Vpu Protein: The Viroporin Encoded by HIV-1
Abstract
:1. Introduction
2. The Role of Vpu Protein in the Spread of HIV-1 from Infected Cells
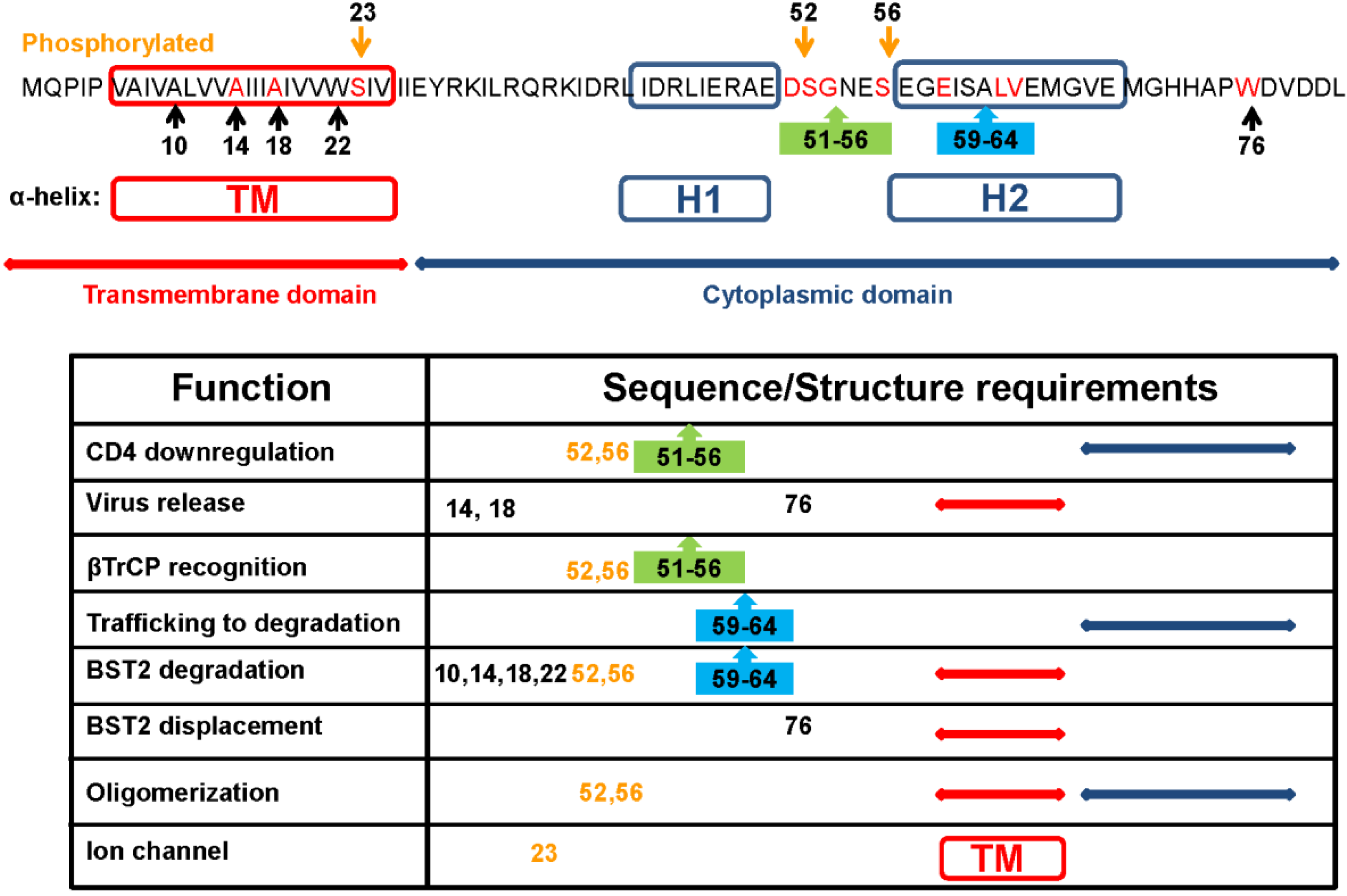
2.1. Downregulation of CD4
2.2. Enhancement of Virus Release
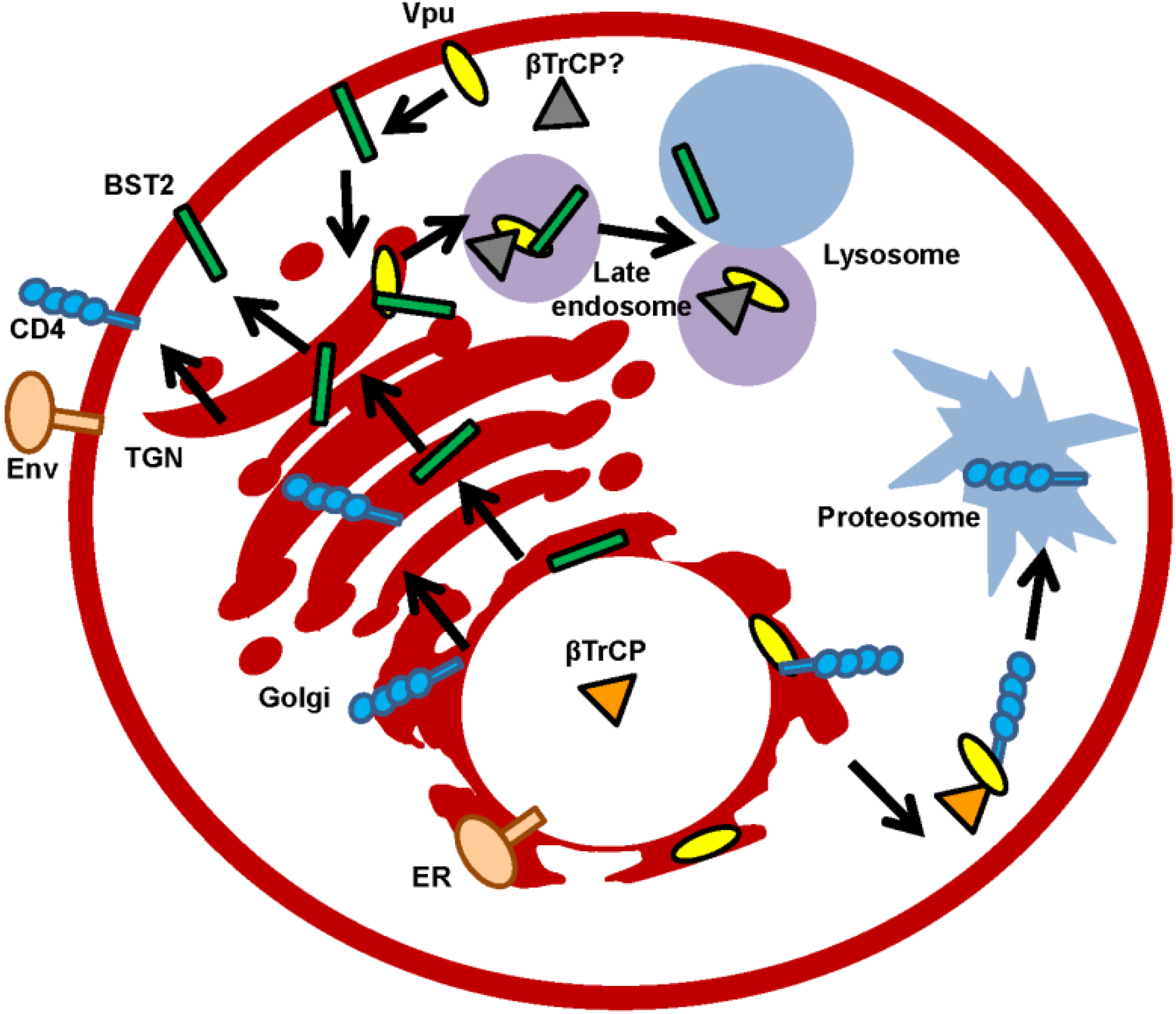
2.2.1. Vpu Antagonizes BST2
2.2.2. Vpu Induces Changes in Ionic Currents of Cellular Membranes
3. Modification of Protein Trafficking and Membrane Integrity
| Protein | Modification | References |
|---|---|---|
| BST2/tetherin/CD317 | downregulation/localization | [24] |
| CCR7 | downregulation | [49] |
| CD1d | downregulation | [50,51] |
| CD4 | downregulation | [11] |
| CD40 | upregulation | [52] |
| CD81 | downregulation | [53] |
| CD155/PVR | downregulation | [54,55] |
| CDL62 | localization | [56] |
| MHC-I | downregulation | [57] |
| NTB-A | localization? | [58,59] |
| AP-1 | localization | [60] |
| βTrCP | localization | [61] |
| TSPANs (25, 26, 28 and 30) | downregulation | [62] |
| TASK-1 | downregulation | [40] |
| Env (HIV-1) | localization | [7,63] |
| Gag (HIV-1) | localization | [63,64] |
| Glycoprotein (SV) | processing/downregulation | [65] |
Vpu Adjusts the Protein Composition and Functioning of Cellular Membranes
4. Conservation and Heterogeneity of the Vpu Protein among Human and Non-Human Primate Isolates
4.1. Conservation of Vpu Functions in Natural Isolates
| Group | CD4 Downregulation | BST2 Downregulation | Localization of Vpu | References |
|---|---|---|---|---|
| P? | no | no | ? | [98,99] |
| N | no | poor | TGN | [100] |
| O | yes | Nef-mediated | ER | [93,99] |
| M | yes | yes | TGN | [100] |
4.2. Sequence Requirement of Vpu Functions
5. Vpu Viroporin as a Therapeutic Target
6. Conclusions and Perspectives
Acknowledgments
Conflicts of Interest
References
- Cohen, A.; Terwilliger, E.F.; Sodroski, J.G.; Haseltine, W.A. Identification of a protein encoded by the vpu gene of HIV-1. Nature 1988, 334, 532–534. [Google Scholar] [CrossRef] [PubMed]
- Strebel, K.; Klimkait, T.; Martin, M.A. A novel gene of HIV-1, vpu, and its 16-kilodalton product. Science 1988, 241, 1221–1223. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, L. Modification of membrane permeability by animal viruses. Adv. Virus Res. 1995, 45, 61–112. [Google Scholar] [PubMed]
- Gonzalez, M.E.; Carrasco, L. Viroporins. FEBS Lett. 2003, 552, 28–34. [Google Scholar] [CrossRef]
- Nieva, J.L.; Madan, V.; Carrasco, L. Viroporins: Structure and biological functions. Nat. Rev. Microbiol. 2012, 10, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.; Felber, B.K.; Fenyo, E.M.; Pavlakis, G.N. Env and Vpu proteins of human immunodeficiency virus type 1 are produced from multiple bicistronic mRNAs. J. Virol. 1990, 64, 5448–5456. [Google Scholar] [PubMed]
- Willey, R.L.; Maldarelli, F.; Martin, M.A.; Strebel, K. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J. Virol. 1992, 66, 7193–7200. [Google Scholar] [PubMed]
- Strebel, K.; Klimkait, T.; Maldarelli, F.; Martin, M.A. Molecular and biochemical analyses of human immunodeficiency virus type 1 Vpu protein. J. Virol. 1989, 63, 3784–3791. [Google Scholar] [PubMed]
- Marassi, F.M.; Ma, C.; Gratkowski, H.; Straus, S.K.; Strebel, K.; Oblatt-Montal, M.; Montal, M.; Opella, S.J. Correlation of the structural and functional domains in the membrane protein Vpu from HIV-1. Proc. Natl. Acad. Sci. USA 1999, 96, 14336–14341. [Google Scholar] [CrossRef] [PubMed]
- Schubert, U.; Bour, S.; Ferrer-Montiel, A.V.; Montal, M.; Maldarelli, F.; Strebel, K. The two biological activities of human immunodeficiency virus type 1 Vpu protein involve two separable structural domains. J. Virol. 1996, 70, 809–819. [Google Scholar] [PubMed]
- Willey, R.L.; Maldarelli, F.; Martin, M.A.; Strebel, K. Human immunodeficiency virus type 1 Vpu protein regulates the formation of intracellular gp160-CD4 complexes. J. Virol. 1992, 66, 226–234. [Google Scholar] [PubMed]
- Geraghty, R.J.; Panganiban, A.T. Human immunodeficiency virus type 1 Vpu has a CD4- and an envelope glycoprotein-independent function. J. Virol. 1993, 67, 4190–4194. [Google Scholar] [PubMed]
- Wray, V.; Federau, T.; Henklein, P.; Klabunde, S.; Kunert, O.; Schomburg, D.; Schubert, U. Solution structure of the hydrophilic region of HIV-1 encoded virus protein U (Vpu) by CD and 1H NMR spectroscopy. Int. J. Pept. Protein Res. 1995, 45, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.; Maldarelli, F.; Karczewski, M.K.; Willey, R.L.; Strebel, K. Human immunodeficiency virus type 1 Vpu protein induces degradation of CD4 in vitro: The cytoplasmic domain of CD4 contributes to Vpu sensitivity. J. Virol. 1993, 67, 3877–3884. [Google Scholar] [PubMed]
- Schubert, U.; Strebel, K. Differential activities of the human immunodeficiency virus type 1-encoded Vpu protein are regulated by phosphorylation and occur in different cellular compartments. J. Virol. 1994, 68, 2260–2271. [Google Scholar] [PubMed]
- Veillette, M.; Desormeaux, A.; Medjahed, H.; Gharsallah, N.E.; Coutu, M.; Baalwa, J.; Guan, Y.; Lewis, G.; Ferrari, G.; Hahn, B.H.; et al. Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. J. Virol. 2014, 88, 2633–2644. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.; Lukhele, S.; Hajjar, F.; Routy, J.P.; Cohen, E.A. HIV Nef and Vpu protect HIV-infected CD4+ T cells from antibody-mediated cell lysis through down-modulation of CD4 and BST2. Retrovirology 2014, 11, e15. [Google Scholar] [CrossRef] [PubMed]
- Balliet, J.W.; Kolson, D.L.; Eiger, G.; Kim, F.M.; McGann, K.A.; Srinivasan, A.; Collman, R. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: Mutational analysis of a primary HIV-1 isolate. Virology 1994, 200, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, R.J.; Talbot, K.J.; Callahan, M.; Harper, W.; Panganiban, A.T. Cell type-dependence for Vpu function. J. Med. Primatol. 1994, 23, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Tokunaga, K.; Kawamura, M.; Adachi, A. Function of human immunodeficiency virus type 1 Vpu protein in various cell types. J. Gen. Virol. 1995, 76, 2717–2722. [Google Scholar] [CrossRef] [PubMed]
- Ewart, G.D.; Sutherland, T.; Gage, P.W.; Cox, G.B. The Vpu protein of human immunodeficiency virus type 1 forms cation-selective ion channels. J. Virol. 1996, 70, 7108–7115. [Google Scholar] [PubMed]
- Deora, A.; Ratner, L. Viral protein U (Vpu)-mediated enhancement of human immunodeficiency virus type 1 particle release depends on the rate of cellular proliferation. J. Virol. 2001, 75, 6714–6718. [Google Scholar] [CrossRef] [PubMed]
- Neil, S.J.; Sandrin, V.; Sundquist, W.I.; Bieniasz, P.D. An interferon-alpha-induced tethering mechanism inhibits HIV-1 and Ebola virus particle release but is counteracted by the HIV-1 Vpu protein. Cell Host Microbe 2007, 2, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Neil, S.J.; Zang, T.; Bieniasz, P.D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 2008, 451, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Kupzig, S.; Korolchuk, V.; Rollason, R.; Sugden, A.; Wilde, A.; Banting, G. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic 2003, 4, 694–709. [Google Scholar] [CrossRef] [PubMed]
- Perez-Caballero, D.; Zang, T.; Ebrahimi, A.; McNatt, M.W.; Gregory, D.A.; Johnson, M.C.; Bieniasz, P.D. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell 2009, 139, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Van, D.N.; Goff, D.; Katsura, C.; Jorgenson, R.L.; Mitchell, R.; Johnson, M.C.; Stephens, E.B.; Guatelli, J. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 2008, 3, 245–252. [Google Scholar]
- Goffinet, C.; Allespach, I.; Homann, S.; Tervo, H.M.; Habermann, A.; Rupp, D.; Oberbremer, L.; Kern, C.; Tibroni, N.; Welsch, S.; et al. HIV-1 antagonism of CD317 is species specific and involves Vpu-mediated proteasomal degradation of the restriction factor. Cell Host Microbe 2009, 5, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Iwabu, Y.; Fujita, H.; Kinomoto, M.; Kaneko, K.; Ishizaka, Y.; Tanaka, Y.; Sata, T.; Tokunaga, K. HIV-1 accessory protein Vpu internalizes cell-surface BST-2/tetherin through transmembrane interactions leading to lysosomes. J. Biol. Chem. 2009, 284, 35060–35072. [Google Scholar] [CrossRef] [PubMed]
- McNatt, M.W.; Zang, T.; Hatziioannou, T.; Bartlett, M.; Fofana, I.B.; Johnson, W.E.; Neil, S.J.; Bieniasz, P.D. Species-specific activity of HIV-1 Vpu and positive selection of tetherin transmembrane domain variants. PLoS Pathog. 2009, 5, e1000300. [Google Scholar] [CrossRef] [PubMed]
- Dube, M.; Roy, B.B.; Guiot-Guillain, P.; Mercier, J.; Binette, J.; Leung, G.; Cohen, E.A. Suppression of Tetherin-restricting activity upon human immunodeficiency virus type 1 particle release correlates with localization of Vpu in the trans-Golgi network. J. Virol. 2009, 83, 4574–4590. [Google Scholar] [CrossRef] [PubMed]
- Lewinski, M.K.; Jafari, M.; Zhang, H.; Opella, S.J.; Guatelli, J. Membrane Anchoring by a C-terminal Tryptophan Enables HIV-1 Vpu to Displace Bone Marrow Stromal Antigen 2 (BST2) from Sites of Viral Assembly. J. Biol. Chem. 2015, 290, 10919–10933. [Google Scholar] [CrossRef] [PubMed]
- Maldarelli, F.; Chen, M.Y.; Willey, R.L.; Strebel, K. Human immunodeficiency virus type 1 Vpu protein is an oligomeric type I integral membrane protein. J. Virol. 1993, 67, 5056–5061. [Google Scholar] [PubMed]
- Pinto, L.H.; Holsinger, L.J.; Lamb, R.A. Influenza virus M2 protein has ion channel activity. Cell 1992, 69, 517–528. [Google Scholar] [CrossRef]
- Grice, A.L.; Kerr, I.D.; Sansom, M.S. Ion channels formed by HIV-1 Vpu: A modelling and simulation study. FEBS Lett. 1997, 405, 299–304. [Google Scholar] [CrossRef]
- Schubert, U.; Ferrer-Montiel, A.V.; Oblatt-Montal, M.; Henklein, P.; Strebel, K.; Montal, M. Identification of an ion channel activity of the Vpu transmembrane domain and its involvement in the regulation of virus release from HIV-1-infected cells. FEBS Lett. 1996, 398, 12–18. [Google Scholar] [CrossRef]
- Coady, M.J.; Daniel, N.G.; Tiganos, E.; Allain, B.; Friborg, J.; Lapointe, J.Y.; Cohen, E.A. Effects of Vpu expression on Xenopus oocyte membrane conductance. Virology 1998, 244, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Marassi, F.M.; Jones, D.H.; Straus, S.K.; Bour, S.; Strebel, K.; Schubert, U.; Oblatt-Montal, M.; Montal, M.; Opella, S.J. Expression, purification, and activities of full-length and truncated versions of the integral membrane protein Vpu from HIV-1. Protein Sci. 2002, 11, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.; Han, J.; Shinlapawittayatorn, K.; Deschenes, I.; Marban, E. Membrane potential depolarization as a triggering mechanism for Vpu-mediated HIV-1 release. Biophys. J. 2010, 99, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.; Seharaseyon, J.; Dong, P.; Bour, S.; Marban, E. Mutual functional destruction of HIV-1 Vpu and host TASK-1 channel. Mol. Cell 2004, 14, 259–267. [Google Scholar] [CrossRef]
- Herrero, L.; Monroy, N.; Gonzalez, M.E. HIV-1 Vpu protein mediates the transport of potassium in Saccharomyces cerevisiae. Biochemistry 2013, 52, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Orenstein, J.; Dimitrov, D.; Martin, M. Cell-to-cell spread of HIV-1 occurs within minutes and may not involve the participation of virus particles. Virology 1992, 186, 712–724. [Google Scholar] [CrossRef]
- Klimkait, T.; Strebel, K.; Hoggan, M.D.; Martin, M.A.; Orenstein, J.M. The human immunodeficiency virus type 1-specific protein Vpu is required for efficient virus maturation and release. J. Virol. 1990, 64, 621–629. [Google Scholar] [PubMed]
- Lu, J.X.; Sharpe, S.; Ghirlando, R.; Yau, W.M.; Tycko, R. Oligomerization state and supramolecular structure of the HIV-1 Vpu protein transmembrane segment in phospholipid bilayers. Protein Sci. 2010, 19, 1877–1896. [Google Scholar] [CrossRef] [PubMed]
- Estrabaud, E.; Le, R.E.; Lopez-Verges, S.; Morel, M.; Belaidouni, N.; Benarous, R.; Transy, C.; Berlioz-Torrent, C.; Margottin-Goguet, F. Regulated degradation of the HIV-1 Vpu protein through a betaTrCP-independent pathway limits the release of viral particles. PLoS Pathog. 2007, 3, e104. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Das, S.R.; Tanwar, C.; Jameel, S. Oligomerization of the human immunodeficiency virus type 1 (HIV-1) Vpu protein—A genetic, biochemical and biophysical analysis. Virol. J. 2007, 4, e81. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.A.; Freed, E.O. The Role of Lipids in Retrovirus Replication. Viruses 2010, 2, 1146–1180. [Google Scholar] [CrossRef] [PubMed]
- Tokarev, A.; Guatelli, J. Misdirection of membrane trafficking by HIV-1 Vpu and Nef: Keys to viral virulence and persistence. Cell. Logist. 2011, 1, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, P.W.; Famiglietti, M.; Sowrirajan, B.; DePaula-Silva, A.B.; Rodesch, C.; Barker, E.; Bosque, A.; Planelles, V. Downmodulation of CCR7 by HIV-1 Vpu results in impaired migration and chemotactic signaling within CD4(+) T cells. Cell Rep. 2014, 7, 2019–2030. [Google Scholar] [CrossRef] [PubMed]
- Moll, M.; Andersson, S.K.; Smed-Sorensen, A.; Sandberg, J.K. Inhibition of lipid antigen presentation in dendritic cells by HIV-1 Vpu interference with CD1d recycling from endosomal compartments. Blood 2010, 116, 1876–1884. [Google Scholar] [CrossRef] [PubMed]
- Bachle, S.M.; Sauter, D.; Sibitz, S.; Sandberg, J.K.; Kirchhoff, F.; Moll, M. Involvement of a C-terminal motif in the interference of primate lentiviral Vpu proteins with CD1d-mediated antigen presentation. Sci. Rep. 2015, 5, 9675. [Google Scholar] [CrossRef] [PubMed]
- Henderson, W.W.; Ruhl, R.; Lewis, P.; Bentley, M.; Nelson, J.A.; Moses, A.V. Human immunodeficiency virus (HIV) type 1 Vpu induces the expression of CD40 in endothelial cells and regulates HIV-induced adhesion of B-lymphoma cells. J. Virol. 2004, 78, 4408–4420. [Google Scholar] [CrossRef] [PubMed]
- Lambele, M.; Koppensteiner, H.; Symeonides, M.; Roy, N.H.; Chan, J.; Schindler, M.; Thali, M. Vpu is the main determinant for tetraspanin downregulation in HIV-1-infected cells. J. Virol. 2015, 89, 3247–3255. [Google Scholar] [CrossRef] [PubMed]
- Matusali, G.; Potesta, M.; Santoni, A.; Cerboni, C.; Doria, M. The human immunodeficiency virus type 1 Nef and Vpu proteins downregulate the natural killer cell-activating ligand PVR. J. Virol. 2012, 86, 4496–4504. [Google Scholar] [CrossRef] [PubMed]
- Bolduan, S.; Reif, T.; Schindler, M.; Schubert, U. HIV-1 Vpu mediated downregulation of CD155 requires alanine residues 10, 14 and 18 of the transmembrane domain. Virology 2014, 464–465, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Vassena, L.; Giuliani, E.; Koppensteiner, H.; Bolduan, S.; Schindler, M.; Doria, M. HIV-1 Nef and Vpu Interfere with L-Selectin (CD62L) Cell Surface Expression To Inhibit Adhesion and Signaling in Infected CD4+ T Lymphocytes. J. Virol. 2015, 89, 5687–5700. [Google Scholar] [CrossRef] [PubMed]
- Kerkau, T.; Bacik, I.; Bennink, J.R.; Yewdell, J.W.; Hunig, T.; Schimpl, A.; Schubert, U. The human immunodeficiency virus type 1 (HIV-1) Vpu protein interferes with an early step in the biosynthesis of major histocompatibility complex (MHC) class I molecules. J. Exp. Med. 1997, 185, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.H.; Sowrirajan, B.; Davis, Z.B.; Ward, J.P.; Campbell, E.M.; Planelles, V.; Barker, E. Degranulation of natural killer cells following interaction with HIV-1-infected cells is hindered by downmodulation of NTB-A by Vpu. Cell Host Microbe 2010, 8, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.; Cohen, E.A. HIV-1 Vpu disarms natural killer cells. Cell Host Microbe 2010, 8, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Weber, E.; Tokarev, A.; Lewinski, M.; Rizk, M.; Suarez, M.; Guatelli, J.; Xiong, Y. Structural basis of HIV-1 Vpu-mediated BST2 antagonism via hijacking of the clathrin adaptor protein complex 1. Elife 2014, 3, e02362. [Google Scholar] [CrossRef] [PubMed]
- Besnard-Guerin, C.; Belaidouni, N.; Lassot, I.; Segeral, E.; Jobart, A.; Marchal, C.; Benarous, R. HIV-1 Vpu sequesters beta-transducin repeat-containing protein (betaTrCP) in the cytoplasm and provokes the accumulation of beta-catenin and other SCFbetaTrCP substrates. J. Biol. Chem. 2004, 279, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Haller, C.; Muller, B.; Fritz, J.V.; Lamas-Murua, M.; Stolp, B.; Pujol, F.M.; Keppler, O.T.; Fackler, O.T. HIV-1 Nef and Vpu are functionally redundant broad-spectrum modulators of cell surface receptors, including tetraspanins. J. Virol. 2014, 88, 14241–14257. [Google Scholar] [CrossRef] [PubMed]
- Van, D.N.; Guatelli, J. HIV-1 Vpu inhibits accumulation of the envelope glycoprotein within clathrin-coated, Gag-containing endosomes. Cell Microbiol. 2008, 10, 1040–1057. [Google Scholar]
- Handley, M.A.; Paddock, S.; Dall, A.; Panganiban, A.T. Association of Vpu-binding protein with microtubules and Vpu-dependent redistribution of HIV-1 Gag protein. Virology 2001, 291, 198–207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gonzalez, M.E.; Carrasco, L. Human immunodeficiency virus type 1 Vpu protein affects Sindbis virus glycoprotein processing and enhances membrane permeabilization. Virology 2001, 279, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Magadan, J.G.; Perez-Victoria, F.J.; Sougrat, R.; Ye, Y.; Strebel, K.; Bonifacino, J.S. Multilayered mechanism of CD4 downregulation by HIV-1 Vpu involving distinct ER retention and ERAD targeting steps. PLoS Pathog. 2010, 6, e1000869. [Google Scholar] [CrossRef] [PubMed]
- Mangeat, B.; Gers-Huber, G.; Lehmann, M.; Zufferey, M.; Luban, J.; Piguet, V. HIV-1 Vpu neutralizes the antiviral factor Tetherin/BST-2 by binding it and directing its beta-TrCP2-dependent degradation. PLoS Pathog. 2009, 5, e1000574. [Google Scholar] [CrossRef] [PubMed]
- Kueck, T.; Neil, S.J. A cytoplasmic tail determinant in HIV-1 Vpu mediates targeting of tetherin for endosomal degradation and counteracts interferon-induced restriction. PLoS Pathog. 2012, 8, e1002609. [Google Scholar] [CrossRef] [PubMed]
- Margottin, F.; Bour, S.P.; Durand, H.; Selig, L.; Benichou, S.; Richard, V.; Thomas, D.; Strebel, K.; Benarous, R. A novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol. Cell 1998, 1, 565–574. [Google Scholar] [CrossRef]
- Douglas, J.L.; Viswanathan, K.; McCarroll, M.N.; Gustin, J.K.; Fruh, K.; Moses, A.V. Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/Tetherin via a {beta}TrCP-dependent mechanism. J. Virol. 2009, 83, 7931–7947. [Google Scholar] [CrossRef] [PubMed]
- Janvier, K.; Pelchen-Matthews, A.; Renaud, J.B.; Caillet, M.; Marsh, M.; Berlioz-Torrent, C. The ESCRT-0 component HRS is required for HIV-1 Vpu-mediated BST-2/tetherin down-regulation. PLoS Pathog. 2011, 7, e1001265. [Google Scholar] [CrossRef] [PubMed]
- Petris, G.; Casini, A.; Sasset, L.; Cesaratto, F.; Bestagno, M.; Cereseto, A.; Burrone, O.R. CD4 and BST-2/tetherin proteins retro-translocate from endoplasmic reticulum to cytosol as partially folded and multimeric molecules. J. Biol. Chem. 2014, 289, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, E.; Andrew, A.J.; Kao, S.; Strebel, K. Vpu enhances HIV-1 virus release in the absence of Bst-2 cell surface down-modulation and intracellular depletion. Proc. Natl. Acad. Sci. USA 2009, 106, 2868–2873. [Google Scholar] [CrossRef] [PubMed]
- Neil, S.J.; Eastman, S.W.; Jouvenet, N.; Bieniasz, P.D. HIV-1 Vpu promotes release and prevents endocytosis of nascent retrovirus particles from the plasma membrane. PLoS Pathog. 2006, 2, e39. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Bhattacharya, J. Evidence that Vpu modulates HIV-1 Gag-envelope interaction towards envelope incorporation and infectivity in a cell type dependent manner. PLoS ONE 2013, 8, e61388. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, M.; Freed, E.O. New insights into HIV assembly and trafficking. Physiology 2011, 26, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Ablan, S.D.; Soheilian, F.; Nagashima, K.; Freed, E.O. Evidence that productive human immunodeficiency virus type 1 assembly can occur in an intracellular compartment. J. Virol. 2009, 83, 5375–5387. [Google Scholar] [CrossRef] [PubMed]
- Votteler, J.; Sundquist, W.I. Virus budding and the ESCRT pathway. Cell Host Microbe 2013, 14, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Casartelli, N.; Sourisseau, M.; Feldmann, J.; Guivel-Benhassine, F.; Mallet, A.; Marcelin, A.G.; Guatelli, J.; Schwartz, O. Tetherin restricts productive HIV-1 cell-to-cell transmission. PLoS Pathog. 2010, 6, e1000955. [Google Scholar] [CrossRef] [PubMed]
- Jolly, C.; Booth, N.J.; Neil, S.J. Cell-cell spread of human immunodeficiency virus type 1 overcomes tetherin/BST-2-mediated restriction in T cells. J. Virol. 2010, 84, 12185–12199. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.B.; Hsu, H.J. Viral channel forming proteins - modeling the target. Biochim. Biophys. Acta 2011, 1808, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Lamb, R.A.; Pinto, L.H. Do Vpu and Vpr of human immunodeficiency virus type 1 and NB of influenza B virus have ion channel activities in the viral life cycles? Virology 1997, 229, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kukol, A.; Arkin, I.T. Vpu transmembrane peptide structure obtained by site-specific fourier transform infrared dichroism and global molecular dynamics searching. Biophys. J. 1999, 77, 1594–1601. [Google Scholar] [CrossRef]
- Moore, P.B.; Zhong, Q.; Husslein, T.; Klein, M.L. Simulation of the HIV-1 Vpu transmembrane domain as a pentameric bundle. FEBS Lett. 1998, 431, 143–148. [Google Scholar] [CrossRef]
- Gonzalez, M.E.; Carrasco, L. The human immunodeficiency virus type 1 Vpu protein enhances membrane permeability. Biochemistry 1998, 37, 13710–13719. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, T.; Routh, A.; Judge, P.J.; Lam, Y.H.; Fischer, D.; Watts, A.; Fischer, W.B. Biophysical characterization of Vpu from HIV-1 suggests a channel-pore dualism. Proteins 2008, 70, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Nieva, J.L.; Madan, V.; Carrasco, L. Viroporins: Structure and biological functions. Nat. Rev. Microbiol. 2012, 10, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, F. Immune evasion and counteraction of restriction factors by HIV-1 and other primate lentiviruses. Cell Host Microbe 2010, 8, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Los Alamos HIV Sequence Database and Analysis Staff. HIV Sequence Compendium 2014; Theoretical Biology and Biophysics Group, Los Alamos National Laboratory: Los Alamos, NM, USA, 2014. Available online: http://www.hiv.lanl.gov/content/sequence/HIV/COMPENDIUM/2014compendium.html (accessed on 30 July 2015).
- Bour, S.; Strebel, K. The human immunodeficiency virus (HIV) type 2 envelope protein is a functional complement to HIV type 1 Vpu that enhances particle release of heterologous retroviruses. J. Virol. 1996, 70, 8285–8300. [Google Scholar] [PubMed]
- Yusim, K.; Kesmir, C.; Gaschen, B.; Addo, M.M.; Altfeld, M.; Brunak, S.; Chigaev, A.; Detours, V.; Korber, B.T. Clustering patterns of cytotoxic T-lymphocyte epitopes in human immunodeficiency virus type 1 (HIV-1) proteins reveal imprints of immune evasion on HIV-1 global variation. J. Virol. 2002, 76, 8757–8768. [Google Scholar] [CrossRef] [PubMed]
- Sauter, D.; Schindler, M.; Specht, A.; Landford, W.N.; Munch, J.; Kim, K.A.; Votteler, J.; Schubert, U.; Bibollet-Ruche, F.; Keele, B.F.; et al. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe 2009, 6, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Vigan, R.; Neil, S.J. Separable determinants of subcellular localization and interaction account for the inability of group O HIV-1 Vpu to counteract tetherin. J. Virol. 2011, 85, 9737–9748. [Google Scholar] [CrossRef] [PubMed]
- Kluge, S.F.; Mack, K.; Iyer, S.S.; Pujol, F.M.; Heigele, A.; Learn, G.H.; Usmani, S.M.; Sauter, D.; Joas, S.; Hotter, D.; et al. Nef proteins of epidemic HIV-1 group O strains antagonize human tetherin. Cell Host Microbe 2014, 16, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Sauter, D. Counteraction of the multifunctional restriction factor tetherin. Front. Microbiol. 2014, 5, e163. [Google Scholar] [CrossRef] [PubMed]
- Plantier, J.C.; Leoz, M.; Dickerson, J.E.; De, O.F.; Cordonnier, F.; Lemee, V.; Damond, F.; Robertson, D.L.; Simon, F. A new human immunodeficiency virus derived from gorillas. Nat. Med. 2009, 15, 871–872. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.M.; Hahn, B.H. Origins of HIV and the AIDS pandemic. Cold Spring Harb. Perspect. Med. 2011, 1, a006841. [Google Scholar] [CrossRef] [PubMed]
- Sauter, D.; Hue, S.; Petit, S.J.; Plantier, J.C.; Towers, G.J.; Kirchhoff, F.; Gupta, R.K. HIV-1 Group P is unable to antagonize human tetherin by Vpu, Env or Nef. Retrovirology 2011, 8, e103. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Lopez, L.A.; Exline, C.M.; Haworth, K.G.; Cannon, P.M. Lack of adaptation to human tetherin in HIV-1 group O and P. Retrovirology 2011, 8, e78. [Google Scholar] [CrossRef] [PubMed]
- Sauter, D.; Unterweger, D.; Vogl, M.; Usmani, S.M.; Heigele, A.; Kluge, S.F.; Hermkes, E.; Moll, M.; Barker, E.; Peeters, M.; et al. Human tetherin exerts strong selection pressure on the HIV-1 group N Vpu protein. PLoS Pathog. 2012, 8, e1003093. [Google Scholar] [CrossRef] [PubMed]
- McNatt, M.W.; Zang, T.; Bieniasz, P.D. Vpu binds directly to tetherin and displaces it from nascent virions. PLoS Pathog. 2013, 9, e1003299. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Guatelli, J.; Lewinski, M.K. Activities of transmitted/founder and chronic clade B HIV-1 Vpu and a C-terminal polymorphism specifically affecting virion release. J. Virol. 2014, 88, 5062–5078. [Google Scholar] [CrossRef] [PubMed]
- Bolduan, S.; Votteler, J.; Lodermeyer, V.; Greiner, T.; Koppensteiner, H.; Schindler, M.; Thiel, G.; Schubert, U. Ion channel activity of HIV-1 Vpu is dispensable for counteraction of CD317. Virology 2011, 416, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Pickering, S.; Hue, S.; Kim, E.Y.; Reddy, S.; Wolinsky, S.M.; Neil, S.J. Preservation of tetherin and CD4 counter-activities in circulating Vpu alleles despite extensive sequence variation within HIV-1 infected individuals. PLoS Pathog. 2014, 10, e1003895. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.E.; Carrasco, L. Viral proteins that enhance membrane permeability. In Viral Membrane Proteins: Structure, Function and Drug Design; Fischer, W.B., Ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2005; pp. 79–90. Available online: http://rd.springer.com/chapter/10.1007/0-387-28146-0_6#page-1 (accessed on 30 July 2015).
- Ewart, G.D.; Mills, K.; Cox, G.B.; Gage, P.W. Amiloride derivatives block ion channel activity and enhancement of virus-like particle budding caused by HIV-1 protein Vpu. Eur. Biophys. J. 2002, 31, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Ewart, G.D.; Nasr, N.; Naif, H.; Cox, G.B.; Cunningham, A.L.; Gage, P.W. Potential new anti-human immunodeficiency virus type 1 compounds depress virus replication in cultured human macrophages. Antimicrob. Agents Chemother. 2004, 48, 2325–2330. [Google Scholar] [CrossRef] [PubMed]
- Khoury, G.; Ewart, G.; Luscombe, C.; Miller, M.; Wilkinson, J. Antiviral efficacy of the novel compound BIT225 against HIV-1 release from human macrophages. Antimicrob. Agents Chemother. 2010, 54, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Luscombe, C.A.; Huang, Z.; Murray, M.G.; Miller, M.; Wilkinson, J.; Ewart, G.D. A novel Hepatitis C virus p7 ion channel inhibitor, BIT225, inhibits bovine viral diarrhea virus in vitro and shows synergism with recombinant interferon-alpha-2b and nucleoside analogues. Antivir. Res. 2010, 86, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Hout, D.R.; Gomez, M.L.; Pacyniak, E.; Gomez, L.M.; Fegley, B.; Mulcahy, E.R.; Hill, M.S.; Culley, N.; Pinson, D.M.; Nothnick, W.; et al. Substitution of the transmembrane domain of Vpu in simian-human immunodeficiency virus (SHIVKU1bMC33) with that of M2 of influenza A results in a virus that is sensitive to inhibitors of the M2 ion channel and is pathogenic for pig-tailed macaques. Virology 2006, 344, 541–559. [Google Scholar] [CrossRef] [PubMed]
- Hout, D.R.; Gomez, L.M.; Pacyniak, E.; Miller, J.M.; Hill, M.S.; Stephens, E.B. A single amino acid substitution within the transmembrane domain of the human immunodeficiency virus type 1 Vpu protein renders simian-human immunodeficiency virus (SHIV(KU-1bMC33)) susceptible to rimantadine. Virology 2006, 348, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Opella, S.J. Conformational changes induced by a single amino acid substitution in the trans-membrane domain of Vpu: Implications for HIV-1 susceptibility to channel blocking drugs. Protein Sci. 2007, 16, 2205–2215. [Google Scholar] [CrossRef] [PubMed]
- Taube, R.; Alhadeff, R.; Assa, D.; Krugliak, M.; Arkin, I.T. Bacteria-based analysis of HIV-1 Vpu channel activity. PLoS ONE 2014, 9, e105387. [Google Scholar] [CrossRef] [PubMed]
- Sauter, D.; Schwarz, S.; Wang, K.; Zhang, R.; Sun, B.; Schwarz, W. Genistein as antiviral drug against HIV ion channel. Planta Med. 2014, 80, 682–687. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, M.E. Vpu Protein: The Viroporin Encoded by HIV-1. Viruses 2015, 7, 4352-4368. https://doi.org/10.3390/v7082824
González ME. Vpu Protein: The Viroporin Encoded by HIV-1. Viruses. 2015; 7(8):4352-4368. https://doi.org/10.3390/v7082824
Chicago/Turabian StyleGonzález, María Eugenia. 2015. "Vpu Protein: The Viroporin Encoded by HIV-1" Viruses 7, no. 8: 4352-4368. https://doi.org/10.3390/v7082824
APA StyleGonzález, M. E. (2015). Vpu Protein: The Viroporin Encoded by HIV-1. Viruses, 7(8), 4352-4368. https://doi.org/10.3390/v7082824






