Integrative Medicine (Herbal Medicine Combined with Drug Therapy) for Behcet’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Methods
2.1. Study Registration and Protocol Information
2.2. Data Sources
2.3. Study Selection
2.3.1. Types of Studies
2.3.2. Types of Participants
- International Study Group (ISG) criteria [18];
- International Criteria for BD (ICBD) criteria [18];
- Criteria of diagnosis and therapeutic effect of diseases and syndromes in traditional Chinese medicine [19];
- Guidelines for the diagnosis and treatment of BD by the Chinese Rheumatology Association (CRA) [20]; and
- Guiding principle of clinical research on new drugs of traditional Chinese medicine [21].
2.3.3. Types of Interventions and Comparison
2.3.4. Types of Outcome Measurements
- Total relative risk (RR): (recovery + marked improvement + improvement)/total number of cases × 100%.
- Recurrence rate.
- Laboratory changes in C-reactive protein (CRP) levels and the erythrocyte sedimentation rate (ESR);
- Symptom score (oral ulcers, genital ulcers, eye inflammation, skin lesions, and arthralgia); and
- Adverse events (AEs).
2.4. Data Extraction and Risk of Bias Assessment
2.4.1. Data Extraction
2.4.2. Risk of Bias
2.4.3. Data Analysis
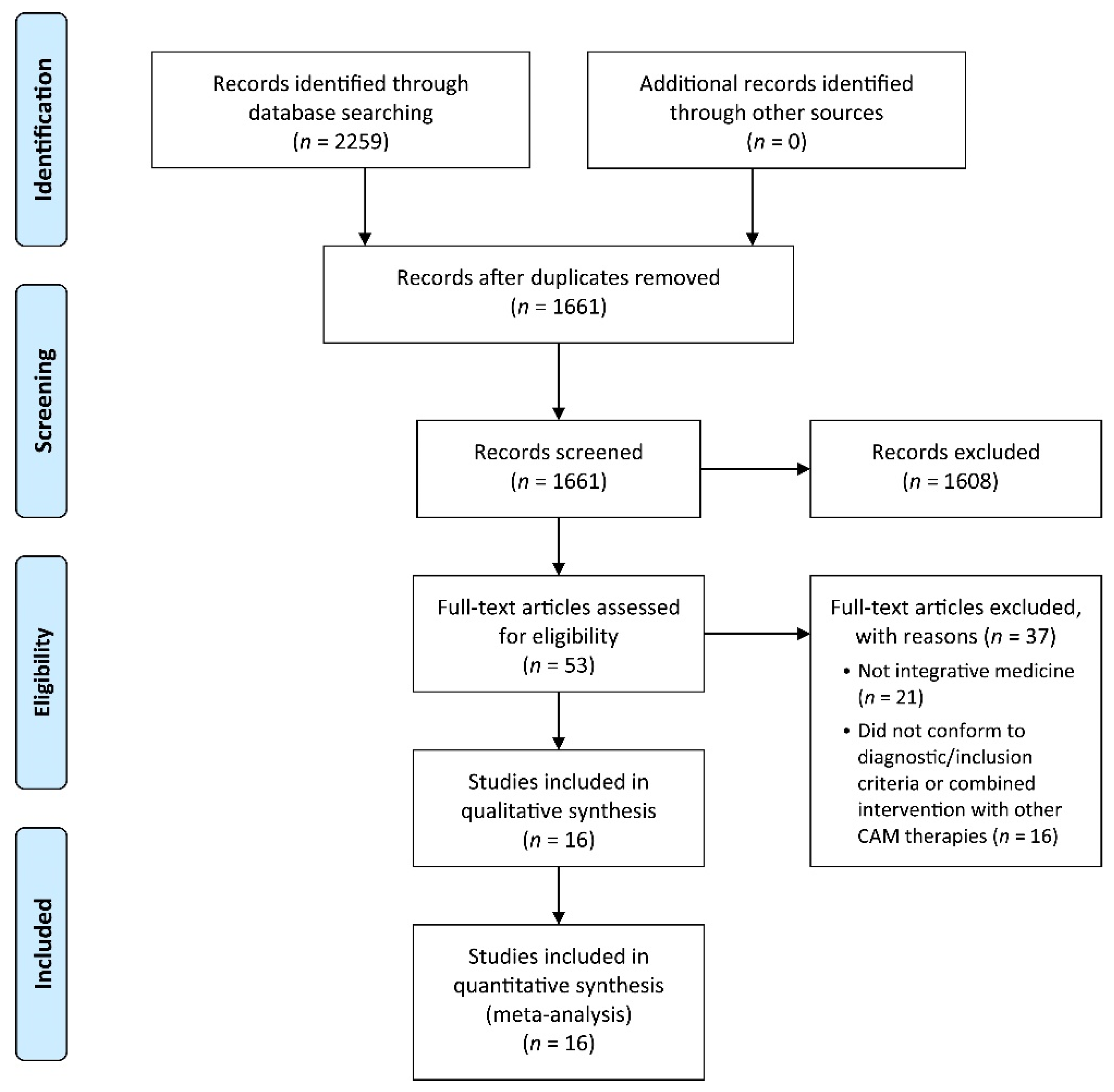
3. Results
3.1. Descriptions of the Included Trials
3.2. Risk of Bias
3.3. Certainty of Evidence
3.4. Outcome Measurements
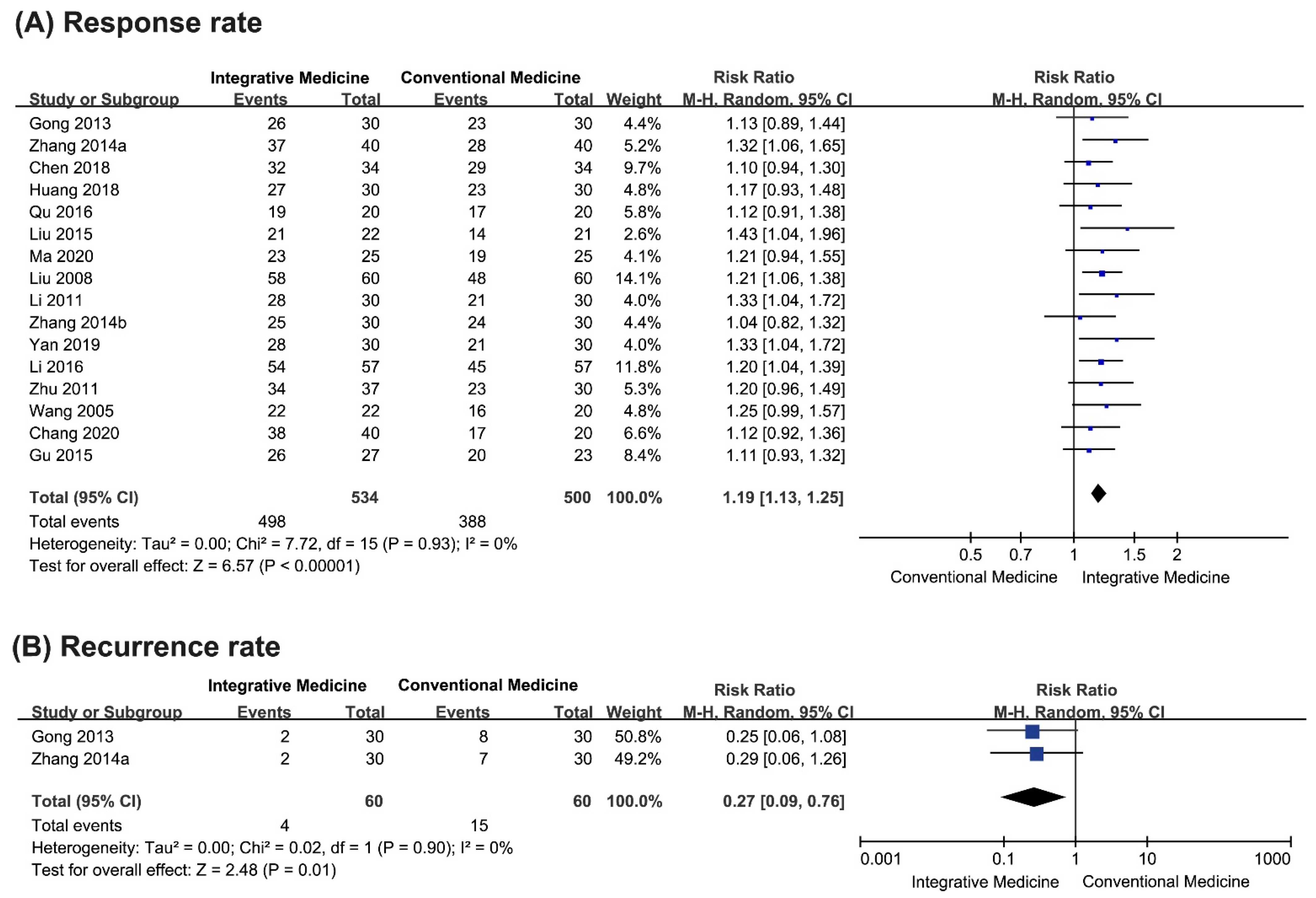
3.4.1. Primary Outcomes
Total Response Rate
Recurrence Rate
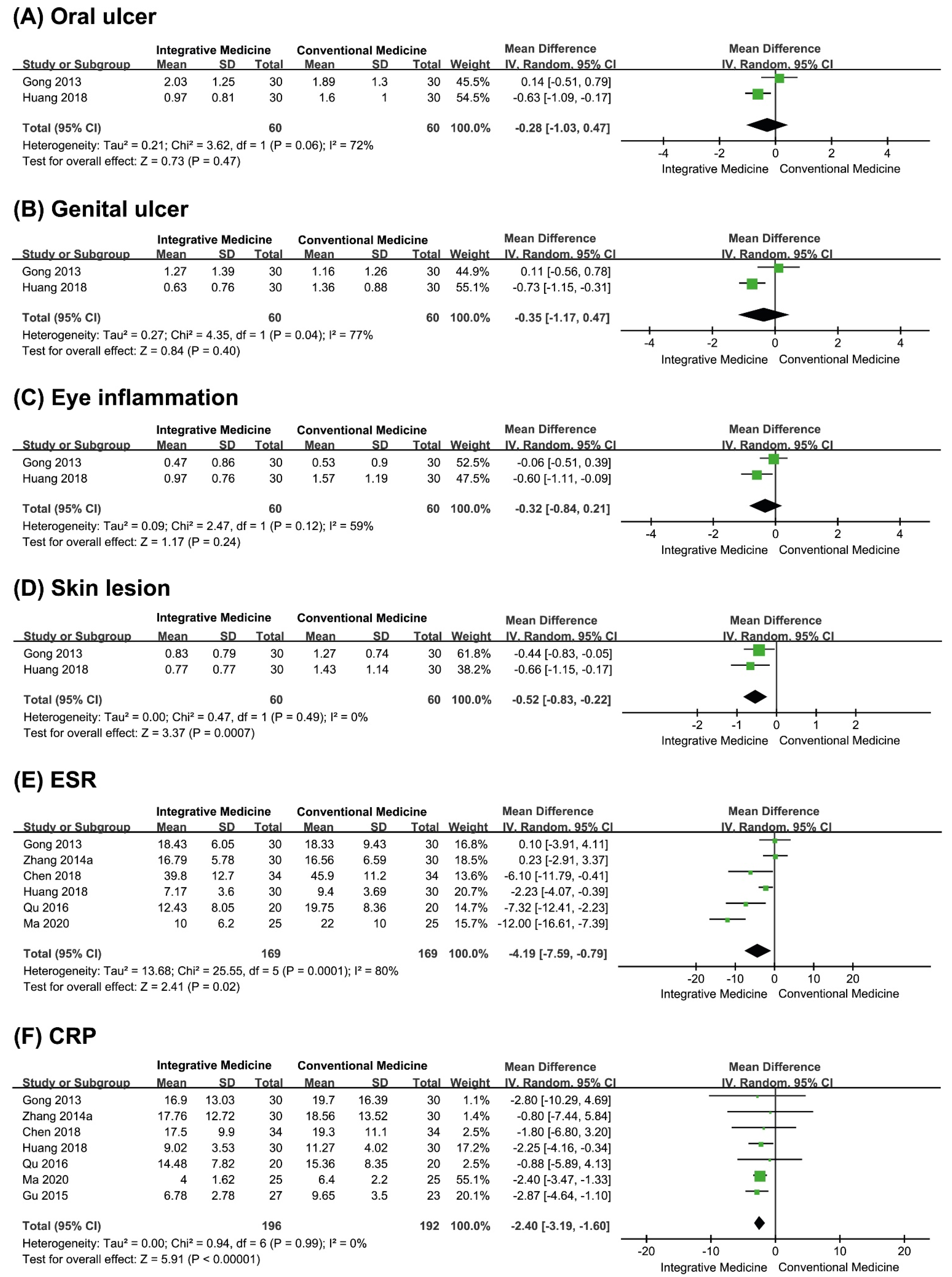
3.4.2. Secondary Outcomes
Symptom Score
- Adverse events
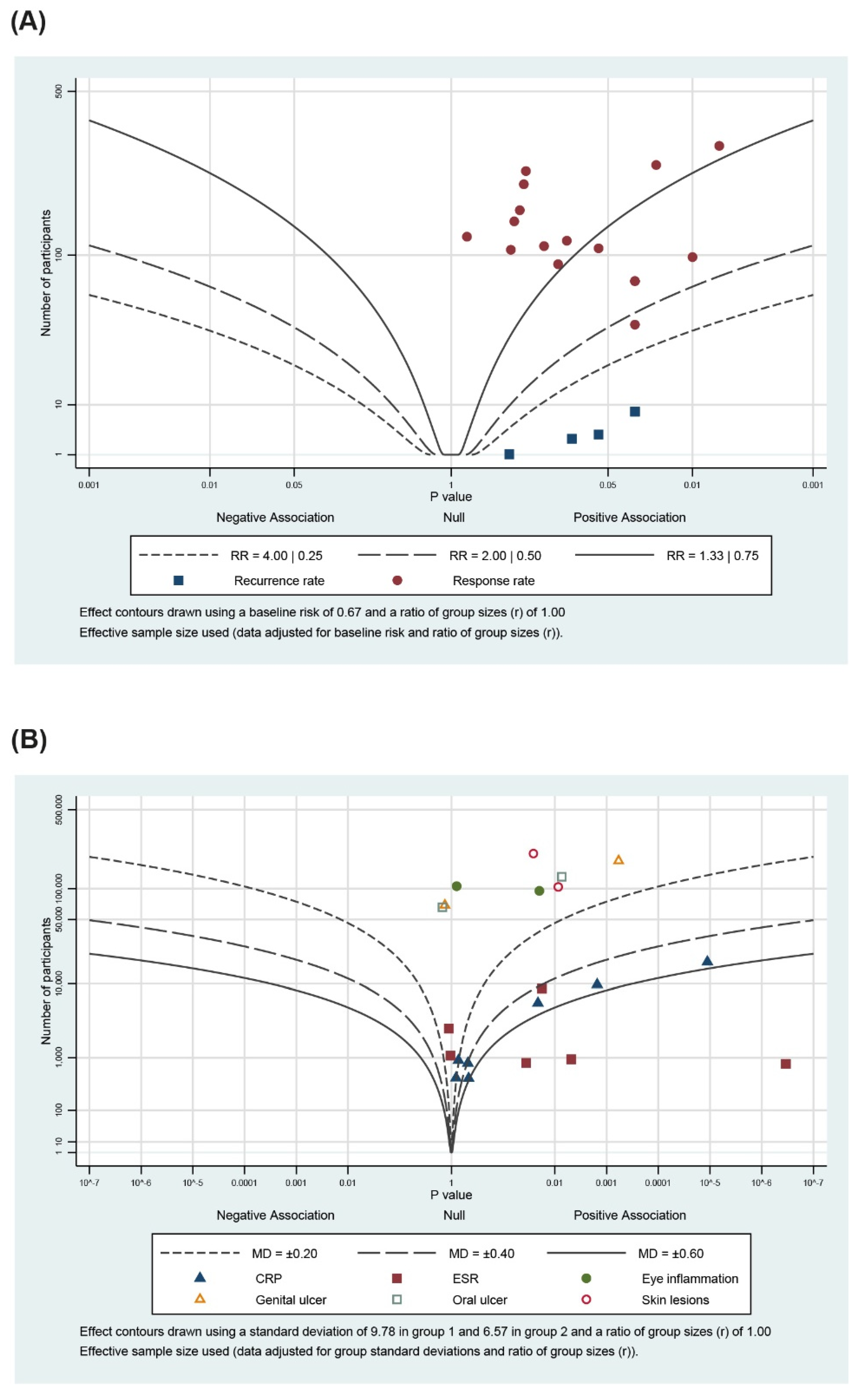
3.5. Albatross Plot
4. Discussion
4.1. Summary of the Main Results
4.2. Quality of the Evidence
4.3. Potential Biases in the Review Process
4.4. Agreements and Disagreements with Other Studies or Reviews
4.5. Implications for Clinical Practice
4.6. Implications for Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, H.; Seo, M.; Ryu, H.; Baek, H. Cross-cultural adaptation and validation of the Behcet’s disease current activity form in Korea. Korean J. Intern. Med. 2015, 30, 714–718. [Google Scholar] [CrossRef]
- Keino, H.; Okada, A. Behcet’s disease: Global epidemiology of an old silk road disease. Br. J. Ophthalmol. 2007, 91, 1573–1574. [Google Scholar] [CrossRef]
- Alpsoy, E.; Zouboulis, C.; Ehrlich, G. Mucocutaneous lesions of Behcet’s disease. Yonsei Med. J. 2007, 48, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Calamia, K.; Wilson, F.; Icen, M.; Crowson, C.; Gabriel, S.; Kremers, H. Epidemiology and clinical characteristics of Behcet’s disease in the US: A population-based study. Arthritis Rheum. 2009, 61, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Maldini, C.; Druce, K.; Basu, N.; LaValley, M.; Mahr, A. Exploring the variability in Behcet’s disease prevalence: A meta-analytical approach. Rheumatology 2018, 57, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, D.; Wechsler, B. Behcet’s disease. Orphanet J. Rare Dis. 2012, 7, 20. [Google Scholar] [CrossRef]
- Nava, F.; Ghilotti, F.; Maggi, L.; Hatemi, G.; Del Bianco, A.; Merlo, C.; Filippini, G.; Tramacere, I. Biologics, colchicine, corticosteroids, immunosuppressants and interferon-alpha for neuro-Behcet’s syndrome. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Saleh, Z.; Arayssi, T. Update on the therapy of behçet disease. Ther. Adv. Chronic. Dis. 2014, 5, 112–134. [Google Scholar] [CrossRef]
- Yurdakul, S.; Mat, C.; Tuzun, Y.; Ozyazgan, Y.; Hamuryudan, V.; Uysal, O.; Senocak, M.; Yazici, H. A double-blind trial of colchicine in Behcet’s syndrome. Arthritis Rheum. 2001, 44, 2686–2692. [Google Scholar] [CrossRef]
- National Center for Complementary and Integrative Health. Complementary, Alternative, or Integrative Health: What’s in a Name? Available online: https://nccih.nih.gov/health/integrative-health (accessed on 22 February 2021).
- Tabish, S. Complementary and alternative healthcare: Is it evidence-based? Int. J. Health Sci. 2008, 2, V–IX. [Google Scholar]
- Wang, J.; Xiong, X. Current situation and perspectives of clinical study in integrative medicine in China. Evid. Based Complement. Altern. Med. 2012, 2012, 268542. [Google Scholar] [CrossRef]
- Sohn, S.; Bang, D.; Lee, S.; Kim, Y.; Lee, E.; Ha, J.; Kim, J.; Choi, S.; Lee, S. Combined treatment with colchicine and herba Taraxaci (tarazacum mongolicum Hand.-mazz.) attenuates Behcet’s disease-like symptoms in mice and influences the expressions of cytokines. Int. Immunopharmacol. 2003, 3, 713–721. [Google Scholar] [CrossRef]
- Sohn, S.; Bang, D.; Lee, S.; Kwon, H.; Lee, E.; Kim, J.; Choi, S.; Lee, S. Combined treatment of colchicine and herbal medicines (Gamichunghyulbohyul-tang of Gamiyongdamsagan-tang) attenuate the Behcet’s disease symptoms in mice. J. Korean Med. 2001, 22, 102–108. [Google Scholar]
- Jun, J.H.; Choi, T.Y.; Lee, H.W.; Ang, L.; Lee, M.S. Herbal Medicine for Behcet’s Disease: A Systematic Review and Meta-Analysis. Nutrients 2020, 13, 46. [Google Scholar] [CrossRef]
- Zhang, Z.; He, D.; Du, H.; Tian, J. Traditional medicine and integrative medicine for Behcet’s disease: A meta analysis and systematic review. Sandong Med. 2014, 54, 91–94. [Google Scholar]
- Zhang, Z.; Xu, J.; He, D. Effectiveness of combined with Chinese medicine and western medicine in the treatment of Behcet’s disease: A meta-analysis. J. Gansu. Univ. Chin. Med. 2015, 32, 50–55. [Google Scholar]
- International Study Group for Behçet’s Disease. Criteria for diagnosis of Behcet’s disease. Lancet 1990, 335, 1078–1080. [Google Scholar] [CrossRef]
- National Administration of Traditional Chinese Medicine. Criteria of Diagnosis and Therapeutic Effect of Diseases and Syndromes in Traditional Chinese Medicine; Nanjing University Publishing House: Nanjing, China, 1994.
- Chinese Rheumatology Association. Chinese guideline for the diagnosis and treatment of Behcet’s disease. Chin. J. Rheumatol. 2011, 15, 345–347. [Google Scholar] [CrossRef]
- Zheng, X. Guiding Principle of Clinical Research on New Drugs of Traditional Chinese Medicine (Trial); China Medical Science Press: Beijing, China, 2002. [Google Scholar]
- Higgins, J.; Altman, D.; Sterne, J. Chapter 8: Assessing risk of bias in included studies. In Cochrane Handbook for Systematic Reviews of Interventions Version 510; Higgins, J., Green, S., Eds.; Wiley: New York, NY, USA, 2015; Available online: http://www.cochrane-handbook.org (accessed on 21 January 2015).
- Gong, Y. Clincal Study of Gancaoxiexintang and Thalidomide in the Treatment of Syndrome of Dampness-Heat in Behcet’s Disease; Shandong University: Jinan, China, 2013; p. 20. [Google Scholar]
- Zhang, Z. Clinical research on treating Behcet’s syndrome with Qingdai San. CJCM 2014, 6, 121–123. [Google Scholar]
- Chen, W.; Su, X.; Shen, P. Clinical observation of Shen’s Shengdi Qinlian Tufuling decoction combined with thalidomide in the treatment of Behcet’s disease. SH J TCM 2018, 52, 51–54. [Google Scholar]
- Huang, H. Treatment of Liver and Spleen Damp Heat Type Behcet’s Syndrome by Huanglian Wendan Decoction; Heilongjiang University: Harbin, China, 2018; p. 25. [Google Scholar]
- Qu, H.; Xi, S.; Cao, Z.; Zeng, Y. Clinical observation of “Yiqi Jiedu Quyu decoction” and thalidomide in treating Behcet’s disease. SH J TCM 2016, 50, 48–50. [Google Scholar]
- Liu, L.; Shao, H.; Gao, M. The Epiglottis Zhuyu decoction on behalf of the tea treatment of oral cavity ulcer curative effect observation of Behcet’s disease. Chin. J. Chin. Ethnomed. Ethnopharm. 2015, 24, 68–70. [Google Scholar]
- Ma, H.; Liang, X.; Ge, X. Clinical observation on thalidomide combined with traditional Chinese medicine in the treatment of Behcet’s Disease. Chin. Med. Mod. Distance Educ. China 2020, 18, 126–129. [Google Scholar]
- Liu, S.; Wang, F. Observation on the curative effect of 60 cases of Behcet’s disease treated with the combination of traditional Chinese and western medicine. Zhejiang J. TCM 2008, 43, 160. [Google Scholar]
- Li, D.; Zhang, J. Observation on 30 cases of Behcet’s disease treated with the combination of traditional Chinese and western medicine. Zhejiang J. TCM 2011, 46, 311. [Google Scholar]
- Zhang, B.; Liu, S.; Peng, X. Clinical observation on treatment of Behcet’s disease with Baitouweng decoction and Yinchenhao decoction combined with hormone. Chin. J. Tradit. Med. Sci. Technol. 2014, 21, 412. [Google Scholar]
- Yan, J. Observation on treatment effect of integrative medicine on Behcet’s disease. J. Pract. TCM 2019, 35, 1496–1497. [Google Scholar]
- Li, H.; Duan, Y.; Liu, S. Clinical effect of Chixiaodou Danggui powder on Behcet’s disease syndrome. Chin. J. Clin. Ration. Drug Use 2016, 9, 58–59. [Google Scholar]
- Zhu, H.; Du, J. 37 cases of Behcet’s disease treated with the combination of traditional Chinese and western medicine. Henan TCM 2011, 31, 1418. [Google Scholar]
- Wang, X.; Meng, H.; Zeng, Z. Observation of therapeutic effect of Leilin deintoxication decoction united with combination of TCM and western medicine on 22 cases of Behect disease. Guiding J. TCM 2005, 11, 27–28. [Google Scholar]
- Chang, J.; Wang, Y.; Hu, C.; Fu, P.; Wang, W.; Gu, Z. Clinical study of Chinese medicine combined with azathioprine tablets in the treatment of Behcet’s disease. Electr. J. Clin. Med. Lit. 2020, 7, 29–30. [Google Scholar]
- Gu, Z.; Wang, Y.; Hao, J.; Cao, Y.; Zeng, X.; Yang, X. Clinical obervation of chinese herbs for dissiating phlegm and removing blood stasis combined with azathioprine tablets on Behect’s disease. Hebei J. TCM 2015, 37, 494–496. [Google Scholar]
- Vickers, A.; Goyal, N.; Harland, R.; Rees, R. Do certain countries produce only positive results? A systematic review of controlled trials. Control. Clin. Trials 1998, 19, 159–166. [Google Scholar] [CrossRef]
- Hatemi, G.; Silman, A.; Bang, D.; Bodaghi, B.; Chamberlain, A.M.; Gul, A.; Houman, M.H.; Kotter, I.; Olivieri, I.; Salvarani, C.; et al. Management of Behcet disease: A systematic literature review for the European League Against Rheumatism evidence-based recommendations for the management of Behcet disease. Ann. Rheum. Dis. 2009, 68, 1528–1534. [Google Scholar] [CrossRef]
- Pan, M.-H.; Chiou, Y.-S.; Tsai, M.-L.; Ho, C.-T. Anti-inflammatory activity of traditional Chinese medicinal herbs. J. Tradit. Complement. Med. 2011, 1, 8–24. [Google Scholar] [CrossRef]
- Ghahremanlo, A.; Boroumand, N.; Ghazvini, K.; Hashemy, S.I. Herbal medicine in oral lichen planus. Phytother Res. 2019, 33, 288–293. [Google Scholar] [CrossRef]
- Ali, S.; Wahbi, W. The efficacy of aloe vera in management of oral lichen planus: A systematic review and meta-analysis. Oral Dis. 2017, 23, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.W.; Hua, H.; Cheung, L.K. Traditional Chinese medicine and oral diseases: Today and tomorrow. Oral Dis. 2011, 17, 7–12. [Google Scholar] [CrossRef]
- Yang, X.L.; Liu, D.; Bian, K.; Zhang, D.D. Study on in vitro anti-inflammatory activity of total flavonoids from Glycyrrhizae Radix et Rhizoma and its ingredients. Zhongguo Zhong Yao Za Zhi 2013, 38, 99–104. (In Chinese) [Google Scholar]
- Li, C.; Lin, G.; Zuo, Z. Pharmacological effects and pharmacokinetics properties of Radix Scutellariae and its bioactive flavones. Biopharm Drug Dispos. 2011, 32, 427–445. [Google Scholar] [CrossRef]
- Li, C.L.; Tan, L.H.; Wang, Y.F.; Luo, C.D.; Chen, H.B.; Lu, Q.; Li, Y.C.; Yang, X.B.; Chen, J.N.; Liu, Y.H.; et al. Comparison of anti-inflammatory effects of berberine, and its natural oxidative and reduced derivatives from Rhizoma Coptidis in vitro and in vivo. Phytomedicine 2019, 52, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef] [PubMed]

| First Author (Year) [Ref] | Sample Size (Randomized/Analyzed) Mean Age/Disease Duration (Years) Diagnostic Criteria | Integrative Medicine (Regimen) | Drug Therapy (Regimen) | Main Outcomes | Results | Adverse Effect |
|---|---|---|---|---|---|---|
| Gong (2013) [23] | 60/60 A: 36.5; B: 36.3/n.r. 1989 ISG; TCM diagnosis | (A) HM (Modified Gancao Xiexin decoction, 2 times daily for 3 months, n = 30), plus (B) | (B) Thalidomide (50 mg, once daily for 3 months, n = 30) | (1) Response rate (2) Recurrence rate (3) Symptom score (4) ESR (5) CRP | (1) RR 1.13 [0.89, 1.44], NS (2) 1 month: RR 0.33 [0.04, 3.03], NS; 2 months: RR 0.25 [0.06, 1.08], NS; 3 months: RR 0.27 [0.08, 0.88], p = 0.03 (3) Oral ulcer: MD 0.14 [−0.51, 0.79], NS; genital ulcer: MD 0.11 [−0.56, 0.78], NS; eye inflammation: MD −0.06 [−0.51, 0.39], NS; skin lesions: MD −0.44 [−0.83, −0.05], p = 0.03 (4) MD 0.10 [−3.91, 4.11], NS (5) MD −2.80 [−10.29, 4.69], NS | Dizziness and drowsiness (A: 1, B: 5); dry mouth and skin (A: 1, B: 3); foreign body sensation of skin (B: 1) |
| Zhang (2014a) [24] | 60/60 A: 23.4; B: 24.8/A: 12.2; B: 12.6 TCM diagnosis | (A) HM (Modified Qingdai san, 2 times daily for 2 months, n = 30), plus (B) | (B) Thalidomide (50 mg, once daily for 2 months, n = 30) | (1) Response rate (2) Recurrence rate (3) ESR (4) CRP | (1) RR 1.32 [1.06, 1.65], p = 0.01 (2) 2 months: RR 0.25 [0.06, 1.08], NS (3) MD 0.23 [−2.91, 3.37], NS (4) MD −0.80 [−7.44, 5.84], NS | Multiple neuritis (B: 1) |
| Chen (2018) [25] | 68/68 A: 38.3; B: 39.5/A: 3.8; B: 3.9 CRA | (A) HM (Shen’s Shengdi Qinlian Tufuling decoction, 2 times daily for 2 months, n = 34), plus (B) | (B) Thalidomide (25–50 mg, once daily for 2 months, n = 34) | (1) Response rate (2) ESR (3) CRP | (1) RR 1.10 [0.94, 1.30], NS (2) MD −6.10 [−11.79, −0.41], p = 0.04 (3) MD −1.80 [−6.80, 3.20], NS | Constipation (A: 1, B: 10); dizziness (A: 1, B: 4); limb numbness (B: 1); nausea (B: 1) |
| Huang (2018) [26] | 60/60 A: 38.0; B: 41.3/A: 5.0; B: 4.8 2013 ICBD; 2002 GCR−TCM | (A) HM (Modified Huanglian Wendan decoction, 2 times daily), plus (B) | (B) Thalidomide (50 mg, 3 times daily; Celecoxib 0.2 g, 2 times daily for 3 months, n = 30) | (1) Response rate (2) Symptom score (3) ESR (4) CRP | (1) RR 1.17 [0.93, 1.48], NS (2) Oral ulcer: MD −0.63 [−1.09, −0.17], p = 0.007; genital ulcer: MD −0.73 [−1.15, −0.31], p = 0.0006; eye inflammation: MD −0.60 [−1.11, −0.09], p = 0.02; skin lesions: MD −0.66 [−1.15, −0.17], p = 0.009 (3) MD −2.23 [−4.07, −0.39], p = 0.02 (4) MD −2.25 [−4.16, −0.34], p = 0.02 | None |
| Qu (2016) [27] | 40/40 A: 37.0; B: 37.3/A: 3.5; B: 3.8 1989 ISG; TCM diagnosis | (A) HM (Modified Yiqi Jiedu Quyu prescription, 2 times daily for 3 months, n = 20), plus (B) | (B) Thalidomide (50 mg, once daily for 3 months, n = 20) | (1) Response rate (2) ESR (3) CRP | (1) RR 1.12 [0.91, 1.38], NS (2) MD −7.32 [−12.41, −2.23], p = 0.005 (3) MD −0.88 [−5.89, 4.13], NS | Constipation (B: 3); dizziness and drowsiness (A: 1, B: 2); hypomenorrhea (B: 1); pruritus (B: 1) |
| Liu (2015) [28] | 46/43 A: 35.8; B: 35.7/A: 3.6; B: 3.6 1989 ISG | (A) HM (Huiyan Zhuyu decoction as tea substitute for 3 months, n = 22), plus (B) | (B) Thalidomide (75 mg, once daily for 3 months, n = 21) | Response rate | RR 1.43 [1.04, 1.96], p = 0.03 | n.r. |
| Ma (2020) [29] | 50/50 A: 37.3; B: 27.9/n.r. 1989 ISG | (A) HM (PI−based prescription for 3 months, n = 25), plus (B) | (B) Thalidomide (50~100 mg, once daily for 3 months, n = 25) | (1) Response rate (2) ESR (3) CRP | (1) RR 1.21 [0.94, 1.55], NS (2) Week 4: MD −1.00 [−4.35, 2.35], NS; week 8: MD −10.00 [−13.98, −6.02], p < 0.001; week 12: MD −12.00 [−16.61, −7.39], p < 0.001 (3) Week 4: MD −2.70 [−3.79, −1.61], p < 0.001; week 8: MD−1.40 [−2.54, −0.26], p = 0.02; week 12: MD −2.40 [−3.47, −1.33], p < 0.001 | Dizziness (A: 2; B: 3); leukocytopenia (B: 1); liver damage (A: 1; B: 2); lower limb numbness (B: 1) |
| Liu (2008) [30] | 100/100 A: 31; B: 32/A: 8.6; B: 8.4 1987 ISG | (A) HM (PI−based prescription, 2 times daily for 3 months, n = 60), plus (B) | (B) Prednisone (0.5~1 mg/kg, once daily for 3 months, n = 40) | Response rate | RR 1.21 [1.06, 1.38], p = 0.006 | n.r. |
| Li (2011) [31] | 60/60 A: 30.0; B: 31.0/A: 7.0; B: 8.0 1990 ISG | (A) HM (PI−based prescription, 2 times daily for 3 months, n = 30), plus (B) | (B) Prednisone (0.5~1 mg/kg, once daily for 3 months, n = 30) | Response rate | RR 1.33 [1.04, 1.72], p = 0.03 | n.r. |
| Zhang (2014b) [32] | 80/80 A: 30.0; B: 32.0/A: 7.0; B: 8.0 1990 ISG | (A) HM (Baitouweng decoction + Yinchenhao decoction, 2 times daily for 3 months, n = 40), plus (B) | (B) Prednisone (0.5~1 mg/kg, once daily for 3 months, n = 40) | Response rate | RR 1.04 [0.82, 1.32], NS | n.r. |
| Yan (2019) [33] | 30/30 A: 30.0; B: 32.0/A: 7.9; B: 8.3 1990 ISG | (A) HM (Duanxia Shenshi decoction + Longdan Xiegan decoction, 2 times daily for 3 months, n = 30), plus (B) | (B) Prednisone (0.5~1 mg/kg, once daily for 3 months, n = 30) | Response rate | RR 1.33 [1.04, 1.72], p = 0.03 | n.r. |
| Li (2016) [34] | 114/114 A: 41.3; B: 41.3/n.r. ISG | (A) HM (Modified Chixiaodou Danggui San, 2 times daily for 2 weeks, n = 57), plus (B) | (B) Prednisone (20~60 mg, once daily; Levamisole 50 mg, 3 times daily for 2 weeks, n = 57) | Response rate | RR 1.20 [1.04, 1.39], p = 0.02 | n.r. |
| Zhu (2011) [35] | 67/67 A: 32.5; B: 30.0/n.r. 1989 ISG | (A) HM (Modified Ziyin Yuyang decoction, 2 times daily for 2 months, n = 37), plus (B) | (B) Prednisone (1 mg/kg once daily) + Cyclophosphamide 200 mg, 3 times weekly for 2 months, n = 30) | Response rate | RR 1.20 [0.96, 1.49], NS | n.r. |
| Wang (2005) [36] | 42/42 A: 34.2; B: 38.7/A: 3.6; B: 3 1989 ISG | (A) HM (Leiling Jiedu decoction, 2 times daily for 2 months, n = 22), plus (B) | (B) Prednisone (30~40 mg, once daily for 2 months) + Dexamethasone (10 mg, once daily for the 1st week, n = 20) | Response rate | RR 1.25 [0.99, 1.57], NS | None |
| Chang (2020) [37] | 60/60 A: 31.4; B: 32.07/A: 2.48; B: 2.51 1989 ISG | (A) HM (Modified Huatan Quyu prescription, 2 times daily for 2 months, n = 40), plus (B) | (B) Azathioprine (100~150 mg, once daily for 2 months, n = 20) | (1) Response rate (2) ESR (3) CRP | (1) RR 1.12 [0.92, 1.36], NS (2) Reported only as p < 0.05 (3) Reported only as p < 0.05 | Leukocytopenia (A: 3; B: 4); liver damage (A: 5; B: 9); nausea and vomiting (A: 2; B: 3); skin rashes (A: 2; B: 2) |
| Gu (2015) [38] | 50/50 A: 30.2; B: 29.6/A: 2.5; B: 2.3 CRA, TCM diagnosis | (A) HM (Modified Huatan Quyu prescription, 2 times daily for 2 months, n = 27), plus (B) | (B) Azathioprine (100 mg, once daily for 2 months, n = 23) | (1) Response rate (2) CRP | (1) RR 1.11 [0.93, 1.32], NS (2) MD −2.87 [−4.64, −1.10], p = 0.002 | Constipation (A: 1); dizziness and headache (B: 2); drowsiness (A: 4); edema (A: 1); leukocytopenia (A: 1, B: 1); loss of appetite (B: 2); nausea and vomiting (B: 3); peripheral sensory neuropathy (A: 2); skin rashes (A: 1, B: 1) |
| Author (Year) [Ref] | Prescription | Composition of Herbs |
|---|---|---|
| Gong (2013) [23] | Modified Gancao Xiexin decoction | Glycyrrhizae Radix et Rhizoma 9 g, Glycyrrhizae Radix et Rhizoma Praeparata 9 g, Scutellariae Radix 9 g, Coptidis Rhizoma 6 g, Angelicae Gigantis Radix 15 g, Astragali Radix 18 g, Coicis Semen 30 g, Zingiberis Rhizoma 6 g, Paeoniae Radix Rubra 9 g, Moutan Cortex 9 g, Lonicerae Flos 20 g, Forsythiae Fructus 9 g, Citri Pericarpium 6 g Modification based on symptoms: [amenorrhea, forgetfulness, insomnia: Ligustri lucidi Fructus 20 g, Polygoni Multiflori Ramulus 20 g]; [nausea, vomiting, loss of appetite: Atractylodis Macrocephalae Rhizoma 15 g, Amomi Fructus 6 g]; [vexing sensation in chest, palms and soles, tidal fever: Anemarrhenae Rhizoma 12 g, Phellodendri Cortex 6 g]; [night sweating or osteopyrexia: Lycii Radicis Cortex12 g] |
| Zhang (2014a) [24] | Modified Qingdai san, topical administration | Indigo Naturalis, Coptidis Rhizoma, Phellodendri Cortex, Natrii Sulfas, Bomeolum |
| Chen (2018) [25] | Shen’s Shengdi Qinlian Tufuling decoction | Rehmanniae Radix 30 g, Scutellariae Radix 30 g, Coptidis Rhizoma 6 g, Smilacis Glabrae Rhizoma 30 g, Caraganae Radix 30 g, Zedoariae Rhizoma 30 g, Paeoniae Radix Rubra 15 g, Moutan Cortex 15 g |
| Huang (2018) [26] | Modified Huanglian Wendan decoction | Pinelliae Rhizoma 25 g, Coptidis Rhizoma 20 g, Bupleuri Radix 10 g, Citri Pericarpium 15 g, Atractylodis Macrocephalae Rhizoma 20 g, Lonicerae Flos 15 g, Smilacis Glabrae Rhizoma 20 g, Plantaginis Semen 10 g, Moutan Cortex 15 g, Glycyrrhizae Radix et Rhizoma 10 g |
| Qu (2016) [27] | Modified Yiqi Jiedu Quyu prescription | Astragali Radix 30 g, Rehmanniae Radix 30 g, Zedoariae Rhizoma 15 g, Scutellariae Radix 30 g, Smilacis Glabrae Rhizoma 30 g, Caraganae Radix 30 g, Rhei Radix et Rhizoma 9 g, Glycyrrhizae Radix et Rhizoma 12 g, Glycyrrhizae Radix et Rhizoma Praeparata 12 g Modification based on symptoms: [erythema in the legs: Ranunculi Tuber 15 g, Forsythiae Fructus 12 g]; [vulvar ulcer: Millettiae Caulis 30 g, Sophorae Radix 15 g]; [redness and pain in eyes: Bupleuri Radix 9 g, Gardeniae Fructus 9 g]; [bitter taste in the mouth: Coptidis Rhizoma 9 g, Lophatheri Herba 9 g]; [dry mouth, mild fever: Anemarrhenae Rhizoma 9 g, Phellodendri Cortex 9 g] |
| Liu (2015) [28] | Huiyan Zhuyu decoction, as tea substitute | Rehmanniae Radix 50 g, Scrophulariae Radix 50 g, Aurantii Fructus 50 g, Persicae Semen 30 g, Angelicae Gigantis Radix 50 g, Carthami Flos 30 g, Bupleuri Radix 30 g, Paeoniae Radix Rubra 30 g, Platycodi Radix 30 g, Glycyrrhizae Radix et Rhizoma 30 g Modification based on PI: [dampness-heat pattern: Taraxaci Herba 20 g, Moutan Cortex 30 g]; [heat toxin pattern: Coicis Semen 50 g, Scutellariae Radix 30 g]; [yin deficiency pattern: Anemarrhenae Rhizoma 50 g, Junci Medulla 10 g, Ecliptae Herba 30 g]; [spleen–stomach yin deficiency pattern: Astragali Radix 50 g, Codonopsis Pilosulae Radix 30 g] |
| Ma (2020) [29] | (1) Modified Wuwei Xiaodu Yin (syndrome of retained dampness toxin) (2) Gancao Xiexin decoction (syndrome of retained dampness-heat) (3) Baihe Dihuang decoction/Modified Zhibai Dihuang decoction (syndrome of yin deficiency with inner heat) | (1) Lonicerae Flos 20 g, Chrysanthemi Flos 20 g, Violae Herba 20 g, Moutan Cortex 15 g, Gentianae Radix 15 g, Bupleuri Radix 15 g, Poria Sclerotium 20 g, Coicis Semen 20 g (2) Glycyrrhizae Radix et Rhizoma 10 g, Scutellariae Radix 15 g, Zingiberis Rhizoma 10 g, Coptidis Rhizoma 10 g, Pinelliae Praeparatum cum Zingiberis 20 g, Zizyphi Fructus 3 pieces (3) Anemarrhenae Rhizoma 20 g, Phellodendron chinense Schneid. 15 g, Lilii Bulbus 20 g, Rehmanniae Radix 20 g, Poria Sclerotium 20 g, Chrysanthemi Flos 20 g, Moutan Cortex 20 g Modification based on symptoms: [swelling and pain in joints: Millettiae Caulis 20 g, Gentiana macrophylla Pallas 20 g, Achyranthes japonica Nakai 20 g, Sinomenium acutum 10 g]; [erythema nodosum: Manis pentadactyla 15 g, Melandrium firmum Rohrbach 15 g, Paeoniae Radix Rubra 20 g]; [redness and blurriness in eyes: Celosia argentea 15 g, Buddleja officinalis Maximowicz 15 g, Gardeniae Fructus 20 g]; [dry stools: Rhei Radix et Rhizoma 8 g, Magnoliae Cortex 15 g]; [insomnia: Polygonum multiflorum 20 g, Ziziphus jujuba Mill.var.spinosa 20 g] |
| Liu (2008) [30] | (1) Xiegan san (syndrome of retained dampness-heat toxin) (2) Danggui Liuhuang decoction (syndrome of yin deficiency with heat toxin) (3) Renshen Maidong san (syndrome of dual deficiency of qi and blood) | (1) Scrophulariae Radix 10 g, Rhei Radix et Rhizoma 10 g, Scutellariae Radix 10 g, Platycodi Radix 10 g, Angelicae Gigantis Radix 10 g, Natrii Sulfas 10 g, Gentianae Radix 10 g, Plantaginis Semen 15 g, Notopterygii Rhizoma 6 g, Anemarrhenae Rhizoma 12 g (2) Angelicae Gigantis Radix 10 g, Rehmanniae Radix 10 g, Rehmanniae Radix Praeparata 10 g, Coptidis Rhizoma 10 g, Scutellariae Radix 10 g, Phellodendri Cortex 10 g, Astragali Radix 20 g (3) Ginseng Radix 10 g, Atractylodis Rhizoma 10 g, Scutellariae Radix 10 g, Anemarrhenae Rhizoma 10 g, Glycyrrhizae Radix et Rhizoma Praeparata 10 g, Bambusae Caulis in Taeniam 10 g, Rehmanniae Radix 12 g, Liriopis Tuber 20 g |
| Li (2011) [31] | (1) Longgan Xiegan decoction (syndrome of retained dampness heat toxin) (2) Zhibai dihuang decoction (syndrome of yin deficiency with heat toxin) (3) Modified Shengmai yin (syndrome of dual deficiency of qi and blood) | (1) Gentianae Radix 12 g, Scutellariae Radix 12 g, Rehmanniae Radix 12 g, Bupleuri Radix 10 g, Bupleuri Radix 10 g, Alismatis Rhizoma 10 g, Plantaginis Semen 10 g, Angelicae Gigantis Radix 10 g, Akebiae Caulis 6 g, Glycyrrhizae Radix et Rhizoma Praeparata 6 g (2) Anemarrhenae Rhizoma 12 g, Phellodendri Cortex 12g, Rehmanniae Radix Praeparata 12 g, Moutan Cortex 12 g, Dioscoreae Rhizoma 12 g, Corni Fructus 10 g, Atractylodis Rhizoma 10 g, Alismatis Rhizoma 10 g (3) Panacis Quinquefolii Radix 6 g, Schizandrae Fructus 6 g, Glycyrrhizae Radix et Rhizoma Praeparata 6 g, Liriopis Tuber 20 g, Astragali Radix 20 g, Ecliptae Herba 20 g |
| Zhang (2014b) [32] | Baitouweng decoction + Yinchenhao decoction | Pulsatillae Radix 12 g, Coptidis Rhizoma 9 g, Phellodendri Cortex 9 g, Fraxini Cortex9 g, Artemisiae Scopariae Herba 30 g, Rhei Radix et Rhizoma9 g, Gardeniae Fructus 9 g |
| Yan (2019) [33] | Duanxia Shenshi decoction + Longdan Xiegan decoction | Atractylodis Rhizoma 6 g, Phellodendri Cortex 9 g, Polyporus 9 g, Atractylodis Rhizoma 9 g, Crataegii Fructus 9 g, Lonicerae Flos 12 g, Ailanthus Altissima Swingle 12 g, Sanguisorbae Radix 9 g, Gentianae Radix 9 g, Angelicae Gigantis Radix 9 g, Gardeniae Fructus 9 g, Akebiae Caulis 6 g, Plantaginis Semen 6 g, Plantaginis Semen 6g, Scutellariae Radix 9 g, Glycyrrhizae Radix et Rhizoma 6 g, Rehmanniae Radix 9 g, Alismatis Rhizoma 9 g |
| Li (2016) [34] | Modified Chixiaodou Danggui San | Phaseoli Semen 15 g, Angelicae Gigantis Radix 15 g, Scutellariae Radix 6 g, Coptidis Rhizoma 6 g, Sophorae Radix 6 g, Plantaginis Semen 6 g, Akebiae Caulis 6 g, Phyllostachys Folium 6 g, Glycyrrhizae Radix et Rhizoma 6 g |
| Zhu (2011) [35] | Modified Ziyin Yuyang decoction | Rehmanniae Radix Praeparata 15 g, Angelicae Gigantis Radix 15 g, Anemarrhenae Rhizoma 10 g, Liriopis Tuber 10 g, Phellodendri Cortex 10 g, Cuscutae Semen 9 g, Ligustri Lucidi Fructus 9 g, Paeoniae Radix Alba 9 g, Moutan Cortex 9 g, Cinnamomi Cortex 6 g, Glycyrrhizae Radix et Rhizoma 6 g Modification based on PI: [dampness-heat pattern: Atractylodis Rhizoma 10 g; qi deficiency pattern: Astragali Radix 15 g] |
| Wang (2005) [36] | Leiling Jiedu decoction | Smilacis Glabrae Rhizoma 15 g, Codonopsis Pilosulae Radix 15 g, Tripterygii Cortex 10 g, Angelicae Gigantis Radix 15 g, Salviae Miltiorrhizae Radix 10 g, Lithospermi Radix 15 g, Rehmanniae Radix 15 g, Oldenlandiae Diffusae Herba 15 g, Glycyrrhizae Radix et Rhizoma 10 g Modification based on PI: [liver–kidney yin deficiency pattern: Rehmanniae Radix Praeparata 10 g, Scrophulariae Radix 10 g, Lycii Fructus 15 g]; [spleen–kidney yang deficiency pattern: Aconiti Iateralis Radix Praeparata 10 g, Cinnamomi Ramulus 10 g]; [dual deficiency of qi and blood pattern: Astragali Radix 10 g, Atractylodis Macrocephalae Rhizoma 10 g] |
| Chang (2020) [37] | Modified Huatan Quyu prescription | Arisaema amurense Maximowicz var. serratum Nakai 9 g, Curcuma phaeocaulis Valeton 10 g, Brassicae Semen 10 g, Pinelliae Rhizoma 12 g, Angelicae Sinensis Radix 12 g, Persicae Semen 9g, Boswellia carterii Birdwood 10 g, Commiphora myrrha 9 g, Zingiberis Rhizoma Recens 15 g, Chuanxiong Rhizoma 6 g, Glycyrrhizae Radix et Rhizoma 30 g, Prunella vulgaris Linné 12 g, Notopterygii Rhizoma seu Radix 15 g |
| Gu (2015) [38] | Modified Huatan Quyu prescription | Pinelliae Rhizoma 15 g, Angelicae Gigantis Radix 9 g, Rehmanniae Radix 9 g, Atractylodis Rhizoma 9 g, Persicae Semen 12 g, Zingiberis Rhizoma Recens 25 g, Paeoniae Radix Rubra 9 g, Cnidii Rhizoma 6 g, Citri Pericarpium 15 g, Glycyrrhizae Radix et Rhizoma 30 g, Commelinae Herba 12 g, Carpesii Fructus 15 g, Euonymi Lignum Suberalatum 12 g, Zaocys Praeparata 12 g Modification based on PI: [liver dampness-heat pattern: Gentianae Radix, Phellodendri Cortex, Phaseoli Semen] Modification based on symptoms: [vulvar ulcer: Kochiae Fructus]; [erosions of anus: Sophorae Fructus Praeparata]; [eye damage: Buddlejae Flos, Cassiae Semen]; [mouth ulcer (external): Bomeolum, Borax, Cinnabaris, Natrii Sulfas Exsiccatus] |
| Integrative Medicine Compared to Drug Therapy for Behcet’s Disease | |||||
|---|---|---|---|---|---|
| Patient or population: Patients with Behcet’s disease Setting: Hospital outpatients (Study design: randomized controlled trial) Intervention: Integrative medicine Comparison: Drug therapy | |||||
| Outcome | № of Participants (Studies) Follow-Up | Certainty of Evidence | Relative Effect * (95% CI) | Anticipated Absolute Effects | |
| Risk with Drug Therapy | Risk Difference with Integrative Medicine | ||||
| Response rate | 1034 (16 RCTs) | ⨁⨁◯◯ LOW a,b | RR 1.19 (1.13 to 1.25) | 776 per 1000 | 147 more per 1000 (101 more to 194 more) |
| Recurrence rate (2 months) | 120 (2 RCTs) | ⨁⨁◯◯ LOW a,b | RR 0.27 (0.09 to 0.76) | 250 per 1000 | 183 fewer per 1000 (228 fewer to 60 fewer) |
| Oral ulcers | 120 (2 RCTs) | ⨁◯◯◯ VERY LOW a,b,c | - | MD 0.28 lower (1.03 lower to 0.47 higher) | |
| Genital ulcers | 120 (2 RCTs) | ⨁◯◯◯ VERY LOW a,b,c | - | MD 0.35 lower (1.17 lower to 0.47 higher) | |
| Eye inflammation | 120 (2 RCTs) | ⨁◯◯◯ VERY LOW a,b,c | - | MD 0.32 lower (0.84 lower to 0.21 higher) | |
| Skin lesions | 120 (2 RCTs) | ⨁⨁◯◯ LOW a,b | - | MD 0.52 lower (0.83 lower to 0.22 lower) | |
| ESR | 338 (6 RCTs) | ⨁◯◯◯ VERY LOW a,b,c | - | MD 4.19 lower (7.59 lower to 0.79 lower) | |
| CRP | 388 (7 RCTs) | ⨁⨁◯◯ LOW a,b | - | MD 2.4 lower (3.19 lower to 1.6 lower) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jun, J.H.; Ang, L.; Choi, T.Y.; Lee, H.W.; Lee, M.S. Integrative Medicine (Herbal Medicine Combined with Drug Therapy) for Behcet’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmaceutics 2021, 13, 476. https://doi.org/10.3390/pharmaceutics13040476
Jun JH, Ang L, Choi TY, Lee HW, Lee MS. Integrative Medicine (Herbal Medicine Combined with Drug Therapy) for Behcet’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmaceutics. 2021; 13(4):476. https://doi.org/10.3390/pharmaceutics13040476
Chicago/Turabian StyleJun, Ji Hee, Lin Ang, Tae Young Choi, Hye Won Lee, and Myeong Soo Lee. 2021. "Integrative Medicine (Herbal Medicine Combined with Drug Therapy) for Behcet’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Pharmaceutics 13, no. 4: 476. https://doi.org/10.3390/pharmaceutics13040476
APA StyleJun, J. H., Ang, L., Choi, T. Y., Lee, H. W., & Lee, M. S. (2021). Integrative Medicine (Herbal Medicine Combined with Drug Therapy) for Behcet’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmaceutics, 13(4), 476. https://doi.org/10.3390/pharmaceutics13040476








