Abstract
Extracellular vesicles (EVs) derived from mesenchymal stromal cells (MSCs) have shown potential for the treatment of tendon and ligament injuries. This approach can eliminate the need to transplant live cells to the human body, thereby reducing issues related to the maintenance of cell viability and stability and potential erroneous differentiation of transplanted cells to bone or tumor. Despite these advantages, there are practical issues that need to be considered for successful clinical application of MSC-EV-based products in the treatment of tendon and ligament injuries. This review aims to discuss the general and tissue-specific considerations for manufacturing MSC-EVs for clinical translation. Specifically, we will discuss Good Manufacturing Practice (GMP)-compliant manufacturing and quality control (parent cell source, culture conditions, concentration method, quantity, identity, purity and impurities, sterility, potency, reproducibility, storage and formulation), as well as safety and efficacy issues. Special considerations for applying MSC-EVs, such as their compatibility with arthroscopy for the treatment of tendon and ligament injuries, are also highlighted.
1. Introduction
1.1. MSCs and MSC-EVs for Tissue Repair and Disease Treatment
Mesenchymal stromal cells (MSCs) are pluripotent, non-hematopoietic stem cells with self-renewal capability. They have immunomodulatory, pro-angiogenic and growth promoting effects and are capable of homing to injured sites. Consequently, there has been an extensive interest in their therapeutic use for tissue repair due to conditions such as neurological disorders [1], liver diseases [2], cardiovascular diseases [3], immune-mediated disorders [4], bone fractures [5] and osteoarthritis [6].
Mounting evidence suggests that MSCs enhanced tissue repair via paracrine factors rather than by direct differentiation [7]. As a consequence, researchers are primarily focusing on MSC secretome, which include soluble proteins, lipids and extracellular vesicles (EVs). EVs are membranous, nano-sized vesicles secreted by all cells. They participate in intercellular communication and possess intrinsic therapeutic activity via addressing their vesicular content of DNA, RNA, proteins and other cellular components from a producer cell to a recipient cell. They can be classified into different types, i.e., exosomes (50–150 nm in diameter), microvesicles (100–1000 nm in diameter) and apoptotic bodies (1–5 μm in diameter), depending on their size and biogenesis. EVs have been shown to modulate the immune response [8,9], potentiate tissue regeneration [10] and function as a potential alternative to stem cell-based therapies [11]. Indeed, MSC-EV-enriched preparations have been shown to be as therapeutically effective as their parent cells in different pre-clinical models in head-to-head comparisons [12,13,14]. Genetically modified EVs can also act as delivery vehicles for therapeutic agents [15]. The capacity of EVs has hence spurred interest in their use both as a delivery system and as a drug for the treatment of various disorders.
1.2. Advantages of MSC-EV-Based Therapeutics
EV-based therapies in tissue repair and regeneration offer numerous advantages, i.e., lower cost, easier storage and improved safety and patient compliance. First, EVs lack self-replicating ability, thereby reducing the risk of malignant transformation and ectopic tissue formation. Second, while MSCs generally have low immunogenicity and are suitable for allogeneic transplantation [16,17,18,19,20,21,22], the lack of immunogenic cell surface proteins in exosomes likely further reduces immunogenic response after allogeneic transplantation and allows them to be used as an off-the-shelf product. Third, the lipid bilayer membrane also enhances the stability and reduces the toxicity of the EV cargo during in vivo delivery. Unlike soluble proteins in the MSC secretome, it is easier to concentrate EVs, though there may be a loss of some potency of the secretome. Moreover, EVs have the intrinsic ability to cross the epithelium and blood-brain barrier, and hence, may be useful for the delivery of drugs with low oral bioavailability and poor epithelial penetration [23,24,25,26]. In addition, the ability to engineer EVs to improve their specific cell targeting capacity or potentiate their therapeutic properties also makes them good drug carriers and therapeutics for tissue repair. EVs derived from MSCs therefore have the potential to serve as an alternative to MSCs in tissue repair. Early clinical trials investigating MSC-EV-based therapeutics have already begun [27,28,29]. Table 1 summarizes completed and ongoing clinical trials of MSC-EVs registered at www.clinicaltrials.gov (accessed on 30 June 2022).

Table 1.
List of clinical trials of MSC-EVs registered at www.clinicaltrials.gov (accessed on 30 June 2022).
2. MSC- and MSC-EV-Based Therapies for Tendon and Ligament Repair
Tendons and ligaments are subject to high tensile loads and are easily torn as a result of overuse or trauma, resulting in significant pain and disability. Together, tendon and ligament injuries account for 30% of all musculoskeletal consultations [30]. More than 32 million acute and chronic tendon and ligament injuries occur annually in the United States [31]. The outcomes of both conservative treatments and surgical repair of tendon are not satisfactory due to the long healing time, scar tissue formation, adhesion, occasional bone formation and high re-rupture rate. Similar to other tissues, the administration of MSCs is a promising approach for tendon, ligament and tendon-to-bone junction repair [32,33,34]. We have shown that the transplantation of tendon-derived stem cells (TDSCs) promotes tendon regeneration and graft healing after anterior cruciate ligament reconstruction (ACLR) [35,36,37]. Recently, there has been considerable interest in studying the effect of MSC-EVs on tendon and ligament repair, with encouraging results (Table 2) [38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61]. However, to date, no clinical trials have been undertaken. As such, the effects of MSC-EVs need to be evaluated in a clinical setting.

Table 2.
Summary of pre-clinical studies investigating the therapeutic effects of MSC-EVs on tendon and ligament repair.
The administration of MSC-EVs was generally found to promote cell proliferation and migration [43,44,47,48,49,53,54,55,58,59], suppress tissue inflammation and apoptosis [40,44,46,48,52,55,58,59], modulate inflammatory response of macrophages [38,39,46,48,52,55], increase collagen deposition [43,45,49], reduce fatty infiltration [50] and promote angiogenesis [52] during tendon and ligament repair. The active molecules in MSC-EVs that contribute to tendon and ligament repair are not entirely known. However, numerous studies have shown that MSC-EVs are rich in miRNA, which contribute to tendon and ligament repair. miRNA sequencing showed that human umbilical cord MSC-derived exosomes (HUMSC-Exos) expressed an antagonist to miR-21a-3p, as miR-21a-3p was under expressed in the exosomes compared to the parent cells. The inhibition of exosomal miR-21a-3p in HUMSC-Exos inactivated RelA/p65 (a core element in the NF-kB pathway involved in inflammation and fibrosis) and reduced the protein expression of Cox2 and α-SMA in rat fibroblasts [40]. Additionally, endoplasmic reticulum stress (ERS)-associated proteins (GRP78, CHOP) and pro-apoptotic protein (Bax) were presented in EVs derived from HUMSCs, potentially explaining their anti-adhesive effect on traumatic tendon injury [41]. In addition, HUMSC-Exos we have been shown to promote tendon healing via miR-27b-3p-mediated suppression of ARHGAP5, resulting in RhoA activation and increased proliferation and migration of primary injured tenocytes [42]. In another study, rat EVs derived from bone marrow-derived stromal cells (BMSC-EVs) were reported to express pro-collagen1A2 and MMP14 proteins, which are important factors for tendon extracellular matrix remodeling. Pro-collagen1A2 was expressed on the membrane surface of BMSC-EVs. Pretreatment of BMSC-EVs with trypsin abrogated their effects on tendon cell proliferation and migration and the expression of collagen type I, suggesting that the biological effects of EVs depended on the interaction of membrane-bound proteins with the recipient tendon cells [43]. Furthermore, miRNA sequencing indicated a significantly higher level of miR-29a-3p in HUMSC-Exos compared to HUMSCs [45]. The level of miR-29a-3p in HUMSC-Exos-treated Achilles tendons was also significantly elevated, and HUMSC-Exos overexpressing miR-29a-3p was found to amplify the effects of HUMSC-Exos on tendon healing in vivo [45]. In addition, exosomes derived from TDSCs (TDSC-Exos) have been found to contain miR-144-3p and enhance tendon repair through miR-144-3p-regulated tenocyte proliferation and migration [49]. Finally, exosomes derived from Scx overexpressing PDGFRα(+) BMSCs reduced osteoclastogenesis and improved tendon–bone healing strength via exosomal miR-6924-5p [56].
3. Translational Gap and Aim of Review
Unlike single active substances, MSCs and their EVs are comprised of pleiotropic bioactive ingredients, representing a major obstacle for the manufacture of reproducible drug products with consistently high efficacy and safety. The content of EVs is affected by the heterogenicity of the donor MSCs. The phenotypes and biological activities of MSCs depend on their origin (biological niche) or the conditions of potential donors (age, diseases, obesity or unknown factors) [62]. The artificial microenvironment of MSCs such as O2 tension, substrate and extracellular matrix cues, culture media, inflammatory stimuli and genetic manipulations can also influence the resulting phenotypes and, hence, paracrine activities [62]. HUMSC-Exos cultured in two different cell culture media showed different surface compositions and cytokine contents [63]. Furthermore, the passage number of MSCs influences the biological activities of EVs. Exosomes isolated from early passage of rat BMSCs exhibited higher neuroprotective potential compared to exosomes derived from later passages [64]. Moreover, the mechanisms of the biogenesis of EVs also influence their content. At least three major subpopulations of EVs produced by different mechanisms, namely exosomes, microvesicles and apoptotic bodies, have been reported. Immortalized E1-MYC 16.3 human embryonic stem cell-derived MSCs were reported to produce at least three distinct 100 nm EV types by different biogenesis pathways that could be distinguished by their membrane lipid composition, proteome and RNA cargos [65]. In one study, BMSC-EVs were fractionated into different density fractions [66]. The different EV gradient fractions were heterogeneous in terms of the quantity and expression of classical exosomal markers. The miRNA and protein profiles of these EV fractions were different, and they also showed differential effects on renal tubular cells in terms of degree of internationalization, stimulation of cell proliferation and inhibition of apoptosis. Therefore, MSC-EVs are heterogeneous, with specific signatures accounting for the biological activity of different subpopulations. EVs produced by a given population of cells cultured under identical conditions are not identical.
Gene therapy medicinal products, somatic cell therapy medicinal products and tissue-engineered products are regulated as advanced therapy products (ATPs) in the authors’ homeland [67] and other places, including Europe (Regulation EC No 1394/2007) [68]. Native cell-free EVs without transgene products are not categorized as ATPs and fall within a regulatory gap. However, EVs are categorized as pharmaceutical products/biological medicinal products, and hence, require clinical trials as investigational new drugs (INDs) before getting marketing approval. To receive an IND, investigators have to demonstrate that their EV-based products fulfill the requirements regarding quality, reproducibility, safety and efficacy. This is especially a challenge for EV-based products due to the heterogeneity and complexity of their composition. While fractionation or sorting may help to identify more homogeneous subpopulations of EVs, careful control of the donor cell source, the cell culture conditions and the EV enrichment process are crucial for clinical translation of EV-based therapies, as they would standardize the EV composition and content.
In this review, we will discuss the general and tendon/ligament-specific considerations of manufacturing MSC-EV-based products for clinical translation. Specially, we will discuss Good Manufacturing Practice (GMP)-compliant manufacturing and quality control (QC), safety and efficacy issues. Special considerations for applying MSC-EVs for the treatment of tendon and ligament injuries are also discussed.
4. Considerations for the Manufacturing, Quality Control, Safety and Efficacy of MSC-EVs
The process is the product. A scalable, reproducible and GMP-compliant manufacturing protocol should be followed to produce MSC-EV therapeutics for the promotion of tendon and ligament healing. Standard operating procedures (SOPs) for MSC-EV manufacturing should be followed and the production process should be documented to ensure high batch-to-batch consistency. Newly produced batches should be compared to previous batches regarding physiochemical properties and biological activity.
Besides controlling the manufacturing process, it is important to specify the release criteria of the physio-chemical-biological properties of MSC-EVs for clinical trials or applications. This is vital for ensuring the efficacy, safety and consistency of MSC-EVs as pharmaceutical products. The release criteria are highly product-specific and depend on the cell source and clinical application. Each MSC-EV preparation for a clinical application has its own specifications. The requirements to examine the physiochemical characteristics and sterility are common to different EV products. However, the requirements for testing the identity, purity, impurities, potency, reproducibility, storage and formulation vary with different MSC-EV preparations. The issues that need to be considered for the manufacturing, QC, safety and efficacy of MSC-EVs are discussed below.
4.1. MSC-EV Manufacturing
4.1.1. Parent Cell Source
The following factors should be considered for the isolation of EV-producing MSCs for therapeutic applications [69].
- Tissue source of MSCs
- Age, medication, and medical history of donor
- Allogeneic versus autologous source
- Any priming of MSCs
- Any genetic modification of MSCs
- MSC culture conditions (MSc isolation procedures, seeding density, culture volume, culture vessel, oxygen level, culture medium, culture time, cell viability, passaging)
- MSC storage and recovery conditions
The source of MSCs for EV production determines the manufacturing and QC strategy of the process and the final product. The primary factors to consider include the tissue origin of the cells, the age and medical history of the donor, whether the cells are autologous or allogeneic and whether the cells have been primed or genetically modified. The plasticity of MSCs isolated from different tissues varies, affecting the properties of the isolated EVs [70,71,72]. The proliferation capacity and yield of MSCs also vary based on the tissue of origin [73,74]. Both proliferation capacity and MSCs yield are important factors for large-scale and affordable production of MSCs and EVs for therapeutic applications. The use of cells isolated from tissue similar to the site of transplantation may have an advantage due to the priming of the cells by the local tissue microenvironment. For instance, TDSCs were reported to show higher proliferation, colony-forming ability and multi-lineage differentiation potential compared to paired BMSCs [73] and to form tendon-like tissue after subcutaneous transplantation to a nude mouse model [75]. MSCs isolated from young and healthy subjects are preferred, as aged MSCs and MSCs derived from diabetic or obese patients have been reported to show reduced activities [76,77,78,79,80,81,82]. Clinical examinations are needed to check for signs or symptoms of communicable diseases among donors. In addition, serological tests of at least the human immunodeficiency virus (types 1 and 2), hepatitis virus (B and C) and Treponema pallidum should be performed [83]. The inclusion and exclusion criteria for MSC donors need to be carefully defined and followed.
MSCs are generally reported to have low immunogenicity, and the use of MSC-EVs further reduces safety concerns. As EVs cannot replicate, the safety concerns of using EVs derived from autologous and allogeneic cells for tissue repair are similar. The use of allogeneic cells allows for the large-scale production of EV-based products as off-the-shelf materials and is, hence, preferred. However, the ease, feasibility and ethical concern of getting allogeneic MSCs from patients not suffering from injuries vary with the tissue of origin of MSCs. The identification of a practical MSC source with high proliferative and multi-lineage differential potential is crucial for large-scale manufacturing of MSC-EVs for clinical application. To use allogeneic MSCs for EV production, a two-tier cell banking system, consisting of a fully characterized master cell bank (MCB) and partially characterized working cell banks (WCB), should be set up [84]. A four-tier cell banking system has also been suggested [85]. However, at the early stages, as a first-in man clinical trial, an approach based on only a well-characterized MCB is acceptable (may add a Post Production Cell Bank (PPCB) as discussed below). The criteria for releasing MSCs for EV production should be clearly defined.
4.1.2. Culture Conditions
In additional to the MSC source, the culture conditions should also be considered. How the cells are manipulated (i.e., any stimulation or pre-treatment), cultured and stored, as well as the experimental conditions (i.e., culture medium composition and lot numbers, passage number, days in culture, seeding density, density/confluence at harvest, culture volume, culture containers, surface coatings, oxygen or other gas tensions and frequency and intervals of harvest) can affect the recovery of MSC-EVs, and, hence, should be documented and remain consistent [86].
For the culture of EV-producing MSCs, a GMP-grade culture system should be considered. Both static systems, like flasks, and dynamic systems, such as bioreactors, can be used for MSC culture. For static systems, both standard tissue culture flasks and special-coated flasks such as CellBIND® flasks have been used to produce GMP-compliant EVs [87]. For large-scale production of MSCs, bioreactors are preferred. The metabolic (glucose and lactate) and physical condition (temperature, pH, oxygen, carbon dioxide) readouts during culturing in bioreactors allows for better control and monitoring of the activities of MSCs, which is beneficial for meeting the regulatory requirements for clinical-grade production. Cell culture conditions such as pH, temperature, pO2, cell culture duration and metabolic activity (such as glucose and lactose), when applicable, should be defined, recorded and compared to the QC acceptance criteria at the manufacturing phase. Regular cell counting and checking of cell viability should be done. The growth rate of MSCs should be monitored by population doubling time and level.
The use of animal origin-free dissociation enzymes and a culture medium of defined composition is preferred for MSC culture and EV enrichment. The use of a xeno-free medium for cell culture has been reported to reduce the doubling time compared to that of cells cultured in research-grade conditions, to increase exosome yield and to remove 97% of the contaminating proteins [88]. EV-depleted medium should be used for EV isolation. The collection of EVs under ideal, serum-free conditions reduces contamination by non-EV proteins and co-isolation of exogenous EVs from serum. However, starvation may affect the physiology of the EV-producing MSCs, and, hence, EV quantity and quality. Human platelet lysate (HPL)-based EV-depleted medium was shown to be suitable for GMP-compliant MSC propagation and MSC-EV enrichment with retained characteristic of MSC surface marker expression, cell morphology, viability and in vitro differentiation potential [89]. In such cases, we recommend including a non-conditioned medium control to assess the contribution of the medium itself to EV production. If priming agents are added to MSCs to improve the quantity and quality of EV production, the concentration of residual priming molecules in the medium after washing and EV yield per cell equivalent (CE) should be documented and compared to the acceptance criteria. The percentage of dead cells at the time of MSC-EV harvest should be indicated, since even a small percentage of dead cells can release apoptotic bodies exceeding the amount of EVs [86].
A cell passage limit for harvesting EVs should be set, as MSCs show cellular senescence and reduced activity during sub-culturing [90]. To test the stability of MSCs during in vitro passaging, a PPCB, consisting of cells at the limit of in vitro passaging for EV production, can be included.
The cryopreservation and thawing methods, as well as the maximum cryopreservation time, should be validated and defined in the manufacturing process. The cell identity, viability and growth rate should be checked after recovery of MSCs from freezing. According to the Food and Drug Administration (FDA) and European Medicines Agency (EMA) guidelines, the minimum acceptance criteria for the viability of MSC-based products and after cell thawing are ≥80% and ≥70%, respectively [91,92].
The identity of MSC culture needs to be checked. The International Society for Cellular Therapy has published a set of minimal criteria for defining MSCs (plastic-adherent, expression of phenotypic markers and ability to differentiate) [93]. In reality, it is well-known that the ISCT criteria are rarely clinically useful, as the markers are not specific to MSCs [94]. For MSCs isolated from specific tissue, additional tissue-specific markers may be included to confirm the cell source. For instance, TDSCs are known to express high levels of scleraxis (Scx) and tendomodulin (Tnmd), which could be added to the marker list for cell identification [94]. In addition, positive expression of major histocompatibility complex (MHC) class I and low/negative expression of MHC II molecules are frequently evaluated due to their roles in immune cell recognition and, thus, their relationship with potential immune response after allogeneic MSC transplantation.
A potency test mimicking the in vivo effects of MSCs is recommended. Mixed lymphocyte reaction (MLR) and similar approaches (peripheral blood mononuclear cell, PBMC stimulation assay) are commonly used by most groups studying MSCs [95]. However, there are concerns about their robustness as potency release tests. Several variations of the standard assays have been proposed to increase robustness, such as measuring MSC-stimulated induction of regulatory T cells (Treg) and assessing markers indicating the immunosuppressive properties of MSCs, including CD200, TNF-αR, IDO and PD-L1, after IFN-γ stimulation [96,97,98,99,100]. Further validation of these tests is needed.
The manufacture and QC of EVs derived from unmodified cells are simpler compared to those for EVs isolated from apparently more complicated, genetically modified cells. More stringent regulations by health authorities are anticipated. The potential risks due to the vector and the specific transgene used for cell transformation should be identified and controlled in the manufacturing and QC procedures. Furthermore, endotoxin, bacterial, fungal, mycoplasma, adventitious viral, genomic stability (such as karyotyping) and in vitro tumorigenicity tests (such as soft agar colony formation assay) of MSC culture should be done.
In summary, throughout the in vitro expansion process, parent MSCs should be characterized at different checkpoints. The QC panel, including cell culture conditions, cryopreservation and thawing procedures, identity, viability, growth rate, purity, impurities, sterility, stability, safety, and potency of parent MSC banking, is shown in Table 3. MSCs are released for EV isolation only when all the QC results comply with the specifications. As with EV preparations, some MSC samples should be retained for analytical (reference samples) and identification purposes (final product).

Table 3.
Suggested QC panel of parent MSC bank for EV production [69,91,92,93,101,102,103,104,105].
4.1.3. Enrichment Method
There is no single optimal method for the concentration or enrichment of MSC-EVs. Different enrichment methods produce different populations [72]. Enrichment methods differ in terms of cost, ease of use, throughput, requirements in time and instrumentation, vesicle loss and the purity of the final product. The MISEV2018 guideline suggests arranging each method on a “recovery vs. specificity” grid [86]. By choosing a highly scalable and automatable concentration method as early as in the pre-clinical stage for MSC-EV isolation, changes in the EV specifications due to the scaling up of the isolation method for clinical trials can be avoided.
Ultracentrifugation (UC) is the most commonly used method for EV enrichment. However, this approach can co-isolate non-EV components, especially for biological fluids such as serum and urine. Due to high shear forces, EVs may aggregate or break, leading to reduced biological activity [106,107]. Ultracentrifugation is time-consuming and has low throughput, limiting its use to small-scale studies. Density gradient centrifugation (DGC) is more efficient for EV isolation which concentrates EVs in a discrete density band. However, the presence of residual gradient material may affect the purity of the finished product. Tangential flow filtration (TFF) is a gentle, size-based fractionation method for EV concentration. It is time-efficient and suitable for large-scale concentration. TFF provides higher EV yields and more effective removal of soluble proteins than enrichment by UC. EVs thus isolated are morphologically intact and display superior batch-to-batch consistency [72,108]. An MSC secretome concentrated by TFF was reported to yield higher protein, lipid, cytokine, and exosome contents than those concentrated by UC [109]. Size-exclusion chromatography (SEC) produces purer EVs. A previous study showed a 100-fold reduction in ferritin, a contaminant, in SEC-concentrated exosomes [110,111].
The enrichment of EVs by precipitation with polymers such as polyethylene glycol (PEG) inevitably leaves residual polymers in the preparation, interfering with downstream characterization and biological activities [111]. EVs prepared by the precipitation method have been shown to have lower biological activities than those enriched by DGC; this might be due to the masking of important EV surface molecules by residual precipitation reagents [111,112] and may render the EVs unusable for therapeutic applications. Moreover, this method is highly non-specific and can easily co-precipitate non-EV components. A combination of different enrichment methods may be used to enhance EV integrity and activities while reducing contamination. For example, SEC has been used to remove gradient material in DGC [113]. TFF combined with SEC has been reported to produce highly pure and functional EVs from large volumes of samples with minimal vesicle loss [114]. Details of the advantages and limitations of different enrichment methods can be found in a recent review [115].
The production and enrichment process, once standardized, should not be easily changed. Any modifications made to any step of the enrichment process need to be validated to confirm that the properties of the MSC-EVs remain unchanged.
4.2. MSC-EV Quality Control
Once the manufacturing procedures of MSC-EVs have been established, a list of QC standards to ensure the quality of the resulting EV preparations produced in different batches should be devised. While the characterization of MSCs and MSC-EVs should be as exhaustive as possible during the product development phase, the tests chosen for routine in-process control, recovery from storage and final product analysis should be technically relevant and feasible in terms of cost, labor and time. The quantity and identity, purity and impurities, sterility, potency, reproducibility, storage and formulation are the most commonly assessed parameters of MSC-EVs.
4.2.1. Quality and Identity
Physicochemical Properties
The physical appearance of MSC-EVs, including the color, uniformity of mass and the presence of visible particles, needs to be noted and reported. General tests, such as pH and osmolality, of MSC-EV solutions are required. For freeze-dried MSC-EVs, the appearance of lyophilizate as well as the dissolution time, color, moisture content and clarity of the reconstituted solution should be recorded in the product development, in-process manufacturing and recovery phases, as well as in the final product.
Particle Size and Concentration
MSC-EV size and concentration, and optionally, zeta potential (ZP), should be documented in the product development, in-process manufacturing and recovery phases, as well as in the final product. The particle size of MSC-EVs can be determined by tunable resistive pulse sensing (TRPS), microfluidic resistive pulse sensing (MRPS), nanoparticle tracking analysis (NTA) or dynamic light scattering (DLS). Additionally, NTA, MRPS and TRPS make it possible to measure particle concentrations. Since these techniques cannot differentiate between EVs and non-EV particles and tend to overestimate EV concentration, unless antibodies staining is allowed, additional tests to validate the successful isolation of MSC-EV particles are needed. Many NTA devices can be used to analyze vesicles labelled with fluorescence dyes, which can increase the specificity of MSC-EV measurements. However, the sensitivity of such devices is limited for small particles. Moreover, only a subset of MSC-EVs can be measured, as there is no universal EV marker. Therefore, it is important to supplement this approach with examinations of MSC-EV morphologies by electron microscopy (EM) in the product development stage. The expression of MSC-EV quantities in cell or EV component (proteins, lipids, RNA) equivalents is helpful in dose-response studies and in gauging the yield of EVs in different batches of MSCs.
Zeta Potential
Zeta potential (ZP) describes the net electrical charge of molecules on the EV surface and is a measure of colloidal stability and aggregation of EVs. The particle environment (presence of charged or uncharged molecules that can adsorb on the particle surface, particle concentration and the pH and ionic strength of the solution) affects the ZP [116]. The ZP of MSC-EVs can be monitored using TRPS/MRPS/NTA/DLS and is expected to be slightly negative [117,118,119]. A stability study based on evaluating the colloidal behavior of MSC-EVs may be useful for identifying the optimal storage conditions.
Morphology
To differentiate EVs from non-EV particles, EM analysis is needed. Transmission electronic microscopy (TEM) shows the typical lipid bilayer of EV as a cup-shaped structure in the resulting image. It can show non-EV substances in the mixture, but it cannot quantify the amount of contaminating soluble factors. EM analysis may not be feasible as a routine test in the in-process manufacturing and recovery phases or in the final product but is recommended at least during the product development phase.
EV Phenotyping
EVs should be phenotyped by the expression of EV protein markers, as suggested by International Society of Extracellular Vesicles [86]. The minimal information for studies of EVs 2018 (MISEV2018) has suggested some criteria for defining EVs [86]. They recommended that at least one positive transmembrane/glycosylphosphatidylinositol (GPI)-anchored protein (e.g., tetraspanins (CD9, CD63, CD81)), one cytosolic protein recovered from EVs (e.g., ESCRT-I/II/III (TSG101) and the accessory protein ALIX, as well as heat shock proteins HSC70 and HSP84) and a negative non-EV protein marker in a specific system (e.g., apolipoprotein A1/2, apolipoprotein B, albumin for plasma) should be assessed. Quantitative analysis of potential contamination based on the presence of proteins in subcellular compartments other than the plasma membrane and endosomes, such as endoplasmic reticulum markers calnexin and GRP94/GP96, can also provide information about the purity of the exosomes. A list of potential contaminants for testing needs to be created in advance, based on the tissue source used for MSC-EV isolation.
Unlike the minimal criteria for defining multipotent MSCs proposed by the International Society for Cellular Therapy [93], the threshold percentage of particles expressing EV markers is not defined by MISEV [86]. Hence, Western blotting of EV markers is still commonly used to confirm EV identity. The result is semi-quantitative and requires large sample volumes, and, hence, is poorly adapted to high throughput analysis. Techniques such as enzyme-linked immunosorbent assay (ELISA), MACSPlex Exosome kit [120], small particle flow cytometry and nano-flow cytometry [121], which enable multiplexing and require only small amounts of EVs for analysis, are preferred for QC of MSC-EVs.
MSC-EV Content
As MSC-EVs carry inherently complex cell-specific cargos of proteins, lipids and genetic materials, standardized characterizations are challenging. In addition to the core signature of highly enriched vesicular proteins, other proteins reflecting the specific parent cell-origin and biogenesis pathway are present. From a therapeutic perspective, this complexity needs to be understood through comprehensive (multi)omic studies [122]. Mass spectrometry (MS)-based proteomics, metabolomics and lipidomics and next-generation sequencing transcriptomics (NGS/RNA-seq) allow high throughput and quantitative studies of EV-derived proteins, metabolites, lipids and RNA species, respectively [123]. These molecules can provide insights into unique biomarkers and the underlying biological mechanisms of MSC-EV-based therapeutics. This information is useful for the development of biomarkers for QC of MSC-EVs of different tissue origins and with different biogenesis pathways.
For routine QC in GMP facilities, it may not be suitable to use the non-targeted profiling approach described above for the measurement of proteins, metabolites, lipids and RNA species in MSC-EVs. As a proxy for the standardization of MSC-EV preparations, the use of simple, reliable and high throughput methods, such as bicinchoninic acid (BCA) assay for total proteins, sulfovanilin assay [124] or Nile red assay [109] for lipids and RiboGreen assay or UV-Vis spectrophotometry (e.g., NanoDrop) for RNA species, may be more appropriate, despite the fact that the sensitivities of these detection methods are limited. It is important to keep in mind that the total protein content is valid only if the MSC-EVs are isolated from cells cultured in a serum-free, chemically defined medium. Some RNA and DNA species may be presented in the MSC-EV preparation as un-encapsulated particles. We recommend measuring the RNA/DNA content in MSC-EV preparations with and without DNase/RNase treatment to differentiate RNA/DNA content in MSC-EVs from impurities in the development, manufacturing and recovery phases, as well as in the final product. For MSC-EV-based products with known RNA molecules, microarrays and real time quantitative reverse transcription polymerase chain reaction (qRT-PCR) can be used to test for the presence of specific RNA species in the development, manufacturing and recovery phase and in the final product.
4.2.2. Purity and Impurities
Purity
Consensus on the definitions and quality metrics of EV purity remain to be established. The particle-to-protein ratio (P/µg protein) [125,126], protein-to-lipid ratio [124,127] and particle-to-RNA ratio [128] have been suggested as indicators of EV purity. Among them, the particle-to-protein ratio is commonly used, because impure preparations are expected to contain non-EV proteins, reducing the particle-to-protein ratio.
Based on the study of cell lines and biological fluids, Webber and Clayton [125] proposed the concentrations >3 × 1010 P/µg, 2 × 109–2 × 1010 P/µg and <1.5 × 109 P/µg to represent highly pure, less pure and impure EV preparations, respectively. However, it was demonstrated that it was difficult to reach the high purity ratio for biological fluids by simple ultracentrifugation and washing. The simple ultracentrifugation and washing of EVs derived from culture cells only reached the less pure ratio. More complex enrichment strategies or additional steps such as further concentration of EVs by DGC are required to achieve high purity; however, these methods may not be suitable for achieving the medium/high throughput required in clinical trials. Affinity capture-based approaches increase the purity of EVs, but the presence of antibodies may affect purity assessments. With the caveats that the particle-to-protein, protein-to-lipid and particle-to-RNA ratios are specific to a given biofluid or type of EV-producing cell and depend heavily on the respective enrichment method, they are useful in assessing batch-to-batch purity in a defined production system. These ratios should therefore be determined in-house for each specific MSC-EV product. Since the particle count obtained by nanoparticle tracking does not differentiate EV from non-EV particles, measuring the ratio of fluorescent EV particles expressing specific EV markers (CD9/CD63/CD81) to protein concentration may improve the specificity, although this method has not been validated.
It is understandable that a less pure MSC-EV preparation may raise concerns about efficacy and safety during clinical applications. While a certain degree of purity of MSC-EVs is definitely required for therapeutic applications, an extraordinarily pure MSC-EV preparation is not necessarily more effective than a less pure one. Additionally, highly pure MSC-EVs are not stable. Moreover, elevated concentration may damage the EVs and remove the loosely associated factors that act in conjunction with them, resulting in a loss of biological effects. Indeed, a recent study showed that the biological effects of MSC-EVs were actually attributable to the contaminating soluble factors present in the EV samples collected using a low-purity method [129]. Therefore, from the cost perspective of manufacturing MSC-EVs and where function is paramount, a less pure MSC-EV preparation may be more efficacious than a highly pure one. Some impurities, such as albumin and fibrinogen, are sometimes added to increase product stability. In cases where the impurities are not expected to have harmful effects on patients, batch-to-batch consistency is more important than reducing the level of impurities.
Impurities
Impurities can be process- or product-related. Process-related impurities include materials from the manufacturing steps, substrates or cell culture supplements. Product-related impurities include MSC-EVs degradation products which appear during manufacturing and storage. An upper limit of impurities should be defined for MSC-EV-based products. Measuring particle concentration and particle size, the concentration of biomarker-positive EVs and the total protein, total lipid and total RNA/DNA contents and their ratios will make it possible to indirectly monitor MSC-EVs and their degradation.
4.2.3. Sterility
Standard microbiological tests determining endotoxin levels, microorganisms, mycoplasma and adventitious viruses, performed by certified laboratories, are mandatory for GMP-compliant MSC-EV manufacturing.
4.2.4. Potency
In vitro biological assays that reflect the proposed/hypothesized mechanisms of action (MoA) of MSC-EVs in a quantitative manner should be developed for QC of the potency of different batches of MSC-EV preparations. Each therapeutic application of MSC-EVs should have its own in vitro potency assays to predict the intended therapeutic effects. Potency assays should be valid, reproducible, specific, sensitive, robust and cost-effective and have well-defined pass/fail criteria to meet the GMP-compliant release guidelines [130]. For instance, MSC-EVs have been shown to promote tendon and ligament healing by promoting tenogenesis, immunosuppression, proliferation and/or angiogenesis [131]. Hence, quantitative analysis of RNA or protein cargos that mediate these therapeutic effects may be one approach for quality control. Due to the complex MoA of MSC-EVs, the use of multiple assays to measure several MSC-EV attributes related to the intended use may be needed. Each individual potency assay should have its own reference standard, i.e., either a validated international or national one for established biochemical assays or one based results of an internally validated reference of MSC-EV preparations for cell-based assays.
4.2.5. Reproducibility
Batch-to-batch reproducibility in the manufacturing process is a key parameter of every pharmaceutical drug. A previous study showed that the batch of secretome, among other factors, including lyophilization, the concentration of excipient and total amount of excipients, is the primary source of variability of the lipid and protein contents and the anti-elastase activity in the production of EVs derived from human adipose-derived stromal cells (ADSC-EVs) [132]. To reduce variability, MSC-EVs isolated from MSCs of different donors or different cell passages can be pooled to compensate for inter-donor differences. The quantity, identity, purity, impurities and potency of different batches of MSC-EVs can then be compared. The pool size is determined by the variability of samples from different donors or passages. Reductions in variability are theoretically the reciprocal of the square root of the number of samples pooled. This equation was confirmed in a previous study after pooling EVs harvested from peripheral blood mononuclear cells (PBMSCs) isolated from different donors [133]. The % deviation was calculated as the standard deviation (SD)/mean × 100%; this can give information about the variability of different batches. In a previous study, a % deviation of less than 10 was regarded as demonstrating low variation [133]. There is a potential safety concern regarding combining EVs from different donors. Strict donor selection criteria and in-process controls such as traceability of the donor should be implemented in the manufacturing process. To enhance traceability, the pooling of MSC-EVs derived from different cell stocks is preferred over the pooling of MSCs from different donors. As there are currently no standards for MSC-EV-based products, a batch developed in-house, shown to be stable and suitable for clinical trials, should be used as an internal reference by which to calibrate the results of different batches and analytical instruments. Potency relative to the in-house reference batch should be reported.
4.2.6. Storage and Formulation
The storage and recovery conditions of isolated MSC-EVs can affect the EV characteristics, including stability, number of particles, aggregation and functions. The stability of biological products decreases over time during storage. The physiochemical and biological properties of MSC-EVs during storage for various times (e.g., 1, 3, 6 months) under accelerated and stress conditions (e.g., temperatures, relative humidity, pH) need to be determined to understand the degradation patterns and to define the limits of stability. EVs are sensitive to changes in pH, which can cause EV aggregation and loss of function. Isotonic buffers are recommended for storing EVs to prevent pH shifts during storage, freezing and thawing. Highly pure EVs may adhere to the surface of the storage container, making them impossible to recover. Low-protein binding synthetic materials should be used as storage containers. Common storage methods of MSC-EVs include freezing at −80 °C and lyophilization. The storage and retrieval conditions of MSC-EVs should be documented. If MSC-EV products are frozen, the number of repeated freeze–thaw cycles should be minimized to prevent EV aggregation, and QC measures should be built in after thawing in the manufacturing process. One or more cryoprotectants are usually added during cryopreservation [134]. Freeze-drying is expected to increase the ease of handling and shelf-life of MSC-EVs compared to suspensions. However, freeze-drying was reported to reduce the amount of proteins and lipids in ADSC-EV preparations [132]. The addition of lyoprotectants such as mannitol, trehalose or sucrose may improve the preservation of EVs under freeze-drying [134]. The choice of excipients can affect the protein and lipid contents, as well as the anti-elastase activity, of MSC-EV preparations [132,135,136] and should be tested. Since excipients affect QC procedures, they should be confirmed early in the manufacturing process.
The route of administration also influences the formulation, efficacy and safety of MSC-EVs. Biomaterials can be added to aid MSC-EV delivery. The kinetics of EV release from biomaterials should be determined. Local administration of MSC-EVs, ideally ultrasound-guided, is preferred, as tendon and ligament injuries are localized, allowing better control and fewer side effects of MSC-EV treatment. Surgeries such as ACLR and rotator cuff repair are assisted with arthroscopy to minimize damage to the surrounding healthy tissue and scarring, shorten recovery time and lower the risk of complications compared to open surgeries. Continuous flow of the irrigation buffer is required to visualize the operated site via an arthroscope. For arthroscopic-assisted tendon and ligament repair, MSC-EVs loaded in pre-formed biomaterial ex vivo are required. This avoids dispersion of MSC-EVs by the irrigation buffer and ensures their successful delivery to the operated site.
The QC panel for MSC-EV manufacturing is shown in Table 4.

Table 4.
Suggested QC panel of MSC-EVs at different stages of product development [68,69,86,87,101,109,115,124,125,126,127,128,137,138,139].
4.3. Safety
Prior to application in clinical trials, the effect of dose and frequency of administration of MSC-EVs on toxicity and immunogenicity need to be evaluated in animals. Phase I clinical trials of pharmacodynamics, pharmacokinetics and potential off-target effects of MSC-EVs at the proposed route of administration are also needed. While MSC-EVs share the complexity of ATP products, they do not fulfill the definition of an ATP unless they contain the transgene-products from genetically modified cells. Despite this, the general scientific principles regarding quality (characterization, potency, reproducibility) and non-clinical and clinical requirements for pharmaceutical products are applicable to MSC-EVs [68]. Autologous MSC-EVs naturally occur in the body, and the release of MSC-EVs is a natural physiological process. Substances in MSC-EVs are also physiological body constituents. There is compelling evidence of a good safety profile of allogeneic MSCs in animal studies [140,141] and clinical trials [142,143]. Derived EVs are, therefore, expected to be safe. There is no evidence that allogeneic EVs cause any adverse events after transplantation in immunocompetent animals [144,145] (Table 2). EVs derived from ADSCs and BMSCs have been found to be non-immunogenic in an in vitro immunogenicity assay [109]. The transplantation of MSC-EVs, if derived from the same origin of target tissue, would further lower safety concerns. Recent clinical reports on the application of allogeneic MSC-EVs for the treatment of a human graft-versus-host disease patient [146], chronic kidney disease patients [147] and patients with COVID-19 [29] showed no adverse side effects, supporting their general safety.
4.4. Efficacy
The availability of animal data demonstrating the efficacy of MSC-EVs in tendon and ligament repair is crucial for subsequent clinical trials. MSC-EVs isolated from different tissues have been shown to promote healing of acute tendon injuries, acute ligament injuries, a failed healing model of tendinopathy and tendon-to-bone junction repair (Table 2). Further research is needed due to the different tissue origins, species, dose and route of administration of MSC-EVs, as well as the different animal models used in previous studies. Data on the dose-frequency response relationship of MSC-EVs are needed to determine the optimal dose for the treatment of specific tendon or ligament injuries. There is no consensus on the best normalization strategy for EV dose (e.g., number of cells or tissue mass), but a rationale should be provided, according to MISEV2018 [86]. As discussed, local injections of MSC-EVs appear to be more suitable for the treatment of local tendon and ligament injuries, and may reduce the dose and manufacturing cost of MSC-EVs in future clinical trials. One pre-clinical study reported systematic intravenous injection of 200 µg protein of BMSC-EVs weekly for the promotion of rotator cuff repair in a rat model [52]. The dose used was high and prohibited future clinical application.
Figure 1 outlines the manufacturing considerations and QC program for the development of GMP-grade MSC-EVs.
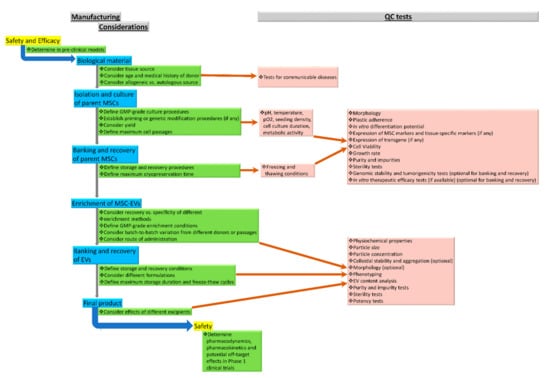
Figure 1.
Manufacturing considerations and QC program for the development of GMP-grade MSC-EVs.
5. Conclusions
In recent years, there has been a boom in research on the effects of MSC-EVs for the treatment of various tendon and ligament injuries. However, none of this research has reached the clinical phase yet. Several key technical challenges need to be overcome for the clinical application of MSC-EVs. First, the biochemical composition of MSC-EVs remains unclear. The production or uptake mechanisms are poorly described, and GMP standards for clinical grade production, storage and recovery are lacking. Second, the drug loading efficiency of EVs is relatively lower than that of liposomes. Third, engineered MSC-EVs containing transgene products are categorized as ATPs, and hence, need to meet more stringent regulatory requirements. Fourth, as the half-life of MSC-EVs is short and they cannot replicate themselves like MSCs, large amounts of MSC-EVs are required for clinical application. The large scale and efficient production of MSC-EVs remains difficult at present. Finally, the physiochemical properties, particle size and concentration, as well as the MSC-EV content, have been the focus of numerous investigations. The MoA or potency of MSC-EVs remains relatively unexplored, resulting in a lack of appropriate functional assays. To overcome the bottleneck for MSC-EV-based therapies, clinical-grade MSCs meeting the requirements for therapeutic use and transplantation are needed for MSC-EV production. Conditions to ensure the sustainable release of MSC-EVs should be established. The specifications of MSC-EVs vary according to the enrichment method. Therefore, it is important to choose a cost-effective, scalable and automatable concentration method as early as possible to avoid changing the method and, hence, MSC-EV specification due to a subsequent scaling up of production. While there is some consensus regarding the transportation and storage of MSC-EVs at −80 °C, this poses challenges and is a cost-ineffective approach. Alternative methods, such as lyophilization, may improve MSC-EV stability during storage and transportation. The route of administration greatly influences the therapeutic efficacy and safety of MSC-EVs and should be determined prior to clinical application. Efforts to develop high-throughput and precise quantification methods for assessing MoA or potency will greatly facilitate the clinical translation of MSC-EV-based therapies. The product is the process. Any slight change in the MSC-EV production process, formulation or storage conditions can influence the composition and biological activity of the finished product. It is, hence, important to build in scalability, reproducibility and GMP concepts, along with QC measures and release criteria to MSC-EV-based products from the time of deciding to manufacture them into pharmaceutical products, or even earlier, at the pre-clinical stage. Researchers have to consider practical issues associated with manufacturing procedures and QC measures, as described in this review, in order to translate the pre-clinical results of MSC-EVs into clinical practice for the treatment of tendon and ligament injuries.
Author Contributions
Conceptualization: P.P.Y.L.; searching of information and drafting of tables: P.P.Y.L., Y.T.L.; writing—original draft preparation: P.P.Y.L.; writing—review & editing: P.P.Y.L.; final approval: P.P.Y.L., Y.T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Center for Neuromusculoskeletal Restorative Medicine (ITC RC/IHK/4/7) supported by the Innovation and Technology Commission-Hong Kong.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Angelina Yui Ling CHU for editing and polishing the English of this manuscript.
Conflicts of Interest
The authors declares no conflict of interest. The funder had no role in the design, execution, interpretation, or writing of the study.
References
- Andrzejewska, A.; Dabrowska, S.; Lukomska, B.; Janowski, M. Mesenchymal Stem Cells for Neurological Disorders. Adv. Sci. 2021, 8, 2002944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Y.; Fan, L.; Zhang, F.; Li, L. The clinical application of mesenchymal stem cells in liver disease: The current situation and potential future. Ann. Transl. Med. 2020, 8, 565. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yu, Y.; Hu, S.; Chen, Y.; Shen, Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Markov, A.; Thangavelu, L.; Aravindhan, S.; Zekiy, A.O.; Jarahian, M.; Chartrand, M.S.; Pathak, Y.; Marofi, F.; Shamlou, S.; Hassanzadeh, A. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res. Ther. 2021, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Sohn, J.; Shen, H.; Langhans, M.T.; Tuan, R.S. Bone marrow mesenchymal stem cells: Aging and tissue engineering applications to enhance bone healing. Biomaterials 2019, 203, 96–110. [Google Scholar] [CrossRef]
- Arshi, A.; Petrigliano, F.A.; Williams, R.J.; Jones, K.J. Stem Cell Treatment for Knee Articular Cartilage Defects and Osteoarthritis. Curr. Rev. Musculoskelet. Med. 2020, 13, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther. 2016, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Marar, C.; Starich, B.; Wirtz, D. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 2021, 22, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Gomzikova, M.O.; James, V.; Rizvanov, A.A. Therapeutic Application of Mesenchymal Stem Cells Derived Extracellular Vesicles for Immunomodulation. Front. Immunol. 2019, 10, 2663. [Google Scholar] [CrossRef] [PubMed]
- Nagelkerke, A.; Ojansivu, M.; van der Koog, L.; Whittaker, T.E.; Cunnane, E.M.; Silva, A.M.; Dekker, N.; Stevens, M.M. Extracellular vesicles for tissue repair and regeneration: Evidence, challenges and opportunities. Adv. Drug Deliv. Rev. 2021, 175, 113775. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Brennan, M.Á.; Lötvall, J.; Breakefield, X.O.; El Andaloussi, S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11, eaav8521. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Grange, C.; Deregibus, M.C.; Calogero, R.A.; Saviozzi, S.; Collino, F.; Morando, L.; Busca, A.; Falda, M.; Bussolati, B.; et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 2009, 20, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Doeppner, T.R.; Herz, J.; Görgens, A.; Schlechter, J.; Ludwig, A.K.; Radtke, S.; de Miroschedji, K.; Horn, P.A.; Giebel, B.; Hermann, D.M. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl. Med. 2015, 4, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, Y.; Sun, S.; Yu, M.; Wang, C.; Pei, X.; Zhu, B.; Wu, J.; Zhao, W. Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology 2012, 17, 493–500. [Google Scholar] [CrossRef]
- Szwedowicz, U.; Łapińska, Z.; Gajewska-Naryniecka, A.; Choromańska, A. Exosomes and Other Extracellular Vesicles with High Therapeutic Potential: Their Applications in Oncology, Neurology, and Dermatology. Molecules 2022, 27, 1303. [Google Scholar] [CrossRef] [PubMed]
- Tasso, R.; Augello, A.; Carida, M.; Postiglione, F.; Tibiletti, M.G.; Bernasconi, B.; Astigiano, S.; Fais, F.; Truini, M.; Cancedda, R.; et al. Development of sarcomas in mice implanted with mesenchymal stem cells seeded onto bioscaffolds. Carcinogenesis 2009, 30, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.T.; Butler, D.L.; Boivin, G.P.; Florer, J.B.; Schantz, E.J.; Wenstrup, R.J. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J. Orthop. Res. 2004, 22, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.A.; Boivin, G.P.; Dressler, M.R.; Smith, F.N.; Young, R.G.; Butler, D.L. Repair of patellar tendon injuries using a cell-collagen composite. J. Orthop. Res. 2003, 21, 420–431. [Google Scholar] [CrossRef]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef]
- Breitbach, M.; Bostani, T.; Roell, W.; Xia, Y.; Dewald, O.; Nygren, J.M.; Fries, J.W.; Tiemann, K.; Bohlen, H.; Hescheler, J.; et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood 2007, 110, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Galiè, M.; Konstantinidou, G.; Peroni, D.; Scambi, I.; Marchini, C.; Lisi, V.; Krampera, M.; Magnani, P.; Merigo, F.; Montani, M.; et al. stem cells share molecular signature with mesenchymal tumor cells and favor early tumor growth in syngeneic mice. Oncogene 2008, 27, 2542–2551. [Google Scholar] [CrossRef] [PubMed]
- Machova Urdzikova, L.; Sedlacek, R.; Suchy, T.; Amemori, T.; Ruzicka, J.; Lesny, P.; Havlas, V.; Sykova, E.; Jendelova, P. Human multipotent mesenchymal stem cells improve healing after collagenase tendon injury in the rat. Biomed. Eng. Online 2014, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.J.; O’Loughlin, A.J.; Samira, L. Exosomes and the blood-brain barrier: Implications for neurological diseases. Ther. Deliv. 2011, 2, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Skog, J.; Wurdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Saint-Pol, J.; Gosselet, F.; Duban-Deweer, S.; Pottiez, G.; Karamanos, Y. Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles. Cells 2020, 9, 851. [Google Scholar] [CrossRef]
- Shi, M.M.; Yang, Q.Y.; Monsel, A.; Yan, J.Y.; Dai, C.X.; Zhao, J.Y.; Shi, G.C.; Zhou, M.; Zhu, X.M.; Li, S.K.; et al. Preclinical efficacy and clinical safety of clinical-grade nebulized allogeneic adipose mesenchymal stromal cells-derived extracellular vesicles. J. Extracell Vesicles 2021, 10, e12134. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Shi, M.M.; Monsel, A.; Dai, C.X.; Dong, X.; Shen, H.; Li, S.K.; Chang, J.; Xu, C.L.; Li, P.; et al. Nebulized exosomes derived from allogeneic adipose tissue mesenchymal stromal cells in patients with severe COVID-19: A pilot study. Stem Cell Res. Ther. 2022, 13, 220. [Google Scholar] [CrossRef]
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef]
- Kaux, J.F.; Forthomme, B.; Goff, C.L.; Crielaard, J.M.; Croisier, J.L. Current opinions on tendinopathy. J. Sports Sci. Med. 2011, 10, 238–253. [Google Scholar]
- Engebretson, B.; Mussett, Z.; Williams, C.; Simmons, A.; Sikavitsas, V. Chapter 12—Tendon Tissue Engineering: Combined Tissue Engineering Approach for the Regeneration of Tendons. In Tendon Regeneration; Gomes, M.E., Reis, R.L., Rodrigues, M.T., Eds.; Academic Press: London, UK, 2015; pp. 321–347. [Google Scholar]
- Hevesi, M.; LaPrade, M.; Saris, D.B.F.; Krych, A.J. Stem cell treatment for ligament repair and reconstruction. Curr. Rev. Musculoskelet. Med. 2019, 12, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P. Stem cell technology for tendon regeneration: Current status, challenges, and future research directions. Stem Cells Cloning 2015, 8, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P.; Wong, O.T. Tendon stem cells: Experimental and clinical perspectives in tendon and tendon-bone junction repair. Muscles Ligaments Tendons J. 2012, 2, 163–168. [Google Scholar] [PubMed]
- Ni, M.; Lui, P.P.; Rui, Y.F.; Lee, Y.W.; Lee, Y.W.; Tan, Q.; Wong, Y.M.; Kong, S.K.; Lau, P.M.; Li, G.; et al. Tendon-derived stem cells (TDSCs) promote tendon repair in a rat patellar tendon window defect model. J. Orthop. Res. 2012, 30, 613–619. [Google Scholar] [CrossRef]
- Lui, P.P.; Wong, O.T.; Lee, Y.W. Application of tendon-derived stem cell sheet for the promotion of graft healing in anterior cruciate ligament reconstruction. Am. J. Sports Med. 2014, 42, 681–689. [Google Scholar] [CrossRef]
- Lui, P.P.; Wong, O.T.; Lee, Y.W. Transplantation of tendon-derived stem cells pre-treated with connective tissue growth factor and ascorbic acid in vitro promoted better tendon repair in a patellar tendon window injury rat model. Cytotherapy 2016, 18, 99–112. [Google Scholar] [CrossRef]
- Chamberlain, C.S.; Clements, A.; Kink, J.A.; Choi, U.; Baer, G.S.; Halanski, M.A.; Hematti, P.; Vanderby, R. Extracellular Vesicle-Educated Macrophages Promote Early Achilles Tendon Healing. Stem Cells 2019, 37, 652–662. [Google Scholar] [CrossRef]
- Shen, H.; Yoneda, S.; Abu-Amer, Y.; Guilak, F.; Gelberman, R.H. Stem cell-derived extracellular vesicles attenuate the early inflammatory response after tendon injury and repair. J. Orthop. Res. 2020, 38, 117–127. [Google Scholar] [CrossRef]
- Yao, Z.; Li, J.; Wang, X.; Peng, S.; Ning, J.; Qian, Y.; Fan, C. MicroRNA-21-3p Engineered Umbilical Cord Stem Cell-Derived Exosomes Inhibit Tendon Adhesion. J. Inflamm. Res. 2020, 13, 303–316. [Google Scholar] [CrossRef]
- Li, J.; Yao, Z.; Xiong, H.; Cui, H.; Wang, X.; Zheng, W.; Qian, Y.; Fan, C. Extracellular vesicles from hydroxycamptothecin primed umbilical cord stem cells enhance anti-adhesion potential for treatment of tendon injury. Stem Cell Res. Ther. 2020, 11, 500. [Google Scholar] [CrossRef]
- Han, Q.; Wang, S.; Chen, D.; Gan, D.; Wang, T. Exosomes derived from human umbilical cord mesenchymal stem cells reduce tendon injuries via the miR-27b-3p/ARHGAP5/RhoA signaling pathway. Acta Biochim. Biophys. Sin. 2022, 54, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Gissi, C.; Radeghieri, A.; Antonetti Lamorgese Passeri, C.; Gallorini, M.; Calciano, L.; Oliva, F.; Veronesi, F.; Zendrini, A.; Cataldi, A.; Bergese, P.; et al. Extracellular vesicles from rat-bone-marrow mesenchymal stromal/stem cells improve tendon repair in rat Achilles tendon injury model in dose-dependent manner: A pilot study. PLoS ONE 2020, 15, e0229914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, H.; Cui, Q.; Han, P.; Yang, S.; Shi, M.; Zhang, T.; Zhang, Z.; Li, Z. Tendon stem cell-derived exosomes regulate inflammation and promote the high-quality healing of injured tendon. Stem Cell Res. Ther. 2020, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Li, J.; Xiong, H.; Cui, H.; Ning, J.; Wang, S.; Ouyang, X.; Qian, Y.; Fan, C. MicroRNA engineered umbilical cord stem cell-derived exosomes direct tendon regeneration by mTOR signaling. J. Nanobiotechnol. 2021, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wang, Q.; Jiang, D. Extracellular vesicles from bone marrow-derived multipotent mesenchymal stromal cells regulate inflammation and enhance tendon healing. J. Transl. Med. 2019, 17, 211. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Cheng, J.; Shi, W.; Ren, B.; Zhao, F.; Shi, Y.; Yang, P.; Duan, X.; Zhang, J.; Fu, X.; et al. Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater. 2020, 106, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, M.; Shi, M.; Zhang, T.; Lu, W.; Yang, S.; Cui, Q.; Li, Z. Adipose-derived mesenchymal stromal cell-derived exosomes promote tendon healing by activating both SMAD1/5/9 and SMAD2/3. Stem Cell Res. Ther. 2021, 12, 338. [Google Scholar] [CrossRef]
- Song, K.; Jiang, T.; Pan, P.; Yao, Y.; Jiang, Q. Exosomes from tendon derived stem cells promote tendon repair through miR-144-3p-regulated tenocyte proliferation and migration. Stem Cell Res. Ther. 2022, 13, 80. [Google Scholar] [CrossRef]
- Wang, C.; Hu, Q.; Song, W.; Yu, W.; He, Y. Adipose Stem Cell-Derived Exosomes Decrease Fatty Infiltration and Enhance Rotator Cuff Healing in a Rabbit Model of Chronic Tears. Am. J. Sports Med. 2020, 48, 1456–1464. [Google Scholar] [CrossRef]
- Han, L.; Liu, H.; Fu, H.; Hu, Y.; Fang, W.; Liu, J. Exosome-delivered BMP-2 and polyaspartic acid promotes tendon bone healing in rotator cuff tear via Smad/RUNX2 signaling pathway. Bioengineered 2022, 13, 1459–1475. [Google Scholar] [CrossRef]
- Huang, Y.; He, B.; Wang, L.; Yuan, B.; Shu, H.; Zhang, F.; Sun, L. Bone marrow mesenchymal stem cell-derived exosomes promote rotator cuff tendon-bone healing by promoting angiogenesis and regulating M1 macrophages in rats. Stem Cell Res. Ther. 2020, 11, 496. [Google Scholar] [CrossRef]
- Fu, G.; Lu, L.; Pan, Z.; Fan, A.; Yin, F. Adipose-derived stem cell exosomes facilitate rotator cuff repair by mediating tendon-derived stem cells. Regen. Med. 2021, 16, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhang, S.; Wang, Y.; Jacobson, D.S.; Reisdorf, R.L.; Kuroiwa, T.; Behfar, A.; Moran, S.L.; Steinmann, S.P.; Zhao, C. Effects of purified exosome product on rotator cuff tendon-bone healing in vitro and in vivo. Biomaterials 2021, 276, 121019. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Kang, X.; Wang, Y.; Bian, X.; He, G.; Zhou, M.; Tang, K. Exosomes Derived from Bone Marrow Stromal Cells (BMSCs) Enhance Tendon-Bone Healing by Regulating Macrophage Polarization. Med. Sci. Monit. 2020, 26, e923328. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Jin, Q.; Ming-Yu, Y.; Yang, H.; Xu, T.; You-Xing, S.; Xu-Ting, B.; Wan, C.; Yun-Jiao, W.; Huan, W.; et al. MiR-6924-5p-rich exosomes derived from genetically modified Scleraxis-overexpressing PDGFRα(+) BMMSCs as novel nanotherapeutics for treating osteolysis during tendon-bone healing and improving healing strength. Biomaterials 2021, 279, 121242. [Google Scholar] [CrossRef]
- Wang, Y.; He, G.; Guo, Y.; Tang, H.; Shi, Y.; Bian, X.; Zhu, M.; Kang, X.; Zhou, M.; Lyu, J.; et al. Exosomes from tendon stem cells promote injury tendon healing through balancing synthesis and degradation of the tendon extracellular matrix. J. Cell. Mol. Med. 2019, 23, 5475–5485. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Wang, Q.; Zhao, Z.; Wu, R.; Wang, M.; Li, J.; Sun, K.; Sun, Z.; Lv, Z.; Xu, J.; et al. Nitric Oxide Nanomotor Driving Exosomes-Loaded Microneedles for Achilles Tendinopathy Healing. ACS Nano. 2021, 15, 13339–13350. [Google Scholar] [CrossRef]
- Zhu, Z.; Gao, R.; Ye, T.; Feng, K.; Zhang, J.; Chen, Y.; Xie, Z.; Wang, Y. The Therapeutic Effect of iMSC-Derived Small Extracellular Vesicles on Tendinopathy Related Pain Through Alleviating Inflammation: An in vivo and in vitro Study. J. Inflamm. Res. 2022, 15, 1421–1436. [Google Scholar] [CrossRef]
- Chamberlain, C.S.; Kink, J.A.; Wildenauer, L.A.; McCaughey, M.; Henry, K.; Spiker, A.M.; Halanski, M.A.; Hematti, P.; Vanderby, R. Exosome-educated macrophages and exosomes differentially improve ligament healing. Stem Cells 2021, 39, 55–61. [Google Scholar] [CrossRef]
- Kornicka-Garbowska, K.; Pędziwiatr, R.; Woźniak, P.; Kucharczyk, K.; Marycz, K. Microvesicles isolated from 5-azacytidine-and-resveratrol-treated mesenchymal stem cells for the treatment of suspensory ligament injury in horse-a case report. Stem Cell Res. Ther. 2019, 10, 394. [Google Scholar] [CrossRef]
- Costa, L.A.; Eiro, N.; Fraile, M.; Gonzalez, L.O.; Saá, J.; Garcia-Portabella, P.; Vega, B.; Schneider, J.; Vizoso, F.J. Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: Implications for further clinical uses. Cell. Mol. Life Sci. 2021, 78, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Rhim, W.K.; Seo, H.J.; Lee, J.Y.; Park, C.G.; Han, D.K. Comparative Analysis of MSC-Derived Exosomes Depending on Cell Culture Media for Regenerative Bioactivity. Tissue Eng. Regen. Med. 2021, 18, 355–367. [Google Scholar] [CrossRef]
- Venugopal, C.; Shamir, C.; Senthilkumar, S.; Babu, J.V.; Sonu, P.K.; Nishtha, K.J.; Rai, K.S.; Dhanushkodi, A. Dosage and Passage Dependent Neuroprotective Effects of Exosomes Derived from Rat Bone Marrow Mesenchymal Stem Cells: An In Vitro Analysis. Curr. Gene Ther. 2017, 17, 379–390. [Google Scholar] [CrossRef]
- Lai, R.C.; Tan, S.S.; Yeo, R.W.; Choo, A.B.; Reiner, A.T.; Su, Y.; Shen, Y.; Fu, Z.; Alexander, L.; Sze, S.K.; et al. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J. Extracell. Vesicles 2016, 5, 29828. [Google Scholar] [CrossRef] [PubMed]
- Collino, F.; Pomatto, M.; Bruno, S.; Lindoso, R.S.; Tapparo, M.; Sicheng, W.; Quesenberry, P.; Camussi, G. Exosome and Microvesicle-Enriched Fractions Isolated from Mesenchymal Stem Cells by Gradient Separation Showed Different Molecular Signatures and Functions on Renal Tubular Epithelial Cells. Stem Cell Rev. Rep. 2017, 13, 226–243. [Google Scholar] [CrossRef] [PubMed]
- Department of Health. Guidance for Cell and Tissue Products. August 2021. Available online: https://www.advancedtherapyinfo.gov.hk/cbb/en/doc/Guidance_for_Cell_and_Tissue_Products.pdf (accessed on 15 June 2022).
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Van Balkom, B.W.M.; Bruno, S.; Choo, A.; Dominici, M.; Gimona, M.; Hill, A.F.; De Kleijn, D.; Koh, M.; Lai, R.C.; et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles 2019, 8, 1609206. [Google Scholar] [CrossRef] [PubMed]
- Pomatto, M.; Gai, C.; Negro, F.; Cedrino, M.; Grange, C.; Ceccotti, E.; Togliatto, G.; Collino, F.; Tapparo, M.; Figliolini, F.; et al. Differential Therapeutic Effect of Extracellular Vesicles Derived by Bone Marrow and Adipose Mesenchymal Stem Cells on Wound Healing of Diabetic Ulcers and Correlation to Their Cargoes. Int. J. Mol. Sci. 2021, 22, 3851. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.; Zhao, B.; Niu, X.; Hu, B.; Li, Q.; Zhang, J.; Ding, J.; Chen, Y.; Wang, Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 2017, 8, 64. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Miller, R.; Stoppato, M.; Sere, Y.Y.; Coles, A.; Didiot, M.C.; Wollacott, R.; Sapp, E.; Dubuke, M.L.; Li, X.; et al. Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Mol. Ther. 2018, 26, 2838–2847. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Lui, P.P.; Rui, Y.F.; Wong, Y.M. Comparison of potentials of stem cells isolated from tendon and bone marrow for musculoskeletal tissue engineering. Tissue Eng. Part A 2012, 18, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, A.; Salama, M.; Zahran, F.; Jones, E.; Badawy, A.; Sobh, M. Characterization of mesenchymal stem cells derived from rat bone marrow and adipose tissue: A comparative study. Int. J. Stem. Cells 2014, 7, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.A.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.M.; Zhang, L.; et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, Y.; Tang, K.; Luo, Y.; Liu, Y.; Chen, W. Downregulation of CITED2 contributes to TGFβ-mediated senescence of tendon-derived stem cells. Cell Tissue Res. 2017, 368, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Inoue, O.; Usui, S.; Takashima, S.I.; Nomura, A.; Yamaguchi, K.; Takeda, Y.; Goten, C.; Hamaoka, T.; Ootsuji, H.; Murai, H.; et al. Diabetes impairs the angiogenic capacity of human adipose-derived stem cells by reducing the CD271+ subpopulation in adipose tissue. Biochem. Biophys. Res. Commun. 2019, 517, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Cianfarani, F.; Toietta, G.; Di Rocco, G.; Cesareo, E.; Zambruno, G.; Odorisio, T. Diabetes impairs adipose tissue-derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen. 2013, 21, 545–553. [Google Scholar] [CrossRef]
- Kornicka, K.; Houston, J.; Marycz, K. Dysfunction of Mesenchymal Stem Cells Isolated from Metabolic Syndrome and Type 2 Diabetic Patients as Result of Oxidative Stress and Autophagy may Limit Their Potential Therapeutic Use. Stem Cell Rev. Rep. 2018, 14, 337–345. [Google Scholar] [CrossRef]
- Abu-Shahba, N.; Mahmoud, M.; El-Erian, A.M.; Husseiny, M.I.; Nour-Eldeen, G.; Helwa, I.; Amr, K.; ElHefnawi, M.; Othman, A.I.; Ibrahim, S.A.; et al. Impact of type 2 diabetes mellitus on the immunoregulatory characteristics of adipose tissue-derived mesenchymal stem cells. Int. J. Biochem. Cell. Biol. 2021, 140, 106072. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Spinetti, G.; Santopaolo, M.; Madeddu, P. Impaired Regeneration Contributes to Poor Outcomes in Diabetic Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 34–44. [Google Scholar] [CrossRef]
- Santopaolo, M.; Sambataro, M.; Spinetti, G.; Madeddu, P. Bone marrow as a target and accomplice of vascular complications in diabetes. Diabetes Metab. Res. Rev. 2020, 36 (Suppl. S1), e3240. [Google Scholar] [CrossRef]
- Wuchter, P.; Bieback, K.; Schrezenmeier, H.; Bornhäuser, M.; Müller, L.P.; Bönig, H.; Wagner, W.; Meisel, R.; Pavel, P.; Tonn, T.; et al. Standardization of Good Manufacturing Practice-compliant production of bone marrow-derived human mesenchymal stromal cells for immunotherapeutic applications. Cytotherapy 2015, 17, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Tigges, J.; Bielec, K.; Brockerhoff, G.; Hildebrandt, B.; Hübenthal, U.; Kapr, J.; Koch, K.; Teichweyde, N.; Wieczorek, D.; Rossi, A.; et al. Academic application of Good Cell Culture Practice for induced pluripotent stem cells. ALTEX 2021, 38, 595–614. [Google Scholar] [CrossRef] [PubMed]
- Pakzad, M.; Hassani, S.N.; Abbasi, F.; Hajizadeh-Saffar, E.; Taghiyar, L.; Fallah, N.; Haghparast, N.; Samadian, A.; Ganjibakhsh, M.; Dominici, M.; et al. A Roadmap for the Production of a GMP-Compatible Cell Bank of Allogeneic Bone Marrow-Derived Clonal Mesenchymal Stromal Cells for Cell Therapy Applications. Stem Cell Rev. Rep. 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lin, E.Y.; Chiou, T.W.; Harn, H.J. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Ci Ji Yi Xue Za Zhi 2019, 32, 113–120. [Google Scholar]
- Andriolo, G.; Provasi, E.; Lo Cicero, V.; Brambilla, A.; Soncin, S.; Torre, T.; Milano, G.; Biemmi, V.; Vassalli, G.; Turchetto, L.; et al. Exosomes from Human Cardiac Progenitor Cells for Therapeutic Applications: Development of a GMP-Grade Manufacturing Method. Front. Physiol. 2018, 9, 1169. [Google Scholar] [CrossRef]
- Pachler, K.; Lener, T.; Streif, D.; Dunai, Z.A.; Desgeorges, A.; Feichtner, M.; Öller, M.; Schallmoser, K.; Rohde, E.; Gimona, M. A Good Manufacturing Practice-grade standard protocol for exclusively human mesenchymal stromal cell-derived extracellular vesicles. Cytotherapy 2017, 19, 458–472. [Google Scholar] [CrossRef]
- Tan, Q.; Lui, P.P.; Rui, Y.F. Effect of in vitro passaging on the stem cell-related properties of tendon-derived stem cells—Implications in tissue engineering. Stem Cells Dev. 2012, 21, 790–800. [Google Scholar] [CrossRef]
- Council of Europe. Nucleated cell count and viability. In The European Pharmacopoeia, 9th ed.; 2.7.29; EDQM: Strasbourg, France, 2017. [Google Scholar]
- Seaver, S. A new United States Pharmacopeia (USP) Chapter 1046: Cell and gene therapy products. Cytotherapy 2000, 2, 45–49. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Lui, P.P. Markers for the identification of tendon-derived stem cells in vitro and tendon stem cells in situ—Update and future development. Stem Cell Res. Ther. 2015, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, J.; Krampera, M. The challenge of defining mesenchymal stromal cell potency assays and their potential use as release criteria. Cytotherapy 2015, 17, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Ketterl, N.; Brachtl, G.; Schuh, C.; Bieback, K.; Schallmoser, K.; Reinisch, A.; Strunk, D. A robust potency assay highlights significant donor variation of human mesenchymal stem/progenitor cell immune modulatory capacity and extended radio-resistance. Stem Cell Res. Ther. 2015, 6, 236. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Vila, I.; Ramírez-Moncayo, C.; Grau-Vorster, M.; Marín-Gallén, S.; Caminal, M.; Vives, J. Optimisation of a potency assay for the assessment of immunomodulative potential of clinical grade multipotent mesenchymal stromal cells. Cytotechnology 2018, 70, 31–44. [Google Scholar] [CrossRef]
- Grau-Vorster, M.; Rodríguez, L.; Del Mazo-Barbara, A.; Mirabel, C.; Blanco, M.; Codinach, M.; Gómez, S.G.; Querol, S.; García-López, J.; Vives, J. Compliance with Good Manufacturing Practice in the Assessment of Immunomodulation Potential of Clinical Grade Multipotent Mesenchymal Stromal Cells Derived from Wharton’s Jelly. Cells 2019, 8, 484. [Google Scholar] [CrossRef]
- De Wolf, C.; van de Bovenkamp, M.; Hoefnagel, M. Regulatory perspective on in vitro potency assays for human mesenchymal stromal cells used in immunotherapy. Cytotherapy 2017, 19, 784–797. [Google Scholar] [CrossRef]
- Guan, Q.; Li, Y.; Shpiruk, T.; Bhagwat, S.; Wall, D.A. Inducible indoleamine 2,3-dioxygenase 1 and programmed death ligand 1 expression as the potency marker for mesenchymal stromal cells. Cytotherapy 2018, 20, 639–649. [Google Scholar] [CrossRef]
- Poupardin, R.; Wolf, M.; Strunk, D. Adherence to minimal experimental requirements for defining extracellular vesicles and their functions. Adv. Drug Deliv. Rev. 2021, 176, 113872. [Google Scholar] [CrossRef]
- Andriolo, G.; Provasi, E.; Brambilla, A.; Lo Cicero, V.; Soncin, S.; Barile, L.; Turchetto, L.; Radrizzani, M. GMP-Grade Methods for Cardiac Progenitor Cells: Cell Bank Production and Quality Control. Methods Mol. Biol. 2021, 2286, 131–166. [Google Scholar]
- Lechanteur, C.; Briquet, A.; Bettonville, V.; Baudoux, E.; Beguin, Y. MSC Manufacturing for Academic Clinical Trials: From a Clinical-Grade to a Full GMP-Compliant Process. Cells 2021, 10, 1320. [Google Scholar] [CrossRef]
- Guadix, J.A.; López-Beas, J.; Clares, B.; Soriano-Ruiz, J.L.; Zugaza, J.L.; Gálvez-Martín, P. Principal Criteria for Evaluating the Quality, Safety and Efficacy of hMSC-Based Products in Clinical Practice: Current Approaches and Challenges. Pharmaceutics 2019, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Robb, K.P.; Fitzgerald, J.C.; Barry, F.; Viswanathan, S. Mesenchymal stromal cell therapy: Progress in manufacturing and assessments of potency. Cytotherapy 2019, 21, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Mol, E.A.; Goumans, M.J.; Doevendans, P.A.; Sluijter, J.P.G.; Vader, P. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomedicine 2017, 13, 2061–2065. [Google Scholar] [CrossRef] [PubMed]
- Nordin, J.Z.; Lee, Y.; Vader, P.; Mäger, I.; Johansson, H.J.; Heusermann, W.; Wiklander, O.P.; Hällbrink, M.; Seow, Y.; Bultema, J.J.; et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine 2015, 11, 879–883. [Google Scholar] [CrossRef]
- Lee, J.H.; Ha, D.H.; Go, H.K.; Youn, J.; Kim, H.K.; Jin, R.C.; Miller, R.B.; Kim, D.H.; Cho, B.S.; Yi, Y.W. Reproducible Large-Scale Isolation of Exosomes from Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells and Their Application in Acute Kidney Injury. Int. J. Mol. Sci. 2020, 21, 4774. [Google Scholar] [CrossRef]
- Bari, E.; Perteghella, S.; Catenacci, L.; Sorlini, M.; Croce, S.; Mantelli, M.; Avanzini, M.A.; Sorrenti, M.; Torre, M.L. Freeze-dried and GMP-compliant pharmaceuticals containing exosomes for acellular mesenchymal stromal cell immunomodulant therapy. Nanomedicine 2019, 14, 753–765. [Google Scholar] [CrossRef]
- Watson, D.C.; Yung, B.C.; Bergamaschi, C.; Chowdhury, B.; Bear, J.; Stellas, D.; Morales-Kastresana, A.; Jones, J.C.; Felber, B.K.; Chen, X.; et al. Scalable, cGMP-compatible purification of extracellular vesicles carrying bioactive human heterodimeric IL-15/lactadherin complexes. J. Extracell. Vesicles 2018, 7, 1442088. [Google Scholar] [CrossRef]
- Gámez-Valero, A.; Monguió-Tortajada, M.; Carreras-Planella, L.; Franquesa, M.L.; Beyer, K.; Borràs, F.E. Size-Exclusion Chromatography-based isolation minimally alters Extracellular Vesicles’ characteristics compared to precipitating agents. Sci. Rep. 2016, 6, 33641. [Google Scholar] [CrossRef]
- Paolini, L.; Zendrini, A.; Di Noto, G.; Busatto, S.; Lottini, E.; Radeghieri, A.; Dossi, A.; Caneschi, A.; Ricotta, D.; Bergese, P. Residual matrix from different separation techniques impacts exosome biological activity. Sci. Rep. 2016, 6, 23550. [Google Scholar] [CrossRef]
- Zhang, X.; Borg, E.G.F.; Liaci, A.M.; Vos, H.R.; Stoorvogel, W. A novel three step protocol to isolate extracellular vesicles from plasma or cell culture medium with both high yield and purity. J. Extracell. Vesicles 2020, 9, 1791450. [Google Scholar] [CrossRef]
- McNamara, R.P.; Caro-Vegas, C.P.; Costantini, L.M.; Landis, J.T.; Griffith, J.D.; Damania, B.A.; Dittmer, D.P. Large-scale, cross-flow based isolation of highly pure and endocytosis-competent extracellular vesicles. J. Extracell. Vesicles 2018, 7, 1541396. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, D.; Mussack, V.; Byrd, J.B. Separation, characterization, and standardization of extracellular vesicles for drug delivery applications. Adv. Drug Deliv. Rev. 2021, 174, 348–368. [Google Scholar] [CrossRef] [PubMed]
- Midekessa, G.; Godakumara, K.; Ord, J.; Viil, J.; Lättekivi, F.; Dissanayake, K.; Kopanchuk, S.; Rinken, A.; Andronowsk, A.; Bhattacharjee, S.; et al. Zeta Potential of Extracellular Vesicles: Toward Understanding the Attributes that Determine Colloidal Stability. ACS Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef]
- Le Saux, S.; Aarrass, H.; Lai-Kee-Him, J.; Bron, P.; Armengaud, J.; Miotello, G.; Bertrand-Michel, J.; Dubois, E.; George, S.; Faklaris, O.; et al. Post-production modifications of murine mesenchymal stem cell (mMSC) derived extracellular vesicles (EVs) and impact on their cellular interaction. Biomaterials 2020, 231, 119675. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Xie, X.; Yuan, J.; Gong, L.; Zhu, Z.; Zhang, J.; Li, H.; Yang, Y.; Wang, Y. Reversing the surface charge of MSC-derived small extracellular vesicles by εPL-PEG-DSPE for enhanced osteoarthritis treatment. J. Extracell. Vesicles 2021, 10, e12160. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Fuzeta, M.; Bernardes, N.; Oliveira, F.D.; Costa, A.C.; Fernandes-Platzgummer, A.; Farinha, J.P.; Rodrigues, C.A.V.; Jung, S.; Tseng, R.J.; Milligan, W.; et al. Scalable Production of Human Mesenchymal Stromal Cell-Derived Extracellular Vesicles Under Serum-/Xeno-Free Conditions in a Microcarrier-Based Bioreactor Culture System. Front. Cell. Dev. Biol. 2020, 8, 553444. [Google Scholar] [CrossRef]
- Koliha, N.; Wiencek, Y.; Heider, U.; Jüngst, C.; Kladt, N.; Krauthäuser, S.; Johnston, I.C.; Bosio, A.; Schauss, A.; Wild, S. A novel multiplex bead-based platform highlights the diversity of extracellular vesicles. J. Extracell. Vesicles 2016, 5, 29975. [Google Scholar] [CrossRef]
- Tian, Y.; Ma, L.; Gong, M.; Su, G.; Zhu, S.; Zhang, W.; Wang, S.; Li, Z.; Chen, C.; Li, L.; et al. Protein Profiling and Sizing of Extracellular Vesicles from Colorectal Cancer Patients via Flow Cytometry. ACS Nano 2018, 12, 671–680. [Google Scholar] [CrossRef]
- Rocha, S.; Carvalho, J.; Oliveira, P.; Voglstaetter, M.; Schvartz, D.; Thomsen, A.R.; Walter, N.; Khanduri, R.; Sanchez, J.C.; Keller, A.; et al. 3D Cellular Architecture Affects MicroRNA and Protein Cargo of Extracellular Vesicles. Adv. Sci. 2018, 6, 1800948. [Google Scholar] [CrossRef]
- Gandham, S.; Su, X.; Wood, J.; Nocera, A.L.; Alli, S.C.; Milane, L.; Zimmerman, A.; Amiji, M.; Ivanov, A.R. Technologies and Standardization in Research on Extracellular Vesicles. Trends Biotechnol. 2020, 38, 1066–1098. [Google Scholar] [CrossRef]
- Osteikoetxea, X.; Balogh, A.; Szabó-Taylor, K.; Németh, A.; Szabó, T.G.; Pálóczi, K.; Sódar, B.; Kittel, Á.; György, B.; Pállinger, É.; et al. Improved characterization of EV preparations based on protein to lipid ratio and lipid properties. PLoS ONE 2015, 10, e0121184. [Google Scholar] [CrossRef] [PubMed]
- Webber, J.; Clayton, A. How pure are your vesicles? J. Extracell. Vesicles 2013, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Maiolo, D.; Paolini, L.; Di Noto, G.; Zendrini, A.; Berti, D.; Bergese, P.; Ricotta, D. Colorimetric nanoplasmonic assay to determine purity and titrate extracellular vesicles. Anal. Chem. 2015, 87, 4168–4176. [Google Scholar] [CrossRef] [PubMed]
- Mihály, J.; Deák, R.; Szigyártó, I.C.; Bóta, A.; Beke-Somfai, T.; Varga, Z. Characterization of extracellular vesicles by IR spectroscopy: Fast and simple classification based on amide and CH stretching vibrations. Biochim. Biophys. Acta Biomembr. 2017, 1859, 459–466. [Google Scholar] [CrossRef]
- Veerman, R.E.; Teeuwen, L.; Czarnewski, P.; Güclüler Akpinar, G.; Sandberg, A.; Cao, X.; Pernemalm, M.; Orre, L.M.; Gabrielsson, S.; Eldh, M. Molecular evaluation of five different isolation methods for extracellular vesicles reveals different clinical applicability and subcellular origin. J. Extracell. Vesicles 2021, 10, e12128. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, T.E.; Nagelkerke, A.; Nele, V.; Kauscher, U.; Stevens, M.M. Experimental artefacts can lead to misattribution of bioactivity from soluble mesenchymal stem cell paracrine factors to extracellular vesicles. J. Extracell. Vesicles 2020, 9, 1807674. [Google Scholar] [CrossRef] [PubMed]
- Gimona, M.; Brizzi, M.F.; Choo, A.B.H.; Dominici, M.; Davidson, S.M.; Grillari, J.; Hermann, D.M.; Hill, A.F.; de Kleijn, D.; Lai, R.C.; et al. Critical considerations for the development of potency tests for therapeutic applications of mesenchymal stromal cell-derived small extracellular vesicles. Cytotherapy 2021, 23, 373–380. [Google Scholar] [CrossRef]
- Lui, P.P.Y. Mesenchymal Stem Cell-Derived Extracellular Vesicles for the Promotion of Tendon Repair—An Update of Literature. Stem Cell Rev. Rep. 2021, 17, 379–389. [Google Scholar] [CrossRef]
- Mocchi, M.; Bari, E.; Marrubini, G.; Bonda, A.F.; Perteghella, S.; Tartara, F.; Cofano, F.; Perna, G.D.; Giovannelli, L.; Mandracchia, D.; et al. Freeze-Dried Mesenchymal Stem Cell-Secretome Pharmaceuticalization: Optimization of Formulation and Manufacturing Process Robustness. Pharmaceutics 2021, 13, 1129. [Google Scholar] [CrossRef]
- Laggner, M.; Gugerell, A.; Bachmann, C.; Hofbauer, H.; Vorstandlechner, V.; Seibold, M.; Gouya Lechner, G.; Peterbauer, A.; Madlener, S.; Demyanets, S.; et al. Reproducibility of GMP-compliant production of therapeutic stressed peripheral blood mononuclear cell-derived secretomes.; a novel class of biological medicinal products. Stem Cell Res. Ther. 2020, 11, 9. [Google Scholar] [CrossRef]
- Bahr, M.M.; Amer, M.S.; Abo-El-Sooud, K.; Abdallah, A.N.; El-Tookhy, O.S. Preservation techniques of stem cells extracellular vesicles: A gate for manufacturing of clinical grade therapeutic extracellular vesicles and long-term clinical trials. Int. J. Vet. Sci. Med. 2020, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Charoenviriyakul, C.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Preservation of exosomes at room temperature using lyophilization. Int. J. Pharm. 2018, 553, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Perteghella, S.; Di Silvestre, D.; Sorlini, M.; Catenacci, L.; Sorrenti, M.; Marrubini, G.; Rossi, R.; Tripodo, G.; Mauri, P.; et al. Production of Mesenchymal Stem/Stromal Freeze-Dried Secretome for Cell-Free Regenerative Nanomedicine: A Validated GMP-Compliant Process. Cells 2018, 7, 190. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Morille, M.; Piffoux, M.; Arumugam, S.; Mauduit, P.; Larghero, J.; Bianchi, A.; Aubertin, K.; Blanc-Brude, O.; Noël, D.; et al. Development of extracellular vesicle-based medicinal products: A position paper of the group “Extracellular Vesicle translatiOn to clinicaL perspectiVEs—EVOLVE France”. Adv. Drug Deliv. Rev. 2021, 179, 114001. [Google Scholar] [CrossRef]
- Rohde, E.; Pachler, K.; Gimona, M. Manufacturing and characterization of extracellular vesicles from umbilical cord-derived mesenchymal stromal cells for clinical testing. Cytotherapy 2019, 21, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Gimona, M.; Pachler, K.; Laner-Plamberger, S.; Schallmoser, K.; Rohde, E. Manufacturing of Human Extracellular Vesicle-Based Therapeutics for Clinical Use. Int. J. Mol. Sci. 2017, 18, 1190. [Google Scholar] [CrossRef]
- Lui, P.P.; Kong, S.K.; Lau, P.M.; Wong, Y.M.; Lee, Y.W.; Tan, C.; Wong, O.T. Allogeneic tendon-derived stem cells promote tendon healing and suppress immunoreactions in hosts: In vivo model. Tissue Eng. Part A 2014, 20, 2998–3009. [Google Scholar] [CrossRef]
- Lui, P.P.; Kong, S.K.; Lau, P.M.; Wong, Y.M.; Lee, Y.W.; Tan, C.; Wong, O.T. Immunogenicity and escape mechanisms of allogeneic tendon-derived stem cells. Tissue Eng. Part A 2014, 20, 3010–3020. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, W.; Lim, C.; Chung, S.G. Treatment of Lateral Epicondylosis by Using Allogeneic Adipose-Derived Mesenchymal Stem Cells: A Pilot Study. Stem Cells 2015, 33, 2995–3005. [Google Scholar] [CrossRef]
- Wang, Y.; Shimmin, A.; Ghosh, P.; Marks, P.; Linklater, J.; Connell, D.; Hall, S.; Skerrett, D.; Itescu, S.; Cicuttini, F.M. Safety, tolerability, clinical, and joint structural outcomes of a single intra-articular injection of allogeneic mesenchymal precursor cells in patients following anterior cruciate ligament reconstruction: A controlled double-blind randomised trial. Arthritis Res. Ther. 2017, 19, 180. [Google Scholar] [CrossRef]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.C.; Gagea, M.; Yang, S.; Blanko, E.V.R.; Peng, Q.; Ma, X.; et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 2018, 3, e99263. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Badawi, M.; Pomeroy, S.; Sutaria, D.S.; Xie, Z.; Baek, A.; Jiang, J.; Elgamal, O.A.; Mo, X.; Perle, K.; et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J. Extracell. Vesicles 2017, 6, 1324730. [Google Scholar] [CrossRef] [PubMed]
- Kordelas, L.; Rebmann, V.; Ludwig, A.K.; Radtke, S.; Ruesing, J.; Doeppner, T.R.; Epple, M.; Horn, P.A.; Beelen, D.W.; Giebel, B. MSC-derived exosomes: A novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 2014, 28, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Nassar, W.; El-Ansary, M.; Sabry, D.; Mostafa, M.A.; Fayad, T.; Kotb, E.; Temraz, M.; Saad, A.N.; Essa, W.; Adel, H. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater. Res. 2016, 20, 21. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).