The Response Mechanism of Plants Under Rock Stress in Karst Ecosystem: A Study Based on the Effects of Aboveground Rocks on Root Phenotypes and Leaf Water Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Measurement of Root Biomass
2.3. Measurement of Root Extension
2.4. Measurement Root of Bifurcation Ratio
2.5. Measurement of Leaf Water Potential
2.6. Statistical Analysis
3. Results
3.1. Root Biomass
3.2. Root Distribution
3.3. Root Bifurcation Ratio
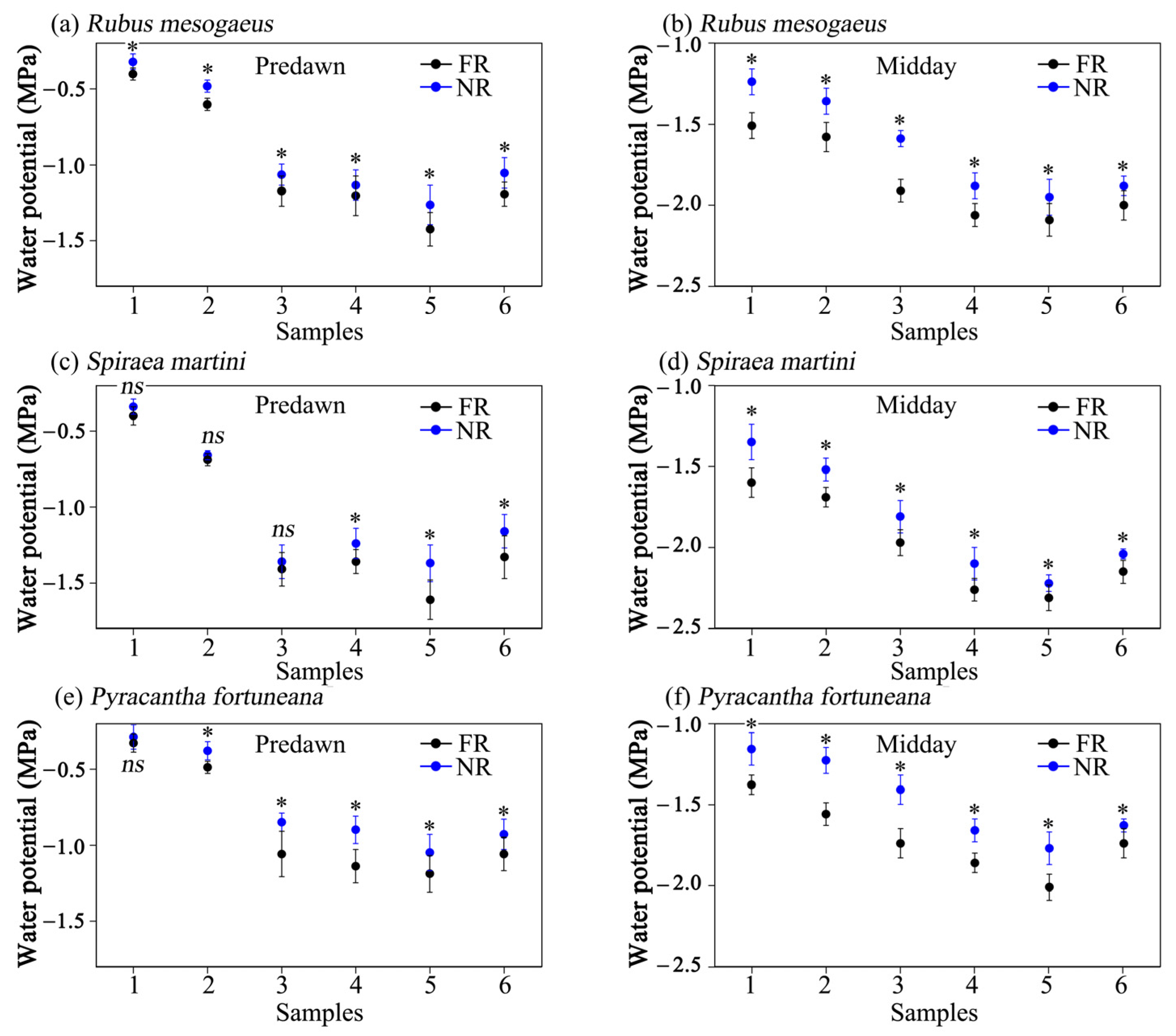
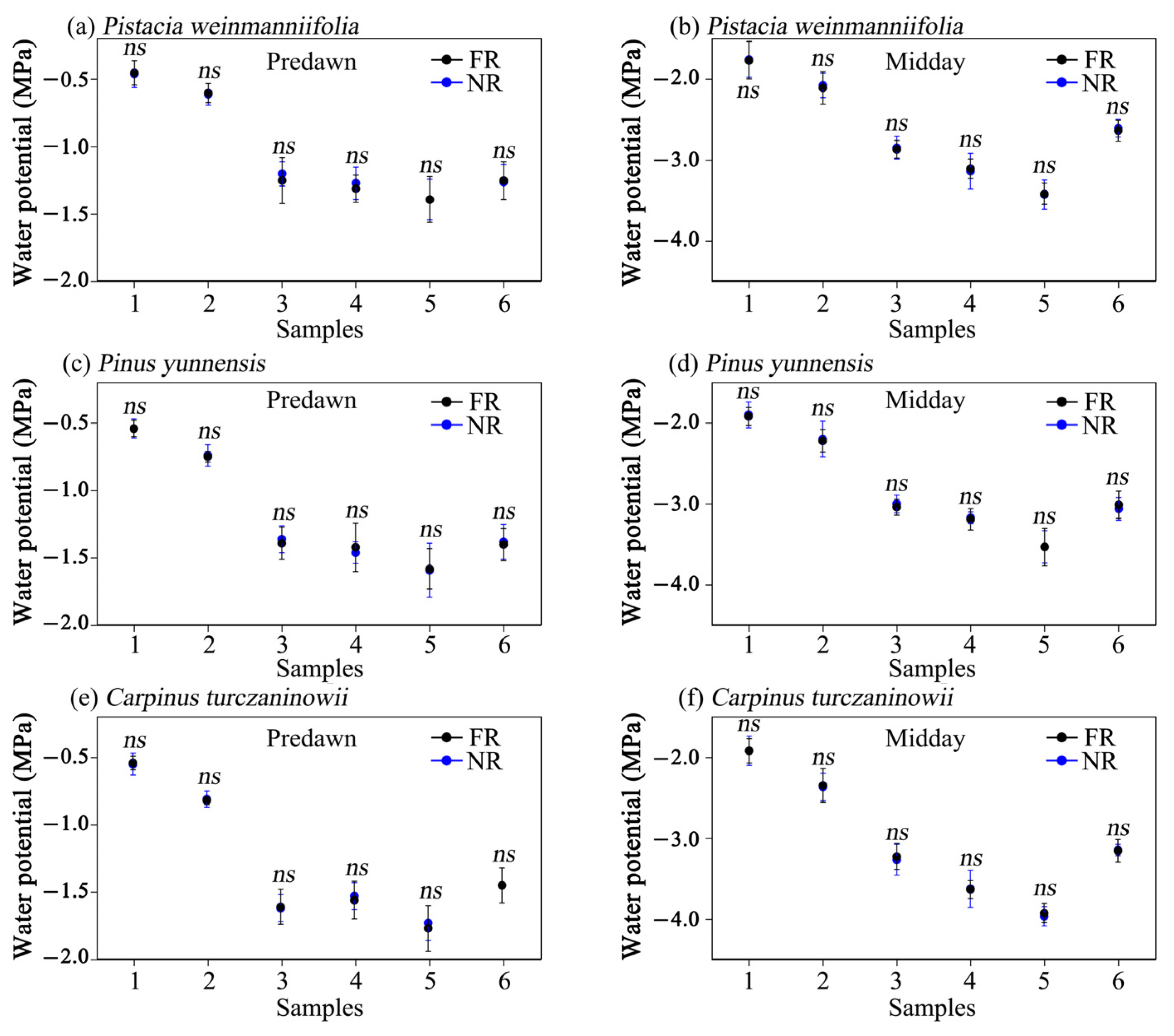
3.4. Leaf Water Potential
4. Discussion
4.1. The Influence of Aboveground Rocks on Plant Roots
4.2. The Influence of Aboveground Rocks on the Leaf Water Potential
4.3. Implication
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, M.W.; He, H.; Jiang, M.; Wang, Z.K.; Li, G.F.; Zhuang, L. Morphological, physiological and metabolomic analysis to unravel the adaptive relationship between root growth of ephemeral plants and different soil habitats. Plant Physiol. Biochem. 2023, 202, 107986. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lin, E.; Liu, H.G.; Li, Y.J.; Li, Z.J.; Cai, R. Water-nitrogen coupling promotes efficient resource utilization by optimizing cotton root morphology under salt stress. Field Crop. Res. 2025, 325, 109830. [Google Scholar] [CrossRef]
- Liu, W.N.; Chen, H.S.; Zou, Q.Y.; Nie, Y.P. Divergent root water uptake depth and coordinated hydraulic traits among typical karst plantations of subtropical China: Implication for plant water adaptation under precipitation changes. Agric. Water Manag. 2021, 249, 106798. [Google Scholar] [CrossRef]
- Peng, L.; Xu, X.J.; Liao, X.F.; Liu, J.M.; Chen, J.Z. Ampelocalamus luodianensis (Poaceae), a plant endemic to karst, adapts to resource heterogeneity in differing microhabitats by adjusting its biomass allocation. Glob. Ecol. Conserv. 2023, 41, e02374. [Google Scholar] [CrossRef]
- Luo, Z.D.; Nie, Y.P.; Ding, Y.L.; Chen, H.S. Replenishment and mean residence time of root-zone water for woody plants growing on rocky outcrops in a subtropical karst critical zone. J. Hydrol. 2021, 603, 127136. [Google Scholar] [CrossRef]
- Nie, Y.P.; Chen, H.S.; Wang, K.L.; Ding, Y.L. Rooting characteristics of two widely distributed woody plant species growing in different karst habitats of southwest China. Plant Ecol. 2014, 215, 1099–1109. [Google Scholar] [CrossRef]
- Jiang, Z.C.; Lian, Y.Q.; Qin, X.Q. Rocky desertification in Southwest China: Impacts, causes, and restoration. Earth Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Zeng, F.M.; Jiang, Z.C.; Shen, L.N.; Chen, W.; Yang, Q.Y.; Zhang, C. Assessment of multiple and interacting modes of soil loss in the karst critical zone, Southwest China (SWC). Geomorphology 2018, 322, 97–106. [Google Scholar] [CrossRef]
- Yan, Y.J.; Dai, Q.H.; Hu, G.; Jiao, Q.; Mei, L.N.; Fu, W.B. Effects of vegetation type on the microbial characteristics of the fissure soil-plant systems in karst rocky desertification regions of SW China. Sci. Total Environ. 2020, 712, 136543. [Google Scholar] [CrossRef]
- Li, Y.; Li, K.P.; Zhou, Q.W.; Zhao, Y.L.; Cai, L.L.; Yang, Z.Y. Spatiotemporal dynamics and similarity in soil moisture in shallow soils on karst slopes. J. Hydrol. 2024, 639, 131655. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Fu, Z.Y.; Wang, F.; Wang, K.L.; Chen, H.S. Soil thickness influences the control effect of micro-topography on subsurface runoff generation in the karst hillslope critical zone. Catena 2024, 239, 107957. [Google Scholar] [CrossRef]
- Li, C.; Zhang, S.Q.; Ding, Y.J.; Ma, S.Y.; Gong, H.Y. Nonlinear influences of climatic, vegetative, geographic and soil factors on soil water use efficiency of global karst landscapes: Insights from explainable machine learning. Sci. Total Environ. 2025, 965, 178672. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, K.L.; Wang, G.P. Dynamic response of soil water to rainfall for different runoff plots on a karst hillslope. Geoderma 2025, 460, 117394. [Google Scholar] [CrossRef]
- Zhao, Z.M.; Shen, Y.X.; Wang, Q.H.; Jiang, R.H. The temporal stability of soil moisture spatial pattern and its influencing factors in rocky environments. Catena 2020, 187, 104418. [Google Scholar] [CrossRef]
- Xiao, L.L.; Li, R.; Cai, F.Y.; Wu, P.P.; Pan, L.H. Effects of vegetation cover and rock embedding landscape types and configurations on regulating runoff generation and sediment yields in karst slope under simulated experiment. J. Hydrol. 2025, 661, 133681. [Google Scholar] [CrossRef]
- Li, Y.Q.; Wang, S.J.; Peng, T.; Zhao, G.Z.; Dai, B. Hydrological characteristics and available water storage of typical karst soil in SW China under different soil–rock structures. Geoderma 2023, 438, 116633. [Google Scholar] [CrossRef]
- Zhou, G.H.; Long, F.Y.; Zu, L.; Jarvie, S.; Peng, Y.; Zang, L.P.; Chen, D.M.; Zhang, G.Q.; Sui, M.Z.; He, Y.J.; et al. Stand spatial structure and microbial diversity are key drivers of soil multifunctionality during secondary succession in degraded karst forests. Sci. Total Environ. 2024, 937, 173504. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.X.; Wang, P.; Ou, H.B.; Wei, S.X.; Wu, L.C.; Jiang, Y.; Wang, R.J.; Liu, X.S.; Wang, Z.H.; Chen, L.J.; et al. Effects of different vegetation restoration on soil nutrients, enzyme activities, and microbial communities in degraded karst landscapes in southwest China. For. Ecol. Manag. 2022, 508, 120002. [Google Scholar] [CrossRef]
- Kumilamba, G.; Liu, S.J.; Wang, Z.J.; Li, J.H.; Zhan, Y.J.; Lu, X.Q. Understory biodiversity in Karst forests: A comparison of pure (Pinus massoniana) and mixed forests in Guiyang, China. For. Ecol. Manag. 2025, 588, 122753. [Google Scholar] [CrossRef]
- Liu, W.N.; Behzad, H.M.; Luo, Z.D.; Huang, L.; Nie, Y.P.; Chen, H.S. Species-Specific Root Distribution and Leaf Iso/Anisohydric Tendencies Shape Transpiration Patterns Across Heterogeneous Karst Habitats. Plant Cell Environ. 2025, 48, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, J.C.; Zhao, Y.J.; Song, H.Y.; Liang, Q.H.; Tao, J.P. Adaption of two grasses to soil thickness variation under different water treatments in a karst region. Acta Ecol. Sin. 2017, 37, 298–306. [Google Scholar]
- Yan, Y.J.; Dai, Q.H.; Yang, Y.Q.; Yan, L.B.; Yi, X.S. Epikarst shallow fissure soil systems are key to eliminating karst drought limitations in the karst rocky desertification area of SW China. Ecohydrology 2022, 15, e2372. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Zhao, P.; Zhang, Z.F.; Li, X.K.; He, C.X.; Zhang, R.Q. Transpiration of Cyclobalanopsis glauca (syn. Quercus glauca) stand measured by sap-flow method in a karst rocky terrain during dry season. Ecol. Res. 2009, 24, 791–801. [Google Scholar] [CrossRef]
- Ding, Y.L.; Nie, Y.P.; Chen, H.S.; Wang, K.L.; Querejeta, J.I. Water uptake depth is coordinated with leaf water potential, water-use efficiency and drought vulnerability in karst vegetation. New Phytol. 2021, 229, 1339–1353. [Google Scholar] [CrossRef] [PubMed]
- Fitter, A.H.; Stickland, T.R.; Harvey, M.L.; Wilson, G.W. Architectural analysis of plant root systems 1. Architectural correlates of exploitation efficiency. New Phytol. 1991, 118, 375–382. [Google Scholar] [CrossRef]
- Fitter, A.H.; Stickland, T.R. Architectural analysis of plant root systems 2. Influence of nutrient supply on architecture in contrasting plant species. New Phytol. 1991, 118, 383–389. [Google Scholar] [CrossRef]
- Fitter, A.H.; Stickland, T.R. Architectural analysis of plant root systems III. Studies on plants under field conditions. New Phytol. 1992, 121, 243–248. [Google Scholar] [CrossRef]
- Gwenzi, W.; Veneklaas, E.J.; Holmes, K.W.; Bleby, T.M.; Phillips, I.R.; Hinz, C. Spatial analysis of fine root distribution on a recently constructed ecosystem in a water-limited environment. Plant Soil 2011, 348, 471–489. [Google Scholar] [CrossRef]
- Hodge, A. The plastic plant: Root responses to heterogeneous supplies of nutrients. New Phytol. 2004, 162, 9–24. [Google Scholar] [CrossRef]
- Huang, T.L.; Tang, L.X.; Chen, L.; Zhang, Q.Y. Root architecture and ecological adaptation strategy of three shrubs in karst area. Sci. Soil Water Conserv. 2019, 17, 89–94. (In Chinese) [Google Scholar]
- Fu, Y.H.; Zhang, D.J.; Xiang, J.; Zhou, Y.; Huang, Z.S.; Yu, L.F. Topological structure of plant roots of different underground habitat profiles in karst areas. Ecol. Environ. Sci. 2022, 31, 865–874. (In Chinese) [Google Scholar]
- Su, L.; Du, H.; Wang, H.; Zeng, F.P.; Song, T.Q.; Peng, W.X.; Chen, L.; Zhang, F. Root architecture of the dominant species in various vegetation restoration processes in karst peak-cluster depression. Acta Bot. Boreali Occident. Sin. 2018, 38, 150–157. (In Chinese) [Google Scholar]
- Peng, X.D.; Wang, X.D.; Dai, Q.H.; Ding, G.J.; Li, C.L. Soil structure and nutrient contents in underground fissures in a rock-mantled slope in the karst rocky desertification area. Environ. Earth Sci. 2020, 79, 3. [Google Scholar] [CrossRef]
- Peng, X.D.; Dai, Q.H.; Ding, G.J.; Shi, D.M.; Li, C.L. The role of soil water retention functions of near-surface fissures with different vegetation types in a rocky desertification area. Plant Soil 2019, 441, 587–599. [Google Scholar] [CrossRef]
- Luo, J.; Luo, W.X.; Liu, J.T.; Wang, Y.J.; Li, Z.F.; Tao, J.P.; Liu, J.C. Karst fissures mitigate the negative effects of drought on plant growth and photosynthetic physiology. Oecologia 2024, 205, 69–80. [Google Scholar] [CrossRef]
- Liu, T.T.; Peng, X.D.; Dai, Q.H.; Xu, S.B. Role of the preferential flow at rock–soil interface in the water leaking process in near-surface fissures filled with soils in the karst rock desertification area. Appl. Water Sci. 2022, 12, 208. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, H.S.; Wang, K.L.; Su, Y.R.; Zhang, J.G.; Yi, A.J. The heterogeneity and its influencing factors of soil nutrients in peak-cluster depression areas of karst region. Agr. Sci. China 2007, 6, 322–329. [Google Scholar] [CrossRef]
- Shen, Y.X.; Wang, D.J.; Chen, Q.Q.; Tang, Y.Y.; Chen, F.J. Large heterogeneity of water and nutrient supply derived from runoff of nearby rock outcrops in karst ecosystems in SW China. Catena 2019, 172, 125–131. [Google Scholar] [CrossRef]
- Li, Q.; Umer, M.; Guo, Y.; Shen, K.P.; Xia, T.T.; Xu, X.Y.; Han, X.; Ren, W.D.; Sun, Y.; Wu, B.L.; et al. Karst soil patch heterogeneity with gravels promotes plant root development and nutrient utilization associated with arbuscular mycorrhizal fungi. Agronomy 2022, 12, 1063. [Google Scholar] [CrossRef]
- Liang, Y.M.; Pan, F.J.; Jiang, Z.C.; Li, Q.; Pu, J.B.; Liu, K.P. Accumulation in nutrient acquisition strategies of arbuscular mycorrhizal fungi and plant roots in poor and heterogeneous soils of karst shrub ecosystems. BMC Plant Biol. 2022, 22, 188. [Google Scholar] [CrossRef]
- Zhao, Z.M.; Zhang, J.; Liu, R. Application of overground rock film mulching (ORFM) technology in karst rocky desertification farmland: Improving soil moisture environment and crop root growth. Agronomy 2024, 14, 1265. [Google Scholar] [CrossRef]
- Fan, C.H.; Zhao, L.S.; Hou, R.; Fang, Q.; Zhang, J.X. Quantitative analysis of rainwater redistribution and soil loss at the surface and belowground on karst slopes at the microplot scale. Catena 2023, 227, 107113. [Google Scholar] [CrossRef]
- Hou, F.; Cheng, J.H.; Zhang, H.; Wang, X.L.; Shi, D.W.; Guan, N. Response of preferential flow to soil–root–rock fragment system in karst rocky desertification areas. Ecol. Indic. 2024, 165, 112234. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Li, Z.; Zhang, J.; Song, H.Y.; Liang, Q.H.; Tao, J.P.; Cornelissen, J.H.C.; Liu, J.C. Do shallow soil, low water availability, or their combination increase the competition between grasses with different root systems in karst soil? Environ. Sci. Pollut. Res. 2017, 24, 10640–10651. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wei, X.; Zhou, Z.; Shao, C.; Su, S. Influence of heterogeneous karst microhabitats on the root foraging ability of Chinese Windmill Palm (Trachycarpus fortunei) seedlings. Int. J. Env. Res. Public Health 2020, 17, 434. [Google Scholar] [CrossRef]
- Ni, J.; Luo, D.H.; Xia, J.; Zhang, Z.H.; Hu, G. Vegetation in karst terrain of southwestern China allocates more biomass to roots. Solid Earth 2015, 6, 799–810. [Google Scholar] [CrossRef]
- Pan, F.J.; Qian, Q.; Liang, Y.M.; Wang, K.L.; Zhang, W. Spatial variations in fine root turnover, biomass, and necromass of two vegetation types in a karst ecosystem, Southwestern China. Forests 2022, 13, 611. [Google Scholar] [CrossRef]
- Shen, Y.X.; Wang, Q.H.; Zhao, Z.M.; Yuan, C. Fine-scale effect of karst rock outcrops on adjacent soil and plant communities in Southwest China. Catena 2022, 219, 106592. [Google Scholar] [CrossRef]
- Moore, O.W.; Buss, H.L.; Green, S.M.; Liu, M.; Song, Z.L. The importance of non-carbonate mineral weathering as a soil formation mechanism within a karst weathering profile in the SPECTRA Critical Zone Observatory, Guizhou Province, China. Acta Geochim. 2017, 36, 566–571. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.M.; Chen, J.Y.; Song, H.Y.; Li, S.H.; Zhao, Y.J.; Tao, J.P.; Liu, J.C. Soil moisture determines horizontal and vertical root extension in the perennial grass Lolium perenne L. growing in karst soil. Front. Plant Sci. 2019, 10, 629. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Liu, Y.G.; Guo, K. Ecophysiological adaptations to drought stress of seedlings of four plant species with different growth forms in karst habitats. Chin. J. Plant Ecol. 2011, 35, 1070. (In Chinese) [Google Scholar]
- Bucci, S.J.; Goldstein, G.; Meinzer, F.C.; Franco, A.C.; Campanello, P.; Scholz, F.G. Mechanisms contributing to seasonal homeostasis of minimum leaf water potential and predawn disequilibrium between soil and plant water potential in Neotropical savanna tree. Trees 2005, 19, 296–304. [Google Scholar] [CrossRef]
- Prior, L.D.; Eamus, D.; Duff, G.A. Seasonal and diurnal patterns of carbon assimilation, stomatal conductance and leaf water potential in Eucalyptus tetrodonta saplings in a wet–dry savanna in northern Australia. Aust. J. Bot. 1997, 45, 241–258. [Google Scholar] [CrossRef]
- Zhao, Z.M.; Shen, Y.X.; Jiang, R.H.; Wang, Q.H. Rock outcrops change infiltrability and water flow behavior in a karst soil. Vadose Zone J. 2020, 19, e20002. [Google Scholar] [CrossRef]
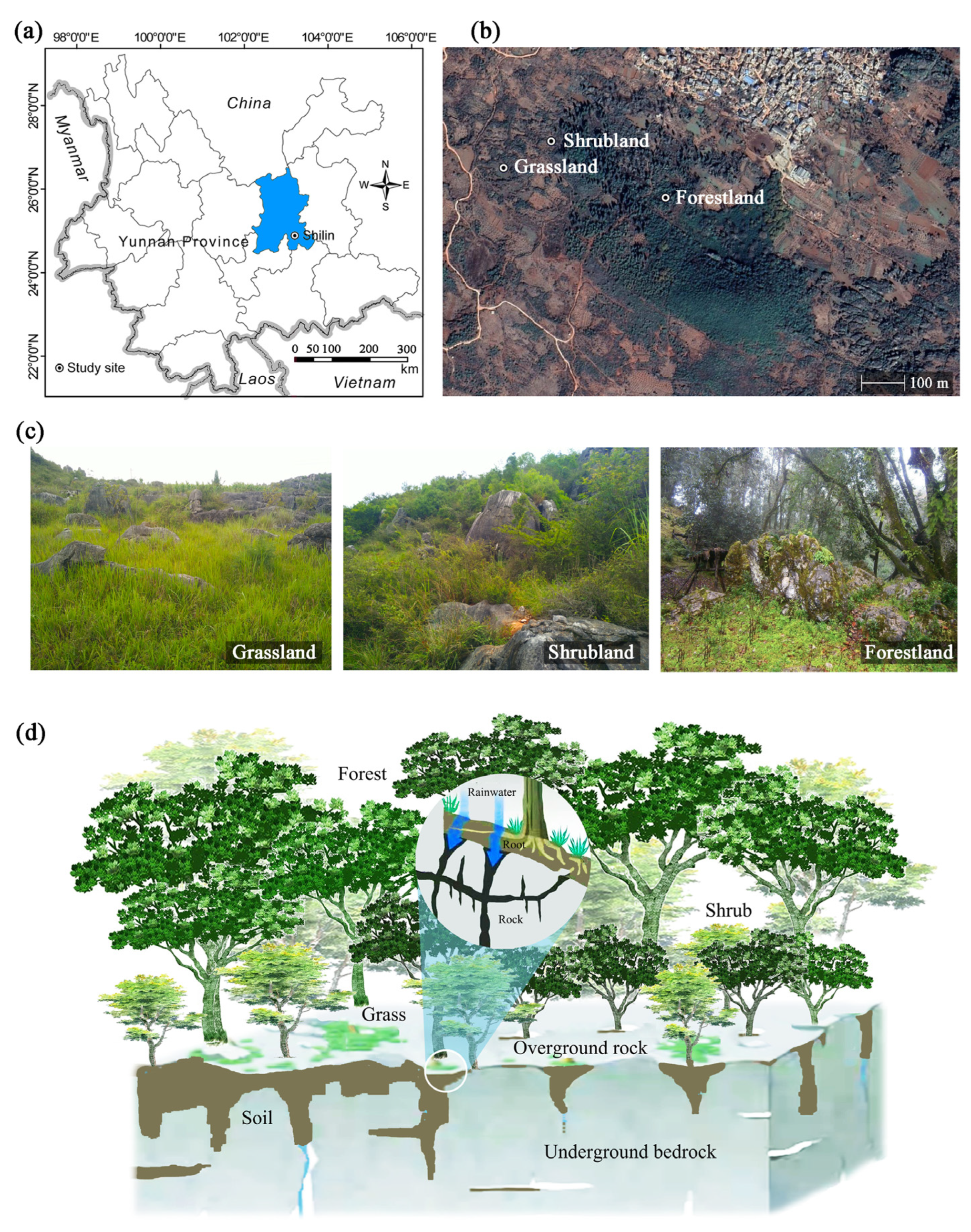
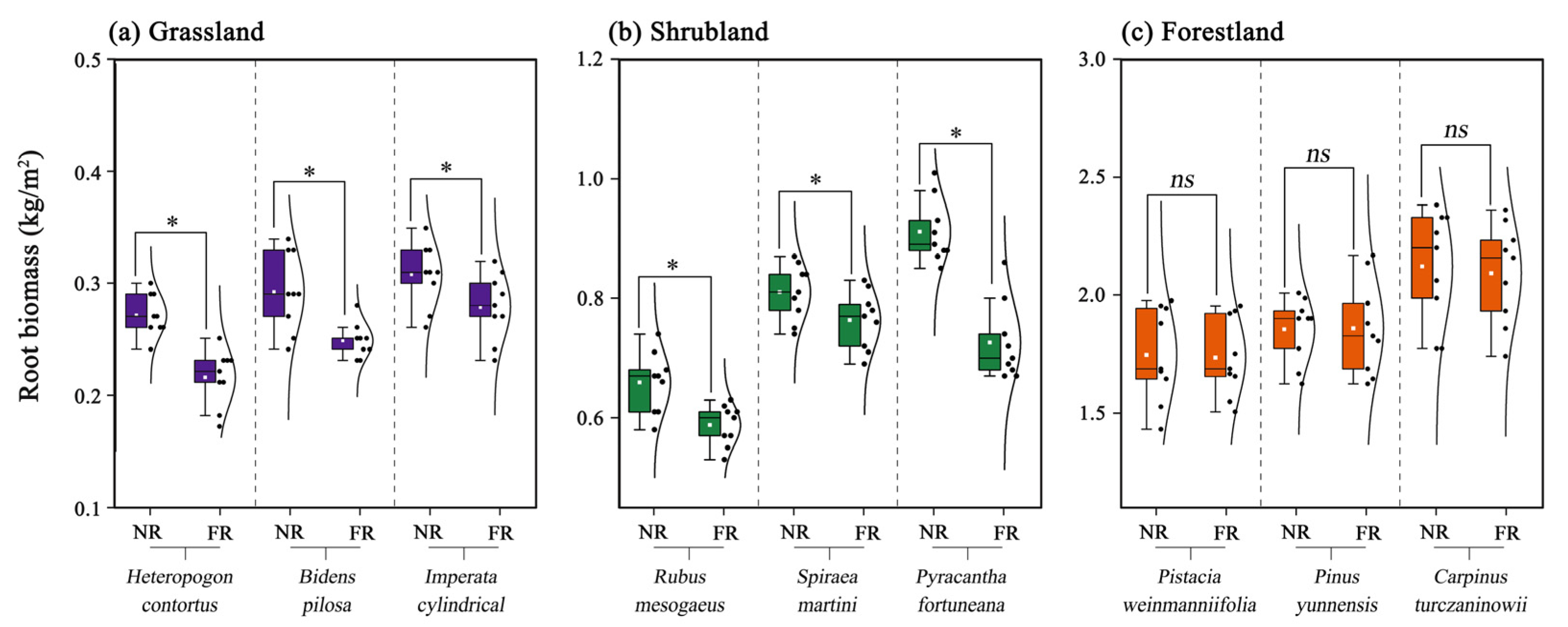
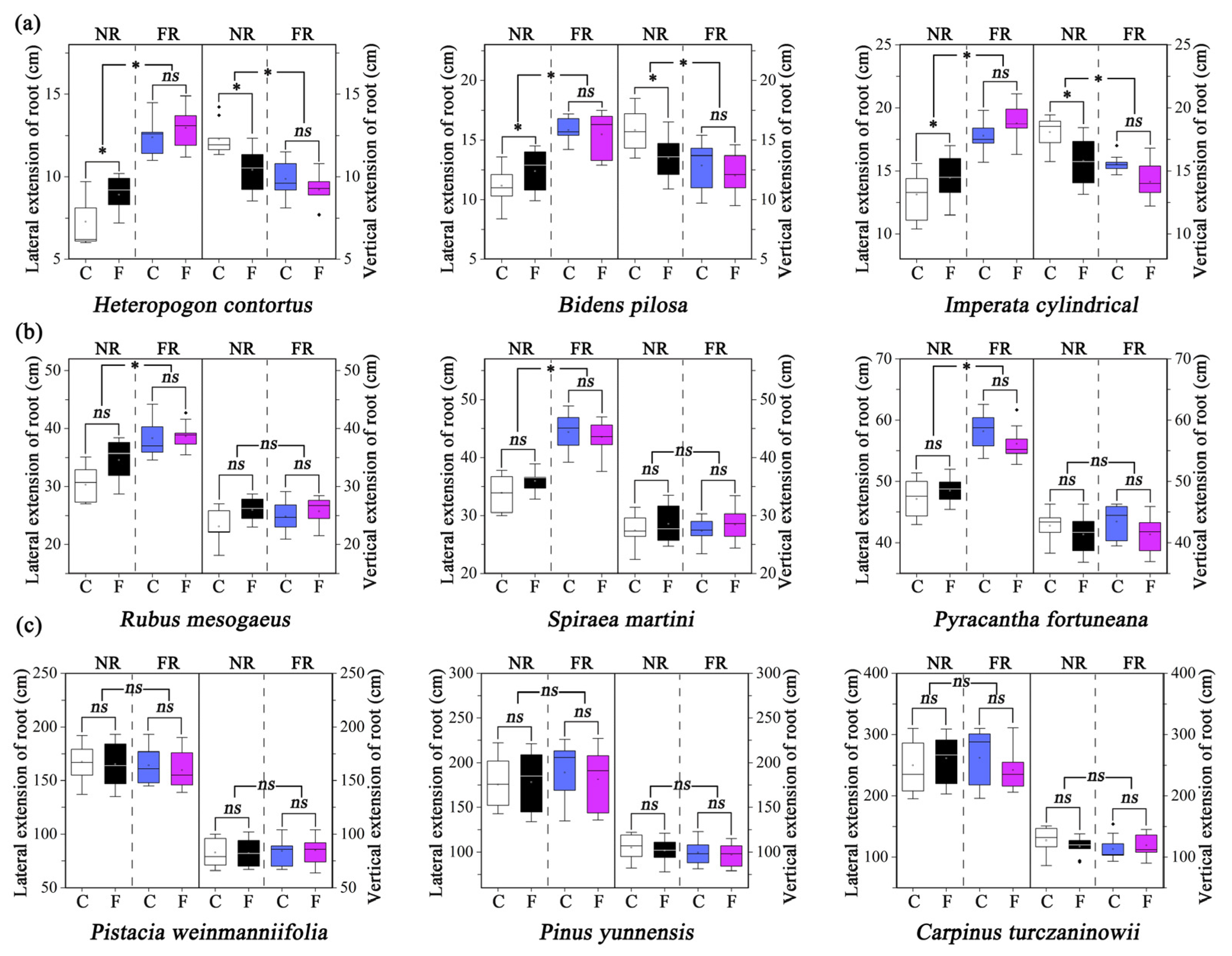
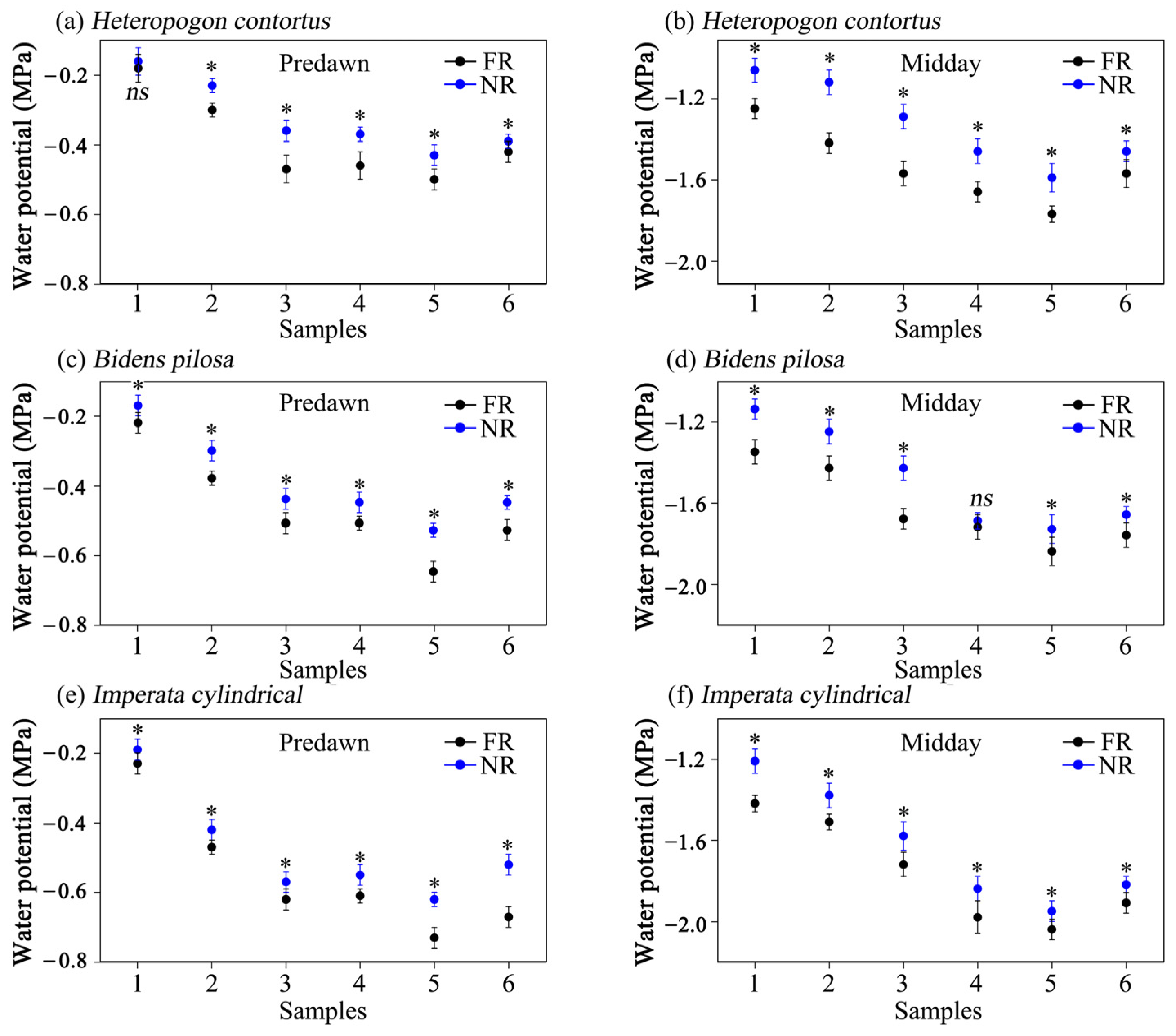
| Species | Treatment | Rb | Ri:Ri+1 | |
|---|---|---|---|---|
| R1:R2 | R2:R3 | |||
| Heteropogon contortus | NR | 2.31 ± 0.22 a | 1.67 ± 0.28 a | 3.22 ± 0.15 a |
| FR | 2.13 ± 0.41 b | 1.52 ± 0.33 b | 3.01 ± 0.55 b | |
| Bidens pilosa | NR | 2.24 ± 0.39 a | 1.60 ± 0.30 a | 3.14 ± 0.54 a |
| FR | 2.04 ± 0.33 b | 1.50 ± 0.36 b | 2.81 ± 0.30 b | |
| Imperata cylindrical | NR | 2.51 ± 0.39 a | 1.92 ± 0.32 a | 3.31 ± 0.52 a |
| FR | 2.16 ± 0.34 b | 1.50 ± 0.32 b | 3.13 ± 0.37 b | |
| ANOVA | Treatment | 0.016 | 0.015 | 0.046 |
| Species | 0.264 | 0.324 | 0.249 | |
| Treatment × Species | 0.727 | 0.282 | 0.864 | |
| Species | Treatment | Rb | Ri:Ri+1 | ||
|---|---|---|---|---|---|
| R1:R2 | R2:R3 | R3:R4 | |||
| Rubus mesogaeus | NR | 3.03 ± 0.23 a | 1.52 ± 0.15 a | 3.04 ± 0.26 a | 6.04 ± 0.66 a |
| FR | 2.72 ± 0.17 b | 1.40 ± 0.14 b | 2.83 ± 0.34 b | 5.09 ± 0.50 b | |
| Spiraea martini | NR | 2.86 ± 0.33 a | 1.39 ± 0.18 a | 2.93 ± 0.42 a | 5.75 ± 0.82 a |
| FR | 2.61 ± 0.20 b | 1.26 ± 0.11 b | 2.61 ± 0.31 b | 5.44 ± 0.64 b | |
| Pyracantha fortuneana | NR | 3.08 ± 0.25 a | 1.54 ± 0.21 a | 3.17 ± 0.20 a | 6.00 ± 0.78 a |
| FR | 2.79 ± 0.23 b | 1.37 ± 0.16 b | 2.93 ± 0.31 b | 5.43 ± 0.82 b | |
| ANOVA | Treatment | <0.001 | 0.002 | 0.004 | 0.003 |
| Species | 0.042 | 0.024 | 0.032 | 0.796 | |
| Treatment × Species | 0.947 | 0.833 | 0.873 | 0.407 | |
| Species | Treatment | Rb | Ri:Ri+1 | |||
|---|---|---|---|---|---|---|
| R1:R2 | R2:R3 | R3:R4 | R4:R5 | |||
| Pistacia weinmanniifolia | NR | 2.17 ± 0.13 a | 1.23 ± 0.09 a | 1.96 ± 0.14 a | 2.68 ± 0.17 a | 3.27 ± 0.22 a |
| FR | 2.17 ± 0.09 a | 1.22 ± 0.06 a | 1.92 ± 0.12 a | 2.70 ± 0.15 a | 3.33 ± 0.19 a | |
| Pinus yunnensis | NR | 2.36 ± 0.19 a | 1.43 ± 0.12 a | 2.13 ± 0.18 a | 2.82 ± 0.26 a | 3.49 ± 0.30 a |
| FR | 2.38 ± 0.15 a | 1.43 ± 0.12 a | 2.15 ± 0.22 a | 2.88 ± 0.14 a | 3.47 ± 0.21 a | |
| Carpinus turczaninowii | NR | 2.56 ± 0.15 a | 1.67 ± 0.09 a | 2.33 ± 0.15 a | 2.97 ± 0.19 a | 3.61 ± 0.31 a |
| FR | 2.47 ± 0.18 a | 1.55 ± 0.11 a | 2.28 ± 0.21 a | 2.85 ± 0.21 a | 3.60 ± 0.35 a | |
| ANOVA | Treatment | 0.560 | 0.155 | 0.535 | 0.790 | 0.868 |
| Species | <0.001 | <0.001 | <0.001 | 0.003 | 0.006 | |
| Treatment × Species | 0.542 | 0.135 | 0.785 | 0.333 | 0.876 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Zhang, J. The Response Mechanism of Plants Under Rock Stress in Karst Ecosystem: A Study Based on the Effects of Aboveground Rocks on Root Phenotypes and Leaf Water Potential. Forests 2025, 16, 1313. https://doi.org/10.3390/f16081313
Zhao Z, Zhang J. The Response Mechanism of Plants Under Rock Stress in Karst Ecosystem: A Study Based on the Effects of Aboveground Rocks on Root Phenotypes and Leaf Water Potential. Forests. 2025; 16(8):1313. https://doi.org/10.3390/f16081313
Chicago/Turabian StyleZhao, Zhimeng, and Jin Zhang. 2025. "The Response Mechanism of Plants Under Rock Stress in Karst Ecosystem: A Study Based on the Effects of Aboveground Rocks on Root Phenotypes and Leaf Water Potential" Forests 16, no. 8: 1313. https://doi.org/10.3390/f16081313
APA StyleZhao, Z., & Zhang, J. (2025). The Response Mechanism of Plants Under Rock Stress in Karst Ecosystem: A Study Based on the Effects of Aboveground Rocks on Root Phenotypes and Leaf Water Potential. Forests, 16(8), 1313. https://doi.org/10.3390/f16081313






