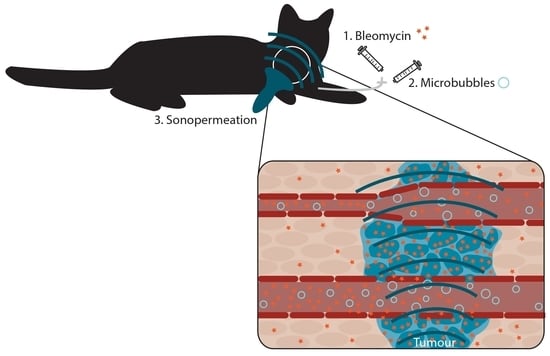
Ultrasound and Microbubbles Mediated Bleomycin Delivery in Feline Oral Squamous Cell Carcinoma—An In Vivo Veterinary Study
Abstract
1. Introduction
2. Methods
2.1. Subjects
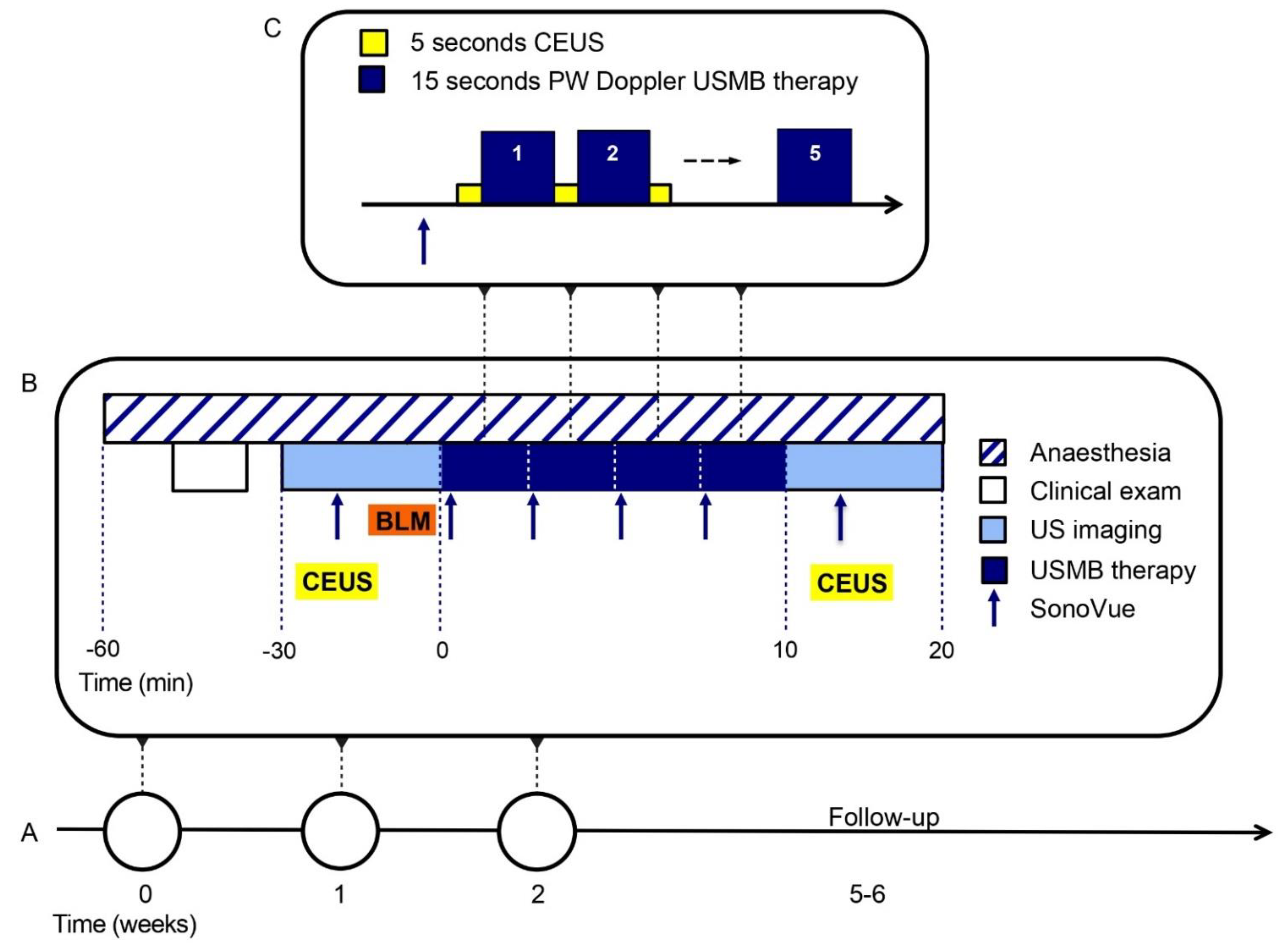
2.2. USMB Treatment
2.3. Other Study Procedures
2.4. Quantitative CEUS Evaluation
3. Results
3.1. Baseline Characteristics
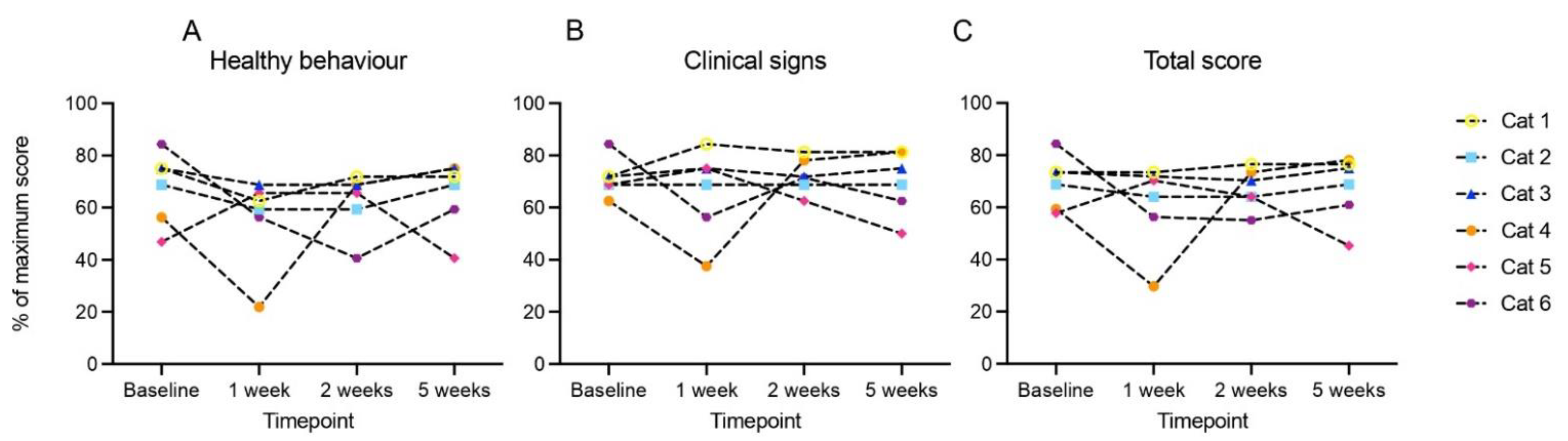
3.2. Bleomycin plus USMB Therapy Was Tolerable
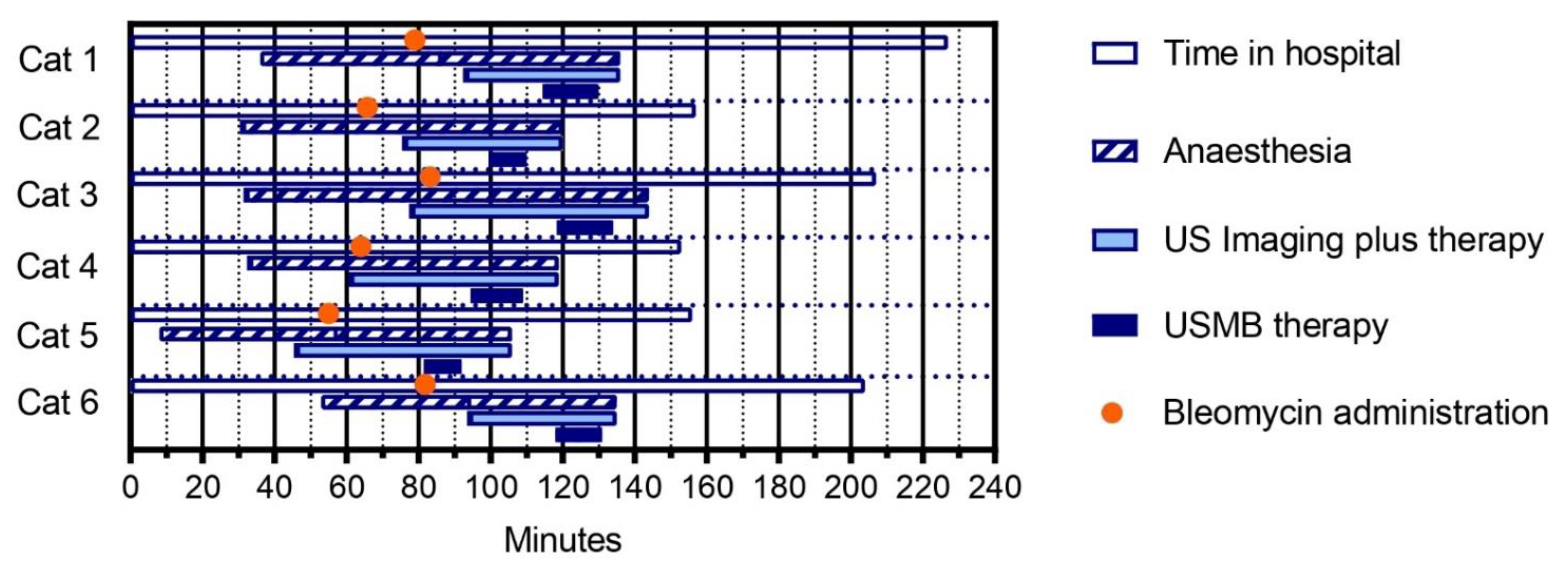
3.3. Three Treatment Sessions of Bleomycin plus USMB Therapy Were Feasible
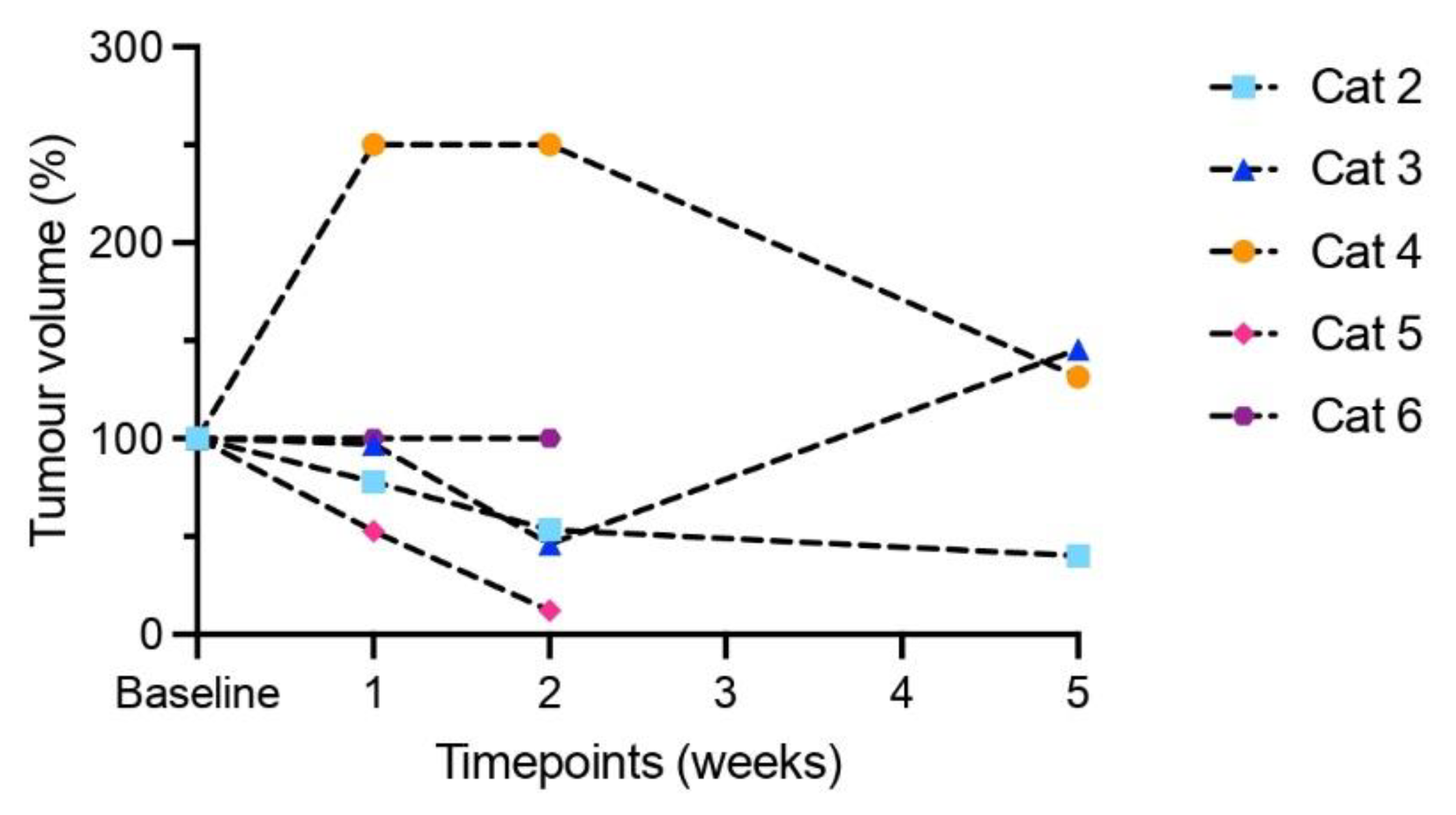
3.4. Modest Clinical Response
3.5. Indication of Increased Tumour Perfusion Assessed by Contrast-Enhanced Ultrasound
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Braakhuis, B.J.; Leemans, C.R.; Visser, O. Incidence and survival trends of head and neck squamous cell carcinoma in the Netherlands between 1989 and 2011. Oral Oncol. 2014, 50, 670–675. [Google Scholar] [CrossRef] [PubMed]
- van der Kamp, M.F.; van Dijk, B.A.C.; Plaat, B.E.C.; van der Laan, B.; Halmos, G.B. To what extent has the last two decades seen significant progress in the management of older patients with head and neck cancer? Eur. J. Surg. Oncol. 2021, 47, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Leeman, J.E.; Li, J.G.; Pei, X.; Venigalla, P.; Zumsteg, Z.S.; Katsoulakis, E.; Lupovitch, E.; McBride, S.M.; Tsai, C.J.; Boyle, J.O.; et al. Patterns of Treatment Failure and Postrecurrence Outcomes Among Patients With Locally Advanced Head and Neck Squamous Cell Carcinoma After Chemoradiotherapy Using Modern Radiation Techniques. JAMA Oncol. 2017, 3, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Rohde, M.; Rosenberg, T.; Pareek, M.; Nankivell, P.; Sharma, N.; Mehanna, H.; Godballe, C. Definition of locally recurrent head and neck squamous cell carcinoma: A systematic review and proposal for the Odense-Birmingham definition. Eur. Arch. Otorhinolaryngol. 2020, 277, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Szturz, P.; Wouters, K.; Kiyota, N.; Tahara, M.; Prabhash, K.; Noronha, V.; Adelstein, D.; Van Gestel, D.; Vermorken, J.B. Low-Dose vs. High-Dose Cisplatin: Lessons Learned From 59 Chemoradiotherapy Trials in Head and Neck Cancer. Front. Oncol. 2019, 9, 86. [Google Scholar] [CrossRef]
- Buchberger, A.M.S.; Strzelczyk, E.A.; Wollenberg, B.; Combs, S.E.; Pickhard, A.; Pigorsch, S.U. Report on Late Toxicity in Head-and-Neck Tumor Patients with Long Term Survival after Radiochemotherapy. Cancers 2021, 13, 4292. [Google Scholar] [CrossRef]
- Nilsen, M.L.; Belsky, M.A.; Scheff, N.; Johnson, J.T.; Zandberg, D.P.; Skinner, H.; Ferris, R. Late and Long-Term Treatment-Related Effects and Survivorship for Head and Neck Cancer Patients. Curr. Treat. Options Oncol. 2020, 21, 92. [Google Scholar] [CrossRef]
- Strojan, P.; Vermorken, J.B.; Beitler, J.J.; Saba, N.F.; Haigentz, M., Jr.; Bossi, P.; Worden, F.P.; Langendijk, J.A.; Eisbruch, A.; Mendenhall, W.M.; et al. Cumulative cisplatin dose in concurrent chemoradiotherapy for head and neck cancer: A systematic review. Head Neck 2016, 38 (Suppl. S1), E2151–E2158. [Google Scholar] [CrossRef]
- Buglione, M.; Alterio, D.; Maddalo, M.; Greco, D.; Gerardi, M.A.; Tomasini, D.; Pegurri, L.; Augugliaro, M.; Marvaso, G.; Turturici, I.; et al. Three weekly versus weekly concurrent cisplatin: Safety propensity score analysis on 166 head and neck cancer patients. Radiat. Oncol. 2021, 16, 239. [Google Scholar] [CrossRef]
- Chong, W.K.; Papadopoulou, V.; Dayton, P.A. Imaging with ultrasound contrast agents: Current status and future. Abdom. Radiol. 2018, 43, 762–772. [Google Scholar] [CrossRef]
- Frinking, P.; Segers, T.; Luan, Y.; Tranquart, F. Three Decades of Ultrasound Contrast Agents: A Review of the Past, Present and Future Improvements. Ultrasound Med. Biol. 2020, 46, 892–908. [Google Scholar] [CrossRef]
- Snipstad, S.; Sulheim, E.; de Lange Davies, C.; Moonen, C.; Storm, G.; Kiessling, F.; Schmid, R.; Lammers, T. Sonopermeation to improve drug delivery to tumors: From fundamental understanding to clinical translation. Expert Opin. Drug Deliv. 2018, 15, 1249–1261. [Google Scholar] [CrossRef]
- Deprez, J.; Lajoinie, G.; Engelen, Y.; De Smedt, S.C.; Lentacker, I. Opening doors with ultrasound and microbubbles: Beating biological barriers to promote drug delivery. Adv. Drug Deliv. Rev. 2021, 172, 9–36. [Google Scholar] [CrossRef] [PubMed]
- Lammertink, B.H.; Bos, C.; van der Wurff-Jacobs, K.M.; Storm, G.; Moonen, C.T.; Deckers, R. Increase of intracellular cisplatin levels and radiosensitization by ultrasound in combination with microbubbles. J. Control Release 2016, 238, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.T.; Wei, K.C.; Liu, H.L. Theranostic Strategy of Focused Ultrasound Induced Blood-Brain Barrier Opening for CNS Disease Treatment. Front. Pharmacol. 2019, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.K.; Tsai, C.L.; Huang, Y.; Hynynen, K. Focused Ultrasound and Microbubbles-Mediated Drug Delivery to Brain Tumor. Pharmaceutics 2020, 13, 15. [Google Scholar] [CrossRef]
- Schoen, S., Jr.; Kilinc, M.S.; Lee, H.; Guo, Y.; Degertekin, F.L.; Woodworth, G.F.; Arvanitis, C. Towards controlled drug delivery in brain tumors with microbubble-enhanced focused ultrasound. Adv. Drug Deliv. Rev. 2022, 180, 114043. [Google Scholar] [CrossRef]
- Dimcevski, G.; Kotopoulis, S.; Bjanes, T.; Hoem, D.; Schjott, J.; Gjertsen, B.T.; Biermann, M.; Molven, A.; Sorbye, H.; McCormack, E.; et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J. Control Release 2016, 243, 172–181. [Google Scholar] [CrossRef]
- Kotopoulis, S.; Dimcevski, G.; Gilja, O.H.; Hoem, D.; Postema, M. Treatment of human pancreatitc cancer using combined ultrasound, microbubbles and gemcitabine: A clinical case study. Med. Phys. 2013, 40, 072902. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Yan, K.; Shen, L.; Yang, W.; Gong, J.; Ding, K. Clinical study of ultrasound and microbubbles for enhancing chemotherapeutic sensitivity of malignant tumors in digestive system. Chin. J. Cancer Res. 2018, 30, 553–563. [Google Scholar] [CrossRef]
- Rix, A.; Piepenbrock, M.; Flege, B.; von Stillfried, S.; Koczera, P.; Opacic, T.; Simons, N.; Boor, P.; Thoroe-Boveleth, S.; Deckers, R.; et al. Effects of contrast-enhanced ultrasound treatment on neoadjuvant chemotherapy in breast cancer. Theranostics 2021, 11, 9557–9570. [Google Scholar] [CrossRef]
- Supsavhad, W.; Dirksen, W.P.; Martin, C.K.; Rosol, T.J. Animal models of head and neck squamous cell carcinoma. Vet. J. 2016, 210, 7–16. [Google Scholar] [CrossRef]
- Cannon, C. Cats, Cancer and Comparative Oncology. Vet. Sci. 2015, 2, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Wypij, J.M. A naturally occurring feline model of head and neck squamous cell carcinoma. Pathol. Res. Int. 2013, 2013, 502197. [Google Scholar] [CrossRef]
- Stebbins, K.E.; Morse, C.C.; Goldschmidt, M.H. Feline Oral Neoplasia: A Ten-Year Survey. Vet. Pathol. 1989, 26, 121–128. [Google Scholar] [CrossRef]
- Martin, C.K.; Tannehill-Gregg, S.H.; Wolfe, T.D.; Rosol, T.J. Bone-invasive oral squamous cell carcinoma in cats: Pathology and expression of parathyroid hormone-related protein. Vet. Pathol. 2011, 48, 302–312. [Google Scholar] [CrossRef]
- Bostock, D.E. The prognosis in cats bearing squamous cell carcinoma. J. Small Anim. Pract. 1972, 13, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Sabhlok, A.; Ayl, R. Palliative radiation therapy outcomes for cats with oral squamous cell carcinoma (1999–2005). Vet. Radiol. Ultrasound 2014, 55, 565–570. [Google Scholar] [CrossRef]
- Withrow, S.J.; Vail, D.M. Withrow and MacEwen’s Small Animal Clinical Oncology; Saunders Elsevier: Philadelphia, PA, USA, 2007; p. 846. [Google Scholar]
- Biller, B.; Berg, J.; Garrett, L.; Ruslander, D.; Wearing, R.; Abbott, B.; Patel, M.; Smith, D.; Bryan, C. 2016 AAHA Oncology Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2016, 52, 181–204. [Google Scholar] [CrossRef]
- Hayes, A.M.; Adams, V.J.; Scase, T.J.; Murphy, S. Survival of 54 cats with oral squamous cell carcinoma in United Kingdom general practice. J. Small Anim. Pract. 2007, 48, 394–399. [Google Scholar] [CrossRef]
- Barabas, K.; Milner, R.; Lurie, D.; Adin, C. Cisplatin: A review of toxicities and therapeutic applications. Vet. Comp. Oncol. 2008, 6, 1–18. [Google Scholar] [CrossRef]
- Tounekti, O.; Pron, G.; Belehradek, J.; Mir, L.M. Bleomycin, an Apoptosis-mimetic Drug That Induces Two Types of Cell Death Depending on the Number of Molecules Internalized. Cancer Res. 1993, 53, 5462–5469. [Google Scholar]
- Mir, L.M.; Tounekti, O.; Orlowski, S. Bleomycin: Revival of an old drug. Gen. Pharmac. 1996, 27, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yan, B.; Gao, L.; Dong, C.; Zhong, J.; D Ortenzio, M.; Nguyen, B.; Seong Lee, S.; Hu, X.; Liang, F. Targeted Delivery of Bleomycin: A Comprehensive Anticancer Review. Curr. Cancer Drug Targets 2016, 16, 509–521. [Google Scholar] [CrossRef]
- Brandt, J.P.; Gerriets, V. Bleomycin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, January 2022; [Updated 29 August 2022]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK555895/ (accessed on 19 January 2023).
- Teissie, J. Electropermeabilization of the cell membrane. In Electroporation Protocols. Methods in Molecular Biology (Methods and Protocols); Li, S., Cutrera, J., Heller, R., Teissie, J., Eds.; Humana Press: New York, NY, USA, 2014; Volume 1121. [Google Scholar]
- Spugnini, E.P.; Baldi, A. Electrochemotherapy in Veterinary Oncology: State-of-the-Art and Perspectives. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Spugnini, E.P.; Pizzuto, M.; Filipponi, M.; Romani, L.; Vincenzi, B.; Menicagli, F.; Lanza, A.; De Girolamo, R.; Lomonaco, R.; Fanciulli, M.; et al. Electroporation Enhances Bleomycin Efficacy in Cats with Periocular Carcinoma and Advanced Squamous Cell Carcinoma of the Head. J. Vet. Intern. Med. 2015, 29, 1368–1375. [Google Scholar] [CrossRef]
- Dotsinsky, I.; Nikolova, B.; Peycheva, E.; Tsoneva, I. New Modality for Electrochemotherapy of Surface Tumors. Biotechnol. Biotechnol. Equip. 2012, 26, 3402–3406. [Google Scholar] [CrossRef]
- Keller, S.B.; Wang, Y.N.; Totten, S.; Yeung, R.S.; Averkiou, M.A. Safety of Image-Guided Treatment of the Liver with Ultrasound and Microbubbles in an in Vivo Porcine Model. Ultrasound Med. Biol. 2021, 47, 3211–3220. [Google Scholar] [CrossRef] [PubMed]
- Seiler, G.S.; Brown, J.C.; Reetz, J.A.; Taeymans, O.; Bucknoff, M.; Rossi, F.; Ohlerth, S.; Alder, D.; Rademacher, N.; Drost, T.; et al. Safety of contrast-enhanced ultrasonography in dogs and cats: 488 cases (2002–2011). J. Am. Vet. Med. Assoc. 2013, 242, 1255–1259. [Google Scholar] [CrossRef]
- Streitberger, A.; Hocke, V.; Modler, P. Measurement of pulmonary transit time in healthy cats by use of ultrasound contrast media “Sonovue(R)”: Feasibility, reproducibility, and values in 42 cats. J. Vet. Cardiol. 2013, 15, 181–187. [Google Scholar] [CrossRef]
- de Brito, M.; Feliciano, M.; Coutinho, L.N.; Uscategui, R.R.; Simoes, A.; Maronezi, M.C.; de Almeida, V.T.; Crivelaro, R.M.; Gasser, B.; Pavan, L.; et al. Doppler and Contrast-Enhanced Ultrasonography of Testicles in Adult Domestic Felines. Reprod. Domest. Anim. 2015, 50, 730–734. [Google Scholar] [CrossRef]
- Streitberger, A.; Modler, P.; Haggstrom, J. Increased normalized pulmonary transit times and pulmonary blood volumes in cardiomyopathic cats with or without congestive heart failure. J. Vet. Cardiol. 2015, 17, 25–33. [Google Scholar] [CrossRef]
- Schweiger, H.; Ohlerth, S.; Gerber, B. Contrast-enhanced ultrasound of both kidneys in healthy, non-anaesthetized cats. Acta Vet. Scand. 2015, 57, 80. [Google Scholar] [CrossRef]
- Stock, E.; Daminet, S.; Paepe, D.; Buresova, E.; Vandermeulen, E.; Smets, P.; Duchateau, L.; Saunders, J.H.; Vanderperren, K. Evaluation of Renal Perfusion in Hyperthyroid Cats before and after Radioiodine Treatment. J. Vet. Intern. Med. 2017, 31, 1658–1663. [Google Scholar] [CrossRef]
- Maciulevičius, M.; Tamosiunas, M.; Venslauskas, M.S.; Satkauskas, S. The relation of Bleomycin Delivery Efficiency to Microbubble Sonodestruction and Cavitation Spectral Characteristics. Sci. Rep. 2020, 10, 7743. [Google Scholar] [CrossRef] [PubMed]
- Lamanauskas, N.; Novell, A.; Escoffre, J.M.; Venslauskas, M.; Satkauskas, S.; Bouakaz, A. Bleomycin delivery into cancer cells in vitro with ultrasound and SonoVue(R) or BR14(R) microbubbles. J. Drug Target 2013, 21, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.O.; Casey, G.D.; Tangney, M.; Cashman, J.; Collins, C.G.; Soden, D.M.; O’Sullivan, G.C. Effective tumor treatment using optimized ultrasound-mediated delivery of bleomycin. Ultrasound Med. Biol. 2008, 34, 406–413. [Google Scholar] [CrossRef] [PubMed]
- de Maar, J.S.; Rousou, C.; van Elburg, B.; Vos, H.J.; Lajoinie, G.P.R.; Bos, C.; Moonen, C.T.W.; Deckers, R. Ultrasound-Mediated Drug Delivery With a Clinical Ultrasound System: In Vitro Evaluation. Front. Pharmacol. 2021, 12, 768436. [Google Scholar] [CrossRef]
- Theilen, G.H.; Madewell, B.R. Veterinary Cancer Medicine, 2nd ed.; Lea & Febiger: Philadelphia, PA, USA, 1979. [Google Scholar]
- Tatlock, S.; Gober, M.; Williamson, N.; Arbuckle, R. Development and preliminary psychometric evaluation of an owner-completed measure of feline quality of life. Vet. J. 2017, 228, 22–32. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Averkiou, M.A.; Correas, J.M.; Lassau, N.; Leen, E.; Piscaglia, F. An EFSUMB introduction into Dynamic Contrast-Enhanced Ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall Med. 2012, 33, 344–351. [Google Scholar] [CrossRef]
- Owen, L.N. TNM Classification of Tumours in Domestic Animals; World Health Organization: Geneva, Switzerland, 1980; pp. 23–24. [Google Scholar]
- Keller, S.B.; Sheeran, P.S.; Averkiou, M.A. Cavitation therapy monitoring of commercial microbubbles with a clinical scanner. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- van Wamel, A.; Kooiman, K.; Harteveld, M.; Emmer, M.; ten Cate, F.J.; Versluis, M.; de Jong, N. Vibrating microbubbles poking individual cells: Drug transfer into cells via sonoporation. J. Control Release 2006, 112, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Čemažar, M.; Miklavčič, D.; Serša, G. Intrinsic sensitivity of tumor cells to bleomycin as an indicator of tumor response to electrochemotherapy. Jpn J. Cancer Res. 1998, 89, 328–333. [Google Scholar] [CrossRef]
- Emanuel, A.L.; Meijer, R.I.; van Poelgeest, E.; Spoor, P.; Serne, E.H.; Eringa, E.C. Contrast-enhanced ultrasound for quantification of tissue perfusion in humans. Microcirculation 2020, 27, e12588. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.M.; Weber, M.A.; Wiesinger, I. Contrast-enhanced ultrasound perfusion imaging of organs. Radiologe 2021, 61 (Suppl. S1), 19–28. [Google Scholar] [CrossRef]
- Barrett, E.J.; Rattigan, S. Muscle perfusion: Its measurement and role in metabolic regulation. Diabetes 2012, 61, 2661–2668. [Google Scholar] [CrossRef]
- Snipstad, S.; Vikedal, K.; Maardalen, M.; Kurbatskaya, A.; Sulheim, E.; Davies, C.L. Ultrasound and microbubbles to beat barriers in tumors: Improving delivery of nanomedicine. Adv. Drug Deliv. Rev. 2021, 177, 113847. [Google Scholar] [CrossRef]
- Kikuchi, M.; Yamane, T.; Shinohara, S.; Fujiwara, K.; Hori, S.Y.; Tona, Y.; Yamazaki, H.; Naito, Y.; Senda, M. 18F-fluoromisonidazole positron emission tomography before treatment is a predictor of radiotherapy outcome and survival prognosis in patients with head and neck squamous cell carcinoma. Ann. Nucl. Med. 2011, 25, 625–633. [Google Scholar] [CrossRef]
- Minchinton, A.I.; Tannock, I.F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Delivery of molecular and cellular medicine to solid tumors. Adv. Drug Deliv. Rev. 2001, 46, 149–168. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Indication/Setting on EPIQ5 or EPIQ7 | Value |
|---|---|---|
| Optimized Pulse Wave (PW) Doppler settings for ultrasound and microbubble (USMB) therapy | ||
| Frequency | C9-2 probe in PW mode | 2.9 MHz |
| Pulse length | Sample volume: 7.5 mm (maximum) | 21 cycles per pulse |
| Pulse repetition frequency | Scale: −4–4 cm/s (minimum) | 0.4 kHz |
| Mechanical index | Relative intensity: −10 dB * | MI 0.3–0.4 at target depth |
| Contrast-enhanced ultrasound (CEUS) settings | ||
| Mechanical index (MI) | MI in CEUS mode <0.1 | CEUS MI = 0.06 |
| Gain | Gain slightly above the noise floor in absence of microbubbles and kept constant | Gain = 45% |
| Dynamic range (compression) | Dynamic range = 50 | |
| Focus position | Focus positioned at the target or a bit deeper (2/3 of image depth) | Adjusted per treatment session and moved during USMB therapy |
| Time gain compensation (TGC) | All switches in central position | All switches in central position |
| Persistence | Off | |
| Patient | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Sex | male | male | male | female | male | female |
| Age at inclusion (years) | 11 | 11 | 18 | 15 | 14 | 15 |
| Body weight at inclusion (kg) | 2.8 | 7.2 | 3.4 | 2.8 | 6.7 | 5.5 |
| TNM stage [57] | T2N1M0 | T2bN0M1 (lungs) | T2bN0M0 | T3bN0Mx | cT3N1Mx | T1N0M0 * |
| Tumour location | tongue, frenulum, and sublingual soft tissue | right maxilla | lip and cheek extending into corner of mouth and caudal maxilla | rostrally in the mouth, infiltrated into mandibula | tongue base and floor of mouth | Sublingual, floor of mouth |
| Supportive care measures | Antibiotics Analgesics (NSAIDs, tramadol) Tube feeding | Antibiotics Analgesics (NSAIDs) | Antibiotics Analgesics (NSAIDs, gabapentin) | Antibiotics Analgesics (NSAIDs, gabapentin) | Antibiotics Analgesics (NSAIDs, buprenorphine) Tube feeding | Antibiotics Analgesics (NSAIDs) Antiemetics (maropitant) mirtazapine |
| Concomitant drugs | Treatment for hyperthyroidism (carbimazole) initiated during study | Treatment for hyperthyroidism (carbimazole) initiated during study | - | - | - | Treatment for hyperthyroidism (thiamazole) initiated before start of study |
| Survival (days) | 46 | 85 | 64 | 56 | 57 | 147 |
| Death | euthanasia | euthanasia | euthanasia | euthanasia | natural death | euthanasia |
| Adverse Event (VCOG CTCAE Version 1.1) | Grade 1 | Grade 2 | Grade 3 | Unknown Grade | Most Likely Related to |
|---|---|---|---|---|---|
| alopecia | 1 | i.v. catheter | |||
| anorexia | 1 | 1 | tumour progression | ||
| cardiac murmur | 1 | comorbidity (hyperthyroidism) | |||
| constipation | 1 | anaesthesia | |||
| dysphagia (fibrosis-like tissue) | 1 | possible reaction to USMB, chemotherapy or previous surgery | |||
| generalized muscle weakness | 1 | tumour progression | |||
| haemorrhage/bleeding | 1 | tumour progression | |||
| hypotension | 1 | anaesthesia | |||
| hypothermia | 1 | anaesthesia | |||
| lethargy/fatigue/decreased general performance | 2 | 3 | anaesthesia or tumour progression | ||
| localized erythema | 1 | i.v. catheter | |||
| oral ulcers | 1 | infection | |||
| pain (most likely at tumour site) | 2 | tumour progression | |||
| ptyalism | 1 | tumour progression | |||
| sinus tachycardia | 1 | possible reaction to USMB or anaesthesia | |||
| skin ulceration | 1 | tumour progression | |||
| soft tissue necrosis | 1 | 2 | tumour progression in cat 1 and 5, possible reaction to USMB or chemotherapy in cat 6 | ||
| vomiting | 1 | anaesthesia | |||
| weight loss | 2 | 1 | tumour progression | ||
| Any adverse event | 15 | 11 | 2 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Maar, J.S.; Zandvliet, M.M.J.M.; Veraa, S.; Tobón Restrepo, M.; Moonen, C.T.W.; Deckers, R. Ultrasound and Microbubbles Mediated Bleomycin Delivery in Feline Oral Squamous Cell Carcinoma—An In Vivo Veterinary Study. Pharmaceutics 2023, 15, 1166. https://doi.org/10.3390/pharmaceutics15041166
de Maar JS, Zandvliet MMJM, Veraa S, Tobón Restrepo M, Moonen CTW, Deckers R. Ultrasound and Microbubbles Mediated Bleomycin Delivery in Feline Oral Squamous Cell Carcinoma—An In Vivo Veterinary Study. Pharmaceutics. 2023; 15(4):1166. https://doi.org/10.3390/pharmaceutics15041166
Chicago/Turabian Stylede Maar, Josanne S., Maurice M. J. M. Zandvliet, Stefanie Veraa, Mauricio Tobón Restrepo, Chrit T. W. Moonen, and Roel Deckers. 2023. "Ultrasound and Microbubbles Mediated Bleomycin Delivery in Feline Oral Squamous Cell Carcinoma—An In Vivo Veterinary Study" Pharmaceutics 15, no. 4: 1166. https://doi.org/10.3390/pharmaceutics15041166
APA Stylede Maar, J. S., Zandvliet, M. M. J. M., Veraa, S., Tobón Restrepo, M., Moonen, C. T. W., & Deckers, R. (2023). Ultrasound and Microbubbles Mediated Bleomycin Delivery in Feline Oral Squamous Cell Carcinoma—An In Vivo Veterinary Study. Pharmaceutics, 15(4), 1166. https://doi.org/10.3390/pharmaceutics15041166