Abstract
Acute Oak Decline (AOD) is a progressive disease affecting oaks across Europe and is increasingly recognised as a threat to the health of forests and urban trees. While the occurrence of this disease has been documented in forest ecosystems, its presence in urban landscapes is still poorly understood. In this study, the occurrence of AOD-associated bacteria (Brenneria goodwinii, Gibbsiella quercinecans, Rahnella victoriana, Lonsdalea quercina) was investigated in Quercus robur and Q. rubra growing in urban areas of Wrocław, Poland. Multiplex real-time PCR analyses confirmed the pathogens in 11 trees, with B. goodwinii being the most common species. Importantly, we provide the first confirmed detection of B. goodwinii in Q. rubra under urban conditions, possibly the first such detection in Europe. The results show the occurrence of AOD-associated pathogens in urban environments, suggesting that such habitats may provide favourable conditions for their occurrence. However, further investigations, including epidemiological and spatial analyses, are needed to clarify whether urban areas contribute to the persistence or spread of these pathogens. Beyond local documentation, our results emphasise the need to include urban ecosystems in AOD surveillance and highlight potential pathways for pathogen adaptation and spread in cities. This work provides new insights into the ecology of AOD in anthropogenically modified habitats and has direct implications for urban tree health monitoring, biodiversity conservation, and the development of integrated management strategies.
1. Introduction
Oaks (Quercus spp.) are key trees in European landscapes, providing habitat for a diverse flora and fauna, and contributing to soil stabilisation, carbon sequestration, and biodiversity conservation [1,2,3,4]. In urban areas, species such as the native English oak (Quercus robur) and the introduced red oak (Quercus rubra) also play an important cultural and ecological role by increasing urban biodiversity, regulating the microclimate, and improving air quality [5]. However, despite their ecological and social importance, oaks in Europe are increasingly threatened by complex decline syndromes caused by both abiotic stress factors and biotic factors [6].
Among these syndromes, Acute Oak Decline (AOD) has emerged as a particularly serious problem. First described in the United Kingdom and later confirmed in other European countries, including Germany, Spain, Poland, Slovakia, France, and Serbia [7,8,9,10,11,12,13], Acute Oak Decline is characterised by bark lesions with dark exudates, wood necrosis, rapid death of the tree crowns, and often by tree mortality [7,14]. Its aetiology involves a polymicrobial bacterial complex dominated by Brenneria goodwinii, Gibbsiella quercinecans, Rahnella victoriana, and Lonsdalea quercina [9,14,15,16,17]. Although the pathogenicity pathways are still being investigated, it is assumed that synergistic bacterial interactions impair host defence and accelerate the decline [14,16].
In addition to biotic factors, a range of abiotic stresses is believed to predispose oaks to decline, including drought, air pollution, soil compaction, limited rooting space, and mechanical injuries [18,19,20]. These stresses are particularly pronounced in urban areas, where trees are subject to chronic disturbance and altered hydrological conditions.
To date, most reports of AOD have come from forests and semi-natural habitats, while the role of urban environments in AOD ecology remains poorly understood [7,21]. This knowledge gap is significant because urban trees may either reflect recent pathogen spread from forests or act as overlooked reservoirs and transmission centres [22]. Clarifying this uncertainty is crucial for understanding the broader epidemiology of AOD and for protecting the ecological and social value of urban oaks.
Based on this gap, we have formulated the following hypotheses:
Hypothesis 1.
AOD-associated bacterial pathogens are present in urban oak populations.
Hypothesis 2.
Their occurrence is related to environmental stress conditions typical of urban habitats.
To test these hypotheses, we conducted a molecular survey of Q. robur and Q. rubra in Wrocław, Poland—a large Central European city characterised by diverse habitat types and strong anthropogenic pressure. By focusing on this urban environment, the study aims to extend current knowledge of AOD ecology beyond forest systems, provide evidence for the role of urban trees in the occurrence of decay-associated bacteria, and identify future strategies for monitoring and managing the health of urban trees.
2. Materials and Methods
2.1. Study Sites and Plant Material
In 2023, a comprehensive survey was carried out to assess the health of oak trees in the city of Wrocław and its immediate surroundings (Figure 1). The aim of the study was to identify symptoms of decay and potential pathogenic factors, with a particular focus on bacterial pathogens. The analysis included two oak species commonly found in the region: English oak (Quercus robur) and red oak (Quercus rubra), both of which play an important role in the structure of urban greenery and in forest and roadside ecosystems.
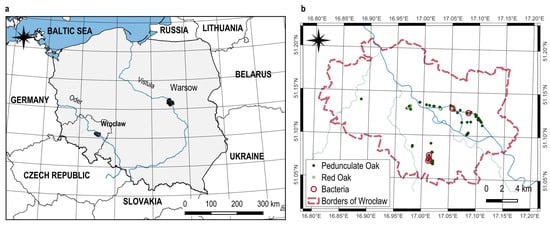
Figure 1.
A sample map containing information about the distribution of trees, divided by oak species. (a) the position of Wroclaw, (b) A sample map containing information about the distribution of trees, divided by oak species.
The selection of trees for the study was opportunistic and based on morphological observations such as leaf discolouration, premature leaf fall, dieback of shoot tips, and the presence of bark and vascular tissue necrosis. Only trees with signs of physiological or pathological stress—indicating a possible bacterial infection or other biotic disorders—were considered (Figure 2).

Figure 2.
Symptoms observed on trees for which the presence of the bacteria was confirmed (a,b) Q. rubro growing on embankments on the Odra river (c,d) Q. robur growing on greenery near recreation area.
The sampling locations were deliberately distributed across both the city centre and the outskirts of Wrocław to ensure the spatial representativeness of the data. The selected sites covered a range of habitat types, from highly urbanised areas such as city parks and roadside tree stands to more semi-natural green spaces, including urban forest fragments and river valleys. This stratified approach enabled a comparative analysis of tree health and pathogen incidence in different urban environments.
Tree condition was evaluated using standardised criteria: (i) crown thinning (estimated visually as percentage of crown loss: <25%, 25%–50%, >50%), (ii) presence and abundance of epicormic shoots, (iii) occurrence of bark exudates or resinous wounds, (iv) fungal fruiting bodies, and (v) insect damage (e.g., Cerambyx cerdo galleries, oviposition holes). Multiple symptoms could be recorded on the same tree. Only symptomatic trees were included, which limits the possibility of comparing pathogen occurrence between declining and apparently healthy individuals. This constraint should be considered when interpreting the results.
Sampling for microbiological analysis followed the methodology described by Tkaczyk et al. [11], previously used in oak dieback studies with bleeding bark cankers. Samples were collected exclusively from trees showing active disease symptoms, such as dark exudates on the trunk or branches, which may indicate the presence of bacterial pathogens. Asymptomatic trees were not included as negative controls in this survey, since the focus of the study was to confirm pathogen presence in visibly declining urban trees. Using sterile swabs, samples were taken directly from the exudates on the tree bark. The samples were then stored at 4 °C until further laboratory analysis.
It should also be noted that the number of Q. rubra individuals surveyed was relatively small (n = 7), which reduces statistical power and prevents drawing robust conclusions about pathogen prevalence in this species.
2.2. Pathogen Detection Procedure
The collected samples were analysed for the presence of four bacterial species associated with Acute Oak Decline (AOD), following the previously published methodology [16]. Although culture-based methods were not applied in this survey due to the technical challenges of isolating AOD-associated bacteria from mixed microbial exudates, we used a validated multiplex real-time PCR assay that ensures high sensitivity and specificity of detection [16]. The material from the swabs was eluted in 1 mL PBS buffer, and the resulting suspension was passed through sterile philtres with a pore-size of 5 µm using a syringe. The filtered samples were centrifuged (Eppendorf MiniSpin®, Eppendorf SE, Hamburg, Germany) at 9000× g for 4 min, after which the supernatant was removed, leaving the bacterial pellet. This procedure was repeated three times, and in the last step, the pellet was resuspended in 50 µL PBS buffer according to common laboratory standards. The prepared samples were shaken with the vortexer and divided into two equal portions of 25 µL—one was used for immediate analysis and the other was frozen at −20 °C for future testing.
Real-time PCR reactions were performed in 0.1 mL QIAGEN tubes using the 1× QUANTUM Probe PCR Master Mix (Syngene, Bangalore, India). Each reaction mix contained 0.1 µM of primers Gq284F and Gq418R, 0.25 µM of primers Bg99F, Bg179R, Rv15F, Rv134R, Lb503F, and Lb634R, and 0.1 µM of a dual-labelled fluorescent probe specific for the pathogens analysed, namely Gq342P, Bg124P, Rv57P, and Lq555p. A volume of 2 µL of bacterial suspension was added to each reaction, and all reactions were performed in triplicate (technical replicates). For each tree, one biological sample was collected per symptomatic site. Sampling sites were stratified across different urban habitats (e.g., parks, river embankments, roadside greenery) to improve representativeness. The study was designed as an occurrence survey to confirm the presence of AOD-associated pathogens in urban trees; therefore, no formal statistical power analysis was conducted. Thermal cycling conditions followed those described by Crampton et al. [16]. PCR products were monitored in real-time by measuring the fluorescence of the reporter dye connected to the TaqMan probe after each amplification cycle.
Real-time PCR analyses were interpreted using a universal detection threshold of Ct ≤ 39. While this approach ensures high sensitivity, we acknowledge that, according to the validation study by Crampton et al. [16], a stricter threshold (Ct < 35) is recommended for B. goodwinii to avoid cross-reactivity. As we used a different master mix (Syngene QUANTUM Probe PCR Master Mix) than the original study, and did not conduct independent sensitivity tests, we adopted Ct ≤ 39 as a conservative threshold for preliminary screening. However, detections between Ct 35–39, particularly for B. goodwinii, should be interpreted with caution and require confirmation by sequencing in future studies.
2.3. Statistical Analysis
To assess the relationship between tree location type and the occurrence of pathogenic bacteria (Brenneria goodwinii, Lonsdalea quercina, Rahnella victoriana, and Gibbsiella quercinecans), Pearson’s chi-square independence tests were applied. For each bacterium, contingency tables were constructed to represent the number of infected and non-infected trees across different location types. Pearson’s chi-square tests were applied to assess whether the distribution of bacterial occurrence differed between tree location types. In cases where contingency tables contained small frequencies (<5 in any cell), the results were interpreted with caution due to limited statistical power. All analyses were performed using TIBCO Statistica® 13.5.0 software.
3. Results
Molecular analyses confirmed the presence of four bacteria associated with Acute Oak Decline (AOD): Brenneria goodwinii (Bg), Lonsdalea quercina (Lq), Rahnella victoriana (Rv), and Gibbsiella quercinecans (Gq). Based on the results of real-time PCR (cycle threshold-Ct ≤ 39), pathogenic bacteria were detected in both Quercus robur and Q. rubra samples.
3.1. Detection Frequency
A total of 56 trees were analysed, including 49 Quercus robur and 7 Quercus rubra. The presence of at least one AOD-associated bacterium was confirmed in 8 Q. robur trees (16.3%) and in 3 Q. rubra trees (42.8%) (Table 1).

Table 1.
Summary of AOD pathogen detections and Ct statistics across oak species (positive detections = Ct ≤ 39; n = number of positive trees).
Real-time PCR analyses (threshold Ct ≤ 39) confirmed the presence of AOD-associated bacteria in symptomatic oaks. Table 1 summarises the individual Ct values, health assessment scores, and habitat types for each sampled tree. The obtained Ct values ranged from 28.2 to 37.9 for Brenneria goodwinii, 29.5–39.7 for Lonsdalea quercina, 32.0–36.8 for Rahnella victoriana, and 32.9–38.5 for Gibbsiella quercinecans. Detection of B. goodwinii in Quercus rubra (Ct range: 31.8–34.3) is of particular importance as the first confirmed report of this pathogen in urban red oak populations in Europe.
In Q. robur, B. goodwinii was the most frequently detected bacterium (n = 6), followed by G. quercinecans (n = 5), R. victoriana (n = 3), and L. quercina (n = 2). Remarkably, all four bacterial species were identified simultaneously in one tree in a recreational green area, and three pathogens were detected in one tree near the river Oder. Several bacteria were detected in three trees, while only one pathogen was found in five trees (Table 2).

Table 2.
Real-time PCR results and health assessment of oak trees in Wrocław. Columns Bg, Lq, Rv, and Gq correspond to Ct values for Brenneria goodwinii, Lonsdalea quercina, Rahnella victoriana, and Gibbsiella quercinecans, respectively.
Among Q. rubra, B. goodwinii was the only pathogen detected in 3 out of 7 trees. A limitation of this study is the relatively small and unbalanced sample size, particularly for Q. rubra (n = 7). As a result, the interpretation of pathogen prevalence and potential host specificity in Q. rubra should be considered preliminary. Nevertheless, the detection of B. goodwinii in this species remains noteworthy as the first documented case under urban conditions in Europe. Future surveys with larger and more balanced sampling will be necessary to confirm the patterns observed here.
Overall, B. goodwinii was the most widespread pathogen, consistent with its known virulence and its role as a core component of the AOD microbiome [15,23]. Its occurrence was closely associated with typical AOD symptoms, including crown dieback and gelatinous stem exudate.
3.2. Habitat Type and Pathogen Detection
Pathogen-positive samples were identified in different urban habitat types, although the frequency of detection and the diversity of pathogens varied considerably between sites (Table 3). The highest number of positive trees was found along river embankments (6 out of 29 samples), where both Q. robur and Q. rubra were affected. In this habitat, three pathogens—Brenneria goodwinii, Gibbsiella quercinecans, and Rahnella victoriana—were detected in this habitat, with some trees harbouring more than one species. There were also signs of the pathogen presence, of pathogens in parks 2 out of 11 trees tested positive, all of which were Q. robur.

Table 3.
Occurrence of pathogens depending on the type of habitat (Bg: Brenneria goodwinii, Gq: Gibbsiella quercinecans, Lq: Lonsdalea quercina, Rv: Rahnella victoriana).
In recreational areas and green spaces along transport routes, fewer but very different pathogens were detected. For example, a single Q. robur tree sampled in a recreational green space tested positive for all four target pathogens (B. goodwinii, G. quercinecans, Lonsdalea quercina, and R. victoriana), suggesting that specific microhabitats may favour complex bacterial interactions. In contrast, no pathogens were detected in Western Park, allotments, informal green spaces, or accompanying green areas, although these sites had very limited sample sizes.
These patterns suggest that the likelihood of pathogen detection is related to habitat characteristics. River embankments and parks—areas with variable hydrological conditions, potential soil compaction, and frequent human activity—may create favourable conditions for pathogen survival or tree susceptibility. The lack of evidence in other habitats could be due to both environmental differences and the small number of trees sampled.
The results of Pearson’s chi-square independence tests did not show any statistically significant relationships between tree location type and the occurrence of the examined bacteria. Therefore, no statistical evidence of an association between pathogen occurrence and habitat type was found. Observed patterns should be regarded as descriptive trends rather than confirmed correlations. For all analyses, the obtained p-values were higher than the assumed significance level of significance level = 0.05 (Brenneria goodwinii: chi-square = 2.30, p = 0.97; Lonsdalea quercina: chi-square = 10.99, p = 0.20; Rahnella victoriana: chi-square = 4.63, p = 0.80; Gibbsiella quercinecans: chi-square = 4.75, p = 0.78), indicating that the distribution of infections caused by individual bacteria did not differ significantly across the studied location types.
3.3. Physiological and Visual Symptoms
The visual assessment of the sampled oaks revealed a variety of symptoms that may indicate physiological stress or progressive decay processes. Among the 56 trees inventoried, the most frequently observed symptoms included crown thinning (in 38 trees, 68%) and the development of epicormic shoots (18 trees, 32%). In addition, signs such as the presence of fungal fruiting bodies (12 trees, 21%) exudation or wounds (9 trees, 16%) and insect activity Cerambyx cerdo were observed in 11 trees (20%).
Crown thinning varied in severity, ranging from slight to extensive reductions. In several cases (e.g., trees 27, 38, 39, 51, 52), crown thinning exceeded 50% and was accompanied by other symptoms such as epicormic shoots, exudation, or fungal fruiting bodies. Epicormic shoots, which are often interpreted as a stress reaction, were observed both in the lower part of the stems and within the crown, and often occurred together with a visible deterioration of the crown condition.
Among the 11 trees where the presence of bacterial pathogens (Brenneria goodwinii, Lonsdalea quercina, Gibbsiella quercinecans and Rahnella victoriana) was confirmed by real-time PCR (Ct lower than 39), crown thinning was observed in 9 individuals (82%). Epicormic shoots were present in 5 of these trees (45%), while visible exudates or resinous wounds were detected in 4 cases (36%). The presence of Cerambyx cerdo was detected in several pathogen-positive trees, which could indicate a facilitating role of insects in the development or aggravation of tree health deterioration.
Fungal pathogens such as Fomitiporia robusta and Fomes fomentarius were detected on 4 trees, three of which tested positive for bacterial DNA. This co-occurrence could indicate a possible synergistic interaction between bacterial and fungal pathogens in the process of tree mortality, although further investigation is required to establish causal relationships.
4. Discussion
The results of this study clearly confirm the presence of bacteria associated with Acute Oak Decline (AOD) in an urban environment. The most frequently detected species was Brenneria goodwinii, with Gibbsiella quercinecans, Lonsdalea quercina and Rahnella victoriana also identified. Statistical analyses revealed no significant associations between tree location and pathogen occurrence, suggesting that the distribution of infections in the surveyed population cannot be explained by habitat type alone. These results represent an important contribution to existing knowledge, as cases of AOD have so far been documented mainly in forests and semi-natural environments [7,9,16].
The dominance of B. goodwinii in the samples analysed is consistent with previous reports identifying this species as a key component of the microbial complex responsible for the development of bark and wood necrosis symptoms in oaks [14,15]. The co-occurrence of G. quercinecans, L. quercina and R. victoriana supports the hypothesis of the polyetiological nature of the disease, in which the ultimate development of symptoms is determined by the synergistic interactions of multiple bacterial species [14]. Such phenomena may explain the observed variability in symptom severity between individual trees and sites, and also point to the need for further research into the functioning of the patabiome in the context of AOD [24].
A novel aspect of the present study is the confirmation of AOD-associated bacteria in an urban environment in Poland. Such environments, characterised by chronic physiological stress factors (including air pollution, soil water deficits, salinity, and mechanical damage), may not only provide favourable conditions for the development of infections, but also potential reservoirs for inoculum [2,4]. Thus far, AOD cases have primarily been associated with forest habitats. However, the presence of these pathogens in cities suggests that they may have greater ecological plasticity than previously thought and that urban areas may act as centres of disease spread into more stable forest ecosystems [14,25]. Of particular significance is the detection of B. goodwinii in Quercus rubra, a widespread species in Central Europe that plays an important role in both managed forests and urban green spaces [26]. This is the first report of the occurrence of this pathogen in red oak in Poland, which expands the known host range of AOD-associated bacteria and emphasises the need to include both native and introduced species in surveillance measures.
The simultaneous occurrence of bacterial and fungal pathogens should also be noted. In several cases, Fomitiporia robusta and Fomes fomentarius—fungi responsible for white rot of the wood—were detected, with three of the affected trees also testing positive for AOD-associated bacteria in PCR tests. This observation could indicate possible interactions between pathogens of different trophic groups, where fungal wood decay pathogens weaken the tissue and thus facilitate bacterial colonisation, while the presence of bacteria may in turn promote further decay and susceptibility of the tree [27]. In addition, recent studies have shown that different white-rot fungi can influence the composition of the associated bacterial communities in the wood, which in turn can influence disease progression [28]. Similar interactions have already been described in forest ecosystems, suggesting that cross-species pathogen complexes may play a key role in the dynamics of oak dieback.
From the perspective of tree disease ecology, it is also important to consider the role of saproxylic insects. In particular, the larvae of Agrilus biguttatus, which have been identified as the main vector of AOD [13,25] initiate the infection process by damaging bark tissue. Under the urban conditions studied, the occurrence of this species was limited, suggesting that infections can also be transmitted via other routes—for example, via mechanical injury to the bark, human activities (tools, maintenance activities), or abiotic factors [18,29]. In addition, the detection of the large capricorn beetle (Cerambyx cerdo) on two trees that tested positive for AOD could also indicate a possible role for this species. Its larvae can facilitate the colonisation of the tissue with pathogens by creating extensive galleries in the wood. Although the association of C. cerdo with AOD needs further investigation, this observation expands the range of possible biotic interactions associated with urban oaks [30,31,32].
No culture-based isolation or sequencing was performed in this study, which is an important limitation, as the applied PCR method does not allow differentiation between living and dead cells. Previous literature has highlighted the difficulties in isolating AOD bacteria due to their slow growth and competition from secondary colonisers [14,16]. Therefore, future research should combine classical microbiological techniques with molecular methods and high-throughput sequencing, which would allow confirmation of the viability of the pathogen and provide deeper insights into the interactions between host and microbiome.
To summarise, this study provides the first evidence for the occurrence of AOD-associated bacteria in an urban environment in Poland. Although statistical tests revealed no significant differences in the distribution of infections between sites, the mere presence of these pathogens in urban conditions is an important warning. These results suggest that cities may serve not only as sites where the disease develops, but also as reservoirs for the inoculum and as potential nodes for the spread of the disease into the natural environment.
A key limitation of this study is the lack of pathogen isolation and sequencing, which prevents confirmation of the viability of detected bacteria and their differentiation from closely related taxa. Moreover, health assessment relied on field-based visual criteria without physiological measurements. Therefore, the results should be considered preliminary and hypothesis-generating. Future research should combine molecular assays with culture-based methods and sequencing of representative isolates to validate these findings.
5. Conclusions
This study provides the first evidence of Acute Oak Decline (AOD) bacterial pathogens in an urban environment in Poland, extending the known ecological range of these microorganisms beyond forest ecosystems. However, the findings should be regarded as preliminary. The lack of pathogen isolation and sequencing, the exclusive focus on symptomatic trees, and the small sample size for Q. rubra limit the strength of the conclusions.
Nevertheless, the detection of B. goodwinii in Q. rubra underlines the potential for AOD pathogens to affect both native and introduced oak species in cities. These results emphasise the need for more comprehensive surveys, combining molecular and culture-based methods, to confirm host-pathogen associations and assess epidemiological significance.
Overall, this work highlights the necessity of including urban trees in AOD surveillance and suggests that urban environments may act as reservoirs or nodes of pathogen spread. Future studies should integrate confirmatory diagnostics, epidemiological modelling, and management-oriented research to safeguard urban oak populations and their ecosystem services.
Author Contributions
Conceptualization, M.T.; methodology, M.T. and K.S.; formal analysis, M.T. and K.S.; investigation, M.T. and R.K.S.; writing—original draft preparation, M.T., R.K.S. and K.S.; writing—review and editing, M.T., R.K.S. and K.S.; visualization, R.K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the authors. The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Peterken, G.F. Natural Woodland: Ecology and Conservation in Northern Temperate Regions; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Jim, C. Physical and chemical properties of a Hong Kong roadside soil in relation to urban tree growth. Urban Ecosyst. 1998, 2, 171–181. [Google Scholar] [CrossRef]
- Roloff, A.; Korn, S.; Gillner, S. The Climate-Species-Matrix to select tree species for urban habitats considering climate change. Urban For. Urban Green. 2009, 8, 295–308. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Aronson, M.F.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; MacIvor, J.S. Biodiversity in the city: Fundamental questions for understanding the ecology of urban green spaces for biodiversity conservation. BioScience 2017, 67, 799–807. [Google Scholar] [CrossRef]
- Konijnendijk, C.C.; Ricard, R.M.; Kenney, A.; Randrup, T.B. Defining urban forestry—A comparative perspective of North America and Europe. Urban For. Urban Green. 2006, 4, 93–103. [Google Scholar] [CrossRef]
- Pautasso, M.; Schlegel, M.; Holdenrieder, O. Forest health in a changing world. Microb. Ecol. 2015, 69, 826–842. [Google Scholar] [CrossRef]
- Denman, S.; Brown, N.; Kirk, S.; Jeger, M.; Webber, J. A description of the symptoms of Acute Oak Decline in Britain and a comparative review on causes of similar disorders on oak in Europe. For. Int. J. For. Res. 2014, 87, 535–551. [Google Scholar] [CrossRef]
- González, A.J.; Ciordia, M. Brenneria goodwinii and Gibbsiella quercinecans isolated from weeping cankers on Quercus robur L. in Spain. Eur. J. Plant Pathol. 2020, 156, 965–969. [Google Scholar] [CrossRef]
- Fernandes, C.; Duarte, L.; Naves, P.; Sousa, E.; Cruz, L. First report of Brenneria goodwinii causing acute oak decline on Quercus suber in Portugal. J. Plant Pathol. 2022, 104, 837–838. [Google Scholar] [CrossRef]
- Tkaczyk, M.; Celma, L.; Ruņģis, D.; Bokuma, G. First Report of Brenneria goodwinii and Gibbsiella quercinecans Bacteria, Detected on Weaken Oak Trees in Poland. Balt. For. 2021, 27, 166–169. [Google Scholar] [CrossRef]
- Tkaczyk, M.; Sikora, K.; Milenković, I. First Report of Bacteria Associated with Bleeding Cankers on Oak Trees in Serbia. For. Pathol. 2025, 55, e70010. [Google Scholar] [CrossRef]
- Tkaczyk, M.; Sikora, K.; Galko, J. First Report of Bacteria Causing Acute Oak Decline on Quercus robur in Slovakia. Eur. J. Plant Pathol. 2024, 169, 113–120. [Google Scholar] [CrossRef]
- Bene, A.; Vergine, M.; Carluccio, G.; Portaccio, L.; Delle Donne, A.; De Bellis, L.; Luvisi, A. Acute Oak Decline-Associated Bacteria: An Emerging Worldwide Threat to Forests. Microorganisms 2025, 13, 1127. [Google Scholar] [CrossRef]
- Denman, S.; Doonan, J.; Ransom-Jones, E.; Broberg, M.; Plummer, S.; Kirk, S.; Scarlett, K.; Griffiths, A.; Kaczmarek, M.; Forster, J.; et al. Microbiome and infectivity studies reveal complex polyspecies tree disease in Acute Oak Decline. ISME J. 2018, 12, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Brady, C.; Denman, S.; Kirk, S.; Venter, S.; Rodríguez-Palenzuela, P.; Coutinho, T. Description of Gibbsiella quercinecans gen. nov., sp. nov., associated with Acute Oak Decline. Syst. Appl. Microbiol. 2010, 33, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Crampton, B.; Plummer, S.; Kaczmarek, M.; McDonald, J.; Denman, S. A multiplex real-time PCR assay enables simultaneous rapid detection and quantification of bacteria associated with acute oak decline. Plant Pathol. 2020, 69, 1301–1310. [Google Scholar] [CrossRef]
- Doonan, J.M.; Broberg, M.; Denman, S.; McDonald, J.E. Host–microbiota–insect interactions drive emergent virulence in a complex tree disease. Proc. R. Soc. B 2020, 287, 20200956. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M.; Lanta, V.; Zverev, V.; Rainio, K.; Kunavin, M.; Zvereva, E. Decreased losses of woody plant foliage to insects in large urban areas are explained by bird predation. Glob. Change Biol. 2017, 23, 4354–4364. [Google Scholar] [CrossRef]
- Brown, N.; Jeger, M.; Kirk, S.; Xu, X.; Denman, S. Epidemiology of Acute Oak Decline in Britain. Forestry 2018, 91, 64–75. [Google Scholar]
- Nawaz, M.; Sun, J.; Shabbir, S.; Khattak, W.A.; Ren, G.; Nie, X.; Bo, Y.; Javed, Q.; Du, D.; Sonne, C. A review of plants strategies to resist biotic and abiotic environmental stressors. Sci. Total Environ. 2023, 900, 165832. [Google Scholar] [CrossRef]
- González, R.; López-López, M.; Biosca, E.; López, F.; Santiago, R.; López, M. First report of bacterial deep bark canker of walnut caused by Brenneria (Erwinia) rubrifaciens in Europe. Plant Dis. 2002, 86, 696. [Google Scholar] [CrossRef]
- Macháčová, M.; Nakládal, O.; Samek, M.; Baťa, D.; Zumr, V.; Pešková, V. Oak decline caused by biotic and abiotic factors in Central Europe: A case study from the Czech Republic. Forests 2022, 13, 1223. [Google Scholar] [CrossRef]
- Denman, S.; Webber, J. Oak declines: New definitions and new episodes in Britain. Q. J. For. 2009, 103, 285–290. [Google Scholar]
- Doonan, J.; Denman, S.; Pachebat, J.; McDonald, J. Genomic analysis of bacteria in the Acute Oak Decline pathobiome. Microb. Genom. 2019, 5, e000240. [Google Scholar] [CrossRef] [PubMed]
- Broberg, M.; Doonan, J.; Mundt, F.; Denman, S.; McDonald, J. Integrated multi-omic analysis of host-microbiota interactions in acute oak decline. Microbiome 2018, 6, 21. [Google Scholar] [CrossRef]
- Brus, R.; Pötzelsberger, E.; Lapin, K.; Brundu, G.; Orazio, C.; Straigyte, L.; Hasenauer, H. Extent, distribution and origin of non-native forest tree species in Europe. Scand. J. For. Res. 2019, 34, 533–544. [Google Scholar] [CrossRef]
- Kotlaba, F. Mushrooms and Other Fungi of Great Britain and Europe. Folia Geobot. Phytotaxon. 1984, 19, 329–331. [Google Scholar]
- Haq, I.; Hillmann, B.; Moran, M.; Willard, S.; Knights, D.; Fixen, K.; Schilling, J. Bacterial communities associated with wood rot fungi that use distinct decomposition mechanisms. ISME Commun. 2022, 2, 26. [Google Scholar] [CrossRef]
- Vuts, J.; Woodcock, C.; Sumner, M.; Caulfield, J.; Reed, K.; Inward, D.; Leather, S.; Pickett, J.; Birkett, M.; Denman, S. Responses of the two-spotted oak buprestid, Agrilus biguttatus (Coleoptera: Buprestidae), to host tree volatiles. Pest Manag. Sci. 2015, 72, 845–851. [Google Scholar] [CrossRef]
- Buse, J.; Schröder, B.; Assmann, T. Modelling habitat and spatial distribution of an endangered longhorn beetle—A case study for saproxylic insect conservation. Biol. Conserv. 2008, 141, 1400–1410. [Google Scholar] [CrossRef]
- Micó, E.; Reboleira, A.; Sánchez, A. Association between Cerambyx welensii and saproxylic beetle diversity in Mediterranean oak woodlands. Insect Conserv. Divers. 2015, 8, 261–270. [Google Scholar]
- Kadej, M.; Zając, K.; Smolis, A.; Tarnawski, D.; Tyszecka, K.; Malkiewicz, A.; Pietraszko, M.; Warchałowski, M.; Gil, R. The great capricorn beetle Cerambyx cerdo L. in south-western Poland–the current state and perspectives of conservation in one of the recent distribution centres in Central Europe. Nat. Conserv. 2017, 19, 111–134. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).