Abstract
Applications of nanoparticles in various fields have been addressed. Nanomaterials serve as carriers for transporting conventional drugs or proteins through lysosomes to various cellular targets. The basic function of lysosomes is to trigger degradation of proteins and lipids. Understanding of lysosomal functions is essential for enhancing the efficacy of nanoparticles-mediated therapy and reducing the malfunctions of cellular metabolism. The lysosomal function is modulated by the movement of ions through various ion channels. Thus, in this review, we have focused on the recruited ion channels for lysosomal function, to understand the lysosomal modulation through the nanoparticles and its applications. In the future, lysosomal channels-based targets will expand the therapeutic application of nanoparticles-associated drugs.
1. Lysosomal Target of Nanoparticles (NPs) and Modulation of NPs for Lysosomal Function
1.1. pH Alteration
The primary function of the lysosome is the degradation of proteins and lipids [1,2]. The regulation of lysosomal pH has been linked to various cellular functions including the degradation of intracellular compartments. For its cellular functions, lysosomal lumen has to be maintained at an acidic pH [3]. Degradation of proteins, which is a crucial function of the lysosome, is carried out by more than 60 kinds of lysosomal hydrolases [4], and these hydrolases are optimized for the highly acidic environment of lysosomes (between pH 4.5 and 5.0) [4,5]. The lysosome as a cellular digestive system eliminates the garbage materials from autophagy and phagocytosis [6,7,8]. Thus, destabilization of lysosomal pH thorough alkalization leads to cellular toxicity and even causes lysosomal storage disease (LSD) [9,10,11]. The application of NPs can mediate various cellular functions by modulating lysosomal pH. Gold NPs (AuNPs) are known to reduce lysosomal activity by alkalization of the lysosomal lumen [11]. This reaction triggers oxidative stress, mitochondrial damage, and decreases cell migration/invasion [11]. In particular, 50-nm sized AuNPs induce autophagosomal accumulation of LC3 and block p62 degradation [12]. Silver NPs (AgNPs) also suppress autophagic responses by decreasing transcription factor EB (TFEB) protein expression, which is followed by lysosomal alkalization [13]. In addition, rare earth oxide NPs (REONPs)-mediated alkalization induces the activation of interleukin-1β IL-1β by an inflammasome [14].
1.2. Cell Viability
The lysosome consists of a typical single phospholipid bilayer to control important cellular functions [15,16]. The lysosomal membrane acts as the connector to contact other compartments such as autophagosome [17,18], mitochondria [19], and endoplasmic reticulum (ER) [20]. On the lysosomal membrane, numerous proteins play important roles such as the mammalian target of rapamycin complex 1 (mTORC1) (nutrient sensing) [21], V-ATPase (Vacuolar type of H+-ATPase) (pH homeostasis) [22], and ion channels/transporters [23]. In addition, deficiency of several lysosomal membrane proteins trigger various diseases such as the Danon disease (lysosome associated membrane proteins, LAMP-2) [24], malignant infantile osteopetrosis (the chloride channel 7, CLC-7) [25], and actin myoclonus-renal failure syndrome (lysosomal integral membrane protein-2) [26]. Damaged lysosome mediates lysosomal membrane permeabilization (LMP), which contributes to cell death [27,28] and induces several diseases such as LSD and other neurodegenerative disease [29,30,31]. Numerous NPs can have membrane damaging effects such as polystyrene NPs (PNPs) [32,33,34], silica dioxide (SiO2) NPs [35], titanium dioxide (TiO2) NPs [36], and Gd2O3:Eu3+ (Gd2O3) NPs [37], and, thus, cause cellular malfunctions. The PNPs (especially positive-charged) block autophagic flux [32], and release cathepsins (proteolytic enzymes), which induce cell death [34]. In addition, the LMP of other NPs reveal NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome (SiO2NPs) [35] and necrosis (Gd2O3 NPs) [37].
1.3. Protein Activity and Expression
Various lysosomal functions are mediated by more than 200 integral lysosomal membrane proteins [4], including (1) the mechanistic target of mTORC1, which is activated by nutrient starvation [28,38], and acts as a negative regulator of autophagy [28,39], and (2) LAMPs, which protect the lysosomal membrane against lysosomal hydrolases not to degrade [40]. NPs induce an inhibitory effect on the mTORC1 pathway to activate autophagy: AgNPs (decreases lysosomal protease activities) [41], Zinc oxide (ZnO) NPs (induces macrophage cell death) [42], and REONPs (induces lysosomal imbalance by TFEB nucleus translocation) [43]. ZnO NPs induce an aberrant expression pattern and de-glycosylation of LAMP-2 by ZnO-induced reactive oxygen species (ROS), which trigger cell death in lung epithelial cells [44]. Additionally, NPs modulate lysosomal motility [45]. Lysosome movement reveals two directions: toward the peripheral cytoplasm (anterograde) [46,47] and juxtanuclear region (retrograde) [48]. To carry out autophagic flux, lysosomes have to move to the juxtanuclear region [22,38], and the dynein complex is the motor protein for retrograde transport [49]. Treatment with carbon nanotubes decreases the expression of synaptosomal-associated protein (SNAP), which is a regulating factor of dynein [50] that blocks retrograde transport and, thus, the autophagic pathway [45]. Taken together, the lysosomal pathways of NPs and occupied proteins may mediate numerous functions. Thus, careful and more extensive consideration of lysosomal-associated NPs needs to be done.
1.4. Accumulation of NPs
Toxic cellular components, such as cytoplasmic macromolecules, damaged or misfolded proteins, and other worn-out organelles, are removed by lysosomes to maintain metabolic homeostasis [3]. Thus, the degradation role of lysosomes is essential for carrying out cellular homeostasis [51] including lipid catabolism [52], cell growth [53], and neurotransmission [54]. However, several NPs interrupt lysosomal degradation and deposit the lysosomal compartment in the cytoplasm. Exposure to AgNPs and copper oxide (CuO) NPs can induce agglomeration of lysosomes and subsequent cellular damage, which leads to cell death in human lung alveolar epithelial cells [55] and human umbilical vein endothelial cells [56]. In addition, NPs can accumulate in lysosomes. SiO2NPs and PNPs impair cell viability and induce lysosomal swelling, which is followed by their accumulation in lysosomes and triggers lysosomal dysfunction and apoptosis [57,58].
2. Regulation of Lysosomal pH and Its Physiological Function
The lysosomal pH gradient is generated and maintained by movement of hydrogen ions (H+) into the lysosomes through the action of vacuolar-type ATPases (V-ATPases) [59], which is supplemented further by movement of other ions [5]. Thus, for effective and continuous movement of H+ into the lysosome, an accompanying counter-ion movement is necessary [5].
The lysosomal V-ATPases consists of two domains: V1 domain, which hydrolyses ATP, and the V0 domain, which translocates H+ ions across the lysosomal membrane [60]. The catalytic domain V1, drives a rotary H+ transport motor by hydrolyzing ATP with translocation of H+ [61,62]. In this case, the V-ATPase rotor is operated in only one direction with an irreversible ATP hydrolysis due to the movement of H+ from cytosol to the lysosomal lumen [5]. The continuous V-ATPase-mediated H+ pumping generates a positive charge in the lysosomal lumen, which inhibits any further movement of H+ [63]. To dissipate this membrane potential, other ions have to be transferred in the opposite direction, and this process is referred to as the counterion flux [5,63]. Counter ion movement is suggested as both entering anions and exiting cations through the lysosomal lumen [5]. One important counter ionic candidate is chloride, transferred by CLC-7, as attenuation of CLC-7 leads to lysosomal dysfunction such as LSD and osteopetrosis [25,64]. Another candidate counter ion is K+, transferred by TMEM175. Its mutation induces neuronal degeneration and LSD [65]. The R740S mutant osteoclasts, mutated in the V-ATPase α3 subunit, possess a higher lysosomal pH, and shows altered mTORC expression (increase in basal protein level and decrease of gene expression) and activity, which, in turn, plays a key role in cell proliferation [57,66]. Additionally, acidification of lysosomes can induce macrophages to secrete N-acetyl-β-D-glucosaminidase through lysosomal exocytosis [67,68], which includes absorption of cytochrome c in rat kidney during renal metabolism [69], and transport of cystine, the product of protein degradation by cathepsin, from lysosomes to cytosol [70]. Thus, alteration of lysosomal pH can be like a commander’s order to modulate the cellular life cycle.
3. Lysosome-Associated Ion Channels for Lysosomal Function
The lysosomal function is modulated by the ion movement and subsequent pH regulation. This movement is accomplished through various ion channels (Figure 1). We have previously reported application of NPs on various channels [71]. In this section, we summarize the recruited channels for lysosomal function to understand the lysosomal modulation through the NPs (Table 1).
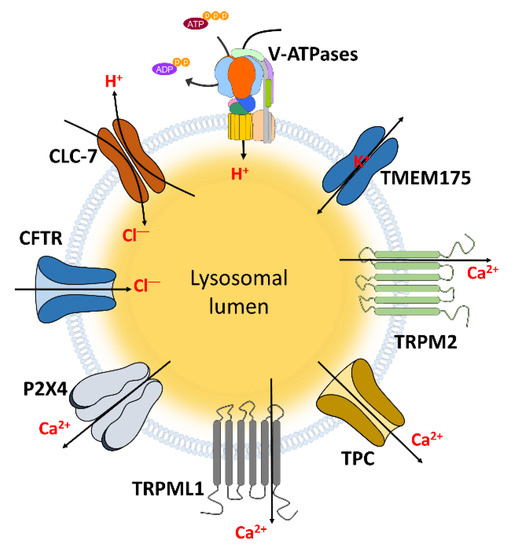
Figure 1.
The channels localized in lysosomal membrane to transport ions. These channels and transporters can regulate lysosomal and cellular functions through transporting and maintaining hydrogen, chloride, Ca2+, and potassium which indicated in Table 1.

Table 1.
The relationship between lysosomal ion channels and cellular functions.
3.1. CLC
CLC channels are the chloride channels that play a critical role in lysosomal function. CLC channels consist of two major isotypes: plasma membrane-associated (CLC-1, -2, and -Ka/-Kb) and intracellular organelle-associated (CLC-3 to CLC-7) [114,115]. Among the intracellular organelle-associated CLC, CLC-3 channel promotes lysosomal acidification and induces bone resorption [72,73]. Deletion of CLC-6 DNA leads to LSD in neuronal cells [74]. Particularly, CLC-7 channel—a chloride/H+ antiporter—is a well characterized CLC channel that serves as a major pathway for chloride ion, and in lysosomes [116,117,118]. As mentioned above, CLC-7 has a regulatory role in lysosomes and inhibition of CLC-7 leads to various diseases such as LSD and osteopetrosis [25,64,81,82,83,84]. Lysosomal acidification is essential for osteoclast-mediated bone resorption. Mutations in the CLC-7 channel can inhibit the lysosomal acidification in an osteoclast [75,76] and trigger osteopetrosis [81,84]. CLC-7-deficient mice show LSD and neurodegeneration, which is followed by retinal degeneration [64,82]. For lysosomal acidification, the CLC-7 channel has to be trafficked to the lysosomes, supported by Ostm1 [119]. Acidification of lysosomes and activation of microglial cells both require CLC-7 channel trafficking to lysosomes for the degradation of amyloid-β peptide (fAβ) deposition, which drives Alzheimer’s disease (AD) [79,80]. Additionally, it has also been reported that deletion of the CLC-7 channel reduces the dentinogenesis and dental bone formation [77,78].
3.2. Cystic Fibrosis (CF) Transmembrane Conductance Regulator (CFTR)
CFTR is an ATP-binding protein, which is regulated by its phosphorylation regulatory (R) domains, and transports chloride among other anions including bicarbonate ion (HCO3−) [120,121,122,123,124]. Mutation of CFTR causes defects in fluid secretion and is responsible for the genetic disease CF [120,124,125]. A prevalent cause of CF results from a deletion of the 508th positioned phenylalanine (ΔF508) even though several other mutations have been identified in CF [120,121,125]. CFTR has been reported to support lysosomal acidification and is localized in intra-organellar components, including ER, Golgi, and endo/lysosomes [126,127]. In CF cells, which have a ΔF508 mutation in CFTR, lysosomal pH is higher than in normal cells [85]. CFTR-null macrophages showed a defective killing function of internalized bacteria by inhibiting phago-lysosomal fusion [86]. Typically, these macrophages kill bacteria by phago-lysosomal ingestion, which is followed by lysosomal acidification [86,127]. This suggests that CFTR-mediated lysosomal acidification can regulate bacteria-killing activity of macrophages. Additionally, activation of CFTR leads to re-acidification of alkalinized lysosomes in retinal pigmented epithelial cells, which suggests it is a useful target for lysosomal clearance [128].
3.3. TRPs
The TRP channels, grouped into six subfamilies of TRPC, TRPV, TRPM, TRPA, TRPP, and TRPML (transient receptor potentials canonical, vanilloid, melastatin, ankyrin, polycystic, and mucolipin, respectively), are cation permeable channels, composed of six transmembrane domains [129,130]. These channels, with their numerous subtypes, have various functions. In particular, TRPM2 and TRPML1-3 play important roles in lysosomes (only four subtypes are localized in the lysosomal membrane) [104,129,131].
3.3.1. TRPM2
The TRPM2 channel is one of the TRPM family cation channels, which is activated by adenosine diphosphate ribose (ADPR) [132,133,134,135], adenine dinucleotide (NAD) [132,136], ROS [135,136,137], and extra/intra-cellular Ca2+ [138,139,140]. TRPM2 is located to numerous tissues and cellular compartments and has various activation mechanisms (Figure 2). Thus, the Ca2+ ion influx through TRPM2 plays multifunctional roles [141,142,143]. TRPM2 is also localized on the lysosomal membrane and modulates cellular functions such as cell migration, cytoskeleton remodeling, and apoptosis [87,88,89]. On the lysosomal membrane of dendritic cells (DC), TRPM2 releases Ca2+ ions to the cytoplasm to mediate optimal DC maturation and DC migration and homing to lymph nodes [87]. H2O2-induced Ca2+ influx increases through lysosomal TRPM2 and triggers actin remodeling, which, subsequently, activates cell migration, even though the extracellular Ca2+ entry does not affect the cytoskeletal remodeling [88]. Additionally, lysosomal TRPM2 Ca2+ ion release in pancreatic β cells induces apoptosis [89]. On the other hand, plasma membrane-localized TRPM2 mediates lysosomal damage via LMP and is associated with NLRP3 inflammasome-activation and mitochondrial fission [90,91].
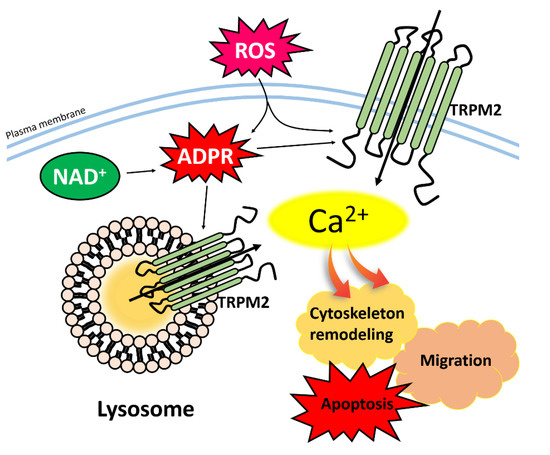
Figure 2.
Activation of TRPM2 channel and cellular function. ADPR, NAD, and ROS induce up-regulation of intracellular Ca2+ concentration through the TRPM2 and, subsequently, mediate with cell migration, cytoskeleton remodeling, and apoptosis. Abbreviations: TRPM2: Transient receptor potentials melastatin 2; ADPR: Adenosine diphosphate ribose; NAD: Adenine dinucleotide; ROS: Reactive oxygen species.
3.3.2. TRPMLs
TRPMLs (all three subtypes, TRPML1-3) are the main cation channels in the endo-lysosomal membrane, and regulate endo-lysosomal cation homeostasis, trafficking, and other cellular functions including intracellular compartment-acidification [104,131,144,145,146,147,148,149,150,151]. At the same time, TRPML1 is the main channel for lysosomal Ca2+ ion releases. TRPML2 and TRPML3 also have important roles in endosomal vesicles: regulation of TRPML2 is involved in the Arf6 recycling pathway [152], innate immune response [153], and B cell development [144,154]. The regulation of TRPML3 is involved in sensing lysosome neutralization [155], hearing functions [156,157], membrane trafficking, and autophagy [158]. Lysosomal Ca2+ ion-release through TRPML1 plays a major role in autophagy, mediated by starvation-induced mTORC1 deactivation and TFEB-induced autophagic gene expression [159,160] with simultaneous regulation of lysosomal acidification [92].
TRPML1 can regulate various cellular functions such as large particle phagocytosis through lysosomes [96], autophagosome biogenesis [161], elimination of bacterial pathogens through lysosome activation [162,163], bone remodeling in osteoclastogenesis [94], gastric acid secretion [93], and coronary arterial myocytes apoptosis [95]. In addition, the TRPML1 can reduce the enlargement of the lysosome by activating calmodulin [164]. Since TRPML1 has numerous functions, its deficiency can trigger various diseases, including stomach hypertrophy and hypergastrinemia [93], LSD [97,98,99], mucolipidosis type IV [100,101,102], Niemann-Pick disease type C (NPC) [97], and AD [103].
3.4. TMEM175
Intra-organelle K+ channel TMEM175 was recently identified in endosomes and lysosomes and is involved in the modulation of luminal pH stability and autophagosomes [165]. Deficiency of TMEM175 results in dysregulated lysosomal pH, impaired autophagosome clearance, and mitochondrial dysfunction in the neuronal system [166]. In addition to TMEM175, Ca2+-activated large conductance K+ channel also localizes to the lysosome and is involved in lysosomal Ca2+ signaling and lipid accumulation [104,105], which suggests lysosomal K+ channels can be considered the new target of neurodegenerative diseases such as LSD.
3.5. Other Ca2+ Channels
3.5.1. Two Pore Channels (TPCs)
TPCs are the key components of Ca2+ signaling in the endo-lysosomal system including TRPML and TRP channels and have been extensively reviewed in various studies [167,168,169]. The TPC1-3 are identified in the endo-lysosome [170,171,172,173,174] and stimulated by nicotinic acid adenine dinucleotide phosphate and phosphatidylinositol 3,5-bisphosphate [171,175,176,177,178]. The roles and pathways of TPCs have been addressed in various organs and biological systems. The inhibition of the TPC channel abolishes the migration of metastatic cancer cells by disrupting the trafficking mechanism of β1-integrin and the formation of leading edges [179]. The TPCs are involved in the autophagic flux of mouse cardiomyocytes [180]. It has been discussed that TPC2 is involved in autophagy progression, cancer cell migration, and cellular pigmentation [106,107,108]. Additionally, signaling events of Parkinson’s disease involve the regulation of TPCs in trafficking [109,110].
3.5.2. P2X4
The P2X4 receptor is expressed ubiquitously in cells from immune, nervous, muscle, and vascular systems [181,182,183]. The P2X4 is stable within the acidic environment of the lysosome and also traffics to the plasma membrane to enhance the phagocytic function [181,184]. P2X4 is activated by ATP and inhibited by the luminal acidic pH in the lysosome [185]. P2X4 consist of an ATP-activated Ca2+ channel and is involved in calmodulin activation to promote endo-lysosomal fusion of intracellular organelles [111,112]. P2X4 is also involved in liver fibrogenesis [113] and alcohol-induced microglial damage [186]. Although P2X4 has been associated with ATP-dependent signaling in the endo-lysosome, further studies are still needed in the future.
4. NP-Induced Proton Sponge Effect through Ion Channels in the Tumor System
Swelling of lysosomes has the potential to increase cellular toxicity by releasing lysosomal compartments and nanoparticles [187,188]. The lysosomal ‘proton sponge effect’ is triggered by the influx of cationic nanoparticles with hydrogen and chloride ions to lysosomes [188]. Accumulated ions in the lysosome may trigger water intake to equilibrate the physiological osmolarity and, subsequently, induce lysosomal rupture [188]. It has been addressed that conceptual use of the lysosomal pH-dependent system and lysosomal rupture develops the self-assembled luminescent AuNPs by the swelling property [189]. In a previous study, we reported that the cationic nanorod conjugated with doxorubicin (DOX) (AuNR-DOX) induced lysosomal swelling and rupture with increased apoptosis (Figure 3) [190]. Lee et al. reported that encapsulated AuNR-DOX in lysosomes is dissociated with DOX by lysosomal hydrolases. A charged linker of AuNR is opened and then recruited negative charged ions such as chloride into the lysosome. The ionic accumulation is developed, and lysosomal rupture occurred. Released chloride from the lysosome through lysosomal rupture activates Ca2+ influx channel TRPM2 in the plasma membrane and, lastly, overload of Ca2+ triggers the enhanced apoptotic effect including the effect of DOX in cancer cells [190]. The intracellular mechanism of nanomaterials and its related channels is now started. However, the effect of nanoparticles on lysosomal ion channels and transporters has still been poorly studied. To use nanomaterials for medicines, understanding the relationship between nanoparticles and lysosomal ion channels has to be expanded.
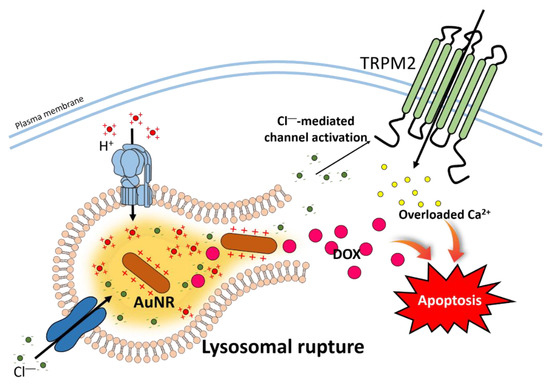
Figure 3.
Schematic cartoon illustrating mechanism of AuNR-DOX-induced apoptosis. The hydrolysis of AuNR-DOX induces AuNR to reflect positive charge and triggers chloride influx into lysosomes. Continued chloride influx leads excessive activation of V-ATPase, and lysosomal swelling and rupture to release DOX and chloride to the cytoplasm. The DOX and Ca2+ through the chloride-activated TRPM2 increase cellular apoptosis. Abbreviation: V-ATPase: Vacuolar type of H+-ATPase.
5. Clinical Application and Limitation of Nanomaterials
As mentioned earlier, NPs have a bio-toxic effect on lysosomes by triggering pH alteration, malfunctions of protein activity, accumulation in lysosomes, and subsequent cell death. We summarized the effect of NPs on cellular functions in Table 2. Accordingly, application of NPs has limitations for nanodrugs and nano-therapies. Thus, recent efforts have challenged to overcome these limitations by maximizing transport ability or reducing cytotoxicity.

Table 2.
The effect of nanoparticles (NPs) on cellular functions.
Nanomaterials can act as the carrier for conventional drugs by transporting drugs or proteins through lysosomes such as AuNRs conjugated with Naja kaouthia protein toxin 1 (NKCT1) (one of the snake toxin protein) [191], silk NPs conjugated with doxorubicin (anti-cancer drugs) [192], and AgNPs conjugated with salinomycin (killing agent for cancer stem cells) [193]. These nanomaterials can maximize drug delivery to reach the lysosome easily, and, subsequently, kill the cancer cells from leukemia [191], breast cancer [192], and ovarian cancer [193]. The “small size” of NPs, which is one of the typical characteristics, can be used to penetrate obstacles that conventional drugs cannot cross, especially the blood brain barrier (BBB) [194]. One of the LSD, Gaucher’s type 3 disease, which occurs by accumulation of glucocerebroside in the brain can be cured by transporting enzymes into the brain [194]. A recent study demonstrated the potential for transporting enzymes across the BBB by using a recombinant arylsulfatase enzyme with polysorbate 80 coated poly-butyl cyanoacrylates NPs [195].
Although biocompatible nanodrugs have been developed, which are made of albumin-based [196,197,198] and lipid-based [199,200] nanoparticles, various studies have attempted to eliminate the toxicity of NPs via conjugation with other materials. For example, iron oxide NPs that induce autophagosome accumulation and impair lysosomes can be rendered bio-safe by coating with poly(lactic-co-glycolic acid) (PLGA) [193]. ZnO NPs and Quantum Dots that induce lysosomal damage with the generation of ROS can be stabilized by coating with α-linolenic acid [201] and 3-mercaptopropionic acid [202]. There are non-toxic nanomaterials that can be degraded into lysosomes, such as nano-diamonds, which are delivered to lysosomes by coating with ubiquitin, to associate with autophagy receptors: sequestosome 1 [203], Ca2+ binding and coiled-coil domain 2 [204,205], and optineurin [206]. Additionally, PLGA NPs are degraded easily in the autophagy pathway [207]. Adjustment of the NPs size can avoid lysosomal accumulation: 60 nm-sized TiO2 NPs are more aggregated and more destabilized in the lysosomal membrane than 180 nm-sized TiO2 in the lysosomes and endosomes [208].
6. Future Perspectives
The primary lysosomal function is to maintain cellular homeostasis. Various attempts of drug delivery systems including nanomaterials and other new paradigms against diseases were engaged (Figure 4). However, a plethora of questions should be answered about nano therapy against lysosomal targets or lysosomal pathways. Although our limited knowledge about the effect of nanomaterials on lysosomal function has been posted, its therapeutic potential cannot be neglected. Nanomaterials are attractive machinery, as carriers for conventional drugs for therapeutic purposes. In addition to the role of the attractive carrier, other unfavorable characteristics of nanomaterials including toxicity should be considered while developing the therapeutic strategies. Understanding the functional support of ion channels or transporters on the lysosome will be expanded further in the coming years and, subsequently, favorable potential of nanomaterial-based therapy will also improve.
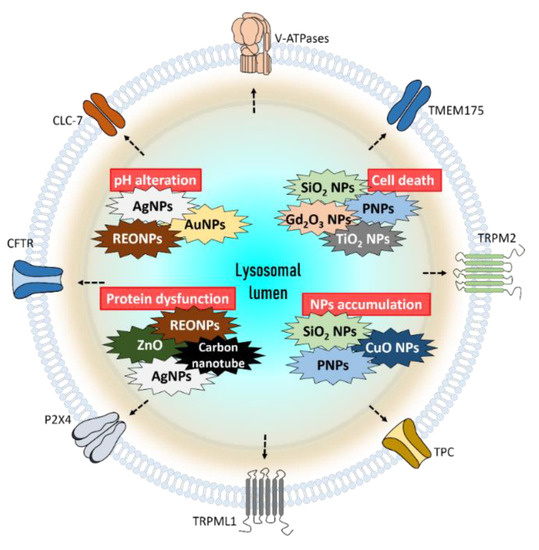
Figure 4.
The summarized role of nanoparticles which effect lysosomes and cellular functions. Exposure to AgNPs, AuNPs, and REONPs induces alkalization of lysosomal lumen. SiO2 NPs, Gd2O3 NPs, PNPs, and TiO2 NPs damage lysosomes, which finally trigger cell death. The lysosomal protein can be damaged by ZnO, REONPs, AgNPs, and carbon nanotubes. Accumulation of SiO2 NPs, PNPs, and CuO NPs can induce lysosomal dysfunction. Abbreviations: REONP: rare earth oxide nanoparticle; PNP: polystyrene nanoparticle
Author Contributions
J.H.H. contributed to the conception and design of the manuscript. D.L. collected the information, drafted the article and figures, and critically revised it for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
The National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT; [NRF-2019R1F1A1046785]) and the grant from the Gachon University Gil Medical Center project [FRD 2018-07] supported this work.
Conflicts of Interest
Authors declare no conflict of interest.
References
- De Duve, C.; Wattiaux, R. Functions of lysosomes. Annu. Rev. Physiol. 1966, 28, 435–492. [Google Scholar] [CrossRef]
- de Duve, C. The lysosome turns fifty. Nat. Cell Biol. 2005, 7, 847–849. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.M.; Zoncu, R. The Lysosome as a Regulatory Hub. Annu. Rev. Cell Dev. Biol. 2016, 32, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Guardia, C.M.; Keren-Kaplan, T.; Bonifacino, J.S. Mechanisms and functions of lysosome positioning. J. Cell Sci. 2016, 129, 4329–4339. [Google Scholar] [CrossRef]
- Mindell, J.A. Lysosomal acidification mechanisms. Annu. Rev. Physiol. 2012, 74, 69–86. [Google Scholar] [CrossRef]
- Walkley, S.U. Pathogenic mechanisms in lysosomal disease: A reappraisal of the role of the lysosome. Acta Paediatr. 2007, 96, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Hipolito, V.E.B.; Ospina-Escobar, E.; Botelho, R.J. Lysosome remodelling and adaptation during phagocyte activation. Cell Microbiol. 2018, 20. [Google Scholar] [CrossRef]
- Herb, M.; Gluschko, A.; Schramm, M. LC3-associated phagocytosis—The highway to hell for phagocytosed microbes. Semin. Cell Dev. Biol. 2019. [Google Scholar] [CrossRef]
- Folts, C.J.; Scott-Hewitt, N.; Proschel, C.; Mayer-Proschel, M.; Noble, M. Lysosomal Re-acidification Prevents Lysosphingolipid-Induced Lysosomal Impairment and Cellular Toxicity. PLoS Biol. 2016, 14, e1002583. [Google Scholar] [CrossRef]
- Stern, S.T.; Adiseshaiah, P.P.; Crist, R.M. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part. Fibre Toxicol. 2012, 9, 20. [Google Scholar] [CrossRef]
- Woldemichael, T.; Rosania, G.R. The physiological determinants of drug-induced lysosomal stress resistance. PLoS ONE 2017, 12, e0187627. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wu, Y.; Jin, S.; Tian, Y.; Zhang, X.; Zhao, Y.; Yu, L.; Liang, X.J. Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. ACS Nano 2011, 5, 8629–8639. [Google Scholar] [CrossRef] [PubMed]
- Miyayama, T.; Fujiki, K.; Matsuoka, M. Silver nanoparticles induce lysosomal-autophagic defects and decreased expression of transcription factor EB in A549 human lung adenocarcinoma cells. Toxicol. Vitr. 2018, 46, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ji, Z.; Qin, H.; Kang, X.; Sun, B.; Wang, M.; Chang, C.H.; Wang, X.; Zhang, H.; Zou, H.; et al. Interference in autophagosome fusion by rare earth nanoparticles disrupts autophagic flux and regulation of an interleukin-1beta producing inflammasome. ACS Nano 2014, 8, 10280–10292. [Google Scholar] [CrossRef]
- Winchester, B.G. Lysosomal membrane proteins. Eur. J. Paediatr. Neurol. 2001, 5, 11–19. [Google Scholar] [CrossRef]
- Schwake, M.; Schroder, B.; Saftig, P. Lysosomal membrane proteins and their central role in physiology. Traffic 2013, 14, 739–748. [Google Scholar] [CrossRef]
- Yu, S.; Melia, T.J. The coordination of membrane fission and fusion at the end of autophagosome maturation. Curr. Opin. Cell Biol. 2017, 47, 92–98. [Google Scholar] [CrossRef]
- Nascimbeni, A.C.; Codogno, P.; Morel, E. Phosphatidylinositol-3-phosphate in the regulation of autophagy membrane dynamics. FEBS J. 2017, 284, 1267–1278. [Google Scholar] [CrossRef]
- Wong, Y.C.; Kim, S.; Peng, W.; Krainc, D. Regulation and Function of Mitochondria-Lysosome Membrane Contact Sites in Cellular Homeostasis. Trends Cell Biol. 2019. [Google Scholar] [CrossRef]
- Helle, S.C.; Kanfer, G.; Kolar, K.; Lang, A.; Michel, A.H.; Kornmann, B. Organization and function of membrane contact sites. Biochim. Biophys. Acta 2013, 1833, 2526–2541. [Google Scholar] [CrossRef]
- Zoncu, R.; Bar-Peled, L.; Efeyan, A.; Wang, S.; Sancak, Y.; Sabatini, D.M. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 2011, 334, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Ostrowski, P.; Jaumouille, V.; Grinstein, S. The position of lysosomes within the cell determines their luminal pH. J. Cell Biol. 2016, 212, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Gu, M.; Xu, H. Lysosomal Ion Channels as Decoders of Cellular Signals. Trends Biochem. Sci. 2019, 44, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Nishino, I.; Fu, J.; Tanji, K.; Yamada, T.; Shimojo, S.; Koori, T.; Mora, M.; Riggs, J.E.; Oh, S.J.; Koga, Y.; et al. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease). Nature 2000, 406, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Kornak, U.; Kasper, D.; Bosl, M.R.; Kaiser, E.; Schweizer, M.; Schulz, A.; Friedrich, W.; Delling, G.; Jentsch, T.J. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 2001, 104, 205–215. [Google Scholar] [CrossRef]
- Berkovic, S.F.; Dibbens, L.M.; Oshlack, A.; Silver, J.D.; Katerelos, M.; Vears, D.F.; Lullmann-Rauch, R.; Blanz, J.; Zhang, K.W.; Stankovich, J.; et al. Array-based gene discovery with three unrelated subjects shows SCARB2/LIMP-2 deficiency causes myoclonus epilepsy and glomerulosclerosis. Am. J. Hum. Genet. 2008, 82, 673–684. [Google Scholar] [CrossRef]
- Wang, F.; Gomez-Sintes, R.; Boya, P. Lysosomal membrane permeabilization and cell death. Traffic 2018, 19, 918–931. [Google Scholar] [CrossRef]
- Mrschtik, M.; Ryan, K.M. Lysosomal proteins in cell death and autophagy. FEBS J. 2015, 282, 1858–1870. [Google Scholar] [CrossRef]
- Serrano-Puebla, A.; Boya, P. Lysosomal membrane permeabilization in cell death: New evidence and implications for health and disease. Ann. N. Y. Acad. Sci. 2016, 1371, 30–44. [Google Scholar] [CrossRef]
- Micsenyi, M.C.; Sikora, J.; Stephney, G.; Dobrenis, K.; Walkley, S.U. Lysosomal membrane permeability stimulates protein aggregate formation in neurons of a lysosomal disease. J. Neurosci. 2013, 33, 10815–10827. [Google Scholar] [CrossRef]
- Venkatesan, R.; Park, Y.U.; Ji, E.; Yeo, E.J.; Kim, S.Y. Malathion increases apoptotic cell death by inducing lysosomal membrane permeabilization in N2a neuroblastoma cells: A model for neurodegeneration in Alzheimer’s disease. Cell Death Discov. 2017, 3, 17007. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Popp, L.; Yang, J.; Kumar, A.; Gangoli, V.S.; Segatori, L. The autophagic response to polystyrene nanoparticles is mediated by transcription factor EB and depends on surface charge. J. Nanobiotechnol. 2015, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Lunova, M.; Prokhorov, A.; Jirsa, M.; Hof, M.; Olzynska, A.; Jurkiewicz, P.; Kubinova, S.; Lunov, O.; Dejneka, A. Nanoparticle core stability and surface functionalization drive the mTOR signaling pathway in hepatocellular cell lines. Sci. Rep. 2017, 7, 16049. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Salvati, A.; Boya, P. Lysosome-dependent cell death and deregulated autophagy induced by amine-modified polystyrene nanoparticles. Open Biol. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Jessop, F.; Hamilton, R.F., Jr.; Rhoderick, J.F.; Fletcher, P.; Holian, A. Phagolysosome acidification is required for silica and engineered nanoparticle-induced lysosome membrane permeabilization and resultant NLRP3 inflammasome activity. Toxicol. Appl. Pharm. 2017, 318, 58–68. [Google Scholar] [CrossRef]
- Popp, L.; Tran, V.; Patel, R.; Segatori, L. Autophagic response to cellular exposure to titanium dioxide nanoparticles. Acta Biomater. 2018, 79, 354–363. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, S.; Duan, J.; Jia, G.; Zhang, J. Europium-doped Gd2O3 nanotubes cause the necrosis of primary mouse bone marrow stromal cells through lysosome and mitochondrion damage. J. Inorg. Biochem. 2015, 146, 28–36. [Google Scholar] [CrossRef]
- Korolchuk, V.I.; Saiki, S.; Lichtenberg, M.; Siddiqi, F.H.; Roberts, E.A.; Imarisio, S.; Jahreiss, L.; Sarkar, S.; Futter, M.; Menzies, F.M.; et al. Lysosomal positioning coordinates cellular nutrient responses. Nat. Cell Biol. 2011, 13, 453–460. [Google Scholar] [CrossRef]
- Noda, T.; Ohsumi, Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J. Biol. Chem. 1998, 273, 3963–3966. [Google Scholar] [CrossRef]
- Eskelinen, E.L. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol. Asp. Med. 2006, 27, 495–502. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, M.; Zhang, T.; Du, E.; Liu, Y.; Qi, S.; Xu, Y.; Zhang, Z. Autophagic effects and mechanisms of silver nanoparticles in renal cells under low dose exposure. Ecotoxicol. Environ. Saf. 2018, 166, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Singh, S.K.; Chauhan, L.K.; Das, M.; Tripathi, A.; Dwivedi, P.D. Zinc oxide nanoparticles induce apoptosis by enhancement of autophagy via PI3K/Akt/mTOR inhibition. Toxicol. Lett. 2014, 227, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Shi, S.S.; Zhang, J.Q.; Zhang, Y.J.; Zhang, L.; Liu, Y.; Jin, P.P.; Wei, P.F.; Shi, R.H.; Zhou, W.; et al. Giant Cellular Vacuoles Induced by Rare Earth Oxide Nanoparticles are Abnormally Enlarged Endo/Lysosomes and Promote mTOR-Dependent TFEB Nucleus Translocation. Small 2016, 12, 5759–5768. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhang, J.; Wang, B.; Xu, G.; Zou, Z. LAMP-2 mediates oxidative stress-dependent cell death in Zn(2+)-treated lung epithelium cells. Biochem. Biophys. Res. Commun. 2017, 488, 177–181. [Google Scholar] [CrossRef]
- Cohignac, V.; Landry, M.J.; Ridoux, A.; Pinault, M.; Annangi, B.; Gerdil, A.; Herlin-Boime, N.; Mayne, M.; Haruta, M.; Codogno, P.; et al. Carbon nanotubes, but not spherical nanoparticles, block autophagy by a shape-related targeting of lysosomes in murine macrophages. Autophagy 2018, 14, 1323–1334. [Google Scholar] [CrossRef]
- Hirokawa, N.; Noda, Y. Intracellular transport and kinesin superfamily proteins, KIFs: Structure, function, and dynamics. Physiol. Rev. 2008, 88, 1089–1118. [Google Scholar] [CrossRef]
- Hollenbeck, P.J.; Swanson, J.A. Radial extension of macrophage tubular lysosomes supported by kinesin. Nature 1990, 346, 864–866. [Google Scholar] [CrossRef]
- Paschal, B.M.; Vallee, R.B. Retrograde transport by the microtubule-associated protein MAP 1C. Nature 1987, 330, 181–183. [Google Scholar] [CrossRef]
- Harada, A.; Takei, Y.; Kanai, Y.; Tanaka, Y.; Nonaka, S.; Hirokawa, N. Golgi vesiculation and lysosome dispersion in cells lacking cytoplasmic dynein. J. Cell Biol. 1998, 141, 51–59. [Google Scholar] [CrossRef]
- Yuzaki, M. Snapin snaps into the dynein complex for late endosome-lysosome trafficking and autophagy. Neuron 2010, 68, 4–6. [Google Scholar] [CrossRef][Green Version]
- Chun, Y.; Kim, J. Autophagy: An Essential Degradation Program for Cellular Homeostasis and Life. Cells 2018, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Ballabio, A. Lysosome: Regulator of lipid degradation pathways. Trends Cell Biol. 2014, 24, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.E.; Zoncu, R. The lysosome as a cellular centre for signalling, metabolism and quality control. Nat. Cell Biol. 2019, 21, 133–142. [Google Scholar] [CrossRef]
- Ferguson, S.M. Neuronal lysosomes. Neurosci. Lett. 2019, 697, 1–9. [Google Scholar] [CrossRef]
- Miyayama, T.; Matsuoka, M. Involvement of lysosomal dysfunction in silver nanoparticle-induced cellular damage in A549 human lung alveolar epithelial cells. J. Occup. Med. Toxicol. 2016, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zou, Z.; Wang, B.; Xu, G.; Wu, Q.; Zhang, Y.; Yuan, Z.; Yang, X.; Yu, C. Lysosomal deposition of copper oxide nanoparticles triggers HUVEC cells death. Biomaterials 2018, 161, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Schutz, I.; Lopez-Hernandez, T.; Gao, Q.; Puchkov, D.; Jabs, S.; Nordmeyer, D.; Schmudde, M.; Ruhl, E.; Graf, C.M.; Haucke, V. Lysosomal Dysfunction Caused by Cellular Accumulation of Silica Nanoparticles. J. Biol. Chem. 2016, 291, 14170–14184. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Bexiga, M.G.; Anguissola, S.; Boya, P.; Simpson, J.C.; Salvati, A.; Dawson, K.A. Time resolved study of cell death mechanisms induced by amine-modified polystyrene nanoparticles. Nanoscale 2013, 5, 10868–10876. [Google Scholar] [CrossRef]
- Ohkuma, S.; Moriyama, Y.; Takano, T. Identification and characterization of a proton pump on lysosomes by fluorescein-isothiocyanate-dextran fluorescence. Proc. Natl. Acad. Sci. USA 1982, 79, 2758–2762. [Google Scholar] [CrossRef]
- Cipriano, D.J.; Wang, Y.; Bond, S.; Hinton, A.; Jefferies, K.C.; Qi, J.; Forgac, M. Structure and regulation of the vacuolar ATPases. Biochim. Biophys. Acta 2008, 1777, 599–604. [Google Scholar] [CrossRef]
- Hirata, T.; Iwamoto-Kihara, A.; Sun-Wada, G.H.; Okajima, T.; Wada, Y.; Futai, M. Subunit rotation of vacuolar-type proton pumping ATPase: Relative rotation of the G and C subunits. J. Biol. Chem. 2003, 278, 23714–23719. [Google Scholar] [CrossRef]
- Yokoyama, K.; Nakano, M.; Imamura, H.; Yoshida, M.; Tamakoshi, M. Rotation of the proteolipid ring in the V-ATPase. J. Biol. Chem. 2003, 278, 24255–24258. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Nayak, S.; Mindell, J.A.; Grabe, M. A model of lysosomal pH regulation. J. Gen. Physiol. 2013, 141, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Kasper, D.; Planells-Cases, R.; Fuhrmann, J.C.; Scheel, O.; Zeitz, O.; Ruether, K.; Schmitt, A.; Poet, M.; Steinfeld, R.; Schweizer, M.; et al. Loss of the chloride channel ClC-7 leads to lysosomal storage disease and neurodegeneration. EMBO J. 2005, 24, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Checchetto, V.; Teardo, E.; Carraretto, L.; Leanza, L.; Szabo, I. Physiology of intracellular potassium channels: A unifying role as mediators of counterion fluxes? Biochim. Biophys. Acta 2016, 1857, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Betz, C.; Hall, M.N. Where is mTOR and what is it doing there? J. Cell Biol. 2013, 203, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Sundler, R. Lysosomal and cytosolic pH as regulators of exocytosis in mouse macrophages. Acta Physiol. Scand. 1997, 161, 553–556. [Google Scholar] [CrossRef]
- Tapper, H.; Sundler, R. Role of lysosomal and cytosolic pH in the regulation of macrophage lysosomal enzyme secretion. Biochem. J. 1990, 272, 407–414. [Google Scholar] [CrossRef]
- Camargo, M.J.; Sumpio, B.E.; Maack, T. Renal hydrolysis of absorbed protein: Influence of load and lysosomal pH. Am. J. Physiol. 1984, 247, F656–F664. [Google Scholar] [CrossRef]
- Smith, M.L.; Greene, A.A.; Potashnik, R.; Mendoza, S.A.; Schneider, J.A. Lysosomal cystine transport. Effect of intralysosomal pH and membrane potential. J. Biol. Chem. 1987, 262, 1244–1253. [Google Scholar]
- Lee, D.; Hong, J.H. Physiological application of nanoparticles in calcium-related proteins and channels. Nanomed. 2019. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, T.; Zhao, Z.; Weinman, S.A. The ClC-3 chloride channel promotes acidification of lysosomes in CHO-K1 and Huh-7 cells. Am. J. Physiol. Cell Physiol. 2002, 282, C1483–C1491. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, F.; Kajiya, H.; Toh, K.; Uchida, S.; Yoshikawa, M.; Sasaki, S.; Kido, M.A.; Tanaka, T.; Okabe, K. Intracellular ClC-3 chloride channels promote bone resorption in vitro through organelle acidification in mouse osteoclasts. Am. J. Physiol. Cell Physiol. 2008, 294, C693–C701. [Google Scholar] [CrossRef] [PubMed]
- Poet, M.; Kornak, U.; Schweizer, M.; Zdebik, A.A.; Scheel, O.; Hoelter, S.; Wurst, W.; Schmitt, A.; Fuhrmann, J.C.; Planells-Cases, R.; et al. Lysosomal storage disease upon disruption of the neuronal chloride transport protein ClC-6. Proc. Natl. Acad. Sci. USA 2006, 103, 13854–13859. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, K.; Gram, J.; Neutzsky-Wulff, A.V.; Jensen, V.K.; Dziegiel, M.H.; Bollerslev, J.; Karsdal, M.A. Characterization of acid flux in osteoclasts from patients harboring a G215R mutation in ClC-7. Biochem. Biophys. Res. Commun. 2009, 378, 804–809. [Google Scholar] [CrossRef]
- Henriksen, K.; Sorensen, M.G.; Jensen, V.K.; Dziegiel, M.H.; Nosjean, O.; Karsdal, M.A. Ion transporters involved in acidification of the resorption lacuna in osteoclasts. Calcif. Tissue Int. 2008, 83, 230–242. [Google Scholar] [CrossRef]
- Wen, X.; Lacruz, R.S.; Paine, M.L. Dental and Cranial Pathologies in Mice Lacking the Cl(-) /H(+) -Exchanger ClC-7. Anat. Rec. 2015, 298, 1502–1508. [Google Scholar] [CrossRef]
- Guo, J.; Bervoets, T.J.; Henriksen, K.; Everts, V.; Bronckers, A.L. Null mutation of chloride channel 7 (Clcn7) impairs dental root formation but does not affect enamel mineralization. Cell Tissue Res. 2016, 363, 361–370. [Google Scholar] [CrossRef][Green Version]
- Murphy, M.P.; LeVine, H., 3rd. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimers Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef]
- Majumdar, A.; Capetillo-Zarate, E.; Cruz, D.; Gouras, G.K.; Maxfield, F.R. Degradation of Alzheimer’s amyloid fibrils by microglia requires delivery of ClC-7 to lysosomes. Mol. Biol. Cell 2011, 22, 1664–1676. [Google Scholar] [CrossRef]
- Jentsch, T.J. Chloride and the endosomal-lysosomal pathway: Emerging roles of CLC chloride transporters. J. Physiol. 2007, 578, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wei, Q.; He, A.; Jia, R.; Xiao, Y. CLC-7: A potential therapeutic target for the treatment of osteoporosis and neurodegeneration. Biochem. Biophys. Res. Commun. 2009, 384, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Weinert, S.; Jabs, S.; Supanchart, C.; Schweizer, M.; Gimber, N.; Richter, M.; Rademann, J.; Stauber, T.; Kornak, U.; Jentsch, T.J. Lysosomal pathology and osteopetrosis upon loss of H+-driven lysosomal Cl- accumulation. Science 2010, 328, 1401–1403. [Google Scholar] [CrossRef]
- Sartelet, A.; Stauber, T.; Coppieters, W.; Ludwig, C.F.; Fasquelle, C.; Druet, T.; Zhang, Z.; Ahariz, N.; Cambisano, N.; Jentsch, T.J.; et al. A missense mutation accelerating the gating of the lysosomal Cl-/H+-exchanger ClC-7/Ostm1 causes osteopetrosis with gingival hamartomas in cattle. Dis. Model. Mech. 2014, 7, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Haggie, P.M.; Verkman, A.S. Unimpaired lysosomal acidification in respiratory epithelial cells in cystic fibrosis. J. Biol. Chem. 2009, 284, 7681–7686. [Google Scholar] [CrossRef] [PubMed]
- Di, A.; Brown, M.E.; Deriy, L.V.; Li, C.; Szeto, F.L.; Chen, Y.; Huang, P.; Tong, J.; Naren, A.P.; Bindokas, V.; et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat. Cell Biol. 2006, 8, 933–944. [Google Scholar] [CrossRef]
- Sumoza-Toledo, A.; Lange, I.; Cortado, H.; Bhagat, H.; Mori, Y.; Fleig, A.; Penner, R.; Partida-Sanchez, S. Dendritic cell maturation and chemotaxis is regulated by TRPM2-mediated lysosomal Ca2+ release. FASEB J. 2011, 25, 3529–3542. [Google Scholar] [CrossRef]
- Li, F.; Abuarab, N.; Sivaprasadarao, A. Reciprocal regulation of actin cytoskeleton remodelling and cell migration by Ca2+ and Zn2+: Role of TRPM2 channels. J. Cell Sci. 2016, 129, 2016–2029. [Google Scholar] [CrossRef]
- Lange, I.; Yamamoto, S.; Partida-Sanchez, S.; Mori, Y.; Fleig, A.; Penner, R. TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci. Signal. 2009, 2, ra23. [Google Scholar] [CrossRef]
- Abuarab, N.; Munsey, T.S.; Jiang, L.H.; Li, J.; Sivaprasadarao, A. High glucose-induced ROS activates TRPM2 to trigger lysosomal membrane permeabilization and Zn(2+)-mediated mitochondrial fission. Sci. Signal. 2017, 10. [Google Scholar] [CrossRef]
- Katsnelson, M.A.; Lozada-Soto, K.M.; Russo, H.M.; Miller, B.A.; Dubyak, G.R. NLRP3 inflammasome signaling is activated by low-level lysosome disruption but inhibited by extensive lysosome disruption: Roles for K+ efflux and Ca2+ influx. Am. J. Physiol. Cell Physiol. 2016, 311, C83–C100. [Google Scholar] [CrossRef] [PubMed]
- Soyombo, A.A.; Tjon-Kon-Sang, S.; Rbaibi, Y.; Bashllari, E.; Bisceglia, J.; Muallem, S.; Kiselyov, K. TRP-ML1 regulates lysosomal pH and acidic lysosomal lipid hydrolytic activity. J. Biol. Chem. 2006, 281, 7294–7301. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.; Zhou, H.; Li, Q.; Muallem, S.; Hofmann, S.L.; Soyombo, A.A. A role for the Ca2+ channel TRPML1 in gastric acid secretion, based on analysis of knockout mice. Gastroenterology 2011, 140, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Erkhembaatar, M.; Gu, D.R.; Lee, S.H.; Yang, Y.M.; Park, S.; Muallem, S.; Shin, D.M.; Kim, M.S. Lysosomal Ca2+ Signaling is Essential for Osteoclastogenesis and Bone Remodeling. J. Bone Min. Res. 2017, 32, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, X.; Walsh, S.W.; Zhang, Y.; Abais, J.M.; Boini, K.M.; Li, P.L. Intracellular two-phase Ca2+ release and apoptosis controlled by TRP-ML1 channel activity in coronary arterial myocytes. Am. J. Physiol. Cell Physiol. 2013, 304, C458–C466. [Google Scholar] [CrossRef] [PubMed]
- Samie, M.; Wang, X.; Zhang, X.; Goschka, A.; Li, X.; Cheng, X.; Gregg, E.; Azar, M.; Zhuo, Y.; Garrity, A.G.; et al. A TRP channel in the lysosome regulates large particle phagocytosis via focal exocytosis. Dev. Cell 2013, 26, 511–524. [Google Scholar] [CrossRef]
- Shen, D.; Wang, X.; Li, X.; Zhang, X.; Yao, Z.; Dibble, S.; Dong, X.P.; Yu, T.; Lieberman, A.P.; Showalter, H.D.; et al. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat. Commun. 2012, 3, 731. [Google Scholar] [CrossRef]
- Weiss, N. Cross-talk between TRPML1 channel, lipids and lysosomal storage diseases. Commun. Integr. Biol. 2012, 5, 111–113. [Google Scholar] [CrossRef]
- Zeevi, D.A.; Frumkin, A.; Bach, G. TRPML and lysosomal function. Biochim. Biophys. Acta 2007, 1772, 851–858. [Google Scholar] [CrossRef]
- Dong, X.P.; Cheng, X.; Mills, E.; Delling, M.; Wang, F.; Kurz, T.; Xu, H. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature 2008, 455, 992–996. [Google Scholar] [CrossRef]
- Miedel, M.T.; Rbaibi, Y.; Guerriero, C.J.; Colletti, G.; Weixel, K.M.; Weisz, O.A.; Kiselyov, K. Membrane traffic and turnover in TRP-ML1-deficient cells: A revised model for mucolipidosis type IV pathogenesis. J. Exp. Med. 2008, 205, 1477–1490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jin, S.; Yi, F.; Li, P.L. TRP-ML1 functions as a lysosomal NAADP-sensitive Ca2+ release channel in coronary arterial myocytes. J. Cell Mol. Med. 2009, 13, 3174–3185. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; McBrayer, M.K.; Wolfe, D.M.; Haslett, L.J.; Kumar, A.; Sato, Y.; Lie, P.P.; Mohan, P.; Coffey, E.E.; Kompella, U.; et al. Presenilin 1 Maintains Lysosomal Ca(2+) Homeostasis via TRPML1 by Regulating vATPase-Mediated Lysosome Acidification. Cell Rep. 2015, 12, 1430–1444. [Google Scholar] [CrossRef]
- Sterea, A.M.; Almasi, S.; El Hiani, Y. The hidden potential of lysosomal ion channels: A new era of oncogenes. Cell Calcium. 2018, 72, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhao, Z.; Li, Q.; Tan, Z. Lysosomal Potassium Channels: Potential Roles in Lysosomal Function and Neurodegenerative Diseases. CNS Neurol. Disord. Drug Targets 2018, 17, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yue, J. TPC2 mediates autophagy progression and extracellular vesicle secretion in cancer cells. Exp. Cell Res. 2018, 370, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Grimm, C.; Bartel, K.; Vollmar, A.M.; Biel, M. Endolysosomal Cation Channels and Cancer-A Link with Great Potential. Pharmaceuticals 2018, 11, 4. [Google Scholar] [CrossRef]
- Lin-Moshier, Y.; Keebler, M.V.; Hooper, R.; Boulware, M.J.; Liu, X.; Churamani, D.; Abood, M.E.; Walseth, T.F.; Brailoiu, E.; Patel, S.; et al. The Two-pore channel (TPC) interactome unmasks isoform-specific roles for TPCs in endolysosomal morphology and cell pigmentation. Proc. Natl. Acad. Sci. USA 2014, 111, 13087–13092. [Google Scholar] [CrossRef]
- Hockey, L.N.; Kilpatrick, B.S.; Eden, E.R.; Lin-Moshier, Y.; Brailoiu, G.C.; Brailoiu, E.; Futter, C.E.; Schapira, A.H.; Marchant, J.S.; Patel, S. Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. J. Cell Sci. 2015, 128, 232–238. [Google Scholar] [CrossRef]
- Rivero-Rios, P.; Gomez-Suaga, P.; Fernandez, B.; Madero-Perez, J.; Schwab, A.J.; Ebert, A.D.; Hilfiker, S. Alterations in late endocytic trafficking related to the pathobiology of LRRK2-linked Parkinson’s disease. Biochem Soc. Trans. 2015, 43, 390–395. [Google Scholar] [CrossRef]
- Cao, Q.; Zhong, X.Z.; Zou, Y.; Murrell-Lagnado, R.; Zhu, M.X.; Dong, X.P. Calcium release through P2X4 activates calmodulin to promote endolysosomal membrane fusion. J. Cell Biol. 2015, 209, 879–894. [Google Scholar] [CrossRef] [PubMed]
- Fois, G.; Winkelmann, V.E.; Bareis, L.; Staudenmaier, L.; Hecht, E.; Ziller, C.; Ehinger, K.; Schymeinsky, J.; Kranz, C.; Frick, M. ATP is stored in lamellar bodies to activate vesicular P2X4 in an autocrine fashion upon exocytosis. J. Gen. Physiol. 2018, 150, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Le Guilcher, C.; Garcin, I.; Dellis, O.; Cauchois, F.; Tebbi, A.; Doignon, I.; Guettier, C.; Julien, B.; Tordjmann, T. The P2X4 purinergic receptor regulates hepatic myofibroblast activation during liver fibrogenesis. J. Hepatol. 2018, 69, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Poroca, D.R.; Pelis, R.M.; Chappe, V.M. ClC Channels and Transporters: Structure, Physiological Functions, and Implications in Human Chloride Channelopathies. Front. Pharm. 2017, 8, 151. [Google Scholar] [CrossRef]
- Stauber, T.; Jentsch, T.J. Sorting motifs of the endosomal/lysosomal CLC chloride transporters. J. Biol. Chem. 2010, 285, 34537–34548. [Google Scholar] [CrossRef]
- Zifarelli, G. A tale of two CLCs: Biophysical insights toward understanding ClC-5 and ClC-7 function in endosomes and lysosomes. J. Physiol. 2015, 593, 4139–4150. [Google Scholar] [CrossRef]
- Graves, A.R.; Curran, P.K.; Smith, C.L.; Mindell, J.A. The Cl-/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature 2008, 453, 788–792. [Google Scholar] [CrossRef]
- Braun, A.P. Identification of ClC-7 as a major pathway for Cl- movement in lysosomes. Channels 2008, 2, 309. [Google Scholar] [CrossRef][Green Version]
- Lange, P.F.; Wartosch, L.; Jentsch, T.J.; Fuhrmann, J.C. ClC-7 requires Ostm1 as a beta-subunit to support bone resorption and lysosomal function. Nature 2006, 440, 220–223. [Google Scholar] [CrossRef]
- Fuller, C.M.; Benos, D.J. Cftr! Am. J. Physiol. 1992, 263, C267–C286. [Google Scholar] [CrossRef]
- Meng, X.; Clews, J.; Kargas, V.; Wang, X.; Ford, R.C. The cystic fibrosis transmembrane conductance regulator (CFTR) and its stability. Cell Mol. Life Sci. 2017, 74, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Moran, O. The gating of the CFTR channel. Cell Mol. Life Sci. 2017, 74, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Borowitz, D. CFTR, bicarbonate, and the pathophysiology of cystic fibrosis. Pediatr. Pulmonol. 2015, 50, S24–S30. [Google Scholar] [CrossRef] [PubMed]
- Csanady, L.; Vergani, P.; Gadsby, D.C. Structure, Gating, and Regulation of the Cftr Anion Channel. Physiol. Rev. 2019, 99, 707–738. [Google Scholar] [CrossRef] [PubMed]
- Riordan, J.R. CFTR function and prospects for therapy. Annu. Rev. Biochem. 2008, 77, 701–726. [Google Scholar] [CrossRef]
- Bradbury, N.A. Intracellular CFTR: Localization and function. Physiol. Rev. 1999, 79, S175–S191. [Google Scholar] [CrossRef]
- Swanson, J. CFTR: Helping to acidify macrophage lysosomes. Nat. Cell Biol. 2006, 8, 908–909. [Google Scholar] [CrossRef]
- Liu, J.; Lu, W.; Guha, S.; Baltazar, G.C.; Coffey, E.E.; Laties, A.M.; Rubenstein, R.C.; Reenstra, W.W.; Mitchell, C.H. Cystic fibrosis transmembrane conductance regulator contributes to reacidification of alkalinized lysosomes in RPE cells. Am. J. Physiol. Cell Physiol. 2012, 303, C160–C169. [Google Scholar] [CrossRef]
- Clapham, D.E.; Runnels, L.W.; Strubing, C. The TRP ion channel family. Nat. Rev. Neurosci. 2001, 2, 387–396. [Google Scholar] [CrossRef]
- Ramsey, I.S.; Delling, M.; Clapham, D.E. An introduction to TRP channels. Annu. Rev. Physiol. 2006, 68, 619–647. [Google Scholar] [CrossRef]
- Bootman, M.D.; Chehab, T.; Bultynck, G.; Parys, J.B.; Rietdorf, K. The regulation of autophagy by calcium signals: Do we have a consensus? Cell Calcium. 2018, 70, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Sano, Y.; Inamura, K.; Miyake, A.; Mochizuki, S.; Yokoi, H.; Matsushime, H.; Furuichi, K. Immunocyte Ca2+ influx system mediated by LTRPC2. Science 2001, 293, 1327–1330. [Google Scholar] [CrossRef] [PubMed]
- Perraud, A.L.; Fleig, A.; Dunn, C.A.; Bagley, L.A.; Launay, P.; Schmitz, C.; Stokes, A.J.; Zhu, Q.; Bessman, M.J.; Penner, R.; et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 2001, 411, 595–599. [Google Scholar] [CrossRef]
- Fleig, A.; Penner, R. Emerging roles of TRPM channels. Novartis. Found. Symp. 2004, 258, 248–258; discussion 258–266. [Google Scholar] [PubMed]
- Wehage, E.; Eisfeld, J.; Heiner, I.; Jungling, E.; Zitt, C.; Luckhoff, A. Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J. Biol. Chem. 2002, 277, 23150–23156. [Google Scholar] [CrossRef]
- Hara, Y.; Wakamori, M.; Ishii, M.; Maeno, E.; Nishida, M.; Yoshida, T.; Yamada, H.; Shimizu, S.; Mori, E.; Kudoh, J.; et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol. Cell 2002, 9, 163–173. [Google Scholar] [CrossRef]
- Kolisek, M.; Beck, A.; Fleig, A.; Penner, R. Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Mol. Cell 2005, 18, 61–69. [Google Scholar] [CrossRef]
- McHugh, D.; Flemming, R.; Xu, S.Z.; Perraud, A.L.; Beech, D.J. Critical intracellular Ca2+ dependence of transient receptor potential melastatin 2 (TRPM2) cation channel activation. J. Biol. Chem. 2003, 278, 11002–11006. [Google Scholar] [CrossRef]
- Starkus, J.; Beck, A.; Fleig, A.; Penner, R. Regulation of TRPM2 by extra- and intracellular calcium. J. Gen. Physiol. 2007, 130, 427–440. [Google Scholar] [CrossRef]
- Csanady, L.; Torocsik, B. Four Ca2+ ions activate TRPM2 channels by binding in deep crevices near the pore but intracellularly of the gate. J. Gen. Physiol. 2009, 133, 189–203. [Google Scholar] [CrossRef]
- Perraud, A.L.; Schmitz, C.; Scharenberg, A.M. TRPM2 Ca2+ permeable cation channels: From gene to biological function. Cell Calcium. 2003, 33, 519–531. [Google Scholar] [CrossRef]
- Sumoza-Toledo, A.; Penner, R. TRPM2: A multifunctional ion channel for calcium signalling. J. Physiol 2011, 589, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Kozai, D.; Kobayashi, R.; Ebert, M.; Mori, Y. Roles of TRPM2 in oxidative stress. Cell Calcium. 2011, 50, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Puertollano, R.; Kiselyov, K. TRPMLs: In sickness and in health. Am. J. Physiol Ren. Physiol. 2009, 296, F1245–F1254. [Google Scholar] [CrossRef]
- Qian, F.; Noben-Trauth, K. Cellular and molecular function of mucolipins (TRPML) and polycystin 2 (TRPP2). Pflug. Arch. 2005, 451, 277–285. [Google Scholar] [CrossRef]
- Dong, X.P.; Wang, X.; Xu, H. TRP channels of intracellular membranes. J. Neurochem. 2010, 113, 313–328. [Google Scholar] [CrossRef]
- Abe, K.; Puertollano, R. Role of TRP channels in the regulation of the endosomal pathway. Physiology 2011, 26, 14–22. [Google Scholar] [CrossRef]
- Grimm, C.; Hassan, S.; Wahl-Schott, C.; Biel, M. Role of TRPML and two-pore channels in endolysosomal cation homeostasis. J. Pharm. Exp. 2012, 342, 236–244. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Wong, C.O.; Zhu, M.X. The role of TRPMLs in endolysosomal trafficking and function. Cell Calcium 2015, 58, 48–56. [Google Scholar] [CrossRef]
- Patel, S.; Cai, X. Evolution of acidic Ca(2)(+) stores and their resident Ca(2)(+)-permeable channels. Cell Calcium 2015, 57, 222–230. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, M.; Yang, Y.; Xu, H. Organellar TRP channels. Nat. Struct. Mol. Biol. 2018, 25, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Karacsonyi, C.; Miguel, A.S.; Puertollano, R. Mucolipin-2 localizes to the Arf6-associated pathway and regulates recycling of GPI-APs. Traffic 2007, 8, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hua, Y.; Vergarajauregui, S.; Diab, H.I.; Puertollano, R. Novel Role of TRPML2 in the Regulation of the Innate Immune Response. J. Immunol. 2015, 195, 4922–4932. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, J.M.; Blomberg, K.E.; Valiaho, J.; Vargas, L.; Heinonen, J.E.; Berglof, A.; Mohamed, A.J.; Nore, B.F.; Vihinen, M.; Smith, C.I. Bruton’s tyrosine kinase: Cell biology, sequence conservation, mutation spectrum, siRNA modifications, and expression profiling. Immunol. Rev. 2005, 203, 200–215. [Google Scholar] [CrossRef]
- Miao, Y.; Li, G.; Zhang, X.; Xu, H.; Abraham, S.N. A TRP Channel Senses Lysosome Neutralization by Pathogens to Trigger Their Expulsion. Cell 2015, 161, 1306–1319. [Google Scholar] [CrossRef]
- Guo, Z.; Grimm, C.; Becker, L.; Ricci, A.J.; Heller, S. A novel ion channel formed by interaction of TRPML3 with TRPV5. PLoS ONE 2013, 8, e58174. [Google Scholar] [CrossRef]
- Di Palma, F.; Belyantseva, I.A.; Kim, H.J.; Vogt, T.F.; Kachar, B.; Noben-Trauth, K. Mutations in Mcoln3 associated with deafness and pigmentation defects in varitint-waddler (Va) mice. Proc. Natl. Acad. Sci. USA 2002, 99, 14994–14999. [Google Scholar] [CrossRef]
- Kim, H.J.; Soyombo, A.A.; Tjon-Kon-Sang, S.; So, I.; Muallem, S. The Ca(2+) channel TRPML3 regulates membrane trafficking and autophagy. Traffic 2009, 10, 1157–1167. [Google Scholar] [CrossRef]
- Medina, D.L.; Di Paola, S.; Peluso, I.; Armani, A.; De Stefani, D.; Venditti, R.; Montefusco, S.; Scotto-Rosato, A.; Prezioso, C.; Forrester, A.; et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 2015, 17, 288–299. [Google Scholar] [CrossRef]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Garcia Arencibia, M.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB links autophagy to lysosomal biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef]
- Scotto Rosato, A.; Montefusco, S.; Soldati, C.; Di Paola, S.; Capuozzo, A.; Monfregola, J.; Polishchuk, E.; Amabile, A.; Grimm, C.; Lombardo, A.; et al. TRPML1 links lysosomal calcium to autophagosome biogenesis through the activation of the CaMKKbeta/VPS34 pathway. Nat. Commun. 2019, 10, 5630. [Google Scholar] [CrossRef]
- Capurro, M.I.; Greenfield, L.K.; Prashar, A.; Xia, S.; Abdullah, M.; Wong, H.; Zhong, X.Z.; Bertaux-Skeirik, N.; Chakrabarti, J.; Siddiqui, I.; et al. VacA generates a protective intracellular reservoir for Helicobacter pylori that is eliminated by activation of the lysosomal calcium channel TRPML1. Nat. Microbiol. 2019, 4, 1411–1423. [Google Scholar] [CrossRef]
- Qi, X.; Man, S.M.; Malireddi, R.K.; Karki, R.; Lupfer, C.; Gurung, P.; Neale, G.; Guy, C.S.; Lamkanfi, M.; Kanneganti, T.D. Cathepsin B modulates lysosomal biogenesis and host defense against Francisella novicida infection. J. Exp. Med. 2016, 213, 2081–2097. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Yang, Y.; Zhong, X.Z.; Dong, X.P. The lysosomal Ca(2+) release channel TRPML1 regulates lysosome size by activating calmodulin. J. Biol. Chem. 2017, 292, 8424–8435. [Google Scholar] [CrossRef] [PubMed]
- Cang, C.; Aranda, K.; Seo, Y.J.; Gasnier, B.; Ren, D. TMEM175 Is an Organelle K(+) Channel Regulating Lysosomal Function. Cell 2015, 162, 1101–1112. [Google Scholar] [CrossRef]
- Jinn, S.; Drolet, R.E.; Cramer, P.E.; Wong, A.H.; Toolan, D.M.; Gretzula, C.A.; Voleti, B.; Vassileva, G.; Disa, J.; Tadin-Strapps, M.; et al. TMEM175 deficiency impairs lysosomal and mitochondrial function and increases alpha-synuclein aggregation. Proc. Natl. Acad. Sci. USA 2017, 114, 2389–2394. [Google Scholar] [CrossRef] [PubMed]
- Tugba Durlu-Kandilci, N.; Ruas, M.; Chuang, K.T.; Brading, A.; Parrington, J.; Galione, A. TPC2 proteins mediate nicotinic acid adenine dinucleotide phosphate (NAADP)- and agonist-evoked contractions of smooth muscle. J. Biol. Chem. 2010, 285, 24925–24932. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.X.; Evans, A.M.; Ma, J.; Parrington, J.; Galione, A. Two-pore channels for integrative Ca signaling. Commun. Integr. Biol. 2010, 3, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Grimm, C.; Butz, E.; Chen, C.C.; Wahl-Schott, C.; Biel, M. From mucolipidosis type IV to Ebola: TRPML and two-pore channels at the crossroads of endo-lysosomal trafficking and disease. Cell Calcium. 2017, 67, 148–155. [Google Scholar] [CrossRef]
- Calcraft, P.J.; Ruas, M.; Pan, Z.; Cheng, X.; Arredouani, A.; Hao, X.; Tang, J.; Rietdorf, K.; Teboul, L.; Chuang, K.T.; et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 2009, 459, 596–600. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Dong, X.P.; Samie, M.; Li, X.; Cheng, X.; Goschka, A.; Shen, D.; Zhou, Y.; Harlow, J.; et al. TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes. Cell 2012, 151, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Cang, C.; Zhou, Y.; Navarro, B.; Seo, Y.J.; Aranda, K.; Shi, L.; Battaglia-Hsu, S.; Nissim, I.; Clapham, D.E.; Ren, D. mTOR regulates lysosomal ATP-sensitive two-pore Na(+) channels to adapt to metabolic state. Cell 2013, 152, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Ogunbayo, O.A.; Zhu, Y.; Shen, B.; Agbani, E.; Li, J.; Ma, J.; Zhu, M.X.; Evans, A.M. Organelle-specific subunit interactions of the vertebrate two-pore channel family. J. Biol. Chem. 2015, 290, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Cang, C.; Aranda, K.; Ren, D. A non-inactivating high-voltage-activated two-pore Na(+) channel that supports ultra-long action potentials and membrane bistability. Nat. Commun. 2014, 5, 5015. [Google Scholar] [CrossRef] [PubMed]
- Ruas, M.; Davis, L.C.; Chen, C.C.; Morgan, A.J.; Chuang, K.T.; Walseth, T.F.; Grimm, C.; Garnham, C.; Powell, T.; Platt, N.; et al. Expression of Ca(2)(+)-permeable two-pore channels rescues NAADP signalling in TPC-deficient cells. EMBO J. 2015, 34, 1743–1758. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Function and dysfunction of two-pore channels. Sci. Signal. 2015, 8, re7. [Google Scholar] [CrossRef]
- Marchant, J.S.; Patel, S. Two-pore channels at the intersection of endolysosomal membrane traffic. Biochem Soc. Trans. 2015, 43, 434–441. [Google Scholar] [CrossRef]
- Grimm, C.; Chen, C.C.; Wahl-Schott, C.; Biel, M. Two-Pore Channels: Catalyzers of Endolysosomal Transport and Function. Front. Pharm. 2017, 8, 45. [Google Scholar] [CrossRef]
- Nguyen, O.N.; Grimm, C.; Schneider, L.S.; Chao, Y.K.; Atzberger, C.; Bartel, K.; Watermann, A.; Ulrich, M.; Mayr, D.; Wahl-Schott, C.; et al. Two-Pore Channel Function Is Crucial for the Migration of Invasive Cancer Cells. Cancer Res. 2017, 77, 1427–1438. [Google Scholar] [CrossRef]
- Garcia-Rua, V.; Feijoo-Bandin, S.; Rodriguez-Penas, D.; Mosquera-Leal, A.; Abu-Assi, E.; Beiras, A.; Maria Seoane, L.; Lear, P.; Parrington, J.; Portoles, M.; et al. Endolysosomal two-pore channels regulate autophagy in cardiomyocytes. J. Physiol. 2016, 594, 3061–3077. [Google Scholar] [CrossRef]
- Qureshi, O.S.; Paramasivam, A.; Yu, J.C.; Murrell-Lagnado, R.D. Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J. Cell Sci. 2007, 120, 3838–3849. [Google Scholar] [CrossRef] [PubMed]
- Suurvali, J.; Boudinot, P.; Kanellopoulos, J.; Ruutel Boudinot, S. P2X4: A fast and sensitive purinergic receptor. Biomed. J. 2017, 40, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Murrell-Lagnado, R.D.; Frick, M. P2X4 and lysosome fusion. Curr. Opin. Pharmacol. 2019, 47, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Toyomitsu, E.; Tsuda, M.; Yamashita, T.; Tozaki-Saitoh, H.; Tanaka, Y.; Inoue, K. CCL2 promotes P2X4 receptor trafficking to the cell surface of microglia. Purinergic Signal. 2012, 8, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zou, Y.; Zhong, X.Z.; Cao, Q.; Zhao, K.; Zhu, M.X.; Murrell-Lagnado, R.; Dong, X.P. P2X4 forms functional ATP-activated cation channels on lysosomal membranes regulated by luminal pH. J. Biol. Chem. 2014, 289, 17658–17667. [Google Scholar] [CrossRef]
- Gofman, L.; Cenna, J.M.; Potula, R. P2X4 receptor regulates alcohol-induced responses in microglia. J. Neuroimmune Pharm. 2014, 9, 668–678. [Google Scholar] [CrossRef]
- Moore, M.N.; Allen, J.I.; McVeigh, A.; Shaw, J. Lysosomal and autophagic reactions as predictive indicators of environmental impact in aquatic animals. Autophagy 2006, 2, 217–220. [Google Scholar] [CrossRef]
- Meng Lin, M.; Kim, H.H.; Kim, H.; Muhammed, M.; Kyung Kim, D. Iron oxide-based nanomagnets in nanomedicine: Fabrication and applications. Nano Rev. 2010, 1. [Google Scholar] [CrossRef]
- Zhu, J.; He, K.; Dai, Z.; Gong, L.; Zhou, T.; Liang, H.; Liu, J. Self-Assembly of Luminescent Gold Nanoparticles with Sensitive pH-Stimulated Structure Transformation and Emission Response toward Lysosome Escape and Intracellular Imaging. Anal. Chem. 2019, 91, 8237–8243. [Google Scholar] [CrossRef]
- Lee, D.U.; Park, J.Y.; Kwon, S.; Park, J.Y.; Kim, Y.H.; Khang, D.; Hong, J.H. Apoptotic lysosomal proton sponge effect in tumor tissue by cationic gold nanorods. Nanoscale 2019, 11, 19980–19993. [Google Scholar] [CrossRef]
- Bhowmik, T.; Gomes, A. NKCT1 (purified Naja kaouthia protein toxin) conjugated gold nanoparticles induced Akt/mTOR inactivation mediated autophagic and caspase 3 activated apoptotic cell death in leukemic cell. Toxicon 2016, 121, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Totten, J.D.; Wongpinyochit, T.; Seib, F.P. Silk nanoparticles: Proof of lysosomotropic anticancer drug delivery at single-cell resolution. J. Drug Target. 2017, 25, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Gurunathan, S. Combination of salinomycin and silver nanoparticles enhances apoptosis and autophagy in human ovarian cancer cells: An effective anticancer therapy. Int. J. Nanomed. 2016, 11, 3655–3675. [Google Scholar] [CrossRef]
- Martin-Banderas, L.; Holgado, M.A.; Duran-Lobato, M.; Infante, J.J.; Alvarez-Fuentes, J.; Fernandez-Arevalo, M. Role of Nanotechnology for Enzyme Replacement Therapy in Lysosomal Diseases. A Focus on Gaucher’s Disease. Curr. Med. Chem. 2016, 23, 929–952. [Google Scholar] [CrossRef] [PubMed]
- Muhlstein, A.; Gelperina, S.; Shipulo, E.; Maksimenko, O.; Kreuter, J. Arylsulfatase A bound to poly(butyl cyanoacrylate) nanoparticles for enzyme replacement therapy—Physicochemical evaluation. Pharmazie 2014, 69, 518–524. [Google Scholar] [PubMed]
- Tan, Y.L.; Ho, H.K. Navigating albumin-based nanoparticles through various drug delivery routes. Drug Discov. Today 2018, 23, 1108–1114. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef]
- An, F.F.; Zhang, X.H. Strategies for Preparing Albumin-based Nanoparticles for Multifunctional Bioimaging and Drug Delivery. Theranostics 2017, 7, 3667–3689. [Google Scholar] [CrossRef]
- Tang, W.L.; Tang, W.H.; Li, S.D. Cancer theranostic applications of lipid-based nanoparticles. Drug Discov. Today 2018, 23, 1159–1166. [Google Scholar] [CrossRef]
- Guven, E. Lipid-based nanoparticles in the treatment of erectile dysfunction. Int J. Impot Res. 2020. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, X.; Gong, Y.; Xiao, A.; Xie, Y.; Liu, L.; Cao, Y. The Interactions between ZnO Nanoparticles (NPs) and alpha-Linolenic Acid (LNA) Complexed to BSA Did Not Influence the Toxicity of ZnO NPs on HepG2 Cells. Nanomaterials 2017, 7, 91. [Google Scholar] [CrossRef]
- Peynshaert, K.; Soenen, S.J.; Manshian, B.B.; Doak, S.H.; Braeckmans, K.; De Smedt, S.C.; Remaut, K. Coating of Quantum Dots strongly defines their effect on lysosomal health and autophagy. Acta Biomater. 2017, 48, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.A.; Outzen, H.; Overvatn, A.; Bjorkoy, G.; Johansen, T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef] [PubMed]
- Thurston, T.L.; Ryzhakov, G.; Bloor, S.; von Muhlinen, N.; Randow, F. The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat. Immunol. 2009, 10, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.W.; Hong, S.B.; Kim, J.H.; Kwon, D.H.; Song, H.K. Structural basis for recognition of autophagic receptor NDP52 by the sugar receptor galectin-8. Nat. Commun. 2013, 4, 1613. [Google Scholar] [CrossRef] [PubMed]
- Wild, P.; Farhan, H.; McEwan, D.G.; Wagner, S.; Rogov, V.V.; Brady, N.R.; Richter, B.; Korac, J.; Waidmann, O.; Choudhary, C.; et al. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 2011, 333, 228–233. [Google Scholar] [CrossRef]
- Zeng, J.; Shirihai, O.S.; Grinstaff, M.W. Degradable Nanoparticles Restore Lysosomal pH and Autophagic Flux in Lipotoxic Pancreatic Beta Cells. Adv. Healthc. Mater. 2019, 8, e1801511. [Google Scholar] [CrossRef]
- Jimeno-Romero, A.; Oron, M.; Cajaraville, M.P.; Soto, M.; Marigomez, I. Nanoparticle size and combined toxicity of TiO2 and DSLS (surfactant) contribute to lysosomal responses in digestive cells of mussels exposed to TiO2 nanoparticles. Nanotoxicology 2016, 10, 1168–1176. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).