Abstract
Severe fever with thrombocytopenia syndrome (SFTS) and Japanese spotted fever (JSF; a spotted fever group rickettsiosis) are tick-borne zoonoses that are becoming a significant public health threat in Japan and East Asia. Strategies for treatment and infection control differ between the two; therefore, initial differential diagnosis is important. We aimed to compare the clinical characteristics of SFTS and JSF based on symptomology, physical examination, laboratory data, and radiography findings at admission. This retrospective study included patients with SFTS and JSF treated at five hospitals in Nagasaki Prefecture, western Japan, between 2013 and 2020. Data from 23 patients with SFTS and 38 patients with JSF were examined for differentiating factors and were divided by 7:3 into a training cohort and a validation cohort. Decision tree analysis revealed leukopenia (white blood cell [WBC] < 4000/μL) and altered mental status as the best differentiating factors (AUC 1.000) with 100% sensitivity and 100% specificity. Using only physical examination factors, absence of skin rash and altered mental status resulted in the best differentiating factors with AUC 0.871, 71.4% sensitivity, and 90.0% specificity. When treating patients with suspected tick-borne infection, WBC < 4000/µL, absence of skin rash, and altered mental status are very useful to differentiate SFTS from JSF.
1. Introduction
Severe fever with thrombocytopenia syndrome (SFTS) is a tick-borne infectious disease caused by the SFTS virus (SFTSV or Dabie bandavirus, a novel genus Banyangvirus of the family Phenuiviridae that was first reported in China in 2011 [1,2]. Since then, many cases have been reported in west Asia, China, Korea, and Japan [3] and are classified as viral hemorrhagic fever [4]. The mortality rate of SFTS ranges from 5–40% [4,5,6,7,8]. Around 60–100 cases of SFTS have been reported annually since 2013 in Japan [9], with endemic areas localized in western Japan.
Spotted fever group rickettsioses are another emerging tick-borne infectious disease that are widely distributed throughout the world, including Rocky Mountain spotted fever in North America, Mediterranean spotted fever on the Mediterranean coast, Queensland tick typhus in Australia, and Japanese spotted fever (JSF) in Japan [10]. JSF is caused by Alphaproteobacteria, belonging to the spotted fever group of the genus Rickettsia [10]. JSF was first reported in Tokushima Prefecture, western Japan, in 1984 [11]. Since then, it has been reported in South Korea, Thailand, the Philippines, and China [12,13], and its related species have also been reported [14,15]. The number of JSF cases in Japan has increased since 2016 to 250–350 cases reported annually [16,17] with a mortality rate from 1–4% [17,18].
Although scrub typhus, another tick-borne infection caused by Orientia tsutsugamushi, is reported in 400–500 cases annually across Japan throughout the year [19], SFTS and JSF are major tick-borne infections especially prevalent in western Japan during the summer season. They are category IV infectious diseases that require the reporting of all cases under the Japanese Infectious Diseases Control Law (criteria for notification: [20,21]). The vector tick species that carry both SFTS and JSF are Haemaphysalis longicornis, H. flava, and Amblyomma testudinarium [1,17,22,23,24], and the two tick-borne infections have similar epidemiological and geographic distributions [16]. Despite similar clinical manifestations such as fever, tick bites, and neurological and gastrointestinal symptoms [5,17,25,26], different treatment regimens and infection-control strategies are applied to each infection; therefore, clinical differentiation between SFTS and JSF is particularly important [4,17]. JSF may be treated with antibiotics; however, effective antiviral therapy has not been established for SFTS. Human-to-human transmission of SFTSV may occur through blood or bodily secretions [27,28,29,30]. Additionally, their diagnosis requires serological or polymerase chain reaction (PCR) testing to confirm the causative pathogens, which are not easily accessed and require time to perform and analyze [8]. Early diagnosis of SFTS and JSF is important due to the potentially fatal nature of the diseases. Differential diagnosis at the initial clinical presentation is useful in planning the management of treatment and infection control.
In this study, we conducted a retrospective comparison of clinical manifestations, laboratory data, and radiologic features of patients with SFTS and JSF hospitalized in Nagasaki, a southwestern prefecture of Japan. Based on decision tree analysis, we identified potential significant factors for differential diagnosis of SFTS and JSF.
2. Materials and Methods
2.1. Study Population and Clinical Data
We retrospectively reviewed the medical records of patients aged ≥20 years diagnosed with laboratory-confirmed SFTS and JSF between January 2013 and December 2020 at five hospitals (two tertiary medical institutions: Nagasaki University Hospital and Sasebo City General Hospital; three secondary medical institutions: Isahaya General Hospital, Sasebo Chuo Hospital, and Nagasaki Rosai Hospital) in Nagasaki Prefecture, Japan. The medical records of all enrolled patients were reviewed. Data on baseline characteristics, clinical presentations, laboratory findings, and radiological findings at admission were collected from the medical records. In-hospital complications and outcomes were assessed.
2.2. Definitions
All definitions used in the study were established before the data analyses. An episode of SFTS was defined as SFTSV, in which viral RNA was confirmed by real-time PCR analysis of a serum sample, as previously described [31]. The diagnosis of JSF was defined as either a 4-fold increase in immunoglobulin G (IgG) in the serum sample with immunofluorescence assay, PCR analysis of a blood sample, or tissue biopsy (eschar) [32]. Altered mental status was defined as Japan coma scale ≥1 points [33]. Acute kidney injury (AKI) was diagnosed according to the Kidney Disease: Improving Global Outcomes definition, as described previously [34]. Chronic kidney disease (CKD) was defined as a reduced glomerular filtration rate (<60 mL/min/1.73 m2) for more than 3 months. [35] Immunosuppressants included corticosteroids and other immunosuppressive agents. Disseminated intravascular coagulation (DIC) scores were calculated using the Japanese Association for Acute Medicine (JAAM) DIC scoring system [36]. Hemophagocytic lymphohistiocytosis (HLH) was defined using HLH-2004 criteria [37].
Complications and treatments were respectively determined as those that developed or were initiated after the initial visit or the date of hospital admission.
2.3. Evaluation of the Chest Radiograph and Computed Tomography (CT)
Two Japanese Respiratory Society (JRS) board-certified pulmonologists each with 15 years of experience reviewed all chest radiographs and CT examinations independently and arrived at a consensus. Cardiomegaly was defined as a cardiothoracic (CTR) ratio > 0.50, based on posterior-anterior (PA) chest radiographs, or a CTR ratio > 0.55 on anterior-posterior (AP) chest radiographs [31]. Abnormalities on chest CT were characterized by consolidation, ground-glass opacity (GGO), centrilobular nodules, interstitial septal thickening, and bronchial wall thickening. The presence of mediastinal lymph node enlargement (>10 mm along the short axis), pleural effusion, pericardial effusion (pericardial thickness of 4 mm or more [31]), hepatomegaly (diameter of >16.0 cm at craniocaudal line [31]), splenomegaly (width measurement of >10.5 cm [31]), and additional lung findings were also recorded.
2.4. Statistical Analysis
The results are expressed as means ± standard deviations and as percentages. Categorical variables were analyzed using Fisher’s exact test, and continuous variables were analyzed using a t-test or Wilcoxon rank sum test. All tests were two-tailed, and differences were considered significant at a p-value < 0.05 or less than the value adjusted by Bonferroni correction.
All cases were divided by 7:3 into a training cohort and a validation cohort. In the training data, a decision tree was built to differentiate SFTS from JSF using variables that were significantly different on bivariate analysis and missing values less than 20%. In addition, we performed a decision tree on each continuous variable one by one to determine cut off values and applied them to categorical data.
The discriminatory power of the differentiation models was evaluated by assessing the area under the receiver operating characteristic curve (AUC), sensitivity, specificity, positive predictive value, and negative predictive value. All analyses were performed using JMP® Pro 16.2.0 predictive analysis software (SAS Institute Inc., Cary, NC, USA).
2.5. Ethical Considerations
The study protocol was approved by the Institutional Review Board of Nagasaki University Hospital (approval number 18121024), Isahaya General Hospital (approval number, 2020-21), Sasebo City General Hospital (approval number 2020-A024), Sasebo Chuo Hospital (approval number 2020-17), and Nagasaki Rosai Hospital (approval number 02003). The institutional review board waived the requirement for informed consent from patients included in this study because of its retrospective design. The summary and information of this study have been disclosed, following the regulations of each participating hospital.
3. Results
3.1. Study Population
Data from a total of 61 hospitalized patients (23 with SFTS and 38 with JSF) at five hospitals during the study period were reviewed. All 23 cases of SFTS were confirmed by PCR testing of blood samples; 2 cases of JSF were confirmed by both the presence of increased Rickettsia japonica antibody levels and PCR test of blood and/or tissue sample, 12 cases by antibody test only, and the remaining 24 cases by PCR testing of blood and/or tissue samples. One case of JSF, diagnosed by antibodies to R. japonica and PCR of blood, was a case of internationally imported infection, with elevated antibodies to R. cornorii and R. africae other than R. japonica, and the coexistence of rickettsiosis infection was suspected.
3.2. Patients’ Baseline Characteristics
The baseline patient characteristics are presented in Table 1. The elderly population was predominant in both the SFTS (mean 70.6 years) and JSF groups (mean 68.8 years). There were no significant differences between the two groups in terms of male sex or underlying diseases. Immunosuppressants were used in two cases of SFTS: corticosteroids and adalimumab. Patients who were farmers, hunters, or living or working in wooded and hilly areas comprised 50.0% of SFTS patients and 27.8% of JSF patients. Patients in both groups were diagnosed frequently in spring and summer, though incidence of SFTS was lower (8.7%) in autumn compared to that of JSF (34.2%). No patients were diagnosed during winter.

Table 1.
Clinical characteristics in patients with SFTS or JSF.
3.3. Patients’ Clinical Symptoms
Table 2 presents the clinical symptoms and physical findings of patients with SFTS or JSF at admission. Approximately 5 and 6 days in patients with SFTS and JSF, respectively, passed between onset of illness and admission. Patients with SFTS were significantly more frequently reporting altered mental status (60.9% vs. 21.1%, p = 0.003) and diarrhea (52.4% vs. 10.5%, p = 0.001) compared to patients with JSF. In contrast, skin rash (34.8% vs. 100%, p < 0.0001) and tick bites (42.9% vs. 88.9%, p = 0.001) were more frequently observed in patients with JSF. With regard to vital signs, most patients in both groups were febrile. There was no difference between the two groups in terms of respiratory rate or SpO2/FiO2 ratio. The qSOFA scores were comparable between the two groups.

Table 2.
Symptoms and physical findings at admission in patients with SFTS or JSF.
3.4. Labortory Findings
The laboratory findings on admission are presented in Table 3. With regard to peripheral blood cell counts, patients with SFTS showed significantly lower numbers of white blood cells (WBCs; mean: 1821.3/μL vs. 7383.9/μL, p < 0.0001) and neutrophils (mean; 1190.8/μL vs. 6176.0/μL, p < 0.0001) compared to those in patients with JSF. Higher atypical lymphocyte ratio (mean: 2.0% vs. 0.2%, p = 0.001) and thrombocytopenia (mean: 58.4 × 103/μL vs. 113.1 × 103/μL, p = 0.001) were observed in the SFTS group. Inflammatory markers such as C-reactive protein (CRP; mean: 0.8 mg/dL vs. 12.3 mg/dL, p < 0.0001), and soluble IL-2 receptor (sIL-2R; mean: 1451.6 IU/L vs. 4521.0 IU/L, p = 0.017) were higher in the JSF group. Highly elevated liver enzymes including aspartate aminotransferase (AST; mean: 328.3 IU/L vs. 88.9 IU/L, p < 0.0001), alanine aminotransferase (ALT; mean: 113.5 IU/L vs. 54.2 IU/L, p = 0.001), and lactate dehydrogenase (LDH; mean: 801.5 IU/L vs. 389.4 IU/L, p = 0.001) were more prevalent in the SFTS group compared to those in the JSF group. Prolonged activated partial thromboplastin time (APTT; mean: 48.0 s vs. 36.9 s, p = 0.001) was seen in the SFTS group compared to the JSF group, though fibrinogen was higher in JFS group (mean: 212.7 mg/dL vs. 382.6 mg/dL, p = 0.001).

Table 3.
Laboratory findings on admission in patients with SFTS or JSF.
3.5. Radiologic Findings
The radiological findings are presented in Table 4. Chest radiograph was performed in 58 patients (95.1%). Chest CT examinations were performed in 22 patients (95.7%) with SFTS and 17 patients (44.7%) with JSF. CT scans were performed at an average of 5–6 days after symptom onset, with an average of 0–1 day after admission with 1 mm (n = 28), 2 mm (n = 5), 3 mm (n = 2), or 5 mm (n = 4) slices at the spine position.

Table 4.
Chest CT findings in patients with SFTS and JSF.
A total of 19 patients with SFTS (86.4%) and 13 patients (76.5%) with JSF had abnormal chest CT findings. Interstitial septal thickening (68.2% vs. 64.7%) and ground glass opacity (GGO) (54.6% vs. 52.9%) were the most frequently found chest CT abnormalities in SFTS and JSF patients, though no differences between groups were identified. Findings of centrilobular nodules and bronchial wall thickening were likely more frequent in the SFTS group (36.4% vs. 11.8%, p = 0.140, 36.4% vs. 5.9%, p = 0.052) than the JSF group. Pleural effusion was likely more frequently detected in patients with JSF than those with SFTS (22.7% vs. 58.8%, p = 0.045). Enlarged mediastinal lymph nodes were detected only in patients with SFTS (13.6%).
3.6. Outcomes and Treatments
Supplementary Table S1 shows the outcomes, complications, and treatments of the patients with SFTS and JSF. The mortality rate of patients with SFTS (26.1%) was higher that of patients with JSF (0.0%) (p = 0.002). The length of hospital stay was longer (mean; 34.5 days vs. 15.9 days, p = 0.015) and the ICU admission rate was higher (mean; 30.4% vs. 7.9%, p = 0.032) in patients with SFTS than those in patients with JSF.
With regard to in-hospital complications, secondary bacterial infections occurring more frequently in the SFTS group than in the JSF group (mean: 26.1% vs. 5.3%, p = 0.044) consisted of bacteremia (n = 4) caused by Streptococcus epidermidis or methicillin-resistant S. capitis, followed by pneumonia (n = 2), and urinary tract infections (n = 1) caused by Pseudomonas aeruginosa. Secondary fungal infections, although not statistically significant, were also more frequent in patients with SFTS (17.4%). These infections include invasive aspergillosis, disseminated trichosporonosis, candidiasis, and pulmonary cryptococcosis. One case of SFTSV meningitis and another case of rickettsial meningitis were observed in the study population. A total of 11 (47.8%) and 16 (69.6%) cases of patients with SFTS developed hemophagocytic lymphohistiocytosis (HLH) or disseminated intravascular coagulation (DIC), respectively. The diagnosis of 9 of 11 cases of HLH in patients with SFTS was made by bone marrow biopsy.
All patients with SFTS were administered antibiotics against rickettsial infections at admission until the SFTSV diagnosis was confirmed. Antiviral agents such as favipiravir and ribavirin were used in five (27.3%) and two (8.7%) patients with SFTS, respectively, based on clinical trials or off-label use [26,38]. All patients with JSF received tetracyclines, including minocycline in 36 cases, doxycycline in 1 case, and a combination of minocycline and doxycycline in 1 case. A total of 12 (31.6%) of the patients with JSF were treated with a combination of quinolones including levofloxacin (n = 9) and ciprofloxacin (n = 3). Corticosteroids, G-CSF, immunoglobulin, and thrombomodulin were more frequently administered to patients with SFTS than to those with JSF, reflecting the severity of the disease. Mechanical ventilation was performed in 27.3% of patients with SFTS, which was higher compared to use in patients with JSF (5.3%).
3.7. Decision Tree Analysis for Differentiation between JSF and SFTS
To investigate factors that could differentiate between SFTS and JSF at the initial hospital visit, all cases were divided into a 7:3 test cohort (n = 44) and a validation cohort (n = 17). A decision tree analysis was performed by applying variables with significant differences in bivariate analysis that excluded variables not available in >20% of patients for a training cohort group. In the training cohort, the final classification using decision tree analysis extracted only two variables: WBC < 4000/μL and altered mental status. A WBC < 4000/μL led to SFTS probability of 100% (16/16). In the case that the patients with WBC > 4000/μL have altered mental status, the possibility of SFTS was 14.3% (Figure 1A). For the validation cohort, the area under the curve (AUC) by the Receiver Operating Characteristic (ROC) of the decision tree was 1.000.
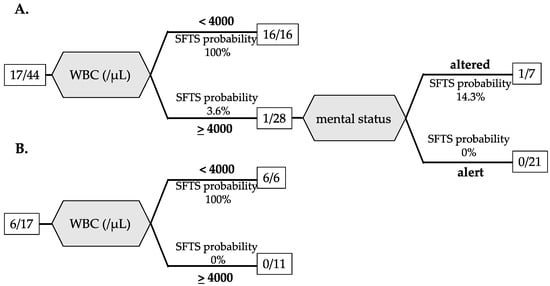
Figure 1.
Decision tree classification of SFTS and JSF based on laboratory data and physical examination for the training cohort (A) and the validation cohort (B). The number in the square is the number of SFTS patients/total patients.
In addition, since blood tests are not available quickly in some situations, another decision tree analysis was performed to distinguish SFST from JSF based only on physical findings with significant differences by bivariate analysis among the training cohort and is shown in Figure 2A. A decision tree analysis using only physical findings extracted two variables: skin rash and altered mental status. Absence of skin rash led to SFTS probability of 100% (12/12). When skin rash was present, altered mental status increased the SFTS probability to 50% (2/4). AUC by ROC of the decision tree was 0.871 with 71.4% sensitivity and 90.0% specificity.
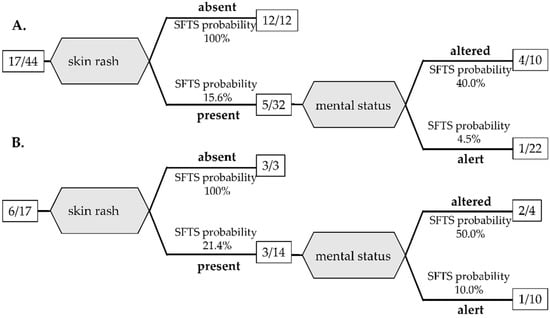
Figure 2.
Decision tree classification of SFTS and JSF based on physical examination for the training cohort (A) and the validation cohort (B). The number in the square is the number of SFTS patients/total patients.
Next, we evaluated each variable for which there was a significant difference in bivariate analysis and in cases with missing values less than 20% in Table 2 and Table 3. In the analysis, continuous variables were changed to categorical data, with cutoff values determined for each using the decision tree analysis among the training cohort group described in the first column of Table 5. AUC, sensitivity, specificity, positive predictive value, and negative predictive values were calculated by applying these variables in the validation cohort group (Table 5). In the validation cohort, WBC count < 4000/μL (p < 0.0001, AUC 1.000), neutrophil count < 2042/μL (p < 0.0001, AUC 1.000), CRP < 4.17 mg/dL (p = 0002, AUC 0.833), and platelet count < 64 × 103/μL (p = 0.003, AUC 0.818) had excellent discriminatory ability with AUC > 0.8.

Table 5.
Sensitivity, specificity, positive predictive value and negative predictive value for differentiating SFTS from JSF.
Due to the small number of patients in the validation cohort, the decision tree analysis derived from all patients in the study and detected AUC, sensitivity, and specificity of each variable are shown in Supplementary Figures S1 and S2, and Supplementary Table S2.
4. Discussion
SFTS and JSF are tick-borne diseases that are geographically, seasonally, and clinically similar [39]. Definitive diagnosis of these diseases is time-consuming, and only a few university hospitals or specialized facilities in Japan are able to perform SFTSV PCR. Differentiating between these two infections is important because their treatment is different, and more importantly, SFTSV is capable of nosocomial transmission [27,28].
The present study revealed that SFTS and JSF did not differ in patients’ underlying conditions; however, many symptoms, such as altered mental status and diarrhea were more frequently found in patients with SFTS. These symptoms may be associated with viremia in SFTS [30,31]. Skin rash and tick bite were frequently found in patients with JFS. Comparison of chest CT findings between SFTS and JSF revealed that bronchogenic infiltration, such as centrilobular nodules and bronchial wall thickening, was likely to occur more frequently in patients with SFTS, which may be due to secondary bacterial or fungal pulmonary infections [31,40]. Notably, abnormal findings on chest CT were found in around 80% of the SFTS group; ground glass opacity, and interstitial septal thickening was detected in more than 50% of patients. Hospitalized patients with SFTS or JSF frequently develop pulmonary edema that may be caused by systemic inflammation [31,40,41,42]. Our study is the first to compare chest radiologic findings between SFTS and JSF; however, the results indicated that radiographical differentiation between these two infectious diseases was difficult.
Many laboratory data values at admission were different between patients with SFTS and those with JSF. Among them, leukopenia of WBC < 4000/µL, as supported by a previous report [8], was the most remarkable variable that was able to distinguish SFTS from JSF with sensitivity of 100%, specificity of 100%, and AUC 1.000. Peripheral blood count is a basic examination that can be performed in most facilities, and results can be obtained within one hour. Altered mental status was detected in more than 60% of SFTS patients in our study, a slight increase from the previously reported 51% [8]. The possibility of SFTS cannot be ruled out if altered mental status is present even when WBC is above 4000 /μL, based on our cohort. If a patient with suspected tick-borne disease has an elevated WBC (≥4000 /μL) but an altered mental status, it is recommended to test for SFTS at the same time as treating rickettsial disease.
We also attempted to differentiate SFTS from JSF based on physical examination alone, assuming a situation where blood tests are not available immediately. Our results showed that the presence or absence of a skin rash was the most important physical finding in differentiating between the two, and the absence of a skin rash was most likely in SFTS patients (100%). Even in the presence of a rash, altered mental status was associated with a high probability of SFTS (50%).
To our best knowledge, only one previous report discussed the differentiation between JSF and SFTS. Kawaguchi et al. [8] reported that CRP level ≤ 1.0 mg/dL was the most useful variable for differentiating SFTS from JSF. The decision tree in our study did not keep CRP in the final node; however, CRP < 4.17 mg/dL itself had sensitivity of 83.3%, specificity of 90.9%, and AUC 0.833 in our study. In Supplementary Table S1, we also performed a decision tree for each variable among all cases in our study, and the cutoff value of CRP 1.78 mg/dL (sensitivity of 87.0%, specificity of 100.0%, and AUC 0.935) for differentiation was similar to that previously reported [8]. Although the incidence of scrub typhus in our study period was low and comparisons could not be made, it is reported that scrub typhus and JSF are both rickettsial diseases and result in similar symptoms and blood test data [43,44]. Table 6 lists previous reports which differentiated between SFTS and other tick-borne infectious diseases such as JSF and scrub typhus. Some previous studies also supported our findings that leukopenia (WBC < 4000 /μL) and altered mental status are significant differentials for SFTS.
Our study had several limitations. First, we enrolled hospitalized patients from secondary and tertiary medical institutions. Among 40 cases of SFTS and 109 cases of JSF diagnosed in our area according to the Infectious Diseases Weekly Report of Nagasaki Prefecture between 2013 and 2020 [45,46], our study included 23/40 (57.5%) of patients with SFTS and 38/109 (34.8%) of patients with JSF. Selection bias, especially for patients with JSF, may have occurred because hospitalized severe cases were mainly included in the study. In fact, our data show a higher percentage with 45.2% of SFTS patients in tertiary medical institutions compared to 21.1% in secondary medical institutions. Second, the data were obtained retrospectively; therefore, missing data values regarding such characteristics as PCT, ferritin, sIL2-R, IgG, and fibrinogen were insufficient for applying to regression analysis and could not be used to determine the significance of these factors in differentiating diseases. In addition, blood tests at the patient’s first visit had variations from the date of infection. This blood time-point of the infection may affect the readout values. Third, scrub typhus, another tick-borne disease, is also prevalent in western Japan, including Nagasaki prefecture, though comparisons could not be made in this study based on the low number of scrub typhus during the study period. In clinical practice, it is necessary to distinguish SFTS from other tick-borne infectious diseases prevalent in the area. A prospective study including mild cases with secured data collection from patients is crucial to substantiate our results and construct a scoring system in real-world practice.

Table 6.
Comparison of studies to differentiate SFTS from other zoonoses including tick-borne.
Table 6.
Comparison of studies to differentiate SFTS from other zoonoses including tick-borne.
| Studies | Study Patients | Differentiation Model | Results |
|---|---|---|---|
| Kim MC et al., 2018. [47] | SFTS (n = 21) scrub typhus (n = 98) | Scoring system (score > 1: SFTS) using 4 factors altered mental status leukopenia (WBC < 4000 /μL) prolonged APTT (>35 s) normal CRP (≤1.0 mg/dL) | score > 1 100% sensitivity 97% specificity AUC 0.995 |
| Sul H et al., 2022. [48] | SFTS (n = 183) scrub typhus (n = 173) | Scoring system (score > 1: SFTS) using 4 factors leukopenia (WBC < 4000 /μL) prolonged APTT (>40 s), normal CRP (≤3.0 mg/dL) elevated CK level (>1000 IU/L) | score > 1 97% sensitivity 98% specificity AUC 0.992 |
| Kawaguchi T et al., 2020. [8] | SFTS (n = 41) JSF (n = 40) | CRP ≤ 1.0 mg/dL | 95% sensitivity 97% specificity AUC 0.96 |
| Heo DH et al., 2020. [49] | SFTS (n = 35) other endemic zoonoses (n = 49) | Scoring system (score ≥ 2: SFTS) using 4 factors neurologic symptom diarrhea leukopenia (WBC < 4000 /μL) normal CRP (<0.5 mg/dL) | score ≥ 2 82.9% sensitivity 95.9% specificity AUC 0.950 |
| Our study | SFTS (n = 23) JSF (n = 38) | Decision tree analysis Pattern A: leukopenia (WBC < 4000 /μL) altered mental status | 100% sensitivity 100% specificity AUC 1.000 |
| Pattern B: absent of skin rash altered mental status | 71.4% sensitivity 90.0% specificity AUC 0.871 |
Abbreviations: SFTS, severe fever with thrombocytopenia syndrome; JSF, Japanese spotted fever; AUC; area under the curve, CI; confidence interval; WBC, white blood cell; APTT, activated partial thromboplastin; CRP, C-reactive protein; CK, creatine kinase.
5. Conclusions
In conclusion, when clinicians differentiate between SFTS and JSF among patients with suspected tick-borne infection, a single item, WBC < 4000/µL, strongly differentiates SFTS from JSF. Additionally, the absence of a skin rash and altered mental status may be helpful for differential diagnosis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v14081807/s1. Supplemental Tables S1 and S2 and Supplemental Figures S1 and S2.
Author Contributions
Conceptualization, K.Y. (Kazuko Yamamoto) and H.M.; methodology, K.Y. (Kazuko Yamamoto); validation, K.Y. (Kazuko Yamamoto) and K.M. (Kouichi Morita); formal analysis, N.N.; investigation, M.T. (Moe Tanaka), H.A., M.Y., A.U., Y.F., S.K. (Shungo Katoh), M.S., S.M., T.K., Y.I., N.A., K.T., S.I., N.I., T.T. (Takahiro Takazono), M.T.(Masato Tashiro), T.T. (Takeshi Tanaka), S.N., K.M. (Konosuke Morimoto), S.K. (Shintaro Kurihara) and A.F.; resources, K.A., K.I. and H.M.; data curation, N.N., K.Y. (Kazuko Yamamoto), M.T. (Moe Tanaka) and K.M. (Kouichi Morita); writing—original draft, N.N.; writing—review and editing, K.Y. (Kazuko Yamamoto); visualization, N.N. and K.Y. (Kazuko Yamamoto); supervision, K.I. and K.Y. (Katsunori Yanagihara); project administration, K.Y. (Kazuko Yamamoto) and H.M.; funding acquisition, K.Y. (Kazuko Yamamoto) and K.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Non-Profit Organization aimed at supporting community medicine research in Nagasaki under grant number 2020 and by AMED under grant number JP21fk0108081.
Institutional Review Board Statement
This multicenter study was conducted in compliance with the principles of the Declaration of Helsinki and was approved by the ethics committees of the participating institutions (approval number:18121024; Nagasaki University Hospital, approval number:2020-21; Sasebo City General Hospital, approval number:2020-A024; Sasebo Chuo Hospital, approval number:2020-17; Nagasaki Rosai Hospital, approval number:02003).
Informed Consent Statement
The requirement for patient consent was waived owing to the retrospective nature of the study, which ensured anonymity.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank Shuntaro Sato, Clinical Research Center, Nagasaki University Hospital, Nagasaki City, Nagasaki, Japan for advising statistical analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yu, X.J.; Liang, M.F.; Zhang, S.Y.; Liu, Y.; Li, J.D.; Sun, Y.L.; Zhang, L.; Zhang, Q.F.; Popov, V.L.; Li, C.; et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 2011, 364, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.H.; Adkins, S.; Alioto, D.; Alkhovsky, S.V.; Amarasinghe, G.K.; Anthony, S.J.; Avsic-Zupanc, T.; Ayllon, M.A.; Bahl, J.; Balkema-Buschmann, A.; et al. 2020 taxonomic update for phylum Negarnaviricota (Riboviria: Orthornavirae), including the large orders Bunyavirales and Mononegavirales. Arch. Virol. 2020, 165, 3023–3072. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; He, B.; Huang, S.-Y.; Wei, F.; Zhu, X.-Q. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect. Dis. 2014, 14, 763–772. [Google Scholar] [CrossRef]
- Saijo, M. Pathophysiology of severe fever with thrombocytopenia syndrome and development of specific antiviral therapy. J. Infect. Chemother. 2018, 24, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Wang, Q.; Cheng, J.; Hu, B.; Li, J.; Zhan, F.; Song, Y.; Guo, D. Current status of severe fever with thrombocytopenia syndrome in China. Virol. Sin. 2017, 32, 51–62. [Google Scholar] [CrossRef]
- Choi, S.J.; Park, S.W.; Bae, I.G.; Kim, S.H.; Ryu, S.Y.; Kim, H.A.; Jang, H.C.; Hur, J.; Jun, J.B.; Jung, Y.; et al. Severe Fever with Thrombocytopenia Syndrome in South Korea, 2013–2015. PLoS Negl. Trop. Dis. 2016, 10, e0005264. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kato, H.; Yamagishi, T.; Shimada, T.; Matsui, T.; Yoshikawa, T.; Kurosu, T.; Shimojima, M.; Morikawa, S.; Hasegawa, H.; et al. Severe Fever with Thrombocytopenia Syndrome, Japan, 2013–2017. Emerg. Infect. Dis. 2020, 26, 692–699. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Umekita, K.; Yamanaka, A.; Hara, S.; Yamaguchi, T.; Inoue, E.; Okayama, A. Impact of C-Reactive Protein Levels on Differentiating of Severe Fever With Thrombocytopenia Syndrome From Japanese Spotted Fever. Open Forum Infect. Dis. 2020, 7, ofaa473. [Google Scholar] [CrossRef]
- National Institute of Infectious Diseases (NIID). Severe Fever With Thrombocytopenia Syndrome(SFTS). Available online: https://www.niid.go.jp/niid/ja/sfts/3143-sfts.html (accessed on 29 June 2022). (In Japanese)
- Parola, P.; Paddock, C.D.; Raoult, D. Tick-borne rickettsioses around the world: Emerging diseases challenging old concepts. Clin. Microbiol. Rev. 2005, 18, 719–756. [Google Scholar] [CrossRef]
- Mahara, F. Japanese spotted fever: Report of 31 cases and review of the literature. Emerg. Infect. Dis. 1997, 3, 105–111. [Google Scholar] [CrossRef]
- Chung, M.H.; Lee, S.H.; Kim, M.J.; Lee, J.H.; Kim, E.S.; Lee, J.S.; Kim, M.K.; Park, M.Y.; Kang, J.S. Japanese spotted fever, South Korea. Emerg. Infect. Dis. 2006, 12, 1122–1124. [Google Scholar] [CrossRef]
- Li, J.; Hu, W.; Wu, T.; Li, H.B.; Hu, W.; Sun, Y.; Chen, Z.; Shi, Y.; Zong, J.; Latif, A.; et al. Japanese Spotted Fever in Eastern China, 2013. Emerg. Infect. Dis. 2018, 24, 2107–2109. [Google Scholar] [CrossRef]
- Takada, N.; Fujita, H.; Kawabata, H.; Ando, S.; Sakata, A.; Takano, A.; Chaithong, U. Spotted fever group Rickettsia sp. closely related to Rickettsia japonica, Thailand. Emerg. Infect. Dis. 2009, 15, 610–611. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.R.; Han, H.J.; Han, F.J.; Zhao, F.M.; Zhang, Z.T.; Xue, Z.F.; Ma, D.Q.; Qi, R.; Zhao, M.; Wang, L.J.; et al. Rickettsia Japonica and Novel Rickettsia Species in Ticks, China. Emerg. Infect. Dis. 2019, 25, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, K.; Aonuma, H.; Kanuka, H. Distribution of tick-borne diseases in Japan: Past patterns and implications for the future. J. Infect. Chemother. 2018, 24, 499–504. [Google Scholar] [CrossRef]
- National Institute of Infectious Diseases (NIID), Japanese spotted fever 1999–2019. Infect. Agent Surveill. Rep. 2020, 41, 133–135.
- Sakabe, S.; Tanaka, H.; Nakanishi, Y.; Toyoshima, H. The clinical course of 239 cases of Japanese spotted fever in Ise Red Cross Hospital, 2006–2019. J. Infect. Chemother. 2022, 28, 211–216. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Infectious Diseases (NIID). Scrub typhus and Japanese spotted fever in Japan 2007–2016. Infect. Agent Surveill. Rep. 2017, 38, 109–112. [Google Scholar]
- National Institute of Infectious Diseases (NIID). Infectious Agent Surveillance Report. Reporting Criteria of Severe Fever with Thrombocytopenia Syndrome (SFTS) Report. Available online: https://www.niid.go.jp/niid/images/iasr/35/408/de4081.pdf (accessed on 29 June 2022).
- National Institute of Infectious Diseases (NIID). Infectious Agent Surveillance Report. Reporting Criteria for Japanese Spotted Fever. Available online: https://www.niid.go.jp/niid/images/iasr/38/448/de4482.pdf (accessed on 29 June 2022).
- Li, Z.; Bao, C.; Hu, J.; Liu, W.; Wang, X.; Zhang, L.; Ji, Z.; Feng, Z.; Li, L.; Shen, A.; et al. Ecology of the Tick-Borne Phlebovirus Causing Severe Fever with Thrombocytopenia Syndrome in an Endemic Area of China. PLoS Negl. Trop. Dis. 2016, 10, e0004574. [Google Scholar] [CrossRef]
- Luo, L.M.; Zhao, L.; Wen, H.L.; Zhang, Z.T.; Liu, J.W.; Fang, L.Z.; Xue, Z.F.; Ma, D.Q.; Zhang, X.S.; Ding, S.J.; et al. Haemaphysalis Longicornis Ticks as Reservoir and Vector of Severe Fever with Thrombocytopenia Syndrome Virus in China. Emerg. Infect. Dis. 2015, 21, 1770–1776. [Google Scholar] [CrossRef]
- Uchida, T.; Yan, Y.; Kitaoka, S. Detection of Rickettsia japonica in Haemaphysalis longicornis ticks by restriction fragment length polymorphism of PCR product. J. Clin. Microbiol. 1995, 33, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Heo, S.T.; Oh, H.; Oh, S.; Lee, K.H.; Yoo, J.R. Prognostic Factors of Severe Fever with Thrombocytopenia Syndrome in South Korea. Viruses 2020, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lu, Q.-B.; Xing, B.; Zhang, S.-F.; Liu, K.; Du, J.; Li, X.-K.; Cui, N.; Yang, Z.-D.; Wang, L.-Y.; et al. Epidemiological and clinical features of laboratory-diagnosed severe fever with thrombocytopenia syndrome in China, 2011–2017: A prospective observational study. Lancet Infect. Dis. 2018, 18, 1127–1137. [Google Scholar] [CrossRef]
- Gai, Z.; Liang, M.; Zhang, Y.; Zhang, S.; Jin, C.; Wang, S.W.; Sun, L.; Zhou, N.; Zhang, Q.; Sun, Y.; et al. Person-to-person transmission of severe fever with thrombocytopenia syndrome bunyavirus through blood contact. Clin. Infect. Dis. 2012, 54, 249–252. [Google Scholar] [CrossRef]
- Kim, W.Y.; Choi, W.; Park, S.W.; Wang, E.B.; Lee, W.J.; Jee, Y.; Lim, K.S.; Lee, H.J.; Kim, S.M.; Lee, S.O.; et al. Nosocomial transmission of severe fever with thrombocytopenia syndrome in Korea. Clin. Infect. Dis. 2015, 60, 1681–1683. [Google Scholar] [CrossRef]
- Yoo, J.R.; Lee, K.H.; Heo, S.T. Surveillance results for family members of patients with severe fever with thrombocytopenia syndrome. Zoonoses Public Health 2018, 65, 903–907. [Google Scholar] [CrossRef]
- Koga, S.; Takazono, T.; Ando, T.; Hayasaka, D.; Tashiro, M.; Saijo, T.; Kurihara, S.; Sekino, M.; Yamamoto, K.; Imamura, Y.; et al. Severe Fever with Thrombocytopenia Syndrome Virus RNA in Semen, Japan. Emerg. Infect. Dis. 2019, 25, 2127–2128. [Google Scholar] [CrossRef]
- Ashizawa, H.; Yamamoto, K.; Ashizawa, N.; Takeda, K.; Iwanaga, N.; Takazono, T.; Sakamoto, N.; Sumiyoshi, M.; Ide, S.; Umemura, A.; et al. Associations between Chest CT Abnormalities and Clinical Features in Patients with the Severe Fever with Thrombocytopenia Syndrome. Viruses 2022, 14, 279. [Google Scholar] [CrossRef]
- Robinson, M.T.; Satjanadumrong, J.; Hughes, T.; Stenos, J.; Blacksell, S.D. Diagnosis of spotted fever group Rickettsia infections: The Asian perspective. Epidemiol. Infect. 2019, 147, e286. [Google Scholar] [CrossRef]
- Yumoto, T.; Naito, H.; Yorifuji, T.; Aokage, T.; Fujisaki, N.; Nakao, A. Association of Japan Coma Scale score on hospital arrival with in-hospital mortality among trauma patients. BMC Emerg. Med. 2019, 19, 65. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Group, K.A.G.W. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit. Care 2013, 17, 204. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Astor, B.C.; Fox, C.H.; Isakova, T.; Lash, J.P.; Peralta, C.A.; Kurella Tamura, M.; Feldman, H.I. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am. J. Kidney Dis. 2014, 63, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Gando, S.; Saitoh, D.; Ogura, H.; Fujishima, S.; Mayumi, T.; Araki, T.; Ikeda, H.; Kotani, J.; Kushimoto, S.; Miki, Y.; et al. A multicenter, prospective validation study of the Japanese Association for Acute Medicine disseminated intravascular coagulation scoring system in patients with severe sepsis. Crit. Care 2013, 17, R111. [Google Scholar] [CrossRef]
- Henter, J.I.; Horne, A.; Arico, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Suemori, K.; Saijo, M.; Yamanaka, A.; Himeji, D.; Kawamura, M.; Haku, T.; Hidaka, M.; Kamikokuryo, C.; Kakihana, Y.; Azuma, T.; et al. A multicenter non-randomized, uncontrolled single arm trial for evaluation of the efficacy and the safety of the treatment with favipiravir for patients with severe fever with thrombocytopenia syndrome. PLoS Negl. Trop. Dis. 2021, 15, e0009103. [Google Scholar] [CrossRef]
- Satoh, M.; Akashi, S.; Ogawa, M.; Wakeyama, T.; Ogawa, H.; Fukuma, A.; Taniguchi, S.; Tani, H.; Kurosu, T.; Fukushi, S.; et al. Retrospective survey of severe fever with thrombocytopenia syndrome in patients with suspected rickettsiosis in Japan. J. Infect. Chemother. 2017, 23, 45–50. [Google Scholar] [CrossRef]
- Bae, S.; Hwang, H.J.; Kim, M.Y.; Kim, M.J.; Chong, Y.P.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; Kim, S.H. Invasive Pulmonary Aspergillosis in Patients With Severe Fever With Thrombocytopenia Syndrome. Clin. Infect. Dis. 2020, 70, 1491–1494. [Google Scholar] [CrossRef]
- Matsuura, H. Japanese spotted fever and rickettsial pneumonia. QJM Int. J. Med. 2021, 114, 261–262. [Google Scholar] [CrossRef]
- Kodama, K.; Noguchi, T.; Chikahira, Y. Japanese spotted fever complicated by acute respiratory failure. Kansenshogaku Zasshi 2000, 74, 162–165. (In Japanese) [Google Scholar] [CrossRef]
- Sando, E.; Suzuki, M.; Katoh, S.; Fujita, H.; Taira, M.; Yaegashi, M.; Ariyoshi, K. Distinguishing Japanese Spotted Fever and Scrub Typhus, Central Japan, 2004–2015. Emerg. Infect. Dis. 2018, 24, 1633–1641. [Google Scholar] [CrossRef]
- Kinoshita, H.; Arima, Y.; Shigematsu, M.; Sunagawa, T.; Saijo, M.; Oishi, K.; Ando, S. Descriptive epidemiology of rickettsial infections in Japan: Scrub typhus and Japanese spotted fever, 2007–2016. Int. J. Infect. Dis. 2021, 105, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki Prefectural Goverment. Prevention of Tick-Borne Infections. Available online: https://www.pref.nagasaki.jp/object/kenkaranooshirase/oshirase/299534.html (accessed on 29 June 2022). (In Japanese)
- Nagasaki Prefectural Goverment. Outbreak of Severe Fever with Thrombocytopenia Syndrome (SFTS) Patients. Available online: https://www.pref.nagasaki.jp/shared/uploads/2021/07/1625713522.pdf (accessed on 29 June 2022). (In Japanese)
- Kim, M.C.; Chong, Y.P.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; Kim, S.H. Differentiation of Severe Fever With Thrombocytopenia Syndrome From Scrub Typhus. Clin. Infect. Dis. 2018, 66, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Sul, H.; Yun, N.R.; Kim, D.-M.; Kim, Y.K.; Kim, J.; Hur, J.; Jung, S.I.; Ryu, S.Y.; Lee, J.Y.; Huh, K.; et al. Development of a Scoring System to Differentiate Severe Fever with Thrombocytopenia Syndrome from Scrub Typhus. Viruses 2022, 14, 1093. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.H.; Kang, Y.M.; Song, K.H.; Seo, J.W.; Kim, J.H.; Chun, J.Y.; Jun, K.I.; Kang, C.K.; Moon, S.M.; Choe, P.G.; et al. Clinical Score System to Differentiate Severe Fever with Thrombocytopenia Syndrome Patients from Patients with Scrub Typhus or Hemorrhagic Fever with Renal Syndrome in Korea. J. Korean Med. Sci. 2020, 35, e77. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).