Abstract
The Enterovirus (EV) and Parechovirus genera of the picornavirus family include many important human pathogens, including poliovirus, rhinovirus, EV-A71, EV-D68, and human parechoviruses (HPeV). They cause a wide variety of diseases, ranging from a simple common cold to life-threatening diseases such as encephalitis and myocarditis. At the moment, no antiviral therapy is available against these viruses and it is not feasible to develop vaccines against all EVs and HPeVs due to the great number of serotypes. Therefore, a lot of effort is being invested in the development of antiviral drugs. Both viral proteins and host proteins essential for virus replication can be used as targets for virus inhibitors. As such, a good understanding of the complex process of virus replication is pivotal in the design of antiviral strategies goes hand in hand with a good understanding of the complex process of virus replication. In this review, we will give an overview of the current state of knowledge of EV and HPeV replication and how this can be inhibited by small-molecule inhibitors.
1. Enterovirus and Parechovirus Associated Diseases
1.2. Enteroviruses
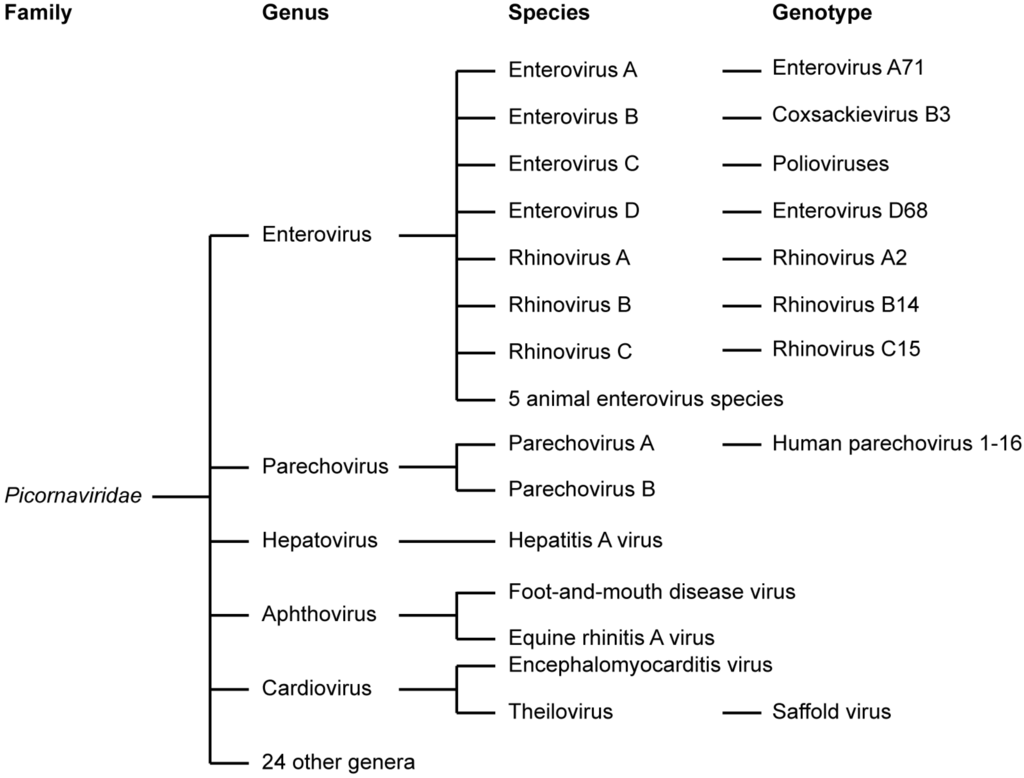
The genus Enterovirus (EV) of the picornavirus family contains many important human pathogens, which are among the most common infections in mankind. Overall, it was estimated that around 10–15 million symptomatic EV infections occur annually in the United States alone [3]. This figure excludes the very prevalent rhinovirus infections. The EVs have been classified into 12 species, including EVs A-D, RV A-C, and five EV species that only infect animals (EV-E to EV-J) (Figure 1) [1,2]. These viruses include coxsackie A and B viruses (CVA and CVB, respectively), echoviruses, polioviruses (PVs), numbered EVs, and rhinoviruses (RV).
EVs are transmitted via the fecal-oral route or via respiratory transmission, depending on the type. EVs have two primary replication sites, the gastrointestinal tract and the respiratory tract, from where the virus can spread to the target organs via the blood circulation. This can result in severe, potentially fatal diseases.
PV is the prototype EV. As in many EV infections, PV infections are mostly clinically mild [4]. However, PV infections can progress to non-paralytic aseptic meningitis (in 1%–2% of cases) or to poliomyelitis, a form of flaccid paralysis (in 0.1%–1% of cases) [4]. Due to intense vaccination programs and surveillance, PV has nearly become extinct, but nevertheless, the virus remains endemic in three countries (Afghanistan, Nigeria and Pakistan) and sporadic PV outbreaks occur.
Coxsackie A and B viruses, echoviruses, and the numbered EVs are associated with a great variety of manifestations, varying from mild respiratory and gastrointestinal infections, herpangina, and hand-foot-and-mouth disease (HFMD), to more severe disease like pleurodynia, hepatitis, myopericarditis, pancreatitis, meningitis, encephalitis, paralysis, and neonatal sepsis leading to mortality [5]. EVs are the most important cause for viral meningitis, accounting for 85%–95% of all cases for which an etiological agent was identified [6].
The genotype/serotype EV-A71 is an emerging pathogen that has caused several outbreaks since the late 1990s. EV-A71 epidemics have been reported worldwide, but mostly in the Asia-Pacific region [7]. HFMD is the most common manifestation of EV-A71 and affects mostly children and infants. However, EV-A71 infections may also result in severe diseases such as pulmonary edema and neurological complications, which may be fatal.
EV-D68 has recently drawn attention because of an outbreak in the United States and to a smaller extent in the rest of the world (e.g., [8,9,10,11]. These EV-D68 infected patients presented with severe respiratory illness. Furthermore, the virus was frequently detected in patients with AFP, suggesting the virus may in rare cases be neurotropic [8,12].
RVs can infect both the upper and the lower respiratory tract and are the major cause of the common cold. Though on the less severe side of the spectrum of the diseases caused by EVs, the common cold results in major costs by, among other things, loss of working days, amounting in the United States to $40 billion annually [13]. In addition to the common cold, RVs can cause severe lower respiratory tract infections, such as pneumonia and bronchiolitis [5]. Moreover, RV infections are a serious threat to patients with asthma, chronic obstructive pulmonary disorder (COPD), or cystic fibrosis in whom respiratory tract infections with RVs can lead to exacerbations [14,15,16,17,18,19,20,21,22,23,24]. RVs are subdivided into the species A, B and C. RV-C has been discovered only recently with the help of molecular diagnostic techniques. Initial studies suggested that RV-C is associated with more severe lower respiratory disease than the other species, but later reports suggest that RV-A may be equally pathogenic [25].
1.3. Parechoviruses
When the HPeVs were first identified they were initially classified in the Enterovirus genus as echovirus 22 and 23 on the basis of cell-culture characteristics. However, phylogenetic analysis showed these viruses to be genetically distinct from any other picornavirus genus [26,27] and these strains were reclassified in a new genus named Parechovirus [28]. Currently, the species Parechovirus A contains 16 HPeV types [1,2]. HPeV prevalence has been underestimated, but current data show that HPeVs are at least as prevalent as EVs [29] and that HPeV is a major pathogen in young children [30]. The most commonly circulating HPeV is HPeV-1, which mainly causes mild gastrointestinal and respiratory disease although sometimes in young children more severe disease can be observed [31]. HPeVs are the second most important cause of viral sepsis-like illness and meningitis in infants [32,33,34]. The majority of these cases are caused by HPeV-3 [33], which is the most pathogenic HPeV type. It is associated with paralysis, neonatal sepsis-like illness and sudden death in infected infants [33,35,36,37,38,39,40,41,42,43]. The HPeVs have received very little attention from the scientific community in the past, but continuing reports of HPeV circulation all over the world are increasing awareness of the significance of this virus group.
2. Enterovirus Replication Cycle
2.1. Enterovirus Virions and Viral Genome Organization
Picornaviruses are small positive-strand RNA viruses. The genome is encapsidated by an icosahedral capsid, forming a virion of around 30 nm in size without an envelope.
The viral genome contains a single open reading frame with highly structured untranslated regions (UTR) at the 5′- and 3′-end and a 3′-poly(A) tail (Figure 2A). The viral genome is uncapped and instead the 5′-end is covalently coupled to the viral protein 3B, in this context usually termed VPg (viral protein genome-linked). The 5′-UTR contains an internal ribosomal entry site (IRES) which mediates cap-independent translation. Overall, the organization of the open reading frame is similar in all picornaviruses, but there are some differences between genera. In the case of EVs, the open reading frame encodes a polyprotein that contains structural proteins (VP1-4) in the P1 region and the nonstructural proteins (2A–2C and 3A–3D) in the P2 and P3 regions (Figure 2B).
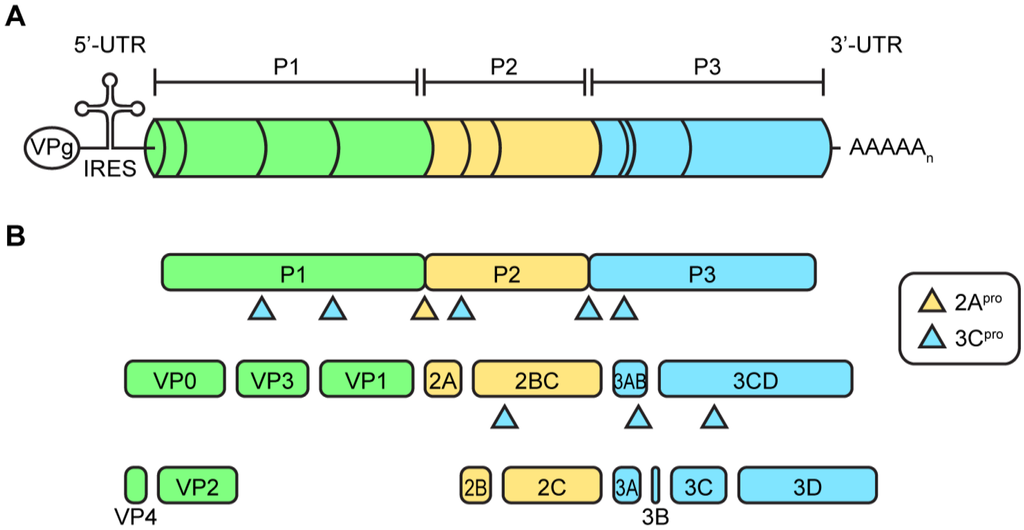
Figure 2.
Enterovirus genome. (A) Depicted is a schematic representation of the enterovirus genome on scale. The enterovirus genome encodes a single polyprotein divided into a P1, P2, and P3 area. At the 5′- and 3′-end the genome contains untranslated regions (UTR), which are highly structured. The 5′-UTR contains an internal ribosomal entry site for cap-independent translation. At the 5′-end, the RNA genome is covalently bound to the viral protein VPg which is used as a primer during RNA replication; (B) The polyprotein is processed into the viral proteins and some stable precursors by the viral proteases 2Apro and 3Cpro (and its precursors).
2.2. Protein Translation and Processing
The EV replication cycle, depicted in Figure 3, is initiated by binding to a receptor. The receptor used differs per virus [53]. For many EVs, the receptor binds at a depression in the capsid called the canyon, which surrounds the fivefold axis of symmetry. Subsequently, the virus is internalized and the viral RNA is released into the cytoplasm. The single polyprotein that is produced, is proteolytically processed by the viral proteases 2Apro and 3Cpro to release the structural and nonstructural viral proteins and some stable precursors (Figure 2B).
Apart from processing of the viral protein, the viral proteases cleave cellular targets, which serves to optimize the environment for viral proliferation. Cleavage of eIF4G and poly(A)-binding protein (PABP) by 2Apro and 3Cpro results in a blockage of translation of cellular proteins, a so-called host shut-off [54,55,56]. In addition, viral proteases cleave several other cellular factors in order to support virus reproduction and/or suppress innate antiviral responses [57,58,59,60,61,62].
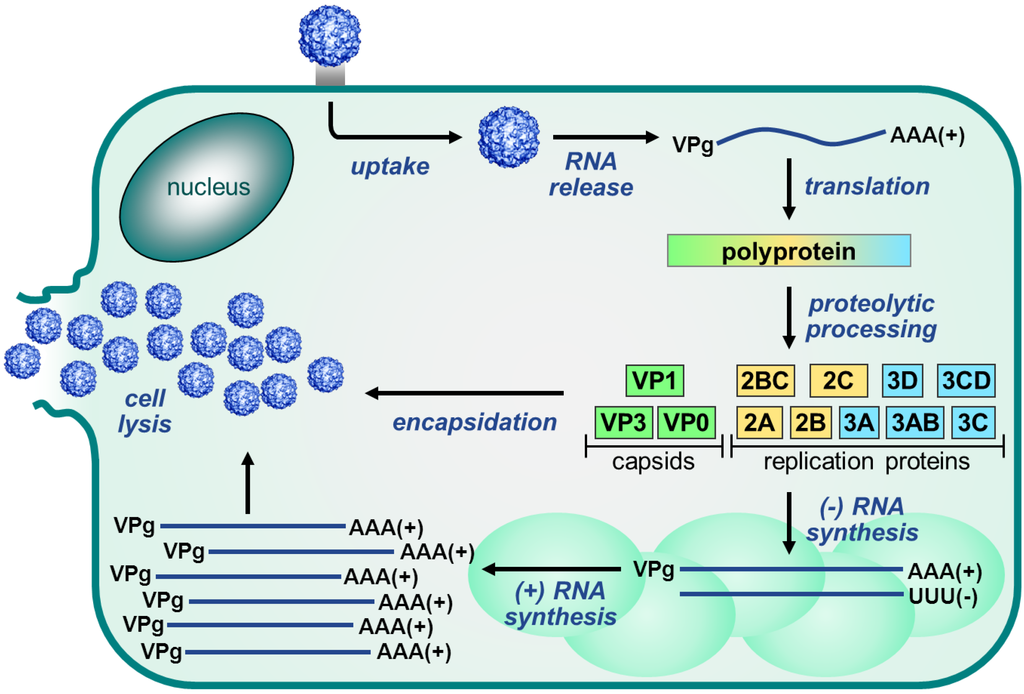
Figure 3.
Enterovirus replication cycle. The Enterovirus replication cycle is initiated by binding of the virus to the receptor and internalization into the cell. Subsequently, the viral RNA genome is released from the virion and translated into a single polyprotein which is then processed by the viral proteases to release the viral proteins. Next, the nonstructural proteins mediate the replication of the RNA genome via a negative-stranded intermediate. This takes place on replication organelles that are formed as a result of a rearrangement of cellular membranes. Newly synthesized positive-stranded RNA molecules can then either enter another round of translation and replication (not depicted) or they can be packaged into the viral capsid proteins to form new infectious virus particles which are released upon cell lysis and through several non-lytic mechanisms.
2.4. The Role of Viral Proteins and Host Factors in Membrane Rearrangements
Typical for positive-strand RNA viruses, replication of the viral RNA takes place on cellular membranes which are drastically reorganized during virus infection [67,68]. In EV-infected cells, both single- and double-membrane structures are observed (Figure 4) [69,70]. Electron tomography studies with PV and CVB3 have revealed that early in virus infection (when RNA replication is already maximal) single-membrane tubular structures are predominant, whereas in later stages these structures appear to flatten, curve, and fuse to form double-membrane vesicles (DMV) [69,70]. These DMVs can then be wrapped by multiple additional cisternae and form multilamellar structures.
The exact origin of the membranes of these organelles is yet unclear. Evidence has been presented that the membranes originated from the early secretory pathway while other data suggested they were derived from the autophagy pathway. The results from the electron tomography studies suggest that there may be some truth in both theories, with the early secretory pathway acting as a source for the single membrane tubules and the autophagy pathway being involved in DMV formation.
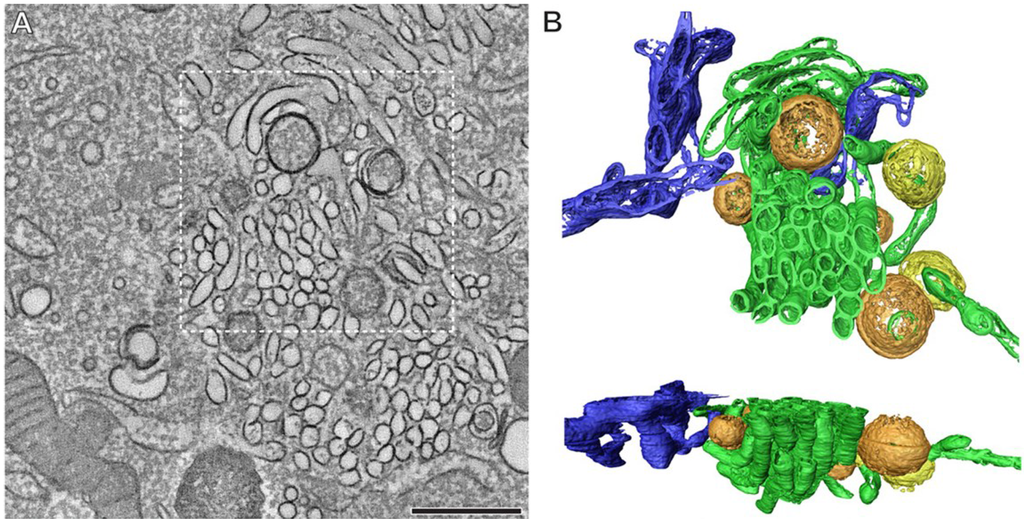
Figure 4.
Extensive membrane rearrangements in Enterovirus-infected cells. An electron tomographic slice through a serial tomogram, bar = 500 nm (A); and top and side views of the surface-rendered model of the boxed area (B) show the presence of single-membrane tubules (green), open (orange) and closed (yellow) double-membrane vesicles in a cell infected with coxsackievirus B3 at 5 h post infection. The ER is depicted in blue. Reprinted from Limpens et al. [70], mBio 2011 with permission from the authors, © 2011 by the American Society for Microbiology.
Important observations that support a role for the early secretory pathway in the membrane rearrangements are that Brefeldin A (BFA), a well-known inhibitor of ER-to-Golgi transport, completely abolishes EV replication [71,72,73,74] and that several proteins from the secretory pathway are essential for virus replication and can be detected on replication organelles. One of these is Golgi-specific BFA-resistance factor 1 (GBF1), which is a target of BFA. In uninfected cells, GBF1 stimulates GTP exchange of the GTPase ADP-ribosylation factor 1 (Arf1), which is localized on the Golgi complex and the ER-Golgi intermediate compartment. Upon activation, Arf1-GTP becomes membrane-bound and mediates the recruitment of effector proteins such as the COP-I coat complex, thereby inducing the formation of secretory vesicles. Arf1 is thus a key regulator of protein and lipid transport within the early secretory pathway. Upon infection, the viral protein 3A recruits GBF1 and indirectly Arf1 to replication organelles (i.e., virus-induced vesicles plus associated replication complexes) through a direct interaction with GBF1 (Figure 5) [71,75,76]. Through a yet unknown mechanism, this leads to the loss of COP-I from membranes, resulting in a disturbance of the secretory pathway and blockage of protein secretion [75,77,78,79]. This impairs the expression of MHC class I on the cell surface and cytokine secretion [80,81], implying that the virus-induced membrane rearrangement not only serves to facilitate viral RNA replication but also to suppress infection-limiting host immune responses.
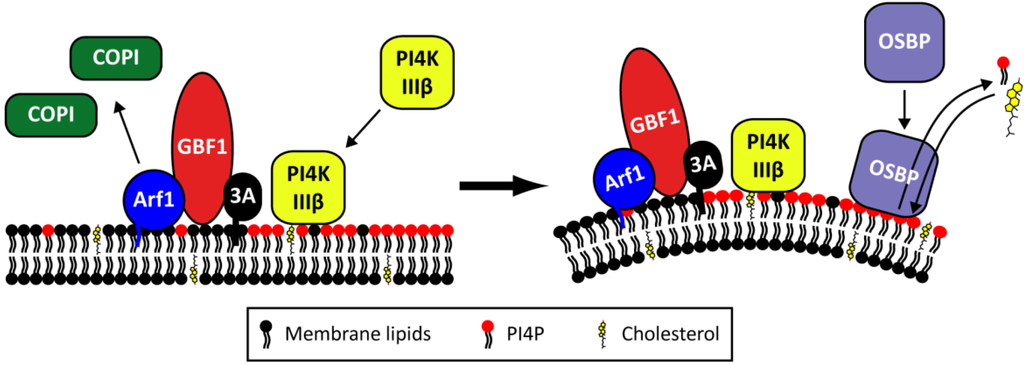
Figure 5.
The proposed role of Golgi-localized host factors in Enterovirus replication. Upon infection, the viral 3A protein recruits GBF1 and indirectly Arf1 to the replication organelles. As a result, COP-I is lost from the membranes. At the same time, PI4KIIIβ is recruited by 3A through a GBF1/Arf-independent mechanism, resulting in an increase in PI4P lipids. OSBP then binds to the PI4P lipids and mediates a PI4P/cholesterol counterflow between the membranes of the replication organelles and the ER.
Phosphatidylinositol-4-kinase III beta (PI4KIIIβ), another Golgi-localized protein, is also an essential host factor for EV replication [77]. PI4KIIIβ is a kinase that synthesizes phosphatidylinositol-4-phosphates (PI4P). As a precursor for PI(4,5)P2, PI4P lipids are part of PI3K and phospholipase C signaling pathways, but PI4P lipids also recruit proteins with a PI4P-binding pleckstrin homology (PH) domain to membranes, thereby regulating membrane biogenesis, lipid homeostasis, and vesicle-mediated trafficking at the Golgi complex [82,83,84]. During infection, PI4KIIIβ is recruited to replication sites by 3A and consequently local levels of PI4P lipids increase (Figure 5) [77]. The mechanism behind the recruitment of PI4KIIIβ by 3A remains to be determined but was shown to be independent of GBF1, Arf1, and ACBD3 [85,86]. In vitro, PI4P lipids specifically bound 3Dpol, suggesting that they may serve to recruit the viral polymerase to replication complexes [77], but firm proof for this idea is lacking. Alternatively, or additionally, the function of PI4P lipids in virus replication may be to recruit PH domain-containing proteins for example with membrane-modifying properties.
One such protein appears to be oxysterol-binding protein (OSBP), a PI4P-binding protein that transports PI4P lipids produced by PI4KIIIβ from the Golgi complex to the ER, in exchange for cholesterol which is transported in the opposite direction [87]. Recent studies by others and ourselves have revealed that OSBP binds to PI4P-enriched replication organelle membranes and mediates a PI4P/cholesterol counterflow between these membranes and the ER (Figure 5) [88,89,90]. As a result, the cholesterol content of membranes of the replication organelles is increased. In addition to this mechanism, increased uptake of cholesterol and a role of endosomal cholesterol have been suggested to contribute to the accumulation of cholesterol in the membranes of the replication [90,91,92]. The cholesterol content of membranes determines the membrane fluidity and formation of lipid microdomains and therefore the virus-induced accumulation of cholesterol may serve to induce the membrane deformations required to generate replication organelles. All in all, it appears that the regulation of PI4P and cholesterol levels is very important to support replication.
DMVs are reminiscent of autophagosomes with respect to their appearance and formation, which originated the idea that the autophagic pathway is involved in the formation of replication organelles. The recent observation that DMVs occur mostly in later stages of infection suggests that this pathway is mostly important in the advanced stages of membrane rearrangements [69,70]. Inhibition of the autophagy pathway impairs viral replication, but only to a modest extent [93,94]. A recent publication has provided evidence that vesicular acidification promotes maturation of PV particles (i.e., VP0 cleavage, see next section), implicating a role for autophagy and DMVs in the last step of the replication cycle [95]. Furthermore, it has been suggested that the DMVs might be involved in non-lytic release of virus particles by fusion with the plasma membrane, challenging the dogma that EVs egress only through cell lysis. Together, these data suggest that the early secretory pathway and the autophagy pathway have a distinct, but important function during EV replication.
Genetic and biochemical evidence suggests that the viral proteins 2BC and 3A are involved in the formation of replication organelles during infection [96,97,98]. These proteins have hydrophobic domains and extensively interact with cellular membranes. 3A is probably important in membrane reorganization through its (direct and indirect) interactions with GBF1, Arf1, and PI4KIIIβ. 2B is a viroporin that enhances the permeability of ER and Golgi membranes [99,100,101,102,103]. Overexpression of 2B leads to disturbed ion homeostasis, impaired transport of proteins through the Golgi complex, and increased targeting of endocytic vesicles to the Golgi complex [78,101,104,105]. How and if these activities are involved in the formation of replication organelles is unknown. 2C has been postulated to contribute to the membrane remodeling by insertion of its hydrophobic domains in the membranes, as well as through its interaction with reticulon proteins [106]. These latter proteins are membrane-shaping proteins that induce and stabilize positive membrane curvature, and may be involved in the formation of the positively curved membranes that are essential for the morphogenesis of the viral replication organelles.
As has become clear from this brief overview, membrane remodeling involves many viral and host proteins and lipids and is a very complicated process that is not completely understood.
2.5. Morphogenesis and Virus Release
Once synthesized, viral RNA of positive polarity is encapsidated by capsid proteins to form new virions. This process is coupled to active replication as only newly synthesized genomes are encapsidated [107,108]. This is not guided by an RNA encapsidation signal or RNA-protein interactions, but rather by an interaction between 2C, which is located in the replication complex, and the capsid protein VP3 [109].
The assembly of new virions (Figure 6) is initiated by the release of the P1 capsid precursor from the polyprotein. This is subsequently folded by the chaperone protein Hsp90 and processed by 3CDpro to release VP0 (the precursor of VP4 and VP2), VP1, and VP3 [110,111]. In a spontaneous process, these capsid proteins assemble to form a protomer. Five protomers together then form a pentamer which in turn assemble to form a provirion. Several, but not all, EVs require the presence of glutathione for the formation and/or stability of the pentamers [112,113,114,115]. The last step is a maturation of the virion by RNA-induced cleavage of VP0 into VP2 and VP4, yielding an infectious virus particle. This process has been suggested to be enhanced by the acidic environment in autophagosome-like DMVs [95].
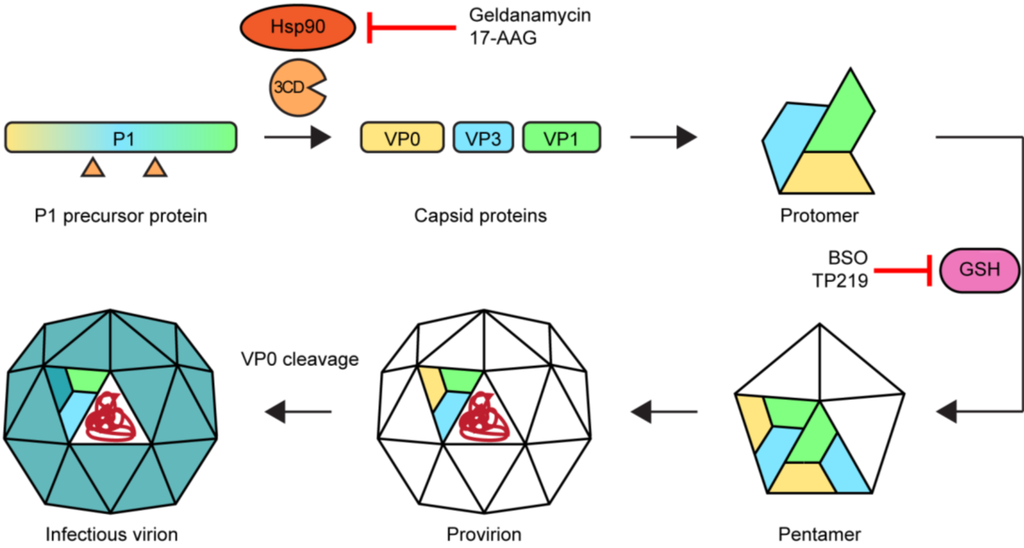
Figure 6.
Morphogenesis of enteroviruses and targets for assembly inhibitors. Hsp90 ensures the proper folding of the P1 precursor protein enabling the cleavage by 3CDpro into capsid proteins VP0, VP3, and VP1 which then form protomers. For part of the EVs, glutathione (GSH) is required either for the transition of protomers into pentamers or for the stabilization of pentamers. Twelve pentamers plus the viral genome (in red) combine to form a provirion, followed by a maturation step in which the VP0 protein is cleaved into VP4 and VP2. Treatment with Hsp90 inhibitors or glutathione depletors results in impaired morphogenesis.
The dogma has always been that newly formed infectious particles are released by lysis of the host cell, but recent reports have suggested additional methods of egress that do not require cell lysis, such as non-lytic release through DMVs and release of phosphatidylserine lipid-enriched vesicles packed with virions [116,117]. This mechanism is reminiscent of the release of hepatitis A virus, another picornavirus, which was recently shown to be released as membrane-wrapped virus particles in membrane structures resembling exosomes [118].
Acknowledgments
The authors thank Jeroen Strating for providing Figure 5. This work was supported by grants from the European Union FP7: Marie Curie IAPP “AIROPico” (grant agreement number 612308) (K.C.W.), Marie Curie Initial Training Network “EUVIRNA” (grant agreement number 264286) (F.J.M.K.) and Large Scale Collaborative Project “SILVER” (grant agreement number 260644) (F.J.M.K.), from the Netherlands Organisation for Scientific Research (NWO): ALW-820.02.018 and VICI-91812628 (F.J.M.K.) and from Crucell (L.L.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
L.L., K.C.W. and F.J.M.K wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Adams, M.J.; King, A.M.; Carstens, E.B. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2013). Arch. Virol. 2013, 158, 2023–2030. [Google Scholar] [CrossRef] [PubMed]
- Knowles, N.J.; Hovi, T.; Hyypiä, T.; King, A.M.; Lindberg, A.M.; Pallansch, M.A.; Palmenberg, A.C.; Simmonds, P.; Skern, T.; Stanway, G.; et al. Picornaviridae. In Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier: San Diego, CA, USA, 2012; pp. 855–880. [Google Scholar]
- Strikas, R.A.; Anderson, L.J.; Parker, R.A. Temporal and geographic patterns of isolates of nonpolio enterovirus in the United States, 1970–1983. J. Infect. Dis. 1986, 153, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Poliomyelitis. In Epidemiology and Prevention of Vaccine-Preventable Diseases; Atkinson, W., Hamborsky, J., Wolfe, S., Eds.; Public Health Foundation: Washington DC, 2012; Volume 12, pp. 249–261. [Google Scholar]
- Tapparel, C.; Siegrist, F.; Petty, T.J.; Kaiser, L. Picornavirus and enterovirus diversity with associated human diseases. Infect. Genet. Evol. 2013, 14, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Rotbart, H.A. Viral meningitis. Semin. Neurol. 2000, 20, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Ooi, M.H.; Wong, S.C.; Lewthwaite, P.; Cardosa, M.J.; Solomon, T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 2010, 9, 1097–1105. [Google Scholar] [CrossRef]
- Greninger, A.L.; Naccache, S.N.; Messacar, K.; Clayton, A.; Yu, G.; Somasekar, S.; Federman, S.; Stryke, D.; Anderson, C.; Yagi, S.; et al. A novel outbreak enterovirus D68 strain associated with acute flaccid myelitis cases in the USA (2012–14): A retrospective cohort study. Lancet. Infect. Dis. 2015, 15, 671–682. [Google Scholar] [CrossRef]
- Meijer, A.; Benschop, K.S.; Donker, G.A.; van der Avoort, H.G. Continued seasonal circulation of enterovirus D68 in the Netherlands, 2011–2014. Euro Surveill. 2014, 19, art 1. [Google Scholar] [CrossRef]
- Midgley, C.M.; Jackson, M.A.; Selvarangan, R.; Turabelidze, G.; Obringer, E.; Johnson, D.; Giles, B.L.; Patel, A.; Echols, F.; Oberste, M.S.; et al. Severe respiratory illness associated with enterovirus D68—Missouri and Illinois, 2014. MMWR. Morb. Mortal. Wkly. Rep. 2014, 63, 798–799. [Google Scholar] [PubMed]
- Bragstad, K.; Jakobsen, K.; Rojahn, A.E.; Skram, M.K.; Vainio, K.; Holberg-Petersen, M.; Hungnes, O.; Dudman, S.G.; Kran, A.-M.B. High frequency of enterovirus D68 in children hospitalised with respiratory illness in Norway, Autumn 2014. Influenza Other Respi. Viruses 2015, 9, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Messacar, K.; Schreiner, T.L.; Maloney, J.A.; Wallace, A.; Ludke, J.; Oberste, M.S.; Nix, W.A.; Robinson, C.C.; Glodé, M.P.; Abzug, M.J.; et al. A cluster of acute flaccid paralysis and cranial nerve dysfunction temporally associated with an outbreak of enterovirus D68 in children in Colorado, USA. Lancet 2015, 385, 1662–1671. [Google Scholar] [CrossRef]
- Fendrick, A.M.; Monto, A.S.; Nightengale, B.; Sarnes, M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch. Intern. Med. 2003, 163, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, G.; Lerikou, M.; Tsiodras, S.; Chranioti, A.; Perros, E.; Anagnostopoulou, U.; Armaganidis, A.; Karakitsos, P. Viral epidemiology of acute exacerbations of chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 2012, 25, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Gern, J.E. The ABCs of rhinoviruses, wheezing, and asthma. J. Virol. 2010, 84, 7418–7426. [Google Scholar] [CrossRef] [PubMed]
- Kherad, O.; Kaiser, L.; Bridevaux, P.-O.; Sarasin, F.; Thomas, Y.; Janssens, J.-P.; Rutschmann, O.T. Upper-respiratory viral infection, biomarkers, and COPD exacerbations. Chest 2010, 138, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Mallia, P.; Message, S.D.; Kebadze, T.; Parker, H.L.; Kon, O.M.; Johnston, S.L. An experimental model of rhinovirus induced chronic obstructive pulmonary disease exacerbations: A pilot study. Respir. Res. 2006, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- McManus, T.E.; Marley, A.-M.; Baxter, N.; Christie, S.N.; O’Neill, H.J.; Elborn, J.S.; Coyle, P.V.; Kidney, J.C. Respiratory viral infection in exacerbations of COPD. Respir. Med. 2008, 102, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.G.; Christodoulou, I.; Rohde, G.; Agache, I.; Almqvist, C.; Bruno, A.; Bonini, S.; Bont, L.; Bossios, A.; Bousquet, J.; et al. Viruses and bacteria in acute asthma exacerbations—A GA2 LEN-DARE systematic review. Allergy 2011, 66, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Seemungal, T.A.; Harper-Owen, R.; Bhowmik, A.; Jeffries, D.J.; Wedzicha, J.A. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur. Respir. J. 2000, 16, 677–683. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burns, J.L.; Emerson, J.; Kuypers, J.; Campbell, A.P.; Gibson, R.L.; McNamara, S.; Worrell, K.; Englund, J.A. Respiratory viruses in children with cystic fibrosis: viral detection and clinical findings. Influenza Other Respi. Viruses 2012, 6, 218–223. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, M.B.; Zerbinati, R.M.; Tateno, A.F.; Oliveira, C.M.; Romão, R.M.; Rodrigues, J.C.; Pannuti, C.S.; da Silva Filho, L.V.F. Rhinovirus C and respiratory exacerbations in children with cystic fibrosis. Emerg. Infect. Dis. 2010, 16, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Kieninger, E.; Singer, F.; Tapparel, C.; Alves, M.P.; Latzin, P.; Tan, H.-L.; Bossley, C.; Casaulta, C.; Bush, A.; Davies, J.C.; et al. High rhinovirus burden in lower airways of children with cystic fibrosis. Chest 2013, 143, 782–790. [Google Scholar] [PubMed]
- Wat, D.; Gelder, C.; Hibbitts, S.; Cafferty, F.; Bowler, I.; Pierrepoint, M.; Evans, R.; Doull, I. The role of respiratory viruses in cystic fibrosis. J. Cyst. Fibros. 2008, 7, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Hayden, F.G.; Turner, R.B. Rhinovirus genetics and virulence: looking for needles in a haystack. Am. J. Respir. Crit. Care Med. 2012, 186, 818–820. [Google Scholar] [CrossRef] [PubMed]
- Joki-Korpela, P.; Hyypiä, T. Parechoviruses, a novel group of human picornaviruses. Ann. Med. 2001, 33, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Stanway, G.; Joki-Korpela, P.; Hyypiä, T. Human parechoviruses—Biology and clinical significance. Rev. Med. Virol. 2000, 10, 57–69. [Google Scholar] [CrossRef]
- Stanway, G.; Brown, F.; Christian, P.; Hovi, T.; Hyypiä, T.; King, A.M.Q.; Knowles, N.J.; Lemon, S.M.; Minor, P.D.; Pallansch, M.A.; et al. Family Picornaviridae. In Virus Taxonomy. Eighth Report of the International Committee on Taxonomy of Viruses; Fauquet, C.M., Mayo, M.A., Maniloff, J., Desselberger, U., Ball, L.A., Eds.; Elsevier/Academic Press: London, UK, 2005; pp. 757–778. [Google Scholar]
- Benschop, K.; Thomas, X.; Serpenti, C.; Molenkamp, R.; Wolthers, K. High prevalence of human Parechovirus (HPeV) genotypes in the Amsterdam region and identification of specific HPeV variants by direct genotyping of stool samples. J. Clin. Microbiol. 2008, 46, 3965–3970. [Google Scholar] [CrossRef] [PubMed]
- Khatami, A.; McMullan, B.J.; Webber, M.; Stewart, P.; Francis, S.; Timmers, K.J.; Rodas, E.; Druce, J.; Mehta, B.; Sloggett, N.A.; et al. Sepsis-like disease in infants due to human parechovirus type 3 during an outbreak in Australia. Clin. Infect. Dis. 2015, 60, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Wildenbeest, J.G.; Wolthers, K.C.; Straver, B.; Pajkrt, D. Successful IVIG treatment of human parechovirus-associated dilated cardiomyopathy in an infant. Pediatrics 2013, 132, e243–e247. [Google Scholar] [CrossRef] [PubMed]
- Benschop, K.S.; Schinkel, J.; Minnaar, R.P.; Pajkrt, D.; Spanjerberg, L.; Kraakman, H.C.; Berkhout, B.; Zaaijer, H.L.; Beld, M.G.H.M.; Wolthers, K.C. Human parechovirus infections in Dutch children and the association between serotype and disease severity. Clin. Infect. Dis. 2006, 42, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Harvala, H.; Robertson, I.; Chieochansin, T.; McWilliam Leitch, E.C.; Templeton, K.; Simmonds, P. Specific association of human parechovirus type 3 with sepsis and fever in young infants, as identified by direct typing of cerebrospinal fluid samples. J. Infect. Dis. 2009, 199, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Wolthers, K.C.; Benschop, K.S.M.; Schinkel, J.; Molenkamp, R.; Bergevoet, R.M.; Spijkerman, I.J.B.; Kraakman, H.C.; Pajkrt, D. Human parechoviruses as an important viral cause of sepsislike illness and meningitis in young children. Clin. Infect. Dis. 2008, 47, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Boivin, G.; Abed, Y.; Boucher, F.D. Human parechovirus 3 and neonatal infections. Emerg. Infect. Dis. 2005, 11, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Renaud, C.; Kuypers, J.; Ficken, E.; Cent, A.; Corey, L.; Englund, J.A. Introduction of a novel parechovirus RT-PCR clinical test in a regional medical center. J. Clin. Virol. 2011, 51, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Sainato, R.; Flanagan, R.; Mahlen, S.; Fairchok, M.; Braun, L. Severe human parechovirus sepsis beyond the neonatal period. J. Clin. Virol. 2011, 51, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Schuffenecker, I.; Javouhey, E.; Gillet, Y.; Kugener, B.; Billaud, G.; Floret, D.; Lina, B.; Morfin, F. Human parechovirus infections, Lyon, France, 2008-10: evidence for severe cases. J. Clin. Virol. 2012, 54, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Selvarangan, R.; Nzabi, M.; Selvaraju, S.B.; Ketter, P.; Carpenter, C.; Harrison, C.J. Human parechovirus 3 causing sepsis-like illness in children from midwestern United States. Pediatr. Infect. Dis. J. 2011, 30, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Verboon-Maciolek, M.A.; Groenendaal, F.; Hahn, C.D.; Hellmann, J.; van Loon, A.M.; Boivin, G.; de Vries, L.S. Human parechovirus causes encephalitis with white matter injury in neonates. Ann. Neurol. 2008, 64, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Walters, B.; Peñaranda, S.; Nix, W.A.; Oberste, M.S.; Todd, K.M.; Katz, B.Z.; Zheng, X. Detection of human parechovirus (HPeV)-3 in spinal fluid specimens from pediatric patients in the Chicago area. J. Clin. Virol. 2011, 52, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Gonzalez, R.; Xie, Z.; Xiao, Y.; Liu, J.; Chen, L.; Liu, C.; Zhang, J.; Ren, L.; Vernet, G.; et al. Human rhinovirus C infections mirror those of human rhinovirus A in children with community-acquired pneumonia. J. Clin. Virol. 2010, 49, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Yuzurihara, S.S.; Ao, K.; Hara, T.; Tanaka, F.; Mori, M.; Kikuchi, N.; Kai, S.; Yokota, S. Human parechovirus-3 infection in nine neonates and infants presenting symptoms of hemophagocytic lymphohistiocytosis. J. Infect. Chemother. 2013, 19, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.A.; Diop, O.M.; Burns, C.C.; Tangermann, R.H.; Wassilak, S.G.F. Tracking progress toward polio eradication—Worldwide, 2013–2014. MMWR. Morb. Mortal. Wkly. Rep. 2015, 64, 415–420. [Google Scholar] [PubMed]
- Bejing Vigoo Biological Co., Ltd. A clinical trial to assess the efficacy and safety of an inactivated vaccine (vero cell) against EV71 in Chinese children aged 6–35 months. Available online: https://clinicaltrials.gov/ct2/show/NCT01508247 (accessed on 27 March 2015).
- Longding Liu, Chinese Academy of Medical Sciences. A protected study of inactivated EV71 vaccine (human diploid cell, KMB-17) in Chinese infants and children. Available online: https://clinicaltrials.gov/ct2/show/NCT01569581 (accessed on 10 March 2015).
- Sinovac Biotech Co., Ltd. An Efficacy Trial in inactivated enterovirus type 71 (EV71) vaccine. Available online: https://clinicaltrials.gov/ct2/show/NCT01507857 (accessed on 27 March 2015).
- Abzug, M.J.; Keyserling, H.L.; Lee, M.L.; Levin, M.J.; Rotbart, H.A. Neonatal enterovirus infection: virology, serology, and effects of intravenous immune globulin. Clin. Infect. Dis. 1995, 20, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Nagington, J. Echovirus 11 infection and prophylactic antiserum. Lancet 1982, 1, 446. [Google Scholar] [CrossRef]
- Wildenbeest, J.G.; van den Broek, P.J.; Benschop, K.S.M.; Koen, G.; Wierenga, P.C.; Vossen, A.C.; Kuijpers, T.W.; Wolthers, K.C. Pleconaril revisited: clinical course of chronic enteroviral meningoencephalitis after treatment correlates with in vitro susceptibility. Antivir. Ther. 2012, 17, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.-H.; Huang, Y.-C.; Chen, M.-C.; Liu, C.-C.; Chiu, N.-C.; Lien, R.; Chang, L.-Y.; Chiu, C.-H.; Tsao, K.-C.; Lin, T.-Y. Effect of intravenous immunoglobulin for neonates with severe enteroviral infections with emphasis on the timing of administration. J. Clin. Virol. 2015, 64, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Enserink, M. Polio endgame. Wanted: Drug for a disappearing disease. Science 2004, 303. [Google Scholar] [CrossRef] [PubMed]
- Tuthill, T.J.; Groppelli, E.; Hogle, J.M.; Rowlands, D.J. Picornaviruses. Curr. Top. Microbiol. Immunol. 2010, 343, 43–89. [Google Scholar] [PubMed]
- Kräusslich, H.G.; Nicklin, M.J.; Toyoda, H.; Etchison, D.; Wimmer, E. Poliovirus proteinase 2A induces cleavage of eucaryotic initiation factor 4F polypeptide p220. J. Virol. 1987, 61, 2711–2718. [Google Scholar] [PubMed]
- Lloyd, R.E.; Grubman, M.J.; Ehrenfeld, E. Relationship of p220 cleavage during picornavirus infection to 2A proteinase sequencing. J. Virol. 1988, 62, 4216–4223. [Google Scholar] [PubMed]
- Joachims, M.; Van Breugel, P.C.; Lloyd, R.E. Cleavage of poly(A)-binding protein by enterovirus proteases concurrent with inhibition of translation in vitro. J. Virol. 1999, 73, 718–727. [Google Scholar] [PubMed]
- Badorff, C.; Lee, G.H.; Lamphear, B.J.; Martone, M.E.; Campbell, K.P.; Rhoads, R.E.; Knowlton, K.U. Enteroviral protease 2A cleaves dystrophin: Evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat. Med. 1999, 5, 320–326. [Google Scholar] [PubMed]
- Barral, P.M.; Morrison, J.M.; Drahos, J.; Gupta, P.; Sarkar, D.; Fisher, P.B.; Racaniello, V.R. MDA-5 is cleaved in poliovirus-infected cells. J. Virol. 2007, 81, 3677–3684. [Google Scholar] [CrossRef] [PubMed]
- Castelló, A.; Alvarez, E.; Carrasco, L. The multifaceted poliovirus 2A protease: Regulation of gene expression by picornavirus proteases. J. Biomed. Biotechnol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Xiao, X.; Xue, Q.; Jin, Q.; He, B.; Wang, J. Cleavage of interferon regulatory factor 7 by enterovirus 71 3C suppresses cellular responses. J. Virol. 2013, 87, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Morosky, S.A.; Delorme-Axford, E.; Dybdahl-Sissoko, N.; Oberste, M.S.; Wang, T.; Coyne, C.B. The coxsackievirus B 3C protease cleaves MAVS and TRIF to attenuate host type I interferon and apoptotic signaling. PLoS Pathog. 2011, 7, e1001311. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xi, X.; Lei, X.; Zhang, X.; Cui, S.; Wang, J.; Jin, Q.; Zhao, Z. Enterovirus 71 protease 2Apro targets MAVS to inhibit anti-viral type I interferon responses. PLoS Pathog. 2013, 9, e1003231. [Google Scholar] [CrossRef] [PubMed]
- Gerber, K.; Wimmer, E.; Paul, A.V. Biochemical and genetic studies of the initiation of human rhinovirus 2 RNA replication: Identification of a cis-replicating element in the coding sequence of 2A(pro). J. Virol. 2001, 75, 10979–10990. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paul, A.V.; Rieder, E.; Kim, D.W.; van Boom, J.H.; Wimmer, E. Identification of an RNA hairpin in poliovirus RNA that serves as the primary template in the in vitro uridylylation of VPg. J. Virol. 2000, 74, 10359–10370. [Google Scholar] [CrossRef] [PubMed]
- Rieder, E.; Paul, A.V.; Kim, D.W.; van Boom, J.H.; Wimmer, E. Genetic and biochemical studies of poliovirus cis-acting replication element cre in relation to VPg uridylylation. J. Virol. 2000, 74, 10371–10380. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Rijnbrand, R.; McKnight, K.L.; Wimmer, E.; Paul, A.; Martin, A.; Lemon, S.M. Sequence requirements for viral RNA replication and VPg uridylylation directed by the internal cis-acting replication element (cre) of human rhinovirus type 14. J. Virol. 2002, 76, 7485–7494. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Bartenschlager, R. Architecture and biogenesis of plus-strand RNA virus replication factories. World J. Virol. 2013, 2, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Belov, G.A.; van Kuppeveld, F.J. (+)RNA viruses rewire cellular pathways to build replication organelles. Curr. Opin. Virol. 2012, 2, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Belov, G.A.; Nair, V.; Hansen, B.T.; Hoyt, F.H.; Fischer, E.R.; Ehrenfeld, E. Complex dynamic development of poliovirus membranous replication complexes. J. Virol. 2012, 86, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Limpens, R.W.; van der Schaar, H.M.; Kumar, D.; Koster, A.J.; Snijder, E.J.; van Kuppeveld, F.J.; Bárcena, M. The transformation of enterovirus replication structures: A three-dimensional study of single- and double-membrane compartments. MBio 2011. [Google Scholar] [CrossRef] [PubMed]
- Belov, G.A.; Feng, Q.; Nikovics, K.; Jackson, C.L.; Ehrenfeld, E. A critical role of a cellular membrane traffic protein in poliovirus RNA replication. PLoS Pathog. 2008, 4, e1000216. [Google Scholar] [CrossRef] [PubMed]
- Gazina, E.V.; Mackenzie, J.M.; Gorrell, R.J.; Anderson, D.A. Differential requirements for COPI coats in formation of replication complexes among three genera of Picornaviridae. J. Virol. 2002, 76, 11113–11122. [Google Scholar] [CrossRef] [PubMed]
- Irurzun, A.; Perez, L.; Carrasco, L. Involvement of membrane traffic in the replication of poliovirus genomes: effects of brefeldin A. Virology 1992, 191, 166–175. [Google Scholar] [CrossRef]
- Lanke, K.H.; van der Schaar, H.M.; Belov, G.A.; Feng, Q.; Duijsings, D.; Jackson, C.L.; Ehrenfeld, E.; van Kuppeveld, F.J. GBF1, a guanine nucleotide exchange factor for Arf, is crucial for coxsackievirus B3 RNA replication. J. Virol. 2009, 83, 11940–11949. [Google Scholar] [CrossRef] [PubMed]
- Wessels, E.; Duijsings, D.; Niu, T.-K.; Neumann, S.; Oorschot, V.M.; de Lange, F.; Lanke, K.H.; Klumperman, J.; Henke, A.; Jackson, C.L.; et al. A viral protein that blocks Arf1-mediated COP-I assembly by inhibiting the guanine nucleotide exchange factor GBF1. Dev. Cell 2006, 11, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Wessels, E.; Duijsings, D.; Lanke, K.H.; van Dooren, S.H.J.; Jackson, C.L.; Melchers, W.J.; van Kuppeveld, F.J. Effects of picornavirus 3A Proteins on Protein Transport and GBF1-dependent COP-I recruitment. J. Virol. 2006, 80, 11852–11860. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hsu, N.-Y.; Ilnytska, O.; Belov, G.; Santiana, M.; Chen, Y.-H.; Takvorian, P.M.; Pau, C.; van der Schaar, H.; Kaushik-Basu, N.; Balla, T.; et al. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell 2010, 141, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Doedens, J.R.; Kirkegaard, K. Inhibition of cellular protein secretion by poliovirus proteins 2B and 3A. EMBO J. 1995, 14, 894–907. [Google Scholar] [PubMed]
- Wessels, E.; Duijsings, D.; Notebaart, R.A.; Melchers, W.J.; van Kuppeveld, F.J. A proline-rich region in the coxsackievirus 3A protein is required for the protein to inhibit endoplasmic reticulum-to-golgi transport. J. Virol. 2005, 79, 5163–5173. [Google Scholar] [CrossRef] [PubMed]
- Deitz, S.B.; Dodd, D.A.; Cooper, S.; Parham, P.; Kirkegaard, K. MHC I-dependent antigen presentation is inhibited by poliovirus protein 3A. Proc. Natl. Acad. Sci. USA 2000, 97, 13790–13795. [Google Scholar] [CrossRef] [PubMed]
- Dodd, D.A.; Giddings, T.H.; Kirkegaard, K. Poliovirus 3A protein limits interleukin-6 (IL-6), IL-8, and beta interferon secretion during viral infection. J. Virol. 2001, 75, 8158–8165. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, G.; Vicinanza, M.; Di Campli, A.; De Matteis, M.A. The multiple roles of PtdIns(4)P—Not just the precursor of PtdIns(4,5)P2. J. Cell Sci. 2008, 121, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.R.; Burd, C.G. Coordination of Golgi functions by phosphatidylinositol 4-kinases. Trends Cell Biol. 2011, 21, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Tirado, F.H.; Bretscher, A. Membrane-trafficking sorting hubs: cooperation between PI4P and small GTPases at the trans-Golgi network. Trends Cell Biol. 2011, 21, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Dorobantu, C.M.; van der Schaar, H.M.; Ford, L.A.; Strating, J.R.; Ulferts, R.; Fang, Y.; Belov, G.; van Kuppeveld, F.J. Recruitment of PI4KIIIβ to coxsackievirus B3 replication organelles is independent of ACBD3, GBF1, and Arf1. J. Virol. 2014, 88, 2725–2736. [Google Scholar] [CrossRef] [PubMed]
- Téoulé, F.; Brisac, C.; Pelletier, I.; Vidalain, P.-O.; Jégouic, S.; Mirabelli, C.; Bessaud, M.; Combelas, N.; Autret, A.; Tangy, F.; et al. The Golgi protein ACBD3, an interactor for poliovirus protein 3A, modulates poliovirus replication. J. Virol. 2013, 87, 11031–11046. [Google Scholar] [CrossRef] [PubMed]
- Mesmin, B.; Bigay, J.; Moser von Filseck, J.; Lacas-Gervais, S.; Drin, G.; Antonny, B. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell 2013, 155, 830–843. [Google Scholar] [CrossRef] [PubMed]
- Strating, J.R.; van der Linden, L.; Albulescu, L.; Bigay, J.; Arita, M.; Delang, L.; Leyssen, P.; van der Schaar, H.M.; Lanke, K.H.; Thibaut, H.J.; et al. Itraconazole Inhibits Enterovirus Replication by Targeting the Oxysterol-Binding Protein. Cell Rep. 2015, 10, 600–615. [Google Scholar] [CrossRef] [PubMed]
- Arita, M. Phosphatidylinositol-4 kinase III beta and oxysterol-binding protein accumulate unesterified cholesterol on poliovirus-induced membrane structure. Microbiol. Immunol. 2014, 58, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Roulin, P.S.; Lötzerich, M.; Torta, F.; Tanner, L.B.; van Kuppeveld, F.J.; Wenk, M.R.; Greber, U.F. Rhinovirus uses a phosphatidylinositol 4-phosphate/cholesterol counter-current for the formation of replication compartments at the ER-Golgi interface. Cell Host Microbe 2014, 16, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Albulescu, L.; Wubbolts, R.; van Kuppeveld, F.J.; Strating, J.R. Cholesterol shuttling is important for RNA replication of coxsackievirus B3 and encephalomyocarditis virus. Cell. Microbiol. 2015, 17, 1144–1156. [Google Scholar] [CrossRef] [PubMed]
- Ilnytska, O.; Santiana, M.; Hsu, N.-Y.; Du, W.-L.; Chen, Y.-H.; Viktorova, E.G.; Belov, G.; Brinker, A.; Storch, J.; Moore, C.; et al. Enteroviruses harness the cellular endocytic machinery to remodel the host cell cholesterol landscape for effective viral replication. Cell Host Microbe 2013, 14, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Zhang, J.; Si, X.; Gao, G.; Mao, I.; McManus, B.M.; Luo, H. Autophagosome supports coxsackievirus B3 replication in host cells. J. Virol. 2008, 82, 9143–9153. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.T.; Giddings, T.H.; Taylor, M.P.; Mulinyawe, S.; Rabinovitch, M.; Kopito, R.R.; Kirkegaard, K. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 2005, 3, e156. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.L.; Jackson, W.T. Intracellular vesicle acidification promotes maturation of infectious poliovirus particles. PLoS Pathog. 2012, 8, e1003046. [Google Scholar] [CrossRef] [PubMed]
- Barco, A.; Carrasco, L. A human virus protein, poliovirus protein 2BC, induces membrane proliferation and blocks the exocytic pathway in the yeast Saccharomyces cerevisiae. EMBO J. 1995, 14, 3349–3364. [Google Scholar] [PubMed]
- Cho, M.W.; Teterina, N.; Egger, D.; Bienz, K.; Ehrenfeld, E. Membrane rearrangement and vesicle induction by recombinant poliovirus 2C and 2BC in human cells. Virology 1994, 202, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Suhy, D.A.; Giddings, T.H.; Kirkegaard, K. Remodeling the endoplasmic reticulum by poliovirus infection and by individual viral proteins: An autophagy-like origin for virus-induced vesicles. J. Virol. 2000, 74, 8953–8965. [Google Scholar] [CrossRef] [PubMed]
- Agirre, A.; Barco, A.; Carrasco, L.; Nieva, J.L. Viroporin-mediated membrane permeabilization. Pore formation by nonstructural poliovirus 2B protein. J. Biol. Chem. 2002, 277, 40434–40441. [Google Scholar] [CrossRef] [PubMed]
- De Jong, A.S.; Wessels, E.; Dijkman, H.B.; Galama, J.M.; Melchers, W.J.; Willems, P.H.; van Kuppeveld, F.J. Determinants for membrane association and permeabilization of the coxsackievirus 2B protein and the identification of the Golgi complex as the target organelle. J. Biol. Chem. 2003, 278, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- De Jong, A.S.; Visch, H.-J.; de Mattia, F.; van Dommelen, M.M.; Swarts, H.G.; Luyten, T.; Callewaert, G.; Melchers, W.J.; Willems, P.H.; van Kuppeveld, F.J. The Coxsackievirus 2B Protein Increases Efflux of Ions from the Endoplasmic Reticulum and Golgi, thereby Inhibiting Protein Trafficking through the Golgi. J. Biol. Chem. 2006, 281, 14144–14150. [Google Scholar] [CrossRef] [PubMed]
- Van Kuppeveld, F.J.; Hoenderop, J.G.; Smeets, R.L.; Willems, P.H.; Dijkman, H.B.; Galama, J.M.; Melchers, W.J. Coxsackievirus protein 2B modifies endoplasmic reticulum membrane and plasma membrane permeability and facilitates virus release. EMBO J. 1997, 16, 3519–3532. [Google Scholar] [CrossRef] [PubMed]
- Aldabe, R.; Carrasco, L. Induction of membrane proliferation by poliovirus proteins 2C and 2BC. Biochem. Biophys. Res. Commun. 1995, 206, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Van Kuppeveld, F.J.; Melchers, W.J.; Kirkegaard, K.; Doedens, J.R. Structure-function analysis of coxsackie B3 virus protein 2B. Virology 1997, 227, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Cornell, C.T.; Kiosses, W.B.; Harkins, S.; Whitton, J.L. Coxsackievirus B3 proteins directionally complement each other to downregulate surface major histocompatibility complex class I. J. Virol. 2007, 81, 6785–6797. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.-F.; Yang, S.-Y.; Wu, B.-W.; Jheng, J.-R.; Chen, Y.-L.; Shih, C.-H.; Lin, K.-H.; Lai, H.-C.; Tang, P.; Horng, J.-T. Reticulon 3 binds the 2C protein of enterovirus 71 and is required for viral replication. J. Biol. Chem. 2007, 282, 5888–5898. [Google Scholar] [CrossRef] [PubMed]
- Molla, A.; Paul, A.V.; Wimmer, E. Cell-free, de novo synthesis of poliovirus. Science 1991, 254, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Nugent, C.I.; Johnson, K.L.; Sarnow, P.; Kirkegaard, K. Functional coupling between replication and packaging of poliovirus replicon RNA. J. Virol. 1999, 73, 427–435. [Google Scholar] [PubMed]
- Liu, Y.; Wang, C.; Mueller, S.; Paul, A.V.; Wimmer, E.; Jiang, P. Direct interaction between two viral proteins, the nonstructural protein 2C and the capsid protein VP3, is required for enterovirus morphogenesis. PLoS Pathog. 2010, 6, e1001066. [Google Scholar] [CrossRef] [PubMed]
- Geller, R.; Vignuzzi, M.; Andino, R.; Frydman, J. Evolutionary constraints on chaperone-mediated folding provide an antiviral approach refractory to development of drug resistance. Genes Dev. 2007, 21, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Ypma-Wong, M.F.; Dewalt, P.G.; Johnson, V.H.; Lamb, J.G.; Semler, B.L. Protein 3CD is the major poliovirus proteinase responsible for cleavage of the P1 capsid precursor. Virology 1988, 166, 265–270. [Google Scholar] [CrossRef]
- Mikami, T.; Satoh, N.; Hatayama, I.; Nakane, A. Buthionine sulfoximine inhibits cytopathic effect and apoptosis induced by infection with human echovirus 9. Arch. Virol. 2004, 149, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Dawson, H. Glutathione is required for efficient production of infectious picornavirus virions. Virology 2006, 353, 258–267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thibaut, H.J.; van der Linden, L.; Jiang, P.; Thys, B.; Canela, M.-D.; Aguado, L.; Rombaut, B.; Wimmer, E.; Paul, A.; Pérez-Pérez, M.-J.; et al. Binding of glutathione to enterovirus capsids is essential for virion morphogenesis. PLoS Pathog. 2014, 10, e1004039. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.-C.; Liu, Y.; Wang, C.; Strauss, M.; Rehage, N.; Chen, Y.-H.; Altan-Bonnet, N.; Hogle, J.; Wimmer, E.; Mueller, S.; et al. An interaction between glutathione and the capsid is required for the morphogenesis of C-cluster enteroviruses. PLoS Pathog. 2014, 10, e1004052. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Du, W.; Hagemeijer, M.C.; Takvorian, P.M.; Pau, C.; Cali, A.; Brantner, C.A.; Stempinski, E.S.; Connelly, P.S.; Ma, H.-C.; et al. Phosphatidylserine Vesicles Enable Efficient En Bloc Transmission of Enteroviruses. Cell 2015, 160, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.M.; Tsueng, G.; Sin, J.; Mangale, V.; Rahawi, S.; McIntyre, L.L.; Williams, W.; Kha, N.; Cruz, C.; Hancock, B.M.; et al. Coxsackievirus B exits the host cell in shed microvesicles displaying autophagosomal markers. PLoS Pathog. 2014, 10, e1004045. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Hensley, L.; McKnight, K.L.; Hu, F.; Madden, V.; Ping, L.; Jeong, S.-H.; Walker, C.; Lanford, R.E.; Lemon, S.M. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature 2013, 496, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Abzug, M.J.; Cloud, G.; Bradley, J.; Sánchez, P.J.; Romero, J.; Powell, D.; Lepow, M.; Mani, C.; Capparelli, E.V.; Blount, S.; et al. Double blind placebo-controlled trial of pleconaril in infants with enterovirus meningitis. Pediatr. Infect. Dis. J. 2003, 22, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Desmond, R.A.; Accortt, N.A.; Talley, L.; Villano, S.A.; Soong, S.-J.; Whitley, R.J. Enteroviral meningitis: Natural history and outcome of pleconaril therapy. Antimicrob. Agents Chemother. 2006, 50, 2409–2414. [Google Scholar] [CrossRef] [PubMed]
- Hayden, F.G.; Coats, T.; Kim, K.; Hassman, H.A.; Blatter, M.M.; Zhang, B.; Liu, S. Oral pleconaril treatment of picornavirus-associated viral respiratory illness in adults: Efficacy and tolerability in phase II clinical trials. Antivir. Ther. 2002, 7, 53–65. [Google Scholar] [PubMed]
- Hayden, F.G.; Herrington, D.T.; Coats, T.L.; Kim, K.; Cooper, E.C.; Villano, S.A.; Liu, S.; Hudson, S.; Pevear, D.C.; Collett, M.; et al. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: Results of 2 double-blind, randomized, placebo-controlled trials. Clin. Infect. Dis. 2003, 36, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Rotbart, H.A.; Webster, A.D. Treatment of potentially life-threatening enterovirus infections with pleconaril. Clin. Infect. Dis. 2001, 32, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Schiff, G.M.; Sherwood, J.R. Clinical activity of pleconaril in an experimentally induced coxsackievirus A21 respiratory infection. J. Infect. Dis. 2000, 181, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Senior, K. FDA panel rejects common cold treatment. Lancet. Infect. Dis. 2002, 2, 264. [Google Scholar] [CrossRef]
- Merck Sharp & Dohme Corp. Effects of Pleconaril Nasal Spray on Common Cold Symptoms and Asthma Exacerbations Following Rhinovirus Exposure. Available online: https://clinicaltrials.gov/ct2/show/NCT00394914 (accessed on 23 June 2015).
- Pleconaril Enteroviral Sepsis Syndrome. Available online: https://clinicaltrials.gov/ct2/show/NCT00031512 (accessed on 28 February 2013).
- Biota Pharmaceuticals HRV Phase IIb Study Achieves Primary Endpoint. Available online: http://www.biota.com.au/uploaded/154/1021819_20hrvphaseiibstudyachieve.pdf (accessed on 28 March 2015).
- Biota Pharmaceuticals Biota Commences Dosing in Vapendavir SPIRITUS Phase 2b Trial. Available online: http://investors.biotapharma.com/releasedetail.cfm?releaseid=899451 (accessed on 28 March 2015).
- Oberste, M.S.; Moore, D.; Anderson, B.; Pallansch, M.A.; Pevear, D.C.; Collett, M.S. In vitro antiviral activity of V-073 against polioviruses. Antimicrob. Agents Chemother. 2009, 53, 4501–4503. [Google Scholar] [CrossRef] [PubMed]
- Buontempo, P.J.; Cox, S.; Wright-Minogue, J.; DeMartino, J.L.; Skelton, A.M.; Ferrari, E.; Albin, R.; Rozhon, E.J.; Girijavallabhan, V.; Modlin, J.F.; et al. SCH 48973: A potent, broad-spectrum, antienterovirus compound. Antimicrob. Agents Chemother. 1997, 41, 1220–1225. [Google Scholar] [PubMed]
- Torres-Torres, S.; Myers, A.L.; Klatte, J.M.; Rhoden, E.E.; Oberste, M.S.; Collett, M.S.; McCulloh, R.J. First use of investigational antiviral drug pocapavir (v-073) for treating neonatal enteroviral sepsis. Pediatr. Infect. Dis. J. 2015, 34, 52–54. [Google Scholar] [CrossRef] [PubMed]
- De Palma, A.M.; Vliegen, I.; De Clercq, E.; Neyts, J. Selective inhibitors of picornavirus replication. Med. Res. Rev. 2008, 28, 823–884. [Google Scholar] [CrossRef] [PubMed]
- Pevear, D.C.; Hayden, F.G.; Demenczuk, T.M.; Barone, L.R.; McKinlay, M.A.; Collett, M.S. Relationship of pleconaril susceptibility and clinical outcomes in treatment of common colds caused by rhinoviruses. Antimicrob. Agents Chemother. 2005, 49, 4492–4499. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, L.; Crump, C.E.; Hayden, F.G. In vitro activity of pleconaril and AG7088 against selected serotypes and clinical isolates of human rhinoviruses. Antivir. Res. 2000, 47, 215–220. [Google Scholar] [CrossRef]
- Ledford, R.M.; Patel, N.R.; Demenczuk, T.M.; Watanyar, A.; Herbertz, T.; Collett, M.S.; Pevear, D.C. VP1 sequencing of all human rhinovirus serotypes: Insights into genus phylogeny and susceptibility to antiviral capsid-binding compounds. J. Virol. 2004, 78, 3663–3674. [Google Scholar] [CrossRef] [PubMed]
- Pevear, D.C.; Tull, T.M.; Seipel, M.E.; Groarke, J.M. Activity of pleconaril against enteroviruses. Antimicrob. Agents Chemother. 1999, 43, 2109–2115. [Google Scholar] [PubMed]
- Benschop, K.S.; Wildenbeest, J.G.; Koen, G.; Minnaar, R.P.; van Hemert, F.J.; Westerhuis, B.M.; Pajkrt, D.; van den Broek, P.J.; Vossen, A.C.; Wolthers, K.C. Genetic and antigenic structural characterization for resistance of echovirus 11 to pleconaril in an immunocompromised patient. J. Gen. Virol. 2015, 96, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Dragovich, P.S.; Prins, T.J.; Zhou, R.; Webber, S.E.; Marakovits, J.T.; Fuhrman, S.A.; Patick, A.K.; Matthews, D.A.; Lee, C.A.; Ford, C.E.; et al. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 4. Incorporation of P1 lactam moieties as L-glutamine replacements. J. Med. Chem. 1999, 42, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- De Palma, A.M.; Pürstinger, G.; Wimmer, E.; Patick, A.K.; Andries, K.; Rombaut, B.; De Clercq, E.; Neyts, J. Potential use of antiviral agents in polio eradication. Emerg. Infect. Dis. 2008, 14, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-C.; Shih, S.-R.; Chang, T.-Y.; Tseng, H.-Y.; Shih, Y.-F.; Yen, K.-J.; Chen, W.-C.; Shie, J.-J.; Fang, J.-M.; Liang, P.-H.; et al. A mammalian cell-based reverse two-hybrid system for functional analysis of 3C viral protease of human enterovirus 71. Anal. Biochem. 2008, 375, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Patick, A.K.; Binford, S.L.; Brothers, M.A.; Jackson, R.L.; Ford, C.E.; Diem, M.D.; Maldonado, F.; Dragovich, P.S.; Zhou, R.; Prins, T.J.; et al. In vitro antiviral activity of AG7088, a potent inhibitor of human rhinovirus 3C protease. Antimicrob. Agents Chemother. 1999, 43, 2444–2450. [Google Scholar] [PubMed]
- Tsai, M.-T.; Cheng, Y.-H.; Liu, Y.-N.; Liao, N.-C.; Lu, W.-W.; Kung, S.-H. Real-time monitoring of human enterovirus (HEV)-infected cells and anti-HEV 3C protease potency by fluorescence resonance energy transfer. Antimicrob. Agents Chemother. 2009, 53, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Patick, A.K.; Brothers, M.A.; Maldonado, F.; Binford, S.; Maldonado, O.; Fuhrman, S.; Petersen, A.; Smith, G.J.; Zalman, L.S.; Burns-Naas, L.A.; et al. In vitro antiviral activity and single-dose pharmacokinetics in humans of a novel, orally bioavailable inhibitor of human rhinovirus 3C protease. Antimicrob. Agents Chemother. 2005, 49, 2267–2275. [Google Scholar] [CrossRef] [PubMed]
- Dragovich, P.S.; Prins, T.J.; Zhou, R.; Johnson, T.O.; Hua, Y.; Luu, H.T.; Sakata, S.K.; Brown, E.L.; Maldonado, F.C.; Tuntland, T.; et al. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 8. Pharmacological optimization of orally bioavailable 2-pyridone-containing peptidomimetics. J. Med. Chem. 2003, 46, 4572–4585. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S.; Maag, D.; Arnold, J.J.; Zhong, W.; Lau, J.Y.; Hong, Z.; Andino, R.; Cameron, C.E. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat. Med. 2000, 6, 1375–1379. [Google Scholar] [PubMed]
- Crotty, S.; Cameron, C.E.; Andino, R. RNA virus error catastrophe: direct molecular test by using ribavirin. Proc. Natl. Acad. Sci. USA 2001, 98, 6895–6900. [Google Scholar] [CrossRef] [PubMed]
- Gazina, E.V.; Smidansky, E.D.; Holien, J.K.; Harrison, D.N.; Cromer, B.A.; Arnold, J.J.; Parker, W.W.; Cameron, C.E.; Petrou, S. Amiloride is a competitive inhibitor of coxsackievirus B3 RNA polymerase. J. Virol. 2011, 85, 10364–10374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van der Linden, L.; Vives-Adrián, L.; Selisko, B.; Ferrer-Orta, C.; Liu, X.; Lanke, K.; Ulferts, R.; De Palma, A.M.; Tanchis, F.; Goris, N.; et al. The RNA template channel of the RNA-dependent RNA polymerase as a target for development of antiviral therapy of multiple genera within a virus family. PLoS Pathog. 2015, 11, e1004733. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.-C.; Chen, T.-C.; Fang, M.-Y.; Yen, K.-J.; Shih, S.-R.; Hsu, J.T.-A.; Tseng, C.-P. Inhibition of enterovirus 71 replication and the viral 3D polymerase by aurintricarboxylic acid. J. Antimicrob. Chemother. 2010, 65, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.A.; Milstrey, K.P.; Trown, P.W. Specific inhibition of viral ribonucleic acid replication by Gliotoxin. Science 1968, 159, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.L.; Carrasco, L. Gliotoxin: inhibitor of poliovirus RNA synthesis that blocks the viral RNA polymerase 3Dpol. J. Virol. 1992, 66, 1971–1976. [Google Scholar] [PubMed]
- Velu, A.B.; Chen, G.-W.; Hsieh, P.-T.; Horng, J.-T.; Hsu, J.T.-A.; Hsieh, H.-P.; Chen, T.-C.; Weng, K.-F.; Shih, S.-R. BPR-3P0128 inhibits RNA-dependent RNA polymerase elongation and VPg uridylylation activities of Enterovirus 71. Antivir. Res. 2014, 112, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-C.; Chang, H.-Y.; Lin, P.-F.; Chern, J.-H.; Hsu, J.T.-A.; Chang, C.-Y.; Shih, S.-R. Novel antiviral agent DTriP-22 targets RNA-dependent RNA polymerase of enterovirus 71. Antimicrob. Agents Chemother. 2009, 53, 2740–2747. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.J.; Flanegan, J.B. Synchronous replication of poliovirus RNA: initiation of negative-strand RNA synthesis requires the guanidine-inhibited activity of protein 2C. J. Virol. 1997, 71, 8482–8489. [Google Scholar] [PubMed]
- Li, J.P.; Baltimore, D. Isolation of poliovirus 2C mutants defective in viral RNA synthesis. J. Virol. 1988, 62, 4016–4021. [Google Scholar] [PubMed]
- Pfister, T.; Jones, K.W.; Wimmer, E. A cysteine-rich motif in poliovirus protein 2C(ATPase) is involved in RNA replication and binds zinc in vitro. J. Virol. 2000, 74, 334–343. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Teterina, N.L.; Kean, K.M.; Gorbalenya, A.E.; Agol, V.I.; Girard, M. Analysis of the functional significance of amino acid residues in the putative NTP-binding pattern of the poliovirus 2C protein. J. Gen. Virol. 1992, 73 (Pt 8), 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Teterina, N.L.; Levenson, E.; Rinaudo, M.S.; Egger, D.; Bienz, K.; Gorbalenya, A.E.; Ehrenfeld, E. Evidence for functional protein interactions required for poliovirus RNA replication. J. Virol. 2006, 80, 5327–5337. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tolskaya, E.A.; Romanova, L.I.; Kolesnikova, M.S.; Gmyl, A.P.; Gorbalenya, A.E.; Agol, V.I. Genetic studies on the poliovirus 2C protein, an NTPase. A plausible mechanism of guanidine effect on the 2C function and evidence for the importance of 2C oligomerization. J. Mol. Biol. 1994, 236, 1310–1323. [Google Scholar] [CrossRef]
- Banerjee, R.; Echeverri, A.; Dasgupta, A. Poliovirus-encoded 2C polypeptide specifically binds to the 3’-terminal sequences of viral negative-strand RNA. J. Virol. 1997, 71, 9570–9578. [Google Scholar] [PubMed]
- Banerjee, R.; Tsai, W.; Kim, W.; Dasgupta, A. Interaction of poliovirus-encoded 2C/2BC polypeptides with the 3’ terminus negative-strand cloverleaf requires an intact stem-loop B. Virology 2001, 280, 41–51. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Banerjee, R.; Dasgupta, A. Interaction of picornavirus 2C polypeptide with the viral negative-strand RNA. J. Gen. Virol. 2001, 82, 2621–2627. [Google Scholar] [PubMed]
- Teterina, N.L.; Gorbalenya, A.E.; Egger, D.; Bienz, K.; Ehrenfeld, E. Poliovirus 2C protein determinants of membrane binding and rearrangements in mammalian cells. J. Virol. 1997, 71, 8962–8972. [Google Scholar] [PubMed]
- Vance, L.M.; Moscufo, N.; Chow, M.; Heinz, B.A. Poliovirus 2C region functions during encapsidation of viral RNA. J. Virol. 1997, 71, 8759–8765. [Google Scholar] [PubMed]
- Verlinden, Y.; Cuconati, A.; Wimmer, E.; Rombaut, B. The antiviral compound 5-(3,4-dichlorophenyl) methylhydantoin inhibits the post-synthetic cleavages and the assembly of poliovirus in a cell-free system. Antivir. Res. 2000, 48, 61–69. [Google Scholar] [CrossRef]
- Li, J.P.; Baltimore, D. An intragenic revertant of a poliovirus 2C mutant has an uncoating defect. J. Virol. 1990, 64, 1102–1107. [Google Scholar] [PubMed]
- Gorbalenya, A.E.; Koonin, E.V. Viral proteins containing the purine NTP-binding sequence pattern. Nucleic Acids Res. 1989, 17, 8413–8440. [Google Scholar] [CrossRef] [PubMed]
- Mirzayan, C.; Wimmer, E. Biochemical studies on poliovirus polypeptide 2C: Evidence for ATPase activity. Virology 1994, 199, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, P.L.; Carrasco, L. Poliovirus protein 2C has ATPase and GTPase activities. J. Biol. Chem. 1993, 268, 8105–8110. [Google Scholar] [PubMed]
- De Palma, A.M.; Heggermont, W.; Lanke, K.; Coutard, B.; Bergmann, M.; Monforte, A.-M.; Canard, B.; De Clercq, E.; Chimirri, A.; Pürstinger, G.; et al. The thiazolobenzimidazole TBZE-029 inhibits enterovirus replication by targeting a short region immediately downstream from motif C in the nonstructural protein 2C. J. Virol. 2008, 82, 4720–4730. [Google Scholar] [CrossRef] [PubMed]
- Hadaschik, D.; Klein, M.; Zimmermann, H.; Eggers, H.J.; Nelsen-Salz, B. Dependence of echovirus 9 on the enterovirus RNA replication inhibitor 2-(α-Hydroxybenzyl)-benzimidazole maps to nonstructural protein 2C. J. Virol. 1999, 73, 10536–10539. [Google Scholar] [PubMed]
- Pincus, S.E.; Diamond, D.C.; Emini, E.A.; Wimmer, E. Guanidine-selected mutants of poliovirus: Mapping of point mutations to polypeptide 2C. J. Virol. 1986, 57, 638–646. [Google Scholar] [PubMed]
- Sadeghipour, S.; Bek, E.J.; McMinn, P.C. Selection and characterisation of guanidine-resistant mutants of human enterovirus 71. Virus Res. 2012, 169, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Agoh, M.; Agoh, Y.; Yoshida, H.; Yoshii, K.; Yoneyama, T.; Hagiwara, A.; Miyamura, T. Mutations in the 2C region of poliovirus responsible for altered sensitivity to benzimidazole derivatives. J. Virol. 2000, 74, 4146–4154. [Google Scholar] [CrossRef] [PubMed]
- Ulferts, R.; van der Linden, L.; Thibaut, H.J.; Lanke, K.H.; Leyssen, P.; Coutard, B.; De Palma, A.M.; Canard, B.; Neyts, J.; van Kuppeveld, F.J. Selective serotonin reuptake inhibitor fluoxetine inhibits replication of human enteroviruses B and D by targeting viral protein 2C. Antimicrob. Agents Chemother. 2013, 57, 1952–1956. [Google Scholar] [CrossRef] [PubMed]
- Wikel, J.H.; Paget, C.J.; DeLong, D.C.; Nelson, J.D.; Wu, C.Y.; Paschal, J.W.; Dinner, A.; Templeton, R.J.; Chaney, M.O.; Jones, N.D.; et al. Synthesis of syn and anti isomers of 6-[[(hydroxyimino)phenyl]methyl]-1-[(1-methylethyl)sulfonyl]-1H-benzimidazol-2-amine. Inhibitors of rhinovirus multiplication. J. Med. Chem. 1980, 23, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Van der Schaar, H.M.; van der Linden, L.; Lanke, K.H.; Strating, J.R.; Pürstinger, G.; de Vries, E.; de Haan, C.A.M.; Neyts, J.; van Kuppeveld, F.J. Coxsackievirus mutants that can bypass host factor PI4KIIIβ and the need for high levels of PI4P lipids for replication. Cell Res. 2012, 22, 1576–1592. [Google Scholar] [CrossRef] [PubMed]
- Brown-Augsburger, P.; Vance, L.M.; Malcolm, S.K.; Hsiung, H.; Smith, D.P.; Heinz, B.A. Evidence that enviroxime targets multiple components of the rhinovirus 14 replication complex. Arch. Virol. 1999, 144, 1569–1585. [Google Scholar] [CrossRef] [PubMed]
- Heinz, B.A.; Vance, L.M. The antiviral compound enviroxime targets the 3A coding region of rhinovirus and poliovirus. J. Virol. 1995, 69, 4189–4197. [Google Scholar] [PubMed]
- Heinz, B.A.; Vance, L.M. Sequence determinants of 3A-mediated resistance to enviroxime in rhinoviruses and enteroviruses. J. Virol. 1996, 70, 4854–4857. [Google Scholar] [PubMed]
- Arita, M.; Kojima, H.; Nagano, T.; Okabe, T.; Wakita, T.; Shimizu, H. Phosphatidylinositol 4-kinase III β is a target of enviroxime-like compounds for antipoliovirus activity. J. Virol. 2011, 85, 2364–2372. [Google Scholar] [CrossRef] [PubMed]
- Van der Schaar, H.M.; Leyssen, P.; Thibaut, H.J.; de Palma, A.; van der Linden, L.; Lanke, K.H.; Lacroix, C.; Verbeken, E.; Conrath, K.; Macleod, A.M.; et al. A novel, broad-spectrum inhibitor of enterovirus replication that targets host cell factor phosphatidylinositol 4-kinase IIIβ. Antimicrob. Agents Chemother. 2013, 57, 4971–4981. [Google Scholar] [CrossRef] [PubMed]
- Hayden, F.G.; Gwaltney, J.M. Prophylactic activity of intranasal enviroxime against experimentally induced rhinovirus type 39 infection. Antimicrob. Agents Chemother. 1982, 21, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.G.; Barrow, G.I.; al-Nakib, W.; Tyrrell, D.A.; DeLong, D.C.; Lenox-Smith, I. Failure to demonstrate synergy between interferon-alpha and a synthetic antiviral, enviroxime, in rhinovirus infections in volunteers. Antivir. Res. 1988, 10, 141–149. [Google Scholar] [CrossRef]
- Levandowski, R.A.; Pachucki, C.T.; Rubenis, M.; Jackson, G.G. Topical enviroxime against rhinovirus infection. Antimicrob. Agents Chemother. 1982, 22, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.D.; Monto, A.S.; DeLong, D.C.; Exelby, A.; Bryan, E.R.; Srivastava, S. Controlled trial of enviroxime against natural rhinovirus infections in a community. Antimicrob. Agents Chemother. 1985, 27, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Phillpotts, R.J.; Jones, R.W.; Delong, D.C.; Reed, S.E.; Wallace, J.; Tyrrell, D.A. The activity of enviroxime against rhinovirus infection in man. Lancet 1981, 1, 1342–1344. [Google Scholar] [CrossRef]
- Phillpotts, R.J.; Wallace, J.; Tyrrell, D.A.; Tagart, V.B. Therapeutic activity of enviroxime against rhinovirus infection in volunteers. Antimicrob. Agents Chemother. 1983, 23, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Lamarche, M.J.; Borawski, J.; Bose, A.; Capacci-Daniel, C.; Colvin, R.; Dennehy, M.; Ding, J.; Dobler, M.; Drumm, J.; Gaither, L.A.; et al. Anti-hepatitis C virus activity and toxicity of type III phosphatidylinositol-4-kinase beta inhibitors. Antimicrob. Agents Chemother. 2012, 56, 5149–5156. [Google Scholar] [CrossRef] [PubMed]
- Arita, M.; Wakita, T.; Shimizu, H. Characterization of pharmacologically active compounds that inhibit poliovirus and enterovirus 71 infectivity. J. Gen. Virol. 2008, 89, 2518–2530. [Google Scholar] [CrossRef] [PubMed]
- Spickler, C.; Lippens, J.; Laberge, M.-K.; Desmeules, S.; Bellavance, É.; Garneau, M.; Guo, T.; Hucke, O.; Leyssen, P.; Neyts, J.; et al. Phosphatidylinositol 4-kinase III beta is essential for replication of human rhinovirus and its inhibition causes a lethal phenotype in vivo. Antimicrob. Agents Chemother. 2013, 57, 3358–3368. [Google Scholar] [CrossRef] [PubMed]
- Arita, M.; Kojima, H.; Nagano, T.; Okabe, T.; Wakita, T.; Shimizu, H. Oxysterol-binding protein family I is the target of minor enviroxime-like compounds. J. Virol. 2013, 87, 4252–4260. [Google Scholar] [CrossRef] [PubMed]
- Albulescu, L.; Strating, J.R.; Thibaut, H.J.; van der Linden, L.; Shair, M.D.; Neyts, J.; van Kuppeveld, F.J. Broad-range inhibition of enterovirus replication by OSW-1, a natural compound targeting OSBP. Antivir. Res. 2015, 117, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Liu, J.O. Recent advances in drug repositioning for the discovery of new anticancer drugs. Int. J. Biol. Sci. 2014, 10, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Tsou, Y.-L.; Lin, Y.-W.; Chang, H.-W.; Lin, H.-Y.; Shao, H.-Y.; Yu, S.-L.; Liu, C.-C.; Chitra, E.; Sia, C.; Chow, Y.-H. Heat shock protein 90: Role in enterovirus 71 entry and assembly and potential target for therapy. PLoS ONE 2013, 8, e77133. [Google Scholar] [CrossRef] [PubMed]
- Bailey, H.H.; Mulcahy, R.T.; Tutsch, K.D.; Arzoomanian, R.Z.; Alberti, D.; Tombes, M.B.; Wilding, G.; Pomplun, M.; Spriggs, D.R. Phase I clinical trial of intravenous l-buthionine sulfoximine and melphalan: an attempt at modulation of glutathione. J. Clin. Oncol. 1994, 12, 194–205. [Google Scholar] [PubMed]
- Bailey, H.H.; Ripple, G.; Tutsch, K.D.; Arzoomanian, R.Z.; Alberti, D.; Feierabend, C.; Mahvi, D.; Schink, J.; Pomplun, M.; Mulcahy, R.T.; et al. Phase I study of continuous-infusion l-S,R-buthionine sulfoximine with intravenous melphalan. J. Natl. Cancer Inst. 1997, 89, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, P.J.; Hamilton, T.C.; LaCreta, F.P.; Gallo, J.M.; Kilpatrick, D.; Halbherr, T.; Brennan, J.; Bookman, M.A.; Hoffman, J.; Young, R.C.; et al. Phase I trial of buthionine sulfoximine in combination with melphalan in patients with cancer. J. Clin. Oncol. 1996, 14, 249–256. [Google Scholar] [PubMed]
- Van de Ven, A.A.; Douma, J.W.; Rademaker, C.; van Loon, A.M.; Wensing, A.M.; Boelens, J.-J.; Sanders, E.A.M.; van Montfrans, J.M. Pleconaril-resistant chronic parechovirus-associated enteropathy in agammaglobulinaemia. Antivir. Ther. 2011, 16, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Wildenbeest, J.G.; Harvala, H.; Pajkrt, D.; Wolthers, K.C. The need for treatment against human parechoviruses: How, why and when? Expert Rev. Anti. Infect. Ther. 2010, 8, 1417–1429. [Google Scholar] [CrossRef] [PubMed]
- Seitsonen, J.; Susi, P.; Heikkilä, O.; Sinkovits, R.S.; Laurinmäki, P.; Hyypiä, T.; Butcher, S.J. Interaction of αVβ3 and αVβ6 integrins with human parechovirus 1. J. Virol. 2010, 84, 8509–8519. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; George, S.; Kusov, Y.; Perbandt, M.; Anemüller, S.; Mesters, J.R.; Norder, H.; Coutard, B.; Lacroix, C.; Leyssen, P.; et al. 3C protease of enterovirus 68: Structure-based design of Michael acceptor inhibitors and their broad-spectrum antiviral effects against picornaviruses. J. Virol. 2013, 87, 4339–4351. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, L.; Ulferts, R.; Nabuurs, S.B.; Kusov, Y.; Liu, H.; George, S.; Lacroix, C.; Goris, N.; Lefebvre, D.; Lanke, K.H.; et al. Application of a cell-based protease assay for testing inhibitors of picornavirus 3C proteases. Antivir. Res. 2014, 103, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.A.; Dragovich, P.S.; Webber, S.E.; Fuhrman, S.A.; Patick, A.K.; Zalman, L.S.; Hendrickson, T.F.; Love, R.A.; Prins, T.J.; Marakovits, J.T.; et al. Structure-assisted design of mechanism-based irreversible inhibitors of human rhinovirus 3C protease with potent antiviral activity against multiple rhinovirus serotypes. Proc. Natl. Acad. Sci. USA 1999, 96, 11000–11007. [Google Scholar] [CrossRef] [PubMed]
- Blom, N.; Hansen, J.; Blaas, D.; Brunak, S. Cleavage site analysis in picornaviral polyproteins: Discovering cellular targets by neural networks. Protein Sci. 1996, 5, 2203–2216. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.H.; Panayiotou, M.; Girling, G.D.; Peard, C.I.; Oikarinen, S.; Hyöty, H.; Stanway, G. Evolution and conservation in human parechovirus genomes. J. Gen. Virol. 2009, 90, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, T.; Emerson, S.U.; Purcell, R.H.; Gauss-Müller, V. Polyprotein processing in echovirus 22: A first assessment. Biochem. Biophys. Res. Commun. 1995, 217, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Coller, B.A.; Chapman, N.M.; Beck, M.A.; Pallansch, M.A.; Gauntt, C.J.; Tracy, S.M. Echovirus 22 is an atypical enterovirus. J. Virol. 1990, 64, 2692–2701. [Google Scholar] [PubMed]
- Samuilova, O.; Krogerus, C.; Pöyry, T.; Hyypiä, T. Specific interaction between human parechovirus nonstructural 2A protein and viral RNA. J. Biol. Chem. 2004, 279, 37822–37831. [Google Scholar] [CrossRef] [PubMed]
- Hyypiä, T.; Horsnell, C.; Maaronen, M.; Khan, M.; Kalkkinen, N.; Auvinen, P.; Kinnunen, L.; Stanway, G. A distinct picornavirus group identified by sequence analysis. Proc. Natl. Acad. Sci. USA 1992, 89, 8847–8851. [Google Scholar] [CrossRef] [PubMed]
- Samuilova, O.; Krogerus, C.; Fabrichniy, I.; Hyypiä, T. ATP hydrolysis and AMP kinase activities of nonstructural protein 2C of human parechovirus 1. J. Virol. 2006, 80, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Tamm, I.; Eggers, H.J. Differences in the selective virus inhibitory action of 2-(α-hydroxybenzyl)-benzimidazole and guanidine HCl. Virology 1962, 18, 439–447. [Google Scholar] [CrossRef]
- Eggers, H.J.; Tamm, I. Spectrum and characteristics of the virus inhibitory action of 2-(α-hydroxybenzyl)-benzimidazole. J. Exp. Med. 1961, 113, 657–682. [Google Scholar] [CrossRef] [PubMed]
- Krogerus, C.; Egger, D.; Samuilova, O.; Hyypiä, T.; Bienz, K. Replication complex of human parechovirus 1. J. Virol. 2003, 77, 8512–8523. [Google Scholar] [CrossRef] [PubMed]
- Snell, N.J. Ribavirin--current status of a broad spectrum antiviral agent. Expert Opin. Pharmacother. 2001, 2, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S.; Saleh, M.-C.; Gitlin, L.; Beske, O.; Andino, R. The poliovirus replication machinery can escape inhibition by an antiviral drug that targets a host cell protein. J. Virol. 2004, 78, 3378–3386. [Google Scholar] [CrossRef] [PubMed]
- Koletsky, A.J.; Harding, M.W.; Handschumacher, R.E. Cyclophilin: distribution and variant properties in normal and neoplastic tissues. J. Immunol. 1986, 137, 1054–1059. [Google Scholar] [PubMed]
- Lin, K.; Gallay, P. Curing a viral infection by targeting the host: the example of cyclophilin inhibitors. Antivir. Res. 2013, 99, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.W.; Giffin, M.J.; Muller, R.; Savage, J.; Lin, Y.C.; Hong, S.; Jin, W.; Whitby, L.R.; Elder, J.H.; Boger, D.L.; et al. Identification of broad-based HIV-1 protease inhibitors from combinatorial libraries. Biochem. J. 2010, 429, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Nkeze, J.; Zhao, R.Y. Effects of HIV-1 protease on cellular functions and their potential applications in antiretroviral therapy. Cell Biosci. 2012, 2, 32. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Su, H.; Zhang, T.; Du, J.; Cui, S.; Yang, F.; Jin, Q. Inhibition of Enterovirus 71 replication by 7-hydroxyflavone and diisopropyl-flavon7-yl Phosphate. PLoS ONE 2014, 9, e92565. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, T.; Du, J.; Cui, S.; Yang, F.; Jin, Q. Anti-enterovirus 71 effects of chrysin and its phosphate ester. PLoS ONE 2014, 9, e89668. [Google Scholar] [CrossRef] [PubMed]
- Tyler, K.L. Rationale for the evaluation of fluoxetine in the treatment of enterovirus D68-associated acute flaccid myelitis. JAMA Neurol. 2015, 72, 493–494. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).