Abstract
Inflammation is a key feature of atherosclerosis. The inflammatory process is involved in all stages of disease progression, from the early formation of plaque to its instability and disruption, leading to clinical events. This strongly suggests that the use of anti-inflammatory agents might improve both atherosclerosis progression and cardiovascular outcomes. Colchicine, an alkaloid derived from the flower Colchicum autumnale, has been used for years in the treatment of inflammatory pathologies, including Gout, Mediterranean Fever, and Pericarditis. Colchicine is known to act over microtubules, inducing depolymerization, and over the NLRP3 inflammasome, which might explain its known anti-inflammatory properties. Recent evidence has shown the therapeutic potential of colchicine in the management of atherosclerosis and its complications, with limited adverse effects. In this review, we summarize the current knowledge regarding colchicine mechanisms of action and pharmacokinetics, as well as the available evidence on the use of colchicine for the treatment of coronary artery disease, covering basic, translational, and clinical studies.
1. Introduction
Cardiovascular (CV) diseases remain the leading cause of mortality worldwide, accounting for up to one third of all registered deaths [1]. The main underlying cause behind cardiovascular disease is atherosclerosis, a chronic inflammatory disease targeting large and medium-sized arteries. In the USA alone, 400,000 people die of coronary artery atherosclerosis and over one million suffer from acute coronary syndrome each year [2]. Management of cardiovascular disease focuses on three main areas: (i) lipid lowering strategies, (ii) control of non-lipid risk factors (such as diabetes, hypertension, obesity, etc.) and (iii) stabilization of the atheromatous plaque, preventing rupture and thrombosis [3]. Current therapies, however, fail to prevent the reoccurrence of ischemic events, a phenomenon known as residual risk [4]. In a ten-year follow-up study of patients post ST elevation myocardial infarction (STEMI), 42% of patients presented with recurrent ischemic events, a risk that was highest during the first year (23.5% per patient/year) even when receiving currently recommended pharmacological treatment [5]. Therefore, the need for new therapies has shifted the focus towards anti-inflammatory drugs than can potentially target the chronic inflammation milieu of atherosclerotic plaques [6].
2. Methods
We conducted a full search of animal and human research, from basic studies to randomized clinical trials and meta-analyses, examining the use of colchicine for the treatment of atherosclerosis and/or coronary artery disease. Medline, Pubmed and Embase databases were searched until May 2022. Two researchers independently screened titles and abstracts of articles for full-text review. After data extraction, two researchers chose the most relevant articles and were in charge of elaborating the initial text, which was then sent to every author for further evaluation. If there were any discrepancies on a specific subject, the topic was re-analyzed, and a consensus was achieved. The final version of the manuscript was approved by every author.
3. Inflammation in Atherosclerosis
Inflammation plays a central role in the pathogenesis of atherosclerosis [7]. Both the innate and adaptative immune responses are involved in the process of atheroma formation, with monocyte/macrophages as key players throughout disease progression [8]. The development of the atherosclerotic plaque starts with the infiltration and accumulation of modified, apolipoprotein B-containing lipoproteins within the vessel wall [9]. Once in the intima layer, oxidized cholesterol in lipoproteins triggers the activation and production of inflammatory mediators in charge of recruiting circulating monocytes to the site of injury [9]. Within the vessel wall, monocytes differentiate to macrophages and engulf modified lipoproteins, becoming foam cells [9]. Foam cells continue to release inflammatory cytokines, in particular TNF-α and interleukin-1β (IL-1β) [10], exacerbating endothelial dysfunction and perpetuating the inflammatory response [11]. Advanced atherosclerotic plaques are characterized by a large lipid-rich core—composed of foam cells, cell debris and extracellular cholesterol—and a fibrous cap, formed by extracellular matrix and smooth muscle cells [12]. In later stages of the disease, macrophages within the plaque release matrix metalloproteinases that target the fibrous cap, destabilizing the plaque and setting the stage for plaque rupture and the consequent ischemic event [13,14] (Figure 1).
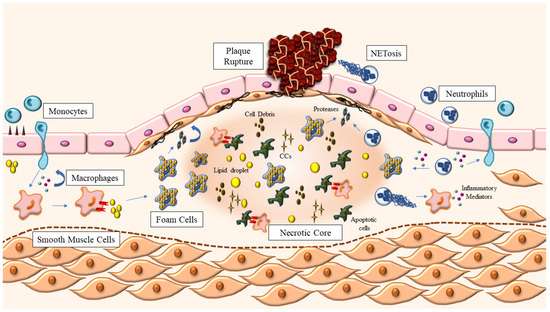
Figure 1.
Atherosclerotic plaque development. Atherosclerosis starts with the accumulation of modified lipoproteins inside the vessel wall, which triggers the recruitment of leukocytes, monocytes and neutrophils from circulation. Once in the intima layer, monocytes differentiate into macrophages, which can now engulf the modified lipoproteins, becoming foam cells. Macrophages also continue to release inflammatory mediators—such as cytokines and chemokines—in response to the increased levels of cholesterol, further amplifying the response. Neutrophils also release pro-inflammatory mediators through granules and NETosis, contributing to an exacerbation of the inflammatory state within the vessel wall. Foam cells, apoptotic cells and cell debris, lipid droplets and extracellular cholesterol crystals (CCs) coalesce in the center of the growing plaque, forming the necrotic core, which is kept stable thanks to the fibrous cap: a structure made of smooth muscle cells and extracellular matrix proteins. The release of proteinases by macrophages and neutrophils weakens the fibrous cap, favoring plaque rupture and the exposure of the contents of the plaque to circulation, triggering blood coagulation and the clinical manifestations of atherosclerosis.
Neutrophils also participate in all stages of atherosclerosis development [15]. Indeed, circulating levels of neutrophils in humans predict future cardiovascular events [16], while in mice they correlate with the size of the developing plaque [17]. Myeloperoxidase (MPO), the main component of neutrophil granules, has been found in atherosclerotic plaques [18]. It has been shown that MPO-induced lipid peroxidation favors foam cell formation [19]. MPO can also activate metalloproteinases, inducing plaque disruption, by the release of reactive oxygen species (ROS) [20]. Similarly, neutrophils are known to release extracellular matrix proteinases that contribute to plaque destabilization [21], like elastase [22] and proteinase-3 [23], locating particularly in rupture-prone areas of the plaque [24,25]. Neutrophil depletion in apolipoprotein E knockout (ApoE KO) mice has been shown to reduce monocyte infiltration and plaque formation in the aorta [26]. In fact, neutrophils can affect monocyte recruitment through several mechanisms [27], including increased expression of adhesion molecules in the endothelium through the release of granule-proteins proteinase 3 and azurocidin [28]. Furthermore, neutrophil extracellular traps (NETs) are web-like structures made of genetic material, histones, MPO and others, which are released upon neutrophil activation [29]. The process of NETs formation is called NETosis and is introduced to discriminate this pathway from other types of cell death [30]. Of note, NETs have been found in atherosclerotic plaques of both mice and humans [31,32,33]. Increased levels of NETosis markers are associated with the severity of coronary atherosclerosis in patients [34]. Similarly, Mangold et al. have shown that the number of NETs and activated neutrophils in patients with acute coronary syndrome (ACS) is related to final infarct size [35]. Finally, autopsied disrupted plaques (i.e., with hemorrhage or erosion) from patients with ACS, presented significantly more neutrophils and NETs compared with plaques without those features [36].
Cholesterol accumulates in atherosclerotic plaques not only in the form of intracellular cholesterol esters but also intra and extracellular cholesterol crystals (CCs) [37]. CCs have been found in all stages of atherosclerotic plaque development [38,39] and have been shown to be associated with plaque rupture [40]. CCs can induce NETosis, which can in turn prime macrophages to synthetize cytokine precursors, such as pro-IL-1β [31]. CCs can also directly activate the nucleotide-binding oligomerization domain-like receptor, pyrin domain-containing 3 (NLRP3) inflammasome in macrophages, resulting in the release of mature IL-1β, a key cytokine involved in the development and disruption of atherosclerotic plaques [37].
4. The NLRP3 Inflammasome
The innate immune system relies on a set of germline-encoded pattern recognition receptors (PRRs) to recognize pathogenic insults as well as defective cells [41]. PRRs detect the presence of pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs), triggering downstream inflammatory pathways leading to removal of the insult and tissue repair [41]. The inflammasomes are cytosolic, multimeric protein complexes that respond to PAMPs and DAMPs [42]. Five members of the PRRs have been confirmed to form inflammasomes, key among them the NLRP3 inflammasome [43]. The NLRP3 inflammasome responds to a wide variety of activators including monosodium urate, extracellular adenosine triphosphate (ATP) and CCs [44]. The basal levels of the components of the NLRP3 inflammasome and their targets are very low [45]. As such, a two-step process of priming and activation is required [46,47]. The priming step is induced by the activation of Toll-like receptors (TLRs) and cytokine receptors, leading to upregulation of the transcription of NLRP3 and pro-IL-1β [48]. Afterwards, further stimuli will promote the inflammasome assembly, resulting in cytokine production [45]. Upon activation, the NLRP3 receptor oligomerizes and interacts with the adaptor protein apoptosis-associated speck-like protein containing a caspase-recruitment domain (ASC), to recruit and activate pro-caspase-1 [49,50]. Active caspase-1 can now cleave pro-IL-1β and pro-IL-18 into their biologically active, highly inflammatory forms [42].
In the context of atherosclerosis, CCs are considered a major driver of NLRP3 inflammasome activation [37]. It has been postulated that CCs activate the inflammasome in a process involving lysosomal damage after phagocytosis [51]. The inefficient clearance of CCs results in the leakage of cathepsin B into the cytoplasm, which in turn can activate the inflammasome complex [52]. Accordingly, the inflammatory response elicited by intraperitoneal CCs injection in WT mice is absent in mice deficient in NLRP3 inflammasome components [38]. Oxidized LDL has also been shown to induce NLRP3 activation through lysosomal disruption via interaction with CD36 [53]. As such, macrophages lacking CD36 fail to release IL-1β, indicating lack of NLRP3 activation [53].
The contribution of IL-1β to atherogenesis has been established by several animal studies, hinting at a role of the NLRP3 inflammasome in disease development [54,55,56]. Bone-marrow deficiency of ASC is associated with reduced vascular inflammation and neointimal formation in a mouse model of vascular injury [57]. LDLR KO mice receiving NLRP3, ASC or IL-1β deficient bone marrow developed significantly reduced atherosclerosis when challenged with an atherogenic diet [38]. Similarly, systemic or bone-marrow deficiency of caspase-1/11 is associated with a reduction in atherosclerotic lesions in both ApoE [58,59] and LDLR KO mice [60]. Conversely, bone marrow transplantation studies in ApoE KO mice have shown that lack of NLRP3, ASC or caspase-1 had no effect on atherosclerotic plaque size, plaque stability or macrophage infiltration [61]. Differences in experimental conditions might explain these contrasting results. In humans, high expression of NLRP3 inflammasome components has been detected in carotid atherosclerotic plaques [62] and high levels of expression of NLRP3 correlate with the severity of coronary artery atherosclerosis [63]. The inflammasome has been shown to be primed in peripheral monocytes from ACS patients compared with controls [64] and levels of NLRP3, IL-1β, IL-18 and other inflammasome components have been found to be elevated in ACS patients compared with controls [65]. Taken together, these studies highlight the important role of IL-1β and the NLRP3 inflammasome in atherosclerosis and mark them as possible targets for the development of new therapeutic strategies.
5. Colchicine
Colchicine is a botanical alkaloid derived from the flower Colchicum autumnale, first described as a medicinal plant in the Ebers papyrus of ancient Egypt in 1550 BC, where it was used for the management of pain and swelling [66]. The colchicine molecule, chemical name N-[(7S)-5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo(a)heptalen-7-yl)acetamide], is composed of three rings [67]. The A (trimethoxyphenyl moiety) and C ring (methoxytropone moiety) are highly involved in binding to tubulin and are maintained in a rigid configuration by B-rings [67]. Modifications to both the A and C rings significantly affect tubulin binding [67,68], while modifications on the B rings are associated with changes in activation energy of the binding and association/dissociation kinetics [69].
Nowadays, colchicine is widely used for the treatment of acute gout flares and Familial Mediterranean Fever (FMF) [70,71]. It has also been used in other inflammatory conditions such as calcium pyrophosphate disease, Adamantiades–Behcet’s syndrome and—in the cardiovascular field—pericarditis [72]. Given the ease of access, low cost and favorable safety profile, colchicine has emerged as a potential oral treatment targeting the inflammatory component of atherosclerosis.
Mechanistically, colchicine acts by inhibiting tubulin polymerization, disrupting the cellular cytoskeleton, and impairing several processes including mitosis, intracellular transport, and phagocytosis [73]. Furthermore, colchicine inhibits neutrophil chemotaxis and adhesion to the inflamed endothelium [74]. At nanoconcentrations, colchicine alters E-selectin distribution on endothelial cells, affecting neutrophil adhesion [75]. On the µM level, colchicine induces L-selectin shedding, preventing recruitment [75]. Paschke and colleagues have also shown that colchicine affects human neutrophil deformability and motility, affecting a key step in inflammatory processes, cell extravasation [76]. Monosodium urate (MSU)-induced superoxide production in neutrophils in vitro is also affected by colchicine treatment [77]. Superoxide production inhibition has also been reported in vivo in MSU-treated peritoneal macrophages, at a dosage significantly lower than the required to affect neutrophil infiltration [78]. Colchicine also modulates TNF-α synthesis by rat liver macrophages [79] and downregulates TNF receptors in both macrophages and endothelial cells [80].
Platelets play a key role in atherosclerosis complications [12]. Early reports have suggested that colchicine might also affect platelet aggregation [81] through mechanisms involving inhibition of cofilin and LIM domain kinase 1 [82]. In FMF patients, colchicine reduced β-thromboglobulin levels, a protein released during platelet activation, with no effect on mean platelet volume, a marker of platelet activity [83]. A more recent experiment by Shah and colleagues showed that colchicine administration to healthy subjects at a clinically relevant dose (1.8 mg single dose) reduced leukocyte-platelet aggregation (both monocyte and neutrophil) as well as levels of surface markers of platelet activity, such as p-selectin and PAC-1 (activated GP IIb/IIIa), with no effect on homotypic platelet aggregation [84]. Likewise, Raju and colleagues have also showed that oral colchicine at a dose of 1 mg did not affect platelet aggregation in response to several stimuli, in patients with acute coronary syndrome [85]. It is possible then that the anti-inflammatory action of colchicine on neutrophils could impact platelet function. NETs can facilitate thrombosis by promoting platelet adhesion, activation, and aggregation, and also the accumulation of prothrombotic factors such as von Willebrand factor and fibrinogen [86]. Interestingly, colchicine has been shown to reduce NETosis induced by CC [87], in neutrophils isolated from individuals with Behcet’s disease [88] and in patients with ACS [89].
6. Colchicine and the NLRP3 Inflammasome
Several studies have confirmed that colchicine limits NLRP3 inflammasome activity. Martinon and colleagues first described inactivation of the NLRP3 inflammasome by colchicine in THP1 cells treated with monosodium urate crystals [90]. In patients with FMF colchicine suppressed IL-1β release from both bone marrow-derived macrophages where the inflammasome was constitutively activated and peripheral blood monocytes [91]. Misawa and colleagues also reported a dose-dependent effect of colchicine on IL-1β production in J774 macrophages treated with various inducers of the NLRP3 inflammasome, including MSU and ATP [92]. Furthermore, colchicine has been described as inhibiting the intracellular transport of ASC, preventing co-localization of NLRP3 components and thus the consequent release of active IL-1β [92]. The pathway through which colchicine inhibits the NLRP3 inflammasome is not clear, however some mechanisms have been proposed. In a model of small intestinal injury, Otani and colleagues showed that colchicine inhibited protein expression of cleaved caspase-1 and IL-1β, without affecting mRNA levels of NLRP3 or IL-1β. Monocyte caspase-1 inhibition has been detected in ACS patients as well [64]. Very recently, using molecular dynamic simulation in a mouse model, it has been proposed that colchicine could interact with the ATP binding region of the NLRP3-NACHT domain, thus potentially precluding inflammasome activation [93]. Similarly, in ATP-mediated NLRP3 inflammasome activation, formation of P2X7 pores is a key step [94]. In this context, Marques-da-Silva et al. demonstrated that colchicine is a strong inhibitor of pore formation in mouse peritoneal macrophages after ATP-induced ethidium bromide permeability, resulting in the release of lower levels of ROS and IL-1β [95]. The same results were also seen in vivo, in mice inoculated with lipopolysaccharide and ATP [95].
The ability of colchicine to interfere with the activation of the NLRP3 inflammasome, in addition to its effect on microtubule formation and neutrophil-mediated inflammation, points towards the use of colchicine as a potential strategy to target the inflammatory component of atherosclerosis.
7. Pharmacokinetics and Safety
Colchicine is rapidly absorbed by the jejunum and ileum with a bioavailability that fluctuates between 24 to 88% [96]. Peak plasma concentrations occur within 0.5–2 h after oral administration, although it can be detected in leukocytes up to 10 days after ingestion [97]. In circulation, approximately 40% is conjugated to plasmatic proteins and the establishment of colchicine–protein complexes in tissues contributes to its large volume distribution [67]. Colchicine is rapidly distributed to peripheral leukocytes and concentration in these cells may exceed those detected in plasma [98]. Most of the drug undergoes enterohepatic recirculation, leading to a second peak in plasma within 6 h of ingestion, and is eliminated through feces and bile [97]. Around 20% of colchicine is eliminated in the urine at 2 h and 30% after 24 h [97]. Colchicine average elimination half-life is 20 h, which can be prolonged by certain pathologies including renal failure and hepatic cirrhosis [99].
Gastrointestinal (GI) intolerance (diarrhea, abdominal pain, vomiting) is the most common side effect of colchicine, affecting around 20% of patients, followed by myalgias [100]. Most of these side effects can be managed with lower daily doses (around 0.5 mg/day) or long-term treatments [100]. Regarding safety, the first meta-analysis that looked at the safety of colchicine use only reported an 83% increased risk of GI side effects, with no evidence of significant serious adverse effects over 824 patient-years [101]. Similar results have been recently reported by Stewart and colleagues in a meta-analysis of 35 randomized controlled trials [102]. Colchicine significantly increased diarrhea (RR 2.4, 95% confidence interval (CI) 1.6–3.7) and other GI events (RR 1.7, 95% CI 1.3–2.3). No increased rate of other adverse events was detected, including infection, hepatic, hematological, muscular, and sensorial events [102]. On the other hand, a pooled analysis of randomized clinical trials comparing low dose colchicine versus placebo showed that colchicine was associated with a significantly higher risk of non-CV death (OR 1.55; 95% CI 1.10 to 2.17; p = 0.01) compared with the placebo group [103]. However, few studies were included and, as stated by the authors, the events reported were low, indicating that the data should be considered with caution [103]. In line with this, a recent retrospective cohort of 24,410 patients with gout showed that colchicine use resulted in an increased risk of pneumonia (adjusted HR, 1.42; 95% CI 1.32–1.53; p < 0.05), related to use duration and accumulated dose [104].
Colchicine interacts with two main proteins that impact its pharmacokinetics and pharmacodynamics: cytochrome P450 3A4 (CYP3A4) and P-glycoprotein. Intestinal and hepatic CYP3A4 metabolize colchicine by demethylation, producing 2- and 3-demethylcolchicine [97]. P-glycoprotein, on the other hand, limits GI availability by extruding colchicine from the GI tract [67]. Adverse reactions have been reported by patients consuming either CYP3A4 inhibitors or P-glycoprotein inhibitors, resulting in altered colchicine metabolism and toxicity [67]. Dose adjustment is recommended in these situations. Some cases of myopathy and/or rhabdomyolysis have been reported for instances when colchicine was used in conjunction with statins, however, they are in general well tolerated when used simultaneously [105]. Death due to colchicine administration has been reported when used in conjunction with clarithromycin, particularly in patients with renal insufficiency [106].
8. Colchicine in Atherosclerosis
8.1. Pre-Clinical Studies
The first animal studies looking at the potential use of colchicine for atherosclerosis showed rather conflicting results. Colchicine administration of high-lipid diet-fed rabbits resulted in a reduction of circulating lipids, restoration of normal triglyceride levels and a protective effect on plaque development in the aorta [107]. On the other hand, colchicine administration on a swine model of balloon-induced atherosclerosis showed the opposite effect, with a mild worsening of plaque development [108]. Though the models used were different, the inconclusive results might have tampered the interest in the usage of this drug for atherosclerosis management. Early in vitro experiments in smooth muscle cells isolated from human atherosclerotic plaques showed that colchicine affected proliferation and migration, which could potentially impact atherosclerosis development [109]. More recently, Huang and colleagues have shown that colchicine administration to hyperlipidemic rats resulted in a reduction in circulating levels of C-reactive protein and lipoprotein associated phospholipase A2, while elevating nitric oxide production, pointing to an improvement in endothelial function. Interestingly, this effect was further enhanced by the concomitant administration of atorvastatin [110]. While Kaminiotis and colleagues have shown that oral administration of colchicine in high-cholesterol diet-fed rabbits showed no effects on atherosclerosis progression [111], Mylonas and colleagues have shown in the same model that colchicine-based anti-inflammatory therapies significantly diminished de novo atherogenesis, decreased triglyceride levels [112,113] and also tampered Krüppel-like factor 4—a transcription factor involved in atheromatosis—overexpression in thoracic aortas [113].
The effect of colchicine in acute myocardial infarction has also been explored. Intraperitoneal administration of colchicine in a mouse model of ischemia/reperfusion resulted in a significant reduction in infarct size 24 h after injury, when administered before reperfusion had been established [114]. Significant improvement in hemodynamic parameters and cardiac fibrosis were also reported [114]. Colchicine has also been shown to be protective when administered after ischemia/reperfusion injury, reducing macrophage infiltration, cardiac remodeling, and dysfunction in a rat model of left coronary artery (LCA) ligation [115]. Similarly, in a mouse model of MI induced by permanent LCA ligation, short-term colchicine administration post MI significantly improved survival, cardiac function and heart failure [116]. This improvement in cardiac performance was associated with a reduction in monocyte and neutrophil infiltration, mRNA expression of inflammatory cytokines and components of the NLRP3 inflammasome 24 h after injury [116]. In SRBI KO/ApoeR61h/h mice, orally administered colchicine resulted in improved survival after feeding with atherogenic diet, however, neither inflammatory markers nor aortic plaque volume were different between the treated and untreated groups (González L, Martínez G, unpublished data).
Finally, the role of colchicine in plaque stabilization has been recently explored by Cecconi and colleagues [117], where atherosclerosis was induced by a high cholesterol diet and balloon endothelial denudation in rabbits. Colchicine treatment reduced the relative increase in aortic wall volume, measured as normalized wall index, and inflammation, measured as 18F-FDG uptake in PET/CT imaging, which could potentially help stabilize the plaque. This effect, however, was only seen in animals with high levels of circulating cholesterol [117]. Table 1 summarizes the available pre-clinical data on colchicine and atherosclerosis.

Table 1.
Pre-clinical studies.
8.2. Translational Studies
In a pilot study including 64 patients with stable coronary artery disease (CAD), Nidorf and colleagues showed that colchicine in low doses decreased high-sensitivity C-reactive protein (hsCRP) levels, a biomarker of inflammation, with no significant side effects [118]. However, the same protective effect was not seen in a pilot randomized controlled trial including patients with acute coronary syndrome (ACS) or stroke, where colchicine failed to reduce hsCRP levels [85]. The different results could be explained by the cause behind hsCRP elevation, which might not be sensitive to colchicine treatment, the dosage used and the context in which the drug was used—acute vs chronic inflammation. A local approach was then used by our group, in which the effect of colchicine on inflammatory cytokine production was assessed in blood samples collected from the coronary sinus [119]. ACS patients were recruited and randomized to receive either colchicine or placebo on top of standard therapy and levels of IL-1β, IL-18 and IL-6 were quantified. Acute colchicine administration resulted in a significant reduction in transcoronary cytokine gradients, suggesting a local intracardiac effect on the NLRP3 inflammasome [119]. In a follow-up study in a different cohort of ACS patients, a significant reduction in the release of IL-1β was observed with colchicine treatment in stimulated peripheral blood monocytes [64]. This reduction was associated with a suppression of monocyte caspase-1 activity. Colchicine was also able to reduce transcoronary and monocyte production of chemokines in treated ACS patients compared with control, which could also positively impact CV outcomes [120]. Furthermore, colchicine treatment suppressed NET production post percutaneous coronary intervention in ACS patients, a process that has been associated with periprocedural MI [89]. Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a cytokine belonging to the TNF family of ligands. Evidence shows that a deficiency in circulating TRAIL is associated with atherosclerotic plaque development, probably by inducing a more dysfunctional type of macrophage, with less migratory capacity, and impaired reverse cholesterol efflux and efferocytosis [121]. Research from our group shows that acute colchicine treatment significantly increases plasma TRAIL levels, purportedly regulating the inhibitory effect of IL-18 upon TRAIL [122].
The anti-inflammatory potential of colchicine in chronic CAD has been recently revaluated. Colchicine—either alone or in combination with methotrexate—did not improve coronary endothelial function in patients with stable CAD, measured through non-invasive MRI [123]. In a proteomics study, serum samples from CAD patients were compared before and 30 days after colchicine treatment. The expression of a total of 37 proteins was reduced, including members of the NLRP3 inflammasome pathway (IL-18, IL-1 receptor antagonist and IL-6), adaptative immune system proteins (C-C motif chemokine 17, CD40 ligand, pro-IL-6) and proteins involved in neutrophil degranulation (myeloperoxidase, myeloblastin and azurocidin among others) [124]. A reduction in median hsCRP has also been reported [124]. In the same cohort of patients, colchicine reportedly affected some biomarkers of inflammation. Colchicine treatment for a year resulted in a reduction of extracellular vesicle (EV) NLRP3 protein but no changes in serum NLRP3 protein levels [125]. Lower levels of hsCRP were also detected but this reduction was not related to EV NLRP3 protein levels [125]. MicroRNAs are known to be involved in multiple pathways driving atherosclerosis development. Barraclough and colleagues recently studied the microRNA signature in ACS patients and how colchicine might affect its expression [126]. Plasma samples collected from the aorta, coronary sinus and right atrium were collected from control, ACS standard therapy and ACS standard therapy plus colchicine patients. A total of 30 miRNAs were significantly elevated in the ACS group compared with controls. In patients with ACS, 12 miRNAs were lower when patients received colchicine and seven of these returned to control levels after colchicine treatment. More importantly, three miRNAs suppressed by colchicine are known to be regulators of inflammatory pathways, indicating that levels of miRNAs could potentially be used to track treatment effectiveness [126].
8.3. Phase 2 Clinical Studies
Colchicine has been shown to be protective in the context of ischemia/reperfusion, in accordance with pre-clinical results. The perioperative administration of colchicine to patients undergoing coronary bypass grafting resulted in a reduction in postoperative levels of myocardial injury biomarkers such as high-sensitivity troponin T (hsTrop) and creatine kinase-myocardial brain fraction (CKmb) [127]. Deftereos and colleagues have also reported beneficial effects of colchicine administration to STEMI patients treated with percutaneous coronary intervention [128]. Colchicine significantly reduced CKmb concentrations as well as infarct size when compared with placebo group [128]. However, the anti-inflammatory effect of colchicine could not be demonstrated in another study including STEMI patients, where hsCRP levels remained unaffected by the treatment [129]. Oral administration of high dose colchicine also demonstrated no effect on infarct size (assessed by cardiac magnetic resonance) in STEMI patients, when administered at reperfusion and for five consecutive days [130].
The anti-inflammatory effect of colchicine treatment in ACS patients seems to be associated with positive changes at atherosclerotic plaque level. In a prospective nonrandomized observational study including 80 patients with recent ACS, colchicine administration was associated with a reduction in low attenuation plaque volume (LAPV)—a measure of plaque instability and predictor of future coronary events [131]. A positive correlation between LAPV and reduced hsCRP levels has also been reported [131]. Furthermore, the addition of colchicine on top of standard therapy in ACS patients (0.5 mg daily for six months) positively impacted the occurrence of major adverse cardiovascular events, improving overall survival in a randomized, placebo-control trial [132]. Reduction in inflammatory markers (hsCRP, IL-6) after colchicine treatment has been reported in chronic coronary artery disease as well [133].
The Colchicine–PCI randomized trial evaluated the effect of colchicine administration before (1–2 h, 1.8 mg) percutaneous coronary intervention on post-PCI myocardial injury, in patients with stable angina (SA) and ACS [134]. Shah and colleagues have reported that preprocedural colchicine did not protect against PCI-related myocardial injury (including PCI-related MI and MACE at 30 days), despite a reduction in IL-6 and hsCRP levels (22–24 h post-PCI) [134]. Dissimilar results have been reported by Cole and colleagues in a similar pilot study, evaluating SA and ACS patients [135]. Colchicine administration prior to PCI intervention (1.5 mg, 6–24 h) significantly reduced major and minor periprocedural MI and injury, especially in NSTEMI patients [135]. Colchicine also significantly reduced pre-PCI inflammatory cytokine levels (IL-6, IL-1β, TNF-α, IFN-γ) and white blood cell counts, with no differences in post-PCI values [136]. Absolute Troponin change was also reportedly lower in the colchicine group [136]. The difference in results might be influenced by both the population studied and, very importantly, the time of colchicine administration.
8.4. Phase 3 Clinical Studies and Meta-Analyses
In the setting of ACS, the COLCOT trial randomized 4745 patients to receive colchicine (0.5 mg BID) or placebo within 30 days post-MI [137]. Colchicine led to a significant reduction of the primary outcome (a composite of death from cardiovascular causes, resuscitated cardiac arrest, myocardial infarction, stroke, or urgent hospitalization for angina leading to coronary revascularization) by 23% (HR 0.77; 95% CI 0.61–0.96; p = 0.02). This was mainly driven by a significant reduction in the incidence of stroke (HR 0.26; 95% CI 0.10–0.70) and urgent hospitalization for angina leading to coronary revascularization (HR 0.50; 95% CI 0.31–0.81) [137]. Interestingly, in a post-hoc analysis of COLCOT, time-to-treatment initiation (i.e., length of time between the index MI and the initiation of colchicine) was inversely correlated with colchicine clinical benefit. Indeed, when administered in-hospital within the first three days after the event, colchicine was associated with a 48% reduction in the risk of ischemic events; which contrasted with a lack of benefit when started later (four to seven days, and seven to thirty days) [138]. The other RCT in the ACS setting, the COPS trial, was an Australian-based study that randomly assigned 795 patients diagnosed with MI or unstable angina to receive colchicine (0.5 mg BID for one month, then 0.5 mg QD for eleven months) vs. placebo [139]. Although the original trial failed to demonstrate a benefit on the one-year primary outcome, an extended 24-month follow-up did show a significant 40% reduction in the composite of all-cause mortality, ACS, ischemia-driven-unplanned-urgent revascularization, and non-cardioembolic ischemic stroke. Of note, just as in COLCOT, the main outcome was driven by a significant reduction in urgent revascularization (HR, 0.19; 95% CI 0.05–0.66; p = 0.009) [140].
In the setting of chronic CAD, the LoDoCo trial randomized 532 patients to receive colchicine 0.5 mg or no colchicine, using an open label design [141]. Colchicine led to a reduction of the primary outcome (composite of ACS, out-of-hospital cardiac arrest, or non-cardioembolic ischemic stroke) of 67% (HR 0.33; 95% CI 0.18–0.59; p < 0.001), due to a significant reduction in the risk of ACS (HR 0.33; 95% CI 0.18–0.63; p < 0.001). This same group published, seven years later, the LoDoCo 2 trial, using a more robust—double blinded, placebo controlled—study design and a 10-fold higher number of patients [142]. In this landmark trial, colchicine led to a reduction in the primary outcome (a composite of cardiovascular death, spontaneous (nonprocedural) myocardial infarction, ischemic stroke, or ischemia-driven coronary revascularization) of 31% (HR 0.69; 95% CI 0.57–0.83; p < 0.001), which was due, again, to a significant reduction of MI (HR 0.7; 95% CI 0.53–0.93; p = 0.01) and also of ischemia-driven coronary revascularization (HR 0.75; 95% CI 0.60–0.94; p = 0.01) [142].
The analysis of the components of ACS in LoDoCo suggested that colchicine reduced the probability of acute coronary events unrelated to stent disease (i.e., in native segments), with lack of effect in the prevention of stent-related disease (i.e., acute stent thrombosis or stent restenosis) [141]. However, in a previous study that included diabetic patients undergoing PCI with bare metal stents, six-month angiographic restenosis rates were reduced by 62% in the group of patients randomized to colchicine as compared with patients in the control group (16% vs. 33%; OR 0.38, 95% CI 0.18–0.79; p = 0.007) [143]. These results may suggest that, along with atheroma plaque stabilization in native coronary arteries, the anti-inflammatory and anti-mitotic effects of colchicine may be equally effective in the prevention of neointimal hyperplasia, the central process in the pathophysiology of in-stent restenosis. However, the effects of colchicine preventing stent related disease in the era of new generation drug eluting stents may be unclear, as the rates of restenosis have decreased significantly [144].
As individual trials suffer from significant heterogeneity regarding the clinical setting (acute versus chronic CAD), treatment (colchicine dose and length of follow-up) and end point definitions, several systematic reviews and meta-analysis have been conducted to the summarize clinical effects of colchicine [145,146,147,148]. In one of these, by Fiolet and colleagues, inclusion criteria were restricted to RCT’s with a minimum follow-up of three months, thus including the five trials mentioned above. In their analysis, colchicine reduced the risk for the primary endpoint—a composite of MI, stroke, or cardiovascular death—by 25% (RR 0.75; 95% CI 0.61–0.92; p = 0.005) with a low between-trial heterogeneity (I2 = 23.9%). Colchicine led to a significant reduction of the individual endpoints MI by 22% (RR 0.78; 95% CI 0.64–0.94; p = 0.010), stroke by 46% (RR 0.54; 95% CI 0.34–0.86; p = 0.009), and coronary revascularization by 23% (RR 0.77; 95% CI 0.66–0.90; p < 0.001) [145]. In subgroup analysis the benefit observed with colchicine was consistent in both acute and chronic coronary syndrome and irrespective of gender [145]. It is noteworthy that the magnitude of the benefit obtained with colchicine in patients with CAD is comparable to that achieved by each of the mainstay therapies for the secondary prevention of CAD—such as antiplatelet agents and statins [149,150]—and has been achieved against a background of optimal treatment with these therapies.
The applicability of these findings may depend upon specific patient subsets. For example, the observed benefit of colchicine may be even higher in patients with diabetes mellitus. In a meta-analysis conducted by Kuzemczak and colleagues, in patients with CAD, the absolute risk reduction of the composite endpoint of MACE achieved by colchicine in patients with diabetes was greater than in patients without diabetes (absolute risk reduction of 3.94% vs. 2.32%, p < 0.001) [151]. On the other hand, as most trials have excluded patients with heart failure and chronic kidney disease, the effects of colchicine for the treatment of CAD in these important populations remain unknown.
Despite these favorable effects seen with colchicine, some trials have documented concerning results regarding non-CV mortality. In the COPS trial the rate of all-cause death was higher in the colchicine group compared with placebo (HR 8.20; 95% CI 1.03–65.61; p = 0.047) due to an increase in non-cardiovascular deaths, which were mostly due to sepsis [139]. Likewise, in the LoDoCo 2 trial there was an increase in non-cardiovascular deaths (HR 1.51; 95% CI 0.99–2.31), but without a parallel increase in severe infections, new cancer diagnosis or severe gastrointestinal adverse effects [142]. Meta-analyses have shown a non-significant lower incidence of cardiovascular mortality (RR 0.82; 95% CI 0.55–1.23; p = 0.339) counterbalanced by a non-significant higher incidence of non-cardiovascular deaths (RR 1.38; 95% CI 0.99–1.92; p = 0.060), with no difference in all-cause mortality (RR 1.08; 95% CI 0.71–1.62; p = 0.726) [145].
Taken together, RCTs show a consistent beneficial effect of colchicine by limiting new cardiovascular events and stroke. However, some barriers remain before a widespread use of colchicine in clinical practice can be adopted. Firstly, and as discussed above, its net effect on mortality is still under scrutiny, with a possible increase in non-cardiovascular deaths, which needs to be clarified in future trials. Secondly, in the era of precision medicine, a more individualized approach may be adopted to target specific populations where colchicine can produce a maximum benefit, such as in those patients with (i) persistent inflammation after the index event (i.e., persistently high hsCRP); (ii) particular markers of excess NLRP3 inflammasome activity (i.e., carriers of the rs10754555 gene variant); or (iii) high-risk of recurrence (such as diabetics). And finally, in the setting of secondary prevention of coronary artery disease, incorporating an additional drug to patients who are already under treatment with multiple medications with proven benefit brings forth the problem of poor adherence, as well as the risk of aggravating polypharmacy in an increasingly elderly and frail population. Table 2 summarizes the available phase 2 and phase 3 data on colchicine and atherosclerosis.

Table 2.
Phase 2 and 3 clinical studies.
9. Conclusions
The inflammatory component of atherosclerosis pathogenesis offers new avenues through which novel therapies can be used and/or developed. Though around for decades, only recently has colchicine been in the eye of scientists and clinicians looking for new therapies for the management of coronary artery disease complications. The intracellular effects of colchicine directly impact key cellular players of inflammation, resulting in protective effects against atherosclerosis development (Figure 2). Translational research and phase 2 and 3 clinical trials have predominantly shown a beneficial effect of colchicine by modulating many underlying processes related to athero-inflammation and resulting in less clinical events. However, the net clinical effect upon mortality is still unclear and new trials must address this issue to be able to finally introduce this long-waited drug into the therapeutic toolkit to treat coronary artery disease.
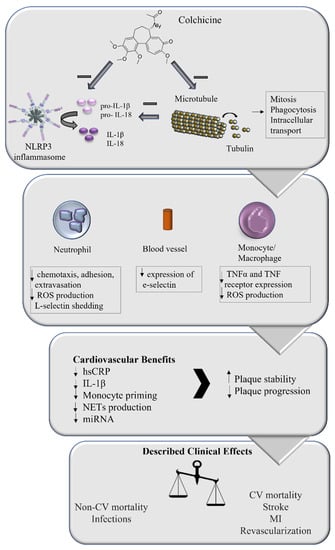
Figure 2.
Role of colchicine in coronary artery disease treatment. Colchicine has been described to affect microtubule stability, impacting several intracellular processes including mitosis, phagocytosis, and intracellular transport. It has also been reported that colchicine affects NLRP3 inflammasome activation, impacting inflammatory cytokines production, both directly and through its action on microtubules. These intracellular effects directly impact the inflammatory response of neutrophils, monocyte/macrophages, and blood vessels, which translates into several cardiovascular benefits. The overall effect on plaque stability and progression impacts the clinical manifestations of atherosclerosis, reducing the incidence of major adverse cardiovascular effects, suggesting that the addition of colchicine to the management of coronary artery disease might be beneficial.
Author Contributions
G.M.R. and P.M.V. designed the study. G.M.R. and L.G. screened the available literature. L.G. and J.F.B. wrote the original draft. G.M.R., M.P.O. and P.M.V. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
GM: MPO: Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) de la Agencia Nacional de Investigación y Desarrollo (ANID)-Proyecto FONDECYT Regular 1210655, LG: ANID, Programa Iniciativa Científica Milenio–ICN2021_004.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. The World Health Report 1999: Making a difference. Health Millions 1999, 25, 3–5. [Google Scholar]
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation 2012, 125, e2–e220. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, K.; Martinez, G.; Patel, S. The Role of Colchicine in Acute Coronary Syndromes. Clin. Ther. 2019, 41, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reith, C.; Armitage, J. Management of residual risk after statin therapy. Atherosclerosis 2016, 245, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Huynh, T.; Montigny, M.; Iftikhar, U.; Gagnon, R.; Eisenberg, M.; Lauzon, C.; Mansour, S.; Rinfret, S.; Afilalo, M.; Nguyen, M.; et al. Recurrent Cardiovascular Events in Survivors of Myocardial Infarction with ST-Segment Elevation (from the AMI-QUEBEC Study). Am. J. Cardiol. 2018, 121, 897–902. [Google Scholar] [CrossRef]
- Khan, R.; Spagnoli, V.; Tardif, J.C.; L’Allier, P.L. Novel anti-inflammatory therapies for the treatment of atherosclerosis. Atherosclerosis 2015, 240, 497–509. [Google Scholar] [CrossRef]
- Geovanini, G.R.; Libby, P. Atherosclerosis and inflammation: Overview and updates. Clin. Sci. 2018, 132, 1243–1252. [Google Scholar] [CrossRef]
- Hansson, G.K.; Libby, P.; Schonbeck, U.; Yan, Z.Q. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ. Res. 2002, 91, 281–291. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Stewart, C.R.; Stuart, L.M.; Wilkinson, K.; van Gils, J.M.; Deng, J.; Halle, A.; Rayner, K.J.; Boyer, L.; Zhong, R.; Frazier, W.A.; et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010, 11, 155–161. [Google Scholar] [CrossRef] [Green Version]
- De Winther, M.P.; Kanters, E.; Kraal, G.; Hofker, M.H. Nuclear factor kappaB signaling in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 904–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Sukhova, G.K.; Schonbeck, U.; Rabkin, E.; Schoen, F.J.; Poole, A.R.; Billinghurst, R.C.; Libby, P. Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation 1999, 99, 2503–2509. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, J.O.; Aikawa, E.; Libby, P.; Vachon, J.R.; Inada, M.; Krane, S.M.; Whittaker, P.; Aikawa, M. Matrix metalloproteinase-13/collagenase-3 deletion promotes collagen accumulation and organization in mouse atherosclerotic plaques. Circulation 2005, 112, 2708–2715. [Google Scholar] [CrossRef] [Green Version]
- Silvestre-Roig, C.; Braster, Q.; Ortega-Gomez, A.; Soehnlein, O. Neutrophils as regulators of cardiovascular inflammation. Nat. Rev. Cardiol. 2020, 17, 327–340. [Google Scholar] [CrossRef]
- Guasti, L.; Dentali, F.; Castiglioni, L.; Maroni, L.; Marino, F.; Squizzato, A.; Ageno, W.; Gianni, M.; Gaudio, G.; Grandi, A.M.; et al. Neutrophils and clinical outcomes in patients with acute coronary syndromes and/or cardiac revascularisation. A systematic review on more than 34,000 subjects. Thromb. Haemost. 2011, 106, 591–599. [Google Scholar] [CrossRef] [Green Version]
- Drechsler, M.; Megens, R.T.; van Zandvoort, M.; Weber, C.; Soehnlein, O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation 2010, 122, 1837–1845. [Google Scholar] [CrossRef]
- Malle, E.; Waeg, G.; Schreiber, R.; Grone, E.F.; Sattler, W.; Grone, H.J. Immunohistochemical evidence for the myeloperoxidase/H2O2/halide system in human atherosclerotic lesions: Colocalization of myeloperoxidase and hypochlorite-modified proteins. Eur. J. Biochem. 2000, 267, 4495–4503. [Google Scholar] [CrossRef]
- Podrez, E.A.; Febbraio, M.; Sheibani, N.; Schmitt, D.; Silverstein, R.L.; Hajjar, D.P.; Cohen, P.A.; Frazier, W.A.; Hoff, H.F.; Hazen, S.L. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J. Clin. Investig. 2000, 105, 1095–1108. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Kassim, S.Y.; Parks, W.C.; Heinecke, J.W. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J. Biol. Chem. 2001, 276, 41279–41287. [Google Scholar] [CrossRef] [Green Version]
- Dorweiler, B.; Torzewski, M.; Dahm, M.; Kirkpatrick, C.J.; Lackner, K.J.; Vahl, C.F. Subendothelial infiltration of neutrophil granulocytes and liberation of matrix-destabilizing enzymes in an experimental model of human neo-intima. Thromb. Haemost. 2008, 99, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, P.A.; Sallenave, J.M. Human neutrophil elastase: Mediator and therapeutic target in atherosclerosis. Int. J. Biochem. Cell Biol. 2008, 40, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Pezzato, E.; Dona, M.; Sartor, L.; Dell’Aica, I.; Benelli, R.; Albini, A.; Garbisa, S. Proteinase-3 directly activates MMP-2 and degrades gelatin and Matrigel; differential inhibition by (-)epigallocatechin-3-gallate. J. Leukoc. Biol. 2003, 74, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Rotzius, P.; Soehnlein, O.; Kenne, E.; Lindbom, L.; Nystrom, K.; Thams, S.; Eriksson, E.E. ApoE(-/-)/lysozyme M(EGFP/EGFP) mice as a versatile model to study monocyte and neutrophil trafficking in atherosclerosis. Atherosclerosis 2009, 202, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Ionita, M.G.; van den Borne, P.; Catanzariti, L.M.; Moll, F.L.; de Vries, J.P.; Pasterkamp, G.; Vink, A.; de Kleijn, D.P. High neutrophil numbers in human carotid atherosclerotic plaques are associated with characteristics of rupture-prone lesions. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1842–1848. [Google Scholar] [CrossRef]
- Zernecke, A.; Bot, I.; Djalali-Talab, Y.; Shagdarsuren, E.; Bidzhekov, K.; Meiler, S.; Krohn, R.; Schober, A.; Sperandio, M.; Soehnlein, O.; et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ. Res. 2008, 102, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Soehnlein, O.; Lindbom, L.; Weber, C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood 2009, 114, 4613–4623. [Google Scholar] [CrossRef]
- Rasmuson, J.; Kenne, E.; Wahlgren, M.; Soehnlein, O.; Lindbom, L. Heparinoid sevuparin inhibits Streptococcus-induced vascular leak through neutralizing neutrophil-derived proteins. FASEB J. 2019, 33, 10443–10452. [Google Scholar] [CrossRef] [Green Version]
- Doring, Y.; Soehnlein, O.; Weber, C. Neutrophil Extracellular Traps in Atherosclerosis and Atherothrombosis. Circ. Res. 2017, 120, 736–743. [Google Scholar] [CrossRef] [Green Version]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Warnatsch, A.; Ioannou, M.; Wang, Q.; Papayannopoulos, V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015, 349, 316–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Megens, R.T.; Vijayan, S.; Lievens, D.; Doring, Y.; van Zandvoort, M.A.; Grommes, J.; Weber, C.; Soehnlein, O. Presence of luminal neutrophil extracellular traps in atherosclerosis. Thromb. Haemost. 2012, 107, 597–598. [Google Scholar] [CrossRef] [PubMed]
- Quillard, T.; Araujo, H.A.; Franck, G.; Shvartz, E.; Sukhova, G.; Libby, P. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: Implications for superficial erosion. Eur. Heart J. 2015, 36, 1394–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borissoff, J.I.; Joosen, I.A.; Versteylen, M.O.; Brill, A.; Fuchs, T.A.; Savchenko, A.S.; Gallant, M.; Martinod, K.; Ten Cate, H.; Hofstra, L.; et al. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2032–2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangold, A.; Alias, S.; Scherz, T.; Hofbauer, M.; Jakowitsch, J.; Panzenbock, A.; Simon, D.; Laimer, D.; Bangert, C.; Kammerlander, A.; et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ. Res. 2015, 116, 1182–1192. [Google Scholar] [CrossRef] [Green Version]
- Pertiwi, K.R.; van der Wal, A.C.; Pabittei, D.R.; Mackaaij, C.; van Leeuwen, M.B.; Li, X.; de Boer, O.J. Neutrophil Extracellular Traps Participate in All Different Types of Thrombotic and Haemorrhagic Complications of Coronary Atherosclerosis. Thromb. Haemost. 2018, 118, 1078–1087. [Google Scholar] [CrossRef]
- Grebe, A.; Latz, E. Cholesterol crystals and inflammation. Curr. Rheumatol. Rep. 2013, 15, 313. [Google Scholar] [CrossRef] [Green Version]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nunez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef] [Green Version]
- Small, D.M. George Lyman Duff memorial lecture. Progression and regression of atherosclerotic lesions. Insights from lipid physical biochemistry. Arteriosclerosis 1988, 8, 103–129. [Google Scholar] [CrossRef] [Green Version]
- Abela, G.S. Cholesterol crystals piercing the arterial plaque and intima trigger local and systemic inflammation. J. Clin. Lipidol. 2010, 4, 156–164. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, B.R.; Kanneganti, T.D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 2021, 22, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Kanneganti, T.D. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J. Cell Biol. 2016, 213, 617–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pope, R.M.; Tschopp, J. The role of interleukin-1 and the inflammasome in gout: Implications for therapy. Arthritis Rheum. 2007, 56, 3183–3188. [Google Scholar] [CrossRef]
- Stutz, A.; Golenbock, D.T.; Latz, E. Inflammasomes: Too big to miss. J. Clin. Investig. 2009, 119, 3502–3511. [Google Scholar] [CrossRef] [Green Version]
- Christgen, S.; Kanneganti, T.D. Inflammasomes and the fine line between defense and disease. Curr. Opin. Immunol. 2020, 62, 39–44. [Google Scholar] [CrossRef]
- Franchi, L.; Munoz-Planillo, R.; Nunez, G. Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 2012, 13, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Mathur, A.; Hayward, J.A.; Man, S.M. Molecular mechanisms of inflammasome signaling. J. Leukoc. Biol. 2018, 103, 233–257. [Google Scholar] [CrossRef]
- Vajjhala, P.R.; Mirams, R.E.; Hill, J.M. Multiple binding sites on the pyrin domain of ASC protein allow self-association and interaction with NLRP3 protein. J. Biol. Chem. 2012, 287, 41732–41743. [Google Scholar] [CrossRef] [Green Version]
- Fernandes-Alnemri, T.; Wu, J.; Yu, J.W.; Datta, P.; Miller, B.; Jankowski, W.; Rosenberg, S.; Zhang, J.; Alnemri, E.S. The pyroptosome: A supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ 2007, 14, 1590–1604. [Google Scholar] [CrossRef]
- Hornung, V.; Bauernfeind, F.; Halle, A.; Samstad, E.O.; Kono, H.; Rock, K.L.; Fitzgerald, K.A.; Latz, E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008, 9, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Schilling, J.D. Lysosomes integrate metabolic-inflammatory cross-talk in primary macrophage inflammasome activation. J. Biol. Chem. 2014, 289, 9158–9171. [Google Scholar] [CrossRef] [Green Version]
- Sheedy, F.J.; Grebe, A.; Rayner, K.J.; Kalantari, P.; Ramkhelawon, B.; Carpenter, S.B.; Becker, C.E.; Ediriweera, H.N.; Mullick, A.E.; Golenbock, D.T.; et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 2013, 14, 812–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirii, H.; Niwa, T.; Yamada, Y.; Wada, H.; Saito, K.; Iwakura, Y.; Asano, M.; Moriwaki, H.; Seishima, M. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 656–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, M.R.; Moehle, C.W.; Johnson, J.L.; Yang, Z.; Lee, J.K.; Jackson, C.L.; Owens, G.K. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J. Clin. Investig. 2012, 122, 70–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P. Interleukin-1 Beta as a Target for Atherosclerosis Therapy: Biological Basis of CANTOS and Beyond. J. Am. Coll. Cardiol. 2017, 70, 2278–2289. [Google Scholar] [CrossRef]
- Yajima, N.; Takahashi, M.; Morimoto, H.; Shiba, Y.; Takahashi, Y.; Masumoto, J.; Ise, H.; Sagara, J.; Nakayama, J.; Taniguchi, S.; et al. Critical role of bone marrow apoptosis-associated speck-like protein, an inflammasome adaptor molecule, in neointimal formation after vascular injury in mice. Circulation 2008, 117, 3079–3087. [Google Scholar] [CrossRef]
- Gage, J.; Hasu, M.; Thabet, M.; Whitman, S.C. Caspase-1 deficiency decreases atherosclerosis in apolipoprotein E-null mice. Can. J. Cardiol. 2012, 28, 222–229. [Google Scholar] [CrossRef]
- Usui, F.; Shirasuna, K.; Kimura, H.; Tatsumi, K.; Kawashima, A.; Karasawa, T.; Hida, S.; Sagara, J.; Taniguchi, S.; Takahashi, M. Critical role of caspase-1 in vascular inflammation and development of atherosclerosis in Western diet-fed apolipoprotein E-deficient mice. Biochem. Biophys. Res. Commun. 2012, 425, 162–168. [Google Scholar] [CrossRef]
- Hendrikx, T.; Jeurissen, M.L.; van Gorp, P.J.; Gijbels, M.J.; Walenbergh, S.M.; Houben, T.; van Gorp, R.; Pottgens, C.C.; Stienstra, R.; Netea, M.G.; et al. Bone marrow-specific caspase-1/11 deficiency inhibits atherosclerosis development in Ldlr(-/-) mice. FEBS J. 2015, 282, 2327–2338. [Google Scholar] [CrossRef]
- Menu, P.; Pellegrin, M.; Aubert, J.F.; Bouzourene, K.; Tardivel, A.; Mazzolai, L.; Tschopp, J. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis 2011, 2, e137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Xie, W.L.; Kong, W.W.; Chen, D.; Qu, P. Expression of the NLRP3 Inflammasome in Carotid Atherosclerosis. J. Stroke Cerebrovasc. Dis. 2015, 24, 2455–2466. [Google Scholar] [CrossRef] [PubMed]
- Paramel Varghese, G.; Folkersen, L.; Strawbridge, R.J.; Halvorsen, B.; Yndestad, A.; Ranheim, T.; Krohg-Sorensen, K.; Skjelland, M.; Espevik, T.; Aukrust, P.; et al. NLRP3 Inflammasome Expression and Activation in Human Atherosclerosis. J. Am. Heart Assoc. 2016, 5, e003031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, S.; Martinez, G.J.; Payet, C.A.; Barraclough, J.Y.; Celermajer, D.S.; Bursill, C.; Patel, S. Colchicine therapy in acute coronary syndrome patients acts on caspase-1 to suppress NLRP3 inflammasome monocyte activation. Clin. Sci. 2016, 130, 1237–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altaf, A.; Qu, P.; Zhao, Y.; Wang, H.; Lou, D.; Niu, N. NLRP3 inflammasome in peripheral blood monocytes of acute coronary syndrome patients and its relationship with statins. Coron. Artery Dis. 2015, 26, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Dasgeb, B.; Kornreich, D.; McGuinn, K.; Okon, L.; Brownell, I.; Sackett, D.L. Colchicine: An ancient drug with novel applications. Br. J. Dermatol. 2018, 178, 350–356. [Google Scholar] [CrossRef]
- D’Amario, D.; Cappetta, D.; Cappannoli, L.; Princi, G.; Migliaro, S.; Diana, G.; Chouchane, K.; Borovac, J.A.; Restivo, A.; Arcudi, A.; et al. Colchicine in ischemic heart disease: The good, the bad and the ugly. Clin. Res. Cardiol. 2021, 110, 1531–1542. [Google Scholar] [CrossRef]
- Cortese, F.; Bhattacharyya, B.; Wolff, J. Podophyllotoxin as a probe for the colchicine binding site of tubulin. J. Biol. Chem. 1977, 252, 1134–1140. [Google Scholar] [CrossRef]
- Pyles, E.A.; Hastie, S.B. Effect of the B ring and the C-7 substituent on the kinetics of colchicinoid-tubulin associations. Biochemistry 1993, 32, 2329–2336. [Google Scholar] [CrossRef]
- Pascart, T.; Richette, P. Colchicine in Gout: An Update. Curr. Pharm. Des. 2018, 24, 684–689. [Google Scholar] [CrossRef]
- Portincasa, P. Colchicine, Biologic Agents and More for the Treatment of Familial Mediterranean Fever. The Old, the New, and the Rare. Curr. Med. Chem. 2016, 23, 60–86. [Google Scholar] [CrossRef] [PubMed]
- Deftereos, S.G.; Beerkens, F.J.; Shah, B.; Giannopoulos, G.; Vrachatis, D.A.; Giotaki, S.G.; Siasos, G.; Nicolas, J.; Arnott, C.; Patel, S.; et al. Colchicine in Cardiovascular Disease: In-Depth Review. Circulation 2022, 145, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.W. The Mechanism of Colchicine Inhibition of Mitosis. I. Kinetics of Inhibition and the Binding of H3-Colchicine. J. Cell Biol. 1965, 25, 145–160. [Google Scholar] [CrossRef]
- Asako, H.; Kubes, P.; Baethge, B.A.; Wolf, R.E.; Granger, D.N. Colchicine and methotrexate reduce leukocyte adherence and emigration in rat mesenteric venules. Inflammation 1992, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Cronstein, B.N.; Molad, Y.; Reibman, J.; Balakhane, E.; Levin, R.I.; Weissmann, G. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J. Clin. Investig. 1995, 96, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Paschke, S.; Weidner, A.F.; Paust, T.; Marti, O.; Beil, M.; Ben-Chetrit, E. Technical advance: Inhibition of neutrophil chemotaxis by colchicine is modulated through viscoelastic properties of subcellular compartments. J. Leukoc. Biol. 2013, 94, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Roberge, C.J.; Gaudry, M.; de Medicis, R.; Lussier, A.; Poubelle, P.E.; Naccache, P.H. Crystal-induced neutrophil activation. IV. Specific inhibition of tyrosine phosphorylation by colchicine. J. Clin. Investig. 1993, 92, 1722–1729. [Google Scholar] [CrossRef] [Green Version]
- Chia, E.W.; Grainger, R.; Harper, J.L. Colchicine suppresses neutrophil superoxide production in a murine model of gouty arthritis: A rationale for use of low-dose colchicine. Br. J. Pharmacol. 2008, 153, 1288–1295. [Google Scholar] [CrossRef] [Green Version]
- Viktorov, A.V.; Yurkiv, V.A. Albendazole and colchicine modulate LPS-induced secretion of inflammatory mediators by liver macrophages. Bull. Exp. Biol. Med. 2011, 151, 683–685. [Google Scholar] [CrossRef]
- Ding, A.H.; Porteu, F.; Sanchez, E.; Nathan, C.F. Downregulation of tumor necrosis factor receptors on macrophages and endothelial cells by microtubule depolymerizing agents. J. Exp. Med. 1990, 171, 715–727. [Google Scholar] [CrossRef] [Green Version]
- Menche, D.; Israel, A.; Karpatkin, S. Platelets and microtubules. Effect of colchicine and D2O on platelet aggregation and release induced by calcium ionophore A23187. J. Clin. Investig. 1980, 66, 284–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cimmino, G.; Tarallo, R.; Conte, S.; Morello, A.; Pellegrino, G.; Loffredo, F.S.; Cali, G.; De Luca, N.; Golino, P.; Trimarco, B.; et al. Colchicine reduces platelet aggregation by modulating cytoskeleton rearrangement via inhibition of cofilin and LIM domain kinase 1. Vascul. Pharmacol. 2018, 111, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Abanonu, G.B.; Daskin, A.; Akdogan, M.F.; Uyar, S.; Demirtunc, R. Mean platelet volume and beta-thromboglobulin levels in familial Mediterranean fever: Effect of colchicine use? Eur. J. Intern. Med. 2012, 23, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Allen, N.; Harchandani, B.; Pillinger, M.; Katz, S.; Sedlis, S.P.; Echagarruga, C.; Samuels, S.K.; Morina, P.; Singh, P.; et al. Effect of Colchicine on Platelet-Platelet and Platelet-Leukocyte Interactions: A Pilot Study in Healthy Subjects. Inflammation 2016, 39, 182–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raju, N.C.; Yi, Q.; Nidorf, M.; Fagel, N.D.; Hiralal, R.; Eikelboom, J.W. Effect of colchicine compared with placebo on high sensitivity C-reactive protein in patients with acute coronary syndrome or acute stroke: A pilot randomized controlled trial. J. Thromb. Thrombolysis 2012, 33, 88–94. [Google Scholar] [CrossRef]
- Moschonas, I.C.; Tselepis, A.D. The pathway of neutrophil extracellular traps towards atherosclerosis and thrombosis. Atherosclerosis 2019, 288, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Munoz, L.E.; Boeltz, S.; Bilyy, R.; Schauer, C.; Mahajan, A.; Widulin, N.; Gruneboom, A.; Herrmann, I.; Boada, E.; Rauh, M.; et al. Neutrophil Extracellular Traps Initiate Gallstone Formation. Immunity 2019, 51, 443–450.e4. [Google Scholar] [CrossRef] [Green Version]
- Safi, R.; Kallas, R.; Bardawil, T.; Mehanna, C.J.; Abbas, O.; Hamam, R.; Uthman, I.; Kibbi, A.G.; Nassar, D. Neutrophils contribute to vasculitis by increased release of neutrophil extracellular traps in Behcet’s disease. J. Dermatol. Sci. 2018, 92, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, K.; Tucker, B.; Kurup, R.; Khandkar, C.; Pandzic, E.; Barraclough, J.; Machet, J.; Misra, A.; Kavurma, M.; Martinez, G.; et al. Colchicine Inhibits Neutrophil Extracellular Trap Formation in Patients with Acute Coronary Syndrome After Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2021, 10, e018993. [Google Scholar] [CrossRef]
- Martinon, F.; Petrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.H.; Wood, G.; Kastner, D.L.; Chae, J.J. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat. Immunol. 2016, 17, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Misawa, T.; Takahama, M.; Kozaki, T.; Lee, H.; Zou, J.; Saitoh, T.; Akira, S. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 2013, 14, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.T.T.; Do, H.M.; Nguyen, T.T. Evaluation of Colchicine’s interaction with the ATP-binding region of mice NLRP3-NACHT domain using molecular docking and dynamics simulation. J. Phys. Conf. Ser. 2022, 2269, 12012. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [Green Version]
- Marques-da-Silva, C.; Chaves, M.M.; Castro, N.G.; Coutinho-Silva, R.; Guimaraes, M.Z. Colchicine inhibits cationic dye uptake induced by ATP in P2X2 and P2X7 receptor-expressing cells: Implications for its therapeutic action. Br. J. Pharmacol. 2011, 163, 912–926. [Google Scholar] [CrossRef] [Green Version]
- Sabouraud, A.; Chappey, O.; Dupin, T.; Scherrmann, J.M. Binding of colchicine and thiocolchicoside to human serum proteins and blood cells. Int. J. Clin. Pharmacol. Ther. 1994, 32, 429–432. [Google Scholar]
- Niel, E.; Scherrmann, J.M. Colchicine today. Jt. Bone Spine 2006, 73, 672–678. [Google Scholar] [CrossRef]
- Terkeltaub, R.A. Colchicine update: 2008. Semin. Arthritis Rheum. 2009, 38, 411–419. [Google Scholar] [CrossRef]
- Putterman, C.; Ben-Chetrit, E.; Caraco, Y.; Levy, M. Colchicine intoxication: Clinical pharmacology, risk factors, features, and management. Semin. Arthritis Rheum. 1991, 21, 143–155. [Google Scholar] [CrossRef]
- Andreis, A.; Imazio, M.; Avondo, S.; Casula, M.; Paneva, E.; Piroli, F.; De Ferrari, G.M. Adverse events of colchicine for cardiovascular diseases: A comprehensive meta-analysis of 14 188 patients from 21 randomized controlled trials. J. Cardiovasc. Med. 2021, 22, 637–644. [Google Scholar] [CrossRef]
- Hemkens, L.G.; Ewald, H.; Gloy, V.L.; Arpagaus, A.; Olu, K.K.; Nidorf, M.; Glinz, D.; Nordmann, A.J.; Briel, M. Colchicine for prevention of cardiovascular events. Cochrane Database Syst. Rev. 2016, 1, CD011047. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Yang, K.C.K.; Atkins, K.; Dalbeth, N.; Robinson, P.C. Adverse events during oral colchicine use: A systematic review and meta-analysis of randomised controlled trials. Arthritis Res. Ther. 2020, 22, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, M.; Princi, G.; Crea, F.; D’Amario, D. Response-letter to the editor: Colchicine and risk of non-cardiovascular death in patients with coronary artery disease: A pooled analysis underlying possible safety concerns. Eur. Heart J. Cardiovasc Pharmacother 2021, 7, e72–e73. [Google Scholar] [CrossRef]
- Tsai, T.L.; Wei, J.C.; Wu, Y.T.; Ku, Y.H.; Lu, K.L.; Wang, Y.H.; Chiou, J.Y. The Association Between Usage of Colchicine and Pneumonia: A Nationwide, Population-Based Cohort Study. Front. Pharmacol. 2019, 10, 908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwier, N.C.; Cornelio, C.K.; Boylan, P.M. A systematic review of the drug-drug interaction between statins and colchicine: Patient characteristics, etiologies, and clinical management strategies. Pharmacotherapy 2022, 42, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Hung, I.F.; Wu, A.K.; Cheng, V.C.; Tang, B.S.; To, K.W.; Yeung, C.K.; Woo, P.C.; Lau, S.K.; Cheung, B.M.; Yuen, K.Y. Fatal interaction between clarithromycin and colchicine in patients with renal insufficiency: A retrospective study. Clin. Infect. Dis. 2005, 41, 291–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojcicki, J.; Hinek, A.; Jaworska, M.; Samochowiec, L. The effect of colchicine on the development of experimental atherosclerosis in rabbits. Pol. J. Pharmacol. Pharm. 1986, 38, 343–348. [Google Scholar]
- Lee, W.M.; Morrison, E.S.; Scott, R.F.; Lee, K.T.; Kroms, M. Effects of methyl prednisolone and colchicine on the development of aortic atherosclerosis in swine. Atherosclerosis 1976, 25, 213–224. [Google Scholar] [CrossRef]
- Bauriedel, G.; Ganesh, S.; Uberfuhr, P.; Welsch, U.; Hofling, B. Growth-inhibiting effect of colchicine on cultured vascular wall myocytes from arteriosclerotic lesions. Z. Kardiol. 1992, 81, 92–98. [Google Scholar]
- Huang, C.; Cen, C.; Wang, C.; Zhan, H.; Ding, X. Synergistic effects of colchicine combined with atorvastatin in rats with hyperlipidemia. Lipids Health Dis. 2014, 13, 67. [Google Scholar] [CrossRef] [Green Version]
- Kaminiotis, V.V.; Agrogiannis, G.; Konstantopoulos, P.; Androutsopoulou, V.; Korou, L.M.; Vlachos, I.S.; Dontas, I.A.; Perrea, D.; Iliopoulos, D.C. Per os colchicine administration in cholesterol fed rabbits: Triglycerides lowering effects without affecting atherosclerosis progress. Lipids Health Dis. 2017, 16, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spartalis, M.; Siasos, G.; Mastrogeorgiou, M.; Spartalis, E.; Kaminiotis, V.V.; Mylonas, K.S.; Kapelouzou, A.; Kontogiannis, C.; Doulamis, I.P.; Toutouzas, K.; et al. The effect of per os colchicine administration in combination with fenofibrate and N-acetylcysteine on triglyceride levels and the development of atherosclerotic lesions in cholesterol-fed rabbits. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7765–7776. [Google Scholar] [CrossRef]
- Mylonas, K.S.; Kapelouzou, A.; Spartalis, M.; Mastrogeorgiou, M.; Spartalis, E.; Bakoyiannis, C.; Liakakos, T.; Schizas, D.; Iliopoulos, D.; Nikiteas, N. KLF4 Upregulation in Atherosclerotic Thoracic Aortas: Exploring the Protective Effect of Colchicine-based Regimens in a Hyperlipidemic Rabbit Model. Ann. Vasc. Surg. 2022, 78, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Akodad, M.; Fauconnier, J.; Sicard, P.; Huet, F.; Blandel, F.; Bourret, A.; de Santa Barbara, P.; Aguilhon, S.; LeGall, M.; Hugon, G.; et al. Interest of colchicine in the treatment of acute myocardial infarct responsible for heart failure in a mouse model. Int. J. Cardiol. 2017, 240, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Taki, J.; Wakabayashi, H.; Hiromasa, T.; Inaki, A.; Ogawa, K.; Shiba, K.; Kinuya, S. Colchicine treatment early after infarction attenuates myocardial inflammatory response demonstrated by (14)C-methionine imaging and subsequent ventricular remodeling by quantitative gated SPECT. Ann. Nucl. Med. 2021, 35, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Fujisue, K.; Sugamura, K.; Kurokawa, H.; Matsubara, J.; Ishii, M.; Izumiya, Y.; Kaikita, K.; Sugiyama, S. Colchicine Improves Survival, Left Ventricular Remodeling, and Chronic Cardiac Function After Acute Myocardial Infarction. Circ. J. 2017, 81, 1174–1182. [Google Scholar] [CrossRef] [Green Version]
- Cecconi, A.; Vilchez-Tschischke, J.P.; Mateo, J.; Sanchez-Gonzalez, J.; Espana, S.; Fernandez-Jimenez, R.; Lopez-Melgar, B.; Fernandez Friera, L.; Lopez-Martin, G.J.; Fuster, V.; et al. Effects of Colchicine on Atherosclerotic Plaque Stabilization: A Multimodality Imaging Study in an Animal Model. J. Cardiovasc. Transl. Res. 2021, 14, 150–160. [Google Scholar] [CrossRef]
- Nidorf, M.; Thompson, P.L. Effect of colchicine (0.5 mg twice daily) on high-sensitivity C-reactive protein independent of aspirin and atorvastatin in patients with stable coronary artery disease. Am. J. Cardiol. 2007, 99, 805–807. [Google Scholar] [CrossRef]
- Martinez, G.J.; Robertson, S.; Barraclough, J.; Xia, Q.; Mallat, Z.; Bursill, C.; Celermajer, D.S.; Patel, S. Colchicine Acutely Suppresses Local Cardiac Production of Inflammatory Cytokines in Patients with an Acute Coronary Syndrome. J. Am. Heart Assoc. 2015, 4, e002128. [Google Scholar] [CrossRef] [Green Version]
- Tucker, B.; Kurup, R.; Barraclough, J.; Henriquez, R.; Cartland, S.; Arnott, C.; Misra, A.; Martinez, G.; Kavurma, M.; Patel, S. Colchicine as a Novel Therapy for Suppressing Chemokine Production in Patients with an Acute Coronary Syndrome: A Pilot Study. Clin. Ther. 2019, 41, 2172–2181. [Google Scholar] [CrossRef]
- Cartland, S.P.; Genner, S.W.; Martinez, G.J.; Robertson, S.; Kockx, M.; Lin, R.C.; O’Sullivan, J.F.; Koay, Y.C.; Manuneedhi Cholan, P.; Kebede, M.A.; et al. TRAIL-Expressing Monocyte/Macrophages Are Critical for Reducing Inflammation and Atherosclerosis. iScience 2019, 12, 41–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cartland, S.P.; Lin, R.C.Y.; Genner, S.; Patil, M.S.; Martinez, G.J.; Barraclough, J.Y.; Gloss, B.; Misra, A.; Patel, S.; Kavurma, M.M. Vascular transcriptome landscape of Trail(-/-) mice: Implications and therapeutic strategies for diabetic vascular disease. FASEB J. 2020, 34, 9547–9562. [Google Scholar] [CrossRef]
- Hays, A.G.; Schar, M.; Barditch-Crovo, P.; Bagchi, S.; Bonanno, G.; Meyer, J.; Afework, Y.; Streeb, V.; Stradley, S.; Kelly, S.; et al. A randomized, placebo-controlled, double-blinded clinical trial of colchicine to improve vascular health in people living with HIV. AIDS 2021, 35, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Opstal, T.S.J.; Hoogeveen, R.M.; Fiolet, A.T.L.; Silvis, M.J.M.; The, S.H.K.; Bax, W.A.; de Kleijn, D.P.V.; Mosterd, A.; Stroes, E.S.G.; Cornel, J.H. Colchicine Attenuates Inflammation Beyond the Inflammasome in Chronic Coronary Artery Disease: A LoDoCo2 Proteomic Substudy. Circulation 2020, 142, 1996–1998. [Google Scholar] [CrossRef] [PubMed]
- Silvis, M.J.M.; Fiolet, A.T.L.; Opstal, T.S.J.; Dekker, M.; Suquilanda, D.; Zivkovic, M.; Duyvendak, M.; The, S.H.K.; Timmers, L.; Bax, W.A.; et al. Colchicine reduces extracellular vesicle NLRP3 inflammasome protein levels in chronic coronary disease: A LoDoCo2 biomarker substudy. Atherosclerosis 2021, 334, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Barraclough, J.Y.; Joglekar, M.V.; Januszewski, A.S.; Martinez, G.; Celermajer, D.S.; Keech, A.C.; Hardikar, A.A.; Patel, S. A MicroRNA Signature in Acute Coronary Syndrome Patients and Modulation by Colchicine. J. Cardiovasc. Pharmacol. Ther. 2020, 25, 444–455. [Google Scholar] [CrossRef]
- Giannopoulos, G.; Angelidis, C.; Kouritas, V.K.; Dedeilias, P.; Filippatos, G.; Cleman, M.W.; Panagopoulou, V.; Siasos, G.; Tousoulis, D.; Lekakis, J.; et al. Usefulness of colchicine to reduce perioperative myocardial damage in patients who underwent on-pump coronary artery bypass grafting. Am. J. Cardiol. 2015, 115, 1376–1381. [Google Scholar] [CrossRef]
- Deftereos, S.; Giannopoulos, G.; Angelidis, C.; Alexopoulos, N.; Filippatos, G.; Papoutsidakis, N.; Sianos, G.; Goudevenos, J.; Alexopoulos, D.; Pyrgakis, V.; et al. Anti-Inflammatory Treatment with Colchicine in Acute Myocardial Infarction: A Pilot Study. Circulation 2015, 132, 1395–1403. [Google Scholar] [CrossRef] [Green Version]
- Akodad, M.; Lattuca, B.; Nagot, N.; Georgescu, V.; Buisson, M.; Cristol, J.P.; Leclercq, F.; Macia, J.C.; Gervasoni, R.; Cung, T.T.; et al. COLIN trial: Value of colchicine in the treatment of patients with acute myocardial infarction and inflammatory response. Arch. Cardiovasc. Dis. 2017, 110, 395–402. [Google Scholar] [CrossRef]
- Mewton, N.; Roubille, F.; Bresson, D.; Prieur, C.; Bouleti, C.; Bochaton, T.; Ivanes, F.; Dubreuil, O.; Biere, L.; Hayek, A.; et al. Effect of Colchicine on Myocardial Injury in Acute Myocardial Infarction. Circulation 2021, 144, 859–869. [Google Scholar] [CrossRef]
- Vaidya, K.; Arnott, C.; Martinez, G.J.; Ng, B.; McCormack, S.; Sullivan, D.R.; Celermajer, D.S.; Patel, S. Colchicine Therapy and Plaque Stabilization in Patients with Acute Coronary Syndrome: A CT Coronary Angiography Study. JACC Cardiovasc. Imaging 2018, 11, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Akrami, M.; Izadpanah, P.; Bazrafshan, M.; Hatamipour, U.; Nouraein, N.; Drissi, H.B.; Manafi, A. Effects of colchicine on major adverse cardiac events in next 6-month period after acute coronary syndrome occurrence; a randomized placebo-control trial. BMC Cardiovasc. Disord. 2021, 21, 583. [Google Scholar] [CrossRef]
- Fiolet, A.T.L.; Silvis, M.J.M.; Opstal, T.S.J.; Bax, W.A.; van der Horst, F.A.L.; Mosterd, A.; de Kleijn, D.; Cornel, J.H. Short-term effect of low-dose colchicine on inflammatory biomarkers, lipids, blood count and renal function in chronic coronary artery disease and elevated high-sensitivity C-reactive protein. PLoS ONE 2020, 15, e0237665. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Pillinger, M.; Zhong, H.; Cronstein, B.; Xia, Y.; Lorin, J.D.; Smilowitz, N.R.; Feit, F.; Ratnapala, N.; Keller, N.M.; et al. Effects of Acute Colchicine Administration Prior to Percutaneous Coronary Intervention: COLCHICINE-PCI Randomized Trial. Circ. Cardiovasc. Interv. 2020, 13, e008717. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.; Htun, N.; Lew, R.; Freilich, M.; Quinn, S.; Layland, J. Colchicine to Prevent Periprocedural Myocardial Injury in Percutaneous Coronary Intervention: The COPE-PCI Pilot Trial. Circ. Cardiovasc. Interv. 2021, 14, e009992. [Google Scholar] [CrossRef]
- Cole, J.; Htun, N.; Lew, R.; Freilich, M.; Quinn, S.; Layland, J. COlchicine to Prevent PeriprocEdural Myocardial Injury in Percutaneous Coronary Intervention (COPE-PCI): A Descriptive Cytokine Pilot Sub-Study. Cardiovasc. Revasc. Med. 2022, 39, 84–89. [Google Scholar] [CrossRef]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Bouabdallaoui, N.; Tardif, J.C.; Waters, D.D.; Pinto, F.J.; Maggioni, A.P.; Diaz, R.; Berry, C.; Koenig, W.; Lopez-Sendon, J.; Gamra, H.; et al. Time-to-treatment initiation of colchicine and cardiovascular outcomes after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). Eur. Heart J. 2020, 41, 4092–4099. [Google Scholar] [CrossRef]
- Tong, D.C.; Quinn, S.; Nasis, A.; Hiew, C.; Roberts-Thomson, P.; Adams, H.; Sriamareswaran, R.; Htun, N.M.; Wilson, W.; Stub, D.; et al. Colchicine in Patients with Acute Coronary Syndrome: The Australian COPS Randomized Clinical Trial. Circulation 2020, 142, 1890–1900. [Google Scholar] [CrossRef]
- Tong, D.C.; Bloom, J.E.; Quinn, S.; Nasis, A.; Hiew, C.; Roberts-Thomson, P.; Adams, H.; Sriamareswaran, R.; Htun, N.M.; Wilson, W.; et al. Colchicine in Patients with Acute Coronary Syndrome: Two-Year Follow-Up of the Australian COPS Randomized Clinical Trial. Circulation 2021, 144, 1584–1586. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Eikelboom, J.W.; Budgeon, C.A.; Thompson, P.L. Low-dose colchicine for secondary prevention of cardiovascular disease. J. Am. Coll. Cardiol. 2013, 61, 404–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Deftereos, S.; Giannopoulos, G.; Raisakis, K.; Kossyvakis, C.; Kaoukis, A.; Panagopoulou, V.; Driva, M.; Hahalis, G.; Pyrgakis, V.; Alexopoulos, D.; et al. Colchicine treatment for the prevention of bare-metal stent restenosis in diabetic patients. J. Am. Coll. Cardiol. 2013, 61, 1679–1685. [Google Scholar] [CrossRef] [Green Version]
- Kereiakes, D.J.; Smits, P.C.; Kedhi, E.; Parise, H.; Fahy, M.; Serruys, P.W.; Stone, G.W. Predictors of death or myocardial infarction, ischaemic-driven revascularisation, and major adverse cardiovascular events following everolimus-eluting or paclitaxel-eluting stent deployment: Pooled analysis from the SPIRIT II, III, IV and COMPARE trials. EuroIntervention 2011, 7, 74–83. [Google Scholar] [CrossRef]
- Fiolet, A.T.L.; Opstal, T.S.J.; Mosterd, A.; Eikelboom, J.W.; Jolly, S.S.; Keech, A.C.; Kelly, P.; Tong, D.C.; Layland, J.; Nidorf, S.M.; et al. Efficacy and safety of low-dose colchicine in patients with coronary disease: A systematic review and meta-analysis of randomized trials. Eur. Heart J. 2021, 42, 2765–2775. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Pascual Figal, D.A.; Bayes-Genis, A.; Emdin, M.; Georgiopoulos, G. Effect of low-dose colchicine in acute and chronic coronary syndromes: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2021, 51, e13464. [Google Scholar] [CrossRef] [PubMed]
- Kofler, T.; Kurmann, R.; Lehnick, D.; Cioffi, G.M.; Chandran, S.; Attinger-Toller, A.; Toggweiler, S.; Kobza, R.; Moccetti, F.; Cuculi, F.; et al. Colchicine in Patients With Coronary Artery Disease: A Systematic Review and Meta-Analysis of Randomized Trials. J. Am. Heart Assoc. 2021, 10, e021198. [Google Scholar] [CrossRef]
- Samuel, M.; Tardif, J.C.; Bouabdallaoui, N.; Khairy, P.; Dubé, M.P.; Blondeau, L.; Guertin, M.C. Colchicine for Secondary Prevention of Cardiovascular Disease: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Can. J. Cardiol. 2021, 37, 776–785. [Google Scholar] [CrossRef]
- Antithrombotic Trialists, C.; Baigent, C.; Blackwell, L.; Collins, R.; Emberson, J.; Godwin, J.; Peto, R.; Buring, J.; Hennekens, C.; Kearney, P.; et al. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009, 373, 1849–1860. [Google Scholar] [CrossRef] [Green Version]
- Cholesterol Treatment Trialists, C.; Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef] [Green Version]
- Kuzemczak, M.; Ibrahem, A.; Alkhalil, M. Colchicine in Patients with Coronary Artery Disease with or Without Diabetes Mellitus: A Meta-analysis of Randomized Clinical Trials. Clin. Drug Investig. 2021, 41, 667–674. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).