The Pharmacological Treatment of Chronic Pain: From Guidelines to Daily Clinical Practice
Abstract
1. Introduction
1.1. NSAIDs
1.2. Opioids
1.3. Central-Nervous-System-Acting Drugs
1.4. Muscle Relaxants
2. Materials and Methods
3. International Guidelines
3.1. Neuropathic and Nociceptive Chronic Pain Treatment
3.2. Neuropathic Chronic Pain
3.3. Nociceptive Chronic Pain
3.4. Nociplastic Pain
4. From Guidelines to Daily Use: The Problem of Safety in Patients with Comorbidity
4.1. Kidney Diseases
4.2. Hepatic Failure
| Drug | Biliary Excretion | Effect in Patients with Hepatic Insufficiency |
|---|---|---|
| Acetaminophen | 1–10% [102] | Contraindicated in patients with severe hepatic insufficiency. Caution is needed in the cases of mild and moderate hepatic insufficiency [63]. |
| Oxycodone | Not clearly estimated but relevant; important hepatic metabolism [73,117] | Oxycodone is contraindicated in patients with moderate–severe hepatic impairment on the label. Some authors refer to the necessity to reduce dosage and prolong intervals [73]. |
| Buprenorphine | 70% [118] | Buprenorphine should be used with caution in cases of mild–moderate hepatic impairment and is contraindicated in severe forms [70]. |
| Fentanyl | Little biliary excretion but strong hepatic metabolism [119] | Concerning fentanyl, dose reduction may be necessary [74]. |
| Hydromorphone | 1% in feces [107] | Hydromorphone is contraindicated in patients with severe hepatic impairment, whereas dose reduction is suggested in those with moderate impairment, and caution is needed in those with mild impairment. It should be avoided in patients with hepatorenal syndrome [108]. |
| Methadone | 10–45% of the metabolite [101] | Methadone is contraindicated in patients with severe hepatic impairment. Caution (lower doses and prolonged intervals between administration) is needed in those with mild–moderate illness, even if other authors describe no dose adjustments [66,104,114]. |
| Tapentadol | 1% [120] | Tapentadol is contraindicated in patients with severe hepatic impairment. No dose adjustments are required in those with mild hepatic impairment, whereas low doses and prolonged dosing intervals are recommended in patients with moderate illness [75]. |
| Tramadol | 10% [121] | Tramadol is not recommended in patients with severe hepatic impairment, and some authors suggest the prolongation of dosing intervals or dose reduction in those with mild–moderate forms [72,114]. American label suggests that the recommended dose for adult patients with severe hepatic impairment is 50 mg every 12 h [109]. |
| Hydrocodone | Low biliary excretion, data not available; relevant hepatic metabolism [71] | Dose reduction [77]. |
| Morphine and codeine | 5–10% morphine in feces [67]; similar percentages for codeine, which is then converted into morphine [106] | Morphine is contraindicated in patients with severe hepatic impairment, and dosage should be reduced by 25% in those with moderate hepatic impairment [67]. Other authors suggest dose reduction and prolongation of dose intervals for oral formulation and dose reduction only for intravenous formulation. It should be avoided in hepatorenal syndrome [114]. Codeine use is not recommended for the possible lack of analgesic effect [114]. |
| Duloxetine | 20% [110] | Duloxetine must not be used in patients with hepatopathy and alterations in hepatic function. Moreover, this drug is hepatotoxic [41,114]. |
| Amitriptyline | Small quantity [31] | Amitriptyline is contraindicated in liver diseases [31]. Other authors suggest its use with caution, even if it is worse-tolerated than nortriptyline and desipramine [114]. |
| Lidocaine 5% patch | Minor quote | Severe hepatic impairment: caution [112]. |
| Tizanidine | 20% [122] | Tizanidine is generally contraindicated in patients with relevant hepatic compromise. It should be used only if the benefit outweighs the risk [46]. |
| Baclofen | 25% [123] | Baclofen is not metabolized by liver, but it is hepatotoxic: caution is needed [44]. |
| Thiocolchicoside | 80% [48] | It may increase liver enzymes or cause hepatic damage [48]. |
| Cyclobenzaprine | Minor quote | It may increase liver enzymes or cause hepatic damage [45]. |
| Eperisone | 24.4% [47] | Eperisone is contraindicated in patients with severe hepatic failure, and caution/dose adjustment may be needed in other forms (maximum 150 mg daily dose) [47]. |
| Pregabalin | None | No dose adjustment [19,30]. |
| Gabapentin | None | No dose adjustment [19,30]. |
4.3. Hypertension
4.4. Bone Fracture
4.5. Cardiovascular Toxicity
4.6. Vertigo
4.7. Respiratory Diseases
4.8. Gastrointestinal Diseases
4.9. Sexual Dysfunctions
4.10. Urinary Symptoms
4.11. Other Clinical Conditions
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised IASP definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Chronic Pain (Primary and Secondary) in over 16s: Assessment of All Chronic Pain and Management of Chronic Primary Pain; National Institute for Health and Care Excellence (NICE): London, UK, 2021. [Google Scholar]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Series Chronic Pain 1 Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Fitzcharles, M.-A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Häuser, W. Nociplastic pain: Towards an understanding of prevalent pain conditions. Lancet 2021, 397, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Gallelli, L.; Galasso, O.; Falcone, D.; Southworth, S.; Greco, M.; Ventura, V.; Romualdi, P.; Corigliano, A.; Terracciano, R.; Savino, R.; et al. The effects of nonsteroidal anti-inflammatory drugs on clinical outcomes, synovial fluid cytokine concentration and signal transduction pathways in knee osteoarthritis. A randomized open label trial. Osteoarthr. Cartil. 2013, 21, 1400–1408. [Google Scholar] [CrossRef]
- Gallelli, L.; Avenoso, T.; Falcone, D.; Palleria, C.; Peltrone, F.; Esposito, M.; De Sarro, G.; Carotenuto, M.; Guidetti, V. Effects of acetaminophen and ibuprofen in children with migraine receiving preventive treatment with magnesium. Headache 2014, 54, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Gallelli, L.; Colosimo, M.; Pirritano, D.; Ferraro, M.; De Fazio, S.; Marigliano, N.M.; De Sarro, G. Retrospective evaluation of adverse drug reactions induced by nonsteroidal anti-inflammatory drugs. Clin. Drug Investig. 2007, 27, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Cryer, B.; Feldman, M. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am. J. Med. 1998, 104, 413–421. [Google Scholar] [CrossRef]
- Walker, C. Are All Oral COX-2 Selective Inhibitors the Same? A Consideration of Celecoxib, Etoricoxib, and Diclofenac. Int. J. Rheumatol. 2018, 2018, 1302835. [Google Scholar] [CrossRef]
- Machado, G.C.; Maher, C.G.; Ferreira, P.H.; Pinheiro, M.B.; Lin, C.W.C.; Day, R.O.; McLachlan, A.J.; Ferreira, M.L. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: Systematic review and meta-analysis of randomised placebo controlled trials. BMJ 2015, 350, h1225. [Google Scholar] [CrossRef]
- Drewes, A.M.; Jensen, R.D.; Nielsen, L.M.; Droney, J.; Christrup, L.L.; Arendt-Nielsen, L.; Riley, J.; Dahan, A. Differences between opioids: Pharmacological, experimental, clinical and economical perspectives. Br. J. Clin. Pharmacol. 2013, 75, 60–78. [Google Scholar] [CrossRef]
- SIGN Scottish Intercollegiate Guidelines Network. Management of Chronic Pain. Available online: http://www.sign.ac.uk/pdf/SIGN136.pdf (accessed on 17 July 2022).
- Tesfaye, S.; Sloan, G.; Petrie, J.; White, D.; Bradburn, M.; Julious, S.; Rajbhandari, S.; Sharma, S.; Rayman, G.; Gouni, R.; et al. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): A multicentre, double-blind, randomise. Lancet 2022, 400, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpaa, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: Systematic review, meta-analysis and updated NeuPSig recommendations. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C. Augmentation of venlafaxine with bupropion: Risks associated with a triple monoamine reuptake inhibition approach to partially responsive depression. J. Clin. Psychiatry 2013, 74, 119–121. [Google Scholar] [CrossRef]
- Mathieson, S.; Lin, C.W.C.; Underwood, M.; Eldabe, S. Pregabalin and gabapentin for pain. BMJ 2020, 369, m1315. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.J.; Weltman, M.; Dember, L.M.; Liebschutz, J.; Jhamb, M. Pain management in patients with chronic kidney disease and end-stage kidney disease. Curr. Opin. Nephrol. Hypertens. 2020, 29, 671–680. [Google Scholar] [CrossRef]
- Robertson, K.; Marshman, L.A.G.; Plummer, D.; Downs, E. Effect of Gabapentin vs Pregabalin on Pain Intensity in Adults with Chronic Sciatica: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 28–34. [Google Scholar] [CrossRef]
- AIFA Agenzia Italiana del Farmaco. Riassunto delle Caratteristiche del Prodotto-Gabapentin. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_001771_038547_RCP.pdf&sys=m0b1l3 (accessed on 24 July 2022).
- AIFA Agenzia Italiana del Farmaco. Riassunto delle Caratteristiche del Prodotto-Pregabalin. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_003891_043719_RCP.pdf&sys=m0b1l3 (accessed on 24 July 2022).
- Di Stefano, G.; La Cesa, S.; Truini, A.; Cruccu, G. Natural history and outcome of 200 outpatients with classical trigeminal neuralgia treated with carbamazepine or oxcarbazepine in a tertiary centre for neuropathic pain. J. Headache Pain 2014, 15, 34. [Google Scholar] [CrossRef]
- Schmitz, B.; Dimova, S.; Zhang, Y.; Chellun, D.; De Backer, M.; Gasalla, T. Tolerability and efficacy of lacosamide and controlled-release carbamazepine monotherapy in patients with newly diagnosed epilepsy and concomitant psychiatric conditions: Post hoc analysis of a prospective, randomized, double-blind trial. Epilepsy Res. 2020, 159, 106220. [Google Scholar] [CrossRef]
- AIFA Agenzia Italiana del Farmaco. Riassunto delle Caratteristiche del Prodotto-Carbamazepina. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_001561_033878_RCP.pdf&sys=m0b1l3 (accessed on 24 July 2022).
- Derry, S.; Asc, R.; Cole, P.; Tan, T.; Ra, M.; Derry, S.; Asc, R.; Cole, P.; Tan, T.; Ra, M. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 1, CD007393. [Google Scholar] [CrossRef]
- Derry, S.; Wiffen, P.J.; Moore, R.A.; Quinlan, J. Topical lidocaine for neuropathic pain in adults. Cochrane Database Syst. Rev. 2014, 2014, CD010958. [Google Scholar] [CrossRef]
- Gilron, I.; Chaparro, L.E.; Tu, D.; Holden, R.R.; Milev, R.; Towheed, T.; Dumerton-Shore, D.; Walker, S. Combination of pregabalin with duloxetine for fibromyalgia: A randomized controlled trial. Pain 2016, 157, 1532–1540. [Google Scholar] [CrossRef]
- Hur, G.; Hwang, E.K.; Moon, J.; Ye, Y.; Shim, J.; Park, H.; Kang, K. Oral Muscle Relaxant May Induce Immediate Allergic Reactions. Yonsei Med. J. 2012, 53, 863–865. [Google Scholar] [CrossRef]
- Kaye, A.D.; Jones, M.R.; Viswanath, O.; Candido, K.D.; Boswell, M.V.; Soin, A.; Sanapati, M.; Harned, M.E.; Simopoulos, T.T.; Sudhir Diwan, S.L.; et al. ASIPP Guidelines for Sedation and Fasting Status of Patients Undergoing Interventional Pain Management Procedures. Available online: https://www.painphysicianjournal.com/linkout?issn=&vol=22&page=201 (accessed on 10 December 2022).
- McDonagh, M.S.; Selph, S.S.; Buckley, D.I.; Holmes, R.S.; Mauer, K.; Ramirez, S.; Hsu, F.C.; Dana, T.; Fu, R.; Chou, R. Nonopioid Pharmacologic Treatments for Chronic Pain; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2020.
- AIFA Agenzia Italiana del Farmaco. Riassunto delle Caratteristiche del Prodotto-Lyrica. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_003199_043740_RCP.pdf&sys=m0b1l3 (accessed on 13 August 2022).
- AIFA Agenzia Italiana del Farmaco. Riassunto delle Caratteristiche del Prodotto-Amitriptilina. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_007046_019906_RCP.pdf&sys=m0b1l3 (accessed on 25 July 2022).
- Dorian, P.; Sellers, E.M.; Reed, K.L.; Warsh, J.J.; Hamilton, C.; Kaplan, H.L.; Fan, T. Amitriptyline and ethanol: Pharmacokinetic and pharmacodynamic interaction. Eur. J. Clin. Pharmacol. 1983, 25, 325–331. [Google Scholar] [CrossRef]
- Kopera, H. Anticholinergic and blood pressure effects of mianserin, amitriptyline and placebo. Br. J. Clin. Pharmacol. 1978, 5, 29S–34S. [Google Scholar] [PubMed]
- Kamińska, K.; Lenda, T.; Konieczny, J.; Wardas, J.; Lorenc-Koci, E. Interactions of the tricyclic antidepressant drug amitriptyline with L-DOPA in the striatum and substantia nigra of unilaterally 6-OHDA-lesioned rats. Relevance to motor dysfunction in Parkinson’s disease. Neurochem. Int. 2018, 121, 125–139. [Google Scholar] [CrossRef]
- Richelson, E. Tricyclic antidepressants and histamine H1 receptors. Mayo Clin. Proc. 1979, 54, 669–674. [Google Scholar] [PubMed]
- Farzam, K.; Tivakaran, V.S. QT Prolonging Drugs. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534864/ (accessed on 14 August 2022).
- Shah, A.; Yousuf, T.; Ziffra, J.; Zaidi, A.; Raghuvir, R. Diphenhydramine and QT prolongation—A rare cardiac side effect of a drug used in common practice. J. Cardiol. Cases 2015, 12, 126–129. [Google Scholar] [CrossRef]
- Francescangeli, J.; Karamchandani, K.; Powell, M.; Bonavia, A. The serotonin syndrome: From molecular mechanisms to clinical practice. Int. J. Mol. Sci. 2019, 20, 2288. [Google Scholar] [CrossRef] [PubMed]
- AIFA Agenzia Italiana del Farmaco. Medrol-Riassunto delle Caratteristiche del Prodotto. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000040_014159_RCP.pdf&sys=m0b1l3 (accessed on 14 August 2022).
- AIFA Agenzia Italiana del Farmaco. Cymbalta-Riassunto delle Caratteristiche del Prodotto. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_001230_036683_RCP.pdf&sys=m0b1l3 (accessed on 14 August 2022).
- AIFA Agenzia Italiana del Farmaco. Riassunto delle Caratteristiche del Prodotto-Duloxetina. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000813_043843_RCP.pdf&sys=m0b1l3 (accessed on 25 July 2022).
- Park, K.; Kim, S.; Ko, Y.J.; Park, B.J. Duloxetine and cardiovascular adverse events: A systematic review and meta-analysis. J. Psychiatr. Res. 2020, 124, 109–114. [Google Scholar] [CrossRef]
- Bixby, A.L.; VandenBerg, A.; Bostwick, J.R. Clinical Management of Bleeding Risk With Antidepressants. Ann. Pharmacother. 2019, 53, 186–194. [Google Scholar] [CrossRef]
- AIFA Agenzia Italiana del Farmaco. Riassunto delle Caratteristiche del Prodotto-Baclofen. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_002322_037930_RCP.pdf&sys=m0b1l3 (accessed on 14 August 2022).
- AIFA Agenzia Italiana del Farmaco. Riassunto delle Caratteristiche del Prodotto-Flexiban. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000143_025327_RCP.pdf&sys=m0b1l3 (accessed on 14 August 2022).
- AIFA Agenzia Italiana del Farmaco. Riassunto delle Caratteristiche del Prodotto-Navizan. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000239_039422_RCP.pdf&retry=0&sys=m0b1l3 (accessed on 14 August 2022).
- AIFA Agenzia Italiana del Farmaco. Expose-Riassunto delle Caratteristiche del Prodotto. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_004375_028631_RCP.pdf&sys=m0b1l3#:~:text=L’eperisonecloridratoèun,deldoloreadessaassociato (accessed on 13 August 2022).
- AIFA Agenzia Italiana del Farmaco. MuscoRil- Riassunto delle caratteristiche del prodotto. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_008055_015896_RCP.pdf&sys=m0b1l3 (accessed on 13 August 2022).
- Colorado Division of Workers’ Compensation. Chronic Pain Disorder Medical Treatment Guideline; Colorado Division of Workers’ Compensation: Denver, CO, USA, 2017; pp. 1–178.
- NIH National Institutes of Health. Metaxalone-Label. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b3a4f6bc-abd4-4b8e-970f-59b3aa6f17a0 (accessed on 14 August 2022).
- NIH National Institutes of Health. Methocarbamol-Label. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=42c0a177-7d62-4bcf-9fce-7dd484cda4d5 (accessed on 14 August 2022).
- Samer, C.F.; Lorenzini, K.I.; Rollason, V.; Daali, Y.; Desmeules, J.A. Applications of CYP450 testing in the clinical setting. Mol. Diagnosis Ther. 2013, 17, 165–184. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.C.; Sajuthi, S.; Deford, P.; Christenson, S.; Rios, C.L.; Montgomery, M.T.; Woodruff, P.G.; Mauger, D.T.; Erzurum, S.C.; Johansson, M.W.; et al. COVID-19–related Genes in Sputum Cells in Asthma. Relationship to Demographic Features and Corticosteroids. Am. J. Respir. Crit. Care Med. 2020, 202, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Awa, K.; Satoh, H.; Hori, S.; Sawada, Y. Prediction of time-dependent interaction of aspirin with ibuprofen using a pharmacokinetic/pharmacodynamic model. J. Clin. Pharm. Ther. 2012, 37, 469–474. [Google Scholar] [CrossRef]
- Di Mizio, G.; Marcianò, G.; Palleria, C.; Muraca, L.; Rania, V.; Roberti, R.; Spaziano, G.; Piscopo, A.; Ciconte, V.; Di Nunno, N.; et al. Drug—Drug Interactions in Vestibular Diseases, Clinical Problems, and Medico-Legal Implications. Int. J. Environ. Res. Public Health 2021, 18, 12936. [Google Scholar] [CrossRef]
- Webster, J. Interactions of NSAIDs with Diuretics and β-Blockers: Mechanisms and Clinical Implications. Drugs 1985, 30, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Chiarella, G.; Marcianò, G.; Viola, P.; Palleria, C.; Pisani, D.; Rania, V.; Casarella, A.; Astorina, A.; Scarpa, A.; Esposito, M.; et al. Nutraceuticals for Peripheral Vestibular Pathology: Properties, Usefulness, Future Perspectives and Medico-Legal Aspects. Nutrients 2021, 13, 3646. [Google Scholar] [CrossRef]
- Moore, N.; Pollack, C.; Butkerait, P. Adverse drug reactions and drug-drug interactions with over-the-counter NSAIDs. Ther Clin Risk Manag. 2015, 11, 1061–1075. [Google Scholar] [CrossRef]
- Kalhor, H.R.; Taghikhani, E. Probe into the Molecular Mechanism of Ibuprofen Interaction with Warfarin Bound to Human Serum Albumin in Comparison to Ascorbic and Salicylic Acids: Allosteric Inhibition of Anticoagulant Release. J. Chem. Inf. Model. 2021, 61, 4045–4057. [Google Scholar] [CrossRef]
- Brouwers, J.R.B.J.; de Smet, P.A.G.M. Pharmacokinetic-Pharmacodynamic Drug Interactions with Nonsteroidal Anti-Inflammatory Drugs. Clin. Pharmacokinet. 1994, 27, 462–485. [Google Scholar] [CrossRef]
- Hersh, E.V.; Pinto, A.; Moore, P.A. Adverse drug interactions involving common prescription and over-the-counter analgesic agents. Clin. Ther. 2007, 29, 2477–2497. [Google Scholar] [CrossRef]
- Radwan, M.A. Zidovudine, Diclofenac and Ketoprofen Pharmacokinetic Interactions in Rats. J. Pharm. Pharmacol. 2010, 52, 665–669. [Google Scholar] [CrossRef] [PubMed]
- AIFA. Agenzia Italiana del Farmaco Riassunto delle Caratteristiche del Prodotto-Tachipirina; AIFA: Rome, Italy, 2022.
- Dordoni, B.; Willson, R.A.; Thompson, R.P.H.; Williams, R. Reduction of Absorption of Paracetamol by Activated Charcoal and Cholestyramine: A Possible Therapeutic Measure. Br. Med. J. 1973, 3, 86–87. [Google Scholar] [CrossRef] [PubMed]
- AIFA Agenzia Italiana del Farmaco. Paracetamolo e Codeina-Riassunto delle Caratteristiche del Prodotto. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_002753_037351_RCP.pdf&sys=m0b1l3 (accessed on 16 August 2022).
- AIFA Agenzia Italiana del Farmaco. Riassunto delle Caratteristiche del Prodotto-Metadone. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000549_029610_RCP.pdf&sys=m0b1l3 (accessed on 16 August 2022).
- AIFA Agenzia Italiana del Farmaco. Riassunto delle Caratteristiche del Prodotto-Morfina Cloridrato. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000141_030677_RCP.pdf&sys=m0b1l3 (accessed on 16 August 2022).
- Inturrisi, C.E.; Jamison, R.N. Clinical pharmacology of opioids for pain. Clin. J. Pain 2002, 18, 3–13. [Google Scholar] [CrossRef]
- Overholser, B.R.; Foster, D.R. Opioid pharmacokinetic drug-drug interactions. Am. J. Manag. Care 2011, 17, 276–287. [Google Scholar]
- AIFA Agenzia Italiana del Farmaco. Buprenorfina-Riassunto delle Caratteristiche del Prodotto. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_002322_039747_RCP.pdf&retry=0&sys=m0b1l3 (accessed on 16 August 2022).
- Cardia, L.; Calapai, G.; Quattrone, D.; Mondello, C.; Arcoraci, V.; Calapai, F.; Mannucci, C.; Mondello, E. Preclinical and clinical pharmacology of hydrocodone for chronic pain: A mini review. Front. Pharmacol. 2018, 9, 1122. [Google Scholar] [CrossRef]
- AIFA Agenzia Italiana del Farmaco. Riassunto delle Caratteristiche del Prodotto-Contramal. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000045_028853_RCP.pdf&sys=m0b1l3 (accessed on 16 August 2022).
- AIFA Agenzia Italiana del Farmaco. Riassunto delle Caratteristiche del Prodotto-Ossicodone. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000549_043927_RCP.pdf&sys=m0b1l3 (accessed on 16 August 2022).
- AIFA Agenzia Italiana del Farmaco. Fentanyl-Riassunto delle Caratteristiche del Prodotto. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_002838_035693_RCP.pdf&sys=m0b1l3 (accessed on 14 July 2022).
- AIFA Agenzia Italiana del Farmaco. Riassunto delle Caratteristiche del Prodotto-Palexia. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_003822_037148_RCP.pdf&sys=m0b1l3 (accessed on 16 August 2022).
- Baldo, B.A. Toxicities of opioid analgesics: Respiratory depression, histamine release, hemodynamic changes, hypersensitivity, serotonin toxicity. Arch. Toxicol. 2021, 95, 2627–2642. [Google Scholar] [CrossRef]
- NIH National Institutes of Health. Hydrocodone and Acetaminophen-Label. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4f505b2a-45a2-4d34-96f6-dedb574cb508 (accessed on 16 August 2022).
- NIH National Institutes of Health. Buprenorphine-Label. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=17ad1a5b-e89f-d5a3-d15b-2eb48bcded7d#Section_7 (accessed on 16 August 2022).
- Dickenson, A.H.; Kress, H.G. Tapentadol: A new option for the treatment of cancer and noncancer pains. J. Pain Res. 2019, 12, 1509–1511. [Google Scholar] [CrossRef]
- Sabbe, J.R.; Sims, P.J.; Sims, M.H. Tramadol-warfarin interaction. Pharmacotherapy 1998, 18, 871–873. [Google Scholar]
- Stevens, A.J.; Woodman, R.J.; Owen, H. The effect of ondansetron on the efficacy of postoperative tramadol: A systematic review and meta-Analysis of a drug interaction. Anaesthesia 2015, 70, 209–218. [Google Scholar] [CrossRef]
- American Society of Anesthesiologists Task Force on Chronic Pain Management; American Society of Regional Anesthesia and Pain Medicine- Practice guidelines for chronic pain management. Anesthesiology 2010, 112, 810–833. [CrossRef]
- National Institute for Health and Care Excellence. Neuropathic Pain in Adults: Pharmacological Management in Non-Specialist Settings; National Institute for Health and Care Excellence (NICE): London, UK, 2013; pp. 1–36. [Google Scholar]
- HCANJ Pain Management Guideline. Available online: https://www.hcanj.org/files/2013/09/Pain-Management-Guidelines-_HCANJ-May-12-final.pdf (accessed on 17 July 2022).
- Pullano, S.A.; Marcianò, G.; Bianco, M.G.; Oliva, G.; Rania, V.; Vocca, C.; Cione, E.; De Sarro, G.; Gallelli, L.; Romeo, P.; et al. FT-IR Analysis of Structural Changes in Ketoprofen Lysine Salt and KiOil Caused by a Pulsed Magnetic Field. Bioengineering 2022, 9, 503. [Google Scholar] [CrossRef]
- Muraca, L.; Scuteri, A.; Burdino, E.; Marcianò, G.; Rania, V.; Catarisano, L.; Casarella, A.; Cione, E.; Palleria, C.; Colosimo, M.; et al. Effectiveness and Safety of a New Nutrient Fixed Combination Containing Pollen Extract plus Teupolioside, in the Management of LUTS in Patients with Benign Prostatic Hypertrophy: A Pilot Study. Life 2022, 12, 965. [Google Scholar] [CrossRef] [PubMed]
- Marcianò, G.; Roberti, R.; Palleria, C.; Mirra, D.; Rania, V.; Casarella, A.; De Sarro, G.; Gallelli, L. SARS-CoV-2 Treatment: Current Therapeutic Options and the Pursuit of Tailored Therapy. Appl. Sci. 2021, 11, 7457. [Google Scholar] [CrossRef]
- Marcianò, G.; Palleria, C.; Casarella, A.; Rania, V.; Basile, E.; Catarisano, L.; Vocca, C.; Bianco, L.; Pelaia, C.; Cione, E.; et al. Effect of Statins on Lung Cancer Molecular Pathways: A Possible Therapeutic Role. Pharmaceuticals 2022, 15, 589. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bauer, B.A.; Wahner-Roedler, D.L.; Chon, T.Y.; Xiao, L. The modified WHO analgesic ladder: Is it appropriate for chronic non-cancer pain? J. Pain Res. 2020, 13, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm. Rep. 2016, 65, 1–49. [Google Scholar] [CrossRef]
- American Geriatric Society. Pharmacological Management of Persistent Pain in Older Persons. J. Am. Geriatr. Soc. 2009, 57, 1331–1346. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Pain Management Best Practices Inter-Agency Task Force Report: Updates, Gaps, Inconsistencies, and Recommendations; U.S. Department of Health & Human Services: Washington, DC, USA, 2019; p. 116.
- Kucharz, E.J.; Szántó, S.; Ivanova Goycheva, M.; Petronijević, M.; Šimnovec, K.; Domżalski, M.; Gallelli, L.; Kamenov, Z.; Konstantynowicz, J.; Radunović, G.; et al. Endorsement by Central European experts of the revised ESCEO algorithm for the management of knee osteoarthritis. Rheumatol. Int. 2019, 39, 1117–1123. [Google Scholar] [CrossRef]
- Rosenquist, R.W.; Benzon, H.T.; Connis, R.T.; De Leon-Casasola, O.A.; Glass, D.D.; Korevaar, W.C.; Mekhail, N.A.; Merrill, D.G.; Nickinovich, D.G.; Rathmell, J.P.; et al. American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine Practice guidelines for chronic pain management. Anesthesiology 2010, 112, 995–1004. [Google Scholar] [CrossRef]
- Chou, R.; Gordon, D.B.; De Leon-Casasola, O.A.; Rosenberg, J.M.; Bickler, S.; Brennan, T.; Carter, T.; Cassidy, C.L.; Chittenden, E.H.; Degenhardt, E.; et al. Management of postoperative pain: A clinical practice guideline from the American pain society, the American society of regional anesthesia and pain medicine, and the American society of anesthesiologists’ committee on regional anesthesia, executive commi. J. Pain 2016, 17, 131–157. [Google Scholar] [CrossRef]
- Xia, W.S.; Peng, Y.N.; Tang, L.H.; Jiang, L.S.; Yu, L.N.; Zhou, X.L.; Zhang, F.J.; Yan, M. Spinal ephrinB/EphB signalling contributed to remifentanil-induced hyperalgesia via NMDA receptor. Eur. J. Pain 2014, 18, 1231–1239. [Google Scholar] [CrossRef]
- Rosen, I.M.; Aurora, R.N.; Kirsch, D.B.; Carden, K.A.; Malhotra, R.K.; Ramar, K.; Abbasi-Feinberg, F.; Kristo, D.A.; Martin, J.L.; Olson, E.J.; et al. Chronic opioid therapy and sleep: An American academy of sleep medicine position statement. J. Clin. Sleep Med. 2019, 15, 1671–1673. [Google Scholar] [CrossRef]
- Palareti, G.; Legnani, C.; Cosmi, B.; Antonucci, E.; Erba, N.; Poli, D.; Testa, S.; Tosetto, A. Comparison between different D-Dimer cutoff values to assess the individual risk of recurrent venous thromboembolism: Analysis of results obtained in the DULCIS study. Int. J. Lab. Hematol. 2016, 38, 42–49. [Google Scholar] [CrossRef]
- Axelrod, D.J.; Reville, B. Using methadone to treat opioid-induced hyperalgesia and refractory pain. J. Opioid Manag. 2007, 3, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Davison, S.N. Clinical pharmacology considerations in pain management in patients with advanced kidney failure. Clin. J. Am. Soc. Nephrol. 2019, 14, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Dean, M. Opioids in renal failure and dialysis patients. J. Pain Symptom Manage. 2004, 28, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Siegers, C.P.; Loeser, W.; Gieselmann, J.; Oltmanns, D. Biliary and renal excretion of paracetamol in man. Pharmacology 1984, 29, 301–303. [Google Scholar] [CrossRef]
- Kacinko, S.L.; Jones, H.E.; Johnson, R.E.; Choo, R.E.; Concheiro-Guisan, M.; Huestis, M.A. Urinary excretion of buprenorphine, norbuprenorphine, buprenorphine-glucuronide, and norbuprenorphine-glucuronide in pregnant women receiving buprenorphine maintenance treatment. Clin. Chem. 2009, 55, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- NIH National Institutes of Health. Methadone-Label. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=802ab399-479b-4271-a2a7-07aadde91cff (accessed on 17 August 2022).
- Hoskin, P.J.; Hanks, G.W. Morphine: Pharmacokinetics and clinical practice. Br. J. Cancer 1990, 62, 705–707. [Google Scholar] [CrossRef]
- NIH National Institutes of Health. Acetaminophen and Codeine. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=97b0ee29-d08e-4d9b-82ed-3ce287dbec28 (accessed on 17 September 2022).
- Abi-aad, K.R.; Derian, A. Hydromorphone. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470393/ (accessed on 17 September 2022).
- AIFA Agenzia Italiana del Farmaco. Riassunto delle Caratteristiche del Prodotto-Jurnista. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_001445_037396_RCP.pdf&sys=m0b1l3 (accessed on 16 August 2022).
- Dhesi, M.; Maldonado, K.A.; Maani, C.V. Tramadol. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- NIH National Institutes of Health. Duloxetine. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2dde979d-b6f8-41d1-96fb-325c75ea3a74#section-12.3 (accessed on 17 September 2022).
- Nagler, E.V.; Webster, A.C.; Vanholder, R.; Zoccali, C. Antidepressants for depression in stage 3–5 chronic kidney disease: A systematic review of pharmacokinetics, ef fi cacy and safety with recommendations by European Renal Best Practice (ERBP)*. Nephrol. Dial. Transpl. 2012, 27, 3736–3745. [Google Scholar] [CrossRef]
- AIFA Agenzia Italiana del Farmaco. Riassunto delle Caratteristiche del Prodotto-Lidocaina. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000045_040335_RCP.pdf&sys=m0b1l3#:~:text=Lidocainacontenutaneicerottidi,quindilariduzionedeldolore (accessed on 17 August 2022).
- Raouf, M.; Atkinson, T.J.; Crumb, M.W.; Fudin, J. Rational dosing of gabapentin and pregabalin in chronic kidney disease. J. Pain Res. 2017, 10, 275–278. [Google Scholar] [CrossRef]
- Dastis, S.N.; Rahier, J.; Lerut, J.; Geubel, A.P. Liver transplantation for nonsteroidal anti-inflammatory drug-induced liver failure: Nimesulide as the first implicated compound. Eur. J. Gastroenterol. Hepatol. 2007, 19, 919–922. [Google Scholar] [CrossRef]
- AIFA Agenzia Italiana del Farmaco Aspirina. Riassunto delle Caratteristiche del Prodotto; AIFA: Rome, Italy, 2021.
- Björnsson, E.S. Hepatotoxicity by drugs: The most common implicated agents. Int. J. Mol. Sci. 2016, 17, 224. [Google Scholar] [CrossRef] [PubMed]
- Huddart, R.; Clarke, M.; Altman, R.B.; Klein, T.E. PharmGKB summary: Oxycodone pathway, pharmacokinetics. Pharm. Genom. 2018, 28, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Ling, W. Buprenorphine for opioid dependence. Expert Rev. Neurother. 2009, 9, 609–616. [Google Scholar] [CrossRef] [PubMed]
- EMA European Medicines Agenecy Effentora-Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/effentora-epar-product-information_en.pdf (accessed on 16 September 2022).
- Fidman, B.; Nogid, A. Role of tapentadol immediate release (Nucynta) in the management of moderate-to-severe pain. Pharm. Ther. 2010, 35, 330–334. [Google Scholar]
- Faria, J.; Barbosa, J.; Moreira, R.; Queirós, O.; Carvalho, F.; Dinis-Oliveira, R.J. Comparative pharmacology and toxicology of tramadol and tapentadol. Eur. J. Pain 2018, 22, 827–844. [Google Scholar] [CrossRef]
- NIH National Institutes of Health. Tizanidine. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7f39499a-98cf-4d13-9b07-ccb5c22e43c8 (accessed on 17 September 2022).
- Ghanavatian, S.; Derian, A. Baclofen. Available online: https://www.ncbi.nlm.nih.gov/books/NBK526037/ (accessed on 17 September 2022).
- Szeto, C.C.; Sugano, K.; Wang, J.G.; Fujimoto, K.; Whittle, S.; Modi, G.K.; Chen, C.H.; Park, J.B.; Tam, L.S.; Vareesangthip, K.; et al. Non-steroidal anti-inflammatory drug (NSAID) therapy in patients with hypertension, cardiovascular, renal or gastrointestinal comorbidities: Joint APAGE/APLAR/APSDE/APSH/APSN/PoA recommendations. Gut 2020, 69, 617–629. [Google Scholar] [CrossRef]
- Ping, F.; Wang, Y.; Wang, J.; Chen, J.; Zhang, W.; Zhi, H.; Liu, Y. Opioids increase hip fracture risk: A meta-analysis. J. Bone Miner. Metab. 2017, 35, 289–297. [Google Scholar] [CrossRef]
- George, M.D.; Baker, J.F.; Leonard, C.E.; Mehta, S.; Miano, T.A.; Hennessy, S. Risk of Nonunion with Nonselective NSAIDs, COX-2 Inhibitors, and Opioids. J. Bone Jt. Surg.—Am. Vol. 2020, 102, 1230–1238. [Google Scholar] [CrossRef]
- Emeny, R.T.; Chang, C.H.; Skinner, J.; O’Malley, A.J.; Smith, J.; Chakraborti, G.; Rosen, C.J.; Morden, N.E. Association of Receiving Multiple, Concurrent Fracture-Associated Drugs with Hip Fracture Risk. JAMA Netw. Open 2019, 2, e1915348. [Google Scholar] [CrossRef]
- Li, L.; Setoguchi, S.; Cabral, H.; Jick, S. Opioid use for noncancer pain and risk of myocardial infarction amongst adults. J. Intern. Med. 2013, 273, 511–526. [Google Scholar] [CrossRef]
- Dam, V.C.J.; Schrier, V.D.R.; Velzen, V.M.; Lemmen, V.M.; Simons, P.; Kuijpers, K.W.K.; Jansen, S.; Kowal, M.A.; Olofsen, E.; Kramers, C.; et al. Inhaled Δ9-tetrahydrocannabinol does not enhance oxycodone- induced respiratory depression: Randomised controlled trial in healthy volunteers. Br. J. Anaesth. 2023, 130, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Lakkad, M.; Martin, B.; Li, C.; Harrington, S.; Dayer, L.; Painter, J.T. The use of gabapentinoids and opioids and risk of developing opioid-induced respiratory depression among older breast cancer survivors with neuropathic pain. J. Cancer Surviv. 2023. [Google Scholar] [CrossRef] [PubMed]
- Jungquist, C.R.; Flannery, M.; Perlis, M.L.; Grace, J.T. Original Article Relationship of Chronic Pain and Opioid Use with Respiratory Disturbance during Sleep. Pain Manag. Nurs. 2012, 13, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L. COX-2: A pivotal enzyme in mucosal protection and resolution of inflammation. Sci. World J. 2006, 6, 577–588. [Google Scholar] [CrossRef]
- Farmer, A.D.; Holt, C.B.; Downes, T.J.; Ruggeri, E.; Del Vecchio, S.; De Giorgio, R. Pathophysiology, diagnosis, and management of opioid-induced constipation. Lancet Gastroenterol. Hepatol. 2018, 3, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Nero, R.; Allen, B.; Hailu, K.; Noor, R.; Theiss, K. Impact of oral naloxegol vs subcutaneous methylnaltrexone in treatment of opioid-induced constipation in the hospital setting. Am. J. Health Syst. Pharm. 2022. [Google Scholar] [CrossRef]
- Takemura, M.; Niki, K.; Miyaguchi, S.; Ueda, M. Naldemedine-laxative combination: Retrospective inpatient study. BMJ Support. Palliat. Care 2022. [Google Scholar] [CrossRef]
- Staats, P.S.; Markowitz, J.; Schein, J. Incidence of Constipation Associated with Long-acting Opioid Therapy: A Comparative Study. South. Med. J. 2004, 97, 129–134. [Google Scholar] [CrossRef]
- Leppert, W.; Zajaczkowska, R.; Wordliczek, J. The role of oxycodone/naloxone in the management of patients with pain and opioid-induced constipation. Expert Opin. Pharmacother. 2019, 20, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Mccabe, M.P.; Sharlip, I.D.; Atalla, E.; Balon, R.; Fisher, A.D.; Laumann, E.; Lee, S.W.; Lewis, R.; Segraves, R.T. Definitions of Sexual Dysfunctions in Women and Men: A Consensus Statement From the Fourth International Consultation on Sexual Medicine 2015. J. Sex. Med. 2016, 13, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Gungor, G.; Perk, H.; Soyupek, S.; Baykal, B.; Demir, M.; Sezer, M.T. Nebivolol protects erectile functions compared to Metoprolol in hypertensive men with atherogenic, venogenic, psychogenic erectile dysfunction. Eur. J. Intern. Med. 2022, 103, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, A.; Perletti, G.; Magri, V.; Stamatiou, K.; Trinchieri, M.; Montanari, E. Drug-induced gynecomastia: A systematic review and meta-analysis of randomized clinical trials. Arch. Ital. Urol. Androl. 2021, 93, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Doherty, R.J.; Wahood, W.; Yolcu, Y.U.; Zreik, J.; Goyal, A.; Gazelka, H.M.; Habermann, E.B.; Sebastian, A.; Bydon, M. Chronic opioid use is associated with increased postoperative urinary retention, length of stay and non-routine discharge following lumbar fusion surgery. Clin. Neurol. Neurosurg. 2020, 197, 106161. [Google Scholar] [CrossRef] [PubMed]
- Kela, I.; Kakarala, C.L.; Hassan, M.; Belavadi, R.; Gudigopuram, S.V.R.; Raguthu, C.C.; Gajjela, H.; Sange, I. Chronic Pain: A Complex Condition With a Multi-Tangential Approach. Cureus 2021, 13, e19850. [Google Scholar] [CrossRef]
- Wiffen, P.J.; Derry, S.; Moore, R.A.; Aldington, D.; Cole, P.; Rice, A.S.C.; Lunn, M.P.T.; Hamunen, K.; Haanpaa, M.; Kalso, E.A. Antiepileptic drugs for neuropathic pain and fibromyalgia—An overview of Cochrane reviews. Cochrane Database Syst. Rev. 2013, 2013, CD010567. [Google Scholar] [CrossRef]
- Salerno, S.M.; Browning, R.; Jackson, J.L. The effect of antidepressant treatment on chronic back pain: A meta-analysis. Arch. Intern. Med. 2002, 162, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Slepukhina, M.A.; Ivashchenko, D.V.; Sheina, M.A.; Muradian, A.A.; Blagovestnov, D.A.; Sychev, D.A. Pain pharmacogenetics. Drug Metab. Pers. Ther. 2020, 35, 20202939. [Google Scholar] [CrossRef]
- Hooten, W.M.; Hu, D.; Cunningham, J.M. Effects of the ABCB1 c.3435C>T (rs1045642) Polymorphism on Heat Pain Perception in Opioid-Free Adults With Chronic Pain. Anesth. Analg. 2021, 133, 1028–1035. [Google Scholar] [CrossRef]
- Bhaskar, A.; Bell, A.; Boivin, M.; Briques, W.; Brown, M.; Clarke, H.; Cyr, C.; Eisenberg, E.; Ferreira, R.; Silva, D.O.; et al. Consensus recommendations on dosing and administration of medical cannabis to treat chronic pain: Results of a modified Delphi process. J. Cannabis Res. 2021, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Aviram, J.; Samuelly-Leichtag, G. Efficacy of cannabis-based medicines for pain management: A systematic review and meta-analysis of randomized controlled trials. Pain Physician 2017, 20, E755–E796. [Google Scholar] [CrossRef] [PubMed]
- Freo, U.; Brugnatelli, V.; Turco, F.; Zanette, G. Analgesic and Antidepressant Effects of the Clinical Glutamate Modulators Acetyl- L -Carnitine and Ketamine. Front. Neurosci. 2021, 15, 584649. [Google Scholar] [CrossRef] [PubMed]
- Cuccurazzu, B.; Bortolotto, V.; Valente, M.M.; Ubezio, F.; Koverech, A.; Canonico, P.L.; Grilli, M. Upregulation of mGlu2 receptors via NF-κB p65 acetylation is involved in the proneurogenic and antidepressant effects of acetyl-L-carnitine. Neuropsychopharmacology 2013, 38, 2220–2230. [Google Scholar] [CrossRef]
- Chiechio, S.; Copani, A.; Iv, R.W.G.; Nicoletti, F. Acetyl-L-carnitine in neuropathic pain: Experimental data. CNS Drugs. 2007, 21, 31–38. [Google Scholar] [CrossRef]
- Parisi, S.; Ditto, M.C.; Borrelli, R.; Fusaro, E. Efficacy of a fixed combination of palmitoylethanolamide and acetyl-l-carnitine (PEA+ALC FC) in the treatment of neuropathies secondary to rheumatic diseases. Minerva Med. 2021, 112, 492–499. [Google Scholar] [CrossRef]
- Rolim, L.C.S.P.; da Silva, E.M.K.; Flumignan, R.L.G.; Abreu, M.M.; Dib, S.A. Acetyl-l-carnitine for the treatment of diabetic peripheral neuropathy. Cochrane Database Syst. Rev. 2019, 2019, CD011265. [Google Scholar] [CrossRef]
- AIFA Agenzia Italiana del Farmaco. Riassunto delle Caratteristiche del Prodotto-Nicetile. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_004375_025369_RCP.pdf&sys=m0b1l3 (accessed on 19 August 2022).
- Knotkova, H.; Hamani, C.; Sivanesan, E.; Le Beuffe, M.F.E.; Moon, J.Y.; Cohen, S.P.; Huntoon, M.A. Neuromodulation for chronic pain. Lancet 2021, 397, 2111–2124. [Google Scholar] [CrossRef]
- Premi, E.; Benussi, A.; La Gatta, A.; Visconti, S.; Costa, A.; Gilberti, N.; Cantoni, V.; Padovani, A.; Borroni, B.; Magoni, M. Modulation of long-term potentiation-like cortical plasticity in the healthy brain with low frequency-pulsed electromagnetic fields. BMC Neurosci. 2018, 19, 34. [Google Scholar] [CrossRef]
- Wuschech, H.; von Hehn, U.; Mikus, E.; Funk, R.H. Effects of PEMF on patients with osteoarthritis: Results of a prospective, placebo-controlled, double-blind study. Bioelectromagnetics 2015, 36, 576–585. [Google Scholar] [CrossRef]
- Maestú, C.; Blanco, M.; Nevado, A.; Romero, J.; Rodríguez-Rubio, P.; Galindo, J.; Lorite, J.B.; De Las Morenas, F.; Fernández-Argüelles, P. Reduction of pain thresholds in fibromyalgia after very low-intensity magnetic stimulation: A double-blinded, randomized placebo-controlled clinical trial. Pain Res. Manag. 2013, 18, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, M.I.; Cole, S.P. Pulsed Magnetic Field Therapy in Refractory Neuropathic Pain Secondary to Peripheral Neuropathy: Electrodiagnostic Parameters—Pilot Study. Neurorehabil. Neural Repair 2004, 18, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Roberti, R.; Marcianò, G.; Casarella, A.; Rania, V.; Palleria, C.; Muraca, L.; Citraro, R.; De Sarro, G.; Serra, R.; Romeo, P.; et al. High-Intensity, Low-Frequency Pulsed Electromagnetic Field as an Odd Treatment in a Patient with Mixed Foot Ulcer: A Case Report. Reports 2022, 5, 3. [Google Scholar] [CrossRef]
- Roberti, R.; Marcianò, G.; Casarella, A.; Rania, V.; Palleria, C.; Vocca, C.; Catarisano, L.; Muraca, L.; Citraro, R.; Romeo, P.; et al. Diamagnetic Therapy in a Patient with Complex Regional Pain Syndrome Type I and Multiple Drug Intolerance: A Case Report. Reports 2022, 5, 18. [Google Scholar] [CrossRef]
- De Sire, A.; Agostini, F.; Lippi, L.; Mangone, M.; Marchese, S.; Cisari, C.; Bernetti, A.; Invernizzi, M. Oxygen—Ozone Therapy in the Rehabilitation Field: State of the Art on Mechanisms of Action, Safety andEffectiveness in Patients with Musculoskeletal Disorders. Biomolecules 2021, 11, 356. [Google Scholar] [CrossRef]
- de C Williams, A.C.; Fisher, E.; Hearn, L.; Eccleston, C. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst. Rev. 2020, 8, CD007407. [Google Scholar] [CrossRef]
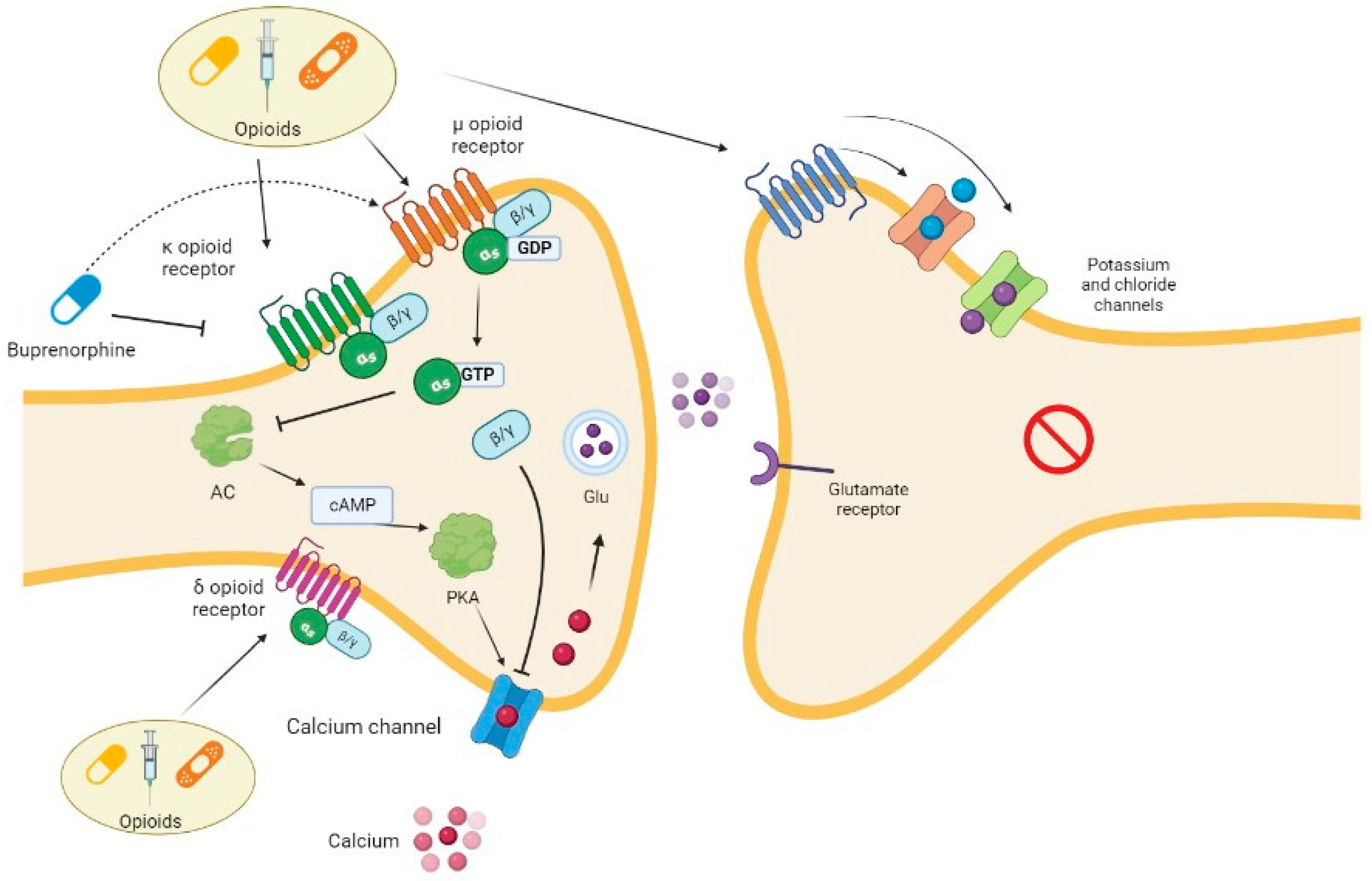

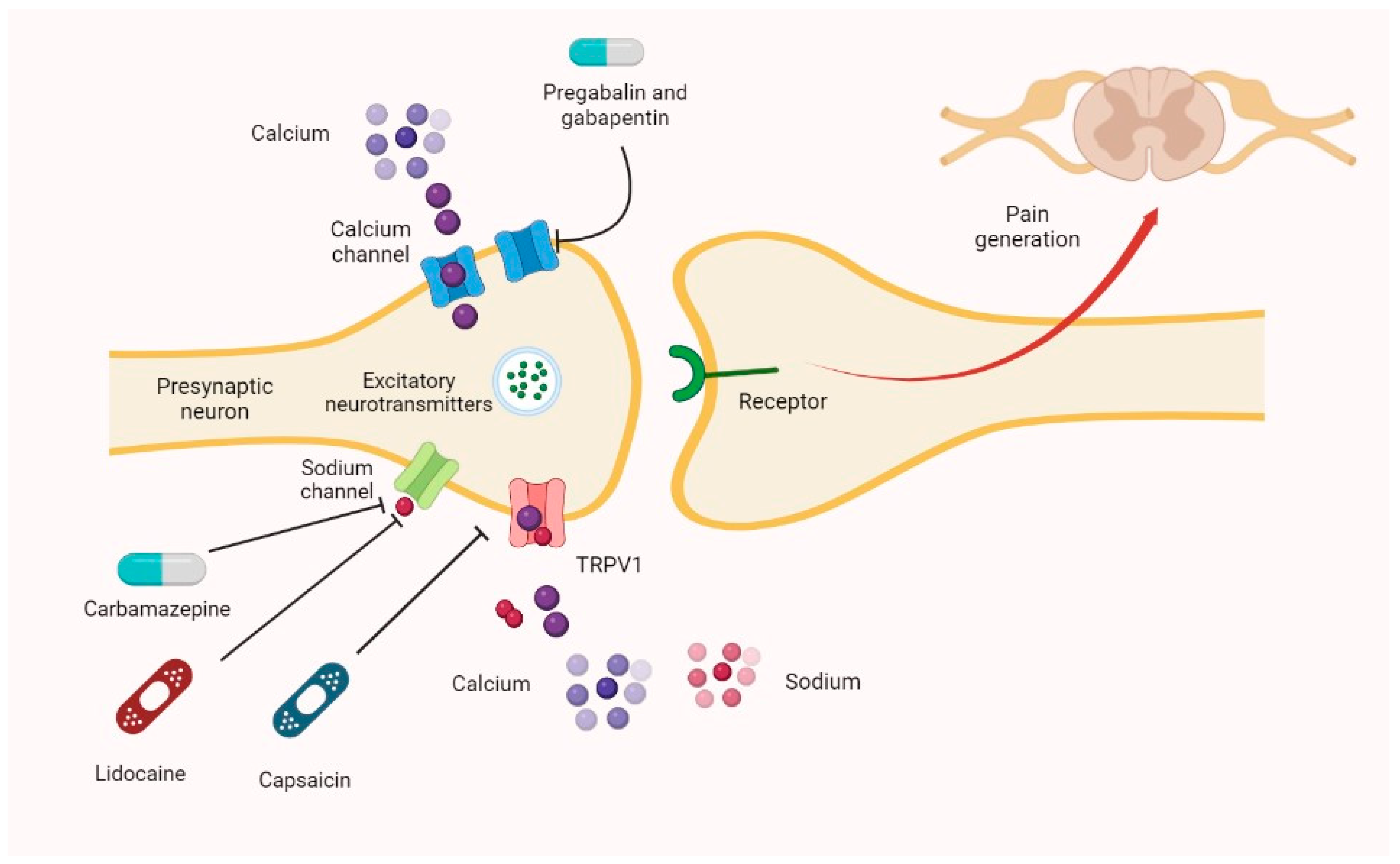
| Characteristic | Opioids |
|---|---|
| Weak | Codeine, tramadol, hydrocodone, and dihydrocodeine |
| Strong | Morphine, oxycodone, fentanyl, buprenorphine, hydromorphone, methadone, and tapentadol |
| Antagonist | Naloxone and naltrexone |
| Nociceptive pain | High activity: codeine, methadone, hydrocodone, hydromorphone, and morphine Low activity: tramadol, oxycodone, and fentanyl |
| Neuropathic pain | Tramadol, oxycodone, fentanyl, buprenorphine, and tapentadol |
| CYP2D6 metabolism | Codeine, tramadol, oxycodone, and hydrocodone |
| CYP3A4 metabolism | Buprenorphine, hydrocodone, methadone, oxycodone, tramadol, and fentanyl |
| Liver conjugation | Buprenorphine, codeine, hydromorphone, morphine, oxycodone, tapentadol, and tramadol |
| Mechanism of action | μ receptor full agonist (morphine, oxycodone, fentanyl, hydromorphone, hydrocodone, methadone, tapentadol, and tramadol) k receptor agonist (oxycodone) μ receptor partial agonist and k receptor antagonist (buprenorphine) SNRI activity (tapentadol and tramadol) |
| Kidney excretion | Buprenorphine (30%), codeine (90%), fentanyl (75%), hydrocodone (6.5% of the parental drug, higher quote including metabolites), hydromorphone (90%), methadone (30%), morphine (90%), oxycodone (80%), tapentadol (99%), and tramadol (90%) |
| Liver excretion | Buprenorphine (70%), codeine (10%), fentanyl (9%), hydrocodone (data not available), hydromorphone (62% of oral dose eliminated by first-pass; 1% in feces), methadone (50%), morphine (10%), oxycodone (20%), and tramadol (10%) |
| Drugs Used for Pain Management | Interacting Drug | Comment |
|---|---|---|
| Anticonvulsants (pregabalin and gabapentin) | CNS-depressing drugs (e.g., opioids) [19,30] and alcohol | Respiratory depression risk. If possible, avoid concomitant use, or reduce dosage. |
| Gabapentin | Antiacids containing aluminum and magnesium [19,30] | Reduction in gabapentin bioavailability. |
| Amitriptyline | CYP2D6, CYP2C19, CYP3A4, and CYP1A2 inhibitors and inducers [31] | Relevant variations in bioavailability are possible. |
| Ethanol [32] | Increase in amitriptyline concentration. | |
| QT-increasing drugs | Risk for arrhythmias [31]. | |
| Valproic acid | Increase in amitriptyline concentration. | |
| Antihypertensive drugs | Risk of further decrease in blood pressure due to α-1 receptor antagonism, but cases of hypertension have been described [31,33]. | |
| Anticholinergic drugs | Increase in the side effects related to anticholinergic actions of amitriptyline [31,33]. | |
| CNS-depressing drugs and alcohol | Increase in CNS depression [31]. | |
| L-DOPA and phenylbutazone | Reduced gastric emptying may arise: L-DOPA and phenylbutazone may be inactivated for this reason. Furthermore, L-DOPA coadministration may facilitate arrhythmias and hypotension [31]. Nevertheless, L-DOPA and amitriptyline coadministration has been associated with better molecular efficacy in Parkinson’s disease [34]. | |
| Antihistamines | Possible increase in QT interval and increased sedation [35,36,37]. | |
| Duloxetine | Other antidepressants or drugs increasing serotonin levels (e.g., tramadol and tapentadol) | Serotonin syndrome risk [38,39]. |
| CYP1A2 and CYP2D6 inhibitors or inducers | Possible variation in duloxetine levels [40]. Contraindicated if CYP1A2 inhibitors are being used in therapy [41]. | |
| CYP2D6 substrates | Increase in these drugs’ levels due to the moderate inhibitory action of duloxetine on CYP2D6 [40]. | |
| CNS-depressing drugs and alcohol | Increase in CNS depression risk [40]. | |
| Antihypertensive drugs | Increase in blood pressure due to the action on noradrenalin reuptake [42]. | |
| Anticoagulants or antiaggregant drugs | Increase in bleeding risk related to action on platelet serotonin [40,43]. | |
| Muscle relaxants | Antihypertensive drugs | Various interactions are described since baclofen, tizanidine, and cyclobenzaprine may decrease blood pressure. No alterations are described with eperisone and thiocolchicoside [44,45,46,47,48]. |
| CNS-depressing drugs and alcohol | Increased CNS depression [49]. | |
| Baclofen | Tricyclic antidepressants | Possible increase in muscular hypotonia risk [44]. |
| Carbidopa and L-DOPA | Worse control of Parkinson’s symptoms. Confusion, hallucinations, and headache [44]. | |
| Lithium | Increase in hyperkinetic symptoms [44]. | |
| Drugs decreasing renal function | Increase in baclofen levels [44]. | |
| Cyclobenzaprine | Structural analog of tricyclic antidepressants [45] | Similar pharmacodynamic actions are expected, including sedation, anticholinergic effects, and blurred vision. |
| Eperisone | Calcium antagonists [47] | Increased calcium antagonists’ effects. |
| Salicylates [47] | Reduced salicylates levels. | |
| Metaxalone | Drugs increasing serotonin levels | Possible risk of serotoninergic syndrome [50]. |
| Methocarbamol | Pyridostigmine | Decreased effect of pyridostigmine in patients with myasthenia [51]. |
| Tizanidine | CYP1A2 inhibitors and inducers [46] | Increased/decreased levels of tizanidine. Contraindicated in presence of CYP1A2 inhibitors [46]. |
| Drugs prolonging QT [46] | Risk for QT prolongations. | |
| Oral contraceptives, verapamil, and cimetidine [46,49] | Possible increase in tizanidine levels. | |
| Beta-blockers or digoxin [46] | Possible increase in hypotension and bradycardia rate. | |
| NSAIDs | CYP2C9 inhibitors/inducers [52,53] | Evaluate dosage increase/reduction. |
| Aspirin and other associated NSAIDs | Risk for reduced effect of aspirin [54]. | |
| Antihypertensive drugs due to kidney damage and inhibition of natriuretic response to diuretic response, impaired synthesis of prostaglandins, sodium and water retention, and suppression of plasma renin activity [55,56] | Increase in blood pressure levels. Minor interactions are described with calcium antagonists [53]. | |
| Anticoagulants, antiaggregant drugs, corticosteroids, SSRIs, and even nutraceuticals/supplements such as Ginkgo Biloba [57,58,59] | Increase in hemorrhagic risk. Warfarin may be released from albumin after NSAID coadministration. Moreover, concomitant CYP2C9 metabolism by the two drugs may affect their concentrations [58,59]. | |
| Lithium, methotrexate, zidovudine, and digoxin | NSAIDs may reduce kidney elimination of some substances including lithium and methotrexate [58,60]. | |
| Probenecid | Aspirin may reduce probenecid effects [58]. | |
| Nephrotoxic medications (e.g., tacrolimus, aminoglycosides, and ciclosporin) [61] | Increased nephrotoxicity. | |
| Zidovudine | Increase in NSAIDs’ plasma levels (diclofenac in particular) and toxicity in animal models [62]. In humans, naproxen modified zidovudine conversion to its glucuronidated metabolite (GZDV) with a transformation in toxic metabolites. | |
| Acetaminophen | Chloramphenicol | Possible increase in chloramphenicol half-life [63]. |
| Drugs slowing or fastening gastric emptying and cholestyramine | Possible increase or reduction in bioavailability. Cholestyramine may reduce paracetamol’s absorption [63,64]. | |
| Hepatotoxic drugs | Increased risk for transaminases increases or liver failure. Phenytoin may reduce paracetamol efficacy and increase liver failure risk [65]. | |
| Opioids | Antidiarrhoeic drugs, | Stypsis [66]. |
| CNS-depressing drugs, and alcohol | CNS depression [67,68]. | |
| CYP3A4 inhibitors/inducers | Buprenorphine, hydrocodone, methadone, oxycodone, tramadol, and fentanyl may be variously involved in these reactions with increases or reductions in drug levels [66,69,70,71,72,73]. | |
| CYP2D6 inhibitors | Codeine, tramadol, oxycodone, and hydrocodone may be involved. Codeine, a prodrug, may be counteracted in its therapeutic action [65,69,71,72,74]. | |
| Methadone | Ammonium chloride | Ammonium chloride may facilitate methadone (a weak base) elimination via its action on urine pH [66]. |
| Anticholinergic drugs | Increase in anticholinergic effects, stypsis in particular [66]. | |
| Desipramine | Increase in desipramine levels [66]. | |
| Didanosine, stavudine, and zidovudine | Methadone may reduce didanosine and stavudine bioavailability, affecting their absorptions and first pass metabolisms. Nevertheless, methadone may increase zidovudine levels, reducing glucuronidation processes and, therefore, its renal clearance [66]. | |
| Octreotide | Possible reduction in analgesic effect. | |
| P-gp inhibitors and inducers | Methadone is a P-gp substrate. Therefore, the inhibition/induction of this protein may result in variations in methadone’s serum levels [66]. | |
| Drugs prolongating QT or antiarrhythmics | Possible risk for arrhythmias [66]. | |
| Morphine | Cimetidine | Reported cases of confusion and respiratory depression [67]. |
| Diuretics | The increase in ADH may contrast the effect of diuretics [67]. | |
| Muscle relaxants/blockers and oral anticoagulants | Morphine may increase the effects of these drugs [67]. | |
| Oxycodone | Anticholinergic drugs | Increase in anticholinergic effects [73]. |
| Fentanyl, methadone, oxycodone, tapentadol, and tramadol | Drugs increasing serotonin levels | Serotonin syndrome risk. Tapentadol has minor action on serotonin reuptake; therefore, the risk of serotonin syndrome is minor, compared with tramadol [72,75]. Methadone has an elevated potential of determining this syndrome [76]. A theoretic risk is also reported for hydrocodone and buprenorphine [77,78]. |
| Tapentadol (alone) | Naproxen and probenecid | These drugs may increase tapentadol levels but without clinical significance. Due to its glucuronidation related metabolism, tapentadol shows few interactions [75,79]. |
| Tramadol (alone) | Warfarin and coumarin derivatives | Possible increase in INR [72,80]. |
| Ondansetron | Possible necessity to increase tramadol dose [72,81]. |
| STEP I | STEP II | STEP III | STEP IV | STEP V | STEP VI | |
|---|---|---|---|---|---|---|
| Nociceptive pain | ||||||
| SIGN [12] | Paracetamol or NSAIDs | Weak opioids or topical NSAIDs | Strong opioids | |||
| CDW [49] | NSAIDs or COX-2 inhibitors | |||||
| AGS [91] | Paracetamol (up to 4 g/day) | NSAIDs | Opioids | |||
| DHHS [92] | Ia: paracetamol Ib: ibuprofen or naproxen Ic: paracetamol plus ibuprofen or naproxen | IIa: codeine IIb: tramadol | IIIa: low-dose morphine or buprenorphine patch (if morphine is ineffective) IIIb: high-dose morphine or 5–30 mg of oxycodone twice a day or fentanyl/buprenorphine patch if morphine is ineffective IIIc: 50 mg of tapentadol twice daily | |||
| ESCEO [93] | Chondroitin sulfate or glucosamine sulfate | Paracetamol or topical NSAIDs | NSAIDs | Intra-articular injection of hyaluronic acid or corticosteroids | Duloxetine | Surgery |
| Neuropathic pain | ||||||
| SIGN [12] | Amitriptyline or gabapentin | Pregabalin | SNRIs | 5% lidocaine | Opioids | 9% capsaicin |
| CDW [49] | Tricyclic antidepressants | Gabapentin/pregabalin or SNRIs (duloxetine) | Other anticonvulsants | Low-dose opioids | ||
| AGS [91] | Duloxetine or pregabalin | |||||
| DHHS [92] | Amitriptyline/imipramine | Gabapentin (1st line) or pregabalin (2nd line) or 0.075% capsaicin cream | Duloxetine or lidocaine plasters (5%-700 mg/plaster) or capsaicin patch (8%-179 mg/plaster) | |||
| Practice [94] | Duloxetine or TCA | Lidocaine or ketamine | ||||
| NICE [2]. | Antidepressants | Gabapentin or pregabalin | ||||
| NeuPSIG [14] | TCA, SNRI, or gabapentin/pregabalin | Tramadol, lidocaine, and capsaicin patches | Opioids or botulin toxin-A | |||
| Drug | Kidney Excretion | Effects in Patients with Renal Failure |
|---|---|---|
| Acetaminophen | 90–99% [102] | Not used with eGFR of <10 mL/min [63,100]. |
| Oxycodone | 50% | Dose adjustments [73]. Some authors consider oxycodone unsafe in patients with advanced kidney failure due to its accumulation risk, interactions, and CYP450 polymorphisms [100]. |
| Buprenorphine | 10–30 [103] | Caution with eGFR of <30 mL/min [70]. |
| Fentanyl | 10% or less of active compound and 75% of the total dose. Metabolites are excreted mainly in urine [74]. | Dose monitoring [74]. |
| Methadone | 20–50% as methadone or its metabolites [101] | Contraindicated in patients with severe kidney impairment [66]. Lower doses and longer intervals between administration in patients with kidney impairment [104]. |
| Morphine | 70–80% [105] | eGFR of 10–50 mL/min: dose reduction of 25%; eGFR of <10 mL/min: dose reduction of 50% [104]. One of the worst options in advanced kidney failure due to accumulation risk [100]. |
| Codeine | Mainly excreted in kidneys [106] | Caution is needed. Davison et al. consider codeine one of the worst options in patients with advanced kidney failure due to CYP2D6 polymorphisms and accumulation risk [100]. |
| Hydromorphone | Most of the dose; 7% unmodified drug [107] | Dose reduction [108]. |
| Hydrocodone | Eliminated with its metabolites, mainly in kidneys, percentage not available [71] | Caution/dose reduction [71,77]. Davison considers it one of the worst options in patients with advanced kidney failure, according to CYP2D6 polymorphism-related and variable responses and possible accumulation risk [100]. |
| Tapentadol | 99% [75] | Not recommended in patients with severe insufficiency [75]. |
| Tramadol | 90% [72] | Prolonged interval between doses; do not use long-release formulation [72]. Increase the interval of administration to 12 h, and limit maximum daily dose to 200 mg [109]. |
| Duloxetine | 70% [110] | eGFR of <30 mL/min: do not use [41]. |
| Amitriptyline | 95% [31] | No dose reduction [31,100,111]. |
| 5% lidocaine patch | >85% | eGFR of <30 mL/min (severe kidney impairment): caution [112]. |
| Tizanidine | 60–70% [46] | eGFR of <25 mL/min: start with 2 mg/day [46]. |
| Baclofen | 75% [44] | Start with lower dosages in all patients with mild–moderate kidney impairment, and use only if benefit outweighs the risk in those with severe kidney impairment [44]. |
| Thiocolchicoside | 20% [48] | No dose adjustments [48]. |
| Cyclobenzaprine | 80% | Low dosage [45]. |
| Eperisone | 76.6% [47] | eGFR of <25 mL/min: low dosage, max. 150 mg daily [47]. |
| Pregabalin | 99% | eGFR of 30–59 mL/min: 300 mg/daily. eGFR of 15–29 mL/min: 150 mg/daily. eGFR of <15 mL/min: 75 mg/daily [113]. |
| Gabapentin | 99% | eGFR of 30–59 mL/min: 1400 mg/daily. eGFR of 15–29 mL/min: 700 mg/daily. eGFR of <15 mL/min: 300 mg/daily [113]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcianò, G.; Vocca, C.; Evangelista, M.; Palleria, C.; Muraca, L.; Galati, C.; Monea, F.; Sportiello, L.; De Sarro, G.; Capuano, A.; et al. The Pharmacological Treatment of Chronic Pain: From Guidelines to Daily Clinical Practice. Pharmaceutics 2023, 15, 1165. https://doi.org/10.3390/pharmaceutics15041165
Marcianò G, Vocca C, Evangelista M, Palleria C, Muraca L, Galati C, Monea F, Sportiello L, De Sarro G, Capuano A, et al. The Pharmacological Treatment of Chronic Pain: From Guidelines to Daily Clinical Practice. Pharmaceutics. 2023; 15(4):1165. https://doi.org/10.3390/pharmaceutics15041165
Chicago/Turabian StyleMarcianò, Gianmarco, Cristina Vocca, Maurizio Evangelista, Caterina Palleria, Lucia Muraca, Cecilia Galati, Francesco Monea, Liberata Sportiello, Giovambattista De Sarro, Annalisa Capuano, and et al. 2023. "The Pharmacological Treatment of Chronic Pain: From Guidelines to Daily Clinical Practice" Pharmaceutics 15, no. 4: 1165. https://doi.org/10.3390/pharmaceutics15041165
APA StyleMarcianò, G., Vocca, C., Evangelista, M., Palleria, C., Muraca, L., Galati, C., Monea, F., Sportiello, L., De Sarro, G., Capuano, A., & Gallelli, L. (2023). The Pharmacological Treatment of Chronic Pain: From Guidelines to Daily Clinical Practice. Pharmaceutics, 15(4), 1165. https://doi.org/10.3390/pharmaceutics15041165







