Autoreactivity of Broadly Neutralizing Influenza Human Antibodies to Human Tissues and Human Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Microarray
2.2. Semiquantitative Score of IHC
2.3. Production of Recombinant Human MAbs
2.4. Autoreactivity Protein Binding by ProtoArray Analysis
2.5. SPR Based HA Binding and Competition Assay
3. Results
3.1. Tissue Microarray Immunohistochemistry for Anti-Influenza Virus Hemagglutinin bNAbs
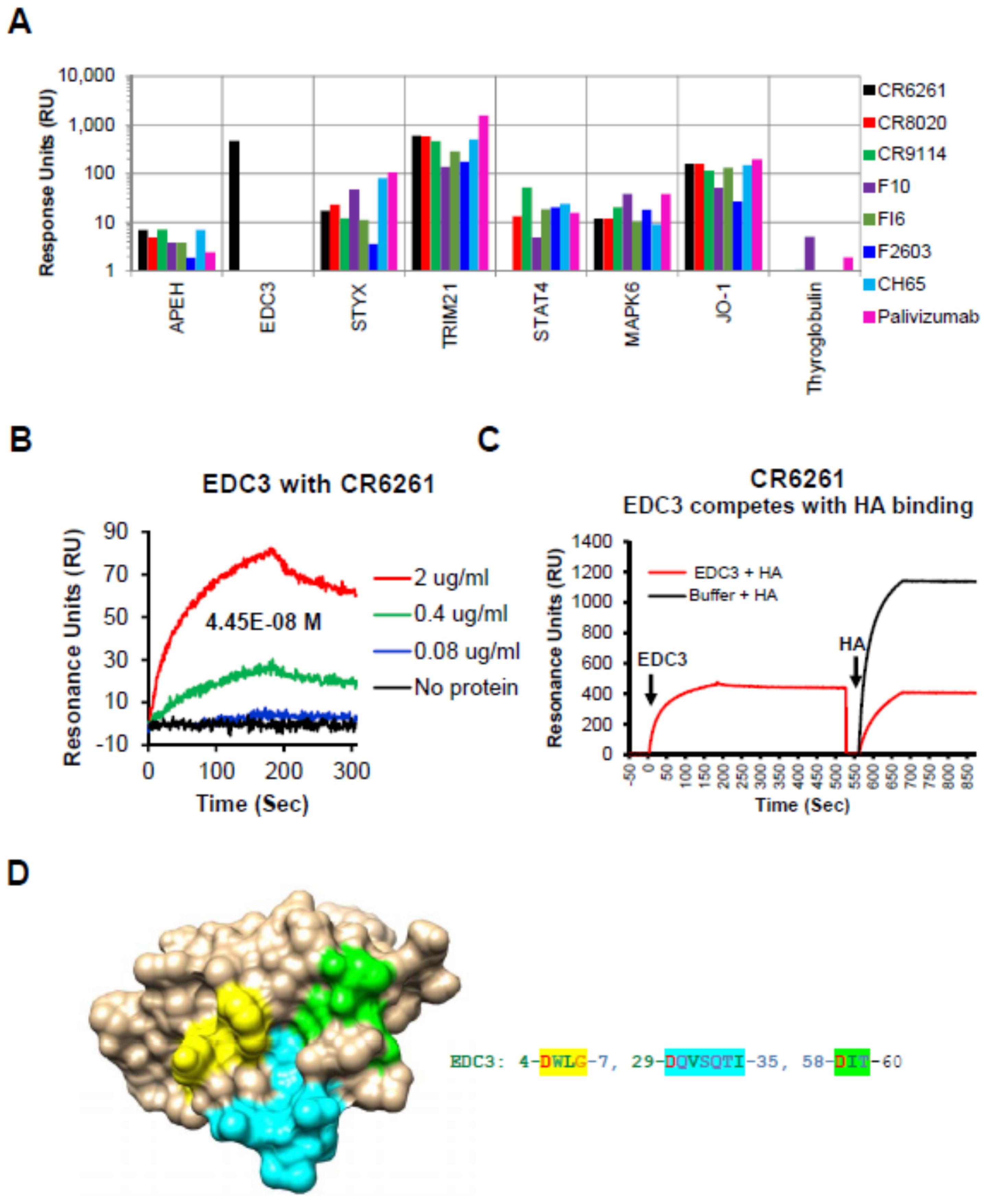
3.2. Autoreactivity to Human Proteins by ProtoArray Analysis
3.3. EDC3 Competes with HA for Binding to MAb CR6261
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krammer, F. Strategies to induce broadly protective antibody responses to viral glycoproteins. Expert Rev. Vaccines 2017, 16, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.I.; Guptill, J.; Naficy, A.; Nachbagauer, R.; Berlanda-Scorza, F.; Feser, J.; Wilson, P.C.; Solorzano, A.; Van der Wielen, M.; Walter, E.B.; et al. Immunogenicity of chimeric haemagglutinin-based, universal influenza virus vaccine candidates: Interim results of a randomised, placebo-controlled, phase 1 clinical trial. Lancet Infect. Dis. 2020, 20, 80–91. [Google Scholar] [CrossRef]
- Khurana, S. Development and regulation of novel influenza virus vaccines: A United States young scientist perspective. Vaccines 2018, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, D.C.; Bhabha, G.; Elsliger, M.A.; Friesen, R.H.; Jongeneelen, M.; Throsby, M.; Goudsmit, J.; Wilson, I.A. Antibody recognition of a highly conserved influenza virus epitope. Science 2009, 324, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, D.C.; Friesen, R.H.; Bhabha, G.; Kwaks, T.; Jongeneelen, M.; Yu, W.; Ophorst, C.; Cox, F.; Korse, H.J.; Brandenburg, B.; et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 2011, 333, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.G.; Xu, H.; Khan, A.R.; O’Donnell, T.; Khurana, S.; King, L.R.; Manischewitz, J.; Golding, H.; Suphaphiphat, P.; Carfi, A.; et al. Preconfiguration of the antigen-binding site during affinity maturation of a broadly neutralizing influenza virus antibody. Proc. Natl. Acad. Sci. USA 2013, 110, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.F.; Huang, Y.; Kaur, K.; Popova, L.I.; Ho, I.Y.; Pauli, N.T.; Henry Dunand, C.J.; Taylor, W.M.; Lim, S.; Huang, M.; et al. Immune history profoundly affects broadly protective B cell responses to influenza. Sci. Transl. Med. 2015, 7, 316ra192. [Google Scholar] [CrossRef] [PubMed]
- Bajic, G.; van der Poel, C.E.; Kuraoka, M.; Schmidt, A.G.; Carroll, M.C.; Kelsoe, G.; Harrison, S.C. Autoreactivity profiles of influenza hemagglutinin broadly neutralizing antibodies. Sci. Rep. 2019, 9, 3492. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Oliver, C.; Prince, G.A.; Hemming, V.G.; Pfarr, D.S.; Wang, S.C.; Dormitzer, M.; O’Grady, J.; Koenig, S.; Tamura, J.K.; et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J. Infect. Dis. 1997, 176, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.H.; Decker, C.J.; Walsh, M.A.; She, M.; Parker, R.; Song, H. Crystal structure of human Edc3 and its functional implications. Mol. Cell. Biol. 2008, 28, 5965–5976. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Garman, L.; Wrammert, J.; Zheng, N.Y.; Capra, J.D.; Ahmed, R.; Wilson, P.C. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat. Protoc. 2009, 4, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Throsby, M.; van den Brink, E.; Jongeneelen, M.; Poon, L.L.; Alard, P.; Cornelissen, L.; Bakker, A.; Cox, F.; van Deventer, E.; Guan, Y.; et al. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS ONE 2008, 3, e3942. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Holl, T.M.; Liu, Y.; Li, Y.; Lu, X.; Nicely, N.I.; Kepler, T.B.; Alam, S.M.; Liao, H.X.; Cain, D.W.; et al. Identification of autoantigens recognized by the 2F5 and 4E10 broadly neutralizing HIV-1 antibodies. J. Exp. Med. 2013, 210, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Foss, S.; Watkinson, R.; Sandlie, I.; James, L.C.; Andersen, J.T. TRIM21: A cytosolic Fc receptor with broad antibody isotype specificity. Immunol. Rev. 2015, 268, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, E.; Friede, M.; Sheikh, M.; Torvaldsen, S.; Newall, A.T. Passive immunization for influenza through antibody therapies, a review of the pipeline, challenges and potential applications. Vaccine 2016, 34, 5442–5448. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, D.; McTamney, P.M.; Yassine, H.M.; Whittle, J.R.; Guo, X.; Boyington, J.C.; Wei, C.J.; Nabel, G.J. Structural and genetic basis for development of broadly neutralizing influenza antibodies. Nature 2012, 489, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Sangesland, M.; Ronsard, L.; Kazer, S.W.; Bals, J.; Boyoglu-Barnum, S.; Yousif, A.S.; Barnes, R.; Feldman, J.; Quirindongo-Crespo, M.; McTamney, P.M.; et al. Germline-encoded affinity for cognate antigen enables vaccine amplification of a human broadly neutralizing response against influenza virus. Immunity 2019, 51, 735–749.e8. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yang, G.; Wiehe, K.; Nicely, N.I.; Vandergrift, N.A.; Rountree, W.; Bonsignori, M.; Alam, S.M.; Gao, J.; Haynes, B.F.; et al. Polyreactivity and autoreactivity among HIV-1 antibodies. J. Virol. 2015, 89, 784–798. [Google Scholar] [CrossRef] [PubMed]
- Abbott, R.K.; Lee, J.H.; Menis, S.; Skog, P.; Rossi, M.; Ota, T.; Kulp, D.W.; Bhullar, D.; Kalyuzhniy, O.; Havenar-Daughton, C.; et al. Precursor frequency and affinity determine B cell competitive fitness in germinal centers, tested with germline-targeting HIV vaccine immunogens. Immunity 2018, 48, 133–146.e6. [Google Scholar] [CrossRef] [PubMed]
| Mean (n = 3) | CR8020 | CR6261 | CR9114 | F10 | FI6 | F2603 | Palivizumab | CH65 | 4E10 | Control |
|---|---|---|---|---|---|---|---|---|---|---|
| Adrenal | 0.00 | 0.17 | 0.67 | 0.50 | 0.17 | 0.50 | 0.00 | 0.17 | 1.00 | 0.00 |
| Bone Marrow | 0.00 | 0.00 | 0.50 | 0.75 | 0.33 | 1.00 | 0.50 | 0.33 | 1.50 | 0.00 |
| Breast | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.67 | 0.00 |
| Brain, Cerebellum | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Brain, Cerebrum | 0.00 | 0.17 | 0.50 | 0.17 | 0.33 | 0.17 | 0.00 | 0.00 | 1.67 | 0.00 |
| Brain, pituitary | 0.00 | 1.33 | 1.67 | 0.00 | 0.17 | 1.50 | 0.00 | 0.00 | 2.83 | 0.00 |
| Colon | 0.00 | 0.00 | 0.17 | 0.00 | 0.00 | 0.17 | 0.00 | 0.00 | 0.83 | 0.00 |
| Oesophagus | 0.00 | 0.17 | 0.00 | 0.00 | 0.00 | 0.17 | 0.33 | 0.00 | 0.17 | 0.00 |
| Heart | 0.00 | 0.50 | 0.33 | 0.50 | 0.17 | 0.50 | 0.17 | 0.00 | 1.00 | 0.00 |
| Kidney | 0.17 | 0.33 | 0.00 | 0.33 | 0.00 | 0.17 | 0.00 | 0.00 | 1.17 | 0.00 |
| Liver | 0.00 | 0.33 | 0.33 | 0.17 | 0.17 | 0.33 | 0.17 | 0.00 | 2.33 | 0.00 |
| Lung | 0.00 | 0.00 | 0.00 | 0.00 | 0.17 | 0.33 | 0.17 | 0.00 | 0.17 | 0.00 |
| Mesothelial Cell | 0.00 | 0.17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.17 | 0.33 | 0.00 |
| Ovary | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.33 | 0.00 |
| Pancreas | 0.00 | 0.67 | 0.67 | 0.17 | 0.00 | 0.33 | 0.17 | 0.17 | 2.50 | 0.00 |
| Peripheral Nerve | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.17 | 0.00 | 0.00 | 0.00 | 0.00 |
| Placenta | 0.00 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.67 | 0.50 | 0.67 | 0.00 |
| Prostate | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 |
| Salivary Gland | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.67 | 0.00 |
| Skin | 0.00 | 0.17 | 0.00 | 0.00 | 0.00 | 0.17 | 0.00 | 0.17 | 0.00 | 0.00 |
| Small Intestine | 0.00 | 0.17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.33 | 0.00 |
| Spleen | 0.00 | 0.33 | 0.17 | 0.00 | 0.00 | 0.17 | 0.00 | 0.00 | 0.33 | 0.00 |
| Skeletal muscle | 0.00 | 0.17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Stomach | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.33 | 0.00 |
| Testis | 0.00 | 0.17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.33 | 0.00 |
| Thymus | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.17 | 0.00 |
| Thyroid | 0.00 | 0.00 | 0.17 | 0.00 | 0.00 | 0.00 | 0.17 | 0.00 | 0.00 | 0.00 |
| Tonsil | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Uterus | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Uterus, Cervix | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khurana, S.; Hahn, M.; Klenow, L.; Golding, H. Autoreactivity of Broadly Neutralizing Influenza Human Antibodies to Human Tissues and Human Proteins. Viruses 2020, 12, 1140. https://doi.org/10.3390/v12101140
Khurana S, Hahn M, Klenow L, Golding H. Autoreactivity of Broadly Neutralizing Influenza Human Antibodies to Human Tissues and Human Proteins. Viruses. 2020; 12(10):1140. https://doi.org/10.3390/v12101140
Chicago/Turabian StyleKhurana, Surender, Megan Hahn, Laura Klenow, and Hana Golding. 2020. "Autoreactivity of Broadly Neutralizing Influenza Human Antibodies to Human Tissues and Human Proteins" Viruses 12, no. 10: 1140. https://doi.org/10.3390/v12101140
APA StyleKhurana, S., Hahn, M., Klenow, L., & Golding, H. (2020). Autoreactivity of Broadly Neutralizing Influenza Human Antibodies to Human Tissues and Human Proteins. Viruses, 12(10), 1140. https://doi.org/10.3390/v12101140




