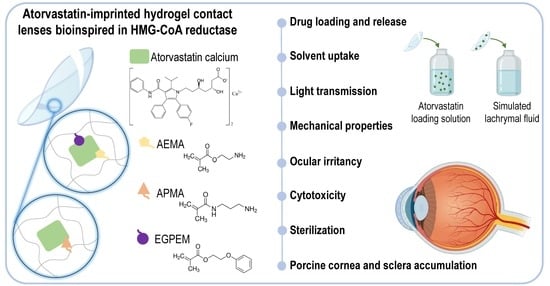
Atorvastatin-Eluting Contact Lenses: Effects of Molecular Imprinting and Sterilization on Drug Loading and Release
Abstract
1. Introduction
2. Materials and Methods
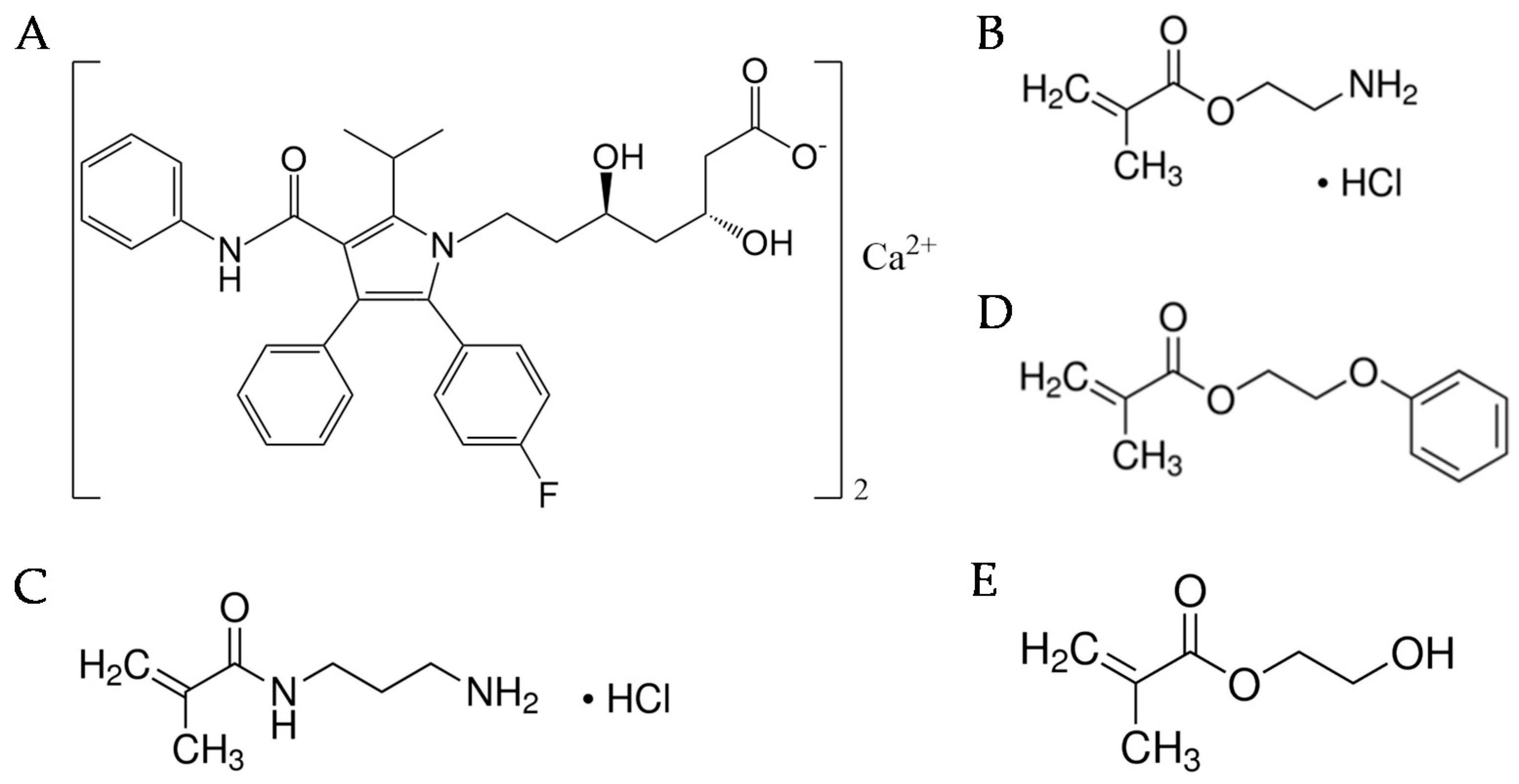
2.1. Materials
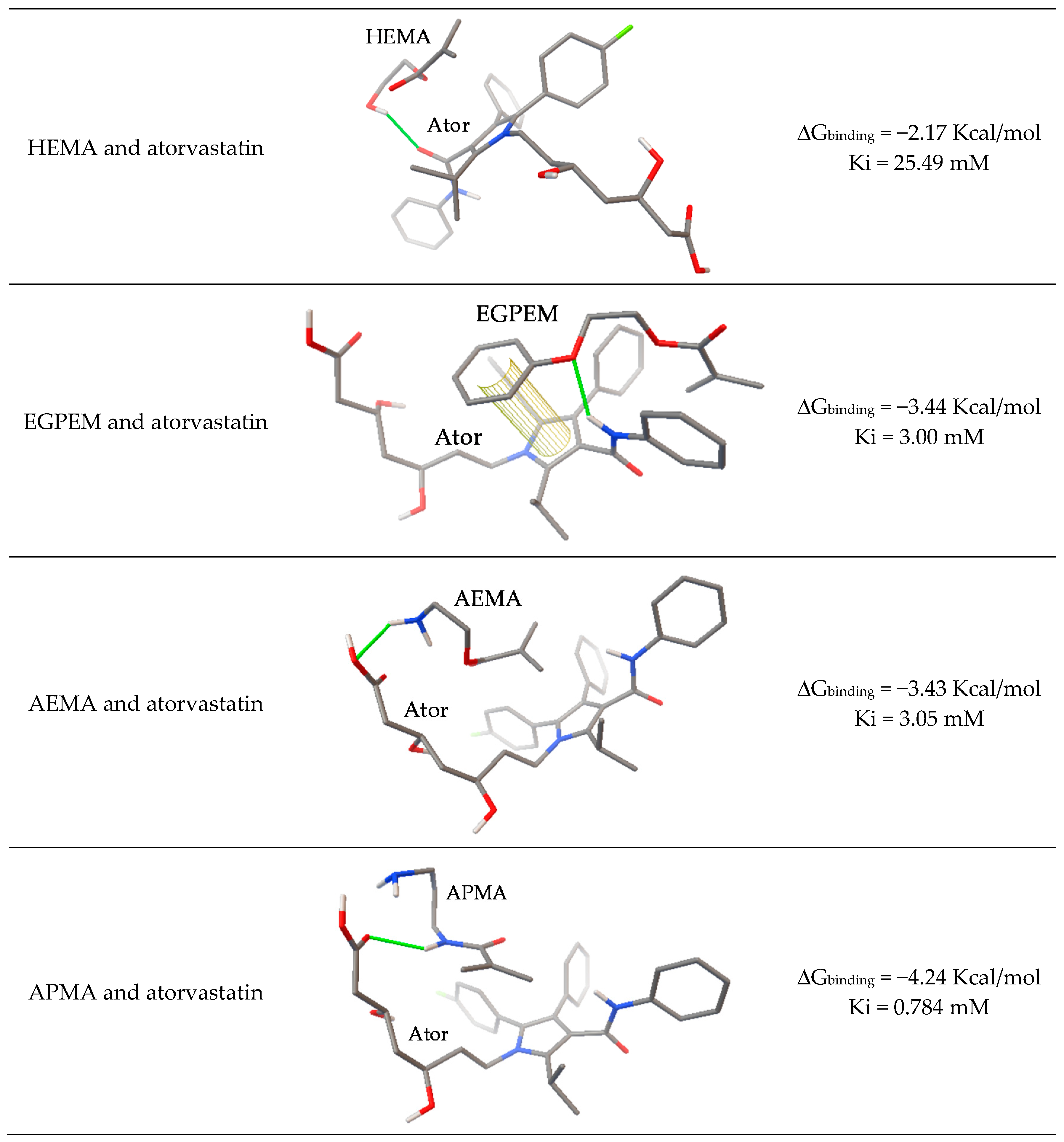
2.2. Computational Modeling
2.3. Hydrogel Preparation
2.4. Hydrogel Characterization
2.5. Atorvastatin Loading and Release
2.6. HET–CAM Test
2.7. Sterilization
2.8. FTIR–ATR Analysis
2.9. Cytocompatibility Studies
2.10. Cornea and Sclera Permeability and Accumulation
2.11. Light Stability of Atorvastatin Calcium Solution
2.12. Statistical Analysis
3. Results and Discussion
3.1. Hydrogels Synthesis and Conditioning
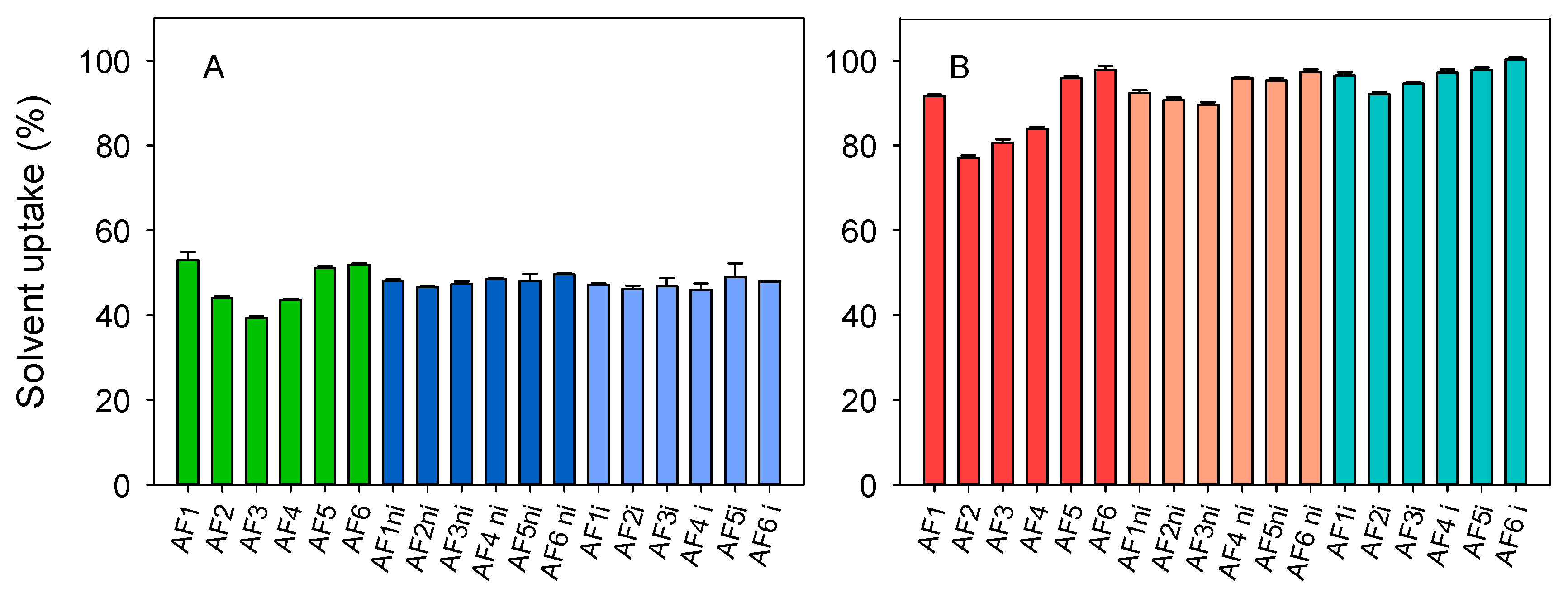
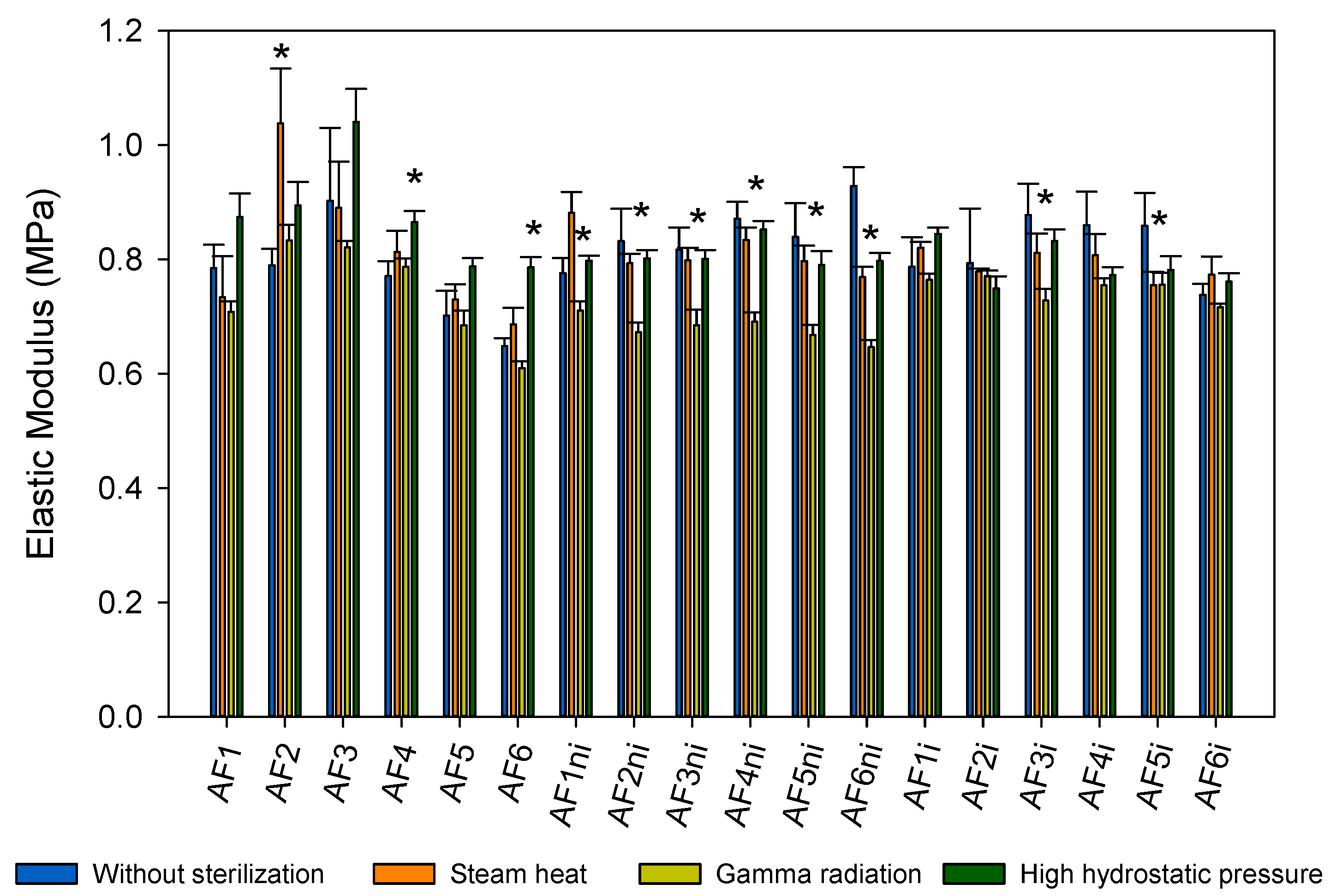
3.2. Hydrogels Characterization
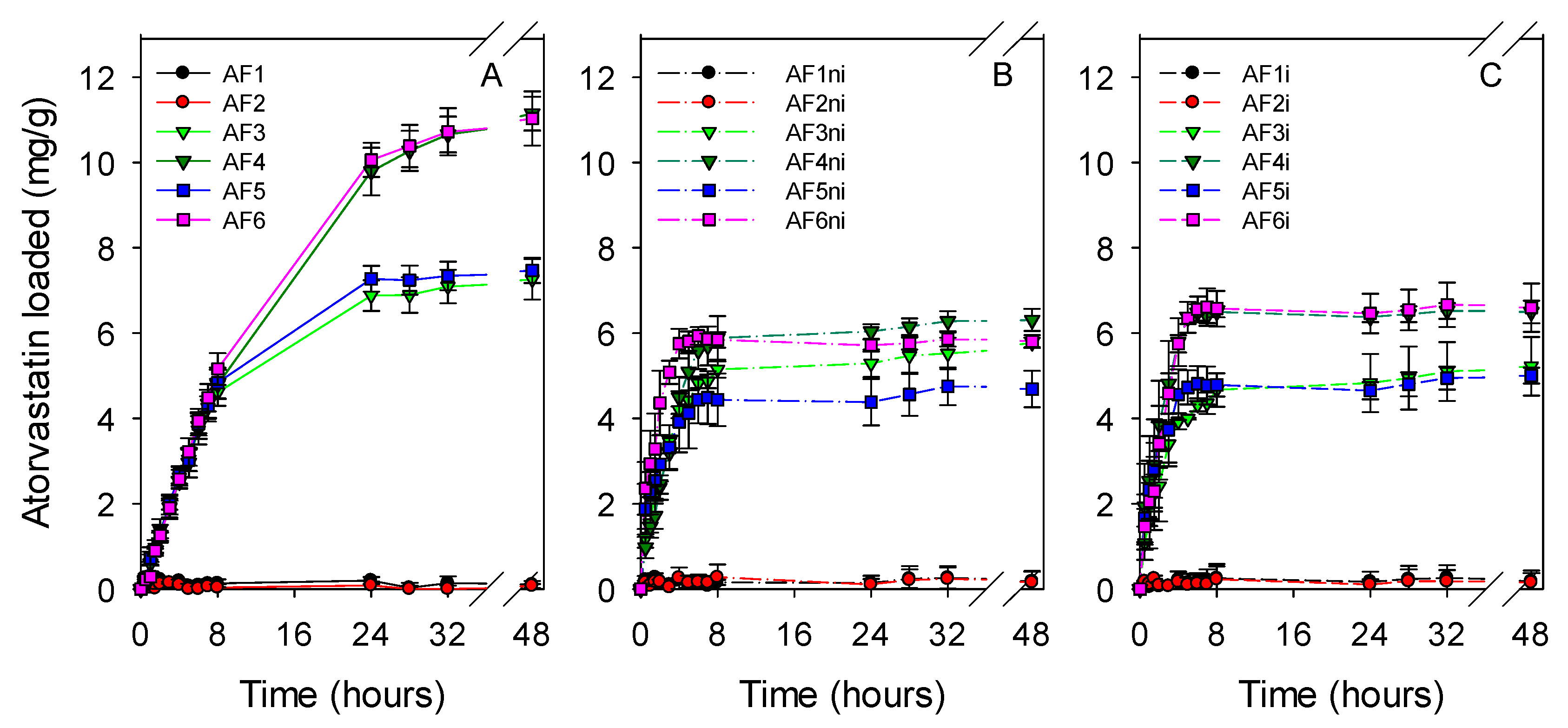
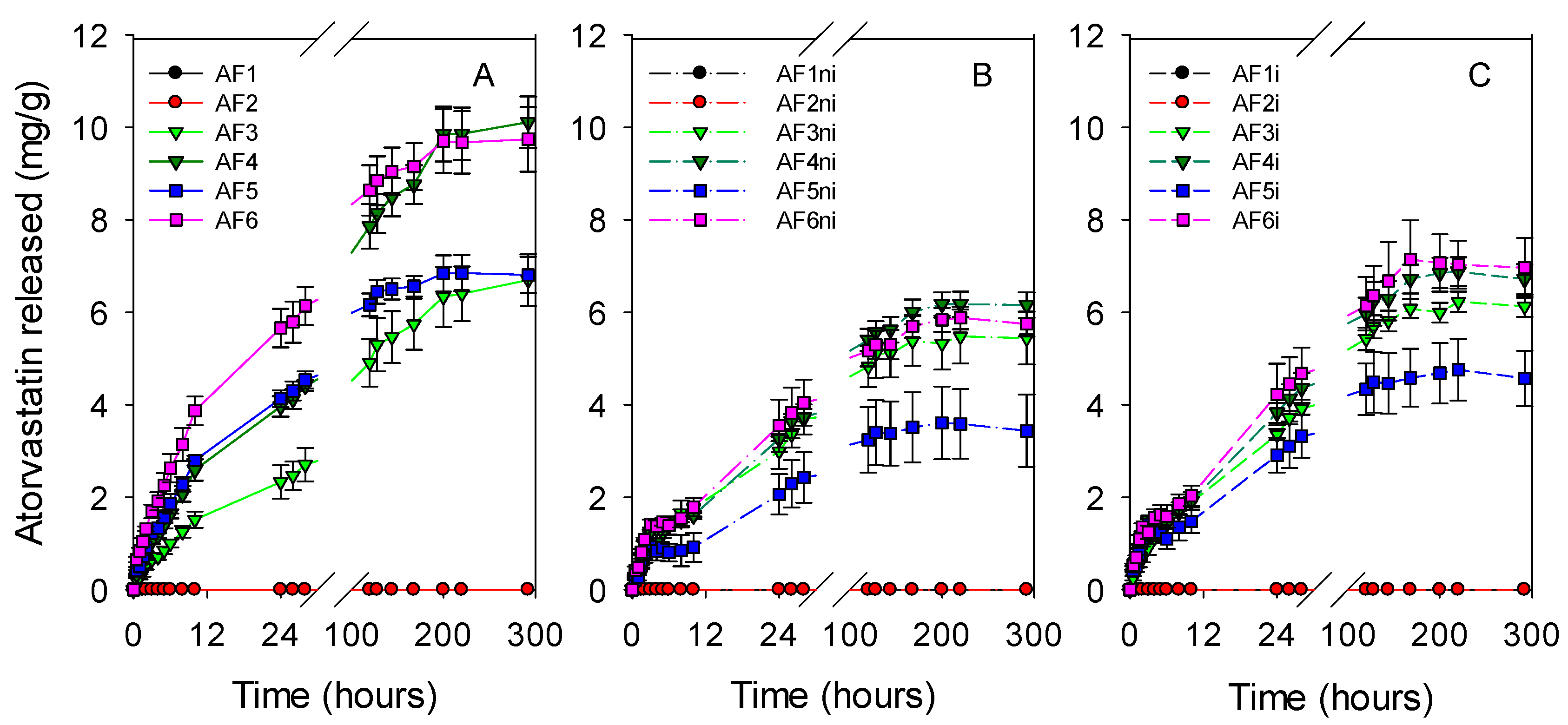
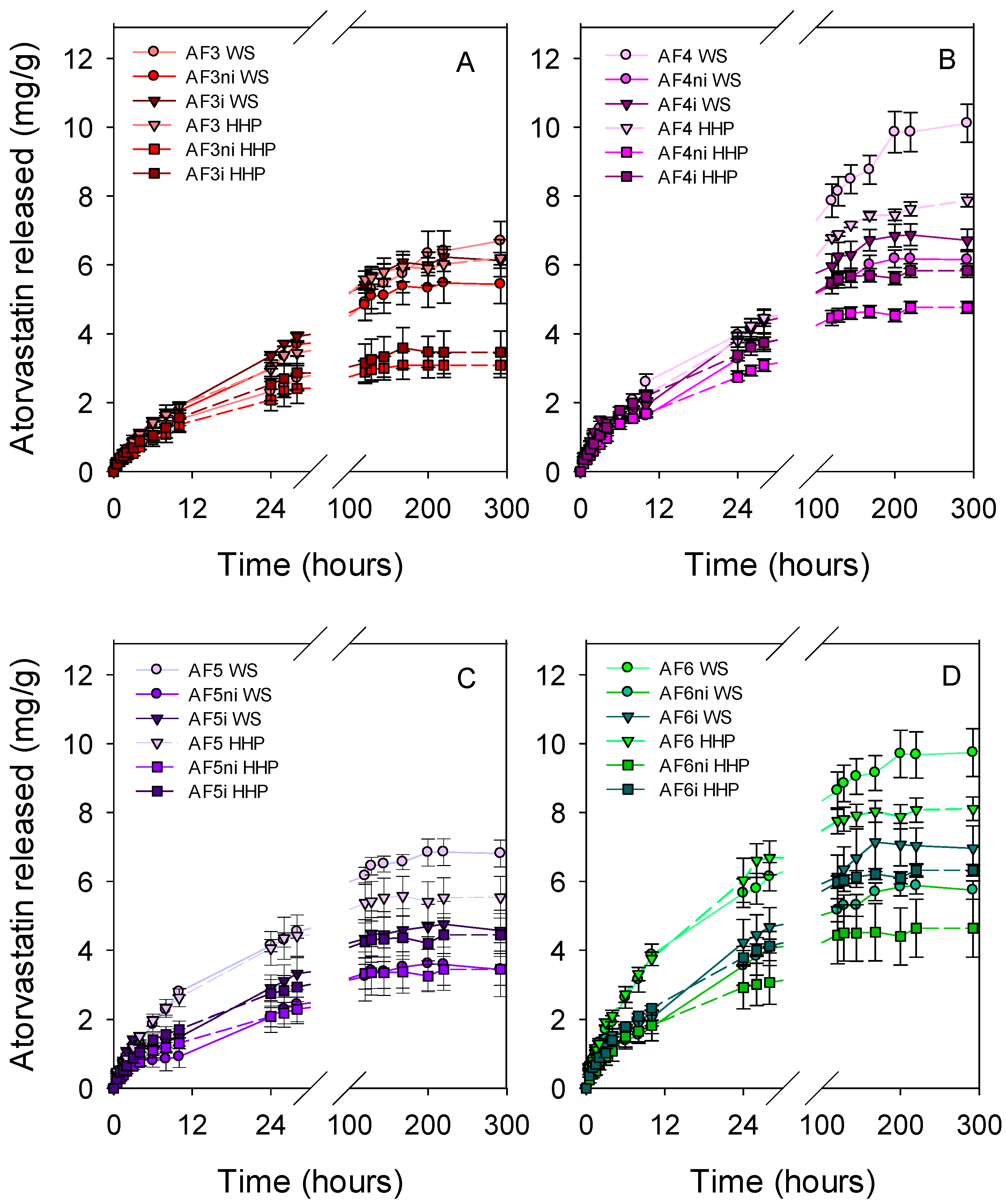
3.3. Atorvastatin Loading and Release
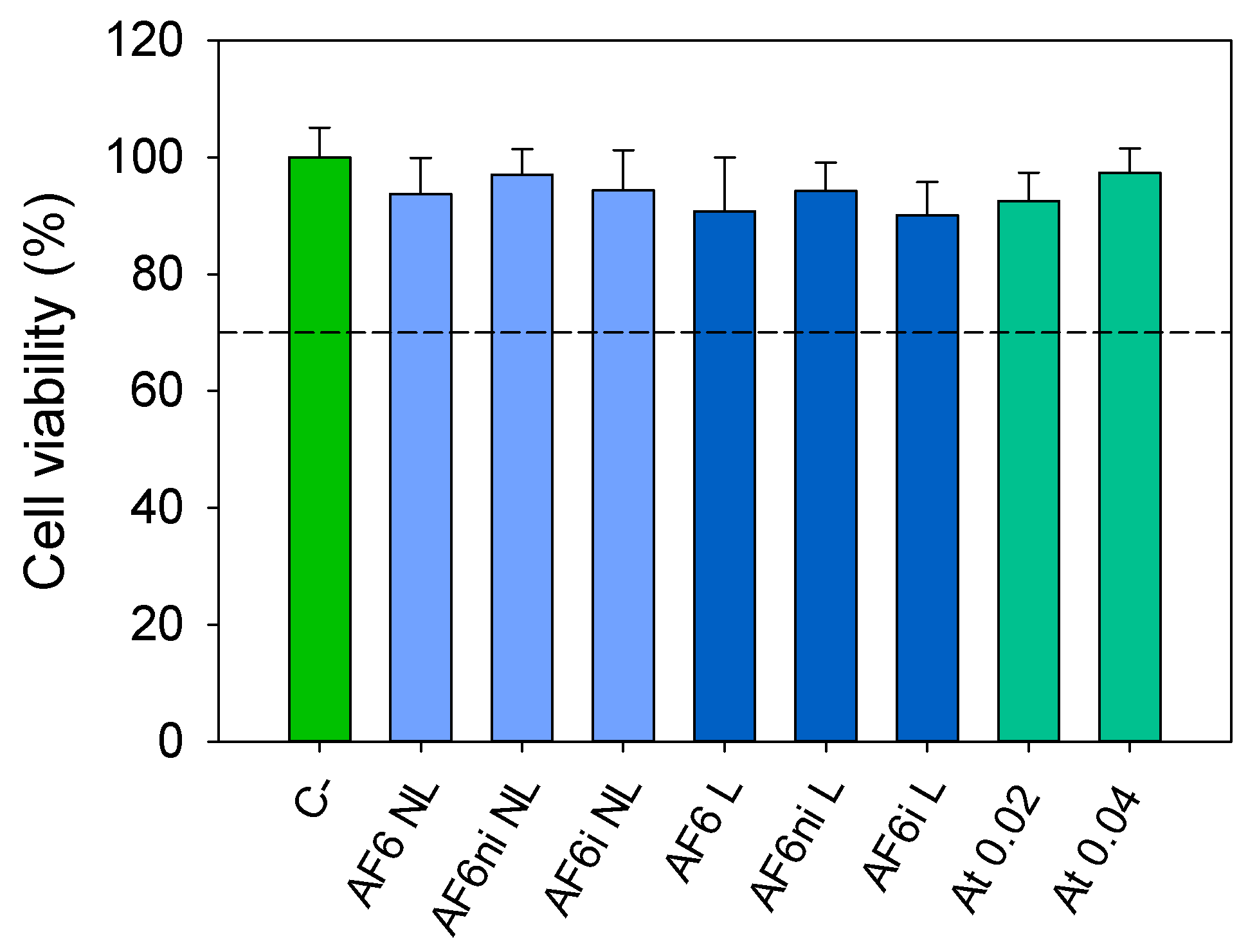
3.4. HET–CAM Test and Cytocompatibility
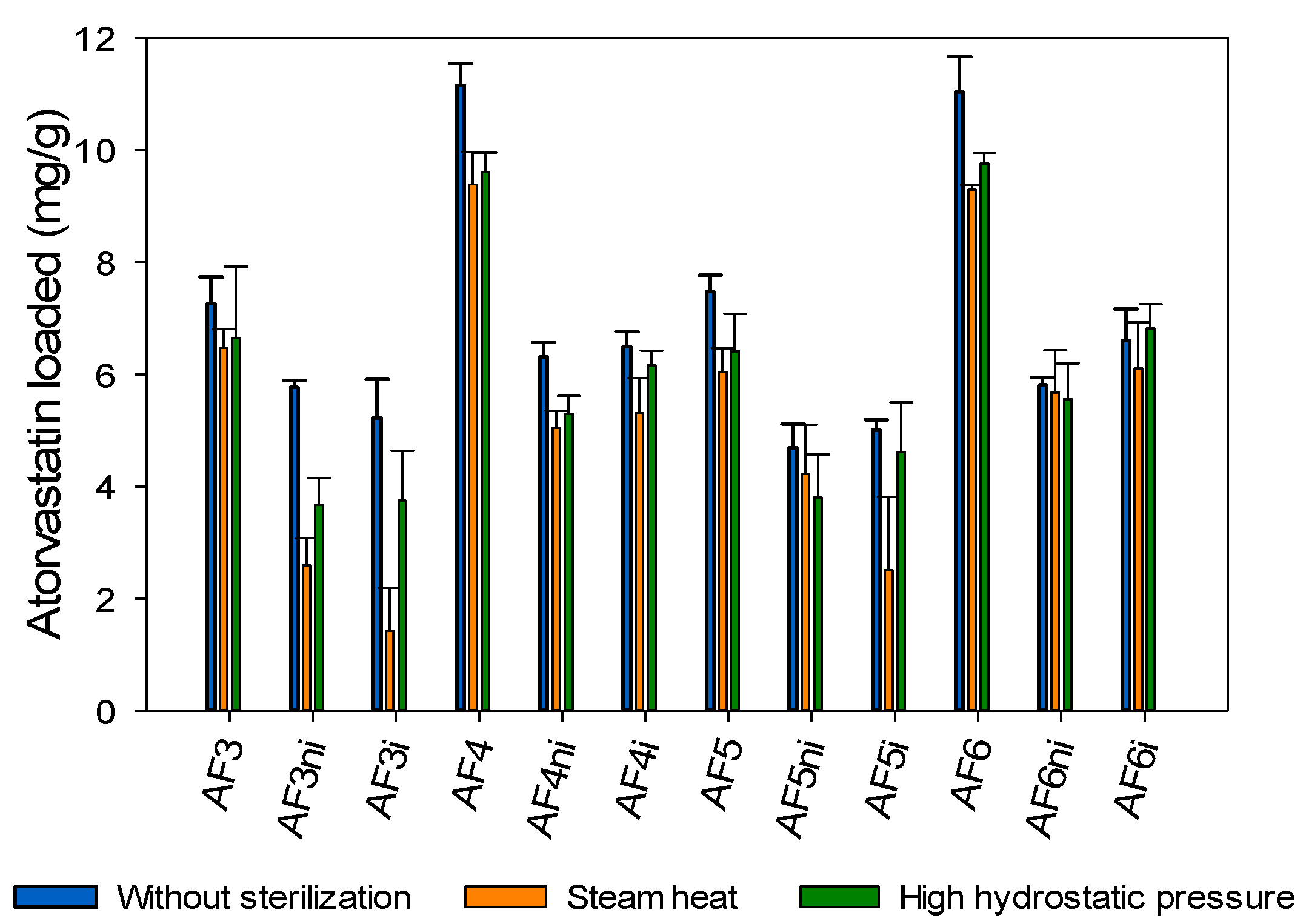
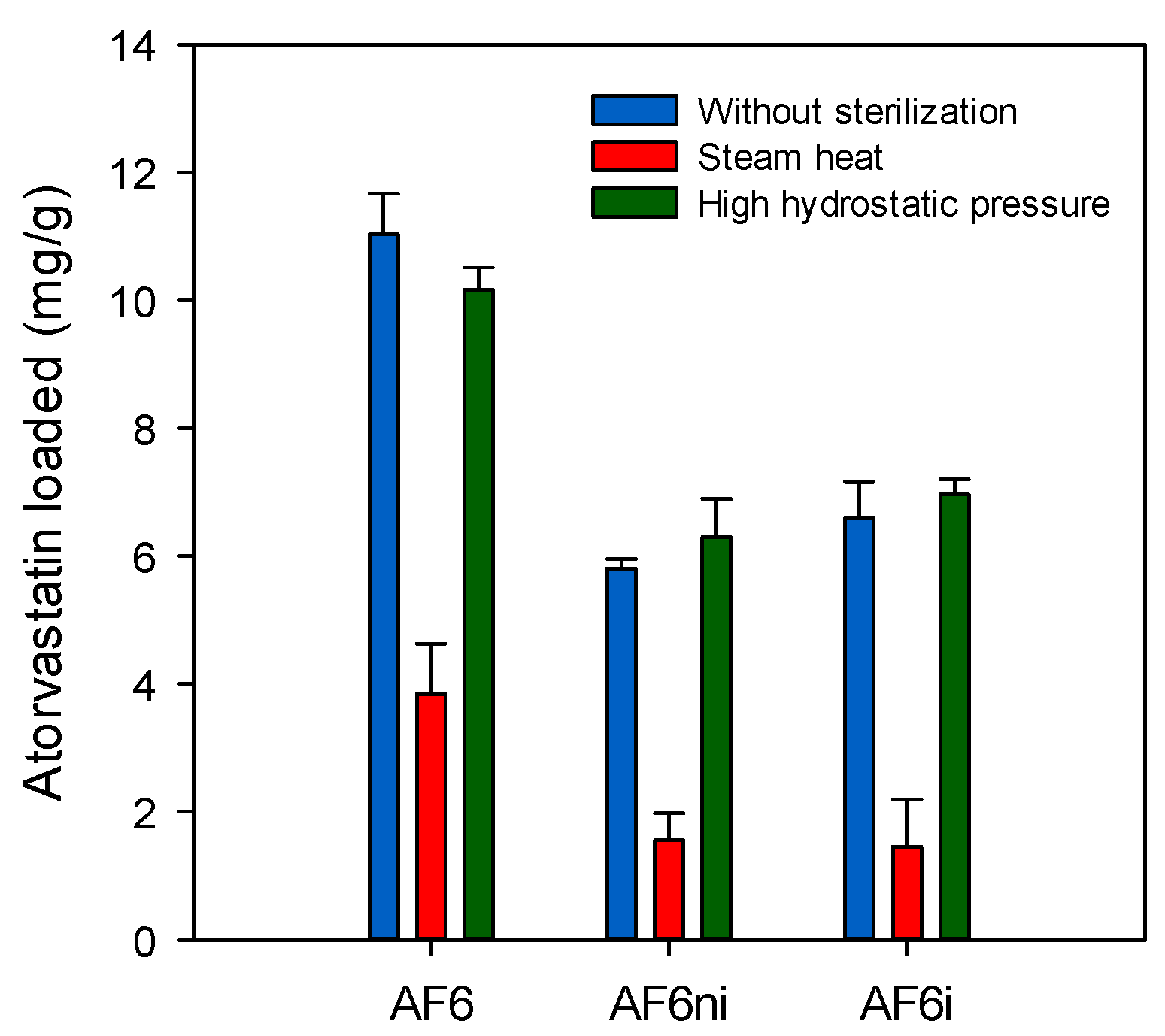
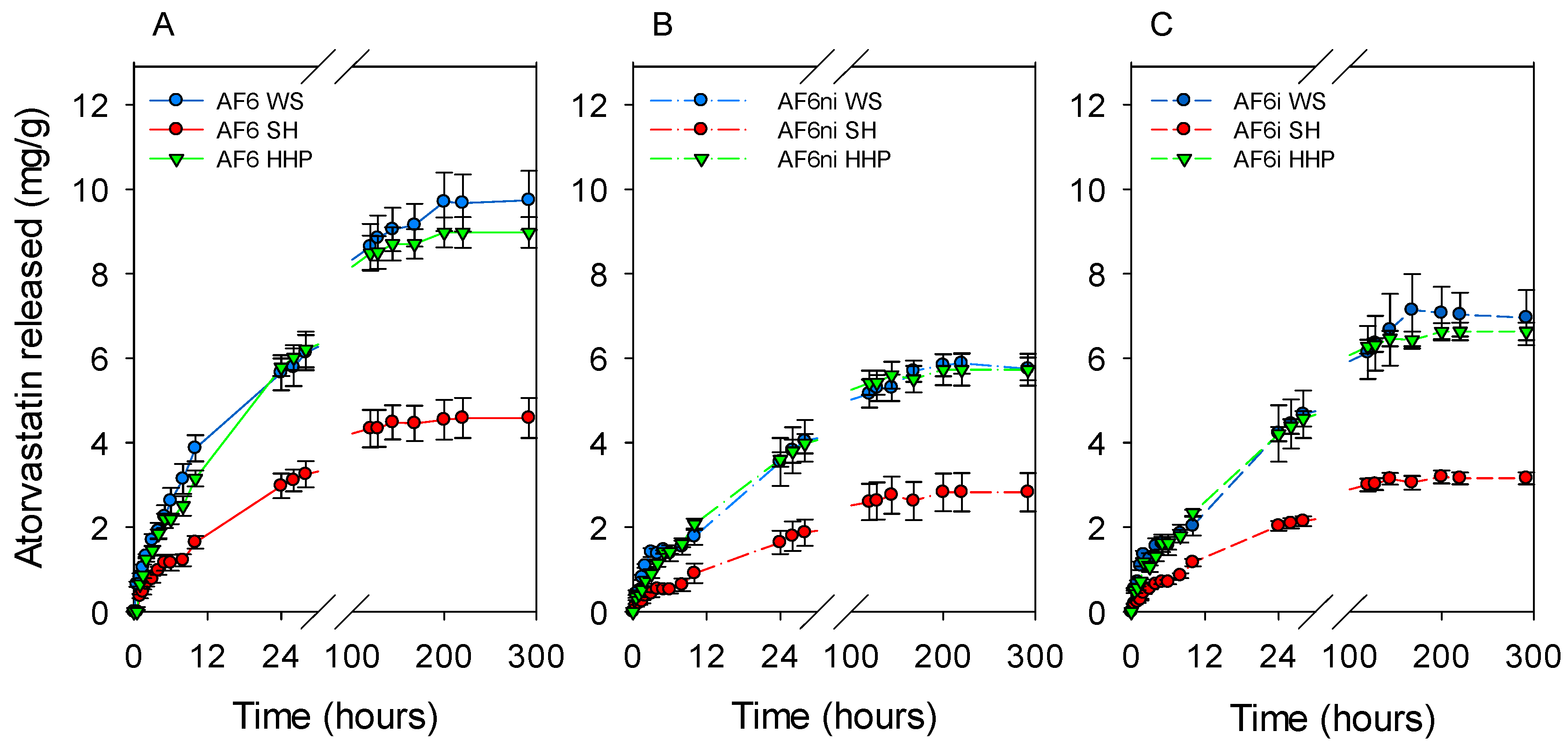
3.5. Effects of Sterilization
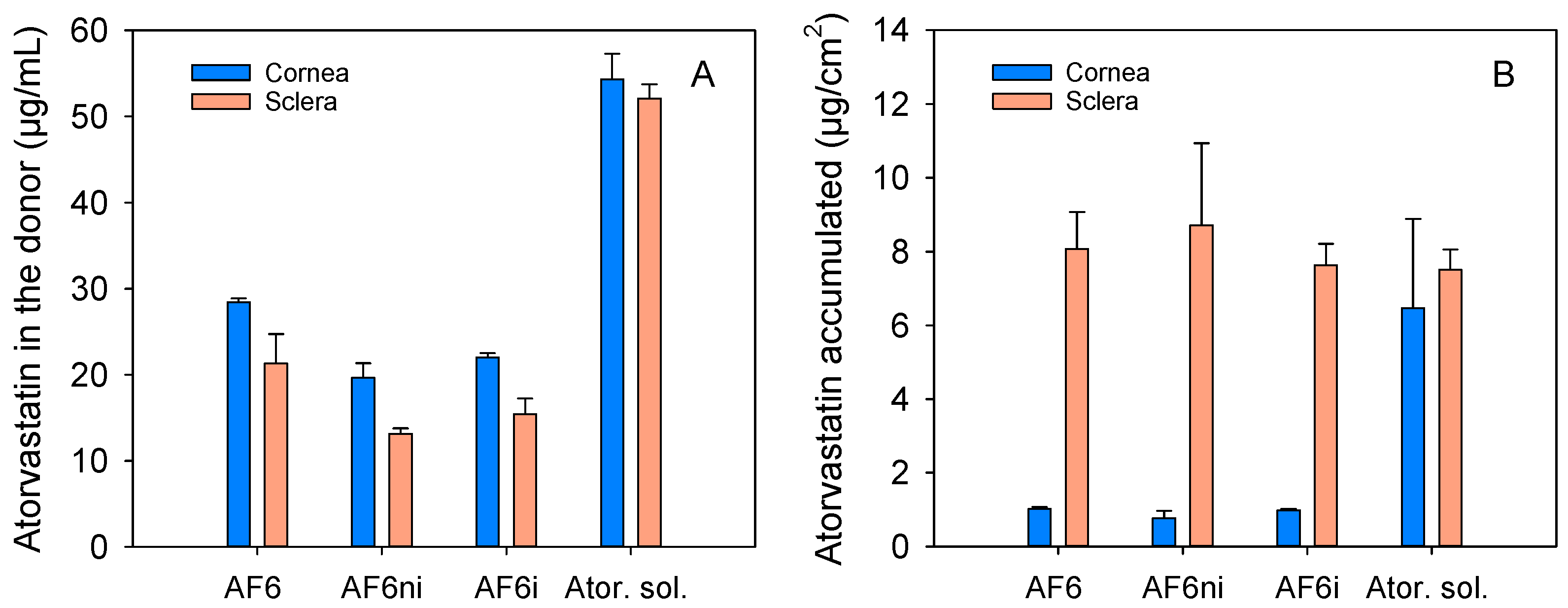
3.6. Cornea and Sclera Permeability and Accumulation
3.7. Stability of Atorvastatin Calcium Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bielecka-Dabrowa, A.; Bytyçi, I.; Von Haehling, S.; Anker, S.; Jozwiak, J.; Rysz, J.; Hernandez, A.V.; Bajraktari, G.; Mikhalidis, D.P.; Banach, M. Association of Statin Use and Clinical Outcomes in Heart Failure Patients: A Systematic Review and Meta-Analysis. Lipids Health Dis. 2019, 18, 1–13. [Google Scholar] [CrossRef]
- Sirtori, C.R. The Pharmacology of Statins. Pharmacol. Res. 2014, 88, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Bedi, O.; Dhawan, V.; Sharma, P.L.; Kumar, P. Pleiotropic Effects of Statins: New Therapeutic Targets in Drug Design. Naunyn. Schmiedebergs. Arch. Pharmacol. 2016, 389, 695–712. [Google Scholar] [CrossRef] [PubMed]
- Gordon, G.M.; Lagier, A.J.; Ponchel, C.; Bauskar, A.; Itakura, T.; Jeong, S.; Patel, N.; Fini, M.E. A Cell-Based Screening Assay to Identify Pharmaceutical Compounds That Enhance the Regenerative Quality of Corneal Repair. Wound Repair Regen. 2016, 24, 89–99. [Google Scholar] [CrossRef]
- Ooi, K.G.J.; Lee, M.H.H.; Burlutsky, G.; Gopinath, B.; Mitchell, P.; Watson, S. Association of Dyslipidaemia and Oral Statin Use, and Dry Eye Disease Symptoms in the Blue Mountains Eye Study. Clin. Exp. Ophthalmol. 2019, 47, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.S.; Poon, K.Y.T. Recent Statin Use and Cataract Surgery. Am. J. Ophthalmol. 2012, 153, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Vejux, A.; Samadi, M.; Lizard, G. Contribution of Cholesterol and Oxysterols in the Physiopathology of Cataract: Implication for the Development of Pharmacological Treatments. J. Ophthalmol. 2011, 2011, 471947. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, T.; Takahashi, A.; Sato, E.; Izumi, N.; Hein, T.W.; Kuo, L.; Yoshida, A. Effect of Systemic Administration of Simvastatin on Retinal Circulation. Arch. Ophthalmol. 2006, 124, 665–670. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Y.; Du, J.; Wang, M.; Zhang, R.; Fu, Y. The Association between Statin Use and Risk of Age-Related Macular Degeneration. Sci. Rep. 2015, 5, 18280. [Google Scholar] [CrossRef] [PubMed]
- Cusick, M.; Chew, E.Y.; Chan, C.C.; Kruth, H.S.; Murphy, R.P.; Ferris, F.L. Histopathology and Regression of Retinal Hard Exudates in Diabetic Retinopathy after Reduction of Elevated Serum Lipid Levels. Ophthalmology 2003, 110, 2126–2133. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, V.; Thapar, S.; Bhansali, A. Lipid-Lowering Drug Atorvastatin as an Adjunct in the Management of Diabetic Macular Edema. Am. J. Ophthalmol. 2004, 137, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Özkiris, A.; Erkiliç, K.; Koç, A.; Mistik, S. Effect of Atorvastatin on Ocular Blood Flow Velocities in Patients with Diabetic Retinopathy. Br. J. Ophthalmol. 2007, 91, 69–73. [Google Scholar] [CrossRef][Green Version]
- Joshi, H.N.; Fakes, M.G.; Serajuddin, A.T.M. Differentiation of 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase Inhibitors by Their Relative Lipophilicity. Pharm. Pharmacol. Commun. 1999, 5, 269–271. [Google Scholar] [CrossRef]
- Ooi, K.G.J.; Khoo, P.; Vaclavik, V.; Watson, S.L. Statins in Ophthalmology. Surv. Ophthalmol. 2019, 64, 401–432. [Google Scholar] [CrossRef]
- Jameel, A.; Ooi, K.G.J.; Jeffs, N.R.; Galatowicz, G.; Lightman, S.L.; Calder, V.L. Statin Modulation of Human T-Cell Proliferation, IL-1 β and IL-17 Production, and IFN- γ T Cell Expression: Synergy with Conventional Immunosuppressive Agents. Int. J. Inflam. 2013, 2013, 43458. [Google Scholar] [CrossRef]
- Ooi, K.G.J.; Wakefield, D.; Billson, F.A.; Watson, S.L. Efficacy and Safety of Topical Atorvastatin for the Treatment of Dry Eye Associated with Blepharitis: A Pilot Study. Ophthalmic Res. 2015, 54, 26–33. [Google Scholar] [CrossRef]
- Vieira-Potter, V.J.; Karamichos, D.; Lee, D.J. Ocular Complications of Diabetes and Therapeutic Approaches. Biomed Res. Int. 2016, 2016, 3801570. [Google Scholar] [CrossRef]
- Wong, T.Y.; Ming, C.; Cheung, G.; Larsen, M.; Sharma, S. Diabetic Retinopathy. Nat. Rev. Disease Primers 2016, 2, 16012. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Titone, R.; Robertson, D.M. The Impact of Hyperglycemia on the Corneal Epithelium: Molecular Mechanisms and Insight. Ocul. Surf. 2019, 17, 644–654. [Google Scholar] [CrossRef]
- Han, S.B.; Yang, H.K.; Hyon, J.Y. Influence of Diabetes Mellitus on Anterior Segment of the Eye. Clin. Interv. Aging 2019, 14, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Patel, D.V.; Mcghee, C.N.; Alany, R.G. New Therapeutic Approaches in the Treatment of Diabetic Keratopathy: A Review. Clin. Exp. Ophthalmol. 2011, 39, 259–270. [Google Scholar] [CrossRef]
- Negi, A.; Vernon, S.A. An Overview of the Eye in Diabetes. J. R. Soc. Med. 2003, 96, 266–272. [Google Scholar] [CrossRef]
- Dogru, M.; Katakami, C.; Inoue, M. Tear Function and Ocular Surface Changes in Noninsulin-Dependent Diabetes Mellitus. Ophthalmology 2001, 108, 586–592. [Google Scholar] [CrossRef]
- Misra, S.L.; Patel, D.V.; McGhee, C.N.J.; Pradhan, M.; Kilfoyle, D.; Braatvedt, G.D.; Craig, J.P. Peripheral Neuropathy and Tear Film Dysfunction in Type 1 Diabetes Mellitus. J. Diabetes Res. 2014, 2014, 848659. [Google Scholar] [CrossRef]
- Phan, C.M.; Bajgrowicz, M.; Gao, H.; Subbaraman, L.N.; Jones, L.W. Release of Fluconazole from Contact Lenses Using a Novel in Vitro Eye Model. Optom. Vis. Sci. 2016, 93, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.M.; Subbaraman, L.; Jones, L. Contact Lenses for Antifungal Ocular Drug Delivery: A Review. Expert Opin. Drug Deliv. 2014, 11, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Varela-Garcia, A.; Gomez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C. Imprinted Contact Lenses for Ocular Administration of Antiviral Drugs. Polymers 2020, 12, 2026. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Anguiano-Igea, S.; Varela-García, A.; Vivero-Lopez, M.; Concheiro, A. Bioinspired Hydrogels for Drug-Eluting Contact Lenses. Acta Biomater. 2019, 84, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P.; Fentzke, R.C.; Bhattacharya, A.; Konar, A.; Hazra, S.; Chauhan, A. In Vitro Drug Release and in Vivo Safety of Vitamin E and Cysteamine Loaded Contact Lenses. Int. J. Pharm. 2018, 544, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Tieppo, A.; White, C.J.; Paine, A.C.; Voyles, M.L.; McBride, M.K.; Byrne, M.E. Sustained in Vivo Release from Imprinted Therapeutic Contact Lenses. J. Control. Release 2012, 157, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Hui, A.; Bajgrowicz-Cieslak, M.; Phan, C.M.; Jones, L. In Vitro Release of Two Anti-Muscarinic Drugs from Soft Contact Lenses. Clin. Ophthalmol. 2017, 11, 1657–1665. [Google Scholar] [CrossRef]
- Ribeiro, A.; Veiga, F.; Santos, D.; Torres-Labandeira, J.J.; Concheiro, A.; Alvarez-Lorenzo, C. Receptor-Based Biomimetic NVP/DMA Contact Lenses for Loading/Eluting Carbonic Anhydrase Inhibitors. J. Memb. Sci. 2011, 383, 60–69. [Google Scholar] [CrossRef]
- González-Chomón, C.; Silva, M.; Concheiro, A.; Alvarez-Lorenzo, C. Biomimetic Contact Lenses Eluting Olopatadine for Allergic Conjunctivitis. Acta Biomater. 2016, 41, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, F.; Concheiro, A.; Alvarez-Lorenzo, C. Epalrestat-Loaded Silicone Hydrogels as Contact Lenses to Address Diabetic-Eye Complications. Eur. J. Pharm. Biopharm. 2018, 122, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, F.; Serro, A.P.; Silva, D.; Concheiro, A.; Alvarez-Lorenzo, C. Hydrogels for Diabetic Eyes: Naltrexone Loading, Release Profiles and Cornea Penetration. Mater. Sci. Eng. C 2019, 105, 110092. [Google Scholar] [CrossRef]
- Varela-Garcia, A.; Concheiro, A.; Alvarez-Lorenzo, C. Cytosine-Functionalized Bioinspired Hydrogels for Ocular Delivery of Antioxidant Transferulic Acid. Biomater. Sci. 2020, 8, 1171–1180. [Google Scholar] [CrossRef]
- Istvan, E.S.; Deisenhofer, J. Structural Mechanism for Statin Inhibition of HMG-CoA Reductase. Science 2001, 292, 1160–1164. [Google Scholar] [CrossRef]
- Galante, R.; Oliveira, A.S.; Topete, A.; Ghisleni, D.; Braga, M.; Pinto, T.J.A.; Colaço, R.; Serro, A.P. Drug-Eluting Silicone Hydrogel for Therapeutic Contact Lenses: Impact of Sterilization Methods on the System Performance. Colloids Surfaces B Biointerf. 2018, 161, 537–546. [Google Scholar] [CrossRef]
- Rogers, W.J. The Effects of Sterilization on Medical Materials and Welded Devices. In Joining and Assembly of Medical Materials and Devices; Zhou, Y., Breyen, M.D., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2013; p. 79. ISBN 9781845695774. [Google Scholar]
- Simmons, A. Future Trends for the Sterilisation of Biomaterials and Medical Devices. In Sterilisation of Biomaterials and Medical Devices; Lerouge, S., Simmons, A., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2012; pp. 310–320. [Google Scholar]
- Chawla, R.; Patil, G.R.; Singh, A.K. High Hydrostatic Pressure Technology in Dairy Processing: A Review. J. Food Sci. Technol. 2011, 48, 260–268. [Google Scholar] [CrossRef]
- Follonier, S.; Panke, S.; Zinn, M. Pressure to Kill or Pressure to Boost: A Review on the Various Effects and Applications of Hydrostatic Pressure in Bacterial Biotechnology. Appl. Microbiol. Biotechnol. 2012, 93, 1805–1815. [Google Scholar] [CrossRef]
- Topete, A.; Pinto, C.A.; Barroso, H.; Saraiva, J.A.; Barahona, I.; Saramago, B.; Serro, A.P. High Hydrostatic Pressure as Sterilization Method for Drug-Loaded Intraocular Lenses. ACS Biomater. Sci. Eng. 2020, 6, 4051–4061. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound Databases. Nucl. Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Pique, M.E.; Lindstrom, W.; Huey, R.; Forli, S.; Hart, W.E.; Halliday, S.; Belew, R.; Olson, A.J. User Guide-AutoDock Version 4.2. Available online: http://autodock.scripps.edu/downloads/faqs-help/manual/autodock-4-2-user-guide/AutoDock4.2.6_UserGuide.pdf (accessed on 10 February 2021).
- Alvarez-Lorenzo, C.; Hiratani, H.; Gómez-Amoza, J.L.; Martínez-Pacheco, R.; Souto, C.; Concheiro, A. Soft Contact Lenses Capable of Sustained Delivery of Timolol. J. Pharm. Sci. 2002, 91, 2182–2192. [Google Scholar] [CrossRef]
- Su, W.F. Radical chain polymerization. In Principles of Polymer Design and Synthesis. Lecture Notes in Chemistry; Su, W.F., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 82, pp. 137–183. [Google Scholar] [CrossRef]
- Kim, S.W.; Bae, Y.H.; Okano, T. Hydrogels: Swelling, Drug Loading, and Release. Pharm. Res. 1992, 9, 283–290. [Google Scholar] [CrossRef]
- Garcia-Valldecabres, M.; López-Alemany, A.; Refojo, M.F. pH Stability of Ophthalmic Solutions. Optometry 2004, 75, 161–168. [Google Scholar] [CrossRef]
- NIH. ICCVAM-Recommended Test Method Protocol: Hen’s Egg Test–Chorioallantoic Membrane (HET-CAM) Test Method. Available online: https://ntp.niehs.nih.gov/iccvam/docs/protocols/ivocular-hetcam.pdf (accessed on 10 February 2021).
- Silva, D.; de Sousa, H.C.; Gil, M.H.; Santos, L.F.; Moutinho, G.M.; Salema-Oom, M.; Alvarez-Lorenzo, C.; Serro, A.P.; Saramago, B. Diclofenac Sustained Release from Sterilised Soft Contact Lens Materials Using an Optimised Layer-by-Layer Coating. Int. J. Pharm. 2020, 585, 119506. [Google Scholar] [CrossRef]
- Martínez-Sancho, C.; Herrero-Vanrell, R.; Negro, S. Study of Gamma-Irradiation Effects on Aciclovir Poly(D,L-Lactic-Co- Glycolic) Acid Microspheres for Intravitreal Administration. J. Control. Release 2004, 99, 41–52. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Schweizer, S.; Nolasco, P.; Barahona, I.; Saraiva, J.; Serro, A.P. Tough and Low Friction Polyvinyl Alcohol Hydrogels Loaded with Anti-Inflammatories for Cartilage Replacement. Lubricants 2020, 8, 36. [Google Scholar] [CrossRef]
- Alvarez-Rivera, F.; Fernández-Villanueva, D.; Concheiro, A.; Alvarez-Lorenzo, C. α-Lipoic Acid in Soluplus® Polymeric Nanomicelles for Ocular Treatment of Diabetes-Associated Corneal Diseases. J. Pharm. Sci. 2016, 105, 2855–2863. [Google Scholar] [CrossRef]
- Varela-Garcia, A.; Concheiro, A.; Alvarez-Lorenzo, C. Soluplus Micelles for Acyclovir Ocular Delivery: Formulation and Cornea and Sclera Permeability. Int. J. Pharm. 2018, 552, 39–47. [Google Scholar] [CrossRef]
- Mustafa, G.; Azeem, A.; Ahmad, F.J.; Khan, Z.I.; Shakeel, F.; Talegaonkar, S. Stability-Indicating RP-HPLC Method for Analysis of Atorvastatin in Bulk Drug, Marketed Tablet and Nanoemulsion Formulation. J. Chil. Chem. Soc. 2010, 55, 184–188. [Google Scholar] [CrossRef]
- Yañez, F.; Chianella, I.; Piletsky, S.A.; Concheiro, A.; Alvarez-Lorenzo, C. Computational Modeling and Molecular Imprinting for the Development of Acrylic Polymers with High Affinity for Bile Salts. Anal. Chim. Acta 2010, 659, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Marć, M.; Kupka, T.; Wieczorek, P.P.; Namieśnik, J. Computational Modeling of Molecularly Imprinted Polymers as a Green Approach to the Development of Novel Analytical Sorbents. Trends Anal. Chem. 2018, 98, 64–78. [Google Scholar] [CrossRef]
- Terracina, J.J.; Bergkvist, M.; Sharfstein, S.T. Computational Investigation of Stoichiometric Effects, Binding Site Heterogeneities, and Selectivities of Molecularly Imprinted Polymers. J. Mol. Model. 2016, 22, 139. [Google Scholar] [CrossRef]
- Chen, J.; Lewis, C.; Balamurugan, D.; Yang, Z.; Ai, L.; Cai, D. Theoretical Analysis of a High Performance Protein Imprint on a Nanosensor. Sens. Biosen. Res. 2016, 7, 12–19. [Google Scholar] [CrossRef]
- Carbonell, T.; Freire, E. Binding Thermodynamics of Statins to HMG-CoA Reductase. Biochemistry 2005, 44, 11741–11748. [Google Scholar] [CrossRef]
- Andrade-Vivero, P.; Fernandez-Gabriel, E.; Alvarez-Lorenzo, C.; Concheiro, A. Improving the Loading and Release of NSAIDs from PHEMA Hydrogels by Copolymerization with Functionalized Monomers. J. Pharm. Sci. 2007, 96, 802–813. [Google Scholar] [CrossRef]
- Refojo, M.F. Hydrophobic Interaction in Poly(2-Hydroxyethyl Methacrylate) Homogeneous Hydrogel. J. Polym. Sci. 1967, 5, 3103–3113. [Google Scholar] [CrossRef]
- Efron, N.; Maldonado-Codina, C. Development of Contact Lenses from a Biomaterial Point of View–Materials, Manufacture, and Clinical Application. In Comprehensive Biomaterials; Ducheyne, P., Healy, K., Hutmacher, D.W., Grainger, D.W., Kirkpatrick, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 517–541. [Google Scholar]
- Ito, K.; Chuang, J.; Alvarez-Lorenzo, C.; Watanabe, T.; Ando, N.; Grosberg, A.Y. Multiple Point Adsorption in a Heteropolymer Gel and the Tanaka Approach to Imprinting: Experiment and Theory. Prog. Polym. Sci. 2003, 28, 1489–1515. [Google Scholar] [CrossRef]
- Malakooti, N.; Alexander, C.; Alvarez-Lorenzo, C. Imprinted Contact Lenses for Sustained Release of Polymyxin B and Related Antimicrobial Peptides. J. Pharm. Sci. 2015, 104, 3386–3394. [Google Scholar] [CrossRef] [PubMed]
- Tieppo, A.; Boggs, A.C.; Pourjavad, P.; Byrne, M.E. Analysis of Release Kinetics of Ocular Therapeutics from Drug Releasing Contact Lenses: Best Methods and Practices to Advance the Field. Contact Lens Ant. Eye 2014, 37, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.L.; Ahearne, M.; Hopkinson, A. An Overview of Current Techniques for Ocular Toxicity Testing. Toxicology 2015, 327, 32–46. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, B.; Kay, G.; Matthews, K.H.; Knott, R.M.; Cairns, D. The Hen’s Egg Chorioallantoic Membrane (HET-CAM) Test to Predict the Ophthalmic Irritation Potential of a Cysteamine-Containing Gel: Quantification Using Photoshop® and ImageJ. Int. J. Pharm. 2015, 490, 1–8. [Google Scholar] [CrossRef]
- Gettings, S.D.; Lordo, R.A.; Demetrulias, J.; Feder, P.I.; Hintze, K.L. Comparison of Low-Volume, Draize and in Vitro Eye Irritation Test Data. I. Hydroalcoholic Formulations. Food Chem. Toxicol. 1996, 34, 737–749. [Google Scholar] [CrossRef]
- ISO 10993-5:2009(en). Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. Available online: https://www.iso.org/obp/ui/#iso:std:iso:10993:-5:ed-3:v1:en (accessed on 10 February 2021).
- McEvoy, B.; Rowan, N.J. Terminal Sterilization of Medical Devices Using Vaporized Hydrogen Peroxide: A Review of Current Methods and Emerging Opportunities. J. Appl. Microbiol. 2019, 127, 1403–1420. [Google Scholar] [CrossRef]
- Kim, E.; Saha, M.; Ehrmann, K. Mechanical Properties of Contact Lens Materials. Eye Contact Lens 2018, 44, S148–S156. [Google Scholar] [CrossRef]
- Perova, T.S.; Vij, J.K.; Xu, H. Fourier Transform Infrared Study of Poly(2-hydroxyethyl methacrylate) PHEMA. Colloid Polym. Sci. 1997, 275, 323–332. [Google Scholar] [CrossRef]
- Bojanowska-Czajka, A.; Kciuk, G.; Gumiela, M.; Borowiecka, S.; Nałęcz-Jawecki, G.; Koc, A.; Garcia-Reyes, J.F.; Ozbay, D.S.; Trojanowicz, M. Analytical, Toxicological and Kinetic Investigation of Decomposition of the Drug Diclofenac in Waters and Wastes Using Gamma Radiation. Environ. Sci. Pollut. Res. 2015, 22, 20255–20270. [Google Scholar] [CrossRef]
- Goel, A.; Baboota, S.; Sahni, J.K.; Srinivas, K.S.; Gupta, R.S.; Gupta, A.; Semwal, V.P.; Ali, J. Development and Validation of Stability-Indicating Assay Method by UPLC for a Fixed Dose Combination of Atorvastatin and Ezetimibe. J. Chromatogr. Sci. 2013, 51, 222–228. [Google Scholar] [CrossRef]
- Topete, A.; Serro, A.P.; Saramago, B. Dual Drug Delivery from Intraocular Lens Material for Prophylaxis of Endophthalmitis in Cataract Surgery. Int. J. Pharm. 2019, 558, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Loch, C.; Zakelj, S.; Kristl, A.; Nagel, S.; Guthoff, R.; Weitschies, W.; Seidlitz, A. Determination of Permeability Coefficients of Ophthalmic Drugs through Different Layers of Porcine, Rabbit and Bovine Eyes. Eur. J. Pharm. Sci. 2012, 47, 131–138. [Google Scholar] [CrossRef]
- Yulianita, R.; Sopyan, I.; Muchtaridi, M. Forced Degradation Study of Statins: A Review. Int. J. Appl. Pharm. 2018, 10, 38–42. [Google Scholar] [CrossRef]
| Hydrogel Code | HEMA (mL) | EGDMA (µL) | EGPEM (µL) | AEMA (mg) | APMA (mg) | Atorvastatin (mg) | AIBN (mg) |
|---|---|---|---|---|---|---|---|
| AF1 | 3 | 12.10 | - | - | - | - | 4.93 |
| AF1ni | 3 | 12.10 | - | - | - | - | 4.93 |
| AF1i | 3 | 12.10 | - | - | - | 12.57 | 4.93 |
| AF2 | 3 | 12.10 | 112.50 | - | - | - | 4.93 |
| AF2ni | 3 | 12.10 | 17.18 | - | - | - | 4.93 |
| AF2i | 3 | 12.10 | 17.18 | - | - | 12.57 | 4.93 |
| AF3 | 3 | 12.10 | 112.50 | 19.90 | - | - | 4.93 |
| AF3ni | 3 | 12.10 | 17.18 | 14.91 | - | - | 4.93 |
| AF3i | 3 | 12.10 | 17.18 | 14.91 | - | 12.57 | 4.93 |
| AF4 | 3 | 12.10 | 112.50 | - | 21.45 | - | 4.93 |
| AF4ni | 3 | 12.10 | 17.18 | - | 16.08 | - | 4.93 |
| AF4i | 3 | 12.10 | 17.18 | - | 16.08 | 12.57 | 4.93 |
| AF5 | 3 | 12.10 | - | 19.90 | - | - | 4.93 |
| AF5ni | 3 | 12.10 | - | 14.91 | - | - | 4.93 |
| AF5i | 3 | 12.10 | - | 14.91 | - | 12.57 | 4.93 |
| AF6 | 3 | 12.10 | - | - | 21.45 | - | 4.93 |
| AF6ni | 3 | 12.10 | - | - | 16.08 | - | 4.93 |
| AF6i | 3 | 12.10 | - | - | 16.08 | 12.57 | 4.93 |
| Hydrogel Code | Amount of Atorvastatin Loaded (mg/g) | KN/W |
|---|---|---|
| AF1 | 0.11 (0.08) | 2.1 (2.3) |
| AF1ni | 0.17 (0.20) | 3.9 (6.2) |
| AF1i | 0.18 (0.21) | 4.1 (6.3) |
| AF2 | 0.09 (0.04) | 1.7 (1.1) |
| AF2ni | 0.16 (0.21) | 3.4 (6.3) |
| AF2i | 0.16 (0.21) | 3.4 (5.6) |
| AF3 | 7.12 (0.38) | 177.6 (11.6) |
| AF3ni | 5.66 (0.12) | 140.9 (3.0) |
| AF3i | 5.22 (0.69) | 130.1 (17.1) |
| AF4 | 11.32 (0.18) | 282.6 (5.5) |
| AF4ni | 6.31 (0.21) | 157.2 (6.5) |
| AF4i | 6.77 (0.27) | 168.7 (6.6) |
| AF5 | 7.50 (0.28) | 187.0 (8.6) |
| AF5ni | 4.69 (0.35) | 116.7 (10.7) |
| AF5i | 5.00 (0.15) | 124.6 (4.7) |
| AF6 | 11.08 (0.63) | 276.4 (19.2) |
| AF6ni | 5.81 (0.12) | 144.7 (3.5) |
| AF6i | 6.69 (0.23) | 166.7 (7.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira-da-Mota, A.F.; Vivero-Lopez, M.; Topete, A.; Serro, A.P.; Concheiro, A.; Alvarez-Lorenzo, C. Atorvastatin-Eluting Contact Lenses: Effects of Molecular Imprinting and Sterilization on Drug Loading and Release. Pharmaceutics 2021, 13, 606. https://doi.org/10.3390/pharmaceutics13050606
Pereira-da-Mota AF, Vivero-Lopez M, Topete A, Serro AP, Concheiro A, Alvarez-Lorenzo C. Atorvastatin-Eluting Contact Lenses: Effects of Molecular Imprinting and Sterilization on Drug Loading and Release. Pharmaceutics. 2021; 13(5):606. https://doi.org/10.3390/pharmaceutics13050606
Chicago/Turabian StylePereira-da-Mota, Ana F., María Vivero-Lopez, Ana Topete, Ana Paula Serro, Angel Concheiro, and Carmen Alvarez-Lorenzo. 2021. "Atorvastatin-Eluting Contact Lenses: Effects of Molecular Imprinting and Sterilization on Drug Loading and Release" Pharmaceutics 13, no. 5: 606. https://doi.org/10.3390/pharmaceutics13050606
APA StylePereira-da-Mota, A. F., Vivero-Lopez, M., Topete, A., Serro, A. P., Concheiro, A., & Alvarez-Lorenzo, C. (2021). Atorvastatin-Eluting Contact Lenses: Effects of Molecular Imprinting and Sterilization on Drug Loading and Release. Pharmaceutics, 13(5), 606. https://doi.org/10.3390/pharmaceutics13050606