Error in Figure
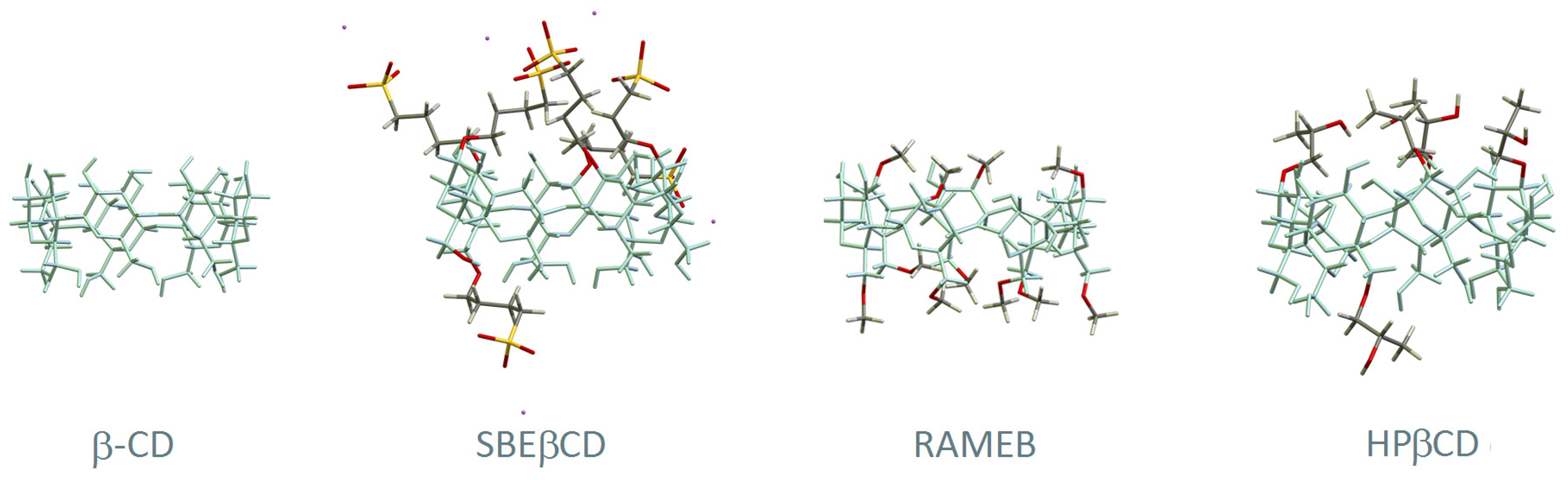
In the original publication [1], there was a mistake in Figure 2 as published. The structures of hydroxypropyl-β-cyclodextrin and sulphobutyl-β-cyclodextrin were not representing the most chemically abundant species that are formed during the reactions of chemical substitution of OH groups by 2-hydroxypropyl and sulfobutyl residues, respectively. The corrected Figure 2 appears below this text.

Figure 2.
Structural representation of β-CD and three of its derivatives: (2-hydroxy)propyl-beta-cyclodextrin (HPβCD), randomly methylated beta-cyclodextrin (RAMEB), and sulfobutyl ether β-CD (SBEβCD). The main skeleton of β-CD is represented in blue and the substituent groups are highlighted with different colours (carbon in grey, oxygen in red, hydrogen in white, sulphur in yellow and sodium in purple).
Figure 2.
Structural representation of β-CD and three of its derivatives: (2-hydroxy)propyl-beta-cyclodextrin (HPβCD), randomly methylated beta-cyclodextrin (RAMEB), and sulfobutyl ether β-CD (SBEβCD). The main skeleton of β-CD is represented in blue and the substituent groups are highlighted with different colours (carbon in grey, oxygen in red, hydrogen in white, sulphur in yellow and sodium in purple).
The authors apologise for any inconvenience caused and state that the scientific conclusions are unaffected. The original publication has also been updated.
Reference
- Braga, S.S.; Barbosa, J.S.; Santos, N.E.; Hyun, S.M.; El-Saleh, F.; Paz, F.A.A. Cyclodextrins in Antiviral Therapeutics and Vaccines. Pharmaceutics 2021, 13, 409. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
