Abstract
Between 2019 and 2023, 163 cases of subgenotype VII.1.1 Newcastle disease virus infection were registered in backyard poultry in the Russian Federation within the framework of epizootiological monitoring. Subgenotype VII.1.1 Newcastle disease virus was reported in a total of 18 different subjects of the Russian Federation. Most of the Newcastle disease outbreaks caused by the viruses of this subgenotype occurred in the autumn and winter period (60%). Further tests allowed for the determination of complete F and HN gene nucleotide sequences for 40 isolates. The results were used to perform the Bayesian analysis of F gene sequences with BEAST v.1.10.4 software. The obtained nucleotide substitution accumulation rates were practically non-dependent on the selected nucleotide substitution model and varied appreciably depending on the applied molecular clock model (0.0018 and 0.002 site-1year-1). The conducted study established that the formation of the ‘Russian’ NDV isolates of subgenotype VII.1.1 followed several stages. In the early 2000s, ancestral viruses belonging to subgenotype VII-d were detected in the Middle East and Eastern Europe. From these, through intermediate forms identified in Iraq around 2007–2008, a group designated as subgenotype VII-L emerged. This group gave rise to two sister clades: the Iranian subgenotype VII-L and the cluster of isolates from Russia and Poland, whose immediate common ancestor likely existed around 2015–2016, probably in Asia.
1. Introduction
Newcastle disease (ND) is a highly contagious viral disease of birds, according to the World Organization for Animal Health (WOAH). Newcastle disease virus (NDV), or Orthoavulavirus javaense, is a member of the genus Orthoavulavirus, the family Paramyxoviridae (ICTV) [1].
NDV is characterized by high genetic diversity. In accordance with the classification proposed by Dimitrov et al. (2019) [2], all NDV isolates are divided into 2 classes—class 1 and class 2, which, in its turn, is subdivided into 21 genotypes (I-XXI). In the past 20 years, the ND outbreaks caused by class 2 genotype VII NDV have been posing the greatest threat to the countries of Europe, Asia, and Africa. This genotype includes subgenotypes VII.1.1, 1.2 and 2 [2]. Most common are subgenotypes VII.1.1 and VII.2. Since the late 1990s, subgenotype VII.1.1 has been detected in China [3,4], Iran [5,6], Taiwan [7], Afghanistan [8], Saudi Arabia [9], Iraq [10], Egypt [11,12,13], Indonesia [14], as well as in Japan [15], Vietnam [16], and Eastern Europe [17]. Subgenotype VII.2 was initially detected in the countries of Southeast Asia (Cambodia, Indonesia, Malaysia) in 2007-2014 [18] and West Africa [19], from where it spread to Mozambique [20], Botswana [21], and Tanzania [22], as well as to the countries of Europe and Asia: Israel, Jordan [23], China [20], Pakistan [24], Oman [25], India [26], Bulgaria, Georgia, and Belgium [17,27].
At different times, subgenotypes VII.1.1 (2000–2010) [28] and VII.2 (2013, 2016–2017) [29,30] were detected in Russia.
Since 2019, all ND outbreaks in the Russian Federation have been caused by NDV isolates of subgenotype VII.1.1 (according to the classification by Dimitrov et al. (2019) [2] or subgenotype VII-L (proposed by Sabouri et al. (2018) [5], according to the classification by Diel et al. (2012) [31]). The viruses of this subgenotype were detected in different regions of the country in 2019–2023. Initially, subgenotype VII-L viruses were described by Iranian researchers in 2017 [6,32], who showed their similarity to and close phylogenetic relationship with the previously known subgenotype VII-d. Subsequently, the authors [33,34] increased the number of the studied isolates of this group, showing their distribution in almost all provinces of Iran.
This paper presents the molecular genetics and evolutionary analysis of subgenotype VII.1.1 NDV isolates detected in Russia in 2019–2023. In addition, the obtained sequences were subjected to Bayesian analysis using BEAST software to determine substitution accumulation rates and the time of the main phylogenetic events.
2. Materials and Methods
2.1. Sampling
In the period from January 2019 to December 2023, the samples of biological material (internal organs, feces, oropharyngeal, and cloacal swabs) from dead poultry and poultry with clinical signs of Newcastle disease were submitted to the National Reference Laboratory for Avian Influenza and Newcastle Disease of the WOAH (Federal State-Financed Institution “Federal Centre for Animal Health” (FGBI “ARRIAH”), Yur′evets, Vladimir, Russia). The samples were collected in the backyards by the specialists of the Territorial Administrations of the Federal Service for Veterinary and Phytosanitary Surveillance (Rosselkhoznadzor) and the Veterinary Departments of the Russian Federation Subjects.
2.2. Sample Preparation
The test material (samples of internal organs, tracheal and cloacal flushes, fecal samples) was crushed (if necessary) using sterile scissors and placed in a plastic container for biomaterial. The resulting mass was weighed and diluted with a sterile phosphate buffer solution to obtain a 10–20% suspension. Next, the suspension was transferred to 1.5 mL and 2 mL tubes and centrifuged at 1000–2000× g for 15 min. The obtained suspensions of organs were used for RNA extraction and virus isolation.
2.3. Virus Isolation
Subgenotype VII.1.1 NDV isolates were detected with RT-PCR in 163 samples from backyard poultry. Each sample was inoculated into 9–11-day-old specific-pathogen-free (SPF) embryonated chicken eggs. The collected allantoic fluid was tested with hemagglutination (HA) assay in accordance with the recommendations of WOAH.
2.4. RNA Extraction
RNA extraction from the allantoic fluid and pathological material was carried out using RNeasy Mini Kits (Qiagen, Hilden, Germany) in accordance with the manufacturer’s instructions.
2.5. Real-Time Reverse Transcription-Polymerase Chain Reaction (Real-Time RT-PCR)
One-stage real-time RT-PCR for matrix gene for the primary screening of the samples was carried out using the reagent kits Promega AMV Reverse Transcriptase and DNA Polymerase (Promega, Madison, WI, USA), Qiagen OneStep RT-PCR Kit (Qiagen, Hilden, Germany), a deoxyribonucleotide triphosphate mixture (Thermo Scientific, Waltham, Massachusetts, USA), solutions of forward (M4100F 5′-AGT-GAT-GTG-CTC-GGA-CCT-TC-3′) and reverse (M4220R 5′-ATC-GTT-TAC-GGA-GAG-GAG-TCC-3′) primers and fluorescent probe (M4169 5′-(FAM)TTC-TCT-AGC-AGT-GGG-ACA-GCC-TGC (RTQ1)-3′) [35]. Temperature and time conditions for real-time RT-PCR were as follows: reverse transcription—30 min at 50 °C, polymerase activation—10 min at 95 °C, then 40 PCR cycles, each consisting of denaturation—10 s at 95 °C, annealing of primers—35 s at 55 °C, and elongation—10 s at 72 °C.
2.6. Reverse Transcription (RT) and Polymerase Chain Reaction (PCR) for Determination of Complete F and HN Gene Sequences
Reverse transcription for cDNA synthesis was carried out using Maxima H Minus Reverse Transcriptase (Thermo Scientific, Waltham, MA, USA), RiboLock RNase Inhibitor (Thermo Scientific, Waltham, MA, USA), a phosphate solution (dNTP) (Thermo Scientific, Waltham, MA, USA), the solution of forward primer VII5 5′-ACCAAACAGAGAATCYGTGAG-3′ and nuclease-free water. Primary PCR (PCR-I) and nested PCR for determination of fusion (F) and heamagglutinin-neuraminidase (HN) gene sequences were carried out using the reagent kits Promega DNA Polymerase (Promega, Madison, WI, USA), DreamTaq DNA Polymerase (Thermo Scientific, Waltham, MA, USA), nuclease-free water, a phosphate solution (dNTP) (Thermo Scientific, USA) and solutions of forward and reverse primers (Table S1).
Temperature and time conditions for primary PCR were as follows: polymerase activation—3 min at 95 °C, then 40 PCR cycles, each consisting of denaturation—20 s at 95 °C, annealing of primers—30 s at 55 °C and elongation—4 min 20 s at 72 °C, followed by additional elongation—10 min at 72 °C. Temperature and time conditions for nested PCR were as follows: polymerase activation—2 min at 95 °C, then 30 PCR cycles, each consisting of denaturation—20 s at 95 °C, annealing of primers—30 s at 55 °C, and elongation—40 s at 72 °C.
2.7. Sequencing
The F and HN gene nucleotide sequences of subgenotype VII.1.1 NDV were determined using an automated ABI Prism 3130xl sequencer and BigDye Terminator Cycle Sequencing kits (Applied Biosystems, Waltham, MA, USA), according to the manufacturer’s instructions. The obtained sequences were edited with BioEdit version 7.2 software, as well as submitted to GenBank (Table 1).

Table 1.
Information on subgenotype VII.1.1 NDV isolates detected in the Russian Federation in 2019–2023.
2.8. Alignment and Editing of Nucleotide Sequences
The alignment and editing of the nucleotide sequences were carried out using ClustalW and BioEdit version 7.2 software. The phylogenetic analysis was carried out with the neighbor-joining method using the HKY model (Kumar S, Stecher G, Li M et al. (2018)) [36]. The robustness of phylogeny was assessed by 500 bootstraps. Discrete gamma distribution was used to model evolutionary rate differences among sites. The analysis was carried out with MEGA X (10.2.6) software.
2.9. Dataset for Phylogenetic and Evolutionary Analysis
Two variants of nucleotide sequence analysis were performed: analysis of a fragment of the F gene of NDV isolates of genotype VII identified in poultry in the Russian Federation since 2003, and analysis of the complete nucleotide sequences of the F and HN isolates identified in the Russian Federation in 2019–2023.
For the phylogenetic analysis, a proprietary database of F gene sequences of NDV genotype VII isolates was used, along with sequences from the dataset “NDV_class_II_pilot_128_May_09_2022” [2], and sequences publicly available in GenBank. Neighbor-joining methods were used to construct phylogenetic trees. Neighbor-joining trees (Maximum Composite Likelihood model) were constructed using MEGA X (10.2.6) with 500 bootstrap replicates [2].
The dataset for the evolutionary analysis included 78 F gene sequences of subgenotype VII.1.1 NDV isolates: 40 sequences obtained during this study, 4 sequences from Iraq, and 34 sequences from Iran described above.
2.10. Evolutionary Analysis of Nucleotide Sequences Using BEAST and BEAUTI Software
To determine the genetic change rates of the isolates and the time of emergence of the Russian branch of subgenotype VII.1.1, the Bayesian analysis of F gene sequences was performed using BEAST (Bayesian Evolutionary Analysis Sampling Trees) v.1.10.4 software. A set of 40 complete F gene sequences of the Russian isolates and Iranian Beh strain (MF417546.1) was analyzed to root the constructed tree. The Russian isolates were grouped into a special group to estimate the time to the most recent common ancestor (TMRCA). GTR and HKY nucleotide substitution models and gamma distribution of substitution rates (five categories), empirical nucleotide frequency, codon splitting into positions 1, 2, and 3, and strict and uncorrelated relaxed molecular clocks were used. Other parameters of the model were set as default. To estimate the parameters of the model, the Monte Carlo Markov Chain was set to 50 million iterations, so that the effective sample size for each parameter was at least 200. To evaluate the parameter estimates from BEAST, Tracer 1.7.2. Software was used (Rambaut et al., 2018) [37].
2.11. Mapping
Maps were created using free Map Chart tools (https://www.mapchart.net/index.html, accessed on 9 September 2025).
3. Results
3.1. Sampling
In the period from 2019 to 2023, 28,776 samples of biological materials were tested for Newcastle disease virus with real-time RT-PCR. During the study, subgenotype VII.1.1 NDV isolates were detected with real-time RT-PCR and RT-PCR in 36 samples in 2019, in 34 samples in 2020, in 8 samples in 2021, in 51 samples in 2022, and in 34 samples in 2023 (in total, 163 samples). The Russian Federation regions in which subgenotype VII.1.1 NDV isolates were detected in 2019–2023 are shown in Figure 1.
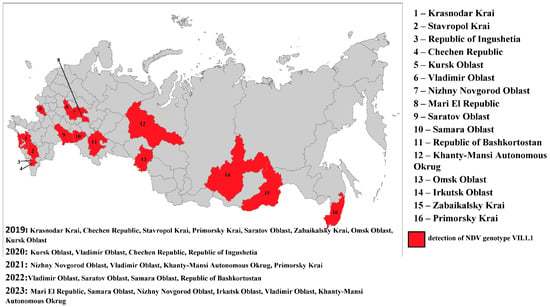
Figure 1.
The Russian Federation regions in which subgenotype VII.1.1 NDV isolates were detected in 2019–2023.
In 2019–2023, subgenotype VII.1.1 viruses were detected in samples collected from backyard poultry. Most of the ND outbreaks caused by the viruses of the said subgenotype occurred in the autumn and winter period (60%). In the Russian Federation, subgenotype VII.1.1 NDV was first detected in the Krasnodar Krai in January 2019. The virus was detected in internal organ samples from non-vaccinated backyard chickens. Then the virus was again isolated from backyard poultry in two other regions in the south of the Russian Federation: in the Chechen Republic in March and in the Stavropol Krai in April. In May, subgenotype VII.1.1 NDV was unexpectedly detected in the Primorsky Krai. The virus was detected in internal organ samples from non-vaccinated chickens. Subsequently, in 2019–2023, the outbreaks of the disease were reported mainly in the autumn and winter period in different geographical locations of the country.
During the work performed, complete F and HN gene nucleotide sequences were determined for 40 isolates. The information on the viruses studied within this work is presented in Table 1.
3.2. Phylogenetic Analysis
To detect closely related NDV isolates that circulated prior to 2019, a phylogenetic analysis of the F gene fragment of isolates identified since 2003 was performed.
A total of 54 nucleotide sequences of the F gene fragment of NDV isolates belonging exclusively to genotype VII were selected from the database of the FGBI “ARRIAH”. For the analysis of the F gene fragment, the most closely related nucleotide sequences from GenBank and the dataset “NDV_class_II_pilot_128_May_09_2022” [2] were used.
Alignment-based analysis of 94 (54 + 40) F gene fragment sequences enabled the identification of genetic groups/subgenotypes of NDVisolates detected in the Russian Federation during the period 2003–2023. It was shown that 56 isolates belonged to subgenotype VII.1.1, which represents a large and genetically diverse group of NDV. This subgenotype includes viruses that caused the fourth ND panzootic and were previously classified under subgenotypes VII-b, VII-d, VII-e, VII-j, and VII-L [2,31]. In contrast, 28 isolates were assigned to subgenotype VII.2 (VII-i, VII-h) [1,30] (Figure 2).
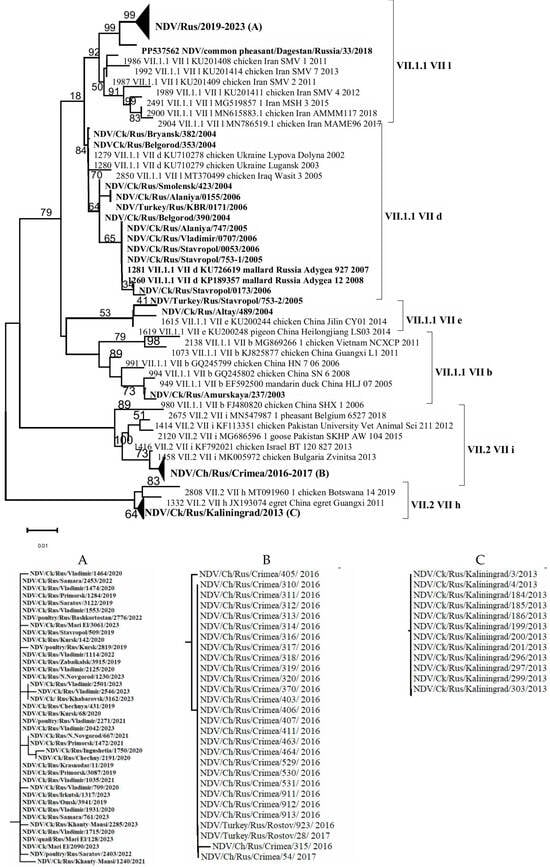
Figure 2.
Phylogenetic tree of NDV isolates of genotype VII (partial F gene ORF nucleotides 212-494). (A–C) Compressed and expanded trees.
As a result of the genetic analysis of NDV isolates collected in the Russian Federation, it was established that isolates belonging to subgenotypes VII-b, VII-d, and VII-e were detected in 2003–2004; in 2005–2006, only subgenotype VII-d was identified; in 2013, only VII-h; in 2016–2017, only VII-i; and in 2019–2023, exclusively subgenotype VII-L. The relatively low number of NDV isolates of subgenotypes VII-h and VII-i detected in 2013 and 2016–2017, respectively, may be attributed to a decrease in the volume of surveillance studies during that period, which likely affected the objectivity of the epizootic picture. On the other hand, a relatively extensive group of closely related isolates from 2019 to 2023 was identified in backyard poultry farms located in various regions of the Russian Federation during comprehensive nationwide surveillance efforts conducted in that period (Figure 1).
It is noteworthy that the VII-d subgenotype isolates detected in 2004–2007 were genetically close to the virulent NDV isolates of subgenotype VII-L identified in 2019–2023. Subgenotype VII-d is globally distributed and has been one of the most widespread circulating subgenotypes among poultry and wild birds since the early 21st century [28]. Phylogenetic analysis of the full coding sequence of the F gene from isolates identified in Bulgaria and Ukraine, conducted by Dimitrov et al. (2016) [28], showed that VII-d viruses replaced strains that had been circulating in Bulgaria prior to 2002. A similar pattern appears to have occurred with NDV isolates in the Russian Federation, and our findings support the conclusions of Dimitrov and colleagues [28].
To enhance the precision of the genetic analysis, complete nucleotide sequences of the F and HN genes were obtained for NDV isolates collected during 2019–2023. These data enabled a comprehensive comparative analysis between the Russian isolates and reference virus sequences available in the ‘NDV_class_II_pilot_128_May_09_2022’ dataset [2] as well as in the GenBank database (ncbi.nlm.nih.gov).
A BLAST (v. 2.16.0 software) search for the most similar sequences revealed that the closest matches to the Russian isolates from 2019 to 2023 were a group of isolates detected in Poland in 2023–2024, showing 99.58–99.16% nucleotide identity in the F gene. In comparison, VII-L subgenotype isolates from Iran, collected between 2011 and 2015, exhibited up to 97.95% identity. A high level of similarity (96.33–97.05%) was also observed when compared with the isolates of the said subgenotype detected in Iran in 2017–2020 and with subgenotype VII-d isolates detected in Iraq in 2005 and 2008 (Figure 3, Figure 4 and Figure S1).
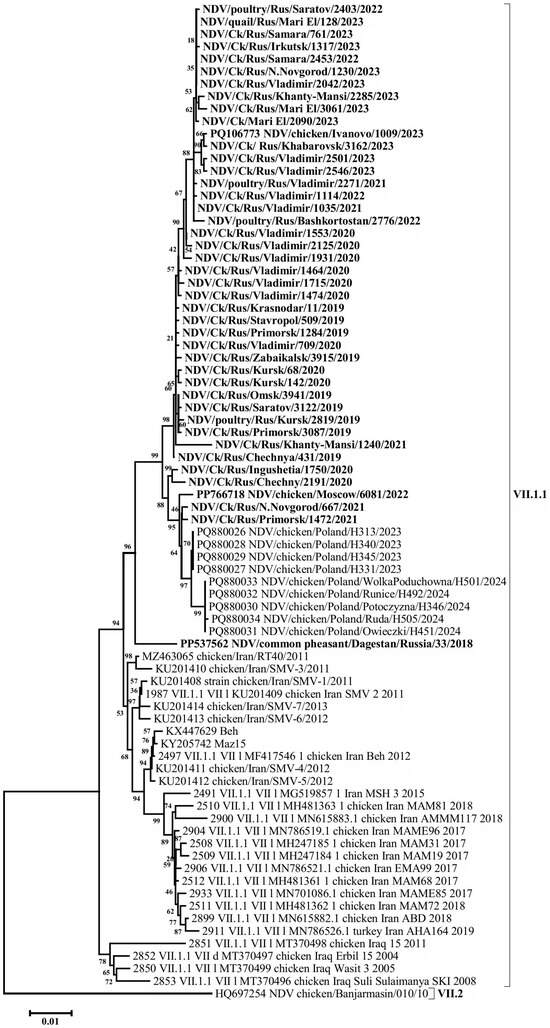
Figure 3.
Phylogenetic tree of NDV isolates of genetic group VII-L (subgenotype VII.1.1) (F gene ORF nucleotides 1-1661).
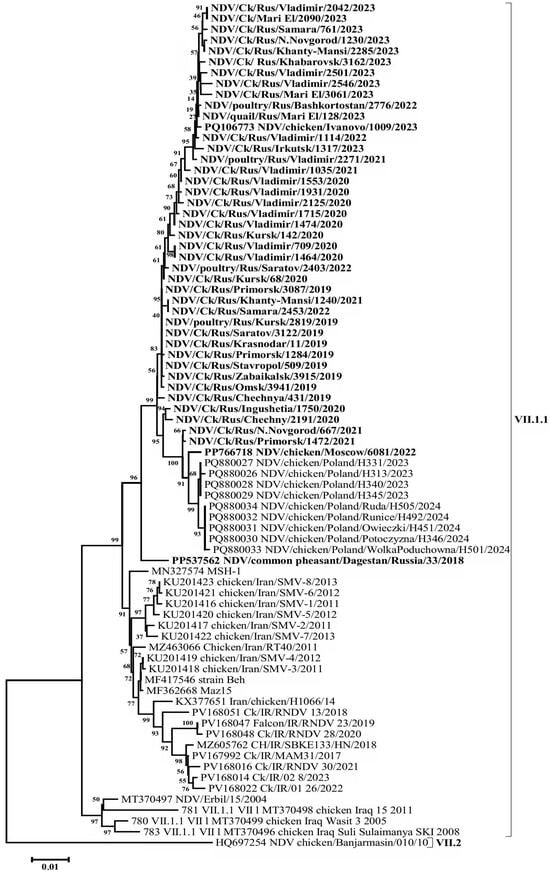
Figure 4.
Phylogenetic tree of NDV isolates of genetic group VII-L (subgenotype VII.1.1) (HN gene ORF nucleotides 1-1713).
Phylogenetic relationships for this group of isolates were determined based on the complete nucleotide sequences of the F and HN genes. The phylogenetic trees constructed for both genes displayed a high degree of congruence, indicating the absence of recombination events—a characteristic feature of the NDV genome. The evolutionary pathway can also be clearly traced. The phylogenetic group of VII-L subgenotype isolates appears to have originated from an ancestral group of VII-d subgenotype isolates that entered the Middle East and Eastern Europe from China in the early 2000s [28], likely via intermediate forms from Iraq. The Russian isolates, along with the closely related Polish isolates (which likely descended from them), and the Iranian VII-L isolates appear to represent sister clades that diverged shortly after the emergence of this subgenotype.
The group of VII.1.1 subgenotype isolates detected in the Russian Federation has a number of specific nucleotide substitutions that place them into a separate genetic lineage within group VII-L. In particular, F gene open reading frame has the following substitutions common to all the Russian Federation isolates: 114A->T, 152A->G, 210C->T, 294C->T, 348T->C, 441T->C, 474T->C, 480A->G, 504C->T, 564C->A, 651G->A, 748T->C, 810T->C, 834A->G, 906T->C, 948A->G, 1008A->C, 1093C->T, 1131C->G, 1217C->A, 1281A->G, 1401T->C, 1455C->A, 1490G->A, 1497T->C (in total, 25 nucleotide substitutions). Most of these substitutions are synonymous transitions (17 substitutions), but synonymous transversions are also present (3 substitutions). At the same time, 5 out of 25 substitutions in the group of the Russian isolates are non-synonymous: 152A->G (N->S), 564C->A (D->E), 1217C->A (G->S), 1455C->A (N->K), 1490G->A (S->N).
The analysis of HN gene ORF nucleotide sequence also revealed substitutions common to all the Russian Federation isolates: 30G->A, 82A->G, 103A->G, 456T->C, 470A->G, 507A->G, 510C->T, 552C->T, 615C->T, 618G->A, 861C->T, 903G->A, 1026C->T, 1066A->G, 1161A->G, 1278A->G, 1450C->T, 1453A->C, 1470G->A, 1490C->A, 1503C->T, 1518C->T, 1530T->C, 1623T->C, 1629T->C, 1684C->T (in total, 26 nucleotide substitutions). As in the case of the F gene, most of these substitutions are synonymous transitions (21 substitutions). It should be noted that 5 out of 26 substitutions are non-synonymous: 82A->G (T->A), 103A->G (M->V), 470A->G (Q->R), 1066A->G (K->E), 1490C->A (A->E).
3.3. Evolutionary Analysis of Nucleotide Sequence Variability of NDVisolates
As part of the study, the Bayesian analysis of the obtained F gene sequences was performed using BEAST software to determine substitution accumulation rates and the time of the main phylogenetic events.
The point mutation accumulation rate in the group of Russian subgenotype VII.1.1 isolates (n = 40) for five years (2019–2023) was determined using BEAST software for GTR and HKY nucleotide substitution models based on the strict and uncorrelated relaxed molecular clock models. The obtained nucleotide substitution accumulation rates were practically non-dependent on the selected nucleotide substitution model and varied appreciably depending on the applied molecular clock model (0.0018 and 0.002 site-1year-1), which was also observed for NDV by other authors for NDV [38] (Table 2).

Table 2.
Substitution accumulation rates and dates of main phylogenetic events for Russian subgenotype VII.1.1 isolates.
4. Discussion
Isolates of subgenotype VII.1.1 (VII-L) first appeared in the Russian Federation in 2019 and began to spread rapidly throughout the country. Isolates of the same genetic group were detected in Poland in 2023–2024. The high level of genetic similarity between the Russian and Polish isolates suggests that they represent a new wave of the extensive epizootic process in Eastern Europe described by Dimitrov et al. [28].
The analysis of identified single nucleotide substitutions indicates a distinct evolutionary pathway of the potential ancestor of the Russian isolate group over a certain period of time, likely around five years. The spread of NDV subgenotype VII.1.1 across the Russian Federation within a single year was rapid and unpredictable. In 2019, outbreaks were recorded in geographically distant regions, ranging from the Caucasus to the Far East. The first three outbreaks occurred in the southern regions of the country, followed by detections in the Far Eastern region, and subsequently in the central part of the country. Comparison of the nucleotide sequences of the detected viruses reveals their close genetic relatedness and descent from a common ancestor. The early branching point of the Russian and Iranian isolate groups, along with the absence of intermediate forms for the initial isolates, suggests the likely existence of a common ancestor in an unknown region of Asia.
The hypothesis of the Middle East as the original source of subgenotype VII.1.1 viruses is supported by the estimated dating of the root node in the phylogenetic tree. For our dataset, the root node dating across different models averaged around 2008–2009, preceding the earliest published isolates from Iran dated to 2010. This indicates an early divergence of the future ‘Russian’ branch of subgenotype VII.1.1. At the same time, the estimated date of the most recent common ancestor of the Russian isolate group (2016–2017) closely corresponds to the detection date of the earliest isolate identified by researchers from Novosibirsk (NDV/common pheasant/Dagestan/Russia/33/2018) in 2018. Together with the phylogenetic data, this supports a virtually monophyletic origin of the group.
The widespread and rapid dissemination of NDV subgenotype VII.1.1 viruses across the Russian Federation, and subsequently Poland, raises several questions regarding the factors facilitating the virus’s spread over considerable distances. The involvement of migratory birds in the transmission of NDV among domestic poultry has been repeatedly discussed in previous studies [39,40,41].
The list of bird species in which NDV genotype VII has been detected is extensive, with chickens being the most frequently studied host. Besides chickens, the list includes turkeys, pheasants, and quail, as well as waterfowl such as mallard, mandarin duck, sheldrake duck, muscovy duck, goose, bean goose, white-fronted goose, common teal, and black swan; near-water birds like crested ibis and egret; raptors including rough-legged buzzard, long-eared owl, Japanese sparrowhawk, sparrowhawk; as well as penguins, parrots, parakeets, peacocks, and ostriches [42,43,44].
NDV genotype VII has also been detected in pigeons [28,34,45]. Pigeons are considered effective carriers and transmitters of subgenotype VII-d viruses to commercially raised broiler chickens kept in open poultry houses [46]. Nevertheless, we did not detect genotype VII viruses in pigeons. Our identification of NDV in pigeons during 2019–2023 revealed only genotypes VI and XXI. Access to domestic poultry in small private farms (SPFs) is not limited to pigeons alone. It may be necessary to broaden the range of bird species utilizing SPFs as a feeding base to identify those involved in the NDV transmission chain.
The direct or indirect influence of humans on the spread of NDV cannot be excluded. The role of anthropogenic factors in the dissemination of NDV related to trade and transport of industrial poultry is supported by several studies from Iran [33,35]. Understanding how humans contribute to the spread of Newcastle disease virus (via trade and/or illegal movement of birds and poultry products) could significantly reduce the economic impact of the disease.
The conducted study established that the formation of the ‘Russian’ NDV isolates of subgenotype VII.1.1 followed several stages. In the early 2000s, ancestral viruses belonging to subgenotype VII-d were detected in the Middle East and Eastern Europe. From these, through intermediate forms identified in Iraq around 2007–2008, a group designated as subgenotype VII-L emerged. This group gave rise to two sister clades: the Iranian subgenotype VII-L and the cluster of isolates from Russia and Poland, whose immediate common ancestor likely existed around 2015–2016, probably in Asia.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v17101319/s1, Figure S1: Phylogenetic tree of NDV isolates (F gene ORF nucleotides 1-1661); Table S1: The structure of primers for amplification and determination of the primary structure of the F and HN genes of the VNB genotype VII.
Author Contributions
Conceptualization, N.A.G., S.N.K., A.A.K. and N.G.Z.; methodology, A.A.K., N.G.Z. and S.N.K.; software, S.N.K. and N.G.Z.; validation, N.A.G., L.O.S., A.V.A. and I.A.C. (Irina A. Chvala); formal analysis, N.A.G., A.V.A. and R.Y.; investigation, L.O.S., A.V.A. and A.A.K.; resources, D.B.A. and I.A.C. (Ilya A. Chvala); data curation, D.B.A. and I.A.C. (Ilya A. Chvala); writing—original draft preparation, N.A.G. and N.G.Z.; writing—review and editing, N.A.G., N.G.Z., S.N.K. and R.Y.; visualization, N.A.G. and S.N.K.; supervision, D.B.A. and I.A.C. (Ilya A. Chvala); project administration, D.B.A. and I.A.C. (Ilya A. Chvala); funding acquisition, D.B.A. and I.A.C. (Ilya A. Chvala). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Federal Service for Veterinary and Phytosanitary Surveillance to implement the objectives of state work “Identification of pathogens of transboundary animal diseases and study of their biological properties” (No. 123032100012-1 (2023) and 124032800009-3 (2024)).
Institutional Review Board Statement
This research was conducted on clinical diagnostic samples submitted for diagnostic testing. No permission was required to use the samples. No work with animals was performed in this study.
Data Availability Statement
The article shows the numbers under which the data was deposited in GenBank.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- International Committee on Taxonomy of Viruses: ICTV. Available online: https://ictv.global/taxonomy/taxondetails?taxnode_id=202401591&taxon_name=Orthoavulavirus%20javaense#release_35 (accessed on 9 September 2025).
- Dimitrov, K.M.; Abolnik, C.; Afonso, C.L.; Albina, E.; Bahl, J.; Berg, M.; Briand, F.X.; Brown, I.H.; Choi, K.S.; Chvala, I.; et al. Updated unified phylogenetic classification system and revised nomenclature for Newcastle disease virus. Infect. Genet. Evol. 2019, 74, 103917. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Cong, Y.; Yin, R.; Sun, Y.; Ding, C.; Yu, S.; Liu, X.; Hu, S.; Qian, J.; Yuan, Q.; et al. Genetic diversity of the genotype VII Newcastle disease virus: Identification of a novel VIIj sub-genotype. Virus Genes 2017, 53, 63–70. [Google Scholar] [CrossRef]
- Wang, J.Y.; Liu, W.H.; Ren, J.J.; Tang, P.; Wu, N.; Wu, H.Y.; Ching, C.D.; Liu, H.J. Characterization of emerging Newcastle disease virus isolates in China. Virol. J. 2015, 12, 119. [Google Scholar] [CrossRef]
- Sabouri, F.; Vasfi Marandi, M.; Bashashati, M. Characterization of a novel VIIl sub-genotype of Newcastle disease virus circulating in Iran. Avian Pathol. 2018, 47, 90–99. [Google Scholar] [CrossRef]
- Makki, F.; Boroomand, Z.; Mayahi, M.; Seyfi Abad Shapouri, M.R. Characterization of Newcastle disease virus in broiler flocks with respiratory symptoms in some provinces of Iran. Mol. Biol. Rep. 2021, 48, 7281–7291. [Google Scholar] [CrossRef]
- Liu, Y.-P.; Chang, C.-Y.; Lee, F.; Chiou, C.-J.; Tsai, H.-J. Phylogenetic analysis of avian paramyxoviruses 1 isolated in Taiwan from 2010 to 2018 and evidence for their intercontinental dispersal by migratory birds. J. Vet. Med. Sci. 2020, 82, 1366–1375. [Google Scholar] [CrossRef]
- Kabir, H.; Hakim, H.; Alizada, M.N.; Hasan, A.; Miyaoka, Y.; Yamaguchi, M.; Shoham, D.; Takehara, K. Isolation, Identification, and Molecular Characterization of Newcastle Disease Virus from Field Outbreaks in Chickens in Afghanistan. Avian Dis. 2022, 66, 176–180. [Google Scholar] [CrossRef]
- Almubarak, A.I.A. Molecular and biological characterization of some circulating strains of Newcastle disease virus in broiler chickens from Eastern Saudi Arabia in 2012–2014. Vet. World 2019, 12, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Al-Zuhariy, M.T.B. Isolation and identification of the Newcastle disease virus from field outbreaks in broiler and layer flocks in Iraq. Open J. Vet. Med. 2017, 41, 23–27. [Google Scholar] [CrossRef]
- Abd Elfatah, K.S.; Elabasy, M.A.; El-Khyate, F.; Elmahallawy, E.K.; Mosad, S.M.; El-Gohary, F.A.; Abdo, W.; Al-Brakati, A.; Seadawy, M.G.; Tahoon, A.E.; et al. Molecular Characterization of Velogenic Newcastle Disease Virus (Sub-Genotype VII.1.1) from Wild Birds, with Assessment of Its Pathogenicity in Susceptible Chickens. Animals 2021, 11, 505. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.M.; Samy, A.; Soliman, M.A.; Arafa, A.; Zanaty, A.; Hassan, M.K.; Sultan, A.H.; Bazid, A.I.; Hussein, A.H. Genotypic and pathogenic characterization of genotype VII Newcastle disease viruses isolated from commercial farms in Egypt and evaluation of heterologous antibody responses. Arch. Virol. 2017, 162, 1985–1994. [Google Scholar] [CrossRef]
- Abd El-Hamid, H.S.; Shafi, M.E.; Albaqami, N.M.; Ellakany, H.F.; Abdelaziz, N.M.; Abdelaziz, M.N.; Abd El-Hack, M.E.; Taha, A.E.; Alanazi, K.M.; Elbestawy, A.R. Sequence analysis and pathogenicity of Avian Orthoavulavirus 1 strains isolated from poultry flocks during 2015–2019. BMC Vet. Res. 2020, 16, 253. [Google Scholar] [CrossRef]
- Putri, N.; Ernawati, R.; Rahmahani, J.; Suwarno, S.; Rantam, F.A. Phylogenetic relationship and genotype variation of six Newcastle disease viruses isolated from duck in Indonesia. Vet. World 2021, 14, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Mase, M.; Inoue, T.; Imada, T. Genotyping of Newcastle Disease Viruses Isolated from 2001 to 2007 in Japan. J. Vet. Med. Sci. 2009, 71, 1101–1104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choi, K.S.; Kye, S.J.; Kim, J.Y.; To, T.L.; Nguyen, D.T.; Lee, Y.J.; Choi, J.G.; Kang, H.M.; Kim, K.I.; Song, B.M.; et al. Molecular epidemiology of Newcastle disease viruses in Vietnam. Trop. Anim. Health Prod. 2013, 46, 271–277. [Google Scholar] [CrossRef]
- Fuller, C.; Löndt, B.; Dimitrov, K.M.; Lewis, N.; van Boheemen, S.; Fouchier, R.; Coven, F.; Goujgoulova, G.; Haddas, R.; Brown, I. An Epizootiological Report of the Re-emergence and Spread of a Lineage of Virulent Newcastle Disease Virus into Eastern Europe. Transbound. Emerg. Dis. 2017, 64, 1001–1007. [Google Scholar] [CrossRef]
- Saputri, M.E.; Poetri, O.N.; Soejoedono, R.D. Phylogenetic studies of Newcastle disease virus isolated from poultry flocks in South Sulawesi Province, Indonesia, in 2019. Adv. Vet. Anim. Res. 2021, 8, 129–137. [Google Scholar] [CrossRef]
- Snoeck, C.J.; Ducatez, M.F.; Owoade, A.A.; Faleke, O.O.; Alkali, B.R.; Tahita, M.C.; Tarnagda, Z.; Ouedraogo, J.B.; Maikano, I.; Mbah, P.O.; et al. Newcastle disease virus in West Africa: New virulent strains identified in non-commercial farms. Arch. Virol. 2009, 154, 47–54. [Google Scholar] [CrossRef]
- Mapaco, L.P.; Monjane, I.V.; Nhamusso, A.E.; Viljoen, G.J.; Dundon, W.G.; Achá, S.J. Phylogenetic analysis of Newcastle disease viruses isolated from commercial poultry in Mozambique (2011–2016). Virus Genes 2016, 52, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Kgotlele, T.; Modise, B.; Nyange, J.F.; Thanda, C.; Cattoli, G.; Dundon, W.G. First molecular characterization of avian paramyxovirus-1 (Newcastle disease virus) in Botswana. Virus Genes 2020, 56, 646–650. [Google Scholar] [CrossRef]
- da Silva, A.P.; Aston, E.J.; Chiwanga, G.H.; Birakos, A.; Muhairwa, A.P.; Kayang, B.B.; Kelly, T.; Zhou, H.; Gallardo, R.A. Molecular Characterization of Newcastle Disease Viruses Isolated from Chickens in Tanzania and Ghana. Viruses 2020, 12, 916. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, A.; Shabbat, M.; Cohen, A.; From, I.; Almog Samina, Y.; Sade Levy, E.; Szer, S.; Zeltcer, N.; Klement, E.; Pima, Y. Point mutation revealed the resurgence of sub-genotype VII.2 Newcastle disease virus in Israel. Avian Pathol. 2022, 51, 236–243. [Google Scholar] [CrossRef]
- Nasir, S.; Wajid, A.; Naureen, A.; Mustafa, A.; Ayub, G.; Ain, Q.; Din, A.M.; Batool, A.; Hussain, T. Isolation and phylogenetic analysis of Avian orthoavulavirus 1 sub-genotypes VII.2 and XXI.1.2 from caged birds in the Lahore district, Pakistan—Short communication. Acta Vet. Hung. 2021, 70, 73–76. [Google Scholar]
- Alsahami, A.A.; Ideris, A.; Omar, A.; Ramanoon, S.Z.; Sadiq, M.B. Isolation, identification and molecular characterization of Newcastle disease viruses in vaccinated chickens from commercial farms in the Sultanate of Oman. Int. J. Vet. Sci. Med. 2018, 6, 248–252. [Google Scholar] [CrossRef]
- Deka, P.; Nath, M.K.; Das, S.; Das, B.C.; Phukan, A.; Lahkar, D.; Bora, B.; Shokeen, K.; Kumar, A.; Deka, P. A study of risk factors associated with Newcastle disease and molecular characterization of genotype XIII Newcastle disease virus in backyard and commercial poultry in Assam, India. Res. Vet. Sci. 2022, 150, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Steensels, M.; Van Borm, S.; Mertens, I.; Houdart, P.; Rauw, F.; Roupie, V.; Snoeck, C.J.; Bourg, M.; Losch, S.; Beerens, N.; et al. Molecular and virological characterization of the first poultry outbreaks of Genotype VII.2 velogenic avian orthoavulavirus type 1 (NDV) in North-West Europe, BeNeLux, 2018. Transbound. Emerg. Dis. 2020, 68, 2147–2160. [Google Scholar] [CrossRef]
- Dimitrov, K.M.; Bolotin, V.; Muzyka, D.; Goraichuk, I.V.; Solodiankin, O.; Gerilovych, A.; Stegniy, B.; Goujgoulova, G.V.; Silko, N.Y.; Pantin-Jackwood, M.J.; et al. Repeated isolation of virulent Newcastle disease viruses of sub-genotype VIId from backyard chickens in Bulgaria and Ukraine between 2002 and 2013. Arch. Virol. 2016, 161, 3345–3353. [Google Scholar] [CrossRef] [PubMed]
- Pchelkina, I.P.; Kolosov, S.N.; Repin, P.I.; Andriyasov, A.V.; Andreychuk, D.B.; Chvala, I.A. Characterization of Newcastle disease virus isolates recovered in the Kaliningrad Oblast in 2013. Proc. Fed. Cent. Anim. Health 2014, 12, 77–85. [Google Scholar]
- Shagurina, Y.V.; Kolosov, S.N.; Andriyasov, A.V.; Chvala, I.A. Genetic analysis of Newcastle disease virus isolates detected in the Republic of Crimea in 2016–2017. In Agrarian Science—Towards Agricultural Production of Siberia, Mongolia, Kazakhstan, Belarus and Bulgaria, Proceedings of the XX International Research-to-Practice Conference, Novosibirsk, Russia, 4–6 October 2017; Volume Part 2; Sibirsky: Novosibirsk, Russia, 2017; pp. 48–50. [Google Scholar]
- Diel, D.G.; da Silva, L.H.; Liu, H.; Wang, Z.; Miller, P.J.; Afonso, C.L. Genetic diversity of avian paramyxovirus type 1: Proposal for a unified nomenclature and classification system of Newcastle disease virus genotypes. Infect. Genet. Evol. 2012, 12, 1770–1779. [Google Scholar] [CrossRef]
- Mayahi, V.; Esmaelizad, M. Molecular evolution and epidemiological links study of Newcastle disease virus isolates from 1995 to 2016 in Iran. Arch. Virol. 2017, 162, 3727–3743. [Google Scholar] [CrossRef]
- Molouki, A.; Mehrabadi, M.H.F.; Bashashati, M.; Akhijahani, M.M.; Lim, S.H.E.; Hajloo, S.A. NDV subgenotype VII(L) is currently circulating in commercial broiler farms of Iran, 2017–2018. Trop. Anim. Health Prod. 2019, 51, 1247–1252. [Google Scholar] [CrossRef]
- Molouki, A.; Sotani, M.; Fallah Mehrabadi, M.H.; Shoushtari, A.; Abtin, A.; Mahmoudzadeh Akhijahani, M.; Abdoshah, M.; Pourbakhsh, S.A.; Allahyari, E.; Ghalyanchilangeroudi, A.; et al. Predominance of Fourth Panzootic Newcastle Disease Virus Subgenotype VII.1.1 in Iran and Its Relation to the Genotypes Circulating in the Region. Curr. Microbiol. 2021, 78, 3068–3078. [Google Scholar] [CrossRef]
- Wise, M.G.; Suarez, D.L.; Seal, B.S.; Pedersen, J.C.; Senne, D.A.; King, D.J.; Kapczynski, D.R.; Spackman, E. Development of a real-time reverse-transcription PCR for detection of newcastle disease virus RNA in clinical samples. J. Clin. Microbiol. 2004, 42, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Shafaati, M.; Ghorbani, M.; Mahmodi, M.; Ebadi, M.; Jalalirad, R. Molecular evaluation and genetic characterisation of Newcastle disease virus’s haemagglutinin-neuraminidase protein isolated from broiler chickens in Iran. Vet. Med. Sci. 2022, 8, 219–228. [Google Scholar] [CrossRef]
- Miller, P.J.; Kim, L.M.; Ip, H.S.; Afonso, C.L. Evolutionary dynamics of Newcastle disease virus. Virology 2009, 391, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.J.; Banks, J.; Collins, M.S.; Manvell, R.J.; Frost, K.M.; Speidel, E.C.; Aldous, E.W. Antigenic and genetic characterisation of Newcastle disease viruses isolated from outbreaks in domestic fowl and turkeys in Great Britain during 1997. Vet. Rec. 1999, 145, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.Q.; Chen, L.G.; Wu, L.L.; Liu, X.F. Newcastle disease in geese: Natural occurrence and experimental infection. Avian Pathol. 2004, 33, 216–221. [Google Scholar] [CrossRef]
- Ramey, A.M.; Reeves, A.B.; Ogawa, H.; Ip, H.S.; Imai, K.; Bui, V.N.; Yamaguchi, E.; Silko, N.Y.; Afonso, C.L. Genetic diversity and mutation of avian paramyxovirus serotype 1 (Newcastle disease virus) in wild birds and evidence for intercontinental spread. Arch. Virol. 2013, 158, 2495–2503. [Google Scholar] [CrossRef]
- Nourani, E.; Kaboli, M.; Collen, B. An Assessment of Threats to Anatidae in Iran. Bird Conserv. Int. 2015, 25, 242–257. [Google Scholar] [CrossRef]
- Karamendin, K.; Kydyrmanov, A.; Kasymbekov, Y.; Daulbayeva, K.; Khan, E.; Seidalina, A.; Sayatov, M.; Gavrilov, A.; Fereidouni, S. Cormorants as Potential Victims and Reservoirs of Velogenic Newcastle Disease Virus (Orthoavulavirus-1) in Central Asia. Avian Dis. 2019, 63, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Goraichuk, I.V.; Gerilovych, A.; Bolotin, V.; Solodiankin, O.; Dimitrov, K.M.; Rula, O.; Muzyka, N.; Mezinov, O.; Stegniy, B.; Kolesnyk, O.; et al. Genetic diversity of Newcastle disease viruses circulating in wild and synanthropic birds in Ukraine between 2006 and 2015. Front. Vet. Sci. 2023, 10, 1026296. [Google Scholar] [CrossRef] [PubMed]
- Ellakany, H.F.; Elbestawy, A.R.; Abd El-Hamid, H.S.; Zedan, R.E.; Gado, A.R.; Taha, A.E.; Soliman, M.A.; Abd El-Hack, M.E.; Swelum, A.A.; Saadeldin, I.M.; et al. Role of Pigeons in the Transmission of Avian Avulavirus (Newcastle Disease-Genotype VIId) to Chickens. Animals 2019, 9, 338. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).