Linking Pollution and Viral Risk: Detection of Dioxins and Coronaviruses in Cats and Dogs
Abstract
1. Introduction
2. Materials and Methods
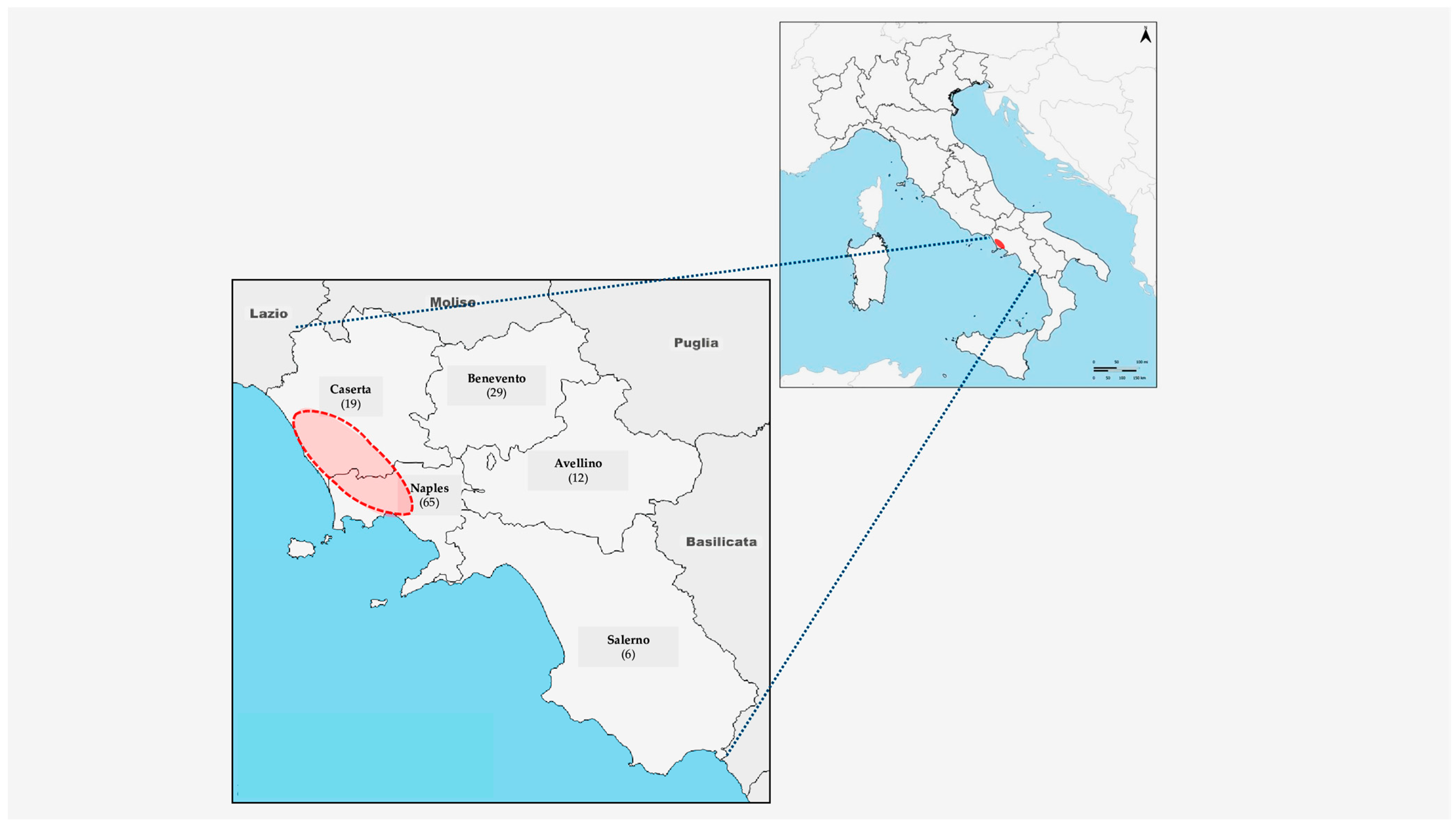
2.1. Sample Collection
2.2. Virological Analysis
2.2.1. Viral Nucleic Acids Extraction
2.2.2. Real-Time PCR for the Detection of Feline Coronavirus
2.2.3. Real-Time PCR for the Detection of Canine Coronavirus
2.2.4. Molecular Typing of CCoV
2.2.5. Digital Droplet PCR (ddPCR)
2.2.6. Virus Isolation
2.3. Chemical Analysis
2.3.1. PCDD/F and DL-PCB Analysis
2.3.2. NDL-PCB Analysis
2.3.3. Instrumental Analysis
2.4. Statistical Analysis
3. Results
3.1. Positivity for Feline Coronavirus (FCoV)
3.2. Positivity for Canine Coronavirus (CCoV)
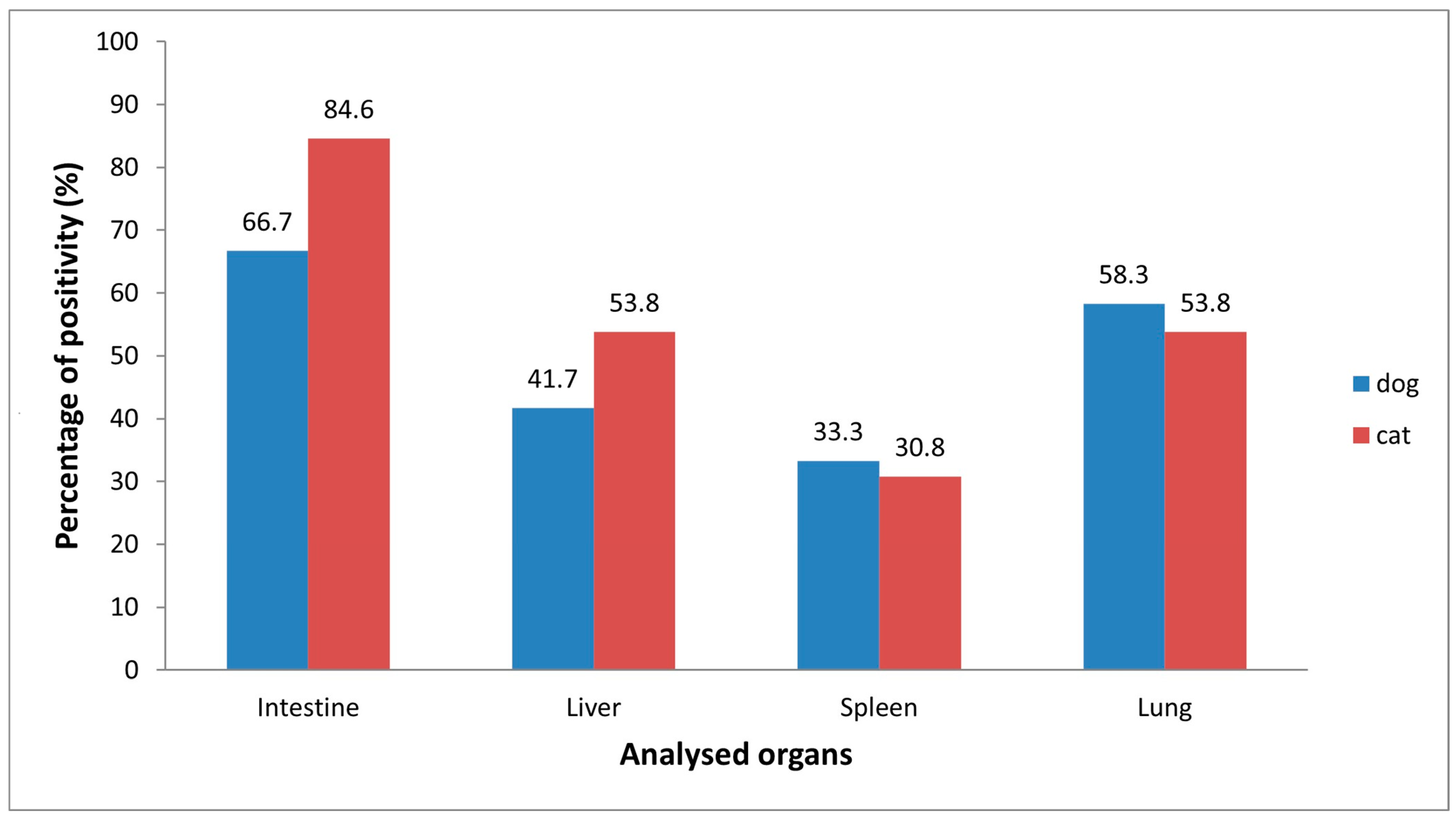
3.3. Viral Isolation by Cell Culture
3.4. Presence of Contaminants in Cats and Dogs Infected/Not Infected by Coronaviruses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Egaña-Marcos, E.; Goñi-Balentziaga, O.; Azkona, G. A Study on the Attachment to Pets Among Owners of Cats and Dogs Using the Lexington Attachment to Pets Scale (LAPS) in the Basque Country. Animals 2025, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Backer, L.C.; Grindem, C.B.; Corbett, W.T.; Cullins, L.; Hunter, J.L. Pet Dogs as Sentinels for Environmental Contamination. Proc. Sci. Total Environ. 2001, 274, 161–169. [Google Scholar] [CrossRef]
- Bischoff, K.; Priest, H.; Mount-Long, A. Animals as Sentinels for Human Lead Exposure: A Case Report. J. Med. Toxicol. 2010, 6, 185–189. [Google Scholar] [CrossRef][Green Version]
- Serpe, F.P.; Fiorito, F.; Esposito, M.; Ferrari, A.; Fracassi, F.; Miniero, R.; Pietra, M.; Roncada, P.; Brambilla, G. Polychlorobiphenyl Levels in the Serum of Cats from Residential Flats in Italy: Role of the Indoor Environment. J. Environ. Sci. Health A Toxic Hazard Subst. Environ. Eng. 2018, 53, 777–785. [Google Scholar] [CrossRef]
- Stern, A.; Andino, R. Viral Evolution. In Viral Pathogenesis; Elsevier: Amsterdam, The Netherlands, 2016; pp. 233–240. [Google Scholar]
- Fiorito, F.; Iovane, V.; Marullo, A.; Costagliola, A.; Granato, G.E.; De Martino, L. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Influences Bovine Herpesvirus 1 Replication through Upregulation of SIRT3 and Cytoskeletal Reorganization. Vet. Res. Commun. 2017, 41, 299–306. [Google Scholar] [CrossRef]
- Eskenazi, B.; Warner, M.; Brambilla, P.; Signorini, S.; Ames, J.; Mocarelli, P. The Seveso Accident: A Look at 40 years of Health Research and Beyond. Environ. Int. 2018, 121, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Bock, K.W. From TCDD-Mediated Toxicity to Searches of Physiologic AHR Functions. Biochem. Pharmacol. 2018, 155, 419–424. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). National Summary Reports on Pesticide Residue Analysis Performed in 2018. EFSA Support. Publ. 2020, 17, 1814E. [Google Scholar] [CrossRef]
- Domingo, J.L. Human Health Risks of Dioxins for Populations Living near Modern Municipal Solid Waste Incinerators. Rev. Environ. Health 2002, 17, 135–148. [Google Scholar] [CrossRef]
- Marquès, M.; Domingo, J.L. Concentrations of PCDD/Fs in Human Blood: A Review of Data from the Current Decade. Int. J. Environ. Res. Public Health 2019, 16, 3566. [Google Scholar] [CrossRef]
- Nadal, M.; Mari, M.; Schuhmacher, M.; Domingo, J.L. Monitoring Dioxins and Furans in Plasma of Individuals Living near a Hazardous Waste Incinerator: Temporal Trend after 20 Years. Environ. Res. 2019, 173, 207–211. [Google Scholar] [CrossRef]
- Domingo, J.L.; Marquès, M.; Mari, M.; Schuhmacher, M. Adverse Health Effects for Populations Living near Waste Incinerators with Special Attention to Hazardous Waste Incinerators. A Review of the Scientific Literature. Environ. Res. 2020, 187, 109631. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Nadal, M.; Rovira, J. Regulatory Compliance of PCDD/F Emissions by a Municipal Solid Waste Incinerator. A Case Study in Sant Adrià de Besòs, Catalonia, Spain. J. Environ. Sci. Health Part A 2024, 59, 273–279. [Google Scholar] [CrossRef]
- Nadal, M.; Marquès, M.; Mari, M.; Rovira, J.; Domingo, J.L. Trends of Polychlorinated Compounds in the Surroundings of a Municipal Solid Waste Incinerator in Mataró (Catalonia, Spain). Assessing Health Risks. Toxics 2020, 8, 111. [Google Scholar] [CrossRef]
- García, F.; Barbería, E.; Torralba, P.; Landin, I.; Laguna, C.; Marquès, M.; Nadal, M.; Domingo, J.L. Decreasing Temporal Trends of Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans in Adipose Tissue from Residents near a Hazardous Waste Incinerator. Sci. Total Environ. 2021, 751, 141844. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Biswas, N.; Alam, M.N. Implications of Xenobiotic-Response Element(s) and Aryl Hydrocarbon Receptor in Health and Diseases. Hum. Cell 2023, 36, 1638–1655. [Google Scholar] [CrossRef] [PubMed]
- Kanan, S.; Samara, F.; Dronjak, L.; Mahasneh, A.; Moyet, M.; Obeideen, K.; Gopal, V. Recent Advances on Dioxin and Furan (Dibenzofuran) Based Pollutants from Analytical, Environmental, and Health Perspectives. Chemosphere 2025, 372, 144120. [Google Scholar] [CrossRef]
- Esposito, M.; Serpe, F.P.; Neugebauer, F.; Cavallo, S.; Gallo, P.; Colarusso, G.; Baldi, L.; Iovane, G.; Serpe, L. Contamination Levels and Congener Distribution of PCDDs, PCDFs and Dioxin-like PCBs in Buffalo’s Milk from Caserta Province (Italy). Chemosphere 2010, 79, 341–348. [Google Scholar] [CrossRef]
- Ssebugere, P.; Sillanpää, M.; Matovu, H.; Mubiru, E. Human and Environmental Exposure to PCDD/Fs and Dioxin-like PCBs in Africa: A Review. Chemosphere 2019, 223, 483–493. [Google Scholar] [CrossRef]
- Alberti, P. The ‘Land of Fires’: Epidemiological Research and Public Health Policy during the Waste Crisis in Campania, Italy. Heliyon 2022, 8, e12331. [Google Scholar] [CrossRef]
- Lambiase, S.; Fiorito, F.; Serpe, F.P.; Trifuoggi, M.; Gallo, P.; Esposito, M. Bioaccumulation of PCDD/Fs and PCBs in Free-Range Hens: Congener Fingerprints and Biotransfer Factors. Chemosphere 2022, 309, 136602. [Google Scholar] [CrossRef]
- Lambiase, S.; Fiorito, F.; Trifuoggi, M.; Gallo, P.; Esposito, M. Levels of PCDD/Fs, PCBs, Metals and Rare Earth Elements in Eggs and Vegetables from Areas with Different Environmental Contamination Impacts in the Campania Region (Southern Italy). Environ. Sci. Pollut. Res. 2024, 31, 55695–55707. [Google Scholar] [CrossRef]
- González, N.; Marquès, M.; Nadal, M.; Domingo, J.L. Meat Consumption: Which Are the Current Global Risks? A Review of Recent (2010–2020) Evidences. Food Res. Int. 2020, 137, 109341. [Google Scholar] [CrossRef]
- Coelho, N.R.; Pimpão, A.B.; Correia, M.J.; Rodrigues, T.C.; Monteiro, E.C.; Morello, J.; Pereira, S.A. Pharmacological Blockage of the AHR-CYP1A1 Axis: A Call for in Vivo Evidence. J. Mol. Med. 2022, 100, 215–243. [Google Scholar] [CrossRef] [PubMed]
- International Agency International Agency for Research on Cancer; World Health Organization. List of Classifications—IARC Monographs on the Identification of Carcinogenic Hazards to Humans; World Health Organization: Geneva, Switzerland, 2025. [Google Scholar]
- Fiorito, F.; Santamaria, R.; Irace, C.; De Martino, L.; Iovane, G. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin and the Viral Infection. Environ. Res. 2017, 153, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; Yang, X.; Bam, M.; Nagarkatti, M.; Nagarkatti, P. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Induces Multigenerational Alterations in the Expression of MicroRNA in the Thymus through Epigenetic Modifications. PNAS Nexus 2023, 2, pgac290. [Google Scholar] [CrossRef]
- Sabuz Vidal, O.; Deepika, D.; Schuhmacher, M.; Kumar, V. EDC-Induced Mechanisms of Immunotoxicity: A Systematic Review. Crit. Rev. Toxicol. 2021, 51, 634–652. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, Y.; Xiao, X.; Liu, A.; Wang, S.; Preston, R.J.S.; Zaytseva, Y.Y.; He, G.; Xiao, W.; Hennig, B.; et al. Inflammation and Cardiometabolic Diseases Induced by Persistent Organic Pollutants and Nutritional Interventions: Effects of Multi-Organ Interactions. Environ. Pollut. 2023, 339, 122756. [Google Scholar] [CrossRef]
- House, R.V.; Lauer, L.D.; Murray, M.J.; Thomas, P.T.; Ehrlich, J.P.; Burleson, G.R.; Dean, J.H. Examination of Immune Parameters and Host Resistance Mechanisms in B6C3F1 Mice Following Adult Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J. Toxicol. Environ. Health 1990, 31, 203–215. [Google Scholar] [CrossRef]
- Burleson, G.R.; Lebrec, H.; Yang, Y.G.; Ibanes, J.D.; Pennington, K.N.; Birnbaum, L.S. Effect of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) on Influenza Virus Host Resistance in Mice. Fundam. Appl. Toxicol. 1996, 29, 40–47. [Google Scholar] [CrossRef]
- Bohn, A.A.; Harrod, K.S.; Teske, S.; Lawrence, B.P. Increased Mortality Associated with TCDD Exposure in Mice Infected with Influenza A Virus Is Not Due to Severity of Lung Injury or Alterations in Clara Cell Protein Content. Chem. Biol. Interact. 2005, 155, 181–190. [Google Scholar] [CrossRef]
- Pokrovsky, A.G.; Cherykh, A.I.; Yastrebova, O.N.; Tsyrlov, I.B. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin as a Possible Activator of HIV Infection. Biochem. Biophys. Res. Commun. 1991, 179, 46–51. [Google Scholar] [CrossRef]
- Gollapudi, S.; Kim, C.H.; Patel, A.; Sindhu, R.; Gupta, S. Dioxin Activates Human Immunodeficiency Virus-1 Expression in Chronically Infected Promonocytic U1 Cells by Enhancing NF-ΚB Activity and Production of Tumor Necrosis Factor-α. Biochem. Biophys. Res. Commun. 1996, 226, 889–894. [Google Scholar] [CrossRef]
- Murayama, T.; Inoue, M.; Nomura, T.; Mori, S.; Eizuru, Y. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Is a Possible Activator of Human Cytomegalovirus Replication in a Human Fibroblast Cell Line. Biochem. Biophys. Res. Commun. 2002, 296, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, F.; Marfè, G.; De Blasio, E.; Granato, G.E.; Tafani, M.; De Martino, L.; Montagnaro, S.; Florio, S.; Pagnini, U. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Regulates Bovine Herpesvirus Type 1 Induced Apoptosis by Modulating Bcl-2 Family Members. Apoptosis 2008, 13, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, F.; Pagnini, U.; De Martino, L.; Montagnaro, S.; Cia, R.; Florio, S.; Pacilio, M.; Fucito, A.; Rossi, A.; Iovane, G.; et al. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Increases Bovine Herpesvirus Type-1 (BHV-1) Replication in Madin-Darby Bovine Kidney (MDBK) Cells in Vitro. J. Cell. Biochem. 2008, 103, 221–233. [Google Scholar] [CrossRef]
- Fiorito, F.; Marfè, G.; Granato, G.E.; Ciarcia, R.; De Blasio, E.; Tafani, M.; Florio, S.; De Martino, L.; Muzi, G.; Pagnini, U.; et al. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Modifies Expression and Nuclear/Cytosolic Localization of Bovine Herpesvirus 1 Immediate-Early Protein (BICP0) during Infection. J. Cell. Biochem. 2010, 111, 333–342. [Google Scholar] [CrossRef]
- Grunewald, M.E.; Shaban, M.G.; Mackin, S.R.; Fehr, A.R.; Perlman, S. Murine Coronavirus Infection Activates the Aryl Hydrocarbon Receptor in an Indoleamine 2,3-Dioxygenase-Independent Manner, Contributing to Cytokine Modulation and Proviral TCDD-Inducible-PARP Expression. J. Virol. 2020, 94, e01743-19. [Google Scholar] [CrossRef]
- Del Sorbo, L.; Cerracchio, C.; Serra, F.; Canzanella, S.; Giugliano, R.; Lambiase, S.; Aránguiz, N.P.; Esposito, M.; Amoroso, M.G.; Fusco, G.; et al. Canine Coronavirus Infection Is Intensified by 2,3,7,8-Tetrachlorodibenzo-p-Dioxin. Arch. Toxicol. 2025, 99, 2211–2223. [Google Scholar] [CrossRef] [PubMed]
- Kenney, S.P.; Wang, Q.; Vlasova, A.; Jung, K.; Saif, L. Naturally Occurring Animal Coronaviruses as Models for Studying Highly Pathogenic Human Coronaviral Disease. Vet. Pathol. 2021, 58, 438–452. [Google Scholar] [CrossRef]
- Kane, Y.; Wong, G.; Gao, G.F. Animal Models, Zoonotic Reservoirs, and Cross-Species Transmission of Emerging Human-Infecting Coronaviruses. Annu. Rev. Anim. Biosci. 2023, 11, 1–31. [Google Scholar] [CrossRef]
- Haake, C.; Cook, S.; Pusterla, N.; Murphy, B. Coronavirus Infections in Companion Animals: Virology, Epidemiology, Clinical and Pathologic Features. Viruses 2020, 12, 1023. [Google Scholar] [CrossRef]
- Le Poder, S. Feline and Canine Coronaviruses: Common Genetic and Pathobiological Features. Adv. Virol. 2011, 2011, 609465. [Google Scholar] [CrossRef]
- Stout, A.E.; André, N.M.; Whittaker, G.R. Feline Coronavirus and Feline Infectious Peritonitis in Nondomestic Felid Species. J. Zoo Wildl. Med. 2021, 52, 14–27. [Google Scholar] [CrossRef]
- Pratelli, A.; Tempesta, M.; Elia, G.; Martella, V.; Decaro, N.; Buonavoglia, C. The Knotty Biology of Canine Coronavirus: A Worrying Model of Coronaviruses’ Danger. Res. Vet. Sci. 2022, 144, 190–195. [Google Scholar] [CrossRef]
- Buonavoglia, C.; Decaro, N.; Martella, V.; Elia, G.; Campolo, M.; Desario, C.; Castagnaro, M.; Tempesta, M. Canine Coronavirus Highly Pathogenic for Dogs. Emerg. Infect. Dis. 2006, 12, 492–494. [Google Scholar] [CrossRef]
- Decaro, N.; Martella, V.; Elia, G.; Campolo, M.; Desario, C.; Cirone, F.; Tempesta, M.; Buonavoglia, C. Molecular Characterisation of the Virulent Canine Coronavirus CB/05 Strain. Virus Res. 2007, 125, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Mari, V.; Campolo, M.; Lorusso, A.; Camero, M.; Elia, G.; Martella, V.; Cordioli, P.; Enjuanes, L.; Buonavoglia, C. Recombinant Canine Coronaviruses Related to Transmissible Gastroenteritis Virus of Swine Are Circulating in Dogs. J. Virol. 2009, 83, 1532–1537. [Google Scholar] [CrossRef]
- Attipa, C.; Gunn-Moore, D.; Mazeri, S.; Epaminondas, D.; Lyraki, M.; Hardas, A.; Loukaidou, S.; Gentil, M. Concerning Feline Infectious Peritonitis Outbreak in Cyprus. Vet. Rec. 2023, 192, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Warr, A.; Attipa, C.; Gunn-Moore, D.; Tait-Burkard, C. FCoV-23 Causing FIP in a Cat Imported to the UK from Cyprus. Vet. Rec. 2023, 193, 414–415. [Google Scholar] [CrossRef] [PubMed]
- Lednicky, J.A.; Tagliamonte, M.S.; White, S.K.; Elbadry, M.A.; Alam, M.M.; Stephenson, C.J.; Bonny, T.S.; Loeb, J.C.; Telisma, T.; Chavannes, S.; et al. Emergence of Porcine Delta-Coronavirus Pathogenic Infections among Children in Haiti through Independent Zoonoses and Convergent Evolution. medRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, A.N.; Toh, T.H.; Lee, J.S.Y.; Poovorawan, Y.; Davis, P.; Azevedo, M.S.P.; Lednicky, J.A.; Saif, L.J.; Gray, G.C. Animal Alphacoronaviruses Found in Human Patients with Acute Respiratory Illness in Different Countries. Emerg. Microbes. Infect. 2022, 11, 699–702. [Google Scholar] [CrossRef]
- Pizzolante, A.; Nicodemo, F.; Pierri, A.; Ferro, A.; Pierri, B.; Buonerba, C.; Albanese, S.; Basso, B.; Cerino, P. Development of a Municipality Index of Environmental Pressure in Campania, Italy. Future Sci. OA 2021, 7, FSO720. [Google Scholar] [CrossRef]
- Amoroso, M.G.; Serra, F.; Esposito, C.; D’Alessio, N.; Ferrara, G.; Cioffi, B.; Anzalone, A.; Pagnini, U.; De Carlo, E.; Fusco, G.; et al. Prevalence of Infection with Porcine Circovirus Types 2 and 3 in the Wild Boar Population in the Campania Region (Southern Italy). Animals 2021, 11, 3215. [Google Scholar] [CrossRef]
- Dye, C.; Helps, C.R.; Siddell, S.G. Evaluation of Real-Time RT-PCR for the Quantification of FCoV Shedding in the Faeces of Domestic Cats. J. Feline Med. Surg. 2008, 10, 167–174. [Google Scholar] [CrossRef]
- Decaro, N.; Mari, V.; Elia, G.; Addie, D.D.; Camero, M.; Lucente, M.S.; Martella, V.; Buonavoglia, C. Recombinant Canine Coronaviruses in Dogs, Europe. Emerg. Infect. Dis. 2010, 16, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Vestri, A.; Marchesano, V.; Rippa, M.; Sagnelli, D.; Picazio, G.; Fusco, G.; Han, J.; Zhou, J.; Petti, L. The Label-Free Detection and Identification of SARS-CoV-2 Using Surface-Enhanced Raman Spectroscopy and Principal Component Analysis. Biosensors 2023, 13, 1014. [Google Scholar] [CrossRef]
- Amoroso, M.G.; Serra, F.; Miletti, G.; Cardillo, L.; de Martinis, C.; Marati, L.; Alfano, F.; Ferrara, G.; Pagnini, U.; De Carlo, E.; et al. A Retrospective Study of Viral Molecular Prevalences in Cats in Southern Italy (Campania Region). Viruses 2022, 14, 2583. [Google Scholar] [CrossRef]
- Sharif, S.; Arshad, S.S.; Hair-Bejo, M.; Omar, A.R.; Zeenathul, N.A.; Hafidz, M.A. Prevalence of Feline Coronavirus in Two Cat Populations in Malaysia. J. Feline Med. Surg. 2009, 11, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Mira, F.; Schirò, G.; Giudice, E.; Purpari, G.; Origgi, F.; Vicari, D.; Di Pietro, S.; Antoci, F.; Gucciardi, F.; Geraci, F.; et al. Viral Pathogens in Domestic Cats in Southern Italy: A Retrospective Analysis in Sicily, 2020–2022. Comp. Immunol. Microbiol. Infect. Dis. 2024, 111, 102209. [Google Scholar] [CrossRef]
- Dong, B.; Zhang, X.; Zhong, X.; Hu, W.; Lin, Z.; Zhang, S.; Deng, H.; Lin, W. Prevalence of Natural Feline Coronavirus Infection in Domestic Cats in Fujian, China. Virol. J. 2024, 21, 2. [Google Scholar] [CrossRef]
- Klein-Richers, U.; Hartmann, K.; Hofmann-Lehmann, R.; Unterer, S.; Bergmann, M.; Rieger, A.; Leutenegger, C.; Pantchev, N.; Balzer, J.; Felten, S. Prevalence of Feline Coronavirus Shedding in German Catteries and Associated Risk Factors. Viruses 2020, 12, 1000. [Google Scholar] [CrossRef]
- Vogel, L.; Van Der Lubben, M.; Te Lintelo, E.G.; Bekker, C.P.J.; Geerts, T.; Schuijff, L.S.; Grinwis, G.C.M.; Egberink, H.F.; Rottier, P.J.M. Pathogenic Characteristics of Persistent Feline Enteric Coronavirus Infection in Cats. Vet. Res. 2010, 41, 71. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.; LaVoy, A.; Evans, S.; Vilander, A.; Webb, C.; Graham, B.; Musselman, E.; LeCureux, J.; Van De Woude, S.; Dean, G.A. Mucosal Immune Response to Feline Enteric Coronavirus Infection. Viruses 2019, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-T.; Chen, P.-Y.; Lo, P.-Y.; Chen, H.-W.; Lin, C.-H. Detection of Feline Coronavirus in Bronchoalveolar Lavage Fluid from Cats with Atypical Lower Airway and Lung Disease: Suspicion of Virus-Associated Pneumonia or Pneumonitis. Animals 2024, 14, 1219. [Google Scholar] [CrossRef]
- Kipar, A.; Meli, M.L.; Baptiste, K.E.; Bowker, L.J.; Lutz, H. Sites of Feline Coronavirus Persistence in Healthy Cats. J. Gen. Virol. 2010, 91, 1698–1707. [Google Scholar] [CrossRef]
- Tasker, S. Diagnosis of Feline Infectious Peritonitis: Update on Evidence Supporting Available Tests. J. Feline Med. Surg. 2018, 20, 228–243. [Google Scholar] [CrossRef]
- Decaro, N.; Buonavoglia, C. Canine Coronavirus: Not Only an Enteric Pathogen. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 1121–1132. [Google Scholar] [CrossRef]
- Pratelli, A.; Martella, V.; Elia, G.; Tempesta, M.; Guarda, F.; Capucchio, M.T.; Carmichael, L.E.; Buonavoglia, C. Severe enteric disease in an animal shelter associated with dual infections by canine adenovirus type 1 and canine coronavirus. J. Vet. Med. B Infect. Dis. Vet. Public Health 2001, 48, 385–392. [Google Scholar] [CrossRef]
- Amer, A.; Siti Suri, A.; Abdul Rahman, O.; Faruku, B.; Saeed, S.; Azmi, T.I. Isolation and molecular characterization of type I and type II feline coronavirus in Malaysia. Virol. J. 2012, 9, 278. [Google Scholar] [CrossRef] [PubMed]
- Tekes, G.; Thiel, H.J. Feline Coronaviruses: Pathogenesis of Feline Infectious Peritonitis. Adv. Virus Res. 2016, 96, 193–218. [Google Scholar]
- Wang, G.; Hu, G.; Liang, R.; Shi, J.; Qiu, X.; Yang, Y.; Jiao, Z.; Chen, Y.; Shen, Z.; Li, M.; et al. Establishment of Full-Length cDNA Clones and an Efficient Oral Infection Model for Feline Coronavirus in Cats. J. Virol. 2021, 95, e0074521. [Google Scholar] [CrossRef]
- Odigie, A.E.; Capozza, P.; Tempesta, M.; Decaro, N.; Pratelli, A. Epidemiological Investigation of Enteric Canine Coronaviruses in Domestic Dogs: A Systematic Review and Meta-Analysis. Res. Vet. Sci. 2024, 174, 105289. [Google Scholar] [CrossRef] [PubMed]
- Mira, F.; Schirò, G.; Lanave, G.; Chiaramonte, G.; Canuti, M.; Giudice, E.; Capozza, P.; Randazzo, V.; Antoci, F.; Raele, D.A.; et al. Molecular Screening and Characterization of Canine Coronavirus Types I and II Strains from Domestic Dogs in Southern Italy, 2019–2021. Transbound. Emerg. Dis. 2024, 2024, 7272785. [Google Scholar] [CrossRef]
- Cardillo, L.; Piegari, G.; Iovane, V.; Viscardi, M.; Alfano, F.; Cerrone, A.; Pagnini, U.; Montagnaro, S.; Galiero, G.; Pisanelli, G.; et al. Lifestyle as Risk Factor for Infectious Causes of Death in Young Dogs: A Retrospective Study in Southern Italy (2015–2017). Vet. Med. Int. 2020, 2020, 6207297. [Google Scholar] [CrossRef] [PubMed]
- Moutinho Costa, E.; Xavier de Castro, T.; de Oliveira Bottino, F.; Nasser Cubel Garcia, R. de C. Molecular Characterization of Canine Coronavirus Strains Circulating in Brazil. Vet. Microbiol. 2014, 168, 8–15. [Google Scholar] [CrossRef]
- Alfano, F.; Fusco, G.; Mari, V.; Occhiogrosso, L.; Miletti, G.; Brunetti, R.; Galiero, G.; Desario, C.; Cirilli, M.; Decaro, N. Circulation of Pantropic Canine Coronavirus in Autochthonous and Imported Dogs, Italy. Transbound. Emerg. Dis. 2020, 67, 1991–1999. [Google Scholar] [CrossRef]
- Zobba, R.; Visco, S.; Sotgiu, F.; Pinna Parpaglia, M.L.; Pittau, M.; Alberti, A. Molecular Survey of Parvovirus, Astrovirus, Coronavirus, and Calicivirus in Symptomatic Dogs. Vet. Res. Commun. 2021, 45, 31–40. [Google Scholar] [CrossRef] [PubMed]
- He, H.J.; Zhang, W.; Liang, J.; Lu, M.; Wang, R.; Li, G.; He, J.W.; Chen, J.; Xing, G.; Chen, Y. Etiology and Genetic Evolution of Canine Coronavirus Circulating in Five Provinces of China, during 2018–2019. Microb. Pathog. 2020, 145, 104209. [Google Scholar] [CrossRef]
- Decaro, N.; Buonavoglia, C. An Update on Canine Coronaviruses: Viral Evolution and Pathobiology. Vet. Microbiol. 2008, 132, 221–234. [Google Scholar] [CrossRef]
- Zappulli, V.; Ferro, S.; Bonsembiante, F.; Brocca, G.; Calore, A.; Cavicchioli, L.; Centelleghe, C.; Corazzola, G.; De Vreese, S.; Gelain, M.E.; et al. Pathology of Coronavirus Infections: A Review of Lesions in Animals in the One-Health Perspective. Animals 2020, 10, 2377. [Google Scholar] [CrossRef]
- Castro, T.X.; De Cubel Garcia, R.C.N.; Gonçalves, L.R.S.; Costa, E.M.; Marcello, G.C.G.; Labarthe, N.V.; Mendes-De-Almeida, F. Clinical, Hematological, and Biochemical Findings in Puppies with Coronavirus and Parvovirus Enteritis. Can. Vet. J. 2013, 54, 885–888. [Google Scholar]
- Evermann, J.F.; Abbott, J.R.; Han, S. Canine Coronavirus-Associated Puppy Mortality without Evidence of Concurrent Canine Parvovirus Infection. J. Vet. Diagn. Investig. 2005, 17, 610–614. [Google Scholar] [CrossRef]
- Ntafis, V.; Mari, V.; Danika, S.; Fragkiadaki, E.; Buonavoglia, C. An Outbreak of Canine Coronavirus in Puppies in a Greek Kennel. J. Vet. Diagn. Investig. 2010, 22, 320–323. [Google Scholar] [CrossRef]
- Zicola, A.; Jolly, S.; Mathijs, E.; Ziant, D.; Decaro, N.; Mari, V.; Thiry, E. Fatal Outbreaks in Dogs Associated with Pantropic Canine Coronavirus in France and Belgium. J. Small Anim. Pract. 2012, 53, 297–300. [Google Scholar] [CrossRef]
- Rowland, H.; Holding, E.; Falces, P.M.; Wissink-Argilaga, N.; Stidworthy, M.F.; Denk, D.; Weir, W.; Krumrie, S.; Dunbar, D.; Hopper, J.S. Canine Coronavirus Subtype 2a Associated with Outbreaks of Fatal Diarrhoea in Bush Dog (Speothos venaticus) Groups. Schweiz. Arch. Tierheilkd. 2021, 2021, 661–671. [Google Scholar] [CrossRef]
- Zappulli, V.; Caliari, D.; Cavicchioli, L.; Tinelli, A.; Castagnaro, M. Systemic Fatal Type II Coronavirus Infection in a Dog: Pathological Findings and Immunohistochemistry. Res. Vet. Sci. 2008, 84, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Healey, A.M.; Fenner, K.N.; O’Dell, C.T.; Lawrence, B.P. Aryl Hydrocarbon Receptor Activation Alters Immune Cell Populations in the Lung and Bone Marrow during Coronavirus Infection. Am. J. Physiol. Lung Cell. Mol. Physiol. 2024, 326, L313–L329. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Rovira, J. Effects of Air Pollutants on the Transmission and Severity of Respiratory Viral Infections. Environ. Res. 2020, 187, 109650. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Marquès, M.; Rovira, J. Influence of Airborne Transmission of SARS-CoV-2 on COVID-19 Pandemic. A Review. Environ. Res. 2020, 188, 109861. [Google Scholar] [CrossRef]
- Pozzer, A.; Dominici, F.; Haines, A.; Witt, C.; Münzel, T.; Lelieveld, J. Regional and Global Contributions of Air Pollution to Risk of Death from COVID-19. Cardiovasc. Res. 2020, 116, 2247–2253. [Google Scholar] [CrossRef]
- Marquès, M.; Domingo, J.L. Positive Association between Outdoor Air Pollution and the Incidence and Severity of COVID-19. A Review of the Recent Scientific Evidences. Environ. Res. 2022, 203, 111930. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Voit-Bak, K.; Schmidt, D.; Morawietz, H.; Bornstein, A.B.; Balanzew, W.; Julius, U.; Rodionov, R.N.; Biener, A.M.; Wang, J.; et al. Is There a Role for Environmental and Metabolic Factors Predisposing to Severe COVID-19? Horm. Metab. Res. 2020, 52, 540–546. [Google Scholar] [CrossRef]
- Barnett-Itzhaki, Z.; Levi, A. Effects of Chronic Exposure to Ambient Air Pollutants on COVID-19 Morbidity and Mortality—A Lesson from OECD Countries. Environ. Res. 2021, 195, 110723. [Google Scholar] [CrossRef] [PubMed]
- Dales, R.; Blanco-Vidal, C.; Romero-Meza, R.; Schoen, S.; Lukina, A.; Cakmak, S. The Association between Air Pollution and COVID-19 Related Mortality in Santiago, Chile: A Daily Time Series Analysis. Environ. Res. 2021, 198, 111284. [Google Scholar] [CrossRef]
- De Angelis, E.; Renzetti, S.; Volta, M.; Donato, F.; Calza, S.; Placidi, D.; Lucchini, R.G.; Rota, M. COVID-19 Incidence and Mortality in Lombardy, Italy: An Ecological Study on the Role of Air Pollution, Meteorological Factors, Demographic and Socioeconomic Variables. Environ. Res. 2021, 195, 110777. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Rothman, K.J.; Cocchio, S.; Narne, E.; Mantoan, D.; Saia, M.; Goffi, A.; Ferrari, F.; Maffeis, G.; Orsini, N.; et al. Associations between Mortality from COVID-19 in Two Italian Regions and Outdoor Air Pollution as Assessed through Tropospheric Nitrogen Dioxide. Sci. Total Environ. 2021, 760, 143355. [Google Scholar] [CrossRef] [PubMed]
- Barreira-Silva, P.; Lian, Y.; Kaufmann, S.H.E.; Moura-Alves, P. The role of the AHR in host-pathogen interactions. Nat. Rev. Immunol. 2024, 25, 178–194. [Google Scholar] [CrossRef]
- Hu, J.; Ding, Y.; Liu, W.; Liu, S. When AHR signaling pathways meet viral infections. Cell Commun. Signal 2023, 21, 42. [Google Scholar] [CrossRef]
- Giovannoni, F.; Li, Z.; Remes-Lenicov, F.; Dávola, M.E.; Elizalde, M.; Paletta, A.; Ashkar, A.A.; Mossman, K.L.; Dugour, A.V.; Figueroa, J.M.; et al. AHR signaling is induced by infection with coronaviruses. Nat. Commun. 2021, 12, 5148. [Google Scholar] [CrossRef]
- Cerracchio, C.; Iovane, V.; Salvatore, M.M.; Amoroso, M.G.; Dakroub, H.; DellaGreca, M.; Nicoletti, R.; Andolfi, A.; Fiorito, F. Effectiveness of the Fungal Metabolite 3-O-Methylfunicone towards Canine Coronavirus in a Canine Fibrosarcoma Cell Line (A72). Antibiotics 2022, 11, 1594. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Du, T.; Wang, J.; Tang, C.; Lei, L.; Yu, W.; Ma, Y.; Huang, P.; Peng, X. Aryl hydrocarbon receptor is a proviral host factor and a candidate pan-SARS-CoV-2 therapeutic target. Sci. Adv. 2023, 9, eadf0211. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Lee, W.S.; Chan, W.O.Y.; He, W.; Mah, M.G.; Yong, C.L.; Ooi, Y.S. Betacoronaviruses SARS-CoV-2 and HCoV-OC43 infections in IGROV-1 cell line require aryl hydrocarbon receptor. Emerg. Microbes Infect. 2023, 12, 2256416. [Google Scholar] [CrossRef] [PubMed]
| FCoV-Infected Cats (n = 13) | Anatomopathological Lesions Observed | Organs Positive for FCoV in Real Time RT-PCR |
|---|---|---|
| (1) | Enteritis and Pneumonia | Intestine, lungs, spleen, liver |
| (2) | Intestine, lungs, spleen, liver | |
| (3) | Intestine, lungs, liver | |
| (4) | Intestine | |
| (5) | Pneumonia | Intestine |
| (6) | Lungs | |
| (7) | Enteritis | Intestine, lungs, spleen, liver |
| (8) | Intestine, lungs, spleen, liver | |
| (9) | Intestine, liver | |
| (10) | Intestine, lungs | |
| (11) | Intestine | |
| (12) | Intestine | |
| (13) | Liver |
| Subgroups | n | Number of Infected Animals | p Value | |
|---|---|---|---|---|
| Sex | Female | 40 | 9 (22.5%) CI 12.1–37.7 | p = 0.22 |
| Male | 40 | 4 (10.0%) CI 3.3–23.6 | ||
| Age 1 | Junior/Adult | 62 | 11 (17.7%) CI 10.3–30.0 | p = 0.72 |
| Kitten | 18 | 2 (11.1%) CI 1.8–34.0 | ||
| Breed | Mixed-Breed | 2 | 0 (0.0%) CI 0.0–70.9 | p = 1.00 |
| Purebred * | 78 | 13 (16.6%) CI 9.8–26.6 | ||
| Lifestyle | Stray | 71 | 11 (15.4%) CI 8.7–25.8 | p = 0.63 |
| Owned | 9 | 2 (22.2%) CI 5.3–55.7 | ||
| Province | Naples | 52 | 10 (19.2%) CI 10.6–32.0 | p = 0.76 |
| Benevento | 17 | 2 (11.7%) CI 2.0–35.5 | ||
| Caserta | 9 | 1 (11.1%) CI 0.0–45.6 | ||
| Salerno | 2 | 0 (0.0%) CI 0.0–70.9 | ||
| Total | 80 | 13 (16.2%) CI 9.6–25.9 | ||
| CCoV-Infected Dogs (n = 12) | Anatomopathological Lesions Observed | Organs Positive for CCoV In Real Time RT-PCR |
|---|---|---|
| (1) | Enteritis and Pneumonia | Intestine, lungs, spleen, liver |
| (2) | Intestine, lungs, spleen, liver | |
| (3) | Intestine, liver | |
| (4) | Lungs | |
| (5) | Pneumonia | Intestine, lungs, liver |
| (6) | Intestine, lungs | |
| (7) | Intestine, lungs | |
| (8) | Lungs | |
| (9) | Spleen | |
| (10) | Enteritis | Intestine, liver |
| (11) | Intestine | |
| (12) | Spleen |
| Animal Characteristics | n | Number of Infected Animals | p Value | |
|---|---|---|---|---|
| Sex | Female | 21 | 7 (33.3%) CI 17.0–54.7 | p = 0.19 |
| Male | 30 | 5 (16.6%) CI 6.8–34.0 | ||
| Age 1 | Junior/Adult | 39 | 5 (12.8%) CI 5.4–28.4 | p < 0.01 |
| Young | 12 | 7 (58.3%) CI 3.8–80.7 | ||
| Breed | Mixed-Breed | 28 | 4 (14.2%) CI 5.0–32.1 | p = 0.10 |
| Purebred * | 23 | 8 (34.7%) CI 18.7–55.2 | ||
| Lifestyle | Stray | 26 | 9 (34.6%) CI 19.3–53.8 | p = 0.09 |
| Owned | 25 | 3 (12.0%) CI 3.3–30.7 | ||
| Province | Naples | 13 | 4 (30.7%) CI 12.3–57.9 | p = 0.12 |
| Benevento | 12 | 1 (8.3%) CI 0.0–37.5 | ||
| Caserta | 10 | 5 (50.0%) CI 23.6–76.3 | ||
| Salerno | 4 | 1 (25.0%) CI 3.4–71.0 | ||
| Avellino | 12 | 1 (8.3%) CI 0.0–37.5 | ||
| Total | 51 | 12 (23.5%) CI 13.8–36.9 | ||
| Liver | Dog (P) | Dog (CN) | Cat (P) | Cat (CN) |
|---|---|---|---|---|
| Congener | n = 9 Mean ± SD | n = 5 Mean ± SD | n = 10 Mean ± SD | n = 8 Mean ± SD |
| PCDD | ||||
| 2,3,7,8-TCDD | 0.0066 ± 0.0073 | ND | 0.0088 ± 0.0093 | 0.0058 ± 0.0029 |
| 12378-PeCDD | ND | ND | ND | 0.038 ± 0.019 |
| 123478-HxCDD | 0.061 ± 0.027 | 0.065 ± 0.028 | 0.055 ± 0.017 | 0.064 ± 0.026 |
| 123678-HxCDD | 0.021 ± 0.0092 | 0.042 ± 0.029 | 0.016 ± 0.011 | 0.025 ± 0.020 |
| 123789-HxCDD | ND | ND | 0.017 ± 0.019 | 0.014 ± 0.0075 |
| 1234678-HpCDD | 0.44 ± 0.31 | 0.95 ± 1.6 | 0.25 ± 0.22 | 0.42 ± 0.61 |
| OCDD | 3.2 ± 4.3 | 2.5 ± 3.9 | 1.6 ± 1.4 | 1.7 ± 1.9 |
| PCDF | ||||
| 2378-TCDF | 0.015 ± 0.0056 | 0.0068 ± 0.0041 | 0.020 ± 0.0086 | 0.024 ± 0.0086 |
| 12378-PeCDF | ND | ND | ND | 0.016 ± 0.0095 |
| 23478-PeCDF | 0.088 ± 0.057 | 0.19 ± 0.14 | 0.095 ± 0.052 | 0.12 ± 0.071 |
| 123478-HxCDF | 0.083 ± 0.058 | 0.19 ± 0.15 | 0.051 ± 0.052 | 0.062 ± 0.027 |
| 123678-HxCDF | 0.050 ± 0.034 | 0.13 ± 0.071 | 0.042 ± 0.046 | 0.049 ± 0.024 |
| 123789-HxCDF | 0.0078 ± 0.0035 | ND | 0.024 ± 0.039 | 0.014 ± 0.0073 |
| 234678-HxCDF | 0.036 ± 0.024 | 0.053 ± 0.026 | 0.084 ± 0.096 | 0.066 ± 0.051 |
| 1234678-HpCDF | 0.12 ± 0.053 | 0.079 ± 0.055 | 0.20 ± 0.22 | 0.20 ± 0.070 |
| 1234789-HpCDF | 0.016 ± 0.0060 | 0.021 ± 0.020 | 0.029 ± 0.050 | 0.022 ± 0.0098 |
| OCDF | 0.13 ± 0.074 | 0.080 ± 0.034 | 1.5 ± 4.0 | 0.15 ± 0.055 |
| DL-PCB | n = 8 | n = 4 | n = 9 | n = 6 |
| PCB 77 | 1.3 ± 0.30 | 1.2 ± 0.51 | 1.5 ± 0.56 | 2.4 ± 1.7 |
| PCB 81 | 0.13 ± 0.088 | 0.077 ± 0.041 | 0.12 ± 0.039 | 0.19 ± 0.12 |
| PCB 126 | 0.73 ± 1.4 | 0.083 ± 0.066 | 0.25 ± 0.31 | 0.16 ± 0.074 |
| PCB 169 | 0.14 ± 0.15 | 0.13 ± 0.064 | 0.19 ± 0.24 | 0.078 ± 0.023 |
| PCB 105 | 7.4 ± 5.2 | 4.6 ± 1.1 | 50.2 ± 47.1 | 24.6 ± 13.1 |
| PCB 114 | 0.84 ± 064 | 0.61 ± 0.23 | 5.9 ± 4.6 | 2.9 ± 0.99 |
| PCB 118 | 23.2 ± 14.7 | 13.5 ± 3.8 | 142.5 ± 121.3 | 68.1 ± 27.6 |
| PCB 123 | 0.50 ± 0.42 | 0.25 ± 0.084 | 0.79 ± 0.78 | 0.55 ± 0.38 |
| PCB 156 | 4.1 ± 3.5 | 2.7 ± 0.33 | 45.8 ± 39.5 | 28.4 ± 33.9 |
| PCB 157 | 1.5 ± 1.7 | 0.80 ± 0.88 | 10.7 ± 9.2 | 4.5 ± 2.8 |
| PCB 167 | 2.6 ± 3.3 | 0.39 ± 0.12 | 11.6 ± 10.8 | 4.2 ± 2.5 |
| PCB 189 | 1.4 ± 2.5 | 1.2 ± 0.55 | 7.9 ± 7.7 | 5.8 ± 9.5 |
| ∑17PCDD/F | 0.070 ± 0.032 | 0.12 ± 0.081 | 0.076 ± 0.046 | 0.089 ± 0.057 |
| ∑12DL-PCB | 0.078 ± 0.14 | 0.012 ± 0.0090 | 0.039 ± 0.038 | 0.022 ± 0.010 |
| ∑PCDD/F + DL-PCB | 0.15 ± 0.17 | 0.15 ± 0.077 | 0.12 ± 0.081 | 0.11 ± 0.048 |
| Liver | Dog (P) | Dog (CN) | Cat (P) | Cat (CN) |
|---|---|---|---|---|
| Congener | n = 8 Mean ± SD | n = 5 Mean ± SD | n = 8 Mean ± SD | n = 6 Mean ± SD |
| NDL-PCB | ||||
| PCB 101 | 0.62 ± 0.60 | 0.58 ± 0.54 | 0.23 ± 0.26 | 0.42 ± 0.46 |
| PCB 28 | 0.90 ± 1.1 | 0.61 ± 0.82 | 0.34 ± 0.51 | 0.81 ± 0.77 |
| PCB 153 | 0.13 ± 0.12 | 0.68 ± 1.3 | 0.45 ± 0.47 | 0.37 ± 0.27 |
| PCB 52 | 1.1 ± 1.3 | 0.92 ± 1.4 | 0.37 ± 0.61 | 0.84 ± 1.0 |
| PCB 138 | 0.083 ± 0.086 | 0.50 ± 0.93 | 0.31 ± 0.36 | 0.29 ± 0.30 |
| PCB 180 | 0.056 ± 0.046 | 0.32 ± 0.60 | 0.22 ± 0.22 | 0.12 ± 0.071 |
| ∑6NDL-PCB | 2.9 ± 3.2 | 3.6 ± 3.7 | 1.9 ± 1.9 | 2.9 ± 2.6 |
| Dog (P) | Dog (CN) | Cat (P) | Cat (CN) |
|---|---|---|---|
| n = 9 | n = 5 | n = 10 | n = 8 |
| 0.0060 | ND < 0.0004 | 0.029 | ND < 0.0008 |
| 0.0070 | 0.0033 | 0.0025 | 0.0090 |
| ND < 0.0007 | ND < 0.0002 | ND < 0.0003 | ND < 0.002 |
| 0.0041 | ND < 0.0003 | 0.0094 | 0.0033 |
| 0.00073 | ND < 0.001 | 0.0055 | ND < 0.0004 |
| 0.0023 | - | ND < 0.0003 | ND < 0.0009 |
| 0.0036 | - | 0.0058 | ND < 0.002 |
| 0.023 | - | 0.0038 | 0.0052 |
| ND < 0.0004 | - | 0.0055 | - |
| - | - | ND < 0.0004 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, F.; Canzanella, S.; Brandi, S.; Picazio, G.; Pugliese, A.M.; Del Sorbo, L.; Miletti, G.; Ragosta, E.; Sannino, E.; Fiorito, F.; et al. Linking Pollution and Viral Risk: Detection of Dioxins and Coronaviruses in Cats and Dogs. Viruses 2025, 17, 1271. https://doi.org/10.3390/v17091271
Serra F, Canzanella S, Brandi S, Picazio G, Pugliese AM, Del Sorbo L, Miletti G, Ragosta E, Sannino E, Fiorito F, et al. Linking Pollution and Viral Risk: Detection of Dioxins and Coronaviruses in Cats and Dogs. Viruses. 2025; 17(9):1271. https://doi.org/10.3390/v17091271
Chicago/Turabian StyleSerra, Francesco, Silvia Canzanella, Sergio Brandi, Gerardo Picazio, Anna Maria Pugliese, Luca Del Sorbo, Gianluca Miletti, Enza Ragosta, Emanuela Sannino, Filomena Fiorito, and et al. 2025. "Linking Pollution and Viral Risk: Detection of Dioxins and Coronaviruses in Cats and Dogs" Viruses 17, no. 9: 1271. https://doi.org/10.3390/v17091271
APA StyleSerra, F., Canzanella, S., Brandi, S., Picazio, G., Pugliese, A. M., Del Sorbo, L., Miletti, G., Ragosta, E., Sannino, E., Fiorito, F., Esposito, M., De Carlo, E., Fusco, G., & Amoroso, M. G. (2025). Linking Pollution and Viral Risk: Detection of Dioxins and Coronaviruses in Cats and Dogs. Viruses, 17(9), 1271. https://doi.org/10.3390/v17091271







