Ballodiolic Acid A and B: Two New ROS, (•OH), (ONOO−) Scavenging and Potent Antimicrobial Constituents Isolated from Ballota pseudodictamnus (L.) Benth.
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Extraction and Isolation of compounds
2.4. Ballodiolic Acid A (1)
2.5. Ballodiolic Acid B (2)
2.6. Ballodiolic Acid (3)
2.7. Ballotenic Acid (4)
2.8. β-amyrin (5)
2.9. Alkaline Hydrolysis of Compounds 1 and 2
2.10. Evaluation of Antioxidative Activity
2.10.1. Measurement of Total ROS Generation Inhibition
2.10.2. Measurement of Hydroxyl Radical Generation inhibition
2.10.3. Measurement of ONOO− Scavenging Activity
2.11. Antimicrobial Screening of Compounds 1–5
2.11.1. Stock Solution Preparation
2.11.2. Anti-Bacterial Assay
2.11.3. Anti-Fungal Assay
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability
Acknowledgments
Conflicts of Interest
References
- Bohme, G.A.; Bon, C.; Lemaire, M.; Reibaud, M.; Piot, O.; Stutzmann, J.M.; Doble, A.; Blanchard, J.C. Altered synaptic plasticity and memory formation in nitric oxide synthase inhibitor-treated rats. Proc. Natl. Acad. Sci. USA 1993, 90, 9191. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Ye, Y.Z.; Anderson, P.J.; Chen, J.; Accavitti, M.A.; Tarpey, M.M.; White, C.R. Biological tyrosine nitration: A pathophysiological function of nitric oxide and reactive oxygen species. Biol. Chem. Hoppe. Seyler. 1994, 375, 81. [Google Scholar]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen elderly study. Lancet 1993, 342, 1007. [Google Scholar] [CrossRef]
- Hunt, E.J.; Lester, C.E.; Lester, P.A.; Tackett, R.L. Effect of St. John’s wort on free radical production. Life Sci. 2001, 69, 181. [Google Scholar] [CrossRef]
- Osman, A.K. Trichome micromorphology of Egyptian Ballota (Lamiaceae) with emphasis on its systematic implication. Pak. J. Bot. 2012, 44, 33. [Google Scholar]
- Nasir, E.; Ali, S.I. Flora of West Pakistan; Fakhri Printing Press: Karachi, Pakistan, 1972; p. 627. [Google Scholar]
- Dulger, B.; Sener, A. Evaluation of antimicrobial activity of Ballota accetabulosa. Afr. J. Microbiol. Res. 2010, 4, 1235. [Google Scholar]
- Yilmaz, B.S.; Altanlar, N.; Citoglu, G.S. Antilisterial activity of Ballota species growing in Turkey. J. Fac. Pharm. 2006, 34, 155. [Google Scholar]
- Savona, G.; Piozzi, F.; Hanson, J.R.; Siverns, M. Structure of three new diterpenoids from Ballota Species. J. Chem. Soc. Perkin Trans. 1977, 1, 322. [Google Scholar] [CrossRef]
- Savona, G.; Piozzi, F.; Hanson, J.R. 13-Hydroxyballonigrinolide, a new diterpenoid from Ballota lanata. Phytochemistry 1978, 17, 213. [Google Scholar] [CrossRef]
- Savona, G.; Piozzi, F.; Marino, M. Rupestralic acid, a new diterpene lactone. Heterocycles 1977, 7, 161. [Google Scholar]
- Bruno, M.; Bondi, M.L.; Piozzi, F.; Arnold, N.A.; Simmonds, M.S. Occurrence of 18-hydroxyballonigrin in Ballota saxatilis ssp.saxatilis from Lebanon. Biochem. Syst. Ecol. 2001, 29, 429. [Google Scholar] [CrossRef]
- Douglas, E.A.; Davies-Coleman, M.T. Transformation of hispanolone from Ballota africana into 15, 16-epoxy-9-hydroxylabda-13(16), 14-diene. South. Afr. J. Chem. 1990, 43, 117. [Google Scholar]
- Savona, G.; Piozzi, F.; Hanson, J.R.; Siverns, M. Structure of ballotinone, a diterpenoid from Ballota nigra. J. Chem. Soc. Perkin Trans. 1976, 1, 1607. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Kupaii, M.; Zdero, M.H. Furanolabdanes and related compounds from Ballota aucheri. Phytochemistry 1991, 31, 344. [Google Scholar] [CrossRef]
- Çitoğlu, G.S.; Sever, B.; Antus, S.; Baitz-Gacs, E.; Altanlar, N. Antifungal activities of flavonoids from Ballota glandulosissima. 2003. Pharmarceutical Biol. 2003, 4, 483. [Google Scholar] [CrossRef]
- Kisiel, W.; Piozzi, F. Tangeretin from Ballota nigra. Pol. J. Chem. 1995, 69, 476. [Google Scholar]
- Ferreres, F.; Tomas-Barberan, F.A.; Tomas-Lorente, F. Lorente Flavonoid compounds from Ballota hirsuta. J. Nat. Prod. 1986, 49, 654. [Google Scholar] [CrossRef]
- Seidel, V.; Bailleul, F.; Libot, F.; Tillequin, F. A phenylpropanoid glycoside from Ballota nigra. Phytochemistry 1997, 44, 691. [Google Scholar] [CrossRef]
- Ullah, N.; Ahmad, I.; Ahmad, N. In vitro antimicrobial, antiprotozoal activities and heavy metals toxicity of different parts of Ballota pseudodictamnus (L.) Benth. Pak. J. Pharm. Sci. 2017, 30, 2203–2209. [Google Scholar]
- Saeed, M.A.; Sabir, A. Irritant potential of some constituents from seed of Caesalpinia bouducella. J. Asian Nat. Prod. Res. 2003, 5, 35. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Farooq, U.; Hussain, J.; Ullah, F.; Nawaz, S.A.; Choudhary, M.I. Two new diterpenoids from Ballota limbate. Chem. Pharm. Bull. 2004, 52, 441. [Google Scholar] [CrossRef]
- Label, C.P.; Bondy, S.C. Sensitive and rapid quantitation of oxygen reactive species formation in rat synaptosomes. Neurochem. Int. 1990, 17, 435–440. [Google Scholar] [CrossRef]
- Nagao, A.; Seki, M.; Kobayashi, H. Inhibition of xanthine oxidase by flavonoids. Biosci. Biotechnol. Biochem. 1999, 63, 1787–1790. [Google Scholar] [CrossRef] [PubMed]
- Kooy, N.W.; Royall, J.A.; Ischiropoulos, H.; Beckman, J.S. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic. Biol. Med. 1994, 16, 149–156. [Google Scholar] [CrossRef]
- Hood, J.R.; Wilkinson, J.M.; Cavanagh, H.M. Evaluation of common antibacterial screening method utilized in essential oil research. J. Essent. Oil Res. 2003, 15, 428. [Google Scholar] [CrossRef]
- Mahesh, B.; Satish, S. Antimicrobial activity of some important medicinal plant against Plants and Human pathogens. J. Agric. Sci. 2008, 4, 839. [Google Scholar]
- Ono, M.; Ito, Y.; Kubo, S.; Nohara, T. Two new iridoids from Vitcis trifoliae Fructus (fruit of Vitex rotundifolia L.). Chem. Pharm. Bull. 1997, 45, 1094. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Cross, R.F.; Palombo, E.A. Identification of antimicrobial component of an ethanolic extract of Australian medicinal plant. Eremophila duttonii. Phytother. Res. 2004, 18, 615–618. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure–activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, J.; Opazo, J.; Mendoza, L.; Urzúa, A.; Wilkens, M. Structure-activity and lipophilicity relationships of selected antibacterial natural flavones and flavanones of Chilean flora. Molecules 2017, 22, 608. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, J.; Li, D.; Shang, C.; Peng, L.; Pan, S. The structure-antifungal activity relationship of 5, 7-dihydroxyflavonoids against Penicillium Italicum. Food Chem. 2017, 224, 26–31. [Google Scholar] [CrossRef]
- Citoğlu, G.S.; Coban, T.; Sever, B.; İşcan, M. Antioxidant properties of Ballota species growing in Turkey. J. Ethnopharmacol. 2004, 92, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Couladis, M.; Tzakou, O.; Verykokidou, E.; Harvala, C. Screening of some Greek aromatic plants for antioxidant activity. Phytother. Res. 2003, 17, 194–195. [Google Scholar] [CrossRef] [PubMed]
- Couladis, M.; Chinou, I.B.; Tzakou, O.; Loukis, A. Composition and antimicrobial activity of the essential oil of Ballota pseudodictamnus L. Bentham. Phytother. Res. 2002, 16, 723–726. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, X.; Xiao, J.; Zhong, Q.; Kuang, Y.; Cao, Y.; Chen, Y. Effects on longevity extension and mechanism of action of carnosic acid in Caenorhabditis elegans. Food Funct. 2019, 10, 1398–1410. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Halliwell, B.; Aeschbach, R.; Loligers, J. Antioxidant and pro-oxidant properties of active rosemary constituents: Carnosol and carnosic acid. Xenobiotica 1992, 22, 257–268. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, X.; Su, Z.; Xiao, J.; Lv, M.; Cao, Y.; Chen, Y. Carnosol improved lifespan and healthspan by promoting antioxidant capacity in Caenorhabditis elegans. Oxid. Med. Cell. Longev. 2019, 2019, 5958043. [Google Scholar] [CrossRef]
- Loussouarn, M.; Krieger-Liszkay, A.; Svilar, L.; Bily, A.; Birtic, S.; Havaux, M. Carnosic acid and carnosol, two major antioxidants of rosemary, act through different mechanisms. Plant. Physiol. 2017, 175, 1381–1394. [Google Scholar] [CrossRef] [PubMed]
- Minnunni, M.; Wolleb, U.; Mueller, O.; Pfeifer, A.; Aeschbacher, H.U. Natural antioxidants as inhibitors of oxygen species induced mutagenicity. Mutat. Res. 1992, 269, 193–200. [Google Scholar] [CrossRef]
- Del Bano, M.J.; Castillo, J.; Benavente-Garcia, O.; Lorente, J.; Martin-Gil, R.; Acevedo, C.; Alcaraz, M. Radioprotective-antimutagenic effects of rosemary phenolics against chromosomal damage induced in human lymphocytes by gamma-rays. J. Agric. Food Chem. 2006, 54, 2064–2068. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Morris-Natschke, S.L.; Lee, K.H. Clerodane diterpenes: Sources, structures, and biological activities. Nat. Prod. Rep. 2016, 33, 1166–1226. [Google Scholar] [CrossRef]
- Kannaste, A.; Laanisto, L.; Pazouki, L.; Copolovici, L.; Suhorutsenko, M.; Azeem, M.; Toom, L.; Borg-Karlson, A.K.; Niinemets, U. Diterpenoid fingerprints in pine foliage across an environmental and chemotypic matrix: Isoabienol content is a key trait differentiating chemotypes. Phytochemistry 2018, 147, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Fahim, F.A.; Esmat, A.Y.; Fadel, H.M.; Hassan, K.F. Allied studies on the effect of Rosmarinus officinalis L. on experimental hepatotoxicity and mutagenesis. Int. J. Food Sci. Nutr. 1999, 50, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Mabkhot, Y.; Badshah, S.L. Bioactivity profile of the diterpene isosteviol and its derivatives. Molecules 2019, 24, 678. [Google Scholar] [CrossRef] [PubMed]
- Ndjoubi, K.O.; Sharma, R.; Badmus, J.A.; Jacobs, A.; Jordaan, A.; Marnewick, J.; Warner, D.F.; Hussein, A.A. Antimycobacterial, cytotoxic, and antioxidant activities of abietane diterpenoids isolated from Plectranthus madagascariensis. Plants 2021, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- González-Cofrade, L.; Oramas-Royo, S.; Cuadrado, I.; Amesty, Á.; Hortelano, S.; Estevez-Braun, A.; de Las Heras, B. Dehydrohispanolone derivatives attenuate the inflammatory response through the modulation of inflammasome activation. J. Nat. Prod. 2020, 83, 2155–2164. [Google Scholar] [CrossRef]
- Núñez, S.; San-Martín, A.; Corsini, G. Antimicrobial activities of diterpenoids and aemisynthetic derivatives from Azorella compacta. J. Chil. Chem. Soc. 2018, 63, 4. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Chen, Y.-W.; Hou, C.-Y. Antioxidant and antibacterial activity of seven predominant terpenoids. Int. J. Food Prop. 2019, 22, 230–238. [Google Scholar] [CrossRef]
- Dickson, R.A.; Houghton, P.; Hylands, P.J. Antibacterial and antioxidant cassane diterpenoids from Caesalpinia benthamiana. Phytochemistry 2007, 68, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, C.E.; Garcia, C.; Pereira, F.; Mota, J.; Pereira, P.; Cebola, M.J.; Reis, C.P.; Correia, I.; Piedade, M.F.; da Piedade, M.E.M.; et al. Characterization of the antimicrobial abietane 7α-Acetoxy-6β-hydroxyroyleanone. Mol. Pharm. 2018, 15, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Proshkina, E.; Plyusnin, S.; Babak, T.; Lashmanova, E.; Maganova, F.; Koval, L.; Platonova, E.; Shaposhnikov, M.; Moskalev, A. Terpenoids as potential geroprotectors. Antioxidants 2020, 9, 529. [Google Scholar] [CrossRef] [PubMed]
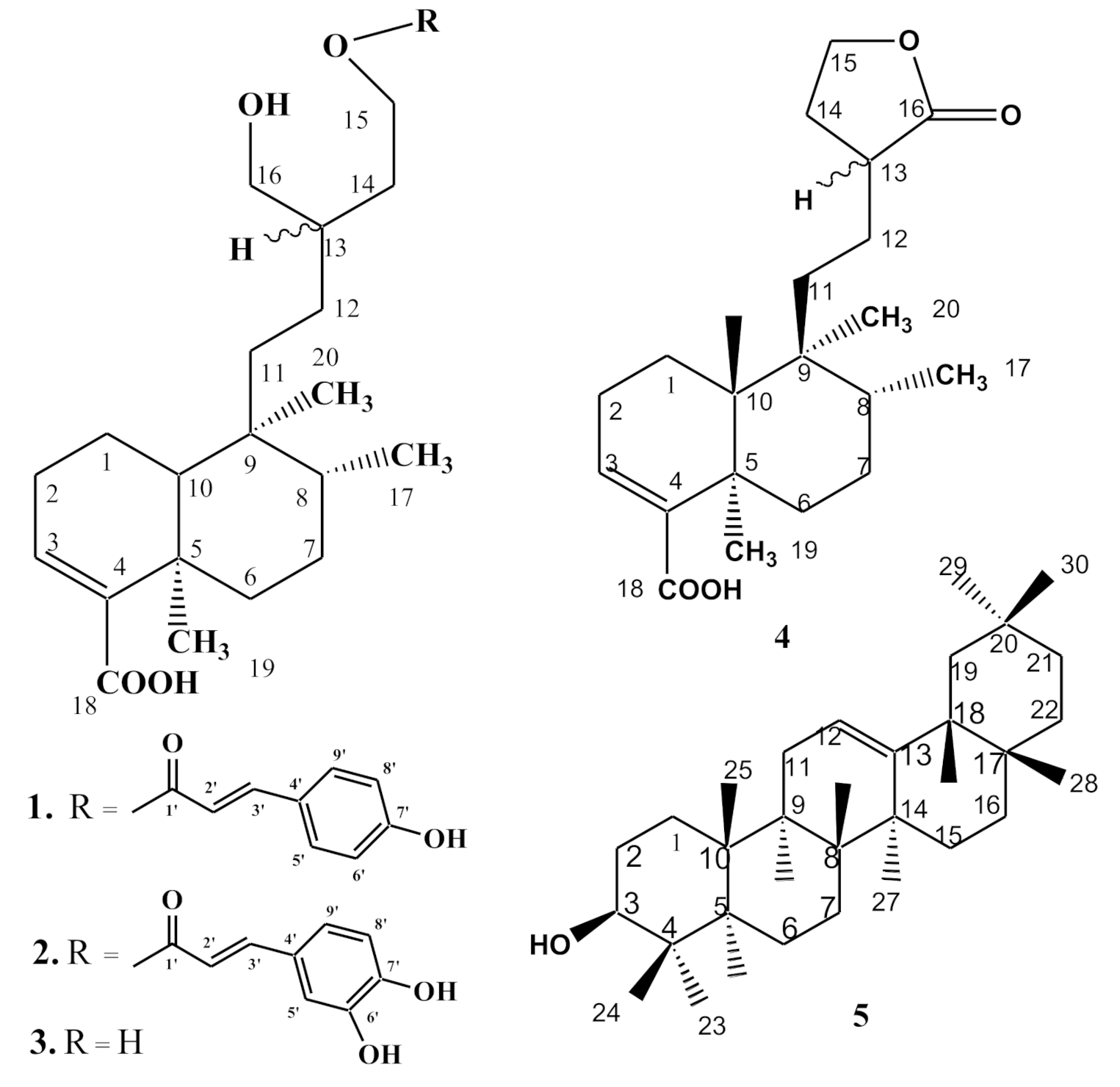
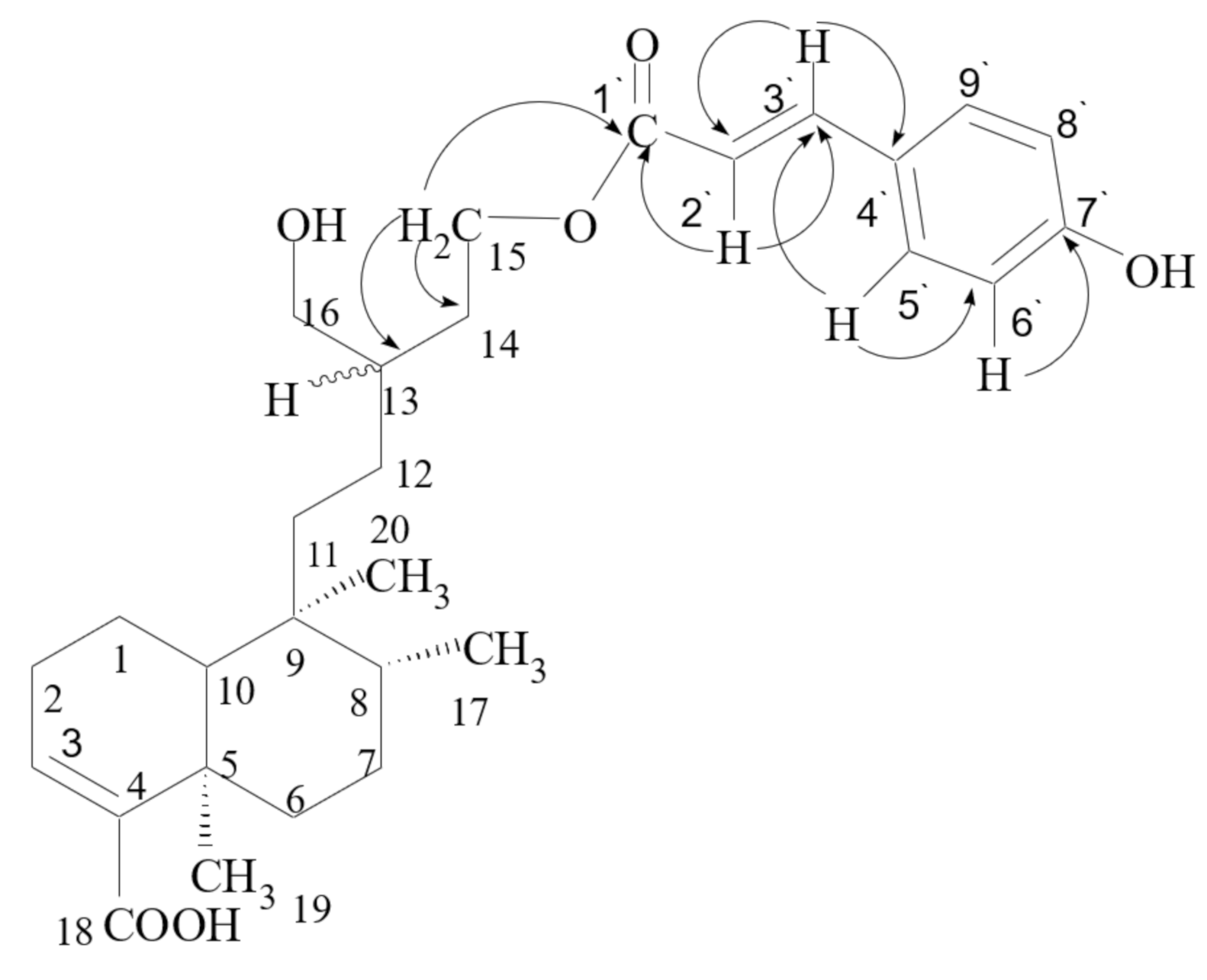
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δ(1H) | δ(13C) | δ (1H) | δ(13C) | δ(1H) | δ(13C)[M1] | |
| 1 | 0.79–0.88 (m) | 17.9 | 0.81–0.90 (m) | 17.6 | 0.80–0.89 (m) | 17.5 |
| 2 | 2.17–2.37 (m) | 27.7 | 2.15–2.33 (m) | 27.5 | 2.14–2.34 (m) | 27.4 |
| 3 | 6.82 (br s) | 141.0 | 6.81 (br s) | 140.8 | 6.79 (br s) | 140.0 |
| 4 | - | 141.9 | - | 141.5 | - | 141.3 |
| 5 | - | 37.2 | - | 37.4 | - | 37.5 |
| 6 | 2.39–2.40 (m) | 35.3 | 2.35–2.38 (m) | 35.5 | 2.35–2.38 (m) | 35.5 |
| 7 | 1.38–1.50 (m) | 27.5 | 1.36–1.47 (m) | 27.3 | 1.38–1.49 (m) | 27.2 |
| 8 | 1.44 (m) | 36.4 | 1.45 (m) | 36.3 | 1.46 (m) | 36.1 |
| 9 | - | 38.5 | - | 38.4 | - | 38.6 |
| 10 | 1.38 (d, 10.3) | 46.8 | 1.35 (d, 10.3) | 46.6 | 1.36 (d, 10.3) | 46.6 |
| 11 | 1.35–1.45 (m) | 35.5 | 1.37–1.43 (m) | 35.7 | 1.36–1.44 (m) | 35.8 |
| 12 | 1.63–1.68 (m) | 24.8 | 1.62–1.70 (m) | 24.7 | 1.61–1.69 (m) | 24.9 |
| 13 | 1.55 (m) | 39.9 | 1.56 (m) | 39.5 | 1.58 (m) | 39.8 |
| 14 | 1.20–1.27 (m) | 29.9 | 1.22–1.28 (m) | 29.6 | 1.23–1.29 (m) | 29.7 |
| 15 | 3.69–4.1 (m) | 62.7 | 3.67–4.1 (m) | 62.3 | 3.65–4.0 (m) | 66.3 |
| 16 | 3.75–3.79 (m) | 61.8 | 3.73–3.78 (m) | 61.5 | 3.70–3.77 (m) | 61.1 |
| 17 | 0.77 (d, 5.1) | 16.3 | 0.78 (d, 5.3) | 16.1 | 0.76 (d, 5.3) | 15.9 |
| 18 | - | 172.7 | - | 172.5 | - | 172.0 |
| 19 | 1.23 (s) | 20.6 | 1.24 (s) | 20.4 | 1.21 (s) | 20.5 |
| 20 | 0.73 (s) | 18.7 | 0.71 (s) | 18.5 | 0.70 (s) | 18.4 |
| 1′ | - | 166.3 | - | 165.9 | - | - |
| 2′ | 6.47 (d, 16.3) | 117.0 | 6.45 (d, 16.2) | 117.3 | - | - |
| 3′ | 7.55 (d, 16.3) | 144.7 | 7.55 (d, 16.2) | 144.9 | - | - |
| 4′ | - | 125.9 | - | 126.4 | - | - |
| 5′ | 7.40 (d, 8.1) | 130.2 | 7.15 (d, 2.3) | 112.9 | - | - |
| 6′ | 6.87 (d, 8.1) | 116.0 | - | 148.2 | - | - |
| 7′ | - | 159.3 | - | 150.3 | - | - |
| 8′ | 6.87 (d, 8.1) | 116.0 | 6.75 (d, 8.1) | 116.0 | - | - |
| 9′ | 7.40 (d, 8.1) | 130.2 | 7.07 (dd, 8.1, 2.3) | 124.1 | - | - |
| Sample | Solvent Fractions | IC50 [µg/mL] a | ||
|---|---|---|---|---|
| •OH b | Total ROS c | ONOO− d | ||
| n-hexane | 79.17 ± 0.05 | >300 | 80.37 ± 0.03 | |
| Root | CHCl3 | 47.28 ± 0.07 | 80.19 ± 0.03 | 41.23 ± 0.07 |
| EtOAc | 19.10 ± 0.05 | 65.13 ± 0.05 | 20.18 ± 0.05 | |
| n-BuOH | 31.51 ± 0.03 | 90.41 ± 0.06 | 61.16 ± 0.04 | |
| H2O | 97.37 ± 0.05 | >400 | 89.12 ± 0.05 | |
| Stem | n-hexane | 83.41 ± 0.03 | >400 | 78.25 ± 0.05 |
| CHCl3 | 55.13 ± 0.05 | 87.21 ± 0.01 | 50.47 ± 0.06 | |
| EtOAc | 29.15 ± 0.06 | 75.09 ± 0.05 | 26.07 ± 0.03 | |
| n-BuOH | 40.09 ± 0.01 | 99.34 ± 0.03 | 60.49 ± 0.05 | |
| H2O | 91.12 ± 0.05 | >400 | 87.17 ± 0.06 | |
| Leaves | n-hexane | 77.39 ± 0.06 | >400 | 85.13 ± 0.07 |
| CHCl3 | 59.25 ± 0.03 | 89.45 ± 0.06 | 52.26 ± 0.05 | |
| EtOAc | 31.15 ± 0.05 | 73.27 ± 0.03 | 30.18 ± 0.01 | |
| n-BuOH | 45.20 ± 0.01 | 99.19 ± 0.05 | 67.38 ± 0.07 | |
| Trolox e | 6.30 ± 0.03 | 40.05 ±0.07 | - | |
| DL-Penicillamine f | - | - | 2.07 ± 0.09 | |
| Compound | IC50[µM] a | ||
| •OH b | Total ROS c | ONOO− d | |
| 1 | 09.15 ± 0.07 | 64.09 ± 0.02 | 8.16 ± 0.01 |
| 2 | 07.22 ± 0.03 | 58.10 ± 0.07 | 6.23 ± 0.04 |
| 3 | 12.17 ± 0.04 | 69.15 ± 0.08 | 10.27 ± 0.02 |
| 4 | 19.08 ± 0.05 | 87.91 ± 0.04 | 21.13 ± 0.09 |
| 5 | 34.10 ± 0.07 | 148.55 ± 0.05 | 69.01 ± 0.05 |
| Trolox e | 2.51 ±0.03 | 35.06 ± 0.07 | - |
| DL-Penicillamine f | - | - | 1.09 ± 0.07 |
| Name of Bacterial Strain | Zone of Inhibition (mm) | |||||
|---|---|---|---|---|---|---|
| Compound 1 | Compound 2 | Compound 3 | Compound 4 | Compound 5 | Standard Drug Levofloxacin | |
| E. coli | 11 ± 0.01 | 12 ± 0.03 | 10 ± 0.01 | 8 ± 0.09 | 7 ± 0.02 | 16 ± 0.01 |
| P. aeruginosa | 8 ± 0.02 | 9 ± 0.02 | 7 ± 0.08 | 5 ± 0.06 | 0 ± 0.01 | 19 ± 0.02 |
| S. typhi | 13 ± 0.09 | 11 ± 0.04 | 9 ± 0.06 | 2 ± 0.04 | 6 ± 0.06 | 24 ± 0.03 |
| B. subtilis | 5 ± 0.053 | 7 ± 0.02 | 4 ± 0.03 | 2 ± 0.05 | 3 ± 0.04 | 22 ± 0.02 |
| S. aureus | 7 ± 0.02 | 9 ± 0.01 | 6 ± 0.01 | 3 ± 0.02 | 5 ± 0.03 | 20 ± 0.01 |
| Name of Fungal Strain | Zone of Inhibition (%) | |||||
|---|---|---|---|---|---|---|
| Compound 1 | Compound 2 | Compound 3 | Compound 4 | Compound 5 | Standard Drug Miconazole | |
| A. flavus | 45% | 47% | 35% | 22% | 25% | 100% |
| F. solani | 30% | 34% | 27% | 9% | 15% | 100% |
| A. fumigatus | 33% | 39% | 30% | 11% | 17% | 100% |
| A. nigar | 21% | 25% | 17% | 15% | 11% | 100% |
| C. glabrata | 25% | 29% | 22% | 17% | 7% | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fozia; Shaheen, A.; Ahmad, I.; Amin, S.B.; Ahmad, N.; Ullah, R.; Bari, A.; Sohaib, M.; Hafiz Majid, M.; Alobaid, A. Ballodiolic Acid A and B: Two New ROS, (•OH), (ONOO−) Scavenging and Potent Antimicrobial Constituents Isolated from Ballota pseudodictamnus (L.) Benth. Pharmaceutics 2021, 13, 402. https://doi.org/10.3390/pharmaceutics13030402
Fozia, Shaheen A, Ahmad I, Amin SB, Ahmad N, Ullah R, Bari A, Sohaib M, Hafiz Majid M, Alobaid A. Ballodiolic Acid A and B: Two New ROS, (•OH), (ONOO−) Scavenging and Potent Antimicrobial Constituents Isolated from Ballota pseudodictamnus (L.) Benth. Pharmaceutics. 2021; 13(3):402. https://doi.org/10.3390/pharmaceutics13030402
Chicago/Turabian StyleFozia, Asmat Shaheen, Ijaz Ahmad, Syed Badar Amin, Nisar Ahmad, Riaz Ullah, Ahmed Bari, Muhammad Sohaib, Mahmood Hafiz Majid, and Abdulrahman Alobaid. 2021. "Ballodiolic Acid A and B: Two New ROS, (•OH), (ONOO−) Scavenging and Potent Antimicrobial Constituents Isolated from Ballota pseudodictamnus (L.) Benth." Pharmaceutics 13, no. 3: 402. https://doi.org/10.3390/pharmaceutics13030402
APA StyleFozia, Shaheen, A., Ahmad, I., Amin, S. B., Ahmad, N., Ullah, R., Bari, A., Sohaib, M., Hafiz Majid, M., & Alobaid, A. (2021). Ballodiolic Acid A and B: Two New ROS, (•OH), (ONOO−) Scavenging and Potent Antimicrobial Constituents Isolated from Ballota pseudodictamnus (L.) Benth. Pharmaceutics, 13(3), 402. https://doi.org/10.3390/pharmaceutics13030402






