Exenatide Microspheres for Monthly Controlled-Release Aided by Magnesium Hydroxide
Abstract
1. Introduction
2. Materials and Methods
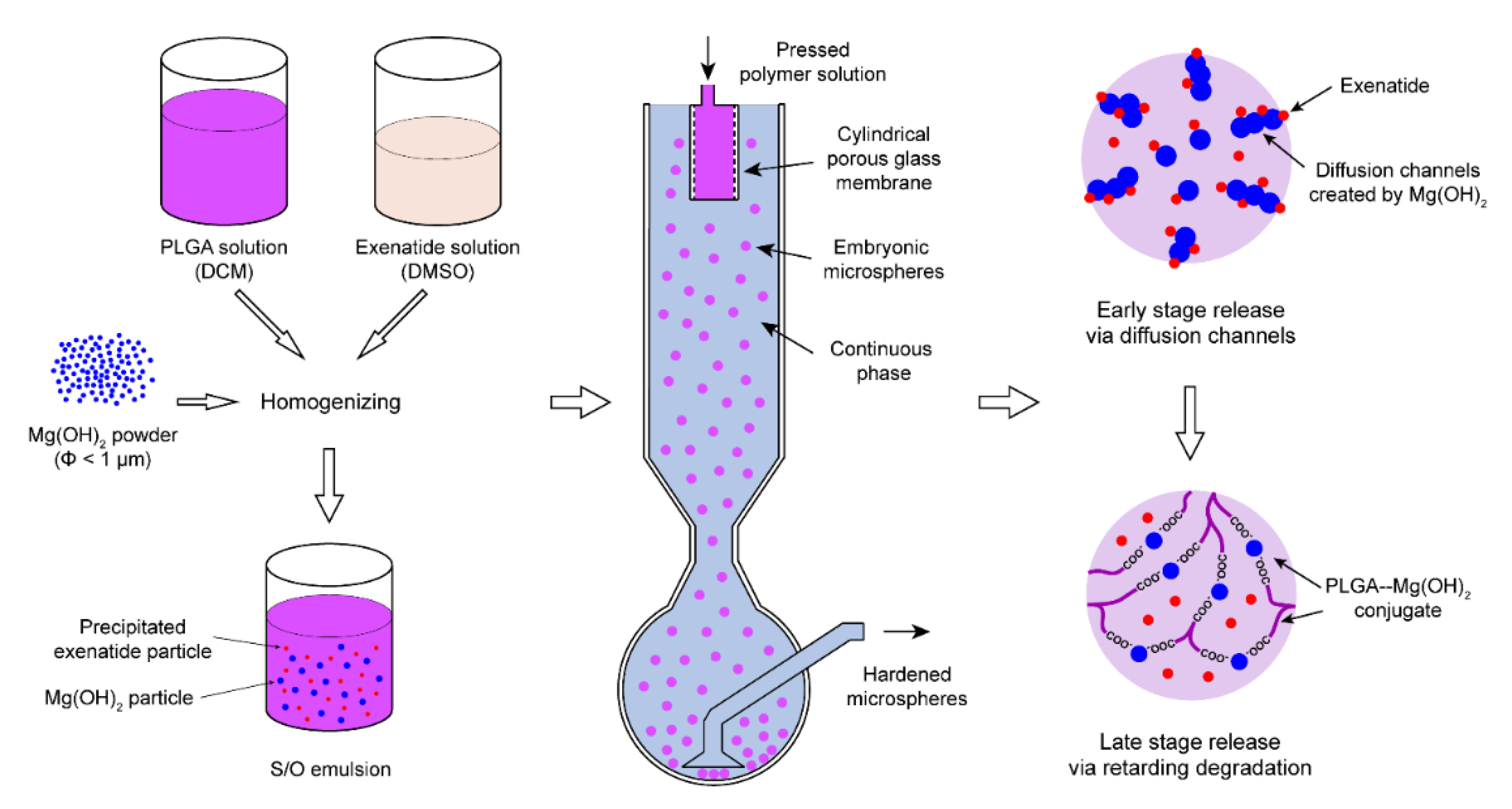
2.1. Preparation of Exenatide Microsphere
2.2. Morphological Characterization of the Microspheres
2.3. Loading Capacity and Encapsulation Efficiency of Exenatide
2.4. In Vitro Release of Exenatide of Various Microsphere Formulations
2.5. Recovery Assay of Magnesium Hydroxide in Degrading Microspheres
2.6. Morphological and Structural Characterization of Magnesium Hydroxide-Loaded Microspheres
2.7. Experimental Animals
2.8. Pharmacokinetic Profiles of Exenatide Microspheres in Rhesus Monkeys
2.9. Efficacy Study in C57 Mice
2.10. Statistical Analysis
3. Results and Discussion
3.1. Morphological and Physical-Chemical Characteristics of the Microspheres
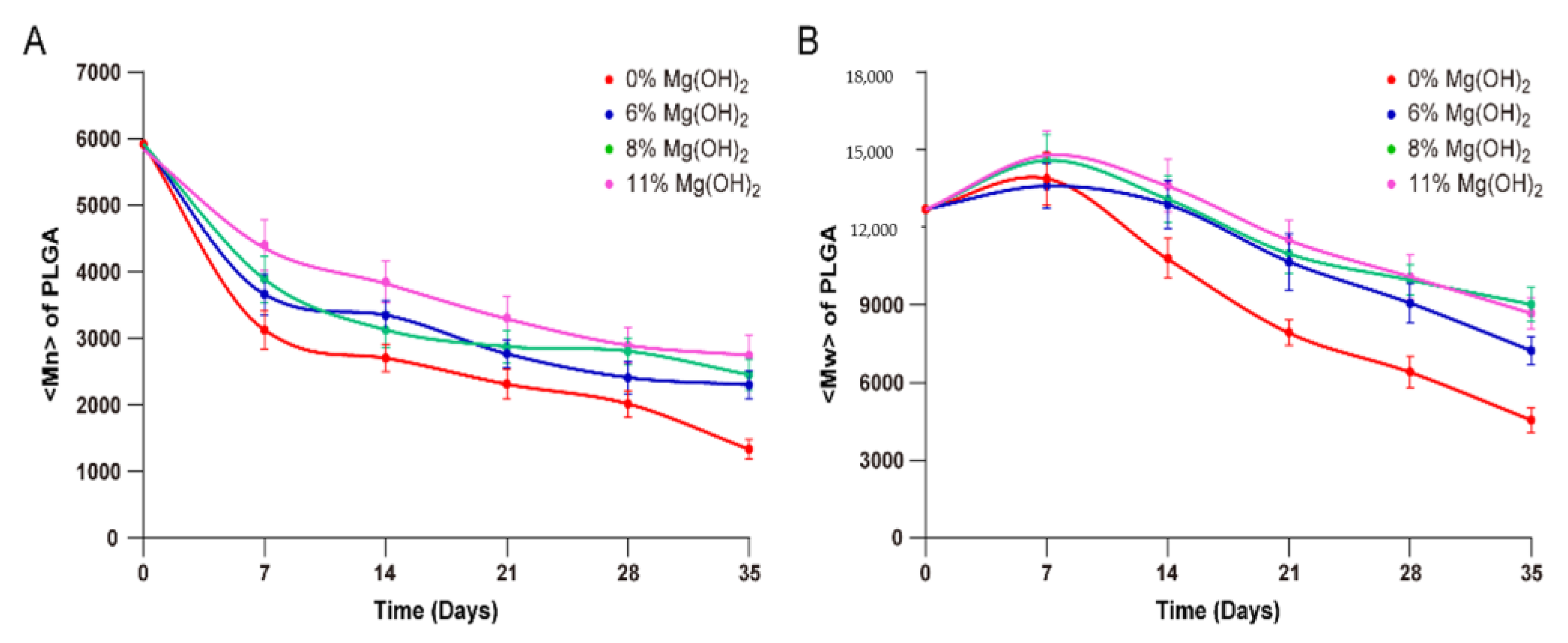
3.2. In Vitro Release Profiles of Exenatide from PLGA Microspheres of Various Formulations
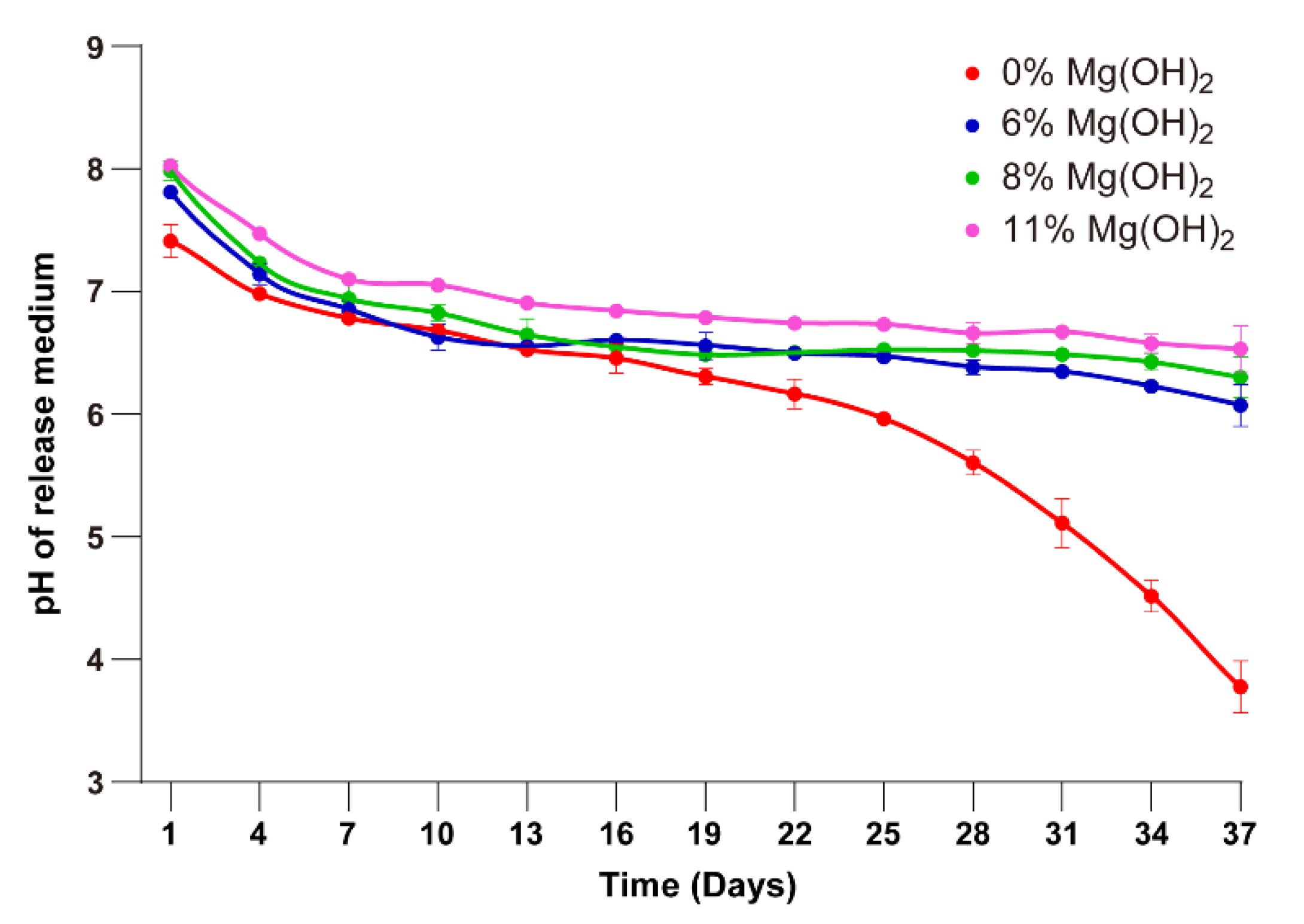
3.3. Solubilization of Magnesium Hydroxide in Degrading Microspheres
3.4. Mechanistic Characterization of Magnesium Hydroxide Loaded Exenatide Microspheres
3.5. Morphological and Structural Characterization of Magnesium Hydroxide-Loaded Exenatide Microspheres
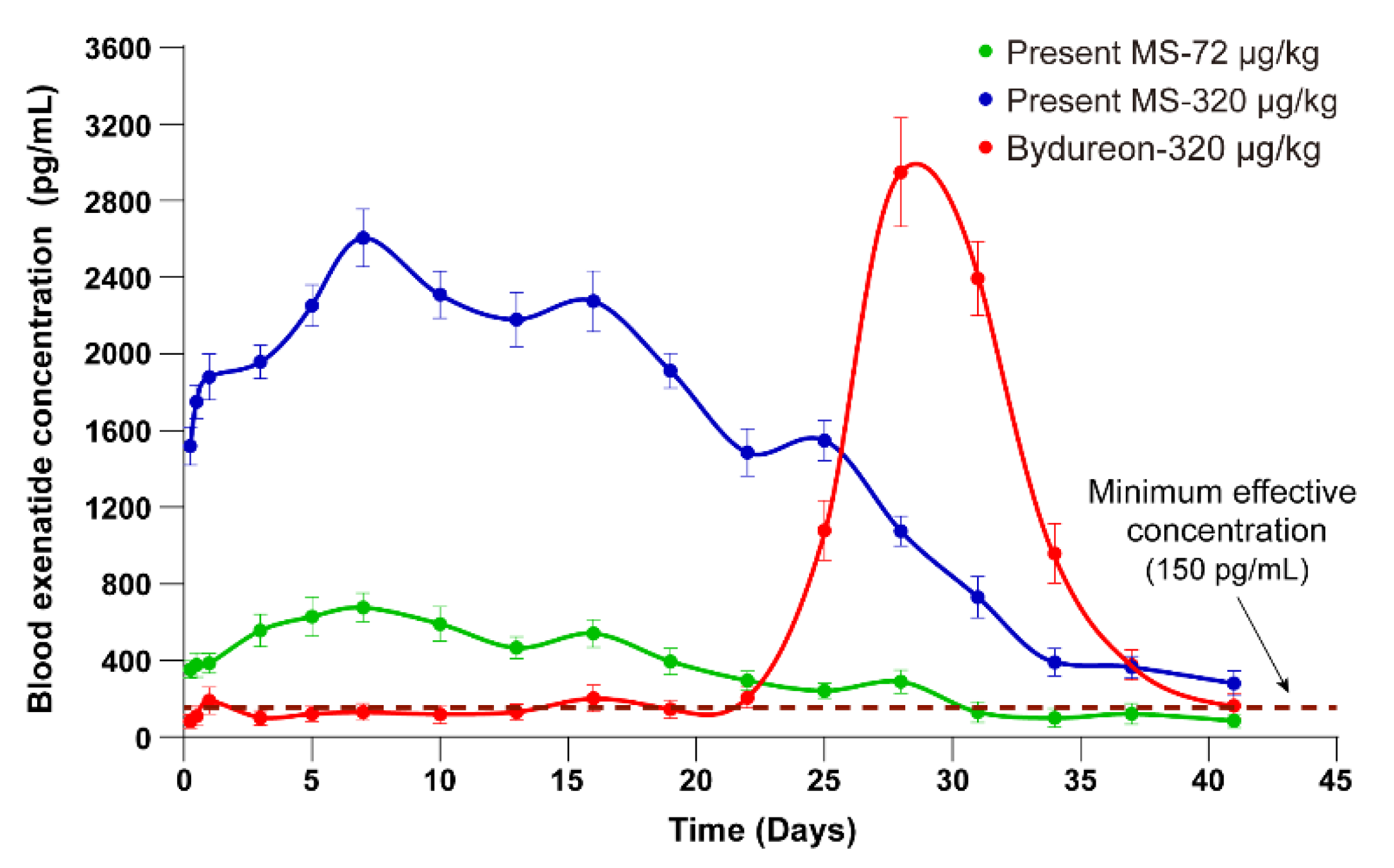
3.6. Pharmacokinetic Profiles of Exenatide in Rhesus Monkeys Injected with Sustained-Release Microspheres
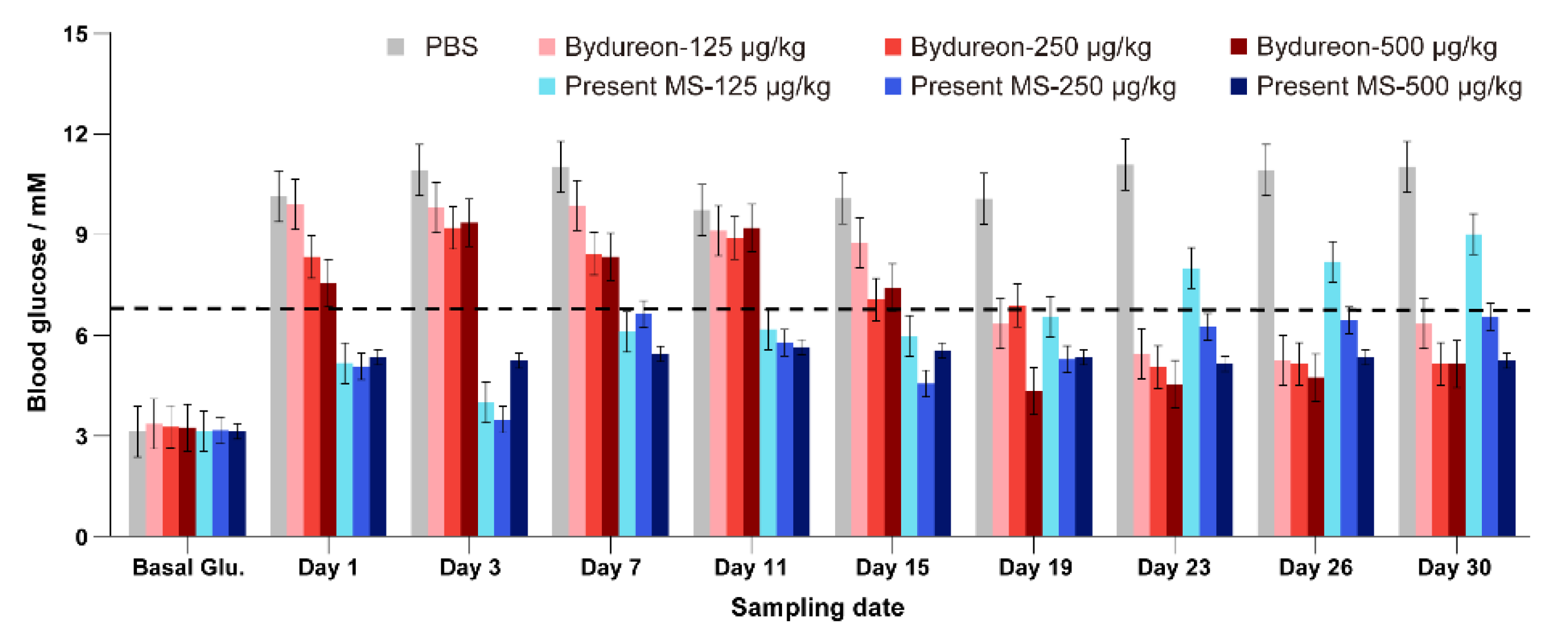
3.7. Hypoglycemic Efficacy of Exenatide Microspheres Examined in C57 Mice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Freiberg, S.; Zhu, X.X. Polymer microspheres for controlled drug release. Int. J. Pharm. 2004, 282, 1–18. [Google Scholar] [CrossRef]
- Kawaguchi, H. Functional polymer microspheres. Prog. Polym. Sci. 2000, 25, 1171–1210. [Google Scholar] [CrossRef]
- Aroda, V.R. A review of GLP-1 receptor agonists: Evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes. Metab. 2018, 20 (Suppl. 1), 22–33. [Google Scholar] [CrossRef]
- Meier, J.J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2012, 8, 728–742. [Google Scholar] [CrossRef]
- Smith, N.K.; Hackett, T.A.; Galli, A.; Flynn, C.R. GLP-1: Molecular mechanisms and outcomes of a complex signaling system. Neurochem. Int. 2019, 128, 94–105. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Rehfeld, J.F.; Knop, F.K.; Asmar, M. Gastrin secretion in normal subjects and diabetes patients is inhibited by glucagon-like peptide 1: A role in the gastric side effects of GLP-1-derived drugs? Scand. J. Gastroenterol. 2019, 54, 1448–1451. [Google Scholar] [CrossRef]
- Knop, F.K.; Brønden, A.; Vilsbøll, T. Exenatide: Pharmacokinetics, clinical use, and future directions. Expert Opin. Pharmacother. 2017, 18, 555–571. [Google Scholar] [CrossRef]
- Chudleigh, R.A.; Platts, J.; Bain, S.C. Comparative Effectiveness of Long-Acting GLP-1 Receptor Agonists in Type 2 Diabetes: A Short Review on the Emerging Data. Diabetes Metab. Syndr. Obes. 2020, 13, 433–438. [Google Scholar] [CrossRef]
- Ruan, S.; Gu, Y.; Liu, B.; Gao, H.; Hu, X.; Hao, H.; Jin, L.; Cai, T. Long-Acting Release Microspheres Containing Novel GLP-1 Analog as an Antidiabetic System. Mol. Pharm. 2018, 15, 2857–2869. [Google Scholar] [CrossRef]
- Li, Y.; Vaughan, K.L.; Tweedie, D.; Jung, J.; Kim, H.K.; Choi, H.I.; Kim, D.S.; Mattison, J.A.; Greig, N.H. Pharmacokinetics of Exenatide in nonhuman primates following its administration in the form of sustained-release PT320 and Bydureon. Sci. Rep. 2019, 9, 17208. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.H. The role of GLP-1 mimetics and basal insulin analogues in type 2 diabetes mellitus: Guidance from studies of liraglutide. Diabetes Obes. Metab. 2012, 14, 304–314. [Google Scholar] [CrossRef]
- Painter, N.A.; Morello, C.M.; Singh, R.F.; McBane, S.E. An evidence-based and practical approach to using BydureonTM in patients with type 2 diabetes. J. Am. Board FAM Med. 2013, 26, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.M.Z.; Patel, U.; Ahmed, I. Development of microspheres for biomedical applications: A review. Prog. Biomater. 2015, 4, 1–19. [Google Scholar] [CrossRef]
- Gu, B.; Sun, X.; Papadimitrakopoulos, F.; Burgess, D.J. Seeing is believing, PLGA microsphere degradation revealed in PLGA microsphere/PVA hydrogel composites. J. Control. Release 2016, 228, 170–178. [Google Scholar] [CrossRef]
- Zolnik, B.S.; Burgess, D.J. Effect of acidic pH on PLGA microsphere degradation and release. J. Control. Release 2007, 122, 338–344. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, Y.; Yuan, W.; Wu, F.; Su, J.; Jin, T. Effect of bases with different solubility on the release behavior of risperidone loaded PLGA microspheres. Colloids Surf B Biointerfaces 2011, 86, 206–211. [Google Scholar] [CrossRef]
- Zhu, G.; Mallery, S.R.; Schwendeman, S.P. Stabilization of proteins encapsulated in injectable poly (lactide-co-glycolide). Nat. Biotechnol. 2000, 18, 52–57. [Google Scholar] [CrossRef]
- Jin, T. Process for Producing Polymeric Microspheres 2019. U.S. Patent Application No. 1,047,101,3B2, 12 November 2019. [Google Scholar]
- Diana, J.N.; Tao, Y.; Du, Q.; Wang, M.; Kumar, C.U.; Wu, F.; Jin, T. PLGA Microspheres of hGH of Preserved Native State Prepared Using a Self-Regulated Process. Pharmaceutics 2020, 12, 683. [Google Scholar] [CrossRef]
- Sadir, S.; Tabassum, S.; Emad, S.; Liaquat, L.; Batool, Z.; Madiha, S.; Shehzad, S.; Sajid, I.; Haider, S. Neurobehavioral and biochemical effects of magnesium chloride (MgCl2), magnesium sulphate (MgSO4) and magnesium-L-threonate (MgT) supplementation in rats: A dose dependent comparative study. Pak. J. Pharm. Sci. 2019, 32 (Suppl. 1), 277–283. [Google Scholar]
- Hayashi, S.I.; Kawamura, H.; Usui, S.; Tominaga, T. Influence of magnesium chloride on the dose-response of polyacrylamide-type gel dosimeters. Radiol. Phys. Technol. 2018, 11, 375–381. [Google Scholar] [CrossRef]
- Kothare, P.A.; Linnebjerg, H.; Isaka, Y.; Uenaka, K.; Yamamura, A.; Yeo, K.P.; de la Peña, A.; Teng, C.H.; Mace, K.; Fineman, M.; et al. Pharmacokinetics, pharmacodynamics, tolerability, and safety of exenatide in Japanese patients with type 2 diabetes mellitus. J. Clin. Pharmacol. 2008, 48, 1389–1399. [Google Scholar] [CrossRef]
- Wysham, C.; Grimm, M.; Chen, S. Once weekly exenatide: Efficacy, tolerability and place in therapy. Diabetes Obes. Metab. 2013, 15, 871–881. [Google Scholar] [CrossRef]



| Formulation | Mg(OH)2 Content (%) | Loading Capacity (%) | Encapsulation Efficiency (%) |
|---|---|---|---|
| Extru-settl-0 | 0 | 3.13 ± 0.03 | 97.01 ± 1.77 |
| Extru-settl-6 | 6 | 2.93 ± 0.03 | 96.63 ± 1.43 |
| Extru-settl-8 | 8 | 2.81 ± 0.01 | 95.49 ± 1.15 |
| Extru-settl-11 | 11 | 2.71 ± 0.01 | 94.92 ± 0.65 |
| W/O/W | 0 | 1.92 ± 0.03 | 59.04 ± 0.31 |
| Incubation Time | 5% Mg(OH)2 | 10% Mg(OH)2 | 15% Mg(OH)2 |
|---|---|---|---|
| 14 days | 0% | 8.9 ± 1.4% | 23.5 ± 3.4% |
| 21 days | 0% | 0% | 9.7 ± 1.9% |
| 28 days | 0% | 0% | 0% |
| Formulation | Mg(OH)2 Load (%) | Mg2+ Content at Day 0 (%) | Mg2+ Content at Day 28 (%) | Fraction of Regained Mg2+ at Day 28 (%) |
|---|---|---|---|---|
| Extru-settl-6 | 6 | 2.29 ± 0.13 | 0.65 ± 0.03 | 28.27 ± 1.40 |
| Extru-settl-8 | 8 | 3.14 ± 0.12 | 0.96 ± 0.05 | 30.64 ± 1.70 |
| Extru-settl-11 | 11 | 4.29 ± 0.22 | 1.33 ± 0.06 | 31.06 ± 1.50 |
| Parameters | Value (Present MS) | Value (BydureonTM) |
|---|---|---|
| CMAX (pg/mL) | 2605 | 3020 |
| DI | 3.47 | 27.71 |
| AUC (pg/d/mL) | 58,658 | 24,097 |
| AUC/dose | 183.3 | 75.3 |
| CMAX (pg/mL) | 2605 | 3020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, Y.; Hu, Z.; Chen, J.; Qin, Y.; Wu, F.; Jin, T. Exenatide Microspheres for Monthly Controlled-Release Aided by Magnesium Hydroxide. Pharmaceutics 2021, 13, 816. https://doi.org/10.3390/pharmaceutics13060816
Ge Y, Hu Z, Chen J, Qin Y, Wu F, Jin T. Exenatide Microspheres for Monthly Controlled-Release Aided by Magnesium Hydroxide. Pharmaceutics. 2021; 13(6):816. https://doi.org/10.3390/pharmaceutics13060816
Chicago/Turabian StyleGe, Yuxuan, Zhenhua Hu, Jili Chen, Yujie Qin, Fei Wu, and Tuo Jin. 2021. "Exenatide Microspheres for Monthly Controlled-Release Aided by Magnesium Hydroxide" Pharmaceutics 13, no. 6: 816. https://doi.org/10.3390/pharmaceutics13060816
APA StyleGe, Y., Hu, Z., Chen, J., Qin, Y., Wu, F., & Jin, T. (2021). Exenatide Microspheres for Monthly Controlled-Release Aided by Magnesium Hydroxide. Pharmaceutics, 13(6), 816. https://doi.org/10.3390/pharmaceutics13060816





