for hydrophobic drug encapsulation [57,104,105].
Heparin-based micelles have been used for the delivery of growth factors and anticancer drugs [182,183,184]. Systems include Tetronic-PCL-heparin micelles (CMC: 0.11 g/L, size ~114 nm), docetaxel-loaded pH-sensitive micelles with deoxycholate, and redox-sensitive micelles synthesized using heparin, β-sitosterol, and cysteamine (encapsulation efficiency: 58.47%) [85]. Other examples involve GA-loaded micelles [114] and heparosan-cholesterol nanocarriers for improved drug uptake [113].
Dextran derivatives have been modified with aldehydes, thiols, acrylates, and other functional groups for drug delivery [116,120,185]. Dextran-based amphiphilic copolymers using PLGA, PCL, and lipids have been developed for loading doxorubicin, docetaxel, and curcumin [186,187,188,189,190,191]. Functionalization with folic acid, cholesterol, and N-isopropylacrylamide has enhanced targeting [84,117,185,192,193]. Synthesis strategies include thiol–disulfide exchange [186], click chemistry [187], and end-to-end coupling [188]. Zwitterionic dextran micelles have also been reported [189].
Alginate, a hydrophilic polymer, requires hydrophobic modification for micelle formation. This has been achieved using long alkyl chains [194,195,196], esterification [197,198], and graft polymerization. Hydrophobic modified alginate was also synthesized by derivatization of sodium alginate with dodecyl glycidyl ether in an aqueous solution [86,199,200] and by esterification of octadecyl chains onto the polysaccharide backbone [201] in a different study. Modified alginate forms micelles with CMCs as low as 0.024 g/L [202] and shows aggregation at concentrations as low as 0.1 mg/mL [203]. Examples include alginate–curcumin micelles for hepatocyte targeting (size ~235 nm, zeta potential −29 mV) [119], calcium-crosslinked spherical micelles (CMC: 0.2 mg/mL) [128], and amphiphilic graft copolymers formed via living radical polymerization [202].
Chitosan’s abundant –NH2 and –OH groups allow for easy chemical modification, affecting its physicochemical and biological properties, particularly its solubility [204,205]. Modifications usually occur at the 2-NH2 position (N-substitution) due to its higher reactivity over the 3-OH and 6-OH groups [206]. Acylation reactions target NH2 (N-acylation), OH (O-acylation), or both (N,O-acylation), using reagents like acyl halides and acid anhydrides in solvents such as pyridine and methanol/water/acetic acid [146,147,149,150,207]. O-acylation requires NH2 protection, often attained using methanesulfonic acid [208], followed by deprotection for N,O-acylation [209].
Alkylation involves Schiff base reactions with aldehydes or ketones [210,211,212], forming –C=N bonds linked to bioactivities [213]. N-alkylation occurs on C2–NH2, and O-alkylation on C3 or C6–OH groups. Due to nucleophilicity, N-alkylation is generally favored. Reaction pathway selection depends on desired functionality, as –NH2 modification influences bioactivity, including antimicrobial properties [214]. Protection/deprotection strategies help preserve chitosan’s biodegradability and safety [205].
Chitosan-based micelles have been developed for hydrophobic drug delivery. Emami et al. [67] synthesized tocopheryl succinate-grafted chitosan oligosaccharide loaded with paclitaxel, enhancing micelle stability and reducing size. Huo et al. [215] created N-octyl-O-glycol chitosan (OGC) micelles for paclitaxel delivery with CMC values between 5.3 and 32.5 mg/L. Guo et al. [216] developed Gal-OCMC-g-SA micelles (160 nm, CAC 0.047 mg/mL) for liver-targeted docetaxel release. Another study used chitosan grafted with deoxycholic acid and N-acetyl-L-cysteine to load quercetin [209]. Jiang et al. [217] combined COS-DOCA and mPEG-PDLLA to produce paclitaxel-loaded mixed micelles (40 nm, 97.09% encapsulation). Examples of chitosan-based micelles developed as drug-delivery systems are summarized in Table 2.
Oral delivery applications also utilize chitosan’s mucoadhesive properties, interacting with mucin via electrostatic and hydrophobic effects [218,219]. Examples include paclitaxel [220], linoleic acid-grafted chitosan oligosaccharide micelles for docetaxel [221], and pH-sensitive N-naphthyl-N,O-succinyl chitosan micelles for meloxicam [222].
Other polysaccharides have also been explored. Pullulan, a microbial exopolysaccharide, has been used in various micelle systems: cholesterol-bearing pullulan [223,224,225,226,227,228], acetylated pullulan [220], poly(L-lactide)-grafted pullulan [118,221], pullulan–desoxycholic acid–PEI [222], and PLGA–grafted pullulan [223]. Galactosylated pullulan-curcumin conjugates have shown targeted delivery to hepatocarcinoma cells [119], and pullulan–tocopherol succinate–folic acid micelles (149.5 nm, –49 mV, CMC: 194.87 μg/mL) were used for epirubicin delivery to Hela and MCF-7 cells [224]. Reduction-sensitive pullulan–stearic acid micelles have also been used for intracellular doxorubicin [225,226,227,228].

Table 2.
Drug-loaded chitosan-based micelles and their important properties.
Table 2.
Drug-loaded chitosan-based micelles and their important properties.
| Name of Amphiphilic Copolymer Micelle | Drug/ Molecule | Size (nm) | ZP (mV) | EE (%) | CMC (mg/mL) | Ref. |
|---|---|---|---|---|---|---|
| Stearyl-grafted chitosan | Atorvastatin | 97.19 | −8.27 | 10.4–35.0 | 6.9 × 10−3–21 × 10−3 | [64] |
| N-phthaloyl-carboxymethyl chitosan | L floxin and BSA | 60–90 30–200 | - | 8.5 52.0 | 0.20 | [112] |
| Cholesteryl hemisuccinate (CHS)-conjugated chitosan | Docetaxel | 303 | +21.3 | NA | NA | [229] |
| Stearic acid-grafted chitosan oligosaccharide | Docetaxel | 20.4 | +53.1 | 55.0 | 0.022 | [68] |
| Water-soluble N-palmitoyl chitosan | Ibuprofen | ~150.0 | - | ~50.0 | 2.0 × 10−3–37.2 × 10−3 | [212] |
| Folate-modified N-Succinyl-N′-Octyl Chitosan | 10 Hydroxyca-camptothecin | 100–200 | −20.0 to +38.0 | 57.0–58.0 | - | [230] |
| N-succinyl-N′-octyl chitosan micelles | Docetaxel | 100–200 | - | 36.4 a | 5.9 × 10−3– 3.1 × 10−2 | [231] |
| Fatty acid grafted chitosan-based copolymer micelles | Cefiximetrihy-drate | 520 | +42 | 38–52 | NA | [111] |
| Grafting oleic acid (OA) on the chitosan (CS) skeleton and penetrating (PEN) and (MAN) conjugation. | pVGF | 199.8 ± 15.7 nm | Positive | NA | NA | [232] |
| Redox-sensitive chitosan derivative (y cholesterol, sulfhydryl, and mPEG (mPEG-CS(SH)-CHO)) | Paclitaxel | 158 | +26.9 | 88.3 | NA | [233] |
| O-methyl-O′-succinylpolyethylene glycol- and oleic acid-grafted chitosan | Camptothecin | 140 nm | Positive | 78 | 0.150–0.147 0.076–0.065 | [234] |
| LA–CMCS (Linoleic acid–carboxymethyl chitosan) | Paclitaxel | 93–119 | −16 to −29 | 56–67 | ~11–18 × 10−3 | [235] |
| CS–g–OA (Oleic acid-grafted chitosan) | Coumarin-6 | 335.5/491 | +20.5 to +38.5 | 29 | 0.5748 | [236] |
| CS–SA–DA (Succinic anhydride and deoxycholic acid-modified chitosan) | Curcumin | 228/269 | −44 to −29 | 80.8 | 0.093 | [237] |
| LCNE–LA (Low-MW chitosan–nicotinic acid–lipoic acid conjugate) | Doxorubicin | 218/254 | +26/35.2 | 92 | 0.1808 | [238] |
EE: encapsulation efficiency; ZP: zeta potential; BSA: bovine serum albumin; a: Loading capacity.
Hydroxyethyl starch (HES), a semi-synthetic polysaccharide, has been esterified with fatty acids (lauric, palmitic, stearic) to form micelles and vesicles (20–350 nm) [106]. Thermo-responsive starches (HBPS), synthesized with butyl glycidyl ether, exhibit temperature-dependent drug release [107]. Amylopectin modified with poly(lactic acid) also forms micelles (20.7–77.2 nm) with adjustable CAC values (0.038–0.190 mg/L) [108].
Importantly, modifying natural polymers like chitosan can significantly alter their properties. For instance, modifying –NH2 groups may reduce pH sensitivity and positive surface charge, affecting bioactivity and drug-delivery performance [239].
6. Characterization of Biopolymers and Their Micelles
Biopolymers, whether in their native (unmodified) or chemically modified forms—as well as the micelles formed from them—are characterized using a range of analytical techniques. These characterizations are essential not only to confirm molecular structure but also to assess surface activity, which plays a crucial role in micelle formation and functionality.
Structural characterization of both unmodified and modified biopolymeric molecules is typically conducted using techniques such as elemental analysis, potentiometric titration, ninhydrin assay, 1H and 13C NMR, HSQC-NMR, Fourier-transform infrared spectroscopy (FTIR), matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS), and thermogravimetric analysis (TGA). For modified biopolymers intended for micelle formation, surface activity characterization is also necessary. Common methods employed for this purpose include surface and interfacial tension measurements, contact angle analysis, and surface energy determination [239,240,241].
Once micelles are formed, further characterization is required to determine their morphological and surface properties. In addition to the aforementioned techniques, specialized tools such as small-angle X-ray scattering (SAXS), dynamic light scattering (DLS), atomic force microscopy (AFM), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and scanning transmission electron microscopy (STEM) are routinely used for comprehensive micellar characterization [239].
In Figure 6, STEM images clearly reveal the formation of spherical, non-porous chitosan micelles with diameters between 30 and 50 nm in aqueous media. Given the relatively high molecular weight of the modified chitosan (~500,000 g mol−1), these structures likely represent coiled aggregates of hydrophobically modified chitosan chains, possibly intercalated with drug molecules. This self-assembly behavior contrasts with more conventional polymeric micelles such as those formed from Pluronic P-123, which typically exhibit smaller diameters (~20 nm) (Figure 2 and Figure 3).
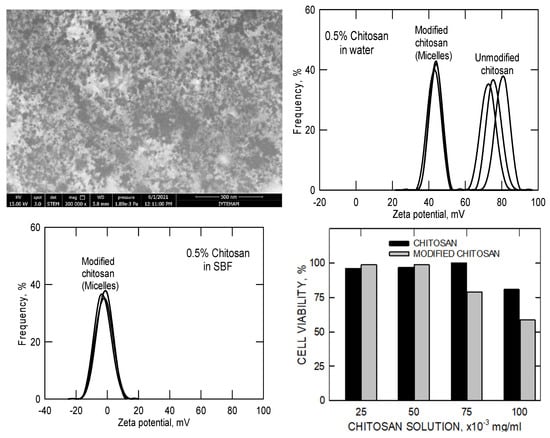
Figure 6.
STEM pictures, zeta potentials, and biocompatibility results of the unmodified (pristine) and modified (hydrophobisized) chitosan molecules in water and in simulated body fluid. (The multiple black lines represent 3 repeats of the same measurements).
The corresponding zeta-potential and cell-viability data further clarify the physicochemical and biological implications of this hydrophobic modification. In aqueous solution, pristine chitosan displays a high positive zeta potential (≈70–90 mV) due to the abundance of protonated amino groups. After partial acylation, the modified chitosan micelles maintain a positive surface charge (≈40–50 mV), indicating that a substantial portion of amine groups remains unreacted and available for electrostatic interactions. When dispersed in simulated body fluid (SBF), the zeta potential shifts toward neutrality and the distribution broadens, reflecting ionic adsorption and screening of surface charges by physiological electrolytes. Although this reduction diminishes colloidal repulsion, it reflects realistic physiological conditions and is characteristic of chitosan-based micellar systems. The accompanying biocompatibility results show that both pristine and modified chitosan maintain high cell viability (>80%) at concentrations up to 50 × 10−3 mg mL−1, with only a slight decrease for the hydrophobically modified samples at higher concentrations. Together, these results demonstrate that partial hydrophobic substitution promotes micellization without compromising biocompatibility, while the remaining positive surface charge contributes to colloidal stability and facilitates potential electrostatic drug loading near physiological pH.
The Krafft temperature is defined as the minimum temperature at which the solubility of an ionic surfactant becomes equal to its critical micelle concentration (CMC); below this temperature, micelles cannot form because the surfactant exists predominantly in a crystalline or precipitated state [242]. This concept originated from studies on low-molecular-weight ionic surfactants that exhibit sharp phase transitions between crystalline and micellar states. In contrast, polymeric or nonionic amphiphiles, such as hydrophobically modified chitosan, do not display a distinct Krafft point. Instead, micellization in these systems is governed mainly by polymer chain mobility, segmental flexibility, and solvent interactions rather than by solubility limits. In the same study [239,241], the micellar dispersions exhibited a pH of approximately 7.0 and a conductivity of 1.51 mS cm−1, consistent with near-neutral, moderately ionic conditions. Although the literature contains limited information on the Krafft temperature of chitosan-based micelles, available data suggest that their effective micellization temperature in simulated body fluid lies below 37 °C. This implies that chitosan micelles remain stable under physiological conditions, retaining their self-assembled structure during circulation and interaction with biological fluids.
7. Stability of Natural Polymeric Micelles
The possible factors, such as chemical structures of molecules, properties of the physiological environment, and the modification type of molecules, that affect the integrity of natural polymeric micelles in blood are summarized in Figure 7. In particular, hydrophobically modified chitosan systems benefit from cooperative hydrophobic association within the shell/core network, whereas classical block-copolymer micelles remain governed by core–corona equilibrium near the CMC.
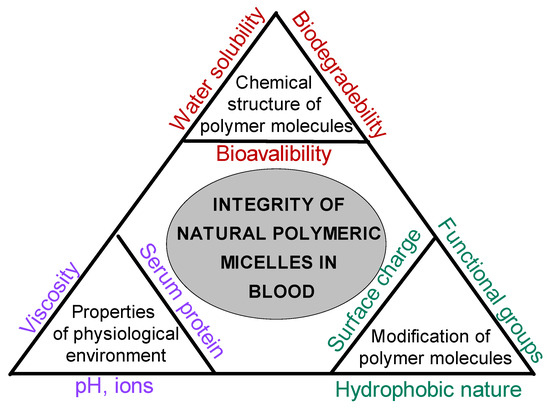
Figure 7.
Factors influencing the integrity of biopolymeric micelles in physiological environments.
The stability of biopolymeric micelles depends on multiple interrelated factors. Therefore, the use of bio-micellar systems in biological applications such as drug delivery requires additional care to take all the changes in the structure into account. One cannot use a single molecule as a reference anymore and expect similar properties from the micelles formed. The properties of micelles could be quite different depending on all the factors mentioned above.
Though a few studies have focused on the stability of the biopolymeric micelles and reported positive findings, the stability of the loaded biopolymeric micelles is usually an issue in the laboratory evaluation of the efficacy of delivery vehicles. Emami et al. [67] have shown that the physical incorporation of α-tocopherol succinate in chitosan-derived polymeric micelles for paclitaxel delivery enhances micelle stability. The improvement is due to the increased hydrophobic interaction between paclitaxel and the micellar core. In their study, to test their stability, micelles were stored at 4 °C for at least three months and their sizes were measured. The authors showed that the incorporation of α-tocopherol succinate into the micellar system produced smaller particles with high stability during storage without affecting the entrapment efficiency.
In another study, Mekhail et al. [64] synthesized and characterized stearyl chitosan and sulfated stearyl chitosan amphiphilic block copolymers. They have shown that polymeric micelles with or without drugs have a negative charge due to the negatively charged stearyl and sulfate groups that prevent the positive charge of the amino groups of chitosan. They concluded that the negative charge of polymeric micelles contributes to the stability of the colloidal micelle solution as a result of electrostatic repulsive forces between the micelles. Ye et al. [68] modified the core of amphiphilic stearic acid-grafted chitosan oligosaccharide (CSO-SA) micelles by the physical solubilization of stearic acid to reduce the burst drug release and enhance the physical stability of CSO-SA micelles. Zhu et al. [230] produced folate-modified N-succinyl-N′-octyl chitosan micelles (folate-SOC) for targeted delivery of 10-Hydroxycamptothecin. The stability of these micelles was tested by suspending these structures in PBS for 30 days and was then characterized. These results showed that folate-modified micelles display better storage stability. Zhu et al. [243] produced water-insoluble anticancer drug gambogic acid-loaded chitosan-based micelles. Their storage stability was tested using lyophilized samples kept at 4 °C for 2 months and they were found to be stable for up to 2 months.
Despite such findings which report positive stability data on biopolymeric micelles, it should be said that the deleterious effects of dilutional factors and the encounter with native blood components that the micelles must endure during intravenous injection must still be considered. The implications of this issue, which were reported by Polat and Polat [14,15,47], have been discussed in detail in their recent ongoing studies [239,240,241].
The authors of this review paper studied the stability of chitosan micelles with dilution and protein interaction by DLS (dynamic light scattering) and the results are presented in Figure 8. It can be seen that the sizes of chitosan micelles do not change even after 1000 rounds of dilution in comparison to P-123 micelles in the case of dilution. However, the structure of natural polymeric micelles becomes unstable in body fluids upon protein interactions.
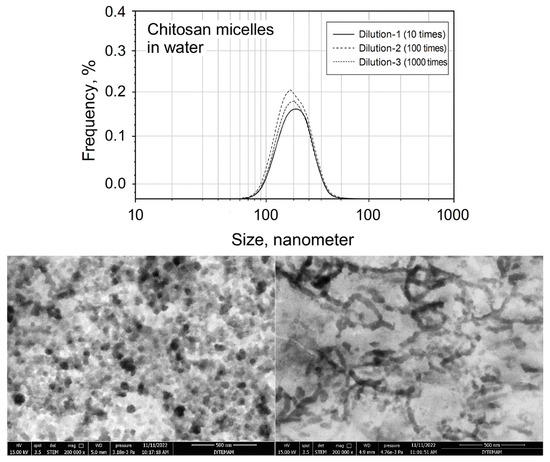
Figure 8.
Dilution-induced disintegration of lipophilic drug-loaded chitosan micelles (upper) and STEM images of drug-loaded chitosan micelles in SBF before dilution (bottom, left) and after 1000 rounds of dilution (bottom, right).
Figure 9 presents a conceptual model summarizing the complex interplay among micelle–micelle, micelle–cell, and micelle–medium–drug interactions that collectively determine drug-delivery performance under physiological conditions. The diagram is not intended as a quantitative or computational model but as a visual framework integrating the key physicochemical and biological factors that control the stability and efficacy of polymeric micellar systems. Along each axis, the figure identifies the dominant interaction modes governing system behavior. The base of the triangle represents micelle–medium–drug interactions, emphasizing the combined influence of environmental conditions (pH, ionic strength, osmolarity, proteins, enzymes, and temperature) and drug–micelle partitioning on colloidal stability, solubilization, and release kinetics. The left axis corresponds to micelle–micelle interactions, where variations in structure, surface charge, and hydrophobicity determine the extent of aggregation or dispersion phenomena typically characterized by techniques such as DLS, ζ-potential, SAXS/SANS, and microscopy. The right axis represents micelle–cell interactions, highlighting how micellar surface properties control cellular attachment, uptake, and biocompatibility, which are experimentally assessed by CLSM, FACS, or cell-viability assays. At the center lies drug-delivery efficacy, reflecting the integrated outcome of these coupled interactions. The model illustrates that premature micelle disintegration, excessive aggregation, or insufficient cell interaction can each compromise delivery performance. Conversely, achieving a balanced interplay among these three domains yields stable, biocompatible micellar systems capable of sustained and targeted drug release. Overall, Figure 9 provides a unified conceptual perspective linking physicochemical stability, environmental responsiveness, and biological interaction to therapeutic performance, thereby guiding the rational design of micellar nanocarriers for clinical use.
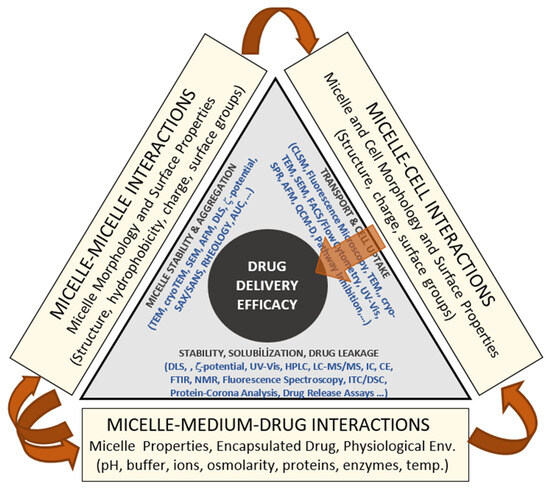
Figure 9.
Conceptual framework linking micellar physicochemical behavior, biological interactions, and environmental stability to overall drug-delivery efficacy.
8. Conclusions
This review has examined the self-assembly behavior of both synthetic and natural polymers into micellar structures capable of encapsulating hydrophobic (water-insoluble) drugs, with a particular focus on their applicability in pharmaceutical drug-delivery systems. Polymeric micelles offer significant advantages for drug delivery, including enhanced cellular uptake efficiency resulting from their nanoscale dimensions. They also exhibit favorable biocompatibility and biodegradability, making them attractive candidates for pharmaceutical applications.
However, natural polymers typically require chemical modification to facilitate self-assembly and micelle formation. The efficiency of micellization and the resulting physicochemical properties of the micelles are strongly influenced by the type and degree of chemical modification applied. Importantly, these modifications may alter key intrinsic properties of the natural polymers—such as pH responsiveness, exemplified by chitosan—which must be carefully considered in the design of drug-delivery systems. Based on the literature reviewed, several key conclusions can be drawn regarding micellar systems that encapsulate drugs through physical entrapment (i.e., without covalent bonding):
- Synthetic polymeric micelles generally lack stability under physiological dilution conditions, leading to disassembly upon administration.
- Natural polymeric micelles tend to exhibit improved stability under dilution compared to synthetic counterparts; however, they remain susceptible to destabilization upon interaction with serum proteins.
- Both synthetic and natural polymeric micelles experience structural compromise in the presence of blood proteins, which poses a significant challenge for systemic administration.
In light of these findings, it is recommended that future research focus on strategies to enhance micelle stability in biological environments. Approaches such as surface modification, cross-linking, or the incorporation of protective coatings may enhance micellar integrity during circulation and prior to cellular uptake.
From a broader conceptual standpoint, the current state of micellar research reveals a transition from empirical formulation toward mechanism-driven design. Future progress will depend on integrating kinetic modeling, interfacial thermodynamics, and in situ characterization techniques to predict and control micelle behavior under physiological conditions. Developing quantitative frameworks that link micelle composition, stability, and drug-release kinetics will be essential for rational optimization. Moreover, the convergence of polymer chemistry, computational modeling, and biophysics is expected to accelerate the development of micelle systems tailored for specific therapeutic routes, oral, transdermal, and intravenous, bridging fundamental science with clinical utility.
These findings highlight that while achieving structural stability under physiological conditions remains a major design challenge, translating these advances into practical therapeutic systems is the next logical step toward real-world application. It should also be noted that comparisons between synthetic and natural polymeric micelles are not always straightforward. The stability and assembly behavior of each system depend strongly on the polymer’s intrinsic molecular parameters, such as molar mass, block-length ratio, degree of hydrophobic modification, and the chemistry of pendant groups, rather than solely on its synthetic or biological origin. Therefore, when assessing or comparing micellar stability, these specific structural features must be considered alongside polymer type, as they often play the dominant role in determining micelle architecture and robustness under physiological conditions.
In recent years, several polymeric micelle systems have progressed from laboratory studies to clinical evaluation. Formulations based on PEG-PLA and PEG-PCL have entered clinical trials for anticancer drugs such as paclitaxel, doxorubicin, and cisplatin, where they have shown improved solubility, reduced toxicity, and better pharmacokinetic profiles. Natural polymer-based micelles, particularly those derived from chitosan, alginate, and fucoidan, are also drawing attention for their biocompatibility and additional biological functions such as mucoadhesion and site-specific targeting. These features make them promising candidates for further clinical translation. Looking ahead, it is expected that hybrid micellar systems combining the structural control of synthetic polymers with the bioactivity of natural materials will play a key role in developing safer and more effective nanomedicines.
Author Contributions
Conceptualization, H.P., M.P. and O.K.P.; methodology, H.P., M.P. and O.K.P.; data curation, H.P. and M.C.E.; writing—original draft preparation, H.P., M.C.E., M.P., K.M.K. and O.K.P.; writing—review and editing, H.P., M.C.E., M.P., K.M.K. and O.K.P.; visualization, H.P. and M.P.; supervision, H.P. and O.K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors thank the Specialist Mutlu Yaman of the Material Research Center of the Integrated Research Centers at Izmir(IZTECH) for performing STEM analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.S.; Chauhan, P.N.; Noolvi, M.N.; Chaturvedi, K.; Ganguly, K.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric micelles: Basic research to clinical practice. Int. J. Pharm. 2017, 532, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Kapse, A.; Anup, N.; Patel, V. Polymeric micelles: A ray of hope among new drug delivery systems. In A Volume in Advances in Pharmaceutical Product Development and Research; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 235–289. [Google Scholar]
- Simões, S.M.; Figueiras, A.R.; Veiga, F.; Concheiro, A.; Alvarez-Lorenzo, C. Polymeric micelles for oral drug administration enabling locoregional and systemic treatments. Expert. Opin. Drug Deliv. 2015, 12, 297–318. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Ramsey, J.; Kabanov, A. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef]
- Duplâtre, G.; Marques, M.F.; da Graça Miguel, M. Size of sodium dodecyl sulfate micelles in aqueous solutions as studied by positron annihilation lifetime spectroscopy. J. Phys. Chem. A 1996, 100, 16608–16612. [Google Scholar] [CrossRef]
- Mazer, N.A.; Benedek, G.B.; Carey, M.C. An investigation of the micellar phase of sodium dodecyl sulfate in aqueous sodium chloride solutions using quasielastic light scattering spectroscopy. J. Phys. Chem. A 1976, 80, 1075–1085. [Google Scholar] [CrossRef]
- Santos, M.S.; Tavares, F.W.; Biscaia, E.C., Jr. Molecular thermodynamics of micellization: Micelle size distributions and geometry transitions. Braz. J. Chem. Eng. 2016, 33, 515–523. [Google Scholar] [CrossRef]
- Movassaghian, S.; Merkel, O.; Torchilin, V. Applications of polymer micelles for imaging and drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 691–707. [Google Scholar] [CrossRef]
- Talelli, M.; Barz, M.; Rijcken, C. Core-crosslinked polymeric micelles: Principles, preparation, biomedical applications, and clinical translation. Nano Today 2015, 10, 93–117. [Google Scholar] [CrossRef]
- Muftuoglu, A.E.; Tasdelen, M.A.; Yagci, Y. Radical Polymerization, Process, and Technology. In Handbook of Vinyl Polymers; Mishra, M., Yagci, Y., Eds.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Subramani, K.; Ahmed, W. Nanoparticulate drug delivery systems for oral cancer treatment. In Emerging Nanotechnologies in Dentistry Processes, Materials and Applications Micro and Nano Technologies; Subramani, K., Ahmed, W., Eds.; William Andrew Publishing: New York, NY, USA, 2012; pp. 333–345. [Google Scholar]
- Atanase, L.I. Micellar drug delivery systems based on natural biopolymers. Polymers 2021, 13, 477. [Google Scholar] [CrossRef]
- Cihan, E.; Polat, M.; Polat, H. Designing of spherical chitosan nano-shells with micellar cores for solvation and safe guarded delivery of strongly lipophilic drugs. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 815–823. [Google Scholar] [CrossRef]
- Polat, H.; Kutluay, G.; Polat, M. Analysis of dilution-induced disintegration of micellar drug carriers in the presence of inter and intra micellar species. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 124989. [Google Scholar] [CrossRef]
- Polat, M.; Kurt, A.N.O.; Ozdamar, B.A.; Polat, H. Synthesis of pristine chitosan foams with enhanced pore structure, surface area, and mechanical for tissue engineering. Mater. Res. Express 2025, 12, 105401. [Google Scholar] [CrossRef]
- Kedar, U.; Phutane, P.; Shidhaye, S. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 714–729. [Google Scholar] [CrossRef] [PubMed]
- Dand, N.M.; Patel, P.B.; Ayre, A.P. Polymeric micelles as a drug carrier for tumor targeting. Chron Young Sci. 2013, 4, 94. [Google Scholar]
- Rapoport, N. Physical stimuli-responsive polymeric micelles for anti-cancer drug delivery. Prog. Polym. Sci. 2007, 32, 962–990. [Google Scholar] [CrossRef]
- Liu, J.; Liang, N.; Li, S. Tumor-targeting and redox-sensitive micelles based on hyaluronic acid conjugate for delivery of paclitaxel. J. Biomater. Appl. 2020, 34, 1458–1469. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, Z.; Shuai, Q. Tumor-targeting peptide-functionalized PEG-PLA micelles for efficient drug delivery. Biomater. Sci. 2020, 8, 2274–2282. [Google Scholar] [CrossRef]
- Yang, Y.; Long, Y.; Wang, Y. Enhanced anti-tumor and anti-metastasis therapy for triple-negative breast cancer by CD44 receptor-targeted hybrid self-delivery micelles. Int. J. Pharm. 2020, 577, 119085. [Google Scholar] [CrossRef]
- Weng, J.; Huang, Z.; Pu, X.; Chen, X.; Yin, G.; Tian, Y.; Song, Y. Preparation of polyethylene glycol-polyacrylic acid block copolymer micelles with pH/hypoxic dual-responsive for tumor. Colloids Surf. B 2020, 191, 110943. [Google Scholar] [CrossRef]
- Yang, H.; Khan, A.; Liu, M.; Fu, M.; Ji, J.; Chi, L.; Zhai, G. Stimuli-responsive polymeric micelles for the delivery of paclitaxel. J. Drug Deliv. Sci. Technol. 2020, 56, 101523. [Google Scholar] [CrossRef]
- Croy, S.R.; Kwon, G.S. Polymeric micelles for drug delivery. Curr. Pharm. Des. 2006, 12, 4669–4684. [Google Scholar] [CrossRef] [PubMed]
- Cormode, D.; Naha, P.; Fayad, Z. Nanoparticle contrast agents for computed tomography: A focus on micelles. Contrast Media Mol. Imaging 2014, 9, 37–52. [Google Scholar] [CrossRef]
- Al Zaki, A.; Joh, D.; Cheng, Z.; De Barros, A.L.; Kao, G.; Dorsey, J.; Tsourkas, A. Gold-loaded polymeric micelles for computed tomography-guided radiation therapy treatment and radiosensitization. ACS Nano 2014, 8, 104–112. [Google Scholar] [CrossRef]
- Annapragada, A.V.; Hoffman, E.; Divekar, A.; Karathanasis, E.; Ghaghada, K.B. High-resolution CT vascular imaging using blood pool contrast agents. Methodist Debakey Cardiovasc. J. 2012, 8, 18–22. [Google Scholar] [CrossRef]
- Owen, S.C.; Chan, D.P.; Shoichet, M.S. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Bader, H.; Ringsdorf, H.; Schmidt, B. Watersoluble polymers in medicine. Angew. Makromolek Chem. 1984, 123, 457–485. [Google Scholar] [CrossRef]
- Glavas, L.; Odelius, K.; Albertsson, A.C. Tuning loading, and release by modification of micelle core crystallinity and preparation. Polym. Adv. Technol. 2015, 26, 880–888. [Google Scholar] [CrossRef]
- Polat, H.; Chander, S. Adsorption of PEO/PPO triblock copolymers and wetting of coal. Colloid Surface A 1999, 146, 199–212. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.; Nawale, R.B.; Kulthe, S.S. Polymeric micelles: General considerations and their applications. Indian J. Pharm. Educ. Res. 2012, 45, 128–138. [Google Scholar]
- Lee, H.; Zeng, F.; Dunne, M.; Allen, C. Methoxy poly (ethylene glycol)-block-poly (δ-valerolactone) copolymer micelles for the formulation of hydrophobic drugs. Biomacromolecules 2005, 6, 3119–3128. [Google Scholar] [CrossRef]
- Mondon, K.; Gurney, R.; Möller, M. Colloidal drug delivery systems-recent advance with polymeric micelles. Chim. Int. J. Chem. 2008, 62, 832–840. [Google Scholar] [CrossRef]
- Cai, S.; Vijayan, K.; Cheng, D.; Lima, E.M.; Discher, D.E. Micelles of different morphologies-advantages of worm-like filomicelles of PEO-PCL in paclitaxel delivery. Pharm. Res. 2007, 24, 2099–2109. [Google Scholar] [CrossRef]
- Maeda, H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J. Control Release 2012, 164, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Jiang, J. pH-sensitive drug loading/releasing in amphiphilic copolymer PAE–PEG: Integrating molecular dynamics and dissipative particle dynamics simulations. J. Control Release 2012, 162, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Siretli, Ç. Synthesis of Silica Nanoparticles with Custom-Made Morphology for Controlled Drug Delivery. Master’s Thesis, İzmir Institute of Technology, Urla-Izmir, Turkey, 2012. [Google Scholar]
- Dobrovolskaia, M.A.; McNeil, S.E. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007, 2, 469–478. [Google Scholar] [CrossRef]
- Abuchowski, A.; Van Es, T.; Palczuk, N.T.; Davis, F.F. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J. Biol. Chem. 1977, 252, 3578–3581. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of engineered nanoparticles for drug delivery. J. Control Release 2013, 166, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Sundar, D.S.; Antoniraj, M.G.; Kumar, C.S.; Mohapatra, S.S.; Houreld, N.N.; Ruckmani, K. Recent trends of biocompatible and biodegradable nanoparticles in drug delivery: A review. Curr. Med. Chem. 2016, 23, 3730–3751. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Liu, J.S.; Wang, J.H.; Zhou, J.; Tang, X.H.; Xu, L.; Shen, T.; Wu, X.Y.; Hong, Z. Enhanced brain delivery of lamotrigine with Pluronic P123-based nanocarrier. Int. J. Nanomed. 2014, 4, 3923–3935. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, D.; Wang, L.; Zhang, J.; Zhang, N. Docetaxel-loaded pluronic p123 polymeric micelles: In vitro and in vivo evaluation. Int. J. Mol. Sci. 2011, 12, 1684–1686. [Google Scholar] [CrossRef]
- Polat, H.; Eren, M.Ç.; Polat, M. The effect of protein BSA on the stability of lipophilic drug (docetaxel)-loaded polymeric micelles. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127712. [Google Scholar] [CrossRef]
- Linse, P.; Malmsten, M. Temperature-dependent micellization in aqueous block copolymer solutions. Macromolecules 1992, 25, 5434–5439. [Google Scholar] [CrossRef]
- Mortensen, K.; Brown, W.; Nordén, B. Inverse melting transition and evidence of three-dimensional cubatic structure in a block-copolymer micellar system. Phys. Rev. Lett. 1992, 68, 2340–2343. [Google Scholar] [CrossRef]
- Alexandridis, P.; Hatton, T.A. Poly (ethylene oxide)-poly (propylene oxide)-poly (ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modeling. Colloids Surf. A Physicochem. Eng. Asp. 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Alexandridis, P. Poly(ethylene oxide) poly(propylene oxide) block copolymer surfactants. J. Colloid Interface Sci. 1997, 2, 478–489. [Google Scholar] [CrossRef]
- Verma, P.; Nath, S.; Singh, P.K.; Kumbhakar, M.; Pal, H. Effects of the block size of pluronic polymers on the water structure in the corona region and its effect on the electron transfer reactions. J. Phys. Chem. B 2008, 112, 6363–6372. [Google Scholar] [CrossRef]
- Sturcova, A.; Schmidt, P.; Dybal, J. Role of hydration and water coordination in micellization of Pluronic block copolymers. J. Colloid Interface Sci. 2010, 352, 415–423. [Google Scholar] [CrossRef]
- Xiong, X.B.; Falamarzian, A.; Garg, S.M.; Lavasanifar, A. Engineering of amphiphilic block copolymers for polymeric micellar drug and gene delivery. J. Control Release 2011, 155, 248–261. [Google Scholar] [CrossRef]
- Kadam, Y.; Yerramilli, U.; Bahadur, A.; Bahadur, P. Micelles from PEO-PPO-PEO block copolymers as nanocontainers for solubilization of a poorly water-soluble drug hydrochlorothiazide. Colloids Surf. B Biointerfaces 2011, 83, 49–57. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Y.; Chen, Y.; Ye, J.; Sha, X.; Fang, X. Multifunctional Pluronic P123/F127 mixed polymeric micelles loaded with paclitaxel for the treatment of multidrug-resistant tumors. Biomaterials 2011, 32, 2894–2906. [Google Scholar] [CrossRef]
- Zhang, M.; Djabourov, M.; Bourgaux, C.; Bouchemal, K. Nanostructured fluids from pluronic® mixtures. Int. J. Pharm. 2013, 454, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Oh, Y.T.; Youn, Y.S.; Nam, M.; Park, B.; Yun, J.; Kim, J.H.; Song, H.T.; Oh, K.T. Binary mixing of micelles using Pluronics for a nano-sized drug delivery system. Colloids Surf. B Biointerfaces 2011, 82, 190–195. [Google Scholar] [CrossRef]
- Zhiqi, H.; Alexandridis, H.P. micellization thermodynamics of pluronic P-123 (EO20PO70EO20) amphiphilic block copolymer in aqueous ethylammonium nitrate (EAN) solutions. Polymers 2018, 10, 32. [Google Scholar]
- Sang, X.; Yang, Q.; Shi, G.; Zhang, L.; Wang, D.; Ni, C. Preparation of pH/redox dual responsive polymeric micelles with enhanced stability and drug-controlled release. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 727–733. [Google Scholar] [CrossRef]
- Hsu, H.J.; Han, Y.; Cheong, M.; Král, P.; Hong, S. Dendritic PEG outer shells enhance serum stability of polymeric micelles. Nanomedicine 2018, 14, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shi, Y.; Kim, J.Y.; Park, K.; Cheng, J.X. Overcoming the barriers in micellar drug delivery: Loading efficiency, in vivo stability, and micelle-cell interaction. Expert. Opin. Drug Deliv. 2012, 7, 49–62. [Google Scholar] [CrossRef]
- Qiu, L.Y.; Bae, Y.H. Polymer architecture and drug delivery. Pharm. Res. 2006, 23, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Mekhail, G.M.; Kamel, A.O.; Awad, G.A.; Mortada, N.D. Anticancer effect of atorvastatin nanostructured polymeric micelles based on stearyl-grafted chitosan. Int. J. Biol. Macromol. 2012, 51, 351–363. [Google Scholar] [CrossRef]
- Diezi, T.A.; Bae, Y.; Kwon, G.S. Enhanced stability of PEG-block-poly (N-hexyl stearate l-aspartamide) micelles in the presence of serum proteins. Mol. Pharm. 2010, 7, 1355–1360. [Google Scholar] [CrossRef]
- Van Domeselaar, G.H.; Kwon, G.S.; Andrew, L.C.; Wishart, D.S. Application of solid phase peptide synthesis to engineering PEO-peptide block copolymers for drug delivery. Colloids Surf. B 2003, 30, 323–334. [Google Scholar] [CrossRef]
- Emami, J.; Rezazadeh, M.; Rostami, M.; Hassanzadeh, F.; Sadeghi, H.; Mostafavi, A.; Minaiyan, M.; Lavasanifar, A. Co-delivery of paclitaxel and α-tocopherol succinate by novel chitosan-based polymeric micelles for improving micellar stability and efficacious combination therapy. Drug Dev. Ind. Pharm. 2015, 41, 1137–1147. [Google Scholar] [CrossRef]
- Ye, Y.Q.; Yang, F.L.; Hu, F.Q.; Du, Y.Z.; Yuan, H.; Yu, H.Y. Core-modified chitosan-based polymeric micelles for controlled release of doxorubicin. Int. J. Pharm. 2008, 352, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Chitkara, D. Structural modifications in polymeric micelles to impart multifunctionality for improved drug delivery. Ther. Deliv. 2016, 7, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M. Polymeric micelles as a new drug carrier system and their required considerations for clinical trials. Expert. Opin. Drug Deliv. 2010, 7, 145–158. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to improve micelle stability for drug delivery. Nano Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef]
- Shuai, X.; Merdan, T.; Schaper, A.K.; Xi, F.; Kissel, T. Core-cross-linked polymeric micelles as paclitaxel carriers. Bioconjug. Chem. 2004, 15, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Cajot, S.; Lautram, N.; Passirani, C.; Jérôme, C. Design of reversibly core cross-linked micelles sensitive to reductive environment. J. Control Release 2011, 152, 30–36. [Google Scholar] [CrossRef]
- Wang, B.; Chen, K.; Yang, R.; Yang, F.; Liu, J. Stimulus-responsive polymeric micelles for the light-triggered release of drugs. Carbohydr. Polym. 2014, 103, 510–519. [Google Scholar] [CrossRef]
- Thurmond, K.B.; Huang, H.; Clark, C.G., Jr.; Kowalewski, T.; Wooley, K.L. Shell cross-linked polymer micelles: Stabilized assemblies with great versatility and potential. Colloids Surf. B Biointerfaces 1999, 16, 45–54. [Google Scholar] [CrossRef]
- Rolland, A.; O’mullane, J.; Goddard, P.; Brookman, L.; Petrak, K. New macromolecular carriers for drugs. I. Preparation and characterization of poly (oxyethylene-b-isoprene-b-oxyethylene) block copolymer aggregates. J. Appl. Polym. Sci. 1992, 44, 1195–1203. [Google Scholar] [CrossRef]
- Wang, Y.C.; Li, Y.; Sun, T.M.; Xiong, M.H.; Wu, J.; Yang, Y.Y.; Wang, J. Core-shell-corona micelle stabilized by reversible cross-linkage for intracellular drug delivery. Macromol. Rapid Commun. 2010, 31, 1201–1206. [Google Scholar] [CrossRef]
- Lee, S.J.; Min, K.H.; Lee, H.J.; Koo, A.N.; Rim, H.P.; Jeon, B.J.; Jeong, S.Y.; Heo, J.S.; Lee, S.C. Ketal cross-linked poly (ethylene glycol)-poly (amino acid) s copolymer micelles for efficient intracellular delivery of doxorubicin. Biomacromolecules 2011, 12, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Dai, Y.; Xia, F.; Zhang, X. Advances in non-covalent crosslinked polymer micelles for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111626. [Google Scholar] [CrossRef]
- Lee, J.; Cho, E.C.; Cho, K. Incorporation and release behavior of hydrophobic drug in functionalized poly (D, L-lactide)-block-poly (ethylene oxide) micelles. J. Control Release 2004, 94, 323–335. [Google Scholar] [CrossRef]
- Glavas, L.; Olsén, P.; Odelius, K.; Albertsson, A.C. Achieving micelle control through core crystallinity. Biomacromolecules 2013, 14, 4150–4156. [Google Scholar] [CrossRef] [PubMed]
- Lavasanifar, A.; Samuel, J.; Kwon, S.G. The effect of alkyl core structure on micellar properties of poly (ethylene oxide)-block-poly(L-aspartamide) derivatives. Colloids Surf. B 2001, 22, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.P.; Guo, X.L.; Liu, G.Q.; Fan, A.; Wang, Z.; Zhao, Y. When self-assembly meets topology: An enhanced micelle stability. Chem. Comm. 2017, 53, 3822–3825. [Google Scholar] [CrossRef]
- Blanco-Fernandez, B.; Concheiro, A.; Makwana, H.; Fernandez-Trillo, F.; Alexander, C.; Alverez-Lorenzo, C. Dually sensitive dextran-based micelles for methotrexate delivery. RSC Adv. 2017, 7, 14448–14460. [Google Scholar] [CrossRef]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.C. Synthesis and characterization of redox-sensitive heparin-β-sitosterol micelles: Their application as carriers for the pharmaceutical agent, doxorubicin, and investigation of their antimetastatic activities in vitro. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 1326–1338. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Z.; Liu, B.; Yang, J.; Yang, X.; Yu, Y. A smart drug delivery system responsive to pH/enzyme stimuli based on hydrophobic modified sodium alginate. Eur. Polym. J. 2020, 133, 109779. [Google Scholar] [CrossRef]
- Han, S.S.; Li, Z.Y.; Zhu, J.Y.; Han, K.; Zeng, Z.Y.; Hong, W.; Li, W.X.; Jia, H.Z.; Liu, Y.; Zhuo, R.X.; et al. Dual-ph sensitive charge-reversal polypeptide micelles for tumor-triggered targeting uptake and nuclear drug delivery. Small 2015, 11, 2443–2454. [Google Scholar] [CrossRef]
- Kadekar, S.; Nawale, G.N.; Rangasami, V.K.; Le Joncour, V.; Laakkonen, P.; Hilborn, J.; Varghese, O.P.; Oommen, O.P. Redox responsive Pluronic micelle mediated delivery of function al. siRNA: A modular nano-assembly for targeted delivery. Biomater. Sci. 2021, 11, 3939–3944. [Google Scholar] [CrossRef]
- Kim, A.; Miura, Y.; Ishii, T.; Mutaf, O.F.; Nishimaya, N.; Cabral, H.; Kataoka, K. Intracellular delivery of charge-converted monoclonal antibodies by combinatorial design of block/homo polyion complex micelles. Biomacromolecules 2016, 17, 446–453. [Google Scholar] [CrossRef]
- Tian, Q.; Fei, C.; Yin, H.; Feng, Y. Stimuli-responsive polymer wormlike micelles. Prog. Polym. Sci. 2019, 89, 108–132. [Google Scholar] [CrossRef]
- Chiang, Y.T.; Yen, Y.W.; Lo, C.L. Reactive oxygen species and glutathione dual redox-responsive micelles for selective cytotoxicity of cancer. Biomaterials 2015, 61, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Sarisozen, C.; Joshi, U.; Mendes, L.P. Stimuli-responsive polymeric micelles for extracellular and intracellular drug delivery. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Makhlouf, A., Abu-Thabit, Y., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 269–304. [Google Scholar]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S. Natural polymers: Polysaccharides and their derivatives for biomedical applications. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 67–89. [Google Scholar]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Silva, T.H.; Alves, A.; Ferreira, B.M.; Oliveria, J.M.; Reys, L.L.; Ferreira, R.J.F.; Sousa, R.A.; Silva, S.S.; Mano, J.F.; Reis, R.L. Materials of marine origin: A review on polymers and ceramics of biomedical interest. Int. Mater. Rev. 2012, 57, 276–306. [Google Scholar] [CrossRef]
- Salerno, A.; Pascual, C.D. Bio-based polymers, supercritical fluids, and tissue engineering. Process Biochem. 2015, 50, 826–838. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Goh, J.C.H.; Teoh, S.H. An introduction to biodegradable materials for tissue engineering applications. Ann. Acad. Med. Singap. 2001, 30, 183–191. [Google Scholar] [CrossRef]
- Garg, T.; Rath, G.; Goyal, A.K. Biomaterials-based nanofiber scaffold: Targeted and controlled carrier for cell and drug delivery. J. Drug Target 2015, 23, 202–221. [Google Scholar]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef]
- Shick, T.; Kadir, A.A.; Ngadiman, N.; Ma’aram, A. A review of biomaterials scaffold fabrication in additive manufacturing for tissue engineering. J. Bioact. Compat. Polym. 2019, 34, 415–435. [Google Scholar] [CrossRef]
- Williams, D.F. Challenges with the development of biomaterials for sustainable tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, L.; Gan, W.; Zhou, J.; Zhang, L. Self-assembled micelles based on hydrophobically modified quaternized cellulose for drug delivery. Colloids Surf. B Biointerfaces 2011, 83, 313–320. [Google Scholar]
- Hsieh, M.F.; Cuong, N.V.; Chen, C.H.; Chen, Y.T.; Yeh, J.M. Nano-sized micelles of block copolymers of methoxy poly (ethylene glycol)-poly(epsilon-caprolactone)-graft-2-hydroxyethyl cellulose for doxorubicin delivery. J. Nanosci. Nanotechnol. 2008, 8, 2362–2368. [Google Scholar]
- Lu, A.; Petit, E.; Jelonek, K.; Orchel, A.; Kasperczyk, J.; Wang, Y.; Su, F.; Li, S. Self-assembled micelles prepared from bio-based hydroxypropyl methylcellulose and polylactide amphiphilic block copolymers for anti-tumor drug release. Int. J. Biol. Macromol. 2020, 154, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Besheer, A.; Hause, G.; Kressler, J.; Mäder, K. Hydrophobically modified hydroxyethyl starch: Synthesis, characterization, and aqueous self-assembly into nano-sized polymeric micelles and vesicles. Biomacromolecules 2007, 8, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Ju, B.; Yan, D.; Zhang, S. Micelles self-assembled from thermoresponsive 2-hydroxy-3-butoxypropyl starches for drug delivery. Carbohydr. Polym. 2012, 87, 1404–1409. [Google Scholar] [CrossRef]
- Lu, W.H.; Zhang, L.M.; Wang, C.; Chen, R.F. Preparation and properties of new micellar drug carriers based on hydrophobically modified amylopectin. Carbohydr. Polym. 2011, 83, 1499–1506. [Google Scholar] [CrossRef]
- Ha, W.; Wu, H.; Wang, X.L.; Peng, S.L.; Ding, L.S.; Zhang, S.; Li, B.J. Self-aggregates of cholesterol-modified carboxymethyl konjac glucomannan conjugate: Preparation, characterization, and preliminary assessment as a carrier of etoposide. Carbohydr. Polym. 2011, 86, 513–519. [Google Scholar]
- Ferreira, S.A.; Pereira, P.; Sampaio, P.; Coutinho, P.J.; Gama, F.M. Supramolecular assembled nanogel made of mannan. J. Colloid Interface Sci. 2011, 361, 97–108. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Parvathy, J.; Nair, A.S. Evaluation of micellar architecture based on functionalized chitosan for the in vitro release of an antibiotic. Des. Monomers Polym. 2016, 19, 99–107. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, L. Self-assembled micelles of N-phthaloyl-carboxymethyl chitosan for drug delivery. Colloids Surf. A Physicochem. Eng. 2009, 337, 21–25. [Google Scholar] [CrossRef]
- Qiu, L.; Shan, X.; Long, M.; Ahmed, K.S.; Zhao, L.; Mao, J.; Zhang, H.; Sun, C.; You, C.; Lv, G.; et al. Elucidation of cellular uptake and intracellular trafficking of heparosan polysaccharide-based micelles in various cancer cells. Int. J. Biol. Macromol. 2019, 130, 755–764. [Google Scholar] [CrossRef]
- Yan, X.; Yang, Y.; He, L.; Peng, D.; Yin, D. Gambogic acid grafted low molecular weight heparin micelles for targeted treatment in a hepatocellular carcinoma model with an enhanced anti-angiogenesis effect. Int. J. Pharm. 2017, 522, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yao, X.; Zhang, Z.; Chen, L.; He, C.; Chen, X. Boronic acid shell-crosslinked dextran-b-PLA micelles for acid-responsive drug delivery. Macromol. Biosci. 2014, 14, 1609–1618. [Google Scholar] [CrossRef]
- Raza, K.; Kumar, N.; Misra, C.; Kaushik, L.; Guru, S.K.; Kumar, P.; Malik, R.; Bhushan, S.; Katare, O.P. Dextran-PLGA-loaded docetaxel micelles with enhanced cytotoxicity and better pharmacokinetic profile. Int. J. Biol. Macromol. 2016, 88, 206–212. [Google Scholar] [CrossRef]
- Chen, X.; Yao, X.; Chen, L. Intracellular pH-sensitive dextran-based micelles as efficient drug delivery platforms. Polym. Int. 2015, 64, 430–436. [Google Scholar] [CrossRef]
- Seo, S.; Lee, C.S.; Jung, Y.S.; Na, K. Thermo-sensitivity and triggered drug release of polysaccharide nanogels derived from pullulan-g-poly (L-lactide) copolymers. Carbohydr. Polym. 2012, 87, 1105–1111. [Google Scholar]
- Sarika, P.R.; James, N.R.; Nishna, N.; Kumar, P.R.A.; Raj, D.K. Galactosylated pullulan-curcumin conjugate micelles for site-specific anticancer activity to hepatocarcinoma cells. Colloids Surf. B Biointerfaces 2015, 133, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.I.; Choi, K.C.; Song, C.E. Doxorubicin release from core-shell type nanoparticles of poly (DL-lactide-co-glycolide)-grafted dextran. Arch. Pharm. Res. 2006, 29, 712–719. [Google Scholar] [CrossRef]
- Shu, G.; Lu, C.; Wang, Z.; Du, Y.; Xu, X.; Xu, M.; Zhao, Z.; Chen, M.; Dai, Y.; Weng, Q.; et al. Fucoidan-based micelles as P-selectin targeted carriers for synergistic treatment of acute kidney injury. Nanomedicine 2021, 32, 102342. [Google Scholar] [CrossRef] [PubMed]
- Phan, N.H.; Ly, T.T.; Pham, M.N.; Luu, T.D.; Vo, T.V.; Tran, P.H.L.; Tran, T.T.D. A comparison of fucoidan conjugated to paclitaxel and curcumin for the dual delivery of cancer therapeutic agents. Anticancer Agents Med. Chem. 2018, 18, 1349–1355. [Google Scholar] [CrossRef]
- Lopez-Heredia, M.A.; Łapa, A.; Mendes, A.C.; Balcaen, L.; Samal, S.K.; Chai, F.; Van der Voort, P.; Stevens, C.; Parakhonskiy, B.; Chronakis, I.S.; et al. Bioinspired, biomimetic, double-enzymatic mineralization of hydrogels for bone regeneration with calcium carbonate. Mater. Lett. 2017, 190, 13–16. [Google Scholar] [CrossRef]
- Awadhiya, A.; Kumar, D.; Rathore, K.; Fatma, B.; Verma, V. Synthesis and characterization of agarose-bacterial cellulose biodegradable composites. Polymer Bull. 2017, 74, 2887–2903. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef]
- Tobacman, J. Review of harmful gastrointestinal effects of carrageenan in animal experiments. Environ. Health Perspect. 2001, 109, 983–994. [Google Scholar] [CrossRef]
- Yu, N.; Li, G.; Gao, Y.; Jiang, H.; Tao, Q. Thermo-sensitive complex micelles from sodium alginate-graft-poly(N-isopropyl acrylamide) for drug release. Int. J. Biol. Macromol. 2016, 86, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, D.; Karabasz, A.; Bzowska, M.; Szuwarzyński, M.; Karewicz, A.; Nowakowska, M. Blood-compatible, stable micelles of sodium alginate-curcumin bioconjugate for anti-cancer applications. Eur. Polym. J. 2019, 13, 208–219. [Google Scholar] [CrossRef]
- Govindharaj, M.; Roopavath, U.; Rath, S. Valorization of discarded Marine Eel fish skin for collagen extraction as a 3D printable blue biomaterial for tissue engineering. J. Clean. Prod. 2019, 210, 412–419. [Google Scholar] [CrossRef]
- Fernández-Cervantes, I.; Rodríguez-Fuentes, N.; León-Deniz, L.V.; Quintana, L.E.A.; Cervantes-Uc, L.M.; Kao, W.H.; Cerón-Espinosa, J.D.; Cauich-Rodríguez, J.V.; Castaño-Meneses, V.M. Cell-free scaffold from jellyfish Cassiopea andromeda (Cnidaria; Scyphozoa) for skin tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110748. [Google Scholar] [CrossRef]
- Coelho, R.C.G.; Marques, A.L.P.; Oliveira, S.M.; Diogo, G.S.; Pirraco, R.P.; Moreira-Silva, J.; Xavier, J.C.; Reis, R.L.; Silva, T.H.; Mano, J.F. Extraction and characterization of collagen from Antarctic and Sub-Antarctic squid and its potential application in hybrid scaffolds for tissue engineering. Mater. Sci. Eng. C Mater. Biol. App. 2017, 78, 787–795. [Google Scholar] [CrossRef]
- Ali, M.; Mousa, H.; Blaudez, F.; El-Sadek, M.S.A.; Mohamed, M.A.; Abdel-Jaber, G.T.; Abdal-hay, A. Dual nanofiber scaffolds composed of polyurethane- gelatin/nylon 6- gelatin for bone tissue engineering. Colloids Surf. A Physicochem. Eng. 2020, 597, 124817. [Google Scholar] [CrossRef]
- Chawla, D.; Kaur, T.; Joshi, A.; Singh, N. 3D bioprinted alginate-gelatin based scaffolds for soft tissue engineering. Int. J. Biol. Macromol. 2019, 144, 560–567. [Google Scholar] [CrossRef]
- Luetchford, K.A.; Chaudhuri, J.B.; De Bank, P.A. Silk fibroin/gelatin microcarriers as scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110116. [Google Scholar] [CrossRef]
- Bonhome-Espinosa, A.B.; Campos, F.; Durand-Herrera, D.; Sanchez, J.D.; Schaub, S.; Durán, J.D.G.; Lopez-Lopez, M.T.; Carriel, V. In vitro characterization of a novel magnetic fibrin-agarose hydrogel for cartilage tissue engineering. J. Mech. Behav. Biomed. Mater. 2020, 104, 103619. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhu, T.; Yu, H.; Zhu, J.; Sun, C.; Wang, J.; Chen, S.; Wang, J.; Guo, X. Potential applications of three-dimensional structure of silk fibroin/poly (ester-urethane) urea nanofibrous scaffold in heart valve tissue engineering. Appl. Surf. Sci. 2018, 447, 269–278. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, C.; Qiao, X.; Liu, T.; Sun, K. Silk fibroin microfibers and chitosan modified poly (glycerol sebacate) composite scaffolds for skin tissue engineering. Polym. Test. 2017, 62, 88–95. [Google Scholar] [CrossRef]
- Zhao, K.; Deng, Y.; Chen, J.C.; Chen, G.Q. Polyhydroxyalkanoate (PHA) scaffolds with good mechanical properties and biocompatibility. Biomaterials 2003, 24, 1041–1045. [Google Scholar] [CrossRef]
- Zhao, J.; Duan, K.; Zhang, J.W.; Xiong, L. The influence of polymer concentrations on the structure and mechanical properties of porous polycaprolactone-coated hydroxyapatite scaffolds. Appl. Surf. Sci. 2010, 256, 4586–4590. [Google Scholar] [CrossRef]
- You, M.; Peng, G.; Li, J.; Ping, M.; Zhihui, W.; Weiliang, S.; Siwu, P.; Chen, G.Q. Chondrogenic differentiation of human bone marrow mesenchymal stem cells on polyhydroxyalkanoate (PHA) scaffolds coated with PHA granule binding protein PhaP fused with RGD peptide. Biomaterials 2011, 32, 2305–2313. [Google Scholar] [CrossRef]
- Corre, Y.M.; Bruzaud, S.; Audic, J.L.; Grohens, Y. Morphology and functional properties of commercial polyhydroxyalkanoates: A comprehensive and comparative study. Polym. Test. 2012, 31, 226–235. [Google Scholar] [CrossRef]
- Märtson, M.; Viljanto, J.; Hurme, T.; Laippala, P.; Saukko, P. Is cellulose sponge degradable or stable as implantation material? An in vivo subcutaneous study in the rat. Biomaterials 1999, 20, 1989–1995. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, Q.; Guo, W.; Terrell, L.; Qureshi, A.T.; Hayes, D.J.; Wu, Q. Electrospun bio-nanocomposite scaffolds for bone tissue engineering by cellulose nanocrystals reinforcing maleic anhydride grafted PLA. ACS Appl. Mater. Interfaces 2013, 5, 3847–3854. [Google Scholar] [CrossRef]
- Wang, T.; Drzal, L.T. Cellulose-nanofiber-reinforced poly (lactic acid) composites prepared by a water-based approach. ACS Appl. Mater. Interfaces 2012, 4, 5079–5085. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Chen, X. Fabrication, applications and challenges of natural biomaterials in tissue engineering. Appl. Mater. Today 2020, 20, 100656. [Google Scholar] [CrossRef]
- Holappa, J.; Nevalainen, T.; Soininen, P.; Másson, M.; Järvinen, T. Synthesis of novel quaternary chitosan derivatives via N-Chloroacyl-6-O-triphenyl methyl chitosans. Biomacromolecules 2006, 7, 407–410. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Zong, Z.; Kimura, Y.; Takahashi, M.; Yamane, H. Characterization of chemical and solid-state structures of acylated chitosans. Polymer 2000, 41, 899–906. [Google Scholar] [CrossRef]
- Zhang, M.; Hirano, S. Novel N-unsaturated fatty acyl and N-trimethylacetyl derivatives of chitosan. Carbohydr. Polym. 1995, 26, 205–209. [Google Scholar] [CrossRef]
- Hirano, S.; Yamaguchi, Y.; Kamiya, M. Novel N-saturated-fatty-acyl derivatives of chitosan soluble in water and in aqueous acid and alkaline solutions. Carbohydr. Polym. 2002, 48, 203–207. [Google Scholar] [CrossRef]
- Yang, J.; Li, J. Self-assembled cellulose materials for biomedicine: A review. Carbohydr. Polym. 2018, 181, 264–274. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, J.; Wang, L.; Ge, W.; Chen, M.; Wang, X.; Sun, R. Preparation of fluorescent core/shell nanoparticles from amphiphilic cellulose-based copolymers for tumor cell imaging. J. Control. Release 2015, 213, e132. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, Y.; Li, D.; Chen, H.; Sun, R.C. Fluorescent amphiphilic cellulose nanoaggregates for sensing trace explosives in aqueous solution. Chem. Comm. 2012, 48, 5569–5571. [Google Scholar] [CrossRef] [PubMed]
- Moccelini, S.K.; Franzoi, A.C.; Vieira, I.C.; Dupont, J.; Scheeren, C.W. A novel support for laccase immobilization: Cellulose acetate modified with ionic liquid and application in biosensor for methyldopa detection. Biosens. Bioelectron. 2011, 26, 3549–3554. [Google Scholar] [CrossRef]
- Tosh, B.; Saikia, C.N.; Dass, N.N. Homogeneous esterification of cellulose in the lithium chloride–N, N-dimethylacetamide solvent system: Effect of temperature and catalyst. Carbohydr. Res. 2000, 327, 345–352. [Google Scholar] [CrossRef]
- Cao, Y.; Li, H.; Zhang, J. Homogeneous synthesis and characterization of cellulose acetate butyrate (CAB) in 1-allyl-3-methylimidazolium chloride (AmimCl) ionic liquid. Ind. Eng. Chem. Res. 2011, 50, 7808–7814. [Google Scholar] [CrossRef]
- Crépy, L.; Miri, V.; Joly, N.; Martin, P.; Lefebvre, J. Effect of side chain length on structure and thermomechanical properties of fully substituted cellulose fatty esters. Carbohydr. Polym. 2011, 83, 1812–1820. [Google Scholar] [CrossRef]
- Cao, X.; Sun, S.; Peng, X.; Zhong, L.; Sun, R.; Jiang, D. Rapid synthesis of cellulose esters by transesterification of cellulose with vinyl esters under the catalysis of NaOH or KOH in DMSO. J. Agric. Food Chem. 2014, 61, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, E.S. Chemical modification of cellulose extracted from sugarcane bagasse: Preparation of hydroxyethyl cellulose. Arab. J. Chem. 2014, 7, 362–371. [Google Scholar] [CrossRef]
- Roy, D.; Guthrie, J.T.; Perrier, S. Graft polymerization: Grafting poly (styrene) from cellulose via reversible addition-fragmentation chain transfer (RAFT) polymerization. Macromolecules 2005, 38, 10363–10372. [Google Scholar] [CrossRef]
- Carlmark, A.; Larsson, E.; Malmström, E. Grafting of cellulose by ring-opening polymerization—A review. Eur. Polym. J. 2012, 48, 1646–1659. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Shu, X.; Shen, Z.; Sun, R.C. Self-assembly and paclitaxel loading capacity of cellulose-graft-poly (lactide) nanomicelles. J. Agric. Food Chem. 2012, 60, 3900–3908. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y.; Sun, R.; Wang, X. Self-assembly and β-carotene loading capacity of hydroxyethyl cellulose-graft-linoleic acid nanomicelles. Carbohydr. Polym. 2016, 145, 56–63. [Google Scholar] [CrossRef]
- Yuan, W.; Yuan, J.; Zhang, F.; Xie, X. Syntheses, characterization, and in vitro degradation of ethyl cellulose-graft-poly(epsilon-caprolactone)-block-poly(L-lactide) copolymers by sequential ring-opening polymerization. Biomacromolecules 2007, 8, 1101–1108. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Shen, Z.; Shu, X.; Sun, R. Preparation of cellulose-graft-poly (ɛ-caprolactone) nanomicelles by homogeneous ROP in ionic liquid. Carbohydr. Polym. 2013, 92, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Carlmark, A.; Malmström, E. Atom transfer radical polymerization from cellulose fibers at ambient temperature. J. Am. Chem. Soc. 2002, 124, 900–901. [Google Scholar] [CrossRef]
- Li, S.; Xiao, M.; Zheng, A.; Xiao, H. Cellulose microfibrils grafted with PBA via surface-initiated atom transfer radical polymerization for biocomposite reinforcement. Biomacromolecules 2011, 12, 3305–3312. [Google Scholar] [CrossRef]
- Zhong, J.F.; Chai, X.S.; Fu, S.Y. Homogeneous grafting poly (methyl methacrylate) on cellulose by atom transfer radical polymerization. Carbohydr. Polym. 2012, 87, 1869–1873. [Google Scholar] [CrossRef]
- Lin, C.; Zhan, H.; Liu, M.; Habibi, Y.; Fu, S.; Lucia, L.A. RAFT synthesis of the cellulose-g-polymethylmethacrylate copolymer in an ionic liquid. J. Appl. Polym. Sci. 2013, 127, 4840–4849. [Google Scholar] [CrossRef]
- Hufendiek, A.; Trouillet, V.; Meier, M.A.; Barner-Kowollik, C. Temperature responsive cellulose-graft-copolymers via cellulose functionalization in an ionic liquid and RAFT polymerization. Biomacromolecules 2014, 15, 2563–2572. [Google Scholar] [CrossRef] [PubMed]
- Barsbay, M.; Kodama, Y.; Güven, O. Functionalization of cellulose with epoxy groups via γ-initiated RAFT-mediated grafting of glycidyl methacrylate. Cellulose 2014, 21, 4067–4079. [Google Scholar] [CrossRef]
- Xiaojun, L.I.; Minghui, Y.I.N.; Zhang, G.; Zhang, F. Synthesis and characterization of novel temperature and pH responsive hydroxylpropyl cellulose-based graft copolymers. Chin. J. Chem. Eng. 2009, 17, 145–149. [Google Scholar]
- Yang, L.; Zhang, J.M.; He, J.S.; Zhang, J.; Gan, Z.H. Synthesis and characterization of temperature-sensitive cellulose-graft-poly (N-isopropylacrylamide) copolymers. Chin. J. Polym. Sci. 2015, 33, 1640–1649. [Google Scholar] [CrossRef]
- Porsch, C.; Hansson, S.; Nordgren, N.; Malmstrom, E. Thermo-responsive cellulose-based architectures: Tailoring LCST using poly (ethylene glycol) methacrylates. Polym. Chem. 2011, 2, 1114–1123. [Google Scholar] [CrossRef]
- Huang, Y.; Kang, H.; Li, G.; Wang, C.; Huang, Y.; Liu, R. Synthesis and photosensitivity of azobenzene functionalized hydroxypropyl cellulose. RSC Adv. 2013, 3, 15909–15916. [Google Scholar] [CrossRef]
- Yang, L.; Kuang, J.; Wang, J.; Li, Z.; Zhang, L. Loading and in vitro controlled release of indomethacin using amphiphilic cholesteryl-bearing carboxymethylcellulose derivatives. Macromol. Biosci. 2008, 10, 279–286. [Google Scholar] [CrossRef]
- Wen, Y.; Oh, J.K. Intracellular delivery cellulose-based bionanogels with dual temperature/pH-response for cancer therapy. Colloids Surf. B Biointerfaces 2015, 133, 246–253. [Google Scholar] [CrossRef]
- Yuan, W.; Zou, H.; Shen, J. Amphiphilic graft copolymers with ethyl cellulose backbone: Synthesis, self-assembly and tunable temperature-CO2 response. Carbohydr. Polym. 2016, 136, 216–223. [Google Scholar] [CrossRef]
- Dou, H.; Jiang, M.; Peng, H.; Chen, D.; Hong, Y. pH-dependent self-assembly: Micellization and micelle-hollow-sphere transition of cellulose-based copolymers. Angew. Chem. Int. Ed. Engl. 2003, 42, 1516–1519. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, F.; Hou, G.; Sun, S. Amphiphilic cellulose: Surface activity and aqueous self-assembly into nano-sized polymeric micelles. React. Funct. Polym. 2008, 68, 981–989. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, L.; Chen, X.; Chu, T.; Guo, Y.; Niu, M. Cationic micelles self-assembled from quaternized cellulose-g-oligo (ε-caprolactone) amphiphilic copolymers. Eur. Polym. J. 2019, 119, 385–392. [Google Scholar] [CrossRef]
- Lee, J.S.; Go, D.H.; Bae, J.W.; Lee, S.J.; Park, K.D. Heparin conjugated polymeric micelle for long-term delivery of basic fibroblast growth factor. J. Control Release 2007, 117, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Liu, Y.; Zhang, H.; Zhang, Z.; Gao, H.; He, Q. Antitumor and antimetastasis activities of heparin-based micelle served as both carrier and drug. ACS Appl. Mater. Interfaces 2016, 8, 9577–9589. [Google Scholar] [CrossRef]
- Abdulkarim, A.; Isa, M.T.; Abdulsalam, S.; Muhammad, A.J.; Ameh, A.O. Extraction and characterisation of chitin and chitosan from mussel shell. Extraction 2013, 3, 108–114. [Google Scholar]
- Situ, J.Q.; Ye, Y.Q.; Zhu, X.L.; Yu, R.S.; You, J.; Yuan, H.; Hu, F.Q.; Du, Y.Z. Specific targeting of A54 homing peptide-functionalized dextran-g-poly (lactic-co-glycolic acid) micelles to tumor cells. Int. J. Nanomed. 2015, 10, 665. [Google Scholar]
- Sun, H.; Guo, B.; Li, X.; Cheng, R.; Meng, F.; Liu, H.; Zhong, Z. Shell-sheddable micelles based on dextran-SS-poly(epsilon-caprolactone) diblock copolymer for efficient intracellular release of doxorubicin. Biomacromolecules 2010, 11, 848–854. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Gao, X.; Yao, X.; Chen, L.; He, C.; Chen, X. Targeted dextran-b-poly (ε-caprolactone) micelles for cancer treatments. RSC Adv. 2015, 5, 18593–18600. [Google Scholar] [CrossRef]
- Li, B.; Wang, Q.; Wang, X.; Wang, C.; Jiang, X. Preparation, drug release and cellular uptake of doxorubicin-loaded dextran-b-poly(ɛ-caprolactone) nanoparticles. Carbohydr. Polym. 2013, 93, 430–437. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.; Hu, P.; Hou, M.; Dong, Y.; Wei, Y. Antifouling zwitterionic dextran micelles for efficient loading DOX. Carbohydr. Polym. 2018, 191, 136–141. [Google Scholar] [CrossRef]
- Du, Y.Z.; Weng, Q.; Yuan, H.; Hu, F.Q. Synthesis and antitumor activity of stearate-g-dextran micelles for intracellular doxorubicin delivery. ACS Nano 2010, 4, 6894–6902. [Google Scholar] [CrossRef]
- Li, S.; Yi, J.; Li, W.; Wang, Z. Synthesis and characterization of three novel amphiphilic dextran self-assembled micelles as potential drug delivery system. J. Mater. Sci. 2017, 52, 12593–12607. [Google Scholar] [CrossRef]
- Varshosaz, J.; Hassanzadeh, F.; Aliabadi, H.S.; Nayebsadrian, M.; Banitalebi, M.; Rostami, M. Synthesis and characterization of folate-targeted dextran/retinoic acid micelles for doxorubicin delivery in acute leukemia. Biomed. Res. Int. 2014, 2014, 525684. [Google Scholar] [CrossRef]
- Yao, X.; Chen, L.; Chen, X.; He, C.; Zheng, H.; Chen, X. Intercellular pH-responsive histidine modified dextran-g-cholesterol micelle for anticancer drug delivery. Colloids Surf. B Biointerfaces 2014, 121, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, S.; Hubert, P.; Lapicque, F.; Payan, E.; Dellacherie, E. Amphiphilic derivatives of sodium alginate and hyaluronate: Synthesis and physicochemical properties of aqueous dilute solutions. Carbohydr. Polym. 2000, 43, 343–349. [Google Scholar] [CrossRef]
- De Boisseson, M.R.; Leonard, M.; Hubert, P.; Marchal, P.; Stequert, A.; Castel, C.; Favre, E.; Dellacherie, E. Physical alginate hydrogels based on hydrophobic or dual hydrophobic/ionic interactions: Bead formation, structure, and stability. J. Colloid Interface Sci. 2004, 273, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Galant, C.; Kjøniksen, A.L.; Nguyen, G.T.; Knudsen, K.D.; Nyström, B. Altering associations in aqueous solutions of a hydrophobically modified alginate in the presence of beta-cyclodextrin monomers. J. Phys. Chem. B 2006, 110, 190–195. [Google Scholar] [CrossRef]
- Broderick, E.; Lyons, H.; Pembroke, T.; Byrne, H.; Murray, B.; Hall, M. The characterization of a novel, covalently modified, amphiphilic alginate derivative, which retains gelling and non-toxic properties. J. Colloid Interface Sci. 2006, 298, 154–161. [Google Scholar] [CrossRef]
- Babak, V.G.; Skotnikova, E.A.; Lukina, I.G.; Pelletier, S.; Hubert, P.; Dellacherie, E. Hydrophobically associating alginate derivatives: Surface tension properties of their mixed aqueous solutions with oppositely charged surfactants. J. Colloid Interface Sci. 2000, 225, 505–510. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.; Zhang, R.; Yuan, S.; Lu, Q.; Yu, Y. Synthesis and micelle properties of the hydrophobic modified alginate. Int. J. Polym. Mater. Polym. 2017, 66, 742–747. [Google Scholar] [CrossRef]
- Yu, Y.; Leng, C.; Liu, Z.; Jıa, F.; Zheng, Y.; Yuan, K.; Yan, S. Preparation and characterization of biosurfactant based on hydrophobically modified alginate. Colloid J. 2014, 76, 622–627. [Google Scholar] [CrossRef]
- Ghahramanpoor, M.K.; Najafabadi, S.A.H.; Abdouss, M.; Bagheri, F.; Eslaminejad, M.B. A hydrophobically-modified alginate gel system: Utility in the repair of articular cartilage defects. J. Mater. Sci. Mater. Med. 2011, 22, 2365. [Google Scholar] [CrossRef] [PubMed]
- Kapishon, V.; Whitney, R.A.; Champagne, P.; Cunningham, M.F.; Neufeld, R.J. Polymerization induced self-assembly of alginate based amphiphilic graft copolymers synthesized by single electron transfer living radical polymerization. Biomacromolecules 2015, 16, 2040–2048. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Kim, D.; Nah, J.W.; Jeon, Y.J. Advances in functionalizing fucoidans and alginates (bio)polymers by structural modifications: A review. Chem. Eng. J. 2019, 355, 33–48. [Google Scholar] [CrossRef]
- Laroche, C.; Delattre, C.; Mati-Baouche, N.; Salah, R.; Ursu, A.V.; Moulti-Mati, F.; Pierre, P.M.G. Bioactivity of chitosan and its derivatives. Curr. Org. Chem. 2018, 22, 641–667. [Google Scholar] [CrossRef]
- Brasselet, C.; Pierre, G.; Dubessay, P.; Dols-Lafargue, M.; Coulon, J.; Maupeu, J.; Vallet-Courbin, A.; de Baynast, H.; Doco, T.; Michaud, P.; et al. Modification of chitosan for the generation of functional derivatives. Appl. Sci. 2019, 9, 1321. [Google Scholar] [CrossRef]
- Xie, Y.; Qiao, H.; Su, Z.; Chen, M.; Ping, Q.; Sun, M. PEGylated carboxymethyl chitosan/calcium phosphate hybrid anionic nanoparticles mediated hTERT siRNA delivery for anticancer therapy. Biomaterials 2014, 35, 7978–7991. [Google Scholar] [CrossRef]
- Pallela, R.; Venkatesan, J.; Bhatnagar, I. Applications of marine collagen-based scaffolds in bone tissue engineering. In Marine biomaterials: Characterization, Isolation and Applications; Kim, S.K., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 519–528. [Google Scholar]
- Tong, Y.; Wang, S.; Xu, J.; Chua, B.; He, C. Synthesis of O, O′-dipalmitoyl chitosan and its amphiphilic properties and capability of cholesterol absorption. Carbohydr. Polym. 2005, 60, 229–233. [Google Scholar] [CrossRef]
- Desbrieres, J.; Martinez, C.; Rinaudo, M. Hydrophobic derivatives of chitosan: Characterization and rheological behaviour. Int. J. Biol. Macromol. 1996, 19, 21–28. [Google Scholar] [CrossRef]
- Sajomsang, W.; Tantayanon, S.; Tangpasuthadol, V.; Thatte, M. Daly, Synthesis and characterization of N-aryl chitosan derivatives. Int. J. Biol. Macromol. 2008, 43, 79–87. [Google Scholar] [CrossRef]
- Sajomsang, W. Synthetic methods and applications of chitosan containing pyridylmethyl moiety and its quaternized derivatives: A review. Carbohydr. Polym. 2010, 80, 631–647. [Google Scholar] [CrossRef]
- Philippova, O.E.; Korchagina, E.V. Chitosan and its hydrophobic derivatives: Preparation and aggregation in dilute aqueous solutions. Polym. Sci. A 2012, 54, 552–572. [Google Scholar] [CrossRef]
- Barbosa, M.; Vale, N.; Costa, F.M.; Martins, M.C.; Gomes, P. Tethering antimicrobial peptides onto chitosan: Optimization of azide-alkyne “click” reaction conditions. Carbohydr. Polym. 2017, 165, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Wang, H.; Wang, L. Physical properties and antibacterial activity of quaternized chitosan/carboxymethyl cellulose blend films. LWT–Food Sci. Technol. 2016, 65, 398–405. [Google Scholar] [CrossRef]
- Huo, M.; Zhang, Y.; Zhou, J.; Zou, A.; Yu, D.; Wu, Y.; Li, J.; Li, H. Synthesis and characterization of low-toxic amphiphilic chitosan derivatives and their application as micelle carrier for antitumor drug. Int. J. Pharm. 2010, 394, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, D.; Li, C.; Jia, L.; Liu, G.; Hao, L.; Zheng, D.; Shen, J.; Li, T.; Guo, Y.; et al. Self-assembled nanoparticles based on galactosylated O-carboxymethyl chitosan-graft-stearic acid conjugates for delivery of doxorubicin. Int. J. Pharm. 2013, 458, 31–38. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, H.; Zhang, X.; Sun, Z.; Wang, F.; Cheng, J.; Xie, H.; Yu, B.; Zhou, L. Deoxycholic acid–modified chitooligosaccharide/mPEG-PDLLA mixed micelles loaded with paclitaxel for enhanced antitumor efficacy. Int. J. Pharm. 2014, 475, 60–68. [Google Scholar] [CrossRef]
- Sonaje, K.; Lin, K.J.; Tseng, M.T.; Wey, S.P.; Su, F.Y.; Chuang, E.Y.; Hsu, C.W.; Chen, C.T.; Sung, H.W.V. Effects of chitosan-nanoparticle-mediated tight junction opening on the oral absorption of endotoxins. Biomaterials 2011, 32, 8712–8721. [Google Scholar] [CrossRef] [PubMed]
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Why is chitosan mucoadhesive. Biomacromolecules 2008, 9, 1837–1842. [Google Scholar] [CrossRef]
- Silva, D.S.; Almeida, A.; Prezotti, F.; Cury, B.; Campana-Filho, S.P.; Sarmento, B. Synthesis and characterization of 3, 6-O, O-dimyristoyl chitosan micelles for oral delivery of paclitaxel. Colloids Surf. B Biointerfaces 2017, 152, 220–228. [Google Scholar] [CrossRef]
- Du, Y.Z.; Wang, L.; Yuan, H.; Hu, F.Q. Linoleic acid-grafted chitosan oligosaccharide micelles for intracellular drug delivery and reverse drug resistance of tumor cells. Int. J. Biol. Macromol. 2011, 48, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Woraphatphadung, T.; Sajomsang, W.; Gonil, P.; Saesoo, P.; Opanasopit, P. Synthesis and characterization of pH-responsive N-naphthyl-N, O-succinyl chitosan micelles for oral meloxicam delivery. Carbohydr. Polym. 2015, 121, 99–106. [Google Scholar] [CrossRef]
- Akiyoshi, K.; Deguchi, S.; Moriguchi, N.; Yamaguchi, S.; Sunamoto, J. Self-aggregates of hydrophobized polysaccharides in water. Formation and characteristics of nanoparticles. Macromolecules 1993, 26, 3062–3068. [Google Scholar] [CrossRef]
- Akiyoshi, K.; Kobayash, S.; Shichibe, S.; Mix, D.; Baudys, M.; Kim, S.W.; Sunamoto, J. Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: Complexation and stabilization of insulin. J. Control Release 1998, 54, 313–320. [Google Scholar] [CrossRef]
- Akiyoshi, K.; Nishikawa, T.; Shichibe, S.; Junzo, S. Stabilization of insulin upon supramolecular complexation with hydrophobized polysaccharide nanoparticle. Chem. Lett. 1995, 24, 707–708. [Google Scholar] [CrossRef]
- Akiyoshi, K.; Yamaguchi, S.; Sunamoto, J. Self-aggregates of hydrophobic polysaccharide derivatives. Chem. Lett. 1991, 20, 1263–1266. [Google Scholar] [CrossRef]
- Morimoto, N.; Nomura, S.I.M.; Miyazawa, N.; Akiyoshi, K. Nanogel engineered designs for polymeric drug delivery. In Polymeric Drug Delivery II; Svenson, S., Ed.; ACS Symposium Series; ACS Publications: Washington, DC, USA, 2006; pp. 88–101. [Google Scholar]
- Zlotnikov, I.D.; Streltsov, D.A.; Ezhov, A.A.; Kudryashova, E.V. Smart pH- and Temperature-Sensitive Micelles Based on Chitosan Grafted with Fatty Acids to Increase the Efficiency and Selectivity of Doxorubicin and Its Adjuvant Regarding the Tumor Cells. Pharmaceutics 2023, 15, 1135. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.F.; Cui, F.; Yin, Y.M.; Cho, H.J.; Kim, D.D. Docetaxel-loaded chitosan-cholesterol conjugate-based self-assembled nanoparticles for overcoming multidrug resistance in cancer cells. Pharmaceutics 2020, 12, 783. [Google Scholar] [CrossRef]
- Zhu, H.; Cao, J.; Cui, S.; Qian, Z.; Gu, Y. Enhanced tumor targeting and antitumor efficacy via hydroxycamptothecin-encapsulated folate-modified N-succinyl-N′-octyl chitosan micelles. J. Pharm. Sci. 2013, 102, 1318–1332. [Google Scholar] [CrossRef]
- Xu, X.; Li, L.; Zhou, J.; Lu, S.; Yang, J.; Yin, X.; Ren, J. Preparation and characterization of N-succinyl-N′-octyl chitosan micelles as doxorubicin carriers for effective anti-tumor activity. Colloids Surf. B Biointerfaces 2007, 55, 222–228. [Google Scholar]
- Gothwal, A.; Lamptey, R.N.L.; Singh, J. Multifunctionalized cationic chitosan polymeric micelles polyplexed with pVGF for noninvasive delivery to the mouse brain through the intranasal route for developing therapeutics for Alzheimer’s Disease. Mol. Pharm. 2023, 20, 3009–3019. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liang, N.; Yan, P.; Kawashima, Y.; Cui, F.; Sun, S. A chitosan-based micellar system as nanocarrier for the delivery of paclitaxel. Polymers 2020, 12, 380. [Google Scholar] [CrossRef]
- Almeida, A.; Araújo, M.; Novoa-Carballal, R.; Andrade, F.; Gonçalves, H.; Reis, R.L.; Lúcio, M.; Schwartz, S., Jr.; Sarmento, B. Novel amphiphilic chitosan micelles as carriers for hydrophobic anticancer drugs. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110920. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, I.; Khalid, M.; Khan, I.U.; Shah, S.N.A.; Nazir, S.; Rehman, M.F.U.; Ranjha, N.M.; Ahmad, M. Conjugated Linoleic Acid–Carboxymethyl Chitosan Polymeric Micelles to Improve the Solubility and Oral Bioavailability of Paclitaxel. Pharmaceutics 2024, 16, 342. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, Q.; Wang, Z.; Mi, Y.; Dong, F.; Tan, W.; Guo, Z. Low molecular weight chitosan based GSH-responsive self-assembled cationic micelle with enhanced anti-tumor effect by combining oxidative damage and chemotherapy. Int. J. Biol. Macromol. 2024, 231, 131736. [Google Scholar] [CrossRef]
- Zegarra-Urquia, C.L.; Zhang, D.; Aguilar, M.R.; García-Fernández, L.; Zaragoza-Contreras, E.A. Development of Fatty Acid-Grafted Chitosan Micellar Systems for the Encapsulation of Hydrophobic Drugs. SSRN 2024, Preprint. Available online: https://ssrn.com/abstract=5601966 (accessed on 16 August 2025).
- Chen, Q.; Jiang, Y.; Yuan, L.; Liu, L.; Zhu, X.; Chen, R.; Wang, Z.; Wu, K.; Luo, H.; Ouyang, Q. Preparation, Characterization, and Antioxidant Properties of Self-Assembled Nanomicelles of Curcumin-Loaded Amphiphilic Modified Chitosan. Molecules 2024, 29, 2693. [Google Scholar] [CrossRef]
- Eren, M.C. Micellar Carrier Systems for Anticancer Drugs Using Natural Polymers. Ph.D. Thesis, Izmir Institute of Technology, Urla-Izmir, Turkey, 2024. [Google Scholar]
- Zeybek, N.; Polat, H.; Gulec, S.; Buyukkileci, A.O. Development of xylan-coated acid-resistant micellar drug carriers for colon-targeted oral delivery. Int. J. Polym. Mater. Polym. Biomater. 2024, 74, 474–483. [Google Scholar] [CrossRef]
- Eren, M.C.; Polat, H.; Polat, M. Investigating the Integrity of Drug-Loaded Chitosan Micelles in Body Fluids Due to Dilution and Protein Interactions. 2025, in preparation to be submitted.
- Manojlović, J.Ž. The Krafft Temperature of Surfactant Solutions. Therm. Sci. 2012, 16 (Suppl. S2), S631–S640. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, C.; Wu, X.; Tang, X.; Ping, Q. Preparation, physical properties, and stability of gambogic acid-loaded micelles based on chitosan derivatives. Drug Dev. Ind. Pharm. 2008, 34, 2–9. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).