Abstract
The pandemic of the coronavirus disease 2019 (COVID-19) represents an unprecedented challenge to identify effective drugs for prevention and treatment. While the world’s attention is focused on news of COVID-19 vaccine updates, clinical management still requires improvement. Due to the similarity of cancer-induced inflammation, immune dysfunction, and coagulopathy to COVID-19, anticancer drugs, such as Interferon, Pembrolizumab or Bicalutamide, are already being tested in clinical trials for repurposing, alone or in combination. Given the rapid pace of scientific discovery and clinical data generated by the large number of people rapidly infected, clinicians need effective medical treatments for this infection.
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has caused catastrophic damage to human life. Since December 2019, the pandemic has spread worldwide and still is ongoing. SARS-CoV-2 primarily infects the upper and lower respiratory tract; however, it can also affect other vital organs. Most people recover from the acute phase of the disease, but some people continue to experience a range of effects for months after recovery. Clinical management is currently focused on supportive care and prevention and control of complications such as acute respiratory distress syndrome (ARDS) [1].
Although the world’s attention is understandably centred on reports of COVID-19 vaccine updates, from supply to administration, the need for treatments cannot be overlooked, as vaccination cannot protect everybody and as infection overwhelms hospitals and nursing homes. When we compare COVID-19 to the common flu, which is routinely targeted and has readily available and effective vaccines, we can see that no vaccine is ideal. Therefore, flu medications are still in high demand to avoid hospitalization and save lives. While the rise of new variants of COVID-19 threatens the efficacy of the available vaccines, it is critical that we must continue researching therapies to minimize hospitalization and cure COVID-19. The world health organization created (WHO) guidelines on using vaccines and antivirals during influenza pandemics to address the shortage of vaccines and antivirals [2]. Demonstrating that with therapy, people can live longer and gain control over the pandemic’s curse, as the likelihood of people becoming ill and spreading the disease decreases. Therapeutics also can be used as prophylactics to prevent hospitalizations and severe cases of the disease.
The food and drug administration (FDA) granted emergency use authorization to two monoclonal antibody treatments for non-hospitalized adults and children over the age of 12 who have mild to moderate COVID-19 symptoms, who are at risk for developing severe COVID-19 or being hospitalized for it. Regeneron’s casirivimab and imdevimab combo and Eli Lilly’s bamlanivimab and etesevimab combination are the two treatmentsPrior approval for the single use of bamlanivmab to treat COVID-19 was withdrawn in April 2021 due to new data revealing minimal efficacy [3]. While these medications can be beneficial, the need for intravenous administration (IV) requires a visit to a clinic or hospital immediately after symptoms appear, which limits their use.
Consequently, effective therapies, which are available to anyone who needs them, must work with various populations and ensure that the responses to the pandemic are globally successful and inclusive. Having both important tools in our arsenal would ensure that most of the population is shielded from the severe effects of COVID-19. However, the development of novel antiviral drugs needs long-term investigation in clinical trials. Therefore, the benefit of repurposing drugs to justify off-label usage is linked to the established safety profile. However, it may vary depending on the disease and the consolidated data on pharmacodynamics, pharmacokinetics and efficacy in phase I–IV trials [4,5]. Some host cell targets that interfere with the viral growth cycle, such as kinases, are commonly shared in the mechanisms of multiple viral infections and other conditions such as cancer, indicating the possibility of translating information through medical disciplines and disease models [6].
Several anticancer compounds were investigated as possible future drugs for COVID-19, among the thousands of coronavirus drugs studied. This article includes anticancer drugs that have already been approved or are being fast-tracked by regulatory authorities, supported by published evidence and used to treat the treatment of cancer patients. In times of crisis, such as COVID-19, drug repurposing is a valuable technique because it provides quick access to agents with not only accessible safety data but also defined manufacturing lines and supply chains, which facilitates the process of discovery. The major limitation of the use of repurposed therapeutics is associated with dosage regimens. Most of the time, effective concentrations needed for antiviral activity are often higher than those clinically attainable under the approved regimens [7].
Drug repurposing is not a reason for designing low-quality clinical trials or emphasizing the bias of early outcomes and uncontrolled cohorts, and it is desirable to use molecules with a specified safety profile. In addition, these therapies have early-and late-phase data on toxicity and complications management, which are especially helpful in the setting of a pandemic versus novel therapies. Several antineoplastic agents have the potential to improve COVID-19 outcomes by using the exact mechanisms and targets used in cancer treatment [8]. These targets are primarily associated with inhibiting cell division, regulating inflammation, and modulating the host-tumor microenvironment.
2. Materials and Methods
Using the OpenData Portal [9] was possible to research COVID-19-related drug repurposing data and experiments for all approved drugs. From this portal were only selected the anticancer drugs tested. Then was searched on the database of clinicaltrials.gov [10], the list of anticancer drugs tested on COVID-19 to see which were listed in clinical trials (20 May 2021). Additionally, searching through the site of European Pharmaceutical Review [11], in the news section, we were able to find more information about repurposed drugs and clinical trials for COVID-19. In the end, it was possible to formulate an updated list of anticancer drug candidates for COVID-19 treatment.
3. Viral, Host and Immune Targets in COVID-19
Antiviral therapy and prevention approaches are focused on (a) inhibiting the replication of the viral genome by either preventing the virus from entering the host cells or suppressing one or more phases of replication; (b) boosting the immune system and producing a type of antiviral memory via vaccination; (c) injection of antiviral antibodies generated in the plasma [12].
SARS-CoV-2 replicates similarly to other Coronaviridae viruses. Coronaviruses can infect the host through both endosomal and non-endosomal (cell surface) routes. The viral protein kinases and their associated signaling cascades have now been targeted in order to reduce coronavirus replication, particularly SARS-CoV-2. The virus can enter the cells via endocytosis or plasma membrane fusion through the interaction between the Spike (S) protein of the virus and angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2) at the target cell [12,13].
After receptor-mediated endocytosis of the virus into the host cells, the virus releases the viral genome (single-stranded positive RNA) and uses the host ribosome to translate into viral polyproteins. Viral proteinases 3CLpro and PLpro cleave viral polyproteins into effector proteins (see Appendix A). RNA-dependent RNA polymerase, in turn, synthesizes a full-length negative-strand RNA template, which is used to make more viral genomic RNA. The viral genome then is synthesized by genomic replication, and four essential structural viral proteins (nucleocapsid (N), spike (S), membrane (M) and envelope (E)) are produced by transcription and translation [14]. The N protein binds genomic RNA, while S, M and E proteins are integrated into the membrane of the endoplasmic reticulum (ER), forming ERGIC—endoplasmic reticulum-Golgi intermediate compartment (also referred to as a vesicular-tubular cluster). The assembled nucleocapsid with helical twisted RNA is encapsulated into the ER lumen, viral progeny is transported by the ERGIC toward the plasma membrane of the host cell, and finally, the daughter virus is released by exocytosis [15].
The SARS-CoV-2 infection activates both innate and adaptive immune responses in the host. Patients with severe COVID-19 have a lower number of natural killer (NK) cells and a higher level of the C-reactive protein. The early failure of antiviral immunity during SARS-CoV-2 infection is correlated with a significant decrease in total T cells and NK cells [16].
Exploring potential clinical targets for COVID-19 attenuation is critical for long-term COVID-19 treatment.
4. Similarities of Cancer Immune Response and COVID-19
Cancer treatment is still a major challenge, but tremendous progress in anticancer drug discovery and development has occurred in the last few decades. The spent decades developing drugs for cancer-induced inflammation, immune dysfunction, and vascularization provided us with a number of drug options that could be useful in the treatment of other diseases.
Patients affected by COVID-19 also display inflammation, immune dysfunction and vascular syndrome dysfunction [17].
Evidence suggests that the immune response to SARS-CoV-2 can play different roles: dysregulated immune responses in critically ill patients with COVID-19 is reflected by lymphopenia, mainly affecting CD4+ T cells, including effector, memory, and regulatory T cells, and decreased IFN-γ expression in CD4+ T cells. Exhaustion of cytotoxic T lymphocytes, activation of macrophages, and a low human leukocyte antigen-DR expression on CD14 monocytes has been noted in patients with COVID-19 [18]. These similarities led scientists to consider anticancer therapy for the management of COVID-19 [8].
Furthermore, the homeostasis maintained by the vascular endothelium in health is affected by COVID-19 infection. In clinical studies, patients with COVID-19 have higher levels of fibrinogen, fibrin degradation products, and D-dimer, which appear to be related to disease severity and thrombotic risk [17]. Since the susceptibility to thrombotic events tends to be, at least in part, linked to inflammation and activation of the innate immune system that can cause systemic coagulation pathways. Therefore, the counterparts between the mechanisms of immunotherapy-related toxicities and the COVID-19 cytokine storm must be well considered in order not to affect the efficiency of the reused drug and increase the risk of the disease.
5. Repurposing Anticancer Drugs against COVID-19
The drug repurposing approach puts the drug discovery process on a fast track. COVID-19 researchers’ attention to its potential growth is wider in a range of different scientific fields. Due to the availability of in-vitro and in-vivo screening data, chemical optimization, toxicity studies, bulk manufacturing, formulation development and pharmacokinetic profiles of FDA-approved drugs, drug development cycles are shortened as all these critical steps can be bypassed [7,8]. In addition, there is no need for larger investments and repurposed drugs are proven to be safe in preclinical models, thus lowering the attrition rates as well. The main advantage of drug repurposing is associated with the established safety of the known candidate compounds. The development time frame and costs are substantially reduced when advancing a candidate into a clinical trial, which is possible without neglecting the comorbidities already associated with certain medications not to aggravate the patient condition provoked by the viral infection [6].
Several drugs that have been approved for cancer indication by the US FDA are now in COVID-19 clinical trials to test their efficiency in reducing mortality and speed up recovery. The following Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6 represent anticancer drugs in clinical trials for COVID-19. In this review, we explore according to different categories of therapies which drugs represent more or fewer advantages for COVID-19. Appendix A is an updated list of all the anticancer drugs we could find or drugs used for the best supportive cancer care, which are being tested on their effectiveness to treat patients with mild to severe SARS-CoV-2.

Table 1.
Anticancer drugs in clinical trials for COVID-19: Interferon-based therapies.

Table 2.
Anticancer drugs in clinical trials for COVID-19: Anti-cytokine agents.

Table 3.
Anticancer drugs in clinical trials for COVID-19: Immune-checkpoint inhibitors.

Table 4.
Anticancer drugs in clinical trials for COVID-19: Hormone therapy.

Table 5.
Anticancer drugs in clinical trials for COVID-19: The inhibitor of elongation factor 1A and the eukaryotic initiation factor 4A.

Table 6.
Anticancer drugs in clinical trials for COVID-19: Blockade of kinase cascades.
5.1. Interferon-Based Therapies
The homeostasis maintained by the vascular endothelium in health is affected by COVID-19 infection. In clinical studies, patients with COVID-19 have higher levels of fibrinogen, fibrin degradation products, and D-dimer, which appear to be related to disease severity and thrombotic risk [19].
SARS-CoV-2 compromises the type 1 interferon antiviral response; therefore, IFN administration seemed a promising approach to stimulate macrophages, which engulf antigens and natural killer cells (NK cells). IFN might be able to strengthen the immune system by activating dormant components [20]. Clinical trials are running to test its effectiveness either alone or in combination with other drugs.
Ribavirin, lopinavir/ritonavir, remdesivir or hydroxychloroquine are some of the drugs tested in combination with IFNs in clinical trials (see Table 1). The study by Hung IF-N et al. demonstrated that early treatment with interferon beta-1b, lopinavir–ritonavir, and ribavirin is safe and highly effective in shortening the duration of the virus shedding, decreasing cytokine responses and allowing patients with mild to moderate disease to be discharged COVID-19 [21].
The problem is that when interferons boost the immune system, COVID-19 are likely to worsen before they improve. Giving anyone an interferon-based drug if they are still on a ventilator and their symptoms are about to overtake them may be fatal. This is why, in the case of viral infections, interferon therapies are usually only used as a last resort [22]. Nonetheless, interferon has already shown success against the antiviral activity, due to their ability to modulate the immune response, which is considered a “standard of care” in suppressing Hepatitis C and B infections [20].
5.2. Anticytokine Agents
The current COVID-19 infection is linked to elevated cytokine levels or hypercytokinemia. Patients who develop cytokine storms quickly experience cardiovascular collapse, multiple organ dysfunction and death [23]. The marked elevation of serum cytokines, especially tumor necrosis factor-alpha, interleukin 17 (IL-17), interleukin 8 (IL-8) and interleukin 6 (IL-6), is seen in patients with COVID-19 who go through pneumonia and hypoxia [24] (Table 2).
The administration of IL-6 blocking agents, such as tocilizumab and siltuximab, has been shown to be effective [25]. Repurposing tocilizumab would be interesting for the prevention or treatment of lung injury caused by COVID-19 since there is currently no effective antiviral therapy. In prospective studies, tocilizumab was linked to a lower relative risk of mortality, but the effects on other outcomes were inconclusive.
The drug siltuximab is a chimeric monoclonal antibody that binds to interleukin-6 (IL-6), preventing binding to soluble and membrane-bound interleukin-6 receptors. Current evidence showed that siltuximab led to a reduced mortality rate from COVID-19 promising to be a possible therapy; however, more studies are necessary [25].
5.3. Immune-Checkpoint Inhibitors
Immune checkpoints are regulatory molecules that are found on the surface of immune cells. When proteins on the surface of immune cells called T cells recognize and bind to partner proteins on other cells, such as tumor cells, immune checkpoints are activated. The T cells receive an “off” signal which may prevent cancer from being destroyed by the immune system. Therefore, immune checkpoint inhibitors are immunotherapy drugs that work by preventing checkpoint proteins from binding to their partner proteins. As a result, the “off” signal is not sent, allowing T cells to kill cancer cells [26,27].
The same principle can be applied for COVID-19 as a potential therapeutic approach (see Table 3). Evidence from preclinical models suggests that blocking programmed death receptor 1 (PD1) protects against RNA virus infections. Among the ICIs, antibodies capable of blocking the pathway of programmed death 1 (PD 1)/PD ligand-1 (PD L1) are promising. PD-1 expression levels on NK cells and T-cells were found to be highly upregulated in COVID-19 patients. When treated with anti-PD 1 and anti-PD L1 antibodies, they regain their T cell competence and effectively counteract viral infection [26,28]. Nivolumab and Pembrolizumab are ICIs that were successfully introduced into the management of various solid cancers, particularly for melanoma [24]. Currently, there is a phase II to trial to access efficacy for COVID-19. Pembrolizumab was tested in combination with tocilizumab [26].
5.4. Hormone Therapy
Androgen deprivation therapy (ADT), also known as androgen suppression therapy, is an antihormone therapy used to treat prostate cancer. Increasing evidence suggests that androgen has the potential to regulate the cellular TMPRSS2 expression and ACE2 [29].
TMPRSS2 is a membrane protease necessary for COVID pathogenesis, which is regulated by androgens. Blocking TMPRSS2 with bicalutamide can reduce viral replication and improve clinical outcomes. These agents may down-regulate TMPRSS2 mRNA and expression resulting in less entry of SARS-CoV-2 entry into cells and thus could arise as promising therapeutic tools in early SARS-CoV-2 infection and COVID-19 [30], see Table 4. A combination of bicalutamide in combination with camostat has the potential to reduce hospitalizations.
Toremifene used in the treatment of advanced breast cancer in postmenopausal women is a first-generation nonsteroidal-selective estrogen receptor modulator. It displays potential effects in blocking various viral infections, including MERS-CoV, SARS-CoV and Ebola virus. Prevents fusion between the viral and endosomal membrane by interacting with and destabilizing the virus membrane glycoprotein and eventually inhibiting viral replication [31]. Moreover, a preliminary study reveals a high potential for the synergistic effects of melatonin and toremifene to reduce viral infection and replication [32].
5.5. Inhibitor of Elongation Factor 1A and the Eukaryotic Initiation Factor 4A
Other molecules revealed potent pre-clinical efficacy against SARS-CoV-2 by inhibiting replication. In the life cycle of SARS-CoV-2, many host proteins play a role, and some are required for viral replication and translation. Drugs that target viral proteins are usually the focus of research, but a complementary approach is to target the required host proteins (Table 5).
Plitidepsin is an inhibitor of elongation factor 1A (eEF1A) and is an authorized drug in Australia for the treatment of multiple myeloma. Antiviral activity of plitidepsin has been analyzed in a human hepatoma cell line infected with the HCoV-229E-GFP virus, a virus similar to the SARS-CoV-2 virus [33]. Clinical studies using this drug are already taking place to assess safety and toxicity profile in patients with COVID-19 who require hospital admission, being the main goal is to select the recommended dose levels of plitidepsin for future phase 2/3 efficacy studies.
Another promising drug being tested in clinical trials is Zotatifin to assess its safety and tolerability. Zotatifin is a selective small-molecule inhibiting the eukaryotic initiation factor 4A (eIF4A), a powerful anti-proliferative target found at the intersection of the RAS and PI3K signaling pathways [34].
5.6. Blockade of Kinase Cascades
To test the hypothesis that PI3K blockade could hamper immune system hyperactivation and thus reduce lung inflammation and interfere with the viral cycle, researchers used one of the most successful targeted strategies in cancer treatment: kinase cascade blockade [35]. In a randomized placebo-controlled phase 2 study, Duvelisib, an orally bioavailable phosphatidylinositol 3-kinase (PI3K) selective inhibitor, is being evaluated for its ability to reduce inflammation in the lungs of patients with severe acute respiratory syndrome coronavirus 2 infections. As has been demonstrated repeatedly for multiple compounds in this pharmacological class, PI3K inhibitors, including the drug duvelisib, can cause lung inflammation and increase the risk of infections, and special caution is required during clinical trials using this class of molecules (Table 6).
On the other hand, Zanubrutinib is an irreversible Bruton tyrosine kinase inhibitor. The aberrant activation of the Bruton tyrosine kinase has a key role in the tumorigenesis of B-cell lymphoma. For COVID-19 evidence suggesting protective effects, a phase II trial is ongoing, aiming to reduce the disease-related immune dysregulation and hyper-inflammation [35].
5.7. Radiation and Prophylactic Vitamin D
Low-dose thoracic irradiation strategies with anti-inflammatory or prophylactic vitamin D have shown antiviral potential. However, there is a lack of direct pre-clinical and clinical evidence for COVID-19 and other therapeutics that may be more accessible, less risky, and less complicated for treatment [36].
Recently, we have acquired an unparalleled knowledge of the molecular processes and immune tolerance mechanisms regulating the occurrence and severity of human neoplasms, contributing to a wide variety of targeted anticancer and immunotherapy treatments [37]. Despite their specificity, however, small-molecule inhibitors and antibody-based therapies cause both on-and off-target effects, including immune-related pneumonia and diabetes, among other conditions, which need to be addressed when translating COVID-19 anticancer therapy. Now it is necessary to continue with clinical trials to overcome the uncertainties about the risks of certain therapeutics and understand which could be more beneficial in a time where vaccines are already available. Therapeutics along with immunization are the key to getting rid of the pandemic.
6. Conclusions
The COVID-19 pandemic has swiftly swept through the world, resulting in huge morbidity and significant mortality. While the news of vaccination brings the promise to the end of the pandemic, the importance of medicines must not be forgotten since it helps to limit the spread of disease and allows both prevention and treatment. Either using repurposed drugs, alone or in combination or even new molecules, the pandemic provides an opportunity to create new models for evaluating novel therapeutic approaches quickly. Due to similarities between cancer and COVID-19, anticancer drugs are repurposed in clinical trials to test their efficacy in targeting inflammation, immune dysfunction, and coagulopathy. Figure 1 illustrates the principal targets of anticancer drugs repurposed in clinical trials for COVID-19.
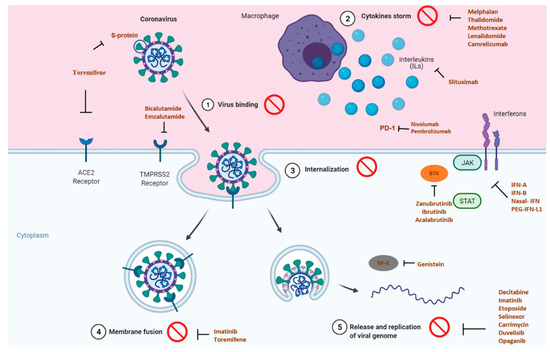
Figure 1.
Principal targets of the anticancer drugs or drugs used for breast cancer supportive care, repurposed in clinical trials for COVID-19, adapted from BioRender templates [38].
Finally, the management of the SARS-CoV-2 pandemic includes multidisciplinary collaboration to identify suitable treatment options for everyone, including and especially countries with limited access to vaccines and people already hospitalized. From the evidence reviewed here, several anticancer drugs seem to retain a promising activity to treat patients with COVID-19.
Author Contributions
B.C. contributed to the conception of the review; the collection, analysis and interpretation of the information included within; the drafting of the review; and critically revising the review for important intellectual content. N.V. contributed to the conception of the review; the collection, analysis and interpretation of the information included within; the drafting of the review; and critically revising the review for important intellectual content; Supervision, N.V.; project administration, N.V.; funding acquisition, N.V. Both authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by “Fundação para a Ciência e Tecnologia” (FCT, Portugal and FEDER—Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020—Operacional Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, in the framework of the project IF/00092/2014/CP1255/CT0004.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
N.V. thanks Fundação para a Ciência e a Tecnologia (FCT, Portugal) for supporting these studies through a project from the National Funds, within CINTESIS, R&D Unit (reference UIDB/4255/2020). The contents of this report are solely the responsibility of the authors and do not necessarily represent the official view of the FCT.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Coronavirus disease 2019 (COVID-19); severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2); acute respiratory distress syndrome (ARDS); world health organization (WHO); the food and drug administration (FDA); interferons (IFNs); transmembrane-serine-protease-2 (TMPRSS2); angiotensin-converting enzyme 2 (ACE2); phosphatidylinositol 3-kinase (PI3K);elongation factor 1A (eEF1A); eukaryotic initiation factor 4A; the eukaryotic initiation factor 4A (eIF4A);programmed death receptor 1 (PD1); programmed death ligand-1 (PD L1); immune-checkpoint inhibitors (ICIs); tumor necrosis factor alpha (TNF-α);interleukin 17 (IL-17); interleukin 8 (IL-8);interleukin 6 (IL-6); Janus kinase (Jak); tyrosine kinases (Tyk); nucleocapsid (N); spike (S); membrane (M); envelope (E); endoplasmic reticulum (ER); endoplasmic reticulum-Golgi intermediate compartment(ERGIC); natural killer (NK)
Appendix A

Table A1.
List of drugs with anticancer effects or used for best supportive cancer care, in clinical studies for the treatment of COVID-19.
Table A1.
List of drugs with anticancer effects or used for best supportive cancer care, in clinical studies for the treatment of COVID-19.
| Anticancer Drug | Viral—Host Targets | Mechanism of Action | Tested in Clinical Trials | NCT Identifier | Combination | Status | Phase | Eligible Population | Primary End-Point | Source (20 May 2021) |
|---|---|---|---|---|---|---|---|---|---|---|
| Acalabrutinib | BTK | Inhibits the activity of BTK and prevents the activation of the B-cell antigen receptor | United states | NCT04380688 | - | Completed | Phase 2 | COVIDCOVID-19 infection | Occurrence of Adverse Events and Serious Adverse Events | [10] |
| Several locations | NCT04346199 | - | Completed | Phase 2 | COVID-19 infection | Subject alive and free of respiratory failure | ||||
| Bevacizumab | VEGF | Vascular permeability inhibition | Yes (France and China) | NCT04275414 | - | Completed | Phase 2 | Severe lung disease or critical disease | Change of PaO2 to FiO2 ratio | [10] |
| NCT04344782 | - | Not yet recruiting | Phase 2 | Severe lung disease | Number of patients who avoid mechanical-assisted ventilation | |||||
| NCT04305106 | - | Recruiting | Not applicable | Disease requiring O2-support | Time to clinical improvement | |||||
| Bicalutamide | Downregulates TMPRSS2 | Binding of androgen receptor | Yes | NCT04509999 | - | Recruiting | Phase 3 | COVID-19 infection, confirmed | COVID-19 symptom relief | [9] |
| NCT04652765 | camostat | Recruting | Phase 1 | COVID-19 infection, confirmed | Reduce number of participants requiring hospitalization | |||||
| Camrelizumab | Immune homeostasis | PD-1/PD-L1 pathway | Yes (China) | ChiCTR2000029806 | - | Recruiting | Not applicable | COVID-19 infection | Proportion of patients with | [10] |
| Blockade | a lung injury score | |||||||||
| - | reduction | |||||||||
| Carrimycin | Inhibit the replication of SARS-CoV-2 in the<break/>cells | Inhibits mTOR pathway | Not provided | NCT04286503 | - | Not yet recruting | Phase 4 | COVID-19 infection | Fever to normal time | [11] |
| NCT04672564 | - | Recruting | Phase 3 | Patient with SARS-CoV-2 infection | Percentage of patients alive without need for supplemental oxygen and ongoing in patient-medical care | |||||
| Decitabine | Nucleic Acid Synthesis Inhibitor | Nucleic acid synthesis inhibitor | Yes (USA) | NCT04482621 | - | Recruting | Phase 2 | COVID infection | Clinical improvement | [9] |
| Duvelisib | PI3K inhibition | Immune homeostasis restoration and viral replication inhibition | Yes (USA) | NCT04372602 | - | Recruting | Phase 2 | Critical disease | Overall survival | [10] |
| NCT04487886 | - | Recruting | Phase 2 | severe COVID-19 who do not require mechanical ventilation | Reduce overall necessity of ventilation | |||||
| Ensifentrine | High selectivity for PDE3 and PDE4 over other enzymes and receptors to minimize off-target effects | Dual inhibitor of phosphodiesterase 3 (PDE3) and 4 (PDE4) | United states | NCT04527471 | None | Active, not recruting | Phase 2 | SARS-CoV-2 infection | Proportion of patients with recovery | [11] |
| Enzalutamide | reduce androgen driven morbidity in COVID-19 | Competitive binder of androgens | Sweden | NCT04475601 | - | Recruting | Phase 2 | SARS-CoV-2 infection | Time to worsening of disease | [9] |
| Etoposide | Topoisomerase II | Inhibits DNA synthesis by forming a complex with topoisomerase II and DNA | United states | NCT04356690 | - | Active, not yet Recruting | Phase 2 | Confirmed COVID-19 infection | Change in pulmonary status | [9] |
| FN-B1A | Jak1 and Tyk2 | Jak1 and Tyk2 | UK | NCT04385095 | - | Recruiting | Phase 2 | COVID-19 infection | Clinical Improvement | [10] |
| Jak1 and Tyk2 | Several locations | NCT04315948 | Lopinavir, ritonavir | Active, not yet Recruiting | Phase 3 | COVID-19 infection | Percentage of subjects reporting severity | |||
| - | Iran | NCT04350671 | Hydroxychloroquine, lopinavir, ritonavir | Enrolling by invitation | Phase 4 | COVID-19 infection | Reduce Mortality | |||
| - | Irna | NCT04350684 | Hydroxychloroquine, lopinavir, ritonavir, umifenovir | Enrolling by invitation | Phase 4 | COVID-19 infection | Time to clinical improvement | |||
| - | Several locations | NCT02735707 | Multifactorial | Recruiting | Phase 4 | COVID-19 infection | All-cause mortality | |||
| Genistein | Inhibition of<break/>both transcription nuclear factor-κB (NF-κB) activation and chemokine-8 secretion | Triggers the ER stress through upregulation of glucose-regulated protein 78 (GRP78) expression | United statwa | NCT04482595 | - | Recruting | Phase 2 | Patients hospitalized for COVID-19 | Change in Diffusing capacity of the lungs for carbon monoxide | [9] |
| Ibrutinib | Inhibition of the Bruton tyrosine kinase | Protection against immune-induced lung injury | Yes (USA) | NCT04375397 | - | Active, not yet Recruiting | Phase 2 | Hospitalised patients with severe pneumonia | Respiratory failure-free survival rate, overall survival | [10] |
| NCT04439006 | - | Recruting | Phase 2 | Patients Requiring Hospitalization | Patients with diminished respiratory failure and death | |||||
| IFN | Jak1 and Tyk2 | Jak1 and Tyk2 | Canada | NCT04354259 | - | Recruiting | Phase 2 | COVID-19 infection | Negative SARS-CoV-2 RNA on nasopharyngeal swab | [10] |
| Jak1 and Tyk2 | Jak1 and Tyk2 | China | NCT04331899 | - | Active, Not yet recruiting | Phase 2 | COVID-19 infection | |||
| IFN beta 1b | Jak1 and Tyk2 | Jak1 and Tyk2 | Hong kong | NCT04647695 | Remdesivir | Recruiting | Phase 2 | high risk of clinical deterioration | Clinical improvement | [10] |
| NCT04494399 | ribavirin | Recruiting | Phase 2 | COVID-19 infection | Reduce hospitalisation | |||||
| IFN-A2B | activate two Jak (Janus kinase) tyrosine kinases (Jak1 and Tyk2) | activate two Jak (Janus kinase) tyrosine kinases (Jak1 and Tyk2) | United States | NCT04349410 | - | Completed | Phase 2/3 | CoVid-19 infection | Improvement in FMTVDM Measurement with nuclear imaging | [10] |
| NCT04379518 | - | Recruiting | Phase 1/2 | Patients with cancer and mild or moderate symptomatic infection | Incidence of adverse events | |||||
| IFN-B1A/B | Jak1 and Tyk2 | Jak1 and Tyk2 | Irnan | NCT04343768 | Hydroxychloroquine, lopinavir, ritonavir | Completed | Phase 2 | COVID-19 infection | Time to clinical improvement | [10] |
| IFN-B1B | Jak1 and Tyk2 | Jak1 and Tyk2 | Hong kong | NCT04350281 | Hydroxychloroquine, lopinavir, ritonavir | Completed | Phase 2 | COVID-19 infection | Time to negative NPS viral load | [10] |
| Jak1 and Tyk2 | Jak1 and Tyk2 | Hong kong | NCT04276688 | Ribavirin, lopinavir, ritonavir | Completed | Phase 2 | COVID-19 infection | Time to negative NPS | ||
| Imatinib | BCR/ABL kinase inhibition | Blockade of cell entry and endosomal trafficking | Yes (France, Spain and USA) | NCT04357613 | - | Not yet Recruitng | Phase 2 | Hospitalised patients | Rate of prevented severe disease worsening | [10] |
| Interferon | Jak1 and Tyk2 | Jak1 and Tyk2 | China | NCT04291729 | Danoprevir, ritonavir | Completed | Phase 4 | COVID-19 infectio | Rate of composite adverse outcome | [10] |
| Lenalidomide | Immunomodulatory agent | substrate specificity of the CRL4CRBN E3 ubiquitin ligase | Spain | NCT04361643 | - | Not yet recruting | Phase 4 | COVID-19 infection | Clinical improvement | [10] |
| Leronlimab | Disruption of the CCL5/RANTES-CCR5 pathway | Immune homeostasis restoration | Yes (USA) | NCT04343651 | - | Active, not recruitng | Phase 2 | Mild/moderate disease | Clinical improvement | [10] |
| NCT04347239 | - | Recruting | Phase 2 | Severe lung disease or critical disease | Overall survival | [10] | ||||
| Masitinib | directly binds to the active site of 3CLpro | Tyrosine kinase inhibitor | Yes (France) | NCT04622865 | Isoquercetin | Recrutiting | Phase 2 | COVID 19 diagnosis | Clinical status of patients at day-15 | [9] |
| Melphalan | anti-inflammatory response | Inhibition of DNA and RNA synthesis by realizing an alkylating peptide | Yes (Russian Federation) | NCT04380376 | - | Recrutiting | Phase 2 | COVID 19 diagnosis | The changes of COVID Ordinal Outcomes Scale | [9] |
| Methotrexate | Immunomodulatory agent | inhibition of folate dependent pathways leading to inhibition of DNA synthesis | France | NCT04481633 | Hydroxychloroquine | Recruiting | Not applicable | COVID-19 infection | Rate of patients with positive anti-COVID19 serology | [10] |
| Nasal IFN-A1B | Jak1 and Tyk2 | Jak1 and Tyk2 | China | NCT04320238 | Anti-thymosin | Recruiting | Phase 3 | Formally serving medical staff in Taihe Hospital | new-onset COVID-19 | [10] |
| Nivolumab | PD-1/PD-L1 pathway blockade | Immune homeostasis restoration | Yes (France and China) | NCT04343144 | - | Not yet recruiting | Phase 2 | Disease requiring O2-support | Time to clinical improvement | [10] |
| NCT04413838 | - | Not yet recruiting | Phase 2 | Obese individuals | Efficacy and safety | [10] | ||||
| NCT04356508 | - | Not yet recruiting | Phase 2 | Clinically stable patients with mild or moderate disease and asymptomatic patients | Viral clearance kinetics | [10] | ||||
| Opaganib | Inhibition of sphingosine kinase-2 | Anti-inflammatory and antiviral properties | Yes (Israel) | NCT04414618 | - | Completed | Phase 2 | Disease requiring O2-support | Measurement of the daily O2 requirements | [10] |
| NCT04467840 | - | Recruting | Phase 2 and 3 | Disease requiring O2-support | Reduce Intubation and mechanical ventilation | |||||
| NCT04435106 | - | Completed | - | severe COVID-19 who required oxygen support via high-flow nasal cannula | Measure the time to weaning from high-flow nasal cannula and | |||||
| Measure the time to breathing ambient | ||||||||||
| NCT04502069 | - | Withdrawn (To be replaced with a randomized placebo-controlled study.) | Phase 1 and 2 | Pneumonia Requiring Oxygen | Time to breathing room air | |||||
| PD-1 blocking antibody | PD-1 | Can prevent the tumor cell from binding PD-1 | Not provided | NCT04268537 | - | Not yet recruiting | Phase 2 | COVID-19 infection | lung injury score | [10] |
| Peg-IFN-L1 | Jak1 and Tyk2 | Jak1 and Tyk2 | United states | NCT04343976 | - | Enrolling by invitation | Phase 2 | COVID-19 infectio | Negative SARS-CoV-2 RNA on nasopharyngeal swab | [10] |
| Peg-IFN-L1A | Jak1 and Tyk2 | Jak1 and Tyk2 | United states | NCT04388709 | - | Withdrawn (Due to the number of competing trials at their site, the study team has closed enrollment and withdrawn this trial.) | Phase 2 | COVID-19 infection | Number of participants with resolution of hypoxia | [10] |
| United states | NCT04344600 | - | Not yet recruiting | Phase 2 | COVID-19 infection | Proportion of participants with no evidence of SARS-CoV-2 infection | ||||
| Pembrolizumab | PD-1/PD-L1 pathway blockade | Immune homeostasis restoration | Yes (Spain) | NCT04335305 | Tocilizumab | Recruiting | Phase 2 | Severe lung disease or critical disease | Percentage of patients with normalisation of SpO2 ≥96% on room air | [10] |
| Plitidepsin | Blockade of eEF1A | Interference with the viral cycle | Yes (Spain) | NCT04382066 | - | Completed | Phase 1 | Hospitalised patients | Frequency of occurrence of Grade 3 or higher AEs | [10] |
| Selinexor | Blockade of nucleocytoplasmic transport | Antiviral and anti-inflammatory properties | Yes (USA, France, Austria, Spain and United Kingdom) | NCT04355676 | - | Withdrawn (No participants enrolled) | Phase 2 | Hospitalised patients with moderate or severe disease | Percentage of participants with at least a two-point improvement in the ordinal scale | [10] |
| NCT04349098 | - | Completed | Phase 2 | Hospitalised patients with severe disease | Improvement | |||||
| NCT04534725 | - | Recruiting | Phase 3 | received cancer related treatment | COVID-19 Prevention and Treatment in Cancer | |||||
| SFX-01 | up-regulates the Nrf2 pathway | Up-regulates the Nrf2 pathway | UK + Evgen Pharma | - | - | Enrolment begins in July (results are expected in 2021) | Phase 2/3 | - | Efficacy at treating ARDS | [11] |
| Siltuximab | Interleukin-6 | Interleukin-6 | Spain | NCT04329650 | - | Recruiting | Phase 2 | Hospitalized patient | Proportion of patients requiring ICU admission at any time | [11] |
| Italy | NCT04322188 | - | Completed | - | COVID-19 infection | mortality in siltuximab treated patients | ||||
| Belgium | NCT04330638 | Anakinra | Active, not recruting | Phase 3 | COVID-19 infection | Time to Clinical Improvement | ||||
| Saudi Arabia | NCT04486521 | tocilizumab | Recruiting | - | COVID-19 infection | Ventilator-Free Days | ||||
| Tamoxifen | Decreased the PGE2 production | Compete with 17β-estradiol (E2) at the receptor site | Egypt | NCT04568096 | - | Not yet recruting | Phase 2 | Adult SARI patients with COVID-19 infection | Lung injury score | [9] |
| Tetrandrine | Ability to block the two-pore channel 2 (TPC2) | Checkpoint inhibitor of the cell cycle | China | NCT04308317 | - | Enrolling by invitation | Phase 4 | COVID-19 infection | Survival rate | [10] |
| Thalidomide | inhibition of inflammatory cytokine production | Inhibit the production of interleukin (IL)-6, | China | NCT04273529 | - | Not yet recruiting | Phase 2 | COVID-19 infection | Time to Clinical recovery | [10] |
| Toremifene | Interaction with coronavirus proteins | Inhibition of viral membranes fusion with host cell endosomes | ? (Not provided) | NCT04531748 | Melatonin | Withdrawn (Funding) | Phase 2 | - | Clinical improvement | [10] |
| Zanubrutinib | Inhibition of the Bruton tyrosine kinase | Protection against immune, lethal and sepsis-induced pulmonary injuries | Yes (USA) | NCT04382586 | - | Completed | Phase 2 | Disease requiring O2-support | Respiratory failure-free survival rate | [10] |
| Zotatifin | Blockade of eIF4A | Inhibition of protein biogenesis | No | NCT04632381 | - | Not yet recruiting | Phase 1 | - | - | [10] |
References
- Zheng, J. SARS-CoV-2: An Emerging Coronavirus that Causes a Global Threat. Int J Biol Sci. 2020, 16, 1678–1685, Published 15 March 2020. [Google Scholar] [CrossRef] [PubMed]
- Drinka, P.J. Influenza vaccination and antiviral therapy: Is there a role for concurrent administration in the institutionalised elderly? Drugs Aging 2003, 20, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Deb, P.; Molla, M.A.; Saif-Ur-Rahman, K. An update to monoclonal antibody as therapeutic option against COVID-19. Biosaf. Health 2021. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Hernandez, J.J.; Pryszlak, M.; Smith, L.; Yanchus, C.; Kurji, N.; Shahani, V.M.; Molinski, S.V. Giving Drugs a Second Chance: Overcoming Regulatory and Financial Hurdles in Repurposing Approved Drugs As Cancer Therapeutics. Front Oncol. 2017, 7, 273. [Google Scholar] [CrossRef]
- Sultana, J.; Crisafulli, S.; Gabbay, F.; Lynn, E.; Shakir, S.; Trifirò, G. Challenges for drug repurposing in the COVID-19 pandemic era. Front. Pharmacol. 2020, 11, 1657. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Gupta, V. Utilizing drug repurposing against COVID-19—Efficacy, limitations, and challenges. Life Sci. 2020, 259, 118275. [Google Scholar] [CrossRef] [PubMed]
- Saini, K.S.; Lanza, C.; Romano, M.; De Azambuja, E.; Cortes, J.; Heras, B.D.L.; De Castro, J.; Saini, M.L.; Loibl, S.; Curigliano, G.; et al. Repurposing anticancer drugs for COVID-19-induced inflammation, immune dysfunction, and coagulopathy. Br. J. Cancer 2020, 123, 694–697. [Google Scholar] [CrossRef]
- National Center for Advancing Translational Sciences|OpenData Portal. Available online: https://opendata.ncats.nih.gov/covid19/databrowser (accessed on 6 February 2021).
- ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/home (accessed on 6 February 2021).
- European Pharmaceutical Review|News. Available online: https://www.europeanpharmaceuticalreview.com/ (accessed on 6 February 2021).
- Richman, D.D.; Nathanson, N. Antiviral Therapy. Viral Pathog. 2016, 271–287. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23. [Google Scholar] [CrossRef]
- Astuti, I.; Ysrafil. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab. Syndr. 2020, 14, 407–412. [Google Scholar] [CrossRef]
- Romano, M.; Ruggiero, A.; Squeglia, F.; Maga, G.; Berisio, R.A. Structural View of SARS-CoV-2 RNA Replication Machinery: RNA Synthesis, Proofreading and Final Capping. Cells 2020, 9, 1267. [Google Scholar] [CrossRef]
- Zingaropoli, M.A.; Perri, V.; Pasculli, P.; Dezza, F.C.; Nijhawan, P.; Savelloni, G.; La Torre, G.; D’Agostino, C.; Mengoni, F.; Lichtner, M.; et al. Major reduction of NKT cells in patients with severe COVID-19 pneumonia. Clin. Immunol. 2021, 222, 108630. [Google Scholar] [CrossRef]
- Jin, Y.; Ji, W.; Yang, H.; Chen, S.; Zhang, W.; Duan, G. Endothelial activation and dysfunction in COVID-19: From basic mechanisms to potential therapeutic approaches. Signal. Transduct. Target. Ther. 2020, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Boechat, J.L.; Chora, I.; Morais, A.; Delgado, L. The immune response to SARS-CoV-2 and COVID-19 immunopathology–current perspectives. Pulmonology 2021. [Google Scholar] [CrossRef] [PubMed]
- Minn, A.J. Interferons and the immunogenic effects of cancer therapy. Trends Immunol. 2015, 36, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.C.; Young, H.A. Interferons: Success in anti-viral immunotherapy. Cytokine Growth Factor Rev. 2014, 25, 369–376. [Google Scholar] [CrossRef]
- Hung, I.F.-N.; Lung, K.-C.; Tso, E.Y.-K.; Liu, R.; Chung, T.W.-H.; Chu, M.-Y.; Ng, Y.-Y.; Lo, J.; Chan, J.; Tam, A.R.; et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial. Lancet 2020, 395, 1695–1704. [Google Scholar] [CrossRef]
- Abdolvahab, M.H.; Moradi-Kalbolandi, S.; Zarei, M.; Bose, D.; Majidzadeh, -A.K.; Farahmand, L. Potential role of interferons in treating COVID-19 patients. Int. Immunopharmacol. 2021, 90, 107171. [Google Scholar] [CrossRef]
- Costela-Ruiz, V.J.; Illescas-Montes, R.; Puerta-Puerta, J.M.; Ruiz, C.; Melguizo-Rodríguez, L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020, 54, 62–75. [Google Scholar] [CrossRef]
- Lacroix, M.; Rousseau, F.; Guilhot, F.; Malinge, P.; Magistrelli, G.; Herren, S.; Jones, S.A.; Jones, G.W.; Scheller, J.; Lissilaa, R.; et al. Novel Insights into Interleukin 6 (IL-6) Cis- and Trans-signaling Pathways by Differentially Manipulating the Assembly of the IL-6 Signaling Complex. J. Biol. Chem. 2020, 290, 26943–26953. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Stewart, I.; Fabbri, L.; Moss, S.; Robinson, K.; Smyth, A.R.; Jenkins, G. Systematic review and meta-analysis of anakinra, sarilumab, siltuximab and tocilizumab for COVID-19. Thorax 2021. [Google Scholar] [CrossRef]
- Sullivan, R.J.; Johnson, D.B.; Rini, B.; Neilan, T.G.; Lovly, C.M.; Moslehi, J.J.; Reynolds, K.L. COVID-19 and immune checkpoint inhibitors: Initial considerations. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Chen, Z.; Wherry, E.J. T cell responses in patients with COVID-19. Nat. Rev. Immunol. 2020, 20, 529–536. [Google Scholar] [CrossRef]
- Saito, N.; Yoshida, K.; Okamoto, M.; Sasaki, J.; Kuroda, C.; Ishida, H.; Ueda, K.; Ideta, H.; Kamanaka, T.; Sobajima, A.; et al. Anti-PD-1 antibody decreases tumour-infiltrating regulatory T cells. BMC Cancer 2019, 20. [Google Scholar] [CrossRef]
- Mollica, V.; Rizzo, A.; Massari, F. The pivotal role of TMPRSS2 in coronavirus disease 2019 and prostate cancer. Future Oncol. 2020, 16, 2029–2033. [Google Scholar] [CrossRef]
- Chakravarty, D.; Nair, S.S.; Hammouda, N.; Ratnani, P.; Gharib, Y.; Wagaskar, V.; Mohamed, N.; Lundon, D.; Dovey, Z.; Kyprianou, N.; et al. Sex differences in SARS-CoV-2 infection rates and the potential link to prostate cancer. Commun. Biol. 2020, 3, 1–12. [Google Scholar] [CrossRef]
- Martin, W.R.; Cheng, F. Repurposing of FDA-Approved Toremifene to Treat COVID-19 by Blocking the Spike Glycoprotein and NSP14 of SARS-CoV-2. J. Proteome Res. 2020, 19, 4670–4677. [Google Scholar] [CrossRef]
- Cheng, F.; Rao, S.; Mehra, R. COVID-19 treatment: Combining anti-inflammatory and antiviral therapeutics using a network-based approach. Cleve Clin. J. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- White, K.M.; Rosales, R.; Yildiz, S.; Kehrer, T.; Miorin, L.; Moreno, E.; Jangra, S.; Uccellini, M.B.; Rathnasinghe, R.; Coughlan, L.; et al. Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science 2021, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Nebigil, C.G.; Moog, C.; Vagner, S.; Benkirane-Jessel, N.; Smith, D.R.; Désaubry, L. Flavaglines as natural products targeting eIF4A and prohibitins: From traditional Chinese medicine to antiviral activity against coronaviruses. Eur. J. Med. Chem. 2020, 203, 112653. [Google Scholar] [CrossRef] [PubMed]
- Sriskantharajah, S.; Hamblin, N.; Worsley, S.; Calver, A.R.; Hessel, E.M.; Amour, A. Targeting phosphoinositide 3-kinase δ for the treatment of respiratory diseases. Ann. N. Y. Acad. Sci. 2013, 1280, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Pioggia, G.; Negrini, S. Vitamin D and Covid-19: An update on evidence and potential therapeutic implications. Clin. Mol. Allergy 2020, 18, 1–8. [Google Scholar] [CrossRef]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Repurposed Drugs with Broad-Spectrum Antiviral Activity, by BioRender.com. Available online: https://app.biorender.com/biorender-templates (accessed on 3 January 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).