Abstract
Lassa fever virus (LASV) can cause life-threatening hemorrhagic fevers for which there are currently no vaccines or targeted treatments. The late Prof. Stefan Kunz, along with others, showed that the high-affinity host receptor for LASV, and other Old World and clade-C New World mammarenaviruses, is matriglycan—a linear repeating disaccharide of alternating xylose and glucuronic acid that is polymerized uniquely on α-dystroglycan by like-acetylglucosaminyltransferase-1 (LARGE1). Although α-dystroglycan is ubiquitously expressed, LASV preferentially infects vascular endothelia and professional phagocytic cells, which suggests that viral entry requires additional cell-specific factors. In this review, we highlight the work of Stefan Kunz detailing the molecular mechanism of LASV binding and discuss the requirements of receptors, such as tyrosine kinases, for internalization through apoptotic mimicry.
1. Introduction
The surfaces of host cells and pathogens are covered with glycoproteins. Glycans are well-known targets for virus binding and entry [1]. We highlight the work of the late Stefan Kunz who showed the surface glycoprotein of Old World, including LASV, and New World clade-C mammarenaviruses, binds matriglycan [2,3,4,5]—a polysaccharide of alternating xylose and glucuronate—on the highly glycosylated mucin-like receptor, dystroglcyan on rodent and human hosts (Figure 1). On the other hand, the spike glycoproteins (GP) of New World mammarenavirus clades use transferrin as a receptor [6,7,8]. We limit the scope of our review to virus binding and entry. Other publications detailing events post cell-entry and broader aspects of arenaviruses have been published [9,10,11,12].
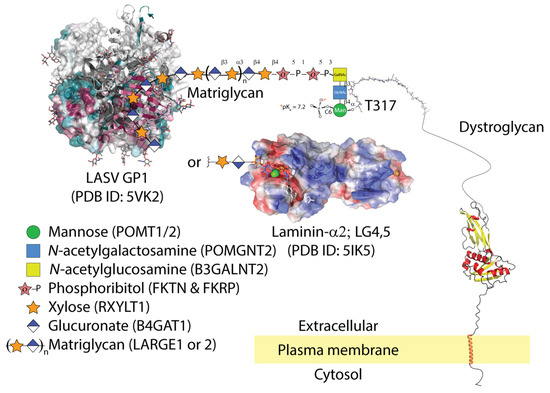
Figure 1.
LASV GP1 is able to bind matriglycan (xylose and glucuronate), but only gains entry to cells that co-express apoptotic phagocytic machinery. The molecular details of LASV GP1 binding to matriglycan are unknown. Matriglycan is polymerized on a primer of extended phosphocore M3 on threonine-317 and possibly 379 of α-dystroglycan. The core M3 trisaccharide is phosphorylated by Protein O-Mannosyl Kinase (POMK); other glycosyltransferases are listed in parentheses next to their corresponding sugars. The conserved surface residues of LASV trimer from 5VK2 are shown as a gradient of magenta (conserved) to green (non-conserved); (accessed on 6 July 2021: https://consurf.tau.ac.il/). LASV GP1 binding displaces LG domains from matriglycan. The semi-transparent electrostatic surface of LG4-5 domains from laminin-2α is shown binding a unit of xylose-glucuronate via calcium (green sphere). Parts of the dystroglycan structure were downloaded from AlphaFold [26].
The high-affinty receptor for the trimeric glycoprotein-1 (GP1) of Old World and clade-C New World mammarenaviruses is matriglycan, which is polymerized on α-dystroglycan post-translationally by like-acetylglucosaminyltransferase (LARGE1) [13]. Dystroglycan consists of α-dystroglycan and β-dystroglycan [14] and is part of the plasma membrane-embedded dystrophin-glycoprotein-complex (DGC), which is ubiquitously expressed but whose composition varies between cell types [15,16,17]. Under normal circumstances, proteins that contain laminin-globular (LG) domains bind matriglycan and components of the DGC bind the actin cytoskeleton such that, at its simplest, the DGC anchors the cytoskeleton to the extracellular matrix (ECM) [18,19,20,21,22]. Genetic mutations that diminish or abolish matriglycan polymerization can cause muscular dystrophies, known as dystroglycanopathies, with or without brain and eye involvement [23,24]. Much like mutations that cause sickle-cell anemia can protect against malarial parasites, LARGE1 alleles have undergone recent positive selection in West African populations where LASV is endemic [25].
Despite ubiquitous expression of matriglycan on dystroglycan throughout human hosts, LASV preferentially infects antigen-presenting cells, including dendritic cells [27], but not muscle cells [28], which suggests that additional factors influence viral entry. The differential manifestations of both genetic and infectious diseases indicate that dystroglycan associates with dynamic, cell-type-specific complexes [29,30] on which pathologies depend. Many reports [31,32,33] have implicated auxiliary cell surface receptors as co-factors for viral entry: the tyrosine kinase family, Tyro3/Axl/MERTK (TAM) or hepatocyte growth factor receptor (HGFR) [34], T-cell immunoglobulin and mucin domain-containing proteins that bind phosphatidylserine, TIM1 and 4 [35], and the lectin family-4 members M and G (CLEC4G and M) that are popularly known as DC-SIGN and LSECtin [33].
LASV is a highly prevalent mammarenavirus in Western Africa, where it infects a few hundred thousand individuals annually [36]. Lassa fever (LF) is associated with high morbidity and a recent average case fatality rate of ~20% in Nigeria [37]. Currently, neither a vaccine nor specific treatments for LF exist. The majority of basic scientific knowledge on LASV entry was generated in the Oldstone laboratory (Scripps Reseach Institute) and much of the detailed analysis was carried out by the late Dr. Stefan Kunz, first as a post-doctoral fellow in the Oldstone laboratory and later as an independent investigator at the University of Lausanne.
Conveniently, lymphocytic choriomeningitis virus (LCMV) and LASV both use α-dystroglycan as a high-affinity receptor [2]. Results from virus overlay protein blot assays (VOPBA), which are like Western blots but use whole virus particles instead of primary antibodies, suggested that an SDS-resistant moiety on α-dystroglycan is responsible for binding virus particles. Additionally, viral infectivity was restored in α-dystroglycan-deficient cells by adenoviral dystroglycan transductions. Purified soluble α-dystroglycan blocked cell entry by acting as a decoy receptor. These initial studies also showed that bacterially expressed α-dystroglycan could not bind virus particles, suggesting that a mammalian-specific post-translational modification, perhaps glycosylation, was crucial to binding [2]. This formed the basis of Dr. Kunz’ invaluable work on mammarenaviral cell entry. Using a series of dystroglycan deletion mutants, he deduced that residues 169–408 of dystroglycan were essential for virus binding and that the viral glycoprotein bound the same post-translational modification as laminin [4]. This work culminated in the understanding that GP1 from LASV, LCMV, Mobala, and Oliveros mammarenavirus [5] bound a chain of alternating xylose and glucuronic acid that is polymerized by LARGE1 on α-dystroglycan residues threonine-317 [13,38]. His conclusions were bosltered by the fact that the overexpression of LARGE1 increased mammarenaviral binding [5,39]. Interestingly, a novel genetic screen using haploid cells confirmed these results [40]. Also, replacing the transmembrane and cytosolic domains of β-dystroglycan did not affect viral entry [41]. This, along with tissue tropism, implicates auxiliary signalling receptors in viral entry. This was the foundation of Dr. Kunz’ independent research.
2. LASV Binds Matriglycan and Is Internalized in Cells Co-Expressing Gas6-Axl
Phosphorylation of intracellular auxiliary receptors is key to LASV cell entry [31,32,42,43]. The application of the generic tyrosine kinase inhibitor genistein prevented virus internalization but not binding [34,42], which suggests that virus binding and internalization are separable. For example, tyrosine-892 on β -dystroglycan is phosphorylated in response to virus binding. The Tyro3/Axl/MERTK (TAM) tyrosine kinases that are expressed on dendritic cells can promote the internalization of virus particles via receptor phosphorylation [17,42].
Axl bound to its co-factor growth arrest-specific protein-6 (Gas6) is expressed in cells that phagocytose apoptotic cells and is key to internalizing other viruses such as Zika, Dengue, and Ebola [32,44,45,46], which use apoptotic mimicry [47,48]. Although there are conflicting results regarding the role of Axl for mammarenavirus cell entry [31,49], Shimojima et al. showed that deletion of the first immunoglobulin-like domain of Axl abolished LASV cell entry [32]. Morizono et al. showed that the presence of Gas6 enhanced LASV infection [33]. A crystal structure of the LG domains from Gas6 in complex with Axl shows the interaction is mediated via an anti-parallel β-zipper and buries a total surface area of ~1100 A2 [50]. The LG domains of laminin-α2 can bind matriglycan through the chelation of calcium via two aspartate residues [51] (Figure 1). There is no evidence that the Gas6 LG domains bind matriglycan like those of laminin-α2 [52]. A more likely scenario is that LASV binds cells that express matriglycan but is only internalized in cells which co-express the Axl phagocytic machinery.
Functionally, deletion or substitution of the ATP binding site (K567M), or phosphotyrosine site (Y821F), of Axl cytoplasmic domains prevents LASV entry, suggesting that Axl tyrosine autophosphorylation is essential for LASV entry [35]. This research suggests that cells co-expressing α-dystroglycan modified with matriglycan, Gas6, and Axl or Tyro3 are highly susceptible to LASV entry, whereas non-phagocytic cells such as myocytes, although they express plenty of matriglycan and can bind virions, are incapable of engulfing LASV particles via apoptotic mimicry [34,47,48].
3. Subunit GP1 of the Viral Spike Glycoprotein Binds Matriglycan at the Interface Formed upon Trimerization
Unlike the calcium chelating mode in which LG domains bind matriglycan [4,51], GP1 from LASV and LCMV, which share ~50% sequence identity, likely bind matriglycan using a region formed at the central surface of the trimer. This deduction comes from the fact that the F260 [L/V/I] substitution in the LCMV GP1 increases the affinity for matriglycan by 2–2.5 orders of magnitude and promotes persistent infection in mice [3]. The residues surrounding leucine-260 are essential for viral fusion [53]. The equivalent leucine residue in the trimeric pre-fusion structure of LASV GP1 also maps to their central interface [54], which hints at the location of matriglycan binding (Figure 2).
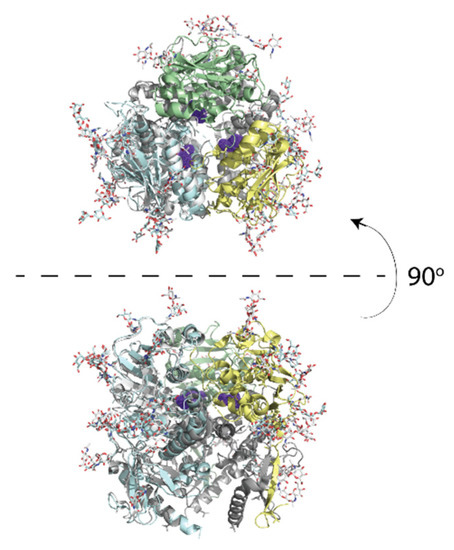
Figure 2.
The crystal structure of LASV GP1 trimer is show in cartoon (PDB ID: 5vk2). Leucine-260 is shown in purple on the LCMV trimer. The upper panel shows the top view; the lower panel shows a side view.
4. Hypothetical Mechanism of Hemorrhage
LASV-infected cells appear to downregulate matriglycan polymerization [55]. The simplest mechanism might be that the spike protein encounters matriglycan that is nascently polymerized on α-dystroglycan in the Golgi as it traverses the secretory pathway. The incidental exposure of GP1 could disrupt and decrease matriglycan polymerization by LARGE1, which might explain the complex formation and self-limiting infection [55]. A decrease in matriglycan expression has been linked to membrane fragility and treatment of muscle cells with inactivated LCMV interferes with membrane integrity [56]. Patients with high viral load can succumb to hemorrhage, although the mechanism of hemorrhage remains unclear. A physical hypothesis is that compromised cell membranes of blood vessels contribute to hemorrhage. A biochemical hypothesis is that interference with vitamin K-dependent protein-S (ProS1), which is another TAM co-receptor, such as Gas6, acts in complex with ProtC to degrade blood clotting factors Va and VII. Interestingly, ProS1 shares domain architecture and 40% sequence identity with Gas6. Moreover, platelets, which express TAMs, are activated by Gas6 [57]. Perturbation of the ProS1-dependent blood coagulation mechanism eventually causes TAM triple-negative mice to hemorrhage [58]. Interference with platelet function may inhibit coagulation and can cause hemorrhage. Both of these physical and biochemical hypotheses remain to be tested experimentally.
5. Conclusions and Remarks
LASV binds host cells via its high-affinity interaction with matriglycan, a repeating disaccharide that is synthesized post-translationally on α-dystroglycan by LARGE1. The spike protein likely binds matriglycan in the central interface formed by trimerization of GP1 monomers. Although virus binding and entry appear to be independent, the former increases the probability of the latter. Viral entry is determined additionally by cell-type-specific factors [17] such as the tyrosine kinase Axl and cell surface lectins. Although studies conflict, many other viruses enter cells using Gas6-Axl mediated apoptotic mimicry [33,44,57,59,60], and this may also explain the cell specificity of the virus. The mechanism of hepatocyte infection and hemorrhage may have to do with parallel tyrosine kinase and LG-domain containing receptors in the liver, such as hepatocyte growth factor receptor (HGFR), and remains to be tested. A decrease in the polymerization of matriglycan may be due to GP1 interacting with the nascent matriglycan polymer on LARGE1-dystroglycan enzyme-substrate complex in the Golgi, which coincidentally prevents further virus entry. Hemorrhage might be the side-effect of conscripting ProS to bind viral glycoprotein resulting in poor blood clotting. We can honor Dr Kunz’ legacy by building on his invaluable work to further explore these unanswered questions.
Author Contributions
S.J. and K.P.C. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by a Paul D. Wellstone Muscular Dystrophy Specialized Research Center grant (1U54NS053672 to K.P.C.). K.P.C. is an investigator of the Howard Hughes Medical Institute.
Institutional Review Board Statement
Not available.
Informed Consent Statement
Not available.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
We are grateful to Amber Mower for assistance with administrative support. We would also like to thank Erhard Hohenester, Juan Carlos de la Torre, Michael Oldstone, Lance Wells, and members of the Campbell lab for their helpful comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lentz, T.L. The recognition event between virus and host cell receptor: A target for antiviral agents. J. Gen. Virol. 1990, 71, 751–766. [Google Scholar] [CrossRef]
- Cao, W.; Henry, M.D.; Borrow, P.; Yamada, H.; Elder, J.H.; Ravkov, E.V.; Nichol, S.T.; Compans, R.W.; Campbell, K.P.; Oldstone, M.B.A. Identification of α-Dystroglycan as a Receptor for Lymphocytic Choriomeningitis Virus and Lassa Fever Virus. Science 1998, 282, 2079–2081. [Google Scholar] [CrossRef] [Green Version]
- Sevilla, N.; Kunz, S.; Holz, A.; Lewicki, H.; Homann, D.; Yamada, H.; Campbell, K.P.; de la Torre, J.C.; Oldstone, M.B.A. Immunosuppression and Resultant Viral Persistence by Specific Viral Targeting of Dendritic Cells. J. Exp. Med. 2000, 192, 1249–1260. [Google Scholar] [CrossRef]
- Kunz, S.; Sevilla, N.; McGavern, D.B.; Campbell, K.P.; Oldstone, M.B.A. Molecular analysis of the interaction of LCMV with its cellular receptor α-dystroglycan. J. Cell Biol. 2001, 155, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Kunz, S.; Rojek, J.M.; Kanagawa, M.; Spiropoulou, C.F.; Barresi, R.; Campbell, K.P.; Oldstone, M.B.A. Posttranslational Modification of Dystroglycan, the Cellular Receptor for Arenaviruses, by the Glycosyltransferase LARGE Is Critical for Virus Binding. J. Virol. 2005, 79, 14282–14296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radoshitzky, S.R.; Abraham, J.; Spiropoulou, C.F.; Kuhn, J.H.; Nguyen, D.; Li, W.; Nagel, J.; Schmidt, P.J.; Nunberg, J.H.; Andrews, N.C.; et al. Transferrin receptor 1 is a cellular receptor for New World haemorrhagic fever arenaviruses. Nature 2007, 446, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, M.L.; Oldenburg, J.; Reignier, T.; Holt, N.; Hamilton, G.A.; Martin, V.K.; Cannon, P.M. New World Clade B Arenaviruses Can Use Transferrin Receptor 1 (TfR1)-Dependent and -Independent Entry Pathways, and Glycoproteins from Human Pathogenic Strains Are Associated with the Use of TfR1. J. Virol. 2008, 82, 938–948. [Google Scholar] [CrossRef] [Green Version]
- Abraham, J.; Corbett, K.D.; Farzan, M.; Choe, H.; Harrison, S.C. Structural basis for receptor recognition by New World hemorrhagic fever arenaviruses. Nat. Struct. Mol. Biol. 2010, 17, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Jae, L.T.; Raaben, M.; Herbert, A.S.; Kuehne, A.I.; Wirchnianski, A.S.; Soh, T.K.; Stubbs, S.H.; Janssen, H.; Damme, M.; Saftig, P.; et al. Lassa virus entry requires a trigger-induced receptor switch. Science 2014, 344, 1506–1510. [Google Scholar] [CrossRef] [Green Version]
- Peng, R.; Xu, X.; Jing, J.; Wang, M.; Peng, Q.; Liu, S.; Wu, Y.; Bao, X.; Wang, P.; Qi, J.; et al. Structural insight into arenavirus replication machinery. Nature 2020, 579, 615–619. [Google Scholar] [CrossRef]
- Pasquato, A.; Fernandez, A.H.; Kunz, S. Studies of Lassa Virus Cell Entry BT—Hemorrhagic Fever Viruses: Methods and Protocols; Salvato, M.S., Ed.; Springer: New York, NY, USA, 2018; pp. 135–155. ISBN 978-1-4939-6981-4. [Google Scholar]
- Bhadelia, N. Understanding Lassa fever. Science 2019, 363, 30. [Google Scholar] [CrossRef]
- Inamori, K.; Yoshida-Moriguchi, T.; Hara, Y.; Anderson, M.E.; Yu, L.; Campbell, K.P. Dystroglycan Function Requires Xylosyl- and Glucuronyltransferase Activities of LARGE. Science 2012, 335, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Ervasti, J.M.; Ohlendieck, K.; Kahl, S.D.; Gaver, M.; Campbell, K.P. Deficiency of a Glycoprotein Component of the Dystrophin Complex in Dystrophic Muscle. Nature 1990, 345, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Durbeej, M.; Henry, M.D.; Ferletta, M.; Campbell, K.P.; Ekblom, P. Distribution of Dystroglycan in Normal Adult Mouse Tissues. J. Histochem. Cytochem. 1998, 46, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Culligan, K.; Ohlendieck, K. Diversity of the Brain Dystrophin-Glycoprotein Complex. J. Biomed. Biotechnol. 2002, 2, 390232. [Google Scholar] [CrossRef] [Green Version]
- Herrador, A.; Fedeli, C.; Radulovic, E.; Campbell, K.P.; Moreno, H.; Gerold, G.; Kunz, S.; Dermody, T.S. Dynamic Dystroglycan Complexes Mediate Cell Entry of Lassa Virus. MBio 2021, 10, e02869-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibraghimov-Beskrovnaya, O.; Ervasti, J.M.; Leveille, C.J.; Slaughter, C.A.; Sernett, S.W.; Campbell, K.P. Primary Structure of Dystrophin-Associated Glycoproteins Linking Dystrophin to the Extracellular Matrix. Nature 1992, 355, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Ervasti, J.M.; Campbell, K.P. A Role for the Dystrophin-Glycoprotein Complex as a Transmembrane Linker Between Laminin and Actin. J. Cell Biol. 1993, 122, 809–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, K.P. Three muscular dystrophies: Loss of cytoskeleton-extracellular matrix linkage. Cell 1995, 80, 675–679. [Google Scholar] [CrossRef] [Green Version]
- Henry, M.D.; Campbell, K.P. Dystroglycan: An extracellular matrix receptor linked to the cytoskeleton. Curr. Opin. Cell Biol. 1996, 8, 625–631. [Google Scholar] [CrossRef]
- Ohlendieck, K. Towards an understanding of the dystrophin-glycoprotein complex: Linkage between the extracellular matrix and the membrane cytoskeleton in muscle fibers. Eur. J. Cell Biol. 1996, 69, 1–10. [Google Scholar]
- Michele, D.E.; Barresi, R.; Kanagawa, M.; Saito, F.; Cohn, R.D.; Satz, J.S.; Dollar, H.; Nishino, I.; Kelley, R.I.; Somer, H.; et al. Post-translational Disruption of Dystroglycan-Ligand Interactions in Congenital Muscular Dystrophies. Nature 2002, 418, 417–422. [Google Scholar] [CrossRef]
- Yoshida-Moriguchi, T.; Campbell, K.P. Matriglycan: A novel polysaccharide that links dystroglycan to the basement membrane. Glycobiology 2015, 25, 702–713. [Google Scholar] [CrossRef] [Green Version]
- Sabeti, P.C.; Varilly, P.; Fry, B.; Lohmueller, J.; Hostetter, E.; Cotsapas, C.; Xie, X.; Byrne, E.H.; McCarroll, S.A.; Gaudet, R.; et al. Genome-wide detection and characterization of positive selection in human populations. Nature 2007, 449, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; ŽíDek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021. [Google Scholar] [CrossRef]
- Baize, S.; Kaplon, J.; Faure, C.; Pannetier, D.; Georges-Courbot, M.-C.; Deubel, V. Lassa Virus Infection of Human Dendritic Cells and Macrophages Is Productive but Fails to Activate Cells. J. Immunol. 2004, 172, 2861–2869. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Urata, S.; Cho, Y.; Ngo, N.; de la Torre, J.C. Cell entry of lymphocytic choriomeningitis virus is restricted in myotubes. Virology 2014, 458–459, 22–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barresi, R. Dystroglycan: From biosynthesis to pathogenesis of human disease. J. Cell Sci. 2006, 119, 199–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durbeej, M.; Campbell, K.P. Biochemical Characterization of the Epithelial Dystroglycan Complex*. J. Biol. Chem. 1999, 274, 26609–26616. [Google Scholar] [CrossRef] [Green Version]
- Fedeli, C.; Torriani, G.; Galan-Navarro, C.; Moraz, M.-L.; Moreno, H.; Gerold, G.; Kunz, S.; Dermody, T.S. Axl Can Serve as Entry Factor for Lassa Virus Depending on the Functional Glycosylation of Dystroglycan. J. Virol. 2021, 92, e01613-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimojima, M.; Ströher, U.; Ebihara, H.; Feldmann, H.; Kawaoka, Y. Identification of Cell Surface Molecules Involved in Dystroglycan-Independent Lassa Virus Cell Entry. J. Virol. 2012, 86, 2067–2078. [Google Scholar] [CrossRef] [Green Version]
- Morizono, K.; Xie, Y.; Olafsen, T.; Lee, B.; Dasgupta, A.; Wu, A.M.; Chen, I.S.Y. The Soluble Serum Protein Gas6 Bridges Virion Envelope Phosphatidylserine to the TAM Receptor Tyrosine Kinase Axl to Mediate Viral Entry. Cell Host Microbe 2011, 9, 286–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oppliger, J.; Torriani, G.; Herrador, A.; Kunz, S.; Dermody, T.S. Lassa Virus Cell Entry via Dystroglycan Involves an Unusual Pathway of Macropinocytosis. J. Virol. 2021, 90, 6412–6429. [Google Scholar] [CrossRef] [Green Version]
- Brouillette, R.B.; Phillips, E.K.; Patel, R.; Mahauad-Fernandez, W.; Moller-Tank, S.; Rogers, K.J.; Dillard, J.A.; Cooney, A.L.; Martinez-Sobrido, L.; Chioma, O.; et al. TIM-1 Mediates Dystroglycan-Independent Entry of Lassa Virus. J. Virol. 2021, 92, e00093-18. [Google Scholar] [CrossRef] [Green Version]
- Ogbu, O.; Ajuluchukwu, E.; Uneke, C.J. Lassa fever in West African sub-region: An overview. J. Vector Borne Dis. 2007, 44, 1. [Google Scholar]
- Yaro, C.A.; Kogi, E.; Opara, K.N.; Batiha, G.E.-S.; Baty, R.S.; Albrakati, A.; Altalbawy, F.M.A.; Etuh, I.U.; Oni, J.P. Infection pattern, case fatality rate and spread of Lassa virus in Nigeria. BMC Infect. Dis. 2021, 21, 149. [Google Scholar] [CrossRef]
- Hara, Y.; Kanagawa, M.; Kunz, S.; Yoshida-moriguchi, T.; Satz, J.S. modification of dystroglycan at Thr-317/319 is required for laminin binding and arenavirus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 17426–17432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojek, J.M.; Spiropoulou, C.F.; Campbell, K.P.; Kunz, S. Old World and Clade C New World Arenaviruses Mimic the Molecular Mechanism of Receptor Recognition Used by α-Dystroglycan’s Host-Derived Ligands. J. Virol. 2007, 81, 5685–5695. [Google Scholar] [CrossRef] [Green Version]
- Jae, L.T.; Raaben, M.; Riemersma, M.; van Beusekom, E.; Blomen, V.A.; Velds, A.; Kerkhoven, R.M.; Carette, J.E.; Topaloglu, H.; Meinecke, P.; et al. Deciphering the Glycosylome of Dystroglycanopathies Using Haploid Screens for Lassa Virus Entry. Science 2013, 340, 479–483. [Google Scholar] [CrossRef] [Green Version]
- Kunz, S.; Campbell, K.P.; Oldstone, M.B.A. α-Dystroglycan can mediate arenavirus infection in the absence of β-dystroglycan. Virology 2003, 316, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Moraz, M.-L.; Pythoud, C.; Turk, R.; Rothenberger, S.; Pasquato, A.; Campbell, K.P.; Kunz, S. Cell entry of Lassa virus induces tyrosine phosphorylation of dystroglycan. Cell. Microbiol. 2013, 15, 689–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojek, J.M.; Moraz, M.-L.; Pythoud, C.; Rothenberger, S.; Van der Goot, F.G.; Campbell, K.P.; Kunz, S. Binding of Lassa virus perturbs extracellular matrix-induced signal transduction via dystroglycan. Cell. Microbiol. 2012, 14, 1122–1134. [Google Scholar] [CrossRef] [Green Version]
- Meertens, L.; Labeau, A.; Dejarnac, O.; Cipriani, S.; Sinigaglia, L.; Bonnet-Madin, L.; Le Charpentier, T.; Hafirassou, M.L.; Zamborlini, A.; Cao-Lormeau, V.-M.; et al. Axl Mediates ZIKA Virus Entry in Human Glial Cells and Modulates Innate Immune Responses. Cell Rep. 2017, 18, 324–333. [Google Scholar] [CrossRef]
- Richard, A.S.; Shim, B.-S.; Kwon, Y.-C.; Zhang, R.; Otsuka, Y.; Schmitt, K.; Berri, F.; Diamond, M.S.; Choe, H. AXL-dependent infection of human fetal endothelial cells distinguishes Zika virus from other pathogenic flaviviruses. Proc. Natl. Acad. Sci. USA 2017, 114, 2024–2029. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Yang, Y.; Yang, Y.; Zou, P.; Chen, J.; He, Y.; Shui, S.; Cui, Y.; Bai, R.; Liang, Y.; et al. AXL promotes Zika virus infection in astrocytes by antagonizing type I interferon signalling. Nat. Microbiol. 2018, 3, 302–309. [Google Scholar] [CrossRef]
- Lemke, G.; Rothlin, C.V. Immunobiology of the TAM receptors. Nat. Rev. Immunol. 2008, 8, 327–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amara, A.; Mercer, J. Viral apoptotic mimicry. Nat. Rev. Microbiol. 2015, 13, 461–469. [Google Scholar] [CrossRef]
- Sullivan, B.M.; Welch, M.J.; Lemke, G.; Oldstone, M.B.A. Is the TAM Receptor Axl a Receptor for Lymphocytic Choriomeningitis Virus? J. Virol. 2013, 87, 4071–4074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, T.; Knyazev, P.G.; Clout, N.J.; Cheburkin, Y.; Göhring, W.; Ullrich, A.; Timpl, R.; Hohenester, E. Structural basis for Gas6–Axl signalling. EMBO J. 2006, 25, 80–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briggs, D.C.; Yoshida-Moriguchi, T.; Zheng, T.; Venzke, D.; Anderson, M.E.; Strazzulli, A.; Moracci, M.; Yu, L.; Hohenester, E.; Campbell, K.P. Structural basis of laminin binding to the LARGE glycans on dystroglycan. Nat. Chem. Biol. 2016, 12, 810–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, T.; Knyazev, P.G.; Cheburkin, Y.; Göhring, W.; Tisi, D.; Ullrich, A.; Timpl, R.; Hohenester, E. Crystal Structure of a C-terminal Fragment of Growth Arrest-specific Protein Gas6: Receptor tyrosine kinase activation by laminin G-like domains. J. Biol. Chem. 2002, 277, 44164–44170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hastie, K.M.; Igonet, S.; Sullivan, B.M.; Legrand, P.; Zandonatti, M.A.; Robinson, J.E.; Garry, R.F.; Rey, F.A.; Oldstone, M.B.; Saphire, E.O. Crystal structure of the prefusion surface glycoprotein of the prototypic arenavirus LCMV. Nat. Struct. Mol. Biol. 2016, 23, 513–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hastie, K.M.; Zandonatti, M.A.; Kleinfelter, L.M.; Heinrich, M.L.; Rowland, M.M.; Chandran, K.; Branco, L.M.; Robinson, J.E.; Garry, R.F.; Saphire, E.O. Structural basis for antibody-mediated neutralization of Lassa virus. Science 2017, 356, 923–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojek, J.M.; Campbell, K.P.; Oldstone, M.B.A.; Kunz, S. Old World Arenavirus Infection Interferes with the Expression of Functional α-Dystroglycan in the Host Cell. Mol. Biol. Cell 2007, 18, 4493–4507. [Google Scholar] [CrossRef] [Green Version]
- Han, R.; Kanagawa, M.; Yoshida-Moriguchi, T.; Rader, E.P.; Ng, R.A.; Michele, D.E.; Muirhead, D.E.; Kunz, S.; Moore, S.A.; Iannaccone, S.T.; et al. Basal lamina strengthens cell membrane integrity via the laminin G domain-binding motif of α-dystroglycan. Proc. Natl. Acad. Sci. USA 2009, 106, 12573–12579. [Google Scholar] [CrossRef] [Green Version]
- Gould, W.R.; Baxi, S.M.; Schroeder, R.; Peng, Y.W.; Leadley, R.J.; Peterson, J.T.; Perrin, L.A. Gas6 receptors Axl, Sky and Mer enhance platelet activation and regulate thrombotic responses. J. Thromb. Haemost. 2005, 3, 733–741. [Google Scholar] [CrossRef]
- Lu, Q.; Lemke, G. Homeostatic Regulation of the Immune System by Receptor Tyrosine Kinases of the Tyro 3 Family. Science 2001, 293, 306–311. [Google Scholar] [CrossRef]
- Masayuki, S.; Ayato, T.; Hideki, E.; Gabriele, N.; Kouki, F.; Tatsuro, I.; Steven, J.; Heinz, F.; Yoshihiro, K. Tyro3 Family-Mediated Cell Entry of Ebola and Marburg Viruses. J. Virol. 2006, 80, 10109–10116. [Google Scholar] [CrossRef] [Green Version]
- Shimojima, M.; Ikeda, Y.; Kawaoka, Y. The Mechanism of Axl-Mediated Ebola Virus Infection. J. Infect. Dis. 2007, 196, S259–S263. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).