Abstract
The type I interferon system plays a critical role in limiting the spread of viral infection. Viruses induce the production of interferon (IFN), which after binding to the IFN-α/β receptor (IFNAR), and triggering of the JAK/STAT signaling cascade, results in the induction of interferon-stimulated genes (ISGs). These ISGs function to inhibit viral replication and to regulate the host immune response. Among these ISGs, the ubiquitin-like molecule, ISG15, is one of the most strongly induced proteins. Similar to ubiquitin, through an IFN induced conjugation cascade, ISG15 is covalently linked to a variety of cellular proteins, suggesting regulation of different cellular processes. Studies performed over the past several years have shown that ISG15 plays a central role in the host’s antiviral response against many viruses. Mice lacking ISG15 display increased susceptibility to multiple viruses. Furthermore, several viruses have developed immune evasion strategies that directly target the ISG15 pathway. Work is now underway to determine the mechanism by which ISG15 functions as an antiviral molecule, such that therapies targeting this pathway can be developed in the future.
1. Introduction—The ISG15 Pathway
ISG15 was first noted in type I interferon (IFN; IFN-α, -β) treated cell lysates in 1984 and was soon after identified as having sequence homology to ubiquitin (reviewed in reference [1]) [2,3]. The crystal structure of ISG15 revealed that it is composed of two domains, each of which assumes a β-grasp fold similar to the ubiquitin structure [2]. ISG15 is one of the most abundantly-induced transcripts upon type I IFN treatment, as well as following TLR ligation and viral infection [3,4]. It is synthesized as a 17 kDa precursor protein that is proteolytically processed into its mature 15 kDa form [3]. Following stimulation with type I IFN, ISG15 exists in three distinct states- free within the cell, released into the extracellular space, or conjugated to target proteins.
Early after its identification, ISG15 was reported to be found in an extracellular form. Stimulation of human monocytes with type I IFN resulted in as much as 50% of the accumulated ISG15 being released into the media [5]. Furthermore, the repeated treatment of patients with interferon-βser resulted in detectable levels of ISG15 in their sera within 48 hours of treatment [5]. ISG15 doesn’t contain a signal sequence; therefore, the mechanism by which it is released remains unknown. Several immunomodulatory activities have been attributed to recombinant or extracellular ISG15 including its ability to induce NK cell proliferation, augment lymphokine-activated-killer (LAK) activity, stimulate the production of IFN-γ, induce DC maturation, and to function as a chemotactic factor for neutrophils [6,7,8]. To date no cell surface receptor has been identified that binds to ISG15; therefore the role that its potential cytokine activity plays in vivo is yet to be ascertained.
Similar to ubiquitin, the mature form of ISG15 contains a C-terminal LRLRGG motif that mediates its covalent conjugation to target proteins, termed ISGylation [9,10]. Within two hours after type I IFN treatment, free ISG15 is detectable in cell lysates, followed by conjugate formation 12–16 hours later [9]. ISGylation utilizes a mechanism similar to ubiquitin, requiring three enzymatic steps (Figure 1). The activating E1 enzyme, Ube1L, was initially identified by Robert Krug and colleagues and functions only in the ISG15 pathway, forming an ATP-dependent thioester bond with ISG15 [11]. The recent generation of an UbE1L knockout mouse has confirmed its role as the ISG15 E1. These mice express free ISG15, but do not form ISG15 conjugates following LPS stimulation or during viral infection [12]. Following activation, ISG15 is transferred to the active-site cysteine of the conjugating E2 enzyme UbcM8/UbcH8 [13,14]. This is followed by the transfer of ISG15 to lysine residues on target proteins by the E3 ligases. To date, three E3 ligases have been shown to interact with the ISG15 pathway and include human HERC5 (Ceb1)/mouse HERC6, HHARI, and Efp [15,16,17,18,19,20]. ISGylation is reversible due to specific removal of ISG15 from conjugated proteins by the deconjugating enzyme Ubp43 (USP18) [21,22]. A screen for additional deubiquitinating enzymes that could target ISG15 conjugates identified USP2, USP5, USP13, and USP14 as potential candidates, whose significance remain to be determined in vivo [23]. While in many ways the ISG15 pathway parallels the ubiquitin conjugation pathway, there are differences that exist. First, all members of the ISG15 conjugation cascade are upregulated by type I IFNs. Second, unlike ubiquitin, in which hundreds of E3s are responsible for the individual substrate specificity, in the ISG15 pathway, Herc5 appears to be a dominant E3 ligase that coordinates the conjugation of the majority of substrates to ISG15. The inhibition of Herc5 expression with siRNA abrogated greater than 90% of the ISG15 conjugate formation that was induced [15]. A recent study by Durfee and colleagues has shown the Herc5 associates with polyribosomes and appears to target newly synthesized proteins, including viral proteins, for modification by ISG15, helping to explain the broad substrate specificity of Herc5 [24].
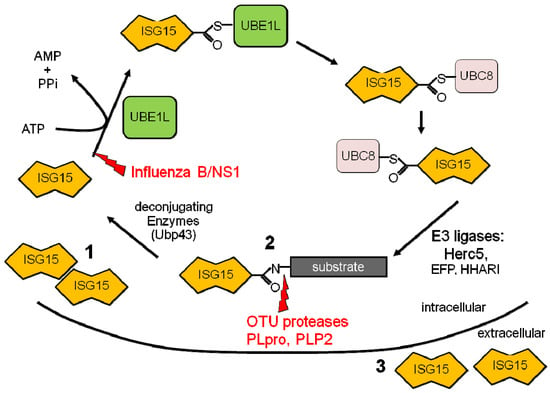
Figure 1.
ISG15 conjugation cascade. Following exposure to IFN a cell will upregulate free intracellular ISG15 (1), conjugate ISG15 to target proteins (2), and release ISG15 into the extracellular space (3). Shown in red are the immune evasion strategies utilized by viruses to circumvent the ISG15 pathway.
Numerous screens have identified nearly 200 candidate ISG15 targets which span a diverse array of biological processes, including protein translation, cell cycle regulation, signal transduction, glycolysis, cell motility, and immune regulation [25,26,27,28]. These targets suggest that ISG15 may be involved in the regulation of a variety of host processes. This has been recently reviewed elsewhere [1]. However, it is still largely unknown what impact ISG15 modification has on a target protein. Unlike ubiquitination, ISGylation does not appear to directly target proteins for proteasome-mediated degradation [29]. Chains of ISG15 do not appear to be formed and no interactions between ISG15 and components of the proteasome have been identified. Based upon the analysis of a limited number of target proteins, ISG15 appears to function by either disrupting the activity of a target protein and/or by altering its localization within the cell [30,31]. The mechanism by which this occurs is largely unknown and will remain an important focus of future investigations. Interestingly, many of the potential target proteins identified include proteins that play an important role in immunity and the host antiviral response, such as STAT1, JAK1, RIG-1, ISG56, PKR, and MxA. The recent observation that Herc5 mediates the ISGylation of actively translated proteins would make many of these IFN induced proteins important targets [24]. It would also provide additional support for ISG15 playing a significant role during viral infection. As discussed below, the importance of ISG15 in the host response to viral infection has been confirmed in (1) studies evaluating the impact of ISG15 overexpression/knockdown on viral growth in vitro; (2) studies of mice deficient in members of the ISG15 conjugation cascade; and (3) the evaluation of viral immune evasion strategies targeting ISG15.
4. Immune Evasion Strategies Targeting ISG15
Host pathways that play a significant role in the antiviral response are often targeted by viruses. Viral immune evasion strategies have been identified that target the ISG15 pathway (Figure 1), providing further support for the importance of this pathway in the host anti-viral response. To date, all of the evasion strategies have targeted ISG15 conjugation by either inhibiting the formation of conjugates or by disrupting the conjugates once formed.
The first identified immune evasion strategy targeting the ISG15 pathway was described by Robert Krug and colleagues while studying interacting partners for the influenza viral protein, NS1 [11]. They determined that while the infection of cells at a high MOI with various influenza B viral strains upregulated free ISG15, no ISG15 conjugates were detected. The mechanism for this inhibition was mediated by the NS1/B gene, which non-covalently bound to ISG15 and inhibited the ability of the ISG15 E1, UbE1L, from interacting with and subsequently activating ISG15, thereby inhibiting conjugate formation [11]. While the NS1 proteins of both influenza A and B viruses are antagonists of IFN, only B/NS1, and not A/NS1 retained the ability to bind to ISG15 and inhibit conjugate formation [11]. Despite this evasion strategy, the ISG15-/- and UbE1L-/- mice are highly susceptible to influenza B virus infection [41,50]. This appears to be due largely to the species specificity of the B/NS1-ISG15 interaction, with B/NS1 binding only to human and non-human primate ISG15, but not to murine or canine ISG15 [20,55]. This may provide important insight into the more restricted host range of influenza B viruses as compared to influenza A viruses.
In addition to influenza B virus, vaccinia virus has also been shown to encode a viral protein that antagonizes ISG15. In this case the E3 protein, which is known to antagonize the host IFN response by several mechanisms (inhibiting PKR, RNaseL and blocking IRF3 activation), was also shown to bind to ISG15 through its C-terminal domain [33]. Once again this protein appears to inhibit ISG15 conjugate formation, although the precise mechanism remains to be determined. In this model, ISG15-/- mice display almost no differences from WT mice following infection with wild type vaccinia virus. However, deletion of the E3 protein from the virus resulted in increased virulence in the ISG15-/- mice, demonstrating its role in the host response, and the efficacy of the immune evasion strategy mounted by vaccinia virus [33].
In contrast to the first two examples, the remaining strategies have taken advantage of the protease activity of different viruses to hydrolyze both ubiquitin and ISG15 from target proteins. The first strategy identified is employed by several viruses with ovarian tumor domain (OTU)-containing proteases [56]. The OTU domain family has recently been identified as deubiquitinating (DUB) enzymes comprised of a group of cysteine proteases which participate in substrate-specific deubiquitination. The L proteins from both Crimean Congo hemorrhagic fever virus (CCHFV) and Dugbe virus (DUGV), as well as the nsP2 proteins from the arteriviruses, equine arteritis virus (EAV) and porcine respiratory and reproductive syndrome virus (PRRSV), contain OTU domains. Unlike their mammalian counterparts (Cezanne and A20) which only had deubiquitinating activity against specific substrates, these viral proteins were found to globally deconjugate both ubiqitin and ISG15 chains, via their cysteine protease activity, but had no activity against SUMO-2 or SUMO-3 conjugates [56]. Expression of these viral OTU domains through either the generation of a transgenic mouse expressing the CCHFV L protein or by overexpressing this protein directly from a recombinant Sindbis virus was able to inhibit the antiviral activity of ISG15 [56]. In addition, these viral OTU proteases were able to inhibit NF-κB dependent signaling [56]. Finally, in addition to OTU domain containing proteases, recent work looking at the papain-like proteases of both the SARS coronavirus (SARS-CoV PLpro) and the human coronavirus NL63 (HCoV-NL63 PLP2) proteins has found that both can deubiquitinate and deISGylate target proteins [57,58]. The significance of this activity during viral infection is still under investigation. In both cases, this viral immune evasion strategy allows these viruses to evade both the IFN and TNFα signaling pathways, both of which play a critical role in the host response to viral infection.
5. Conclusion
Significant progress has been made in identifying the components of the ISG15 conjugation cascade, understanding how these proteins are induced during viral infection, and identifying potential targets for ISGylation. While ISG15 may regulate a variety of cellular processes, it appears to play a critical role in the host response to viral infection. This is supported by studies in ISG15-/- and UbE1L-/- mice, and also in the identification of viral immune evasion strategies that target the ISG15 pathway. Several viral systems appear to be regulated by ISG15, based upon both in vitro and in vivo studies. Recent efforts have begun to dissect the mechanism by which ISG15 is regulating these responses. However, despite this progress many questions still remain and will be the focus of future investigations. First, for many of the viral systems, observations made from either overexpression studies or knockdown studies will need to be confirmed in vivo. Second, additional mechanistic studies will be necessary to understand how ISG15 is regulating such a diverse group of viruses. Only after this information is in hand will we be able to develop therapeutics that target this pathway. Third, while viral proteins can be ISGylated, at least during influenza virus infection, it remains unclear if the antiviral effects of ISG15 are due solely to modification of viral proteins, or if the modification of host proteins also contributes to this antiviral effect. Fourth, the report by Durfee et al. suggests that only actively translated proteins will be targeted for ISGylation, at least by Herc5 [24]. These would include the many genes that are induced by type I IFN, and will be expressed in cells which are not virally infected. The impact of ISGylation on these proteins and what is their fate remains unanswered. Fifth, to date the antiviral activities of ISG15 and the identified viral immune evasion strategies have targeted ISG15 conjugation. However, studies with the Ebola VLPs suggest that free, unconjugated ISG15 may mediate its antiviral effects [32,39]. Unanchored ubiquitin chains have recently been shown to modify both the NF-kB and RIG-I signaling pathways [59,60]. Therefore, evaluating the potential activity of unconjugated ISG15, both within the cell, and that which is released from the cell, will be of interest. Finally, IFNs have pleotropic effects on the immune response. Determining whether ISG15 plays a role in these responses could have important consequences for the development of future therapeutic and vaccine strategies.
References and Notes
- Jeon, Y.J.; Yoo, H.M.; Chung, C.H. ISG15 and immune diseases. Biochim. Biophys. Acta. 2010, 1802, 485–496. [Google Scholar] [CrossRef]
- Narasimhan, J.; Wang, M.; Fu, Z.; Klein, J.M.; Haas, A.L.; Kim, J.J. Crystal structure of the interferon-induced ubiquitin-like protein ISG15. J. Biol. Chem. 2005, 280, 27356–27365. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.L.; Narasimhan, J.; Mende-Mueller, L.; Haas, A.L. Precursor processing of pro-ISG15/UCRP, an interferon-beta-induced ubiquitin-like protein. J. Biol. Chem. 1999, 274, 25061–25068. [Google Scholar] [CrossRef] [PubMed]
- Der, S.D.; Zhou, A.; Williams, B.R.; Silverman, R.H. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc. Natl. Acad. Sci. U. S. A. 1998, 95, 15623–15628. [Google Scholar] [CrossRef] [PubMed]
- D’Cunha, J.; Ramanujam, S.; Wagner, R.J.; Witt, P.L.; Knight, E., Jr.; Borden, E.C. In vitro and in vivo secretion of human ISG15, an IFN-induced immunomodulatory cytokine. J. Immunol. 1996, 157, 4100–4108. [Google Scholar] [CrossRef] [PubMed]
- Padovan, E.; Terracciano, L.; Certa, U.; Jacobs, B.; Reschner, A.; Bolli, M.; Spagnol, G.C.; Borden, E.C.; Heberer, M. Interferon stimulated gene 15 constitutively produced by melanoma cells induces e-cadherin expression on human dendritic cells. Cancer Res. 2002, 2, 3453–3458. [Google Scholar]
- D’Cunha, J.; Knight, E., Jr.; Haas, A.L.; Truitt, R.L.; Borden, E.C. Immunoregulatory properties of ISG15, an interferon-induced cytokine. Proc. Natl. Acad. Sci. U. S. A. 1996, 93, 211–215. [Google Scholar] [CrossRef]
- Owhashi, M.; Taoka, Y.; Ishii, K.; Nakazawa, S.; Uemura, H.; Kambara, H. Identification of a ubiquitin family protein as a novel neutrophil chemotactic factor. Biochem. Biophys. Res. Commun. 2003, 309, 533–539. [Google Scholar] [CrossRef]
- Loeb, K.R.; Haas, A.L. The interferon-inducible 15-kDa ubiquitin homolog conjugates to intracellular proteins. J. Biol. Chem. 1992, 267, 7806–7813. [Google Scholar] [CrossRef]
- Narasimhan, J.; Potter, J.L.; Haas, A.L. Conjugation of the 15-kDa interferon-induced ubiquitin homolog is distinct from that of ubiquitin. J. Biol. Chem. 1996, 271, 324–330. [Google Scholar] [CrossRef]
- Yuan, W.; Krug, R.M. Influenza B virus NS1 protein inhibits conjugation of the interferon (IFN)-induced ubiquitin-like ISG15 protein. EMBO J. 2001, 20, 362–371. [Google Scholar] [CrossRef]
- Kim, K.I.; Yan, M.; Malakhova, O.; Luo, J.K.; Shen, M.F.; Zou, W.; de la Torre, J.C.; Zhang, D.E. Ube1L and protein ISGylation are not essential for alpha/beta interferon signaling. Mol. Cell. Biol. 2006, 26, 72–479. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.I.; Giannakopoulos, N.V.; Virgin, H.W.; Zhang, D.E. Interferon-inducible ubiquitin E2, Ubc8, is a conjugating enzyme for protein ISGylation. Mol. Cell. Biol. 2004, 24, 9592–9600. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Beaudenon, S.L.; Kelley, M.L.; Waddell, M.B.; Yuan, W.; Schulman, B.A.; Huibregtse, J.M.; Krug, R.M. The UbcH8 ubiquitin E2 enzyme is also the E2 enzyme for ISG15, an IFN-alpha/beta-induced ubiquitin-like protein. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 7578–7582. [Google Scholar] [CrossRef] [PubMed]
- Dastur, A.; Beaudenon, S.; Kelley, M.; Krug, R.M.; Huibregtse, J.M. Herc5, an interferon-induced HECT E3 enzyme, is required for conjugation of ISG15 in human cells. J. Biol. Chem. 2006, 281, 4334–4338. [Google Scholar] [CrossRef]
- Nakasato, N.; Ikeda, K.; Urano, T.; Horie-Inoue, K.; Takeda, S. Inoue, S. A ubiquitin E3 ligase Efp is up-regulated by interferons and conjugated with ISG15. Biochem. Biophys. Res. Commun. 2006, 351, 540–546. [Google Scholar] [CrossRef]
- Takeuchi, T.; Inoue, S.; Yokosawa, H. Identification and Herc5-mediated ISGylation of novel target proteins. Biochem. Biophys. Res. Commun. 2006, 348, 473–477. [Google Scholar] [CrossRef]
- Wong, J.J.; Pung, Y.F.; Sze, N.S.; Chin, K.C. HERC5 is an IFN-induced HECT-type E3 protein ligase that mediates type I IFN-induced ISGylation of protein targets. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 10735–10740. [Google Scholar] [CrossRef]
- Zou, W.; Zhang, D.E. The interferon-inducible ubiquitin-protein isopeptide ligase (E3) EFP also functions as an ISG15 E3 ligase. J. Biol. Chem. 2006, 281, 3989–3994. [Google Scholar] [CrossRef]
- Versteeg, G.A.; Hale, B.G.; Van Boheemen, S.; Wolff, T.; Lenschow, D.J.; Garcia-Sastre, A. Species-specific antagonism of host ISGylation by the influenza B virus NS1 protein. J. Virol. 2010, 84, 5423–5430. [Google Scholar] [CrossRef]
- Kim, K.I.; Zhang, D.E. UBP43, an ISG15-specific deconjugating enzyme: expression, purification, and enzymatic assays. Meth. Enzymology 2005, 398, 491–499. [Google Scholar]
- Malakhov, M.P.; Malakhova, O.A.; Kim, K.I.; Ritchie, K.J.; Zhang, D.E. UBP43 (USP18) specifically removes ISG15 from conjugated proteins. J. Biol. Chem. 2002, 277, 9976–9981. [Google Scholar] [CrossRef]
- Catic, A.; Fiebiger, E.; Korbel, G.A.; Blom, D.; Galardy, P.J.; Ploegh, H.L. Screen for ISG15-crossreactive deubiquitinases. PLoS ONE 2007, 2, e679. [Google Scholar] [CrossRef] [PubMed]
- Durfee, L.A.; Lyon, N.; Seo, K.; Huibregtse, J.M. The ISG15 conjugation system broadly targets newly synthesized proteins: implications for the antiviral function of ISG15. Mol. Cell. 2010, 38, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Giannakopoulos, N.V.; Luo, J.K.; Papov, V.; Zou, W.; Lenschow, D.J.; Jacobs, B.S.; Borden, E.C.; Li, J.; Virgin, H.W.; Zhang, D.E. Proteomic identification of proteins conjugated to ISG15 in mouse and human cells. Biochem. Biophys. Res. Commun. 2005, 336, 496–506. [Google Scholar] [CrossRef]
- Zhao, C.; Denison, C.; Huibregtse, J.M.; Gygi, S.; Krug, R.M. Human ISG15 conjugation targets both IFN-induced and constitutively expressed proteins functioning in diverse cellular pathways. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 10200–10205. [Google Scholar] [CrossRef]
- Malakhov, M.P.; Kim, K.I.; Malakhova, O.A.; Jacobs, B.S.; Borden, E.C.; Zhang, D.E. High-throughput immunoblotting. Ubiquitiin-like protein ISG15 modifies key regulators of signal transduction. J. Biol. Chem. 2003, 278, 16608–16613. [Google Scholar] [CrossRef]
- Pattyn, E.; Verhee, A.; Uyttendaele, I.; Piessevaux, J.; Timmerman, E.; Gevaert, K.; Vandekerckhove, J.; Peelman, F.; Tavernier, J. HyperISGylation of Old World monkey ISG15 in human cells. PLoS ONE 2008, 3, e2427. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.L.; Hassel, B.A. Proteasomes modulate conjugation to the ubiquitin-like protein, ISG15. J. Biol. Chem. 2003, 278, 1594–1602. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Choi, J.S.; Lee, J.Y.; Yu, K.R.; Kim, S.M.; Ka, S.H.; Oh, K.H.; Kim, K.I.; Zhang, D.E.; Bang, O.S.; Chung, C.H. ISG15 modification of filamin B negatively regulates the type I interferon-induced JNK signalling pathway. EMBO Rep. 2009, 10, 374–380. [Google Scholar] [CrossRef]
- Zou, W.; Papov, V.; Malakhova, O.; Kim, K.I.; Dao, C.; Li, J.; Zhang, D.E. ISG15 modification of ubiquitin E2 Ubc13 disrupts its ability to form thioester bond with ubiquitin. Biochem. Biophys. Res. Commun. 2005, 336, 61–68. [Google Scholar] [CrossRef]
- Okumura, A.; Pitha, P.M.; Harty, R.N. ISG15 inhibits Ebola VP40 VLP budding in an L-domain-dependent manner by blocking Nedd4 ligase activity. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 3974–3979. [Google Scholar] [CrossRef]
- Guerra, S.; Caceres, A.; Knobeloch, K.P.; Horak, I.; Esteban, M. Vaccinia virus E3 protein prevents the antiviral action of ISG15. PLoS Pathog. 2008, 4, e1000096. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, T.Y.; Zhao, C.; Krug, R.M. Interferon-induced ISG15 conjugation inhibits influenza A virus gene expression and replication in human cells. J. Virol. 2009, 83, 5971–5977. [Google Scholar] [CrossRef]
- Hsiao, N.W.; Chen, J.W.; Yang, T.C.; Ortoff, G.M.; Wu, Y.Y.; Lai, C.H.; Lan, Y.C.; Lin, C.W. ISG15 over-expression inhibits replication of the Japanese encephalitis virus in human medulloblastoma cells. Antivir. Res. 2010, 85, 504–511. [Google Scholar] [CrossRef]
- Shi, H.X.; Yang, K.; Liu, X.; Liu, X.Y.; Wei, B.; Shan, Y.F.; Zhu, L.H.; Wang, C. Positive regulation of interferon regulatory factor 3 activation by Herc5 via ISG15 modification. Mol. Cell. Biol. 2010, 30, 2424–2436. [Google Scholar] [CrossRef] [PubMed]
- Pincetic, A.; Kuang, Z.; Seo, E.J.; Leis, J. The interferon-induced gene ISG15 blocks retrovirus release from cells late in the budding process. J. Virol. 2010, 84, 4725–4736. [Google Scholar] [CrossRef]
- Okumura, A.; Lu, G.; Pitha-Rowe, I.; Pitha, P.M. Innate antiviral response targets HIV-1 release by the induction of ubiquitin-like protein ISG15. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Malakhova, O.A.; Zhang, D.E. ISG15 inhibits Nedd4 ubiquitin E3 activity and enhances the innate antiviral response. J. Biol. Chem. 2008, 283, 8783–8787. [Google Scholar] [CrossRef]
- Broering, R.; Zhang, X.; Kottilil, S.; Trippler, M.; Jiang, M.; Lu, M.; Gorken, G.; Schlaak, J.F. The interferon stimulated gene 15 functions as a proviral factor for the hepatitis C virus and as a regulator of the IFN response. Gut 2010, 59, 1111–1119. [Google Scholar] [CrossRef]
- Lenschow, D.J.; Lai, C.; Frias-Staheli, N.; Giannakopoulos, N.V.; Lutz, A.; Wolff, T.; Osiak, A.; Levine, B.; Schmidt, R.F.; Garcia-Sastre, A.; Leib, D.A.; Pekosz, A.; Knobelock, K.P.; Horak, I.; Virgin, H.W. IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza, herpes, and Sindbis viruses. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 1371–1376. [Google Scholar] [CrossRef]
- Osiak, A.; Utermohlen, O.; Niendorf, S.; Horak, I.; Knobeloch, K.P. ISG15, an interferon-stimulated ubiquitin-like protein, is not essential for STAT1 signaling and responses against vesicular stomatitis and lymphocytic choriomeningitis virus. Mol. Cell. Biol. 2005, 25, 6338–6345. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, K.J.; Malakhov, M.P.; Hetherington, C.J.; Zhou, L.; Little, M.T.; Malakhova, O.A.; Sipe, J.C.; Orkin, S.H.; Zhang, D.E. Dysregulation of protein modification by ISG15 results in brain cell injury. Genes Dev. 2002, 16, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- Malakhova, O.A.; Yan, M.; Malakhov, M.P.; Yuan, Y.; Ritchie, K.J.; Kim, K.I.; Peterson, L.F.; Shuai, K.; Zhang, D.E. Protein ISGylation modulates the JAK-STAT signaling pathway. Genes Dev. 2003, 17, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, K.J.; Hahn, C.S.; Kim, K.I.; Yan, M.; Rosario, D.; Li, L.; de la Torre, J.C.; Zhang, D.E. Role of ISG15 protease UBP43 (USP18) in innate immunity to viral infection. Nat. Med. 2004, 10, 1374–1378. [Google Scholar] [CrossRef]
- Knobeloch, K.P.; Utermohlen, O.; Kisser, A.; Prinz, M.; Horak, I. Reexamination of the role of ubiquitin-like modifier ISG15 in the phenotype of UBP43-deficient mice. Mol. Cell. Biol. 2005, 25, 11030–11034. [Google Scholar] [CrossRef]
- Malakhova, O.A.; Kim, K.I.; Luo, J.K.; Zou, W.; Kumar, K.G.; Fuchs, S.Y.; Shuai, K.; Zhang, D.E. UBP43 is a novel regulator of interferon signaling independent of its ISG15 isopeptidase activity. EMBO J. 2006, 25, 2358–2367. [Google Scholar] [CrossRef]
- Giannakopoulos, N.V.; Arutyunova, E.; Lai, C.; Lenschow, D.J.; Haas, A.L.; Virgin, H.W. ISG15 Arg151 and the ISG15-conjugating enzyme UbE1L are important for innate immune control of Sindbis virus. J. Virol. 2009, 83, 1602–1610. [Google Scholar] [CrossRef]
- Lenschow, D.J.; Giannakopoulos, N.V.; Gunn, L.J.; Johnston, C.; O’Guin, A.K.; Schmidt, R.E.; Levine, B.; Virgin, H.W. Identification of interferon-stimulated gene 15 as an antiviral molecule during Sindbis virus infection in vivo. J. Virol. 2005, 79, 13974–13983. [Google Scholar] [CrossRef]
- Lai, C.; Struckhoff, J.J.; Schneider, J.; Martinez-Sobrido, L.; Wolff, T.; Garcia-Sastre, A.; Zhang, D.E.; Lenschow, D.J. Mice lacking the ISG15 E1 enzyme UbE1L demonstrate increased susceptibility to both mouse-adapted and non-mouse-adapted influenza B virus infection. J. Virol. 2009, 83, 1147–1151. [Google Scholar] [CrossRef]
- Tang, Y.; Zhong, G.; Zhu, L.; Liu, X.; Shan, Y.; Feng, H.; Bu, Z.; Chen, H.; Wang, C. Herc5 attenuates influenza A virus by catalyzing ISGylation of viral NS1 protein. J. Immunol. 2010, 184, 5777–5790. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Hsiang, T.Y.; Kuo, R.L.; Krug, R.M. ISG15 conjugation system targets the viral NS1 protein in influenza A virus-infected cells. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 2253–2258. [Google Scholar] [CrossRef]
- Lu, G.; Reinert, J.T.; Pitha-Rowe, I.; Okumura, A.; Kellum, M.; Knobeloch, K.P.; Hassel, B.; Pitha, P.M. ISG15 enhances the innate antiviral response by inhibition of IRF-3 degradation. Cell. Mol. Biol. 2006, 52, 29–41. [Google Scholar] [PubMed]
- Takeuchi, T.; Iwahara, S.; Saeki, Y.; Sasajima, H.; Yokosawa, H. Link between the ubiquitin conjugation system and the ISG15 conjugation system: ISG15 conjugation to the UbcH6 Ubiquitin E2 enzyme. J. Biochem. 2005, 138, 711–719. [Google Scholar] [CrossRef]
- Sridharan, H.; Zhao, C.; Krug, R.M. Species specificity of the NS1 protein of influenza B virus: NS1 binds only human and non-human primate ubiquitin-like ISG15 proteins. J. Biol. Chem. 2010, 285, 7852–7856. [Google Scholar] [CrossRef]
- Frias-Staheli, N.; Giannakopoulos, N.V.; Kikkert, M.; Taylor, S.L.; Bridgen, A.; Paragas, J.; Richt, J.A.; Rowland, R.R.; Schmaljohn, C.S.; Lenschow, D.J.; Snijder, E.J.; Garcia-Sastre, A.; Virgin, H.W. Ovarian tumor domain-containing viral proteases evade ubiquitin- and ISG15-dependent innate immune responses. Cell Host Microbe 2007, 2, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Clementz, M.A.; Chen, Z.; Banach, B.S.; Wang, Y.; Sun, L.; Ratia, K.; Baez-Santos, Y.M.; Wang, J.; Takayama, J.; Ghosh, A.K.; Li, K.; Mesecar, A.D.; Baker, S.C. Deubiquitinating and interferon antagonism activities of coronavirus papain-like proteases. J. Virol. 2010, 84, 4619–4629. [Google Scholar] [CrossRef]
- Lindner, H.A.; Lytvyn, V.; Qi, H.; Lachance, P.; Ziomek, E.; Menard, R. Selectivity in ISG15 and ubiquitin recognition by the SARS coronavirus papain-like protease. Arch. Biochem. Biophys. 2007, 466, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Sun, L.; Jiang, X.; Chen, S.; Hou, F.; Adhikan, A.; Xu, M.; Chen, Z.J. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell 2010, 141, 315–330. [Google Scholar] [CrossRef]
- Xia, Z.P.; Sun, L.; Chen, X.; Pineda, G.; Jiang, X.; Adhikari, A.; Zeng, W.; Chen, Z.J. Direct activation of protein kinases by unanchored polyubiquitin chains. Nature 2009, 461, 114–119. [Google Scholar] [CrossRef]
- Kim, J.H.; Luo, J.K.; Zhang, D.E. The level of hepatitis B virus replication is not affected by protein ISG15 modification but is reduced by inhibition of UBP43 (USP18) expression. J. Immunol. 2008, 181, 6467–6472. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2010 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/3.0/).
