How Does the Addition of Kollidon®VA64 Inhibit the Recrystallization and Improve Ezetimibe Dissolution from Amorphous Solid Dispersions?
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Spray Drying (SD)
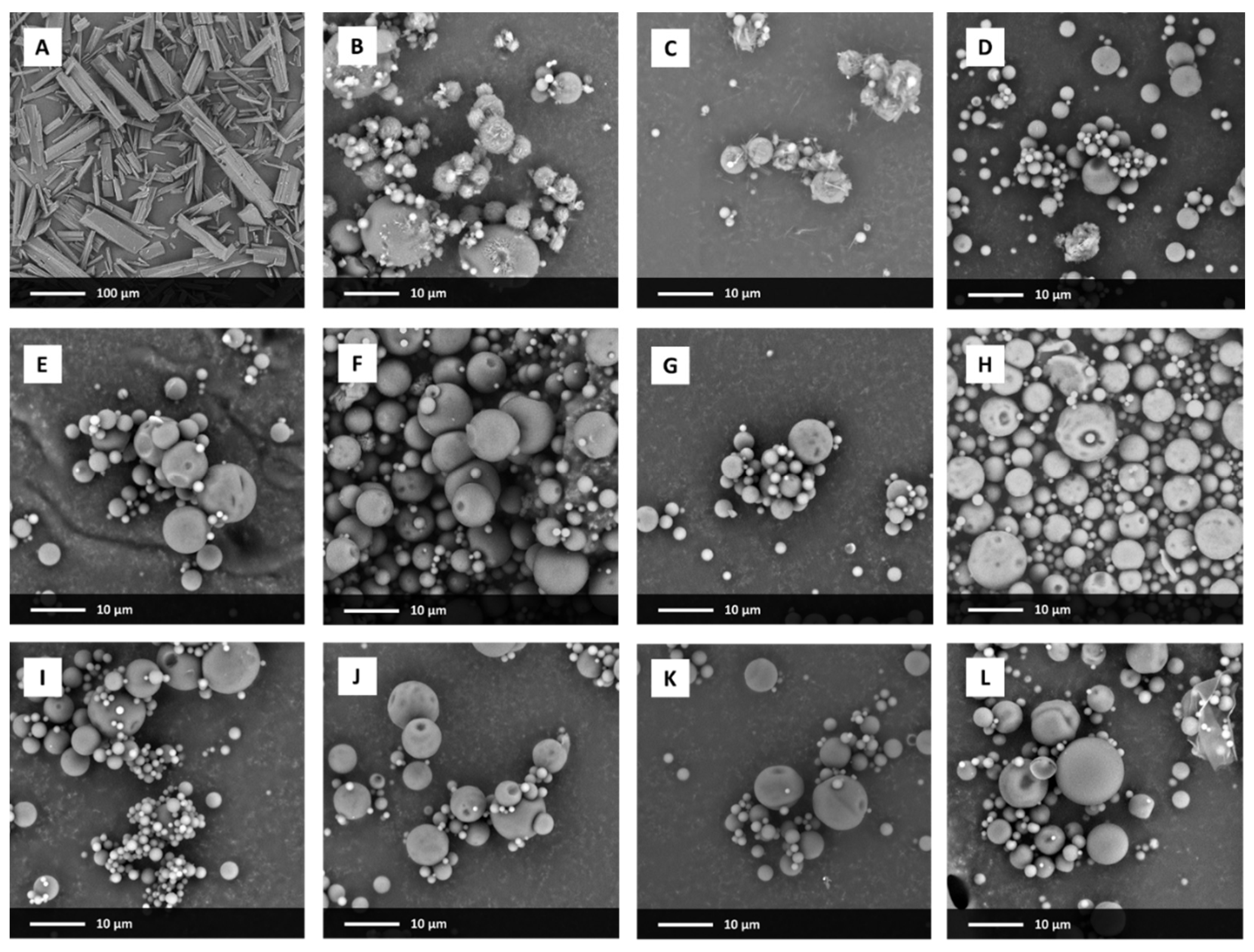
2.3. Scanning Electron Microscopy (SEM)
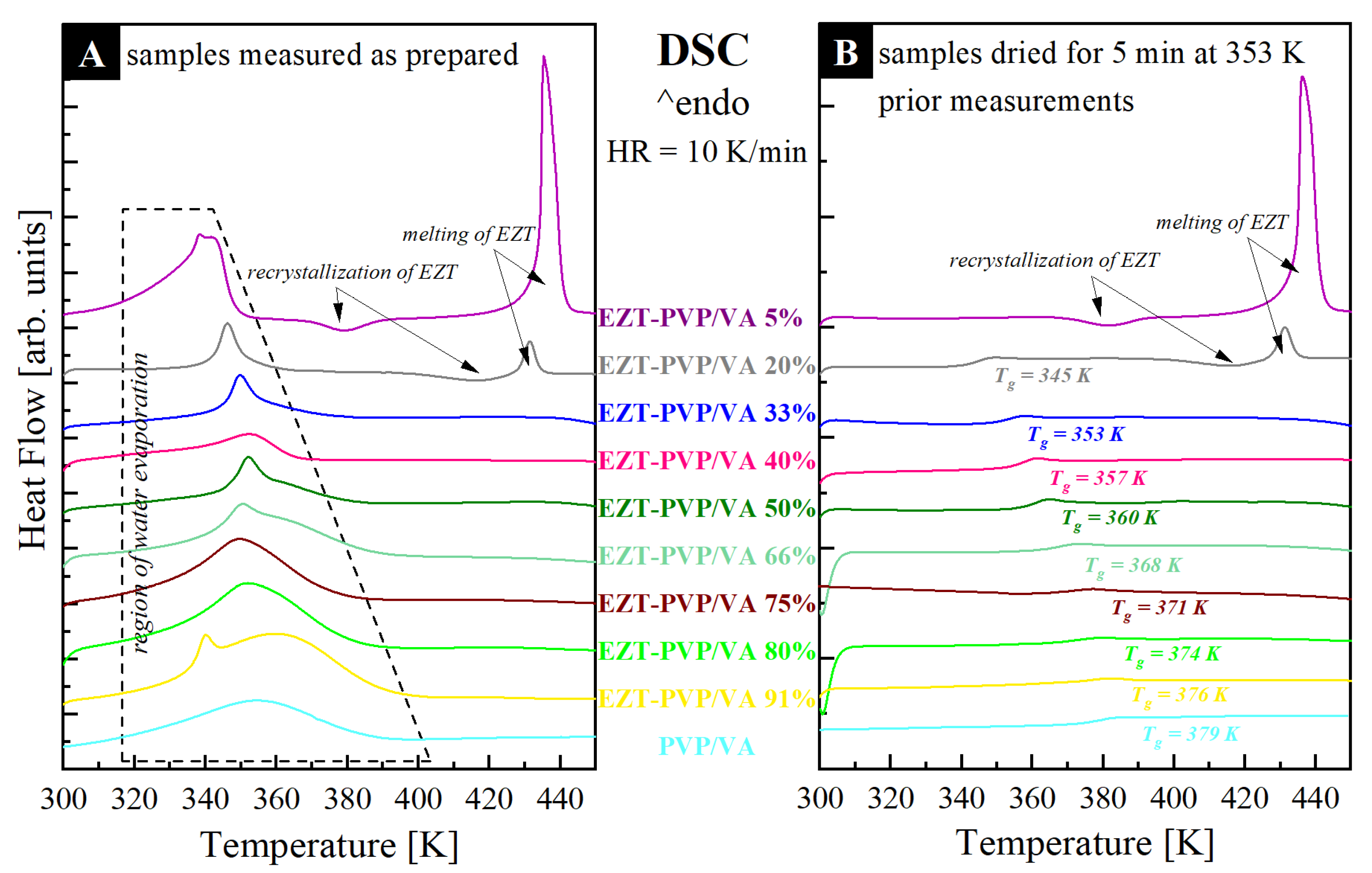
2.4. Differential Scanning Calorimetry (DSC)
2.5. Powder X-ray Diffraction (PXRD)
2.6. Laser Diffraction Measurements
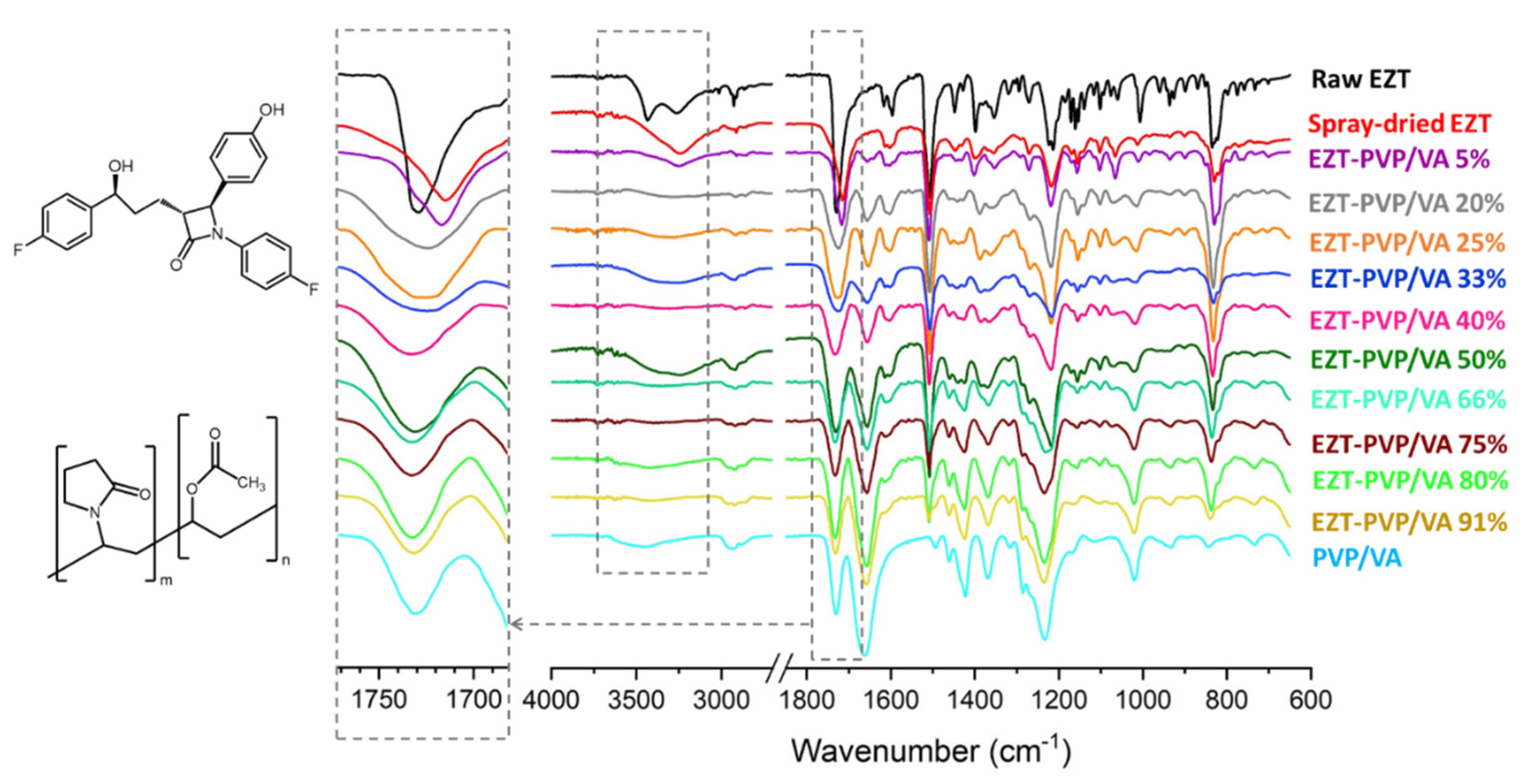
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
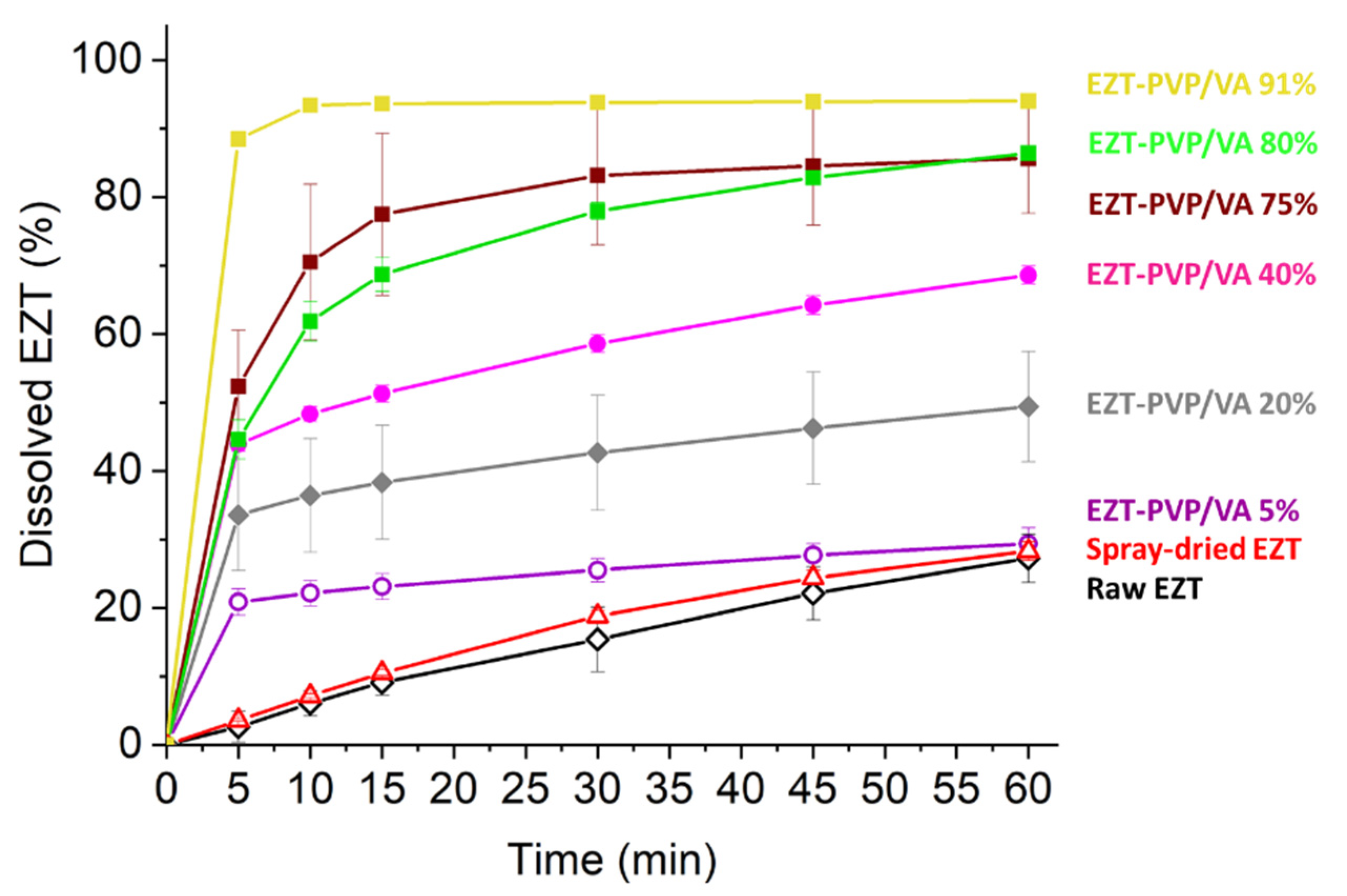
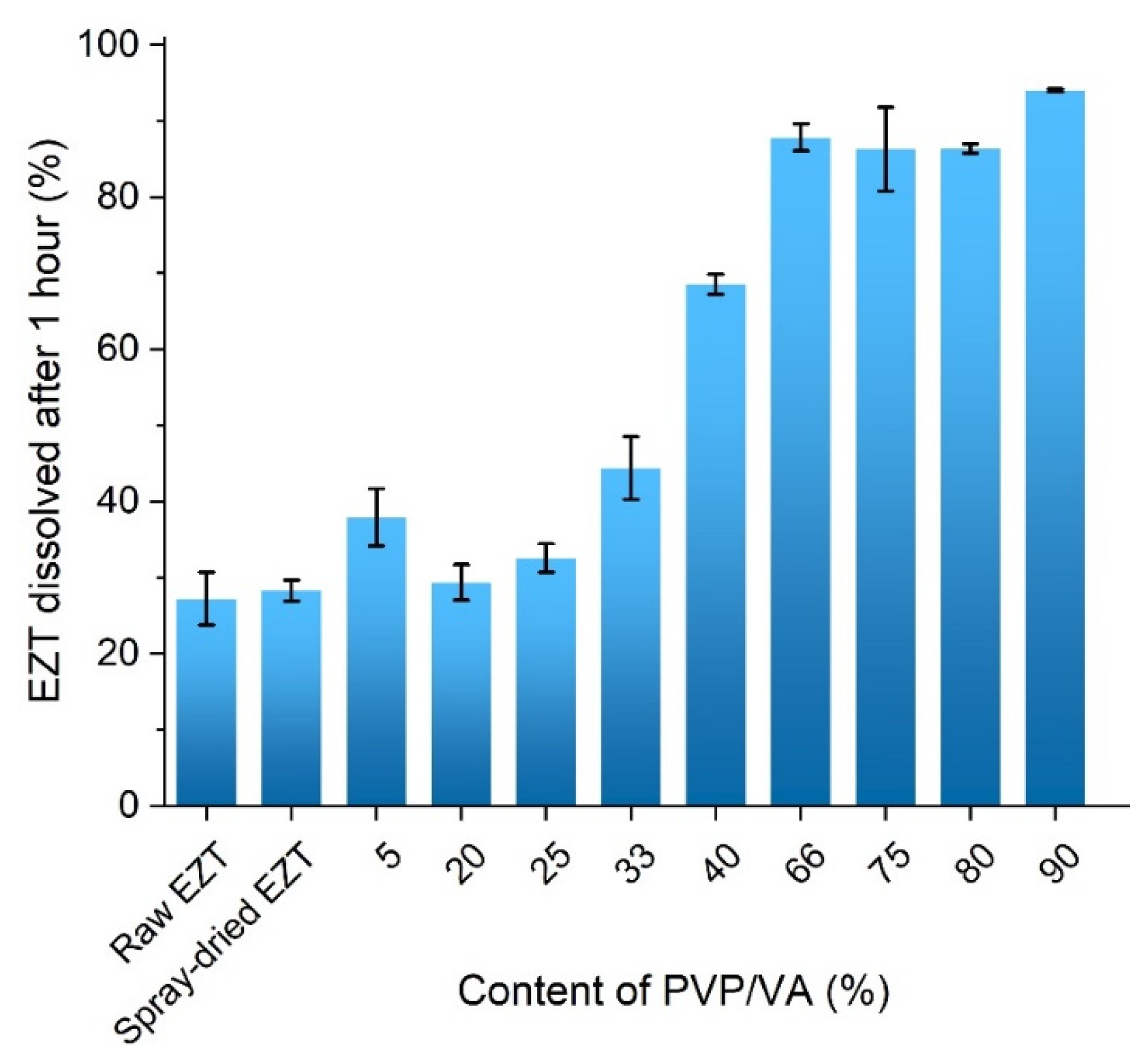
2.8. Dissolution Study
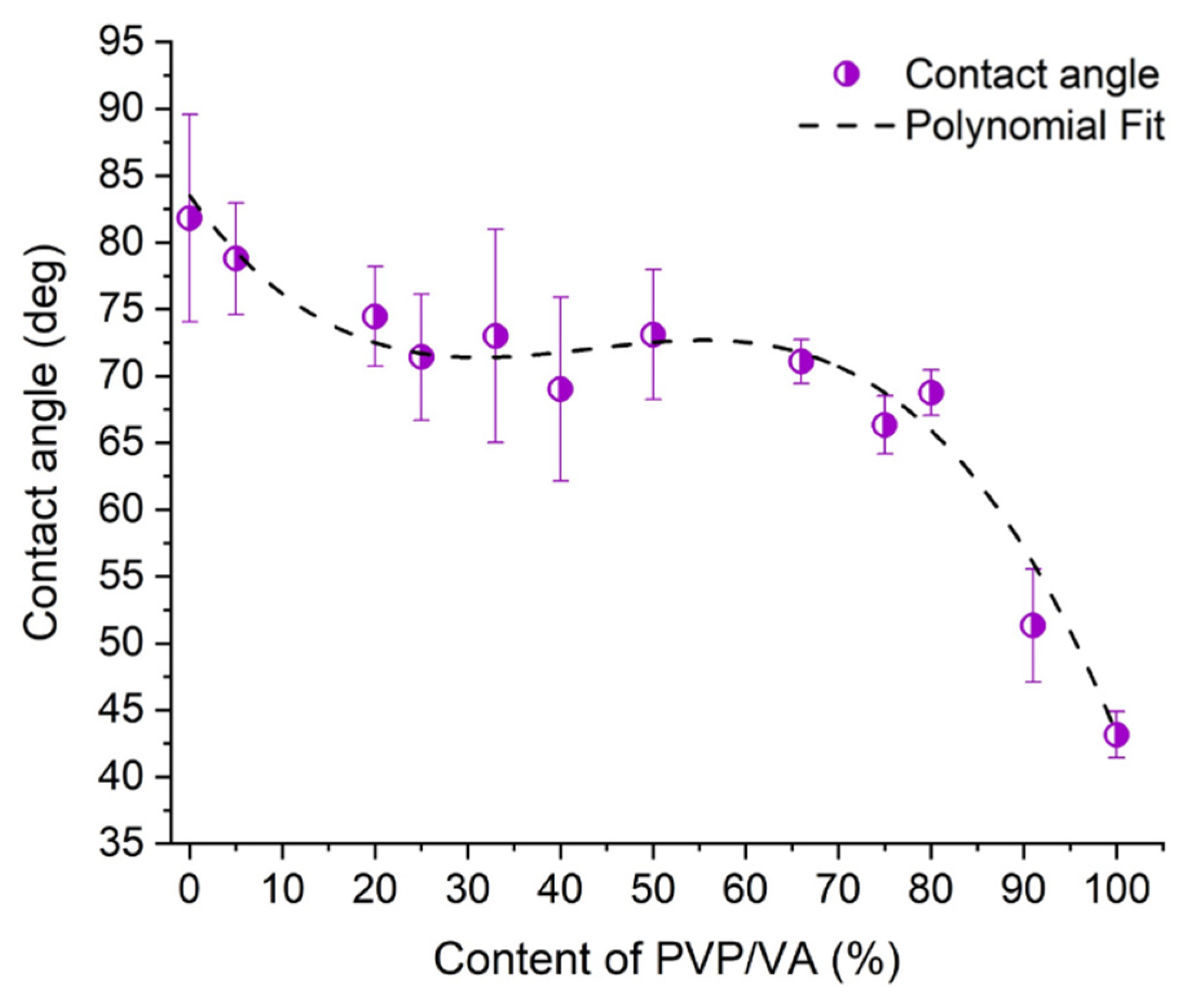
2.9. Wettability Study
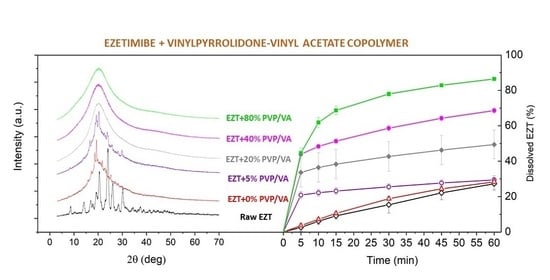
3. Results
3.1. Particle Size and Morphology
3.2. Molecular Structure and Interactions
3.3. Thermal Properties
3.4. Wettability Study
3.5. Dissolution Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–17858. [Google Scholar] [CrossRef]
- Russell, C.; Sheth, S.; Jacoby, D. A Clinical Guide to Combination Lipid-Lowering Therapy. Curr. Atheroscler. Rep. 2018, 20, 19. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef]
- Alenghat, F.J.; Davis, A.M. Management of Blood Cholesterol. JAMA 2019, 321, 800. [Google Scholar] [CrossRef]
- Kosoglou, T.; Statkevich, P.; Johnson-Levonas, A.O.; Paolini, J.F.; Bergman, A.J.; Alton, K.B. Ezetimibe: A Review of its Metabolism, Pharmacokinetics and Drug Interactions. Clin. Pharmacokinet. 2005, 44, 467–494. [Google Scholar] [CrossRef]
- Clader, J.W. Ezetimibe and other Azetidinone Cholesterol Absorption Inhibitors. Curr. Top. Med. Chem. 2005, 5, 243–256. [Google Scholar] [CrossRef]
- Nutescu, E.A.; Shapiro, N.L. Ezetimibe: A Selective Cholesterol Absorption Inhibitor. Pharmacotherapy 2003, 23, 1463–1474. [Google Scholar] [CrossRef]
- Shimpi, M.R.; Childs, S.L.; Boström, D.; Velaga, S.P. New cocrystals of ezetimibe with L-proline and imidazole. Crys. Eng. Comm. 2014, 16, 8984–8993. [Google Scholar] [CrossRef]
- Mulye, S.P.; Jamadar, S.A.; Karekar, P.S.; Pore, Y.V.; Dhawale, S.C. Improvement in physicochemical properties of ezetimibe using a crystal engineering technique. Powder Technol. 2012, 222, 131–138. [Google Scholar] [CrossRef]
- Parmar, K.R.; Shah, S.R.; Sheth, N.R. Preparation, Characterization, and In Vitro Evaluation of Ezetimibe Binary Solid Dispersions with Poloxamer 407 and PVP K30. J. Pharm. Innov. 2011, 6, 107–114. [Google Scholar] [CrossRef]
- Parmar, K.R.; Shah, S.R.; Sheth, N.R. Studies in Dissolution Enhancement of Ezetimibe by Solid Dispersions in Combination with a Surface Adsorbent. Dissolut. Technol. 2011, 18, 55–61. [Google Scholar] [CrossRef]
- Gulsun, T.; Gursoy, R.N.; Oner, L. Design and Characterization of Nanocrystal Formulations Containing Ezetimibe. Chem. Pharm. Bull. 2011, 59, 41–45. [Google Scholar] [CrossRef]
- Ha, E.-S.; Kim, J.-S.; Baek, I.-h.; Hwang, S.-J.; Kim, M.-S. Enhancement of dissolution and bioavailability of ezetimibe by amorphous solid dispersion nanoparticles fabricated using supercritical antisolvent process. J. Pharm. Investig. 2015, 45, 641–649. [Google Scholar] [CrossRef]
- Taupitz, T.; Dressman, J.B.; Klein, S. New formulation approaches to improve solubility and drug release from fixed dose combinations: Case examples pioglitazone/glimepiride and ezetimibe/simvastatin. Eur. J. Pharm. Biopharm. 2013, 84, 208–218. [Google Scholar] [CrossRef]
- Taylor, L.S.; Zhang, G.G.Z. Physical chemistry of supersaturated solutions and implications for oral absorption. Adv. Drug Deliv. Rev. 2016, 101, 122–142. [Google Scholar] [CrossRef]
- Zografi, G.; Newman, A. Interrelationships Between Structure and the Properties of Amorphous Solids of Pharmaceutical Interest. J. Pharm. Sci. 2017, 106, 5–27. [Google Scholar] [CrossRef]
- Anderson, B.D. Predicting Solubility/Miscibility in Amorphous Dispersions: It Is Time to Move Beyond Regular Solution Theories. J. Pharm. Sci. 2018, 107, 24–33. [Google Scholar] [CrossRef]
- Newman, A.; Knipp, G.; Zografi, G. Assessing the performance of amorphous solid dispersions. J. Pharm. Sci. 2012, 101, 1355–1377. [Google Scholar] [CrossRef]
- Rumondor, A.C.F.; Ivanisevic, I.; Bates, S.; Alonzo, D.E.; Taylor, L.S. Evaluation of Drug-Polymer Miscibility in Amorphous Solid Dispersion Systems. Pharm. Res. 2009, 26, 2523–2534. [Google Scholar] [CrossRef] [PubMed]
- Ivanisevic, I. Physical Stability Studies of Miscible Amorphous Solid Dispersions. J. Pharm. Sci. 2010, 99, 4005–4012. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J.H. Strategies to Address Low Drug Solubility in Discovery and Development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef] [PubMed]
- Rumondor, A.C.F.; Marsac, P.J.; Stanford, L.A.; Taylor, L.S. Phase Behavior of Poly(vinylpyrrolidone) Containing Amorphous Solid Dispersions in the Presence of Moisture. Mol. Pharm. 2009, 6, 1492–1505. [Google Scholar] [CrossRef] [PubMed]
- Marsac, P.J.; Shamblin, S.L.; Taylor, L.S. Theoretical and Practical Approaches for Prediction of Drug–Polymer Miscibility and Solubility. Pharm. Res. 2006, 23, 2417–2426. [Google Scholar] [CrossRef]
- Konno, H.; Taylor, L.S. Influence of different polymers on the crystallization tendency of molecularly dispersed amorphous felodipine. J. Pharm. Sci. 2006, 95, 2692–2705. [Google Scholar] [CrossRef] [PubMed]
- Vojinović, T.; Medarević, D.; Vranić, E.; Potpara, Z.; Krstić, M.; Djuriš, J.; Ibrić, S. Development of ternary solid dispersions with hydrophilic polymer and surface adsorbent for improving dissolution rate of carbamazepine. Saudi Pharm. J. 2018, 26, 725–732. [Google Scholar] [CrossRef]
- Trasi, N.S.; Taylor, L.S. Effect of polymers on nucleation and crystal growth of amorphous acetaminophen. Cryst. Eng. Commun. 2012, 14, 5188–5197. [Google Scholar] [CrossRef]
- Paudel, A.; Worku, A.; Meeus, J.; Guns, S.; Mooter, G. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: Formulation and process considerations. Int. J. Pharm. 2013, 453, 253–284. [Google Scholar] [CrossRef]
- Cal, K.; Sollohub, K. Spray drying technique. I: Hardware and process parameters. J. Pharm. Sci. 2010, 99, 575–586. [Google Scholar] [CrossRef]
- Fong, S.Y.; Ibisogly, A.; Bauer-Brandl, A. Solubility enhancement of BCS class II drug by solid phospholipid dispersions: Spray drying versus freeze-drying. Int. J. Pharm. 2015, 496, 382–391. [Google Scholar] [CrossRef]
- Wlodarski, K.; Sawicki, W.; Kozyra, A.; Tajber, L. Physical stability of solid dispersions with respect to thermodynamic solubility of tadalafil in PVP-VA. Eur. J. Pharm. Biopharm. 2015, 96, 237–246. [Google Scholar] [CrossRef]
- Sóti, P.L.; Bocz, K.; Pataki, H.; Eke, Z.; Farkas, A.; Verreck, G.; Kiss, É.; Fekete, P.; Vigh, T.; Wagner, I.; et al. Comparison of spray drying, electroblowing and electrospinning for preparation of eudragit E and itraconazole solid dispersions. Int. J. Pharm. 2015, 494, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Szafraniec, J.; Antosik, A.; Knapik-Kowalczuk, J.; Gawlak, K.; Kurek, M.; Szlęk, J.; Jamróz, W.; Paluch, M.; Jachowicz, R. Molecular Disorder of Bicalutamide-Amorphous Solid Dispersions Obtained by Solvent Methods. Pharmaceutics 2018, 10, 194. [Google Scholar] [CrossRef] [PubMed]
- Szafraniec-Szczęsny, J.; Antosik-Rogóż, A.; Knapik-Kowalczuk, J.; Kurek, M.; Szefer, E.; Gawlak, K.; Chmiel, K.; Peralta, S.; Niwiński, K.; Pielichowski, K.; et al. Compression-Induced Phase Transitions of Bicalutamide. Pharmaceutics 2020, 12, 438. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Van denMooter, A.G. Spray drying formulation of amorphous solid dispersions. Adv. Drug Deliv. Rev. 2016, 100, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Vo, C.L.-N.; Park, C.; Lee, B.-J. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2013, 85, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Smithey, P.G.D.; Taylor, L. Amorphous solid dispersions: An enabling formulation technology for oral delivery of poorly water soluble drugs. AAPS Newsmag. 2013, 16, 11–14. [Google Scholar]
- Califice, A.; Michel, F.; Dislaire, G.; Pirard, E. Influence of particle shape on size distribution measurements by 3D and 2D image analyses and laser diffraction. Powder Technol. 2013, 237, 67–75. [Google Scholar] [CrossRef]
- Reddy, B.P.; Reddy, K.R.; Reddy, R.R.; Reddy, D.M.; Reddy, K.S.C. Ezetimibe. Polymorphs. Patent WO2005009955A1, 2 March 2005. [Google Scholar]
- Knapik, J.; Wojnarowska, Z.; Grzybowska, K.; Hawelek, L.; Sawicki, W.; Wlodarski, K.; Markowski, J.; Paluch, M. Physical Stability of the Amorphous Anticholesterol Agent (Ezetimibe): The Role of Molecular Mobility. Mol. Pharm. 2014, 11, 4280–4290. [Google Scholar] [CrossRef]
- Knapik, J.; Wojnarowska, Z.; Grzybowska, K.; Jurkiewicz, K.; Tajber, L.; Paluch, M. Molecular Dynamics and Physical Stability of Coamorphous Ezetimib and Indapamide Mixtures. Mol. Pharm. 2015, 12, 3610–3619. [Google Scholar] [CrossRef]
- Baghel, S.; Cathcart, H.; O'Reilly, N.J. Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. J. Pharm. Sci. 2015, 105, 2527–2544. [Google Scholar] [CrossRef]
- Prajapati, P.; Pandey, J.; Shimpi, M.R.; Srivastava, A.; Tandon, P.; Velaga, S.P.; Sinha, K. Combined spectroscopic and quantum chemical studies of ezetimibe. J. Mol. Struct. 2016, 1225, 193–203. [Google Scholar] [CrossRef]
- Dahlberg, C.; Millqvist-Fureby, A.; Schuleit, M.; Furó, I. Polymer-drug interactions and wetting of solid dispersions. Eur. J. Pharm. Sci. 2010, 39, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Puri, K.; Dantuluri, A.K.; Kumar, M.; Karar, N.; Bansal, A.K. Wettability and surface chemistry of crystalline and amorphous forms of a poorly water soluble drug. Eur. J. Pharm. Sci. 2010, 40, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Karavas, E.; Ktistis, G.; Xenakis, A.; Georgarakis, E. Effect of hydrogen bonding interactions on the release mechanism of felodipine from nanodispersions with polyvinylpyrrolidone. Eur. J. Pharm. Biopharm. 2006, 63, 103–114. [Google Scholar] [CrossRef]
- Mehatha, A.S.; Suryadevara, V.; Lankapalli, S.R.; Deshmukh, A.M.; Sambath, L.P. Formulation and Optimization of Ezetimibe Containing Solid Dispersions Using Kollidon VA64. Turk. J. Pharm. Sci. 2014, 11, 113–126. [Google Scholar]
- Gil, E.C.; Colarte, A.I.; Sampedro, J.L.L.; Bataille, B. Subcoating with Kollidon VA 64 as water barrier in a new combined native dextran/HPMC-cetyl alcohol controlled release tablet. Eur. J. Pharm. Biopharm. 2008, 69, 303–311. [Google Scholar] [CrossRef]
- Jadhav, P.; Gokarna, V.; Deshpande, V.; Vavia, P. Bioavailability Enhancement of Olmesartan Medoxomil Using Hot-Melt Extrusion: In-Silico, In-Vitro, and In-Vivo Evaluation. AAPS Pharm. Sci. Tech. 2020, 21, 1–17. [Google Scholar] [CrossRef]
- Ziaee, A.; Albadarin, A.B.; Padrela, L.; Faucher, A.; O’Reilly, E.; Walker, G. Spray drying ternary amorphous solid dispersions of ibuprofen—An investigation into critical formulation and processing parameters. Eur. J. Pharm. Biopharm. 2017, 120, 43–51. [Google Scholar] [CrossRef]
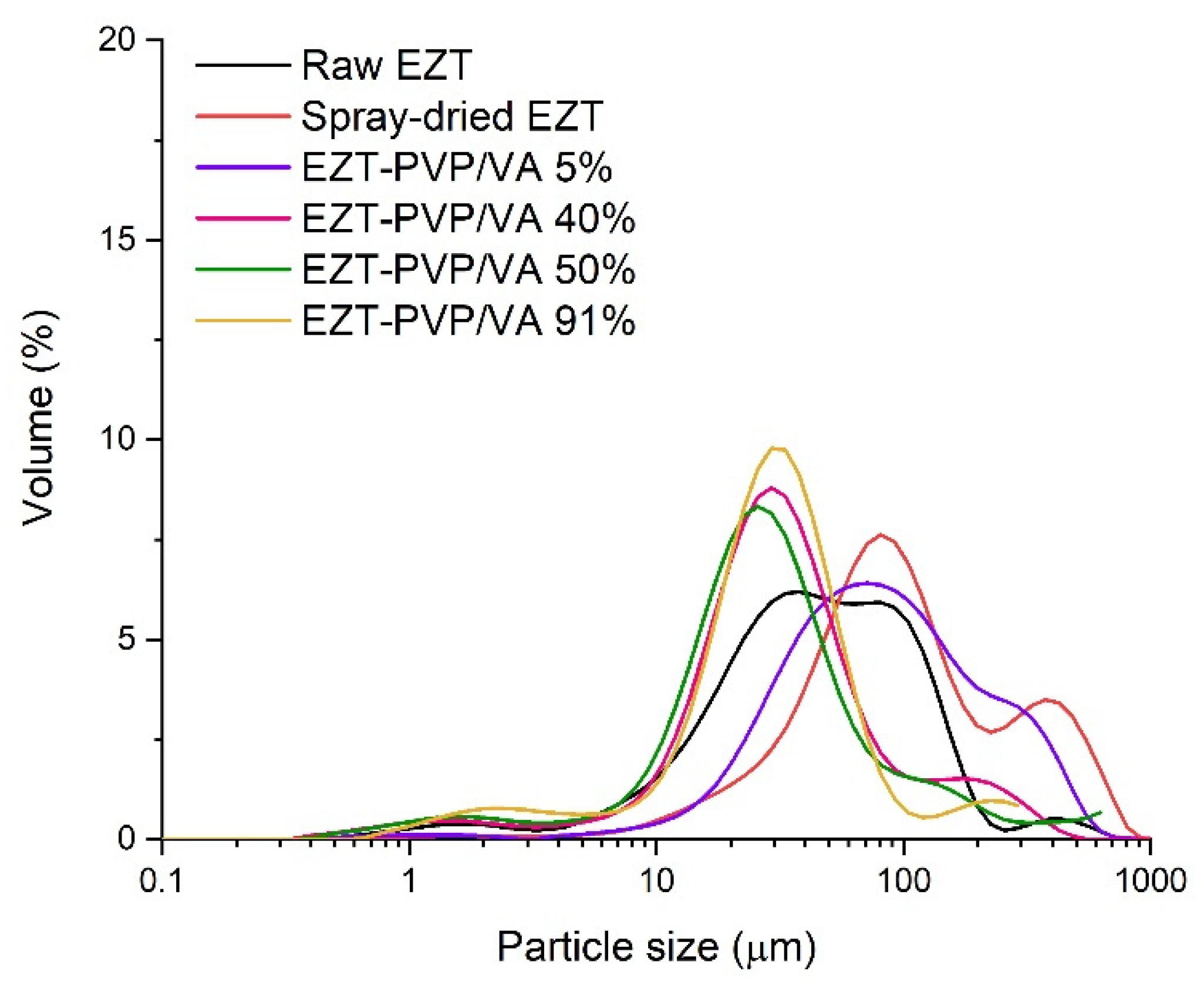

| System | Dv(50) ± SD (µm) | Span 1 |
|---|---|---|
| Raw EZT | 43.7 | 2.7 |
| Spray-dried EZT | 91.0 | 4.1 |
| EZT-PVP/VA 5% | 78.9 | 3.3 |
| EZT-PVP/VA 20% | 34.8 | 3.1 |
| EZT-PVP/VA 25% | 37.0 | 2.3 |
| EZT-PVP/VA 33% | 36.0 | 2.1 |
| EZT-PVP/VA 40% | 30.4 | 3.5 |
| EZT-PVP/VA 50% | 27.8 | 4.7 |
| EZT-PVP/VA 66% | 41.6 | 1.7 |
| EZT-PVP/VA 75% | 51.8 | 1.6 |
| EZT-PVP/VA 80% | 41.8 | 1.8 |
| EZT-PVP/VA 91% | 31.4 | 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szafraniec-Szczęsny, J.; Antosik-Rogóż, A.; Kurek, M.; Gawlak, K.; Górska, A.; Peralta, S.; Knapik-Kowalczuk, J.; Kramarczyk, D.; Paluch, M.; Jachowicz, R. How Does the Addition of Kollidon®VA64 Inhibit the Recrystallization and Improve Ezetimibe Dissolution from Amorphous Solid Dispersions? Pharmaceutics 2021, 13, 147. https://doi.org/10.3390/pharmaceutics13020147
Szafraniec-Szczęsny J, Antosik-Rogóż A, Kurek M, Gawlak K, Górska A, Peralta S, Knapik-Kowalczuk J, Kramarczyk D, Paluch M, Jachowicz R. How Does the Addition of Kollidon®VA64 Inhibit the Recrystallization and Improve Ezetimibe Dissolution from Amorphous Solid Dispersions? Pharmaceutics. 2021; 13(2):147. https://doi.org/10.3390/pharmaceutics13020147
Chicago/Turabian StyleSzafraniec-Szczęsny, Joanna, Agata Antosik-Rogóż, Mateusz Kurek, Karolina Gawlak, Anna Górska, Sebastian Peralta, Justyna Knapik-Kowalczuk, Daniel Kramarczyk, Marian Paluch, and Renata Jachowicz. 2021. "How Does the Addition of Kollidon®VA64 Inhibit the Recrystallization and Improve Ezetimibe Dissolution from Amorphous Solid Dispersions?" Pharmaceutics 13, no. 2: 147. https://doi.org/10.3390/pharmaceutics13020147
APA StyleSzafraniec-Szczęsny, J., Antosik-Rogóż, A., Kurek, M., Gawlak, K., Górska, A., Peralta, S., Knapik-Kowalczuk, J., Kramarczyk, D., Paluch, M., & Jachowicz, R. (2021). How Does the Addition of Kollidon®VA64 Inhibit the Recrystallization and Improve Ezetimibe Dissolution from Amorphous Solid Dispersions? Pharmaceutics, 13(2), 147. https://doi.org/10.3390/pharmaceutics13020147