Elucidating Alterations in Viral and Human Gene Expression Due to Human Papillomavirus Integration by Using Multimodal RNA Sequencing
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Clinical Samples
2.2. HPV Genotyping
2.3. Single-Strand CAGE and Total RNA-Seq
2.4. Mapping, Quantifying, and Visualizing HPV Transcriptomes
2.5. Identification of Viral-Human Chimeric RNA and HPV ISs
2.6. Human Gene Expression Analysis of Total RNA-Seq
2.7. Quantification of CAGE Reads at the HPV ISs
3. Results
3.1. Transcriptome Analysis of HPV-Derived Transcripts Using CAGE and Total RNA-Seq
3.2. Identification of Chimeric RNA
3.3. Transcriptome Upregulation of the Chimeric Sites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BINDS | Basis for Supporting Innovative Drug Discovery and Life Science |
| CAGE | cap analysis of gene expression |
| CIN | Cervical intraepithelial neoplasia |
| HPV | human papillomavirus |
| IGV | Integrative Genomics Viewer |
| IS | Integration Site |
| NCBI | National Center for Biotechnology Information |
| NGS | next-generation sequencing |
| ORF | open reading frame |
| PCR | polymerase chain reaction |
| RNA-seq | RNA sequencing |
| RIN | RNA integrity number |
| TAD | Topologically associating domain |
| TSS | transcription start sites |
References
- Lowy, D.R.; Schiller, J.T. Reducing HPV-associated cancer globally. Cancer Prev. Res. 2012, 5, 18–23. [Google Scholar] [CrossRef]
- Yim, E.-K.; Park, J.-S. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res. Treat. 2005, 37, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Human papillomavirus-associated cancers—United States, 2004–2008. Morb. Mortal. Wkly. Rep. 2012, 61, 258–261. [Google Scholar]
- Doorbar, J. Molecular biology of human papillomavirus infection and cervical cancer. Clin. Sci. 2006, 110, 525–541. [Google Scholar] [CrossRef]
- Münger, K.; Scheffner, M.; Huibregtse, J.M.; Howley, P.M. Interactions of HPV E6 and E7 oncoproteins with tumour suppressor gene products. Cancer Surv. 1992, 12, 197–217. [Google Scholar]
- Jeon, S.; Allen-Hoffmann, B.L.; Lambert, P.F. Integration of human papillomavirus type 16 into the human genome correlates with a selective growth advantage of cells. J. Virol. 1995, 69, 2989–2997. [Google Scholar] [CrossRef]
- McBride, A.A.; Warburton, A. The role of integration in oncogenic progression of HPV-associated cancers. PLOS Pathog. 2017, 13, e1006211. [Google Scholar] [CrossRef]
- Wentzensen, N.; Vinokurova, S.; von Knebel Doeberitz, M. Systematic review of genomic integration sites of human papillomavirus genomes in epithelial dysplasia and invasive cancer of the female lower genital tract. Cancer Res. 2004, 64, 3878–3884. [Google Scholar] [CrossRef]
- Bodelon, C.; Untereiner, M.E.; Machiela, M.J.; Vinokurova, S.; Wentzensen, N. Genomic characterization of viral integration sites in HPV-related cancers. Int. J. Cancer 2016, 139, 2001–2011. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Integrated Genomic and Molecular Characterization of Cervical Cancer. Nature 2017, 543, 378–384. [Google Scholar] [CrossRef]
- Hu, Z.; Zhu, D.; Wang, W.; Li, W.; Jia, W.; Zeng, X.; Ding, W.; Yu, L.; Wang, X.; Wang, L.; et al. Genome-wide profiling of HPV integration in cervical cancer identifies clustered genomic hot spots and a potential microhomology-mediated integration mechanism. Nat Genet. 2015, 47, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Rusan, M.; Li, Y.Y.; Hammerman, P.S. Genomic landscape of human papillomavirus-associated cancers. Clin. Cancer Res. 2015, 21, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Akagi, K.; Li, J.; Broutian, T.R.; Padilla-Nash, H.; Xiao, W.; Jiang, B.; Rocco, J.W.; Teknos, T.N.; Kumar, B.; Wangsa, D.; et al. Genome-wide analysis of HPV integration in human cancers reveals recurrent, focal genomic instability. Genome Res. 2014, 24, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Liu, Y.; Shi, S.; Zhang, R.; Zhang, T.; Xu, Q.; Zhu, P.; Chen, X.; Lu, F. Long-distance interaction of the integrated HPV fragment with MYC gene and 8q24.22 region upregulating the allele-specific MYC expression in HeLa cells. Int. J. Cancer 2017, 141, 540–548. [Google Scholar] [CrossRef]
- Kamal, M.; Lameiras, S.; Deloger, M.; Morel, A.; Vacher, S.; Lecerf, C.; Dupain, C.; Jeannot, E.; Girard, E.; Baulande, S.; et al. Human papilloma virus (HPV) integration signature in Cervical Cancer: Identification of MACROD2 gene as HPV hot spot integration site. Br. J. Cancer 2021, 124, 777–785. [Google Scholar] [CrossRef]
- Shiraki, T.; Kondo, S.; Katayama, S.; Waki, K.; Kasukawa, T.; Kawaji, H.; Kodzius, R.; Watahiki, A.; Nakamura, M.; Arakawa, T.; et al. Cap analysis gene expression for high-throughput analysis of transcriptional starting point and identification of promoter usage. Proc. Natl. Acad. Sci. USA 2003, 100, 15776–15781. [Google Scholar] [CrossRef]
- Andersson, R.; Gebhard, C.; Miguel-Escalada, I.; Hoof, I.; Bornholdt, J.; Boyd, M.; Chen, Y.; Zhao, X.; Schmidl, C.; Suzuki, T.; et al. An atlas of active enhancers across human cell types and tissues. Nature 2014, 507, 455–461. [Google Scholar] [CrossRef]
- Kouno, T.; Moody, J.; Kwon, A.T.-J.; Shibayama, Y.; Kato, S.; Huang, Y.; Böttcher, M.; Motakis, E.; Mendez, M.; Severin, J.; et al. C1 CAGE detects transcription start sites and enhancer activity at single-cell resolution. Nat. Commun. 2019, 10, 360. [Google Scholar] [CrossRef]
- Taguchi, A.; Nagasaka, K.; Plessy, C.; Nakamura, H.; Kawata, Y.; Kato, S.; Hashimoto, K.; Nagamatsu, T.; Oda, K.; Kukimoto, I.; et al. Use of Cap Analysis Gene Expression to detect human papillomavirus promoter activity patterns at different disease stages. Sci. Rep. 2020, 10, 17991. [Google Scholar] [CrossRef]
- Brant, A.C.; Menezes, A.N.; Felix, S.P.; de Almeida, L.M.; Sammeth, M.; Moreira, M.A.M. Characterization of HPV integration, viral gene expression and E6E7 alternative transcripts by RNA-Seq: A descriptive study in invasive cervical cancer. Genomics 2019, 111, 1853–1861. [Google Scholar] [CrossRef]
- Nishiwaki, M.; Yamamoto, T.; Tone, S.; Murai, T.; Ohkawara, T.; Matsunami, T.; Koizumi, M.; Takagi, Y.; Yamaguchi, J.; Kondo, N.; et al. Genotyping of human papillomaviruses by a novel one-step typing method with multiplex PCR and clinical applications. J. Clin. Microbiol. 2008, 46, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Azuma, Y.; Kusumoto-Matsuo, R.; Takeuchi, F.; Uenoyama, A.; Kondo, K.; Tsunoda, H.; Nagasaka, K.; Kawana, K.; Morisada, T.; Iwata, T.; et al. Human papillomavirus genotype distribution in cervical intraepithelial neoplasia grade 2/3 and invasive cervical cancer in Japanese women. Jpn. J. Clin. Oncol. 2014, 44, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Morioka, M.S.; Kawaji, H.; Nishiyori-Sueki, H.; Murata, M.; Kojima-Ishiyama, M.; Carninci, P.; Itoh, M. Cap analysis of gene expression (CAGE): A quantitative and genome-wide assay of transcription start sites. Methods Mol. Biol. 2020, 2120, 277–301. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdottir, H.; Turner, D.; Mesirov, J.P. igv.js: An embeddable JavaScript implementation of the Integrative Genomics Viewer (IGV). Bioinformatics 2023, 39, 830. [Google Scholar] [CrossRef]
- Kinjo, S.; Monma, N.; Misu, S.; Kitamura, N.; Imoto, J.; Yoshitake, K.; Gojobori, T.; Ikeo, K. Maser: One-Stop Platform for NGS Big Data from Analysis to Visualization. Database 2018, 2018. [Google Scholar] [CrossRef]
- Trapnell, C.; Hendrickson, D.G.; Sauvageau, M.; Goff, L.; Rinn, J.L.; Pachter, L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 2013, 31, 46–53. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.O.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Yu, L.; Majerciak, V.; Zheng, Z.-M. HPV16 and HPV18 genome structure, expression, and post-transcriptional regulation. Int. J. Mol. Sci. 2022, 23, 4943. [Google Scholar] [CrossRef] [PubMed]
- Warburton, A.; Redmond, C.J.; Dooley, K.E.; Fu, H.; Gillison, M.L.; Akagi, K.; Symer, D.E.; Aladjem, M.I.; McBride, A.A. HPV integration hijacks and multimerizes a cellular enhancer to generate a viral-cellular super-enhancer that drives high viral oncogene expression. PLOS Genet. 2018, 14, e1007179. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Majerciak, V.; Lobanov, A.; Mirza, S.; Band, V.; Liu, H.; Cam, M.; Hughes, S.H.; Lowy, D.R.; Zheng, Z.-M.; et al. HPV oncogenes expressed from only one of multiple integrated HPV DNA copies drive clonal cell expansion in cervical cancer. mBio 2024, 15, e0072924. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.C.; Phelps, W.C.; Lindgren, V.; Braun, M.J.; Gonda, M.A.; Howley, P.M. Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J. Virol. 1987, 61, 962–971. [Google Scholar] [CrossRef]
- Jeon, S.; Lambert, P.F. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: Implications for cervical carcinogenesis. Proc. Natl. Acad. Sci. USA 1995, 92, 1654–1658. [Google Scholar] [CrossRef]
- Romanczuk, H.; Howley, P.M. Disruption of either the E1 or the E2 regulatory gene of human papillomavirus type 16 increases viral immortalization capacity. Proc. Natl. Acad. Sci. USA 1992, 89, 3159–3163. [Google Scholar] [CrossRef]
- Pett, M.R.; Herdman, M.T.; Palmer, R.D.; Yeo, G.S.H.; Shivji, M.K.; Stanley, M.A.; Coleman, N. Selection of cervical keratinocytes containing integrated HPV16 associates with episome loss and an endogenous antiviral response. Proc. Natl. Acad. Sci. USA 2006, 103, 3822–3827. [Google Scholar] [CrossRef]
- Vinokurova, S.; Wentzensen, N.; Kraus, I.; Klaes, R.; Driesch, C.; Melsheimer, P.; Kisseljov, F.; Dürst, M.; Schneider, A.; von Knebel Doeberitz, M. Type-dependent integration frequency of human papillomavirus genomes in cervical lesions. Cancer Res. 2008, 68, 307–313. [Google Scholar] [CrossRef]
- Baba, S.; Taguchi, A.; Kawata, A.; Hara, K.; Eguchi, S.; Mori, M.; Adachi, K.; Mori, S.; Iwata, T.; Mitsuhashi, A.; et al. Differential expression of human papillomavirus 16-, 18-, 52-, and 58-derived transcripts in cervical intraepithelial neoplasia. Virol. J. 2020, 17, 32. [Google Scholar] [CrossRef]
- Groves, I.J.; Drane, E.L.A.; Michalski, M.; Monahan, J.M.; Scarpini, C.G.; Smith, S.P.; Bussotti, G.; Várnai, C.; Schoenfelder, S.; Fraser, P.; et al. Short- and long-range cis interactions between integrated HPV genomes and cellular chromatin dysregulate host gene expression in early cervical carcinogenesis. PLoS Pathog. 2021, 17, e1009875. [Google Scholar] [CrossRef]
- Holmes, A.; Lameiras, S.; Jeannot, E.; Marie, Y.; Castera, L.; Sastre-Garau, X.; Nicolas, A. Mechanistic signatures of HPV insertions in cervical carcinomas. NPJ Genom. Med. 2016, 1, 16004. [Google Scholar] [CrossRef]
- Li, W.; Qi, Y.; Cui, X.; Huo, Q.; Zhu, L.; Zhang, A.; Tan, M.; Hong, Q.; Yang, Y.; Zhang, H.; et al. Characteristic of HPV Integration in the Genome and Transcriptome of Cervical Cancer Tissues. Biomed. Res. Int. 2018, 2018, 6242173. [Google Scholar] [CrossRef]
- Elix, C.; Pal, S.K.; Jones, J.O. The role of peroxisome proliferator-activated receptor gamma in prostate cancer. Asian J. Androl. 2018, 20, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, D.J.; Missiaen, R.; Skuli, N.; Steger, D.J.; Simon, M.C. Cell-Intrinsic Tumorigenic Functions of PPARγ in Bladder Urothelial Carcinoma. Mol. Cancer Res. 2021, 19, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Justilien, V.; Regala, R.P.; Tseng, I.-C.; Walsh, M.P.; Batra, J.; Radisky, E.S.; Murray, N.R.; Fields, A.P. Matrix metalloproteinase-10 is required for lung cancer stem cell maintenance, tumor initiation and metastatic potential. PLoS ONE 2012, 7, e35040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, C.; Chang, Y.; Zhang, Z.; Hu, Y.; Zhang, F.; Lu, Y.; Zheng, L.; Zhang, W.; Li, X.; et al. Long non-coding RNA CASC11 interacts with hnRNP-K and activates the WNT/β-catenin pathway to promote growth and metastasis in colorectal cancer. Cancer Lett. 2016, 376, 62–73. [Google Scholar] [CrossRef]
- Zamani, M.; Foroughmand, A.-M.; Hajjari, M.-R.; Bakhshinejad, B.; Johnson, R.; Galehdari, H. CASC11 and PVT1 spliced transcripts play an oncogenic role in colorectal carcinogenesis. Front. Oncol. 2022, 12, 954634. [Google Scholar] [CrossRef]
- Wang, B.; Liu, M.; Zhuang, R.; Jiang, J.; Gao, J.; Wang, H.; Chen, H.; Zhang, Z.; Kuang, Y.; Li, P. Long non-coding RNA CCAT2 promotes epithelial-mesenchymal transition involving Wnt/β-catenin pathway in epithelial ovarian carcinoma cells. Oncol. Lett. 2018, 15, 3369–3375. [Google Scholar] [CrossRef]
- Ling, H.; Spizzo, R.; Atlasi, Y.; Nicoloso, M.; Shimizu, M.; Redis, R.S.; Nishida, N.; Gafà, R.; Song, J.; Guo, Z.; et al. CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic progression and chromosomal instability in colon cancer. Genome Res. 2013, 23, 1446–1461. [Google Scholar] [CrossRef]
- Varfolomeev, E.; Blankenship, J.W.; Wayson, S.M.; Fedorova, A.V.; Kayagaki, N.; Garg, P.; Zobel, K.; Dynek, J.N.; Elliott, L.O.; Wallweber, H.J.A.; et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell 2007, 131, 669–681. [Google Scholar] [CrossRef]
- Kotelevets, L.; Chastre, E. A New Story of the Three Magi: Scaffolding Proteins and lncRNA Suppressors of Cancer. Cancers 2021, 13, 4264. [Google Scholar] [CrossRef]
- Li, X.; Jin, F.; Li, Y. A novel autophagy-related lncRNA prognostic risk model for breast cancer. J Cell. Mol. Med. 2021, 25, 4–14. [Google Scholar] [CrossRef]
- Zhou, B.; Hao, Q.; Liang, Y.; Kong, E. Protein palmitoylation in cancer: Molecular functions and therapeutic potential. Mol. Oncol. 2023, 17, 3–26. [Google Scholar] [CrossRef]
- Kultti, A.; Zhao, C.; Singha, N.C.; Zimmerman, S.; Osgood, R.J.; Symons, R.; Jiang, P.; Li, X.; Thompson, C.B.; Infante, J.R.; et al. Accumulation of extracellular hyaluronan by hyaluronan synthase 3 promotes tumor growth and modulates the pancreatic cancer microenvironment. Biomed. Res. Int. 2014, 2014, 817613. [Google Scholar] [CrossRef]
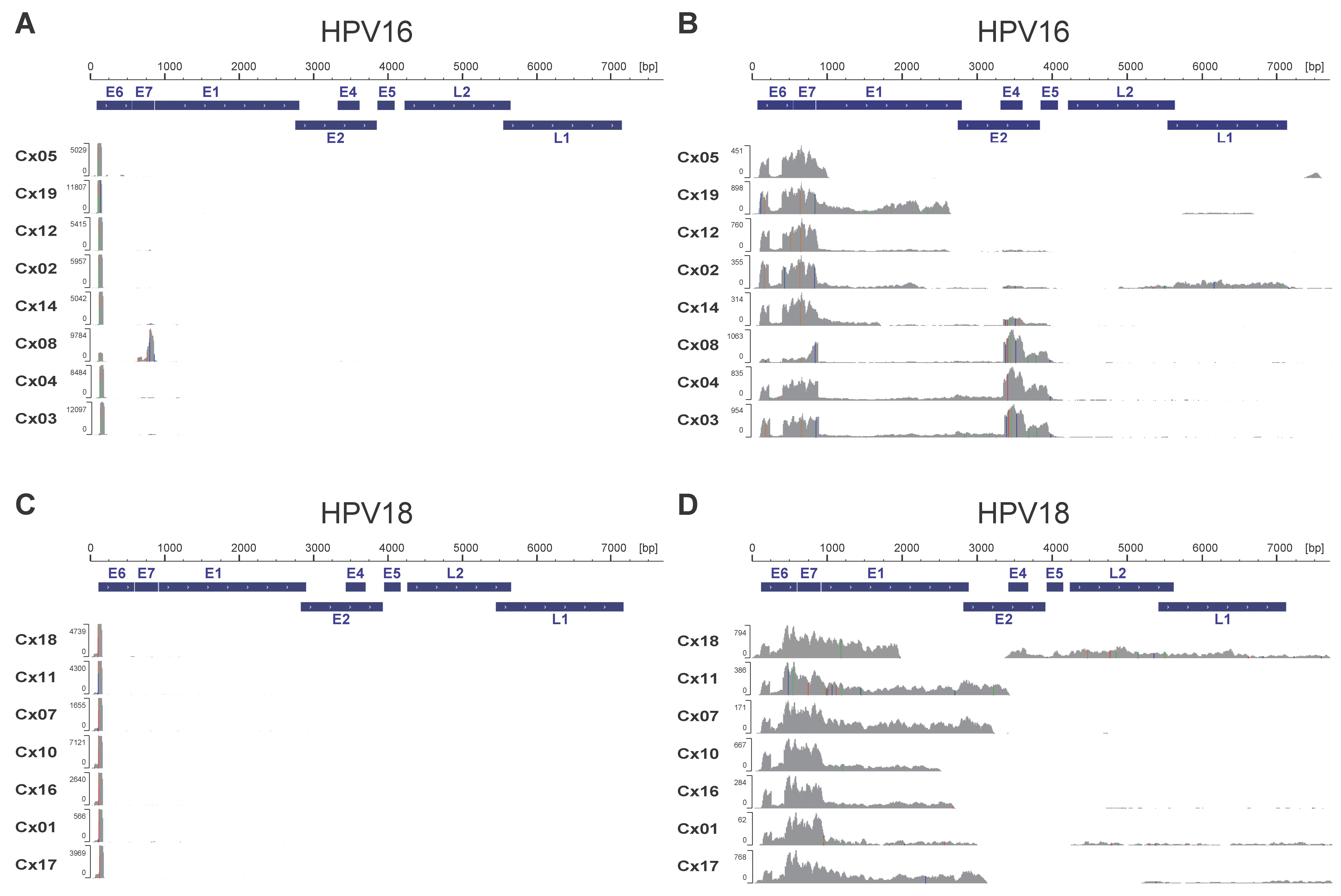
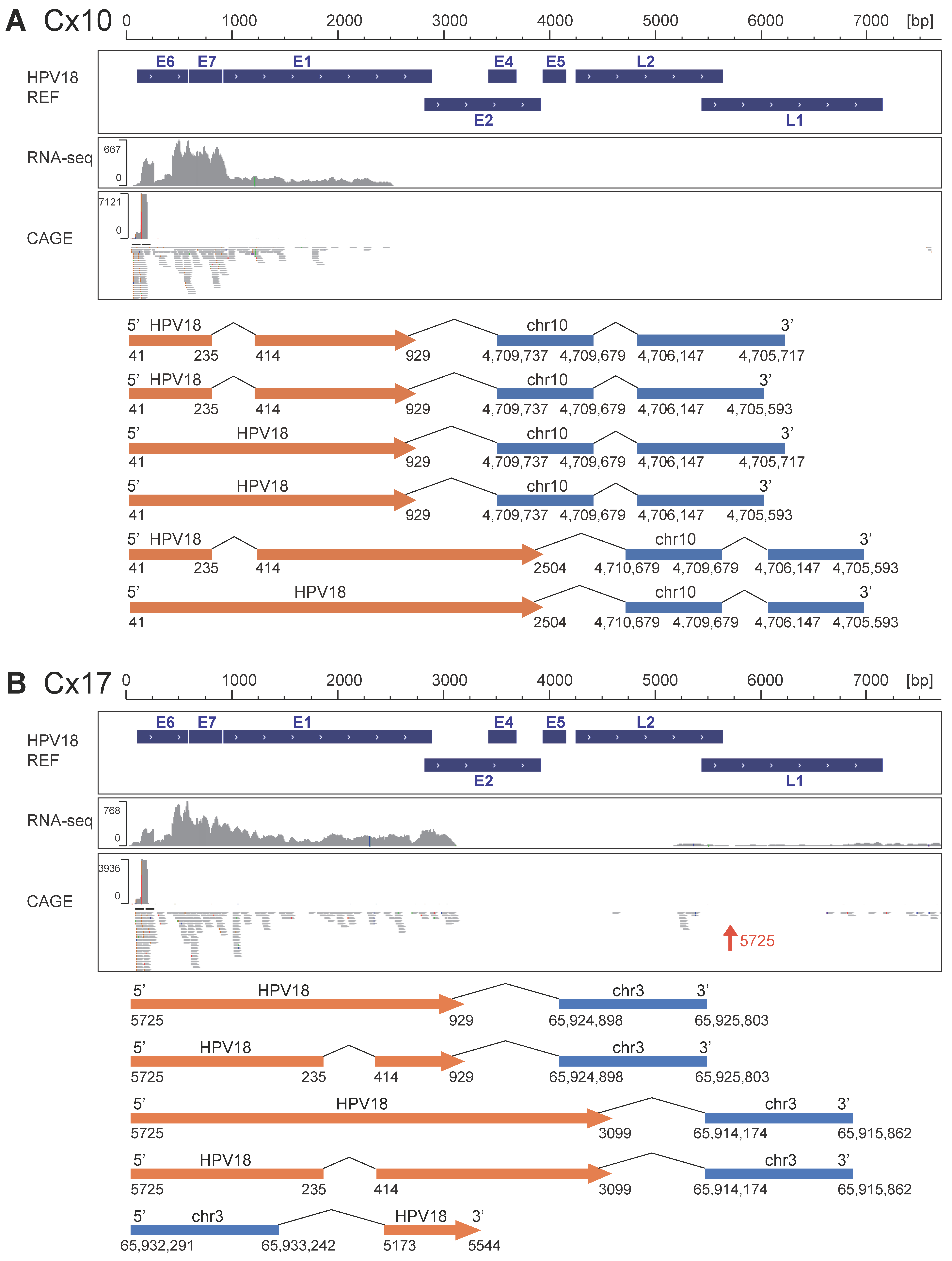
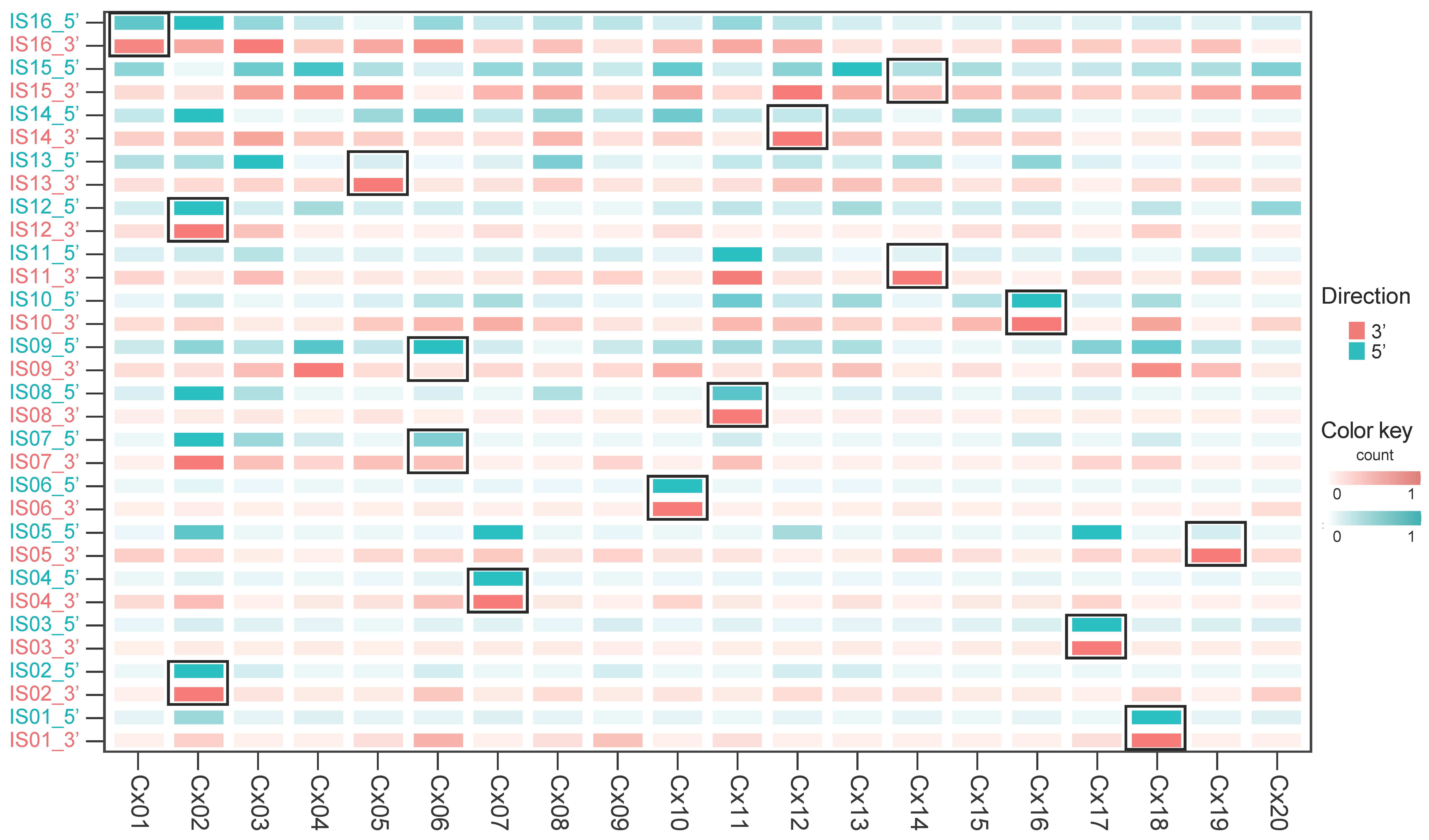
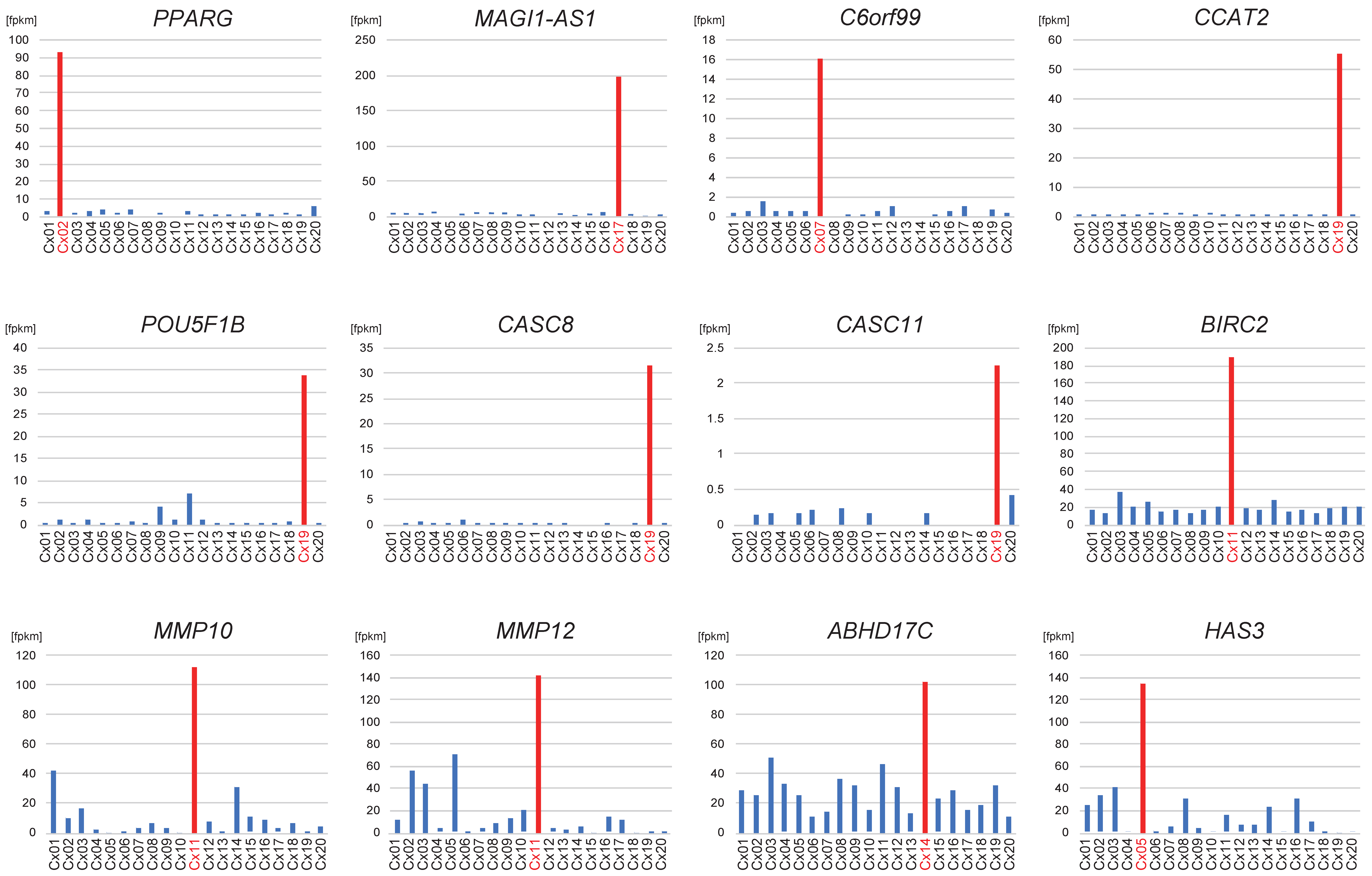
| Sample ID | Age | Histology | T Stage | LNM | HPV Type | Mapped HPV Type | Total RNA-Seq Reads Mapped to HPV Genome | CAGE Reads Mapped to HPV Genome | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Read Number | Mapping Rate (%) | Read Number | Mapping Rate (%) | |||||||
| Cx01 | 67 | SCC | 2b | 0 | 18 | 18 | 397 | 0.002 | 614 | 0.002 |
| Cx02 | 37 | SCC | 2a | 0 | 16(33) | 16 | 2589 | 0.011 | 6167 | 0.018 |
| 33 | 0 | 0 | 0 | 0 | ||||||
| Cx03 | 58 | SCC | 2b | 1 | 16 | 16 | 7086 | 0.033 | 13,206 | 0.045 |
| Cx04 | 35 | ADS | 1b1 | 0 | 16 | 16 | 6091 | 0.028 | 9421 | 0.031 |
| Cx05 | 36 | SCC | 2a2 | 0 | 16(52) | 16 | 1645 | 0.008 | 5235 | 0.016 |
| 52 | 0 | 0 | 0 | 0 | ||||||
| Cx06 | 70 | SCC | 2a2 | 1 | 52 | 52 | 2717 | 0.012 | 5021 | 0.015 |
| Cx07 | 34 | ADC | 1b2 | 1 | 18 | 18 | 1626 | 0.007 | 1895 | 0.006 |
| Cx08 | 32 | SCC | 1b2 | 1 | 16 | 16 | 4586 | 0.021 | 17,587 | 0.061 |
| Cx09 | 35 | ADS | 1b1 | 0 | 16 | 16 | 3 | 0.000 | 160 | 0.000 |
| Cx10 | 50 | SCC | 2b | 0 | 18 | 18 | 3970 | 0.017 | 8162 | 0.027 |
| Cx11 | 32 | ADS | 1b2 | 0 | 18 | 18 | 3232 | 0.015 | 4805 | 0.013 |
| Cx12 | 48 | SCC | 2b | 0 | 16 | 16 | 2927 | 0.014 | 5687 | 0.021 |
| Cx13 | 43 | SCC | 1b1 | 0 | 58 | 58 | 1445 | 0.007 | 2351 | 0.007 |
| Cx14 | 31 | SCC | 2a | 1 | 16 | 16 | 1546 | 0.007 | 5494 | 0.017 |
| Cx15 | 48 | ADC | 1a1 | 0 | 16 | 16 | 2 | 0.000 | 57 | 0.000 |
| Cx16 | 29 | SCC | 2b | 1 | 18 | 18 | 1679 | 0.008 | 2997 | 0.009 |
| Cx17 | 37 | SCC | 1b | 0 | 18 | 18 | 6584 | 0.031 | 4576 | 0.014 |
| Cx18 | 62 | ADC | 2b | 1 | 18 | 18 | 10,630 | 0.052 | 5641 | 0.016 |
| Cx19 | 41 | ADC | 1b2 | 1 | 16 | 16 | 6905 | 0.036 | 12,314 | 0.045 |
| Cx20 | 67 | ADC | 2b | 1 | 45 | 45 | 0 | 0 | 0 | 0 |
| Sample ID | BINDS ID | HPV Type | TSS Type | Total RNA-Seq Type | Chimeric RNA | Integration Location | Upregulated Genes Around IS | Number of Types of Chimeric RNAs | IS ID | TSS Activation Pattern Around IS | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Start from Human Genome | Start from HPV Genome | |||||||||||
| Start from HPV Early Promoter | Start from HPV Other Promoters | ||||||||||||
| Cx02 | BINDS 025-002 | 16 | A | I | + | chr3: 12,405,935 | PPARG | 9 | 3 | 5, nt25 | 1, nt1377 | IS 02 | bidirectional |
| + | chr16: 68,398,335 | IS 12 | bidirectional | ||||||||||
| Cx05 | BINDS 025-005 | 16 | A | I | + | chr16: 69,114,338 | HAS3 | 2 | 0 | 2, nt27 | 0 | IS 13 | unidirectional |
| Cx12 | BINDS 025-016 | 16 | A | I | + | chr18: 63,381,192 | 4 | 0 | 4, nt106 | 0 | IS 14 | unidirectional | |
| Cx14 | BINDS 025-018 | 16 | A | I | + | chr15: 80,711,958 | ABHD17C | 9 | 3 | 6, nt99 | 0 | IS 11 | unidirectional |
| + | chr19: 1,879,919 | IS 15 | none | ||||||||||
| Cx19 | BINDS 025-024 | 16 | A | I | + | chr8: 127,417,949 | CASC8 CCAT2 POU5F1B CASC11 | 8 | 1 | 6, nt25 | 1, nt6033 | IS 05 | unidirectional |
| Cx01 | BINDS 025-001 | 18 | A | I | + | chrX: 46,716,828 | 2 | 0 | 2, nt120 | 0 | IS 16 | none | |
| Cx07 | BINDS 025-008 | 18 | A | I | + | chr6: 158,894,981 | C6orf99 | 5 | 0 | 5, nt43 | 0 | IS 04 | bidirectional |
| Cx10 | BINDS 025-012 | 18 | A | I | + | chr10: 4,705,593 | 6 | 0 | 6, nt41 | 0 | IS 06 | bidirectional | |
| Cx11 | BINDS 025-015 | 18 | A | I | + | chr11: 102,862,906 | MMP12 MMP10 BIRC2 | 26 | 0 | 26, nt76 | 0 | IS 08 | unidirectional |
| Cx16 | BINDS 025-020 | 18 | A | I | + | chr15: 71,524,938 | 7 | 1 | 6, nt59 | 0 | IS 10 | bidirectional | |
| Cx17 | BINDS 025-022 | 18 | A | I | + | chr3: 65,914,174 | MAGI1-AS1 | 5 | 1 | 0 | 4, nt5725 | IS 03 | bidirectional |
| Cx18 | BINDS 025-023 | 18 | A | I | + | chr2: 176,845,914 | 6 | 1 | 0 | 5, nt6613 | IS 01 | bidirectional | |
| Cx06 | BINDS 025-007 | 52 | A | II | + | chr11: 4,631,073 | 4 | 0 | 4, nt7726 | 0 | IS 07 | none | |
| + | chr12: 52,897,678 | IS 09 | none | ||||||||||
| Cx03 | BINDS 025-003 | 16 | A | II | − | 0 | |||||||
| Cx04 | BINDS 025-004 | 16 | A | II | − | 0 | |||||||
| Cx08 | BINDS 025-010 | 16 | B | II | − | 0 | |||||||
| Cx13 | BINDS 025-017 | 58 | A | II | − | 0 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamai, K.; Kinjo, S.; Taguchi, A.; Nagasaka, K.; Yoshimoto, D.; Duong, A.Q.; Yamamoto, Y.; Iuchi, H.; Mori, M.; Sone, K.; et al. Elucidating Alterations in Viral and Human Gene Expression Due to Human Papillomavirus Integration by Using Multimodal RNA Sequencing. Viruses 2025, 17, 1344. https://doi.org/10.3390/v17101344
Tamai K, Kinjo S, Taguchi A, Nagasaka K, Yoshimoto D, Duong AQ, Yamamoto Y, Iuchi H, Mori M, Sone K, et al. Elucidating Alterations in Viral and Human Gene Expression Due to Human Papillomavirus Integration by Using Multimodal RNA Sequencing. Viruses. 2025; 17(10):1344. https://doi.org/10.3390/v17101344
Chicago/Turabian StyleTamai, Kana, Sonoko Kinjo, Ayumi Taguchi, Kazunori Nagasaka, Daisuke Yoshimoto, Anh Quynh Duong, Yoko Yamamoto, Hitoshi Iuchi, Mayuyo Mori, Kenbun Sone, and et al. 2025. "Elucidating Alterations in Viral and Human Gene Expression Due to Human Papillomavirus Integration by Using Multimodal RNA Sequencing" Viruses 17, no. 10: 1344. https://doi.org/10.3390/v17101344
APA StyleTamai, K., Kinjo, S., Taguchi, A., Nagasaka, K., Yoshimoto, D., Duong, A. Q., Yamamoto, Y., Iuchi, H., Mori, M., Sone, K., Hamada, M., Kawana, K., Ikeo, K., Hirota, Y., & Osuga, Y. (2025). Elucidating Alterations in Viral and Human Gene Expression Due to Human Papillomavirus Integration by Using Multimodal RNA Sequencing. Viruses, 17(10), 1344. https://doi.org/10.3390/v17101344








