Abstract
Insect hemocytes are the only immune cells that can mount a humoral and cellular immune response. Despite the critical involvement of hemocytes in immune responses against bacteria, fungi, and parasites in mosquitoes, our understanding of their antiviral potential is still limited. It has been shown that hemocytes express humoral factors such as TEP1, PPO, and certain antimicrobial peptides that are known to restrict viral infections. Insect hemocytes also harbor the major immune pathways, such as JAK/STAT, TOLL, IMD, and RNAi, which are critical for the control of viral infection. Recent research has indicated a role for hemocytes in the regulation of viral infection through RNA interference and autophagy; however, the specific mechanism by which this regulation occurs remains uncharacterized. Conversely, some studies have suggested that hemocytes act as agonists of arboviral infection because they lack basal lamina and circulate throughout the whole mosquito, likely facilitating viral dissemination to other tissues such as salivary glands. In addition, hemocytes produce arbovirus agonist factors such as lectins, which enhance viral infection. Here, we summarize our current understanding of hemocytes’ involvement in viral infections.
1. Introduction
Since the discovery that mosquitoes can transmit infectious diseases, hundreds of pathogens, such as parasites and viruses, have been identified as etiologic agents of important human illnesses [1]. Aedes mosquitoes are the vectors for many arboviral diseases, such as dengue, Zika, Chikungunya, yellow fever, Mayaro virus, and others, all of which have a significant impact on human health [2,3]. For many years, the primary vectors, Aedes aegypti and Aedes albopictus, were restricted geographically to parts of Africa and Asia. However, both species have great adaptability to many environments, and globalization and global warming have facilitated the invasion and establishment of these species on every continent except Antarctica [4,5,6,7,8,9]. As a consequence, dengue prevalence has increased about 30-fold during the last 50 years, and it is now considered the most important mosquito-borne viral disease. An estimated 400 million dengue cases annually occur globally, and more than half of the world’s population is at risk of infection [10,11].
In the last decades, significant efforts have been focused on eradicating mosquito-borne diseases; however, diseases such as malaria remains the cause of more than 200 million cases and about half a million deaths each year. The success in reducing malaria cases since 2000 is largely attributable to vector control [2,12]: insecticides and mosquito repellents have been the main tools used to successfully limit vector populations and pathogen transmission, but unfortunately mosquitoes have developed resistance to these insecticides, which has led to a re-emergence of vector-borne disease [13]. As an alternative strategy to overcome these obstacles, the use of genetically engineered mosquitoes to suppress the vector population or reduce vector competence is currently being evaluated [14].
In the field, only about 1% of mosquitoes actually contract arboviral infections, mainly thanks to the mosquitoes’ immune responses, which are a critical factor in limiting viral infection [15,16]. The mosquito immune response involves cellular and humoral responses that eliminate pathogens such as bacteria, fungi, parasites, and viruses. The humoral response includes the release of antimicrobial peptides (AMPs), prophenoloxidase (PPO), and opsonins such as thioester-containing proteins (TEPs). Most of these antimicrobial molecules are produced by epithelial cells, mainly in the fat bodies; the exceptions are PPO and the TEPs, which are exclusively produced by hemocytes [17]. In addition, hemocytes are responsible for the mosquito’s cellular immune response, which involves phagocytosis, encapsulation, and nodulation [18]. In the case of viruses, the antiviral immune responses involve apoptosis of infected cells and virus RNA targeting by the RNA interference (RNAi) pathway [19,20,21]. Other than the RNAi pathway, most of the known immune processes are modulated by four major signaling pathways: TOLL, IMD, JAK/STAT, and JNK [21]. During the past two decades, the molecular mechanisms of various immune pathways have been uncovered, specifically by studying the immune responses in the midgut and the salivary glands, which are the critical organs for the development and transmission of human pathogens in the mosquito [22,23]. Nevertheless, it is well known that hemocytes are exclusive producers of critical molecules, such as TEP1, PPO, and sP22D, which limit Plasmodium development in the mosquito [24,25,26,27]. It is very likely that hemocytes are involved in the antiviral immune response. However, this aspect of hemocytes is poorly understood. In the present review, we discuss the existing research that supports an antiviral role for hemocytes.
4. Humoral Factors Produced by Hemocytes Regulate Viral Infections
4.1. Prophenoloxidase
Hemocytes produce several soluble molecules, such as AMPs, enzymes, and opsonins, to eliminate pathogens circulating in the hemolymph. PPO is produced exclusively by hemocytes, and along with its role in pathogen defense, it is also involved in coagulation, cuticle hardening, and pigmentation [98,99,100]. The PPO system is one of the most important mechanisms for the elimination of bacteria, fungi, and Plasmodium. Interestingly, PPO upregulation and activation have been reported in Ae. aegypti, Armigeres subalbatus, and Lymantria dispar infected with Semliki forest virus, SINV, and baculovirus Lymantria dispar multiple nucleocapsid nucleopolyhedrovirus (LdMNPV), respectively. In addition, knockdown or inhibition of PPO increases the viral load and mortality in insects, suggesting that PPO is involved in the antiviral immune response [33,34,35]. It has been suggested that PPO suppresses viral infection by killing infected cells (damaged by cell lysis) through melanization [101], or through recognition of the glycoprotein of the viral envelope by lectins that activate the PPO cascade [102]. However, the mechanism remains unknown, and it may be specific for a particular virus type and insect species.
4.2. Antimicrobial Peptides (AMPs)
The AMPs are the most conserved humoral effectors of immune systems. They are present in all living organisms, but in insects they display one of the broadest degrees of diversity and highest levels of abundance [103,104]. In insects, the AMPs are produced by various tissues, with fat bodies and hemocytes being the most common sources [105]. The AMPs exhibit activity against almost all kinds of pathogens, including viruses [104,106]. Interestingly, recent reports have shown that DENV infection in Ae. aegypti and various mosquito cell lines induces overexpression of AMPs such as defensins, cecropins, gambicin, diptericin, and attacin [69,107,108]. In addition, it has been reported that a knockdown of cecropin and defensin genes increases the viral load in Ae. aegypti, suggesting an antiviral role for these AMPs [107]. Even though there is no evidence thus far to suggest that mosquito hemocytes express AMPs as an antiviral mechanism of defense, they express most of the antimicrobial peptides, including cecropins and defensins [86,109,110,111,112]. In B. mori, baculovirus infection induces upregulation of cecropins in hemocytes, which probably have antiviral functions [61]. It would not be a surprise for mosquito hemocytes to play a similar role; however, more studies are needed to address this hypothesis.
4.3. Pattern Recognition Receptors (Opsonins)
The innate immune response starts with the recognition of non-self molecules by pattern recognition receptors (PRRs). Insects, which lack a classic adaptive immunity, have instead developed a broad repertoire of PRRs that recognize common structures in pathogens known as pathogen-associated molecular patterns (PAMPs). These PAMPs include the lipopolysaccharide of Gram-negative bacteria, peptidoglycan of Gram-positive bacteria, β-1,3-glucan in fungi, and the dsRNA or ssDNA of viruses. After PAMPs have been recognized, PRRs act as opsonins, or they activate and regulate the immune response through immune signaling pathways that trigger the production of humoral factors, phagocytosis, encapsulation, and nodulation (reviewed in [113,114]). Curiously, mosquito hemocytes express the most common PRRs as well as some exclusive PRRs that are involved in phagocytosis, melanization, or nodulation [112]. It is also interesting that hemocytes have been reported to express PRRs that participate in viral elimination, but they also express other PRRs that play an opposite role, facilitating viral infections.
In insects, one of the most important PRRs is the family of thioester-containing proteins (TEPs), which are essential for antibacterial defense [115,116,117]. In mosquitoes, TEPs are a critical factor in the elimination of Plasmodium during the early stages of infection [24,35,118,119]. Hemocytes haves been suggested as the main producers of TEPs [24,120]. Interestingly, a recent study has shown that knockdown of TEP1 and TEP2 results in higher titers of DENV2 and WNV in Ae. aegypti [121,122]. Overexpression of TEP1 also suppresses DENV2 infection [122], suggesting an antagonist role for Ae. aegypti TEP1 during DENV infection. Similarly, in Ae. aegypti, a macroglobulin complement-related factor (AaMCR) and scavenger receptor-C (AaSR-C) have an antagonist role against DENV1-4 and yellow fever virus (YFV), and they are more highly expressed in hemocytes than in any other tissue [107]. It has been suggested that TEPs provide resistance to flavivirus infection by activating the TOLL, JAK/STAT, and IMD pathways, as well as producing AMPs [92,93,107,121,123]. TEP1, in cooperation with other proteins, can regulate mechanisms such as melanization, AMP expression, and phagocytosis, which can have an impact on viral infection.
Lectins are very important to the insect immune response because they can recognize and bind to carbohydrates in the walls of microorganisms, mainly bacteria. Lectins are involved in the processes of pathogen elimination, including opsonization, PPO activation, encapsulation, nodulation, and agglutination. However, they can also act as agonists with regard to some pathogens (reviewed in [124]). For example, it is well known that in An. gambiae, P. falciparum evades the immune response by recruiting CTL4 and CTLM2A [125,126]. In addition, several reports have described lectins as a critical factor in the establishment of arboviral infection in mosquitoes, because they facilitate the virus’s entry into mosquito cells (reviewed in [127]). In Ae. aegypti, nine mosquito galactose-specific C-type lectins (mosGCTLs) have been identified that facilitate DENV2 infection, with mosGCLT-3, which is highly expressed on hemocytes, being the most critical [42]. In a similar way, mosGCLT-1 has been implicated in facilitating WNV infection in Ae. aegypti and Culex quinquefasciatus and is highly expressed in hemocytes and salivary glands as well [41]. Both mosGCLT-1 and mosGCLT-3 bind to protein E of WNV and DENV2, forming a complex lectin/protein E in the hemolymph [41,42]. Then binding of mosGCLT-1 to protein E is recognized by tyrosine phosphatase 1 (mosPTP-1), which is expressed in most tissues, facilitating viral entry into multiple mosquito tissues [41]. Even though there is as yet no supporting evidence, it is probable that mosGCLT-3 and other lectins undergo similar interactions with other receptors.
Lectins can recognize the carbohydrates of viral envelopes, which can lead to the activation of multiple immune response mechanisms, including the PPO cascade and phagocytosis. However, viruses have developed strategies to evade the immune system, in this case using lectins as a receptor for endocytosis and cell invasion. On the other hand, hemocytes express other antagonist PRRs that are conducive to viral elimination, such as TEP. Studies in this area have shown that hemocytes express genes with opposite functions against viruses, and it is probable that hemocytes can play agonist or antagonist roles, depending on many factors that influence the mosquito’s immune response (including the type of virus and insect, the degree of fitness, and the composition of the microbiota). For instance, it is well known that the mosquito microbiota plays an important role in susceptibility to viral infection [128]. In Ae. aegypti, mosGCLTs are important contributors to homeostasis of the microbiota, and their expression is regulated by its composition [129]. Thus, an alteration in the mosquito microbiota can alter the expression of mosGCLTs and antagonist factors in the hemocytes, which can cause a switch in the hemocytes from agonist to antagonist (or vice versa) with regard to arboviral infections.
6. Concluding Remarks
Despite the many advances that have been made in elucidating the molecular basis of mosquito immunology, the mechanisms governing its cellular immune responses against viral infection are still far from clear. Hemocytes are an extremely vital part of the mosquito’s immune system and are involved in both cellular and humoral responses. They exclusively produce several immune factors that are critical for the elimination of human pathogens. Here, we have discussed some of the possible ways that hemocytes participate in the immune response against viruses. Interestingly, the information available suggests that hemocytes could be involved in the elimination of viruses as well as in protecting them from elimination and promoting infection (Figure 1). Hemocyte function can be influenced by external factors, such as the nature of the microbiota, the viral load, fitness, and other factors, which can cause the scale to lean toward an agonistic or antagonistic effect on the viruses. However, it is clear that more research is needed to thoroughly assess the role(s) of hemocytes during viral infections.
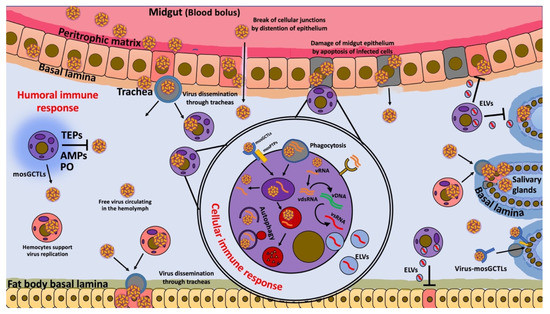
Figure 1.
Hemocytes’ immune response during viral infections. Hypothetical model of possible functions of hemocytes during viral infection. Blood meal contains viruses that trigger the production of digestive enzymes and midgut distention, which disrupt cellular junctions and cause apoptosis in midgut epithelium cells (gray square cells). These effects facilitate virus dissemination, allowing infection of cells and trachea via gaps in the cellular junctions and via apoptosis of cells. Viruses released from the midgut epithelium can be freely transported in the hemolymph or by infected hemocytes (circular purple (uninfected) and pink cells (infected)) that are distributed in the flowing hemolymph to secondary tissues, such as fat bodies and salivary glands, which receive the virus through the tracheal system. Hemocytes can facilitate the entry of a virus into cells of various tissues through the production of mosquito galactose-specific C-type lectins (mosGCTLs) and protein tyrosine phosphatases (mosPTPs). However, hemocytes also produce phenoloxidase (PO), antimicrobial peptides (AMPs), and thioester-containing proteins (TEPs), which are involved in virus elimination. In addition, in the later stages of infection, hemocytes take up free viruses by endocytosis or phagocytosis of apoptotic cells, together with cellular debris containing virus and dsRNA; they then develop viral small interfering RNAs (vsRNAs) that are released by exosome-like vesicles (ELVs) to enter infected cells and confer virus resistance through the RNAi pathway. Finally, hemocytes can also eliminate viruses by autophagy.
For many years, one of the main limitations in hemocyte research was the scarcity of sensitive and accurate tools to examine these cells. Adult mosquitoes have only 2000–5000 hemocytes, so the use of suitable strategies and technologies is particularly critical. Recently, many new tools have been developed for the study of hemocytes, such as intravital staining, which allows the monitoring of hemocytes in vivo [32]. Single-cell RNA sequencing has allowed the identification of different subpopulations in mosquitoes and their specific mechanisms of immune response against pathogens such as Plasmodium [109,110,111]. Furthermore, clodronate liposomes have been used to deplete phagocytic hemocytes in An. gambiae, Ae. aegypti, and Drosophila [119,152]. Finally, a promoter of Drosophila hemolectin driving mosquito hemocyte-specific expression has been reported [44]. These new tools will help us to address many questions about hemocyte biology, as well as to identify new strategies for vector-borne disease control.
Author Contributions
V.C.-J. and G.D. contributed to the conception, writing and critical revision of the manuscript; V.C.-J., C.V.T. and S.D. contributed to the drafting, writing and critical revision of the manuscript; V.C.-J. and G.D. supervised and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by the National Institutes of Health grant R01AI141532.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Deborah McClellan for editorial assistance.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Huang, Y.-J.S.; Higgs, S.; Vanlandingham, D.L. Arbovirus-Mosquito Vector-Host Interactions and the Impact on Transmission and Disease Pathogenesis of Arboviruses. Front. Microbiol. 2019, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A.; Gubler, D.J.; Weaver, S.C.; Monath, T.P.; Heymann, D.L.; Scott, T.W. Epidemic Arboviral Diseases: Priorities for Research and Public Health. Lancet Infect. Dis. 2017, 17, e101–e106. [Google Scholar] [CrossRef]
- Girard, M.; Nelson, C.B.; Picot, V.; Gubler, D.J. Arboviruses: A Global Public Health Threat. Vaccine 2020, 38, 3989–3994. [Google Scholar] [CrossRef]
- Tran, B.-L.; Tseng, W.-C.; Chen, C.-C.; Liao, S.-Y. Estimating the Threshold Effects of Climate on Dengue: A Case Study of Taiwan. Int. J. Environ. Res. Public Health 2020, 17, 1392. [Google Scholar] [CrossRef] [PubMed]
- Lwande, O.W.; Obanda, V.; Lindström, A.; Ahlm, C.; Evander, M.; Näslund, J.; Bucht, G. Globe-Trotting Aedes Aegypti and Aedes Albopictus: Risk Factors for Arbovirus Pandemics. Vector-Borne Zoonotic Dis. 2020, 20, 71–81. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and Future Spread of the Arbovirus Vectors Aedes Aegypti and Aedes Albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef]
- Braack, L.; Gouveia de Almeida, A.P.; Cornel, A.J.; Swanepoel, R.; de Jager, C. Mosquito-Borne Arboviruses of African Origin: Review of Key Viruses and Vectors. Parasites Vectors 2018, 11, 29. [Google Scholar] [CrossRef]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The Global Distribution of the Arbovirus Vectors Aedes Aegypti and Ae. Albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Ebi, K.L.; Nealon, J. Dengue in a Changing Climate. Environ. Res. 2016, 151, 115–123. [Google Scholar] [CrossRef]
- World Health Organization. Guidance Framework for Testing Genetically Modified Mosquitoes, 2nd ed.; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-002523-3.
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The Global Distribution and Burden of Dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-004049-6.
- Liu, N. Insecticide Resistance in Mosquitoes: Impact, Mechanisms, and Research Directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Dong, Y.; Simões, M.L.; Dimopoulos, G. Mosquito Transgenesis for Malaria Control. Trends Parasitol. 2021, 38, 54–66. [Google Scholar] [CrossRef]
- Souza-Neto, J.A.; Powell, J.R.; Bonizzoni, M. Aedes Aegypti Vector Competence Studies: A Review. Infect. Genet. Evol. 2019, 67, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Palomares, L.A.; Moreno-García, M.; Lanz-Mendoza, H.; Salazar, M.I. Molecular Basis for Arbovirus Transmission by Aedes Aegypti Mosquitoes. Intervirology 2018, 61, 255–264. [Google Scholar] [CrossRef] [PubMed]
- King, J.G. Developmental and Comparative Perspectives on Mosquito Immunity. Dev. Comp. Immunol. 2020, 103, 103458. [Google Scholar] [CrossRef]
- Hillyer, J.F.; Strand, M.R. Mosquito Hemocyte-Mediated Immune Responses. Curr. Opin. Insect Sci. 2014, 3, 14–21. [Google Scholar] [CrossRef]
- Sim, S.; Jupatanakul, N.; Dimopoulos, G. Mosquito Immunity against Arboviruses. Viruses 2014, 6, 4479–4504. [Google Scholar] [CrossRef]
- Marques, J.T.; Imler, J.-L. The Diversity of Insect Antiviral Immunity: Insights from Viruses. Curr. Opin. Microbiol. 2016, 32, 71–76. [Google Scholar] [CrossRef]
- Tikhe, C.V.; Dimopoulos, G. Mosquito Antiviral Immune Pathways. Dev. Comp. Immunol. 2021, 116, 103964. [Google Scholar] [CrossRef]
- Dong, Y.; Simões, M.L.; Dimopoulos, G. Versatile Transgenic Multistage Effector-Gene Combinations for Plasmodium Falciparum Suppression in Anopheles. Sci. Adv. 2020, 6, eaay5898. [Google Scholar] [CrossRef]
- Smith, R.C.; Vega-Rodriguez, J.; Jacobs-Lorena, M. The Plasmodium Bottleneck: Malaria Parasite Losses in the Mosquito Vector. Mem. Do Inst. Oswaldo Cruz 2014, 109, 644–661. [Google Scholar] [CrossRef]
- Levashina, E.A.; Moita, L.F.; Blandin, S.; Vriend, G.; Lagueux, M.; Kafatos, F.C. Conserved Role of a Complement-like Protein in Phagocytosis Revealed by DsRNA Knockout in Cultured Cells of the Mosquito, Anopheles Gambiae. Cell 2001, 104, 709–718. [Google Scholar] [CrossRef]
- Danielli, A.; Loukeris, T.G.; Lagueux, M.; Muller, H.-M.; Richman, A.; Kafatos, F.C. A Modular Chitin-Binding Protease Associated with Hemocytes and Hemolymph in the Mosquito Anopheles Gambiae. Proc. Natl. Acad. Sci. USA 2000, 97, 7136–7141. [Google Scholar] [CrossRef]
- Hernández-Martínez, S.; Lanz, H.; Rodríguez, M.H.; Torres, J.A.; Adolfo, M.-P.; Tsutsumi, V. Morphological and Cytochemical Characterization of Female Anopheles Albimanus (Diptera: Culicidae) Hemocytes. J. Med. Entomol. 1999, 36, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, J.F.; Schmidt, S.L.; Christensen, B.M. Rapid Phagocytosis and Melanization of Bacteria and Plasmodium Sporozoites by Hemocytes of the Mosquito Aedes Aegypi. J. Parasitol. 2003, 89, 62–69. [Google Scholar] [CrossRef]
- Gaudet, R.G.; Bradfield, C.J.; MacMicking, J.D. Evolution of Cell-Autonomous Effector Mechanisms in Macrophages versus Non-Immune Cells. In Myeloid Cells in Health and Disease; Gordon, S., Ed.; ASM Press: Washington, DC, USA, 2017; pp. 615–635. ISBN 978-1-68367-066-7. [Google Scholar]
- Eleftherianos, I.; Heryanto, C.; Bassal, T.; Zhang, W.; Tettamanti, G.; Mohamed, A. Haemocyte-mediated Immunity in Insects: Cells, Processes and Associated Components in the Fight against Pathogens and Parasites. Immunology 2021, 164, 401–432. [Google Scholar] [CrossRef]
- Castillo, J.C.; Robertson, A.E.; Strand, M.R. Characterization of Hemocytes from the Mosquitoes Anopheles Gambiae and Aedes Aegypti. Insect Biochem. Mol. Biol. 2006, 36, 891–903. [Google Scholar] [CrossRef]
- King, J.G.; Hillyer, J.F. Spatial and Temporal in Vivo Analysis of Circulating and Sessile Immune Cells in Mosquitoes: Hemocyte Mitosis Following Infection. BMC Biol. 2013, 11, 55. [Google Scholar] [CrossRef]
- King, J.G.; Hillyer, J.F. Infection-Induced Interaction between the Mosquito Circulatory and Immune Systems. PLoS Pathog. 2012, 8, e1003058. [Google Scholar] [CrossRef]
- Yan, Y.; Hillyer, J.F. The Immune and Circulatory Systems Are Functionally Integrated across Insect Evolution. Sci. Adv. 2020, 6, eabb3164. [Google Scholar] [CrossRef]
- Sigle, L.T.; Hillyer, J.F. Mosquito Hemocytes Preferentially Aggregate and Phagocytose Pathogens in the Periostial Regions of the Heart That Experience the Most Hemolymph Flow. Dev. Comp. Immunol. 2016, 55, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.C.; Ferreira, A.B.B.; Trisnadi, N.; Barillas-Mury, C. Activation of Mosquito Complement Antiplasmodial Response Requires Cellular Immunity. Sci. Immunol. 2017, 2, eaal1505. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, J.F.; Pass, G. The Insect Circulatory System: Structure, Function, and Evolution. Annu. Rev. Entomol. 2020, 65, 121–143. [Google Scholar] [CrossRef]
- Keddie, B.A.; Aponte, G.W.; Volkman, L.E. The Pathway of Infection of Autographa Californica Nuclear Polyhedrosis Virus in an Insect Host. Science 1989, 243, 1728–1730. [Google Scholar] [CrossRef] [PubMed]
- Engelhard, E.K.; Kam-Morgan, L.N.; Washburn, J.O.; Volkman, L.E. The Insect Tracheal System: A Conduit for the Systemic Spread of Autographa Californica M Nuclear Polyhedrosis Virus. Proc. Natl. Acad. Sci. USA 1994, 91, 3224–3227. [Google Scholar] [CrossRef]
- Trudeau, D.; Washburn, J.O.; Volkman, L.E. Central Role of Hemocytes in Autographa Californica M Nucleopolyhedrovirus Pathogenesis in Heliothis Virescens and Helicoverpa Zea. J. Virol. 2001, 75, 996–1003. [Google Scholar] [CrossRef]
- Salazar, M.I.; Richardson, J.H.; Sánchez-Vargas, I.; Olson, K.E.; Beaty, B.J. Dengue Virus Type 2: Replication and Tropisms in Orally Infected Aedes Aegypti Mosquitoes. BMC Microbiol. 2007, 7, 9. [Google Scholar] [CrossRef]
- Cheng, G.; Cox, J.; Wang, P.; Krishnan, M.N.; Dai, J.; Qian, F.; Anderson, J.F.; Fikrig, E. A C-Type Lectin Collaborates with a CD45 Phosphatase Homolog to Facilitate West Nile Virus Infection of Mosquitoes. Cell 2010, 142, 714–725. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, F.; Liu, J.; Xiao, X.; Zhang, S.; Qin, C.; Xiang, Y.; Wang, P.; Cheng, G. Transmission-Blocking Antibodies against Mosquito C-Type Lectins for Dengue Prevention. PLoS Pathog. 2014, 10, e1003931. [Google Scholar] [CrossRef]
- Parikh, G.R.; Oliver, J.D.; Bartholomay, L.C. A Haemocyte Tropism for an Arbovirus. J. Gen. Virol. 2009, 90, 292–296. [Google Scholar] [CrossRef]
- Pondeville, E.; Puchot, N.; Parvy, J.-P.; Carissimo, G.; Poidevin, M.; Waterhouse, R.M.; Marois, E.; Bourgouin, C. Hemocyte-Targeted Gene Expression in the Female Malaria Mosquito Using the Hemolectin Promoter from Drosophila. Insect Biochem. Mol. Biol. 2020, 120, 103339. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Balaraman, V.; Kantor, A.M.; Lin, J.; Grant, D.G.; Held, N.L.; Franz, A.W.E. Chikungunya Virus Dissemination from the Midgut of Aedes Aegypti Is Associated with Temporal Basal Lamina Degradation during Bloodmeal Digestion. PLoS Negl. Trop. Dis. 2017, 11, e0005976. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.M.; Ehrlich, H.Y.; Magalhaes, T.; Miller, M.R.; Conway, P.J.; Bransfield, A.; Misencik, M.J.; Gloria-Soria, A.; Warren, J.L.; Andreadis, T.G.; et al. Successive Blood Meals Enhance Virus Dissemination within Mosquitoes and Increase Transmission Potential. Nat. Microbiol. 2020, 5, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Romoser, W.S.; Wasieloski, L.P.; Pushko, P.; Kondig, J.P.; Lerdthusnee, K.; Neira, M.; Ludwig, G.V. Evidence for Arbovirus Dissemination Conduits from the Mosquito (Diptera: Culicidae) Midgut. J. Med. Entomol. 2004, 41, 9. [Google Scholar] [CrossRef]
- Raquin, V.; Lambrechts, L. Dengue Virus Replicates and Accumulates in Aedes Aegypti Salivary Glands. Virology 2017, 507, 75–81. [Google Scholar] [CrossRef]
- Franz, A.; Kantor, A.; Passarelli, A.; Clem, R. Tissue Barriers to Arbovirus Infection in Mosquitoes. Viruses 2015, 7, 3741–3767. [Google Scholar] [CrossRef]
- Wang, H.; Gort, T.; Boyle, D.L.; Clem, R.J. Effects of Manipulating Apoptosis on Sindbis Virus Infection of Aedes Aegypti Mosquitoes. J. Virol. 2012, 86, 6546–6554. [Google Scholar] [CrossRef]
- Dong, S.; Kantor, A.M.; Lin, J.; Passarelli, A.L.; Clem, R.J.; Franz, A.W.E. Infection Pattern and Transmission Potential of Chikungunya Virus in Two New World Laboratory-Adapted Aedes Aegypti Strains. Sci. Rep. 2016, 6, 24729. [Google Scholar] [CrossRef]
- Cui, Y.; Grant, D.G.; Lin, J.; Yu, X.; Franz, A.W.E. Zika Virus Dissemination from the Midgut of Aedes Aegypti Is Facilitated by Bloodmeal-Mediated Structural Modification of the Midgut Basal Lamina. Viruses 2019, 11, 1056. [Google Scholar] [CrossRef]
- Merwaiss, F.; Filomatori, C.V.; Susuki, Y.; Bardossy, E.S.; Alvarez, D.E.; Saleh, M.-C. Chikungunya Virus Replication Rate Determines the Capacity of Crossing Tissue Barriers in Mosquitoes. J. Virol. 2021, 95, e01956-20. [Google Scholar] [CrossRef]
- Hillyer, J.F.; Barreau, C.; Vernick, K.D. Hemocoel, Efficiency of Salivary Gland Invasion by Malaria Sporozoites Is Controlled by Rapid Sporozoite Destruction in the Mosquito. Int. J. Parasitol. 2007, 37, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Fink, K.; Ng, C.; Nkenfou, C.; Vasudevan, S.G.; van Rooijen, N.; Schul, W. Depletion of Macrophages in Mice Results in Higher Dengue Virus Titers and Highlights the Role of Macrophages for Virus Control. Eur. J. Immunol. 2009, 39, 2809–2821. [Google Scholar] [CrossRef] [PubMed]
- Tassetto, M.; Kunitomi, M.; Andino, R. Circulating Immune Cells Mediate a Systemic RNAi-Based Adaptive Antiviral Response in Drosophila. Cell 2017, 169, 314–325.e13. [Google Scholar] [CrossRef] [PubMed]
- Nainu, F.; Tanaka, Y.; Shiratsuchi, A.; Nakanishi, Y. Protection of Insects against Viral Infection by Apoptosis-Dependent Phagocytosis. J. Immunol. 2015, 195, 5696–5706. [Google Scholar] [CrossRef]
- Lamiable, O.; Arnold, J.; de Faria, I.J.D.S.; Olmo, R.P.; Bergami, F.; Meignin, C.; Hoffmann, J.A.; Marques, J.T.; Imler, J.-L. Analysis of the Contribution of Hemocytes and Autophagy to Drosophila Antiviral Immunity. J. Virol. 2016, 90, 5415–5426. [Google Scholar] [CrossRef]
- Leite, T.H.J.F.; Ferreira, Á.G.A.; Imler, J.-L.; Marques, J.T. Distinct Roles of Hemocytes at Different Stages of Infection by Dengue and Zika Viruses in Aedes Aegypti Mosquitoes. Front. Immunol. 2021, 12, 660873. [Google Scholar] [CrossRef]
- Li, T.; Xia, Y.; Xu, X.; Wei, G.; Wang, L. Functional Analysis of Dicer-2 Gene in Bombyx Mori Resistance to BmNPV Virus. Arch. Insect Biochem. Physiol. 2020, 105, e21724. [Google Scholar] [CrossRef]
- Feng, M.; Xia, J.; Fei, S.; Peng, R.; Wang, X.; Zhou, Y.; Wang, P.; Swevers, L.; Sun, J. Identification of Silkworm Hemocyte Subsets and Analysis of Their Response to Baculovirus Infection Based on Single-Cell RNA Sequencing. Front. Immunol. 2021, 12, 1521. [Google Scholar] [CrossRef]
- Jiang, L. Insights Into the Antiviral Pathways of the Silkworm Bombyx Mori. Front. Immunol. 2021, 12, 325. [Google Scholar] [CrossRef]
- Dong, Y.; Dong, S.; Dizaji, N.B.; Rutkowski, N.; Pohlenz, T.; Myles, K.; Dimopoulos, G. The Aedes Aegypti SiRNA Pathway Mediates Broad-Spectrum Defense against Human Pathogenic Viruses and Modulates Antibacterial and Antifungal Defenses. PLoS Biol. 2022, 20, e3001668. [Google Scholar] [CrossRef]
- Hess, A.M.; Prasad, A.N.; Ptitsyn, A.; Ebel, G.D.; Olson, K.E.; Barbacioru, C.; Monighetti, C.; Campbell, C.L. Small RNA Profiling of Dengue Virus-Mosquito Interactions Implicates the PIWI RNA Pathway in Anti-Viral Defense. BMC Microbiol. 2011, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, M.; Almire, F.; Kean, J.; Donald, C.L.; McDonald, A.; Wee, B.; Lauréti, M.; Varjak, M.; Terry, S.; Vazeille, M.; et al. The Aedes Aegypti Domino Ortholog P400 Regulates Antiviral Exogenous Small Interfering RNA Pathway Activity and Ago-2 Expression. mSphere 2020, 5, e00081-20. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, O.; Niu, J.; Nji Tizi Taning, C. RNAi in Insects: A Revolution in Fundamental Research and Pest Control Applications. Insects 2020, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Tomoyasu, Y.; Miller, S.C.; Tomita, S.; Schoppmeier, M.; Grossmann, D.; Bucher, G. Exploring Systemic RNA Interference in Insects: A Genome-Wide Survey for RNAi Genes in Tribolium. Genome Biol. 2008, 9, 1–22. [Google Scholar] [CrossRef]
- Joga, M.R.; Zotti, M.J.; Smagghe, G.; Christiaens, O. RNAi Efficiency, Systemic Properties, and Novel Delivery Methods for Pest Insect Control: What We Know So Far. Front. Physiol. 2016, 7, 553. [Google Scholar] [CrossRef] [PubMed]
- Angleró-Rodríguez, Y.I.; Tikhe, C.V.; Kang, S.; Dimopoulos, G. Aedes Aegypti Toll Pathway Is Induced through DsRNA Sensing in Endosomes. Dev. Comp. Immunol. 2021, 122, 104138. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Pan, P.C.; Govind, S. A Role for the Drosophila Toll/Cactus Pathway in Larval Hematopoiesis. Development 1998, 125, 1909–1920. [Google Scholar] [CrossRef]
- Minakhina, S.; Tan, W.; Steward, R. JAK/STAT and the GATA Factor Pannier Control Hemocyte Maturation and Differentiation in Drosophila. Dev. Biol. 2011, 352, 308–316. [Google Scholar] [CrossRef]
- Minakhina, S.; Steward, R. Melanotic Mutants in Drosophila: Pathways and Phenotypes. Genetics 2006, 174, 253–263. [Google Scholar] [CrossRef]
- Brutscher, L.M.; Daughenbaugh, K.F.; Flenniken, M.L. Antiviral Defense Mechanisms in Honey Bees. Curr. Opin. Insect Sci. 2015, 10, 71–82. [Google Scholar] [CrossRef]
- Ferreira, Á.G.; Naylor, H.; Esteves, S.S.; Pais, I.S.; Martins, N.E.; Teixeira, L. The Toll-Dorsal Pathway Is Required for Resistance to Viral Oral Infection in Drosophila. PLoS Pathog. 2014, 10, e1004507. [Google Scholar] [CrossRef] [PubMed]
- He, Y.J.; Lu, G.; Qi, Y.H.; Zhang, Y.; Zhang, X.D.; Huang, H.J.; Zhuo, J.C.; Sun, Z.T.; Yan, F.; Chen, J.P.; et al. Activation of Toll Immune Pathway in an Insect Vector Induced by a Plant Virus. Front. Immunol. 2021, 11, 3494. [Google Scholar] [CrossRef] [PubMed]
- Zambon, R.A.; Nandakumar, M.; Vakharia, V.W.; Wu, L.P. The Toll Pathway Is Important for an Antiviral Response in Drosophila. Proc. Natl. Acad. Sci. USA 2005, 102, 7257–7262. [Google Scholar] [CrossRef] [PubMed]
- The Aedes Aegypti Toll Pathway Controls Dengue Virus Infection PLOS Pathogens. Available online: https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1000098 (accessed on 1 August 2022).
- Barletta, A.B.F.; Saha, B.; Trisnadi, N.; Talyuli, O.; Raddi, G.; Barillas-Mury, C. Hemocyte Differentiation to the Megacyte Lineage Enhances Mosquito Immunity against Plasmodium. bioRxiv 2022. preprint. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Visweswariah, S.S. Intramacrophage ROS Primes the Innate Immune System via JAK/STAT and Toll Activation. Cell Rep. 2020, 33, 108368. [Google Scholar] [CrossRef]
- Pan, X.; Zhou, G.; Wu, J.; Bian, G.; Lu, P.; Raikhel, A.S.; Xi, Z. Wolbachia Induces Reactive Oxygen Species (ROS)-Dependent Activation of the Toll Pathway to Control Dengue Virus in the Mosquito Aedes Aegypti. Proc. Natl. Acad. Sci. USA 2012, 109, E23–E31. [Google Scholar] [CrossRef]
- Dudzic, J.P.; Hanson, M.A.; Iatsenko, I.; Kondo, S.; Lemaitre, B. More Than Black or White: Melanization and Toll Share Regulatory Serine Proteases in Drosophila. Cell Rep. 2019, 27, 1050–1061.e3. [Google Scholar] [CrossRef]
- Zhang, K.; Shen, L.; Wang, X.; Yang, H.; Zhang, X.; Pan, G.; Li, C.; Ji, H.; Abbas, M.N.; Li, C.; et al. Scavenger Receptor C Regulates Antimicrobial Peptide Expression by Activating Toll Signaling in Silkworm, Bombyx Mori. Int. J. Biol. Macromol. 2021, 191, 396–404. [Google Scholar] [CrossRef]
- Engineered Aedes Aegypti JAK/STAT Pathway-Mediated Immunity to Dengue Virus PLOS Neglected Tropical Diseases. Available online: https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0005187 (accessed on 1 August 2022).
- Bang, I.S. JAK/STAT Signaling in Insect Innate Immunity. Entomol. Res. 2019, 49, 339–353. [Google Scholar] [CrossRef]
- Barillas-Mury, C.; Han, Y.-S.; Seeley, D.; Kafatos, F.C. Anopheles Gambiae Ag-STAT, a New Insect Member of the STAT Family, Is Activated in Response to Bacterial Infection. EMBO J. 1999, 18, 959–967. [Google Scholar] [CrossRef]
- Yan, Y.; Sigle, L.T.; Rinker, D.C.; Estévez-Lao, T.Y.; Capra, J.A.; Hillyer, J.F. The IMD and JNK Pathways Drive the Functional Integration of the Immune and Circulatory Systems of Mosquitoes. bioRxiv 2022. preprint. [Google Scholar] [CrossRef]
- Costa, A.; Jan, E.; Sarnow, P.; Schneider, D. The Imd Pathway Is Involved in Antiviral Immune Responses in Drosophila. PLoS ONE 2009, 4, e7436. [Google Scholar] [CrossRef] [PubMed]
- Avadhanula, V.; Weasner, B.P.; Hardy, G.G.; Kumar, J.P.; Hardy, R.W. A Novel System for the Launch of Alphavirus RNA Synthesis Reveals a Role for the Imd Pathway in Arthropod Antiviral Response. PLoS Pathog. 2009, 5, e1000582. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.; Freisinger, T.; Ishii, K.; Okado, K.; Shinzawa, N.; Fukumoto, S.; Kanuka, H. Activation of Imd Pathway in Hemocyte Confers Infection Resistance through Humoral Response in Drosophila. Biochem. Biophys. Res. Commun. 2013, 430, 1120–1125. [Google Scholar] [CrossRef]
- Sherri, N.; Salloum, N.; Mouawad, C.; Haidar-Ahmad, N.; Shirinian, M.; Rahal, E.A. Epstein-Barr Virus DNA Enhances Diptericin Expression and Increases Hemocyte Numbers in Drosophila Melanogaster via the Immune Deficiency Pathway. Front. Microbiol. 2018, 9, 1268. [Google Scholar] [CrossRef]
- Tafesh-Edwards, G.; Eleftherianos, I. JNK Signaling in Drosophila Immunity and Homeostasis. Immunol. Lett. 2020, 226, 7–11. [Google Scholar] [CrossRef]
- Chowdhury, A.; Modahl, C.M.; Tan, S.T.; Xiang, B.W.W.; Missé, D.; Vial, T.; Kini, R.M.; Pompon, J.F. JNK Pathway Restricts DENV2, ZIKV and CHIKV Infection by Activating Complement and Apoptosis in Mosquito Salivary Glands. PLoS Pathog. 2020, 16, e1008754. [Google Scholar] [CrossRef]
- Garver, L.S.; de Almeida Oliveira, G.; Barillas-Mury, C. The JNK Pathway Is a Key Mediator of Anopheles Gambiae Antiplasmodial Immunity. PLoS Pathog. 2013, 9, e1003622. [Google Scholar] [CrossRef]
- Hernández-Martínez, S.; Lanz-Mendoza, H.; Martínez-Barnetche, J.; Rodríguez, M.H. Antimicrobial Properties of Anopheles Albimanus Pericardial Cells. Cell Tissue Res. 2013, 351, 127–137. [Google Scholar] [CrossRef]
- Cardoso-Jaime, V.; Maya-Maldonado, K.; Celestino-Montes, A.; Tsutsumi, V.; Hernández-Martínez, S. Lysozyme C-1 Gene Is Overexpressed in Anopheles Albimanus Pericardial Cells after an Immune Challenge. Dev. Comp. Immunol. 2021, 114, 103830. [Google Scholar] [CrossRef]
- Cevik, D.; Acker, M.; Michalski, C.; Jacobs, J.R. Pericardin, a Drosophila Collagen, Facilitates Accumulation of Hemocytes at the Heart. Dev. Biol. 2019, 454, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Gera, J.; Budakoti, P.; Suhag, M.; Mandal, L.; Mandal, S. Physiological ROS Controls Upd3-Dependent Modeling of ECM to Support Cardiac Function in Drosophila. Sci. Adv. 2022, 8, eabj4991. [Google Scholar] [CrossRef] [PubMed]
- Cerenius, L.; Söderhäll, K. Immune Properties of Invertebrate Phenoloxidases. Dev. Comp. Immunol. 2021, 122, 104098. [Google Scholar] [CrossRef] [PubMed]
- Eleftherianos, I.; Revenis, C. Role and Importance of Phenoloxidase in Insect Hemostasis. J. Innate. Immun. 2011, 3, 28–33. [Google Scholar] [CrossRef] [PubMed]
- González-Santoyo, I.; Córdoba-Aguilar, A. Phenoloxidase: A Key Component of the Insect Immune System: Biochemical and Evolutionary Ecology of PO. Entomol. Exp. Appl. 2012, 142, 1–16. [Google Scholar] [CrossRef]
- McNeil, J.; Cox-Foster, D.; Slavicek, J.; Hoover, K. Contributions of Immune Responses to Developmental Resistance in Lymantria Dispar Challenged with Baculovirus. J. Insect Physiol. 2010, 56, 1167–1177. [Google Scholar] [CrossRef]
- Rodriguez-Andres, J.; Rani, S.; Varjak, M.; Chase-Topping, M.E.; Beck, M.H.; Ferguson, M.C.; Schnettler, E.; Fragkoudis, R.; Barry, G.; Merits, A.; et al. Phenoloxidase Activity Acts as a Mosquito Innate Immune Response against Infection with Semliki Forest Virus. PLoS Pathog. 2012, 8, e1002977. [Google Scholar] [CrossRef]
- Yi, H.-Y.; Chowdhury, M.; Huang, Y.-D.; Yu, X.-Q. Insect Antimicrobial Peptides and Their Applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef]
- Wang, G. (Ed.) Antimicrobial Peptides: Discovery, Design and Novel Therapeutic Strategies; Advances in molecular and cellular microbiology; CABI: Wallingford, UK; Cambridge, MA, USA, 2010; ISBN 978-1-84593-657-0. [Google Scholar]
- Hillyer, J.F. Mosquito Immunity. In Invertebrate Immunity; Söderhäll, K., Ed.; Advances in Experimental Medicine and Biology; Springer US: Boston, MA, USA, 2010; Volume 708, pp. 218–238. ISBN 978-1-4419-8058-8. [Google Scholar]
- Feng, M.; Fei, S.; Xia, J.; Labropoulou, V.; Swevers, L.; Sun, J. Antimicrobial Peptides as Potential Antiviral Factors in Insect Antiviral Immune Response. Front. Immunol. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, Y.; Zhang, X.; Wang, J.; Li, Z.; Pang, X.; Wang, P.; Cheng, G. Complement-Related Proteins Control the Flavivirus Infection of Aedes Aegypti by Inducing Antimicrobial Peptides. PLoS Pathog. 2014, 10, e1004027. [Google Scholar] [CrossRef]
- Liu, W.-Q.; Chen, S.-Q.; Bai, H.-Q.; Wei, Q.-M.; Zhang, S.-N.; Chen, C.; Zhu, Y.-H.; Yi, T.-W.; Guo, X.-P.; Chen, S.-Y.; et al. The Ras/ERK Signaling Pathway Couples Antimicrobial Peptides to Mediate Resistance to Dengue Virus in Aedes Mosquitoes. PLoS Negl. Trop. Dis. 2020, 14, e0008660. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Mohammed, M.; Franzén, O.; Ankarklev, J.; Smith, R.C. Single-Cell Analysis of Mosquito Hemocytes Identifies Signatures of Immune Cell Subtypes and Cell Differentiation. eLife 2021, 10, e66192. [Google Scholar] [CrossRef] [PubMed]
- Raddi, G.; Barletta, A.B.F.; Efremova, M.; Ramirez, J.L.; Cantera, R.; Teichmann, S.A.; Barillas-Mury, C.; Billker, O. Mosquito Cellular Immunity at Single-Cell Resolution. Science 2020, 369, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Severo, M.S.; Landry, J.J.M.; Lindquist, R.L.; Goosmann, C.; Brinkmann, V.; Collier, P.; Hauser, A.E.; Benes, V.; Henriksson, J.; Teichmann, S.A.; et al. Unbiased Classification of Mosquito Blood Cells by Single-Cell Genomics and High-Content Imaging. Proc Natl Acad Sci USA 2018, 115, E7568–E7577. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Fuchs, J.F.; Mayhew, G.F.; Yu, H.E.; Christensen, B.M. Tissue-Enriched Expression Profiles in Aedes Aegypti Identify Hemocyte-Specific Transcriptome Responses to Infection. Insect Biochem. Mol. Biol. 2012, 42, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Zhang, R.; Zhang, J. The Diversity of Pattern Recognition Receptors (PRRs) Involved with Insect Defense against Pathogens. Curr. Opin. Insect Sci. 2019, 33, 105–110. [Google Scholar] [CrossRef]
- Dubovskiy, I.; Kryukova, N.; Glupov, V.V.; Ratcliffe, N. Encapsulation and Nodulation in Insects. Invertebr. Surviv. J. 2016, 13, 229–246. [Google Scholar] [CrossRef]
- Shokal, U.; Eleftherianos, I. Evolution and Function of Thioester-Containing Proteins and the Complement System in the Innate Immune Response. Front. Immunol. 2017, 8, 759. [Google Scholar] [CrossRef]
- Blandin, S. Thioester-Containing Proteins and Insect Immunity. Mol. Immunol. 2004, 40, 903–908. [Google Scholar] [CrossRef]
- Williams, M.; Baxter, R. The Structure and Function of Thioester-Containing Proteins in Arthropods. Biophys. Rev. 2014, 6, 261–272. [Google Scholar] [CrossRef]
- Blandin, S.; Shiao, S.-H.; Moita, L.F.; Janse, C.J.; Waters, A.P.; Kafatos, F.C.; Levashina, E.A. Complement-Like Protein TEP1 Is a Determinant of Vectorial Capacity in the Malaria Vector Anopheles Gambiae. Cell 2004, 116, 661–670. [Google Scholar] [CrossRef]
- Kwon, H.; Smith, R.C. Chemical Depletion of Phagocytic Immune Cells in Anopheles Gambiae Reveals Dual Roles of Mosquito Hemocytes in Anti- Plasmodium Immunity. Proc Natl Acad Sci USA 2019, 116, 14119–14128. [Google Scholar] [CrossRef] [PubMed]
- Bou Aoun, R.; Hetru, C.; Troxler, L.; Doucet, D.; Ferrandon, D.; Matt, N. Analysis of Thioester-Containing Proteins during the Innate Immune Response of Drosophila Melanogaster. J. Innate. Immun. 2011, 3, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.-C.; Li, H.-H.; Li, J.-C.; Liu, W.-L.; Chen, C.-H.; Shiao, S.-H. A Thioester-Containing Protein Controls Dengue Virus Infection in Aedes Aegypti Through Modulating Immune Response. Front. Immunol. 2021, 12, 670122. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Liu, L.; Wang, P.; Zhang, Y.; Zhao, Y.O.; Colpitts, T.M.; Feitosa, F.; Anderson, J.F.; Fikrig, E. An In Vivo Transfection Approach Elucidates a Role for Aedes Aegypti Thioester-Containing Proteins in Flaviviral Infection. PLoS ONE 2011, 6, e22786. [Google Scholar] [CrossRef] [PubMed]
- Souvannaseng, L.; Hun, L.V.; Baker, H.; Klyver, J.M.; Wang, B.; Pakpour, N.; Bridgewater, J.M.; Napoli, E.; Giulivi, C.; Riehle, M.A.; et al. Inhibition of JNK Signaling in the Asian Malaria Vector Anopheles Stephensi Extends Mosquito Longevity and Improves Resistance to Plasmodium Falciparum Infection. PLoS Pathog. 2018, 14, e1007418. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; You, M.; Rao, X.-J.; Yu, X.-Q. Insect C-Type Lectins in Innate Immunity. Dev. Comp. Immunol. 2018, 83, 70–79. [Google Scholar] [CrossRef]
- Simões, M.L.; Mlambo, G.; Tripathi, A.; Dong, Y.; Dimopoulos, G. Immune Regulation of Plasmodium Is Anopheles Species Specific and Infection Intensity Dependent. mBio 2017, 8, e01631-17. [Google Scholar] [CrossRef]
- Simões, M.L.; Dong, Y.; Mlambo, G.; Dimopoulos, G. C-Type Lectin 4 Regulates Broad-Spectrum Melanization-Based Refractoriness to Malaria Parasites. PLoS Biol. 2022, 20, e3001515. [Google Scholar] [CrossRef]
- Adelman, Z.; Myles, K. The C-Type Lectin Domain Gene Family in Aedes Aegypti and Their Role in Arbovirus Infection. Viruses 2018, 10, 367. [Google Scholar] [CrossRef]
- Caragata, E.P.; Tikhe, C.V.; Dimopoulos, G. Curious Entanglements: Interactions between Mosquitoes, Their Microbiota, and Arboviruses. Curr. Opin. Virol. 2019, 37, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Xiao, X.; Liu, Y.; Zhang, R.; Liu, J.; Liu, Q.; Wang, P.; Cheng, G. Mosquito C-Type Lectins Maintain Gut Microbiome Homeostasis. Nat. Microbiol. 2016, 1, 16023. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Orme, M.; Meier, P. Inhibitor of Apoptosis Proteins in Drosophila: Gatekeepers of Death. Apoptosis 2009, 14, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Clem, R.J. Defining the Core Apoptosis Pathway in the Mosquito Disease Vector Aedes Aegypti: The Roles of Iap1, Ark, Dronc, and Effector Caspases. Apoptosis 2011, 16, 105–113. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, K.; Olson, B.J.S.C.; Huang, N.; Unis, D.; Clem, R.J. Rapid Selection against Arbovirus-Induced Apoptosis during Infection of a Mosquito Vector. Proc. Natl. Acad. Sci. USA 2015, 112, E1152–E1161. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.; Ramirez, J.L.; Dimopoulos, G. Dengue Virus Infection of the Aedes Aegypti Salivary Gland and Chemosensory Apparatus Induces Genes That Modulate Infection and Blood-Feeding Behavior. PLoS Pathog. 2012, 8, e1002631. [Google Scholar] [CrossRef]
- Kelly, E.M.; Moon, D.C.; Bowers, D.F. Apoptosis in Mosquito Salivary Glands: Sindbis Virus-Associated and Tissue Homeostasis. J. Gen. Virol. 2012, 93, 2419–2424. [Google Scholar] [CrossRef]
- Vijayakumar, K.; Cho, G. Autophagy: An Evolutionarily Conserved Process in the Maintenance of Stem Cells and Aging. Cell Biochem. Funct. 2019, 37, 452–458. [Google Scholar] [CrossRef]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in Healthy Aging and Disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef]
- Kroemer, G.; Mariño, G.; Levine, B. Autophagy and the Integrated Stress Response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.; Sándor, G.O.; Juhász, G. Autophagy Maintains Stem Cells and Intestinal Homeostasis in Drosophila. Sci. Rep. 2018, 8, 4644. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.L.; Baehrecke, E.H. Growth Arrest and Autophagy Are Required for Salivary Gland Cell Degradation in Drosophila. Cell 2007, 131, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Bryant, B.; Raikhel, A.S. Programmed Autophagy in the Fat Body of Aedes Aegypti Is Required to Maintain Egg Maturation Cycles. PLoS ONE 2011, 6, e25502. [Google Scholar] [CrossRef] [PubMed]
- Gunay, B.; Goncu, E. Role of Autophagy in Midgut Stem Cells of Silkworm Bombyx Mori, during Larval–Pupal Metamorphosis. Arch Insect Biochem. Physiol. 2021, 108, e21832. [Google Scholar] [CrossRef]
- Tian, L.; Ma, L.; Guo, E.; Deng, X.; Ma, S.; Xia, Q.; Cao, Y.; Li, S. 20-Hydroxyecdysone Upregulates Atg Genes to Induce Autophagy in the Bombyx Fat Body. Autophagy 2013, 9, 1172–1187. [Google Scholar] [CrossRef]
- Shiba, H.; Yabu, T.; Sudayama, M.; Mano, N.; Arai, N.; Nakanishi, T.; Hosono, K. Sequential Steps of Macroautophagy and Chaperone-Mediated Autophagy Are Involved in the Irreversible Process of Posterior Silk Gland Histolysis during Metamorphosis of Bombyx Mori. J. Exp. Biol. 2016, 219, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Tindwa, H.; Jo, Y.H.; Patnaik, B.B.; Lee, Y.S.; Kang, S.S.; Han, Y.S. Molecular Cloning and Characterization of Autophagy-Related Gene TmATG8 in Listeria-Invaded Hemocytes of Tenebrio Molitor. Dev. Comp. Immunol. 2015, 51, 88–98. [Google Scholar] [CrossRef]
- Barletta, A.B.F.; Silva, M.C.L.N.; Sorgine, M.H.F. Validation of Aedes Aegypti Aag-2 Cells as a Model for Insect Immune Studies. Parasites Vectors 2012, 5, 148. [Google Scholar] [CrossRef]
- Brackney, D.E.; Correa, M.A.; Cozens, D.W. The Impact of Autophagy on Arbovirus Infection of Mosquito Cells. PLoS Negl. Trop. Dis. 2020, 14, e0007754. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Smartt, C.T. Activation of the Autophagy Pathway Decreases Dengue Virus Infection in Aedes Aegypti Cells. Parasites Vectors 2021, 14, 551. [Google Scholar] [CrossRef] [PubMed]
- Henderson, C.; Brustolin, M.; Hegde, S.; Dayama, G.; Lau, N.; Hughes, G.L.; Bergey, C.; Rasgon, J.L. Mayaro Virus Infection Elicits an Innate Immune Response in Anopheles Stephensi. bioRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Wang, L.-L.; Wang, X.-R.; Wei, X.-M.; Huang, H.; Wu, J.-X.; Chen, X.-X.; Liu, S.-S.; Wang, X.-W. The Autophagy Pathway Participates in Resistance to Tomato Yellow Leaf Curl Virus Infection in Whiteflies. Autophagy 2016, 12, 1560–1574. [Google Scholar] [CrossRef]
- Wang, S.; Guo, H.; Zhu-Salzman, K.; Ge, F.; Sun, Y. PEBP Balances Apoptosis and Autophagy in Whitefly upon Arbovirus Infection. Nat. Commun. 2022, 13, 846. [Google Scholar] [CrossRef] [PubMed]
- Ramesh Kumar, J.; Smith, J.P.; Kwon, H.; Smith, R.C. Use of Clodronate Liposomes to Deplete Phagocytic Immune Cells in Drosophila Melanogaster and Aedes Aegypti. Front. Cell Dev. Biol. 2021, 9, 627976. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).