Anticoronaviral Activity of the Natural Phloroglucinols, Dryocrassin ABBA and Filixic Acid ABA from the Rhizome of Dryopteris crassirhizoma by Targeting the Main Protease of SARS-CoV-2
Abstract
:1. Introduction
2. Materials and Methods
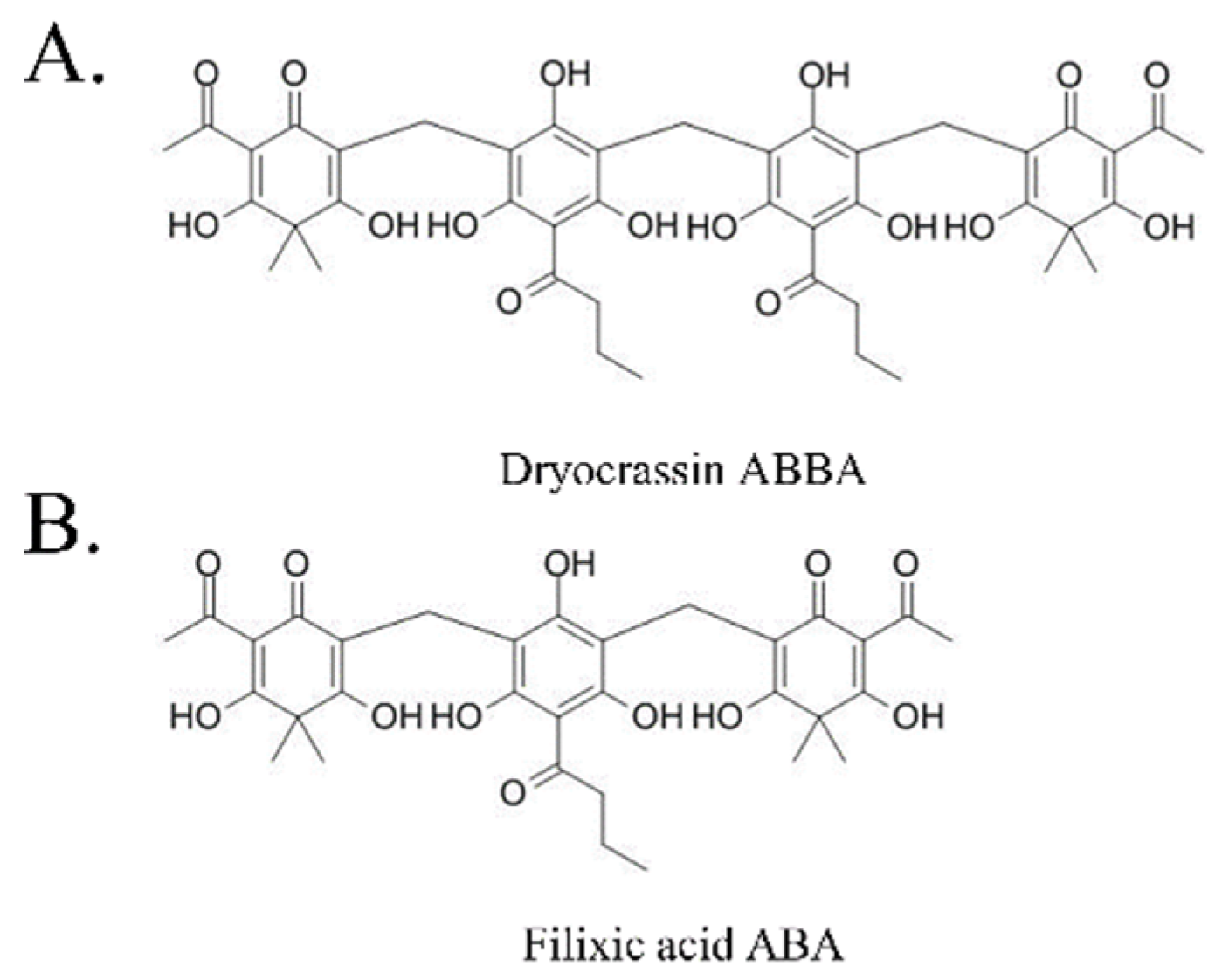
2.1. Test Compounds
2.2. SARS-CoV-2 Mpro Activity Assay
2.3. Cells and Viruses
2.4. Immunofluorescence-Based Antiviral Assays
2.5. Five-Day Repeated-Dose Toxicity Study
2.6. Liver Microsomal Metabolic Stability Assays
2.7. Human Ether-a-Go-Go-Related Gene (hERG) K+ Channel Activity Assays
2.8. Plasma Stability Assays
2.9. Cytochrome P-450 (CYP450) Enzyme Inhibition Assays
2.10. Pharmacokinetic Studies in Mice
2.11. Statistical Analysis
3. Results
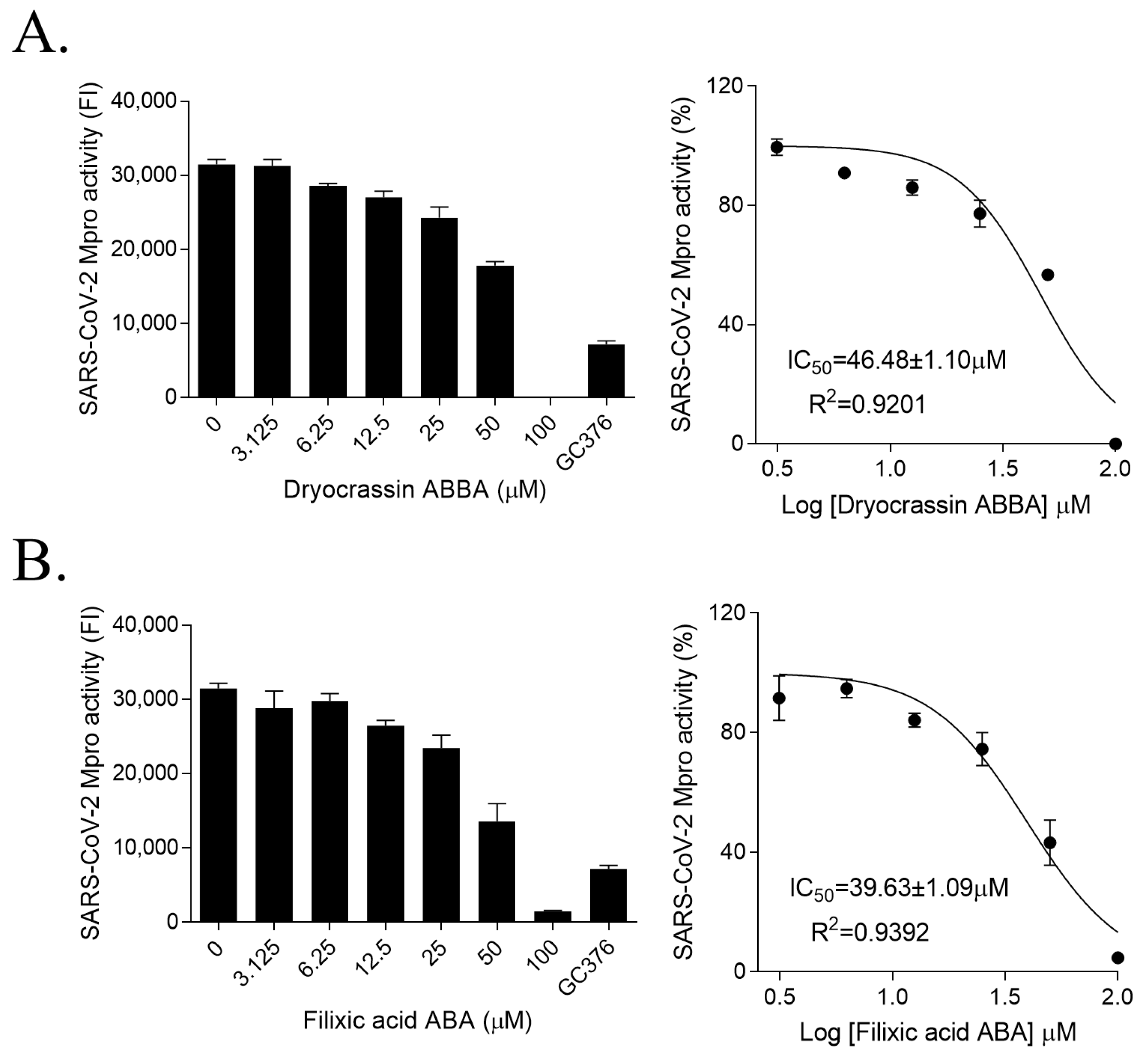
3.1. SARS-CoV-2 Mpro Inhibitory Activity of Dryocrassin ABBA and Filixic Acid ABA
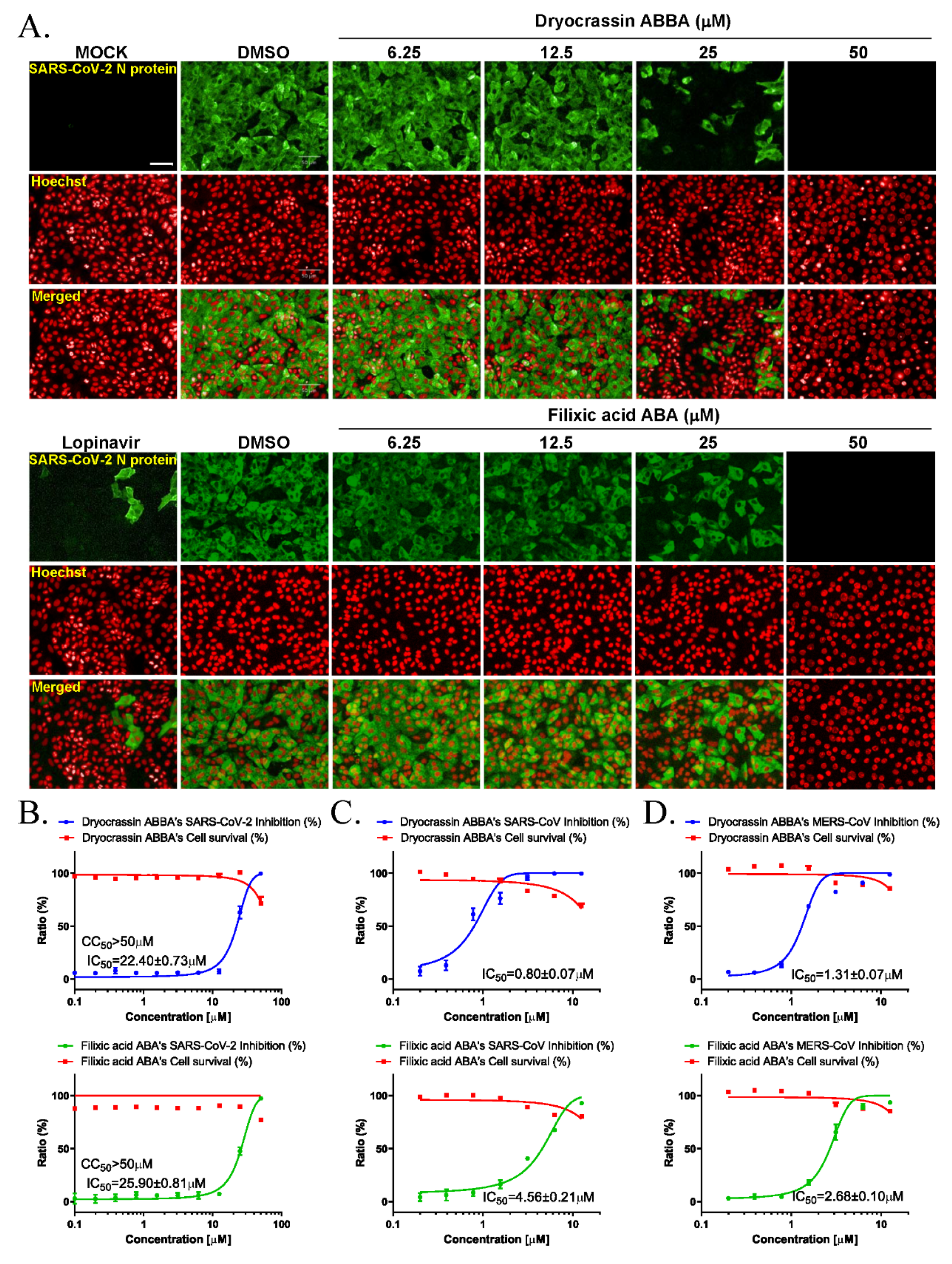
3.2. Anti-SARS-CoV-2 Activity of Dryocrassin ABBA and Filixic Acid ABA
3.3. Broad-Spectrum Anticoronaviral Activity of Dryocrassin ABBA and Filixic Acid ABA
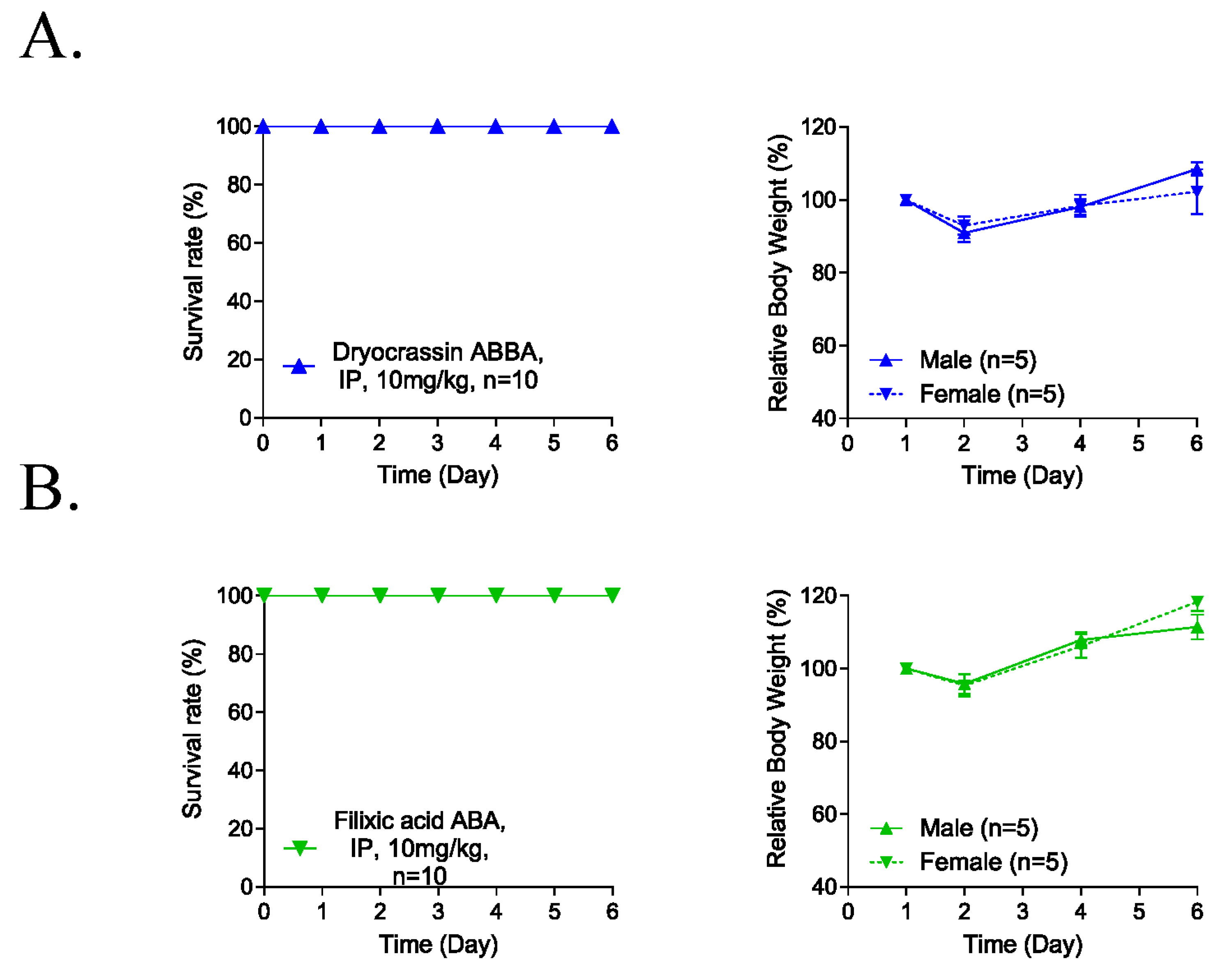
3.4. Five-Day Repeated-Dose Toxicity of Dryocrassin ABBA and Filixic Acid ABA
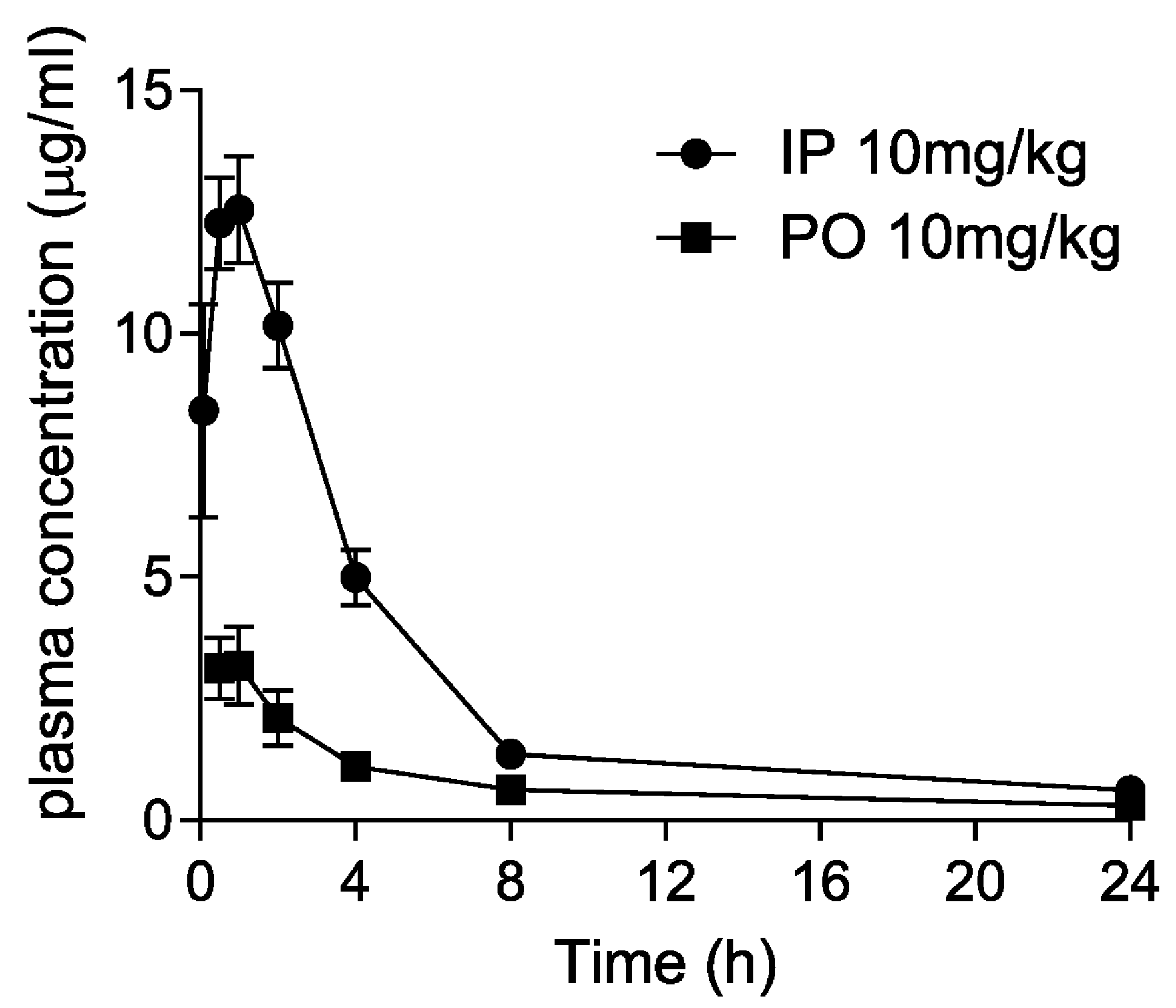
3.5. Pharmacokinetics of Dryocrassin ABBA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3CLpro | 3C-like protease |
| CC50 | Half-maximal cytotoxic concentration |
| CoV | Coronavirus |
| COVID-19 | Coronavirus disease 2019 |
| CYP450 | Cytochrome P-450 |
| hERG | Human ether-a-go-go-related gene |
| IC50 | Half-maximal inhibitory concentration |
| MERS-CoV | Middle East respiratory syndrome coronavirus |
| Mpro | Main protease |
| NA | Neuraminidase |
| ORF | Open reading frame |
| SARS-CoV | Severe acute respiratory syndrome coronavirus |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
References
- World Health Organization. Coronavirus Disease (COVID-19) Pandemic. Available online: https://covid19.who.int/ (accessed on 21 December 2021).
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, J.Y.; Yang, J.S.; Kim, J.W.; Kim, V.N.; Chang, H. The Architecture of SARS-CoV-2 Transcriptome. Cell 2020, 181, 914–921.e910. [Google Scholar] [CrossRef]
- Owen, D.R.; Allerton, C.M.; Anderson, A.S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; Boras, B.; Cardin, R.D.; Carlo, A.; Coffman, K.J. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 2021, 374, 1586–1593. [Google Scholar]
- Breidenbach, J.; Lemke, C.; Pillaiyar, T.; Schäkel, L.; Al Hamwi, G.; Diett, M.; Gedschold, R.; Geiger, N.; Lopez, V.; Mirza, S. Targeting the Main Protease of SARS-CoV-2: From the Establishment of High Throughput Screening to the Design of Tailored Inhibitors. Angew. Chem. Int. Ed. 2021, 60, 10423–10429. [Google Scholar] [CrossRef]
- Suda, G.; Sakamoto, N. Recent advances in the treatment of hepatitis C virus infection for special populations and remaining problems. J. Gastroenterol. Hepatol. 2021, 36, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Voshavar, C. Protease Inhibitors for the Treatment of HIV/AIDS: Recent Advances and Future Challenges. Curr. Top. Med. Chem. 2019, 19, 1571–1598. [Google Scholar] [CrossRef]
- Hattori, S.-i.; Higashi-Kuwata, N.; Hayashi, H.; Allu, S.R.; Raghavaiah, J.; Bulut, H.; Das, D.; Anson, B.J.; Lendy, E.K.; Takamatsu, Y. A small molecule compound with an indole moiety inhibits the main protease of SARS-CoV-2 and blocks virus replication. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Rut, W.; Groborz, K.; Zhang, L.; Sun, X.; Zmudzinski, M.; Pawlik, B.; Wang, X.; Jochmans, D.; Neyts, J.; Młynarski, W. SARS-CoV-2 M pro inhibitors and activity-based probes for patient-sample imaging. Nat. Chem. Biol. 2021, 17, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Nho, Y.H.; Yun, S.K.; Hwang, Y.S. Anti-invasive and Anti-tumor Effects of Dryopteris crassirhizoma Extract by Disturbing Actin Polymerization. Integr. Cancer 2019, 18, 1534735419851197. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lee, G.J.; Yoon, D.H.; Yu, T.; Oh, J.; Jeong, D.; Lee, J.; Kim, S.H.; Kim, T.W.; Cho, J.Y. ERK1- and TBK1-targeted anti-inflammatory activity of an ethanol extract of Dryopteris crassirhizoma. J. Ethnopharmacol 2013, 145, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Ban, S.H.; Kim, J.E.; Pandit, S.; Jeon, J.G. Influences of Dryopteris crassirhizoma extract on the viability, growth and virulence properties of Streptococcus mutans. Molecules 2012, 17, 9231–9244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, B.; Chi, C.; Fu, Y.W.; Zhang, Q.Z.; Wang, G.X. In vivo anthelmintic effect of flavonol rhamnosides from Dryopteris crassirhizoma against Dactylogyrus intermedius in goldfish (Carassius auratus). Parasitol. Res. 2013, 112, 4097–4104. [Google Scholar] [CrossRef]
- Maryam, M.; Te, K.K.; Wong, F.C.; Chai, T.T.; Gary, K.; Gan, S.C. Antiviral activity of traditional Chinese medicinal plants Dryopteris crassirhizoma and Morus alba against dengue virus. J. Integr. Agric. 2020, 19, 1085–1096. [Google Scholar] [CrossRef]
- Wang, J.; Yan, Y.T.; Fu, S.Z.; Peng, B.; Bao, L.L.; Zhang, Y.L.; Hu, J.H.; Zeng, Z.P.; Geng, D.H.; Gao, Z.P. Anti-Influenza Virus (H5N1) Activity Screening on the Phloroglucinols from Rhizomes of Dryopteris crassirhizoma. Molecules 2017, 22, 431. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Ye, F.; Feng, Y.; Yu, F.; Wang, Q.; Wu, Y.; Zhao, C.; Sun, H.; Huang, B.; Niu, P.; et al. Both Boceprevir and GC376 efficaciously inhibit SARS-CoV-2 by targeting its main protease. Nat. Commun. 2020, 11, 4417. [Google Scholar] [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Leist, S.R.; Schafer, A.; Won, J.; Brown, A.J.; Montgomery, S.A.; Hogg, A.; Babusis, D.; Clarke, M.O.; et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020, 11, 222. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.H.; Min, J.S.; Jeon, S.; Lee, J.; Kim, S.; Park, T.; Park, D.; Jang, M.S.; Park, C.M.; Song, J.H.; et al. Lycorine, a non-nucleoside RNA dependent RNA polymerase inhibitor, as potential treatment for emerging coronavirus infections. Phytomedicine 2021, 86, 153440. [Google Scholar] [CrossRef]
- Wu, L.; Chen, Y.; Ma, Y.; Yang, Z.; Yang, N.; Deng, W.; Chen, Y.; Sun, Y.; Li, Y.; Lin, L. Clinical practice guideline on treating influenza in adult patients with Chinese patent medicines. Pharmacol. Res. 2020, 160, 105101. [Google Scholar] [CrossRef]
- Gao, D.; Niu, M.; Wei, S.-z.; Zhang, C.-e.; Zhou, Y.-f.; Yang, Z.-w.; Li, L.; Wang, J.-b.; Zhang, H.-z.; Zhang, L. Identification of a pharmacological biomarker for the bioassay-based quality control of a thirteen-component TCM formula (Lianhua Qingwen) used in treating influenza A virus (H1N1) infection. Front. Pharmacol. 2020, 11, 746. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.Y.; Gu, S.; Alemi, S.F. Chinese herbal medicine for COVID-19: Current evidence with systematic review and meta-analysis. J. Integr. Med. 2020, 18, 385. [Google Scholar] [CrossRef] [PubMed]
- Runfeng, L.; Yunlong, H.; Jicheng, H.; Weiqi, P.; Qinhai, M.; Yongxia, S.; Chufang, L.; Jin, Z.; Zhenhua, J.; Haiming, J.; et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharm. Res. 2020, 156, 104761. [Google Scholar] [CrossRef] [PubMed]
- Min, B.S.; Tomiyama, M.; Ma, C.M.; Nakamura, N.; Hattori, M. Kaempferol acetylrhamnosides from the rhizome of Dryopteris crassirhizoma and their inhibitory effects on three different activities of human immunodeficiency virus-1 reverse transcriptase. Chem. Pharm. Bull. 2001, 49, 546–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Miyashiro, H.; Nakamura, N.; Hattori, M. Two new triterpenes from the Rhizome of Dryopteris crassirhizoma, and inhibitory activities of its constituents on human immunodeficiency virus-1 protease. Chem. Pharm. Bull. 2008, 56, 711–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Gao, L.; Si, J.; Sun, Y.; Liu, J.; Cao, L.; Feng, W.H. Inhibition of porcine reproductive and respiratory syndrome virus replication by flavaspidic acid AB. Antivir. Res. 2013, 97, 66–73. [Google Scholar] [CrossRef]
| MS (%) (Remaining after 30 min) | hERG Inhibition | Plasma Stability (% Remaining) | CYP450 Inhibition (IC50, μM) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mice | Rat | Hum | Hum | Rat | 1A2 | 2C9 | 2C19 | 2D6 | 3A4 | |
| 68.3 ± 2.1 | >99 | >99 | 7.27% (10 μM) IC50 > 50 μM | 29.67 ± 8.37 | 67.00 ± 7.29 | 14.7 | 0.4 | 16.1 | 13.5 | 8.33 |
| Parameters (n = 3) | IP (10 mg/kg) | PO (10 mg/kg) |
|---|---|---|
| Tmax (h) | 1.17 ± 0.76 | 0.67 ± 0.29 |
| Cmax (μg/mL) | 12.81 ± 1.79 | 3.64 ± 0.57 |
| T1/2 (h) | 5.5 ± 0.56 | 12.6 ± 3.76 |
| AUC (μg∙h/mL) | 65.96 ± 8.63 | 19.3 ± 1.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.-H.; Jeon, S.; Lee, J.; Kim, S.; Jang, M.S.; Park, C.M.; Song, J.H.; Kim, H.R.; Kwon, S. Anticoronaviral Activity of the Natural Phloroglucinols, Dryocrassin ABBA and Filixic Acid ABA from the Rhizome of Dryopteris crassirhizoma by Targeting the Main Protease of SARS-CoV-2. Pharmaceutics 2022, 14, 376. https://doi.org/10.3390/pharmaceutics14020376
Jin Y-H, Jeon S, Lee J, Kim S, Jang MS, Park CM, Song JH, Kim HR, Kwon S. Anticoronaviral Activity of the Natural Phloroglucinols, Dryocrassin ABBA and Filixic Acid ABA from the Rhizome of Dryopteris crassirhizoma by Targeting the Main Protease of SARS-CoV-2. Pharmaceutics. 2022; 14(2):376. https://doi.org/10.3390/pharmaceutics14020376
Chicago/Turabian StyleJin, Young-Hee, Sangeun Jeon, Jihye Lee, Seungtaek Kim, Min Seong Jang, Chul Min Park, Jong Hwan Song, Hyoung Rae Kim, and Sunoh Kwon. 2022. "Anticoronaviral Activity of the Natural Phloroglucinols, Dryocrassin ABBA and Filixic Acid ABA from the Rhizome of Dryopteris crassirhizoma by Targeting the Main Protease of SARS-CoV-2" Pharmaceutics 14, no. 2: 376. https://doi.org/10.3390/pharmaceutics14020376
APA StyleJin, Y.-H., Jeon, S., Lee, J., Kim, S., Jang, M. S., Park, C. M., Song, J. H., Kim, H. R., & Kwon, S. (2022). Anticoronaviral Activity of the Natural Phloroglucinols, Dryocrassin ABBA and Filixic Acid ABA from the Rhizome of Dryopteris crassirhizoma by Targeting the Main Protease of SARS-CoV-2. Pharmaceutics, 14(2), 376. https://doi.org/10.3390/pharmaceutics14020376






