Abstract
The Pinus yunnanensis forest of southwestern China represents a unique and ecologically critical vegetation type, historically shaped by fire disturbances. To mitigate catastrophic wildfire risks, prescribed burning has been widely implemented as a management tool in these ecosystems. However, its effects on plant community structure and biodiversity remain insufficiently quantified. To investigate the specific changes in plant community characteristics caused by prescribed burning, this study was conducted in the Pinus yunnanensis forest in Zhaobi Hill, Xinping county. Our results revealed that prescribed burning induced differential effects on understory communities while exerting negligible effects on canopy tree composition. In the shrub layer, the number of shrub species decreased from 26 to 20, accompanied by a complete extirpation of arboreal saplings. Dominance hierarchies shifted markedly, transitioning from Lithocarpus mairei and Pinus yunnanensis regeneration cohorts in unburned plots to fire-adapted species Duhaldea cappa and Craibiodendron stellatum. Concomitantly, the average height of shrubs had a significant reduction in burning plots. Contrastingly, the number of herb species increased from 30 to 37 in burning plots, with non-significant alterations in abundance, height, and importance values. Prescribed burning significantly decreases the α species diversity of shrubs, but only has minimal effects on the α species diversity indices of herbs. Overall, prescribed burning appears to be the primary factor affecting the species diversity index of shrubs, while altitude, forest structure, and soil nutrient content exert greater influences on the species diversity index of the herbaceous layer. Prescribed burning was the dominant factor shaping the community structure and species diversity of the shrub layer, and the missing saplings of trees in the shrub layer might influence future forest succession in the long term.
1. Introduction
Pinus yunnanensis is a unique and indigenous coniferous tree species found in southwestern China. The Pinus yunnanensis forest represents the dry subtropical regions of western China and is widely spread across the Yunnan-Guizhou Plateau, with central Yunnan being at its core area [1]. This forest type is abundant in Yunnan Province and has significant carbon storage potential [2,3]. While most of the Pinus yunnanensis forests today are man-made, having been established through air-seeding in the late 1970s, a few original pristine forests still exist in remote areas of northwestern and central Yunnan [2]. The Pinus yunnanensis forest represents a category of forests that have evolved from subtropical evergreen broad-leaved forests as a result of prolonged droughts and frequent wildfires [1,2,3]. This forest type is particularly vulnerable to fire disturbances due to its inherent characteristics and environmental conditions. Specifically, the natural shedding of lower branches by Pinus yunnanensis trees facilitates fire propagation, while the dry and warm climate of the regions where these forests are located further exacerbates their susceptibility to fire [4,5]. Prescribed burning, a widely used technique in various regions, refers to controlled fires that aim to reduce fire hazards in forest ecosystems and modify the characteristics of fire disturbances by reducing the fuel loads on forest surfaces [6,7,8,9,10,11,12,13]. This practice is commonly employed in different ecosystems, including the South African fynbos [7], woodlands and forests of Australia [9,10,11,12,13], fire-prone areas in Mediterranean regions [14,15], fire-suppressed forests in the USA [16,17,18,19,20], and fire-prone ecosystems in China [3,21,22,23].
Prescribed burning has an impact on the structure of plant communities and the diversity of species in forests, and these effects vary depending on the different plant strata and types of vegetation [10,12,13,17,18,22,24,25,26,27,28,29,30,31,32,33]. Although low-intensity prescribed burning has minimal effects on the overstory strata of forests and herbaceous plants, it significantly reduces the cover and stem density of shrubs in the midstory [6,17,18,19,20,23,24,25,27,28,29,33]. Frequent prescribed burning has been found to have detrimental effects on the frequency and relative abundance of important shrubs in fynbos ecosystems [18,24]. However, in dry sclerophyll forests in southeastern Australia, burning may have a positive impact on the species richness of plants less than 1 m in height, while negatively affecting taller plants. It could also reduce the density and canopy cover of the overstory, leading to the loss of woody seedlings and saplings, but increasing the abundance and richness of understory species [18]. In broadleaf forests, burning has been shown to significantly enhance the richness of vascular plants, exotic plants, and herbs [27,34]. Similarly, in oak savanna ecosystems, burning has been observed to increase canopy openness and the percentage of vegetative cover in the understory [29]. On the other hand, the effects of burning on the diversity of vascular plant species and exotic species in Arizona Ponderosa pine forests are more subtle [28]. Understory vegetation plays a crucial role in various aspects of the forest ecosystem, including nutrient cycling, water conservation, forest climate regulation, and animal habitats [17,18], and the changes in the community structure and species diversity in the understory are essential for the overall stability of the forest ecosystem.
Prescribed burning primarily affects understory plants, while its impact on the overstory is generally minimal [22,24,34]. The changes in understory vegetation structure caused by burning may influence the future development of the forest ecosystem [18]. While Pinus yunnanensis forests generally have a lower abundance of understory vegetation, the importance of understory for the stability of forest ecosystems should not be overlooked [3]. However, the effects of prescribed burning on the species composition and diversity of the understory in Pinus yunnanensis forests remain uncertain. We hypothesized that prescribed burning would significantly affect the species composition and plant heights of the understory vegetation, and the impact of burning on the canopy strata of Pinus yunnanensis forests should be minor. It is possible that these effects could be influenced by other factors, such as altitude and soil nutrient levels. To investigate this further, we conducted a comprehensive plant study in both the prescribed burning area and an adjacent unburned area in the Pinus yunnanensis forests of central Yunnan. Our study aimed to address the following questions: (1) How does prescribed burning influence the plant community structure and composition of Pinus yunnanensis forests? (2) What are the differential effects of prescribed burning on the arboreal, shrub, and herbaceous layers within Pinus yunnanensis forests? (3) To what extent do other factors, such as altitude or soil nutrient levels, interact with the effects of prescribed burning on the plant community structure of the understory in this region?
2. Materials and Methods
2.1. Study Area
This study was conducted in Pinus yunnanensis forests, which were aerially seeded in Zhaobi Hill, Xinping county, Yunnan province, southwestern China (Figure 1). The region has a subtropical humid monsoon climate with distinct wet and dry seasons, an average annual temperature of 18.1 °C, an annual mean precipitation of 869 mm, and an annual total sunshine duration of 2838 h. The dominant soil type in this region is red soil, and the Pinus yunnanensis forest was established through afforestation by aerial seeding in the 1970s. The vegetation community of the forest includes three layers: the arboreal layer, the shrub layer, and the herb layer. The arboreal layer includes Pinus yunnanensis, Keteleeria evelyniana, Cyclobalanopsis glaucoides, Castanopsis orthacantha, Castanopsis delavayi, and Quercus acutissima. The shrub layer and herb layer constitute the understory; plants in the shrub layer include Vaccinium duclouxii, V. fragile, Lyonia ovalifolia, Craibiodendron stellatum, and Duhaldea cappa, and the herb layer is mainly composed of Anaphalis sp., Arundinella setosa, Capillipedium assimile, Elsholtzia rugulosa, E. bodinieri, Eulalia pallens, E. quadrinervis, Hedyotis sp., Leontopodium sp., Pteridium aquilinum, Erigeron sp., Pseudognaphalium sp., and Laggera alata. Pinus yunnanensis forests are middle-aged and originated from natural Pinus yunnanensis forests enhanced by aerial seeding and management measures during the 1970s. Prescribed burning has been a consistent practice in this region since 2016 as a preventative measure against forest fires (Figure 2). These prescribed burns typically occur in late January or early February, with low-intensity fires being utilized between 2016 and 2019 and discontinued in 2020 and 2021. The burned area is restored for three years after the last prescribed burning period.
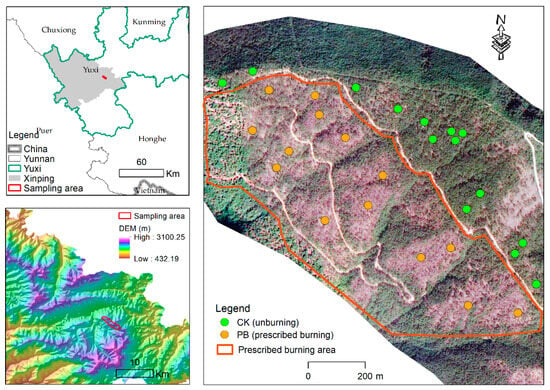
Figure 1.
Sampling spots in the study area.

Figure 2.
The understory plant characteristics between unburned areas and prescribed burning areas.
2.2. Field Measurements
In January 2022, field surveys were conducted in unburned and planned burned areas. Fourteen sample plots were established in each area, with each plot consisting of three 20 m × 20 m subplots. The distance between each plot was approximately 200–500 m, while the distance between each subplot was less than 100 m. This resulted in a total of 84 subplots. All subplots had a similar aspect, and other basic information, such as altitude, slope, and canopy density, showed minimal variation between prescribed burning and unburned plots (Figure 1 and Figure 2). The survey plots were strategically placed at least 50 m from the road, with elevations ranging from 1950 to 2200 m. These plots were predominantly located on sunny and moderate slopes (Table 1). Within each 20 m × 20 m plot, the diameters at breast height (DBH) of individual plants, with a DBH greater than 2 cm, were measured and recorded. Additionally, tree height and height under lower branches in the tree layer were recorded. According to the methods for large forest plot surveys [35,36], in each 20 m × 20 m plot, five 5 m × 5 m quadrats were set in a clover shape to investigate the height, crown breadth, and number of plant species in the shrub layer. Furthermore, within each of the five shrub quadrats, five 1 m × 1 m sub-quadrats were set to investigate the number and coverage of plants in the herbaceous layer. Lastly, five soil samples were collected from the 0–10 cm soil layer to measure the chemical stoichiometric characteristics of soil C, N, P, and K.

Table 1.
Basic information of sample plots.
2.3. Laboratory Analysis
All trees and shrubs in all sample plots were classified based on their respective species, following the guidelines provided in the Yunnan Flora and the unpublished Xinping Flora. For herbs, identification was carried out at the specific species level, and when it was not possible to identify the species of the herbaceous plant, only the genus was identified (Table A2 and Table A3).
The sampled soils underwent air-drying and were subsequently passed through a US Standard 60 mesh to exclude small rocks and fine roots. The resulting mineral soils were then analyzed for total soil carbon (TC), soil organic carbon (SOC), total nitrogen (TN), hydrolysable nitrogen (A–N), available potassium (A–K), and soil available phosphorus (A–P). Additionally, the C/N ratio was calculated based on the total carbon and total nitrogen content of the soils. The TC analysis was conducted using a ZYT-2000 analyzer, while SOC was measured using a Dichromate titration method. TN and A–N were determined using the SKD-200 analyzer. A–K was measured using the FP6410 analyzer, and A-P was determined using a UV-visible spectrophotometer analyzer.
2.4. Statistical Analysis
To evaluate the structure and composition of the Pinus yunnanensis forest community, plant importance value, species abundance, and height were measured. The α diversity indices, such as the Shannon–Winner diversity index (H′), Simpson’s dominance index (D), Pielou’s evenness index (EH), and Margalef’s richness index (F), were calculated to assess community diversity. Additionally, beta diversity indices, including the Jaccard index (βj), Cody index (βc), and Morisita–Horn index (CMH), were used. For the shrub layer, H′, D, EH, and F were utilized to measure species diversity. Due to the challenges in accurately counting the number of herbs, H′, D, and EH were employed to assess the species diversity in the herb layers. The diversity index was calculated using the methodology described in Hill [37] and Ma et al. [38].
where indicates the proportion of plant species i in a specific sample site, denotes the count of individual plants belonging to species i, S denotes the number of plant species in a sample site, and N denotes the total count of all plant species in the sample site. In the equations of βc, βj, and CMH, represents the number of species increasing along the habitat gradient H, represents the number of species decreasing along the same gradient, a represents the number of species in sample A, b represents the number of species in sample B, j represents the number of species shared by both sample A and sample B, and and represent the coverage of individual plants belonging to species i in sample A and sample B, respectively. Different values of the Jaccard similarity index (βj) represent different meanings. Values of 0 to 0.25 indicate extremely dissimilar community compositions, 0.25 to 0.50 represents moderately dissimilar, 0.50 to 0.75 indicates moderately similar, and 0.75 to 1.00 represents extremely similar communities. The Morisita–Horn index (CMH) represents the coefficient of similarity between communities undergoing planned burning, while the Cody index (βc) represents the dissimilarity of species composition.
We utilized the Kruskal–Wallis (KW) test to identify disparities in species abundance, coverage, height, and species diversity index. Additionally, we employed NMDS to explore differences in understory community structure between the burned area and the unburned area. We identified the influential factors of α species diversity by using Spearman correlation. Furthermore, we conducted Multivariate Analysis of Variance (MANOVA) to analyze the relative contribution of each factor to the α diversity indexes. Finally, we investigated the associations between species composition and various environmental factors, including fires, altitudes, upper vegetation characteristics, and soil nutrients, using the structural equation model (SEM).
NMDS is used to visually evaluate the structure of plant communities and their environmental resources (such as soil organic layer thickness, moisture content, pH, total carbon, nitrogen, and phosphorus). NMDS is a widely used multivariate analysis method, suitable for complex iterative sorting analysis of nonlinear data. It reflects the relationships between entities in a geometric space using the distances between points in a low-dimensional space through dimensional reduction and sorting, and could simultaneously handle multiple dependent and independent variables and visually display the relationships between variables in a sorting plot.
SEM is a multivariate statistical method used to establish, estimate, and test causal relationships. Compared to traditional statistical methods, SEM can study both observable variables and variables that cannot be directly observed (latent variables), and can indicate direct or indirect relationships between variables. SEM could handle multiple dependent variables simultaneously and visually display the relationships between variables in a path diagram. SEM is used to quantitatively evaluate the direct and indirect impacts of factors such as burning and topography on the surface plant communities of Pinus yunnanensis forests and conduct path analysis.
All analyses were performed in R version 4.2.1 [39]. The WX test was conducted using the “Wilcox.test” package, the NMDS analyses were performed using the “vegan” package, Spearman correlation was employed using the “cor.test” package, MANOVA was executed using the “car” and “MASS” packages, and the SEM analysis was conducted using the “sem” package.
3. Results
There were 63 recorded species from 21 plant families in our sample plots. There were 53 species from 13 families in unburned plots, and 59 species from 19 families in burned plots. The most abundant families were Asteraceae (19.44%), Fagaceae (16.67%), and Poaceae (12.50%) in all the sample plots, and the most abundant families were similar in unburned plots and burned plots. The responses of community characteristics and species composition of the Pinus yunnanensis forest to prescribed burning were varied in the arborous layer, shrub layer, and herbaceous layer.
3.1. Effects of Prescribed Burning on Community Characteristics and Species Composition of the Arboreal Layer
There was no difference in the tree species composition of the arboreal layer between prescribed burning (PB) and unburned areas (CK). The sample plots of both PB and CK included Pinus yunnanensis, Keteleeria evelyniana, Quercus acutissima, and Anneslea fragrans. Pinus yunnanensis was the only dominant tree species, and the number of Keteleeria evelyniana, Quercus acutissima, and Anneslea fragrans was almost negligible.
The KW test showed that the DBH (diameters at breast height) and the height of trees (height) were similar between the burned and unburned areas, though the DBH and height were slightly larger in the burned area than in the unburned area (Table A2, Figure 3a,b). On the other hand, the heights under the branch in the tree layers were higher in the prescribed burning plots than in the unburned plots. The mean shrub heights in the unburned plots and prescribed burning plots were 5.68 m and 6.77 m, respectively, indicating a statistically significant difference between the two groups (Figure 3c).
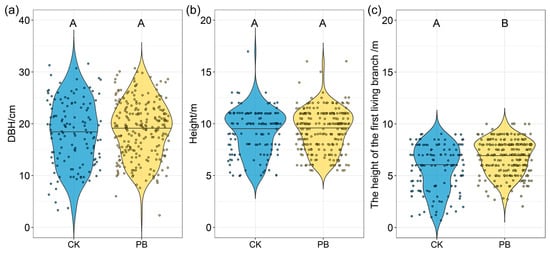
Figure 3.
(a) The diameter at breast height (DBH), (b) height, and (c) the height of the first living branch of the arboreal layer between understory plant characteristics in unburned areas and prescribed burning areas. The Wilcoxon test was used to assess differences. Bars labeled with the same letter are not significantly different (p ≥ 0.05).
3.2. Effects of Prescribed Burning on the Composition and Structure of Understory Layers
3.2.1. Effects of Prescribed Burning on the Composition and Structure of Shrubs
Although prescribed burning decreased the average height and coverage of shrubs in understory vegetation (Figure 4), it altered the species community composition of shrubs (Figure 5). Prescribed burning significantly decreased the species richness of shrubs (Figure 6). The number of plant species in the shrub layer decreased from 26 in the unburned plots to 20 after prescribed burning. In addition, the abundance of plants within the shrub layer was also affected (Figure 7a). Tetrastigma yunnanense, Lithocarpus mairei, and saplings of five tree species, including Pinus yunnanensis, Quercus acutissima, Quercus variabilis, Castanopsis delavayi, and Schima wallichii, disappeared from the burned plots. The abundance of Morella rubra, Vaccinium fragile, and Keteleeria evelyniana significantly declined. On the other hand, Melastoma polyanthum, Raphiolepis indica, and Osteomeles schwerinae were newly discovered in the burned plots. Compared to the unburned plots, only Duhaldea cappa showed a significant increase in abundance, while the other co-existing species, in both burned and unburned plots, did not show such a substantial change.
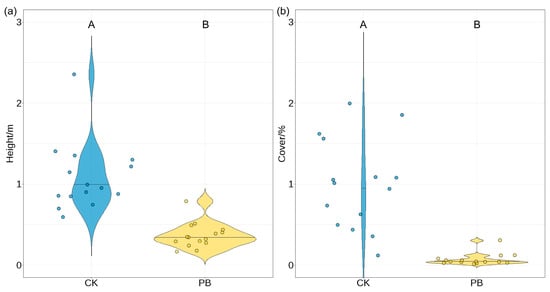
Figure 4.
(a) The average height and (b) coverage of shrubs in the understory vegetation. The Wilcoxon test was used to assess differences. Bars labeled with the same letter are not significantly different (p ≥ 0.05).
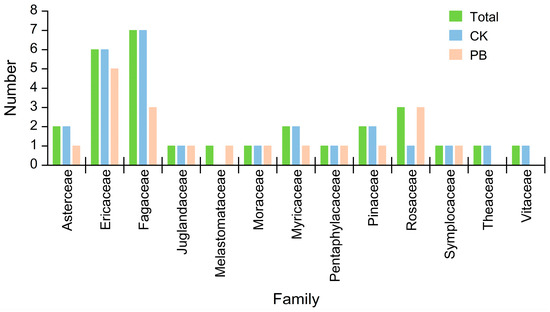
Figure 5.
The family number of shrubs in the understory vegetation.
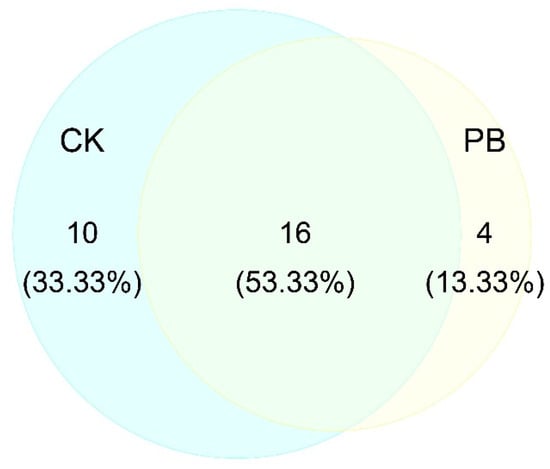
Figure 6.
The number of plant species of shrub layers in unburned (CK) and prescribed burning (PB) sampling sites.
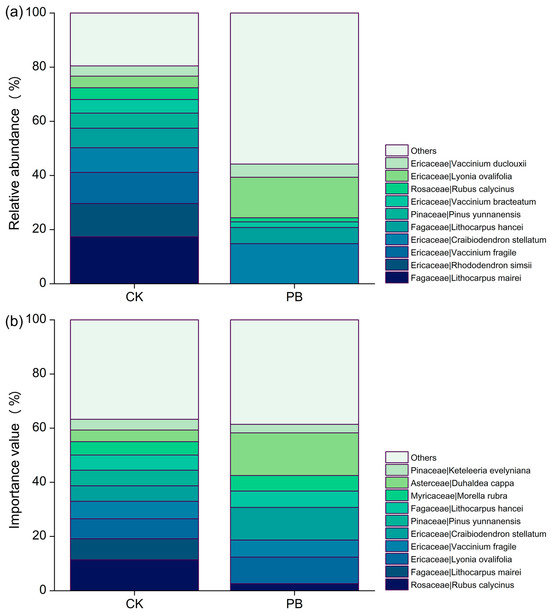
Figure 7.
The top ten genera of (a) plant abundance and (b) importance in the shrub layers.
Prescribed burning also resulted in a reduction in the height of most plants in the shrub layer. Vaccinium duclouxii, Vaccinium fragile, Rubus calycinus, Lyonia ovalifolia, Keteleeria evelyniana, Symplocos sumuntia, Vaccinium bracteatum, Engelhardtia spicata, and Anneslea fragrans showed significantly lower heights in the burned plots compared to the unburned plots. However, Cyclobalanopsis glaucoides and Ficus tikoua experienced a significant increase in height, excluding the newly found shrubs. Overall, prescribed burning led to a considerable decrease in the average height of the shrub layer, decreasing from 84.60 ± 28.83 cm in the unburned plots to 50.42 ± 27.71 cm in the burned plots (Table A4).
The importance value (IV) of many plants in the shrub layer showed changes in the prescribed burning plots compared to the unburned plots, although most of these changes were not easily noticeable (Figure 7b). However, the IV of Duhaldea cappa and Craibiodendron stellatum exhibited a significant increase in the prescribed burning plots, while Lyonia ovalifolia and Vaccinium fragile decreased. The dominant plants in the shrub layer also shifted from Lithocarpus mairei and Lyonia ovalifolia in the unburned area to Duhaldea cappa in the prescribed burning area. Furthermore, the NMDS analysis results provided further evidence of the notable impact of prescribed burning on the vegetation in the shrub layer. This assertion is supported by the graphical representation of NMDS at a 95% confidence interval. It is worth mentioning that the stress value obtained from ranking the NMDS for the shrub layer was 0.135, indicating that NMDS can yield accurate results (Figure 8).
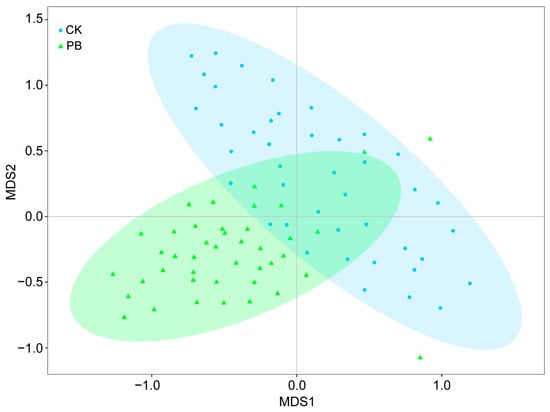
Figure 8.
Two–dimensional NMDS visualization of community composition in the shrub layer. CI = 95%, C represents unburned plots, and PB represents prescribed burning plots.
3.2.2. Effects of Prescribed Burning on the Composition and Structure of the Herb Layers
Prescribed burning resulted in an increase in the number of herb species from 30 to 37 (Figure 9). Three species, including Crassocephalum crepidioides, Cyperus rotundus, and Gamochaeta pensylvanica, disappeared in the burned plots. On the other hand, 15 species, such as Parochetus communis, Themeda triandra, Erigeron canadensis, Gerbera delavayi, Centella asiatica, Clinopodium repens, Lespedeza bicolor, Asteraceae japonica, Taraxacum mongolicum, Scutellaria indica, Desmodium concinnum, Hedyotis diffusa, Bothriochloa ischaemum, Imperata cylindrica, and Ainsliaea yunnanensis, were only found in the burned plot. The majority of the newly–found species belonged to the Poaceae and Asteraceae families (Figure 10).
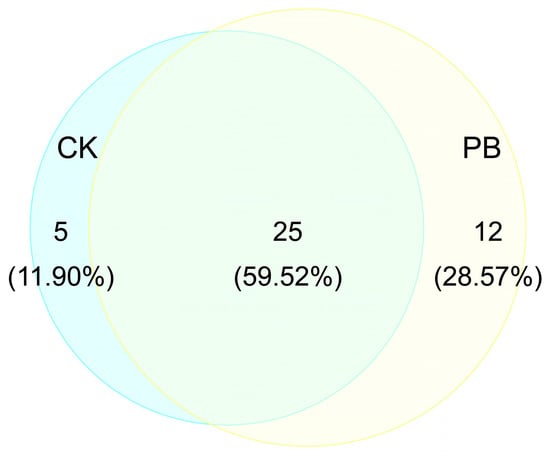
Figure 9.
The number of plant species in the herb layers in unburned (CK) and prescribed burning (PB) sampling sites.
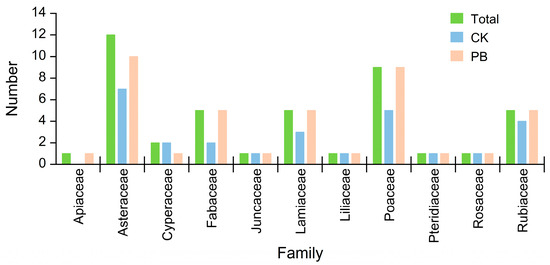
Figure 10.
The family number of herbs in understory vegetation.
Prescribed burning had minimal impact on the abundance of herbs. Apart from the newly discovered species, no other species showed a significant increase in abundance. Only Pteridium aquilinum exhibited a significant decrease in abundance, apart from undiscovered species (Figure 11a). The average height of herb layers in unburned plots increased from 25.83 ± 9.61 cm to 34.36 ± 22.28 cm in the burned plots (Figure 12 and Table A5). The burning treatment partially altered the importance values of the herb layer plants. In the unburned plots, the species with the highest importance value (IV) was Arundinella setosa, while in the burned plots, it was Elsholtzia rugulosa. Except for Eulalia quadrinervis, Hedyotis uncinella, Pteridium aquilinum, and Desmodium microphyllum, which were no longer present, and the newly added species, most of the coexisting species in the burned and unburned plots showed no significant difference in their importance values (Figure 12b).
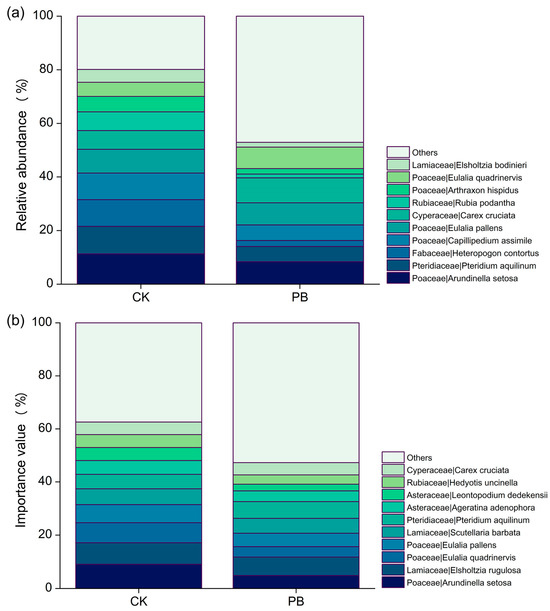
Figure 11.
The top ten genera of (a) plant abundance and (b) importance in the herb layers.
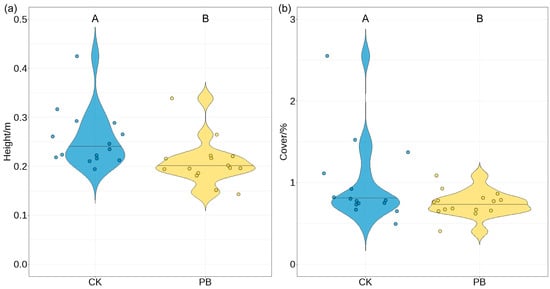
Figure 12.
(a) The average height and (b) coverage of herbs in understory vegetation. The Wilcoxon test was used to assess differences. Bars labeled with the same letter are not significantly different (p ≥ 0.05).
The NMDS results strongly indicate that the impact of planned burning on herbaceous-layer plants can be disregarded. This is confirmed by the graphical representation of NMDS and the 95% confidence interval. The stress values assigned to the NMDS of the herb layer were remarkably low (0.186), which highlights the effectiveness of NMDS in producing accurate results (Figure 13).
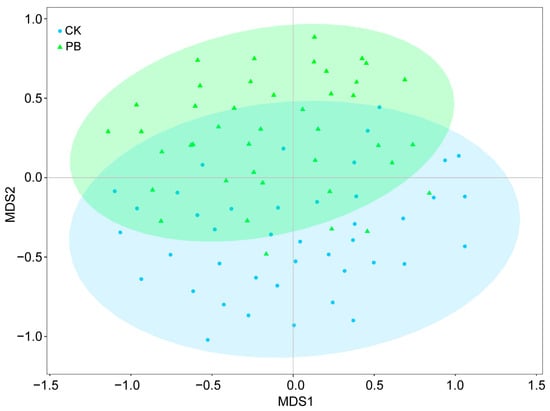
Figure 13.
Two–dimensional NMDS visualization of community composition in the herb layer. CI = 95%, C represents unburned plots, and PB represents prescribed burning plots.
3.3. Effects of Prescribed Burning on Diversity Indices of Understory Species
The effects of prescribed burning on species diversity indices differed between shrub layers and herb layers (Table 2 and Figure 14). Prescribed burning had a significant impact on the Shannon–Winner diversity index (H′), Simpson’s dominance index (D), Pielou’s evenness index (EH), and Margalef’s richness index (F) of shrub layers (Figure 14a and Table A6), resulting in a decrease in these indices. However, the positive effects of prescribed burning on the H′ and D of herb layers were limited, and it had slightly negative effects on the EH of herb layers (Figure 14b and Table A7).

Table 2.
Effects of prescribed burning on the beta diversity index of the understory.
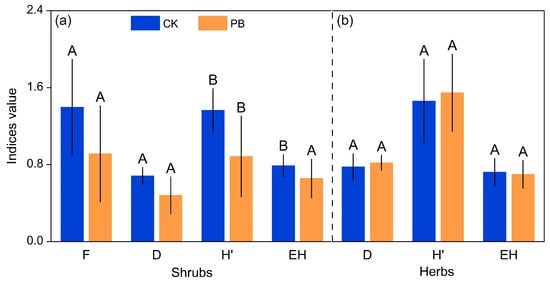
Figure 14.
Diversity indexes of (a)the shrub layer and (b) the herbs layer in burned plots and unburned plots. The Wilcoxon test was used to assess differences. Bars labeled with the same letter are not significantly different (p ≥ 0.05).
Different β diversity indices showed varying responses in herbs and shrubs to prescribed burning. The Jaccard index (βj) indicated that there was a moderate similarity in the number of plant species between the areas subjected to prescribed burning and those that were not burned, with slightly higher similarity in the herbaceous layer compared to the shrub layer. On the other hand, the Cody index (βc) suggested a higher species turnover in the herbaceous layer compared to the shrub layer. However, the Morisita–Horn index (CMH) indicated a higher similarity in the herbaceous layer in both the burned and unburned areas.
3.4. Analysis of Major Factors Affecting the Species Biodiversity of the Understory
There were notable differences in influential factors between the shrub and herb layers (Figure 15). The MANOVA showed that the diversity index of the shrub layer calculated by Shannon’s index was mainly affected by soil pH, which accounted for 26.08% of its importance. Similarly, Margalef’s index was primarily influenced by pH, with an importance value of 29.81%. When considering Pielou’s index, the dominant influencing factor was found to be altitude, with an importance of 19.53%. On the other hand, fire regimes emerged as the most influential factor for Simpson’s index, with an importance value of 19.92%. Moving on to the herb layer, both Pielou’s index and Shannon’s index were predominantly affected by tree diameter at breast height, with importance values of 30.57% and 26.25%, respectively. Lastly, Simpson’s index in the herb layer was primarily influenced by altitude, with an importance value of 17.51%.
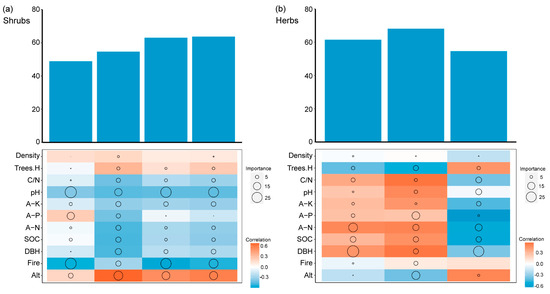
Figure 15.
Spearman’s correlation coefficients of (a) the shrub layer and (b) the herb layer between the species diversity index and influencing factors. Density represents tree density, Trees.H represents tree height, C/N represents soil total carbon/total nitrogen, A-K represents soil available potassium, A-P represents soil available phosphorus, A-N represents soil hydrolyzable nitrogen, SOC represents soil organic carbon, DBH represents tree diameter at breast height, Fire represents prescribed burning, and Alt represents altitude. The size of the circle represents the importance of each variable. Orange represents a positive correlation; blue represents a negative correlation. The darker the color, the stronger the correlation. The bar graph represents the total explanatory power of canopy structure factors, environmental factors, and soil nutrient factors on plant diversity variation.
The structural equation model (SEM) revealed the relationships among α species diversity indexes of the understory vegetation and the possible influencing factors (Figure 16). For the shrub layer (Figure 16a), significant negative correlations existed between Simpson’s index and Shannon’s index and fire (prescribed burning), with path coefficients of −0.25 and −0.34 (p < 0.01), respectively (Figure 15a). Additionally, Margalef’s index, Simpson’s index, Shannon’s index, and Pielou’s index displayed significant correlations with soil pH, with path coefficients of −0.52, −0.50, −0.47, and −0.34 (p < 0.05), respectively. Similarly, Simpson’s index, Shannon’s index, and Pielou’s index exhibited positive correlations with altitude, with path coefficients of 0.44, 0.37, and 0.54 (p < 0.01), respectively. For the herb layer (Figure 16b), Simpson’s index had a significant correlation with fire (prescribed burning) and altitude, with path coefficients of 0.46 and 0.72 (p < 0.001), respectively. Additionally, Shannon’s index was found to be significantly influenced by tree diameter at breast height and soil pH, with path coefficients of 0.78 and 0.31 (p < 0.001), respectively, and Pielou’s index was also significantly correlated with tree diameter at breast height and soil A-N, with path coefficients of 0.72 and 0.44 (p < 0.01), respectively (Figure 15b).
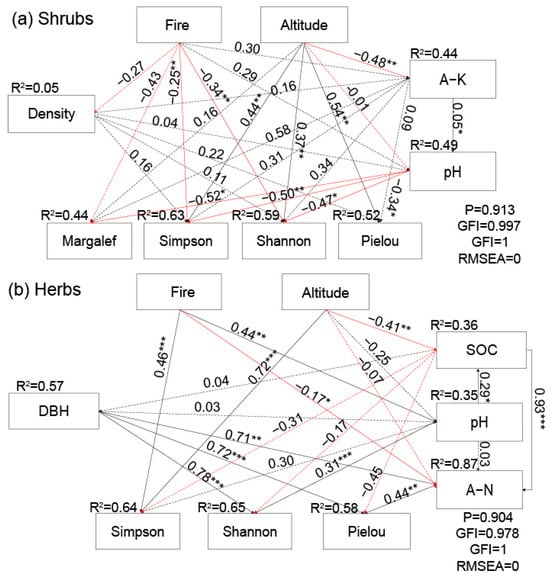
Figure 16.
Relationships among varied factors for (a) the shrub layer and (b) the herb layer, respectively. Density represents the tree density of the overstory, DBH represents tree diameter at breast height, Fire represents prescribed burning, A-K represents soil available potassium, SOC represents soil organic carbon, and A-N represents soil hydrolyzable nitrogen. Red represents a negative correlation, black represents a positive correlation, and dashed lines represent insignificant correlations. Solid lines represent significant causal relationships between two variables, with significance levels of p < 0.05 (*), 0.01 (**), and 0.001 (***).
4. Discussion
Prescribed burning had minimal impacts on overstory trees of the Pinus yunnanensis forest [21,29,40]. Our findings further demonstrate that prescribed burning exerts no significant alteration on the floristic composition of the arboreal stratum in Pinus yunnanensis forests, with its primary measurable impact limited to an increase in the height below branches. the increase in the height of the first branch of Pinus yunnanensis can be attributed to two additional factors. The warm and dry climate characteristic of the southwestern region, combined with low-intensity fires, has altered the micro-environment within forest gaps, leading to elevated temperatures and reduced humidity. These conditions enhance transpiration rates and induce the natural shedding of lower branches. Furthermore, Pinus yunnanensis exhibits intense intraspecific competition and a pronounced self-pruning effect, which results in excessive canopy closure during the seedling stage. Insufficient light and nutrients cause the lower branches and leaves to fall off. The species’ strong pioneer traits enable the upper canopy to dominate, further suppressing the growth of lower branches. Additionally, the increase in the height of the first living branch of Pinus yunnanensis, coupled with the reduction in shrub layer height following prescribed burning, has collectively diminished the vertical continuity of the Pinus yunnanensis forest in burned plots, thereby significantly reducing the likelihood of crown fires.
Environmental changes and biological characteristics jointly drive the increase in branches below the height. However, the effects of prescribed burning on shrubs were relatively larger [41,42]. Prescribed burning altered species composition in the shrub layer of the understory, which was similar to the effects of frequency fire on shrubby dry sclerophyll forests of southeastern Australia [41], Mediterranean-climate shrublands [8], understory vegetation of Arizona ponderosa pine forests [43], a Pinus halepensis forest in northeastern Spain [42], and Appalachian oak-dominated forests [44].
The varied impacts of prescribed burning on the understory of forests might be because of the differences in forest types and the post-fire time [25,44,45,46]. We found that three years after prescribed burning, species richness of the shrub layer in the prescribed burning plots was still clearly lower than that in unburned plots, which was similar to the effects of prescribed burning on the understory of Pinus halepensis forests in northeastern Spain [11]. On the contrary, understory species richness of Appalachian oak-dominated forests was not affected by burning [44]. Prescribed burning shifted the dominant species from Lithocarpus mairei and Lyonia ovalifolia in the unburned area to Duhaldea cappa in our study, consistent with the influence of periodic burning on the understory composition of an open New England woodland [46]. Saplings of Pinus yunnanensis, Schima wallichii, Quercus acutissima, and Lithocarpus mairei missing from the understory of Pinus yunnanensis forest after prescribed burning may affect forest regeneration processes and the future of the forest ecosystem [14,44].
Prescribed burning decreased the height of shrub-layer species, with frequent burning resulting in a decline in species richness for species taller than 1 m and a relative increase of smaller species less than this height in Pinus yunnanensis forest. Even low-intensity, frequent burning can destroy seedlings, and shorter intervals between burns leave insufficient time for tall shrub species to recover [42,47]. In contrast, the number of individual shrubs and species of shrubs is higher in areas of high-intensity fire. Low-intensity fires reduce fuel loads, thereby enhancing light availability and mineral nutrient effectiveness for understory vegetation [48]. Moreover, prescribed burning increases the heterogeneity of ground-level light intensity and soil conditions in forests, which promotes herbaceous plant diversity [49]. Prescribed burning also positively influences litter decomposition, enabling seeds of many plant species to reach the soil layer and facilitating their establishment and growth [12]. Additionally, low-intensity prescribed fires break seed dormancy mechanisms, stimulating germination processes in various plant species [3,50].
We found that compared with the unburned plots, the abundance of Fagaceae species in the shrub layer decreased after the planned burn, while the abundance of Fabaceae species significantly increased (Table A4). This result is similar to those of Knapp et al. [17] and Amoako et al. [51]. Most Fabaceae plants have strong drought and fire resistance. After fire disturbance or other environmental disturbances, although the total nitrogen content in the soil may decrease, the available nitrogen content increases, providing favorable conditions for nitrogen fixation through root nodules in Fabaceae plants, thereby accelerating their growth and increasing the probability of re-sprouting [52,53,54]. Though seed germination of Pinus yunnanensis is stimulated by high temperature and most species in semi-humid evergreen broad-leaved forests can resprout after fire [3], frequent burning may destroy regenerating saplings in shrub and herb layers, thereby altering the species community composition of shrubs. Prescribed burning is not appropriate for young forests. Low-intensity fires tend to significantly consume low shrubs, the herbaceous layer, and the litter layer [47].
Prescribed burning has enhanced the species richness of herbaceous plants in Pinus yunnanensis forests, with the highest increase observed in pioneer plants belonging to the Compositae and Gramineae families. These findings align with the effects of frequent fires on herbaceous vegetation in upland oak forests in the eastern United States [45]. The dominant species in the herb layer remained unchanged in the prescribed burning plots, while the changes in herbaceous understory abundance and height were also minimal, resembling patterns observed in Ponderosa Pine forests [40,55,56]. Herb abundance and diversity in Mediterranean pine forests increased significantly within 8 to 9 years of the planned burns [14]. Vegetative fuels rearrange over short intervals after fire, and herbaceous and ground apomictic cover in southeastern Australia is higher in low-intensity fire areas [50]. Long-term, low-intensity forest fires can be effective in reducing ground fuel loads and increasing canopy openness by reducing plant continuity vertically and horizontally, removing some shrub and tree seedlings, which contributes to herbaceous plant abundance and cover and reduces the incidence of crown fires [49,57]. Prescribed burning substantially elevated the importance value of ferns while diminishing that of Ageratina adenophora (see Table A5). Wang et al. [58] noted that, despite low-intensity fires suppressing the growth of invasive species, they are insufficient to completely eradicate Ageratina adenophora.
Fires could directly impact the structure of forest plant communities and indirectly influence them by altering soil nutrient content [59,60,61]. Fire can significantly alter the physical, chemical, and biological properties of soil, thereby indirectly influencing the structure and function of forest plant communities. Fire can change the physical, chemical, and biological properties of soils [62], and frequent wildfires limit the rooting activity of vegetation, resulting in a significant loss of soil organic carbon SOC, a decrease in elemental N concentrations, and a significant decrease in phosphorus, potassium, sulfur, and others. An evaluation of cation exchange capacity can be found in Refs. [63,64,65]. However, low-intensity fire has a positive effect on promoting plant community regeneration, and Scharenbroch et al. [59] found that low-intensity forest fires resulted in significant increases in soil pH, conductivity, Ca2+, Mg2+, K+, Na+, NO3−, total nitrogen, and organic carbon in the understory of oak (Quercus) forests in the United States. Moreover, the combustion process promotes the mineralization of organic phosphorus and releases a large amount of soluble inorganic phosphorus, thereby enhancing the absorption and utilization efficiency of nutrients by vegetation and soil microorganisms [66,67]. Our study also indicated that fire is the most influential factor affecting species diversity in the shrub layer: the α diversity index of the shrub layer exhibited a significant correlation with soil pH, A-K, and A-P content, while the α species diversity of the herbaceous layer was closely related to soil pH, SOC, and A-N.
Fires are a key factor influencing the succession of forest ecosystems, and the high temperatures at which forest fires occur can cause massive mortality of soil microorganisms, which in turn alters the composition and community characteristics of soil microorganisms [68,69]. Low-intensity burning induces the decomposition of nutrients in plants and litter into soil organic matter, which increases the soil organic carbon content and thus promotes the propagation and activity of microbial communities [49,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73]. However, we only considered abiotic factors when exploring the driving factors affecting the species diversity of understory vegetation, and we should comprehensively consider both biotic and abiotic factors in the follow-up study to better understand the differences and patterns of changes in species diversity and carbon stocks in the understory vegetation after the fire, and to further understand the effects of forest fire disturbance on the composition, structure, and succession of Pinus yunnanensis communities to provide a scientific basis for the protection, restoration, and management of Pinus yunnanensis forests after fire.
5. Conclusions
We investigated the impact of prescribed burning on plant community structure characteristics of the understory in the Pinus yunnanensis forests of central Yunnan. We compared the plant community structure in a prescribed burning area with an unburned area. Results showed:
- (1)
- Prescribed burning had little impact on the arboreal layer, only increasing the height under the living branches.
- (2)
- Prescribed burning induced a marked reduction in shrub layer species richness. Burning significantly altered the structure of the understory vegetation community in the shrub layers of the Pinus yunnanensis forests, declining from 26 taxa in unburned plots to 20 in prescribed burning plots, and it triggered a species shift in dominance hierarchies, with Lithocarpus mairei being supplanted by the fire-adapted species Duhaldea cappa, and the complete extirpation of arboreal saplings. Concomitantly, all biodiversity indices exhibited pronounced declines: the Shannon–Wiener diversity index (H′), Simpson’s dominance index (D), and Margalef’s richness index (F) were significantly reduced post-burn. Furthermore, vertical stratification was simplified, as evidenced by a substantial decrease in mean shrub-layer height.
- (3)
- Prescribed burning exhibited a modest augmentation in herbaceous species richness (30 to 37 taxa) yet manifested negligible impacts on herb-layer structural parameters. Comparative analyses revealed non-significant alterations in species abundance distributions, vertical stratification (herb height), and α diversity indices.
- (4)
- Our findings demonstrated that prescribed burning differentially modified the community structure of the understory vegetation in Pinus yunnanensis forest, with pronounced alterations observed in the shrub layer. The compositional shifts in both shrub and herbaceous strata exhibited dependent variability mediated by interacting biotic and abiotic covariates. Prescribed burning is the predominant determinant of the shrub-layer community. Conversely, herbaceous species diversity displayed stronger covariation with canopy architectural parameters and soil nutrient gradients.
Author Contributions
Conceptualization, X.L., Q.W. and H.P.; data curation, H.P.; investigation, X.L., H.P., H.Y., A.Y. and J.W.; methodology, X.L., Q.W., Y.X. and H.P.; formal analysis, X.L., H.P. and Y.P.; writing—original draft, X.L., H.P. and Y.X.; investigation, X.L. and Q.W.; project administration, X.L. and Q.W.; supervision and funding acquisition, X.L. and Q.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant numbers: 32360395, 31901322, 32160376, 202202AD080010, YNWR-QNBJ-2019-244).
Data Availability Statement
There are no known competing financial interests or personal relationships that could have affected this work.
Acknowledgments
Thanks are due to Jun Pu and Xiyan Zhang in Forest Fire Prevention Office of Xinping Forestry Bureau, Yunnan province, China.
Conflicts of Interest
The author declares no conflicts of interest.
Appendix A

Table A1.
CBI index estimation.
Table A1.
CBI index estimation.
| Description of Fire Damage Characteristics | Fire Severity Scale | ||||||
|---|---|---|---|---|---|---|---|
| No Effect | Low | Moderate | High | ||||
| 0 | 0.5 | 1 | 1.5 | 2.0 | 2.5 | 3.0 | |
| A. Surface fuels and the soil layer | |||||||
| Litter/light fuel consumed | Unchanged | - | 50% Litter | 80% Litter | 100% Litter | >80% Light Fuel | 98% Light fuel |
| Duff | Unchanged | - | Light char | - | 50% Loss | - | Consumed |
| Medium fuel 7.6–20.3 cm | Unchanged | 10% Consumed | 20% Consumed | - | 40% Consumed | - | >60% Consumed |
| Heavy fuel > 20.3 cm | Unchanged | - | 10% Loss | - | 25% Loss | - | 40% Loss |
| Soil & rock cover/color | Unchanged | - | 10% Changed | - | 40% Changed | - | >80% Changed |
| B. Herbs, low shrubs, and trees less than 1 m | |||||||
| % Foliage altered | Unchanged | - | 30% | - | 80% | 95% | 100%, Branch loss |
| Frequency % living | 100% | - | 90% | - | 30% | <20% | <1% |
| Colonizers | Unchanged | - | Low | - | Moderate | High–low | 100% |
| Species composition | Unchanged | - | Little Change | - | Moderate change | - | High change |
| C. Tall shrubs and trees 1 to 5 m | |||||||
| % Foliage altered | Unchanged | 10% | 20% | - | 60–90% | 95% | Branch Loss |
| Frequency % living | 100% | 95% | 90% | - | 30% | 15% | <1% |
| Colonizers | Unchanged | - | 15% | - | 70% | 90% | 100% |
| Species composition | Unchanged | - | Little change | - | Moderate change | - | High change |
| D. Canopy layer (>5 m) | |||||||
| % Green | 100% | 90% | 80% | - | 40% | >10% | - |
| % Black | Unchanged | - | 5–20% | - | 60% | >85% | 100%, Branch loss |
| % Brown | Unchanged | - | 10% | - | 40–80% | <40% or >80% | - |
| % Canopy mortality | Unchanged | 5% | 15% | - | 60% | 80% | 100% |
| Char height | Unchanged | - | 1.5 m | - | 2.8 m | - | >5 m |

Table A2.
Species family genus in the shrub layer of Pinus yunnanensis forests.
Table A2.
Species family genus in the shrub layer of Pinus yunnanensis forests.
| Species | Family Name | Generic Name | Group |
|---|---|---|---|
| Vernonia cumingiana | Asterceae | Vernonia | Native perennial shrub |
| Duhaldea cappa | Asterceae | Duhaldea | Native perennial shrub |
| Craibiodendron stellatum | Ericaceae | Craibiodendron | Native perennial shrub |
| Vaccinium bracteatum | Ericaceae | Vaccinium | Native perennial shrub |
| Rhododendron simsii | Ericaceae | Rhododendron | Native perennial shrub |
| Lyonia ovalifolia | Ericaceae | Lyonia | Native perennial shrub |
| Vaccinium fragile | Ericaceae | Vaccinium | Native perennial shrub |
| Vaccinium duclouxii | Ericaceae | Vaccinium | Native perennial shrub |
| Campylotropis hirtella | Fabaceae | Campylotropis | Native perennial shrub |
| Lithocarpus mairei | Fagaceae | Lithocarpus | Native perennial shrub |
| Cyclobalanopsis glaucoides | Fagaceae | Cyclobalanopsis | Native perennial shrub |
| Quercus acutissima | Fagaceae | Quercus | Native perennial shrub |
| Castanopsis delavayi | Fagaceae | Castanopsis | Native perennial shrub |
| Lithocarpus hancei | Fagaceae | Lithocarpus | Native perennial shrub |
| Quercus variabilis | Fagaceae | Quercus | Native perennial shrub |
| Engelhardtia spicata | Juglandaceae | Engelhardtia | Native perennial shrub |
| Melastoma polyanthum | Melastomataceae | Melastoma | Native perennial shrub |
| Ficus tikoua | Moraceae | Ficus | Native perennial shrub |
| Morella rubra | Myricaceae | Morella | Native perennial shrub |
| Myrica nana | Myricaceae | Myrica | Native perennial shrub |
| Anneslea fragrans | Pentaphylacaceae | Anneslea | Native perennial shrub |
| Pinus yunnanensis | Pinaceae | Pinus | Native perennial shrub |
| Keteleeria evelyniana | Pinaceae | Keteleeria | Native perennial shrub |
| Raphiolepis indica | Rosaceae | Raphiolepis | Native perennial shrub |
| Osteomeles schwerinae | Rosaceae | Osteomeles | Native perennial shrub |
| Rubus calycinus | Rosaceae | Rubus | Native perennial shrub |
| Symplocos sumuntia | Symplocaceae | Symplocos | Native perennial shrub |
| Schima wallichii | Theaceae | Schima | Native perennial shrub |
| Tetrastigma yunnanense | Vitaceae | Tetrastigma | Native perennial shrub |

Table A3.
Species family genus in the herb layer of Pinus yunnanensis forests.
Table A3.
Species family genus in the herb layer of Pinus yunnanensis forests.
| Species | Family Name | Generic Name | Group |
|---|---|---|---|
| Centella asiatica | Apiaceae | Centella | Native perennial herb |
| Ageratina adenophora | Asteraceae | Ageratina | Native perennial herb |
| Pseudognaphalium adnatum | Asteraceae | Pseudognaphalium | Native perennial herb |
| Leontopodium dedekensii | Asteraceae | Leontopodium | Native perennial herb |
| Anaphalis margaritacea | Asteraceae | Anaphalis | Native perennial herb |
| Erigeron canadensis | Asteraceae | Erigeron | Native perennial herb |
| Laggera alata | Asteraceae | Laggera | Native perennial herb |
| Gerbera delavayi | Asteraceae | Gerbera | Native perennial herb |
| Crassocephalum crepidioides | Asteraceae | Crassocephalum | Native perennial herb |
| Asteraceae japonica | Asteraceae | Asteraceae | Native perennial herb |
| Taraxacum mongolicum | Asteraceae | Taraxacum | Native perennial herb |
| Gamochaeta pensylvanica | Asteraceae | Gamochaeta | Native perennial herb |
| Ainsliaea yunnanensis | Asteraceae | Ainsliaea | Native perennial herb |
| Carex cruciata | Cyperaceae | Carex | Native perennial herb |
| Cyperus rotundus | Cyperaceae | Cyperus | Native perennial herb |
| Parochetus communis | Fabaceae | Parochetus | Native perennial herb |
| Desmodium microphyllum | Fabaceae | Desmodium | Native perennial herb |
| Heteropogon contortus | Fabaceae | Heteropogon | Native perennial herb |
| Lespedeza bicolor | Fabaceae | Lespedeza | Native perennial herb |
| Desmodium concinnum | Fabaceae | Desmodium | Native perennial herb |
| Juncus effusus | Juncaceae | Juncus | Native perennial herb |
| Elsholtzia rugulosa | Lamiaceae | Elsholtzia | Native perennial herb |
| Elsholtzia bodinieri | Lamiaceae | Elsholtzia | Native perennial herb |
| Clinopodium repens | Lamiaceae | Clinopodium | Native perennial herb |
| Scutellaria indica | Lamiaceae | Scutellaria | Native perennial herb |
| Scutellaria barbata | Lamiaceae | Scutellaria | Native perennial herb |
| Ophiopogon japonicus | Liliaceae | Ophiopogon | Native perennial herb |
| Arundinella setosa | Poaceae | Arundinella | Native perennial herb |
| Eulalia pallens | Poaceae | Eulalia | Native perennial herb |
| Eulalia quadrinervis | Poaceae | Eulalia | Native perennial herb |
| Themeda triandra | Poaceae | Themeda | Native perennial herb |
| Capillipedium assimile | Poaceae | Capillipedium | Native perennial herb |
| Arthraxon hispidus | Poaceae | Arthraxon | Native perennial herb |
| Bothriochloa ischaemum | Poaceae | Bothriochloa | Native perennial herb |
| Miscanthus sinensis | Poaceae | Miscanthus | Native perennial herb |
| Imperata cylindrica | Poaceae | Imperata | Native perennial herb |
| Pteridium aquilinum | Pteridiaceae | Pteridium | Native perennial herb |
| Duchesnea indica | Rosaceae | Duchesnea | Native perennial herb |
| Hedyotis uncinella | Rubiaceae | Hedyotis | Native perennial herb |
| Hedyotis auricularia | Rubiaceae | Hedyotis | Native perennial herb |
| Rubia podantha | Rubiaceae | Rubia | Native perennial herb |
| Galium bungei | Rubiaceae | Galium | Native perennial herb |
| Hedyotis diffusa | Rubiaceae | Hedyotis | Native perennial herb |

Table A4.
The importance value, plant abundance, and height of the shrub layer in prescribed burning and unburned plots.
Table A4.
The importance value, plant abundance, and height of the shrub layer in prescribed burning and unburned plots.
| Species | Abundance | Height (cm) | Importance Value (IV) | |||
|---|---|---|---|---|---|---|
| CK | PB | CK | PB | CK | PB | |
| Craibiodendron stellatum | 12.57 ± 11.82 | 11.08 ± 9.05 | 75.42 ± 44.9 | 54.92 ± 28.1 | 0.35 ± 0.05 | 0.46 ± 0.15 * |
| Lithocarpus mairei | 24.00 ± 15.66 | - ** | 127.72 ± 64.20 | - ** | 0.47 ± 0.05 | - ** |
| Pinus yunnanensis | 6.88 ± 7.69 | - | 51.04 ± 15.97 | - | 0.35 ± 0.07 | - |
| Morella rubra | 3.50 ± 2.50 | 0.64 ± 0.98 | 204.24 ± 95.18 | 114.89 ± 91.46 | 0.30 ± 0.08 | 0.22 ± 0.10 |
| Anneslea fragrans | 1.50 ± 0.50 | 1.46 ± 0.86 | 43.50 ± 22.50 | 22.50 ± 2.50 * | 0.14 ± 0.02 | 0.13 ± 0.03 |
| Engelhardtia spicata | 3.33 ± 3.30 | 0.35 ± 0.14 | 106.33 ± 33.99 | 43.00 ± 0.82 ** | 0.16 ± 0.03 | 0.10 ± 0.01 |
| Schima wallichii | 3.00 ± 2.00 | - | 30.50 ± 24.50 | - | 0.10 ± 0.03 | - |
| Vaccinium bracteatum | 7.00 ± 5.10 | 1.00 ± 1.56 | 71.44 ± 20.86 | 36.25 ± 16.14 * | 0.22 ± 0.01 | 0.19 ± 0.10 |
| Symplocos sumuntia | 0.50 ± 0.50 | 0.17 ± 0.37 | 200.00 ± 12.50 | 65.00 ± 5.60 ** | 0.13 ± 0.01 | 0.04 ± 0.02 |
| Keteleeria evelyniana | 1.33 ± 0.94 | 1.05 ± 0.43 | 89.50 ± 57.50 | 22.33 ± 3.80 ** | 0.24 ± 0.11 | 0.12 ± 0.04 |
| Cyclobalanopsis glaucoides | 1.50 ± 0.50 | 0.43 ± 0.25 | 200.60 ± 119.40 | 210.00 ± 190.00 | 0.14 ± 0.08 | 0.24 ± 0.15 |
| Quercus acutissima | 1.67 ± 0.94 | - | 75.78 ± 19.51 | - | 0.11 ± 0.02 | - |
| Castanopsis delavayi | 2.00 ± 1.00 | - | 72.17 ± 48.17 | - | 0.19 ± 0.13 | - |
| Lithocarpus hancei | 10.00 ± 5.00 | 4.50 ± 3.50 | 65.90 ± 15.30 | 40.81 ± 4.19 | 0.34 ± 0.03 | 0.23 ± 0.04 |
| Quercus variabilis | 1.00 ± 0.30 | - | 315.00 ± 13.50 | - | 0.15 ± 0.02 | - |
| Melastoma polyanthum | - | 0.14 ± 0.35 | - | 65.00 ± 6.80 | 0.07 ± 0.01 | |
| Raphiolepis indica | - | 0.37 ± 0.17 | - | 30.00 ± 0.50 | - | 0.16 ± 0.05 |
| Osteomeles schwerinae | - | 1.00 ± 0.03 | - | 105.00 ± 11.40 | - | 0.13 ± 0.02 |
| Tetrastigma yunnanense | 1.00 ± 0.02 | - | 5.00 ± 0.90 | - | 0.13 ± 0.01 | - |
| Vernonia cumingiana | 0.50 ± 0.34 | - | 243.00 ± 13.50 | - | 0.07 ± 0.02 | - |
| Myrica nana | 0.67 ± 0.47 | - | 246.50 ± 9.50 | - | 0.20 ± 0.01 | - |
| Rhododendron simsii | 17.00 ± 0.60 | - | 53.59 ± 1.60 | - | 0.19 ± 0.04 | - |
| Duhaldea cappa | 4.00 ± 2.00 | 33.45 ± 25.87 ** | 53.29 ± 17.48 | 44.23 ± 17.75 | 0.26 ± 0.07 | 0.60 ± 0.14 ** |
| Lyonia ovalifolia | 5.92 ± 4.68 | 8.18 ± 11.22 | 99.44 ± 77.75 | 55.02 ± 27.58 * | 0.45 ± 0.13 | 0.37 ± 0.08 * |
| Vaccinium fragile | 15.9 ± 12.21 | 2.80 ± 4.94 * | 20.76 ± 11.33 | 14.31 ± 3.5 * | 0.39 ± 0.12 | 0.24 ± 0.12 ** |
| Campylotropis hirtella | 0.50 ± 0.50 | 0.99 ± 0.86 | 21.00 ± 1.50 | 20.25 ± 0.75 | 0.06 ± 0.02 | 0.20 ± 0.10 |
| Vaccinium duclouxii | 5.25 ± 4.97 | 3.64 ± 1.38 | 51.60 ± 16.37 | 27.18 ± 3.70 * | 0.21 ± 0.05 | 0.16 ± 0.01 |
| Rubus calycinus | 6.00 ± 0.40 | 1.12 ± 0.50 | 34.80 ± 3.70 | 18.00 ± 0.90 ** | 0.69 ± 0.04 | 0.10 ± 0.02 |
| Ficus tikoua | 1.00 ± 0.20 | 1.00 ± 0.03 | 7.00 ± 0.50 | 9.00 ± 0.50 | 0.03 ± 0.01 | 0.05 ± 0.02 |
** Represents p ≤ 0.05, * represents 0.05 < p ≤ 0.10, and “-” indicates that the species was not found in sample plots.

Table A5.
The importance value, coverage, and height of herbs in prescribed burning areas and in unburned areas.
Table A5.
The importance value, coverage, and height of herbs in prescribed burning areas and in unburned areas.
| Species | Abundance | Height (cm) | Importance Value (IV) | |||
|---|---|---|---|---|---|---|
| Unburned | PB | Unburned | PB | Unburned | PB | |
| Elsholtzia rugulosa | 9.01 ± 6.96 | 6.83 ± 3.50 | 31.14 ± 14.18 | 30.83 ± 11.74 | 0.46 ± 0.06 | 0.49 ± 0.02 |
| Pteridium aquilinum | 30.59 ± 24.48 | 11.71 ± 10.43 * | 60.66 ± 27.85 | 57.96 ± 21.96 * | 0.31 ± 0.12 | 0.45 ± 0.05 ** |
| Arundinella setosa | 33.75 ± 26.40 | 17.37 ± 7.72 | 35.96 ± 18.53 | 68.94 ± 32.78 * | 0.52 ± 0.13 | 0.35 ± 0.13 |
| Ageratina adenophora | 11.56 ± 8.02 | 4.53 ± 9.07 | 28.70 ± 14.11 | 16.16 ± 7.07 | 0.30 ± 0.03 | 0.29 ± 0.07 |
| Hedyotis uncinella | 1.29 ± 1.04 | 1.45 ± 0.57 | 8.38 ± 4.69 | 7.63 ± 4.07 | 0.28 ± 0.04 | 0.25 ± 0.03 * |
| Eulalia pallens | 26.57 ± 24.53 | 17.10 ± 12.55 | 54.17 ± 26.05 | 74.66 ± 43.48 | 0.39 ± 0.15 | 0.36 ± 0.10 |
| Eulalia quadrinervis | 15.68 ± 11.01 | 16.66 ± 9.49 | 23.25 ± 11.62 | 29.42 ± 21.91 | 0.43 ± 0.11 | 0.28 ± 0.11 * |
| Parochetus communis | - | 1.65 ± 1.42 ** | - | 2.73 ± 0.30 ** | - | 0.34 ± 0.01 ** |
| Carex cruciata | 20.92 ± 19.34 | 19.22 ± 11.5 | 25.92 ± 9.46 | 36.35 ± 9.34 | 0.27 ± 0.07 | 0.33 ± 0.05 |
| Themeda triandra | - | 22.40 ± 12.61 ** | - | 65.75 ± 33.8 ** | - | 0.33 ± 0.12 ** |
| Capillipedium assimile | 29.61 ± 12.25 | 12.06 ± 7.31 | 45.67 ± 17.17 | 47.25 ± 16.18 | 0.25 ± 0.06 | 0.26 ± 0.06 |
| Hedyotis auricularia | 0.57 ± 0.49 | 0.77 ± 0.65 | 5.33 ± 4.03 | 9.00 ± 8.39 | 0.18 ± 0.03 | 0.18 ± 0.03 |
| Pseudognaphalium adnatum | 1.15 ± 0.81 | 1.31 ± 0.43 | 6.33 ± 4.03 | 3.88 ± 0.22 | 0.14 ± 0.06 | 0.16 ± 0.04 |
| Leontopodium dedekensii | 6.16 ± 5.14 | 0.64 ± 0.47 | 18.57 ± 5.95 | 17.81 ± 14.93 | 0.28 ± 0.16 | 0.18 ± 0.01 |
| Desmodium microphyllum | 1.68 ± 1.55 | 0.82 ± 0.40 | 5.75 ± 2.75 | 22.97 ± 19.92 | 0.08 ± 0.02 | 0.16 ± 0.03 * |
| Heteropogon contortus | 29.77 ± 27.67 | 4.60 ± 3.78 | 27.92 ± 1.56 | 11.50 ± 4.50 | 0.26 ± 0.12 | 0.11 ± 0.02 |
| Juucus effusus | 13.38 ± 4.49 | 1.84 ± 1.19 | 12.17 ± 1.03 | 15.75 ± 8.61 | 0.22 ± 0.01 | 0.15 ± 0.01 |
| Anaphalis margaritacea | 3.44 ± 1.68 | 1.52 ± 1.29 | 8.88 ± 6.62 | 11.25 ± 8.32 | 0.17 ± 0.03 | 0.16 ± 0.06 |
| Erigeron canadensis | - | 1.48 ± 0.99 ** | - | 5.78 ± 2.27 ** | - | 0.19 ± 0.01 ** |
| Duchesnea indica | 0.09 ± 0.05 | 3.07 ± 2.66 | 2.00 ± 0.40 | 5.78 ± 2.64 | 0.05 ± 0.02 | 0.19 ± 0.02 |
| Elsholtzia bodinieri | 14.34 ± 13.90 | 3.67 ± 2.33 | 5.38 ± 2.22 | 6.57 ± 2.56 | 0.15 ± 0.04 | 0.17 ± 0.06 |
| Rubia podantha | 20.81 ± 0.60 | 2.92 ± 0.56 | 39.00 ± 0.80 | 5.88 ± 2.13 | 0.15 ± 0.02 | 0.12 ± 0.03 |
| Galium bungei | 2.03 ± 1.20 | 1.84 ± 1.36 | 4.00 ± 1.00 | 3.83 ± 1.55 | 0.10 ± 0.01 | 0.12 ± 0.01 |
| Laggera alata | 3.95 ± 3.34 | 1.36 ± 1.02 | 29.50 ± 14.50 | 22.00 ± 19.91 | 0.10 ± 0.01 | 0.13 ± 0.06 |
| Ophiopogon japonicus | 0.09 ± 0.03 | 1.92 ± 1.52 | 20.00 ± 0.40 | 18.25 ± 6.25 | 0.05 ± 0.01 | 0.08 ± 0.01 |
| Gerbera delavayi | - | 6.85 ± 6.19 | - | 4.50 ± 2.04 | - | 0.12 ± 0.07 |
| Arthraxon hispidus | 17.24 ± 0.70 | 4.24 ± 0.52 | 30.00 ± 0.80 | 20.00 ± 1.70 | 0.11 ± 0.02 | 0.09 ± 0.01 |
| Centella asiatica | - | 0.03 ± 0.01 | - | 3.00 ± 0.40 | - | 0.04 ± 0.01 |
| Crassocephalum crepidioides | 0.06 ± 0.05 | - | 7.00 ± 0.40 | - | 0.03 ± 0.01 | - |
| Lespedeza bicolor | - | 0.90 ± 0.40 | - | 10.67 ± 5.3 | - | 0.07 ± 0.03 |
| Asteraceae japonica | - | 0.67 ± 0.01 | - | 3.00 ± 2.00 | - | 0.05 ± 0.01 |
| Clinopodium repens | - | 1.33 ± 0.20 | - | 4.00 ± 0.30 | - | 0.06 ± 0.03 |
| Taraxacum mongolicum | - | 0.36 ± 0.10 | - | 3.00 ± 0.06 | - | 0.03 ± 0.04 |
| Scutellaria indica | - | 0.34 ± 0.20 | - | 2.50 ± 0.03 | - | 0.03 ± 0.01 |
| Desmodium concinnum | - | 0.07 ± 0.04 | - | 10.00 ± 0.70 | - | 0.02 ± 0.02 |
| Hedyotis diffusa | - | 0.54 ± 0.30 | - | 4.00 ± 0.05 | - | 0.03 ± 0.01 |
| Bothriochloa ischaemum | - | 0.02 ± 0.01 | - | 14.00 ± 2.50 | - | 0.02 ± 0.01 |
| Miscanthus sinensis | - | 4.07 ± 0.30 | - | 40.00 ± 3.20 | - | 0.06 ± 0.03 |
| Cyperus rotundus | 0.46 ± 0.30 | - | 3.00 ± 0.20 | - | 0.05 ± 0.01 | - |
| Gamochaeta pensylvanica | 1.13 ± 0.4 | - | 3.50 ± 0.5 | - | 0.05 ± 0.02 | - |
| Imperata cylindrica | - | 20.53 ± 19.00 | - | 34.17 ± 20.17 | - | 0.09 ± 0.04 |
| Scutellaria barbata | 3.27 ± 2.95 | 3.23 ± 2.87 | 19.67 ± 8.96 | 31.80 ± 3.66 | 0.34 ± 0.01 | 0.40 ± 0.07 |
| Ainsliaea yunnanensis | - | 0.50 ± 0.03 | - | 17.00 ± 4.10 | - | 0.20 ± 0.01 |
** Represents p ≤ 0.05, * represents 0.05 < p ≤ 0.10, and “-” indicates that the species was not found in sample plots.

Table A6.
The multiple regression equation of α diversity indexes for the shrub layer.
Table A6.
The multiple regression equation of α diversity indexes for the shrub layer.
| Shrub | Equation Model | R2 | AIC |
|---|---|---|---|
| Shannon | Shannon = 5.595 × Alt + 0.467 × A-K + 0.103 × Density − 0.180 × Fire − 4.283 × pH − 0.180 × A-P − 0.251 × BA | 0.630 | −74.463 |
| Simpson | Simpson = 3.160 × Alt + 0.067 × Density + 0.240 × A-K − 0.082 × Fire − 0.073 × A-P − 0.120 × BA − 2.356 × pH | 0.637 | −106.208 |
| Pielou | Pielou = 2.782 × Alt + 0.151 × A-K − 1.528 × pH − 0.108 × A-P − 0.241 × Trees.H − 0.103 × DBH | 0.546 | −105.754 |
| Margalef | Margalef = 4.733 × Alt + 0.600 × A-K − 0.251 × Fire − 4.817 × pH − 0.273 × BA | 0.489 | −57.260 |
Alt represents altitude, Fire represents prescribed burning, Trees.H represents tree height, Density represents tree density, DBH represents tree diameter at breast height, BA represents tree basal area, A-P represents soil available phosphorus, and A-K represents soil available potassium.

Table A7.
The multiple regression equation of α diversity indexes for the herb layer.
Table A7.
The multiple regression equation of α diversity indexes for the herb layer.
| Herb | Equation Model | R2 | AIC |
|---|---|---|---|
| Shannon | Shannon = −0.181 × SOC + 0.635 × DBH + 0.131 × Density + 1.224 × pH | 0.681 | −90.089 |
| Simpson | Simpson = 1.436 × Alt + 0.071 × Fire + 0.441 × pH − 0.071 × SOC | 0.547 | −136.768 |
| Pielou | Pielou = 0.278 × DBH + 0.186 × A-N + 0.056 × Density − 0.192 × SOC | 0.615 | −124.213 |
Alt represents altitude, Fire represents prescribed burning, DBH represents tree diameter at breast height, Density represents tree density, SOC represents soil organic carbon, and A-N represents soil hydrolyzable nitrogen.
References
- Wu, Z.Y.; Zhu, Y.C. Yunnan Vegetation; Beijing Science Publishing House: Beijing, China, 1987; pp. 401–466. [Google Scholar]
- Jin, Z.Z.; Peng, J. Pinus yunnanensis; Yunnan Science & Technology Press: Kunming, China, 2004; pp. 401–446. [Google Scholar]
- Su, W.H.; Shi, Z.; Zhou, R.; Zhao, Y.J.; Zhang, G.F. The role of fire in the Central Yunnan Plateau ecosystem, southwestern China. For. Ecol. Manag. 2015, 356, 22–30. [Google Scholar] [CrossRef]
- Tian, X.R.; Shu, L.F.; Zhao, F.J.; Wang, M.Y. Impacts of Climate Change on Forest Fire Danger in China. Linye Kexue 2017, 53, 159–169. [Google Scholar]
- Ma, Z.G.; Wang, J.X.; Mou, K.H.; Yang, D.G.; Su, Y.M. An Initial Exploration of Forest Fires and Fire Hazard Zoning in Pinus yunnanensis forest Area. J. Sichuan For. Sci. Technol. 1991, 23–30+46. [Google Scholar]
- Fernandes, P.M.; Botelho, H.S. A review of prescribed burning effectiveness in fire hazard reduction. Int. J. Wildland Fire 2003, 12, 117–128. [Google Scholar] [CrossRef]
- Boer, M.M.; Sadler, R.J.; Wittkuhn, R.S.; McCaw, L.; Grierson, P.F. Long-term impacts of prescribed burning on regional extent and incidence of wildfires—Evidence from 50 years of active fire management in SW Australian forests. For. Ecol. Manag. 2009, 259, 132–142. [Google Scholar] [CrossRef]
- Van Wilgen, B.W.; Forsyth, G.G.; De Klerk, H.; Das, S.; Khuluse, S.; Schmitz, P. Fire management in Mediterranean-climate shrublands: A case study from the Cape fynbos, South Africa. J. Appl. Ecol. 2010, 47, 631–638. [Google Scholar] [CrossRef]
- Burrows, N.; McCaw, L. Prescribed burning in southwestern Australian forests. Front. Ecol. Environ. 2013, 11, e25–e34. [Google Scholar] [CrossRef]
- Fernandes, P.M.; Davies, G.M.; Ascoli, D.; Fernández, C.; Moreira, F.; Rigolot, E.; Stoof, C.R.; Vega, J.A.; Molina, D. Prescribed burning in southern Europe: Developing fire management in a dynamic landscape. Front. Ecol. Environ. 2013, 11, e4–e14. [Google Scholar] [CrossRef]
- McCaw, W.L. Managing forest fuels using prescribed fire–a perspective from southern Australia. For. Ecol. Manag. 2013, 294, 217–224. [Google Scholar] [CrossRef]
- Holland, G.J.; Clarke, M.F.; Bennett, A.F. Prescribed burning consumes key forest structural components: Implications for landscape heterogeneity. Ecol. Appl. 2017, 27, 845–858. [Google Scholar] [CrossRef]
- Bradshaw, S.; Dixon, K.; Lambers, H.; Cross, A.; Bailey, J.; Hopper, S. Understanding the long-term impact of prescribed burning in mediterranean-climate biodiversity hotspots, with a focus on south-western Australia. Int. J. Wildland Fire 2018, 27, 643–657. [Google Scholar] [CrossRef]
- Casals, P.; Valor, T.; Besalú, A.; Molina-Terrén, D. Understory fuel load and structure eight to nine years after prescribed burning in Mediterranean pine forests. For. Ecol. Manag. 2016, 362, 156–168. [Google Scholar] [CrossRef]
- Duane, A.; Aquilué, N.; Canelles, Q.; Morán-Ordoñez, A.; De Cáceres, M.; Brotons, L. Adapting prescribed burns to future climate change in Mediterranean landscapes. Sci. Total Environ. 2019, 677, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Brockway, D.G.; Lewis, C.E. Long-term effects of dormant-season prescribed fire on plant community diversity, structure and productivity in a longleaf pine wiregrass ecosystem. For. Ecol. Manag. 1997, 96, 167–183. [Google Scholar] [CrossRef]
- Knapp, B.O.; Stephan, K.; Hubbart, J.A. Structure and composition of an oak-hickory forest after over 60 years of repeated prescribed burning in Missouri, USA. For. Ecol. Manag. 2015, 344, 95–109. [Google Scholar] [CrossRef]
- Kobziar, L.N.; Godwin, D.; Taylor, L.; Watts, A.C. Perspectives on trends, effectiveness, and impediments to prescribed burning in the southern US. Forests 2015, 6, 561–580. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Cao, J.B.; Zhou, L.X.; Li, F.; Fu, S.L. Effects of prescribed burning on carbon accumulation in two paired vegetation sites in subtropical China. For. Ecosyst. 2019, 6, 26. [Google Scholar] [CrossRef]
- Regmi, A.; Kreye, M.M.; Kreye, J.K. Forest landowner demand for prescribed fire as an ecological management tool in Pennsylvania, USA. For. Policy Econ. 2023, 148, 102902. [Google Scholar] [CrossRef]
- Franklin, S.B.; Robertson, P.A.; Fralish, J.S. Prescribed burning effects on upland Quercus forest structure and function. For. Ecol. Manag. 2003, 184, 315–335. [Google Scholar] [CrossRef]
- Wang, Q.H.; Shan, Q.H.; Gong, J.P.; Pu, J.; Kou, W.L.; Xu, W.H.; Wang, H.Y. A Study on Prescribed Burning in Pure Forest of Pinus yunnanensis Franch in Central Yunnan Province. Acta Agric. Univ. Jiangxiensis 2018, 40, 235–240. [Google Scholar]
- Dou, X.; Yu, H.Z.; Wang, J.Y.; Li, F.; Liu, Q.; Sun, L.; Hu, T.X. Effect of prescribed burning on the small-scale spatial heterogeneity of soil microbial biomass in Pinus koraiensis and Quercus mongolica forests of China. J. For. Res. 2023, 34, 609–622. [Google Scholar] [CrossRef]
- van Wilgen, B.W. Fire management in species-rich Cape fynbos shrublands. For. Ecol. Manag. 2013, 11, e35–e44. [Google Scholar] [CrossRef]
- Eales, J.; Haddaway, N.R.; Bernes, C.; Cooke, S.J.; Jonsson, B.G.; Kouki, J.; Petrokofsky, G.; Taylor, J.J. What is the effect of prescribed burning in temperate and boreal forest on biodiversity, beyond pyrophilous and saproxylic species? A systematic review. Environ. Evid. 2018, 7, 19. [Google Scholar] [CrossRef]
- Mahood, A.L.; Balch, J.K. Repeated fires reduce plant diversity in low-elevation Wyoming big sagebrush ecosystems (1984–2014). Ecosphere 2019, 10, e02591. [Google Scholar] [CrossRef]
- Barefoot, C.R.; Willson, K.G.; Hart, J.L.; Schweitzer, C.J.; Dey, D.C. Effects of thinning and prescribed fire frequency on ground flora in mixed Pinus-hardwood stands. For. Ecol. Manag. 2019, 432, 729–740. [Google Scholar] [CrossRef]
- Koivula, M.; Vanha-Majamaa, I. Experimental evidence on biodiversity impacts of variable retention forestry, prescribed burning, and deadwood manipulation in Fennoscandia. Ecol. Process 2020, 9, 11. [Google Scholar] [CrossRef]
- Bassett, T.J.; Landis, D.A.; Brudvig, L.A. Effects of experimental prescribed fire and tree thinning on oak savanna understory plant communities and ecosystem structure. For. Ecol. Manag. 2020, 464, 118047. [Google Scholar] [CrossRef]
- Zald, H.S.; Kerns, B.K.; Day, M.A. Limited effects of long-term repeated season and interval of prescribed burning on understory vegetation compositional trajectories and indicator species in ponderosa pine forests of Northeastern Oregon, USA. Forests 2020, 11, 834. [Google Scholar] [CrossRef]
- Roberton, B.; Rebar, D. Timing of prescribed burns impacts plant diversity but not investment in pollinator recruitment in a tallgrass prairie. Ecosphere 2022, 13, e3914. [Google Scholar] [CrossRef]
- Kumar, R. Response of understorey vegetation in chir pine forests to prescribed burning in Shiwalik region of Himalaya. J. Environ. Biol. 2022, 43, 622–630. [Google Scholar] [CrossRef]
- Vaughan, M.C.; Hagan, D.L.; Bridges Jr, W.C.; Barrett, K.; Norman, S.; Coates, T.A.; Klein, R. Effects of burn season on fire-excluded plant communities in the southern Appalachian Mountains, USA. For. Ecol. Manag. 2022, 516, 120244. [Google Scholar] [CrossRef]
- Céspedes, B.; Torres, I.; Pérez, B.; Luna, B.; Moreno, J.M. Burning season does not affect post-fire regeneration but fire alters the balance of the dominant species in a seeder-dominated M editerranean shrubland. Appl. Veg. Sci. 2014, 17, 711–725. [Google Scholar] [CrossRef]
- Condit, R. Tropical Forest Census Plots: Methods and Results from Barro Colorado Island, Panama and a Comparison with Other Plots; Springer Science & Business Media: New York, NY, USA, 1998; pp. 91–92. [Google Scholar]
- Fang, J.Y.; Wang, X.P.; Shen, Z.H.; Tang, Z.Y.; He, J.S.; Yu, D.; Jiang, Y.; Wang, Z.H.; Zhen, C.Y.; Zhu, J.L.; et al. Methods and protocols for plant community inventory. Biodivers. Sci. 2009, 17, 533–548. [Google Scholar]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Ma, K.P.; Liu, C.R.; Liu, Y.M. Measurement of biotic community diversity:II β diversity. Biodivers. Sci. 1995, 3, 38–43. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 10 April 2022).
- Cowman, D.; Russell, W. Fuel load, stand structure, and understory species composition following prescribed fire in an old-growth coast redwood (Sequoia sempervirens) forest. Fire Ecol. 2021, 17, 17. [Google Scholar] [CrossRef]
- Penman, T.D.; Binns, D.L.; Shiels, R.J.; Allen, R.M.; Kavanagh, R.P. Changes in understorey plant species richness following logging and prescribed burning in shrubby dry sclerophyll forests of south-eastern Australia. Austral Ecol. 2008, 33, 197–210. [Google Scholar] [CrossRef]
- Fuentes, L.; Duguy, B.; Nadal-Sala, D. Short-term effects of spring prescribed burning on the understory vegetation of a Pinus halepensis forest in Northeastern Spain. Sci. Total Environ. 2018, 610, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.F.; Sieg, C.H.; Dickson, B.G.; Saab, V. Exotic plant species diversity: Influence of roads and prescribed fire in Arizona ponderosa pine forests. Rangel. Ecol. Manag. 2008, 61, 284–293. [Google Scholar] [CrossRef]
- Perles, S.J.; Niu, X.M.; Ruth, A.D.; Gibbons, L.D. Initial conditions influence effects of prescribed burns and deer exclosure fences on tree regeneration and understory diversity in Appalachian oak-dominated forests. For. Ecol. Manag. 2021, 495, 119353. [Google Scholar] [CrossRef]
- Burton, J.A.; Hallgren, S.W.; Fuhlendorf, S.D.; Leslie, D.M. Understory response to varying fire frequencies after 20 years of prescribed burning in an upland oak forest. Plant Ecol. 2011, 212, 1513–1525. [Google Scholar] [CrossRef]
- Borden, C.G.; Duguid, M.C.; Ashton, M.S. The legacy of fire: Long-term changes to the forest understory from periodic burns in a New England oak-hickory forest. Fire Ecol. 2021, 17, 24. [Google Scholar] [CrossRef]
- Baeza, M.J.; De Luís, M.; Raventós, J.; Escarré, A. Factors influencing fire behaviour in shrublands of different stand ages and the implications for using prescribed burning to reduce wildfire risk. J. Environ. Econ. Manag. 2002, 65, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.E.; Robles, M.D. Tamm review: The effects of prescribed fire on wildfire regimes and impacts: A framework for comparison. For. Ecol. Manag. 2020, 475, 118435. [Google Scholar] [CrossRef]
- Bowles, M.L.; Jacobs, K.A.; Mengler, J.L. Long-term changes in an oak forest’s woody understory and herb layer with repeated burning1. J. Torrey Bot. Soc. 2007, 134, 223–237. [Google Scholar] [CrossRef]
- Gordon, C.E.; Nolan, R.H.; Boer, M.M.; Bendall, E.R.; Williamson, J.S.; Price, O.F.; Kenny, B.J.; Taylor, J.E.; Denham, A.J.; Bradstock, R.A. Severe and Short Interval Fires Rearrange Dry Forest Fuel Arrays in South-Eastern Australia. Fire 2024, 7, 130. [Google Scholar] [CrossRef]
- Amoako, E.E.; Issifu, H.; Husseini, R. The effects of prescribed dry season burning on woody species composition, Mole National Park, Ghana. Trop. Conserv. Sci. 2023, 16, 19400829231164936. [Google Scholar] [CrossRef]
- Kutiel, P.; Naveh, Z. The effect of fire on nutrients in a pine forest soil. Plant Soil 1987, 104, 269–274. [Google Scholar] [CrossRef]
- Ouédraogo, A.; Thiombiano, A. Regeneration pattern of four threatened tree species in Sudanian savannas of Burkina Faso. Agrofor. Syst. 2012, 86, 35–48. [Google Scholar] [CrossRef]
- Han, Y.; Köster, K.; Dou, X.; Wang, J.; Yu, C.; Hu, H.; Ding, Y.; Hu, T. Prescribed burning reshapes the relationship between soil chemical properties and understory plant biodiversity. Catena 2024, 246, 108478. [Google Scholar] [CrossRef]
- Kerns, B.K.; Thies, W.G.; Niwa, C.G. Season and severity of prescribed burn in ponderosa pine forests: Implications for understory native and exotic plants. Ecoscience 2006, 13, 44–55. [Google Scholar] [CrossRef]
- Springer, J.D.; Stoddard, M.T.; Rodman, K.C.; Huffman, D.W.; Fornwalt, P.J.; Pedersen, R.J.; Laughlin, D.C.; McGlone, C.M.; Daniels, M.L.; Fulé, P.Z. Increases in understory plant cover and richness persist following restoration treatments in Pinus ponderosa forests. J Appl. Ecol. 2024, 61, 25–35. [Google Scholar] [CrossRef]
- Agee, J.K.; Skinner, C.N. Basic principles of forest fuel reduction treatments. For. Ecol. Manag. 2005, 211, 83–96. [Google Scholar] [CrossRef]
- Wang, J.M.; Li, S.F.; Xu, F.D.; Wang, Y.; Su, J.R. Effects of Prescribed Burning on the Community Structure and Species Diversity in Pinus kesiya var. langbianensis Primary Forest. J. Northwest For. Univ. 2020, 35, 62–67. [Google Scholar]
- Scharenbroch, B.C.; Nix, B.; Jacobs, K.; Bowles, M. Two decades of low-severity prescribed fire increases soil nutrient availability in a Midwestern, USA oak (Quercus) forest. Geoderma 2012, 183, 80–91. [Google Scholar] [CrossRef]
- Weiser, F.; Sauer, A.; Gettueva, D.; Field, R.; Irl, S.D.; Vetaas, O.; Chiarucci, A.; Hoffmann, S.; Fernández-Palacios, J.M.; Otto, R. Impacts of forest fire on understory species diversity in Canary pine ecosystems on the island of La Palma. Forests 2021, 12, 1638. [Google Scholar] [CrossRef]
- López-Cruz, S.d.C.; Aryal, D.R.; Velázquez-Sanabria, C.A.; Guevara-Hernández, F.; Venegas-Sandoval, A.; Casanova-Lugo, F.; La, O.-A.M.A.; Venegas-Venegas, J.A.; Reyes-Sosa, M.B.; Pinto-Ruiz, R. Effect of prescribed burning on tree diversity, biomass stocks and soil organic carbon storage in tropical highland forests. Forests 2022, 13, 2164. [Google Scholar] [CrossRef]
- Mataix-Solera, J.; Cerdà, A.; Arcenegui, V.; Jordán, A.; Zavala, L. Fire effects on soil aggregation: A review. Earth Sci. Rev. 2011, 109, 44–60. [Google Scholar] [CrossRef]
- Neill, C.; Patterson III, W.A.; Crary Jr, D.W. Responses of soil carbon, nitrogen and cations to the frequency and seasonality of prescribed burning in a Cape Cod oak-pine forest. For. Ecol. Manag. 2007, 250, 234–243. [Google Scholar] [CrossRef]
- Girona-García, A.; Galarza, R.Z.; Mora, J.L.; Armas-Herrera, C.M.; Martí, C.; Ortiz-Perpiñá, O.; Badía-Villas, D. Effects of prescribed burning for pasture reclamation on soil chemical properties in subalpine shrublands of the Central Pyrenees (NE-Spain). Sci. Total Environ. 2018, 644, 583–593. [Google Scholar] [CrossRef]
- Quigley, K.M.; Kolka, R.; Sturtevant, B.R.; Dickinson, M.B.; Kern, C.C.; Donner, D.M.; Miesel, J.R. Prescribed burn frequency, vegetation cover, and management legacies influence soil fertility: Implications for restoration of imperiled pine barrens habitat. For. Ecol. Manag. 2020, 470, 118163. [Google Scholar] [CrossRef]
- Salgado, L.; Alvarez, M.; Díaz, A.; Gallego, J.; Forján, R. Impact of wildfire recurrence on soil properties and organic carbon fractions. J. Environ. Manag. 2024, 354, 120293. [Google Scholar] [CrossRef] [PubMed]
- Merino, A.; Jiménez, E.; Fernández, C.; Fontúrbel, M.T.; Campo, J.; Vega, J.A. Soil organic matter and phosphorus dynamics after low intensity prescribed burning in forests and shrubland. J. Environ. Econ. Manag. 2019, 234, 214–225. [Google Scholar] [CrossRef] [PubMed]
- He, T.H.; Lamont, B.B.; Pausas, J.G. Fire as a key driver of Earth’s biodiversity. Biol. Rev. 2019, 94, 1983–2010. [Google Scholar] [CrossRef]
- Pérez-Valera, E.; Verdú, M.; Navarro-Cano, J.; Goberna, M. Soil microbiome drives the recovery of ecosystem functions after fire. Soil Biol. Biochem. 2020, 149, 107948. [Google Scholar] [CrossRef]
- Hartshorn, A.S.; Coetsee, C.; Chadwick, O.A. Pyromineralization of soil phosphorus in a South African savanna. Chem. Geol. 2009, 267, 24–31. [Google Scholar] [CrossRef]
- Chen, S.F.; Chen, F.; Suo, A.L.; Feng, H.Y.; Gong, J.W.; Liu, X.D. Investigation of soil microbial community composition in Pinus tabulaeformis forests after different fire severities. Acta Ecol. Sinica 2025, 45, 4223–4236. [Google Scholar]
- Cai, H.; Li, D.; Han, Y.; Hu, T.; Yang, G.; Sun, L. Changes in above-and below-ground biodiversity mediate understory biomass response to prescribed burning in Northeast China. Plant Soil 2024, 1–15. [Google Scholar] [CrossRef]
- Revillini, D.; David, A.S.; Menges, E.S.; Main, K.N.; Afkhami, M.E.; Searcy, C.A. Microbiome-mediated response to pulse fire disturbance outweighs the effects of fire legacy on plant performance. New Phytol. 2022, 233, 2071–2082. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).