Abstract
Anelloviruses have a broad mammalian host range, including Torque teno felis virus (FcTTV), a felid-associated member that remains undercharacterized. This is the first comprehensive study of FcTTV in domestic cats in Central Asia. We analyzed blood samples from 206 domestic cats from the large city of Astana, Kazakhstan, collected in 2023–2024. Using nested PCR we identified 63 FcTTV-positive samples (30.6% prevalence), and the sequences were compared to global reference strains. Potential demographic associations (sex and age) were assessed. The study revealed an overall FcTTV prevalence of 30.6%. Infection rates showed no significant sex-related differences: ages varied 4–168 months. ORF1 sequencing revealed multiple FcTTV variants in 27% of samples, with no demographic links. Phylogenetic analysis revealed distinct patterns at both nucleotide and amino acid levels: 3 groups of nucleotide sequences (max divergence 21.68%; intra-cluster 5.15–6.8%), and 3 clusters of amino acid sequences (max divergence 16.81%; intra-cluster 2.82–6.68%). Deletions were found in ORF1 in some variants. Global phylogeny aligned clusters with Asian/European strains (90–98% identity), confirming FcTTV1 affiliation and inter-regional transmission. Our study of FcTTV in Kazakhstan reveals moderate virus prevalence with considerable genetic diversity across viral strains and frequent co-infections with multiple variants.
1. Introduction
The Anelloviridae family is represented by small, non-enveloped viruses, which harbor negative-sense single-stranded DNA genomes of fairly compact size (1.6–3.9 kilobases). Viruses in this family show broad vertebrate host ranges, including humans; nevertheless, most species exhibit marked host specificity. Anelloviruses dominate the human blood virome, comprising over 70% of all viral sequences detected in healthy individuals [1]. This strikingly high prevalence establishes Anelloviridae as the dominant human virome [2]. The archetypal Torque teno virus (TTV) was first discovered in 1997 in a patient with post-transfusion hepatitis of unknown etiology [3]. Since its discovery, TTV taxonomy has undergone multiple revisions and expansions. The advent of high-throughput sequencing (NGS) and metagenomic approaches has particularly accelerated this process [4,5,6]. The Anelloviridae family (ICTV 2024) contains 34 genera and 173 species [7]. The species-level classification is based on the level of nucleotide homology in the ORF1 gene: strains with an identity below 69% are considered to belong to different species [8,9].
Despite the high prevalence of anelloviruses in humans and other mammals, the role of these viruses as causative agents of diseases is not yet clear. During the first decade after the discovery of TTV, a significant number of studies focused on identifying associations with diseases and substantiating TTV as a potential etiological agent [10], including in viral hepatitis [11,12], autoimmune diseases [13], and multiple sclerosis [14]. On the other side, the near-universal prevalence of anelloviruses in humans (approaching 100% in adults) has prompted the ‘beneficial virome’ hypothesis, which postulates that these viruses may be beneficial by stimulating the immune system in early childhood. Consequently, TTV has emerged as a biomarker of the immune status, with a clinical utility in monitoring transplant recipients. The TTV viral load provides an assessment of the immune system state upon organ transplant [15,16].
Although domestic cats are the most prevalent companion animals and live in close contact with humans, data on anellovirus circulation in cats remain limited [17,18,19,20]. Better characterization of these viruses in cats is essential to resolve Anelloviridae diversity, host specificity, and their possible zoonotic significance. Comprehensive FcTTV monitoring helps to evaluate the clinical significance of this virus, including risks to pet owners.
Here we present the first study of FcTTV prevalence and genetic diversity in Kazakhstan’s domestic cats.
2. Materials and Methods
2.1. Samples and Data Collection
This study analyzed whole blood samples collected from 206 domestic cats examined at two veterinary clinics in Astana between September 2023 and December 2024. Demographic data included age (median: 60 months; range: 4–168 months; n = 101) and sex (53.6% female, 46.4% male; n = 166), with most animals being mixed-breed (n = 196) and a subset diagnosed with infectious diseases (n = 16: panleukopenia, n = 10; rhinotracheitis, n = 1; peritonitis, n = 5). Samples were collected in K2EDTA vacuum tubes, stored at +4–8 °C during transport (≤24 h), and processed following ethical approval from the S. Seifullin Kazakh Agrotechnical University (protocol code 1, dated 9 January 2022).
2.2. DNA Isolation
DNA isolation was performed using the method described by Kanai et al. [21], with minor modifications. Blood samples (including clotted and pre-frozen; volume 200 µL) were transferred to sterile 1.5 mL microcentrifuge tubes containing 250 µL of lysis buffer (360 µg/mL of proteinase K, 150 mM NaCl, 50 mM EDTA, 2% SDS). Incubation was carried out at 60 °C for 3 h with periodic stirring on a vortex. After lysis, 150 µL of 6 M NaCl and 600 µL of chloroform-isoamyl alcohol (in a 24:1 ratio) were added to the tubes, then thoroughly mixed. Centrifugation was performed at 1677× g for 5 min for phase separation. The upper phase containing nucleic acids was transferred to a new tube with 800 µL of 90% ethanol for DNA precipitation. The samples were centrifuged at the same parameters, after which the precipitate was washed with 70% ethanol. The resulting precipitate was dried at room temperature and resuspended in 100 µL of TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0).
2.3. PCR Testing
Detection of FcTTV was performed using nested polymerase chain reaction (PCR) with primers described by Veronika Jarošová and co-authors [18]. Primers P1a (5′-AGTAAGTACACTGACGAATGGCTGA-3′) and P2a (5′-CAGTTACCACAGTTCGAGGTCGT-3′) were used for the first round of amplification, and P3a (5′-ACTGGTGACAGGACGCGA-3′) and P4a (5′-TGCGGAGACAAGTTGCTTCC-3′) were used for the second round. PCR amplification was performed in 25 µL reaction mixtures containing 10 pmol of each primer, 10 mM Tris-HCl (pH 8.8 at 25 °C), 50 mM KCl, 0.08% (v/v) Nonidet P40, 2.5 mM MgCl2, 200 µM of each dNTP, 1.5 U Hot Start Taq DNA polymerase (BiolabMix, Novosibirsk, Russia), and either 5 µL of template DNA (first round) or 3 µL of PCR product (second round). Reactions were carried out in a Mastercycler ProS thermal cycler (Eppendorf, Hamburg, Germany) with an initial denaturation at 95 °C for 5 min, followed by 32 cycles of 95 °C for 30 s, 57 °C for 30 s, and 72 °C for 50 s, and a final extension at 72 °C for 5 min. Amplification products were analyzed by electrophoresis on 1.5% agarose gels stained with ethidium bromide and visualized under UV light.
2.4. Sequencing ORF1
Complete ORF1 was amplified using nested PCR using primers P1 (5′-TCGCACGTCCTGTCACCAGT-3′) and P2 (5′-GGAAGCAACTTGTCTCCGCA-3′) for the first round of amplification, and primers P3 (5′-TCAGCCATTCGTCAGTGTACTTACT-3′) and P2 (5′- GGAAGCAACTTGTCTCCGCA-3′) [18]. PCR amplification was performed in 25 µL reactions containing 12.5 µL of 2× BioMaster HS-Taq PCR-Sp (BiolabMix, Novosibirsk, Russia), 1 µL (10 pmol/µL) of each round-specific primer, either 5 µL of template DNA (first round) or 3 µL of PCR product (second round), and nuclease-free water to adjust the final volume. Thermal cycling was conducted on a Mastercycler ProS (Eppendorf, Hamburg, Germany) with an initial denaturation at 95 °C for 5 min, followed by 32 cycles of 95 °C for 30 s, 63 °C (first round) or 61 °C (second round) for 20 s, and 72 °C for 3 min, with a final extension at 72 °C for 15 min. The resulting ~1750 bp amplicons were purified using AMPure XP beads (Beckman Coulter, Beverly, MA, USA) at a 1:1 ratio, followed by library preparation with the Illumina DNA Prep kit (Illumina, San Diego, CA, USA) and sequencing on the MiSeq platform using a 300-cycle reagent kit.
Raw sequence data were first quality-checked using FastQC v0.12.1 (https://github.com/s-andrews/FastQC, accessed on 8 September 2025), followed by read trimming and quality filtering with Seqtk v1.3 (https://github.com/lh3/seqtk, accessed on 8 September 2025) and Sickle v1.33 (https://github.com/najoshi/sickle, accessed on 8 September 2025) to remove adapters and low-quality bases. High-quality reads were assembled de novo using the MEGAHIT v1.2.9 [22] metagenomic data assembler. The assembler was chosen due to its ability to generate long and accurate contigs from short reads, especially those obtained from co-infected samples [23,24]. Subsequently, the obtained contigs were analyzed to identify and retrieve complete FcTTV ORF1 sequences.
2.5. Phylogenetic Analysis
Phylogenetic relationships were inferred by maximum likelihood (ML) under the Hasegawa–Kishino–Yano (HKY) substitution model [25], which was identified as the best-fit model using the “Find Best DNA/Protein Models” function in MEGA X [26]. Bootstrap support values are based on 1000 replicates. Evolutionary rate variation among sites was modeled using a discrete Gamma distribution with 5 categories (+G parameter).
3. Results
3.1. Prevalence and Genetic Diversity of FcTTV in Domestic Cats
FcTTV DNA was detected in 30.6% (63/206) of sampled cats. Among PCR-positive animals with recorded sex (n = 58), the distribution was nearly equal between females (48.3%, 28/58) and males (51.7%, 30/58), with no significant sex-based difference (χ2 = 0.069, p = 0.793). For age-stratified analysis (n = 43), infected cats ranged from 4 to 168 months old (median: 72 months). Concurrent infections were rare, with only three cases identified: two feline infectious peritonitis (FIP) diagnoses and one parvovirus enteritis.
Despite positive results in short-amplicon diagnostic PCR, amplification of the full-length ORF1 failed in 26/63 FcTTV-positive samples (41.3%), possibly due to low target copy number, template DNA degradation, and/or suboptimal long-amplicon PCR efficiency. Full-length ORF1 sequencing was successful in 37/63 FcTTV-positive samples (58.7%), revealing coinfection with two distinct viral variants in 10 samples (27%). No correlations were found between variant coinfection and host age or sex.
Phylogenetic analysis of nucleotide sequences (n = 47) identified three major clusters comprising 35 genetic variants (Figure 1a, Table S1), with maximum inter-cluster divergence of 18.0%. Cluster-specific divergence levels were 5.9% (Cluster 1), 6.4% (Cluster 2), and 4.9% (Cluster 3), with ORF1 lengths of 1311 bp (Clusters 1–2) and 1302 bp (Cluster 3).
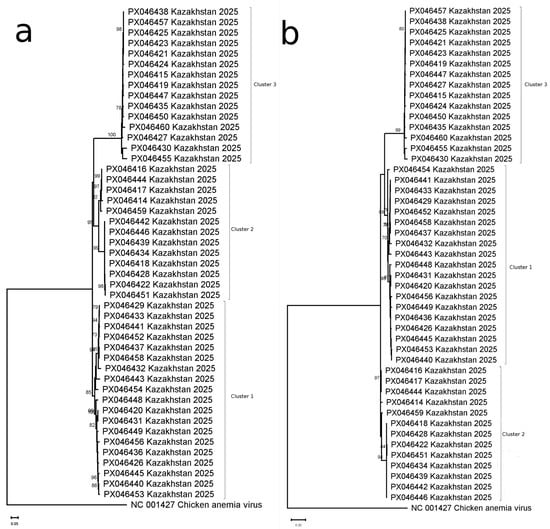
Figure 1.
Phylogenetic analysis of FcTTV variants. (a) Nucleotide-based tree constructed from 47 complete ORF1 sequences (derived from 37 samples, including 10 with confirmed co-infections). (b) Corresponding amino acid-based phylogeny from the same sample set. Both trees demonstrate clustering patterns of viral variants, with co-infected cases marked.
Amino acid analysis confirmed this virus genotypic structure (Figure 1b), resolving 33 variants with maximum divergence of 28.9% (Cluster 1: 8.5%; Cluster 2: 10%; Cluster 3: 6.4%). Notably, Cluster 3 variants lacked three amino acids present in the reference strain from Genbank (AB076003) (deletions were found in positions 44, 275, and 276), which was verified by read mapping on ORF1 assembly.
3.2. FcTTV Isolates in Kazakhstan as Compared to Global Diversity
FcTTV sequences from Astana showed maximum divergence 17.09% from reference AB076003 and thus were classified as genotype 1 (FcTTV1). At the same time, isolates from Kazakhstan show grouping into three clusters with distinct geographic associations: Cluster 1 strains (93.5–98% identity) aligned with AB076003 and variants from China (HM142588) and the Czech Republic (KM229764, KM229765); Cluster 2 (94.1–95.7% identity) included a 2010 Chinese strain (HM142589); and Cluster 3 (91.2–97.8% identity) grouped with six Chinese and one Czech variant. In contrast, French sequences formed a separate branch with lower identity (83–94.5%), suggesting regional divergence alongside historical cross-continental transmission (Figure 2, Table S1).
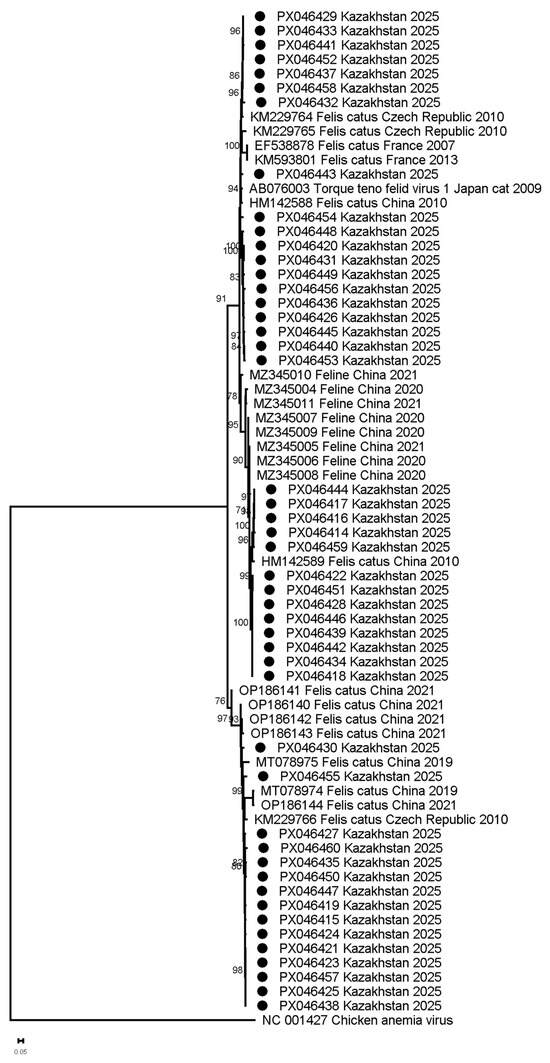
Figure 2.
Maximum-likelihood phylogeny of FcTTV ORF1 nucleotide sequences. (Isolates from Kazakhstan indicated by circles; reference sequences from GenBank are labeled with accession numbers. The tree was constructed using HKY + G model (1000 bootstrap replicates) with scale bar showing substitutions/site. Reference strains include AB076003 (prototype) and representative Asian/European variants.
4. Discussion
The current study is the first of its kind on the prevalence and genetic diversity of TTV among domestic cats in Kazakhstan. Recently, the number of studies on FcTTV in domestic cats has significantly declined due to a lack of targeted research. The scientific focus has shifted toward anellovirus species in other host animals [27,28,29]. However, the topic remains under-researched, as previous studies have been geographically limited to the Czech Republic and Shanghai [17,18]. Our study, which revealed a prevalence of 30.6% and a genetic divergence of up to 21.68% in ORF1, contributes to this area by highlighting regional specificities in Kazakhstan and confirming the need for monitoring for veterinary epidemiology with the expansion of the studied animal species and sampling range.
A study of the carriage of Torque teno felis virus (FcTTV) in the Astana cat population revealed a prevalence of 30.6% (63/206 samples), which is consistent with previously obtained prevalence values among domestic cats in Shanghai (36.67%) and the Czech Republic (33.63%) [17,18]. FcTTV infection rates observed in domestic cats appear moderate when contrasted with reports of near-universal (up to 100%) anellovirus prevalence in certain human and ani-mal populations [30,31]. TTV prevalence exhibits marked interspecies variation, with particularly wide ranges observed in domestic pigs (TTSuV: 17–100% across and within countries) [32,33,34], whereas in domestic dogs the virus is present in 7–38% [35]. However, keeping pets in confined and stressful conditions often results in decreased immunity, which may explain the high rates of anellovirus infection. In wild animals, TTV infection also varies, with wild pigs infected worldwide ranging from 32% to 66% [36], 87% of wild forest mice in the UK and 100% of forest martens are infected with TTV [37,38]. In stool samples from mouse-like rodents from 6 provinces of China, TTV was detected in 29.1% of samples. [39]. The consistent TTV prevalence among domestic cats across three countries (30.6–36.67%) presents intriguing epidemiological stability compared to other species. However, current infection rates may underestimate true prevalence due to: (1) non-standardized detection methods, and (2) high genetic diversity enabling variant escape from conventional PCR assays—a known challenge in anellovirus surveillance [10]. Our diversity estimates also should be interpreted with caution. Targeted PCR can underrepresent phylogenetically distant lineages, co-infections, and rare variants due to primer bias, template integrity requirements, and limitations of long-amplicon PCR. Unbiased virome/metagenomic sequencing is needed to capture the full spectrum of circulating anelloviruses.
The balanced sex distribution among infected cats (females: 48.3%, males: 51.7%; p = 0.793) aligns with established anellovirus epidemiology, where host sex typically shows no association with infection status [40]. Infected cats showed a higher median age (72 months vs. 60 months in the general population), consistent with the chronic infection pattern observed for TTV in humans and other mammals, where prevalence correlates positively with host age [41,42,43].
We detected coinfection with multiple FcTTV1 variants in 27% (10/37) of sequenced samples, revealing substantial mixed infections in Astana’s cat population. This frequency aligns with global anellovirus patterns, where coinfection rates often surpass single-variant infections, facilitating viral diversification through recombination and enhanced replication dynamics [36,44]. Similar evidence of recombination, both within and between different groups of anelloviruses, has been observed in wild cats, supporting a long history of host–virus coevolution [45]. In pigs (TTSuV), coinfections are even more common than infections with a single variant [44], and mixed infections with different genotypes are common in humans [46].
Phylogenetic analysis of ORF1 sequences identified three distinct FcTTV clusters among Astana isolates, exhibiting diversity but consistent with a genetic variation within a single species [9]. Phylogenetic analysis incorporating multiple reference strains from GenBank revealed distinct geographic patterns: two clusters contained both Asian (Kazakhstan, China) and European (Czech Republic) variants (90–98% identity), while the third cluster comprised exclusively Asian strains (>95% identity to Chinese isolates, 2010–2023). This phylogeographic structure suggests inter-regional transmission possibly linked to international cat trade, and localized evolution of the Asian cluster [47].
5. Conclusions
This first comprehensive study of Torque teno felis virus (FcTTV) in domestic cats in Central Eurasia reveals high prevalence (30.6%), significant genetic diversity (up to 21.68% ORF1 divergence), and frequent co-infections (27% of sequenced samples), which complements the epidemiological picture of anellovirus infection. Phylogenetic evidence of shared FcTTV genotypes between Asian and European strains suggests global prevalence, supposedly the result of the international trade of cat breeds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v17091265/s1, Table S1: Nucleotide sequence divergence in the ORF1 region between FcTTV1 strains detected in Astana and globally circulating variants; Table S2: Level of divergence in the amino acid sequence of ORF1 among FcTTV strains circulating in Astana.
Author Contributions
Conceptualization, G.Y. and A.S. (Alexandr Shevtsov); methodology, G.Y., B.A. and A.A.; software, A.S. (Alexandr Shevtsov) and A.A.; validation, G.Y., B.A., A.A. and S.A.; formal analysis, A.S. (Alexandr Shevtsov), B.A., A.A. and A.S. (Alexandr Shustov); investigation, G.Y. and S.B. (Symbat Bolysbekkyzy); resources, G.Y.; data curation, A.S. (Alexandr Shevtsov), A.S. (Alexandr Shustov), S.B. (Salima Baduanova) and A.A.; writing—original draft preparation, G.Y., B.A., A.S. (Alexandr Shevtsov) and E.S.; writing—review and editing, A.S. (Alexandr Shustov); visualization, A.S. (Alexandr Shevtsov) and E.S.; supervision, A.S. (Alexandr Shustov); project administration, G.Y.; funding acquisition, G.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan, Grant No. AP19679977 «Study of species diversity and genetic features of viral and bacterial pathogens of infectious diseases of pets in Astana».
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of S. Seifullin Kazakh Agrotechnical University (protocol code 1, dated 9 January 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data from this project is publicly available NCBI, GenBank Ac#: PX046414-PX046460.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Feng, B.; Liu, B.; Cheng, M.; Dong, J.; Hu, Y.; Jin, Q.; Yang, F. An atlas of the blood virome in healthy individuals. Virus Res. 2023, 323, 199004. [Google Scholar] [CrossRef]
- Timmerman, A.L.; Schönert, A.L.; van der Hoek, L. Anelloviruses versus human immunity: How do we control these viruses? FEMS Microbiol. Rev. 2024, 48, fuae005. [Google Scholar] [CrossRef]
- Nishizawa, T.; Okamoto, H.; Konishi, K.; Yoshizawa, H.; Miyakawa, Y.; Mayumi, M. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem. Biophys. Res. Commun. 1997, 241, 92–97. [Google Scholar] [CrossRef]
- Biagini, P. Classification of TTV and related viruses (anelloviruses). TT Viruses Still Elus. Hum. Pathog. 2009, 331, 21–33. [Google Scholar]
- Nokhova, A.R.; Dubovitskiy, N.A.; Derko, A.A.; Khozyainova, A.A.; Kurskaya, O.G.; Shestopalov, A.M.; Sharshov, K.A. Genetic characteristics of anelloviruses detected in individual viromes of children with acute respiratory symptoms using the metagenomic approach. Acta Virol. 2025, 69, 13512. [Google Scholar] [CrossRef]
- Okamoto, H. History of discoveries and pathogenicity of TT viruses. TT Viruses Still Elus. Hum. Pathog. 2009, 331, 1–20. [Google Scholar]
- Simmonds, P.; Adriaenssens, E.M.; Lefkowitz, E.J.; Oksanen, H.M.; Siddell, S.G.; Zerbini, F.M.; Alfenas-Zerbini, P.; Aylward, F.O.; Dempsey, D.M.; Dutilh, B.E. Changes to virus taxonomy and the ICTV Statutes ratified by the International Committee on Taxonomy of Viruses (2024). Arch. Virol. 2024, 169, 236. [Google Scholar] [CrossRef]
- Varsani, A.; Kraberger, S.; Opriessnig, T.; Maggi, F.; Celer, V.; Okamoto, H.; Biagini, P. Anelloviridae taxonomy update 2023. Arch. Virol. 2023, 168, 277. [Google Scholar] [CrossRef] [PubMed]
- Varsani, A.; Opriessnig, T.; Celer, V.; Maggi, F.; Okamoto, H.; Blomström, A.-L.; Cadar, D.; Harrach, B.; Biagini, P.; Kraberger, S. Taxonomic update for mammalian anelloviruses (family Anelloviridae). Arch. Virol. 2021, 166, 2943–2953. [Google Scholar] [CrossRef] [PubMed]
- Brani, P.; Manzoor, H.Z.; Spezia, P.G.; Vigezzi, A.; Ietto, G.; Dalla Gasperina, D.; Minosse, C.; Bosi, A.; Giaroni, C.; Carcano, G. Torque teno virus: Lights and shades. Viruses 2025, 17, 334. [Google Scholar] [CrossRef] [PubMed]
- Hazanudin, S.N.; Othman, Z.; Sekawi, Z.; Kqueen, C.; Rasdi, R. Torque Teno Virus and Hepatitis: A review on correlation. Life Sci. Med. Biomed. 2019, 3, 31. [Google Scholar] [CrossRef]
- Hu, Z.-J.; Lang, Z.-W.; Zhou, Y.-S.; Yan, H.-P.; Huang, D.-Z.; Chen, W.-R.; Luo, Z.-X. Clinicopathological study on TTV infection in hepatitis of unknown etiology. World J. Gastroenterol. 2002, 8, 288. [Google Scholar] [CrossRef]
- Gergely, P., Jr.; Perl, A.; Poór, G. Possible pathogenic nature of the recently discovered TT virus: Does it play a role in autoimmune rheumatic diseases? Autoimmun. Rev. 2006, 6, 5–9. [Google Scholar] [CrossRef]
- Mancuso, R.; Saresella, M.; Hernis, A.; Agostini, S.; Piancone, F.; Caputo, D.; Maggi, F.; Clerici, M. Torque teno virus (TTV) in multiple sclerosis patients with different patterns of disease. J. Med. Virol. 2013, 85, 2176–2183. [Google Scholar] [CrossRef]
- Kelly, E.; Awan, A.; Sweeney, C.; Wildes, D.; De Gascun, C.; Hassan, J.; Riordan, M. Torque Teno Virus Loads as a Marker of Immunosuppression in Pediatric Kidney Transplant Recipients. Pediatr. Transplant. 2024, 28, e14857. [Google Scholar] [CrossRef]
- Redondo, N.; Navarro, D.; Aguado, J.M.; Fernández-Ruiz, M. Viruses, friends, and foes: The case of Torque Teno Virus and the net state of immunosuppression. Transpl. Infect. Dis. 2022, 24, e13778. [Google Scholar] [CrossRef]
- Gao, J.; Liu, C.; Yi, J.; Shi, Y.; Li, H.; Liu, H. Genomic Characteristics of Feline Anelloviruses Isolated from Domestic Cats in Shanghai, China. Vet. Sci. 2023, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Jarošová, V.; Hrazdilová, K.; Filipejová, Z.; Schánilec, P.; Celer, V. Whole genome sequencing and phylogenetic analysis of feline anelloviruses. Infect. Genet. Evol. 2015, 32, 130–134. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Wang, Y.; Liu, Z.; Li, J.; Guo, L.; Yang, S.; Shen, Q.; Zhao, X.; Cui, L. Identification and genomic characterization of a novel species of feline anellovirus. Virol. J. 2016, 13, 146. [Google Scholar] [CrossRef]
- Zhu, C.; Shan, T.; Cui, L.; Luo, X.; Liu, Z.; Tang, S.; Liu, Z.; Yuan, C.; Lan, D.; Zhao, W. Molecular detection and sequence analysis of feline Torque teno virus (TTV) in China. Virus Res. 2011, 156, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Kanai, N.; Fujii, T.; Saito, K.; Tokoyama, T. Rapid and simple method for preparation of genomic DNA from easily obtainable clotted blood. J. Clin. Pathol. 1994, 47, 1043–1044. [Google Scholar] [CrossRef]
- Li, D.; Luo, R.; Liu, C.-M.; Leung, C.-M.; Ting, H.-F.; Sadakane, K.; Yamashita, H.; Lam, T.-W. MEGAHIT v1.0: A fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods 2016, 102, 3–11. [Google Scholar] [CrossRef]
- Molina-Mora, J.A.; Cordero-Laurent, E.; Calderón-Osorno, M.; Chacón-Ramírez, E.; Duarte-Martínez, F. Metagenomic pipeline for identifying co-infections among distinct SARS-CoV-2 variants of concern: Study cases from Alpha to Omicron. Sci. Rep. 2022, 12, 9377. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhang, L.; Zhang, X.; Yun, F.; Chang, Y.; Tuersun, A.; Aisaiti, K.; Ma, Z. Metagenome-assembled viral genomes analysis reveals diversity and infectivity of the RNA virome of gerbillinae species. Viruses 2022, 14, 356. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Kishino, H.; Yano, T.-A. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J. Mol. Evol. 1985, 22, 160–174. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Cavalcante, L.T.; Cosentino, M.A.; D’arc, M.; Moreira, F.R.; Mouta, R.; Augusto, A.M.; Troccoli, F.; Soares, M.A.; Santos, A.F. Characterization of a new anellovirus species infecting an ocelot (Leopardus pardalis) in Brazil. Genet. Mol. Biol. 2023, 46, e20230015. [Google Scholar] [CrossRef] [PubMed]
- Di Profio, F.; Sarchese, V.; Fruci, P.; Aste, G.; Martella, V.; Palombieri, A.; Di Martino, B. Exploring the enteric virome of cats with acute gastroenteritis. Vet. Sci. 2023, 10, 362. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, L.; Tu, Z.; Sun, S.; Sun, Y.; Yi, L.; Tu, C.; He, B. Virome profiling of an amur leopard cat reveals multiple anelloviruses and a bocaparvovirus. Vet. Sci. 2022, 9, 640. [Google Scholar] [CrossRef]
- Al-Qahtani, A.A.; Alabsi, E.S.; AbuOdeh, R.; Thalib, L.; El Zowalaty, M.E.; Nasrallah, G.K. Prevalence of anelloviruses (TTV, TTMDV, and TTMV) in healthy blood donors and in patients infected with HBV or HCV in Qatar. Virol. J. 2016, 13, 208. [Google Scholar] [CrossRef]
- Sabbaghian, M.; Gheitasi, H.; Shekarchi, A.A.; Tavakoli, A.; Poortahmasebi, V. The mysterious anelloviruses: Investigating its role in human diseases. BMC Microbiol. 2024, 24, 40. [Google Scholar] [CrossRef] [PubMed]
- Rammohan, L.; Xue, L.; Wang, C.; Chittick, W.; Ganesan, S.; Ramamoorthy, S. Increased prevalence of torque teno viruses in porcine respiratory disease complex affected pigs. Vet. Microbiol. 2012, 157, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, H.; Yang, X.; Guan, Z.; Zhou, Y. Molecular detection of Torque teno virus in different breeds of swine. Virol. J. 2011, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.-T.; Giménez-Lirola, L.; Huang, Y.-W.; Meng, X.-J.; Halbur, P.G.; Opriessnig, T. The prevalence of Torque teno sus virus (TTSuV) is common and increases with the age of growing pigs in the United States. J. Virol. Methods 2012, 183, 40–44. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Ahn, H.-S.; Han, S.-H.; Go, H.-J.; Kim, D.-H.; Kim, J.-H.; Lee, J.-B.; Park, S.-Y.; Song, C.-S.; Lee, S.-W. Genetic analysis of torque teno canis virus identified in Republic of Korea. Vet. Sci. 2022, 9, 693. [Google Scholar] [CrossRef]
- Li, X.; Tavares, Y.; Carneiro, C.M.; Phillips, C.; Subramaniam, K.; Lednicky, J.; Boughton, R.K.; Pepin, K.M.; Miller, R.S.; VerCauteren, K.C. Whole genome characterization of Torque teno sus virus 1 (TTSuV1) in wild and domestic pigs: Insights into genetic classification, host differentiation, and intra-host variation. Front. Microbiol. 2025, 16, 1585558. [Google Scholar] [CrossRef]
- Nishiyama, S.; Dutia, B.M.; Stewart, J.P.; Meredith, A.L.; Shaw, D.J.; Simmonds, P.; Sharp, C.P. Identification of novel anelloviruses with broad diversity in UK rodents. J. Gen. Virol. 2014, 95, 1544–1553. [Google Scholar] [CrossRef]
- van den Brand, J.M.; van Leeuwen, M.; Schapendonk, C.M.; Simon, J.H.; Haagmans, B.L.; Osterhaus, A.D.; Smits, S.L. Metagenomic analysis of the viral flora of pine marten and European badger feces. J. Virol. 2012, 86, 2360–2365. [Google Scholar] [CrossRef]
- Xiong, Y.-Q.; Mo, Y.; Chen, M.-J.; Cai, W.; He, W.-Q.; Chen, Q. Detection and phylogenetic analysis of torque teno virus (TTV) carried by murine rodents and house shrews in China. Virology 2018, 516, 189–195. [Google Scholar] [CrossRef]
- Venkataraman, T.; Swaminathan, H.; Arze, C.A.; Jacobo, S.M.; Bhattacharyya, A.; David, T.; Nawandar, D.M.; Delagrave, S.; Mani, V.; Yozwiak, N.L. Comprehensive profiling of antibody responses to the human anellome using programmable phage display. Cell Rep. 2022, 41, 111754. [Google Scholar] [CrossRef]
- Focosi, D.; Spezia, P.G.; Macera, L.; Salvadori, S.; Navarro, D.; Lanza, M.; Antonelli, G.; Pistello, M.; Maggi, F. Assessment of prevalence and load of torquetenovirus viraemia in a large cohort of healthy blood donors. Clin. Microbiol. Infect. 2020, 26, 1406–1410. [Google Scholar] [CrossRef]
- Li, X.; Parker, B.M.; Boughton, R.K.; Beasley, J.C.; Smyser, T.J.; Austin, J.D.; Pepin, K.M.; Miller, R.S.; Vercauteren, K.C.; Wisely, S.M. Torque Teno sus virus 1: A potential surrogate pathogen to study pig-transmitted transboundary animal diseases. Viruses 2024, 16, 1397. [Google Scholar] [CrossRef] [PubMed]
- Väisänen, E.; Kuisma, I.; Mäkinen, M.; Ilonen, J.; Veijola, R.; Toppari, J.; Hedman, K.; Söderlund-Venermo, M. Torque teno virus primary infection kinetics in early childhood. Viruses 2022, 14, 1277. [Google Scholar] [CrossRef] [PubMed]
- Blois, S.; Mallus, F.; Liciardi, M.; Pilo, C.; Camboni, T.; Macera, L.; Maggi, F.; Manzin, A. High prevalence of co-infection with multiple Torque teno sus virus species in Italian pig herds. PLoS ONE 2014, 9, e113720. [Google Scholar] [CrossRef] [PubMed]
- Kraberger, S.; Serieys, L.E.; Richet, C.; Fountain-Jones, N.M.; Baele, G.; Bishop, J.M.; Nehring, M.; Ivan, J.S.; Newkirk, E.S.; Squires, J.R. Complex evolutionary history of felid anelloviruses. Virology 2021, 562, 176–189. [Google Scholar] [CrossRef]
- Maggi, F.; Andreoli, E.; Lanini, L.; Fornai, C.; Vatteroni, M.; Pistello, M.; Presciuttini, S.; Bendinelli, M. Relationships between total plasma load of torquetenovirus (TTV) and TTV genogroups carried. J. Clin. Microbiol. 2005, 43, 4807–4810. [Google Scholar] [CrossRef]
- Lipinski, M.J.; Froenicke, L.; Baysac, K.C.; Billings, N.C.; Leutenegger, C.M.; Levy, A.M.; Longeri, M.; Niini, T.; Ozpinar, H.; Slater, M.R. The ascent of cat breeds: Genetic evaluations of breeds and worldwide random-bred populations. Genomics 2008, 91, 12–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).