Abstract
This review describes the recently FDA-approved drugs (in the year 2022). Many of these products contain active moieties that FDA had not previously approved, either as a single ingredient or as part of a combination. These products frequently provide important new therapies for patients with multiple unmet diseases. The diverse small molecules are described according to the date of approval and their syntheses is discussed. This review comprises classical chemical scaffolds together with innovative drugs such as a deuterium-containing drug.
1. Introduction
The constant research for innovative therapies leads every year to molecules that are approved by the United States Food and Drug Administration (FDA), a federal agency of the U.S. Department of Health and Human Services.
Novel building blocks and their connections have been explored much in recent years allowing drug hunters to explore a much bigger chemical space [1].
Small molecule drugs are organic compounds with low molecular weight. For a long time, they have been the backbone of the pharmaceutical industry. There are several benefits that make small molecules important drugs in therapy, such as the possibility of oral administration and cell membrane permeability which allow them to reach specific tissue and targets. For this purpose, small molecule drugs can be designed in order to acquire, for example, specific affinity and selectivity (target, tissue penetration and distribution) [2].
The goal of this review is to highlight the chemical entities approved in the year 2022 for clinical use. The approved drugs (and their synthesis) are listed according to their chronological approval date [3]. This paper focuses specifically on small molecules, as high-molecular weight peptides, vaccines and other biotechnological drugs are beyond the scope of this work. Interestingly, from the perspective of the functional group characterizing the molecules herein discussed, four contain a sulfonamide functionality, one is a macrocycle and two are steroidal structures. Two radioisotope/contrast agents and one deuterium-containing drug were approved. These latter surely represent a very innovative starting point for future drug discovery efforts. Halogenated (mainly chlorinated and fluorinated) drugs still characterize a consistent percentage of the approved drugs, underlining the importance of halogens in drug discovery [4]. All the structures contain at least one aromatic ring except for two drugs which possess steroid-like structure.
2. FDA-Approved Drugs in 2022
2.1. Daridorexant
Approved at the beginning of 2022 (7 January 2022) and launched by Idorsia U.S. [5], daridorexant (11) (brand name Quviviq®) is a 1,2,3-triazole derivative with an expected global sales to 2026 of $1052 million [6]. The mechanism of action of daridorexant in the treatment of insomnia is presumed to be through antagonism of orexin receptors. The orexin neuropeptide signaling system plays a role in wakefulness [7]. The synthesis [8] is reported in Scheme 1, and when performed at the kilogram-scale it has moderate yield: 50% [8].
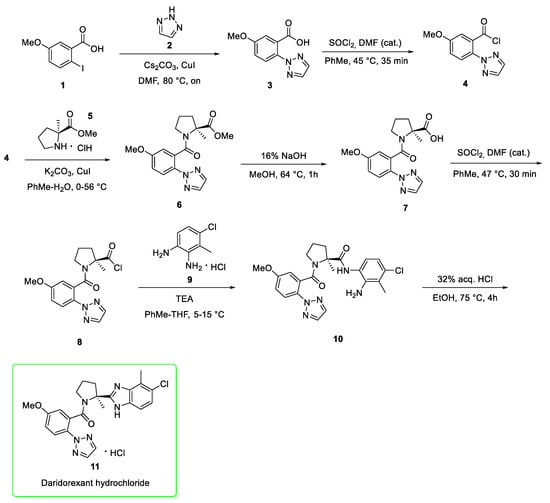
Scheme 1.
Synthesis of daridorexant hydrochloride (11) [8].
The first step involves the reaction between 2-iodo-5-methoxybenzoic acid (1) and 1,2,3-triazole (2) in a copper-mediated Ullmann-like coupling reaction that affords the triazole derivative 3. After the activation of carboxylic acid by thionyl chloride to generate the acyl chloride 4, the amidation with the (S)-proline derivative 5 afforded the amide 6. The alkaline hydrolysis of 6 provided the carboxylic acid 7, in turn converted into the corresponding acyl chloride 8 which upon treatment of the 4-chloro-3-methylbenzene-1,2-diamine (9) in presence of triethylamine gave the bis-amide 10. The treatment of 10 with hydrochloric acid afforded Daridorexant (11) in the form of hydrochloric salt.
2.2. Abrocitinib
Approved in January 2022 (14 January 2022), abrocitinib (brand name Cibinqo®) is a selective Janus kinase 1 (JAK1) inhibitor effective and safe for the treatment of atopic dermatitis (AD), with good oral bioavailability as well as lack of immunogenicity, addressing some of the limitations of biologic drugs currently available. It is expected that the global sales forecast in 2026 will be $760 million [6]. Abrocitinib was developed by Pfizer to treat moderate to severe AD patients [9]. Its synthesis (Scheme 2) [10] begins with the reductive amination between benzyl (3-oxocyclobutyl)carbamate and methylamine to afford diasteroselectively the desired cis cyclobuthyl amine derivative 13. A high selectivity and purity biocatalityc method to obtain amine 13 was later reported in a green-fashion [11]. Derivative 13 was nucleophilically added to the tosyl-protected pyrrolo [2,3-d]pyrimidine 14 (the electron-withdrawing effect of the tosyl group allowed the nucleophilic aromatic substitution reaction to occur readily in high yield) in the presence of diisopropylethylamine (DIPEA) to give the intermediate 15 which underwent the deprotection of carboxylbenzyl protecting group under acidic conditions (HBr) to afford 16. The free primary amino group of 16 reacted with the propane-1-sulfonyl chloride 17 to provide the sulfonamide 18. The last stage (yield: 74%) involved the removal of tosyl group by using lithium hydroxide or sodium hydroxide in ethanol to generate Abrocitinib (19).
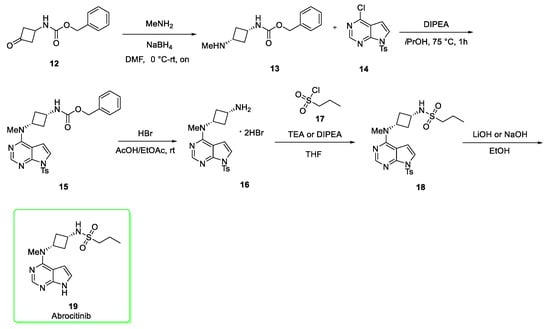
Scheme 2.
Synthesis of abrocitinib (19) [10].
2.3. Mitapivat
Approved in February 2022 (17 February 2022) and developed by Agios Pharmaceuticals, mitapivat (25, brand name Pyrukynd®) was approved for the treatment of hereditary hemolytic anemias [12]. It is expected that the global sales forecast in 2026 will be $511 million [6]. This molecule is an allosteric activator of the pyruvate kinase enzyme and it is chemically a sulfonamide. Its synthesis [13], reported in Scheme 3, begins with ethyl-4-aminobenzoate (20) that nucleophilically attacks, under alkaline conditions in pyridine, the quinoline-based sulfonyl chloride (21) to afford the sulfonamide intermediate 22. After the alkaline hydrolysis the carboxylic acid 23 was obtained and then amidated using 1,1′-carbonyldiimidazole (CDI) and (cyclopropylmethyl)piperazine dihydrochloride (24) in dimethylacetamide (DMA) to give mitapivat (25); in this last step the yield reported was 90% [13].
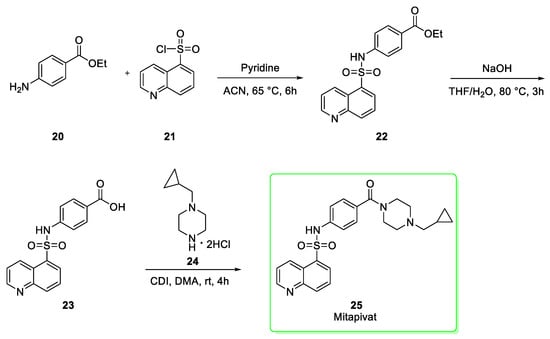
Scheme 3.
Synthesis of mitapivat (25) [13].
2.4. Pacritinib
Pacritinib (36, brand name Vonjo®) was authorized (28 February 2022) for the treatment of high-risk primary or secondary myelofibrosis in adults with low platelets [14].
Discovered and synthesized by S*BIO Pte Ltd. (Singapore), it is a Janus kinase 2 (JAK2) inhibitor [15] with an expected sales forecast in 2026 of $496 million [6].
The synthesis of pacritinib (Scheme 4) [16] starts with the nucleophilic attack of 2-hydroxy-5-nitrobenzaldehyde (26) to 1-bromo-2-chloroethane (27) in the presence of potassium carbonate to afford the aromatic ether 28. The reduction of the aldehydic group of 28 afforded the primary alcohol 29 which was then subjected to the reaction with allyl bromide 30 in the presence of tetrabutylammonium hydrogensulfate (TBAHSO4) to give the diether 31. The nitro group of 31 was reduced to amine under iron/ammonium chloride conditions to afford the amine 32. After the nucleophilic aromatic substitution, the ring-closing metathesis (RCM) between 32 and the chloropyrimidine 33 afforded the macrocycle 34 via ruthenium-based approach using the Zhan catalyst 1B. The macrocycle was obtained as inseparable mixtures of approximately 85:15 trans:cis geometry [16]. Pacritinib (36) was eventually converted via nucleophilically addition of pyrrolidine 35 to the chloride 34; in this step a 83% yield was reached [16].
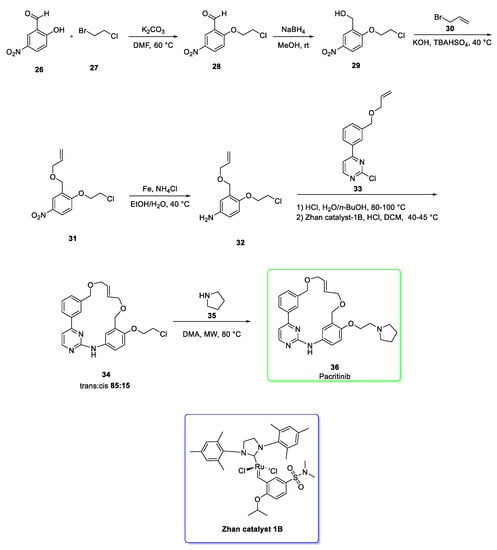
Scheme 4.
Synthesis of pacritinib (36) [16] and the structure of Zhan catalyst (blue box).
2.5. Ganaxolone
Approved in March 2022 (18 March 2022), ganaxolone (41) was authorized for the treatment of seizures in cyclin-dependent kinase-like 5 deficiency disorders [3]. The mechanism of action of Ganaxolone is yet unknown. Most probably it modulates (positive allosteric modulator) both synaptic and extrasynaptic GABAA receptors to normalize over-excited neurons [17,18].
It is expected that the global sales forecast in 2026 will be $434 million [6].
Developed by Marinus Pharmaceuticals, ganaxolone (brand name Ztalmy®) possesses a steroidal structure and its synthesis [19] (Scheme 5) starts from the precursor pregnenolone 37. The initial chemoselective hydrogenation catalyzed by palladium on carbon afforded 38 which is oxidized under hypochlorite conditions to afford the corresponding ketone 39.
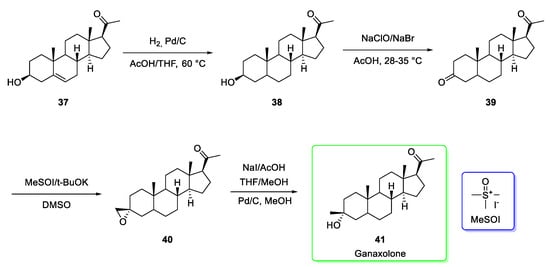
Scheme 5.
Synthesis of ganaxolone (41) [19] and the structure of trimethylsulfoxonium iodide (MeSOI, blue box), the agent used to introduce epoxide ring.
In the following steps, the epoxide formation mediated by trimethylsulfoxonium iodide (MeSOI) led to 40, then treated with sodium iodide (NaI) followed by treatment with methanol under palladium-catalysis to give ganaxolone (41). The reported yield of the last stage was 90% [19].
2.6. Lutetium (177Lu) Vipivotide Tetraxetan
Lutetium (177Lu) vipivotide tetraxetan (53) (brand name Pluvicto®) is a urea-based inhibitor of the prostate-specific membrane antigen (PSMA). It is expected that the global sales forecast in 2026 will be $851 million [6].
Mechanism of action arises from lutetium-177 that delivers its beta-minus emission to PSMA-expressing cells [20].
Developed by Advanced Accelerator Applications, it has been authorized in March 2022 (23 March 2022) for the treatment of prostate-specific membrane antigen-positive metastatic castration-resistant prostate cancer where other therapies failed [3].
Its structure is composed of PSMA-617, a human prostate-specific membrane antigen (PSMA)-targeting ligand, conjugated to the beta-emitting radioisotope lutetium, Lu 177 (177Lu) [21]. The synthetic pathway for PSMA-617 (Scheme 6) proceeds via solid-phase peptide chemistry [22,23]. The first step involves the conversion of the amino group of the bis-tert-butyl protected L-glutamate 42 to isocyanate 43 by means of triphosgene. Subsequently, a resin-immobilized (2-chloro-tritylresin) ε-allyloxycarbonyl protected lysine 44 was added to afford the urea 45. The allyloxy protecting group was cleaved by using tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4) and morpholine to give compound 46 which was condensed with Fmoc-3-(2-naphthyl)-L-alanine (Fmoc-2-Nal-OH, 47) via HBTU activation and later treated with a solution of piperidine to afford the naphthyl-based pseudopeptide 48. The free amino group of 48 was condensed with trans-4-(Fmoc-aminomethyl)cyclohexanecarboxylic acid 49 followed by deprotection of Fmoc to give 50. The free aminomethyl group of 50 was eventually coupled with tri-tert-butyl 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate [tris-(tert-but)DOTA, 51] to provide PSMA-617 (52).
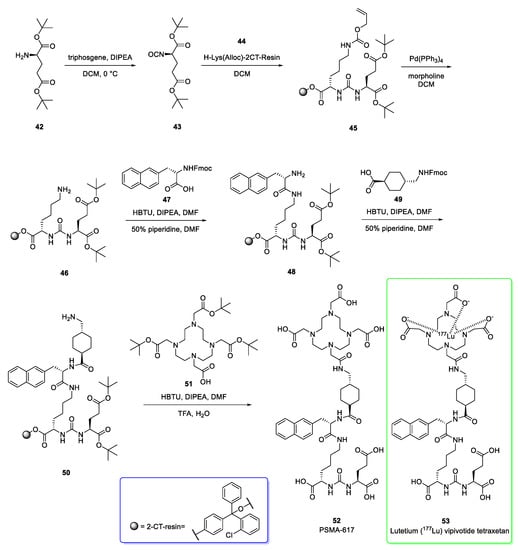
Scheme 6.
Synthesis of PSMA-617 (52) [22,23]. The structure of the 2-chloro-tritylresin (2-CT- resin) is reported as well (blue box). Lutetium (177Lu) vipivotide tetraxetan is shown.
2.7. Oteseconazole
The antifungal oteseconazole (67, brand name Vivjoa®) was approved in April 2022 (26 April 2022) with the indication of reducing the incidence of recurrent vulvovaginal candidiasis (RVVC) in females with a history of RVVC who are not of reproductive potential [3].
Launched by Mycovia Pharmaceuticals [24], it is a tetrazole-based compound inhibiting the enzyme CYP51, known as 14α demethylase, leading to fungal destruction.
Its synthesis (Scheme 7) [25,26] starts from the reaction between 2,5-dibromopyridine 54 and ethyl bromodifluoroacetate via a copper catalyzed nucleophilic addition to afford the ester 55. This was subjected to nucleophilic attack of the lithiated l-bromo-2,4-difluorobenzene (56) at −65 °C to afford derivative 57. The following Henry reaction provided the mixture of nitro alcohols 59 (with desired R chirality) and 60 (S enantiomer of 59) in the presence of 5% mol of organocatalyst 58. The enantiomeric ratio of the couple 59:60 was 90:10. The organocatalyst 58 proved to be the most effective in stereoselectivity among the many others tested. The subsequent catalytic hydrogenation (using the platinum catalyst Noblyst® P8071, 61) of the mixture of nitro alcohols led to the corresponding amines 62 and 63. The conversion of 62 and 63 to the corresponding tetrazoles 64 and 65 was mediated by trimethylsilyl azide and trimethyl orthoformate in the presence of acetic acid. The last step involves a Suzuki coupling between the aryl bromide of 64 and 65 and the boronic ester 66 catalyzed by (diphenylphosphino) ferrocene] dichloropalladium (II) to generate the desired oteseconazole 67. The authors claim that an enantioenriched (95.9%) form of 67 was obtained via diastereomeric recrystallisation with (S)-Camphor sulfonic acid [25].
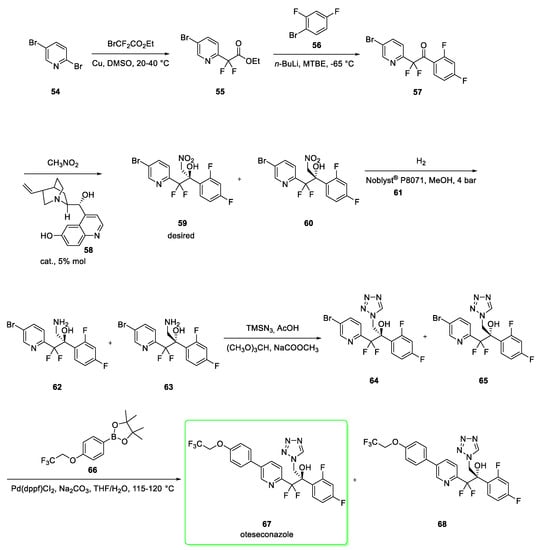
Scheme 7.
Synthesis of oteseconazole (67) [25,26].
2.8. Mavacamten
Mavacamten (75, brand name Camzyos®) was approved in April 2022 (28 April 2022) for the treatment of certain classes of obstructive hypertrophic cardiomyopathy [3]. It is an orally active cardiac myosin inhibitor developed by MyoKardia [27]. It is expected that the global sales forecast in 2028 will be $1.658 billion [28].
Its synthesis (Scheme 8) [29] starts from isopropylamine (69) which upon reaction with trimethylsilyl isocyanate 70 is converted to isopropylurea 71. The reaction between 71 and dimethyl malonate in the presence of sodium methoxide as base gave 1-isopropyl barbituric acid 72. The subsequent conversion to chloride derivative 73 is mediated by POCl3 using triethylbenzylammonium chloride (TEBAC) as phase transfer catalyst. The last stage is the nucleophilic addition of (S)-methylbenzylamine (74) to 73 in order to afford mavacamten 75. Last step yield was 69% [29].
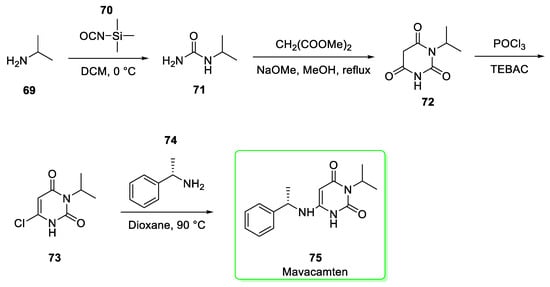
Scheme 8.
Synthesis of mavacamten (75) [29].
2.9. Vonoprazan (in Combination with Amoxicillin, and Clarithromycin)
Vonoprazam (in combination with amoxicillin and clarithromycin, brand name Voquezna®) was approved in May 2022 (3 May 2022) for the treatment of Helicobacter pylori infection [1]. It is expected that the global sales forecast in 2028 will be $869 million [28]. Launched by Takeda, it is a an orally bioavailable potassium-competitive acid blocker (P-CAB) [30]. P-CABs reversibly inhibit gastric acid secretion by competing with the K+ on the luminal surface, preventing the acid secretion [31]. The inhibition of gastric secretion combined to the antibacterial activities of amoxicillin and clarithromycin makes Voquezna® a synergistically active “combo”.
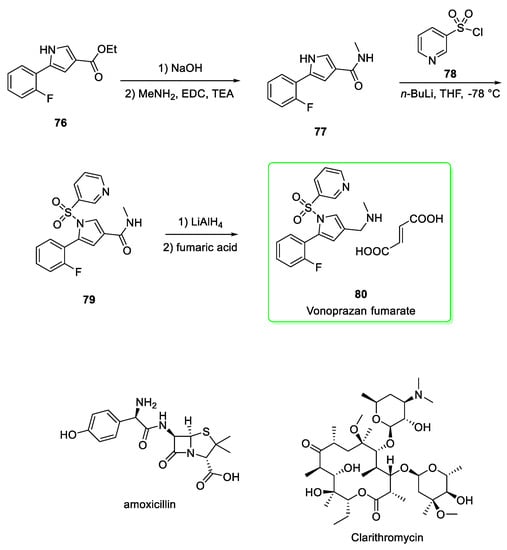
A very practical synthetic pathway (Scheme 9) for the preparation of vonoprazan fumarate [32] starts from the alkaline hydrolysis of the fluoroaryl-pyrrole 76 followed by the activation of carboxylic acid by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and the coupling with methylamine to afford amide 77. The deprotonation of pyrrole-NH by n-butyllithium and the attack to pyridine-3-sulfonyl chloride 78 provided the sulfonamide 79. The subsequent reduction of amide to amine, addition of fumaric acid and the recrystallization from methanol yielded vonoprazan fumarate 80.
Scheme 9.
Synthesis of vonoprazan fumarate (80) [32]. The structure of amoxicillin and clarithromycin are reported for completeness in the lower part of the Scheme.
Interestingly, no product was observed if other bases such as NaH, t-BuOK, or TEA were used instead of n-butyllithium in the sulfonamidation step. The authors highlight that other reducing agents such as BH3, Red-Al and NaBH4-BF3 were ineffective in the reduction stage from amide to amine. Overall yield was 41.3% [32].
2.10. Tapinarof
Tapinarof (brand name Vtama®) was approved in May 2022 (23 May 2022) for the treatment of plaque psoriasis [1]. It is an aryl hydrocarbon receptor (AhR) agonist that is being developed by Dermavant Sciences Inc. (a subsidiary of Roivant Sciences Inc., Basel, Switzerland) [33]. It is a naturally derived small molecule produced by bacterial symbionts of entomopathogenic nematodes [34].
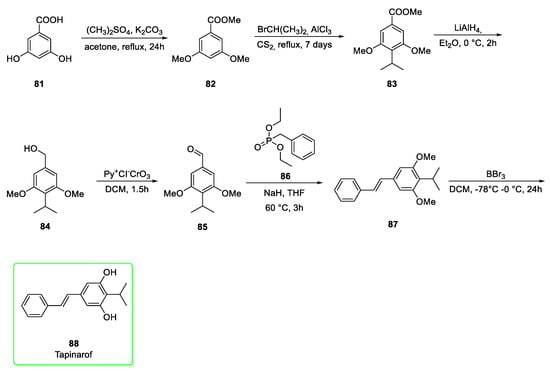
Tapinarof is synthesized (Scheme 10) [35] starting from the commercially available 3,4-dihydroxybenzoic acid 81. The methylation step by using dimethyl sulfate afforded the trimethylated derivative 82. The subsequent Friedel–Crafts alkylation of 82 with isopropyl bromide in carbon disulfide afforded the isopropyl intermediate 83. Ester of 83 was reduced to alcohol 84 by LiAlH4 and later oxidized to aldehyde (85) by using pyridinium chlorochromate. The Horner–Wadsworth–Emmons (HWE) reaction between 85 and diethyl benzylphosphonate ester (86) yielded the E-alkene 87. The demethylation step mediated by BBr3 gave tapinarof (88). Last step yield was 95%.
Scheme 10.
Synthesis of tapiranof (88) [35].
2.11. Deucravacitinib
Deucravacitinib (brand name Sotyktu®) was approved in September 2022 (9 September 2022) and for the treatment, as in the case of tapinarof, of plaque psoriasis [1]. It is expected that the global sales forecast in 2026 will be $1.12 billion [36].
It is a deuterated drug launched by Bristol Meyers Squibb [35] selectively targeting tyrosine kinase 2 (TYK2), a member of the Janus family of kinases (JAK). The incorporation of deuterium, the authors say, is important, to “slow down the production of an otherwise promiscuous metabolite”. Metabolic stability is an important parameter to take into account and novel deuterium-based drugs can appear in the next years. Moreover, C–D bonds are shorter and at times more resistant than C–H bonds [37,38].
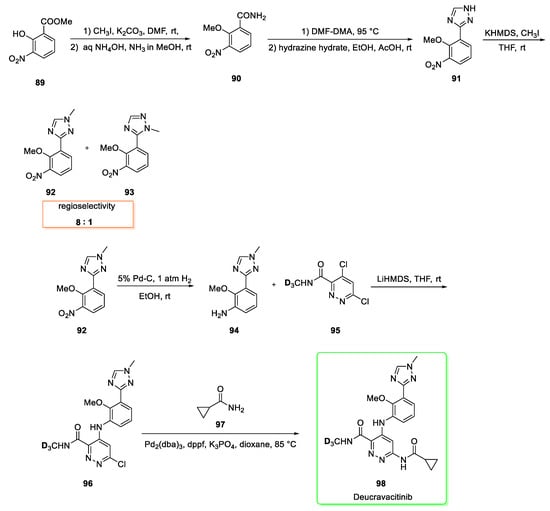
Its synthesis (Scheme 11) [38] starts from methyl-2-hydroxy-3- nitrobenzoate 89 which is firstly methylated with methyl iodide and later converted into amide by ammonolysis to afford the intermediate 90. The treatment of 90 with DMF-DMA followed by condensation with hydrazine hydrate yielded the triazole 91 which was then methylated in presence of methyl iodide and potassium hexamethylsilazide to generate compound 92 in a good regioselectivity (8:1) over compound 93.
Scheme 11.
Synthesis of deucravacitinib (98) [38].
Pure compound 92 was subjected to catalytic hydrogenation (Pd-C, H2) to give the substituted aniline 94 which was then mixed with the pyridazine 95 [39] in the presence of lithium hexamethyldisilazide as a base to afford the diaryl aniline 96. The last stage involves a palladium-catalyzed Buchwald−Hartwig reaction between 96 and cyclopropyl amide 97 to form Deucravacitinib (98). The reaction was optimized and it was found that a combination of 1,1′-bis(dicyclohexylphosphino)ferrocene (dppf), tris(dibenzylideneacetone)dipalladium(0) [Pd2(dba)3] and aqueous potassium triphosphate resulted in a better yield and milder conditions if compared to the triad XantPhos/Pd2(dba)3/Cs2CO3. Last step yield was 76%.
2.12. Gadopiclenol
Approved in September 2022 (21 September 2022), gadopiclenol is used to detect and visualize lesions, together with Magnetic Resonance Imaging (MRI), with abnormal vascularity in the central nervous system and the body [1].
Gadopiclenol (brand name Elucirem® [1]) is a macrocyclic gadolinium-based contrast agent (GBCA) having high relaxivity properties, which was designed to increase lesion detection and characterization by magnetic resonance imaging [40]. It was launched by Bracco [41].
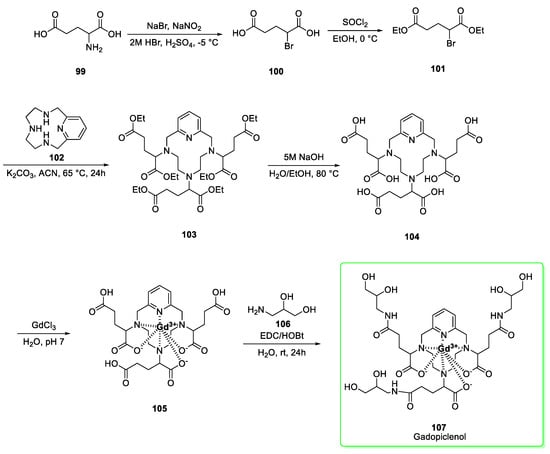
Its synthetic preparation (Scheme 12) [42] originates with racemic glutamic acid. The amino group is diazotated and attacked by bromide to afford derivative 100. The conversion of 100 to the diethyl-ester 101 was obtained via using thionyl chloride in ethanol. The subsequent nucleophilic substitution between 101 and 3,6,9-triaza-1(2,6)-pyridinacyclodecaphane (102) yielded the intermediate 103 which was subjected to alkaline hydrolysis to afford the exa-acid 104. The following complexation with gadolinium by adding gadolinium (III) chloride afforded the intermediate 105 which was converted to the isomeric gadopiclenol (107) by condensation (EDC/HOBt conditions) of the free carboxylic acid groups with isoserinol (106). Last step yield was 78%.
Scheme 12.
Synthesis of gadopiclenol isomeric mixture (107) [42].
2.13. Oomidenepag Isopropyl
Oomidenepag isopropyl (brand name Omlonti® [1]) was approved in September 2022 (22 September 2022) with the indication of reducing elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension [1]. It is expected that the global sales forecast in 2026 will be $121 million [36].
It is a selective prostaglandin E2 receptor agonist with a non-prostaglandin structure that is being developed by Ube Industries and Santen Pharmaceutical in Japan, Singapore and the USA [43]. Oomidenepag isopropyl is a prodrug being converted in vivo into its active form, oomidenepag [44].
Its synthesis (Scheme 13) [45,46,47] originates from the preparation of the pyrazole synthon 110. This was prepared by nucleophilic addition of substituted benzylamine 108 to pyridine-3-sulfonyl chloride (109) in the presence of triethylamine. In parallel, the initial alkylation of BOC-protected aniline 111 with tert-butyl 2-bromoacetate (112) was carried out in presence of sodium hydride. The resulting 113 which was reduced (calcium hydride and NaBH4 conditions) at the ester functionality to afford alcohol 114. The subsequent Mitsunobu reaction between 110 and 114 mediated by tributylphosphine and tetramethylazodicarboxamide (TMAD) afforded the substituted sulfonamide 115. The removal of BOC-protecting group of 115 afforded 116 with the simultaneous hydrolysis at the tert-butyl ester moiety. The final stage is given by the esterification with isopropanol to afford oomidenepag isopropyl (117).
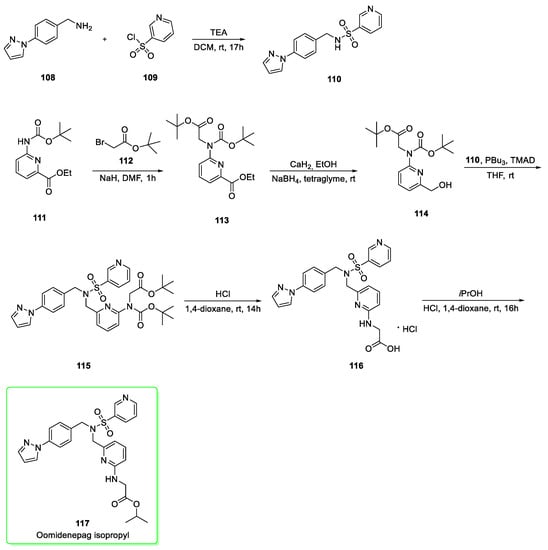
Scheme 13.
Synthesis of oomidenepag isopropyl (117) [45,46,47].
2.14. Taurursodiol (in Combination with Sodium Phenylbutyrate)
Approved (brand name Relyvrio® [1]) on 29 September 2022 for amyotrophic lateral sclerosis (ALS) in combination with sodium phenylbutyrate, taurursodiol is a tauro-bile acid conjugate. It is expected that the global sales forecast in 2026 will be $1.095 billion [36]. Combination product Relyvrio® is postulated to have a synergistic effect that can reduce neuronal death by simultaneous inhibition of endoplasmic reticulum and mitochondrial stress [48].
Taurursodiol can be obtained from ursodeoxycholic acid (118). This latter is naturally present in bears’ livers and related carnivores [49]. Otherwise, semisynthetic ursodeoxycholic acid is obtained in 30% of yield [50]. A convenient synthesis of taurursodiol (Scheme 14) proceeds via microwave [51]. The condensation between the carboxylic acid of ursodeoxycholic acid (118) and taurine (119) by adding N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ), triethylamine (TEA) or N-Methylmorpholine (NMM) in ethanol under microwave afford taurursodiol (120). Last stage yield was 67%.
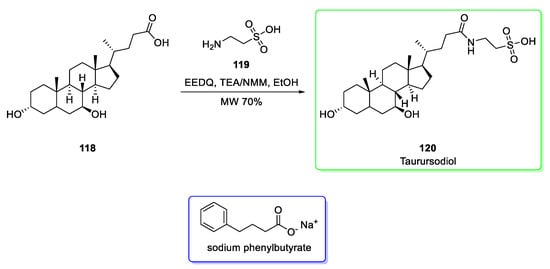
Scheme 14.
Synthesis of taurursodiol (120) obtained via MW from ursodeoxycholic acid [51]. Structure of sodium phenylbutyrate is reported (blue box).
2.15. Futibatinib
Approved on 30 September 2022 for the treatment of intrahepatic cholangiocarcinoma harboring fibroblast growth factor receptor 2 (FGFR2) gene fusions or other rearrangements [1], futibatinib (brand name Lytgobi® [1]) is a highly selective irreversible fibroblast growth factor receptor (FGFR1–4) inhibitor [52]. Constitutive FGFR signaling can support the proliferation and survival of malignant cells.
Its structure contains a pyrazolo-pyrimidine scaffold and the synthesis (Scheme 15) [53,54] starts from 3-amino-1H-pyrazole-4-carbonitrile (121) which is transformed into pyrazolo-pyrimidine 123 by addition of formamide (122). The following conversion to iodo-derivative 124 was mediated by N-iodosuccinimide (NIS) in DMF. Sonogashira coupling [triethylamine, cuprous iodide and diphenylphosphino)ferrocene]dichloropalladium (II)] between halide 124 and alkyne 125 provided the derivative 126. The subsequent nucleophilic attack (resulting in inversion of configuration) of 126 to mesyl-protected alcohol 127 afforded the pyrrolidine derivative 128 which was subjected to BOC-removal by addition of HCl to give 129. The final step involves another nucleophilic attack (acylation) of 129 to acryloyl chloride (130) followed by neutralization with sodium bicarbonate to yield futibatinib (131).
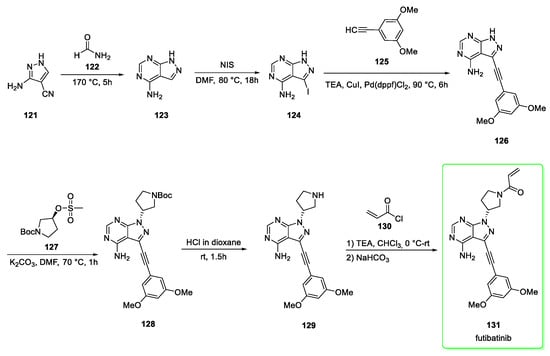
Scheme 15.
Synthesis of futibatinib (131) [53,54].
3. Sulfonamide: An Historical Re-Occurring Moiety in the FDA-Approved Drug List
Sulfonamide-containing drugs (four entities) are arising among the approved molecules in 2022. Sulfonamides were among the first examples of carboxylic acid isosteres to show utility in drug design [55].
In the previous year (2021), only odevixibat (brand name Bylvay®, Figure 1), a drug containing cyclic sulfonamide, was approved for the treatment of pruritus [56], while in 2020 no sulfonamide-containing drugs were approved by FDA [57].
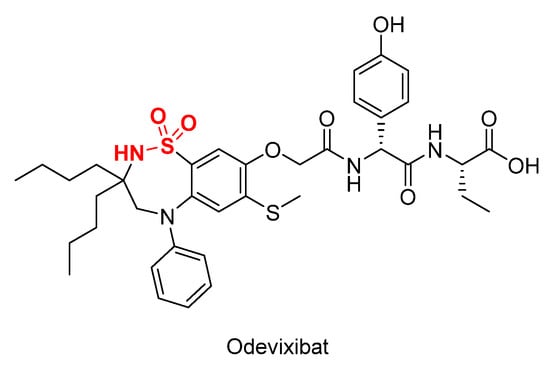
Figure 1.
Chemical structure of odevixibat. The sulfonamide moiety is shown in red.
The approved sulfonamides in 2022 are a Janus kinase inhibitor (abrocitinib, 19), a pyruvate kinase enzyme activator (mitapivat, 25), a potassium-competitive acid blocker (P-CAB) represented by vonoprazan (vonoprazan fumarate, 80) and a selective prostaglandin E2 receptor agonist (oomidenepag isopropyl, 117). None of these molecules (Figure 2) are cyclic sulfonamides (also known as sultams) differing from odevixibat approved in 2021. Sultams are often incorporated into the target molecules as a stable lactam equivalent [58].
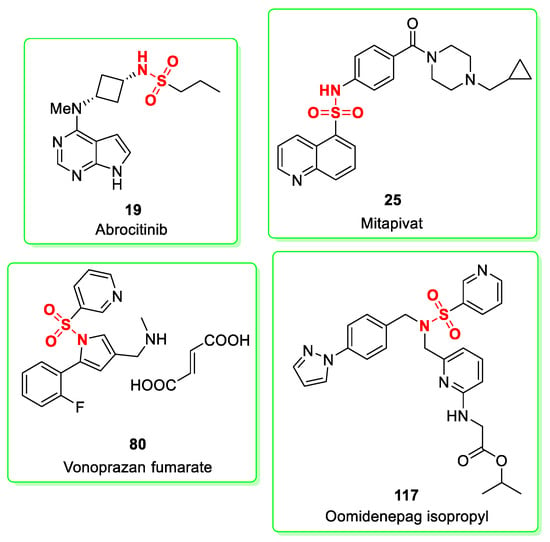
Figure 2.
Chemical structure of approved sulfonamides in 2022. Abrocitinib (19), mitapivat (25), vonoprazan fumarate (80) and oomidenepag isopropyl (117). The sulfonamide moieties are shown in red.
Historically, the first sulfonamide drug was prontosil rubrum (Figure 3). This compound was first synthesized by Bayer chemists Josef Klarer and Fritz Mietzsch as part of a research program designed to find dyes that might act as antibacterial drugs in the body [59]. It was the year 1932. Later on, sulfonamides have been broadly used as antibacterial agents given their similarity to p-aminobenzoic acid (PABA) in the synthesis of folic acid which is essential for the further production of DNA in the bacteria [60]. Another well-known explored field has been the use of sulfonamides and isosteres as inhibitors of metalloenzyme carbonic anhydrase [61].
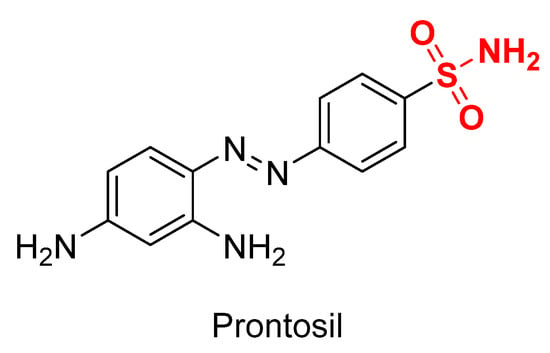
Figure 3.
Chemical structure of prontosil. The sulfonamide moiety is shown in red.
Interestingly, none of the sulfonamides approved by FDA in 2022 belong to the last two groups of inhibitors, creating novel opportunities in hitting novel and diverse targets.
Moreover, a key-feature that would help in the design of novel bioactive compounds is the fact that sulfonamides possess high hydrolytic stability [62].
4. Concluding Remarks
In this work, all small molecule drugs that were approved by the FDA in 2022 were discussed. For each compound, the biological activity and the chemical synthesis were provided. It appears that sulfonamide-containing drugs are dominating the year 2022 with four molecules approved. Their physicochemical and pharmacodynamic properties still play an important role many years after the discovery of the first sulfonamides endowed with antibacterial activities.
Deuterium-containing drug deucravacitinib represents, on the other hand, an example of novel approaches in drug discovery incorporating an unusual isotope to provide drug stability.
The discovery of pyrimidinedione mavacamten for the treatment of cardiomyopathy and the huge forecasted sales (Table S1) for this molecule stress the importance of finding new active molecules in the field of cardiovascular diseases.
The combination of drugs, for example vonoprazan (in combination with amoxicillin and clarithromycin) and taurursodiol (in combination with sodium phenylbutyrate) is still a valid weapon for the treatment of unmet medical diseases.
The 15 FDA-approved small molecules (Figure S1) in 2022 are examples of classical and innovative drug discovery approaches which are paving the way for future, exciting approvals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics14112538/s1, Table S1: Table describing the names of the 15 molecules approved by FDA in 2022. Figure S1: Global sales forecast in 2026 (* 2028 for some drugs).
Author Contributions
D.B.T. and L.S. contributed to the conception of this review. D.B.T. and L.S. analyzed the literatures and wrote the manuscript. D.B.T., L.S. and C.S. completed figures drawing. D.B.T., L.S., C.S., O.R., L.B. and F.M. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
University of Perugia “Fondo per la Ricerca di Base 2020” and Project “DELPHI Star Labs” in the frame of the financial support “Dipartimenti di Eccellenza”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hunting for Drugs in Chemical Space. Available online: https://cen.acs.org/pharmaceuticals/drug-discovery/Hunting-drugs-chemical-space/100/i23 (accessed on 24 October 2022).
- Small Molecules. Available online: https://www.astrazeneca.com/r-d/next-generation-therapeutics/small-molecule.html (accessed on 27 October 2022).
- Novel Drug Approvals for 2022|FDA. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2022 (accessed on 24 October 2022).
- Benedetto Tiz, D.; Bagnoli, L.; Rosati, O.; Marini, F.; Sancineto, L.; Santi, C. New Halogen-Containing Drugs Approved by FDA in 2021: An Overview on Their Syntheses and Pharmaceutical Use. Molecules 2022, 27, 1643. [Google Scholar] [CrossRef] [PubMed]
- Mignot, E.; Mayleben, D.; Fietze, I.; Leger, D.; Zammit, G.; Bassetti, C.L.A.; Pain, S.; Kinter, D.S.; Roth, T. Safety and Efficacy of Daridorexant in Patients with Insomnia Disorder: Results from Two Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trials. Lancet Neurol. 2022, 21, 125–139. [Google Scholar] [CrossRef]
- Urquhart, L. FDA New Drug Approvals in Q1 2022. Nat. Rev. Drug Discov. 2022, 21, 329. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T. The Role of Orexin in Motivated Behaviours. Nat. Rev. Neurosci. 2014, 15, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Kocienski, P. Synthesis of Daridorexant. Synfacts 2021, 17, 0244. [Google Scholar] [CrossRef]
- Napolitano, M.; Fabbrocini, G.; Ruggiero, A.; Marino, V.; Nocerino, M.; Patruno, C. The Efficacy and Safety of Abrocitinib as a Treatment Option for Atopic Dermatitis: A Short Report of the Clinical Data. Drug Des. Dev. Ther. 2021, 15, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.L.; Kaila, N.; Strohbach, J.W.; Trzupek, J.D.; Brown, M.F.; Flanagan, M.E.; Mitton-Fry, M.J.; Johnson, T.A.; TenBrink, R.E.; Arnold, E.P.; et al. Identification of N-{Cis-3-[Methyl(7H-Pyrrolo[2,3-d]Pyrimidin-4-Yl)Amino]Cyclobutyl}propane-1-Sulfonamide (PF-04965842): A Selective JAK1 Clinical Candidate for the Treatment of Autoimmune Diseases. J. Med. Chem. 2018, 61, 1130–1152. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Karmilowicz, M.J.; Burke, D.; Burns, M.P.; Clark, L.A.; Connor, C.G.; Cordi, E.; Do, N.M.; Doyle, K.M.; Hoagland, S.; et al. Biocatalytic Reductive Amination from Discovery to Commercial Manufacturing Applied to Abrocitinib JAK1 Inhibitor. Nat. Catal. 2021, 4, 775–782. [Google Scholar] [CrossRef]
- Al-Samkari, H.; van Beers, E.J. Mitapivat, a Novel Pyruvate Kinase Activator, for the Treatment of Hereditary Hemolytic Anemias. Ther. Adv. Hematol. 2021, 12, 204062072110660. [Google Scholar] [CrossRef] [PubMed]
- Sizemore, J.; Guo, L.; Mirmehrabi, M.; Su, Y. Crystalline Forms of N-(4-(4-(Cyclopropylmethyl) Piperazine-1-Carbonyl)Phenyl)Quinoline-8-Sulfonamide. WO2019104134A1, 31 May 2019. [Google Scholar]
- Verstovsek, S.; Komrokji, R.S. A Comprehensive Review of Pacritinib in Myelofibrosis. Future Oncol. 2015, 11, 2819–2830. [Google Scholar] [CrossRef]
- Hart, S.; Goh, K.C.; Novotny-Diermayr, V.; Tan, Y.C.; Madan, B.; Amalini, C.; Ong, L.C.; Kheng, B.; Cheong, A.; Zhou, J.; et al. Pacritinib (SB1518), a JAK2/FLT3 Inhibitor for the Treatment of Acute Myeloid Leukemia. Blood Cancer J. 2011, 1, e44. [Google Scholar] [CrossRef] [PubMed]
- William, A.D.; Lee, A.C.-H.; Blanchard, S.; Poulsen, A.; Teo, E.L.; Nagaraj, H.; Tan, E.; Chen, D.; Williams, M.; Sun, E.T.; et al. Discovery of the Macrocycle 11-(2-Pyrrolidin-1-Yl-Ethoxy)-14,19-Dioxa-5,7,26-Triaza-Tetracyclo[19.3.1.1(2,6).1(8,12)]Heptacosa-1(25),2(26),3,5,8,10,12(27),16,21,23-Decaene (SB1518), a Potent Janus Kinase 2/Fms-Like Tyrosine Kinase-3 (JAK2/FLT3) Inhibitor for the Treatment of Myelofibrosis and Lymphoma. J. Med. Chem. 2011, 54, 4638–4658. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.B.; Wood, P.L.; Wieland, S.; Hawkinson, J.E.; Belelli, D.; Lambert, J.J.; White, H.S.; Wolf, H.H.; Mirsadeghi, S.; Tahir, S.H.; et al. Characterization of the Anticonvulsant Properties of Ganaxolone (CCD 1042; 3alpha-Hydroxy-3beta-Methyl-5alpha-Pregnan-20-One), a Selective, High-Affinity, Steroid Modulator of the Gamma-Aminobutyric Acid(A) Receptor. J. Pharmacol. Exp. Ther. 1997, 280, 1284–1295. [Google Scholar] [PubMed]
- Ganaxolone. Available online: https://www.drugs.com/monograph/ganaxolone.html (accessed on 15 November 2022).
- Reddy, D. Neurosteroid Compounds and Methods for Their Preparation and Use in Treating Central Nervous System Disorders. WO2019209850A1, 31 October 2019. [Google Scholar]
- Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215833s000lbl.pdf (accessed on 24 October 2022).
- Keam, S.J. Lutetium Lu 177 Vipivotide Tetraxetan: First Approval. Mol. Diagn. Ther. 2022, 26, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Benešová, M.; Schäfer, M.; Bauder-Wüst, U.; Afshar-Oromieh, A.; Kratochwil, C.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Preclinical Evaluation of a Tailor-Made DOTA-Conjugated PSMA Inhibitor with Optimized Linker Moiety for Imaging and Endoradiotherapy of Prostate Cancer. J. Nucl. Med. 2015, 56, 914–920. [Google Scholar] [CrossRef]
- Eder, M.; Schäfer, M.; Bauder-Wüst, U.; Hull, W.-E.; Wängler, C.; Mier, W.; Haberkorn, U.; Eisenhut, M. 68 Ga-Complex Lipophilicity and the Targeting Property of a Urea-Based PSMA Inhibitor for PET Imaging. Bioconjugate Chem. 2012, 23, 688–697. [Google Scholar] [CrossRef]
- Hoy, S.M. Oteseconazole: First Approval. Drugs 2022, 82, 1017–1023. [Google Scholar] [CrossRef]
- Wirth, D.D.; Yates, C.M.; Hoekstra, W.J. Antifungal Compound Process. WO2017049096A1, 23 March 2017. [Google Scholar]
- Wirth, D.D.; Yates, C.M.; Hoekstra, W.J.; Bindl, M.F.; Hartmann, E. Antifungal Compound Process. WO2017049080A1, 23 March 2017. [Google Scholar]
- Keam, S.J. Mavacamten: First Approval. Drugs 2022, 82, 1127–1135. [Google Scholar] [CrossRef]
- Urquhart, L. FDA New Drug Approvals in Q2 2022. Nat. Rev. Drug Discov. 2022, 21, 550. [Google Scholar] [CrossRef]
- Oslob, J.; Anderson, R.; Aubele, D.; Evanchik, M.; Fox, J.C.; Kane, B.; Lu, P.; McDowell, R.; Rodriguez, H.; Song, Y.; et al. Pyrimidinedione Compounds. USOO9585883B2, 7 March 2017. [Google Scholar]
- Garnock-Jones, K.P. Vonoprazan: First Global Approval. Drugs 2015, 75, 439–443. [Google Scholar] [CrossRef]
- Sachs, G.; Shin, J.M.; Hunt, R. Novel Approaches to Inhibition of Gastric Acid Secretion. Curr. Gastroenterol. Rep. 2010, 12, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.-Y.; Zeng, H.; Yao, K.; Li, J.-Q.; Liu, Y. Novel and Practical Synthesis of Vonoprazan Fumarate. Synth. Commun. 2017, 47, 1169–1174. [Google Scholar] [CrossRef]
- Keam, S.J. Tapinarof Cream 1%: First Approval. Drugs 2022, 82, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Richardson, W.H.; Schmidt, T.M.; Nealson, K.H. Identification of an Anthraquinone Pigment and a Hydroxystilbene Antibiotic from Xenorhabdus Luminescens. Appl. Environ. Microbiol. 1988, 54, 1602–1605. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Webster, J.; Li, J.; Hu, K.; Zhu, J. Anti-Inflammatory and Psoriasis Treatment and Protein Kinase Inhibition by Hydroxyltilbenes and Novel Stilbene Derivatives and Analogues. US20030171429A, 11 September 2003. [Google Scholar]
- Brown, A. FDA New Drug Approvals in Q3 2022. Nat. Rev. Drug Discov. 2022, 21, 788. [Google Scholar] [CrossRef]
- Mullard, A. First de Novo Deuterated Drug Poised for Approval. Nat. Rev. Drug Discov. 2022, 21, 623–625. [Google Scholar] [CrossRef]
- Wrobleski, S.T.; Moslin, R.; Lin, S.; Zhang, Y.; Spergel, S.; Kempson, J.; Tokarski, J.S.; Strnad, J.; Zupa-Fernandez, A.; Cheng, L.; et al. Highly Selective Inhibition of Tyrosine Kinase 2 (TYK2) for the Treatment of Autoimmune Diseases: Discovery of the Allosteric Inhibitor BMS-986165. J. Med. Chem. 2019, 62, 8973–8995. [Google Scholar] [CrossRef]
- Moslin, R.; Zhang, Y.; Wrobleski, S.T.; Lin, S.; Mertzman, M.; Spergel, S.; Tokarski, J.S.; Strnad, J.; Gillooly, K.; McIntyre, K.W.; et al. Identification of N -Methyl Nicotinamide and N -Methyl Pyridazine-3-Carboxamide Pseudokinase Domain Ligands as Highly Selective Allosteric Inhibitors of Tyrosine Kinase 2 (TYK2). J. Med. Chem. 2019, 62, 8953–8972. [Google Scholar] [CrossRef]
- Robic, C.; Port, M.; Rousseaux, O.; Louguet, S.; Fretellier, N.; Catoen, S.; Factor, C.; Le Greneur, S.; Medina, C.; Bourrinet, P.; et al. Physicochemical and Pharmacokinetic Profiles of Gadopiclenol: A New Macrocyclic Gadolinium Chelate With High T1 Relaxivity. Investig. Radiol. 2019, 54, 475–484. [Google Scholar] [CrossRef]
- Gadopiclenol: Another Milestone Achieved|Bracco Corporate. Available online: https://www.bracco.com/en/news/gadopiclenol-another-milestone-achieved (accessed on 24 October 2022).
- Napolitano, R.; Lattuada, L.; Baranyai, Z.; Guidolin, N.; Marazzi, G. Gadolinium Bearing Pcta-Based Contrast Agents. WO2020030618A1, 13 February 2020. [Google Scholar]
- Duggan, S. Omidenepag Isopropyl Ophthalmic Solution 0.002%: First Global Approval. Drugs 2018, 78, 1925–1929. [Google Scholar] [CrossRef]
- Aihara, M.; Ropo, A.; Lu, F.; Kawata, H.; Iwata, A.; Odani-Kawabata, N.; Shams, N. Intraocular Pressure-Lowering Effect of Omidenepag Isopropyl in Latanoprost Non-/Low-Responder Patients with Primary Open-Angle Glaucoma or Ocular Hypertension: The FUJI Study. Jpn. J. Ophthalmol. 2020, 64, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, M.; Yoneda, K.; Okanari, E.; Shigetomi, M. Pharmaceutical Composition for Treating or Preventing Glaucoma. WO2010113957A1, 7 October 2010. [Google Scholar]
- Iwamura, A.; Tanaka, M.; Katsube, T.; Shigetomi, M.; Okasei, E.; Tokunaga, H. Medicine Containing Pyridylaminoacetic Acid Compound. JP2011057633A, 24 March 2011. [Google Scholar]
- Iwamura, R.; Tanaka, M.; Katsube, T.; Shigetomi, M.; Okanari, E.; Tokunaga, Y.; Fujiwara, H. Pyridylaminoacetic Acid Compound. WO2009113600A1, 17 September 2009. [Google Scholar]
- Relyvrio (Sodium Phenylbutyrate and Taurursodiol) FDA Approval History. Available online: https://www.drugs.com/history/relyvrio.html (accessed on 25 October 2022).
- Hagey, L.; Crombie, D.; Espinosa, E.; Carey, M.; Igimi, H.; Hofmann, A. Ursodeoxycholic Acid in the Ursidae: Biliary Bile Acids of Bears, Pandas, and Related Carnivores. J. Lipid Res. 1993, 34, 1911–1917. [Google Scholar] [CrossRef]
- Tonin, F.; Arends, I.W.C.E. Latest Development in the Synthesis of Ursodeoxycholic Acid (UDCA): A Critical Review. Beilstein J. Org. Chem. 2018, 14, 470–483. [Google Scholar] [CrossRef] [PubMed]
- Dayal, B.; Bhojawala, J.; Rapole, K.R.; Pramanik, B.N.; Ertel, N.H.; Shefer, S.; Salen, G. Chemical Synthesis, Structural Analysis, and Decomposition of N-Nitroso Bile Acid Conjugates. Bioorganic Med. Chem. 1996, 4, 885–890. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Bahleda, R.; Hierro, C.; Sanson, M.; Bridgewater, J.; Arkenau, H.-T.; Tran, B.; Kelley, R.K.; Park, J.O.; Javle, M.; et al. Futibatinib, an Irreversible FGFR1–4 Inhibitor, in Patients with Advanced Solid Tumors Harboring FGF / FGFR Aberrations: A Phase I Dose-Expansion Study. Cancer Discov. 2022, 12, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Geng, M.; Wang, Y.; Ai, J.; Fan, J.; Dai, Y.; Ding, J. New Compound Having Fgfr Inhibitory Activity and Preparation and Application Thereof. WO2017215485A1, 21 December 2017. [Google Scholar]
- Sootome, H. Therapeutic Agent for Fgfr Inhibitor-Resistant Cancer. WO2015008844A1, 22 January 2015. [Google Scholar]
- Ballatore, C.; Huryn, D.M.; Smith, A.B. Carboxylic Acid (Bio)Isosteres in Drug Design. ChemMedChem 2013, 8, 385–395. [Google Scholar] [CrossRef]
- Novel Drug Approvals for 2021|FDA. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2021 (accessed on 25 October 2022).
- Novel Drug Approvals for 2020|FDA. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2020 (accessed on 24 October 2022).
- Zhong, D.; Wu, D.; Zhang, Y.; Lu, Z.; Usman, M.; Liu, W.; Lu, X.; Liu, W.-B. Synthesis of Sultams and Cyclic N -Sulfonyl Ketimines via Iron-Catalyzed Intramolecular Aliphatic C–H Amidation. Org. Lett. 2019, 21, 5808–5812. [Google Scholar] [CrossRef]
- Mondal, S.; Malakar, S. Synthesis of Sulfonamide and Their Synthetic and Therapeutic Applications: Recent Advances. Tetrahedron 2020, 76, 131662. [Google Scholar] [CrossRef]
- Ovung, A.; Bhattacharyya, J. Sulfonamide Drugs: Structure, Antibacterial Property, Toxicity, and Biophysical Interactions. Biophys. Rev. 2021, 13, 259–272. [Google Scholar] [CrossRef]
- Carta, F.; Supuran, C.T.; Scozzafava, A. Sulfonamides and Their Isosters as Carbonic Anhydrase Inhibitors. Future Med. Chem. 2014, 6, 1149–1165. [Google Scholar] [CrossRef]
- Białk-Bielińska, A.; Stolte, S.; Matzke, M.; Fabiańska, A.; Maszkowska, J.; Kołodziejska, M.; Liberek, B.; Stepnowski, P.; Kumirska, J. Hydrolysis of Sulphonamides in Aqueous Solutions. J. Hazard. Mater. 2012, 221–222, 264–274. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).