Abstract
The transmission of pathogens through contact with contaminated surfaces is an important route for the spread of infections. The recent outbreak of COVID-19 highlights the necessity to attenuate surface-mediated transmission. Currently, the disinfection and sanitization of surfaces are commonly performed in this regard. However, there are some disadvantages associated with these practices, including the development of antibiotic resistance, viral mutation, etc.; hence, a better strategy is necessary. In recent years, peptides have been studied to be utilized as a potential alternative. They are part of the host immune defense and have many potential in vivo applications in drug delivery, diagnostics, immunomodulation, etc. Additionally, the ability of peptides to interact with different molecules and membrane surfaces of microorganisms has made it possible to exploit them in ex vivo applications such as antimicrobial (antibacterial and antiviral) coatings. Although antibacterial peptide coatings have been studied extensively and proven to be effective, antiviral coatings are a more recent development. Therefore, this study aims to highlight antiviral coating strategies and the current practices and application of antiviral coating materials in personal protective equipment, healthcare devices, and textiles and surfaces in public settings. Here, we have presented a review on potential techniques to incorporate peptides in current surface coating strategies that will serve as a guide for developing cost-effective, sustainable and coherent antiviral surface coatings. We further our discussion to highlight some challenges of using peptides as a surface coating material and to examine future perspectives.
1. Introduction
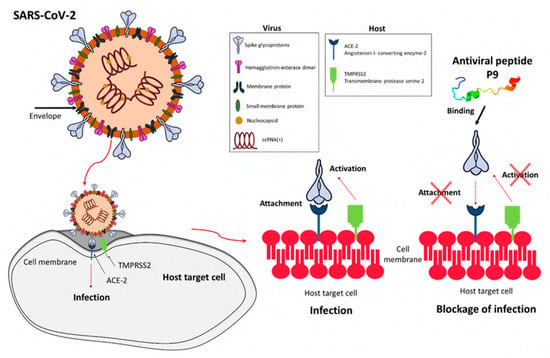
In light of the continuing global pandemic caused by COVID-19, there is a greater focus on implementing the safest possible procedures to prevent the transmission of viruses. The COVID-19 pandemic affected people on a global scale and had lasting repercussions for individuals, communities, and societies in terms of health, economics, society, culture, etc. Therefore, preventing the transmission of viruses is essential for protecting human health, easing the burden on healthcare systems, and maintaining economic stability. Viruses are self-contained biological organisms consisting of a DNA or RNA core surrounded by a protein shell [1]. Viruses can be classified by their nucleic acid (RNA or DNA) [2], lipid membrane [3], shape [4], etc. Lipid membrane classification is the most common, and they can be classified as either enveloped, meaning that their viral particle is surrounded by a lipid membrane, or non-enveloped. Viruses cannot multiply until they infect a host cell [5]. Envelopes are often formed from the plasma membrane of the host cell during budding, the process by which viruses leave their host cell [6]. The envelope is then modified by the addition of proteins (glycoproteins) in the form of spikes, which aid in the virus’s entry into host cells and, in conjunction with the envelope, play a variety of roles in virus–host interactions [7]. This mechanism is demonstrated in Figure 1. While the envelope is important for the process of budding from the cell, it also facilitates structural flexibility and serves to mask capsid spike antigens from antibodies produced by the host [8]. Non-enveloped viruses can invade cells via a number of endocytic pathways that ultimately result in plasma membrane penetration, or via the internal penetration of membranes [9]. This capability to avoid the host’s immune response could be a key factor in the spread of viral infections.
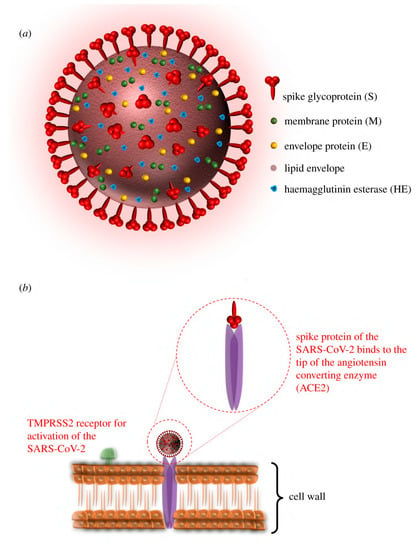
Figure 1.
Illustration of (a) the structure and proteins of the coronavirus, and (b) the mechanism for cell entry via spike proteins (adapted with permission from Aydogdu et al. [10]).
Viruses are capable of rapid mutation when they enter and proliferate within their host. The virus is transferred and propagated among humans by various routes, including air, direct contact, body fluid transmission, and indirectly through contaminated shared surfaces. In addition, many viruses are transmitted to humans by animals or insects. Respiratory viruses such as influenza [11] and SARS-CoV [12] are spread through droplets expelled by an infected host into the air. As observed with SARS-CoV-2, this mode of respiratory virus transmission is fast and difficult to contain [13]. This transmission is shown in Figure 2. Unlike viruses, when bacteria infect a human they tend to stay in one region and spread from there, resulting in what is called a “local infection” [1,14]. Because of this, it is far simpler to treat bacterial infections with novel antimicrobials than it is to treat viral infections. Due to the rapid mutation of the virus’s structure, the creation of viral vaccines is time-consuming and associated with failure risk. In addition to creating conventional vaccinations, novel materials and coatings with broad potency against multiple microorganisms are necessary.
Figure 2.
Schematic representation of how respiratory viruses spread through droplets (adapted with permission from Otter et al. [15]).
It is well-established that contaminated surfaces are a significant factor in the dissemination of viral diseases [16,17]. Surface contact can spread viruses such as influenza, Hepatitis B, respiratory syncytial virus (RSV), rhinoviruses, noroviruses, and coronaviruses [18,19]. Because of their ability to cause severe disease and other health problems in humans, viruses have long been considered a growing threat to society [20]. For example, the coronavirus disease (COVID-19) has emerged as a catastrophic threat to global human health [21]. A recent outbreak of Ebola hemorrhagic fever (also known as EHF) (2014) has had a devastating impact on the living species in Africa [22,23]. In recent years, there has been rising concern over the emergence of novel, more dangerous viruses, especially with SARS [24]. This has rekindled interest in the quest to develop surfaces (antimicrobial surfaces) that can inhibit the propagation of viruses and other microorganisms. While several antibacterial coatings have been developed and put into commercial use, relatively little is known about antiviral coatings [25]. Antiviral surface studies are often restricted by the complexity of viral structures, various varieties of viruses, and a lack of molecular understanding of these non-living particles.
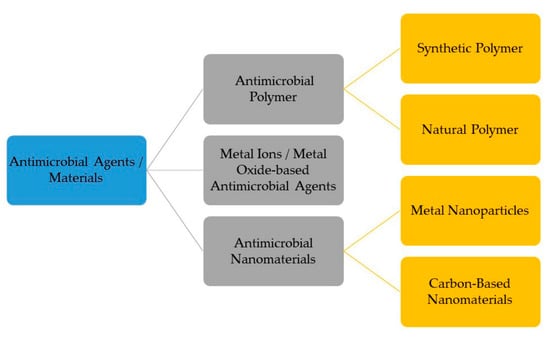
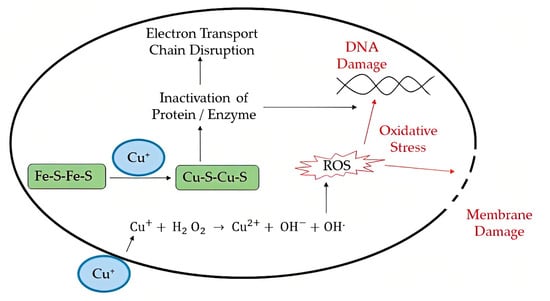
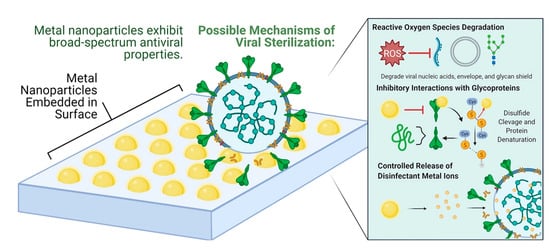
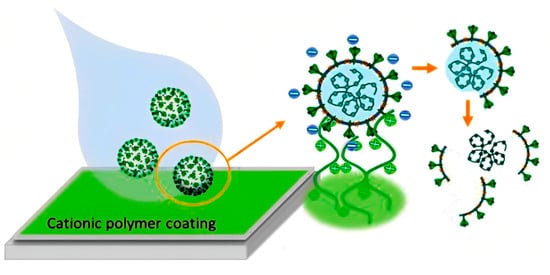
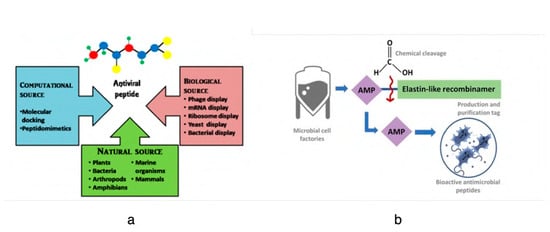
Depending on the conditions, respiratory viruses can live on inanimate environmental surfaces for a long time [17]. The prevalence and spread of infectious viruses can be mitigated through effective cleaning and disinfection methods [26]. There are many different types of antimicrobial coatings, but they all serve the same purpose: to prevent the growth of viruses and other microorganisms on the treated surface. It is important that any antimicrobial coating be able to cover any surface regardless of the applied environmental conditions mechanically, and that it be able to inactivate any virus or microorganism quickly without leaving detrimental consequences for the consumers [27]. Many kinds of substances have been considered for use as antimicrobial coatings. Each option comes with its own set of benefits and drawbacks. For example, metal nanoparticles such as silver nanoparticles are a type of antimicrobial agent normally immobilized or adsorbed onto the target surface. However, silver nanoparticles could be harmful to other life forms. Low-dose exposure to silver nanoparticles causes oxidative stress and mitochondrial dysfunction, according to an in vitro toxicity assessment in rat liver cells [28]. Additionally, silver nanoparticles were found to be harmful to mouse germline stem cells in vitro, where they disrupted mitochondrial function and induced cell membrane leakage [29,30,31]. Copper, on the other hand, may be easily incorporated, e.g., as an alloy or coating, into commonly touched hard surfaces such as door accessories, faucets, stair banisters, and steadying poles in transportation [32]. However, the potential adverse effects of relatively high copper usage must be carefully considered. Neurodegenerative diseases such as Alzheimer’s and Parkinson’s may have a connection to copper toxicity, or copper may contribute to the development of these diseases [33]. One of the most important factors to consider while deploying antimicrobial surfaces is the potential for unintended environmental impacts due to leaching [34]. Nature can serve as a source of inspiration for the development of antiviral techniques, and biopolymers originating from natural sources may provide a promising avenue, similar to the discovery of naturally occurring antibiotics such as antimicrobial peptides (AMPs) [5]. The skin acts as a physical barrier to protect against the outside environment and as the body’s first line of defense against pathogenic microorganisms such as bacteria, viruses, and fungi. The epithelial surface of the skin contains the cellular and performed biochemical components that make up the innate immune system. In the skin, soluble peptides known as antimicrobial peptides (AMPs) play a crucial role in the innate immune system’s defense against pathogens [35]. In this review, the peptides that have potency against viruses will be termed antiviral peptides (AVP).
Antiviral peptides (AVPs) are short chains of amino acids that have the ability to inhibit viral infections by acting on different stages of the virus life cycle. AVPs belong to the broad class of antimicrobial peptides (AMPs), which are part of the immune system of all living organisms. AVPs are gaining prominence as novel therapeutic targets, as the peptides have antiviral efficacy to inhibit viral infection directly and indirectly. Studies suggest that AVPs have the capacity to target various steps in the viral life cycle, from attachment to the host cells to the viral replication system in the host. However, the mechanism of action and target inhibition sites of these AVPs in the viral replication cycle varies depending on the type of peptide and the viral pathogen. AVPs can be obtained from either natural, synthetic, or recombinant sources. Synthetic AVPs are created by artificially adding chemical groups or amino acids to the naturally occurring peptide sequences. As with AMPs, the naturally occurring AVPs have a net positive charge and are cationic and amphipathic in nature. These AVPs can come from various sources, including plants, bacteria, arthropods, amphibians, marine animals, and mammals, with a wide range of action mechanisms.
Due to low toxicity, high specificity, and negligible side effects, AVPs have become attractive novel therapeutic options. The surface material, its characteristics, and the environment around it are all important contributors to long-term viral persistence. The type of surface, its porosity, and its adsorption sites can all have an impact on how long viruses remain on surfaces. Physical factors such as temperature, humidity, and surface roughness also contribute to virus persistence. Viral persistence may also be facilitated by biological variables, including the presence of other bacteria, biofilms, or biological fluids such as saliva or mucus droplets. Lastly, chemical factors such as pH, reactive species, or the presence of organic materials can also make it worse as it has been shown that many viruses can be stabilized by the presence of organic materials such as lipids and proteins in the environment [36]. It is important to tailor the surface coatings specifically for the virus type because each type interacts with the surface differently. Material properties, along with environmental factors, should be considered for designing efficient antiviral coatings. This literature review focuses on peptides, which are natural substances, and their antimicrobial properties, primarily to bring attention to the antiviral peptide as a novel material in coating strategies. The current coating techniques and the potential of antiviral peptides in surface coatings are discussed in Section 2 and Section 4, respectively. In addition, the potential challenges of working with antiviral peptides and their future prospects are described.
5. Challenges with Peptide Coatings
Despite the obvious benefits of employing these peptides, there are still a number of challenges in the clinical development of peptide-based anti-infection medicines and coatings. The drawbacks include the enzymatic breakdown of peptides in body fluids, the potential toxicity to host tissue cells or surrounding microorganisms, low water-solubility due to the presence of hydrophobic residue, and high synthesis and handling costs. Additionally, the peptides might lose activity when exposed to environmental factors such as high temperature or UV light exposure while immobilizing peptides on the surface. These peptides mostly have smaller amino acid sequences, and synthetic and recombinantly designed peptides contain even smaller sequences. Then, in vitro and in vivo stability validation in a physiologically simulated environment is necessary before designing antiviral peptide-functionalized coatings. In the presence of human blood serum immobilized peptides seem to aggregate, whereas in water peptide structure is not altered [155]. The increased ionic strength of serum thus affects the stability of the peptide [156]. However, higher ionic strength increased the antibacterial properties of the LL-37 peptide [157]. With peptides such as HDPs, a fine balance in concentration is required and above that range the peptides might be toxic to the nearby cells. In therapeutic applications, coating nanoparticles with peptides can improve the distribution, half-life, and lower toxicity of these peptides [75].
Peptide-conjugated surface coatings are proven to be more stable than release-based coatings and free-soluble peptides. Due to the presence of a cleavage site with high arginine and lysine content, these cationic peptides have a shorter half-life of a few hours. This challenge can be mitigated by engineering the arginine residues with α-amino-3-guanidino-propionic acid (Agp) [158]. It is promising that RRP9W4N synthetic peptide, when conjugated to an elastin-like polypeptide (ELP) surface, could maintain stability for up to 24 h when incubated in human serum media [155]. Despite these somewhat stable surfaces, the stability of the coating techniques outlined in Section 4 needs to be further studied in order to be improved for practical use. Numerous researchers are attempting to immobilize different antimicrobial peptides on surfaces, including glass, latex, polyethylene, paper, and other materials. The current situation and potential of the usage of all antiviral agents in PPE, face masks, and public areas were summarized by Rakowska et al. [5].
Immobilizing peptides on a surface is not straightforward, as different technical applications have distinct demands for surface materials. Different peptide properties such as specificity against the target virus, orientation during immobilization, selection of spacer or linker, optimal exposure, and not disturbing the secondary structure should be carefully considered. The surface properties, such as porosity, surface charge, energy, etc., can also greatly influence the adhesion and stability of the coating. The functional groups of the peptide play a significant role in selection. As a result, selecting the appropriate immobilization method necessitates taking into account both the material and the peptide being immobilized. It is obvious that both material science and biotechnology will contribute significantly to the creation of novel and realistic coating strategies to contain viral outbreaks. Alongside bacteria, broad-spectrum antiviral approaches should also be researched and investigated thoroughly to help us prepare for and overcome potential viral pandemic challenges in the future.
6. Conclusions
Viral outbreaks entail widespread illness, death, and economic disruption. The focus is usually placed on developing antiviral drugs to curb the impact. However, it has come to the knowledge of the scientific community that infected surfaces contribute greatly to the spread of the virus, and necessary measures need to be taken. The application of disinfectants for surfaces in places such as hospitals and public settings is not a long-lasting option. Conventional surface coatings also have limitations, for example cytotoxicity, susceptibility to microbial fouling, etc., that make them unsuitable for healthcare devices and sensitive surfaces.
Therefore, the introduction of novel materials to develop more effective coatings is necessary. This study aims to present an overview and provide a broad understanding of the current state of knowledge and practices in the area of antiviral coatings to identify areas where further research is needed. To develop a benign, environmentally sustainable and cost-effective coating, a rigorous understanding of their working principles as well as their pros and cons is necessary. As discussed above, current antimicrobial coatings have some limitations that can be overcome by utilizing antimicrobial peptides. This has the potential to achieve tunable surface chemistry that renders enhanced efficacy against microorganisms. However, extensive study is required to make it cost-effective for large-scale production and establish it as a sustainable surface coating material.
Author Contributions
Writing—original draft preparation: M.J., P.B., M.T.I. Writing—review and editing: M.J., P.B., M.T.I., R.P. Supervision: R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
In this review paper, no new data was generated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gelderblom, H.R. Chapter 41: Structure and classification of viruses. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Anderson, T.F. The reactions of bacterial viruses with their host cells. Bot. Rev. 1949, 15, 464–505. [Google Scholar] [CrossRef]
- Lwoff, A.; Tournier, P. The classification of viruses. Annu. Rev. Microbiol. 1966, 20, 45–74. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.H. Bacteriophages; Citeseer, Inter-Science Publishers: New York, NY, USA, 1959. [Google Scholar]
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Zhang, J.; Gilmore, I.S. Antiviral surfaces and coatings and their mechanisms of action. Commun. Mater. 2021, 2, 53. [Google Scholar] [CrossRef]
- Sun, X.; Whittaker, G.R. Role for Influenza Virus Envelope Cholesterol in Virus Entry and Infection. J. Virol. 2003, 77, 12543–12551. [Google Scholar] [CrossRef] [PubMed]
- Bosch, B.J.; van der Zee, R.; de Haan, C.A.; Rottier, P.J.M. The Coronavirus Spike Protein Is a Class I Virus Fusion Protein: Structural and Functional Characterization of the Fusion Core Complex. J. Virol. 2003, 77, 8801–8811. [Google Scholar] [CrossRef] [PubMed]
- Wisskirchen, K.; Lucifora, J.; Michler, T.; Protzer, U. New pharmacological strategies to fight enveloped viruses. Trends Pharmacol. Sci. 2014, 35, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.S. Virus entry: Molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2004, 2, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Aydogdu, M.O.; Altun, E.; Chung, E.; Ren, G.; Homer-Vanniasinkam, S.; Chen, B.; Edirisinghe, M. Surface interactions and viability of coronaviruses. J. R. Soc. Interface 2021, 18, 20200798. [Google Scholar] [CrossRef] [PubMed]
- Brankston, G.; Gitterman, L.; Hirji, Z.; Lemieux, C.; Gardam, M. Transmission of influenza A in human beings. Lancet Infect. Dis. 2007, 7, 257–265. [Google Scholar] [CrossRef]
- Kutter, J.S.; de Meulder, D.; Bestebroer, T.M.; Lexmond, P.; Mulders, A.; Richard, M.; Fouchier, R.A.; Herfst, S. SARS-CoV and SARS-CoV-2 are transmitted through the air between ferrets over more than one meter distance. Nat. Commun. 2021, 12, 1653. [Google Scholar] [CrossRef]
- Kim, Y.I.; Kim, S.G.; Kim, S.M.; Kim, E.H.; Park, S.J.; Yu, K.M.; Chang, J.H.; Kim, E.J.; Lee, S.; Casel, M.A.B.; et al. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe 2020, 27, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Durmus Tekir, S.; Cakir, T.; Ulgen, K. Infection Strategies of Bacterial and Viral Pathogens through Pathogen–Human Protein–Protein Interactions. Front. Microbiol. 2012, 3, 870. [Google Scholar] [CrossRef] [PubMed]
- Otter, J.A.; Donskey, C.; Yezli, S.; Douthwaite, S.; Goldenberg, S.D.; Weber, D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: The possible role of dry surface contamination. J. Hosp. Infect. 2016, 92, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Gralinski, L.E.; Menachery, V.D. Return of the Coronavirus: 2019-nCoV. Viruses 2020, 12, 135. [Google Scholar] [CrossRef]
- Boone, S.A.; Gerba, C.P. Significance of Fomites in the Spread of Respiratory and Enteric Viral Disease. Appl. Environ. Microbiol. 2007, 73, 1687–1696. [Google Scholar] [CrossRef]
- MacDonald, N.E.; Hall, C.B.; Suffin, S.C.; Alexson, C.; Harris, P.J.; Manning, J.A. Respiratory Syncytial Viral Infection in Infants with Congenital Heart Disease. N. Engl. J. Med. 1982, 307, 397–400. [Google Scholar] [CrossRef]
- Winther, B.; McCue, K.; Ashe, K.; Rubino, J.R.; Hendley, J.O. Environmental contamination with rhinovirus and transfer to fingers of healthy individuals by daily life activity. J. Med. Virol. 2007, 79, 1606–1610. [Google Scholar] [CrossRef]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging Infectious Diseases of Wildlife—Threats to Biodiversity and Human Health. Science 2000, 287, 443–449. [Google Scholar] [CrossRef]
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef]
- Muyembe-Tamfum, J.; Mulangu, S.; Masumu, J.; Kayembe, J.; Kemp, A.; Paweska, J.T. Ebola virus outbreaks in Africa: Past and present. Onderstepoort J. Veter Res. 2012, 79, 8. [Google Scholar] [CrossRef]
- Safari, S.; Baratloo, A.; Rouhipour, A.; Ghelichkhani, P.; Yousefifard, M. Ebola Hemorrhagic Fever as a Public Health Emergency of International Concern: A Review Article. Emergency 2015, 3, 3–7. [Google Scholar] [PubMed]
- Phua, J.; Weng, L.; Ling, L.; Egi, M.; Lim, C.-M.; Divatia, J.V.; Shrestha, B.R.; Arabi, Y.M.; Ng, J.; Gomersall, C.D.; et al. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir. Med. 2020, 8, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.E.; Dorhoi, A.; Hotchkiss, R.S.; Bartenschlager, R. Host-directed therapies for bacterial and viral infections. Nat. Rev. Drug Discov. 2017, 17, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Pemmada, R.; Zhu, X.; Dash, M.; Zhou, Y.; Ramakrishna, S.; Peng, X.; Thomas, V.; Jain, S.; Nanda, H.S. Science-Based Strategies of Antiviral Coatings with Viricidal Properties for the COVID-19 Like Pandemics. Materials 2020, 13, 4041. [Google Scholar] [CrossRef] [PubMed]
- Sassi, H.P.; Sifuentes, L.Y.; Koenig, D.W.; Nichols, E.; Clark-Greuel, J.; Wong, L.F.; McGrath, K.; Gerba, C.P.; Reynolds, K.A. Control of the spread of viruses in a long-term care facility using hygiene protocols. Am. J. Infect. Control 2015, 43, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Hess, K.; Gear Hart, J.M.; Geiss, K.T.; Schlarger, J.J. In Vitro Toxicity of Nanoparticles in BRL 3a Rat Liver Cells. Toxicol Vitr. 2005, 19, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Shirvanimoghaddam, K.; Akbari, M.K.; Yadav, R.; Al-Tamimi, A.K.; Naebe, M. Fight against COVID-19: The case of antivi-ral surfaces. APL Mater. 2021, 9, 031112. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.A.; Lynch, I. Mechanisms of silver nanoparticle release, transformation and tox-icity: A critical review of current knowledge and recommendations for future studies and applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
- Pohanka, M. Copper and copper nanoparticles toxicity and their impact on basic functions in the body. Bratisl. Med. J. 2019, 120, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.A.; Arellano, C.; Pardo, M.; Vera, R.; Gálvez, R.; Cifuentes, M.; Berasain, M.A.; Gómez, M.; Ramírez, C.; Vidal, R.M. Antimicrobial properties of a novel copper-based composite coating with potential for use in healthcare facilities. Antimicrob. Resist. Infect. Control 2019, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Roby, K.D.; Di Nardo, A. Innate immunity and the role of the antimicrobial peptide cathelicidin in inflammatory skin dis-ease. Drug Discov. Today Dis. Mech. 2013, 10, e79–e82. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.E.; Solomon, K.R. Acidity as a factor in leaching of copper, chromium and arsenic from CCA-treated dimension lumber. Environ. Toxicol. Chem. 1990, 9, 1331–1337. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Chakhalian, D.; Shultz, R.B.; Miles, C.E.; Kohn, J. Opportunities for biomaterials to address the challenges ofCOVID-19. J. Biomed. Mater. Res. Part A 2020, 108, 1974–1990. [Google Scholar] [CrossRef]
- Nasri, N.; Rusli, A.; Teramoto, N.; Jaafar, M.; Ku Ishak, K.M.; Shafiq, M.D.; Abdul Hamid, Z.A. Past and Current Progress in the Development of Antiviral/Antimicrobial Polymer Coating towards COVID-19 Prevention: A Review. Polymers 2021, 13, 4234. [Google Scholar] [CrossRef]
- Govind, V.; Bharadwaj, S.; Ganesh, M.R.S.; Vishnu, J.; Shankar, K.V.; Shankar, B.; Rajesh, R. Antiviral properties of copper and its alloys to inactivate covid-19 virus: A review. Biometals 2021, 34, 1217–1235. [Google Scholar] [CrossRef]
- Imani, S.M.; Ladouceur, L.; Marshall, T.; Maclachlan, R.; Soleymani, L.; Didar, T.F. Antimicrobial nanomaterials and coatings: Current mechanisms and future perspectives to control the spread of viruses including SARS-CoV-2. ACS Nano 2020, 14, 12341–12369. [Google Scholar] [CrossRef]
- Rifkind, J.M.; Shin, Y.A.; Heim, J.M.; Eichhorn, G.L. Cooperative disordering of single-stranded polynucleotides through copper crosslinking. Biopolym. Orig. Res. Biomol. 1976, 15, 1879–1902. [Google Scholar] [CrossRef]
- Lin, N.; Verma, D.; Saini, N.; Arbi, R.; Munir, M.; Jovic, M.; Turak, A. Antiviral nanoparticles for sanitizing surfaces: A roadmap to self-sterilizing against COVID-19. Nano Today 2021, 40, 101267. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.P.; Silveira, A.P.; Bonatto, C.C.; Reis, I.G.; Milreu, P.V. Silver nanoparticles as antimicrobial agents: Past, present, and future. In Nanostructures for Antimicrobial Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 577–596. [Google Scholar]
- Gurunathan, S.; Qasim, M.; Choi, Y.; Do, J.T.; Park, C.; Hong, K.; Kim, J.H.; Song, H. Antiviral potential of nanoparti-cles—Can nanoparticles fight against coronaviruses? Nanomaterials 2020, 10, 1645. [Google Scholar] [CrossRef] [PubMed]
- Vasudev, M.C.; Koerner, H.; Singh, K.M.; Partlow, B.P.; Kaplan, D.L.; Gazit, E.; Bunning, T.J.; Naik, R.R. Vertically Aligned Peptide Nanostructures Using Plasma-Enhanced Chemical Vapor Deposition. Biomacromolecules 2014, 15, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Pal, U.; Bayan, S.; Mondal, S.; Ghosh, R.; Darbar, S.; Saha-Dasgupta, T.; Ray, S.K.; Pal, S.K. Nanoceutical Fabric Prevents COVID-19 Spread through Expelled Respiratory Droplets: A Combined Computational, Spectroscopic, and Antimicrobial Study. ACS Appl. Bio Mater. 2021, 4, 5471–5484. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.J.; Slaine, P.D.; Keltie, E.; Palit, S.; McKinnell, S.L.; Longpre, B.E.; Ko, K.R.; Green, J.; Markle, G.; Kim, J.S.; et al. Non-woven textiles formed from contact drawn poly (ethylene oxide) fibers provide tunable filtration and virucidal proper-ties via entrapment of silver nanoparticles. ACS Appl. Polym. Mater. 2021, 3, 4245–4255. [Google Scholar] [CrossRef]
- Assis, M.; Simoes, L.G.P.; Tremiliosi, G.C.; Coelho, D.; Minozzi, D.T.; Santos, R.I.; Vilela, D.C.; Santos, J.R.d.; Ribeiro, L.K.; Rosa, I.L.V.; et al. SiO2-Ag composite as a highly virucidal material: A roadmap that rapidly eliminates SARS-CoV-2. Nanomaterials 2021, 11, 638. [Google Scholar] [CrossRef]
- Chiome, T.J.; Srinivasan, A. Use of antiviral nanocoating in personal protective wear. Int. J. Health Allied Sci. 2020, 9, 62–67. [Google Scholar]
- Ferrari, A.C.; Bonaccorso, F.; Fal’Ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.L.; Palermo, V.; Pugno, N.; et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar] [CrossRef]
- Legge, E.J.; Ahmad, M.; Smith, C.T.; Brennan, B.; Mills, C.A.; Stolojan, V.; Pollard, A.J.; Silva, S.R.P. Physicochemical charac-terisation of reduced graphene oxide for conductive thin films. RSC Adv. 2018, 8, 37540–37549. [Google Scholar] [CrossRef]
- Ye, S.; Shao, K.; Li, Z.; Guo, N.; Zuo, Y.; Li, Q.; Lu, Z.; Chen, L.; He, Q.; Han, H. Antiviral Activity of Graphene Oxide: How Sharp Edged Structure and Charge Matter. ACS Appl. Mater. Interfaces 2015, 7, 21571–21579. [Google Scholar] [CrossRef]
- Ziem, B.; Rahn, J.; Donskyi, I.; Silberreis, K.; Cuellar, L.; Dernedde, J.; Keil, G.; Mettenleiter, T.C.; Haag, R. Polyvalent 2D Entry Inhibitors for Pseudorabies and African Swine Fever Virus. Macromol. Biosci. 2017, 17, 1600499. [Google Scholar] [CrossRef] [PubMed]
- Elazzazy, A.M.; Elbeshehy, E.K.; Betiha, M.A. In vitro assessment of activity of graphene silver composite sheets against multidrug-resistant bacteria and Tomato Bushy Stunt Virus. Trop. J. Pharm. Res. 2018, 16, 2705–2711. [Google Scholar] [CrossRef]
- Reina, G.; Iglesias, D.; Samorì, P.; Bianco, A. Graphene: A Disruptive Opportunity for COVID-19 and Future Pandemics? Adv. Mater. 2021, 33, 2007847. [Google Scholar] [CrossRef] [PubMed]
- Linklater, D.P.; Baulin, V.A.; Juodkazis, S.; Ivanova, E.P. Mechano-bactericidal mechanism of graphene nanomaterials. Interface Focus 2018, 8, 20170060. [Google Scholar] [CrossRef]
- Jain, A.; Duvvuri, L.; Farah, S.; Beyth, N.; Domb, A.; Khan, W. Antimicrobial polymers. Adv. Healthc. Mater. 2014, 3, 1969–1985. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Mouritz, A.P.; Galos, J.; Linklater, D.P.; Ladani, R.B.; Kandare, E.; Crawford, R.J..; Ivanova, E.P. Towards antiviral polymer composites to combat COVID-19 transmission. Nano Select 2021, 2, 2061. [Google Scholar] [CrossRef]
- Farshbaf, M.; Davaran, S.; Zarebkohan, A.; Annabi, N.; Akbarzadeh, A.; Salehi, R. Significant role of cationic polymers in drug delivery systems. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1872–1891. [Google Scholar] [CrossRef]
- Kopecˇek, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef]
- Malmsten, M. Antimicrobial and antiviral hydrogels. Soft Matter. 2011, 7, 8725–8736. [Google Scholar] [CrossRef]
- Thormar, H.; Bergsson, G.; Gunnarsson, E.; Georgsson, G.; Witvrouw, M.; Steingrimsson, O.; De Clercq, E.; Kristmundsdót-tir, T. 976 Hydrogels containing monocaprin have potent microbicidal activities against sexually transmitted viruses and bacteria in vitro. Sex. Transm. Infect. 1999, 75, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Ji, E.; Corbitt, T.S.; Parthasarathy, A.; Schanze, K.S.; Whitten, D.G. Light and Dark-Activated Biocidal Activity of Conjugated Polyelectrolytes. ACS Appl. Mater. Interfaces 2011, 3, 2820–2829. [Google Scholar] [CrossRef] [PubMed]
- Chemburu, S.; Corbitt, T.S.; Ista, L.K.; Ji, E.; Fulghum, J.; Lopez, G.P.; Ogawa, K.; Schanze, K.S.; Whitten, D.G. Light-Induced Biocidal Action of Conjugated Polyelectrolytes Supported on Colloids. Langmuir 2008, 24, 11053–11062. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Z.; Zhu, J.; Tang, Y.; Canady, T.D.; Chi, E.Y.; Schanze, K.S.; Whitten, D.G. Dark Antimicrobial Mechanisms of Cationic Phenylene Ethynylene Polymers and Oligomers against Escherichia coli. Polymers 2011, 3, 1199–1214. [Google Scholar] [CrossRef]
- Boarino, A.; Wang, H.; Olgiati, F.; Artusio, F.; Özkan, M.; Bertella, S.; Razza, N.; Cagno, V.; Luterbacher, J.S.; Klok, H.-A.; et al. Lignin: A Sustainable Antiviral Coating Material. ACS Sustain. Chem. Eng. 2022, 10, 14001–14010. [Google Scholar] [CrossRef]
- Hosseini, M.; Behzadinasab, S.; Benmamoun, Z.; Ducker, W.A. The viability of SARS-CoV-2 on solid surfaces. Curr. Opin. Colloid Interface Sci. 2021, 55, 101481. [Google Scholar] [CrossRef]
- Patel, S.; Akhtar, N. Antimicrobial peptides (AMPs): The quintessential ‘offense and defense’ molecules are more than antimicrobials. Biomed. Pharmacother. 2017, 95, 1276–1283. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef]
- Chianese, A.; Zannella, C.; Monti, A.; De Filippis, A.; Doti, N.; Franci, G.; Galdiero, M. The Broad-Spectrum Antiviral Poten-tial of the Amphibian Peptide AR-23. Int. J. Mol. Sci. 2022, 23, 883. [Google Scholar] [CrossRef]
- Suda, S.; Field, D.; Barron, N. Antimicrobial peptide production and purification. In Protein Chromatography: Methods and Protocols; Springer Nature: Clifton, NJ, USA, 2017; pp. 401–410. [Google Scholar]
- Makowski, M.; Silva, Í.C.; Pais do Amaral, C.; Gonçalves, S.; Santos, N.C. Advances in lipid and metal nanoparticles for antimicrobial peptide delivery. Pharmaceutics 2019, 11, 588. [Google Scholar] [CrossRef] [PubMed]
- Freitas, E.D.; Bataglioli, R.A.; Oshodi, J.; Beppu, M.M. Antimicrobial peptides and their potential application in antiviral coating agents. Colloids Surf. B Biointerfaces 2022, 217, 112693. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, G.; Gabrani, R. Antiviral Peptides: Identification and Validation. Int. J. Pept. Res. Ther. 2020, 27, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.; da Costa, A.; Dias, S.C.; Casal, M.; Machado, R. Production and Purification of Two Bioactive Antimicrobial Peptides Using a Two-Step Approach Involving an Elastin-Like Fusion Tag. Pharmaceuticals 2021, 14, 956. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current ap-plications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Rothan, H.A.; Mohamed, Z.; Suhaeb, A.M.; Rahman, N.A.; Yusof, R. Antiviral cationic peptides as a strategy for innovation in global health therapeutics for dengue virus: High yield production of the biologically active recombinant plectasin pep-tide. OMICS J. Integr. Biol. 2013, 17, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Ajingi, Y.S.; Rukying, N.; Aroonsri, A.; Jongruja, N. Recombinant active Peptides and their Therapeutic functions. Curr. Pharm. Biotechnol. 2022, 23, 645–663. [Google Scholar] [CrossRef]
- Kumar, R.; Ali, S.A.; Singh, S.K.; Bhushan, V.; Mathur, M.; Jamwal, S.; Mohanty, A.K.; Kaushik, J.K.; Kumar, S. Antimicrobial Peptides in Farm Animals: An Updated Review on Its Diversity, Function, Modes of Action and Therapeutic Prospects. Vet. Sci. 2020, 7, 206. [Google Scholar] [CrossRef]
- Yacoub, T.; Rima, M.; Karam, M.; Sabatier, J.-M.; Fajloun, Z. Antimicrobials from Venomous Animals: An Overview. Molecules 2020, 25, 2402. [Google Scholar] [CrossRef]
- Mulder, K.C.L.; Elima, L.A.; Miranda, V.J.; Dias, S.C.; Franco, O.L. Current scenario of peptide-based drugs: The key roles of cationic antitumor and antiviral peptides. Front. Microbiol. 2013, 4, 321. [Google Scholar] [CrossRef]
- Sørensen, O.E.; Follin, P.; Johnsen, A.H.; Calafat, J.; Tjabringa, G.S.; Hiemstra, P.S.; Borregaard, N. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood J. Am. Soc. Hematol. 2001, 97, 3951–3959. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M.; Gennaro, R.; Skerlavaj, B.; Tomasinsig, L.; Circo, R. Cathelicidin Peptides as Candidates for a Novel Class of Antimicrobials. Curr. Pharm. Des. 2002, 8, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Gordon, Y.J.; Huang, L.C.; Romanowski, E.G.; Yates, K.A.; Proske, R.J.; McDermott, A.M. Human Cathelicidin (LL-37), a Multifunctional Peptide, is Expressed by Ocular Surface Epithelia and has Potent Antibacterial and Antiviral Activity. Curr. Eye Res. 2005, 30, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial Peptides in Health and Disease. N. Engl. J. Med. 2002, 347, 1199–1200. [Google Scholar] [CrossRef]
- Fabisiak, A.; Murawska, N.; Fichna, J. LL-37: Cathelicidin-related antimicrobial peptide with pleiotropic activity. Pharmacol. Rep. 2016, 68, 802–808. [Google Scholar] [CrossRef]
- Nizet, V.; Ohtake, T.; Lauth, X.; Trowbridge, J.; Rudisill, J.; Dorschner, R.A.; Pestonjamasp, V.; Piraino, J.; Huttner, K.; Gallo, R.L. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 2001, 414, 454–457. [Google Scholar] [CrossRef]
- Bandurska, K.; Berdowska, A.; Barczyn’ska-Felusiak, R.; Krupa, P. Unique features of human cathelicidin LL-37. Biofactors 2015, 41, 289–300. [Google Scholar] [CrossRef]
- Bals, R.; Wilson, J.M. Cathelicidins—A family of multifunctional antimicrobial peptides. Cell. Mol. Life Sci. CMLS 2003, 60, 711–720. [Google Scholar] [CrossRef]
- Sousa, F.H.; Casanova, V.; Findlay, F.; Stevens, C.; Svoboda, P.; Pohl, J.; Proudfoot, L.; Barlow, P.G. Cathelicidins display con-served direct antiviral activity towards rhinovirus. Peptides 2017, 95, 76–83. [Google Scholar] [CrossRef]
- Bergman, P.; Walter-Jallow, L.; Broliden, K.; Agerberth, B.; Söderlund, J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr. HIV Res. 2007, 5, 410–415. [Google Scholar] [CrossRef]
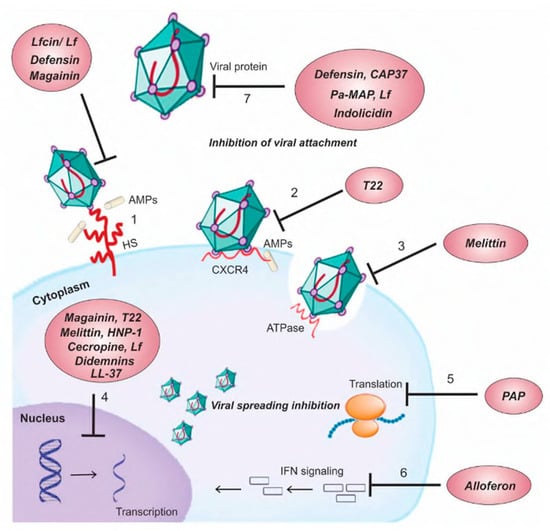
- Xu, D.; Lu, W. Defensins: A Double-Edged Sword in Host Immunity. Front. Immunol. 2020, 11, 764. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.S.; Wiens, M.E.; Smith, J.G. Antiviral Mechanisms of Human Defensins. J. Mol. Biol. 2013, 425, 4965–4980. [Google Scholar] [CrossRef] [PubMed]
- Casanova, V.; Sousa, F.H.; Shakamuri, P.; Svoboda, P.; Buch, C.; D’Acremont, M.; Christophorou, M.A.; Pohl, J.; Stevens, C.; Barlow, P.G. Citrullination Alters the Antiviral and Immunomodulatory Activities of the Human Cathelicidin LL-37 During Rhinovirus Infection. Front. Immunol. 2020, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Kalenik, B.M.; Góra-Sochacka, A.; Sirko, A. Β-defensins—Underestimated peptides in influenza combat. Virus Res. 2018, 247, 10–14. [Google Scholar] [CrossRef]
- Tripathi, S.; Tecle, T.; Verma, A.; Crouch, E.; White, M.; Hartshorn, K. The human cathelicidin LL-37 inhibits influenza A viruses through a mechanism distinct from that of surfactant protein D or defensins. J. Gen. Virol. 2013, 94, 40–49. [Google Scholar] [CrossRef]
- Aloul, K.M.; Nielsen, J.E.; Defensor, E.B.; Lin, J.S.; Fortkort, J.A.; Shamloo, M.; Cirillo, J.D.; Gombart, A.F.; Barron, A.E. Upregulating Human Cathelicidin Antimicrobial Peptide LL-37 Expression May Prevent Severe COVID-19 Inflammatory Responses and Reduce Microthrombosis. Front. Immunol. 2022, 13, 880961. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Li, D.; Wei, D.Q.; Zhao, J.; Wang, J. Human intestinal defensin 5 inhibits SARS-CoV-2 invasion by cloaking ACE2. Gastroenterology 2020, 159, 1145–1147. [Google Scholar] [CrossRef]
- Kudryashova, E.; Zani, A.; Vilmen, G.; Sharma, A.; Lu, W.; Yount, J.S.; Kudryashov, D.S. Inhibition of SARS-CoV-2 infection by human defensin HNP1 and retrocyclin RC-101. J. Mol. Biol. 2022, 434, 167225. [Google Scholar] [CrossRef]
- Tonk, M.; Ržek, D.; Vilcinskas, A. Compelling evidence for the activity of antiviral peptides against SARS-CoV-2. Viruses 2021, 13, 912. [Google Scholar] [CrossRef]
- Tangpricha, V.; Judd, S.E.; Ziegler, T.R.; Hao, L.; Alvarez, J.A.; Fitzpatrick, A.M.; McComsey, G.A.; Eckard, A.R. LL-37 Concentrations and the Relationship to Vitamin D, Immune Status, and Inflammation in HIV-Infected Children and Young Adults. AIDS Res. Hum. Retrovir. 2014, 30, 670–676. [Google Scholar] [CrossRef]
- Wang, G.; Watson, K.M.; Buckheit, R.W., Jr. Anti-Human Immunodeficiency Virus Type 1 Activities of Antimicrobial Peptides Derived from Human and Bovine Cathelicidins. Antimicrob. Agents Chemother. 2008, 52, 3438–3440. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.M.; Hong, T.; Boo, L.M.; Nguyen, T.; Zhao, C.; Bristol, G.; Zack, J.A.; Waring, A.J.; Yang, O.O.; Lehrer, R.I. Retrocy-clin: A primate peptide that protects cells from infection by T-and M-tropic strains of HIV-1. Proc. Natl. Acad. Sci. USA 2002, 99, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury—Nucleoside Analogues. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547852/ (accessed on 1 May 2020).
- Taylor, R.; Kotian, P.; Warren, T.; Panchal, R.; Bavari, S.; Julander, J.; Dobo, S.; Rose, A.; El-Kattan, Y.; Taubenheim, B.; et al. BCX4430–a broad-spectrum antiviral adenosine nucleoside analog under development for the treatment of Ebola virus disease. J. Infect. Public Health 2016, 9, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Warren, T.K.; Jordan, R.; Lo, M.K.; Ray, A.S.; Mackman, R.L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H.C.; et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016, 531, 381–385. [Google Scholar] [CrossRef]
- Yu, Y.; Cooper, C.L.; Wang, G.; Morwitzer, M.J.; Kota, K.; Tran, J.P.; Bradfute, S.B.; Liu, Y.; Shao, J.; Zhang, A.K.; et al. Engi-neered human cathelicidin antimicrobial peptides inhibit Ebola virus infection. iScience 2020, 23, 100999. [Google Scholar] [CrossRef]
- He, M.; Zhang, H.; Li, Y.; Wang, G.; Tang, B.; Zhao, J.; Huang, Y.; Zheng, J. Cathelicidin-Derived Antimicrobial Peptides Inhibit Zika Virus Through Direct Inactivation and Interferon Pathway. Front. Immunol. 2018, 9, 722. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, E.H.; O’Neal, J.T.; Dale, G.; Holthausen, D.J.; Bowen, J.R.; Quicke, K.M.; Skountzou, I.; Gopal, S.; George, S.; et al. The amphibian peptide Yodha is virucidal for Zika and dengue viruses. Sci. Rep. 2021, 11, 602. [Google Scholar] [CrossRef]
- Krepstakies, M.; Lucifora, J.; Nagel, C.-H.; Zeisel, M.B.; Holstermann, B.; Hohenberg, H.; Kowalski, I.; Gutsmann, T.; Baumert, T.F.; Brandenburg, K.; et al. A New Class of Synthetic Peptide Inhibitors Blocks Attachment and Entry of Human Pathogenic Viruses. J. Infect. Dis. 2012, 205, 1654–1664. [Google Scholar] [CrossRef]
- Bastarrachea, L.J.; Goddard, J.M. Antimicrobial Coatings with Dual Cationic and N-Halamine Character: Characterization and Biocidal Efficacy. J. Agric. Food Chem. 2015, 63, 4243–4251. [Google Scholar] [CrossRef]
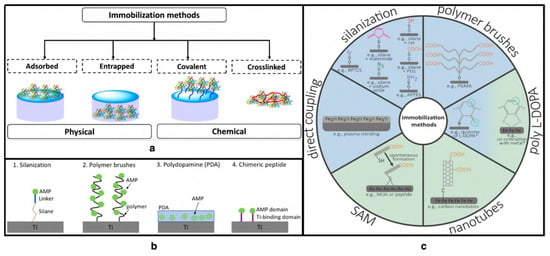
- Bagheri, M.; Beyermann, M.; Dathe, M. Immobilization Reduces the Activity of Surface-Bound Cationic Antimicrobial Peptides with No Influence upon the Activity Spectrum. Antimicrob. Agents Chemother. 2009, 53, 1132–1141. [Google Scholar] [CrossRef]
- Imam, H.T.; Marr, P.C.; Marr, A.C. Enzyme entrapment, biocatalyst immobilization without covalent attachment. Green Chem. 2021, 23, 4980–5005. [Google Scholar] [CrossRef]
- Andrea, A.; Molchanova, N.; Jenssen, H. Antibiofilm Peptides and Peptidomimetics with Focus on Surface Immobilization. Biomolecules 2018, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Stillger, L.; Müller, D. Peptide-coating combating antimicrobial contaminations: A review of covalent immobilization strategies for industrial applications. J. Mater. Sci. 2022, 57, 10863–10885. [Google Scholar] [CrossRef]
- Yu, K.; Lo, J.C.Y.; Mei, Y.; Haney, E.F.; Siren, E.; Kalathottukaren, M.T.; Hancock, R.E.; Lange, D.; Kizhakkedathu, J.N. Toward Infection-Resistant Surfaces: Achieving High Antimicrobial Peptide Potency by Modulating the Functionality of Polymer Brush and Peptide. ACS Appl. Mater. Interfaces 2015, 7, 28591–28605. [Google Scholar] [CrossRef] [PubMed]
- Hilpert, K.; Elliott, M.; Jenssen, H.; Kindrachuk, J.; Fjell, C.D.; Körner, J.; Winkler, D.F.; Weaver, L.L.; Henklein, P.; Ulrich, A.S.; et al. Screening and Characterization of Surface-Tethered Cationic Peptides for Antimicrobial Activity. Chem. Biol. 2009, 16, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Carvalho, I.F.; Montelaro, R.C.; Gomes, P.; Martins, M.C.L. Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 2011, 7, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Pinto, S.; Evangelista, M.B.; Gil, H.; Kallip, S.; Ferreira, M.G.; Ferreira, L. High-density antimicrobial peptide coating with broad activity and low cytotoxicity against human cells. Acta Biomater. 2016, 33, 64–77. [Google Scholar] [CrossRef]
- Jeong, G.M.; Seong, H.; Im, S.G.; Sung, B.H.; Kim, S.C.; Jeong, K.J. Coating of an antimicrobial peptide on solid substrate via initiated chemical vapor deposition. J. Ind. Eng. Chem. 2018, 58, 51–56. [Google Scholar] [CrossRef]
- Steven, M.D.; Hotchkiss, J.H. Covalent immobilization of an antimicrobial peptide on poly(ethylene) film. J. Appl. Polym. Sci. 2008, 110, 2665–2670. [Google Scholar] [CrossRef]
- Rapsch, K.; Bier, F.F.; Tadros, M.; Von Nickisch-Rosenegk, M. Identification of Antimicrobial Peptides and Immobilization Strategy Suitable for a Covalent Surface Coating with Biocompatible Properties. Bioconjugate Chem. 2014, 25, 308–319. [Google Scholar] [CrossRef]
- Chen, G.; Zhou, M.; Chen, S.; Lv, G.; Yao, J. Nanolayer biofilm coated on magnetic nanoparticles by using a dielectric barrier discharge glow plasma fluidized bed for immobilizing an antimicrobial peptide. Nanotechnology 2009, 20, 465706. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lee, C.W.; Kim, H.J.; Jung, H.-H.; Kim, J.I.; Shin, S.Y.; Shin, S.-H. Structural analysis and mode of action of BMAP-27, a cathelicidin-derived antimicrobial peptide. Peptides 2019, 118, 170106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mulvenon, A.; Makarov, E.; Wagoner, J.; Knibbe, J.; Kim, J.O.; Osna, N.; Bronich, T.K.; Poluektova, L.Y. Antiviral peptide nanocomplexes as a potential therapeutic modality for HIV/HCV co-infection. Biomaterials 2013, 34, 3846–3857. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Willcox, M.; Cole, N.; Ho, K.K.; Rasul, R.; Denman, J.; Kumar, N. Characterization of chemoselective surface attachment of the cationic peptide melimine and its effects on antimicrobial activity. Acta Biomater. 2012, 8, 4371–4379. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.M.; Maia, S.R.; Gomes, P.A.; Martins, M.C.L. Dhvar5 antimicrobial peptide (AMP) chemoselective covalent im-mobilization results on higher antiadherence effect than simple physical adsorption. Biomaterials 2015, 52, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.; Mishra, A. Exploring potential of glass surface immobilized short antimicrobial peptide (AMP) as antibacterial coatings. Mater. Today Proc. 2021, 49, 1367–1377. [Google Scholar] [CrossRef]
- Appendini, P.; Hotchkiss, J.H. Surface modification of poly(styrene) by the attachment of an antimicrobial peptide. J. Appl. Polym. Sci. 2001, 81, 609–616. [Google Scholar] [CrossRef]
- Héquet, A.; Humblot, V.; Berjeaud, J.-M.; Pradier, C.-M. Optimized grafting of antimicrobial peptides on stainless steel surface and biofilm resistance tests. Colloids Surfaces B Biointerfaces 2011, 84, 301–309. [Google Scholar] [CrossRef]
- Stepulane, A.; Rajasekharan, A.K.; Andersson, M. Multifunctional Surface Modification of PDMS for Antibacterial Contact Killing and Drug-Delivery of Polar, Nonpolar, and Amphiphilic Drugs. ACS Appl. Bio Mater. 2022, 5, 5289–5301. [Google Scholar] [CrossRef]
- Yasir, M.; Dutta, D.; Hossain, K.R.; Chen, R.; Ho, K.K.K.; Kuppusamy, R.; Clarke, R.J.; Kumar, N.; Willcox, M.D.P. Mechanism of Action of Surface Immobilized Antimicrobial Peptides Against Pseudomonas aeruginosa. Front. Microbiol. 2020, 10, 3053. [Google Scholar] [CrossRef]
- Atefyekta, S. Antibacterial Surface Coatings for Biomedical Applications. Ph.D. Thesis, Chalmers Tekniska Hogskola, Göteborg, Sweden, 2016. [Google Scholar]
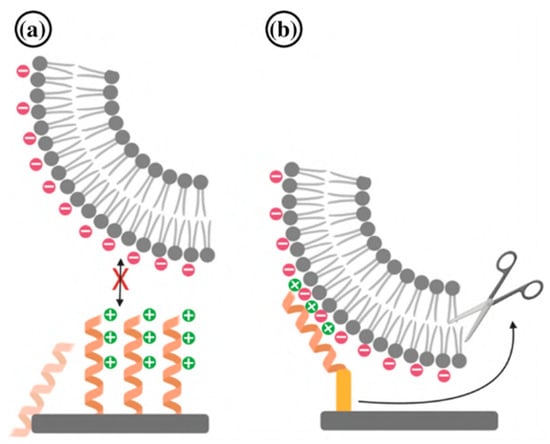
- Townsend, L.; Williams, R.L.; Anuforom, O.; Berwick, M.R.; Halstead, F.; Hughes, E.; Stamboulis, A.; Oppenheim, B.; Gough, J.; Grover, L.; et al. Antimicrobial peptide coatings for hydroxyapatite: Electrostatic and covalent attachment of antimicrobial peptides to surfaces. J. R. Soc. Interface 2017, 14, 20160657. [Google Scholar] [CrossRef]
- Xiao, M.; Jasensky, J.; Gerszberg, J.; Chen, J.; Tian, J.; Lin, T.; Lu, T.; Lahann, J.; Chen, Z. Chemically immobilized anti-microbial peptide on polymer and self-assembled monolayer substrates. Langmuir 2018, 34, 12889–12896. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.; Kooi, S.; Chang, S.; Sedransk, K.; Gleason, K. Initiated chemical vapor deposition of antimicrobial polymer coatings. Biomaterials 2006, 28, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Soliman, W.; Bhattacharjee, S.; Kaur, K. Adsorption of an Antimicrobial Peptide on Self-Assembled Monolayers by Molecular Dynamics Simulation. J. Phys. Chem. B 2010, 114, 11292–11302. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Kobe, A.C.; Sang, T.; Aparicio, C. Unraveling dominant surface physicochemistry to build antimicrobial peptide coatings with supramolecular amphiphiles. Nanoscale 2020, 12, 20767–20775. [Google Scholar] [CrossRef] [PubMed]
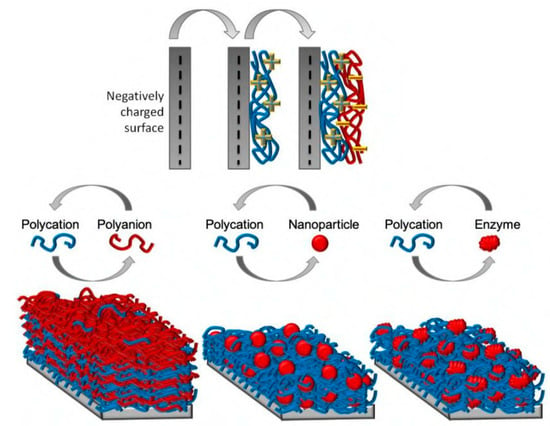
- Escobar, A.; Muzzio, N.; Moya, S.E. Antibacterial Layer-by-Layer Coatings for Medical Implants. Pharmaceutics 2020, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Fleming, K.E.; Chuang, H.F.; Chau, T.M.; Loose, C.R.; Stephanopoulos, G.N.; Hammond, P.T. Controlling the release of peptide antimicrobial agents from surfaces. Biomaterials 2010, 31, 2348–2357. [Google Scholar] [CrossRef]
- Cao, M.; Zhao, W.; Wang, L.; Li, R.; Gong, H.; Zhang, Y.; Xu, H.; Lu, J. CAS: 528: DC% 2BC1cXht1eksb7L: Graphene ox-ide-assisted accumulation and layer-by-layer assembly of antibacterial peptide for sustained release applications. ACS Appl. Mater. Interfaces 2018, 10, 24937–24946. [Google Scholar] [CrossRef]
- Otto, D.P.; de Villiers, M.M. Layer-by-layer nanocoating of antiviral polysaccharides on surfaces to prevent coronavirus infections. Molecules 2020, 25, 3415. [Google Scholar] [CrossRef]
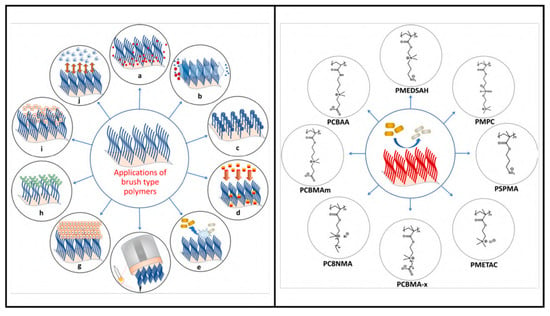
- Açarı, İ.K.; Sel, E.; Özcan, İ.; Ateş, B.; Köytepe, S.; Thakur, V.K. Chemistry and engineering of brush type polymers: Perspective towards tissue engineering. Adv. Colloid Interface Sci. 2022, 305, 102694. [Google Scholar] [CrossRef]
- Muszanska, A.K.; Rochford, E.T.J.; Gruszka, A.; Bastian, A.A.; Busscher, H.J.; Norde, W.; Van Der Mei, H.C.; Herrmann, A. Antiadhesive Polymer Brush Coating Functionalized with Antimicrobial and RGD Peptides to Reduce Biofilm Formation and Enhance Tissue Integration. Biomacromolecules 2014, 15, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Song, L.; Luan, S.; Xin, Z.; Du, S.; Shi, H.; Yuan, S.; Yang, Y.; Yin, J. A hierarchical polymer brush coating with du-al-function antibacterial capability. Colloids Surf. B Biointerfaces 2017, 150, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, J.; Xue, Y.; Ding, T.; Zhu, S.; Mao, M.; Zhang, L.; Han, Y. Polymer brush grafted antimicrobial peptide on hydroxyapatite nanorods for highly effective antibacterial performance. Chem. Eng. J. 2021, 423, 130133. [Google Scholar] [CrossRef]
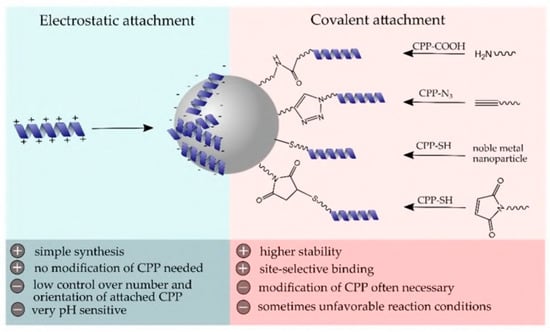
- Alghrair, Z.K.; Fernig, D.G.; Ebrahimi, B. Enhanced inhibition of influenza virus infection by peptide–noble-metal nano-particle conjugates. Beilstein J. Nanotechnol. 2019, 10, 1038–1047. [Google Scholar] [CrossRef]
- Baram-Pinto, D.; Shukla, S.; Richman, M.; Gedanken, A.; Rahimipour, S.; Sarid, R. Surface-modified protein nanospheres as potential antiviral agents. Chem. Commun. 2012, 48, 8359–8361. [Google Scholar] [CrossRef]
- Gessner, I.; Neundorf, I. Nanoparticles Modified with Cell-Penetrating Peptides: Conjugation Mechanisms, Physicochemical Properties, and Application in Cancer Diagnosis and Therapy. Int. J. Mol. Sci. 2020, 21, 2536. [Google Scholar] [CrossRef]
- da Costa, A.; Pereira, A.M.; Sampaio, P.; Rodríguez-Cabello, J.C.; Gomes, A.C.; Casal, M.; Machado, R. Protein-Based Films Functionalized with a Truncated Antimicrobial Peptide Sequence Display Broad Antimicrobial Activity. ACS Biomater. Sci. Eng. 2021, 7, 451–461. [Google Scholar] [CrossRef]
- Lima, L.F.; Sousa, M.G.D.C.; Rodrigues, G.R.; de Oliveira, K.B.S.; Pereira, A.M.; da Costa, A.; Machado, R.; Franco, O.L.; Dias, S.C. Elastin-like Polypeptides in Development of Nanomaterials for Application in the Medical Field. Front. Nanotechnol. 2022, 1169, 31. [Google Scholar] [CrossRef]
- Atefyekta, S.; Pihl, M.; Lindsay, C.; Heilshorn, S.C.; Andersson, M. Antibiofilm elastin-like polypeptide coatings: Functionality, stability, and selectivity. Acta Biomater. 2019, 83, 245–256. [Google Scholar] [CrossRef]
- Dominy, B.N.; Perl, D.; Schmid, F.X.; Brooks III, C.L. The effects of ionic strength on protein stability: The cold shock protein family. J. Mol. Biol. 2002, 319, 541–554. [Google Scholar] [CrossRef]
- Strömstedt, A.A.; Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Evaluation of Strategies for Improving Proteolytic Resistance of Antimicrobial Peptides by Using Variants of EFK17, an Internal Segment of LL-37. Antimicrob. Agents Chemother. 2009, 53, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Knappe, D.; Henklein, P.; Hoffmann, R.; Hilpert, K. Easy Strategy To Protect Antimicrobial Peptides from Fast Degradation in Serum. Antimicrob. Agents Chemother. 2010, 54, 4003–4005. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).