Impact of the Interaction of Hepatitis B Virus with Mitochondria and Associated Proteins
Abstract
1. Introduction
2. Methods
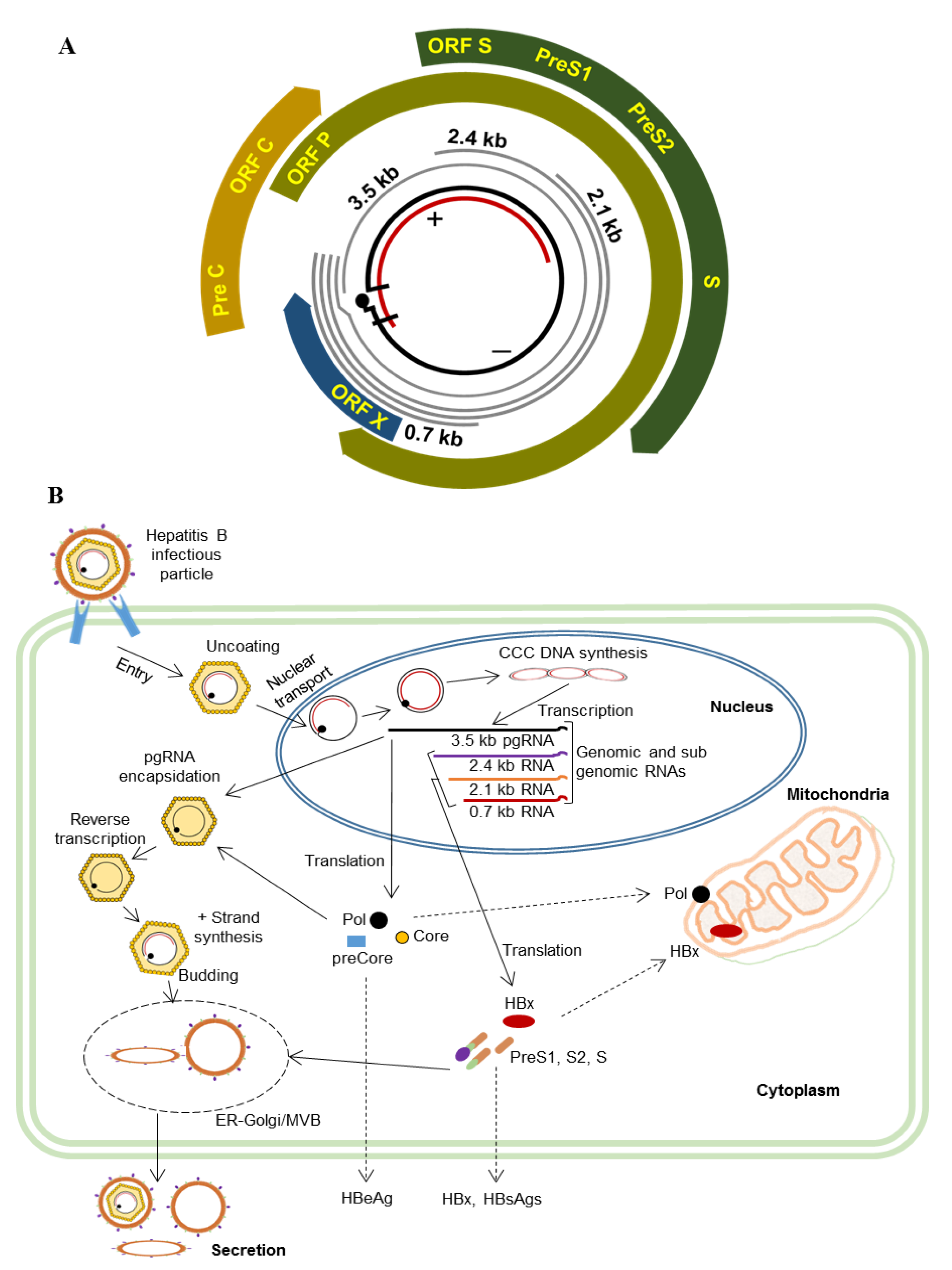
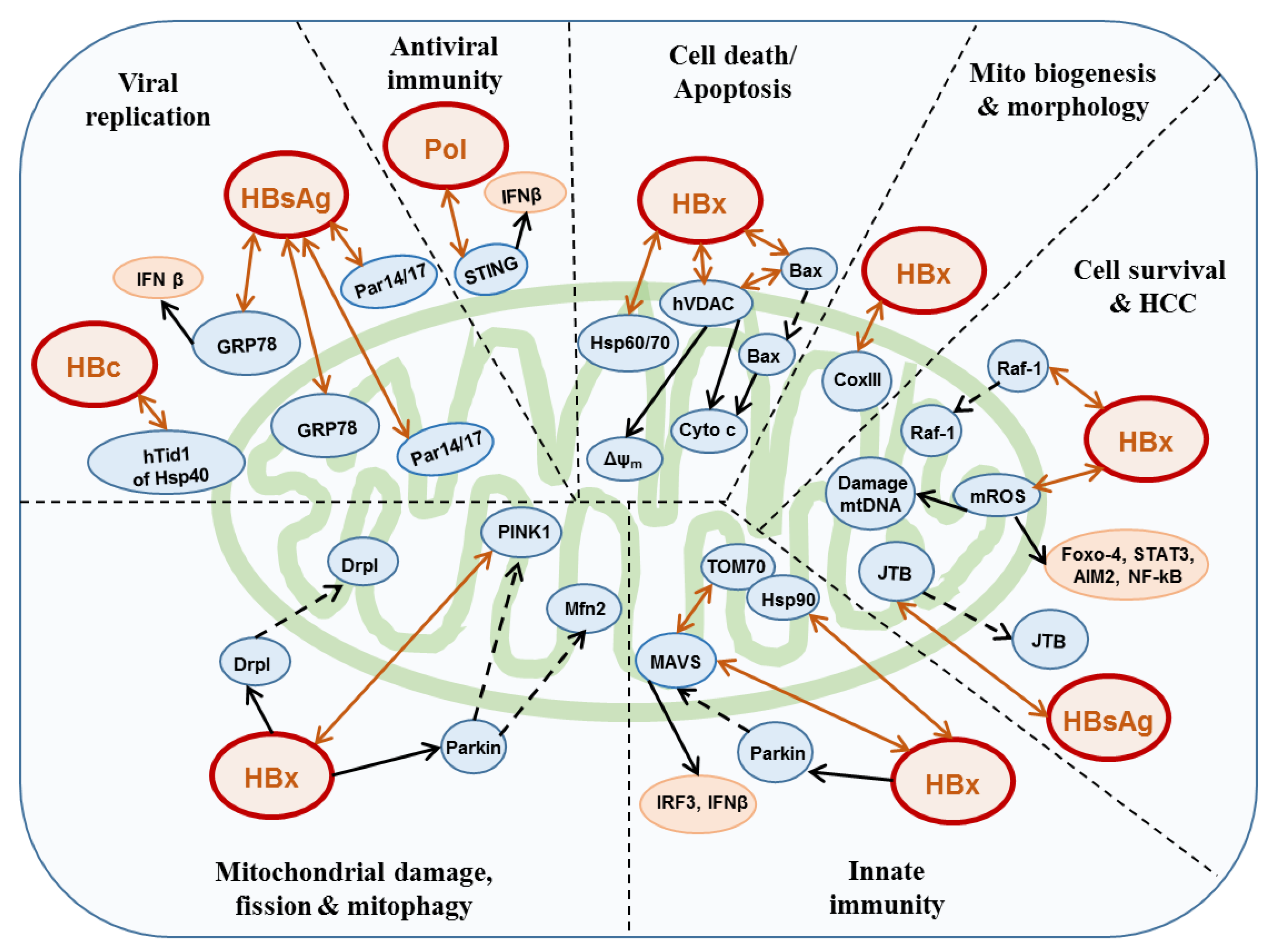
3. HBV and Mitochondria
3.1. HBx
3.2. Polymerase
3.3. HBsAg
3.4. Core
4. Conclusions and Perspectives
Author Contributions
Conflicts of Interest
References
- Endo, T.; Yamano, K. Multiple pathways for mitochondrial protein traffic. Boil. Chem. 2009, 390, 723–730. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis*. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Rizzuto, R.; Hajnoczky, G.; Su, T.P. MAM: More than just a housekeeper. Trends Cell Biol. 2009, 19, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Degli Esposti, D.; Hamelin, J.; Bosselut, N.; Saffroy, R.; Sebagh, M.; Pommier, A.; Martel, C.; Lemoine, A. Mitochondrial Roles and Cytoprotection in Chronic Liver Injury. Biochem. Res. Int. 2012, 2012, 1–16. [Google Scholar] [CrossRef]
- West, A.P.; Shadel, G.S.; Ghosh, S. Mitochondria in innate immune responses. Nat. Rev. Immunol. 2011, 11, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Syed, G.H.; Kim, S.-J.; Siddiqui, A. Mitochondrial dynamics and viral infections: A close nexus. Biochim. Biophys. Acta (BBA) Bioenerg. 2015, 1853, 2822–2833. [Google Scholar] [CrossRef]
- Kim, S.-J.; Khan, M.; Quan, J.; Till, A.; Subramani, S.; Siddiqui, A. Hepatitis B Virus Disrupts Mitochondrial Dynamics: Induces Fission and Mitophagy to Attenuate Apoptosis. PLoS Pathog. 2013, 9, e1003722. [Google Scholar] [CrossRef]
- Anand, S.K.; Tikoo, S.K. Viruses as Modulators of Mitochondrial Functions. Adv. Virol. 2013, 2013, 1–17. [Google Scholar] [CrossRef]
- El-Serag, H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012, 142, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.G.; Ueda, K. A meta-analysis on genetic variability of RT/HBsAg overlapping region of hepatitis B virus (HBV) isolates of Bangladesh. Infect. Agents Cancer 2019, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Tsurimoto, T.; Matsubara, K. Three envelope proteins of hepatitis B virus: Large S, middle S, and major S proteins needed for the formation of Dane particles. J. Virol. 1991, 65, 3521–3529. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.G.; Ueda, K. Investigation of a Novel Hepatitis B Virus Surface Antigen (HBsAg) Escape Mutant Affecting Immunogenicity. PLoS ONE 2017, 12, e0167871. [Google Scholar] [CrossRef]
- Delius, H.; Gough, N.M.; Cameron, C.H.; Murray, K. Structure of the hepatitis B virus genome. J. Virol. 1983, 47, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Sprengel, R.; Kühn, C.; Will, H.; Schaller, H. Comparative sequence analysis of duck and human hepatitis B virus genomes. J. Med Virol. 1985, 15, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.G.; Mahmud, M.; Nazir, K.H.M.N.H.; Ueda, K. PreS1 Mutations Alter the Large HBsAg Antigenicity of a Hepatitis B Virus Strain Isolated in Bangladesh. Int. J. Mol. Sci. 2020, 21, 546. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhong, G.; Xu, G.; He, W.; Jing, Z.; Gao, Z.; Huang, Y.; Qi, Y.; Peng, B.; Wang, H.; et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 2012, 1, e00049. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.; Nassal, M. Hepatitis B virus replication. World J. Gastroenterol. 2007, 13, 48–64. [Google Scholar] [CrossRef]
- Grimm, D.; Thimme, R.; Blum, H.E. HBV life cycle and novel drug targets. Hepatol. Int. 2011, 5, 644–653. [Google Scholar] [CrossRef]
- Prange, R. Host factors involved in hepatitis B virus maturation, assembly, and egress. Med. Microbiol. Immunol. 2012, 201, 449–461. [Google Scholar] [CrossRef]
- Unchwaniwala, N.; Sherer, N.M.; Loeb, D.D. Hepatitis B Virus Polymerase Localizes to the Mitochondria, and Its Terminal Protein Domain Contains the Mitochondrial Targeting Signal. J. Virol. 2016, 90, 8705–8719. [Google Scholar] [CrossRef]
- Shirakata, Y.; Koike, K. Hepatitis B Virus X Protein Induces Cell Death by Causing Loss of Mitochondrial Membrane Potential. J. Boil. Chem. 2003, 278, 22071–22078. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, Z.; Huh, K.-W.; Lasher, R.; Siddiqui, A. Hepatitis B Virus X Protein Colocalizes to Mitochondria with a Human Voltage-Dependent Anion Channel, HVDAC3, and Alters Its Transmembrane Potential. J. Virol. 2000, 74, 2840–2846. [Google Scholar] [CrossRef] [PubMed]
- Henkler, F.; King, I.A.; Hoare, J.; McGarvey, M.J.; Waseem, N.; Koshy, R.; Goldin, R.D. Intracellular localization of the hepatitis B virus HBx protein. J. Gen. Virol. 2001, 82, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Gehring, A.; Bertoletti, A.; Tavis, J.E. Host Factor-Targeted Hepatitis B Virus Therapies. Intervirology 2014, 57, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, M.J.; Navas-Martin, S. Hepatitis B and C virus hepatocarcinogenesis: Lessons learned and future challenges. Cancer Lett. 2011, 305, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, M.J.; Schneider, R.J. The Enigmatic X Gene of Hepatitis B Virus. J. Virol. 2004, 78, 12725–12734. [Google Scholar] [CrossRef]
- Rawat, S.; Clippinger, A.J.; Bouchard, M.J. Modulation of Apoptotic Signaling by the Hepatitis B Virus X Protein. Viruses 2012, 4, 2945–2972. [Google Scholar] [CrossRef]
- Casciano, J.C.; Bouchard, M.J. Hepatitis B virus X protein modulates cytosolic Ca2+ signaling in primary human hepatocytes. Virus Res. 2018, 246, 23–27. [Google Scholar] [CrossRef]
- Takada, S.; Shirakata, Y.; Kaneniwa, N.; Koike, K. Association of hepatitis B virus X protein with mitochondria causes mitochondrial aggregation at the nuclear periphery, leading to cell death. Oncogene 1999, 18, 6965–6973. [Google Scholar] [CrossRef]
- Huh, K.-W.; Siddiqui, A. Characterization of the mitochondrial association of hepatitis B virus X protein, HBx. Mitochondrion 2002, 1, 349–359. [Google Scholar] [CrossRef]
- Adachi, M.; Higuchi, H.; Miura, S.; Azuma, T.; Inokuchi, S.; Saito, H.; Kato, S.; Ishii, H. Bax interacts with the voltage-dependent anion channel and mediates ethanol-induced apoptosis in rat hepatocytes. Am. J. Physiol. Liver Physiol. 2004, 287, G695–G705. [Google Scholar] [CrossRef]
- Clippinger, A.J.; Bouchard, M.J. Hepatitis B Virus HBx Protein Localizes to Mitochondria in Primary Rat Hepatocytes and Modulates Mitochondrial Membrane Potential. J. Virol. 2008, 82, 6798–6811. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, J.M.; Soane, L. Multiple Functions of BCL-2 Family Proteins. Cold Spring Harb. Perspect. Boil. 2013, 5, a008722. [Google Scholar] [CrossRef] [PubMed]
- Li, S.K.; Ho, S.F.; Tsui, K.W.; Fung, K.P.; Waye, M.M. Identification of functionally important amino acid residues in the mitochondria targeting sequence of Hepatitis B virus X protein. Virology 2008, 381, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Waris, G.; Huh, K.-W.; Siddiqui, A. Mitochondrially Associated Hepatitis B Virus X Protein Constitutively Activates Transcription Factors STAT-3 and NF-κB via Oxidative Stress. Mol. Cell. Boil. 2001, 21, 7721–7730. [Google Scholar] [CrossRef]
- Jung, S.-Y.; Kim, Y.-J. C-terminal region of HBx is crucial for mitochondrial DNA damage. Cancer Lett. 2013, 331, 76–83. [Google Scholar] [CrossRef]
- He, P.; Zhang, D.; Li, H.; Yang, X.; Li, D.; Zhai, Y.; Ma, L.; Feng, G. Hepatitis B virus X protein modulates apoptosis in human renal proximal tubular epithelial cells by activating the JAK2/STAT3 signaling pathway. Int. J. Mol. Med. 2013, 31, 1017–1029. [Google Scholar] [CrossRef]
- Li, L.; Hann, H.-W.; Wan, S.; Hann, R.S.; Wang, C.; Lai, Y.; Ye, X.; Evans, A.; Myers, R.E.; Ye, Z.; et al. Cell-free circulating mitochondrial DNA content and risk of hepatocellular carcinoma in patients with chronic HBV infection. Sci. Rep. 2016, 6, 23992. [Google Scholar] [CrossRef]
- Chen, T.; Xun, Z.; Lin, J.; Fu, Y.; Wu, W.; Fu, X.; Hu, Y.; Zeng, Y.; Ou, Q. Association between mitochondrial DNA content and baseline serum levels of HBsAg in chronic hepatitis B infection. J. Med. Virol. 2017, 89, 1958–1962. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, Y.; Liu, J.; Liu, H.; Ge, N.; Yang, H.; Zhang, H.; Xing, J. Association of mitochondrial DNA content in peripheral blood leukocyte with hepatitis B virus-related hepatocellular carcinoma in a Chinese Han population. Cancer Sci. 2011, 102, 1553–1558. [Google Scholar] [CrossRef]
- Wang, C.; Hann, H.-W.; Hann, R.S.; Wan, S.; Myers, R.E.; Ye, Z.; Xing, J.; Yang, H. Circulating Mitochondrial DNA Content Associated with the Risk of Liver Cirrhosis: A Nested Case–Control Study. Dig. Dis. Sci. 2015, 60, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Nomoto, S.; Fujii, T.; Kaneko, T.; Takeda, S.; Inoue, S.; Kanazumi, N.; Nakao, A. Correlation between copy number of mitochondrial DNA and clinico-pathologic parameters of hepatocellular carcinoma. Eur. J. Surg. Oncol. (EJSO) 2006, 32, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Fisicaro, P.; Barili, V.; Montanini, B.; Acerbi, G.; Ferracin, M.; Guerrieri, F.; Salerno, D.; Boni, C.; Massari, M.; Cavallo, M.C.; et al. Targeting mitochondrial dysfunction can restore antiviral activity of exhausted HBV-specific CD8 T cells in chronic hepatitis B. Nat. Med. 2017, 23, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.I.; Hwang, J.M.; Im, J.H.; Lee, Y.I.; Kim, N.S.; Kim, D.G.; Yu, D.Y.; Moon, H.B.; Park, S.K. Human Hepatitis B Virus-X Protein Alters Mitochondrial Function and Physiology in Human Liver Cells. J. Boil. Chem. 2004, 279, 15460–15471. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Siddiqui, A. Hepatitis B Virus X Protein Stimulates the Mitochondrial Translocation of Raf-1 via Oxidative Stress. J. Virol. 2007, 81, 6757–6760. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.-Y.; Fang, X.-F.; Zou, L.-Y.; Huang, Y.-H.; Chen, Z.-X.; Li, D.; Zhou, L.-Y.; Chen, H.; Wang, X.-Z. The co-localization of HBx and COXIII upregulates COX-2 promoting HepG2 cell growth. Int. J. Oncol. 2014, 45, 1143–1150. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, X.Z.; Tao, Q.M.; Chen, Z.X.; Li, D.; Lin, N. A novel hepatitis B virus X?interactive protein: Cytochrome C oxidase III. J. Gastroenterol. Hepatol. 2006, 21, 711–715. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.-Z.; Yu, J.-P.; Chen, Z.-X.; Huang, Y.-H.; Tao, Q.-M. Cytochrome C oxidase III interacts with hepatitis B virus X protein in vivo by yeast two-hybrid system. World J. Gastroenterol. 2004, 10, 2805–2808. [Google Scholar] [CrossRef]
- Zou, L.-Y.; Zheng, B.-Y.; Fang, X.-F.; Li, D.; Huang, Y.-H.; Chen, Z.-X.; Zhou, L.-Y.; Wang, X.-Z. HBx co-localizes with COXIII in HL-7702 cells to upregulate mitochondrial function and ROS generation. Oncol. Rep. 2015, 33, 2461–2467. [Google Scholar] [CrossRef]
- Yoo, Y.-S.; Park, Y.-J.; Lee, H.-S.; Oanh, N.T.K.; Cho, M.-Y.; Heo, J.; Lee, E.-S.; Cho, H.; Park, Y.-Y. Mitochondria ubiquitin ligase, MARCH5 resolves hepatitis B virus X protein aggregates in the liver pathogenesis. Cell Death Dis. 2019, 10, 938. [Google Scholar] [CrossRef]
- Yonashiro, R.; Ishido, S.; Kyo, S.; Fukuda, T.; Goto, E.; Matsuki, Y.; Ohmura-Hoshino, M.; Sada, K.; Hotta, H.; Yamamura, H.; et al. A novel mitochondrial ubiquitin ligase plays a critical role in mitochondrial dynamics. EMBO J. 2006, 25, 3618–3626. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-Y.; Nguyen, O.T.K.; Kang, H.; Cho, H. MARCH5-mediated quality control on acetylated Mfn1 facilitates mitochondrial homeostasis and cell survival. Cell Death Dis. 2014, 5, e1172. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, S.; Tokuyama, T.; Yonashiro, R.; Inatome, R.; Yanagi, S. Roles of mitochondrial ubiquitin ligase MITOL/MARCH5 in mitochondrial dynamics and diseases. J. Biochem. 2014, 155, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Karbowski, M.; Neutzner, A.; Youle, R.J. The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J. Cell Boil. 2007, 178, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Qu, L.; Yan, S. Cyclooxygenase-2 promotes tumor growth and suppresses tumor immunity. Cancer Cell Int. 2015, 15, 106. [Google Scholar] [CrossRef]
- Park, M.H.; Hong, J.T. Roles of NF-κB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef]
- Man, S.M.; Zhu, Q.; Zhu, L.; Liu, Z.; Karki, R.; Malik, A.; Sharma, D.; Li, L.; Malireddi, R.S.; Gurung, P.; et al. Critical Role for the DNA Sensor AIM2 in Stem Cell Proliferation and Cancer. Cell 2015, 162, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-F.; Ou-Yang, F.; Hung, J.-Y.; Liu, J.-C.; Wang, H.; Wang, S.-C.; Hou, M.-F.; Hortobagyi, G.N.; Hung, M.-C. AIM2 suppresses human breast cancer cell proliferation in vitro and mammary tumor growth in a mouse model. Mol. Cancer Ther. 2006, 5, 1–7. [Google Scholar] [CrossRef]
- Chen, S.-L.; Liu, L.-L.; Lu, S.-X.; Luo, R.-Z.; Wang, C.-H.; Wang, H.; Cai, S.-H.; Yang, X.; Xie, D.; Zhang, C.Z.; et al. HBx-mediated decrease of AIM2 contributes to hepatocellular carcinoma metastasis. Mol. Oncol. 2017, 11, 1225–1240. [Google Scholar] [CrossRef]
- McClain, S.L.; Clippinger, A.J.; Lizzano, R.; Bouchard, M.J. Hepatitis B Virus Replication Is Associated with an HBx-Dependent Mitochondrion-Regulated Increase in Cytosolic Calcium Levels. J. Virol. 2007, 81, 12061–12065. [Google Scholar] [CrossRef] [PubMed]
- Scaglioni, P.P.; Melegari, M.; Wands, J.R. Posttranscriptional regulation of hepatitis B virus replication by the precore protein. J. Virol. 1997, 71, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, S.Y.; Kim, J.; Lee, H.; Choi, M.; Kim, J.K.; Ahn, J.K. Hepatitis B virus X protein induces apoptosis by enhancing translocation of Bax to mitochondria. IUBMB Life 2008, 60, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.Y.; Li, D.; Cai, D.E.; Huang, X.Y.; Zheng, B.Y.; Huang, Y.H.; Chen, Z.X.; Wang, X.Z. Hepatitis B virus X protein sensitizes HL-7702 cells to oxidative stress-induced apoptosis through modulation of the mitochondrial permeability transition pore. Oncol. Rep. 2017, 37, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Dudek, J. Role of Cardiolipin in Mitochondrial Signaling Pathways. Front. Cell Dev. Boil. 2017, 5, 90. [Google Scholar] [CrossRef]
- You, D.-G.; Cho, Y.Y.; Lee, H.-R.; Lee, J.-H.; Yu, S.J.; Yoon, J.-H.; Yoo, Y.D.; Kim, Y.J.; Lee, G.Y. Hepatitis B virus X protein induces size-selective membrane permeabilization through interaction with cardiolipin. Biochim. Biophys. Acta (BBA) Biomembr. 2019, 1861, 729–737. [Google Scholar] [CrossRef]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.-F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Boil. 2010, 8, e1000298. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Li, D.; Chen, Z.-X.; Huang, Y.-H.; Gao, W.-Y.; Zheng, B.-Y.; Wang, X.-Z. Hepatitis B Virus X protein elevates Parkin-mediated mitophagy through Lon Peptidase in starvation. Exp. Cell Res. 2018, 368, 75–83. [Google Scholar] [CrossRef]
- Ogura, N.; Ogawa, K.; Watashi, K.; Ito, T.; Wakita, T. Novel stable HBV producing cell line systems for expression and screening antiviral inhibitor of hepatitis B virus in human hepatoma cell line. Biochem. Biophys. Res. Commun. 2018, 498, 64–71. [Google Scholar] [CrossRef]
- Chi, H.-C.; Chen, S.-L.; Lin, S.-L.; Tsai, C.-Y.; Chuang, W.-Y.; Lin, Y.-H.; Huang, Y.-H.; Tsai, M.-M.; Yeh, C.-T.; Lin, K.-H. Thyroid hormone protects hepatocytes from HBx-induced carcinogenesis by enhancing mitochondrial turnover. Oncogene 2017, 36, 5274–5284. [Google Scholar] [CrossRef]
- Wang, C.; Yang, W.; Yan, H.X.; Luo, T.; Zhang, J.; Tang, L.; Wu, F.Q.; Zhang, H.L.; Yu, L.X.; Zheng, L.Y.; et al. Hepatitis B virus X (HBx) induces tumorigenicity of hepatic progenitor cells in 3,5-diethoxycarbonyl-1,4-dihydrocollidine-treated HBx transgenic mice. Hepatology 2012, 55, 108–120. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kanai, F.; Kawakami, T.; Tateishi, K.; Ijichi, H.; Kawabe, T.; Arakawa, Y.; Kawakami, T.; Nishimura, T.; Shirakata, Y.; et al. Interaction of the hepatitis B virus X protein (HBx) with heat shock protein 60 enhances HBx-mediated apoptosis. Biochem. Biophys. Res. Commun. 2004, 318, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Sun, D.C.; Lou, S.; Bo, X.C.; Lu, Z.; Qian, X.H.; Wang, S.Q. HBx protein of hepatitis B virus (HBV) can form complex with mitochondrial HSP60 and HSP70. Arch. Virol. 2005, 150, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Patel, H.V.; Ridley, R.G.; Freeman, K.B.; Gupta, R.S. Mitochondrial import of the human chaperonin (HSP60) protein. Biochem. Biophys. Res. Commun. 1990, 169, 391–396. [Google Scholar] [CrossRef]
- Azem, A.; Oppliger, W.; Lustig, A.; Jenö, P.; Feifel, B.; Schatz, G.; Horst, M. The mitochondrial hsp70 chaperone system. Effect of adenine nucleotides, peptide substrate, and mGrpE on the oligomeric state of mhsp70. J. Boil. Chem. 1997, 272, 20901–20906. [Google Scholar] [CrossRef]
- Mueller, J.W.; Bayer, P. Small Family with Key Contacts: Par14 and Par17 Parvulin Proteins, Relatives of Pin1, Now Emerge in Biomedical Research. Perspect. Med. Chem. 2008, 2, 11–20. [Google Scholar] [CrossRef]
- Rulten, S.; Thorpe, J.; Kay, J. Identification of Eukaryotic Parvulin Homologues: A New Subfamily of Peptidylprolyl cis–trans Isomerases. Biochem. Biophys. Res. Commun. 1999, 259, 557–562. [Google Scholar] [CrossRef]
- Saeed, U.; Kim, J.; Piracha, Z.Z.; Kwon, H.; Jung, J.; Chwae, Y.-J.; Park, S.; Shin, H.-J.; Kim, K. Parvulin 14 and Parvulin 17 Bind to HBx and cccDNA and Upregulate Hepatitis B Virus Replication from cccDNA to Virion in an HBx-Dependent Manner. J. Virol. 2019, 93, e01840-18. [Google Scholar] [CrossRef]
- Vazquez, C.; Horner, S.M. MAVS Coordination of Antiviral Innate Immunity. J. Virol. 2015, 89, 6974–6977. [Google Scholar] [CrossRef]
- Seth, R.B.; Sun, L.; Ea, C.K.; Chen, Z.J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 2005, 122, 669–682. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Wei, B.; Shi, H.-X.; Shan, Y.-F.; Wang, C. Tom70 mediates activation of interferon regulatory factor 3 on mitochondria. Cell Res. 2010, 20, 994–1011. [Google Scholar] [CrossRef]
- Fan, A.C.Y.; Bhangoo, M.K.; Young, J.C. Hsp90 Functions in the Targeting and Outer Membrane Translocation Steps of Tom70-mediated Mitochondrial Import. J. Boil. Chem. 2006, 281, 33313–33324. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Ni, C.; Song, T.; Liu, Y.; Yang, X.; Zheng, Z.; Jia, Y.; Yuan, Y.; Guan, K.; Xu, Y.; et al. The Hepatitis B Virus X Protein Disrupts Innate Immunity by Downregulating Mitochondrial Antiviral Signaling Protein. J. Immunol. 2010, 185, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Syed, G.H.; Kim, S.-J.; Siddiqui, A. Hepatitis B Virus-Induced Parkin-Dependent Recruitment of Linear Ubiquitin Assembly Complex (LUBAC) to Mitochondria and Attenuation of Innate Immunity. PLoS Pathog. 2016, 12, e1005693. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Jung, S.Y.; Hodgson, A.J.; Madden, C.R.; Qin, J.; Slagle, B.L. Hepatitis B Virus Regulatory HBx Protein Binds to Adaptor Protein IPS-1 and Inhibits the Activation of Beta Interferon. J. Virol. 2011, 85, 987. [Google Scholar] [CrossRef]
- Chung, K.K.K.; Thomas, B.; Li, X.; Pletnikova, O.; Troncoso, J.C.; Marsh, L.; Dawson, V.L.; Dawson, T.M. Nitrosylation of Parkin Regulates Ubiquitination and Compromises Parkin’s Protective Function. Science 2004, 304, 1328. [Google Scholar] [CrossRef]
- Suslov, A.; Boldanova, T.; Wang, X.; Wieland, S.; Heim, M.H. Hepatitis B Virus Does Not Interfere With Innate Immune Responses in the Human Liver. Gastroenterology 2018, 154, 1778–1790. [Google Scholar] [CrossRef]
- Kornyeyev, D.; Ramakrishnan, D.; Voitenleitner, C.; Livingston, C.M.; Xing, W.; Hung, M.; Kwon, H.J.; Fletcher, S.P.; Beran, R.K. Spatiotemporal Analysis of Hepatitis B Virus X Protein in Primary Human Hepatocytes. J. Virol. 2019, 93, e00248-19. [Google Scholar] [CrossRef]
- Keasler, V.V.; Hodgson, A.J.; Madden, C.R.; Slagle, B.L. Hepatitis B virus HBx protein localized to the nucleus restores HBx-deficient virus replication in HepG2 cells and in vivo in hydrodynamically-injected mice. Virology 2009, 390, 122–129. [Google Scholar] [CrossRef]
- Harrison, A.; Lemey, P.; Hurles, M.; Moyes, C.; Horn, S.; Pryor, J.; Malani, J.; Supuri, M.; Masta, A.; Teriboriki, B.; et al. Genomic Analysis of Hepatitis B Virus Reveals Antigen State and Genotype as Sources of Evolutionary Rate Variation. Viruses 2011, 3, 83–101. [Google Scholar] [CrossRef]
- Jones, S.A.; Clark, D.N.; Cao, F.; Tavis, J.E.; Hu, J. Comparative analysis of hepatitis B virus polymerase sequences required for viral RNA binding, RNA packaging, and protein priming. J. Virol. 2014, 88, 1564–1572. [Google Scholar] [CrossRef]
- Cho, G.; Park, S.-G.; Jung, G. Localization of HSP90 Binding Sites in the Human Hepatitis B Virus Polymerase. Biochem. Biophys. Res. Commun. 2000, 269, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ryu, W.-S. Hepatitis B Virus Polymerase Blocks Pattern Recognition Receptor Signaling via Interaction with DDX3: Implications for Immune Evasion. PLoS Pathog. 2010, 6, e1000986. [Google Scholar] [CrossRef] [PubMed]
- Park, S.G.; Jung, G. Human Hepatitis B Virus Polymerase Interacts with the Molecular Chaperonin Hsp60. J. Virol. 2001, 75, 6962–6968. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Chen, J.; Wu, M.; Chen, H.; Kato, N.; Yuan, Z. Hepatitis B virus polymerase inhibits RIG-I- and Toll-like receptor 3-mediated beta interferon induction in human hepatocytes through interference with interferon regulatory factor 3 activation and dampening of the interaction between TBK1/IKK and DDX3. J. Gen. Virol. 2010, 91, 2080–2090. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Chen, J.; Li, Y.; Wang, W.; Du, X.; Song, W.; Zhang, W.; Lin, L.; Yuan, Z. Hepatitis B virus polymerase disrupts K63-linked ubiquitination of STING to block innate cytosolic DNA-sensing pathways. J. Virol. 2015, 89, 2287–2300. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, M.-M.; Wang, Y.-Y.; Shu, H.-B. TRIM32 Protein Modulates Type I Interferon Induction and Cellular Antiviral Response by Targeting MITA/STING Protein for K63-linked Ubiquitination*. J. Boil. Chem. 2012, 287, 28646–28655. [Google Scholar] [CrossRef]
- Glebe, D.; Urban, S. Viral and cellular determinants involved in hepadnaviral entry. World J. Gastroenterol. 2007, 13, 22–38. [Google Scholar] [CrossRef]
- Sun, F.-C.; Wei, S.; Li, C.-W.; Chang, Y.-S.; Chao, C.-C.; Lai, Y.-K. Localization of GRP78 to mitochondria under the unfolded protein response. Biochem. J. 2006, 396, 31–39. [Google Scholar] [CrossRef]
- Lazăr, C.; Macovei, A.; Petrescu, S.; Branza-Nichita, N. Activation of ERAD Pathway by Human Hepatitis B Virus Modulates Viral and Subviral Particle Production. PLoS ONE 2012, 7, e34169. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, J.; Chan, H.L.Y.; Chen, Y.-C.; Wang, H.; Chen, Y.; Chan, C.-Y.; Go, M.Y.Y.; Tsai, S.-N.; Ngai, S.-M.; et al. Glucose-regulated protein 78 is an intracellular antiviral factor against hepatitis B virus. Mol. Cell. Proteom. 2009, 8, 2582–2594. [Google Scholar] [CrossRef]
- Cho, D.-Y.; Yang, G.-H.; Ryu, C.J.; Hong, H.J. Molecular Chaperone GRP78/BiP Interacts with the Large Surface Protein of Hepatitis B Virus In Vitro and In Vivo. J. Virol. 2003, 77, 2784–2788. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gao, Z.; Niu, X.; Zhang, Q.; Chen, H.; Gao, A.; Qi, S.; Xiang, R.; Belting, M.; Zhang, S. Mitochondria chaperone GRP75 moonlighting as a cell cycle controller to derail endocytosis provides an opportunity for nanomicrosphere intracellular delivery. Oncotarget 2017, 8, 58536–58552. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, R. Hsp70 Family Member, mot-2/mthsp70/GRP75, Binds to the Cytoplasmic Sequestration Domain of the p53 Protein. Exp. Cell Res. 2002, 274, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Ge, Y.; Qi, Y.; Shi, Z.; Jiao, Y.; Qi, X.; Zhai, X.; Wang, H. Identification of GRP75 as a novel PreS1 binding protein using a proteomics strategy. Braz. J. Microbiol. 2010, 41, 512–518. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patient, R.; Hourioux, C.; Roingeard, P. Morphogenesis of hepatitis B virus and its subviral envelope particles. Cell. Microbiol. 2009, 11, 1561–1570. [Google Scholar] [CrossRef]
- Huovila, A.P.; Eder, A.M.; Fuller, S.D. Hepatitis B surface antigen assembles in a post-ER, pre-Golgi compartment. J. Cell Boil. 1992, 118, 1305–1320. [Google Scholar] [CrossRef]
- Chua, P.K.; Wang, R.Y.-L.; Lin, M.-H.; Masuda, T.; Suk, F.-M.; Shih, C. Reduced Secretion of Virions and Hepatitis B Virus (HBV) Surface Antigen of a Naturally Occurring HBV Variant Correlates with the Accumulation of the Small S Envelope Protein in the Endoplasmic Reticulum and Golgi Apparatus. J. Virol. 2005, 79, 13483–13496. [Google Scholar] [CrossRef]
- Gong, X.; Zhu, Y.; Dong, J.; Chen, J.; You, J.; Zheng, Q.; Rao, Z.; Mao, Q.; Jiang, J. Small hepatitis B surface antigen interacts with and modulates enoyl-coenzyme A hydratase expression in hepatoma cells. Arch. Virol. 2013, 158, 1065–1070. [Google Scholar] [CrossRef]
- Xiao, C.-X.; Yang, X.-N.; Huang, Q.-W.; Zhang, Y.-Q.; Lin, B.-Y.; Liu, J.-J.; Liu, Y.-P.; Jazag, A.; Guleng, B.; Ren, J.-L. ECHS1 acts as a novel HBsAg-binding protein enhancing apoptosis through the mitochondrial pathway in HepG2 cells. Cancer Lett. 2013, 330, 67–73. [Google Scholar] [CrossRef]
- Liu, Y.-P.; Yang, X.-N.; Jazag, A.; Pan, J.-S.; Hu, T.-H.; Liu, J.-J.; Guleng, B.; Ren, J.-L. HBsAg Inhibits the Translocation of JTB into Mitochondria in HepG2 Cells and Potentially Plays a Role in HCC Progression. PLoS ONE 2012, 7, e36914. [Google Scholar] [CrossRef]
- Wong, N.; Chan, A.; Lee, S.-W.; Lam, E.; To, K.-F.; Lai, P.B.-S.; Li, X.-N.; Liew, C.-T.; Johnson, P.J. Positional mapping for amplified DNA sequences on 1q21-q22 in hepatocellular carcinoma indicates candidate genes over-expression. J. Hepatol. 2003, 38, 298–306. [Google Scholar] [CrossRef]
- Holmes, K.; Shepherd, D.A.; Ashcroft, A.E.; Whelan, M.; Rowlands, D.J.; Stonehouse, N.J. Assembly Pathway of Hepatitis B Core Virus-like Particles from Genetically Fused Dimers. J. Boil. Chem. 2015, 290, 16238–16245. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Standring, D.N. Hepatitis B virus capsid particles are assembled from core-protein dimer precursors. Proc. Natl. Acad. Sci. USA 1992, 89, 10046–10050. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.L.; Fu, Y.M.; Xu, Z.X.; Zou, Y.; Deng, K. Hepatitis B virus core protein dimerdimer interface is critical for viral replication. Mol. Med. Rep. 2019, 19, 262–270. [Google Scholar] [CrossRef]
- Syken, J.; De-Medina, T.; Münger, K. TID1, a human homolog of the Drosophila tumor suppressor l(2)tid, encodes two mitochondrial modulators of apoptosis with opposing functions. Proc. Natl. Acad. Sci. USA 1999, 96, 8499–8504. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.M.; Münger, K. Depletion of physiological levels of the human TID1 protein renders cancer cell lines resistant to apoptosis mediated by multiple exogenous stimuli. Oncogene 2004, 23, 8419–8431. [Google Scholar] [CrossRef] [PubMed]
- Trentin, G.; He, Y.; Wu, D.; Tang, D.; Rozakis-Adcock, M. Identification of an hTid-1 mutation which sensitizes gliomas to apoptosis. FEBS Lett. 2004, 578, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.-Y.; Kim, S.-B.; Ahn, B.-Y. Negative regulation of hepatitis B virus replication by cellular Hsp40/DnaJ proteins through destabilization of viral core and X proteins. J. Gen. Virol. 2006, 87, 1883–1891. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.G.; Akter, S.; Ohsaki, E.; Ueda, K. Impact of the Interaction of Hepatitis B Virus with Mitochondria and Associated Proteins. Viruses 2020, 12, 175. https://doi.org/10.3390/v12020175
Hossain MG, Akter S, Ohsaki E, Ueda K. Impact of the Interaction of Hepatitis B Virus with Mitochondria and Associated Proteins. Viruses. 2020; 12(2):175. https://doi.org/10.3390/v12020175
Chicago/Turabian StyleHossain, Md. Golzar, Sharmin Akter, Eriko Ohsaki, and Keiji Ueda. 2020. "Impact of the Interaction of Hepatitis B Virus with Mitochondria and Associated Proteins" Viruses 12, no. 2: 175. https://doi.org/10.3390/v12020175
APA StyleHossain, M. G., Akter, S., Ohsaki, E., & Ueda, K. (2020). Impact of the Interaction of Hepatitis B Virus with Mitochondria and Associated Proteins. Viruses, 12(2), 175. https://doi.org/10.3390/v12020175





