Abstract
Exogenous retroviruses are subclassified into seven genera and include viruses that cause diseases in humans. The viral Gag and Gag-Pro-Pol polyproteins are processed by the retroviral protease in the last stage of replication and inhibitors of the HIV-1 protease are widely used in AIDS therapy. Resistant mutations occur in response to the drug therapy introducing residues that are frequently found in the equivalent position of other retroviral proteases. Therefore, besides helping to understand the general and specific features of these enzymes, comparative studies of retroviral proteases may help to understand the mutational capacity of the HIV-1 protease.
1. Introduction
All replication competent retroviruses code for a protease (PR) that has an essential function in viral replication, therefore the PR of human retroviruses, especially that of human immunodeficiency virus type 1 (HIV-1), is a good and well-explored target for antiviral therapy. Currently retroviruses are classified into seven genera: the phylogenetic tree created for these viruses on the basis of the protease sequences is shown in Figure 1: this relationship is similar to that established based on the whole sequences [].
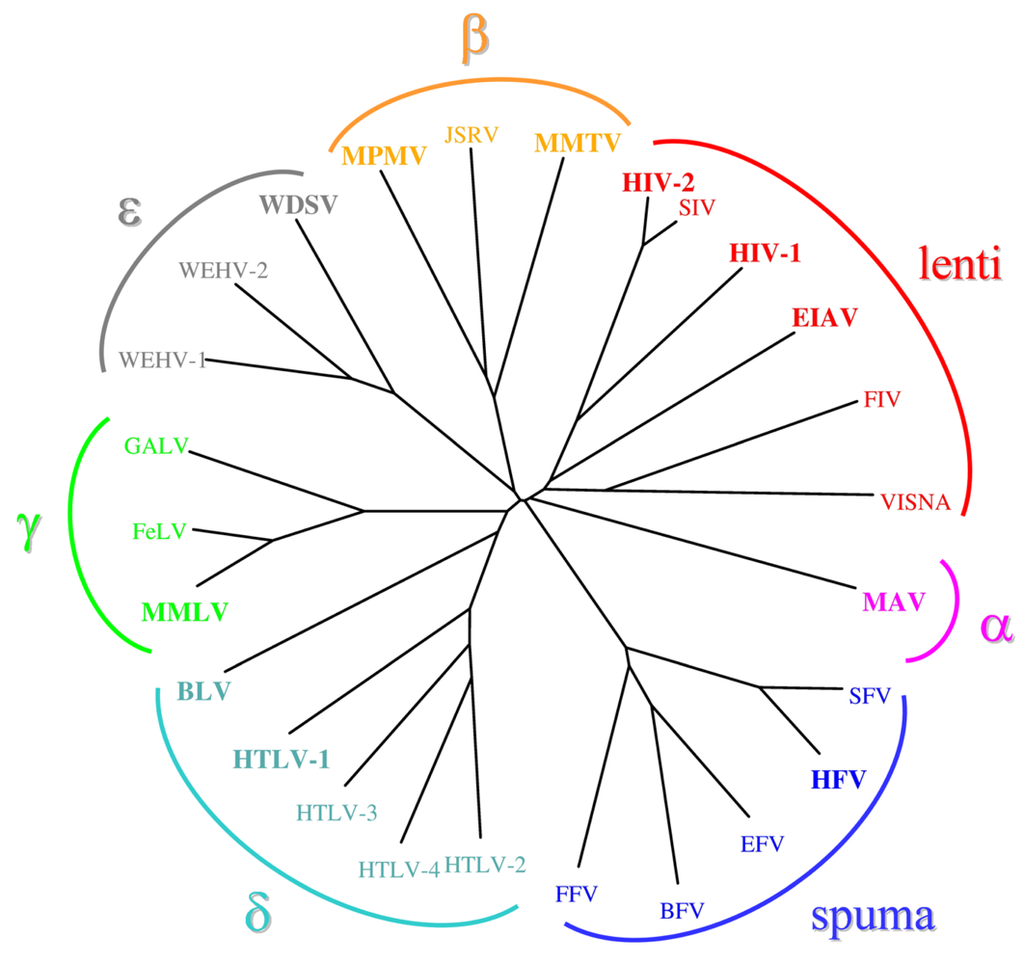
Figure 1.
Phylogenetic relationship of retroviral proteases. Sequence alignment and the phylogenetic tree was made by ClustalW and Phylip programs, respectively. Abbreviations used: BLV, bovine leukemia virus; BFV, bovine foamy virus; EIAV, equine infectious anemia virus; EFV, equine foamy virus; FeLV, feline leukemia virus; FFV, feline foamy virus; FIV, feline immunodeficiency virus; GALV, gibbon-ape leukemia virus; HIV, human immunodeficiency virus; HTLV, human T-lymphotropic virus; HFV, human foamy virus; JSRV, jaagsiekte sheep retrovirus; MAV, myeloblastosis associated virus; MMLV, Moloney murine leukemia virus; MMTV, mouse mammary tumor virus; MPMV, Mason-Pfizer monkey virus; SIV, simian immunodeficiency virus; SFV, simian foamy virus; WDSV, walleye dermal sarcoma virus; WEHV, walleye epidermal hyperplasia virus; Proteases reviewed in this paper are in larger bold.
The viral PR plays a critical role at the last stage of viral replication by processing of the Gag and Gag-Pro-Pol polyproteins at a limited number of sites (Figure 2), by converting the morphologically distinct “immature” virions into “mature” ones []. Based on the sites of processing, it is not possible to give a consensus substrate sequence (unlike e.g., for caspases), and this is a general characteristic of the retroviral proteases. Nevertheless, based on similarities in amino acid sequences, primate lentiviral (HIV-1, HIV-2, SIV) cleavage sites were grouped into three classes [], while analysis of a broader range of retroviral protease cleavage site sequences suggested two types of cleavage sites []. Later systematic specificity studies on HIV-1 PR also verified the existence of two types of cleavage sites, type 1 - having an aromatic residue and Pro - (highlighted in red in Figure 2), and type 2 having hydrophobic residues (excluding Pro) at the site of cleavage [,], defined as P1 and P1’ positions [], respectively. These classical cleavage type sites also showed different preferences for the P2 and P2' positions [,].
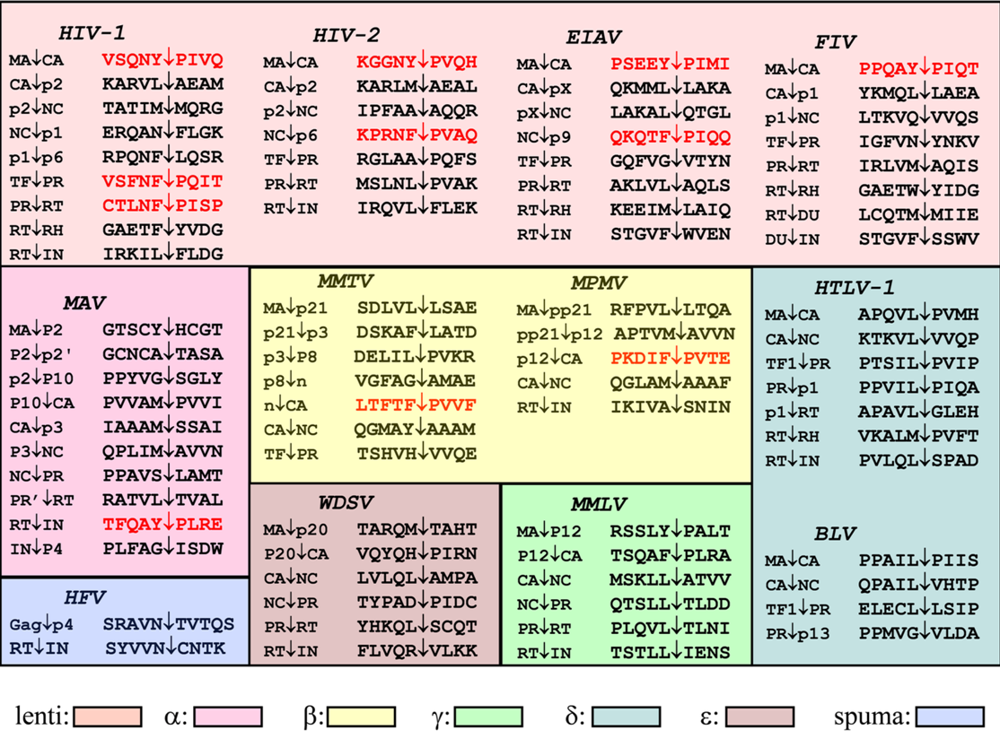
Figure 2.
Naturally occurring cleavage sites in retroviral Gag and Gag-Pro-Pol polyproteins. The site cleaved by the cognate retroviral protease is indicated by an arrow. Type 1 cleavage sites are in red. Abbreviations used: CA, capsid; NC, nucleocapsid; TF, transframe protein; PR, protease; RH, RNaseH; IN, integrase; DU, dUTPase. Proteins and peptides with unidentified functions are abbreviated with the size of the protein in kDa (e.g., p12 is a protein having 12 kDa, while pp refers to phosphoprotein), as pX, or by the letter n.
The type 1 cleavage site was thought to be important in defining specificity. With the exception of pepsin, cellular proteases are not capable to efficiently process peptide bonds at the imino side of Pro residue. The unique nature of Pro at the P1’ position of retroviral cleavage sites was recognized even before the discovery of the PR and implied that these sites should be processed by a virally-coded enzyme []. It should be noted, that many of the cleavage sites do not fit into these classifications (e.g., they contain polar residue at P1 or P1’ or contain Pro after a nonaromatic residue, see Figure 2), therefore this classification might be an oversimplification.
The PR is coded in the so called pro gene, that is either in frame with the Gag and Pol (as in case of MAV) or is produced with a stop codon suppression (exemplified by MMLV), frameshift mechanism (exemplified by HIV-1) or by a splicing event (as it is the case of the HFV PR). As examples, the organization of the Gag-Pro-Pol proteins of HIV-1 HTLV-1, MAV and HFV is provided in Figure 3.
The way of PR synthesis appears to be in a good correlation with the activity of the enzymes: while proteases produced by frameshifting or stop codon suppression, and therefore being present only in 5-20% amount compared to the Gag typically have high specific activity, the protease of MAV produced in frame of Gag and therefore being in an equivalent amount with this substrate has a substantially lower specific activity (see below). The PR is an aspartyl protease, acts as a homodimer, as described in detail by Weber et al. in this issue []. Structure-based sequence comparison of the proteases reviewed in this paper is provided in Figure 4.
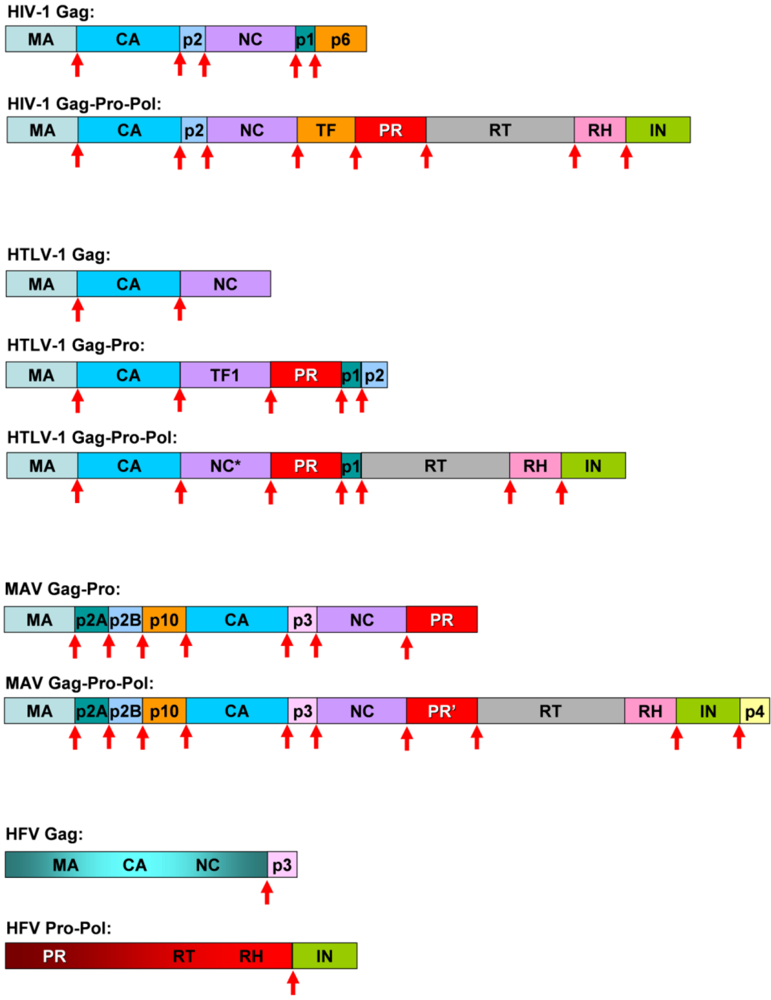
Figure 3.
Organization of the Gag-Pro-Pol proteins in lentiviral HIV-1, deltaretroviral HTLV-1, alpharetroviral MAV and spumaretroviral HFV. Sites of PR processing are indicated by arrows.
Specificity studies of retroviral proteases typically utilize protein substrates or oligopeptides. As crystal structures have been determined for various retroviral proteases [] or molecular models could be built with high certainty, specificity studies have been frequently complemented with molecular modeling to understand the molecular basis of PR specificity. However, as no generally accepted and widely used standard assay conditions have been established in the retroviral protease field, and the PR is highly sensitive to the pH, ionic strength and type of the substrate (e.g., protein versus peptide, or the presence of ionizable side chains such as Glu), it is difficult to quantitatively compare data published by different laboratories. Furthermore, while it has been a commonly used strategy to compare the specificity of a PR of a given retrovirus to that of another one (typically to that of HIV-1), only a few studies dealt with the comparison of more enzymes. In this review, after the description of the specificity results regarding some representative proteases, the comparative studies involving a set of proteases will be summarized.

Figure 4.
Structure-based sequence alignment of the retroviral proteases. Active site aspartate residues are in red, amino acid residues involved in substrate binding are in blue and underlined.
2. HIV-1 protease
By far the most of our knowledge on the specificity of retroviral proteases has been obtained by studying that of HIV-1 PR as described in several reviews []. In type 1 cleavage sites of primate lentiviruses (HIV-1, HIV-2 and SIV), there is a preference for Asn at P2 and beta-branched hydrophobic residue (Val or Ile) at P2’, while in type 2 cleavage sites the P2 residue is typically beta branched and the P2’ residue is Glu or Gln (Figure 2). Although only the CA↓p2 site of HIV-1 contains the charged Glu at P2’, this residue was suggested to play a regulatory role in the viral protein processing, as cleavage at this site is accelerated by lower pH []. A schematic diagram of the substrate binding site of HIV-1 PR with modeled interaction of the bound residues of the MA↓CA cleavage site is given in Figure 5
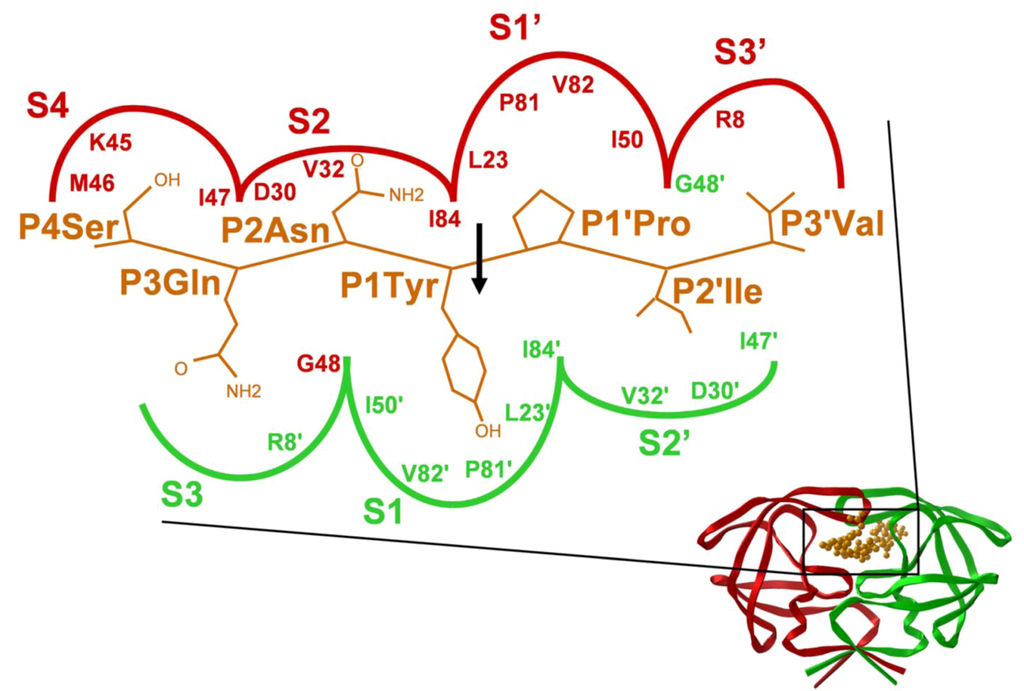
Figure 5.
Schematic representation of the HIV-1 MA↓CA cleavage site substrate in the S4 S3' subsites of HIV-1 PR. The indicated substrate sequence was modeled into the binding site of the crystallographic structure of the PR. The relative size of each subsite is indicated approximately by the area enclosed by the curved line.
The substrate binds to the enzyme in an extended beta conformation, and it is anchored by several hydrogen bonds: a very similar binding mode is observed with the inhibitors of the PR. The HIV-1 PR recognizes at least seven substrate residues, from P4 to P3’ and each amino acid side chain of the substrate fits in successive subsites (S4 to S3') formed by PR residues (Figure 5). Although the HIV-1 PR (similarly to the proteases of other retroviruses) is a symmetrical dimer of two identical subunits, the residues of naturally occurring cleavage sites do not show symmetrical arrangements (Figure 2) and no obvious symmetrical substrate preference has been observed for the specificity of HIV-1 PR []. Nevertheless, modeling showed that the same residues of the two enzyme subunits interact with the appropriate substrate residues at both sides of the scissile bond (Figure 5). Based on detailed specificity studies as well as HIV-1 PR-inhibitor crystal structures, there appear to be a very strong sequence context dependence of the specificity of HIV-1 PR, that also provides an explanation for the lack of consensus sequences, and appears to be a general feature for the retroviral proteases. As the substrate binding pockets are overlapping, interactions could occur between the side chains of the substrate [], and these interactions could lead to alternative positioning of a given side chain [] causing an energetically non-additive nature of the ligand side chain-binding pocket interactions []. Crystallographic studies of active site mutant HIV-1 PR complexed with substrate peptides [] and of active enzyme complexed with substrate analogs [] suggested the molecular basis of this phenomenon. Unlike in the Gag and Gag-Pro-Pol cleavage sites, cellular protein cleavage sites frequently contain charged residues, especially Glu at P2’ []. The reason for the markedly different subsite preference in viral and cellular proteins of the HIV-1 PR is not known, but might at least partially be due to the different assay conditions applied. In spite of the strong sequence context-dependent nature of the HIV-1 PR specificity, the basic features of the substrate binding subsites could be determined independently of the substrates used. The S4 subsite is close to the protein surface and partly exposed to solvent, and the HIV-1 S4 binding site prefers small, even hydrophilic residues. The S3-S3’ region is generally hydrophobic, although the naturally occurring cleavage sites contain at least two polar (or even charged) residues at some of these positions (Figure 2). It should be taken into account, that these regions are expected to be linker sequences between protein domains of Gag(-Pro-Pol), a function that requires a certain degree of hydrophilic nature of the given sequence. Besides the hydrophobicity, the size of residues as well as the presence or absence of branching (especially beta branches) appear to be important in determining specificity. The S3 (like S3’) subsite is relatively large, and is partly exposed to solvent at the surface of the enzyme. The P3 side chain may be positioned either to interact with the more polar residues of the PR surface (as shown in Figure 5), or to interact with the hydrophobic internal residues of the enzyme: various residues are accepted at this position by HIV-1 PR. Studies with type 1 peptide substrates indicated a preference for small residues like Cys or Asn at P2 position and preference for beta-branched Val or Ile at P2' position [], while studies with type 2 peptides showed preference for beta-branched residues, especially for Val at P2, and Glu for P2' [], in good agreement with the type 1/type 2 cleavage site classification. Further studies revealed, that in case of tight packing of both S1 and S1' with Tyr residues, a relatively larger residue at either P2 or P2' is preferable, but not at both positions [].
As a consequence of the error-prone nature of the reverse transcriptase, HIV-1 (as typically other retroviruses) is a quasi-species, having natural sequence variations throughout its genome, including the pro gene (Figure 6). Furthermore, HIV-1 PR mutates extensively as a response of the selective pressure exerted by the PR inhibitors used in drug therapy. Unlike the natural variations, these mutations causing drug resistance tend to occur in the substrate binding region of the protease (reviewed in []) and frequently the resistant mutations introduce residues that can be found at the equivalent position of other retroviral proteases (Figure 6), therefore comparative study of retroviral proteases is a promising approach not only to recognize general and specific features of the PR, but also to discover the mutational capacity of the HIV-1 PR. Approximately half of the residues have been found to be mutated as a consequence of the protease inhibitor applications, and have been implicated in drug resistance. Structural analysis of mutant proteases are reviewed in this issue of Viruses []. As the drug-resistant mutations typically occur in the substrate binding region, they are expected to change the specificity of the enzyme, as verified by various studies, and reviewed in []. However, an important feature of the drug resistant mutants is that they are still capable to cleave the viral processing sites fairly efficiently. A property, termed vitality was introduced as a measure of the selective advantage of different mutants in the presence of a given inhibitor []. Crystallographic analysis of HIV-1 PR substrate and inhibitor complexes suggested that the inhibitor envelope (the space filled by the inhibitor molecule) protrudes from the substrate envelope, therefore the protease is capable to mutate interacting residues into smaller ones to substantially alter the inhibitor binding without dramatically affecting the substrate binding and hydrolysis [].
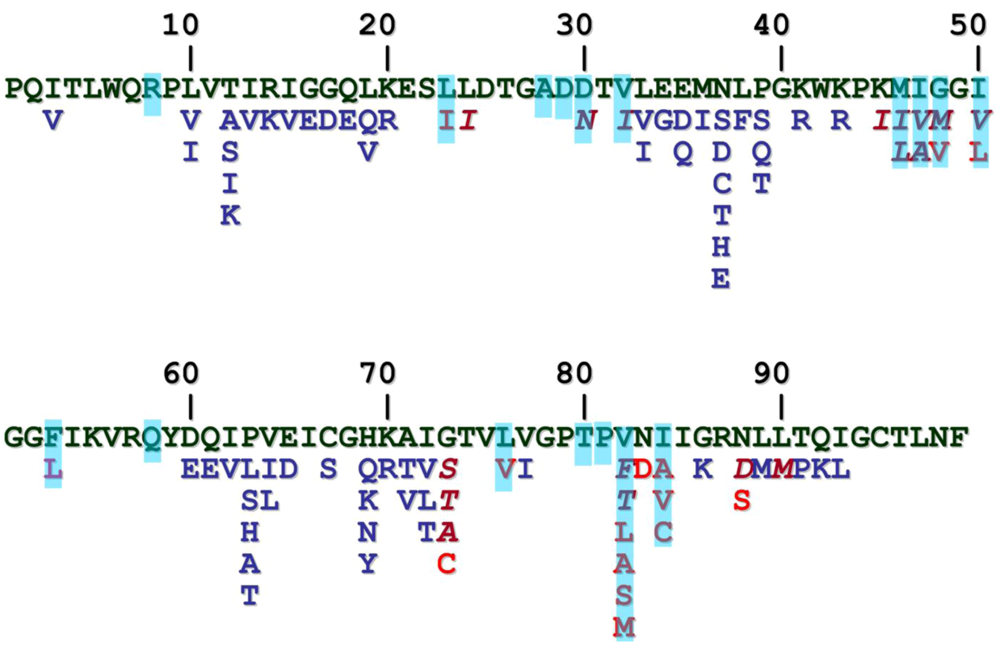
Figure 6.
Sequence polymorphism and drug-resistant mutations of HIV-1 PR. Sequence of the HIV-1HXB2 PR is in green, natural variations are in blue, resistant mutations are in red. Those residues that can be observed at the equivalent position of another retroviral PR are shown in italics. Residues that are involved in ligand binding are marked with blue background.
3. HIV-2 protease
In comparison with HIV-1, HIV-2 is less pathogenic and less transmissible, with lower viral loads while asymptomatic and a slower progression to AIDS []. HIV-2 PR, like the HIV-1 enzyme, was able to cleave all oligopeptides representing the main Gag-Pro-Pol cleavage sites in HIV-1 and in HIV-2 [], and similar result was also observed with in vitro polyprotein processing [,]. Although initial reports suggested substantial differences in the specificity of HIV proteases [,], subsequent studies suggested that the specificity of these two enzymes are rather similar [,,] in good agreement with their very similar substrate binding sites: they differ in only four residues appearing as conservative substitutions (Val32Ile, Ile47Val, Leu76Met, Val82Ile) with the gain or loss of methyl groups. Specificity studies in combination with molecular modeling have revealed the importance of these residues to explain differences in substrate specificity between HIV-1 and HIV-2 proteases [,,]. Although clinical inhibitors of HIV-1 PR typically less potent against HIV-2 PR [], they can be utilized in anti-HIV-2 therapy, as reviewed recently with various other aspects of HIV-2 PR []. Another primate lentivirus for which the specificity has been studied is that of SIV. The specificity of the enzyme appeared to be identical to that of HIV-2, in good agreement with the high homology: the two enzymes have almost identical sequences []. Although the resistant mutations in HIV-2 PR are also expected to change the enzyme specificity, as it is the case with HIV-1 PR, this has not been studied so far.
Acknowledgments
The critical reading of the manuscript by Peter Bagossi and his help in preparing the figures is greatly acknowledged. Author’s research was supported by the Hungarian Science and Research Fund (OTKA K68288).
Referneces
- Weiss, R.A. The discovery of endogenous retroviruses. Retrovirology 2006, 3, 67. [Google Scholar] [CrossRef] [PubMed]
- Oroszlan, S.; Luftig, R.B. Retroviral proteinases. Curr. Top. Microbiol. Immunol. 1990, 157, 153–185. [Google Scholar] [PubMed]
- Henderson, L.E.; Benveniste, R.E.; Sowder, R.; Copeland, T.D.; Schultz, A.M.; Oroszlan, S. Molecular characterization of gag proteins from simian immunodeficiency virus (SIVMne). J. Virol. 1988, 62, 2587–2595. [Google Scholar] [PubMed]
- Pettit, S.C.; Simsic, J.; Loeb, D.D.; Everitt, L.; Hutchison 3rd, C.A.; Swanstrom, R. Analysis of retroviral protease cleavage sites reveals two types of cleavage sites and the structural requirements of the P1 amino acid. J. Biol. Chem. 1991, 266, 14539–14547. [Google Scholar] [PubMed]
- Griffiths, J.T.; Phylip, L.H.; Konvalinka, J.; Strop, P.; Gustchina, A.; Wlodawer, A.; Davenport, R.J.; Briggs, R.; Dunn, B.M.; Kay, J. Different requirements for productive interaction between the active site of HIV-1 proteinase and substrates containing -hydrophobic*hydrophobic- or -aromatic*pro- cleavage sites. Biochemistry 1992, 31, 5193–51200. [Google Scholar] [CrossRef] [PubMed]
- Tözsér, J.; Bagossi, P.; Weber, I.T.; Louis, J.M.; Copeland, T.D.; Oroszlan, S. Studies on the symmetry and sequence context dependence of the HIV-1 proteinase specificity. J. Biol. Chem. 1997, 272, 16807–16814. [Google Scholar] [CrossRef] [PubMed]
- Schechter, I.; Berger, A. On the size of the active site in proteases. I. Papain. Biochem. Biophys. Res. Commun. 1967, 27, 157–162. [Google Scholar] [CrossRef]
- Amino- and carboxyl-terminal amino acid sequences of proteins coded by gag gene of murine leukemia virus. Proc. Natl. Acad. Sci. USA 1978, 75, 1404–1408. [CrossRef]
- Weber, I.T.; Agniswamy, J. HIV-1 Protease: Structural Perspectives on Drug Resistance. Viruses 2009, 1, 1110–1136. [Google Scholar] [CrossRef]
- Dunn, B.M.; Goodenow, M.M.; Gustchina, A.; Wlodawer, A. Retroviral proteases. Genome Biol. 2002, 3, reviews3006.1–reviews3006.7. [Google Scholar] [CrossRef]
- Tomasselli, A.G.; Heinrikson, R.L. Specificity of retroviral proteases: an analysis of viral and nonviral protein substrates. Methods Enzymol. 1994, 241, 279–301. [Google Scholar] [PubMed]
- Dunn, B.M.; Gustchina, A.; Wlodawer, A.; Kay, J. Subsite preferences of retroviral proteinases. Methods Enzymol. 1994, 241, 254–278. [Google Scholar] [PubMed]
- Louis, J.M.; Weber, I.T.; Tözsér, J.; Clore, G.M.; Gronenborn, A.M. HIV-1 protease: maturation, enzyme specificity, and drug resistance. Adv. Pharmacol. 2000, 49, 111–146. [Google Scholar] [PubMed]
- Tözsér, J.; Oroszlan, S. Proteolytic events of HIV-1 replication as targets for therapeutic intervention. Curr. Pharm. Des. 2003, 9, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Pettit, S.C.; Moody, M.D.; Wehbie, R.S.; Kaplan, A.H.; Nantermet, P.V.; Klein, C.A.; Swanstrom, R. The p2 domain of human immunodeficiency virus type 1 Gag regulates sequential proteolytic processing and is required to produce fully infectious virions. J. Virol. 1994, 68, 8017–8027. [Google Scholar] [PubMed]
- Boross, P.; Bagossi, P.; Copeland, T.D.; Oroszlan, S.; Louis, J.M.; Tözsér, J. Effect of substrate residues on the P2' preference of retroviral proteinases. Eur. J. Biochem. 1999, 264, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Prabu-Jeyabalan, M.; Nalivaika, E.; Schiffer, C.A. Substrate shape determines specificity of recognition for HIV-1 protease: analysis of crystal structures of six substrate complexes. Structure 2002, 10, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Boross, P.I.; Wang, Y.F.; Gaddis, L.; Liu, F.; Chen, X.; Tözsér, J.; Harrison, R.W.; Weber, I.T. Molecular basis for substrate recognition and drug resistance from 1.1 to 1.6 angstroms resolution crystal structures of HIV-1 protease mutants with substrate analogs. FEBS J. 2005, 272, 5265–5277. [Google Scholar] [CrossRef] [PubMed]
- Tözsér, J.; Weber, I.T.; Gustchina, A.; Bláha, I.; Copeland, T.D.; Louis, J.M.; Oroszlan, S. Kinetic and modeling studies of S3-S3' subsites of HIV proteinases. Biochemistry 1992, 31, 4793–4800. [Google Scholar] [CrossRef] [PubMed]
- Shafer, R.W.; Schapiro, J.M. HIV-1 drug resistance mutations: an updated framework for the second decade of HAART. AIDS Rev. 2008, 10, 67–84. [Google Scholar] [PubMed]
- Weber, I.T.; Zhang, Y.; Tözsér, J. HIV-1 Protease and AIDS Therapy. Lendeckel U.; Hooper, M.N., Ed.; Springer: Berlin, Germany, 2009; pp. 25–45. [Google Scholar]
- Gulnik, S.V.; Suvorov, L.I.; Liu, B.; Yu, B.; Anderson, B.; Mitsuya, H.; Erickson, J.W. Kinetic characterization and cross-resistance patterns of HIV-1 protease mutants selected under drug pressure. Biochemistry 1995, 34, 9282–9287. [Google Scholar] [CrossRef] [PubMed]
- King, N.M.; Prabu-Jeyabalan, M.; Nalivaika, E.A.; Schiffer, C.A. Combating susceptibility to drug resistance: lessons from HIV-1 protease. Chem. Biol. 2004, 11, 1333–1338. [Google Scholar] [PubMed][Green Version]
- Bock, P.J.; Markovitz, D.M. Infection with HIV-2. AIDS 2001, 15, S35–S45. [Google Scholar] [CrossRef] [PubMed]
- Tözsér, J.; Bláha, I.; Copeland, T.D.; Wondrak, E.M.; Oroszlan, S. Comparison of the HIV-1 and HIV-2 proteinases using oligopeptide substrates representing cleavage sites in Gag and Gag-Pol polyproteins. FEBS Lett. 1991, 281, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.C.; Carr, S.F.; Jarnagin, K.; Kirsher, S.; Barnett, J.; Chow, J.; Chan, H.W.; Chen, M.S.; Medzihradszky, D.; Yamashiro, D.; et al. Synthetic HIV-2 protease cleaves the GAG precursor of HIV-1 with the same specificity as HIV-1 protease. Arch. Biochem. Biophys. 1990, 277, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Pichuantes, S.; Babé, L.M.; Barr, P.J.; DeCamp, D.L.; Craik, C.S. Recombinant HIV2 protease processes HIV1 Pr53gag and analogous junction peptides in vitro. J. Biol. Chem. 1990, 265, 13890–13898. [Google Scholar] [PubMed]
- Tomasselli, A.G.; Hui, J.O.; Sawyer, T.K.; Staples, D.J.; Bannow, C.; Reardon, I.M.; Howe, W.J.; DeCamp, D.L.; Craik, C.S.; Heinrikson, R.L. Specificity and inhibition of proteases from human immunodeficiency viruses 1 and 2. J. Biol. Chem. 1990, 265, 14675–14683. [Google Scholar] [PubMed]
- Poorman, R.A.; Tomasselli, A.G.; Heinrikson, R.L.; Kézdy, F.J. A cumulative specificity model for proteases from human immunodeficiency virus types 1 and 2, inferred from statistical analysis of an extended substrate database. J. Biol. Chem. 1991, 266, 14554–14561. [Google Scholar] [PubMed]
- Tözsér, J.; Gustchina, A.; Weber, I.T.; Blaha, I.; Wondrak, E.M.; Oroszlan S. Studies on the role of the S4 substrate binding site of HIV proteinases. FEBS Lett. 1991, 279, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Bagossi, P.; Cheng, Y.S.; Oroszlan, S.; Tözsér, J. Comparison of the specificity of homo- and heterodimeric linked HIV-1 and HIV-2 proteinase dimers. Protein Eng. 1998, 11, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Brower, E.T.; Bacha, U.M.; Kawasaki, Y.; Freire, E. Inhibition of HIV-2 protease by HIV-1 protease inhibitors in clinical use. Chem Biol Drug Des. 2008, 71, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Arias, L.; Tözsér, J. HIV-1 protease inhibitors: effects on HIV-2 replication and resistance. Trends Pharmacol Sci. 2008, 29, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Tomasselli, A.G.; Bannow, C.A.; Deibel Jr., M.R.; Hui, J.O.; Zurcher-Neely, H.A.; Reardon, I.M.; Smith, C.W.; Heinrikson, R.L. Chemical synthesis of a biotinylated derivative of the simian immunodeficiency virus protease. Purification by avidin affinity chromatography and autocatalytic activation. J. Biol. Chem. 1992, 267, 10232–10237. [Google Scholar] [PubMed]
- Rushlow, K.; Peng, X.X.; Montelaro, R.C.; Shih, D.S. Expression of the protease gene of equine infectious anemia virus in Escherichia coli: formation of the mature processed enzyme and specific cleavage of the gag precursor. Virology 1992, 188, 396–401. [Google Scholar] [CrossRef] [PubMed]
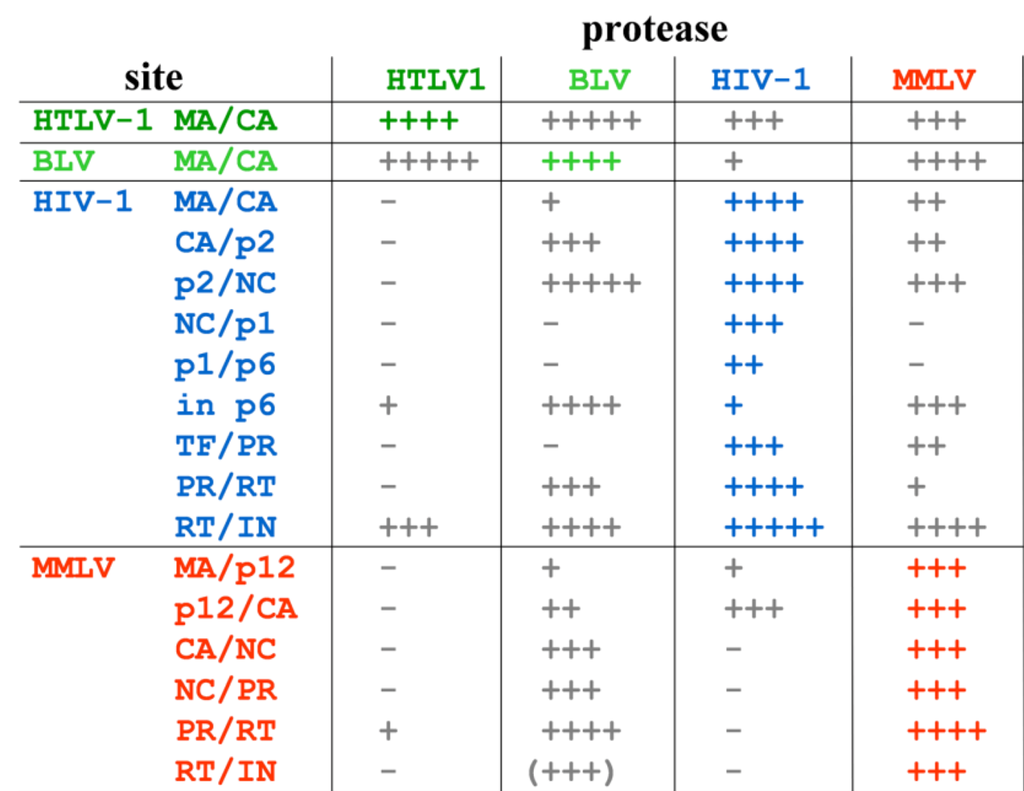
- Luukkonen, B.G.; Tan, W.; Fenyö, E.M.; Schwartz, S. Analysis of cross reactivity of retrovirus proteases using a vaccinia virus-T7 RNA polymerase-based expression system. J. Gen. Virol. 1995, 76, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.J.; Bur, D.; Wlodawer, A.; Gustchina, A.; Payne, S.L.; Dunn, B.M.; Kay, J. Expression, characterisation and mutagenesis of the aspartic proteinase from equine infectious anaemia virus. Eur. J. Biochem. 1996, 241, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Weber, I.T.; Tözsér, J.; Wu, J.; Friedman, D.; Oroszlan S. Molecular model of equine infectious anemia virus proteinase and kinetic measurements for peptide substrates with single amino acid substitutions. Biochemistry 1993, 32, 3354–3362. [Google Scholar] [CrossRef] [PubMed]
- Tözsér, J.; Friedman, D.; Weber, I.T.; Bláha, I.; Oroszlan, S. Studies on the substrate specificity of the proteinase of equine infectious anemia virus using oligopeptide substrates. Biochemistry 1993, 32, 3347–3353. [Google Scholar] [CrossRef] [PubMed]
- Wlodawer, A.; Gustchina, A.; Reshetnikova, L.; Lubkowski, J.; Zdanov, A.; Hui, K.Y.; Angleton, E.L.; Farmerie, W.G.; Goodenow, M.M.; Bhatt, D.; et al. Structure of an inhibitor complex of the proteinase from feline immunodeficiency virus. Nat. Struct. Biol. 1995, 2, 480–488. [Google Scholar] [CrossRef]
- Lin, Y.C.; Beck, Z.; Lee, T.; Le, V.D.; Morris, G.M.; Olson, A.J.; Wong, C.H.; Elder, J.H. Alteration of substrate and inhibitor specificity of feline immunodeficiency virus protease. J. Virol. 2000, 74, 4710–4720. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beck, Z.Q.; Lin, Y.C.; Elder, J.H. Molecular basis for the relative substrate specificity of human immunodeficiency virus type 1 and feline immunodeficiency virus proteases. J. Virol. 2001, 75, 9458–9469. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Beck, Z.; Morris, G.M.; Olson, A.J.; Elder, J.H. Structural basis for distinctions between substrate and inhibitor specificities for feline immunodeficiency virus and human immunodeficiency virus proteases. J. Virol. 2003, 77, 6589–6600. [Google Scholar] [CrossRef] [PubMed]
- Schnölzer, M.; Rackwitz, H.R.; Gustchina, A.; Laco, G.S.; Wlodawer, A.; Elder, J.H.; Kent, S.B. Comparative properties of feline immunodeficiency virus (FIV) and human immunodeficiency virus type 1 (HIV-1) proteinases prepared by total chemical synthesis. Virology 1996, 224, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Bagossi, P.; Sperka, T.; Fehér, A.; Kádas, J.; Zahuczky, G.; Miklóssy, G.; Boross, P.; Tözsér, J. Amino acid preferences for a critical substrate binding subsite of retroviral proteases in type 1 cleavage sites. J. Virol. 2005, 79, 4213–4218. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Jaskólski, M.; Rao, J.K.; Leis, J.; Wlodawer, A. Crystal structure of a retroviral protease proves relationship to aspartic protease family. Nature 1989, 337, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Konvalinka, J.; Horejsí, M.; Andreánsky, M.; Novek, P.; Pichová, I.; Bláha, I.; Fábry, M.; Sedlácek, J.; Foundling, S.; Strop, P. An engineered retroviral proteinase from myeloblastosis associated virus acquires pH dependence and substrate specificity of the HIV-1 proteinase. EMBO J. 1992, 11, 1141–1144. [Google Scholar] [PubMed]
- Grinde, B.; Cameron, C.E.; Leis, J.; Weber, I.T.; Wlodawer, A.; Burstein, H.; Bizub, D.; Skalka, A.M. Mutations that alter the activity of the Rous sarcoma virus protease. J. Biol. Chem. 1992, 267, 9481–9490. [Google Scholar] [PubMed]
- Grinde, B.; Cameron, C.E.; Leis, J.; Weber, I.T.; Wlodawer, A.; Burstein, H.; Skalka AM. Analysis of substrate interactions of the Rous sarcoma virus wild type and mutant proteases and human immunodeficiency virus-1 protease using a set of systematically altered peptide substrates. J. Biol. Chem. 1992, 267, 9491–9498. [Google Scholar] [PubMed]
- Cameron, C.E.; Grinde, B.; Jacques, P.; Jentoft, J.; Leis, J.; Wlodawer, A.; Weber, I.T. Comparison of the substrate-binding pockets of the Rous sarcoma virus and human immunodeficiency virus type 1 proteases. J. Biol. Chem. 1993, 268, 11711–11720. [Google Scholar] [PubMed]
- Cameron, C.E.; Ridky, T.W.; Shulenin, S.; Leis, J.; Weber, I.T.; Copeland, T.; Wlodawer, A.; Burstein, H.; Bizub-Bender, D.; Skalka, A.M. Mutational analysis of the substrate binding pockets of the Rous sarcoma virus and human immunodeficiency virus-1 proteases. J. Biol. Chem. 1994, 269, 11170–11177. [Google Scholar] [PubMed]
- Strop, P.; Konvalinka, J.; Stys, D.; Pavlickova, L.; Blaha, I.; Velek, J.; Travnicek, M.; Kostka, V.; Sedlacek, J. Specificity studies on retroviral proteinase from myeloblastosis-associated virus. Biochemistry 1991, 30, 3437–3443. [Google Scholar] [CrossRef] [PubMed]
- Tözsér, J.; Bagossi, P.; Weber, I.T; Copeland, T.D.; Oroszlan, S. Comparative studies on the substrate specificity of avian myeloblastosis virus proteinase and lentiviral proteinases. J. Biol. Chem. 1996, 271, 6781–6788. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Arias, L.; Young, M.; Oroszlan, S. Purification and characterization of the mouse mammary tumor virus protease expressed in Escherichia coli. J. Biol. Chem. 1992, 267, 24134–24139. [Google Scholar] [PubMed]
- Hrusková-Heidingsfeldová, O.; Andreansky, M.; Fábry, M.; Bláha, I.; Strop, P.; Hunter, E. Cloning, bacterial expression, and characterization of the Mason-Pfizer monkey virus proteinase. J. Biol. Chem. 1995, 270, 15053–15058. [Google Scholar] [CrossRef] [PubMed]
- Rumlová, M.; Ruml, T.; Pohl, J.; Pichová, I. Specific in vitro cleavage of Mason-Pfizer monkey virus capsid protein: evidence for a potential role of retroviral protease in early stages of infection. Virology 2003, 310, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Tözsér, J.; Shulenin, S.; Kádas, J.; Boross, P.; Bagossi, P.; Copeland, T.D.; Nair, B.C.; Sarngadharan, M.G.; Oroszlan, S. Human immunodeficiency virus type 1 capsid protein is a substrate of the retroviral proteinase while integrase is resistant toward proteolysis. Virology 2003, 310, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaka, Y.; Katoh, I.; Copeland, T.D.; Oroszlan, S. Murine leukemia virus protease is encoded by the gag-pol gene and is synthesized through suppression of an amber termination codon. Proc. Natl. Acad. Sci. USA 1985, 82, 1618–1622. [Google Scholar] [CrossRef]
- Menendez-Arias, L.; Tözsér, J.; Oroszlan, S. Moloney murine leukemia virus retropepsin, 2ndBarrett, A.J., Rawlings, N.D., Woessner, J.F., Eds.; Elsevier: London, UK, 2004; pp. 176–178. [Google Scholar]
- Menendez-Arias, L.; Gotte, D.; Oroszlan, S. Moloney murine leukemia virus protease: bacterial expression and characterization of the purified enzyme. Virology 1993, 196, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Fehér, A.; Boross, P.; Sperka, T.; Miklóssy, G.; Kádas, J.; Bagossi, P.; Oroszlan, S.; Weber, I.T.; Tözsér, J. Characterization of the murine leukemia virus protease and its comparison with the human immunodeficiency virus type 1 protease. J. Gen. Virol. 2006, 87, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Arias, L.; Weber, I.T.; Soss, J.; Harrison, R.W.; Gotte, D.; Oroszlan, S. Kinetic and modeling studies of subsites S4-S3' of Moloney murine leukemia virus protease. J. Biol. Chem. 1994, 269, 16795–16801. [Google Scholar] [PubMed]
- Cannon, K.; Qin, L.; Schumann, G.; Boeke, J.D. Moloney murine leukemia virus protease expressed in bacteria is enzymatically active. Arch. Virol. 1998, 143, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.M.; Harrod, R.; Franchini, G. Molecular biology and pathogenesis of the human T-cell leukaemia/lymphotropic virus Type-1 (HTLV-1). Int. J. Exp. Pathol. 2001, 82, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Hrusková-Heidingsfeldová, O.; Bláha, I.; Urban, J.; Strop, P.; Pichová, I. Substrates and inhibitors of human T-cell leukemia virus type 1 (HTLV-1) proteinase. Leukemia 1997, 11, 45–46. [Google Scholar] [PubMed]
- Sperka, T.; Miklóssy, G.; Tie, Y.; Bagossi, P.; Zahuczky, G.; Boross, P.; Matúz, K.; Harrison, R.W.; Weber, I.T.; Tözsér, J. Bovine leukemia virus protease: comparison with human T-lymphotropic virus and human immunodeficiency virus proteases. J. Gen. Virol. 2007, 88, 2052–2063. [Google Scholar] [CrossRef] [PubMed]
- Kádas, J.; Weber, I.T.; Bagossi, P.; Miklóssy, G.; Boross, P.; Oroszlan, S.; Tözsér, J. Narrow substrate specificity and sensitivity toward ligand-binding site mutations of human T-cell Leukemia virus type 1 protease. J. Biol. Chem. 2004, 279, 27148–27157. [Google Scholar] [CrossRef] [PubMed]
- Zahuczky, G.; Boross, P.; Bagossi, P.; Emri, G.; Copeland, T.D.; Oroszlan, S.; Louis, J.M.; Tözsér, J. Cloning of the bovine leukemia virus proteinase in Escherichia coli and comparison of its specificity to that of human T-cell leukemia virus proteinase. Biochim. Biophys. Acta. 2000, 1478, 1–8. [Google Scholar] [PubMed]
- Flügel, R.M.; Pfrepper, K.I. Proteolytic processing of foamy virus Gag and Pol proteins. Curr. Top. Microbiol. Immunol. 2003, 277, 63–88. [Google Scholar] [PubMed]
- Khan, A.S. Simian foamy virus infection in humans: prevalence and management. Expert Rev. Anti Infect. Ther. 2009, 7, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Pfrepper, K.I.; Flügel, R.M. Molecular characterization of proteolytic processing of the Gag proteins of human spumaretrovirus. Methods Mol. Biol. 2005, 304, 435–444. [Google Scholar] [PubMed]
- Roy, J.; Linial, M.L. Role of the foamy virus Pol cleavage site in viral replication. J. Virol. 2007, 81, 4956–4962. [Google Scholar] [CrossRef] [PubMed]
- Konvalinka, J.; Löchelt, M.; Zentgraf, H.; Flügel, R.M.; Kräusslich, H.G. Active foamy virus proteinase is essential for virus infectivity but not for formation of a Pol polyprotein. J. Virol. 1995, 69, 7264–7268. [Google Scholar] [PubMed]
- Fenyöfalvi, G.; Bagossi, P.; Copeland, T.D.; Oroszlan, S.; Boross, P.; Tözsér, J. Expression and characterization of human foamy virus proteinase. FEBS Lett. 1999, 462, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Eizert, H.; Bander, P.; Bagossi, P.; Sperka, T.; Miklóssy, G.; Boross, P.; Weber, I.T.; Tözsér, J. Amino acid preferences of retroviral proteases for amino-terminal positions in a type 1 cleavage site. J. Virol. 2008, 82, 10111–10117. [Google Scholar] [CrossRef] [PubMed]
- Fodor, S.K.; Vogt, V.M. Characterization of the protease of a fish retrovirus, walleye dermal sarcoma virus. J. Virol. 2002, 76, 4341–4349. [Google Scholar] [CrossRef] [PubMed]
- Holzschu, D.L.; Martineau, D.; Fodor, S.K.; Vogt, V.M.; Bowser, P.R.; Casey, J.W. Nucleotide sequence and protein analysis of a complex piscine retrovirus, walleye dermal sarcoma virus. J. Virol. 1995, 69, 5320–5331. [Google Scholar] [PubMed]
- Konvalinka, J.; Heuser, A.M.; Hruskova-Heidingsfeldova, O.; Vogt, V.M.; Sedlacek, J.; Strop, P.; Kräusslich, H.G. Proteolytic processing of particle-associated retroviral polyproteins by homologous and heterologous viral proteinases. Eur. J. Biochem. 1995, 228, 191–188. [Google Scholar] [CrossRef] [PubMed]
- Snásel, J.; Shoeman, R.; Horejsí, M.; Hrusková-Heidingsfeldová, O.; Sedlácek, J.; Ruml, T.; Pichová, I. Cleavage of vimentin by different retroviral proteases Arch. Biochem. Biophys. 2000, 377, 241–245. [Google Scholar] [CrossRef]
- Alvarez, E.; Castelló, A.; Menéndez-Arias, L.; Carrasco, L. HIV protease cleaves poly(A)-binding protein. Biochem. J. 2006, 396, 219–226. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.